Abstract
Background:
Congenital heart disease (CHD) accounts for approximately 40% of deaths in United States (US) children with birth defects. Previous US data from 1999–2006 demonstrated an overall decrease in CHD mortality. Our study aimed to assess current trends in US mortality related to CHD from infancy to adulthood over the last 19 years and determine differences by sex and race/ethnicity.
Methods:
We conducted an analysis of death certificates from 1999–2017 to calculate annual CHD mortality by age at death, race/ethnicity, and sex. Population estimates used as denominators in mortality rate calculations for infants were based on National Center for Health Statistics live birth data. Mortality rates in individuals ≥1 year of age used US Census Bureau bridged-race population estimates as denominators. We used joinpoint regression to characterize temporal trends in all-cause mortality, mortality directly attributable to and related to CHD by age, race/ethnicity, and sex.
Results:
There were 47.7 million deaths with 1 in 814 deaths due to CHD (n=58,599). While all-cause mortality decreased 16.4% across all ages, mortality resulting from CHD declined 39.4% overall. The mean annual decrease in CHD mortality was 2.6%, with the largest decrease for those age >65 years. The age-adjusted mortality rate decreased from 1.37 to 0.83 per 100,000. Males had higher mortality due to CHD than females throughout the study, although both sexes declined at a similar rate (~40% overall), with a 3–4% annual decrease between 1999 and 2009, followed by a slower annual decrease of 1.4% through 2017. Mortality resulting from CHD significantly declined among all races/ethnicities studied, although disparities in mortality persisted for non-Hispanic blacks versus non-Hispanic whites (mean annual decrease 2.3% versus 2.6%, respectively; age-adjusted mortality rate 1.67 to 1.05 versus 1.35 to 0.80 per 100,000, respectively).
Conclusions:
While overall US mortality due to CHD has decreased over the last 19 years, disparities in mortality persist for males compared to females and for non-Hispanic blacks compared to non-Hispanic whites. Determining factors that contribute to these disparities such as access to quality care, timely diagnosis, and maintenance of insurance will be important moving into the next decade.
Keywords: Congenital Heart Disease, Mortality, Health disparities, Pediatric, Lifespan
INTRODUCTION
Congenital heart disease (CHD) is the leading cause of birth defect-related mortality, accounting for approximately 40% of deaths in children with birth defects in the United States and worldwide.1–4 Prior studies demonstrated decreasing mortality due to CHD in the United States over time, likely secondary to marked advances in disease recognition and improved medical and surgical care across the lifespan.5,6 The most comprehensive assessment in the United States to date was a population-based study evaluating CHD mortality from 1999 through 2006.5 This study demonstrated decreasing mortality over time among all race/ethnicities and both sexes; however, disparities persisted, with higher mortality described in non-Hispanic (NH) blacks compared to NH whites and in males compared to females throughout the lifespan. Other studies have shown the increased mortality for NH black children has been predominantly in the lowest mortality CHD lesions, suggesting an influence of sociodemographic factors.7,8 While children of Hispanic ethnicity have not demonstrated the same disparities in CHD mortality in population-based studies, subgroup analysis of Hispanics with CHD in the United States demonstrated higher mortality in infants of Mexican mothers than those of NH white mothers.9
More recent population-based CHD mortality data are lacking. Advances in CHD care are ongoing and include a dramatic increase in prenatal diagnosis, prevention of preterm and early-term deliveries, national collaborative quality initiatives in perioperative care of CHD, and public reporting of many CHD outcomes.10–17 Increased attention has been paid to sex, racial/ethnic, and sociodemographic disparities in care of people affected by CHD across the age spectrum.18 Our study aims to update prior data on temporal trends in mortality related to CHD in the United States by examining mortality over the last 19 years overall, by age and CHD lesion, to determine if mortality is continuing to decline, and to evaluate if prior disparities noted across race/ethnicity and sex are decreasing.
METHODS
Mortality (numerator) data
All data used in this project are free of charge and publicly available for download from https://www.cdc.gov/nchs, and the analytical methods are available from the corresponding author on reasonable request. We conducted a 19-year analysis of Multiple Cause of Death (MCOD) data files compiled and produced annually by the National Center for Health Statistics at the Centers for Disease Control and Prevention.19 As our study was based on analyses that used publicly available, de-identified MCOD and census data, it was deemed exempt by the Baylor College of Medicine Institutional Review Board. The MCOD files are based on death certificates filed by registries in all 50 states and the District of Columbia. Deaths among nonresident aliens, nationals living abroad, residents of United States territories, and fetal deaths are excluded from MCOD files. Each record is derived from a death certificate containing a single underlying cause of death (UCOD), up to 20 contributing causes of death, and demographic and geographic data on each decedent. The UCOD is assigned based on the World Health Organization’s definition of “the disease or injury which initiated the train of morbid events leading directly to death, or the circumstances of the accident or violence which produced the fatal injury.”20 As is the case for all deaths from 1999 and beyond, the UCOD and all listed contributing causes of death were captured using International Classification of Diseases, Tenth Revision (ICD-10) codes. For each decedent, we also ascertained sex (male, female), age in years (categorized as <1, 1 to 4, 5 to 17, 18 to 34, 35 to 49, 50 to 64, and ≥65), race/ethnicity, and associated CHD. Race and ethnicity are reported as separate variables on the death certificate; the final racial/ethnic category, defined to be consistent with categories used in bridged-race population estimates,21 was first based on ethnicity (Hispanic or NH) and the NH group was further subdivided by race (NH white, NH black, and NH other). Specific CHD lesion mortality was assessed overall in the lifespan, as well as in the infant period. Median age at death was also assessed for the study period.
In addition to deaths from any cause (all-cause mortality), we used the UCOD and contributing causes of death to define several CHD-specific mortality subgroups. Mortality resulting from CHD was defined as a death certificate in which the UCOD field contained an ICD-10 code in the Q20–Q26 range that is indicative of a CHD (Table I in the Supplement). CHD-related deaths were a broader category in which either the UCOD or any contributing cause of death had a CHD-related ICD-10 code.
Population (denominator) data
Population estimates used as denominators in the calculation of mortality rates for decedents less than one year of age were based on live birth data downloaded from the National Center for Health Statistics.22 For decedents one year of age and older, population estimates used as denominators in mortality rate calculation were bridged-race estimates from the United States Census Bureau.21 Population counts for 1999 were intercensal estimates based on the 1990 and 2000 decennial census; population counts for 2000 and 2010 were based on the decennial census conducted in their respective years; population counts for 2001–2009 were revised intercensal estimates based on the 2000 and 2010 decennial census; and population counts for 2011–2017 were postcensal estimates, combining the 2010 decennial census data with birth, death, migration, and net international immigration data.
Statistical analyses
For each year of the study, and across strata of sex and race/ethnicity, we calculated age-specific death rates for 1) all-cause mortality, 2) mortality due to CHD, and 3) CHD-related mortality. Rates were calculated as the number of deaths per 1,000 live births for infants <1 year or per 100,000 population for ages ≥1 year. The population subgroups of sex and race/ethnicity whose mortality was being compared can differ in their age distributions, and age is strongly positively correlated with mortality. Therefore, to facilitate comparison across subgroups and from year to year, we also calculated age-adjusted mortality rates using the direct standardization method in which age-specific death rates were weighted according to the 2000 Standard Population.23
We assessed temporal trends in all-cause mortality, mortality resulting directly due to and related to CHD by age, race/ethnicity, sex, and specific CHD. We then estimated and characterized temporal trends in mortality across the entire 19-year study period using joinpoint regression, which is particularly effective at identifying changes in the temporal trends of events over time.24 The first step in joinpoint regression is to fit annual mortality rate data to a straight line – one with zero joinpoints – that assumes a single trend best characterizes how rates change over the entire assessment period. Then, a joinpoint is added to the model at the most likely inflection point at which the trend changes, and a Monte Carlo permutation test is used to determine whether the added joinpoint improves model fit; if so, the joinpoint will be incorporated.24 The addition and assessment of additional joinpoints proceeds in an iterative fashion until a final model is selected with an optimal number of joinpoints. In the final model, each joinpoint corresponds to a statistically significant change in the mortality trend, and how mortality changes within each distinct time interval is characterized with an annual percent change (APC) and its associated 95% confidence interval (CI). Joinpoint regression also provides an estimate of the average APC (AAPC), which is a single value that describes trends in mortality over the entire study period, even when there are significant changes in the trend over time.24 To compare two sets of trend data (e.g., men and women) we used a test of parallelism to assess whether their joinpoint-estimated regression mean functions were parallel, with the p-value of the test determined using the permutation distribution of the test statistic. To assess the extent to which race/ethnic disparities in all-cause and CHD mortality were changing during the study period, we calculated age-standardized mortality rate ratios with 95% confidence intervals? (e.g., comparing NH blacks to NH whites) and infant mortality rate ratios for each year of the study.
To maintain consistency with previously published research on CHD-related mortality in the United States, our base case analyses included all deaths associated with CHD. However, as a sensitivity analysis, in an attempt to assess CHD mortality independent of prematurity and extracardiac birth defects, we re-ran our analyses after excluding as a CHD-related death any case with either prematurity, an extracardiac defect, or genetic syndrome as a contributing cause of death (Figure I in the Supplement). Considering the large proportion of deaths due to CHD that are documented as “unspecified CHD”, a subgroup which may have comparatively lower accuracy, we conducted another sensitivity analysis in which results were analyzed excluding those with unspecified CHD.
All statistical tests were performed with SAS version 9.4 (Cary, NC) and the Joinpoint Regression Program version 4.7.0.0.25
RESULTS
All cause and age-adjusted CHD mortality
Overall all-cause and CHD mortality
In the United States between 1999 and 2017, there were approximately 2.5 million deaths annually with 3,084 per year due to CHD, representing 1 in 814 deaths. While the all-cause, age-adjusted mortality rate decreased on average by 1.1% per year (Table 1), the mean annual decrease in mortality due to CHD and CHD-related mortality was faster, at 2.6% and 2.0%, respectively. Among all CHD-related deaths, CHD was listed as the UCOD 65.2% of the time (Table II in the Supplement), decreasing from 68.9% in 1999 to 60.9% in 2017 (Table III in the Supplement).
Table 1.
