Abstract
Context:
Goal-concordant care is an important indicator of high-quality care in serious illness.
Objectives:
To estimate the prevalence of patient-reported receipt of goal-concordant care among seriously ill outpatients and identify factors associated with absence of patient-reported goal-concordance.
Methods:
Analysis of enrollment surveys from a multi-center cluster-randomized trial of outpatients with serious illness. Patients reported their prioritized healthcare goal and the focus of their current medical care; these items were matched to define receipt of goal-concordant care.
Results:
Of 405 patients with a prioritized healthcare goal, 58% reported receipt of goal-concordant care, 17% goal-discordant care, and 25% were uncertain of the focus of their care. Patient-reported receipt of goal-concordance differed by patient goal. For patients who prioritized extending life, 86% reported goal-concordant care, 2% goal-discordant care, and 12% were uncertain of the focus of their care. For patients who prioritized relief of pain and discomfort, 51% reported goal-concordant care, 21% goal-discordant care, and 28% were uncertain of the focus of their care. Patients who prioritized a goal of relief of pain and discomfort were more likely to report goal-discordant care than patients who prioritized a goal of extending life (RRR 22.20; 95%CI 4.59, 107.38).
Conclusion:
Seriously ill outpatients who prioritize a goal of relief of pain and discomfort are less likely to report receipt of goal-concordant care than patients who prioritize extending life. Future interventions designed to improve receipt of goal-concordant care should focus on identifying patients who prioritize relief of pain and discomfort and promoting care aligned with that goal.
Keywords: goal-concordant care, goals-of-care, goals, values, preferences, palliative care, serious illness, end-of-life, advance care planning (ACP)
INTRODUCTION
Patients, families, and clinicians agree that seriously ill patients ought to receive goal-concordant care, that is, medical care that aligns with patients’ values, goals, and life priorities.1,2 Goal-concordant care has been rated as an indicator of high-quality care in serious illness and an important outcome defining successful advance care planning.3-5 Despite its importance, no single measurement strategy has received widespread support.3,6-8 Several strategies to measure goal-concordant care in serious illness have been used, each with limitations.3,8 Surveying bereaved caregivers' impressions of goal-concordant care is limited by potentially inadequate caregiver knowledge of patient goals and by recall bias.3,8-10 The comparison of advance directives with subsequent care documented in the electronic health record (EHR) is limited by the inherent vagueness of advance directives, poor documentation in the EHR, and potential changes in goals over time.11-13 Moreover, retrospective assessment of the EHR for alignment between patients’ goals and received treatments may be difficult because raters often disagree about the goals that treatments aim to achieve.8,14 In addition, none of these strategies allow for assessment of goal-concordant care in real-time, thus limiting opportunities to improve communication and delivery of goal-concordant care for the patients in whom the measure was assessed.
One strategy to assess goal-concordant care for seriously ill patients in real-time was developed in the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT).15-17 In this strategy, patients first indicated their prioritized healthcare goal, choosing between extending life versus relief of pain and discomfort; they then indicated whether the medical care they were currently receiving was best described as focusing on extending life or relief of pain and discomfort. Matching responses to these two items defined goal-concordant care. While this strategy has limitations, including that it is solely based on patients’ perceptions of the focus of their care, unique advantages such as real-time assessment and firsthand knowledge of patients' goals at the time of assessment make it an attractive option. Moreover, patients’ perceptions of whether the focus of their care is consistent with their goals may be as important as whether the focus of their care is consistent with their goals. Using this strategy to assess goal-concordant care, the SUPPORT investigators found about 60% of seriously ill hospitalized patients (or their surrogates) reported goal-concordant care, with differential reports of concordance by goal: 86% of patients whose top priority was extending life and 41% of patients whose top priority was relief of pain and discomfort reported goal-concordant care.17
To our knowledge, this strategy has not been used among outpatients with serious illness, a setting and time of great opportunity to improve communication and delivery of goal-concordant care for patients, many of whom may not desire hospitalization or aggressive treatments.18 The aim of this study was two-fold: 1) to estimate the prevalence of patient-reported receipt of goal-concordant care among seriously ill outpatients; and 2) to identify factors associated with the absence of patient-reported goal-concordant care.
METHODS
Study Design
This study is a secondary analysis of data collected from patient self-reported questionnaires collected at enrollment of a multi-center cluster-randomized trial of a patient and clinician communication priming intervention designed to improve goals-of-care communication in seriously ill outpatients.19-23 Institutional review boards at all sites approved the study, and all participants provided written informed consent.
Population and Setting
The parent randomized trial includes clinicians in outpatient settings and their patients with serious illness who were recruited from two large health systems in the Pacific Northwest. Eligible clinicians were physicians or nurse practitioners who provided primary or specialty care and who had five or more eligible patients in their patient panel. Clinicians were recruited by phone and in person between February 2014 and November 2015. Using clinic schedules and the EHR, consecutive eligible patients cared for by the participating clinician were identified and recruited between March 2014 and May 2016. Eligible patients were 18 years of age or older, had at least two visits with the participating clinician in the prior 18 months, and had one or more qualifying diagnosis or condition. Qualifying diagnoses and conditions were chosen to identify patients with a median estimated survival of approximately 2 years or for whom a goals-of-care discussion would be indicated.24-28 Eligible diagnoses included: (1) advanced cancer, defined by either metastatic cancer or inoperable lung cancer; (2) chronic lung disease, defined by either COPD with FEV1 <35% predicted or oxygen dependence, restrictive lung disease with TLC <50% predicted, or cystic fibrosis with FEV1 <30% predicted; (3) heart failure, defined by either NYHA Class III or IV heart failure, pulmonary arterial hypertension with six minute walk distance of <250 meters, LVAD, or ICD implant; (4) liver failure, defined by either Child’s Class C cirrhosis or MELD score >17; and (5) renal failure, defined as dialysis-dependent renal failure and diabetes. Eligible conditions included: (1) age 75 years or older and at least one life-limiting chronic illness (defined as any diagnosis listed above that was not severe enough for outright eligibility); (2) age 90 years or older; (3) hospitalization in the prior 18 months with a life-limiting chronic illness; and (4) Charlson comorbidity score of 6 or higher.
