Abstract
Integrins mediate adhesion of cells to substrates and maintain tissue integrity by facilitating mechanotransduction between cells, the extracellular matrix, and gene expression in the nucleus. Changes in integrin expression in corneal epithelial cells and corneal endothelial cells impacts their adhesion to the epithelial basement membrane (EpBM) and Descemet’s membrane, respectively. Integrins also play roles in assembly of basement membranes by both activating TGFβ1 and other growth factors. Over the past two decades, this knowledge has been translated into methods to grow corneal epithelial and endothelial cells in vitro for transplantation in the clinic thereby transforming clinical practice and quality of life for patients. Current knowledge on the expression and function of the integrins that mediate adhesion to the basement membrane expressed by corneal epithelial and endothelial cells in health and disease is summarized. This is the first review to discuss similarities and differences in the integrins expressed by both cell types.
Keywords: integrins, epithelial basement membrane, Descemet’s membrane, corneal epithelial cells, corneal endothelial cells
1. Introduction
The cornea has a stratified squamous epithelium on its outer apical surface and a simple squamous epithelium on its inner surface exposed to the aqueous humor. Underneath these two epithelia are two basement membranes with distinct compositions. The epithelial basement membrane (EpBM) is produced primarily by secretion of extracellular matrix (ECM) proteins by the basal cells of the corneal epithelium with some ECM contributed by anterior stromal cells. Whereas the corneal epithelial basal cells are highly proliferative, corneal endothelial cells proliferate rarely in the adult human cornea, and over time with aging, there are fewer of these cells present lining the inner surface of the cornea. The endothelial cell basement membrane is called Descemet’s membrane (DM). A schematic showing epithelial and endothelial cell adhesion to their respective basement membranes is presented in Figure 1. The term “Bowman’s layer” refers not to the EpBM but to the acellular layer of ECM under the EpBM which is present in the human and avian but not in the rabbit or mouse cornea (Wilson, 2020).
Figure 1. Schematic comparing corneal epithelium-EpBM and corneal endothelium-DM binding interactions.
(A) Both hemidesmosomes and focal adhesions mediate cell adhesion between the corneal epithelium and EpBM. Type I hemidesmosomes contain integrin α6β4, plectin, BP230, and BP180/type XVII collagen that links laminin-332 (LN332) in the EpBM to keratin cytoskeletal proteins present in the cytoplasm. Focal adhesions at the corneal epithelium-EpBM interface consist of integrin α3β1 or α2β1 bound to fibronectin, laminin, or collagen and associate with cytoplasmic integrin-binding proteins (e.g., talin (TLN), paxillin (PXN), focal adhesion kinase (FAK), and vinculin (VCL)) that are involved in downstream signaling pathways related to cell survival and migration. (B) Focal adhesions formed at the corneal endothelium-DM interface are composed by integrins α3β1 or α6β1 that bind to laminin-511 (LN511) or −411 (LN411) present in the DM. Cytoplasmic integrin-binding proteins localized at the plasma membrane bind to cytoskeletal proteins and influence actin dynamics.
The corneal epithelium is subjected to forces caused by blinking and eye rubbing, and the epithelial cells adhere to the EpBM primarily via intermediate filament based hemidesmosomes. By contrast, the corneal endothelial cells function as pumps to maintain hydration in the cornea and move nutrients from the aqueous humor, into their cytoplasm, and release them into DM where they can remain bound to proteoglycans or diffuse into the corneal stroma. Because they are located inside the anterior chamber, the corneal endothelial cells do not need to provide the same barrier function that the epithelial cells provide at the anterior surface nor are they frequently injured so maintaining strong robust adhesion to DM is not as important as it is for the corneal epithelial cells. The two cell types adhere to their substrates via distinct integrin heterodimers; they secrete different amounts and types of ECM proteins which the integrins on their basal surfaces assemble into basement membranes with distinct properties.
In this review, after a brief introduction to integrins, we describe the current state of knowledge on the role that integrins play in mediating adhesion of corneal epithelial and endothelial cells to their basement membranes. For a discussion of Bowman’s layer and Descemet’s membrane, please see recent reviews by Wilson, et al. (de Oliveira and Wilson, 2020; Wilson, 2020).
2. Integrins: an overview
Bidirectional communication between the ECM and cells is mediated by biophysical cues and mechanotransduction pathways in a process known as ‘dynamic reciprocity’ (Bornstein and McPherson, 1982). This interplay was described by Mina Bissell and colleagues (Bissell et al., 1982) and provided a mechanism for how different cellular populations may arise within a tissue. During development, progenitor cells remain in close proximity to each other and yet may differentiate into completely different cell types depending on the surrounding ECM, hormones, growth factors, and biophysical cues present in the microenvironment that contribute to epigenetic modifications and cell specialization (Bissell et al., 1982; Bissell and Aggeler, 1987; Levin and Stevenson, 2012; Crowder et al., 2016). This mechanism is particularly important during embryonic development and cancer metastasis, which are both associated with significant changes in the ECM that occur in parallel to cellular transformation (Lin and Bissell, 1993). In dynamic reciprocity, conformational changes in integrin structure alter cellular interactions with the ECM and basement membrane proteins, e.g., fibronectin, collagen, laminin, and heparan sulfate proteoglycans, allowing both ‘outside-in’ and ‘inside-out’ signaling (Takagi et al., 2002). Signal transduction pathways associated with cell proliferation, survival, and differentiation, may then be activated by interactions between the cytoskeleton and transcriptional and translational proteins in the nucleus. In turn, as the ECM influences cell signaling, the cell layer may also alter the secretion and deposition of ECM and basement membrane proteins, thus invoking a reciprocity that defines the constantly evolving and aging tissue structure.
The process of ‘dynamic reciprocity’ is fundamental in corneal biology. In a tissue predominately composed by ECM proteins whose organization and maintenance are crucial for tissue transparency and proper refraction, biomechanical signaling via integrins plays a major role in maintaining tissue integrity. The theory of dynamic reciprocity hypothesized in 1982 that proteins with the biomechanical properties of integrins existed; four years later the first integrin was cloned (Tamkun et al., 1986). The laboratories of Richard Hynes at MIT and Martin Hemler and Tim Springer at Harvard Medical School realized after comparing data from chicken β1 integrin with DNA sequences from human immune cells the complexity of the integrin family (for historical perspective see (Hynes, 2004)). Integrins are integral membrane, single pass, αβ heterodimers expressed on the surfaces of both adherent and circulating cells. The β1 integrin subunit would turn out to form heterodimers with over 10 different α chains. The family size would expand dramatically over the next several decades and include another 7 β chains (β2, β3, β4, β5, β6, β7, and β8) complementing 18 α chains. Work in basic cell biology labs would go on to show that integrins function as the major mechanotransducers in tissues to regulate tissue integrity in part by functioning as cell signaling hubs at sites of cell:substrate adhesions.
In the early 1990’s, integrins began to be characterized in the corneal epithelium (Stepp et al., 1990; Grushkin-Lerner and Trinkaus-Randall, 1991; Tervo et al., 1991; Stepp et al., 1993) and stroma (Lauweryns et al., 1991; Garana et al., 1992; Masur et al., 1993). Over time, integrins would also be studied on corneal endothelial cells as well (Okumura et al., 2015), but difficulty growing the cells limited discoveries to organotypic cell cultures (Zieske et al., 1994).
3. Corneal epithelial cell integrins associated with the EpBM
The ECM composition of the EpBM in healthy corneas was compared to that of corneas from patients with diabetes and diabetic retinopathy (Ljubimov et al., 1998). Using newly characterized monoclonal antibodies against individual laminin chains, these researchers showed that the EpBM contains several laminin chains (α1, α5, β1, γ1). LNs are trimers containing α, β, and γ chains; using the revised nomenclature (LNαβγ) these data indicate that the EpBM contains LN111 and LN511. They also showed the presence of LN332 using an antibody that recognizes the LN332 complex. In addition to these LNs, the EpBM also expresses entactin/nidogen, various collagen IV chains (α1, α2, α3, α4, α5, α6), type VII collagen, perlecan, tenascin C, and fibrillin-1. The composition of ECM proteins in basement membranes in the body vary in tissue specific ways; the relative amount of each ECM protein varies with aging and in various pathologies. In a subsequent study, Kabosova and colleagues (2007) compared human infant and adult corneal EpBM and found variations in composition of the EpBM at the center compared to the limbus and between infants and adults (Kabosova et al., 2007). Since the cornea epithelial progenitor and stem cells reside at the limbus, these data show that the limbal niche had a unique ECM composition. Research using model organisms has shown that when basement membranes form during development and when they are regenerated during wound repair, epithelial cells interact directly with LNs; type IV collagen and perlecan assemble on the scaffold formed by integrins and dystrogylcan binding to LNs (Hohenester and Yurchenco, 2013; Pozzi et al., 2017; Pastor-Pareja, 2020).
