Abstract
Drosophila melanogaster express vesicular transporters for the storage of neurotransmitters acetylcholine, biogenic amines, GABA, and glutamate. The large array of powerful molecular-genetic tools available in Drosophila enhance the use of this model organism for studying transporter function and regulation.
Introduction
Here we provide an overview of vesicular neurotransmitter transporters in Drosophila and include additional background for readers unfamiliar with Drosophila as a model system. Advantages of Drosophila melanogaster for the study of neurotransmitter transporters as well as other transporters include their low cost, a short lifespan, and an ever-growing array of powerful molecular-genetic tools (Bellen et al., 2010; Ugur et al., 2016).
As in mammals, Drosophila vesicular neurotransmitter transporters localize to the membranes of secretory vesicles and are responsible for transport and storage of neurotransmitters into the vesicle lumen (Blakely and Edwards, 2012). Additionally, like mammals, Drosophila vesicular transporters are required for the storage of monoamines in synaptic vesicles (SVs) as well as large dense core vesicles (LDCVs) which also store and release peptide neurotransmitters (Nassel, 2018) (Fig 1). Unlike mammals which express two vesicular monoamine (VMAT) and three vesicular glutamate transporter (VGLUT) genes, flies express a single ortholog of VMAT and VGLUT, as well as a vesicular GABA (VGAT) and vesicular acetylcholine (VAChT) transporter.
Fig 1. Drosophila Vesicular neurotransmitter transporters.
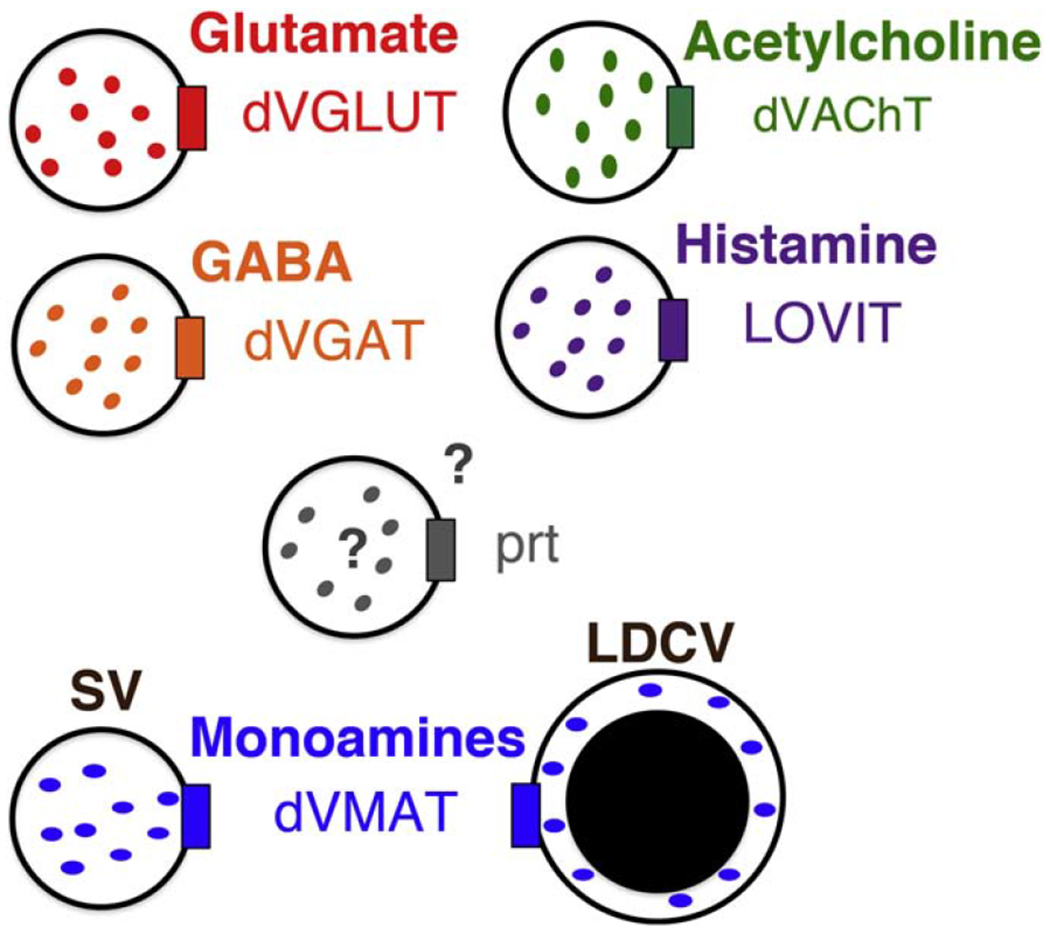
Vesicular neurotransmitter transporters expressed in the fly include one variant each of VGLUT, VGAT, VMAT and VAChT. VMAT localizes to both synaptic and dense core vesicles (SVs and LDCVs respectively) while VGLUT, VAChT and VACHT are primarily confined to SVs. Additional vesicular transporters that are expressed in flies but not mammals include the orphan transporter portabella (prt) and the histamine transporter, LOVIT.
Drosophila VMAT (dVMAT) and dVAChT are members of the SLC18 subfamily of the major facilitator superfamily (MFS), while dVGAT and dVGLUT are members of the SLC32 and SLC17 subfamilies, respectively (Martin and Krantz, 2014). Another member of SLC17 has also been identified in mammals as a vesicular ATP or nucleotide transporter (VNUT) (Sawada et al., 2008); a possible VNUT ortholog is present in the fly genome but has not yet been characterized (Moriyama et al., 2017). In addition to orthologs of VMAT and VAChT, flies express another SLC18 member that is not present in mammals, portabella (prt) (Brooks et al., 2011) and, to date, its substrate remains unknown. Finally, a member of the SLC45 family of putative sugar transporters primarily responsible for proton-mediated sugar transport in mammals, was recently identified as a vesicular histamine transporter in the Drosophila visual system (Xu and Wang, 2019).
A Brief Fly Neurobiology Primer
Fly Life Cycle
Flies pass through several distinct developmental stages prior to adulthood including one day as an embryo (Hartenstein and Wodarz, 2013; Menon et al., 2013), five days through three successive larval stages known as instars and five days as pupae. Vesicular transporters are generally expressed beginning in the late embryonic stage as the nervous system matures and neurons differentiate from precursor cells. Fly neurons support action potentials and calcium-mediated release at the nerve terminal similar to mammals. Unlike mammals, however, most terminals in the fly possess a characteristic presynaptic specialization known as a T-bar, around which SVs cluster. By contrast, LDCVs do not cluster near T-bars and fewer T-bars are found in terminals that contain primarily LDCVs (Atwood et al., 1993; Jia et al., 1993; Karsai et al., 2013).
In flies and other invertebrates, neuronal somata and processes are generally localized to stereotypic positions, both centrally and in the periphery. In the embryo and larva, the central nervous system includes the segmented ventral nerve cord and the bulbous, anterior structure variously referred to as “the larval brain” or the “fused subesophogeal and supraesophageal ganglia.” (Fig 2A).
Fig 2. Basic neuroanatomy of larval and adult Drosophila.
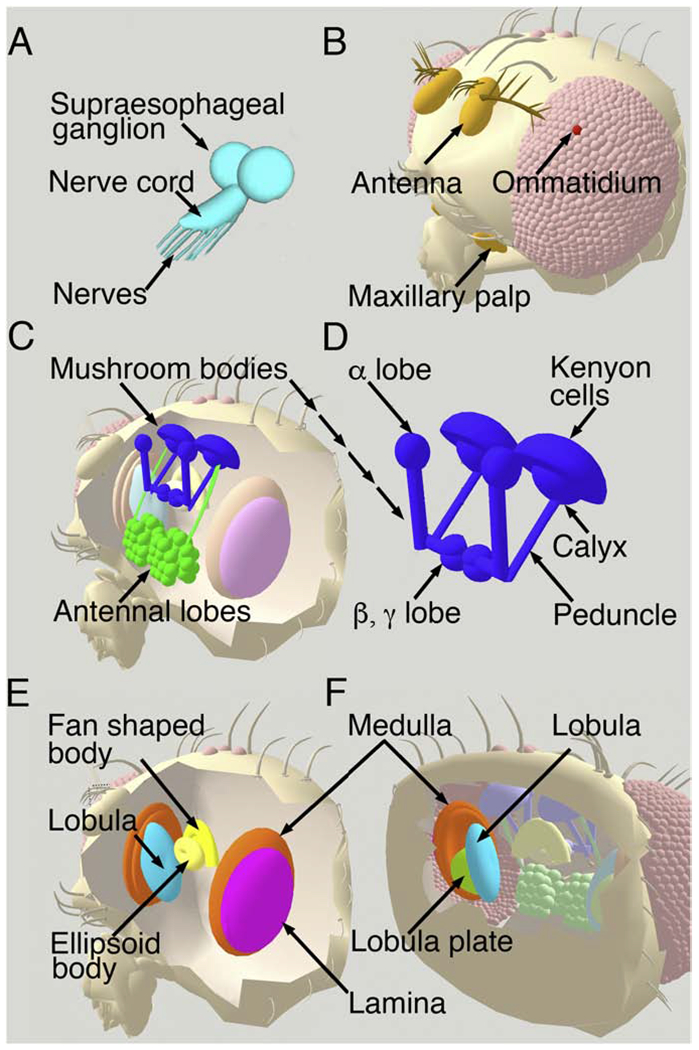
A) The central nervous system of Drosophila larva is composed of the ventral nerve cord and the fused sub- and supra-esophageal ganglia, labeled here as the supraesophageal ganglion. It is sometimes loosely referred to as the brain or developing optic lobes. Nerve bundles emanating from the nerve cord contain both efferent processes from motoneurons as well afferents from sensory cells. The adult central nervous system includes neurons within the head capsule (B-F) and the thoracic nerve cord (not shown) as well as peripheral sensory neurons that send afferents into the CNS (not shown) and a loose network of neurons associated with viscera (not shown). B) The external anatomy of the head capsule includes a large number of small and large bristles (micro and macrochaetae); only a few are shown. Both the maxillary palp and the distal portion of the antenna contain olfactory neurons that project to the antennal lobes. The retina of the eye is composed of several hundred clusters of photoreceptors known as ommatidia, each composed of eight individual photoreceptor cells. A single ommatidium is highlighted. C) Each of the two bilaterally symmetric antennal lobes (green) are composed of clusters of neurons arranged into glomeruli, represented here as spheres. Projections from the antennal lobes (green) innervate the mushroom bodies (blue) as well as regions of the central brain that are not shown. D) The mushroom bodies are composed of a dorsal “cap” containing the cell bodies of the Kenyon cells. The dendrites of the Kenyon cells make up the Calyx of the mushroom bodies and receive inputs from the antennal lobes. The Kenyon cell axons continue anteriorly and ventrally through the Peduncle and terminate in the vertically oriented α lobes and the horizontally oriented β and γ lobes. Front (E) and back (F) views of the head show the optic ganglia including the lamina (pink), medulla (orange), lobula (light blue) and lobula plate (green). Cells in the first two optic ganglia--the lamina and medulla-- receive input from photoreceptor cells and send processes to the third and fourth optic ganglia--the lobula and lobula plate-- for higher order processing. Two elements of the central complex are shown: the fan shaped body and ellipsoid body (both yellow).