Temporal Trends in All-Cause Mortality and Mortality Resulting From CHD, by Age, Race/Ethnicity, and Sex, United States, 1999–2017
| Population subgroup | CHD listed as underlying cause of death | All-Cause | ||||||
|---|---|---|---|---|---|---|---|---|
| Mortality rate * | 99–17 AAPC (95% CI) | Mortality rate * | 1999–2017 average annual percent change (95% CI) | |||||
| 1999 | 2006 | 2017 | 1999 | 2006 | 2017 | |||
| All ages | ||||||||
| Overall | 1.37 | 1.03 | 0.83 | −2.6 (−2.9, −2.2) | 875.20 | 791.26 | 732.07 | −1.1 (−1.2, −0.9) |
| Race/ethnicity | ||||||||
| NH white | 1.35 | 1.01 | 0.80 | −2.6 (−3.0, −2.2) | 859.58 | 788.72 | 755.15 | −0.8 (−1.0, −0.7) |
| NH black | 1.67 | 1.41 | 1.05 | −2.3 (−2.8, −1.8) | 1149.35 | 1018.60 | 882.25 | −1.5 (−1.7, −1.3) |
| Hispanic | 1.20 | 0.86 | 0.81 | −2.2 (−2.8, −1.5) | 676.26 | 603.83 | 525.64 | −1.5 (−1.7, −1.3) |
| NH other | 0.85 | 0.73 | 0.55 | −2.9 (−3.4, −2.3) | 570.67 | 499.98 | 438.59 | −1.4 (−1.6, −1.2) |
| Sex | ||||||||
| Male | 1.47 | 1.16 | 0.92 | −2.4 (−2.8, −2.0) | 1066.58 | 942.84 | 864.63 | −1.2 (−1.4, −1.1) |
| Female | 1.26 | 0.91 | 0.74 | −2.7 (−3.2, −2.3) | 733.61 | 671.75 | 619.86 | −1.0 (−1.2, −0.7) |
| Age at death, <1 y | ||||||||
| Overall | 45.14 | 37.42 | 29.52 | −2.2 (−2.6, −1.8) | 704.86 | 667.58 | 577.92 | −1.0 (−1.4, −0.7) |
| Race/ethnicity | ||||||||
| NH white | 44.18 | 35.12 | 26.89 | −2.6 (−2.9, −2.2) | 577.44 | 558.89 | 469.52 | −1.0 (−1.7, −0.3) |
| NH black | 56.70 | 53.77 | 40.26 | −1.8 (−2.3, −1.3) | 1458.00 | 1377.77 | 1199.01 | −1.0 (−2.2, 0.2) |
| Hispanic | 45.08 | 36.62 | 33.66 | −1.8 (−2.6, −1.1) | 574.89 | 548.40 | 530.69 | −0.5 (−0.7, −0.4) |
| NH other | 28.73 | 25.52 | 17.77 | −3.2 (−4.2, −2.3) | 463.49 | 430.20 | 334.07 | −1.9 (−2.4, −1.3) |
| Sex | ||||||||
| Male | 48.60 | 40.90 | 30.34 | −2.4 (−2.7, −2.1) | 771.14 | 730.26 | 630.44 | −1.1 (−1.5, −0.7) |
| Female | 41.51 | 33.77 | 28.67 | −2.1 (−2.6, −1.7) | 635.35 | 601.79 | 522.87 | −1.0 (−1.4, −0.6) |
| Age at death, 1–4 y | ||||||||
| Overall | 1.43 | 1.14 | 0.95 | −2.0 (−2.7, −1.2) | 34.22 | 29.13 | 24.25 | −1.9 (−2.1, −1.7) |
| Race/ethnicity | ||||||||
| NH white | 1.14 | 0.96 | 0.68 | −2.4 (−3.6, −1.2) | 30.13 | 25.65 | 22.42 | −1.6 (−1.8, −1.3) |
| NH black | 2.43 | 1.76 | 1.67 | −1.8 (−3.1, −0.4) | 54.59 | 47.44 | 40.15 | −1.5 (−2.1, −0.9) |
| Hispanic | 1.54 | 1.16 | 0.96 | −2.1 (−3.4, −0.8) | 30.87 | 26.52 | 19.40 | −2.8 (−3.5, −2.1) |
| NH other | 1.55 | 1.26 | 1.17 | −1.2 (−3.4, 1.1) | 31.04 | 24.82 | 19.82 | −2.4 (−3.0, −1.7) |
| Sex | ||||||||
| Male | 1.63 | 1.21 | 1.03 | −2.4 (−3.1, −1.6) | 37.92 | 31.29 | 27.28 | −1.9 (−2.1, −1.7) |
| Female | 1.23 | 1.07 | 0.87 | −1.5 (−2.7, −0.3) | 30.35 | 26.88 | 21.08 | −1.9 (−2.2, −1.7) |
| Age at death, 5–17 y | ||||||||
| Overall | 0.46 | 0.3 | 0.28 | −3.0 (−3.6, −2.3) | 27.20 | 23.11 | 19.42 | −1.8 (−2.3, −1.3) |
| Race/ethnicity | ||||||||
| NH white | 0.42 | 0.30 | 0.25 | −3.3 (−4.1, −2.5) | 26.14 | 21.99 | 18.61 | −1.8 (−2.4, −1.1) |
| NH black | 0.69 | 0.42 | 0.49 | −2.1 (−3.3, −1.0) | 37.32 | 31.49 | 29.59 | −1.2 (−2.2, −0.2) |
| Hispanic | 0.41 | 0.20 | 0.22 | −2.6 (−4.1, −1.1) | 22.62 | 20.72 | 15.99 | −2.0 (−3.2, −0.8) |
| NH other | 0.38 | 0.23 | 0.38 | −1.4 (−3.6, 1.0) | 21.91 | 18.97 | 15.04 | −1.9 (−3.3, −0.4) |
| Sex | ||||||||
| Male | 0.58 | 0.33 | 0.35 | −2.9 (−3.6, −2.1) | 33.48 | 28.91 | 24.23 | −1.7 (−2.6, −0.9) |
| Female | 0.35 | 0.26 | 0.21 | −3.1 (−3.8, −2.3) | 20.57 | 17.03 | 14.41 | −1.8 (−2.6, −1.0) |
| Age at death, 18–34 y | ||||||||
| Overall | 0.61 | 0.49 | 0.36 | −3.3 (−3.8, −2.8) | 97.15 | 103.7 | 115.03 | 1.1 (0.3, 1.8) |
| Race/ethnicity | ||||||||
| NH white | 0.65 | 0.48 | 0.35 | −3.8 (−4.4, −3.2) | 86.99 | 98.90 | 120.84 | 1.9 (1.4, 2.4) |
| NH black | 0.71 | 0.74 | 0.50 | −2.8 (−3.8, −1.8) | 174.00 | 169.41 | 163.95 | −0.2 (−1.0, 0.6) |
| Hispanic | 0.44 | 0.37 | 0.37 | −1.8 (−3.5, 0.0) | 83.36 | 85.98 | 83.10 | 0.1 (−1.3, 1.5) |
| NH other | 0.44 | 0.40 | 0.18 | −1.6 (−3.7, 0.6) | 62.60 | 60.71 | 65.00 | 0.3 (−0.1, 0.8) |
| Sex | ||||||||
| Male | 0.67 | 0.66 | 0.49 | −2.6 (−3.3, −1.9) | 136.15 | 148.45 | 160.97 | 1.0 (0.4, 1.6) |
| Female | 0.56 | 0.31 | 0.23 | −4.7 (−5.4, −4.0) | 57.11 | 57.7 | 67.35 | 0.9 (0.6, 1.2) |
| Age at death, 35–49 y | ||||||||
| Overall | 0.64 | 0.48 | 0.38 | −2.2 (−2.7, −1.7) | 243.12 | 246.08 | 235.24 | −0.2 (−0.5, 0.1) |
| Race/ethnicity | ||||||||
| NH white | 0.69 | 0.54 | 0.46 | −1.5 (−2.1, −0.8) | 219.33 | 239.87 | 251.40 | 0.8 (0.5, 1.1) |
| NH black | 0.70 | 0.45 | 0.36 | −2.5 (−3.7, −1.2) | 464.41 | 410.49 | 349.13 | −1.6 (−2.2, −1.0) |
| Hispanic | 0.39 | 0.35 | 0.28 | −2.7 (−4.1, −1.2) | 190.06 | 169.58 | 151.86 | −1.3 (−1.9, −0.6) |
| NH other | 0.32 | 0.25 | 0.12 | −2.4 (−4.6, −0.2) | 140.36 | 136.67 | 128.63 | −0.6 (−1.1, −0.1) |
| Sex | ||||||||
| Male | 0.69 | 0.54 | 0.46 | −1.7 (−2.3, −1.1) | 313.10 | 309.14 | 295.93 | −0.3 (−0.6, −0.1) |
| Female | 0.60 | 0.42 | 0.32 | −2.8 (−3.6, −2.0) | 174.40 | 183.98 | 175.21 | 0.0 (−0.3, 0.4) |
| Age at death, 50–64 y | ||||||||
| Overall | 0.65 | 0.46 | 0.4 | −2.4 (−3.4, −1.4) | 795.63 | 737.44 | 751.54 | −0.3 (−0.4, −0.2) |
| Race/ethnicity | ||||||||
| NH white | 0.67 | 0.47 | 0.45 | −1.9 (−3.1, −0.8) | 753.99 | 714.15 | 762.83 | 0.1 (−0.1, 0.3) |
| NH black | 0.74 | 0.70 | 0.37 | −3.5 (−5.6, −1.3) | 1368.62 | 1198.37 | 1099.00 | −1.2 (−1.4, −1.0) |
| Hispanic | 0.38 | 0.31 | 0.24 | −2.8 (−4.9, −0.7) | 596.78 | 537.36 | 496.07 | −1.1 (−1.2, −0.9) |
| NH other | 0.47 | 0.19 | 0.20 | −3.4 (−6.3, −0.3) | 474.91 | 411.44 | 407.04 | −0.7 (−0.9, −0.5) |
| Sex | ||||||||
| Male | 0.72 | 0.53 | 0.46 | −1.4 (−2.2, −0.5) | 991.35 | 923.47 | 938.71 | −0.3 (−0.4, −0.2) |
| Female | 0.59 | 0.41 | 0.35 | −3.0 (−3.7, −2.2) | 612.43 | 562.25 | 574.93 | −0.3 (−0.5, −0.2) |
| Age at death, ≥65 y | ||||||||
| Overall | 1.45 | 0.83 | 0.64 | −4.2 (−4.9, −3.5) | 5165.06 | 4734.20 | 4065.00 | −1.5 (−1.6, −1.4) |
| Race/ethnicity | ||||||||
| NH white | 1.50 | 0.87 | 0.69 | −3.9 (−4.6, −3.2) | 5257.74 | 4909.71 | 4285.54 | −1.3 (−1.4, −1.2) |
| NH black | 1.39 | 0.86 | 0.43 | −5.0 (−6.8, −3.2) | 5771.03 | 5042.22 | 4203.58 | −1.8 (−2.0, −1.5) |
| Hispanic | 1.15 | 0.30 | 0.50 | −5.4 (−9.1, −1.6) | 3464.59 | 3148.16 | 2817.09 | −1.2 (−1.4, −1.0) |
| NH other | 0.42 | 0.65 | 0.52 | −4.1 (−6.4, −1.7) | 2915.30 | 2635.32 | 2350.02 | −1.2 (−1.3, −1.1) |
| Sex | ||||||||
| Male | 1.27 | 0.81 | 0.63 | −3.7 (−5.2, −2.2) | 5635.32 | 5002.52 | 4322.99 | −1.5 (−1.7, −1.3) |
| Female | 1.59 | 0.84 | 0.65 | −4.5 (−5.7, −3.2) | 4836.98 | 4537.22 | 3859.25 | −1.3 (−1.5, −1.0) |
Footnote: CI, confidence interval
Mortality rates are calculated as the number of deaths per 100,000 population for all age groups one year or older and as the number of deaths per 100,000 live births for the infant (<1 year) age group. Mortality rates for “all ages” are age-adjusted; mortality rates for individual age groups are crude (age-specific).
