Abstract
Studying communities at different stages of urbanisation and industrialisation can teach us how timing and intensity of light affects the circadian clock under real-life conditions. We have previously described a strong tendency towards morningness in the Baependi Heart Study, located in a small rural town in Brazil. Here, we tested the hypothesis that this morningness tendency is associated with early circadian phase based on objective measurements (as determined by dim light melatonin onset, DLMO, and activity) and light exposure. We also analysed how well the previously collected chronotype questionnaire data was able to predict these DLMO values. The average DLMO observed in 73 participants (40 female) was 20:03±01:21, SD, with an earlier average onset in men (19:38±01:16) than in women (20:24±01:21; p≤0.01). However, men presented larger phase angle between DLMO and sleep onset time as measured by actigraphy (4.11 hours vs 3.16 hours; p≤0.01). Correlational analysis indicated associations between light exposure, activity rhythms, and DLMO, such that early DLMO was observed in participants with higher exposure to light, higher activity and earlier light exposure. The strongest significant predictor of DLMO was morningness-eveningness questionnaire (MEQ) (beta=−0.35, p≤0.05), followed by age (beta=−0.47, p≤0.01). Sex, light exposure, and variables derived from the Munich Chronotype Questionnaire were not significant predictors. Our observations demonstrate that both early sleep patterns and earlier circadian phase have been retained in this small rural town in spite of availability of electrification, in contrast to metropolitan post-industrial areas.
Keywords: Actigraphy, Circadian rhythms, Neuroendocrinology, Phase angle, Sleep-wake rhythm
Introduction
Circadian rhythms are observed as physical, mental, and behavioural daily oscillations. The circadian pacemaker is differentially sensitive to the resetting effects of retinal light exposure depending upon the circadian phase at which the light exposure occurs. Light exposure during biological evening delays the phase of the human circadian pacemaker, whilst light exposure in the early hours of the morning advances it.1,2
Dim light melatonin onset (DLMO) is considered the most reliable measure of circadian phase in humans3,4 and is determined as the point in time where melatonin levels rise above a set threshold, which typically occurs 2–3 h before the habitual onset of nocturnal sleep.5 DLMO assessment is typically performed in small numbers of individuals, and most studies to date have been performed in higher-income countries in laboratory settings. Obtaining this gold standard measure of circadian timing from a variety of populations can help us better understand variability in circadian timing, and ultimately the multitude of ways in which it relates to health.
Until recently, human activity was largely constrained by the natural photoperiod. The generally accepted view is that this was radically changed by industrialisation, which enabled both production and leisure during the hours of natural darkness under increasingly more affordable artificial light. Although the process was not studied in real time, there is a general consensus that this development has delayed bedtimes and circadian phase, although it is still controversial whether it has also resulted in a reduction of total sleep time.6 The importance of studying communities at different stages of the path towards urbanisation and industrialisation is becoming increasingly recognised, with a number of key recent publications providing important data points.6–12
Studying a cohort in a rural town in South-Eastern Brazil, the Baependi Heart Study Cohort, and comparing it to metropolitan populations, we have previously described a strong tendency towards early chronotype (morningness).13 Baependi is a small rural town (population: 19,148 habitants14) in the state of Minas Gerais in Brazil (21.95° S, 44.88° W). Duration of daylight in Baependi ranges from 10:47 (sunrise 06:37 and sunset 17:25) to 13:27h (sunrise 06:18 and sunset 19:46 during Daylight Savings Time, which has subsequently been abolished in Brazil but was still in operation during the study period). Baependi has followed a stable upward trajectory in Municipal Human Development Index – MHDI (a measure that assesses the same dimensions as the global HDI - health, education and income — on a scale between 0 and 1), which has increased from 0.430 to 0.681 between 1991 and 2010. The lack of knowledge about how the quantity of daylight, including mixed day/electric light conditions, impacts circadian entrainment and if this effect is dependent on the moment of the day has recently been identified as a key research priority. In spite of universal access to electricity in Baependi, both diurnal preference/chronotype13 and sleep timing16 are still significantly earlier than in metropolitan areas. We hypothesized that these observations were associated with an earlier circadian phase and high exposure to natural light/dark cycle. Testing this hypothesis required the assessment of the circadian phase under habitual sleep/wake conditions (i.e. not sleeping in a laboratory). The main purpose of this study was to investigate circadian phase of healthy adults who were following their habitual sleep-wake schedule. We investigated the associations between DLMO, and chronotype, sleep/wake cycle, activity, and light exposure, by objective means of actigraphy recording.
Methods
Participants
We collected saliva samples from 76 participants (42 female; ages 19–60 years). The study protocol conformed to the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the Hospital das Clínicas, University of São Paulo, Brazil. All procedures were conducted with informed and written consent of the participants. The methodology for recruitment has been described previously.17,18 Briefly, all extended family members aged above 18 years of probands who had initially been randomly selected from 11 out of 12 census districts in Baependi were initially invited to participate in the full study.
Protocol
Sampling for DLMO was scheduled for Tuesdays, Wednesdays, and Thursdays, between 26 August 2016 and 28 September 2016 (before the switch to daylight savings time). Participants were invited to participate through a telephone interview and those who reported being prescribed beta-blockers or antidepressants as well as those reporting shift work or sleep disorders were excluded. All participants were asked to maintain their habitual sleep-wake pattern for a minimum of 7 days before the DLMO assessment and were instructed to wear an actigraph device for a fourteen-day period, starting from the day of the saliva collection. Saliva sampling took place in the research station, located in an easily accessible sector of the town, where all questionnaire-based assessments were also conducted. The sunrise times during the months of August and September ranged from 05:42 to 06:14h, while the sunset time ranged from 17:48 to 17:57h, resulting in a day length range of 11:34 to 12:14 hours.
Measurements
Chronotype questionnaires
The following questionnaires were administered to the participants between March and November 2016:
The Morningness-Eveningness Questionnaire (MEQ),19 consisting of 19 items that measure a participant’s preference for the timing of daily activities. Lower scores indicate evening preference and higher scores morning preference.
The Munich Chronotype questionnaire (MCTQ), which focuses primarily on sleep timing, has 14 questions that assess the regularity of the participant`s sleep timing on workdays and work-free days. Chronotype is estimated as the midpoint of sleep on work-free days minus half of the difference between sleep duration on work-free days and average sleep duration of the week to control for sleep debt (mid-point of sleep on work-free days, sleep corrected, MSFsc). The following measurements from the MCTQ were also analysed, SOw: sleep onset workdays; SOf: sleep onset work-free days; SEw: local time of getting out of bed workdays; SEf: local time of getting out of bed work-free days; SDw: sleep duration workdays; SD: sleep duration work-free days; mid-sleep phase during working days (MSW) and mid-sleep phase during free-days (MSF).
