Abstract
The 2015 HRS/EHRA/APHRS/SOLAECE Expert Consensus Statement on Optimal Implantable Cardioverter-Defibrillator Programming and Testing provided guidance on bradycardia programming, tachycardia detection, tachycardia therapy, and defibrillation testing for implantable cardioverter-defibrillator (ICD) patient treatment. The 32 recommendations represented the consensus opinion of the writing group, graded by Class of Recommendation and Level of Evidence. In addition, Appendix B provided manufacturer-specific translations of these recommendations into clinical practice consistent with the recommendations within the parent document. In some instances, programming guided by quality evidence gained from studies performed in devices from some manufacturers was translated such that this programming was approximated in another manufacturer’s ICD programming settings. The authors found that the data, although not formally tested, were strong, consistent, and generalizable beyond the specific manufacturer and model of ICD. As expected, because these recommendations represented strategic choices to balance risks, there have been reports in which adverse outcomes were documented with ICDs programmed to Appendix B recommendations. The recommendations have been reviewed and updated to minimize such adverse events. Notably, patients who do not receive unnecessary ICD therapy are not aware of being spared potential harm, whereas patients in whom their ICD failed to treat life-threatening arrhythmias have their event recorded in detail. The revised recommendations employ the principle that the randomized trials and large registry data should guide programming more than anecdotal evidence. These recommendations should not replace the opinion of the treating physician who has considered the patient’s clinical status and desired outcome via a shared clinical decision-making process.
Keywords: Antitachycardia pacing, Bradycardia mode and rate, Defibrillation testing, Implantable cardioverter-defibrillator, Programming, Sudden cardiac death, Tachycardia detection, Tachycardia therapy, Ventricular tachycardia, Ventricular fibrillation
Document Reviewers: Serge Boveda, MD, PhD; Michael R. Gold, MD, PhD, FHRS; Roberto Keegan, MD; Valentina Kutyifa, MD, PhD, FHRS, FESC, FACC; Chu-Pak Lau, MD, FHRS, CCDS; Mark A. McGuire, MBBS, PhD; Siva K. Mulpuru, MD, FHRS, CCDS; David J. Slotwiner, MD, FHRS; William Uribe, MD, MBA, FHRS.
TABLE OF CONTENTS
Abstract………………………………………In this issue
Manufacturer-Specific Translation of ICD Programming Recommendations: Abbott (Formerly St. Jude Medical)………………………………………....In this issue
Manufacturer-Specific Translation of ICD Programming Recommendations: BIOTRONIK……………….In this issue
Manufacturer-Specific Translation of ICD Programming Recommendations: Boston Scientific……………In this issue
Manufacturer-Specific Translation of ICD Programming Recommendations: Medtronic…………………..In this issue
Manufacturer-Specific Translation of ICD Programming Recommendations: MicroPort CRM (Formerly LivaNova and Sorin Group)………………………………...In this issue
Appendix 1 Author disclosure table………….In this issue
Appendix 2 Reviewer disclosure table………..In this issue
Manufacturer-specific translation of ICD programming recommendations‡
‡The manufacturer-specific programming settings/choices set forth below are based on a compilation of clinical expertise and clinical trial data as reported in the 2015 HRS/EHRA/APHRS/SOLAECE Expert Consensus Statement on Optimal Implantable Cardioverter-Defibrillator Programming and Testing, of which this Appendix B is a part. These recommended settings/choices represent a diligent and good faith effort on the part of the writing committee to translate the consensus statement recommendations to device settings/choices for the four specified clinical issues/implantable cardioverter-defibrillator (ICD) therapies where the writing committee considered that there was sufficient consensus and supporting data to make recommendations intended to improve the safety, morbidity, and mortality profile of patients with these clinical issues/ICD therapies. They are the recommendations of the writing committee only. They do not represent the position or recommendations of HRS, EHRA, LAHRS (formerly SOLAECE), or APHRS, nor are they the manufacturer’s nominal settings or the precise programming tested during clinical trials of these devices, nor are they necessarily the settings/choices that would be recommended by the manufacturer. These recommended settings/choices are not applicable in all circumstances. As stated in the Introduction to the consensus statement, “The care of individual patients must be provided in context of their specific clinical condition and the data available on that patient.” Each treating physician must carefully consider the circumstances of their individual patient and determine whether these recommended settings/choices are appropriate to their patient’s circumstances.