All-cause and CHD mortality by sex
Males, compared to females, experienced higher all-cause, age-adjusted mortality (925.5 vs. 661.1 per 100,000) and age-adjusted mortality due to CHD (1.13 vs. 0.93 per 100,000). When evaluating temporal trends, age-adjusted mortality decreased from 1999 and 2017 for both males and females, and this decline was more pronounced for mortality due to CHD than for all-cause mortality (Table 1). Trends in age-adjusted CHD mortality were similar for males and females, with a 3–4% annual decrease between 1999 and 2009, followed by a slower annual decrease of 1.4% through 2017 (Figure 1).
Figure 1. Temporal trends in age-adjusted all-cause mortality (top) and CHD-specific mortality (bottom), by sex, 1999–2017.
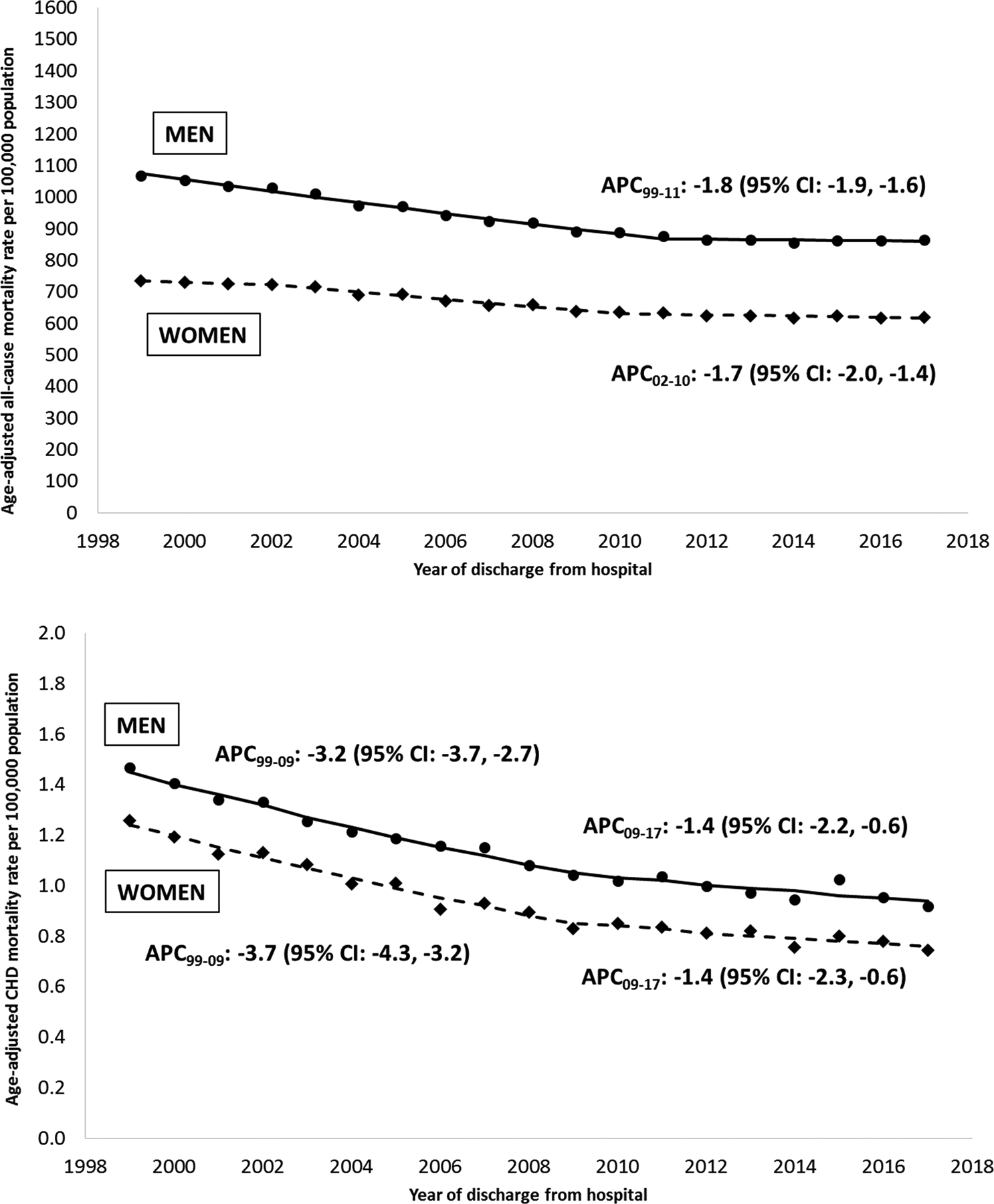
Footnotes: APC, annual percent change; CHD, congenital heart defect
Only APCs that reflect a statistically significant temporal trend in mortality are presented as text. Differences by gender: Tests of parallelism revealed men and women had non-parallel regression mean functions for all-cause mortality (p=0.008) and CHD-specific mortality (p=0.004). Differences by cause: Tests of parallelism revealed no differences between all-cause and CHD-specific mortality for either men (p=0.420) or women (p=0.158).
All-cause and CHD mortality by race/ethnicity
Age-adjusted mortality decline from 1999 to 2017 was more pronounced for mortality due to CHD than for all-cause mortality for all race/ethnicities (Figure 2). Age-adjusted all-cause mortality demonstrated that NH blacks experienced more pronounced decreases in rates of mortality (from 1149.4 to 882.3 per 100,000) than NH whites (from 859.6 to 755.2 per 100,000) and Hispanics (676.3 to 525.6 per 100,000, Table 1). In contrast, NH blacks experienced a similar rate of decline in CHD-specific age-adjusted mortality at 2.3% annually, compared to 2.6% for NH whites (Figure 2). Whereas the age-adjusted mortality rate ratio comparing NH blacks to NH whites has decreased from 1.34 to 1.17 for all-cause mortality, the disparity for CHD-specific mortality has actually risen from 1.24 in 1999 to 1.31 in 2017 without a consistent temporal trend (Figure 3). Among Hispanics, although decreases in age-adjusted CHD mortality mirrored NH whites from 1999–2009, since then the static rate among Hispanics and the ongoing decline among NH whites has resulted in a Hispanic:NH white age-standardized mortality rate ratio changing from 0.89 (95% CI: 0.80, 0.99) in 1999 to 1.01 (95% CI: 0.92, 1.12) in 2017 (Figure 3).
Figure 2. Temporal trends in age-adjusted all-cause mortality (top) and CHD-specific mortality (bottom), by race/ethnicity, 1999–2017.
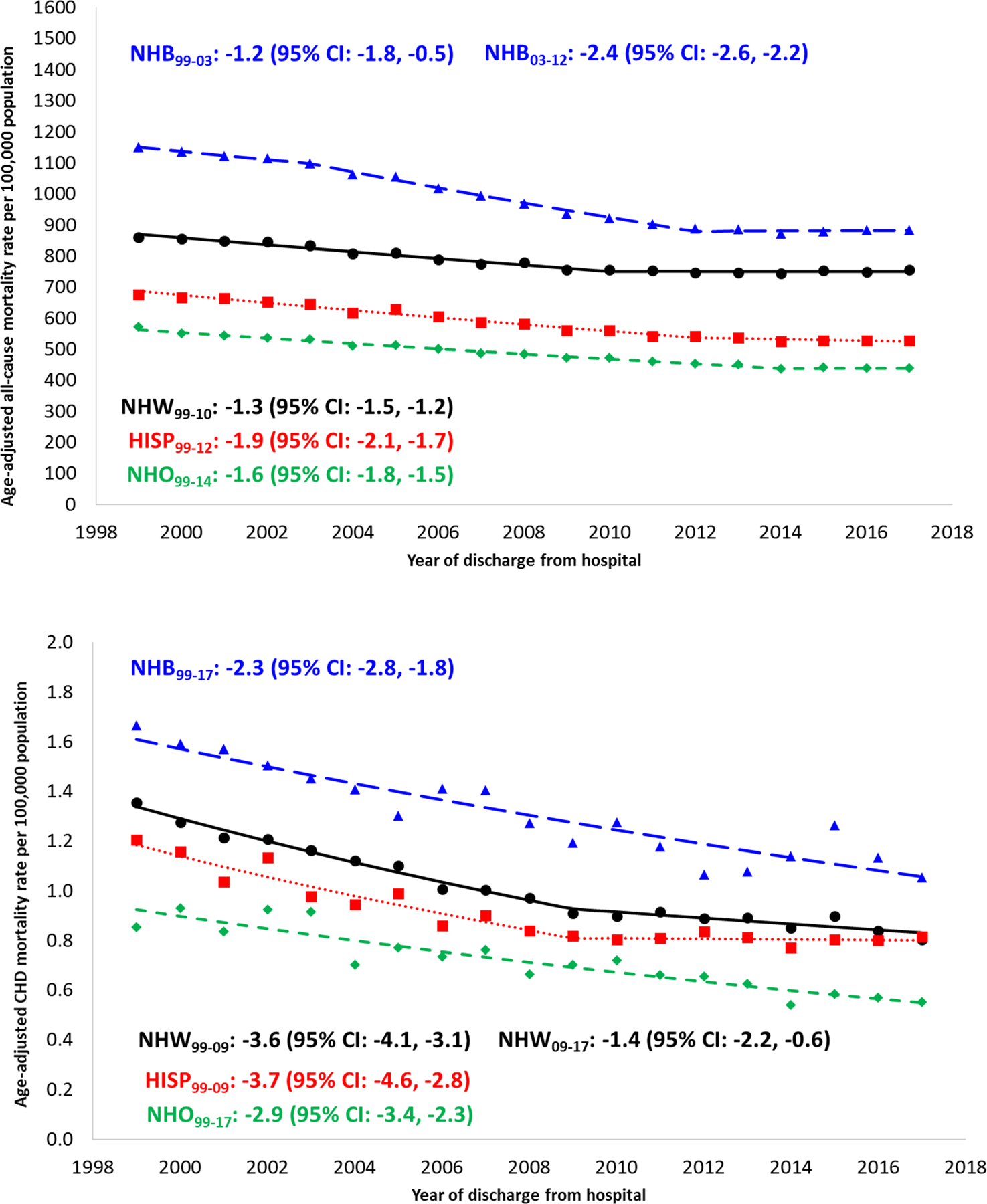
Footnotes: APC, annual percent change; CHD, congenital heart defect
Only APCs that reflect a statistically significant temporal trend in mortality are presented as text. Differences by race/ethnicity: Tests of parallelism revealed that NH blacks (p<0.001), Hispanics (p<0.001), and NH other (p=0.005) had non-parallel regression mean functions for all-cause mortality compared to NH whites. NH blacks (p=0.029) and Hispanics (p=0.024) had non-parallel regression mean functions for CHD-specific mortality compared to NH whites. NH other did not have regression lines that departed from parallelism compared to NH whites (p=0.089). Differences by cause: Tests of parallelism revealed no differences between all-cause and CHD-specific mortality for either NH whites (p=0.206), NH blacks (p=0.432), Hispanics (p=0.511), or NH other (p=0.162).
