Introduction
Sporotrichosis is a subcutaneous mycosis caused by thermodimorphic fungi of Sporothrix schenckii complex and is usually transmitted as a result of traumatic inoculation of these microorganisms; infection can produce a number of manifestations.1,2 In Brazil, sporotrichosis has been a major public health issue, with multiple outbreaks occurring because of zoonotic transmission, including contact with cats, and S brasiliensis is the most prevalent agent in Rio de Janeiro State.3,4 Immunocompromised individuals are thought to be at increased risk of developing a more severe clinical presentation.5 Disseminated sporotrichosis in immunocompetent individuals as result of contact with cats has becoming increasingly reported.6,7 Historically, cases of sporotrichosis in the Brazilian northeast have been infrequently reported, and this was not thought to be an endemic area for this mycosis. Herein, we report 4 cases of disseminated sporotrichosis in patients in a public hospital in Pernambuco. Publication of this case series report was granted institutional review board approval by the Ethics Committee in Research at the Federal University of Pernambuco, Brazil.
Case reports
Case 1
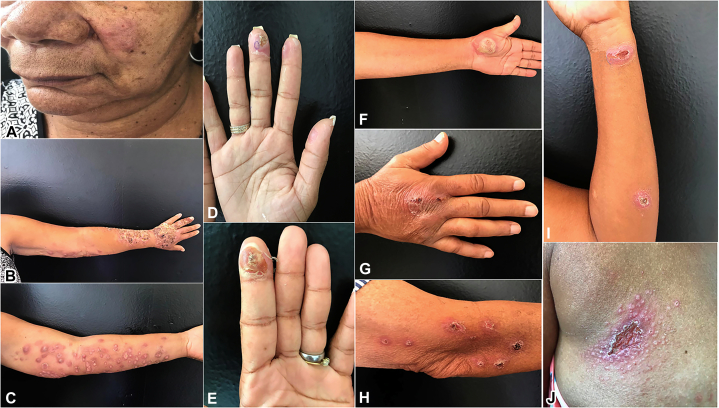
A 61-year-old woman with diabetes mellitus presented with ulcerated nodules on the upper aspect of the limbs and on the trunk and face. These lesions followed from scratches of a cat, and had been present for approximately 20 weeks. The patient did not report any other form of trauma. More specifically, she denied any contact with plants. Clinical examination revealed numerous verrucous and ulcerated plaques on the left arm, and an ulcerated nodule on the face (Fig 1, A, B, and C). Purulence was also noted on physical examination, and the patient reported recent fevers and chills; therefore, there was concern about a secondary bacterial infection. HIV testing result was negative. Itraconazole treatment was started with 200 mg/d, combined with clindamycin and ciprofloxacin to treat a secondary bacterial infection. The patient continued to be febrile, and there was edema of the upper portion of the limb and purulence. Because of the lack of improvement, the patient was hospitalized and treated with a combination of 10 days of amphotericin B deoxycholate (50 mg/d) and daptomycin. Amphotericin B deoxycholate monotherapy was continued for 4 weeks (cumulative dose of 1.4 g during 28 days), with visible improvement. The patient was then discharged with itraconazole 400 mg/d. One month later, she presented with new lesions on the left forearm and gastrointestinal complaints (epigastric pain and diarrhea). Potassium iodide 3.5 g/d was started and the patient continued to receive a dose of itraconazole 200 mg/d. She continued this regimen for 22 weeks, by which time the lesions had entirely resolved.
Fig 1.
Disseminated sporotrichosis lesions. A, B, and C, Patient 1 with lesions on the face and right upper aspect of her arm. D and E, Patient 2 with lesions on the fingertips of the hands. F, G, and H, Patient 3 with lesions on the hands, right arm, and elbow. I and J, Patient 4 with lesions on the upper portion of the body and left forearm.
Case 2
A 26-year-old woman presented with a 40-day history of lesions on her arms and hands that began after 2 bites from her ill cat when she was administering medication. On examination, 2 erythematous ulcers were present on the fingers (Fig 1, D and E), as well as lesions on the upper portion of the arms and enlarged axillary and epitrochlear lymph nodes. She was admitted to the hospital and began receiving ciprofloxacin and itraconazole. Distal bone damage was ruled out by magnetic resonance imaging, which revealed only edema of soft tissues. HIV testing result was negative, and the patient did not receive immunosuppressive medications. She was then treated with itraconazole 200 mg/d for 16 weeks, and there was resolution by the end of that period.