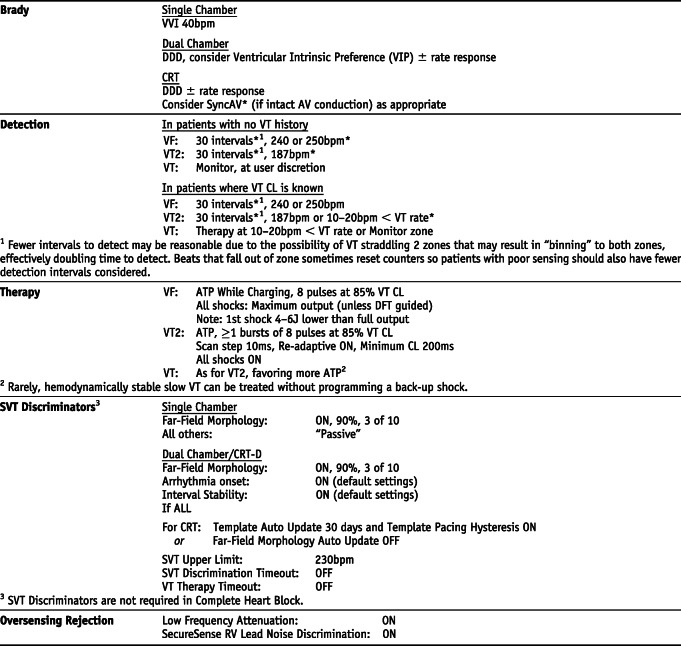
Abbott (Formerly St. Jude Medical) *Settings that are not nominal are marked with an asterisk
Manufacturer-specific translation of ICD programming recommendations‡
‡The manufacturer-specific programming settings/choices set forth below are based on a compilation of clinical expertise and clinical trial data as reported in the 2015 HRS/EHRA/APHRS/SOLAECE Expert Consensus Statement on Optimal Implantable Cardioverter-Defibrillator Programming and Testing, of which this Appendix B is a part. These recommended settings/choices represent a diligent and good faith effort on the part of the writing committee to translate the consensus statement recommendations to device settings/choices for the four specified clinical issues/implantable cardioverter-defibrillator (ICD) therapies where the writing committee considered that there was sufficient consensus and supporting data to make recommendations intended to improve the safety, morbidity, and mortality profile of patients with these clinical issues/ICD therapies. They are the recommendations of the writing committee only. They do not represent the position or recommendations of HRS, EHRA, LAHRS (formerly SOLAECE), or APHRS, nor are they the manufacturer’s nominal settings or the precise programming tested during clinical trials of these devices, nor are they necessarily the settings/choices that would be recommended by the manufacturer. These recommended settings/choices are not applicable in all circumstances. As stated in the Introduction to the consensus statement, “The care of individual patients must be provided in context of their specific clinical condition and the data available on that patient.” Each treating physician must carefully consider the circumstances of their individual patient and determine whether these recommended settings/choices are appropriate to their patient’s circumstances.