In the corneal epithelium, several integrins are expressed. In human and mouse central cornea, α2β1, α3β1, αvβ5, and α6β4 all have a polarized localization within the epithelium with expression highest in the basal cells and tapering off so that apical squames do not express integrins. Most integrins localize to the basal aspect of the basal cells where they mediate adhesion to the EpBM. α6β4 mediates adhesion to the EpBM via hemidesmosomes whereas other integrins including α3β1 and αvβ5 mediate adhesion via focal adhesions which are actin based. Integrins are synthesized and assembled into heterodimers in the endoplasmic reticulum and Golgi. They are then transported via endosomes to the basolateral region of basal cells where the endosomes fuse with basolateral membranes. Integrins are then transported within the cell membrane to the basal cell surface.
We know that the integrins expressed at the EpBM are capable of mediating adhesion of corneal epithelial cells to collagens, fibronectins, laminins, and vitronectin. When integrin expression decreases, cells become less proliferative and less adhesive to the underlying basement membrane. Integrins bound to the ECM exert mechanical forces on the matrix. Those forces are transmitted via integrin cytoplasmic domains to the cytoskeleton and the nucleus where they regulate gene expression. By pulling on EpBM proteins as they are secreted by the epithelial cells, integrins induce the unfolding of cryptic sites in the ECM proteins that allow assembly of the ECM proteins with one another to make up the EpBM. As ECM proteins spontaneously assemble into the EpBM, changes in their conformation alter the forces applied to the integrins bound to them. This process both activates the integrin and alters signaling to the cytoskeleton and can expose proteolytically sensitive sites leading to integrin ectodomain cleavage.
Our understanding of the functions of integrins in the EpBM has relied on studies of genetically engineered mice, gene mutations in humans, and various human pathologies. Below, we describe our current understanding focusing on integrins that mediate adhesion to the EpBM. Table 1 lists the integrins that mediate epithelial cell adhesion to the EpBM.
Table 1.
Integrins expressed on corneal epithelial cells
| Corneal epithelial cell integrins adhering to the epithelial basement membrane | ||||
|---|---|---|---|---|
| Integrin Family | Subunit | Gene | Heterodimer | Known Ligands |
| β4 ITGB4 | α6 | ITGA6 | α6β4 | Laminin 332 |
| β1 ITGB1 | α2 | ITGA2 | α2β1 | Laminin, Collagen |
| α3 | ITGA3 | α3β1 | Laminin, FN, TSP-1, Nidogen, Collagen type IV | |
| αv ITGAV | β1 | ITGB1 | αvβ1 | Laminin, FN |
| β5 | ITGB5 | αvβ5 | FN, Laminin, Vitronectin | |
| β6 | ITGB6 | αvβ6 | Vitronectin, Fibronectin | |
(Abbreviations: Fibronectin (FN), Thrombospondin-1 (TSP-1)).
α6β4
In 1990, we showed that α6β4 was an integral membrane component of the hemidesmosome (HD) (Stepp et al., 1990). HDs come in two types. Corneal and epidermal HDs are type I HDs. They contain a specific set of components: α6β4, plectin, BP230, and BP180 (type XVII collagen) bound to LN332 in the basement membrane. Type II HDs contain α6β4 and plectin bound to LN332 (Litjens et al., 2006) and are present in simple epithelial cells, myoepithelial cells, and cultured cells. When β4 integrin null mice were generated, pups developed but their skin shed after birth and pups died (Dowling et al., 1996). Later studies showed that loss of β4 integrin also lead to defects in vascular endothelial cells (Wang et al., 2012) and peripheral nerves (Van der Zee et al., 2008). Due to the absence of LN332 at these sites, the α6β4 containing cell substrate adhesions formed by vascular endothelial and neuronal cells are considered a subclass of the type II HD. α6 integrin was also deleted in mice; like β4 null mice, the mice lost their epidermis after birth (Georges-Labouesse et al., 1996). In vitro studies of cells from the epidermis of these mice showed increased TGFβ1 signaling and accelerated terminal differentiation of keratinocytes (Rodius et al., 2007). Mutations in genes that encode α6, β4, or other proteins present in the hemidesmosome cause epidermolysis bullosa- a pathology that can exhibit mild and fatal forms depending on the severity of the mutation and which leads to skin blistering (Hashimoto et al., 2016) and ocular surface defects (Jonsson et al., 2015).
Debridement injury to the mouse cornea can be performed so that the EpBM remains attached to the corneal stroma or the EpBM can be removed at the time of surgery (Pal-Ghosh et al., 2004). When we compared the wound response to these two types of injury, we found that leaving the EpBM behind impaired wound resolution and contributed to the formation of recurrent erosions (Pal-Ghosh et al., 2011b). When the EpBM is left intact, epithelial cells and resident immune cells are forced to degrade it to allow for the formation of new hemidesmosomes with LN332 and type VII collagen. The erosions form, in part, due to proteolytic cleavage of the extracellular domain of β4 integrin by MMPs (Pal-Ghosh et al., 2011a).
Interest in adult stem cells in the skin and treating corneal epithelial stem cell deficiency in the cornea led several groups to show that α6β4 is expressed at high levels on epithelial stem and progenitor cells in the skin (Jones and Watt, 1993; Kaur and Li, 2000; Tani et al., 2000) and cornea (Pajoohesh-Ganji and Stepp, 2005). These discoveries led directly to improvements in the quality of the epidermal and corneal epithelial cells produced in vitro for use in patients with severe burns and treating corneal stem cell deficiencies (Ahmad et al., 2007; Hayashi et al., 2008).
In vivo, corneal epithelial stem cells expressing high levels of α6β4 are located at the limbus (Pajoohesh-Ganji and Stepp, 2005; Polisetti et al., 2016). Differences in EpBM composition at the limbus compared to the central cornea in humans had been shown by (Ljubimov et al., 1995; Ljubimov et al., 1998) and confirmed by Schlötzer-Schrehardt (Schlotzer-Schrehardt et al., 2007). Using this knowledge, optimized ECM coated surfaces are now used to enhance expansion of epithelial stem cells in vitro for transplantation (Polisetti et al., 2017). These types of studies are increasing our understanding of the cell biology mediating corneal epithelial cell adhesion to the EpBM and the role it plays in maintaining the stem and progenitor cells at the limbus.
Recurrent erosions in the mouse can also develop because of a delay in the ability of corneal epithelial basal cells expressing high levels of α6β4 to down regulate their proliferation after debridement injury (Pal-Ghosh et al., 2011b). Inhibiting cell proliferation with a single treatment with mitomycin C restored epithelial integrity and allowed the corneal sensory nerves to reinnervate the tissue (Pal-Ghosh et al., 2016).
Clinical interest in α6β4 increased after studies lead by C. Stephen Foster showed that patients with cicatricial pemphigoid have autoantibodies against β4 integrin (Tyagi et al., 1996; Rashid et al., 2013). These antibodies bind to the basal surface of corneal epithelial cells and prevent them from interacting with the ECM proteins in the EpBM eventually leading to blistering, inflammation, and severe damage to the ocular surface. Treatments aimed at reducing inflammation often improve these symptoms. Bullous pemphigoid is a similar autoimmune disease but instead of autoantibodies against β4 integrin, patients have autoantibodies against BP230 (also called dystonin), BP180 or type XVII collagen. In some cases, patients that present with pemphigoid have inherited mutations in one or more of the hemidesmosomal or desmosomal proteins (Goletz et al., 2017).
α3β1
α3β1 is one of the major integrins in corneal epithelial basal cells. While α6β4 expression increases after corneal epithelial debridement injury, α3β1 expression remains constant (Stepp et al., 1993). Like α6β4, α3β1 adheres to LN332 in the EpBM (Belkin and Stepp, 2000). In vitro studies have shown that α3β1 competes with α6β4 for LN332 via a mechanism referred to as trans-dominant inhibition by competing for cytoskeletal proteins (Diaz-Gonzalez et al., 1996; Hodivala-Dilke et al., 1998). When LN332 is bound to α3β1, it is subjected to mechanical forces exerted primarily via the actin cytoskeleton whereas when LN332 is bound to α6β4, it is subjected to mechanical forces exerted primarily via the intermediate filaments (keratins). While α6β4:LN332 adhesions form hemidesmosomes, α3β1 adhesions are called focal adhesions. Mice engineered to lack α3β1 have defective basement membrane assembly leading to skin and kidney defects (DiPersio et al., 1997). Recently, a mutation in α3 integrin was shown to cause renal developmental defects involving glomerular basement membrane defects (Shukrun et al., 2014). When α3β1 integrin is deleted specifically from mouse skin and mice used in skin tumor studies, α6β4+ positive stem cells left their niche and terminally differentiate leading to smaller tumor sizes (Sachs et al., 2012). These studies together highlight the importance of α3β1 and α6β4 in maintaining cell adhesion within the limbal niche and in response to injury.