Dim-light melatonin onset (DLMO) assessment
Participants were invited to the research station for one evening, arriving at 1730h. The saliva sampling started 4.5 hours before the mean bedtime of the population, as described previously.16 During the phase assessment, participants remained awake from 1800h to 2300h in dim light (<10 lux), as verified at the level of the participant’s neck with an actigraphy monitor (ActTrust AT0503, Condor Instruments, São Paulo, Brazil). Participants were seated in comfortable chairs and had access to toilet facilities (also <10 lux). They spent the sampling period watching a dimmed TV (<10 lux) and talking to each other and supervising staff. A research assistant was always present to make sure that participants were not falling asleep. Participants provided a 2 mL saliva sample every 60 minutes (1800, 1900, 2000, 2100, 2200 and 2300h, respectively) directly into a centrifuge tube. Snacks and water were provided after each sampling, and participants rinsed their mouths with water 10 minutes before the collection of each sample. Samples were immediately frozen at −20°C after the collection and shipped on dry ice to the UK to be assayed for melatonin. All samples from an individual participant were assayed in the same batch. DLMO was defined as the point in time (as determined with linear interpolation) when the melatonin concentration exceeded the threshold of 3 pg/ml. DLMO was used as the marker of circadian phase in each participant.21 Three out of the 76 participants were excluded because their salivary melatonin levels did not exceed the threshold.
Actigraphy protocol
Participants were instructed to wear an actimetry device that contained an accelerometer and also light and temperature sensors (ActTrust AT0503) on their non-dominant wrist on the outside of any long-sleeved clothing for a fourteen-day period. The Proportional Integration Mode (PIM) algorithm was used to derive a measure of user activity from acceleration readings. The PIM data with epochs of 60 s were integrated every hour to generate 24 epochs of 3600 s each day. Participants were instructed to use the event button to report any time they removed the actigraph and the times they went to bed and woke up. All data files were visually screened for sufficient wear time and then processed for analysis. A day was considered valid if it was recorded with no more than 1 hour of non-valid signal (i.e. non-wear periods). Out of the total of 76 participants with DLMO, 67 presented valid actigraphy recordings, with full 14-day records available from a majority of participants (n=53). For eight participants, 10 to 13 days of valid recordings were available, for a further five, six days, and for one, five days.
Sleep variables
For each day, we manually identified a main rest interval as the primary sleep period in agreement with standard procedures in sleep actigraphy.22,23 All valid days of recording were averaged to produce the variables related to sleep. The actigraph software, ActStudio, provided an estimation of the sleep duration (SD), sleep onset time (SO), and sleep offset time (SE) based on a modified version of the Cole-Kripke algorithm (Condor Instruments).
Non-parametric variables
We applied a non-parametric approach to describe the 24-hour rest-activity profile of the participants, as daily-rest-activity pattern did not perfectly follow the sinusoidal waveform assumed by the cosinor parametric method. We selected 5 consecutive valid days, starting at midnight of the first day of recording, in order to have a consistent number of days across all participants. The following non-parametric variables were derived from the data: M10 (a measure of total activity in most active 10-hour period, indicating diurnal motor activity), L5 (a measure of total motor activity in the least active 5-hour period, indicating nocturnal motor activity), IV (intradaily variability), IS (interdaily stability), F5 (beginning of L5, representing a measure of the beginning of rest phase), F10 (beginning of M10, representing a measure of the beginning of active phase), and RA (relative amplitude). M16 (a measure of total light exposure in the 16-hour period with maximum illumination, indicating diurnal light exposure). M3i (total light exposure within 3-hour post sunrise).24 Phase angle was calculated as the difference between DLMO and sleep onset, or the difference between DLMO and F5, or difference between DLMO and F10.
Data Analysis
Data are presented as mean±SD unless otherwise specified. Normal distribution was confirmed by the Kolmogorov–Smirnov test for all variables of interest. Data were log10 transformed prior to statistical analyses in the case of non-normal distribution (M3i and SEw, L5, IV and IS). A General Linear Model (GLM) was used for comparisons of DLMO, MEQ, MCTQ and actigraphy parameters between sexes, controlling by age. The partial eta-squared (η2p) was used as estimates of effect size. For all participants, initial Pearson’s correlations were calculated to determine associations between DLMO, chronotype (MEQ and mid-sleep), sleep parameters, light exposure (M3i), and activity (M10). As a second step, a multiple linear regression analysis was performed with MEQ, MSw, MSf, SOw, SOf, M10, M3i, age, and sex together to assess the accuracy of these variables in predicting DLMO, using the enter method. The Durbin-Watson statistic was applied to test for autocorrelation in the residuals. A p value of ≤0.05 was considered significant. Statistical analysis was performed using SPSS (Version 25) (IBM, Armonk, NY) and graphs were obtained with RStudio (Version 1.1.463) using the ggplot2 package.25
Results
Demographic information (including official urban/rural residential zone classification)13,26, circadian parameters, and results derived from the chronotype questionnaires are provided in Table 1. The 73 participants (aged from 19 to 60 years) included 33 men (aged 38±10) and 40 women (aged 36±12).
Table 1:
Demographics, DLMO, and questionnaire-derived sleep parameters
| Demographics | Total (n=73) | Female (n=40) | Male (n=33) |
|---|---|---|---|
| Age, years | 36.4±10.9 | 35.5±10.9 | 37.6±10.1 |
| Body mass index | 24.7±5.8 | 24.4±7.1 | 25.1±4.0 |
| Residential zone (Urban/Rural) | 63/10 | 37/3 | 26/7 |
| Employed, n (%) | 59 (80.8%) | 32 (80.0%) | 27 (81.8%) |
| DLMO (hh:min) | 20:03±01:21 | 20:24±01:21 | 19:38±01:16** |
| MEQ Score (n=56; 25 Male) | 59.1±9.2 | 57.9±9.6 | 60.6±8.7 |
| MCTQ (n=64; 29 Male) | |||
| SOw (hh:min) | 23:15±01:20 | 23:29±01:12 | 23:00±01:26 |
| SOf (hh:min) | 23:40±01:15 | 23:53±01:17 | 23:24±01:11 |
| SEw (hh:min) | 06:13±00:47 | 06:21±00:46 | 06:03±00:48 |
| SEf (hh:min) | 07:43±01:31 | 07:53±01:33 | 7:32±01:28 |
| SDw (hh:min) | 06:57±01:15 | 06:52±01:17 | 07:03±01:14 |
| SDf (hh:min) | 08:03±01:29 | 08:00±01:44 | 8:07±01:09 |
| MSFsc (hh:min) | 03:16±01:05 | 03:25±01:07 | 03:05±01:02 |
Data are presented as mean±SD; MEQ: Morningness-Eveningness Questionnaire score; MCTQ: Munich Chronotype questionnaire score; SOw: sleep onset workdays; SOf: sleep onset work-free days; SEw: sleep offset workdays; SEf: sleep offset work-free days; SDw: sleep duration workdays; SD: sleep duration work-free days; MSFsc: mid-sleep. GLM p-values:
p<0.01.
Dim light melatonin onset profiles, chronotype and questionnaire-derived sleep characteristics.