During pupation, the nervous system is completely remodeled in parallel to the reorganization of the body plan from larva to adult. A variety of excellent resources describing adult fly neuroanatomy are available online including http://www.virtualflybrain.org/site/vfb_site/overview.htm. A simplified overview is shown in Fig 2. Major structures serving sensory functions within the adult brain include the optic ganglia (composed of the lamina, medulla, lobula and lobula plate) (Melnattur and Lee, 2011) and the antennal lobes which receive olfactory input from the antenna and maxillary palp (Masse et al., 2009; Mu et al., 2012; Wilson, 2013). The antennal lobes (roughly analogous to the mammalian olfactory bulb), and comprising cholinergic projection neurons, innervate the mushroom bodies (MBs) (functionally analogous to the mammalian hippocampus), a structure required for learning, memory and the integration of sensory information(Cognigni et al., 2018; Guven-Ozkan and Davis, 2014; Heisenberg, 2003; Iniguez et al., 2013; Ito et al., 1998; Kahsai and Zars, 2011). Additional morphologically distinct and functionally important structures in the adult central brain include the fan shaped body and the ellipsoid body; both are elements of the central complex, which is associated with coordination of movement and other behaviors (Lebestky et al., 2009; Liu et al., 2012; Seelig and Jayaraman, 2013; Strauss, 2002; Ueno et al., 2012)
Fly Neurotransmitters
Drosophila use many of the same neurotransmitters as mammals including the monoamines dopamine (DA), serotonin (5HT) and histamine (Monastirioti, 1999). Histamine has been studied extensively in insects because of its role in the visual system and is synthesized by the enzyme histidine decarboxylase (Burg et al., 1993).
Unlike mammals, flies and other insects do not appear to synthesize more than trace quantities of the adrenergic neurotransmitters noradrenaline and adrenaline. Rather, two structurally similar molecules, tyramine and octopamine (OA), serve as the major adrenergic transmitters in Drosophila (Borowsky et al., 2001; Roeder, 2005). Their biosynthesis requires the initial conversion of tyrosine to tyramine by Tyrosine decarboxylase (Tdc) (Cole et al., 2005) (Fig 3). Hydroxylation at the β carbon catalyzed by Tyramine β Hydroxylase (TβH) converts tyramine to OA (Monastirioti et al., 1996) (Fig 3). Hydroxylation of the β carbon to generate OA is analogous to the conversion of DA to NE in mammals. As in mammals, flies synthesize DA via Tyrosine Hydroxylase (TH) and DOPA Decarboxylase (DDC) (Fig 3) (Birman et al., 1994). Also, like mammals, Drosophila synthesize GABA and acetylcholine (ACh) via glutamate via Glutamic Acid Decarboxylase (GAD) (Jackson et al., 1990) and Choline Acetyl Transferase (ChAT) respectively (Kitamoto et al., 1998). The genomic organization of the cholinergic locus is remarkably conserved, with the vesicular acetylcholine transporter (VAChT) contained within an intron of ChAT in flies as well as mammals (Kitamoto et al., 1998).
Fig 3. Biosynthesis of dopamine, tyramine and octopamine.
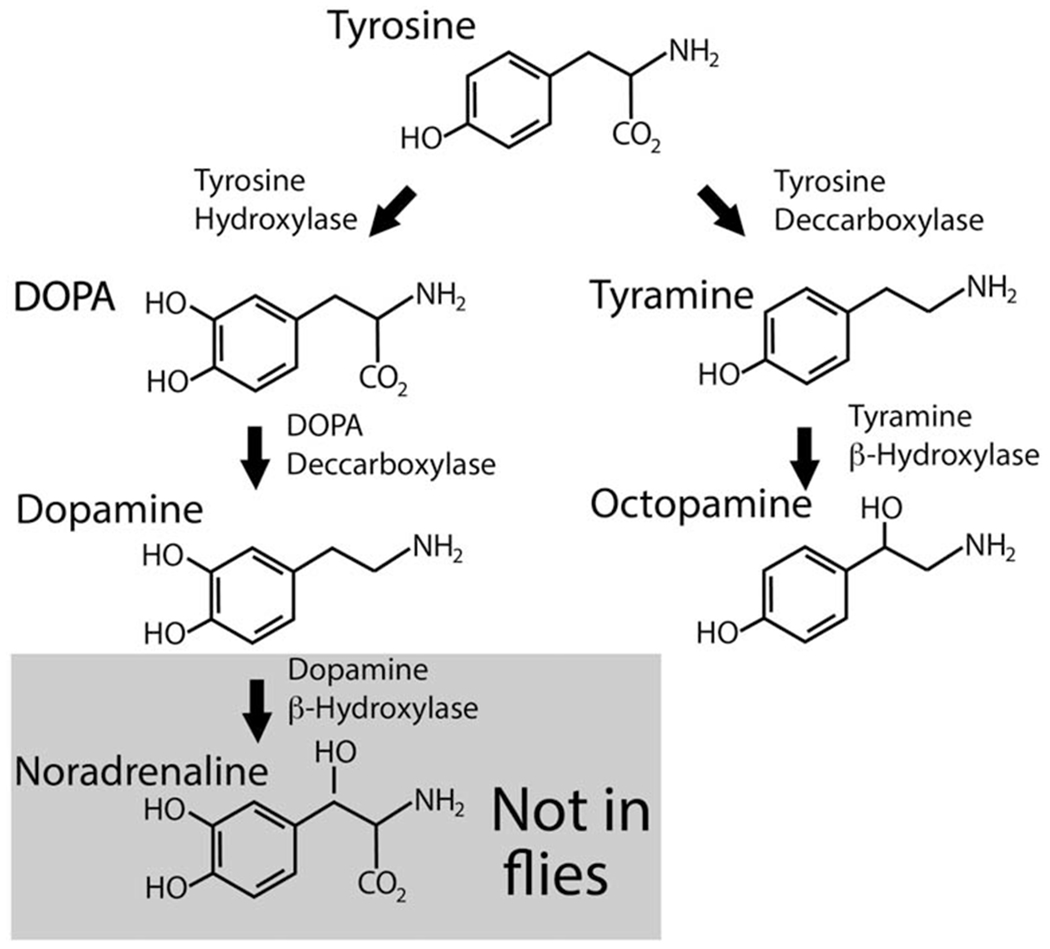
The major adrenergic neurotransmitters in the fly are tyramine and octopamine. Flies synthesize tyramine and octopamine using tyrosine decarboxylase to first convert tyrosine to tyramine. Tyramine β-Hydroxlase generates octopamine from tyramine. This is distinct from the evolutionarily conserved pathway used by both flies and mammals to generate dopamine via the successive actions of Tyrosine hydroxylase and Dopa decarboxylase (DDC) also known as Aromatic amino acid decarboxylase (AADC).
Both glutamate and ACh act as excitatory transmitters in the fly, but unlike mammals, glutamate rather than ACh is released at the fly neuromuscular junction (NMJ) (Jan and Jan, 1976). In addition, glutamate also functions as an inhibitory transmitter in flies through activation of a glutamate-gated chloride channel (Liu and Wilson, 2013; Rohrbough and Broadie, 2002). GABA appears to function strictly as an inhibitory transmitter in the fly (Lee et al., 2003).
Neurotransmitter Catabolism
Catabolism of neurotransmitters in Drosophila has, in general, received less attention than analogous processes in mammals, but in some instances, may be more dependent on conjugation than oxidation. For example, the enzyme ebony conjugates DA to β-alanine in the fly visual system (Suh and Jackson, 2007). Histamine also undergoes conjugation by ebony in a complex process that, at least in the visual system, involves transport of metabolites in and out of glia, analogous to glutamate recycling in mammals (Edwards and Meinertzhagen, 2010). (Fig 4). Histamine that is released from fly photoreceptors is taken up by glia by an unknown transporter. Within glial cells, ebony conjugates histamine to β-alanine to generate carcinine (Borycz et al., 2002; Richardt et al., 2002). Carcinine is then transported out of glia via an unknown mechanism and taken up into photoreceptors by the recently defined Carcinine Transporter (CarT), a member of the SLC22 family of the MFS (Chaturvedi et al., 2016; Stenesen et al., 2015; Xu et al., 2015). It is reconverted into histamine in neurons by the cysteine peptidase tan (True et al., 2005; Wagner et al., 2007). Additional cells in the visual system may also contribute to histamine recycling and metabolism (Borycz et al., 2012; Romero-Calderon et al., 2008).