Recently, it has been confirmed that limbal epithelial stem/progenitor cells are maintained in the limbal niche by α3β1 and α6β4 integrins and their extracellular ligands LNα1, LNα2, LNα3 and LNα5 (Polisetti et al., 2016). Since LNα5 has been shown to represent a signature component specifically produced by epithelial progenitor cells, recombinant LN511 has been used to enhance in vitro expansion of limbal epithelial stem cells (Polisetti et al., 2017). Consistently, functional blocking of α3β1 and α6β4 integrins suppressed adhesion and proliferation of cells on LN511-coated surfaces.
Figure 2 shows a TEM image of the epithelial cells: EpBM interface, shows hemidesmosomes, and highlights the localization and expression of ECM proteins in the basement membrane and α3 and β4 integrins in the epithelium.
Figure 2. Hemidesmosomes contain LN332 and α6β4 integrin at the EpBM and α3β1 integrin adjacent in focal contacts.
A. i. TEM image showing HDs at the corneal epithelial:basement membrane interface in a control mouse cornea; asterisks highlight HDs. ii and iii: The localization of LN332 and perlecan are shown in the cornea. While LN332 is exclusive to the EpBM, perlecan localizes to both EpBM and DM. Images in A are modified from Sta.Iglesia and Stepp (2000) (Sta Iglesia and Stepp, 2000) with permission from ARVO, as the copyright holder. B. i The localization of α3 (green) and β4 (red) integrin in control corneas is shown; in ii and iii, integrins are shown separately. Note that the localization of α3 integrin is not restricted exclusively to the basal cell layer but extends apically into suprabasal cells. By contrast, β4 integrin is more restricted. Compared to the EpBM, DM does not show any evidence for expression of β4 integrin and minimal expression of α3 integrin. Bar, 50 μm. Image modified from (Pal-Ghosh et al., 2004) with permission from ARVO, as the copyright holder. In Aii and iii and Bi-iii, asterisks indicate the apical surface of the corneal epithelium and arrows indicate DM.
αv family integrins (αvβ1, αvβ5, αvβ6)
αv forms heterodimers with multiple β subunits (β1, β3, β5, β6, and β8). The “v” designation in αv integrins refers to vitronectin which is one of the αv integrin ligands. αv integrins adhere to the tripeptide sequence arginine-glycine-aspartic acid or RGD on extracellular matrix proteins. Vitronectin, abundant in serum, and fibronectin have RGD sequences and bind to αv integrins. In skin and cornea, αvβ5 and αvβ6 are the major forms yet some αvβ1 may also be present.
αv family integrins were shown to regulate the activation of latent TGFβ1 by binding to an RGD sequence in the TGFβ1 latency associated protein (Annes et al., 2004; Munger and Sheppard, 2011). Detailed studies of the mechanism of activation of TGFβ1 by αv integrin have led to numerous insights into fibrosis, epithelial-mesenchymal transition (EMT), and cancer metastasis (Massague, 2012; Meng et al., 2016). While TGFβ1 induces fibrosis in stromal cells, in keratinocytes, TGFβ1 modulates the secretion and assembly of the ECM proteins that make up the EpBM (Stepp et al., 2007).
While αvβ5 has been studied in skin (Janes and Watt, 2004; Jiang et al., 2014) and cornea (Stepp et al., 2002), more research has been performed in retinal pigment epithelial (RPE) cells. Studies using β5 null mice showed that αvβ5 mediates adhesion of RPE cells to Bruch’s membrane as well as the phagocytosis of shed rod and cone outer segments (Nandrot et al., 2004). Mice lacking β5 integrin also fail to regulate shedding of rod and cone outer segments in a circadian manner (Nandrot and Finnemann, 2006). While it hasn’t been studied, it is likely that αvβ5 regulates phagocytosis in corneal epithelial cells that express it.
In normal mice, quiescent epithelial cells switch from expressing αvβ5 to αvβ6 after injury which enhances their migration (Jiang et al., 2014). Mice lacking expression of β6 have delayed wound healing in the skin (Eslami et al., 2009; Duperret et al., 2016) and cornea (Blanco-Mezquita et al., 2011). These mice also show reduced fibrosis after wounding due to impaired activation of latent TGFβ1 (Wu et al., 2019).
Basement membranes have been shown to function as reservoirs or sponges for growth factors secreted by the corneal epithelial cells (Vlodavsky et al., 1991; Dowd et al., 1999). Growth factors bind with variable affinities to heparan binding sites present on many ECM proteins. When the cornea is injured and the EpBM is left intact, TGFβ1 accumulates in the EpBM after corneal injury; if the EpBM has been removed at the time of injury, TGFβ1 diffuses into the stroma (Stramer et al., 2003). By controlling the activation of TGFβ1 secreted by corneal epithelial basal cells, αv family integrins regulate wound resolution and fibrosis.
4. Integrin-mediated Signaling at Descemet’s Membrane
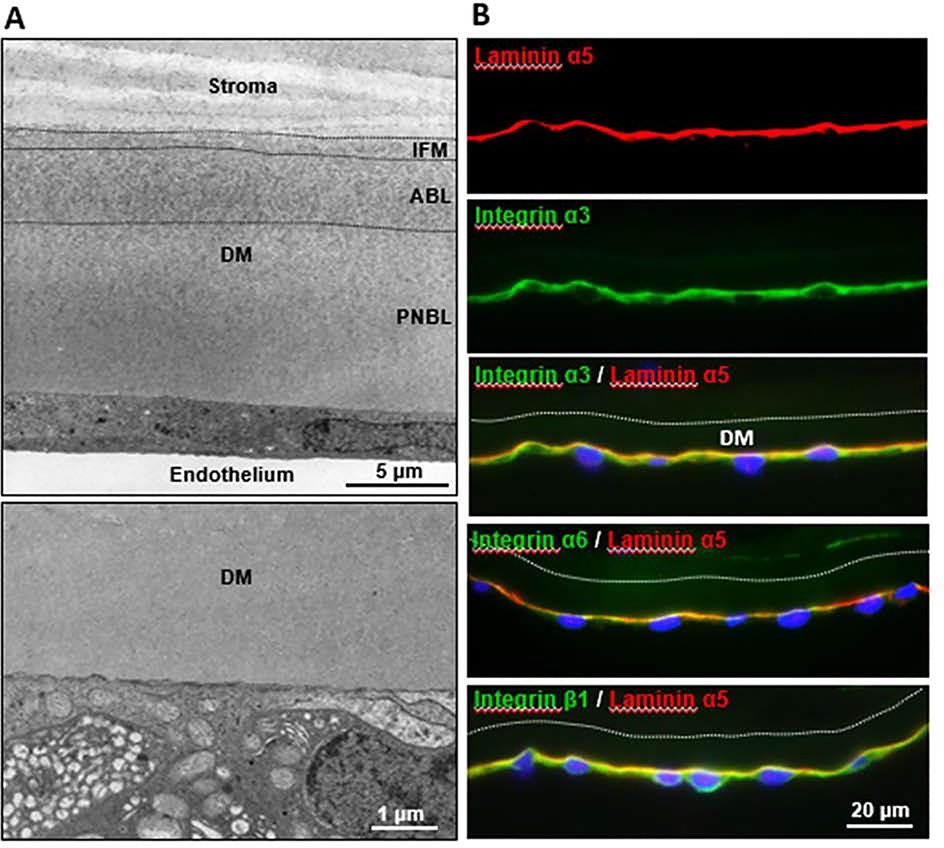
Similar to the anterior cornea, integrins play an essential role in mediating attachment of the endothelium to the posterior elastic lamina known as DM that resides between the endothelial layer and posterior stroma. In humans, the acellular DM is 10–12 μm thick on average and composed of laminin, collagen types IV and VIII, nidogen, fibronectin, and perlecan, which may bind to integrins present on the plasma membrane of corneal endothelial cells. DM is nearly 7 times stiffer than the EpBM with average elastic moduli of 50 kPa (± 17.8 kPa) and 7.5 kPa (± 4.2 kPa), respectively (Last et al., 2009). Visualized by electron microscopy, DM is composed of an anterior banded (fetal) layer, approx. 3 μm in thickness, and a posterior nonbanded (postnatal layer) layer; DM is attached to the corneal stroma by a narrow transitional zone of amorphous ECM termed the interfacial matrix (Murphy et al., 1984; Sawada et al., 1990); a TEM image is presented in Figure 3A. The high abundance of collagen type VIII appears to be specific to the DM compared to other basement membranes found in the body (Kapoor et al., 1988). Contrary to the EpBM which becomes thinner with age, the postnatal DM is known to thicken by 3- to 4-fold from birth to old age due to the accumulation of non-striated collagen fibrils deposited on the striated collagen lamellae with a distinct periodic banding of 110 nm formed in utero (Murphy et al., 1984). Apart from providing structural integrity of the cornea, DM has been suggested to play a role in corneal hydration, endothelial cell differentiation, and maintenance of the corneal curvature. Studies have also suggested that the DM limits exposure of the corneal stroma to pro-fibrotic growth factors that are highly abundant in the aqueous humor, such as TGF-β2, which may promote corneal haze following photorefractive keratectomy or riboflavin-UV-A corneal crosslinking [reviewed in (Wilson, 2019)].