Figure 3. Age-standardized mortality rate ratios, all ages, 1999–2017.
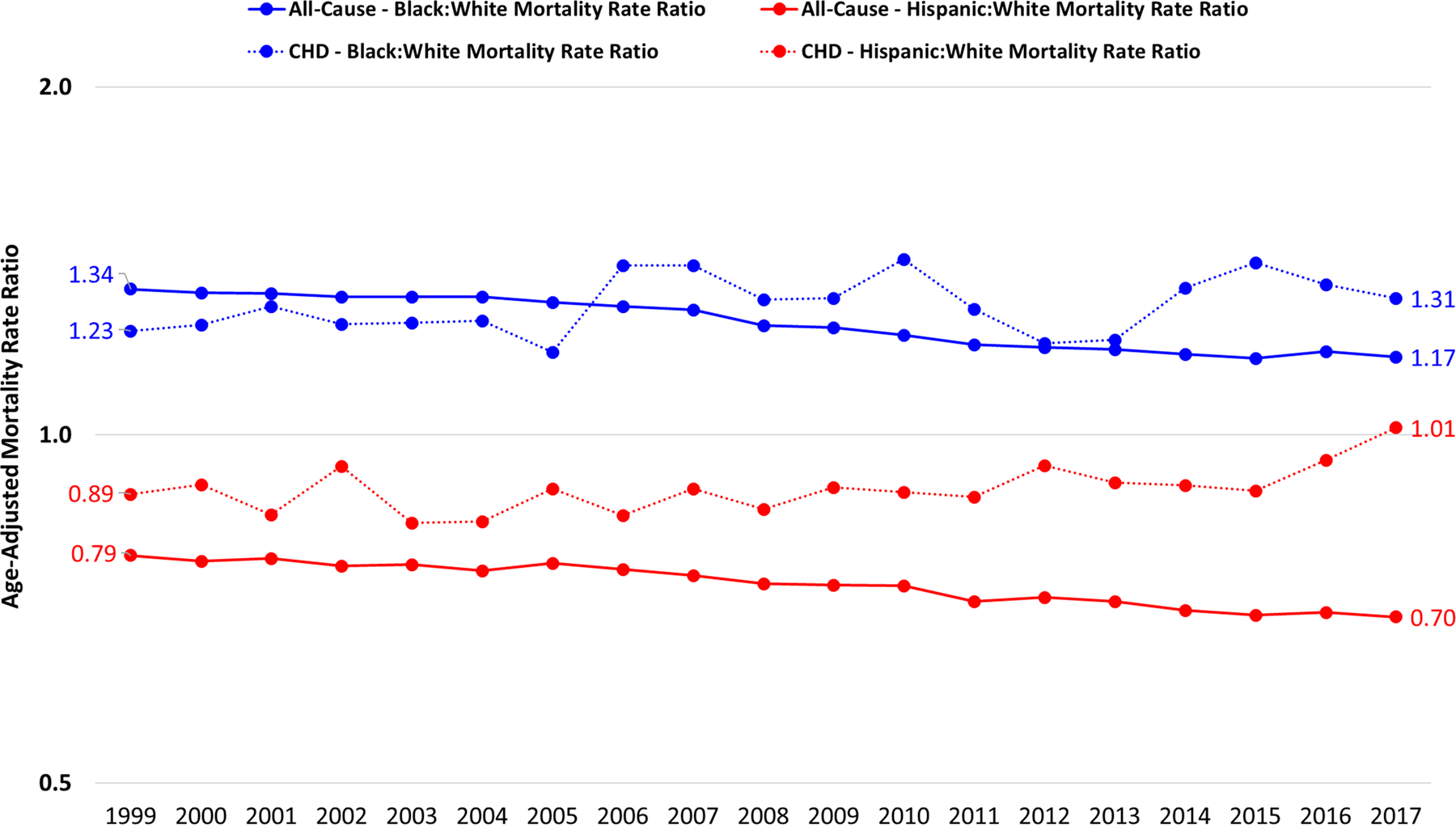
Footnotes: Rate ratios were calculated as the age-standadrized mortality rate in one racial/ethnic group (e.g., non-Hispanic blacks) divided by the age-standadrized mortality rate in the reference racial/ethnic group (e.g., non-Hispanic whites). This was done separately for all-cause mortality and mortality due to CHD.
CHD mortality by CHD lesion
Overall, the largest number of deaths in which a CHD was listed as the UCOD was “other specified CHD” and “unspecified CHD”. Over the 19-year period, the lesions with the highest percentage of death that occurred during infancy were anomalous pulmonary venous connection (89.4%), hypoplastic left heart syndrome (86.1%), and common truncus (e.g. truncus arteriosus, 77.8%) (Table 2) When examining temporal trends, infant mortality due to CHD had a statistically significant decrease for most CHD lesions (Table 2). The highest mean annual decrease in infant mortality by lesion over the 19-year period was for ventricular septal defect (5.6%), aortic valve anomalies (4.9%) and common ventricle/double inlet left ventricle (3.5%). For most CHD lesions, we saw an increase in the median age at death between the early (1999–2006) and late (2007–2017) time periods. Of note, for the lesions associated with the highest infant mortality, anomalous pulmonary venous connection and common truncus, the median age at death did not change between these two time periods, and remained at 30 days. For hypoplastic left heart syndrome, the median age at death increased by 53% (from 14 days to 30 days). Even when infant deaths were excluded, this tendency for increased median age at death over time was also observed for all lesions except common truncus (Table 2).
Table 2.
Temporal Trends in Infant Mortality Resulting From CHD, by CHD lesion, 1999–2017
| Defect | Deaths in all age groups | Deaths during infancy | % deaths that occur during infancy | 99–17 AAPC in infant mortality (95% CI) | Among all CHD-related deaths | Excluding infants aged < 1 year | ||
|---|---|---|---|---|---|---|---|---|
| Median Age at Death 1999–2006 | Median Age at Death 2007–2017 | Median Age at Death 1999–2006 | Median Age at Death 2007–2017 | |||||
| Anomalous pulmonary venous connection | 661 | 591 | 89.4 | −1.0 (−2.8, 0.9) | 30 days | 30 days | 1 year | 34 years |
| Aortic valve anomalies | 946 | 449 | 47.5 | −4.9 (−6.5, −3.2) | 152 days | 23 years | 45 years | 58 years |
| Atrioventricular septal defect | 1033 | 606 | 58.7 | 0.6 (−2.5, 3.8) | 183 days | 213 days | 22 years | 32 years |
| Coarctation of the aorta | 898 | 517 | 57.6 | −3.2 (−5.0, −1.3) | 61 days | 152 days | 38 years | 41 years |
| Common truncus | 796 | 619 | 77.8 | −1.9 (−5.1, 1.4) | 30 days | 30 days | 22 years | 19 years |
| Common ventricle/double inlet ventricle | 482 | 260 | 53.9 | −3.5 (−5.2, −1.7) | 183 days | 335 days | 17 years | 18 years |
| Ebstein anomaly | 850 | 485 | 57.1 | −1.7 (−2.7, −0.6) | 30 days | 91 days | 41 years | 48 years |
| Hypoplastic left heart syndrome | 6536 | 5626 | 86.1 | −2.8 (−3.6, −1.9) | 14 days | 30 days | 2 years | 4 years |
| Pulmonary artery atresia/stenosis | 1493 | 855 | 57.3 | −1.6 (−2.9, −0.3) | 183 days | 213 days | 29 years | 35 years |
| Pulmonary valve anomalies | 172 | 128 | 74.4 | 0.7 (−2.7, 4.2) | 30 days | 61 days | 27 years | 33 years |
| Tetralogy of Fallot | 3103 | 1176 | 37.9 | −0.9 (−3.2, 1.5) | 8 years | 16 years | 34 years | 38 years |
| Transposition of the great arteries | 2165 | 1243 | 57.4 | −3.1 (−4.3, −2.0) | 61 days | 213 days | 23 years | 30 years |
| Tricuspid valve anomalies | 439 | 165 | 37.6 | −2.2 (−5.7, 1.5) | 12 years | 17 years | 23 years | 31 years |
| Ventricular septal defect | 2613 | 592 | 22.7 | −5.6 (−7.5, −3.7) | 44 years | 51 years | 60 years | 61 years |
| Other specified congenital heart disease | 10900 | 2311 | 21.2 | −2.0 (−2.8, −1.2) | 36 years | 39 years | 44 years | 47 years |
| Unspecified congenital heart disease | 20663 | 11134 | 53.9 | −1.9 (−2.3, −1.4) | 213 days | 244 days | 26 years | 30 years |
Footnote: AAPC, average annual percent change; CI, confidence interval
Only deaths in which the CHD is listed as the underlying cause of death are included in this table.
Median age at death is calculated among all cases, not just deaths during infancy. Expressed in days when less than 1 year, otherwise expressed in years.
Age-specific CHD mortality
Infant mortality
Death in infancy accounted for 47.7% (n=27,962) of all deaths due to CHD. Infant mortality was consistently higher in males than in females throughout the study period though decreases in mortality over time were similar between the sexes (Table 1). Although all race/ethnic groups experienced overall reductions in CHD-specific infant mortality, NH black infants experienced the highest mortality due to CHD, followed by Hispanics (Table 1, Figure 4). The annual percent decrease during the study period for NH blacks was less (1.8%) than the decrease among NH whites (2.6%). Hispanics, despite experiencing the most significant improvements from 1999–2004, have had unchanged CHD-specific infant mortality for over a decade (Figure 4). These differences in temporal trends have resulted in mortality rate ratios for NH blacks and Hispanics that reflect slightly worsening disparities in CHD-specific infant mortality (Figure 5).
Figure 4. Temporal trends in all-cause infant mortality (top) and CHD-specific infant mortality (bottom), by race/ethnicity, 1999–2017.