Fig 4. Histamine recycling in the fly visual system.
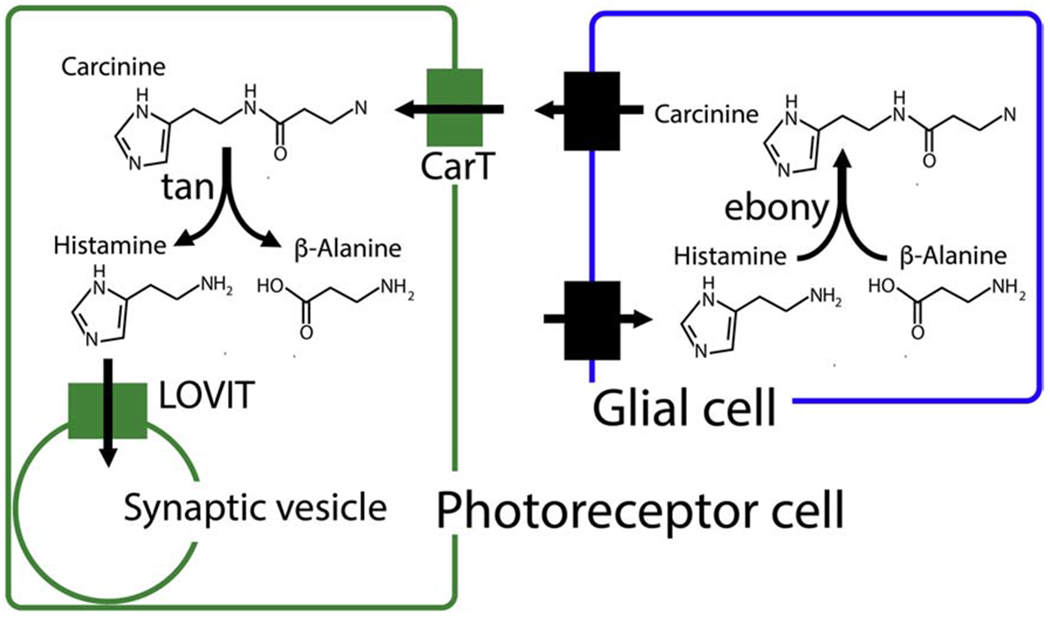
Histamine is stored in synaptic vesicles within photoreceptor cells by the vesicular transporter LOVIT. After exocytotic release, histamine is taken up by glia via an unknown transporter and conjugated to β-alanjne by the enzyme ebony to generate carcinine. Carcinine leaves glia via an unknown mechanism and is transported into photoreceptor cells by CarT, where it is converted to histamine by the peptidase tan.
Flies do not appear to express an ortholog of the enzyme monoamine oxidase (MAO) which, in mammals, mediates the degradation of serotonin, dopamine and noradrenalin (Roelofs and Van Haastert, 2001). However, biochemical studies have demonstrated oxidative activity in fly homogenates and it is possible that oxidation plays a role in the degradation of some neurotransmitters in flies as in mammals (Chaudhuri et al., 2007; Wang et al., 2011; Yellman et al., 1997).
Expression of the plasma membrane serotonin and DA transporters in aminergic neurons suggests that, like mammals, serotonin and DA are likely to be recycled in presynaptic release sites (Bang et al., 2011; Giang et al., 2011). By contrast, plasma membrane GABA and glutamate transporters are expressed in Drosophila glia indicating that, as in mammals, glia likely play a prominent role in recycling these neurotransmitters (Neckameyer and Cooper, 1998; Parinejad et al., 2016; Seal et al., 1998; Soustelle et al., 2002; Stork et al., 2014).
Drosophila Vesicular Neurotransmitter Transporters
dVAChT
VAChT is widely expressed throughout the fly CNS, and more recently, has been shown to be expressed in the fly mushroom bodies (Barnstedt et al., 2016; Boppana et al., 2017; Pankova and Borst, 2017). As noted above, the mushroom bodies are critical for learning and memory in insects and are composed of several thousand intrinsic Kenyon cells (KCs). Earlier ultrastructural data on KC dendrites suggested that afferent processes, but not the KCs themselves, were cholinergic (Yasuyama et al., 2002). By contrast, more recent work using antibodies to dVAChT as well as epitope tagging of the endogenous VAChT locus have clearly shown that a large population of KCs can store and release ACh (Barnstedt et al., 2016; Boppana et al., 2017; Pankova and Borst, 2017). The functional importance of ACh release from KCs was demonstrated in multiple ways including the response of post-synaptic mushroom body output neurons to ACh, memory deficits following RNAi-mediated knock down of dVAChT in KCs and blockade of KC output with cholinergic drugs (Barnstedt et al., 2016).
In addition to RNA interference (RNAi) knock down studies (Barnstedt et al., 2016), both overexpression and genetic mutations in the endogenous dVAChT locus have been used to investigate its function. VAChT overexpression in Drosophila leads to reduced lifespan, age-dependent defects in locomotion as well as severe deficits in learning and memory (Showell et al., 2020). The findings on cognitive impairment mirrors prior studies in mice (Kolisnyk et al., 2013) and indicate that adverse effects may be associated with an increase in VAChT activity.
dVAChT loss of function mutants include dVAChT1 which is embryonic lethal and (Showell et al., 2020)putative null, and the weaker, “hypomorphic” allele dVAChT2. dVAChT2 survives through the second larval stage but locomotes slower than wild type animals (Kitamoto et al., 2000), consistent with the identified role for ACh in the larval locomotive circuit (Baines, 2003; Malloy et al., 2019). Heterozygous dVAChT mutants survive to adulthood, but show subtle neuronal defects (Kitamoto et al., 2000). In particular, electrophysiological analysis of adult dVAChT heterozygotes suggests that during periods of sustained vesicle release, at least one circuit in the adult CNS fails to maintain normal levels of ACh release (Kitamoto et al., 2000). One mechanism underlying this change could be reduced numbers of VAChT molecules on each SV (Prado et al., 2013). In addition, it is likely that under conditions of reduced VAChT expression in both flies and mammals, some vesicles are likely to be devoid of VAChT or any luminal ACh (Prado et al., 2013). The contribution of each mechanism to the phenotype of specific mutants may vary depending on the rate of SV recycling and as well as the mechanism(s) responsible for determining when a vesicle is “full” (Williams, 1997).
Previous models proposed to determine when a vesicle is full include a static “set point” model and a more dynamic equilibrium model in which the balance between uptake and leakage regulates loading (Williams, 1997). The equilibrium model predicts that changes in the expression of vesicular transporters – the molecules that mediate loading – might change the amount of transmitter in each vesicle. In one recent study, increased levels of VAChT driven by overexpression of a transgene failed to show an increase in the amplitude of miniature electrophysiological potentials (“minis”) which represent the post-synaptic response to a single SV (Cash et al., 2016). This was interpreted as supporting a set point model in which each vesicle contains fixed amount of neurotransmitter, at least in the central cholinergic synapses that were examined (Cash et al., 2016). However, in other circuits and systems, changes in vesicular transporter expression have been suggested to modify vesicular neurotransmitter content (Daniels et al., 2004; Lima et al., 2010; Song et al., 1997). Together, these data suggest the possibility that perhaps only a subset of neurons have the ability to increase (or decrease) the complement of vesicular transporters on each vesicle and thereby increase vesicle loading.
Another variable contributing to loading SVs is the intrinsic activity of each individual transporter. Further structure-functional analysis of dVAChT has demonstrated that, even if there is a set point in some cholinergic vesicles in the fly, it might be reset by altering transporter activity. This was recently shown by manipulating a polyglutamine region in the C-terminal cytoplasmic tail of dVAChT (Vernon et al., 2019). Wild type VAChT contains thirteen glutamines at this site and addition of one glutamine reduced both mini frequency and amplitude; conversely, a reduction from thirteen to twelve glutamines increased vesicle loading (Vernon et al., 2019). Since the polyglutamine repeat is restricted to insect species, this mechanism would not extend to mammalian VAChT. It nonetheless represents an interesting example of mechanisms that may regulate vesicular transporter activity and vesicle loading. Since the polyglutamine region in dVAChT is not directly involved in transport, some form of intramolecular interaction may be required. This could conceivably involve sites similar to those required for the G-protein mediated regulation of mammalian VMAT (Ahnert-Hilger et al., 1998; Brunk et al., 2006).
In an additional structure function study of dVAChT by the same group, a Y49N mutation identified as conferring insecticide resistance was shown to increase spontaneous mini frequency but dampen trains of evoked release (Vernon et al., 2018). The two phenomena might be causally related since an increase in the spontaneous release of vesicles could deplete the pool available for evoked release. Since Y49 is found within a putative trafficking motif (Vernon et al., 2018), it is tempting to hypothesize that the increase in minis could be caused by an alteration in VAChT trafficking. Indeed, increased trafficking to SVs might increase the number of vesicles that contain a single VAChT or possibly increase the number of VAChT molecules per SV.
dVGLUT
dVGLUT is expressed in all glutamatergic neurons in the larva and adult fly including the glutamatergic motor neurons innervating the NMJ (Daniels et al., 2004; Daniels et al., 2008). The membrane topology of dVGLUT has been examined using a series of epitope tags predicted to reside on the luminal and cytosolic loops between predicted transmembrane domains as well as at the N- and C-termini (Fei et al., 2007). Deletion of the cytosolic C-terminus abrogates lethality caused by dVGLUT over-expression, clearly implicating an important functional role for this domain including trafficking of the transporter to SVs (Grygoruk et al., 2010). These data are consistent with the presence of critical trafficking motifs that reside in the C-termini of mammalian VGLUTs (Foss et al., 2013; Voglmaier et al., 2006). However, the baseline localization of dVGLUT to SVs appears to be surprisingly unaffected by deletion of the C-terminus (Grygoruk et al., 2010) and studies of mammalian VGLUT trafficking have identified trafficking motifs in both the N and C termini (Foss et al., 2013; Voglmaier et al., 2006). It is possible that the contribution of specific domains to VGLUT trafficking could vary between cell types or species.