Figure 3. Integrin containing focal adhesions can be visualized at the corneal endothelial: DM interface.
A. TEM image at top showing the ultrastructure of Descemet’s membrane (DM) composed of an interfacial matrix (IFM) mediating attachment to the corneal stroma, anterior banded layer (ABL) and posterior nonbanded layer (PNBL). (Re-produced from (Schlötzer-Schrehardt and Kruse, 2016) with permission). TEM image at bottom showing endothelial-DM interface with electron-dense focal adhesions (arrows). B. Immunofluorescence images of corneal tissue sections showing co-localization of laminin α5 and integrins α3, α6 and β1 along the endothelial-DM interface. Protocol for the immunolabelling method and antibody information available in (Polisetti et al., 2017).
In terms of downstream signaling, activation of integrins by the ECM is dependent on a class of cytoskeletal proteins, known as talins, which function as essential adaptor proteins that mediate binding of the cytoplasmic integrin β-subunit to actin bundles (Tadokoro et al., 2003). Talins contain at least two integrin-binding subunits and may recruit additional cytoskeletal proteins, including vinculin that also binds to actin filaments to increase contractile forces at focal adhesions. Downstream signaling of integrin-activation involves mitogen activated protein kinase, a major regulator of cell proliferation, differentiation, and apoptosis, and focal adhesion kinase, which influences cell survival and migration. Phosphorylated focal adhesion kinase then activates Src, phosphoinositide 3-kinase, and phospholipase C-γ, among others that promote cell survival (via Akt signaling). In addition, the cytoplasmic Rho-GTPases are regulators of cytoskeletal dynamics and often described as molecular switches due to their ability to activate multiple downstream pathways. One notable effector of Rho, Rho-associated kinase (ROCK), has been shown to be an important modulator of actin-myosin dynamics via phosphorylation of myosin light chain phosphatase and LIM kinase, which leads to cofilin deactivation and actin polymerization (Maekawa et al., 1999). An interplay between the Rho/ROCK pathway and TGF-β is evident as cell contraction requires ROCK activity independent of α-smooth muscle actin expression (Miura et al., 2006; Kita et al., 2008). These pathways play an important role in cell migration, adhesion, and contractility, as well as cell survival and proliferation. ROCK-targeted therapeutics aim to selectively modulate these processes in the context of disease (e.g., glaucoma or Fuchs’ endothelial corneal dystrophy), injury (e.g., corneal scarring), or surgery (e.g., Descemet’s Stripping Endothelial Keratoplasty) and will be further discussed in Section 6.
5. Endothelial Cell Integrins
The corneal endothelium is essential in regulating water flux between the aqueous humor and the corneal stroma in order to maintain corneal deturgescence, which limits hydration of the cornea. Primary corneal endothelial cells are suspended in G1-phase of the cell cycle and remain non-proliferative in vivo. Methods for culturing corneal endothelial cells in vitro have shown an ability to promote proliferation using growth factors, in addition to fibronectin or collagen type I or IV coatings to allow cell attachment to traditional polystyrene culture plates or polydimethylsiloxane substrates (Engler et al., 2009; Palchesko et al., 2015). Recent focus has been placed on developing viable substrates for the cultivation and expansion of corneal endothelial cells for transplantation in corneal dystrophies affecting endothelial function, such as Fuch’s endothelial corneal dystrophy, congenital hereditary endothelial dystrophy, and posterior polymorphous corneal dystrophy. The integrins that appear to be relevant for maintaining and promoting maturation of primary human corneal endothelial cells may be inferred from this work. Table 2 shows the integrins expressed by corneal endothelial cells as they interface with DM. Biosynthetic coatings of various polymers and protein-based substrates have shown favorable cellular responses that promote cell attachment, survival, proliferation, and terminal differentiation of corneal endothelial cells. An example is inclusion of the RGD (Arg-Gly-Asp) and DGEA (Asp-Gly-Glu-Ala) α2β1 integrin binding sites on a biosynthetic substratum of poly-ε-lysine hydrogels with octanedioic acid crosslinks, which appears to improve endothelial cell binding, proliferation, and maturation in vitro (Kennedy et al., 2019). Human corneal endothelial cells may also be cultured on other protein or polymer based-approaches, including biocompatible silk protein films that may be surface-coated to promote cell attachment (Ramachandran et al., 2020). Many of these polymers naturally lack cell binding domains, which may be useful in an effort to customize coatings to favor select cell interactions (e.g., endothelial cells), while limiting the binding of other cell types (e.g., lymphocytes) and reduce the likelihood of rejection and promote cell integration.
Table 2.
Integrins expressed on corneal endothelial cells
| Corneal endothelial cell integrins adhering to Descemet’s Membrane | ||||
|---|---|---|---|---|
| Integrin Family | Subunit | Gene | Heterodimer | Known Ligands |
| β1 ITGB1 | α2 | ITGA2 | α2β1 | Laminin, Collagen |
| α3 | ITGA3 | α3β1 | Laminin, FN, TSP-1, Nidogen, Collagen type IV | |
| α4 | ITGA4 | α4β1 | FN-IIIA | |
| α5 | ITGA5 | α5β1 | Collagen, Laminin | |
| α6 | ITGA6 | α6β1 | Collagen, Laminin, FN | |
| αv ITGAV | β1 | ITGB1 | αvβ1 | Laminin, FN |
| β3 | ITGB3 | αvβ3 | FN, Laminin, Vitronectin | |
| β5 | ITGB5 | αvβ5 | FN, Laminin, Vitronectin | |
| β6 | ITGB6 | αvβ6 | FN, Laminin, Vitronectin | |
(Abbreviations: Fibronectin (FN), thrombospondin-1 (TSP-1)).
Maintenance of the corneal endothelium is also dependent on mechanotransduction pathways mediated by the basement membrane. The corneal endothelium and DM have a direct relationship regulated by the stiffness of the substratum influenced by the biochemical composition of the posterior DM. Mechanotransduction has been shown to favor mesenchymal stem cell differentiation to corneal endothelial cells solely by varying the substratum topography and stiffness (Gutermuth et al., 2019). Micropatterning of cell culture substrates to create surfaces similar to hexagonal structures seen in DMs have also shown to favor corneal endothelial cell binding and maturation based on increased tight junctional marker expression. These studies indicate that topography of the substratum can regulate cell phenotype (Muhammad et al., 2015). These findings may be related to the dynamic spatial orientation of integrins that may adapt based on the composition and organization of the substratum.
In terms of specific integrins involved in cell binding, integrins α3β1 and α6β1 appear to mediate binding interactions between primary human corneal endothelial cells and LN511 in vitro (Okumura et al., 2015). LNα5, LNβ1, LNβ2 and LNγ1 chains were also found along the endothelial-DM interface in vivo, suggesting that LN511 and LN521 represent the major LN forms at the endothelial face of DM. The predominant integrin chains expressed on the mRNA and protein level in endothelial cells in vivo comprise integrin α3, α6, β1 and β5, although integrins α1, α2, α4, α5, α10, α11 and αv were also weakly expressed (Okumura et al., 2015). In vitro, functional blocking of α3β1 and α6β1 integrins suppressed phosphorylation of focal adhesion kinase (FAK) and cell adhesion to LN511-coated surfaces, indicating that laminins regulate endothelial cell adhesion through binding to integrin α3β1 and α6β1. Figure 3B shows the localization of major integrins α3, α6, and β1 in corneal endothelial cells at DM.