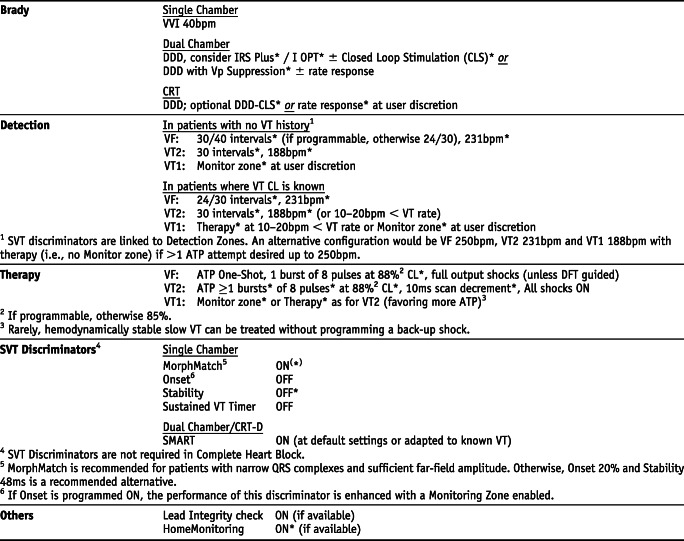
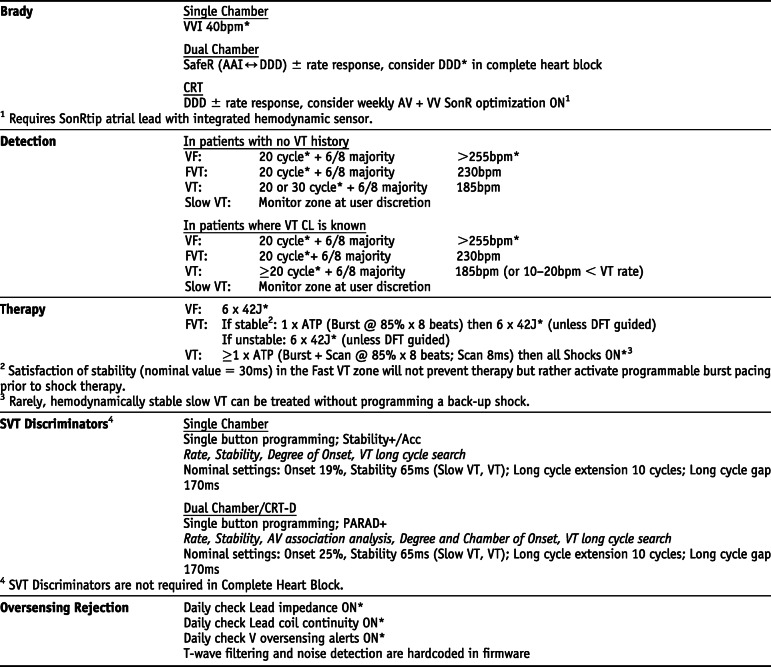
BIOTRONIK *Settings that are not nominal are marked with an asterisk
Manufacturer-specific translation of ICD programming recommendations‡
‡The manufacturer-specific programming settings/choices set forth below are based on a compilation of clinical expertise and clinical trial data as reported in the 2015 HRS/EHRA/APHRS/SOLAECE Expert Consensus Statement on Optimal Implantable Cardioverter-Defibrillator Programming and Testing, of which this Appendix B is a part. These recommended settings/choices represent a diligent and good faith effort on the part of the writing committee to translate the consensus statement recommendations to device settings/choices for the four specified clinical issues/implantable cardioverter-defibrillator (ICD) therapies where the writing committee considered that there was sufficient consensus and supporting data to make recommendations intended to improve the safety, morbidity, and mortality profile of patients with these clinical issues/ICD therapies. They are the recommendations of the writing committee only. They do not represent the position or recommendations of HRS, EHRA, LAHRS (formerly SOLAECE), or APHRS, nor are they the manufacturer’s nominal settings or the precise programming tested during clinical trials of these devices, nor are they necessarily the settings/choices that would be recommended by the manufacturer. These recommended settings/choices are not applicable in all circumstances. As stated in the Introduction to the consensus statement, “The care of individual patients must be provided in context of their specific clinical condition and the data available on that patient.” Each treating physician must carefully consider the circumstances of their individual patient and determine whether these recommended settings/choices are appropriate to their patient’s circumstances.