Data Collection
Data for this secondary analysis were obtained from patient questionnaires completed at enrollment (i.e., prior to the intervention) and from the EHR.
Outcome
The primary outcome was patient-reported receipt of goal-concordant care among patients with a prioritized healthcare goal. This was determined by matching patient responses on two survey items developed for the SUPPORT study.15-17 The first item asked patients about their current prioritized goal: "If you had to make a choice at this time, would you prefer a plan of medical care that focuses on extending life as much as possible, even if it means having more pain and discomfort, or would you want a plan of medical care that focuses on relieving pain and discomfort as much as possible, even if that means not living as long?" The second item asked patients about the focus of their current care: "Using those same categories, which of the following best describes the focus of the medical care you are currently receiving?" For both questions, patients could choose one of the two options (i.e., extending life… or relief of pain and discomfort…) or "I don't know/not sure". Although many patients may value both extending life and relief of discomfort, the forced choice structure of the question helps identify a patient’s top priority.29,30
Matching the three responses to these two questions resulted in nine possible response combinations (Table 1). We considered patients who were unsure of their prioritized goal to be ineligible for these analyses, as it is impossible to report receipt of goal-concordant care without a clear goal.6 For the remaining six possible combinations, we defined three groups: goal-concordant, goal-discordant, and focus-uncertain. We defined goal-concordant care as a match between a patient’s endorsed goal and perception of the focus of care received, goal-discordant care as a mismatch between a patient’s endorsed goal and perception of the focus of care received, and focus-uncertain as patients who reported a goal but were unsure of the focus of their care.
Table 1.
Coding of patient response combinations to prioritized healthcare goal and focus of current care questionsa
| Prioritized Healthcare Goal |
Focus of Current Care | ||
|---|---|---|---|
| Extending Life | Relief of Pain and Discomfort |
Unsure | |
| Extending Life | Goal-Concordant | Goal-Discordant | Focus-Uncertain |
| Relief of Pain and Discomfort | Goal-Discordant | Goal-Concordant | Focus-Uncertain |
| Unsure | Ineligible | Ineligible | Ineligible |
Goal-concordant group bolded. See text for exact wording of survey questions.
Exposures
We selected eleven exposure variables a priori to be parsimonious and minimize multiple comparisons. Because of their associations with treatment preferences in prior studies,11 the following patient demographics were selected: age, gender, race/ethnicity (non-Hispanic white vs. minority), marital/partner status (partner vs. other), level of education, and self-perceived health status (rated as "poor", "fair", "good", "very good", or "excellent"). Additionally, we included a qualifying diagnosis of advanced cancer as a dichotomous variable. We hypothesized patients with advanced cancer may be less likely to report goal-concordant care than other eligible patients because the distinct pressure patients with advanced cancer and their clinicians feel to stay positive and "fight" may uniquely undermine and delay honest discussions about goals of care.31-36
We also examined prior goals-of-care discussions, hypothesizing that patients who have discussed their goals previously may be more likely to report receipt of goal-concordant care.22,37,38 We used two items from the enrollment questionnaires: 1) the occurrence of prior discussion(s) with the patient's enrolled clinician; and 2) the occurrence of prior discussion(s) with family or friends. For the prior discussion with their enrolled clinician, patients were asked: "Have you ever discussed with this doctor, in a face-to-face discussion, the kind of medical care you would want if you were too sick to speak for yourself?" Response options were "yes", "no", and "I don't know". The latter two were collapsed into a single response category to create a dichotomous variable. For prior discussions with family or friends, patients were asked: "Have you discussed with a family member or friend who is involved in your care the kind of medical care you would want if you were too sick to speak for yourself?" Response options were "yes", "no", "I don't know", and "not applicable, I don't have a family member or friend involved in my care at this time". We collapsed the latter three options into a single response category to create a dichotomous variable. These questions were previously validated.39-42
We also hypothesized that patients with higher levels of trust in their clinician would be more likely to report receipt of goal-concordant care.43 To measure trust, we used the single item from the short-form Wake Forest Trust-in-Physician Scale that assesses overall trust: "All in all, you have complete trust in this doctor".44 Responses ranged from 0 (strongly disagree) to 4 (strongly agree), and we dichotomized the responses to “less than complete trust” (ratings of 0-3) and “complete trust” (rating of 4). We have used this item previously.19
Finally, we examined whether a patient’s prioritized goal (extending life vs. relief of pain and discomfort) was associated with patient-reported receipt of goal-concordant care. Based on a prior study of seriously ill hospitalized patients,17 we hypothesized goal-concordant care would be more commonly reported by patients who prioritized a goal of extending life.
Statistical Analysis
We report complete case data from the sample of patients with a prioritized healthcare goal. To estimate confidence intervals around proportions of goals, perceptions of the focus of care, and goal-concordant and goal-discordant care, we ran intercept-only logit models and converted the logits to estimated proportions. To examine the association between exposure variables and the trichotomous outcome (i.e., goal-concordant, goal-discordant, focus-uncertain), we fit a single multivariate adjusted multinomial logistic regression model including all a priori exposure variables. We selected goal-concordant as the reference group for the multinomial model. Multinomial regression was chosen because there is no inherent ordering to the categories of the trichotomous outcome. All models clustered patients under clinicians to adjust standard errors for lack of independence between patients treated by the same clinician. We accepted a two-sided p-value of <0.05 as evidence of statistical significance. We used Stata Version 15.1 for all analyses.