Primary human corneal endothelial cells are known to be susceptible to undergoing EMT. The integrin subunit α2 is upregulated in endothelial cells expressing EMT-markers (Toda et al., 2016). Laminin-411 and −511 also appear important for primary human corneal endothelial cell binding by minimizing the number of cells undergoing EMT (Toda et al., 2016). This phenotypic switching has made transplantations of endothelial cell suspensions more difficult. Research has focused on developing therapeutics to improve endothelial cell binding and maturation post-transplantation.
6. Therapeutics that modulate ROCK
By targeting downstream effectors of integrin-mediated signaling, such as Rho/ROCK, fibrosis can be attenuated (Tada et al., 2001; Yamamoto et al., 2012; Zhou et al., 2013). Inhibition of Rho/ROCK signaling has also been shown to promote retention of stem-like properties and proliferation of retinal pigment epithelial cells cultured in vitro by inhibiting EMT suggesting a direct interplay of signaling pathways involved in proliferation and differentiation (Croze et al., 2014; Croze et al., 2016). This work provides evidence that therapeutic modulation of integrin-mediated signaling may aid in promoting cell viability, adhesion, and recovery, and may translate to improved tissue regeneration following transplantation.
In terms of applications in the cornea, studies in vitro have shown favorable corneal endothelial cell attachment to substrates using a synthetic ROCK inhibitor, Y-27632 (Okumura et al., 2012), which targets both isoforms of ROCK (ROCK1 and ROCK2) (Ishizaki et al., 2000). The improved cell adhesion with ROCK inhibition may be a result of increased cell spreading that is apparent in 2D cultures (Croze et al., 2016) and occurs with a change in distribution of focal adhesions and stress fibers that result from altered cofilin activity. Eyedrops of Y-27632 applied to a primate model with corneal endothelial damage showed improved endothelial cell proliferation and functional recovery based on decreased corneal edema (Okumura et al., 2013). A small clinical study with 8 patients (4 with late-stage Fuchs’ dystrophy) who were given eyedrops 6 times per day showed reduced edema and thickness. This improvement was also seen in some patients with central corneal edema but not in those with diffuse corneal edema (Okumura et al., 2013). Given the potential role for isoform-dependent functions of ROCK on actin dynamics and maintaining stemness in certain cell types, further studies evaluating targeted therapeutics for a select isoform (ROCK1 or ROCK2) may be important for clinical applications (Newell-Litwa et al., 2015). Studies of an approved glaucoma treatment and ROCK1 inhibitor, ripasudil, have also shown favorable clinical results in patients with Fuchs’ dystrophy (Moloney et al., 2017; Macsai and Shiloach, 2019). This work is a significant advance in efforts to identify druggable targets to regulate cell pluripotency, EMT, and adhesion by focusing on integrin-mediated signaling to improve the outcome of corneal endothelial cell transplantations to promote cell attachment and survival. The Rho/ROCK pathway has also been implicated in TGF-β-promoted contraction in fibrotic conditions, such as proliferative diabetic retinopathy and vitreo-retinopathy (Kita et al., 2008).
7. Endothelial Cell Integrins and Disease
Degeneration of the corneal endothelium and underlying DM during Fuchs’ endothelial cell dystrophy leads to corneal edema and haze development. Thickening of DM is a characteristic of Fuchs’ progression and is attributed to elevated EMT by the endothelial layer and the formation of ECM deposits, known as guttae, which range from 5 μm to 50 μm in diameter (Xia et al., 2016). The role of biophysical changes in the DM leading to cellular pathology is apparent in Fuchs’ dystrophy (Ali et al., 2016). Early-onset Fuchs’ is associated with COL8a mutations (L450W and Q455K) that leads to altered collagen type VIII accumulation in the anterior DM (Gottsch et al., 2005a; Gottsch et al., 2005b). These mutations are associated with irregular fibril spacing and softening of the DM that may lead to altered endothelial cell function and precedes the increased ECM deposition and DM-thickening that follows in later stages of Fuchs’ dystrophy. COL8a2 mutant mouse models of early-onset Fuchs’ (COL8a2L450W/L450W and COL8a2Q455K/Q455K) have shown a >50% decrease in DM stiffness by 10 months of age which appears to correlate with a loss in endothelial cell counts (Leonard et al., 2019).
Altered integrin expression has been identified in a Fuchs’ tissue-engineered model. A 2-fold reduction in ITGA10 was detected in Fuchs’ corneal endothelial cells in this tissue-engineered system (Goyer et al., 2018). Using real-time PCR array technology, Weller et al. showed upregulation of integrin α1, α3, α4, αL, β1, β3 and β4 in explanted endothelial cell-DM specimens from Fuchs’ patients compared to normal specimens (Weller et al., 2014). A genome-wide association study of Fuchs’ patients and control individuals identified LAMC1 (laminin subunit γ 1) and KANK4 (isoform belonging to the obscure Kank class of proteins involved in stress fiber formation) (Zhu et al., 2008) as potential risk factors for Fuchs’ development (Afshari et al., 2017). Further studies are warranted to characterize integrin expression in the Fuchs’ cornea to determine how interactions between the corneal endothelium and DM affect pathogenesis and progression of the disease.
If we apply the theory of dynamic reciprocity to Fuchs’ dystrophy, differences in the mechanical forces that integrins exert on their substrate would induce guttae formation by altering the forces the cells exert on DM. Integrins, as surface proteins, are druggable targets and there are peptides and antibodies that can be used either block integrin function or induce integrin activation. Select targeting of integrin-mediated signaling to promote tissue regeneration in the absence of EMT or excessive ECM deposition relies primarily on the tissue and disease status. Blocking integrin-activation following corneal wounding leads both to reduced corneal epithelial cell migration following wounding (Blanco-Mezquita et al., 2011) and a parallel delay in stromal scar development (Wu et al., 2019) suggesting that this therapeutic approach may be more beneficial to decrease TGFβ1-driven fibrosis and/or inhibit EMT. During fibrotic conditions, targeting specific integrins has shown some success in certain tissues. For example, global knockout of ανβ6 leads to reduced scar tissue development in chemical injury models or genetic pro-fibrotic models of the lung, kidney, liver, and skin (reviewed in (Henderson and Sheppard, 2013)). In these tissues, development of fibrosis is blunted by inhibiting ανβ6-mediated activation and release of TGFβ1 that occurs following binding of the integrin to the RGD-sequence found in the latency-associated peptide linked to the pro-TGFβ1 form. Likewise, targeting the α3β1 integrin has also shown inhibition of fibrosis in alveolar epithelial cells by preventing EMT and myofibroblast differentiation that requires binding interactions between α3β1 and the formation of a phospho-SMAD2 complex with β-catenin (Kim et al., 2009). This approach targeting select integrins or downstream pathways, such as ROCK, may be a useful means to prevent fibrosis and EMT in various tissues, including the cornea.
8. Summary
Since integrins were first characterized, they have been found to be critical for maintaining tissue architecture in the cornea. Basic and translational research on integrins has contributed new clinical treatments for blinding corneal pathologies and a more complete understanding of the impact of mechanotransduction on corneal homeostasis. Yet, corneal pathology continues to lead to corneal blindness and loss of quality of life; new treatments are needed. Dynamic reciprocity suggests that integrins are involved. While much has been learned about integrins, additional knowledge of the roles of integrins and their adhesion to the EpBM and DMs in health and disease is greatly needed.
Highlights.
Current knowledge on the expression and function of the integrins that mediate adhesion to the basement membranes expressed by corneal epithelial and endothelial cells in health and disease is summarized. This is the first review to discuss similarities and differences in the integrins expressed by both cell types.