Similar to studies of Drosophila and mammalian VAChT, miniature end plate potentials have been used to examine neurotransmitter content in dVGLUT mutants (Daniels et al., 2006). In mutant flies expressing reduced levels of dVGLUT, mini frequency was also reduced, suggesting that some exocytosed SVs lacked dGVLUT and were therefore “empty.” More surprisingly, the size of the remaining minis, an indication of SV filling, was not altered in the mutant. These data suggest that a reduced number of VGLUT molecules, and perhaps only one, may suffice to fill a vesicle under some conditions (Daniels et al., 2006). However, using other techniques, recent estimates of VGLUT copies per vesicle in wild type mammalian preparations have ranged from 4 to 14 (Mutch et al., 2011; Takamori et al., 2006).
Over-expression of dVGLUT at high levels using relatively strong GAL4 drivers is larval lethal (Daniels et al., 2011). Over-expression using a weaker driver allows survival through adulthood allowing the study of the adult phenotype (Daniels et al., 2011). Interestingly, the surviving adults show large lacunae in their CNS, possibly the result of glutamate-mediated excitotoxicity (Daniels et al., 2011). Consistent with the observation that dVGLUT over expression causes functional changes in SV homeostasis, electrophysiological studies of dVGLUT over-expression in larva show an increase in quantal size, as well as large spontaneous events at the larval NMJ (Daniels et al., 2004; Daniels et al., 2011), a phenotype similar to that seen with over-expression of vesicular transporters in mammals (Edwards, 2007; Wilson et al., 2005).
dVMAT
dVMAT encodes at least two splice variants, dVMAT-Λ and -B, which differ at their C-termini (Greer et al., 2005). dVMAT-A is expressed in both larvae and adults in all dopaminergic, serotonergic and octopaminergic cells (Chang et al., 2006; Greer et al., 2005). Selective rescue of dVMAT function in either individual or multiple aminergic systems within a dVMAT null mutant background was used to determine the respective contributions of dopaminergic, serotonergic and/or octopaminergic/tyraminergic neurons to amine-dependent behaviors (Chen et al., 2013). In addition to supporting known roles of each neurotransmitter in behavior, this analysis provides a useful approach to define new roles that might be difficult to study using biosynthetic mutants (Adamo et al., 1995; Alekseyenko et al., 2010; Bacon et al., 1995; Fox et al., 2006; Koon et al., 2011; Lee et al., 2009; Monastirioti et al., 1996).
In contrast to dVMAT-A and mammalian VMATs, dVMAT-B is expressed in a subset of glia rather than neurons (Romero-Calderón et al., 2008). Its function in the optic lobes may include storage of histamine or its metabolite carcinine (Romero-Calderón et al., 2008). Although mammalian glia likely take up amines via low affinity mechanisms (Dahlin et al., 2007; Yoshikawa et al., 2013), the expression of a specific amine transporter in glia is unusual and further studies of dVMAT-B may help determine the role of glia in the recycling of histamine and perhaps other biogenic amines.
dVMAT mutants show a number of behavioral deficits consistent with the loss of exocytotic amine release in the nervous system (Chen et al., 2013; Simon et al., 2009). Similar to mouse knockouts, mutant tissue levels of amines are dramatically reduced, presumably due to the degradation of amines that are not sequestered in secretory vesicles (Fon et al., 1997; Simon et al., 2009). Under standard fly culture conditions, loss of dVMAT is lethal, but reducing the density of the culture can increase viability, in at least some genetic backgrounds, thus allowing for use of dVMAT mutants in behavioral assays. dVMAT mutants show a dramatic decrease in baseline larval locomotion (Simon et al., 2009), mirroring previously demonstrated effects of dVMAT blockade by reserpine in flies with wild type dVMAT expression (Pendleton et al., 2000).
Both mutation of dVMAT and pharmacologic inhibition with reserpine cause increased sleep (Nall and Sehgal, 2013). In contrast, amphetamines decrease sleep in flies (Andretic et al., 2005), presumably via release of DA (Pizzo et al., 2013). In each case, changes in sleep were distinguished from changes in locomotion using previously established criteria (Hendricks et al., 2000; Shaw et al., 2000). The circuits underlying these effects remain unclear but could potentially be generally relevant to the behavioral effects of stimulants in mammals.
“Genetic rescue” experiments in which dVMAT mutant transgenes were expressed in a dVMAT null mutant background have provided a convenient method for analyzing the effects of the mutant alleles. Mutation of signals in the C-terminus of dVMAT-A blocks endocytosis in cultured cells and disrupts trafficking to SVs when expressed in vivo (Grygoruk et al., 2010). This also leads to a corresponding increase in the localization of dVMAT to LDCVs (Grygoruk et al., 2014). Genetic rescue with dVMAT trafficking mutants differentially affected subsets of aminergic circuits, with some showing profound deficits and others showing little or no change relative to wild type (Grygoruk et al., 2014). Further studies of the circuits disrupted by dVMAT trafficking mutants may shed light on the function of amine release from SVs versus LDCVs, a topic that remains poorly understood. Additional genetic rescue experiments in the dVMAT null background have been used to visualize subpopulations of monoaminergic terminals labeled with fluorescent false neurotransmitters (FFNs). This combination of genetics with imaging and pharmacology enabled dissection of the mechanisms underlying the actions of amphetamines in vivo within whole intact fly brains (Aguilar et al., 2017; Freyberg et al., 2016). These studies also demonstrated that the tandem activities of both the Drosophila dopamine transporter (dDAT) and dVMAT are essential for the ability of amphetamines to redistribute DA out of the vesicle lumen into the cytoplasm and eventually out of the terminals – a key mechanism for these drugs’ psychostimulant effects (Freyberg et al., 2016).
Flies over-expressing dVMAT in DA and 5HT cells show an increase in motor activity and a blunted behavioral response to cocaine (Chang et al., 2006). Likewise, administration of cocaine increases motor activity in the fly (Bainton et al., 2000; McClung and Hirsh, 1998; Torres and Horowitz, 1998). Similar to amphetamines (Andretic et al., 2005), over-expression of dVMAT in cells producing both 5HT and DA increases male courtship behavior (Chang et al., 2006). Importantly, the effects of dVMAT as well as dVGLUT over-expression demonstrate that the changes in neurotransmitter release seen with overexpression of vesicular transporters in vitro (Pothos et al., 2000; Song et al., 1997) can have significant downstream behavioral sequelae in an intact organism.
Over-expression of dVMAT as well as loss of function alleles have been used to explore in vivo the role of DA homeostasis in neurodegenerative processes relevant to Parkinson’s disease (PD) (Lawal et al., 2010; Sang et al., 2007). DA has a high oxidative potential, in part through the formation of dopamine quinone, which may form adducts with DNA (Stokes et al., 1996; Stokes et al., 1999) and conjugate to proteins implicated in genetic forms of PD (Conway et al., 2001; Hastings et al., 1996; Van Laar et al., 2009). Thus, it has been suggested that an increase in VMAT activity may have neuroprotective effects (reviewed in (Guillot and Miller, 2009)). This has been tested in vitro in cell cultures using mammalian VMAT and dVMAT (Mosharov et al., 2009; Park et al., 2007), and in vivo in the fly (Inamdar et al., 2013; Lawal et al., 2010; Sang et al., 2007). Over-expression of dVMAT rescues the loss of DA neurons caused by either a genetic or chemical insult (Inamdar et al., 2013; Lawal et al., 2010; Sang et al., 2007). Conversely, loss of dVMAT activity increases the toxicity of both genetic and chemical insults to DA cells (Inamdar et al., 2013; Lawal et al., 2010; Sang et al., 2007). Similar to studies of VMATs in mammals (Caudle et al., 2007; Mosharov et al., 2009; Sulzer et al., 2000), these studies suggest the possibility that drugs which increase VMAT activity could show neuroprotective effects in models of PD. Accordingly, a mutant showing diminished levels of dVMAT expression (i.e., a “functional hypomorph”) was used to screen for drugs that might increase dVMAT activity or otherwise increase vesicular amine storage (Lawal et al., 2014). The results of this screen yielded several molecules that a priori would not be classified as aminergic but could indirectly modify dVMAT activity (Lawal et al., 2014). Similar screens might be used to identify drugs that can directly or indirectly modify the activity of other transporters.
dVGAT
Like mammals, the Drosophila genome contains a single vesicular GABA transporter gene (dVGAT) (Fei et al., 2010). dVGAT appears to be expressed in all GABAergic neurons in fly larva since it precisely co-localizes with GABA in the ventral nerve cord, and is also expressed in most if not all adult GABAergic neurons (Enell et al., 2007; Fei et al., 2010). Mutation of dVGAT causes developmental lethality (Fei et al., 2010). To overcome this potential limitation, inducible expression of a dVGAT transgene has been used to restore dVGAT function during development and thereby allow adult behavioral studies. One adult phenotype demonstrated in the conditional rescue line was a surprisingly specific defect in the detection of small objects in the fly’s visual field (Fei et al., 2010). Other aspects of reduced GABAergic signaling in this transgenic model remain to be explored.