RESULTS
Nine hundred and seventeen patients were identified as eligible for the randomized trial and 537 enrolled (Figure 1). Five hundred and twenty-eight of these patients answered both questions required to assess goal-concordant care. One hundred and twenty-one (23%) patients were unsure of their prioritized healthcare goal and excluded. Of the remaining patients, two were excluded for lack of response to all the a priori selected exposure variables, resulting in a complete case sample of 405 patients with a prioritized healthcare goal. These 405 patients had a median age of 76 years and about half were women. Most patients were non-Hispanic and white (78%). The most common qualifying diagnosis was advanced cancer (16%; Table 2).
Figure 1. Patient Flow Diagram.
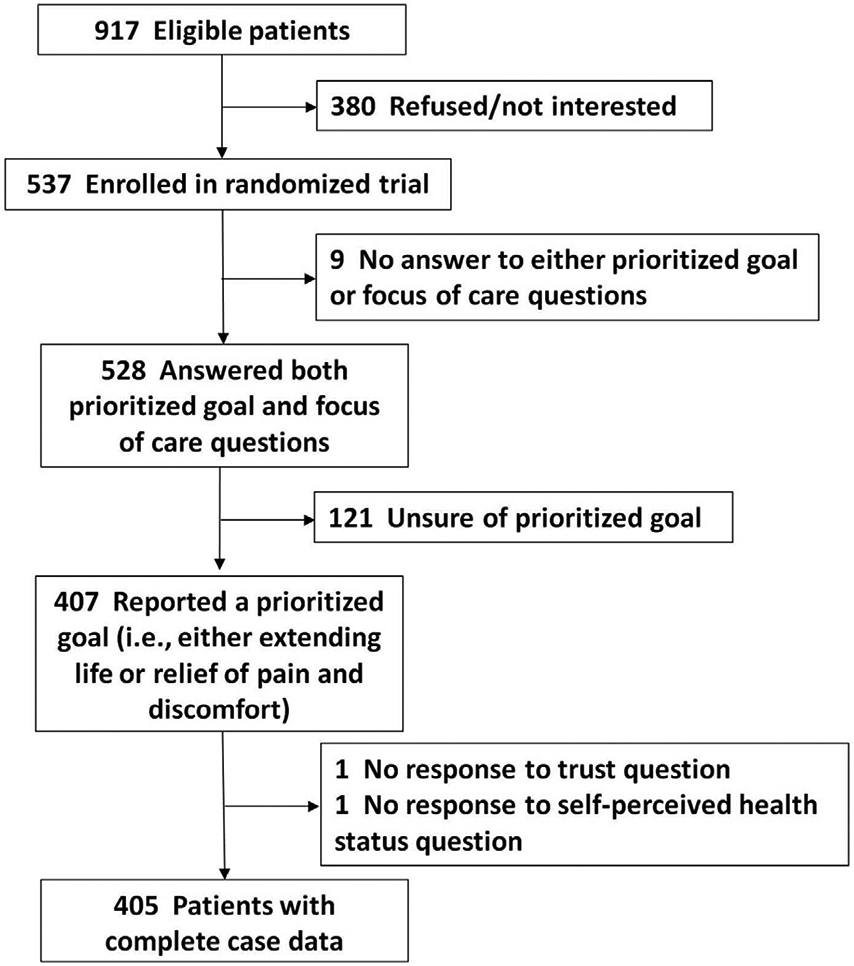
Table 2.
Characteristics of patients with a prioritized healthcare goal, by concordance group
| Characteristic | Total | Goal-Concordant | Goal-Discordant | Focus-Uncertain |
|---|---|---|---|---|
| Total, n (%) | 405 (100.0) | 236 (58.3) | 69 (17.0) | 100 (24.7) |
| Age (median, IQR) | 76.3 (17.5) | 75.3 (18.0) | 73.9 (16.9) | 78.6 (14.1) |
| Female | 198 (48.9) | 103 (43.6) | 34 (49.3) | 61 (61.0) |
| Racial/ethnic minority | 91 (22.5) | 55 (23.3) | 18 (26.1) | 18 (18.0) |
| Currently married or living with partner | 174 (43.0) | 103 (43.6) | 31 (44.9) | 40 (40.0) |
| Level of education | ||||
| 8th grade or less | 9 (2.2) | 4 (1.7) | 3 (4.4) | 2 (2.0) |
| Some high school | 26 (6.4) | 16 (6.8) | 2 (2.9) | 8 (8.0) |
| High school diploma or equivalent | 54 (13.3) | 39 (16.5) | 4 (5.8) | 11 (11.0) |
| Trade school or some college | 169 (41.7) | 99 (42.0) | 25 (36.2) | 45 (45.0) |
| 4-yr college degree | 70 (17.3) | 39 (16.5) | 17 (24.6) | 14 (14.0) |
| Some graduate school | 17 (4.2) | 8 (3.4) | 5 (7.3) | 4 (4.0) |
| Graduate degree | 60 (14.8) | 31 (13.1) | 13 (18.8) | 16 (16.0) |
| Self-perceived health status | ||||
| Poor | 63 (15.6) | 38 (16.1) | 6 (8.7) | 19 (19.0) |
| Fair | 122 (30.1) | 68 (28.8) | 25 (36.2) | 29 (29.0) |
| Good | 142 (35.1) | 88 (37.3) | 21 (30.4) | 33 (33.0) |
| Very good | 59 (14.6) | 33 (14.0) | 12 (17.4) | 14 (14.0) |
| Excellent | 19 (4.7) | 9 (3.8) | 5 (7.3) | 5 (5.0) |
| Charlson comorbidity score, median (IQR) | 7 (3) | 7 (2) | 7 (3) | 7 (2.5) |
| Qualifying diagnoses and conditions | ||||
| Diagnoses | ||||
| Advanced cancer | 63 (15.6) | 31 (13.1) | 21 (30.4) | 11 (11.0) |
| Chronic lung disease | 39 (9.6) | 25 (10.6) | 7 (10.1) | 7 (7.0) |
| Heart failure | 29 (7.2) | 19 (8.1) | 3 (4.4) | 7 (7.0) |
| Liver failure | 2 (0.5) | 2 (0.9) | 0 (0.0) | 0 (0.0) |
| Renal failure | 19 (4.7) | 11 (4.7) | 2 (2.9) | 6 (6.0) |