Mean DLMO time (h±min) was 20:03±01:21 (Table 1). The GLM analysis adjusted by age revealed a sex effect [F(1,72)=6.82; p=0.01=; observed power=0.73]. The effect size, as indexed by partial eta-squared (η2p), was 0.10. DLMO time was significantly earlier in the male participants (19:38±01:16 vs 20:24±01:21, p=0.01); 95%CI [11.4, 85.4]. To explore our hypothesis that the relatively greater morningness in the Baependi population is associated with a correspondingly early circadian phase, we present in Figure 1 a comparison between DLMO time from our dataset and previously published studies performed in non-clinical populations of similar age and an unrestricted sleep schedule.
Figure 1:
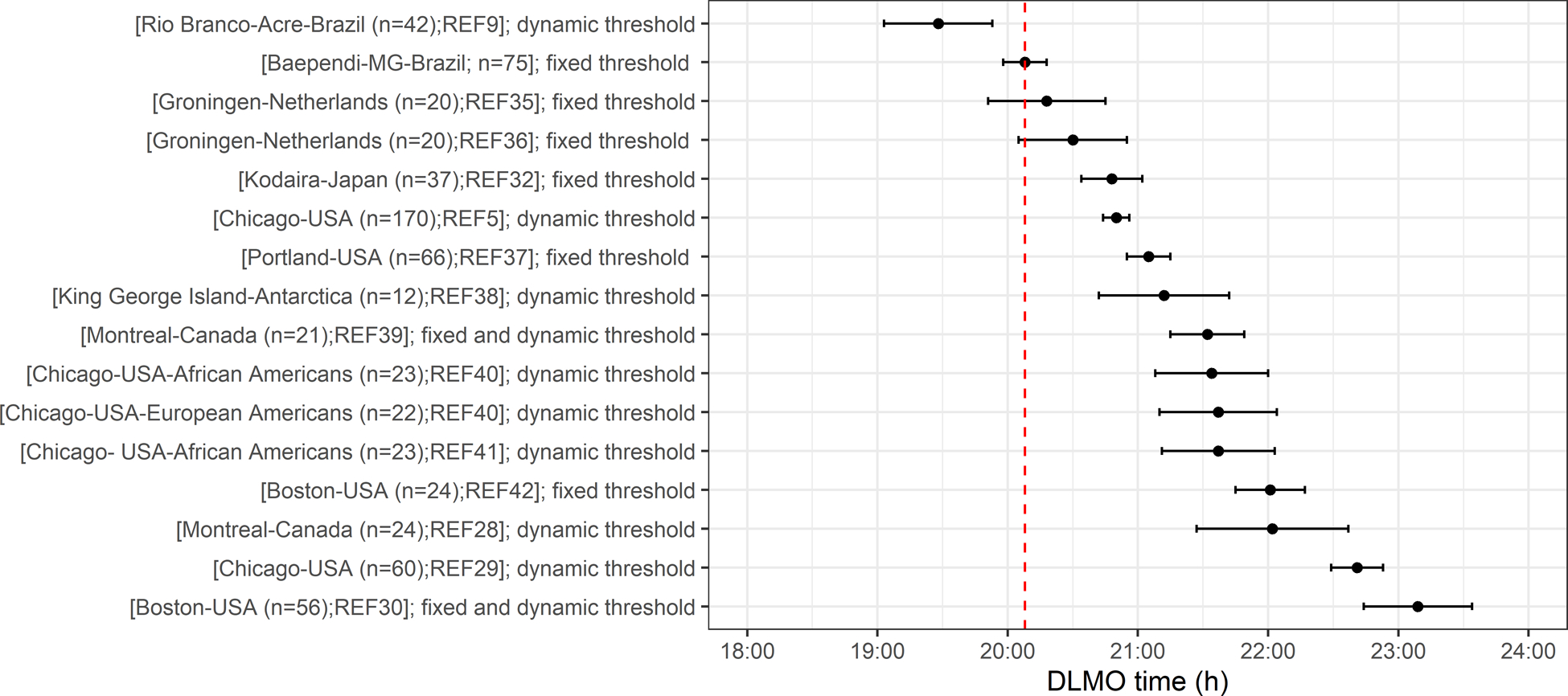
DLMO for the Baependi population (mean is marked with red dotted line) compared with published DLMO data from other adult populations9,28–30,32,35–42. Data are presented as mean±SEM. Different methodologies for determining DLMO are shown as follows: Dynamic threshold refers to defining DLMO based on two standard deviations above the mean of three or more pre-rise values or from parameters describing a fitted curve to the time series of melatonin samples. Fixed threshold refers to analyzing DLMO based on a time of attaining a 1 or 3 pg/ml level for saliva or a comparable 10 pg/ml for plasma. The threshold used in the present study was 3 pg/ml.
Two different assessments for chronotype were considered for this analysis, MEQ score and the phase of entrainment (the time of MSFsc) derived from the MCTQ. No significant sex difference was observed for MEQ score or MSFsc (Table 1). Furthermore, sleep timing assessed by the MCTQ was similar between females and males.
The GLM repeated measure analysis adjusted by age revealed differences in sleep end [F(1,63)=16.3; p=0.01; observed power=0.98, η2p = 0.22], and sleep duration [F(1,63)=14.2; p=0.01; observed power=0.95, η2p = 0.20]. In general, participants reported waking up earlier on work days (06:13±00:47 vs 7:47±01:31, p=0.01) compared to work-free days; 95%CI [73.6, 115.2]. In addition, participants slept longer on work-free days (8:06±1:29) compared to work days (6:57±1:15); 95%CI. [49, 91.8]. The relationship between DLMO time and MCTQ sleep onset and offset variables is presented in Figure 2A.