It is not clear whether Drosophila, like mammals, use glycine as a neurotransmitter and whether dVGAT could also serve to store glycine. Since the structure of β-alanine is intermediate between glycine and GABA and mammalian and VGAT has been shown to transport β-alanine in vitro (Juge et al., 2013), it is also possible that dVGAT could play a role in β-alanine storage or metabolism in the fly. To date, such a possibility has not yet been tested. As noted above, conjugation of β-alanine to histamine is also required for histamine recycling in the fly (Borycz et al., 2002), and conjugation of β-alanine is important for DA metabolism in both the cuticle and the central nervous system (Suh and Jackson, 2007; Wright, 1987). This opens the door to further investigations of the intersections between dVGAT, histamine, and DA.
portabella
Drosophila and some other insects express portabella, an additional vesicular transporter that appears to be absent from mammalian genomes (Brooks et al., 2011; Lawal and Krantz, 2013). Similar to VAChT, prt is expressed in the KCs within the mushroom bodies (Brooks et al., 2011). Mutation of prt results in a peculiar defect in sexual behavior, characterized primarily by an inability of males to maintain their position during copulation (Brooks et al., 2011). The primary structure of the prt protein is most similar to dVMAT and it is possible that the substrate is also similar to known monoamines; however, the biosynthetic enzymes for DA, 5HT, OA and histamine are not expressed in KCs, suggesting that prt may transport a novel neurotransmitter (Brooks et al., 2011). Alternatively, it is possible that prt may act primarily to regulate the function of VAChT, similar to the proposed role of some other co-expressed vesicular transporters (see below).
LOVIT
In Drosophila, histamine functions as the primary neurotransmitter in photoreceptor cells (Nassel, 1999; Sarthy, 1991; Stuart et al., 2007). In mammals, VMATs are thought to be responsible for histamine storage but dVMAT is not expressed in fly photoreceptors (Chang et al., 2006). Recently, a member of the SLC45 family of putative sugar transporters, SLC45A2, has been implicated in vesicular histamine transport and storage, particularly in the context of modulation of fly vision, and thus renamed Loss of Visual Transmission (LOVIT) (Xu and Wang, 2019). LOVIT is highly expressed in the terminals of photoreceptors and localized to SVs. Mutation of lovit resulted in loss of histamine in photoreceptor terminals, disrupting the response of postsynaptic neurons to light stimuli in adult flies and also ablating phototactic behavior (Xu and Wang, 2019). Conversely, the expression of LOVIT in the photoreceptors of lovit mutant flies restored the response of postsynaptic neurons to light stimuli. Together, these data indicate that LOVIT is responsible for vesicular storage and release of histamine in the photoreceptor cells of Drosophila. It is possible that LOVIT is also responsible for vesicular storage of histamine in other cells in the fly but this remains unclear.
Co-release of neurotransmitters and neuropeptides
In mammals, it is well established that neurons can co-release classical neurotransmitters and neuropeptides from LDCVs (Granger et al., 2017). A recent review summarized the colocalization of neuropeptides and neurotransmitters in fly neurons regulating behaviors such as sleep, olfaction, feeding and developmental processes (Nassel, 2018). In addition, a recent report suggested that a receptor protein tyrosine phosphatase Ptp4E may post-transcriptionally up-regulate neuropeptide content in dense core vesicles (Tao et al., 2019). Interestingly, Ptp4E deficiency was also reported to upregulate presynaptic dVMAT expression and activity at the same octopaminergic synapses (Tao et al., 2019). Future studies of this relatively simple circuit may be useful to explore mechanisms that may govern the storage and co-release both classical neurotransmitters and neuropeptides.
Co-expression of vesicular transporters
Multiple studies in both invertebrates and mammals indicate that more than one classical neurotransmitter can be stored and released from the same neuron. In most cases, this requires the co-expression of two vesicular transporters within the same cell (Granger et al., 2017; Hnasko and Edwards, 2011; Munster-Wandowski et al., 2016). In some cases, vesicular transporters may reside on the same vesicle; however, the context in which occurs remains an active topic of investigation and may vary across both synapses and cell types (Galvan and Gutierrez, 2017; Morales and Margolis, 2017; Silm et al., 2019).
In addition to co-release, co-localization on the same vesicle allows regulatory interactions between vesicular transporters (Munster-Wandowski et al., 2016). The molecular genetic tools available in the fly are well suited to explore this phenomenon. Similar to findings in mammals, dVGLUT is expressed in a subset of DA neurons in the adult fly central brain (Aguilar et al., 2017; Hnasko et al., 2012; Trudeau and El Mestikawy, 2018). Recent work has shown that depolarization of these cells causes an increase in the DA content of SVs immediately prior to exocytosis (Aguilar et al., 2017). The pH gradient across the vesicle membrane (ΔpH) is the primary driving force for dVMAT mediated loading of DA into SVs (Hnasko and Edwards, 2012), and dVGLUT was required to increase the ΔpH that was in turn responsible for increased DA loading and release (Aguilar et al., 2017). These results suggest a unique role for dVGLUT in tuning vesicular DA release to meet the demands of neuronal activity.
In addition to dVGLUT’s role in the regulation of intraluminal SV pH, recent work has shown that following its localization to the plasma membrane during exocytosis, dVGLUT can modulate proton efflux from nerve terminals (Rossano et al., 2017). Indeed, dVGLUT’s previously unrecognized ability to extrude protons may be critical for maintaining a stable cytoplasmic pH despite the movement of protons out of SVs required for dVMAT-dependent neurotransmitter transport into vesicles (Rossano et al., 2017).
dVGLUT expression in DA neurons also has been implicated in modulating their vulnerability to neurodegeneration. Heterologous over-expression of dVGLUT in DA neurons induced cell death in subsets of DA neurons including those innervating the central complex (Steinkellner et al., 2018). This phenomenon is analogous to experiments using mammalian DA neurons in which VGLUT2 expression was demonstrated to play a role in DA neuron vulnerability to neurotoxic insults (Shen et al., 2018; Steinkellner et al., 2018). Interestingly, recent work in mammals also showed that, although almost all DA neurons express VGLUT2 early in life, most repress its expression by adulthood with the exception of a relatively small population of DA neurons in the mouse ventral tegmental area (Steinkellner et al., 2018). It remains unclear whether a similar developmental repression of dVGLUT expression occurs in fly DA neurons. Nevertheless, these findings suggest that overriding the endogenous regulation via ectopic VGLUT expression is selectively toxic to adult DA neurons that normally repress or, at a minimum, are not equipped to express significant levels of VGLUT2 or dVGLUT.
Summary
Despite significant differences in anatomy, many of the molecular and cellular aspects of neurotransmitter storage in flies and mammals are similar. The abundance of molecular-genetic tools as well their low cost and short lifespan make Drosophila an attractive model for the study of vesicular transporters and their regulation.
Highlights.
Drosophila melanogaster express orthologs of mammalian vesicular transporters
Drosophila also express some vesicular transporters not found in mammals
Mutants and transgenes available in Drosophila can be used to study transporter function
Acknowledgements:
This work was supported the NIMH MH107390 (DEK), MH114017 (DEK), the John F. and Nancy A. Emmerling Fund of The Pittsburgh Foundation (Z.F.). the NIA K01AG049055-05 (HOL), 3K01AG049055-04S1 (HOL) grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adamo SA, Linn CE, and Hoy RR. 1995. The role of neurohormonal octopamine during ‘fight or flight’ behaviour in the field cricket Gryllus bimaculatus. J Exp Biol. 198:1691–1700. [DOI] [PubMed] [Google Scholar]
- Aguilar JI, Dunn M, Mingote S, Karam CS, Farino ZJ, Sonders MS, Choi SJ, Grygoruk A, Zhang Y, Cela C, Choi BJ, Flores J, Freyberg RJ, McCabe BD, Mosharov EV, Krantz DE, Javitch JA, Sulzer D, Sames D, Rayport S, and Freyberg Z. 2017. Neuronal Depolarization Drives Increased Dopamine Synaptic Vesicle Loading via VGLUT. Neuron. 95:1074–1088 e1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnert-Hilger G, Nurnberg B, Exner T, Schafer T, and Jahn R. 1998. The heterotrimeric G protein Go2 regulates catecholamine uptake by secretory vesicles. Embo J. 17:406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseyenko OV, Lee C, and Kravitz EA. 2010. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PloS one. 5:e10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretic R, van Swinderen B, and Greenspan RJ. 2005. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 15:1165–1175. [DOI] [PubMed] [Google Scholar]
- Atwood HL, Govind CK, and Wu CF. 1993. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 24:1008–1024. [DOI] [PubMed] [Google Scholar]
- Bacon JP, Thompson KS, and Stern M. 1995. Identified octopaminergic neurons provide an arousal mechanism in the locust brain. J Neurophysiol. 74:2739–2743. [DOI] [PubMed] [Google Scholar]
- Baines RA 2003. Postsynaptic protein kinase A reduces neuronal excitability in response to increased synaptic excitation in the Drosophila CNS. J Neurosci. 23:8664–8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LTY, Singh CM, Moore MS, Neckameyer WS, and Heberlein U. 2000. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr. Biol 10:187–194. [DOI] [PubMed] [Google Scholar]