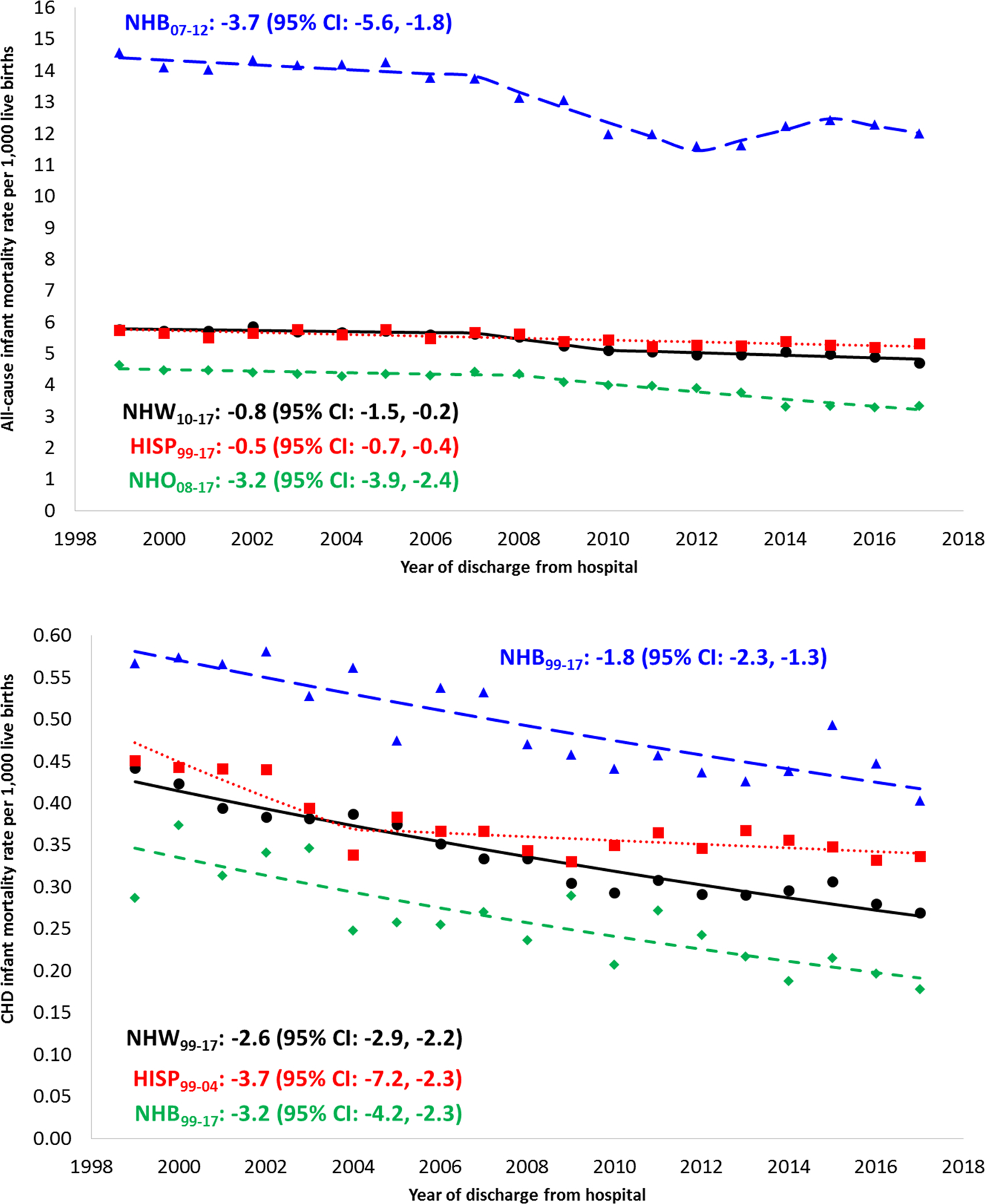
Footnotes: APC, annual percent change; CHD, congenital heart defect
Only APCs that reflect a statistically significant temporal trend in mortality are presented as text. Differences by race/ethnicity: Tests of parallelism revealed that Hispanics (p<0.001) and NH other (p=0.003) had non-parallel regression mean functions for all-cause mortality compared to NH whites. NH blacks did not have regression lines that departed from parallelism compared to NH whites (p=0.054). Only Hispanics (p=0.024) had a non-parallel regression mean function for CHD-specific mortality compared to NH whites. NH blacks (p=0.175) and NH other (p=0.094) did not have regression lines that departed from parallelism compared to NH whites. Differences by cause: Tests of parallelism revealed significant differences between all-cause and CHD-specific mortality for NH whites (p=0.002), Hispanics (p=0.015), or NH other (p=0.048), but not for NH blacks (p=0.051).
Figure 5. Infant mortality rate ratios, all ages, 1999–2017.
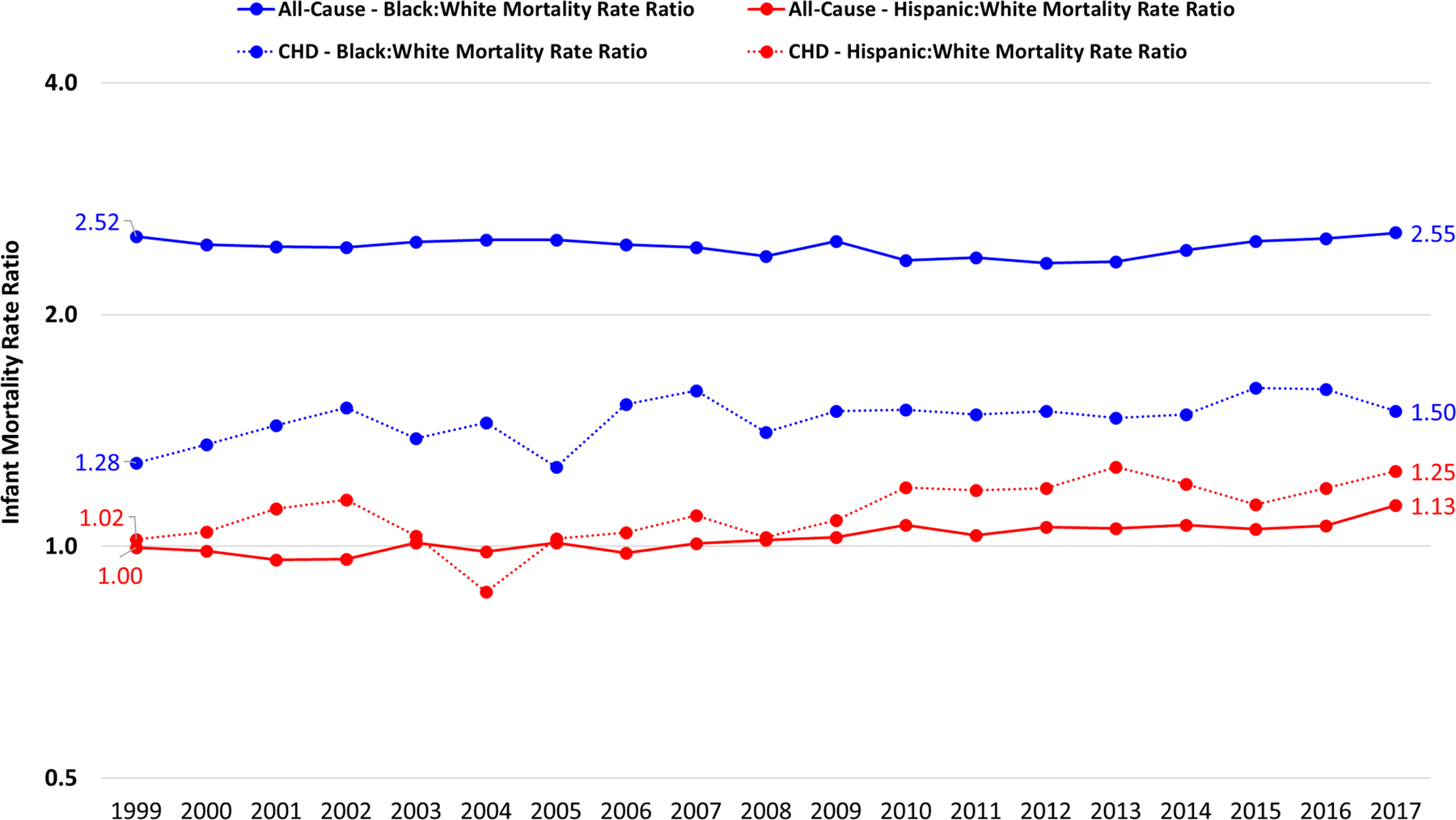
Footnotes: Rate ratios were calculated as the infant mortality rate in one racial/ethnic group (e.g., non-Hispanic blacks) divided by the infant mortality rate in the reference racial/ethnic group (e.g., non-Hispanic whites). This was done separately for all-cause infant mortality and infant mortality due to CHD.
Childhood mortality
For those age 1–17 years, mortality due to CHD decreased between 1999 and 2017. Among children aged 1 to 4 years mortality decreased from 1.43 to 0.95 per 100,000, with similar findings in higher rates of mortality for males and for NH blacks and Hispanics. Among children 5–17 years old, the age group with the lowest CHD-specific mortality, rates decreased from 0.46 to 0.28 per 100,000. While there were higher rates of mortality for males and for NH blacks, there were lower mortality rates for Hispanics (Table II in the Supplement). It was during childhood when the highest proportion of CHD-related deaths had the CHD listed as the UCOD (Table II in the Supplement).
Adult mortality
Adult mortality resulting from CHD decreased among all adults during the study period, with the most pronounced decreases in the oldest (>65 years) age group (Table 1). In younger adults (18–34 years), NH whites experienced the most significant decreases over time, whereas NH blacks and Hispanics tended to have higher-magnitude decreases among older adults (Table 1).
Comparing all age-specific CHD mortality
Unlike the characteristic J-shaped association observed between age and all-cause mortality, mortality due to CHD was at least 30-fold higher during infancy than any other age group (Figure 6). CHD mortality decreased through the 5–17 year old age group, followed by a subsequent increase with older age groups. The largest decrease in mortality due to CHD was among individuals ≥ 65 years (−4.2% each year); whereas in 1999 they had the second-highest mortality of any age group (1.5 per 100,000) and comparable with early childhood CHD mortality (1.4 per 100,000), by 2017, mortality in this oldest age group was 40% lower than among children aged 1–4 years (Figure 6). It is noteworthy that all seven age groups experienced at least a 2% average annual decrease in mortality due to CHD.
Figure 6. All-cause mortality (top) and CHD-specific mortality (bottom), by age group, 1999 and 2017.