| Conditions | ||||
| Age 75+ yrs with chronic life-limiting illness | 150 (37.0) | 85 (36.0) | 24 (34.8) | 41 (41.0) |
| Age 90+ yrs | 32 (7.9) | 18 (7.6) | 1 (1.5) | 13 (13.0) |
| Hospitalized with serious condition in 18 months before study enrollment | 71 (17.5) | 45 (19.1) | 16 (23.2) | 10 (10.0) |
| Charlson comorbidity score of 6+ | 332 (82.0) | 190 (80.5) | 57 (82.6) | 85 (85.0) |
| Reported prior goals-of-care discussion with family or friend | 322 (79.5) | 197 (83.5) | 53 (76.8) | 72 (72.0) |
| Reported prior goals-of-care discussion with enrolled clinician | 122 (30.1) | 78 (33.1) | 22 (31.9) | 22 (22.0) |
| Complete trust in enrolled clinician | 318 (78.5) | 189 (80.1) | 53 (76.8) | 76 (76.0) |
Patient-reported goals, focus of care, and goal-concordant care
Of the 405 patients with a prioritized healthcare goal, 318 (79%; 95% CI 74-82%) prioritized relief of pain and discomfort, while 87 (21%; 95% CI 18-26%) prioritized extending life (Figure 2). One hundred and sixty-three (40%; 95% CI 36-45%) patients perceived the focus of their current care to be relieving pain and discomfort, while 142 (35%; 95% CI 30-40%) perceived the focus to be extending life and 100 (25%; 95% CI 21-29%) were unsure. Fifty-eight percent (95% CI 54-62%) reported receipt of goal-concordant care, while 17% (95% CI 14-21%) reported goal-discordant care. For patients with a prioritized goal of extending life, 86% (95% CI 77-92%) reported goal-concordant care, while 2% (95% CI 1-9%) reported goal-discordant care and 12% (95% CI 6-20%) were uncertain of the focus of their care. Among patients with a prioritized goal of relief of pain and discomfort, 51% (95% CI 46-55%) reported goal-concordant care, while 21% (95% CI 17-26%) reported goal-discordant care and 28% (95% CI 24-33%) expressed uncertainty about the focus of their care (Figure 2).
Figure 2. Sunburst chart of prioritized healthcare goal (inner circle) and perception of focus of current care (outer ring).a.
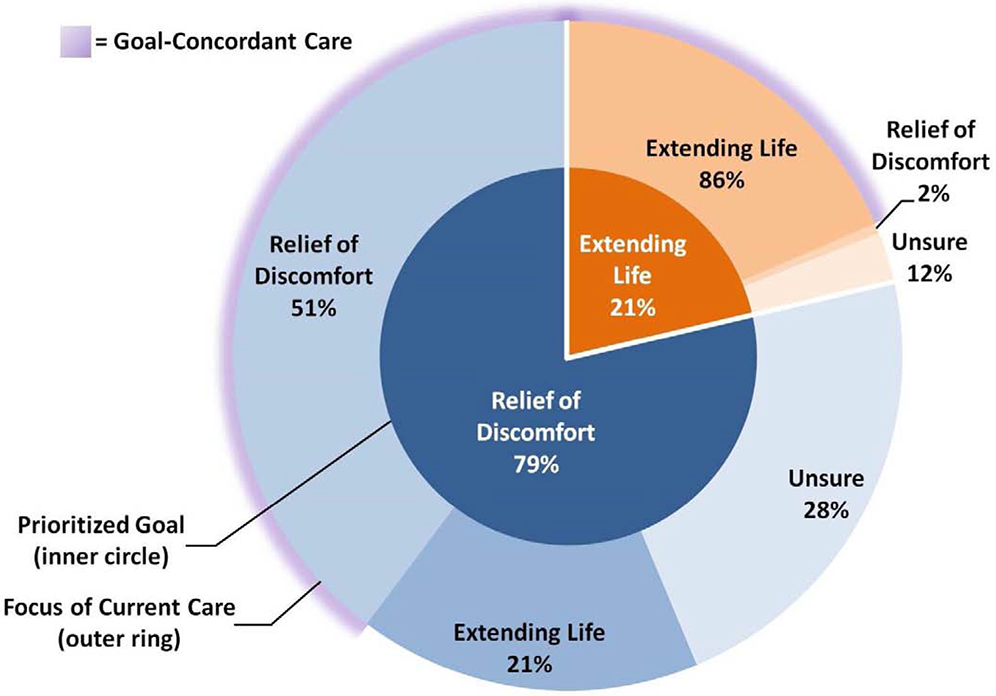
a. Inner circle shows the prioritized healthcare goal of 405 seriously ill patients (white font). Outer ring shows these patients' perception of the focus of their current medical care within each goal group (black font), with blue shades representing a prioritized goal of relief of pain and discomfort and orange shades representing a prioritized goal of extending life. Outer ring shows within goal group percentages of focus of care (e.g., 51% of patients who prioritized a goal of relief of pain and discomfort also perceived relieving pain and discomfort as the focus of their current care). Purple glow represents patient-reported receipt of goal-concordant care.