- Bang S, Hyun S, Hong ST, Kang J, Jeong K, Park JJ, Choe J, and Chung J. 2011. Dopamine signalling in mushroom bodies regulates temperature-preference behaviour in Drosophila. PLoS Genet. 7:e1001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstedt O, Owald D, Felsenberg J, Brain R, Moszynski JP, Talbot CB, Perrat PN, and Waddell S. 2016. Memory-Relevant Mushroom Body Output Synapses Are Cholinergic. Neuron. 89:1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen HJ, Tong C, and Tsuda H. 2010. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat Rev Neurosci. 11:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birman S, Morgan B, Anzivino M, and Hirsh J. 1994. A novel and major isoform of tyrosine hydroxylase in Drosophila is generated by alternative RNA processing. J. Biol. Chem 269:26559–26567. [PubMed] [Google Scholar]
- Blakely RD, and Edwards RH. 2012. Vesicular and plasma membrane transporters for neurotransmitters. Cold Spring Harb Perspect Biol. 4:22199021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boppana S, Kendall N, Akinrinsola O, White D, Patel K, and Lawal H. 2017. Immunolocalization of the vesicular acetylcholine transporter in larval and adult Drosophila neurons. Neurosci Lett. 643:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, and Gerald C. 2001. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 98:8966–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borycz J, Borycz JA, Edwards TN, Boulianne GL, and Meinertzhagen IA. 2012. The metabolism of histamine in the Drosophila optic lobe involves an ommatidial pathway: beta-alanine recycles through the retina. J Exp Biol. 215:1399–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borycz J, Borycz JA, Loubani M, and Meinertzhagen IA. 2002. tan and ebony genes regulate a novel pathway for transmitter metabolism at fly photoreceptor terminals. J Neurosci. 22:10549–10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks ES, Greer CL, Romero-Calderon R, Serway CN, Grygoruk A, Haimovitz JM, Nguyen BT, Najibi R, Tabone CJ, Steven de Belle J, and Krantz DE. 2011. A Putative Vesicular Transporter Expressed in Drosophila Mushroom Bodies that Mediates Sexual Behavior May Define a Neurotransmitter System. Neuron. 72:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk I, Blex C, Rachakonda S, Holtje M, Winter S, Pahner I, Walther DJ, and Ahnert-Hilger G. 2006. The first luminal domain of vesicular monoamine transporters mediates G-protein-dependent regulation of transmitter uptake. J Biol Chem. 281:33373–33385. [DOI] [PubMed] [Google Scholar]
- Burg MG, Sarthy PV, Koliantz G, and Pak WL. 1993. Genetic and molecular identification of a Drosophila histidine decarboxylase gene required in photoreceptor transmitter synthesis. Embo J. 12:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash F, Vernon SW, Phelan P, Goodchild J, and Baines RA. 2016. Central cholinergic synaptic vesicle loading obeys the set-point model in Drosophila. J Neurophysiol. 115:843–850. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, and Miller GW. 2007. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 27:8138–8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H-Y, Grygoruk A, Brooks ES, Ackerson LC, Maidment NT, Bainton RJ, and Krantz DE. 2006. Over-expression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Molecular Psychiatry. 11:99–113. [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, Luan Z, Guo P, and Li HS. 2016. Drosophila Vision Depends on Carcinine Uptake by an Organic Cation Transporter. Cell Rep. 14:2076–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A, Bowling K, Funderburk C, Lawal H, Inamdar A, Wang Z, and O’Donnell JM. 2007. Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J Neurosci. 27:2457–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Ng F, Lebestky T, Grygoruk A, Djapri C, Lawal HO, Zaveri HA, Mehanzel F, Najibi R, Seidman G, Murphy NP, Kelly RL, Ackerson LC, Maidment NT, Jackson FR, and Krantz DE. 2013. Dispensable, Redundant, Complementary, and Cooperative Roles of Dopamine, Octopamine, and Serotonin in Drosophila melanogaster. Genetics. 193:159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognigni P, Felsenberg J, and Waddell S. 2018. Do the right thing: neural network mechanisms of memory formation, expression and update in Drosophila. Curr Opin Neurobiol. 49:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, and Hirsh J. 2005. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 280:14948–14955. [DOI] [PubMed] [Google Scholar]
- Conway KA, Rochet JC, Bieganski RM, and Lansbury PT Jr. 2001. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 294:1346–1349. [DOI] [PubMed] [Google Scholar]
- Dahlin A, Xia L, Kong W, Hevner R, and Wang J. 2007. Expression and immunolocalization of the plasma membrane monoamine transporter in the brain. Neuroscience. 146:1193–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Collins CA, Chen K, Gelfand MV, Featherstone DE, and DiAntonio A. 2006. A single vesicular glutamate transporter is sufficient to fill a synaptic vesicle. Neuron. 49:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Collins CA, Gelfand MV, Dant J, Brooks ES, Krantz DE, and DiAntonio A. 2004. Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J Neurosci. 24:10466–10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Gelfand MV, Collins CA, and DiAntonio A. 2008. Visualizing glutamatergic cell bodies and synapses in Drosophila larval and adult CNS. J Comp Neurol. 508:131–152. [DOI] [PubMed] [Google Scholar]
- Daniels RW, Miller BR, and DiAntonio A. 2011. Increased vesicular glutamate transporter expression causes excitotoxic neurodegeneration. Neurobiol Dis. 41:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RH 2007. The neurotransmitter cycle and quantal size. Neuron. 55:835–858. [DOI] [PubMed] [Google Scholar]
- Edwards TN, and Meinertzhagen IA. 2010. The functional organisation of glia in the adult brain of Drosophila and other insects. Prog Neurobiol. 90:471–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enell L, Hamasaka Y, Kolodziejczyk A, and Nassel DR. 2007. gamma-Aminobutyric acid (GABA) signaling components in Drosophila: immunocytochemical localization of GABA(B) receptors in relation to the GABA(A) receptor subunit RDL and a vesicular GABA transporter. J Comp Neurol. 505:18–31. [DOI] [PubMed] [Google Scholar]
- Fei H, Chow DM, Chen A, Romero-Calderón R, Ong WS, Ackerson LC, Maidment NT, Simpson JH, Frye MA, and Krantz DE. 2010. Mutation of the Drosophila vesicular GABA transporter disrupts visual figure detection. J Exp Biol. 213:1717–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei H, Karnezis T, Reimer RJ, and Krantz DE. 2007. Membrane topology of the Drosophila vesicular glutamate transporter. J. Neurochem 101:1662–1671. [DOI] [PubMed] [Google Scholar]
- Fon EA, Pothos EN, Sun B-C, Kileen N, Sulzer D, and Edwards RH. 1997. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 19:1271–1283. [DOI] [PubMed] [Google Scholar]
- Foss SM, Li H, Santos MS, Edwards RH, and Voglmaier SM. 2013. Multiple Dileucine-like Motifs Direct VGLUT1 Trafficking. J Neurosci. 33:10647–10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox LE, Soll DR, and Wu CF. 2006. Coordination and modulation of locomotion pattern generators in Drosophila larvae: effects of altered biogenic amine levels by the tyramine Beta hydroxlyase mutation. J Neurosci. 26:1486–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyberg Z, Sonders MS, Aguilar JI, Hiranita T, Karam CS, Flores J, Pizzo AB, Zhang Y, Farino ZJ, Chen A, Martin CA, Kopajtic TA, Fei H, Hu G, Lin YY, Mosharov EV, McCabe BD, Freyberg R, Wimalasena K, Hsin LW, Sames D, Krantz DE, Katz JL, Sulzer D, and Javitch JA. 2016. Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain. Nat Commun. 7:10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan EJ, and Gutierrez R. 2017. Target-Dependent Compartmentalization of the Corelease of Glutamate and GABA from the Mossy Fibers. J Neurosci. 37:701–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giang T, Ritze Y, Rauchfuss S, Ogueta M, and Scholz H. 2011. The serotonin transporter expression in Drosophila melanogaster. J Neurogenet. 25:17–26. [DOI] [PubMed] [Google Scholar]
- Granger AJ, Wallace ML, and Sabatini BL. 2017. Multi-transmitter neurons in the mammalian central nervous system. Curr Opin Neurobiol. 45:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer CL, Grygoruk A, Patton DE, Ley B, Romero-Calderon R, Chang H-Y, Houshyar R, Bainton RJ, DiAntonio A, and Krantz DE. 2005. A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin and octopamine. J Neurobiol. 64:239–258. [DOI] [PubMed] [Google Scholar]
- Grygoruk A, Chen A, Martin CA, Lawal HO, Fei H, Gutierrez G, Biedermann T, Najibi R, Hadi R, Chouhan AK, Murphy NP, Schweizer FE, Macleod GT, Maidment NT, and Krantz DE. 2014. The redistribution of Drosophila vesicular monoamine transporter mutants from synaptic vesicles to large dense-core vesicles impairs amine-dependent behaviors. J Neurosci. 34:6924–6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygoruk A, Fei H, Daniels RW, Miller BR, Diantonio A, and Krantz DE. 2010. A tyrosine-based motif localizes a Drosophila vesicular transporter to synaptic vesicles in vivo. J Biol Chem. 285:6867–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot TS, and Miller GW. 2009. Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol Neurobiol. 39:149–170. [DOI] [PubMed] [Google Scholar]
- Guven-Ozkan T, and Davis RL. 2014. Functional neuroanatomy of Drosophila olfactory memory formation. Learn Mem. 21:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein V, and Wodarz A. 2013. Initial neurogenesis in Drosophila. Wiley Interdiscip Rev Dev Biol. 2:701–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings TG, Lewis DA, and Zigmond MJ. 1996. Reactive dopamine metabolites and neurotoxicity: implications for Parkinson’s disease. Adv. Exp. Med. Biol 387:97–106. [DOI] [PubMed] [Google Scholar]