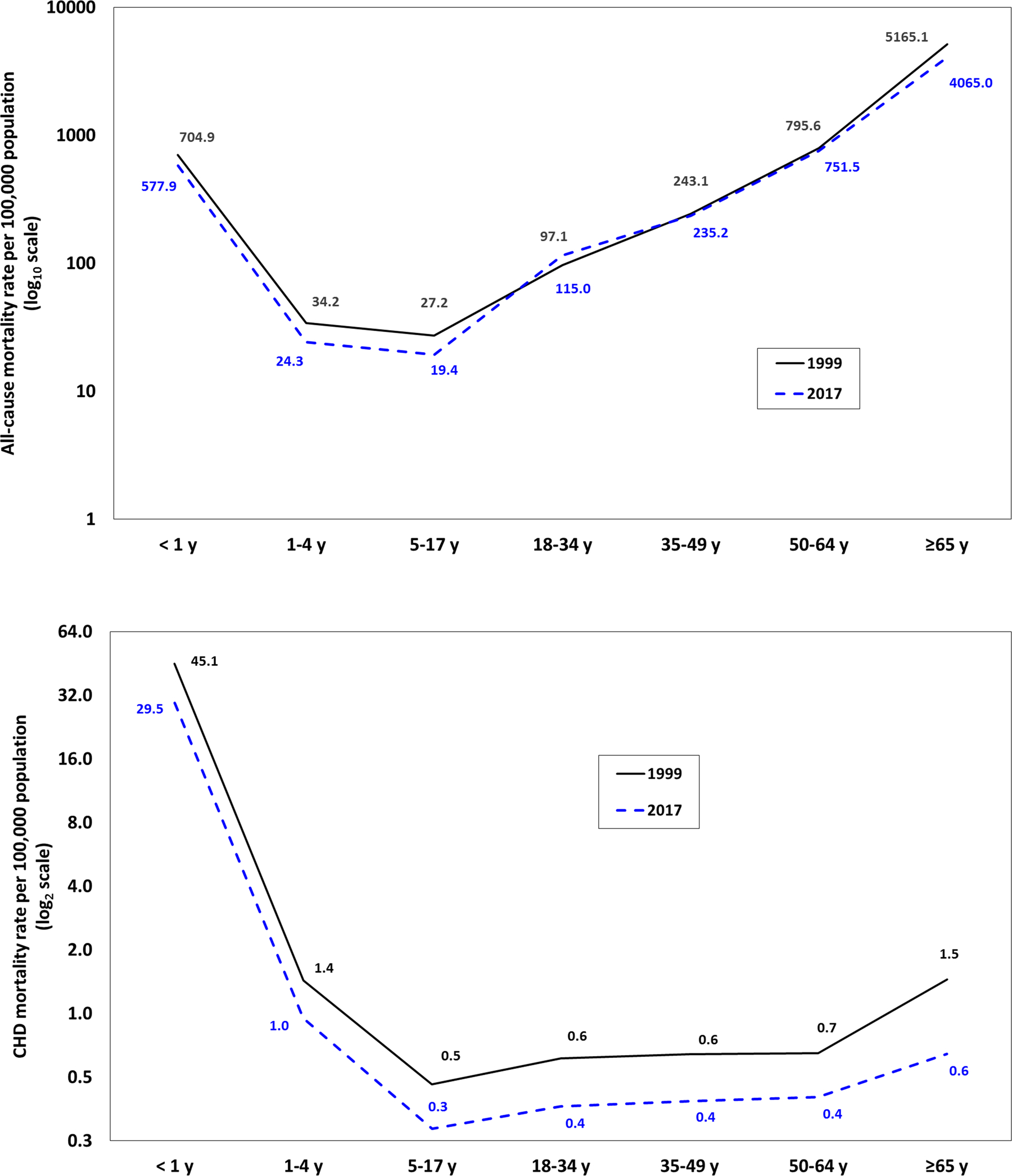
Footnotes: Due to the large differences in the magnitude of all-cause versus CHD-specific mortality rates, to visualize and compare their respective distributions by age, all-cause mortality rates were plotted on the Y-axis with a log base 10 transformation (common logarithm), whereas CHD-specific mortality rates were plotted on the Y-axis with a log base 2 transformation (binary logarithm).
Underlying causes of death for deaths in which CHD was listed as a contributing cause
Among infants, Trisomy 13, 18, and 21 were listed as the UCOD for more than 30% of all deaths in which a CHD was documented as a contributing (but not the underlying) cause of death (Table IV in the Supplement). Other common UCODs included respiratory distress syndrome, congenital diaphragmatic hernia, necrotizing enterocolitis (NEC), hypoplasia and dysplasia of lung, and bacterial sepsis. Trisomy 21 was the top UCOD for all age groups through age 49, after which acute myocardial infarction was the most common UCOD. Trisomy 18 was more commonly listed as a UCOD in female (24.5%) than male (13.5%) infants, and NEC was more common in males, but there were no other pronounced differences in causes of infant death by sex or race/ethnicity (Table V in the Supplement). Among decedents age 1 year or older, compared to other race/ethnic groups, NH whites with CHD were more likely to die from acute myocardial infarction, whereas NH blacks were more likely to die from hypertensive heart disease without heart failure.
Sensitivity analyses
Among all age groups combined, 7,169 (12.3%) of the 58,599 deaths due to CHD had prematurity (infants only), an extracardiac defect, or a genetic condition listed as a contributing cause of death (Figure I in the Supplement). We found no appreciable differences in comparison of rates by decedent characteristics or across time when these cases were excluded from the numerator. However, among infants, nearly 19% of deaths due to CHD had one of these conditions listed as a contributing cause. When these cases were excluded from the numerator, the annual percent decrease in CHD specific infant mortality was mildly more pronounced (−2.9 [95% CI, −3.1 to −2.6]) than when they were included (−2.2 [95% CI, −2.6 to −1.8]; P=0.047). Also, when excluding deaths in which the UCOD was unspecified CHD, although we observed an expected decrease in the mortality rate, comparisons of temporal trends by sex or race/ethnicity did not change (Figures II, III in the Supplement).
DISCUSSION
This work provides a contemporary update to previous studies on CHD mortality by age, sex and race/ethnicity in the United States.5,6,26,27 Overall during the 19-year study period, CHD mortality decreased 39.4%, a more pronounced change relative to the 24% drop observed in the prior study’s timeframe (1999–2006). Nearly half (47.7%) of all CHD mortality occurred during infancy, which has decreased only 0.4% compared to the prior study. Nearly one-quarter of all CHD mortality (23.5%) occurred among those >18 years, and the median age at death for most CHD lesions increased over time, likely secondary to advances in surgical and medical care.
Since the last year of the prior national assessment of CHD mortality, overall CHD mortality rates for males and females have decreased. However, comparing the average APC in morality between males and females in the past 19 years, the difference in these 2 groups has not changed significantly throughout the lifespan, from birth to those >65 years of age. This differs from other studies that have shown increased mortality for females in infancy and childhood. At least two large studies have shown risk of death is increased in early infancy for girls after high-risk cardiac operations.28,29 Another study demonstrates an increased risk of death from CHD in females among children surviving their initial congenital heart surgery.30 On the contrary, a large study in adults with CHD did not show differences in mortality between men and women.31 Some differences may be attributable to the distribution of CHD lesion subtypes between the sexes, and certain higher-risk lesions being present in specific sexes.32 For example, female infants have lower rates of severe CHD than males, thus contributing to lower CHD-related mortality as a proportion of all-cause mortality. However, higher mortality exists in female infants that do have severe CHD requiring high-risk procedures.28,29 Currently, little is known about the underlying mechanisms of sex differences in outcomes, and genetic, hormonal, behavioral, or other undefined factors must be evaluated.32 Given our findings, sex should be considered in effect modification of certain conditions and in risk stratification of conditions where evidence of sex-related increased risk of morbidity and mortality exists.32
Our study describes current nationwide data on racial/ethnic mortality disparities in CHD populations.5,6 When removing the impact of CHD deaths for which prematurity or extracardiac birth defects contribute, racial disparity remains most pronounced between NH blacks and NH whites less than one year of age. Disconcertingly, NH Black:NH white disparity in CHD mortality persists through 35 years of age. Also concerning was our novel finding that Hispanic mortality changed from 11% less likely to die from CHD than NH whites in 1999 to a mortality rate equal to NH whites in 2017. Determination of the multifactorial nature of these finding may help to reduce ongoing disparities among NH blacks, and may serve as a guide among Hispanic populations with CHD, who are at continued risk for health disparities as the most uninsured group in the United States.33
Racial/ethnic disparities and associated difference in mortality rates are not isolated to individuals affected by CHD. Children of different racial and ethnic backgrounds have increased mortality in other congenital defects and chronic diseases.34 One United States study showed children of NH black and Hispanic mothers had significantly higher mortality risk in children less than 8 years old for one-third to one-half of the 21 analyzed birth defects.26 Interestingly, five defects were of cardiac origin for NH black children and four for Hispanic children. Despite improvement in overall CHD (and other chronic disease) mortality, persistence of racial/ethnic mortality disparities make clear multi-level (population, systemic, institutional, and individual) contributions continue facilitating its existence.
At the population level, concerns regarding access to quality care exist. Families from rural communities experience a higher risk of a missed prenatal CHD diagnosis.35 Furthermore, concentrated CHD care at specialized pediatric cardiac centers allows advanced expertise and resources to manage complex patients. A recent study demonstrates lack of proximity to a “top 50 center” results in an infant mortality rate 28% greater than for infants living proximal to a center.36
At the systemic level, national health policies protecting the insurance status of patients with CHDs, including the elimination of insurance exclusion due to preexisting conditions or lifetime caps, are critical to reducing racial/ethnic disparities throughout the lifespan.33 Furthermore, with increasing numbers of individuals with CHD surviving into adulthood, assuring access to high quality inpatient and outpatient adult CHD care and increasing the number of adult CHD providers is critical to long term reduction in racial/ethnic disparities in CHD mortality.37,38
At an institutional level, expertise in CHD surgery, surgical volume, and CHD perioperative care are important factors for reducing racial/ethnic disparities, although patients with lower socioeconomic status may be referred to low-performing hospitals.39–41 One multicenter database study demonstrated that Hispanic ethnicity confers increased odds of experiencing a CHD-related complication, and NH blacks, compared to NH whites, had higher mortality rates after experiencing a CHD-related complication.42 Additionally, implicit bias – attitudes or stereotypes affecting understanding, action, and decisions in an unconscious manner – may play a role in racial/ethnic disparities. One multicenter study demonstrated that after adjusting for age, sex, genetic syndrome, surgery risk category, insurance type, and hospital of surgery, the relative risk of death was 1.32 for NH blacks (CI, 1.14–1.52) compared with NH whites.43 Understanding referral patterns, CHD center quality, and implementing implicit bias training are all necessary to reduce racial/ethnic disparities in CHD mortality.
At the individual level, while earlier studies showed that maternal race/ethnicity was a predictor of increased risk of mortality,8 several recent studies have demonstrated socioeconomic mediators play a role in differences noted in racial/ethnic mortality and CHD. One study showed that maternal education and insurance status explained 33.2% and 27.6% of the relationship between NH whites versus Hispanics and poor clinical outcomes.44 Another recent study demonstrates adjusting for insurance type reduced the black-white disparity in CHD mortality risk by 50%.45 Transportation, opportunity costs, and knowledge of the need for ongoing outpatient care may contribute to racial/ethnic disparities. One study showed that NH whites with CHD have an elevated risk of lapse in outpatient clinical care follow-up.46 Other studies have shown many of the racial differences in mortality were in the outpatient setting. One statewide study noted that differences in mortality between NH blacks and NH whites were most notably observed during the postneonatal period and early childhood.47 Other studies focus on neighborhood level factors after hospital discharge, demonstrating that CHD patients from low-income neighborhoods (which are disproportionately NH black and Hispanic) have a higher mortality risk and utilize more inpatient resources compared to higher income communities48 and that this morality risk persists even when adjusting for individual-level characteristics.49 Establishing programs to assist with maintenance long-term outpatient care, mitigating neighborhood level risks, and providing patient/parent education about the need for lifelong care plays a major role in reducing racial/ethnic disparities.
Other interesting findings from our data surround differences based on age. The finding that the largest decrease in CHD-associated death was among individuals >65 years may appear counter-intuitive. Utilization of specific ICD-10 codes in this population may have changed over time; however, we posit instead that this finding reflects lower complexity CHD and increased beneficial intervention in the older population over time. Studies suggest that the population >65 years has a relatively higher prevalence of low complexity CHD like valvar disease and atrial septal defects compared to younger cohorts.50–52 Multiple authors demonstrated that despite stable prevalence of CHD in patients >65 years old, there has been increased utilization of surgical and transcatheter procedures (e.g. surgical and transcatheter aortic valve replacement for bicuspid aortic valve) and a concomitant decrease in lesion-specific and intervention-specific mortality over the same time period.52–54
Unfortunately, much of CHD mortality is documented as unspecified CHD (35%), particularly during infancy (40%), making it challenging to draw reliable conclusions regarding the impact of specific CHD lesions over time. However, we consistently demonstrate decline in CHD lesion-specific infant mortality in the 19-year period, with a median age of death consistently increasing in recent years. Additionally, understanding the contribution of co-morbid or underlying conditions causing mortality in someone with CHD is important to adequately characterize mortality across the lifespan. While CHD as the UCOD declined over the time period, chromosomal abnormalities, infection, and necrotizing enterocolitis were the most common UCODs for CHD-related deaths among infants, and Down syndrome and myocardial infarction were the most common UCODs among CHD-related deaths in persons aged 1–49 and ≥50 years, respectively.