Acknowledgements
We dedicate this review to our friend and colleague James D. Zieske with whom we carried out many of the studies we cite here. His contributions to the field have been huge and he is missed. In addition, we also want to thank everyone who we have taught, trained, and collaborated with over the years. Each one pushed us to think more clearly and deeply. This work was supported by R01EY08512 (MAS), R01EY005665 (JDZ), and 5T32EY007145–20.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afshari NA, Igo RP, Morris NJ, Stambolian D, Sharma S, Pulagam VL, Dunn S, Stamler JF, Truitt BJ, Rimmler J, Kuot A, Croasdale CR, Qin X, Burdon KP, Riazuddin SA, Mills R, Klebe S, Minear MA, Zhao J, Balajonda E, Rosenwasser GO, Baratz KH, Mootha VV, Patel SV, Gregory SG, Bailey-Wilson JE, Price MO, Price FW, Craig JE, Fingert JH, Gottsch JD, Aldave AJ, Klintworth GK, Lass JH, Li Y-J, Iyengar SK, 2017. Genome-wide association study identifies three novel loci in Fuchs endothelial corneal dystrophy. Nature Communications 8, 14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S, Stewart R, Yung S, Kolli S, Armstrong L, Stojkovic M, Figueiredo F, Lako M, 2007. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem cells (Dayton, Ohio) 25, 1145–1155. [DOI] [PubMed] [Google Scholar]
- Ali M, Raghunathan V, Li JY, Murphy CJ, Thomasy SM, 2016. Biomechanical relationships between the corneal endothelium and Descemet’s membrane. Experimental eye research 152, 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annes JP, Chen Y, Munger JS, Rifkin DB, 2004. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. The Journal of cell biology 165, 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkin AM, Stepp MA, 2000. Integrins as receptors for laminins. Microscopy research and technique 51, 280–301. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Aggeler J, 1987. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Progress in clinical and biological research 249, 251–262. [PubMed] [Google Scholar]
- Bissell MJ, Hall HG, Parry G, 1982. How does the extracellular matrix direct gene expression? Journal of Theoretical Biology 99, 31–68. [DOI] [PubMed] [Google Scholar]
- Blanco-Mezquita JT, Hutcheon AE, Stepp MA, Zieske JD, 2011. alphaVbeta6 integrin promotes corneal wound healing. Investigative ophthalmology & visual science 52, 8505–8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P, McPherson J, 1982. Synthesis and secretion of structural macromolecules by endothelial cells in culture, Pathobiology of the Endothelial Cell. Academic Press, New York, pp. 215–228. [Google Scholar]
- Crowder SW, Leonardo V, Whittaker T, Papathanasiou P, Stevens MM, 2016. Material Cues as Potent Regulators of Epigenetics and Stem Cell Function. Cell Stem Cell 18, 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croze RH, Buchholz DE, Radeke MJ, Thi WJ, Hu Q, Coffey PJ, Clegg DO, 2014. ROCK Inhibition Extends Passage of Pluripotent Stem Cell-Derived Retinal Pigmented Epithelium. Stem cells translational medicine 3, 1066–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croze RH, Thi WJ, Clegg DO, 2016. ROCK Inhibition Promotes Attachment, Proliferation, and Wound Closure in Human Embryonic Stem Cell-Derived Retinal Pigmented Epithelium. Transl Vis Sci Technol 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira RC, Wilson SE, 2020. Descemet’s membrane development, structure, function and regeneration. Experimental eye research 197, 108090. [DOI] [PubMed] [Google Scholar]
- Diaz-Gonzalez F, Forsyth J, Steiner B, Ginsberg MH, 1996. Trans-dominant inhibition of integrin function. Molecular biology of the cell 7, 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO, 1997. alpha3beta1 Integrin is required for normal development of the epidermal basement membrane. The Journal of cell biology 137, 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd CJ, Cooney CL, Nugent MA, 1999. Heparan sulfate mediates bFGF transport through basement membrane by diffusion with rapid reversible binding. The Journal of biological chemistry 274, 5236–5244. [DOI] [PubMed] [Google Scholar]
- Dowling J, Yu QC, Fuchs E, 1996. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. The Journal of cell biology 134, 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duperret EK, Natale CA, Monteleon C, Dahal A, Ridky TW, 2016. The integrin alphav-TGFbeta signaling axis is necessary for epidermal proliferation during cutaneous wound healing. Cell cycle (Georgetown, Tex.) 15, 2077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C, Kelliher C, Speck CL, Jun AS, 2009. Assessment of attachment factors for primary cultured human corneal endothelial cells. Cornea 28, 1050–1054. [DOI] [PubMed] [Google Scholar]
- Eslami A, Gallant-Behm CL, Hart DA, Wiebe C, Honardoust D, Gardner H, Hakkinen L, Larjava HS, 2009. Expression of integrin alphavbeta6 and TGF-beta in scarless vs scar-forming wound healing. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 57, 543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garana RM, Petroll WM, Chen WT, Herman IM, Barry P, Andrews P, Cavanagh HD, Jester JV, 1992. Radial keratotomy. II. Role of the myofibroblast in corneal wound contraction. Investigative ophthalmology & visual science 33, 3271–3282. [PubMed] [Google Scholar]
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M, 1996. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nature genetics 13, 370–373. [DOI] [PubMed] [Google Scholar]
- Goletz S, Zillikens D, Schmidt E, 2017. Structural proteins of the dermal-epidermal junction targeted by autoantibodies in pemphigoid diseases. Experimental dermatology 26, 1154–1162. [DOI] [PubMed] [Google Scholar]
- Gottsch JD, Sundin OH, Liu SH, Jun AS, Broman KW, Stark WJ, Vito EC, Narang AK, Thompson JM, Magovern M, 2005a. Inheritance of a novel COL8A2 mutation defines a distinct early-onset subtype of fuchs corneal dystrophy. Investigative ophthalmology & visual science 46, 1934–1939. [DOI] [PubMed] [Google Scholar]
- Gottsch JD, Zhang C, Sundin OH, Bell WR, Stark WJ, Green WR, 2005b. Fuchs corneal dystrophy: aberrant collagen distribution in an L450W mutant of the COL8A2 gene. Investigative ophthalmology & visual science 46, 4504–4511. [DOI] [PubMed] [Google Scholar]
- Goyer B, Theriault M, Gendron SP, Brunette I, Rochette PJ, Proulx S, 2018. Extracellular Matrix and Integrin Expression Profiles in Fuchs Endothelial Corneal Dystrophy Cells and Tissue Model. Tissue engineering. Part A 24, 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grushkin-Lerner LS, Trinkaus-Randall V, 1991. Localization of integrin and syndecan in vivo in a corneal epithelial abrasion and keratectomy. Current eye research 10, 75–85. [DOI] [PubMed] [Google Scholar]
- Gutermuth A, Maassen J, Harnisch E, Kuhlen D, Sauer-Budge A, Skazik-Voogt C, Engelmann K, 2019. Descemet’s Membrane Biomimetic Microtopography Differentiates Human Mesenchymal Stem Cells Into Corneal Endothelial-Like Cells. Cornea 38, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Hirako Y, Tsuruta D, 2016. beta4 integrin in hereditary and acquired mucocutaneous diseases. Experimental dermatology 25, 267–268. [DOI] [PubMed] [Google Scholar]
- Hayashi R, Yamato M, Saito T, Oshima T, Okano T, Tano Y, Nishida K, 2008. Enrichment of corneal epithelial stem/progenitor cells using cell surface markers, integrin alpha6 and CD71. Biochemical and biophysical research communications 367, 256–263. [DOI] [PubMed] [Google Scholar]
- Henderson NC, Sheppard D, 2013. Integrin-mediated regulation of TGFβ in fibrosis. Biochimica et biophysica acta 1832, 891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, DiPersio CM, Kreidberg JA, Hynes RO, 1998. Novel roles for alpha3beta1 integrin as a regulator of cytoskeletal assembly and as a trans-dominant inhibitor of integrin receptor function in mouse keratinocytes. The Journal of cell biology 142, 1357–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenester E, Yurchenco PD, 2013. Laminins in basement membrane assembly. Cell adhesion & migration 7, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, 2004. The emergence of integrins: a personal and historical perspective. Matrix biology : journal of the International Society for Matrix Biology 23, 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, Narumiya S, 2000. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Molecular pharmacology 57, 976–983. [PubMed] [Google Scholar]
- Janes SM, Watt FM, 2004. Switch from alphavbeta5 to alphavbeta6 integrin expression protects squamous cell carcinomas from anoikis. The Journal of cell biology 166, 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Teng M, Guo X, Zhang D, Zhang Q, Zhang J, Huang Y, 2014. Switch from alphavbeta5 to alphavbeta6 integrin is required for CD9-regulated keratinocyte migration and MMP-9 activation. FEBS letters 588, 4044–4052. [DOI] [PubMed] [Google Scholar]
- Jones PH, Watt FM, 1993. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73, 713–724. [DOI] [PubMed] [Google Scholar]