Case 3
A 64-year-old man presented with a 4-week history of erythematous nodules and ulcers with overlying crust on the hands, with lesions measuring 0.5 to 1.2 cm in diameter, as well as with lesions on the right arm and elbow (Fig 1, F, G, and H). The patient did not report fevers, chills, or other systemic symptoms. He had a history of type 1 diabetes, which was well controlled. He began receiving itraconazole 100 mg/d and local heat 2 to 3 times per day for 15 days, with noticeable improvement. After 1 month of this regimen, the patient presented with polymorphous erythema of the hands and feet, with spontaneous resolution in 10 days. He was treated with itraconazole for 23 weeks.
Case 4
A 46-year-old woman presented with multiple cutaneous lesions on her back and left arm, and on her face (Fig 1, I and J). On physical examination, there were ulcerated nodules on the wrist and forearm, as well as lymphangitis. On the face, there was a 3.5 × 2.0-cm ulcerated plaque, with satellites papules, besides nodular lesions on her face. HIV testing result was negative. These lesions had been present for 8 weeks and appeared after her ill cat scratched her. She denied any systemic symptoms. She then began receiving itraconazole 100 mg/d for 13 weeks, which led to resolution of her symptoms.
Methods
We carried out a punch biopsy, and a swab of the suppurative exudate was performed. Direct microscopy was performed. Tissue fragment and secretion were seeded on potato dextrose agar plates and incubated for 7 days at 25°C (77°F), fungal cultures were isolated, and micromorphologic features were observed via optical microscope. Histopathology was performed by hematoxylin-eosin staining.
Sporothrix species genomic DNA was obtained and partial sequencing of the calmodulin gene (CAL) was performed.1 Basic local alignment search tool (http://www.ncbi.nlm.nih.gov/BLAST) analysis was carried out and phylogeny was performed in MEGA7 (Pennsylvania State University, PA). The sequences were deposited in GenBank (Table I).
Table I.
Minimal inhibitory concentration of 4 antifungals against Sporothrix brasiliensis isolated from humans
| Case (isolate) | GenBank accession number | ITC, μg/mL | Amph B, μg/mL | TBF, μg/mL | KI, μg/mL |
|---|---|---|---|---|---|
| 1 (HCPE 1030) | MH881062 | 0.0625 | 0.5 | 0.125 | >64 |
| 2 (URM 8079) | MH881046 | 0.0625 | 0.5 | 8 | >64 |
| 3 (URM 7971) | MH881050 | 0.125 | 1 | 0.125 | >64 |
| 4 (URM 8077) | MH881044 | >16 | 2 | 0.125 | >64 |
Amph B, Amphotericin B; HCPE, Hospital das Clínicas de Pernambuco; ITC, itraconazole; KI, potassium iodide; TBF, terbinafine; URM, University of Recife Mycology.
Broth microdilution method was performed to verify the isolates' resistance profile, according to the reference method.8
Results
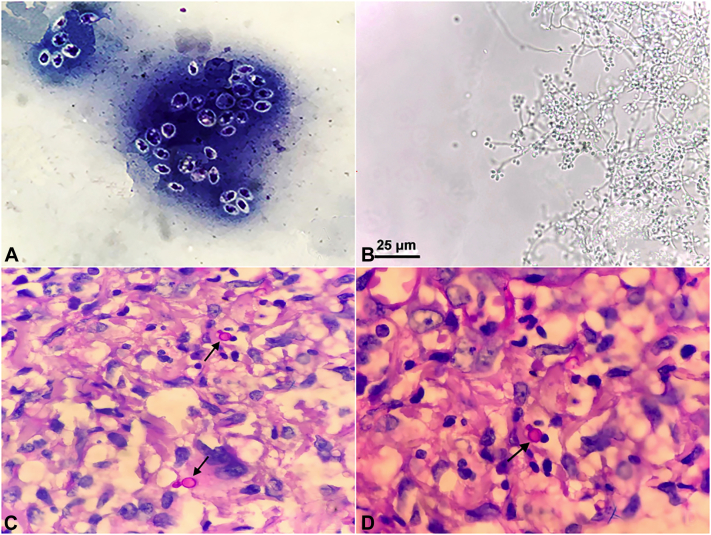
Yeast-like cells were observed in direct microscopic examination (Fig 2, A), and micromorphology from the cultures showed thin and hyaline septate mycelia with delicate conidiophores, finishing at an expanded denticulate vesicle at the apex and bearing the floral aspect typical of Sporothrix species (Fig 2, B). Histopathology showed blastospore yeastlike cells (Fig 2, C and D).