Figure 2:
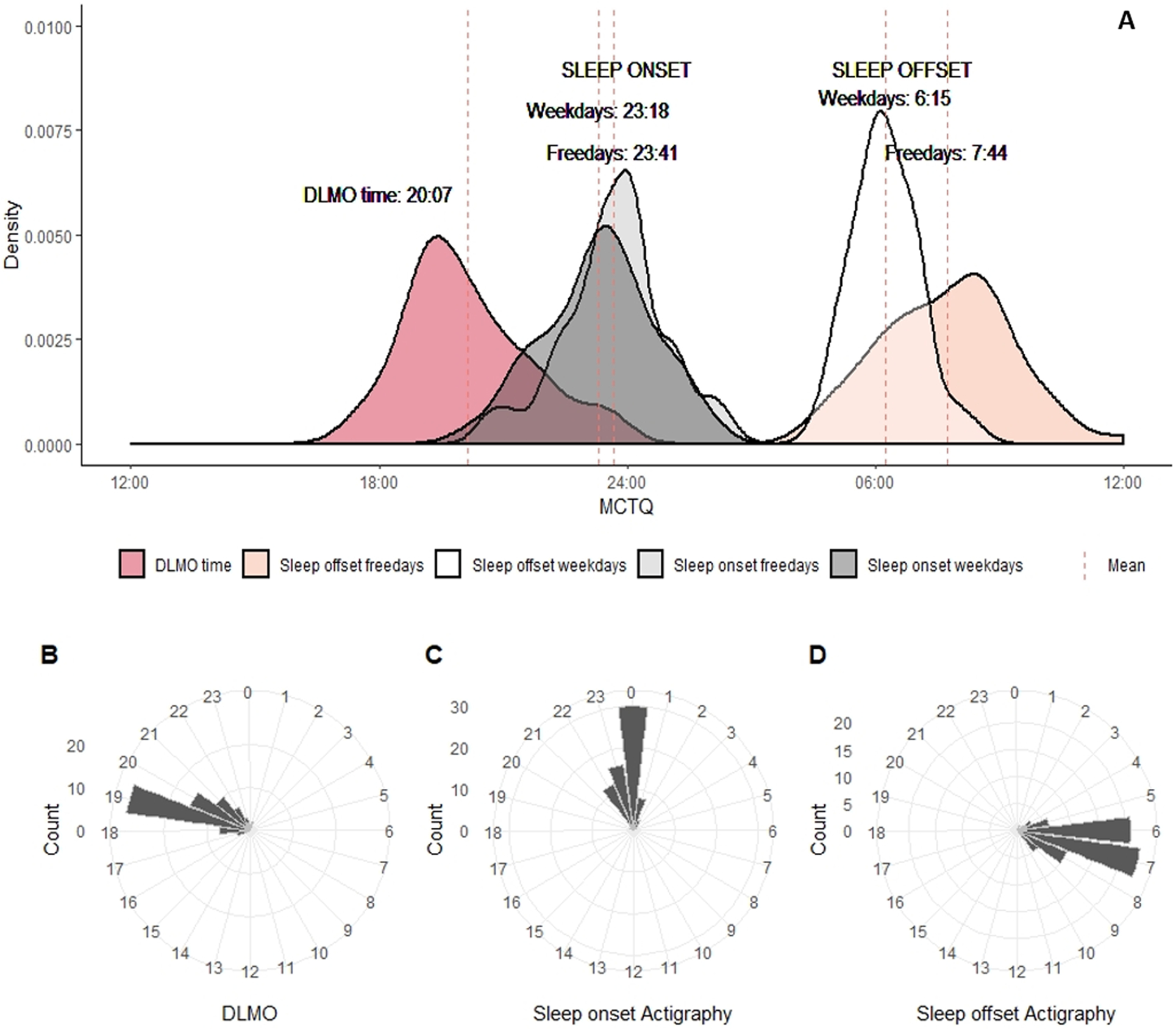
Relationship between DLMO time and sleep schedules obtained from MCTQ and actigraphy. (A) Density plots representing DLMO time compared with reported sleep habits at work days and work-free days from MCTQ; (B) Daily hour circular plots representing DLMO time, (C) sleep onset from actigraphy, (D) sleep offset from actigraphy. Bar heights are proportional to the number of individuals with DLMO, sleep onset and offset time from actigraphy within each hour.
To test for associations between DLMO and chronotype and sleep timing, correlational analysis was performed. We found that DLMO significantly correlated with MEQ (r=−0.30, p=0.02=), the higher the MEQ scores, the earlier the DLMO, as shown in Figure 3A. MSW and MSF also correlated with DLMO (r=0.25, p=0.04= and r=0.27, p=0.03=), but not MSFsc (=0.25, p=0.13=.), the later the DLMO, the later the mid-sleep phase, as shown in Figure 3B–D. Similar results were found for SOf, where later DLMO time was associated with later sleep onset on work-free days (r=0.27, p=0.03=.), as shown in Figure 3E–F.
Figure 3:
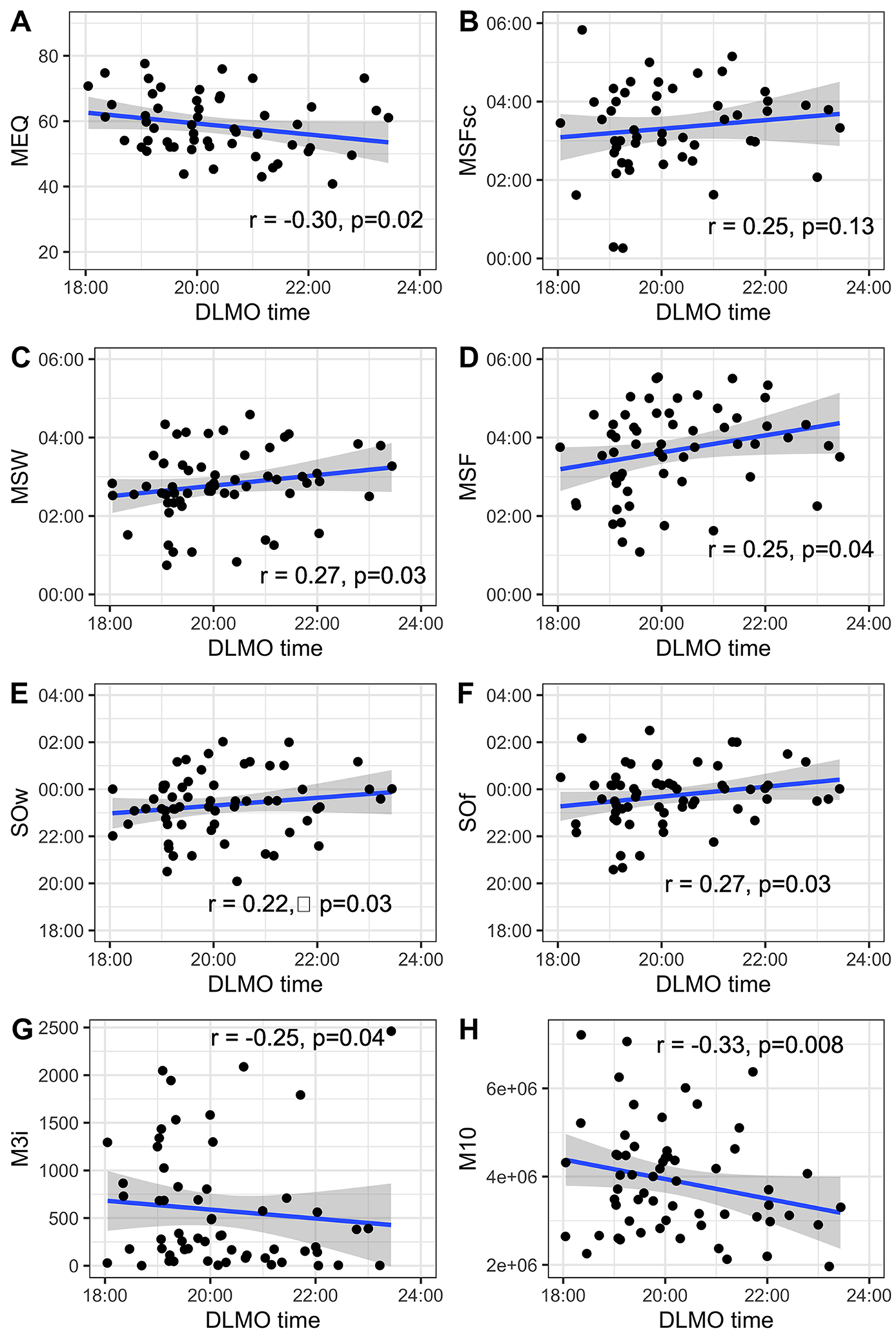
Associations between DLMO and (A) morningness-eveningness questionnaire score (MEQ), (B) mid-sleep on free days corrected for sleep debts on workdays (MSFsc), (C) mid-sleep on workdays (MSW), (D) mid-sleep on work-free days (MSF), (E) sleep onset weekdays (SOw), (F) sleep onset on work-free days (SOf), (G) total light exposure within 3 hour post sunrise (M3i), (H) diurnal motor activity (M10). The blue line represents the linear association between each pair of variables. Pearson’s correlation coefficients and p-values for each analysis are shown in the corresponding graph.