- Heisenberg M 2003. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 4:266–275. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, and Pack AI. 2000. Rest in Drosophila is a sleep-like state. Neuron. 25:129–138. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, and Edwards RH. 2011. Neurotransmitter Corelease: Mechanism and Physiological Role. Annu Rev Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, and Edwards RH. 2012. Neurotransmitter corelease: mechanism and physiological role. Annual review of physiology. 74:225–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Hjelmstad GO, Fields HL, and Edwards RH. 2012. Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J Neurosci. 32:15076–15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamdar AA, Hossain MM, Bernstein AI, Miller GW, Richardson JR, and Bennett JW. 2013. Fungal-derived semiochemical 1-octen-3-ol disrupts dopamine packaging and causes neurodegeneration. Proc Natl Acad Sci U S A. 110:19561–19566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez J, Schutte SS, and O’Dowd DK. 2013. Cav3-type alpha1T calcium channels mediate transient calcium currents that regulate repetitive firing in Drosophila antennal lobe PNs. J Neurophysiol. 110:1490–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Suzuki K, Estes P, Ramaswami M, Yamamoto D, and Strausfeld NJ. 1998. The organization of extrinsic neurons and their implications in the functional roles of the mushroom bodies in Drosophila melanogaster Meigen. Learn Mem. 5:52–77. [PMC free article] [PubMed] [Google Scholar]
- Jackson FR, Newby LM, and Kulkarni SJ. 1990. Drosophila GABAergic systems: sequence and expression of glutamic acid decarboxylase. J Neurochem. 54:1068–1078. [DOI] [PubMed] [Google Scholar]
- Jan LY, and Jan YN. 1976. L-glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol. 262:215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia XX, Gorczyca M, and Budnik V. 1993. Ultrastructure of neuromuscular junctions in Drosophila: comparison of wild type and mutants with increased excitability. J Neurobiol. 24:1025–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N, Omote H, and Moriyama Y. 2013. Vesicular GABA transporter (VGAT) transports beta-alanine. J Neurochem. 127:482–486. [DOI] [PubMed] [Google Scholar]
- Kahsai L, and Zars T. 2011. Learning and memory in Drosophila: behavior, genetics, and neural systems. Int Rev Neurobiol. 99:139–167. [DOI] [PubMed] [Google Scholar]
- Karsai G, Pollak E, Wacker M, Vomel M, Selcho M, Berta G, Nachman RJ, Isaac RE, Molnar L, and Wegener C. 2013. Diverse in- and output polarities and high complexity of local synaptic and non-synaptic signaling within a chemically defined class of peptidergic Drosophila neurons. Front Neural Circuits. 7:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T, Wang W, and Salvaterra PM. 1998. Structure and organization of the Drosophila cholinergic locus. J Biol Chem. 273:2706–2713. [DOI] [PubMed] [Google Scholar]
- Kitamoto T, Xie X, Wu CF, and Salvaterra PM. 2000. Isolation and characterization of mutants for the vesicular acetylcholine transporter gene in Drosophila. J. Neurobiol 42:161–171. [PubMed] [Google Scholar]
- Kolisnyk B, Guzman MS, Raulic S, Fan J, Magalhaes AC, Feng G, Gros R, Prado VF, and Prado MA. 2013. ChAT-ChR2-EYFP mice have enhanced motor endurance but show deficits in attention and several additional cognitive domains. J Neurosci. 33:10427–10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koon AC, Ashley J, Barria R, DasGupta S, Brain R, Waddell S, Alkema MJ, and Budnik V. 2011. Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat Neurosci. 14:190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal HO, Chang HY, Terrell AN, Brooks ES, Pulido D, Simon AF, and Krantz DE. 2010. The Drosophila vesicular monoamine transporter reduces pesticide-induced loss of dopaminergic neurons. Neurobiol Dis. 40:102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal HO, and Krantz DE. 2013. SLC18: Vesicular neurotransmitter transporters for monoamines and acetylcholine. Mol Aspects Med. 34:360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal HO, Terrell A, Lam HA, Djapri C, Jang J, Hadi R, Roberts L, Shahi V, Chou MT, Biedermann T, Huang B, Lawless GM, Maidment NT, and Krantz DE. 2014. Drosophila modifier screens to identify novel neuropsychiatric drugs including aminergic agents for the possible treatment of Parkinson’s disease and depression. Mol Psychiatry. 19:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebestky T, Chang JS, Dankert H, Zelnik L, Kim YC, Han KA, Wolf FW, Perona P, and Anderson DJ. 2009. Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron. 64:522–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Su H, and O’Dowd DK. 2003. GABA receptors containing Rdl subunits mediate fast inhibitory synaptic transmission in Drosophila neurons. J Neurosci. 23:4625–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HG, Rohila S, and Han KA. 2009. The octopamine receptor OAMB mediates ovulation via Ca2+/calmodulin-dependent protein kinase II in the Drosophila oviduct epithelium. PloS one. 4:e4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima F, Prado VF, Prado MA, and Kushmerick C. 2010. Quantal release of acetylcholine in mice with reduced levels of the vesicular acetylcholine transporter. J Neurochem. 113:943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Placais PY, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, and Tanimoto H. 2012. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 488:512–516. [DOI] [PubMed] [Google Scholar]
- Liu WW, and Wilson RI. 2013. Glutamate is an inhibitory neurotransmitter in the Drosophila olfactory system. Proc Natl Acad Sci U S A. 110:10294–10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy CA, Somasundaram E, Omar A, Bhutto U, Medley M, Dzubuk N, and Cooper RL. 2019. Pharmacological identification of cholinergic receptor subtypes: modulation of locomotion and neural circuit excitability in Drosophila larvae. Neuroscience. 411:47–64. [DOI] [PubMed] [Google Scholar]
- Martin CA, and Krantz DE. 2014. Drosophila melanogaster as a genetic model system to study neurotransmitter transporters. Neurochem Int. 73:71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse NY, Turner GC, and Jefferis GS. 2009. Olfactory information processing in Drosophila. Curr Biol. 19:R700–713. [DOI] [PubMed] [Google Scholar]
- McClung C, and Hirsh J. 1998. Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr Biol. 8:109–112. [DOI] [PubMed] [Google Scholar]
- Melnattur KV, and Lee CH. 2011. Visual circuit assembly in Drosophila. Dev Neurobiol. 71:1286–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon KP, Carrillo RA, and Zinn K. 2013. Development and plasticity of the Drosophila larval neuromuscular junction. Wiley Interdiscip Rev Dev Biol. 2:647–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastirioti M 1999. Biogenic amine systems in the fruit fly Drosophila melanogaster. Microscop Res Tech. 45:106–121. [DOI] [PubMed] [Google Scholar]
- Monastirioti M, Linn CEJ, and White K. 1996. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J. Neurosci 16:3900–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, and Margolis EB. 2017. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 18:73–85. [DOI] [PubMed] [Google Scholar]
- Moriyama Y, Hiasa M, Sakamoto S, Omote H, and Nomura M. 2017. Vesicular nucleotide transporter (VNUT): appearance of an actress on the stage of purinergic signaling. Purinergic Signal. 13:387–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, and Sulzer D. 2009. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 62:218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu L, Ito K, Bacon JP, and Strausfeld NJ. 2012. Optic glomeruli and their inputs in Drosophila share an organizational ground pattern with the antennal lobes. J Neurosci. 32:6061–6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster-Wandowski A, Zander JF, Richter K, and Ahnert-Hilger G. 2016. Co-existence of Functionally Different Vesicular Neurotransmitter Transporters. Front Synaptic Neurosci. 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutch SA, Kensel-Hammes P, Gadd JC, Fujimoto BS, Allen RW, Schiro PG, Lorenz RM, Kuyper CL, Kuo JS, Bajjalieh SM, and Chiu DT. 2011. Protein quantification at the single vesicle level reveals that a subset of synaptic vesicle proteins are trafficked with high precision. J Neurosci. 31:1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nall AH, and Sehgal A. 2013. Small-molecule screen in adult Drosophila identifies VMAT as a regulator of sleep. J Neurosci. 33:8534–8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassel DR 2018. Substrates for Neuronal Cotransmission With Neuropeptides and Small Molecule Neurotransmitters in Drosophila. Front Cell Neurosci. 12:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nässel DR 1999. Histamine in the brain of insects: A review. Microscop. Res. Tech 44:121–136. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, and Cooper RL. 1998. GABA transporters in Drosophila melanogaster: molecular cloning, behavior, and physiology. Invert Neurosci. 3:279–294. [DOI] [PubMed] [Google Scholar]
- Pankova K, and Borst A. 2017. Transgenic line for the identification of cholinergic release sites in Drosophila melanogaster. J Exp Biol. 220:1405–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parinejad N, Peco E, Ferreira T, Stacey SM, and van Meyel DJ. 2016. Disruption of an EAAT-Mediated Chloride Channel in a Drosophila Model of Ataxia. J Neurosci. 36:7640–7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SS, Schulz EM, and Lee D. 2007. Disruption of dopamine homeostasis underlies selective neurodegeneration mediated by alpha-synuclein. The European journal of neuroscience. 26:3104–3112. [DOI] [PubMed] [Google Scholar]
- Pendleton RG, Rasheed A, and Hillman R. 2000. Effects of adrenergic agents on locomotor behavior and reproductive development in Drosophila. Drug Development Research. 50:142–146. [Google Scholar]
- Pizzo AB, Karam CS, Zhang Y, Yano H, Freyberg RJ, Karam DS, Freyberg Z, Yamamoto A, McCabe BD, and Javitch JA. 2013. The membrane-raft protein Flotillin-1 is essential in dopamine neurons for amphetamine-induced behavior in Drosophila. . Molecular Psychiatry. 18:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EN, Larsen KE, Krantz DE, Liu Y, Haycock JW, Setlik W, Gershon MD, Edwards RH, and Sulzer D. 2000. Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J. Neurosci 20:7297–7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado VF, Roy A, Kolisnyk B, Gros R, and Prado MA. 2013. Regulation of cholinergic activity by the vesicular acetylcholine transporter. Biochem J. 450:265–274. [DOI] [PubMed] [Google Scholar]
- Richardt A, Rybak J, Stortkuhl KF, Meinertzhagen IA, and Hovemann BT. 2002. Ebony protein in the Drosophila nervous system: optic neuropile expression in glial cells. J Comp Neurol. 452:93–102. [DOI] [PubMed] [Google Scholar]