Fig 2.
A, Panoptic coloring preparation evidencing yeastlike cells of Sporothrix brasiliensis. B, Lactophenol preparation evidencing S brasiliensis. C and D, Hematoxylin-eosin stain preparation evidencing blastospore yeastlike cells of S brasiliensis (arrows).
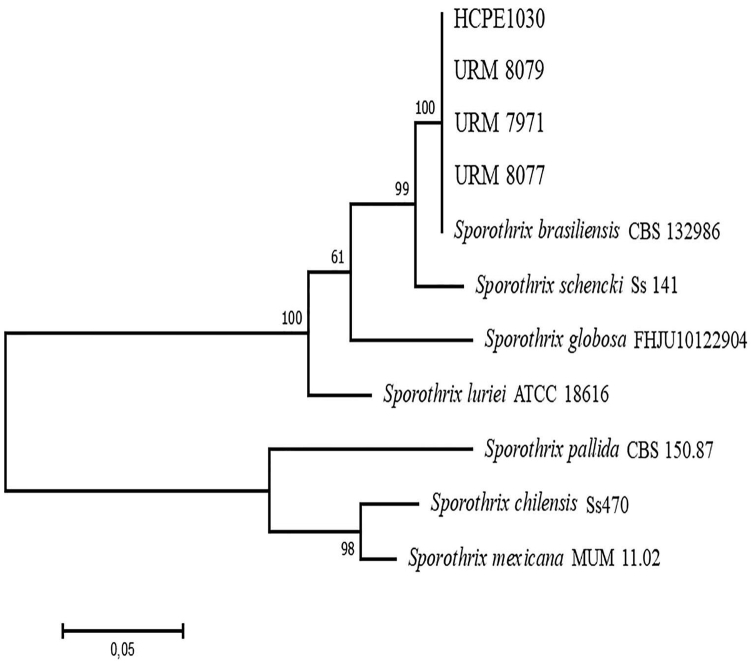
Basic local alignment search tool analysis suggested S brasiliensis as the etiologic agent in all cases, and phylogeny confirmed species identification (Fig 3). All isolates were susceptible to amphotericin B. The isolates for cases 2 and 4 were, respectively, terbinafine and itraconazole resistant, according to the reference method. Potassium iodide had no in vitro activity against all isolates (Table I).
Fig 3.
Maximum likelihood phylogenetic tree showing evolutionary relationship between Sporothrix pathogenic species.
Discussion
Despite the morphologic similarity among S schenckii complex species, previous studies suggest S brasiliensis is the most virulent among the reported sporotrichosis species and is related to atypical presentations, even in immunocompetent individuals. Disseminated lesions can occur, followed by hypersensitivity reactions, and community outbreaks have been reported.3,7,9 Sporotrichosis has a global distribution, and there have been an increasing number of cases reported in the last decades. In Brazil, zoonotic transmission as a result of contact with felines is thought to be the primary cause of S brasiliensis sporotrichosis in humans, with 4669 cases occurred in Rio de Janeiro from 1998 to 2016.10,11 Sporotrichosis is also well described in Rio Grande do Sul State, and as in Rio de Janeiro, contact with cats is thought to be play an important role in transmission.12
The patients described in cases 2 and 4 live in Olinda County, in nearby districts (approximately 3.1 km), whereas the patient described in case 3 lives in Jaboatão dos Guararapes County, 26 km away from Olinda. Both cities are in the metropolitan region of Recife, capital of Pernambuco State. Also, the patient described in case 1 lives in Nazaré da Mata, north countryside zone of Pernambuco, 46.7 km away from Jaboatão dos Guararapes and 77.8 km away from Olinda.
Disseminated sporotrichosis commonly occurs in patients with low numbers of CD4+ cells, such as chemotherapy patients.5,7 Nevertheless, case reports have been published of the occurrence of disseminated lesions in immunocompetent individuals.6,7 In our study, the 4 patients were HIV negative. Multiple cutaneous lesions are usually related to multiples bites or scratches,5 as occurred in cases 2 and 4.