Actigraphy derived variables and Dim Light Melatonin Onset
Table 2 summarises the actigraphy-derived variables. Sex differences were not observed for sleep onset time, measured through actigraphy. Females presented longer total sleep time in comparison to males (7.11 hours vs. 6.39 hours [F(1,66)=5.48; p=0.02.; observed power=0.64, η2p = 0.08]. Figure 2B–D shows circular histograms to illustrate the relationship between DLMO and sleep onset and offset obtained by actigraphy. GLM analysis revealed a sex effect on sleep onset phase angle (i.e., the timing of sleep onset relative to DLMO) [F(1,66)=7.26; p=0.01; observed power=0.75, η2p = 0.10]. The phase angle to sleep onset observed with actigraphy was larger in men than in women (4.1 hours vs 3.2 hours, p=0.009.; 95%CI [17.1, 115.2]). Figure 4 depicts the individual differences in DLMO and phase-relation with nighttime sleep. Figure 5A presents the mean profile of the activity pattern comparing both sexes. The GLM analysis revealed a sex effect on diurnal motor activity (M10) [F(1,62)=21.55; p=0.001; observed power=0.99, η2p = 0.26]. Figure 5B represents the mean profile of light exposure. GLM analysis also revealed a sex effect on diurnal light exposure (M16lu) [F(1,57)=11.13; p=0.002=0.; observed power=0.90, η2p = 0.17] and on light exposure within the first 3 hours after sunrise (M3i) [F(1,61)=10.95; p=0.002; observed power=0.90, η2p = 0.16]. In summary, male participants were significantly more active (4.7×106 ± 1.3×106 vs 3.3×106 ± 0.9×106, p=0.001; 95%CI [0.74×106, 1.87×106]) and were exposed to higher amounts of light (3808 vs 3195, p=0.002; 95%CI [242.9, 974.2]), mainly during the first 3 hours after sunrise (877 vs 359, p=0.002; 95%CI [196.5, 797.4]). Correlational analysis indicated association between DLMO and higher light exposure (r=−0.25, p=0.04=) and activity (r=−0.33, p=0.008; Figure 3G–H). Furthermore, we also observed associations between DLMO and the rest onset phase angle (i.e., the timing of the beginning of rest phase relative to DLMO; r=−0.74, p=0.01), and the active onset phase angle (i.e., the timing of the beginning of the active phase relative to DLMO; r=0.61, p=0.01).
Table 2:
Activity, light, and sleep characteristics derived from actigraphy
| Total (n=67) | Female (n=35) | Male (n=32) | |
|---|---|---|---|
| Sleep onset time (hh:min) | 23:46±01:09 | 23:42±01:12 | 23:50±01:05 |
| Sleep offset time (hh:min) | 06:56±01:20 | 7:10±01:22 | 6:41±01:17 |
| Sleep duration (hh:min) | 06:56±00:53 | 7:11±00:44 | 6:39±00:57* |
| Diurnal motor activity | 3.9 × 106 ± 1.2 × 106 | 3.3 × 106 ± 0.9 × 106 | 4.7 × 106 ± 1.3 × 106 ** |
| Nocturnal motor activity | 3.7 × 104 ± 2.0 × 104 | 3.5 × 104 ± 1.9 × 104 | 4.1 × 104 ± 2.1 × 104 |
| Intradaily variability | 0.93±0.30 | 0.97±0.17 | 0.90±0.40 |
| Interdaily stability | 0.60±0.49 | 0.61±0.49 | 0.60±0.49 |
| Relative amplitude | 0.9764±0.0154 | 0.9770±0.01573 | 0.9759±0.01528 |
| Beginning of rest phase | 24:47±01:13 | 24:53±01:24 | 24:40±00:59 |
| Beginning of active phase | 08:39±01:55 | 08:57±02:00 | 08:19±01:49 |
| Diurnal light exposure | 349.2±747.1 | 3195.1±602.9 | 3808.1±763.1** |
| Total light exposure within 3-hour post sunrise | 601.6±654.2 | 359.1±520.1 | 877.5±689.2** |
| Phase angle between DLMO and sleep onset | 3:42±1:46 | 3:16±1:53 | 4:11±1:31** |
| Phase angle between DLMO and beginning of rest phase | 4:43±1:49 | 3:25±2:00 | 5:02±1:33 |
| Phase angle between DLMO and beginning of active phase | 11:24±2:26 | 11:29±2:32 | 11:18±2:21 |
Data are presented as mean±SD; GLM:
p<0.01.
Figure 4:
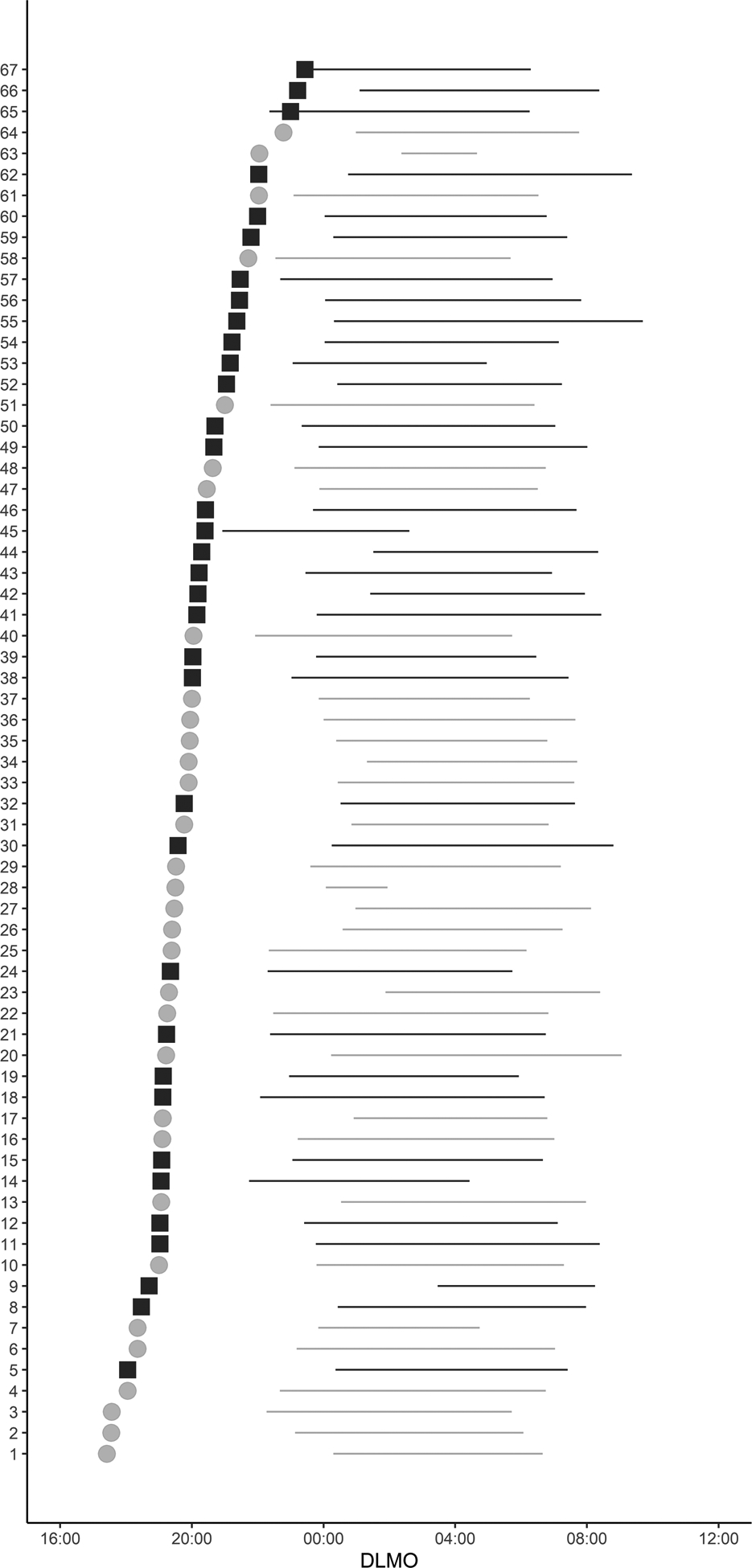
Individual differences in DLMO and its relationship to habitual nighttime sleep. DLMO time is represented by filled squares for women (black) and filled circles for men (grey). To represent the habitual sleep schedule the average of sleep episodes obtained using actigraphy plotted using horizontal bars. Bar heights are proportional to the number of individuals with DLMO, sleep onset and offset time from actigraphy within each hour. Data are ordered from earliest to latest DLMO. Four participants were excluded from the figure due to missing actigraphy data.