Characteristics associated with the outcome
The adjusted multivariate multinomial logistic regression model is shown in Table 3. Older patients were less likely to report receipt of goal-discordant care (RRR 0.96 per year; 95% CI 0.94, 0.99). Patients more likely to report receipt of goal-discordant care included those with better self-perceived health status (RRR 1.32 per healthier status level; 95% CI 1.00, 1.73), advanced cancer (RRR 2.11; 95% CI 1.02, 4.36), and a prioritized goal of relief of pain and discomfort (RRR 22.20; 95% CI 4.59, 107.38). Patients more likely to report uncertainty about the focus of their care included female patients (RRR 1.96; 95% CI 1.16, 3.30) and those with a prioritized goal of relief of pain and discomfort (RRR 3.69; 95% CI 1.67, 8.14).
Table 3.
Adjusted multivariate multinomial logistic regression examining associations with patient-reported receipt of goal-discordant care and uncertain focus of care, among patients with a prioritized healthcare goal (n=405)a
| Goal-Discordant ∣ Goal-Concordant | Focus-Uncertain ∣ Goal-Concordant | |||
|---|---|---|---|---|
| Characteristic | RRR (95% CI) | p-value | RRR (95% CI) | p-value |
| Patient characteristics | ||||
| Age (per year) | 0.964 (0.939, 0.989) | 0.005 | 1.006 (0.981, 1.032) | 0.627 |
| Female | 1.336 (0.775, 2.302) | 0.297 | 1.957 (1.159, 3.302) | 0.012 |
| Racial/ethnic minority | 1.696 (0.825, 3.488) | 0.151 | 0.982 (0.522, 1.849) | 0.956 |
| Currently married or living with partner | 0.997 (0.543, 1.832) | 0.993 | 1.147 (0.624, 2.111) | 0.659 |
| Education (per rising level) | 1.192 (0.955, 1.488) | 0.121 | 1.015 (0.848, 1.214) | 0.872 |
| Self-perceived health status (per healthier status level) | 1.316 (1.000, 1.731) | 0.050 | 1.004 (0.781, 1.290) | 0.975 |
| Advanced cancer | 2.112 (1.024, 4.356) | 0.043 | 0.822 (0.385, 1.754) | 0.613 |
| Prioritized goal is relief of pain and discomfort | 22.197 (4.588, 107.384) | <0.001 | 3.689 (1.671, 8.143) | 0.001 |
| Communication processes | ||||
| Prior discussion with family or friend | 0.662 (0.323, 1.358) | 0.261 | 0.594 (0.341, 1.033) | 0.065 |
| Prior discussion with enrolled clinician | 1.075 (0.572, 2.018) | 0.822 | 0.636 (0.350, 1.156) | 0.138 |
| Complete trust in enrolled clinician | 0.803 (0.361, 1.785) | 0.590 | 0.777 (0.425, 1.421) | 0.413 |
Multivariate multinomial logistic regression with patients clustered under clinicians. Reference group is goal-concordant care. Bold items represent exposure variables that met the prespecified level of statistical significance (p-value <0.05). RRR is relative risk ratio.
DISCUSSION
In this sample of seriously ill outpatients with prioritized healthcare goals, 60% of patients reported receipt of goal-concordant care. Non-concordance was composed of a sizable portion of patients reporting goal-discordant care and a sizable portion reporting uncertainty of the focus of their care. In addition, we found that patient-reported goal-concordance differed substantially by patients' prioritized goal. While 85% of patients who prioritized extending life reported goal-concordant care, only half of patients who prioritized relief of pain and discomfort reported goal-concordant care. This finding is similar to findings in SUPPORT nearly thirty years ago.17 Moreover, 1 in 5 patients with a top priority of relief of pain and discomfort reported goal-discordant care, and the relative risk ratio of goal-discordant care increased by a factor of more than twenty for these patients as compared to patients who prioritized extending life. Nearly 30% of patients with a prioritized goal of relief of pain and discomfort reported uncertainty of the focus of their care, and the relative risk ratio of uncertainty increased by a factor of more than three for these patients as compared to patients who prioritized extending life. It is disappointing that despite the advances in palliative care over the past 30 years, the proportion of patients reporting receipt of goal-concordant care is similar.
The substantial difference in patient-reported receipt of goal-concordant care between patients who prioritized relief of pain and discomfort and those who prioritized extending life has several potential explanations. First, evidence that clinicians often lack understanding of patient treatment preferences45-51 suggests poor understanding of patient goals may also be common. Clinicians with poor understanding of patient goals may defer to the default practice of delivering care focused on extending life. As a result, patients who prioritize the goal of extending life may receive goal-concordant care irrespective of clinician understanding of their goal, while patients who prioritize the goal of relief of pain and discomfort may only receive goal-concordant care if their clinicians know their goal and forego the default practice. Future studies should identify ways to improve clinicians’ understanding of patient goals and, in so doing, improve the matching of goals to treatment. Second, even when clinicians understand patient goals, they may struggle to develop and implement a plan of care focused on relief of pain and discomfort. Lack of confidence in the ability to deliver multiple aspects of palliative care is commonly described as a major barrier to delivery of primary palliative care among non-palliative care specialists.52 Further training in primary palliative care may be needed to ensure that the delivery of goal-concordant care is routine practice for all clinicians treating patients with serious illness.53 Third, some patients may struggle to accurately perceive the focus of their care when that focus is no longer on default life-extending care. This may be related to clinicians inadequately communicating the focus of care54 or patients misunderstanding the focus of care. While it is possible that some patients may be receiving goal-concordant care without being aware of it, we believe it is important patients understand how the treatments they receive are guided by their goals.