Guidelines for the treatment of cutaneous sporotrichosis suggest 200 mg of itraconazole for 2 to 4 weeks daily, and 400 mg when there is a poor initial response. In refractory cases, different treatment options can be considered. These include 5 drops of saturated solution of potassium iodide 3 times daily, increasing to 40 to 50 drops (as tolerated by patients) and 500 mg of terbinafine (twice daily) when the patient cannot tolerate other treatments.13 For disseminated or extracutaneous sporotrichosis, amphotericin B at 3 to 5 mg/kg/d is the recommended schedule. For pregnant patients, azoles are not recommended, and amphotericin deoxycholate (0.7-1 mg/kg/d) or liposomal (3-5 mg/kg/d) is a good option. Hyperthermia is also recommended for pregnant patients.13
Itraconazole remains the first therapeutic choice for cutaneous sporotrichosis treatment,2 and in Rio de Janeiro, where S brasiliensis is prevalent, treatment with itraconazole starting with 100 to 400 mg for adults is often recommended.14 In our study, a dose of 100 to 200 mg/d led to clinical improvement, except in case 1, in which, although the patient was immunocompetent, achieving a therapeutic cure was challenging, which may have been a result of secondary bacterial infection; therefore, other treatments were used.
To the best of our knowledge, we describe the first cases of disseminated cutaneous human sporotrichosis caused by S brasiliensis in Pernambuco State. S brasiliensis has previously been described as the cause of sporotrichosis in other regions in Brazil. Itraconazole and potassium iodide, associated with thermotherapy, are good therapeutic choices in disseminated cutaneous cases of sporotrichosis caused by S brasiliensis, especially if there is evidence of hematogenic dissemination, and amphotericin B can be used in refractory cases or cases with visceral involvement.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Marimon R., Serena C., Gené J., Cano J., Guarro J. Sporothrix brasiliensis, S. globosa, and S. mexicana three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:198–206. doi: 10.1128/JCM.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahajan V. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract. 2014;2014:272376. doi: 10.1155/2014/272376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodrigues A.M., de Melo Teixeira M., de Hoog G.S. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl Trop Dis. 2013;7:e2281. doi: 10.1371/journal.pntd.0002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira S.A., Gremião I.D., Kitada A.A., Boechat J.S., Viana P.G., Schubach T.M. The epidemiological scenario of feline sporotrichosis in Rio de Janeiro, State of Rio de Janeiro, Brazil. Rev Soc Bras Med Trop. 2014;47:392–393. doi: 10.1590/0037-8682-0092-2013. [DOI] [PubMed] [Google Scholar]
- 5.Bonifaz A., Tirado-Sánchez A. Cutaneous disseminated and extracutaneous sporotrichosis: current status of a complex disease. J Fungi. 2017;3:2–13. doi: 10.3390/jof3010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panduro N.T., Alvarez M.I.R. Unusual presentation of disseminated cutaneous sporotrichosis in an immunocompetent patient. JAAD Case Rep. 2018;79:AB307. [Google Scholar]
- 7.Almeida-Paes R., de Oliveira M.M.E., Freitas D.F.S., do Valle A.C.F., Zancopé-Oliveira R.M., Gutierrez-Galhardo M.C. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis. 2014;8:e3094. doi: 10.1371/journal.pntd.0003094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute CLSI reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard. Second edition. 2008. clsi.org/media/1455/m38a2_sample.pdf Available at: Accessed September 2, 2020.
- 9.Arrillaga-Moncrief I., Capilla J., Mayayo E. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect. 2009;15:651–655. doi: 10.1111/j.1469-0691.2009.02824.x. [DOI] [PubMed] [Google Scholar]
- 10.Chakrabarti A., Bonifaz A., Gutierrez-Galhardo M.C., Mochizuki T., Li S. Global epidemiology of sporotrichosis. Med Mycol. 2014;53:3–14. doi: 10.1093/mmy/myu062. [DOI] [PubMed] [Google Scholar]
- 11.Gremião I.D.F., Miranda L.H.M., Reis E.G., Rodrigues A.M., Pereira S.A. Zoonotic epidemic of sporotrichosis: cat to human transmission. PLoS Pathog. 2017;13:e1006077. doi: 10.1371/journal.ppat.1006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poester V.R., Mattei A.S., Madrid I.M. Sporotrichosis in Southern Brazil, towards an epidemic? Zoonoses Public Health. 2018;65:815–821. doi: 10.1111/zph.12504. [DOI] [PubMed] [Google Scholar]
- 13.Kauffman C.A., Bustamante B., Chapman S.W., Pappas P.G. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:1255–1265. doi: 10.1086/522765. [DOI] [PubMed] [Google Scholar]
- 14.Orofino-Costa R., Macedo P.M., Rodrigues A.M., Bernardes-Engemann A.R. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92:606–620. doi: 10.1590/abd1806-4841.2017279. [DOI] [PMC free article] [PubMed] [Google Scholar]