Figure 5:
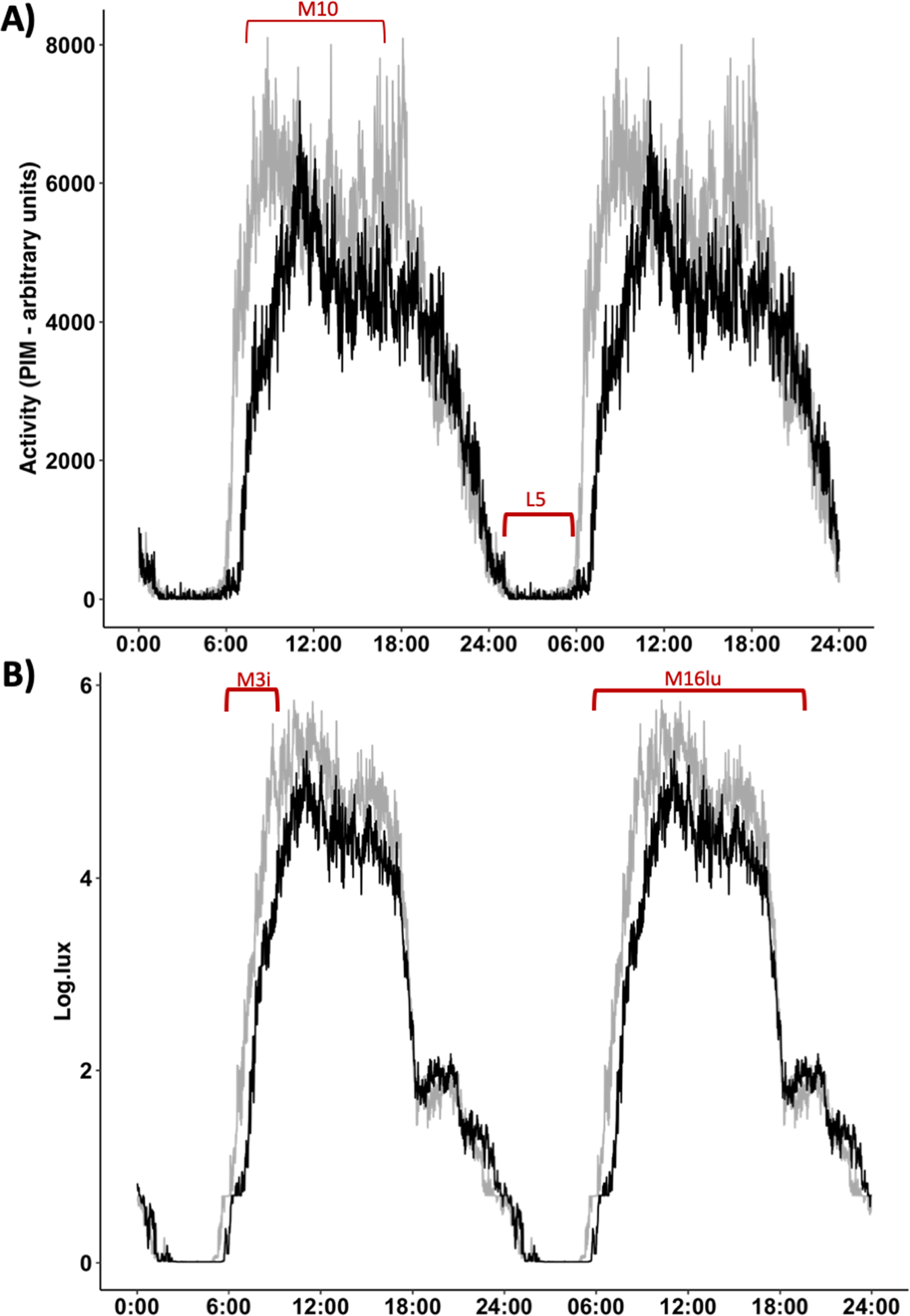
Double plotted 24-h average activity (arbitrary units) and light exposure (lux) measured in 60-s epochs using actigraphy devices. Means of each epoch for 5 days of recordings are presented. Men (grey) begin their activity earlier than women (black) and display higher amount of activity during the most active ten-hour period (M10) (A). Light exposure during the first three hours after sunrise was higher for men in comparison to women, and total light exposure during consecutive 16 hours (M16lu) also was higher for men in comparison to women (B).
Light exposure and activity patterns in the population were also associated with earlier sleep. We found that light exposure within the first 3 hours after sunrise (M3i) correlated with objective sleep onset (r=−0.53; p=0.001=), MSF (r=−0.39; p=0.01) and MSFsc (r=−0.36; p=0.01), and RA (r=0.52; p=0.01) as shown in Supplemental Figure 1A–D. Similarly, activity during the most active ten-hour period (M10) was correlated with objective sleep onset (r=−0.31; p=0.003), MSF (r=−0.47; p=0.001), as well MSFsc (r=−0.48; p=0.001), and RA (r=−0.41; p=0.01), as shown in Supplemental Figure 1E–H.