- Jonsson F, Bystrom B, Davidson AE, Backman LJ, Kellgren TG, Tuft SJ, Koskela T, Ryden P, Sandgren O, Danielson P, Hardcastle AJ, Golovleva I, 2015. Mutations in collagen, type XVII, alpha 1 (COL17A1) cause epithelial recurrent erosion dystrophy (ERED). Human mutation 36, 463–473. [DOI] [PubMed] [Google Scholar]
- Kabosova A, Azar DT, Bannikov GA, Campbell KP, Durbeej M, Ghohestani RF, Jones JC, Kenney MC, Koch M, Ninomiya Y, Patton BL, Paulsson M, Sado Y, Sage EH, Sasaki T, Sorokin LM, Steiner-Champliaud MF, Sun TT, Sundarraj N, Timpl R, Virtanen I, Ljubimov AV, 2007. Compositional differences between infant and adult human corneal basement membranes. Investigative ophthalmology & visual science 48, 4989–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor R, Sakai LY, Funk S, Roux E, Bornstein P, Sage EH, 1988. Type VIII collagen has a restricted distribution in specialized extracellular matrices. The Journal of cell biology 107, 721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Li A, 2000. Adhesive properties of human basal epidermal cells: an analysis of keratinocyte stem cells, transit amplifying cells, and postmitotic differentiating cells. The Journal of investigative dermatology 114, 413–420. [DOI] [PubMed] [Google Scholar]
- Kennedy S, Lace R, Carserides C, Gallagher AG, Wellings DA, Williams RL, Levis HJ, 2019. Poly-ε-lysine based hydrogels as synthetic substrates for the expansion of corneal endothelial cells for transplantation. Journal of Materials Science: Materials in Medicine 30, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, Frank JA, Brumwell AN, Wheeler SE, Kreidberg JA, Chapman HA, 2009. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest 119, 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T, Hata Y, Arita R, Kawahara S, Miura M, Nakao S, Mochizuki Y, Enaida H, Goto Y, Shimokawa H, Hafezi-Moghadam A, Ishibashi T, 2008. Role of TGF-beta in proliferative vitreoretinal diseases and ROCK as a therapeutic target. Proceedings of the National Academy of Sciences of the United States of America 105, 17504–17509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last JA, Liliensiek SJ, Nealey PF, Murphy CJ, 2009. Determining the mechanical properties of human corneal basement membranes with atomic force microscopy. Journal of structural biology 167, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauweryns B, van den Oord JJ, Volpes R, Foets B, Missotten L, 1991. Distribution of very late activation integrins in the human cornea. An immunohistochemical study using monoclonal antibodies. Investigative ophthalmology & visual science 32, 2079–2085. [PubMed] [Google Scholar]
- Leonard BC, Jalilian I, Raghunathan VK, Wang W, Jun AS, Murphy CJ, Thomasy SM, 2019. Biomechanical changes to Descemet’s membrane precede endothelial cell loss in an early-onset murine model of Fuchs endothelial corneal dystrophy. Experimental eye research 180, 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Stevenson CG, 2012. Regulation of cell behavior and tissue patterning by bioelectrical signals: challenges and opportunities for biomedical engineering. Annual review of biomedical engineering 14, 295–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CQ, Bissell MJ, 1993. Multi-faceted regulation of cell differentiation by extracellular matrix. The FASEB Journal 7, 737–743. [DOI] [PubMed] [Google Scholar]
- Litjens SH, de Pereda JM, Sonnenberg A, 2006. Current insights into the formation and breakdown of hemidesmosomes. Trends in cell biology 16, 376–383. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Burgeson RE, Butkowski RJ, Michael AF, Sun TT, Kenney MC, 1995. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Laboratory investigation; a journal of technical methods and pathology 72, 461–473. [PubMed] [Google Scholar]
- Ljubimov AV, Huang ZS, Huang GH, Burgeson RE, Gullberg D, Miner JH, Ninomiya Y, Sado Y, Kenney MC, 1998. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 46, 1033–1041. [DOI] [PubMed] [Google Scholar]
- Macsai MS, Shiloach M, 2019. Use of Topical Rho Kinase Inhibitors in the Treatment of Fuchs Dystrophy After Descemet Stripping Only. Cornea 38, 529–534. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S, 1999. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science (New York, N.Y.) 285, 895–898. [DOI] [PubMed] [Google Scholar]
- Massague J, 2012. TGFbeta signalling in context. Nature reviews. Molecular cell biology 13, 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur SK, Cheung JK, Antohi S, 1993. Identification of integrins in cultured corneal fibroblasts and in isolated keratocytes. Investigative ophthalmology & visual science 34, 2690–2698. [PubMed] [Google Scholar]
- Meng XM, Nikolic-Paterson DJ, Lan HY, 2016. TGF-beta: the master regulator of fibrosis. Nature reviews. Nephrology 12, 325–338. [DOI] [PubMed] [Google Scholar]
- Miura M, Hata Y, Hirayama K, Kita T, Noda Y, Fujisawa K, Shimokawa H, Ishibashi T, 2006. Critical role of the Rho-kinase pathway in TGF-beta2-dependent collagen gel contraction by retinal pigment epithelial cells. Experimental eye research 82, 849–859. [DOI] [PubMed] [Google Scholar]
- Moloney G, Petsoglou C, Ball M, Kerdraon Y, Hollhumer R, Spiteri N, Beheregaray S, Hampson J, D’Souza M, Devasahayam RN, 2017. Descemetorhexis Without Grafting for Fuchs Endothelial Dystrophy-Supplementation With Topical Ripasudil. Cornea 36, 642–648. [DOI] [PubMed] [Google Scholar]
- Muhammad R, Peh GS, Adnan K, Law JB, Mehta JS, Yim EK, 2015. Micro- and nano-topography to enhance proliferation and sustain functional markers of donor-derived primary human corneal endothelial cells. Acta biomaterialia 19, 138–148. [DOI] [PubMed] [Google Scholar]
- Munger JS, Sheppard D, 2011. Cross talk among TGF-beta signaling pathways, integrins, and the extracellular matrix. Cold Spring Harbor perspectives in biology 3, a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C, Alvarado J, Juster R, 1984. Prenatal and postnatal growth of the human Descemet’s membrane. Investigative ophthalmology & visual science 25, 1402–1415. [PubMed] [Google Scholar]
- Nandrot EF, Finnemann SC, 2006. Altered rhythm of photoreceptor outer segment phagocytosis in beta5 integrin knockout mice. Advances in experimental medicine and biology 572, 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC, 2004. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. The Journal of experimental medicine 200, 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell-Litwa KA, Badoual M, Asmussen H, Patel H, Whitmore L, Horwitz AR, 2015. ROCK1 and 2 differentially regulate actomyosin organization to drive cell and synaptic polarity. The Journal of cell biology 210, 225–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura N, Kakutani K, Numata R, Nakahara M, Schlotzer-Schrehardt U, Kruse F, Kinoshita S, Koizumi N, 2015. Laminin-511 and −521 enable efficient in vitro expansion of human corneal endothelial cells. Investigative ophthalmology & visual science 56, 2933–2942. [DOI] [PubMed] [Google Scholar]
- Okumura N, Koizumi N, Kay EP, Ueno M, Sakamoto Y, Nakamura S, Hamuro J, Kinoshita S, 2013. The ROCK inhibitor eye drop accelerates corneal endothelium wound healing. Investigative ophthalmology & visual science 54, 2493–2502. [DOI] [PubMed] [Google Scholar]
- Okumura N, Koizumi N, Ueno M, Sakamoto Y, Takahashi H, Tsuchiya H, Hamuro J, Kinoshita S, 2012. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. The American journal of pathology 181, 268–277. [DOI] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Stepp MA, 2005. In search of markers for the stem cells of the corneal epithelium. Biology of the cell 97, 265–276. [DOI] [PubMed] [Google Scholar]
- Pal-Ghosh S, Blanco T, Tadvalkar G, Pajoohesh-Ganji A, Parthasarathy A, Zieske JD, Stepp MA, 2011a. MMP9 cleavage of the beta4 integrin ectodomain leads to recurrent epithelial erosions in mice. Journal of cell science 124, 2666–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Ghosh S, Pajoohesh-Ganji A, Brown M, Stepp MA, 2004. A mouse model for the study of recurrent corneal epithelial erosions: alpha9beta1 integrin implicated in progression of the disease. Investigative ophthalmology & visual science 45, 1775–1788. [DOI] [PubMed] [Google Scholar]
- Pal-Ghosh S, Pajoohesh-Ganji A, Tadvalkar G, Kyne BM, Guo X, Zieske JD, Stepp MA, 2016. Topical Mitomycin-C enhances subbasal nerve regeneration and reduces erosion frequency in the debridement wounded mouse cornea. Experimental eye research 146, 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Ghosh S, Pajoohesh-Ganji A, Tadvalkar G, Stepp MA, 2011b. Removal of the basement membrane enhances corneal wound healing. Experimental eye research 93, 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchesko RN, Lathrop KL, Funderburgh JL, Feinberg AW, 2015. In Vitro Expansion of Corneal Endothelial Cells on Biomimetic Substrates. Scientific reports 5, 7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Pareja JC, 2020. Atypical basement membranes and basement membrane diversity - what is normal anyway? Journal of cell science 133. [DOI] [PubMed] [Google Scholar]