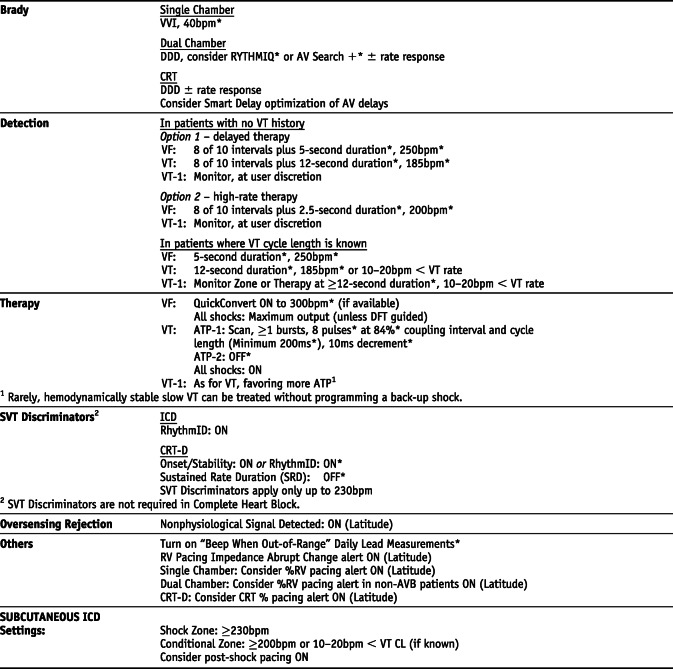
Boston Scientific *Settings that are not nominal are marked with an asterisk
Manufacturer-specific translation of ICD programming recommendations‡
‡The manufacturer-specific programming settings/choices set forth below are based on a compilation of clinical expertise and clinical trial data as reported in the 2015 HRS/EHRA/APHRS/SOLAECE Expert Consensus Statement on Optimal Implantable Cardioverter-Defibrillator Programming and Testing, of which this Appendix B is a part. These recommended settings/choices represent a diligent and good faith effort on the part of the writing committee to translate the consensus statement recommendations to device settings/choices for the four specified clinical issues/implantable cardioverter-defibrillator (ICD) therapies where the writing committee considered that there was sufficient consensus and supporting data to make recommendations intended to improve the safety, morbidity, and mortality profile of patients with these clinical issues/ICD therapies. They are the recommendations of the writing committee only. They do not represent the position or recommendations of HRS, EHRA, LAHRS (formerly SOLAECE), or APHRS, nor are they the manufacturer’s nominal settings or the precise programming tested during clinical trials of these devices, nor are they necessarily the settings/choices that would be recommended by the manufacturer. These recommended settings/choices are not applicable in all circumstances. As stated in the Introduction to the consensus statement, “The care of individual patients must be provided in context of their specific clinical condition and the data available on that patient.” Each treating physician must carefully consider the circumstances of their individual patient and determine whether these recommended settings/choices are appropriate to their patient’s circumstances.