As DLMO was correlated with light exposure, chronotype, and sleep timing, we applied a regression analysis to assess which of these variables would better predict the DLMO timing. The model using MEQ, MSF, MSW, SOw, SOf, M10 and light exposure during the 3 hours after sunrise (M3i), age and sex to predict DLMO explained 44% of the variance in DLMO (p=0.02). The strongest significant predictor of DLMO was MEQ (beta = −0.63, p=0.004), followed by age (beta = 0.52, p=0.01); Durbin-Watson = 1.69. Sex, light exposure, sleep onset and mid-sleep were not significant predictors.
Discussion
We show that the tendency towards morningness in Baependi cohort is not only behavioural, but has physiological correlates. Notably, to the best of our knowledge, this is the largest study accessing the circadian phase under habitual sleep/wake schedules using the gold standard measure, DLMO, and describing its association with sleep and nonparametric derived variables from actigraphy during a short and defined period (the one larger sample presented in Figure 1 represents pooled data from multiple studies performed over a number of years in the same location)5. Based on methodological differences and on the large inter-individual differences in melatonin production,5 the comparison between DLMO time across different studies requires some caution, and the figure has been annotated with information about the different methods used in each study. Our observations confirm the hypothesis that morningness in Baependi is associated with an early phase in objective measurements (DLMO, actigraphy) and with early light exposure. The only previously reported population-based DLMO earlier than the one observed here was observed in rubber tappers living in the Amazonian rainforest.9
Additionally, we present objective data describing the sleep characteristics (timing and duration), light exposure, and activity patterns of the same subset of participants from the Baependi Heart Study. Together, the DLMO evaluation and sleep description by actigraphy are in agreement with our previous findings described based on self-reports of both morningness-eveningness,13 and sleep timing.16 Located 1.5° North of the Tropic of Capricorn, the Baependi population is extensively exposed to the natural light/dark cycle, with high exposure to morning sunlight, as we will discuss below.
The role of light as zeitgeber is very well established, and humans naturally synchronize to sun time. We have demonstrated clear associations between DLMO and light exposure pattern (the diurnal light exposure - M16lu) and higher exposure to light in the early morning (M3i). This is consistent with circadian phase, and thus DLMO, being entrained to the solar light-dark cycle.1,2 The non-parametric analysis of actigraphy allows us to infer properties of circadian timing system. According to Gonçalves and colleagues,24 the most active 10-hour period and the least active 5-hour period represent key behavioural outputs of the circadian timing, and indices of the synchronisation of the circadian timing system to the external world. We found robust correlation between M10 and DLMO, as well as between M10 and sleep onset. The evidence of association between the nonparametric variables and DLMO substantially support the Gonçalves model.24
We observed earlier average DLMO times in men than in women. This was concomitant with earlier activity and higher morning light exposure. Thus, our study does not provide any evidence suggesting that this difference is biological as opposed to behaviour-dependent. Indeed, our findings contrast to the published literature, which either reports no sex difference in DLMO or earlier timings in women.5,28–31
DLMO is an extremely precise measure of circadian phase. Although we were able to collect it from a relatively large sample of the population, collecting DLMO data is resource intensive and not feasible in many studies. We therefore sought to investigate how well the questionnaire data from the same population were able to predict our measured DLMO values. Only two studies published to date have compared MEQ, MCTQ, and DLMO in the same individuals. Kitamura and colleagues32 assessed DLMO in 37 middle-aged adults (both sexes) from hourly saliva samples that the participants individually collected unsupervised in their homes. The results indicated a stronger association between MSFsc and DLMO than MEQ and DLMO, suggesting that MSFsc better reflects endogenous circadian timing compared with the MEQ score. Another study conducted by Kantermann and colleagues33 assessed DLMO in more controlled sampling conditions, but included not only normal controls (n=36) but also individuals diagnosed with delayed sleep phase disorder (DSPD) reported similar results. MEQ is a scale that is based on questions around preferred timings of sleep and waking activities, whereas MCTQ asks about actual timings during weeks and weekends. As DLMO was assessed during work days, the phase of entrainment reported by the MSFsc was not associated with the biological marker of phase (DLMO). Our results highlight that diurnal preference (MEQ) better reflects endogenous circadian timing compared with MCTQ in the conditions reported here. A possible explanation is that the chronotype measured by MCTQ will be more likely to change depending on social cues (work or entertainment)34. It has been proposed that industrialisation has caused a chronic phase delay of rest-activity rhythms8,10–12. A phase difference in sleep timing has been described when groups of people were compared who lived in very similar conditions apart from the presence or absence of electricity6,8,9. The only population study showing an earlier average DLMO than what we report here was performed in participants living without access to electricity (see Figure 1)9. Although fully electrified, Baependi is a small rural town whose inhabitants experience a high degree of exposure to the daylight. In summary, we observed an early circadian phase in Baependi, which is consistent with multiple previous behavioural observations of sleep-wake timing and chronotype.
Supplementary Material
Supplemental Figure 1: Associations between light exposure within the first 3 hours after sunrise (M3i) and (A) sleep onset time obtained using actigraphy, (B) mid-sleep on free days (MSF), (C) mid-sleep on free days corrected for sleep debts on workdays (MSFsc); Associations between activity (M10) with (E) sleep onset time obtained using actigraphy, (F) mid-sleep on free days (MSF), (G) mid-sleep on free days corrected for sleep debts on workdays (MSFsc). The blue line represents the linear association between each pair of variables. Pearson’s correlation coefficients and p values for each analysis are shown in the corresponding graph.