- Polisetti N, Sorokin L, Okumura N, Koizumi N, Kinoshita S, Kruse FE, Schlötzer-Schrehardt U, 2017. Laminin-511 and −521-based matrices for efficient ex vivo-expansion of human limbal epithelial progenitor cells. Scientific reports 7, 5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisetti N, Zenkel M, Menzel-Severing J, Kruse FE, Schlötzer-Schrehardt U, 2016. Cell Adhesion Molecules and Stem Cell-Niche-Interactions in the Limbal Stem Cell Niche. Stem cells (Dayton, Ohio) 34, 203–219. [DOI] [PubMed] [Google Scholar]
- Pozzi A, Yurchenco PD, Iozzo RV, 2017. The nature and biology of basement membranes. Matrix biology : journal of the International Society for Matrix Biology 57–58, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran C, Gupta P, Hazra S, Mandal BB, 2020. In Vitro Culture of Human Corneal Endothelium on Non-Mulberry Silk Fibroin Films for Tissue Regeneration. Translational Vision Science & Technology 9, 12–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid KA, Foster CS, Ahmed AR, 2013. Identification of epitopes within integrin beta4 for binding of auto-antibodies in ocular cicatricial and mucous membrane pemphigoid: preliminary report. Investigative ophthalmology & visual science 54, 7707–7716. [DOI] [PubMed] [Google Scholar]
- Rodius S, Indra G, Thibault C, Pfister V, Georges-Labouesse E, 2007. Loss of alpha6 integrins in keratinocytes leads to an increase in TGFbeta and AP1 signaling and in expression of differentiation genes. Journal of cellular physiology 212, 439–449. [DOI] [PubMed] [Google Scholar]
- Sachs N, Secades P, van Hulst L, Kreft M, Song JY, Sonnenberg A, 2012. Loss of integrin alpha3 prevents skin tumor formation by promoting epidermal turnover and depletion of slow-cycling cells. Proceedings of the National Academy of Sciences of the United States of America 109, 21468–21473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada H, Konomi H, Hirosawa K, 1990. Characterization of the collagen in the hexagonal lattice of Descemet’s membrane: its relation to type VIII collagen. The Journal of cell biology 110, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Dietrich T, Saito K, Sorokin L, Sasaki T, Paulsson M, Kruse FE, 2007. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Experimental eye research 85, 845–860. [DOI] [PubMed] [Google Scholar]
- Schlötzer-Schrehardt U, Kruse FE, 2016. Anatomy and Physiology: Considerations in Relation to Transplantation, in: Hjortdal J (Ed.), Corneal Transplantation. Springer International Publishing, Cham, pp. 9–22. [Google Scholar]
- Shukrun R, Vivante A, Pleniceanu O, Vax E, Anikster Y, Dekel B, Lotan D, 2014. A human integrin-alpha3 mutation confers major renal developmental defects. PloS one 9, e90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sta Iglesia DD, Stepp MA, 2000. Disruption of the basement membrane after corneal débridement. Investigative ophthalmology & visual science 41, 1045–1053. [PubMed] [Google Scholar]
- Stepp MA, Gibson HE, Gala PH, Iglesia DD, Pajoohesh-Ganji A, Pal-Ghosh S, Brown M, Aquino C, Schwartz AM, Goldberger O, Hinkes MT, Bernfield M, 2002. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. Journal of cell science 115, 4517–4531. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Liu Y, Pal-Ghosh S, Jurjus RA, Tadvalkar G, Sekaran A, Losicco K, Jiang L, Larsen M, Li L, Yuspa SH, 2007. Reduced migration, altered matrix and enhanced TGFbeta1 signaling are signatures of mouse keratinocytes lacking Sdc1. Journal of cell science 120, 2851–2863. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Spurr-Michaud S, Gipson IK, 1993. Integrins in the wounded and unwounded stratified squamous epithelium of the cornea. Investigative ophthalmology & visual science 34, 1829–1844. [PubMed] [Google Scholar]
- Stepp MA, Spurr-Michaud S, Tisdale A, Elwell J, Gipson IK, 1990. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proceedings of the National Academy of Sciences of the United States of America 87, 8970–8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME, 2003. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Investigative ophthalmology & visual science 44, 4237–4246. [DOI] [PubMed] [Google Scholar]
- Tada S, Iwamoto H, Nakamuta M, Sugimoto R, Enjoji M, Nakashima Y, Nawata H, 2001. A selective ROCK inhibitor, Y27632, prevents dimethylnitrosamine-induced hepatic fibrosis in rats. Journal of Hepatology 34, 529–536. [DOI] [PubMed] [Google Scholar]
- Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA, 2003. Talin binding to integrin beta tails: a final common step in integrin activation. Science (New York, N.Y.) 302, 103–106. [DOI] [PubMed] [Google Scholar]
- Takagi J, Petre BM, Walz T, Springer TA, 2002. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110, 599–611. [DOI] [PubMed] [Google Scholar]
- Tamkun JW, DeSimone DW, Fonda D, Patel RS, Buck C, Horwitz AF, Hynes RO, 1986. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell 46, 271–282. [DOI] [PubMed] [Google Scholar]
- Tani H, Morris RJ, Kaur P, 2000. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proceedings of the National Academy of Sciences of the United States of America 97, 10960–10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo K, Tervo T, van Setten GB, Virtanen I, 1991. Integrins in human corneal epithelium. Cornea 10, 461–465. [DOI] [PubMed] [Google Scholar]
- Toda M, Ueno M, Yamada J, Hiraga A, Tanaka H, Schlotzer-Schrehardt U, Sotozono C, Kinoshita S, Hamuro J, 2016. The Different Binding Properties of Cultured Human Corneal Endothelial Cell Subpopulations to Descemet’s Membrane Components. Investigative ophthalmology & visual science 57, 4599–4605. [DOI] [PubMed] [Google Scholar]
- Tyagi S, Bhol K, Natarajan K, Livir-Rallatos C, Foster CS, Ahmed AR, 1996. Ocular cicatricial pemphigoid antigen: partial sequence and biochemical characterization. Proceedings of the National Academy of Sciences of the United States of America 93, 14714–14719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Zee CE, Kreft M, Beckers G, Kuipers A, Sonnenberg A, 2008. Conditional deletion of the Itgb4 integrin gene in Schwann cells leads to delayed peripheral nerve regeneration . The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 11292–11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I, Fuks Z, Ishai-Michaeli R, Bashkin P, Levi E, Korner G, Bar-Shavit R, Klagsbrun M, 1991. Extracellular matrix-resident basic fibroblast growth factor: implication for the control of angiogenesis. Journal of cellular biochemistry 45, 167–176. [DOI] [PubMed] [Google Scholar]
- Wang L, Dong Z, Zhang Y, Miao J, 2012. The roles of integrin beta4 in vascular endothelial cells. Journal of cellular physiology 227, 474–478. [DOI] [PubMed] [Google Scholar]
- Weller JM, Zenkel M, Schlötzer-Schrehardt U, Bachmann BO, Tourtas T, Kruse FE, 2014. Extracellular matrix alterations in late-onset Fuchs’ corneal dystrophy. Investigative ophthalmology & visual science 55, 3700–3708. [DOI] [PubMed] [Google Scholar]
- Wilson SE, 2019. Coordinated Modulation of Corneal Scarring by the Epithelial Basement Membrane and Descemet’s Basement Membrane. Journal of refractive surgery (Thorofare, N.J. : 1995) 35, 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, 2020. Bowman’s layer in the cornea- structure and function and regeneration. Experimental eye research 195, 108033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Hutcheon AEK, Sriram S, Tran JA, Zieske JD, 2019. Initiation of fibrosis in the integrin Alphavbeta6 knockout mice. Experimental eye research 180, 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia D, Zhang S, Nielsen E, Ivarsen AR, Liang C, Li Q, Thomsen K, Hjortdal JØ, Dong M, 2016. The Ultrastructures and Mechanical Properties of the Descement’s Membrane in Fuchs Endothelial Corneal Dystrophy. Scientific reports 6, 23096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Quantock AJ, Young RD, Okumura N, Ueno M, Sakamoto Y, Kinoshita S, Koizumi N, 2012. A selective inhibitor of the Rho kinase pathway, Y-27632, and its influence on wound healing in the corneal stroma. Molecular vision 18, 1727–1739. [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ, 2013. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest 123, 1096–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Kakinuma N, Wang Y, Kiyama R, 2008. Kank proteins: a new family of ankyrin-repeat domain-containing proteins. Biochimica et biophysica acta 1780, 128–133. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Mason VS, Wasson ME, Meunier SF, Nolte CJ, Fukai N, Olsen BR, Parenteau NL, 1994. Basement membrane assembly and differentiation of cultured corneal cells: importance of culture environment and endothelial cell interaction. Experimental cell research 214, 621–633. [DOI] [PubMed] [Google Scholar]