In addition to a prioritized goal of relief of pain and discomfort, we found several other factors were associated with patients' reporting goal-discordant care, albeit with smaller effect sizes. Patients with better self-perceived health status were more likely to report goal-discordant care. Clinicians may be less likely to prompt goals-of-care discussions with patients who project better self-perceived health status or may assume these patients prioritize extending life. However, research suggests self-perceived health status is not significantly associated with the prioritized goals of seriously ill patients.23 We also found patients with advanced cancer were more likely to report goal-discordant care. This may relate to goals-of-care discussions often beginning late in the illness course of patients with advanced cancer36 or a focus on aggressive treatments in effort to maintain patient hope.31 Moreover, honest goals-of-care discussions may be undermined by the pressure patients with advanced cancer feel to stay positive and "fight".32-35 Finally, we found younger patients were more likely to report goal-discordant care, perhaps because these patients or their clinicians are less likely to prompt goals-of-care discussions as compared to older patients.
While the majority of patients with a prioritized goal believed they understood the focus of their care, about a quarter of patients were unsure. We found female patients were more likely to report feeling uncertain about the focus of their care. Further work is needed to better understand this finding. It is possible greater uncertainty could result from gender-related differences in time spent discussing the focus of care with their clinicians. Another possibility is that women may express less confidence in their knowledge than men.55 Although differences in confidence are rarely reflective of differences in objective knowledge,55 this may have contributed to more women expressing uncertainty.
The factors we identified as associated with goal-discordant care or uncertainty with the focus of care are important to help identify patients at risk for these undesirable outcomes; however, they are not modifiable risk factors. We were surprised to find that neither prior discussions nor trust in clinicians were associated with reports of goal-concordant care. There are several possible explanations for these results. First, we did not have information on the quality of prior discussions. It is possible poor-quality discussions could have minimal effects on patient-reported goal-concordant care. Second, the timing of discussions in relation to our measure of goal-concordance was unknown. It is possible that patients’ goals or foci of care changed over time. Third, patients were only asked about prior discussions with their enrolled clinician and therefore do not include prior discussions with other clinicians. Finally, the strong ceiling effect of trust, even as a dichotomized single item, may have precluded its ability to discriminate levels of trust.
This study has several important limitations. First, discussing goals-of-care with patients is different than responding to survey questions. Survey questions may oversimplify complex patient perspectives. In this study, we did not explore how patients interpreted the goal question or the focus of care question, although both were developed and cognitively tested for patients for the SUPPORT study.15,16 Second, we relied on patients’ perception of the focus of their care, which may not necessarily be reflective of the actual focus of their care. While we believe patients’ perception is important in itself, future studies could consider alternative approaches toward understanding the focus of care, including surveying clinicians. Third, measuring goal-concordant care remains challenging with no single method widely accepted. While the method we used has limitations, we think it provides an important contribution to our understanding of patient-reported goal-concordant care in serious illness. Fourth, patients in our study were seriously, but not terminally ill, as only 40 patients died during the 6-month follow-up of the randomized trial. It is possible goals of care and receipt of goal-concordant care for terminally ill patients may be different than for seriously ill patients. Fifth, although our participation rate was relatively high at 58%, non-response bias could be present. Finally, although multi-centered, our study took place in one region of the US with mostly white, non-Hispanic patients and may not generalize to other regions or populations.
In this study, we assessed patient-reported receipt of goal-concordant care among adults with serious illness. We found 60% of patients reported goal-concordant care, and that goal-concordance differed substantially by patients' goal, with patients prioritizing relief of pain and discomfort far less likely to report goal-concordant care than patients prioritizing extending life. Further work is needed to better understand the gaps that contribute to this difference. To maximize goal-concordant care, clinicians should work to understand patients' goals, find ways to build skills and confidence in delivering palliative care, and explicitly describe to patients how treatment choices reflect patients' goals.
ACKNOWLEDGEMENTS
Contributors: none
- Funders:
- Patient-Centered Outcomes Research Institute® (PCORI®) Award (IH-12-11-4596)
- Grant from the Cambia Health Foundation
- University of Washington Pulmonary and Critical Care T32 # HL 007287-39
- University of Washington Palliative Care T32 # HL 125195-04
Prior Presentation: none
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLAIMER
The statements presented in this work are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
CONFLICTS OF INTEREST
Drs. Engelberg and Curtis report grant funding from PCORI during the conduct of the study. The remaining authors report no conflicts of interest.
Matthew E. Modes, MD, MPP = no conflicts of interest
Susan R. Heckbert, MD, PhD = no conflicts of interest
Ruth A. Engelberg, PhD = Dr. Engelberg reports grant funding from PCORI during the conduct of the study.