Acknowledgments
The authors are thankful to the Municipal Council of Baependi for logistical support and assistance with field work, the dedicated staff at the field station and the population of Baependi for their participation in the Baependi Heart Study. This study was supported by awards from the Academy of Medical Sciences/Newton International Fellowship to F Ruiz (NIF004\1030), from CNPq to H Vallada (400791/2015-5), from FAPESP to AC Pereira, JE Krieger, and M Pedrazzoli (grants 2007/58150-7, 2010/51010-8, 2013/17368-0), from Fundação Zerbini and Hospital Samaritano, by the Global Innovation Initiative to M von Schantz (jointly funded by the British Council and the UK Department of Business and Skills), by the National Institutes of Health (R01HL 141881), and from the Institute of Advanced Studies of University of Surrey for F Beijamini.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
REFERENCES
- 1.Eastman CI, Martin SK. How to use light and dark to produce circadian adaptation to night shift work. Ann Med. 1999;31(2):87–98. [DOI] [PubMed] [Google Scholar]
- 2.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(Pt 3):945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewy AJ. Melatonin as a marker and phase-resetter of circadian rhythms in humans. Adv Exp Med Biol. 1999;460:425–434. [DOI] [PubMed] [Google Scholar]
- 4.Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17(2):181–193. [DOI] [PubMed] [Google Scholar]
- 5.Burgess HJ, Fogg LF. Individual differences in the amount and timing of salivary melatonin secretion. PLoS One. 2008;3(8):e3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beale AD, Pedrazzoli M, Goncalves B, et al. Comparison between an African town and a neighbouring village shows delayed, but not decreased, sleep during the early stages of urbanisation. Sci Rep. 2017;7(1):5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peixoto CA, da Silva AG, Carskadon MA, Louzada FM. Adolescents living in homes without electric lighting have earlier sleep times. Behav Sleep Med. 2009;7(2):73–80. [DOI] [PubMed] [Google Scholar]
- 8.de la Iglesia HO, Fernandez-Duque E, Golombek DA, et al. Access to Electric Light Is Associated with Shorter Sleep Duration in a Traditionally Hunter-Gatherer Community. J Biol Rhythms. 2015;30(4):342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno CR, Vasconcelos S, Marqueze EC, et al. Sleep patterns in Amazon rubber tappers with and without electric light at home. Sci Rep. 2015;5:14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yetish G, Kaplan H, Gurven M, et al. Natural sleep and its seasonal variations in three pre-industrial societies. Curr Biol. 2015;25(21):2862–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samson DR, Manus MB, Krystal AD, Fakir E, Yu JJ, Nunn CL. Segmented sleep in a nonelectric, small-scale agricultural society in Madagascar. Am J Hum Biol. 2017;29(4). [DOI] [PubMed] [Google Scholar]
- 12.Pilz LK, Levandovski R, Oliveira MAB, Hidalgo MP, Roenneberg T. Sleep and light exposure across different levels of urbanisation in Brazilian communities. Sci Rep. 2018;8(1):11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Schantz M, Taporoski TP, Horimoto AR, et al. Distribution and heritability of diurnal preference (chronotype) in a rural Brazilian family-based cohort, the Baependi study. Sci Rep. 2015;5:9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.IBGE. Instituto Brasileiro de Geografia e Estatistica. Estimativa de populacao dos Estados e Municipios - prazos e procedimentos. https://www.ibge.gov.br/cidades-e-estados/mg/baependi.html. 2020. [Google Scholar]
- 15.Münch M, Wirz-Justice A, Brown SA, et al. The Role of Daylight for Humans: Gaps in Current Knowledge. Clocks & Sleep. 2020;2: 61–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beijamini F, Knutson KL, Lorenzi-Filho G, et al. Timing and quality of sleep in a rural Brazilian family-based cohort, the Baependi Heart Study. Sci Rep. 2016;6:39283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Oliveira CM, Pereira AC, de Andrade M, Soler JM, Krieger JE. Heritability of cardiovascular risk factors in a Brazilian population: Baependi Heart Study. BMC Med Genet. 2008;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egan KJ, von Schantz M, Negrao AB, et al. Cohort profile: the Baependi Heart Study-a family-based, highly admixed cohort study in a rural Brazilian town. BMJ Open. 2016;6(10):e011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 20.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18(1):80–90. [DOI] [PubMed] [Google Scholar]
- 21.Arendt J Melatonin: characteristics, concerns, and prospects. J Biol Rhythms. 2005;20(4):291–303. [DOI] [PubMed] [Google Scholar]
- 22.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 23.Marino M, Li Y, Rueschman MN, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goncalves BS, Adamowicz T, Louzada FM, Moreno CR, Araujo JF. A fresh look at the use of nonparametric analysis in actimetry. Sleep Med Rev. 2015;20:84–91. [DOI] [PubMed] [Google Scholar]
- 25.Wickham H ggplot2 In: Elegant Graphics for Data Analysis. 2 ed.: Springer International Publishing; 2016. [Google Scholar]
- 26.IBGE. Classifiação e caracterização dos espaços rurais e urbanos do Brasil. In: Instituto Brasileiro de Geografia e Estatística 2017: https://www.ibge.gov.br/apps/rural_urbano/. [Google Scholar]
- 27.Roenneberg T, Kumar CJ, Merrow M. The human circadian clock entrains to sun time. Curr Biol. 2007;17(2):R44–45. [DOI] [PubMed] [Google Scholar]
- 28.Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in morningness-eveningness. J Biol Rhythm. 2004;19(3):248–257. [DOI] [PubMed] [Google Scholar]
- 29.Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res. 2005;14(3):229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cain SW, Dennison CF, Zeitzer JM, et al. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms. 2010;25(4):288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Reen E, Sharkey KM, Roane BM, et al. Sex of college students moderates associations among bedtime, time in bed, and circadian phase angle. J Biol Rhythms. 2013;28(6):425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitamura S, Hida A, Aritake S, et al. Validity of the Japanese version of the Munich ChronoType Questionnaire. Chronobiol Int. 2014;31(7):845–850. [DOI] [PubMed] [Google Scholar]
- 33.Kantermann T, Sung H, Burgess HJ. Comparing the Morningness-Eveningness Questionnaire and Munich ChronoType Questionnaire to the Dim Light Melatonin Onset. J Biol Rhythms. 2015;30(5):449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roenneberg T, Pilz LK, Zerbini G, EC. W. Chronotype and Social Jetlag: A (Self-) Critical Review. Biology (Basel). 2019;8(3):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wams EJ, Woelders T, Marring I, et al. Linking Light Exposure and Subsequent Sleep: A Field Polysomnography Study in Humans. Sleep. 2017;40(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woelders T, Beersma DGM, Gordijn MCM, Hut RA, Wams EJ. Daily Light Exposure Patterns Reveal Phase and Period of the Human Circadian Clock. J Biol Rhythms. 2017;32(3):274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emens JS, Yuhas K, Rough J, Kochar N, Peters D, Lewy AJ. Phase angle of entrainment in morning- and evening-types under naturalistic conditions. Chronobiol Int. 2009;26(3):474–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva A, Simón D, Pannunzio B, Casaravilla C, Díaz A, Tassino B. Chronotype-Dependent Changes in Sleep Habits Associated with Dim Light Melatonin Onset in the Antarctic Summer. Clocks & Sleep. 2019;1(3):352–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawinska A, Dumont M, Selmaoui B, Paquet J, Carrier J. Are modifications of melatonin circadian rhythm in the middle years of life related to habitual patterns of light exposure? J Biol Rhythms. 2005;20(5):451–460. [DOI] [PubMed] [Google Scholar]
- 40.Eastman CI, Suh C, Tomaka VA, Crowley SJ. Circadian rhythm phase shifts and endogenous free-running circadian period differ between African-Americans and European-Americans. Sci Rep. 2015;5:8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eastman CI, Tomaka VA, Crowley SJ. Circadian rhythms of European and African-Americans after a large delay of sleep as in jet lag and night work. Sci Rep. 2016;6:36716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pullman RE, Roepke SE, Duffy JF. Laboratory validation of an in-home method for assessing circadian phase using dim light melatonin onset (DLMO). Sleep Med. 2012;13(6):703–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Associations between light exposure within the first 3 hours after sunrise (M3i) and (A) sleep onset time obtained using actigraphy, (B) mid-sleep on free days (MSF), (C) mid-sleep on free days corrected for sleep debts on workdays (MSFsc); Associations between activity (M10) with (E) sleep onset time obtained using actigraphy, (F) mid-sleep on free days (MSF), (G) mid-sleep on free days corrected for sleep debts on workdays (MSFsc). The blue line represents the linear association between each pair of variables. Pearson’s correlation coefficients and p values for each analysis are shown in the corresponding graph.