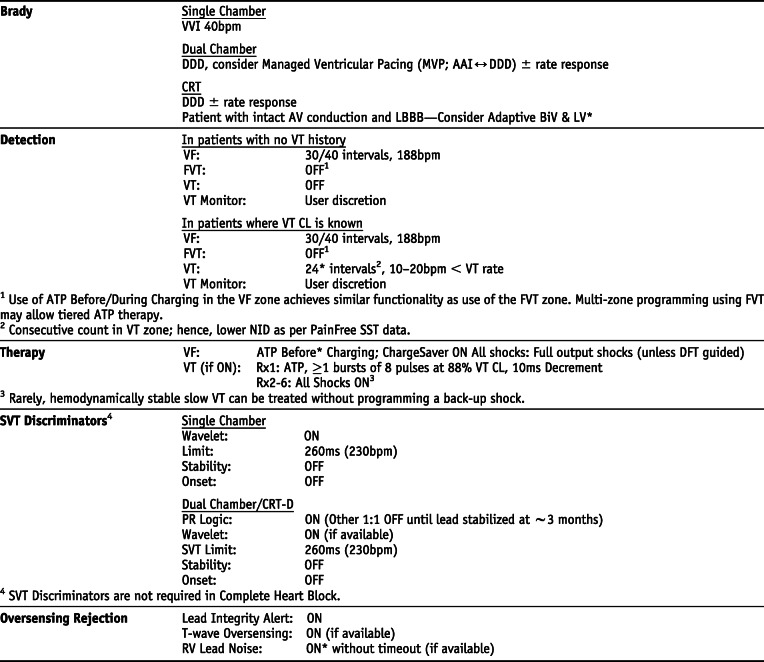
Medtronic *Settings that are not nominal are marked with an asterisk
Manufacturer-specific translation of ICD programming recommendations‡
‡The manufacturer-specific programming settings/choices set forth below are based on a compilation of clinical expertise and clinical trial data as reported in the 2015 HRS/EHRA/APHRS/SOLAECE Expert Consensus Statement on Optimal Implantable Cardioverter-Defibrillator Programming and Testing, of which this Appendix B is a part. These recommended settings/choices represent a diligent and good faith effort on the part of the writing committee to translate the consensus statement recommendations to device settings/choices for the four specified clinical issues/implantable cardioverter-defibrillator (ICD) therapies where the writing committee considered that there was sufficient consensus and supporting data to make recommendations intended to improve the safety, morbidity, and mortality profile of patients with these clinical issues/ICD therapies. They are the recommendations of the writing committee only. They do not represent the position or recommendations of HRS, EHRA, LAHRS (formerly SOLAECE), or APHRS, nor are they the manufacturer’s nominal settings or the precise programming tested during clinical trials of these devices, nor are they necessarily the settings/choices that would be recommended by the manufacturer. These recommended settings/choices are not applicable in all circumstances. As stated in the Introduction to the consensus statement, “The care of individual patients must be provided in context of their specific clinical condition and the data available on that patient.” Each treating physician must carefully consider the circumstances of their individual patient and determine whether these recommended settings/choices are appropriate to their patient’s circumstances.
MicroPort CRM (Formerly LivaNova and Sorin Group) *Settings that are not nominal are marked with an asterisk
Appendix
Appendix 1.
Author disclosure table
| Writing group member | Employment | Honoraria/Speaking/Consulting | Speakers’ bureau | Research* | Fellowship support* | Ownership/Partnership/Principal/Majority stockholder | Stock or stock options | Intellectual property/Royalties | Other |
|---|---|---|---|---|---|---|---|---|---|
| Martin K. Stiles, MBChB, PhD, FHRS (Chair) | Waikato Hospital, Hamilton, New Zealand | None | None | None | None | None | None | None | None |
| Laurent Fauchier, MD, PhD | Centre Hospitalier Universitaire Trousseau, Université François Rabelais, Tours, France |
1: BMS/Pfizer; 1: Boehringer Ingelheim; 1: Medtronic; 1: Novartis; 2: Bayer HealthCare |
None | None | None | None | None | None | None |
| Carlos A. Morillo, MD, FHRS | Libin Cardiovascular Institute of Alberta, University of Calgary, Calgary, Canada |
1: Abbott; 1: Bayer; 1: BMS/Pfizer; 1: Medtronic; 1: Servier |
None | None | None | None | None | None | None |
| Bruce L. Wilkoff, MD, FHRS, CCDS | Cleveland Clinic, Cleveland, Ohio |
1: Abbott Vascular; 2: Medtronic; 2: Philips |
None | None | None | None | None | None | None |
Number value: 0 = $0; 1 = ≤ $10,000; 2 = > $10,000 to ≤ $25,000; 3 = > $25,000 to ≤ $50,000; 4 = > $50,000 to ≤ $100,000; 5 = > $100,000
*Research and fellowship support are classed as programmatic support. Sources of programmatic support are disclosed but are not regarded as a relevant relationship with industry for writing group members or reviewers
Appendix 2.