Elizabeth L. Nielsen, MPH = no conflicts of interest
J. Randall Curtis, MD, MPH = Dr. Curtis reports grant funding from PCORI during the conduct of the study.
Erin K. Kross, MD = no conflicts of interest
REFERENCES
- 1.Dzau VJ, McClellan MB, McGinnis JM, et al. Vital Directions for Health and Health Care: Priorities From a National Academy of Medicine Initiative. JAMA 2017;317:1461–70. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine . Committee on Approaching Death: Addressing Key End-of-Life Issues ib Dying in America : improving quality and honoring individual preferences near the end of life. Washington, D.C.: Washington, D.C. : The National Academies Press; 2015. [PubMed] [Google Scholar]
- 3.Sanders JJ, Curtis JR, Tulsky JA. Achieving Goal-Concordant Care: A Conceptual Model and Approach to Measuring Serious Illness Communication and Its Impact. J Palliat Med 2018;21:S17–s27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sudore RL, Heyland DK, Lum HD, et al. Outcomes That Define Successful Advance Care Planning: A Delphi Panel Consensus. J Pain Symptom Manage 2018;55:245–55.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dy SM, Kiley KB, Ast K, et al. Measuring what matters: top-ranked quality indicators for hospice and palliative care from the American Academy of Hospice and Palliative Medicine and Hospice and Palliative Nurses Association. J Pain Symptom Manage 2015;49:773–81. [DOI] [PubMed] [Google Scholar]
- 6.Halpern SD. Goal-Concordant Care - Searching for the Holy Grail. N Engl J Med 2019;381:1603–6. [DOI] [PubMed] [Google Scholar]
- 7.Unroe KT, Hickman SE, Torke AM. Care Consistency With Documented Care Preferences: Methodologic Considerations for Implementing the "Measuring What Matters" Quality Indicator. J Pain Symptom Manage 2016;52:453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson SB, Butow PN, Kerridge I, Bell ML, Tattersall MHN. How Well Do Current Measures Assess the Impact of Advance Care Planning on Concordance Between Patient Preferences for End-of-Life Care and the Care Received: A Methodological Review. J Pain Symptom Manage 2018;55:480–95. [DOI] [PubMed] [Google Scholar]
- 9.Haines L, Rahman OK, Sanders JJ, Johnson K, Kelley A. Factors That Impact Family Perception of Goal-Concordant Care at the End of Life. J Palliat Med 2019;22:927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khandelwal N, Curtis JR, Freedman VA, et al. How Often Is End-of-Life Care in the United States Inconsistent with Patients' Goals of Care? J Palliat Med 2017;20:1400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auriemma CL, Nguyen CA, Bronheim R, et al. Stability of end-of-life preferences: a systematic review of the evidence. JAMA Intern Med 2014;174:1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamas D, Panariello N, Henrich N, et al. Advance Care Planning Documentation in Electronic Health Records: Current Challenges and Recommendations for Change. J Palliat Med 2018;21:522–8. [DOI] [PubMed] [Google Scholar]
- 13.Lee RY, Brumback LC, Sathitratanacheewin S, et al. Association of Physician Orders for Life-Sustaining Treatment With ICU Admission Among Patients Hospitalized Near the End of Life. JAMA 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnbull AE, Sahetya SK, Colantuoni E, Kweku J, Nikooie R, Curtis JR. Inter-Rater Agreement of Intensivists Evaluating the Goal Concordance of Preference-Sensitive ICU Interventions. J Pain Symptom Manage 2018;56:406–13.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA 1995;274:1591–8. [PubMed] [Google Scholar]
- 16.Kreling B, Robinson DK, Bergner M. Data collection strategies in SUPPORT. J Clin Epidemiol 1990;43 Suppl:5s–9s. [DOI] [PubMed] [Google Scholar]
- 17.Teno JM, Fisher ES, Hamel MB, Coppola K, Dawson NV. Medical care inconsistent with patients' treatment goals: association with 1-year Medicare resource use and survival. J Am Geriatr Soc 2002;50:496–500. [DOI] [PubMed] [Google Scholar]
- 18.Steinhauser KE, Christakis NA, Clipp EC, McNeilly M, McIntyre L, Tulsky JA. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA 2000;284:2476–82. [DOI] [PubMed] [Google Scholar]
- 19.Coats H, Downey L, Sharma RK, Curtis JR, Engelberg RA. Quality of Communication and Trust in Patients With Serious Illness: An Exploratory Study of the Relationships of Race/Ethnicity, Socioeconomic Status, and Religiosity. J Pain Symptom Manage 2018;56:530–40.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtis JR, Downey L, Back AL, et al. Effect of a Patient and Clinician Communication-Priming Intervention on Patient-Reported Goals-of-Care Discussions Between Patients With Serious Illness and Clinicians: A Randomized Clinical Trial. JAMA Intern Med 2018;178:930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fakhri S, Engelberg RA, Downey L, et al. Factors Affecting Patients' Preferences for and Actual Discussions About End-of-Life Care. J Pain Symptom Manage 2016;52:386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modes ME, Engelberg RA, Downey L, Nielsen EL, Curtis JR, Kross EK. Did a Goals-of-Care Discussion Happen? Differences in the Occurrence of Goals-of-Care Discussions as Reported by Patients, Clinicians, and in the Electronic Health Record. J Pain Symptom Manage 2019;57:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modes ME, Engelberg RA, Downey L, et al. Toward Understanding the Relationship Between Prioritized Values and Preferences for Cardiopulmonary Resuscitation Among Seriously Ill Adults. J Pain Symptom Manage 2019;58:567–77.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cholongitas E, Papatheodoridis GV, Vangeli M, Terreni N, Patch D, Burroughs AK. Systematic review: The model for end-stage liver disease--should it replace Child-Pugh's classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther 2005;22:1079–89. [DOI] [PubMed] [Google Scholar]
- 25.Connors AF Jr., Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996;154:959–67. [DOI] [PubMed] [Google Scholar]