As with any study, there are limitations when using death certificate data, particularly related to CHD mortality; therefore, our findings should be interpreted with caution.26,55 First, previous studies have shown poor (~50%) sensitivity for recording common cardiac diseases states, possibly related to inadequate training of physicians in death certificate completion.55 A 2017 death certificate study assessed errors in the cause and manner of death fields completed by non–Medical Examiners and found 51% of death certificates had major errors regardless of whether or not completers were physicians.56 An Australian study investigated sensitivity and specificity of the national mortality codes in identifying cardiovascular disease deaths and found that the coding underestimated the true proportion of CHD and stroke deaths by 13.6% and 50.8%, respectively.57 While using UCOD has limitations, a similar methodology was used in many analyses, including the prior study by Gilboa et al.5
In our study, the most commonly listed cause of a CHD-related death from birth to 64 years was unspecified CHD, and 33% of all mortality with a CHD as the UCOD was coded as unspecified CHD, making it challenging to associate specific diseases as the cause of death. While there are rules surrounding “acceptable” causal relationships between cause-of-death codes to ultimately select the correct UCOD, the data are imperfect and limited by what was recorded on the death certificate. ICD-9 and ICD-10 codes have remained an imperfect tool to accurately classify CHD, resulting in challenges as they contribute to the classification of the MCOD data. One study from 2018 evaluated accuracy of ICD-9 coding versus medical records for adults with CHD, and found misclassifications occurred in 23% of patients, and the overall accuracy of a CHD diagnosis was 48.7%.58 Although studies examining the use of ICD-9 and ICD-10 codes for CHD have demonstrated variable accuracy depending on specific lesion and setting,58–62 to our knowledge, no studies suggest coding accuracy has changed over time and no studies have specifically examined accuracy relating to use of the codes on the death certificate. However, ICD-10 codes have been used on death certificates since 1999 and the primary source of data for mortality statistics for the United States.26
While ICD-10 added more categories for CHD increasing specificity for a number of phenotypes, the classification of CHD causes of death in MCOD data remains and imperfect tool for classifying CHD mortality and was not designed with rigorous etiologic research in mind. Additionally, death certificate data do not allow for assessment of other factors, including late disease presentation, early or late postoperative deaths, or location of received care. These factors all contribute to CHD mortality.
Overall US mortality attributable to CHD has decreased over the past 19 years; however, disparities in mortality persist for males in comparison with females, for NH Blacks in comparison with NH Whites, and for Hispanics who have unchanged CHD-specific infant mortality in the last decade compared with decreasing rates in NH Whites. Patient sex should be considered in risk stratification for conditions in which there is evidence of sex-related increased morbidity and mortality risk. Racial disparities in CHD mortality persist in the current era and are most notable between infancy and 34 years. Persistence in, and sometimes exacerbation of, disparities despite improvement in overall CHD mortality in all race/ethnic groups makes clear other contributing factors play a role, including contributions at the population, systemic, institutional, and individual levels. Moving forward, these factors must be taken into account to mitigate the gap in sex differences and racial disparities in patients with CHD.
Supplementary Material
CLINICAL PERSPECTIVE
What is new?
Overall US mortality due to congenital heart disease (CHD) throughout the lifespan has decreased over the last 19 years, with the highest mortality rate in infants (<1 year).
Disparities in mortality due to CHD persist with men having higher mortality than women, non-Hispanic blacks having higher mortality compared to non-Hispanic whites, and Hispanics having unchanged CHD-specific infant mortality in the last decade compared with decreasing rates in non-Hispanic Whites.
For those <50 years old with CHD as a contributing cause of death, associated genetic abnormalities are the leading underlying cause of death; myocardial infarction was the leading underlying cause of death in those 50 years and older.
What are the clinical implications?
Strategies should be employed to reduce disparities in mortality among non-Hispanic black infants, children and young adults.
Individuals living longer with CHD need coaching regarding healthy lifestyle, activity, and health care practices.
Individuals with CHD need access to programs that assist with maintenance of long-term outpatient care and patient/parent education regarding the need for lifelong care are imperative
- Multi-level changes are needed:
- Population: Ensure access to quality care in rural and urban areas.
- Systemic: Implement public policies to protect insurance coverage throughout the lifespan.
- Institutional: Eliminate referral and provider bias; create quality CHD centers.
- Individual: Ensure patient transportation, education, insurance coverage, and appropriate transfer from pediatric to adult care.
ACKNOWLEDGEMENTS
None
SOURCES OF FUNDING
This project was supported by grant number K23 HL127164 (principal investigator: KNL) and grant number K23 HL127266 (principal investigator: SAM) from the National Institutes of Health/National Heart Lung and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- AAPC
average annual percent change
- APC
annual percent change
- CHD
congenital heart disease
- CI
confidence interval
- ICD-10
International Classification of Diseases, Tenth Revision
- MCOD
Multiple Cause of Death
- NH
non-Hispanic
- UCOD
underlying cause of death
Footnotes
DISCLOSURES
The authors have no financial relationships relevant to this article to disclose.
SUPPLEMENTAL MATERIALS
Supplemental Tables I-V
Supplemental Figures I-III
REFERENCES
- 1.Yang Q, Khoury MJ, Mannino D. Trends and patterns of mortality associated with birth defects and genetic diseases in the United States, 1979–1992: An analysis of multiple-cause mortality data. Genet Epidemiol. 1997;14:493–505. [DOI] [PubMed] [Google Scholar]
- 2.Petrini J, Damus K, Johnston RB. An overview of infant mortality and birth defects in the United States. Teratology. 1997;56:8–10. [DOI] [PubMed] [Google Scholar]
- 3.Roncancio CP, Misnaza SP, Peña IC, Prieto FE, Cannon MJ, Valencia D. Trends and characteristics of fetal and neonatal mortality due to congenital anomalies, Colombia 1999–2008. J Maternal-fetal Neonatal Medicine Official J European Assoc Périnat Medicine Fed Asia Ocean Périnat Soc Int Soc Périnat Obstetricians. 2017;31:1748–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Global Health Estimates 2016: Disease Burden by Cause, Age, Sex, by Country and by Region 2000–2016. https://www.who.int/healthinfo/global_burden_disease/GlobalDALY_method_2000_2016.pdf?ua=1 Published, Geneva, 2018. Accessed May 15, 2019. [Google Scholar]
- 5.Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality Resulting From Congenital Heart Disease Among Children and Adults in the United States, 1999 to 2006. Circulation. 2010;122:2254–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality Associated With Congenital Heart Defects in the United States. Circulation. 2001;103:2376–2381. [DOI] [PubMed] [Google Scholar]
- 7.Pace ND, Oster ME, Forestieri NE, Enright D, Knight J, Meyer RE. Sociodemographic Factors and Survival of Infants With Congenital Heart Defects. Pediatrics. 2018;142:e20180302. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Liu G, Druschel CM, Kirby RS. Maternal Race/Ethnicity and Survival Experience of Children with Congenital Heart Disease. J Pediatrics. 2013;163:1437–1442.e2. [DOI] [PubMed] [Google Scholar]
- 9.Lopez KN, Nembhard WN, Wang Y, Liu G, Kucik JE, Copeland G, Gilboa SM, Kirby RS, Canfield M. Birth defect survival for hispanic subgroups. Birth Defects Res. 2018;110:352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlo WF, Cnota JF, Dabal RJ, Anderson JB. Practice trends over time in the care of infants with hypoplastic left heart syndrome: A report from the National Pediatric Cardiology Quality Improvement Collaborative. Congenit Heart Dis. 2017;12:315–321. [DOI] [PubMed] [Google Scholar]
- 11.Komisar J, Srivastava S, Geiger M, Doucette J, Ko H, Shenoy J, Shenoy R. Impact of changing indications and increased utilization of fetal echocardiography on prenatal detection of congenital heart disease. Congenit Heart Dis. 2016;12:67–73. [DOI] [PubMed] [Google Scholar]
- 12.Hoerst A, Bakar A, Cassidy SC, Clabby M, Grippo ED, Graupe M, Harahsheh AS, Hlavacek AM, Hart SA, Kipps AK, et al. , (PAC3) PACCC. Variation in care practices across pediatric acute care cardiology units: Results of the Pediatric Acute Care Cardiology Collaborative (PAC3) hospital survey. Congenit Heart Dis. 2019;14:419–426. [DOI] [PubMed] [Google Scholar]
- 13.Gaies M, Ghanayem NS, Alten JA, Costello JM, Lasa JJ, Chanani NK, Shin AY, Retzloff L, Zhang W, Pasquali SK, et al. Variation in Adjusted Mortality for Medical Admissions to Pediatric Cardiac ICUs. Pediatric Critical Care Medicine J Soc Critical Care Medicine World Fed Pediatric Intensive Critical Care Soc. 2018;20:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickey PA, Connor JA, Cherian KM, Jenkins K, Doherty K, Zhang H, Gaies M, Pasquali S, Tabbutt S, Louis JDS, et al. International quality improvement initiatives. Cardiol Young. 2017;27:S61–S68. [DOI] [PubMed] [Google Scholar]
- 15.Lihn SL, Kugler JD, Peterson LE, Lannon CM, Pickles D, Beekman RH. Transparency in a Pediatric Quality Improvement Collaborative: A Passionate Journey by NPC-QIC Clinicians and Parents. Congenit Heart Dis. 2015;10:572–580. [DOI] [PubMed] [Google Scholar]
- 16.Overman DM, Jacobs ML, O’Brien JE, Kumar SR, Mayer JE, Ebel A, Clarke DR, Jacobs JP. Ten Years of Data Verification: The Society of Thoracic Surgeons Congenital Heart Surgery Database Audits. World J Pediatric Congenit Hear Surg. 2019;10:454–463. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs JP. The science of assessing the outcomes and improving the quality of the congenital and paediatric cardiac care. Curr Opin Cardiol. 2015;30:100–111. [DOI] [PubMed] [Google Scholar]
- 18.Nembhard WN, Salemi JL, Ethen MK, Fixler DE, DiMaggio A, Canfield MA. Racial/Ethnic Disparities in Risk of Early Childhood Mortality Among Children With Congenital Heart Defects. Pediatrics. 2011;127:e1128–e1138. [DOI] [PubMed] [Google Scholar]
- 19.National Center for Health Statistics. Vital Statistics Online Data Portal: Mortality Multiple Cause Files. https://www.cdc.gov/nchs/data_access/VitalStatsOnline.htm Published 2019. Accessed February 12, 2019.