- Roeder T 2005. Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol. 50:447–477. [DOI] [PubMed] [Google Scholar]
- Roelofs J, and Van Haastert PJ. 2001. Genes lost during evolution. Nature. 411:1013–1014. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, and Broadie K. 2002. Electrophysiological analysis of synaptic transmission in central neurons of Drosophila larvae. J Neurophysiol. 88:847–860. [DOI] [PubMed] [Google Scholar]
- Romero-Calderón R, Uhlenbrock G, Boryz J, Simon AF, Grygoruk A, Yee SK, Shyer A, Ackerson LC, Maidment NT, Meinertzhagen IA, Hovemann BT, and Krantz DE. 2008. A glial variant of the vesicular monoamine transporter is required to store histamine in the Drosophila visual system. PLoS Genet. 4(11):e1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossano AJ, Kato A, Minard KI, Romero MF, and Macleod GT. 2017. Na(+) /H(+) exchange via the Drosophila vesicular glutamate transporter mediates activity-induced acid efflux from presynaptic terminals. J Physiol. 595:805–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang TK, Chang HY, Lawless GM, Ratnaparkhi A, Mee L, Ackerson LC, Maidment NT, Krantz DE, and Jackson GR. 2007. A Drosophila model of mutant human parkin-induced toxicity demonstrates selective loss of dopaminergic neurons and dependence on cellular dopamine. J Neurosci. 27:981–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy PV 1991. Histamine: a neurotransmitter candidate for Drosophila photoreceptors. J Neurochem. 57:1757–1768. [DOI] [PubMed] [Google Scholar]
- Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, and Moriyama Y. 2008. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A. 105:56835686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal B, Daniels GM, Wolfgang WJ, Forte MA, and Amara SG. 1998. Identification and characterization of a cDNA encoding a neuronal glutamate transporter from Drosophila melanogaster. Receptors Channels. 6:51–64. [PubMed] [Google Scholar]
- Seelig JD, and Jayaraman V. 2013. Feature detection and orientation tuning in the Drosophila central complex. Nature. 503:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, and Tononi G. 2000. Correlates of sleep and waking in Drosophila melanogaster. Science. 287:1834–1837. [DOI] [PubMed] [Google Scholar]
- Shen H, Marino RAM, McDevitt RA, Bi GH, Chen K, Madeo G, Lee PT, Liang Y, De Biase LM, Su TP, Xi ZX, and Bonci A. 2018. Genetic deletion of vesicular glutamate transporter in dopamine neurons increases vulnerability to MPTP-induced neurotoxicity in mice. Proc Natl Acad Sci U S A. 115:E11532–E11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showell SS, Martinez Y, Gondolfo S, Boppana S, and Lawal HO. 2020. Overexpression of the vesicular acetylcholine transporter disrupts cognitive performance and causes age-dependent locomotion decline in Drosophila. Mol Cell Neurosci. 105:103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silm K, Yang J, Marcott PF, Asensio CS, Eriksen J, Guthrie DA, Newman AH, Ford CP, and Edwards RH. 2019. Synaptic Vesicle Recycling Pathway Determines Neurotransmitter Content and Release Properties. Neuron. 102:786–800 e785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AF, Daniels R, Romero-Calderón R, Grygoruk A, Chang HY, Najibi R, Shamouelian D, Salazar E, Solomon M, Ackerson LC, Maidment NT, Diantonio A, and Krantz DE. 2009. Drosophila vesicular monoamine transporter mutants can adapt to reduced or eliminated vesicular stores of dopamine and serotonin. Genetics. 181:525–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H-J, Ming G-L, Fon E, Bellocchio E, Edwards RH, and Poo M-M. 1997. Expression of a putative vesicular acetylcholine transporter facilitates quantal transmitter packaging. Neuron. 18:815–826. [DOI] [PubMed] [Google Scholar]
- Soustelle L, Besson MT, Rival T, and Birman S. 2002. Terminal glial differentiation involves regulated expression of the excitatory amino acid transporters in the Drosophila embryonic CNS. Dev Biol. 248:294–306. [DOI] [PubMed] [Google Scholar]
- Steinkellner T, Zell V, Farino ZJ, Sonders MS, Villeneuve M, Freyberg RJ, Przedborski S, Lu W, Freyberg Z, and Hnasko TS. 2018. Role for VGLUT2 in selective vulnerability of midbrain dopamine neurons. J Clin Invest. 128:774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenesen D, Moehlman AT, and Kramer H. 2015. The carcinine transporter CarT is required in Drosophila photoreceptor neurons to sustain histamine recycling. Elife. 4:e10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes AH, Brown BG, Lee CK, Doolittle DJ, and Vrana KE. 1996. Tyrosinase enhances the covalent modification of DNA by dopamine. Brain Res Mol Brain Res. 42:167–170. [DOI] [PubMed] [Google Scholar]
- Stokes AH, Hastings TG, and Vrana KE. 1999. Cytotoxic and genotoxic potential of dopamine. J Neurosci Res. 55:659–665. [DOI] [PubMed] [Google Scholar]
- Stork T, Sheehan A, Tasdemir-Yilmaz OE, and Freeman MR. 2014. Neuron-glia interactions through the Heartless FGF receptor signaling pathway mediate morphogenesis of Drosophila astrocytes. Neuron. 83:388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R 2002. The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol. 12:633–638. [DOI] [PubMed] [Google Scholar]
- Stuart AE, Borycz J, and Meinertzhagen IA. 2007. The dynamics of signaling at the histaminergic photoreceptor synapse of arthropods. Prog Neurobiol. 82:202–227. [DOI] [PubMed] [Google Scholar]
- Suh J, and Jackson FR. 2007. Drosophila ebony activity is required in glia for the circadian regulation of locomotor activity. Neuron. 55:435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Bogulavsky J, Larsen KE, Buhr G, Karatekin E, Kleinman MH, Turro N, Krantz D, Edwards RH, Greene LA, and Zecca L. 2000. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc. Natl. Acad. Sci. USA 97:11869–11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmuller H, Heuser J, Wieland F, and Jahn R. 2006. Molecular anatomy of a trafficking organelle. Cell. 127:831–846. [DOI] [PubMed] [Google Scholar]
- Tao J, Bulgari D, Berkhoudt DA, Calderon MJ, Watkins SC, Fonseca Velez HJ, Sabeva N, Deitcher DL, and Levitan ES. 2019. Drosophila Ptp4E regulates vesicular packaging for monoamine-neuropeptide co-transmission. J Cell Sci. 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres G, and Horowitz JM. 1998. Activating properties of cocaine and cocaethylene in a behavioral preparation of Drosophila melanogaster. Synapse. 29:148–161. [DOI] [PubMed] [Google Scholar]
- Trudeau LE, and El Mestikawy S. 2018. Glutamate Cotransmission in Cholinergic, GABAergic and Monoamine Systems: Contrasts and Commonalities. Front Neural Circuits. 12:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True JR, Yeh SD, Hovemann BT, Kemme T, Meinertzhagen IA, Edwards TN, Liou SR, Han Q, and Li J. 2005. Drosophila tan encodes a novel hydrolase required in pigmentation and vision. PLoS Genet. 1:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Tomita J, Tanimoto H, Endo K, Ito K, Kume S, and Kume K. 2012. Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat Neurosci. 15:1516–1523. [DOI] [PubMed] [Google Scholar]
- Ugur B, Chen K, and Bellen HJ. 2016. Drosophila tools and assays for the study of human diseases. Disease models & mechanisms. 9:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laar VS, Mishizen AJ, Cascio M, and Hastings TG. 2009. Proteomic identification of dopamine-conjugated proteins from isolated rat brain mitochondria and SH-SY5Y cells. Neurobiol Dis. 34:487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon SW, Goodchild J, and Baines RA. 2018. The VAChTY49N mutation provides insecticide-resistance but perturbs evoked cholinergic neurotransmission in Drosophila. PloS one. 13:e0203852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon SW, Goodchild J, and Baines RA. 2019. A Poly-Glutamine Region in the Drosophila VAChT Dictates Fill-Level of Cholinergic Synaptic Vesicles. eNeuro. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmaier SM, Kam K, Yang H, Fortin DL, Hua Z, Nicoll RA, and Edwards RH. 2006. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron. 51:71–84. [DOI] [PubMed] [Google Scholar]
- Wagner S, Heseding C, Szlachta K, True JR, Prinz H, and Hovemann BT. 2007. Drosophila photoreceptors express cysteine peptidase tan. J Comp Neurol. 500:601–611. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ferdousy F, Lawal H, Huang Z, Daigle JG, Izevbaye I, Doherty O, Thomas J, Stathakis DG, and O’Donnell JM. 2011. Catecholamines up integrates dopamine synthesis and synaptic trafficking. J Neurochem. 119:1294–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J 1997. How does a vesicle know it is full? Neuron. 18:683–686. [DOI] [PubMed] [Google Scholar]
- Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, Erickson JD, and Liu G. 2005. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 25:6221–6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI 2013. Early olfactory processing in Drosophila: mechanisms and principles. Annu Rev Neurosci. 36:217–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TR 1987. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv. Genet 24:127–222. [PubMed] [Google Scholar]
- Xu Y, An F, Borycz JA, Borycz J, Meinertzhagen IA, and Wang T. 2015. Histamine Recycling Is Mediated by CarT, a Carcinine Transporter in Drosophila Photoreceptors. PLoS Genet. 11:e1005764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, and Wang T. 2019. LOVIT Is a Putative Vesicular Histamine Transporter Required in Drosophila for Vision. Cell Rep. 27:1327–1333 e1323. [DOI] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA, and Schurmann FW. 2002. Synaptic organization of the mushroom body calyx in Drosophila melanogaster. J Comp Neurol. 445:211–226. [DOI] [PubMed] [Google Scholar]
- Yellman C, Tao H, He B, and Hirsh J. 1997. Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc Natl Acad Sci U S A. 94:4131–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Naganuma F, Iida T, Nakamura T, Harada R, Mohsen AS, Kasajima A, Sasano H, and Yanai K. 2013. Molecular mechanism of histamine clearance by primary human astrocytes. Glia. 61:905–916. [DOI] [PubMed] [Google Scholar]