Reviewer disclosure table
| Peer reviewer | Employment | Honoraria/Speaking/Consulting | Speakers’ bureau | Research* | Fellowship support* | Ownership/Partnership/Principal/Majority stockholder | Stock or stock options | Intellectual property/Royalties | Other |
|---|---|---|---|---|---|---|---|---|---|
| Serge Boveda, MD, PhD | Cardiology Department, Clinique Pasteur, Toulouse, France |
1: Boston Scientific; 1: Medtronic; 1: MicroPort |
None | None | None | None | None | None | None |
| Michael R. Gold, MD, PhD, FHRS | Medical University of South Carolina, Charleston, South Carolina |
1: Abbott Vascular; 1: EBR Systems; 1: Medtronic; 2: Boston Scientific |
None | None | None | None | None | None | 1: ABIM |
| Roberto Keegan, MD | Hospital Privado del Sur and Hospital Español, Bahia Blanca, Argentina | None | None | None | None | None | None | None | None |
| Valentina Kutyifa, MD, PhD, FHRS, FESC, FACC | University of Rochester Medical Center, Rochester, New York; Adjunct Position at Semmelweis University Heart Center, Budapest, Hungary |
1: Biotronik; 1: ZOLL Medical Corporation |
None |
5: Biotronik; 5: Boston Scientific; 5: ZOLL Medical Corporation |
None | None | None | None | None |
| Chu-Pak Lau, MD, FHRS, CCDS | The University of Hong Kong, Hong Kong, Hong Kong | None | None | None | None | None | None | None | None |
| Mark A. McGuire, MBBS, PhD | Heart Rhythm Centre, Newtown, Australia | 1: Medtronic | None | None | None | None | None | None | None |
| Siva K. Mulpuru, MD, FHRS, CCDS | Mayo Clinic Arizona, Phoenix, Arizona | None | 0: Medtronic | None | None | None | None | None | None |
| David J. Slotwiner, MD, FHRS | Weill Cornell Medical College, New York, New York | None | None | None | None | None | None | None | None |
| William Uribe, MD, MBA, FHRS | CES Cardiología, Medellin, Colombia |
1: Abbot Laboratories; 1: Pfizer |
None | None | None | None | None | None | None |
Number value: 0 = $0; 1 = ≤ $10,000; 2 = > $10,000 to ≤ $25,000; 3 = > $25,000 to ≤ $50,000; 4 = > $50,000 to ≤ $100,000; 5 = > $100,000
ABIM = American Board of Internal Medicine
* Research and fellowship support are classed as programmatic support. Sources of programmatic support are disclosed but are not regarded as a relevant relationship with industry for writing group members or reviewers
Footnotes
Martin K. Stiles is the Chair. He is the Representative of the Asia Pacific Heart Rhythm Society (APHRS)
Laurent Fauchier is the Representative of the European Heart Rhythm Association (EHRA)
Carlos A. Morillo is the Representative of the Latin American Heart Rhythm Society (LAHRS)
Bruce L. Wilkoff is the Representative of the Heart Rhythm Society (HRS)
Developed in partnership with and endorsed by the European Heart Rhythm Association (EHRA), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS). For copies of this document, please contact the Elsevier Inc. Reprint Department (reprints@elsevier.com). Permissions: Multiple copies, modification, alteration, enhancement, and/or distribution of this document are not permitted without the express permission of the Heart Rhythm Society. Instructions for obtaining permission are located at https://www.elsevier.com/about/our-business/policies/copyright/permissions. This article has been copublished in Heart Rhythm, Europace, and the Journal of Arrhythmia. Correspondence: Heart Rhythm Society, 1325 G Street NW, Suite 400, Washington, DC 20005. E-mail address: clinicaldocs@hrsonline.org.
Change history
4/16/2020
Springer Nature’s version of this paper was updated to present the correct author list, author affiliations, and correct formatting and location of sections, tables, and figures.