- 20.International statistical classification of diseases and related health problems 10th revision Volume 2 Instruction manual. Geneva: World Health Organization; 2011. Available from: http://www.who.int/classifications/icd/ICD10Volume2_en_2010.pdf Accessed September 10, 2019. [Google Scholar]
- 21.National Center for Health Statistics. U.S. Census Populations With Bridged Race Categories. https://www.cdc.gov/nchs/nvss/bridged_race.htm. Published 2019. Accessed February 27, 2019.
- 22.National Center for Health Statistics. Vital Statistics Online Data Portal: Birth Data Files. https://www.cdc.gov/nchs/data_access/VitalStatsOnline.htm#Births. Published 2019. Accessed February 12, 2019.
- 23.National Center for Health Statistics. Year 2000 Standard Population for the United States. https://wonder.cdc.gov/wonder/help/mcd.html#2000%20Standard%20Population. Published 2019. Accessed March 3, 2019.
- 24.Kim H, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. [DOI] [PubMed] [Google Scholar]
- 25.National Cancer Institute. Statistical Methodology and Applications Branch and Data Modeling Branch, Surveillance Research Program. 2011. [Google Scholar]
- 26.Nembhard WN, Pathak EB, Schocken DD. Racial/ethnic disparities in mortality related to congenital heart defects among children and adults in the United States. Ethnic Dis. 2008;18:442–449. [PubMed] [Google Scholar]
- 27.Collins JW, Soskolne G, Rankin KM, Ibrahim A, Matoba N. African-American:White Disparity in Infant Mortality due to Congenital Heart Disease. J Pediatrics. 2017;181:131–136. [DOI] [PubMed] [Google Scholar]
- 28.Kochilas LK, Vinocur JM, Menk JS. Age-dependent sex effects on outcomes after pediatric cardiac surgery. J Am Heart Assoc. 2014;3:e000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marelli A, Gauvreau K, Landzberg M, Jenkins K. Sex Differences in Mortality in Children Undergoing Congenital Heart Disease Surgery: A United States Population-Based Study. Circulation. 2010;122:S234–S240. [DOI] [PubMed] [Google Scholar]
- 30.McCracken C, Spector LG, Menk JS, Knight JH, Vinocur JM, Thomas AS, Oster ME, Louis JDS, Moller JH, Kochilas L. Mortality Following Pediatric Congenital Heart Surgery: An Analysis of the Causes of Death Derived From the National Death Index. J Am Heart Assoc. 2018;7:e010624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verheugt CL, Uiterwaal CSPM, van der Velde ET, Meijboom FJ, Pieper PG, Vliegen HW, van Dijk APJ, Bouma BJ, Grobbee DE, Mulder BJM. Gender and outcome in adult congenital heart disease. Circulation. 2008;118:26–32. [DOI] [PubMed] [Google Scholar]
- 32.D’Alto M, Budts W, Diller GP, Mulder B, Assenza GE, Oreto L, Ciliberti P, Bassareo PP, Gatzoulis MA, Dimopoulos K. Does gender affect the prognosis and risk of complications in patients with congenital heart disease in the modern era? Int J Cardiol. 2019;290:156–161. [DOI] [PubMed] [Google Scholar]
- 33.Artiga S, Orgera K, Damico A. Changes in Health Coverage by Race and Ethnicity since the ACA, 2010 – 2018. 2020. Kaiser Family Foundation, Disparities Policy. https://www.kff.org/disparities-policy/issue-brief/changes-in-health-coverage-by-race-and-ethnicity-since-the-aca-2010-2018 Accessed September 10, 2019.
- 34.Flores G, Escala MK, Hall BG. Dead wrong: the growing list of racial/ethnic disparities in childhood mortality. J Pediatrics. 2015;166:790–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill GD, Block JR, Tanem JB, Frommelt MA. Disparities in the prenatal detection of critical congenital heart disease. Prenatal Diag. 2015;35:859–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaltman JR, Burns KM, Pearson GD, Goff DC, Evans F. Disparities in Congenital Heart Disease Mortality Based on Proximity to a Specialized Pediatric Cardiac Center. Circulation. 2020:141:1034–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime Prevalence of Congenital Heart Disease in the General Population From 2000 to 2010. Circulation. 2014;130:749–756. [DOI] [PubMed] [Google Scholar]
- 38.Mylotte D, Pilote L, Ionescu-Ittu R, Abrahamowicz M, Khairy P, Therrien J, Mackie AS, Marelli A. Specialized Adult Congenital Heart Disease Care. Circulation. 2014;129:1804–1812. [DOI] [PubMed] [Google Scholar]
- 39.LaPar DJ, Ghanta RK, Kern JA, Crosby IK, Rich JB, Speir AM, Kron IL, Ailawadi G, Initiative I for the VCSQ. Hospital Variation in Mortality From Cardiac Arrest After Cardiac Surgery: An Opportunity for Improvement? Ann Thorac Surg. 2014;98:534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preston L, Turner J, Booth A, O’Keeffe C, Campbell F, Jesurasa A, Cooper K, Goyder E. Is there a relationship between surgical case volume and mortality in congenital heart disease services? A rapid evidence review. Bmj Open. 2015;5:e009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oster ME, Strickland MJ, Mahle WT. Impact of prior hospital mortality versus surgical volume on mortality following surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2011;142:882–886. [DOI] [PubMed] [Google Scholar]
- 42.Chan T, Lion KC, Mangione-Smith R. Racial Disparities in Failure-to-Rescue among Children Undergoing Congenital Heart Surgery. J Pediatrics. 2015;166:812–818.e4. [DOI] [PubMed] [Google Scholar]
- 43.Oster ME, Strickland MJ, Mahle WT. Racial and ethnic disparities in post-operative mortality following congenital heart surgery. J Pediatrics. 2011;159:222–226. [DOI] [PubMed] [Google Scholar]
- 44.Peyvandi S, Baer RJ, Moon-Grady AJ, Oltman SP, Chambers CD, Norton ME, Rajagopal S, Ryckman KK, Jelliffe-Pawlowski LL, Steurer MA. Socioeconomic Mediators of Racial and Ethnic Disparities in Congenital Heart Disease Outcomes: A Population-Based Study in California. J Am Heart Assoc. 2018;7:e010342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kucik JE, Cassell CH, Alverson CJ, Donohue P, Tanner JP, Minkovitz CS, Correia J, Burke T, Kirby RS. Role of health insurance on the survival of infants with congenital heart defects. Am J Public Health. 2014;104:e62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson JL, Morack J, Harris M, DeSalvo J, Daniels CJ, Chisolm DJ. Racial disparities in clinic follow-up early in life among survivors of congenital heart disease. Congenit Heart Dis. 2018;14:305–310. [DOI] [PubMed] [Google Scholar]
- 47.Nembhard WN, Xu P, Ethen MK, Fixler DE, Salemi JL, Canfield MA. Racial/ethnic disparities in timing of death during childhood among children with congenital heart defects. Birth Defects Res Part Clin Mol Teratol. 2013;97:628–640. [DOI] [PubMed] [Google Scholar]
- 48.Anderson BR, Fieldston ES, Newburger JW, Bacha EA, Glied SA. Disparities in Outcomes and Resource Use After Hospitalization for Cardiac Surgery by Neighborhood Income. Pediatrics. 2018;141:e20172432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kucik JE, Nembhard WN, Donohue P, Devine O, Wang Y, Minkovitz CS, Burke T. Community socioeconomic disadvantage and the survival of infants with congenital heart defects. Am J Public Health. 2014;104:e150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu C, Moore BM, Kotchetkova I, Cordina RL, Celermajer DS. Causes of death in a contemporary adult congenital heart disease cohort. Hear Br Cardiac Soc. 2018;104:1678–1682. [DOI] [PubMed] [Google Scholar]
- 51.Oliver JM, Gallego P, Gonzalez AE, Garcia-Hamilton D, Avila P, Alonso A, Ruiz-Cantador J, Peinado R, Yotti R, Fernandez-Aviles F. Impact of age and sex on survival and causes of death in adults with congenital heart disease. Int J Cardiol. 2017;245:119–124. [DOI] [PubMed] [Google Scholar]
- 52.Afilalo J, Therrien J, Pilote L, Ionescu-Ittu R, Martucci G, Marelli AJ. Geriatric congenital heart disease: burden of disease and predictors of mortality. J Am Coll Cardiol. 2011;58:1509–1515. [DOI] [PubMed] [Google Scholar]
- 53.Soud M, Al-khadra Y, Darmoch F, Pacha HM, Fanari Z, Alraies Mc. Transcatheter aortic valve replacement in patients with bicuspid aortic valve stenosis: National trends and in-hospital outcomes. Avicenna J Medicine. 2020;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Opotowsky AR, Perlstein T, Landzberg MJ, Colan SD, O’Gara PT, Body SC, Ryan LF, Aranki S, Singh MN. A shifting approach to management of the thoracic aorta in bicuspid aortic valve. J Thorac Cardiovasc Surg. 2012;146:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lakkireddy DR, Gowda MS, Murray CW, Basarakodu KR, Vacek JL. Death certificate completion: How well are physicians trained and are cardiovascular causes overstated? Am J Medicine. 2004;117:492–498. [DOI] [PubMed] [Google Scholar]
- 56.McGivern L, Shulman L, Carney JK, Shapiro S, Bundock E. Death Certification Errors and the Effect on Mortality Statistics. Public Heal Reports Wash D C 1974. 2017;132:33354917736514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harriss LR, Ajani AE, Hunt D, Shaw J, Chambers B, Dewey H, Frayne J, Beauchamp A, Duvé K, Giles GG, et al. Accuracy of national mortality codes in identifying adjudicated cardiovascular deaths. Aust Nz J Publ Heal. 2011;35:466–476. [DOI] [PubMed] [Google Scholar]
- 58.Khan A, Ramsey K, Ballard C, Armstrong E, Burchill LJ, Menashe V, Pantely G, Broberg CS. Limited Accuracy of Administrative Data for the Identification and Classification of Adult Congenital Heart Disease. J Am Heart Assoc. 2018;7(2)e007378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen S, Jannot A-S, Iserin L, Bonnet D, Burgun A, Escudié J-B. Accuracy of claim data in the identification and classification of adults with congenital heart diseases in electronic medical records. Arch Cardiovasc Dis. 2019;112:31–43. [DOI] [PubMed] [Google Scholar]
- 60.Steiner JM, Kirkpatrick JN, Heckbert SR, Habib A, Sibley J, Lober W, Curtis JR. Identification of adults with congenital heart disease of moderate or great complexity from administrative data. Congenit Heart Dis. 2017;13:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broberg C, McLarry J, Mitchell J, Winter C, Doberne J, Woods P, Burchill L, Weiss J. Accuracy of Administrative Data for Detection and Categorization of Adult Congenital Heart Disease Patients from an Electronic Medical Record. Pediatr Cardiol. 2014;36:719–725. [DOI] [PubMed] [Google Scholar]
- 62.Pasquali SK, Peterson ED, Jacobs JP, He X, Li JS, Jacobs ML, Gaynor JW, Hirsch JC, Shah SS, Mayer JE. Differential Case Ascertainment in Clinical Registry Versus Administrative Data and Impact on Outcomes Assessment for Pediatric Cardiac Operations. Ann Thorac Surg. 2013;95:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