- 26.McMurray JJ, Pfeffer MA. Heart failure. Lancet 2005;365:1877–89. [DOI] [PubMed] [Google Scholar]
- 27.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29. [DOI] [PubMed] [Google Scholar]
- 28.Steinhauser KE, Clipp EC, Hays JC, et al. Identifying, recruiting, and retaining seriously-ill patients and their caregivers in longitudinal research. Palliat Med 2006;20:745–54. [DOI] [PubMed] [Google Scholar]
- 29.Finkelstein EA, Bilger M, Flynn TN, Malhotra C. Preferences for end-of-life care among community-dwelling older adults and patients with advanced cancer: A discrete choice experiment. Health Policy 2015;119:1482–9. [DOI] [PubMed] [Google Scholar]
- 30.Flynn TN, Bilger M, Malhotra C, Finkelstein EA. Are Efficient Designs Used in Discrete Choice Experiments Too Difficult for Some Respondents? A Case Study Eliciting Preferences for End-of-Life Care. Pharmacoeconomics 2016;34:273–84. [DOI] [PubMed] [Google Scholar]
- 31.Bluhm M, Connell CM, De Vries RG, Janz NK, Bickel KE, Silveira MJ. Paradox of Prescribing Late Chemotherapy: Oncologists Explain. J Oncol Pract 2016;12:e1006–e15. [DOI] [PubMed] [Google Scholar]
- 32.Byrne A, Ellershaw J, Holcombe C, Salmon P. Patients' experience of cancer: evidence of the role of 'fighting' in collusive clinical communication. Patient Educ Couns 2002;48:15–21. [DOI] [PubMed] [Google Scholar]
- 33.Haines I The war on cancer: time for a new terminology. Lancet 2014;383:1883. [DOI] [PubMed] [Google Scholar]
- 34.Morrell L, Ii SS, Wordsworth S, Wilson R, Rees S, Barker R. Cancer as the "perfect storm"? A qualitative study of public attitudes to health conditions. Health Sci Rep 2018;1:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metaphors Nicholas G. and malignancy: making sense of cancer. Curr Oncol 2013;20:e608–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mack JW, Cronin A, Taback N, et al. End-of-life care discussions among patients with advanced cancer: a cohort study. Ann Intern Med 2012;156:204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. Bmj 2010;340:c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008;300:1665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Au DH, Udris EM, Engelberg RA, et al. A randomized trial to improve communication about end-of-life care among patients with COPD. Chest 2012;141:726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curtis JR, Engelberg RA, Nielsen EL, Au DH, Patrick DL. Patient-physician communication about end-of-life care for patients with severe COPD. Eur Respir J 2004;24:200–5. [DOI] [PubMed] [Google Scholar]
- 41.Curtis JR, Patrick DL, Caldwell E, Greenlee H, Collier AC. The quality of patient-doctor communication about end-of-life care: a study of patients with advanced AIDS and their primary care clinicians. Aids 1999;13:1123–31. [DOI] [PubMed] [Google Scholar]
- 42.Engelberg R, Downey L, Curtis JR. Psychometric characteristics of a quality of communication questionnaire assessing communication about end-of-life care. J Palliat Med 2006;9:1086–98. [DOI] [PubMed] [Google Scholar]
- 43.Street RL Jr., Makoul G, Arora NK, Epstein RM. How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Educ Couns 2009;74:295–301. [DOI] [PubMed] [Google Scholar]
- 44.Dugan E, Trachtenberg F, Hall MA. Development of abbreviated measures to assess patient trust in a physician, a health insurer, and the medical profession. BMC Health Serv Res 2005;5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coppola KM, Ditto PH, Danks JH, Smucker WD. Accuracy of primary care and hospital-based physicians' predictions of elderly outpatients' treatment preferences with and without advance directives. Arch Intern Med 2001;161:431–40. [DOI] [PubMed] [Google Scholar]
- 46.Downey L, Au DH, Curtis JR, Engelberg RA. Life-sustaining treatment preferences: matches and mismatches between patients' preferences and clinicians' perceptions. J Pain Symptom Manage 2013;46:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer GS, Tulsky JA, Rose MR, Siminoff LA, Arnold RM. Patient knowledge and physician predictions of treatment preferences after discussion of advance directives. J Gen Intern Med 1998;13:447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattimore TJ, Wenger NS, Desbiens NA, et al. Surrogate and physician understanding of patients' preferences for living permanently in a nursing home. J Am Geriatr Soc 1997;45:818–24. [DOI] [PubMed] [Google Scholar]
- 49.Teno JM, Hakim RB, Knaus WA, et al. Preferences for cardiopulmonary resuscitation: physician-patient agreement and hospital resource use. The SUPPORT Investigators. J Gen Intern Med 1995;10:179–86. [DOI] [PubMed] [Google Scholar]
- 50.Wenger NS, Phillips RS, Teno JM, et al. Physician understanding of patient resuscitation preferences: insights and clinical implications. J Am Geriatr Soc 2000;48:S44–51. [DOI] [PubMed] [Google Scholar]
- 51.O'Donnell H, Phillips RS, Wenger N, Teno J, Davis RB, Hamel MB. Preferences for cardiopulmonary resuscitation among patients 80 years or older: the views of patients and their physicians. J Am Med Dir Assoc 2003;4:139–44. [DOI] [PubMed] [Google Scholar]
- 52.Carey ML, Zucca AC, Freund MA, Bryant J, Herrmann A, Roberts BJ. Systematic review of barriers and enablers to the delivery of palliative care by primary care practitioners. Palliat Med 2019;33:1131–45. [DOI] [PubMed] [Google Scholar]
- 53.Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med 2013;368:1173–5. [DOI] [PubMed] [Google Scholar]
- 54.Jerant AF, Azari RS, Nesbitt TS, Meyers FJ. The TLC model of palliative care in the elderly: preliminary application in the assisted living setting. Ann Fam Med 2004;2:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ehrlinger J, Dunning D. How chronic self-views influence (and potentially mislead) estimates of performance. J Pers Soc Psychol 2003;84:5–17. [PubMed] [Google Scholar]


