Introduction
Sweet syndrome, also known as acute febrile neutrophilic dermatosis, was first described by Sweet in 1964.1 It is usually characterized by rapid onset of erythematous nodules or plaques with predominantly neutrophilic-dense infiltrates in the dermis. Fever and neutrophilia are usually present.2 Sweet syndrome can be classified into classic, malignancy-associated, and drug-induced subtypes.2,3 The latter has been reported to account for 1% to 26% of cases.4 Several medications are associated with drug-induced Sweet syndrome and the most frequently implicated is granulocyte-colony stimulating factor.5 We report a case of drug-induced Sweet syndrome in which lamotrigine was the main suspect drug, with excellent response to drug discontinuation.
Case report
A 28-year-old Chilean man presented to his local hospital with a 4-day history of facial edema and eruptive erythematous tender plaques in the head and neck, with associated fever (temperature 38.8°C/101.8°F). Some lesions were in the upper and lower lips. He had a history of epilepsy and long-term treatment with clobazam 5 mg daily and phenytoin 100 mg 3 times daily since childhood. Five months before consultation, he discontinued phenytoin and replaced it with lamotrigine 25 mg, increasing its dosage progressively up to 100 mg daily 1 week before consulting. The dosage of clobazam was not changed.
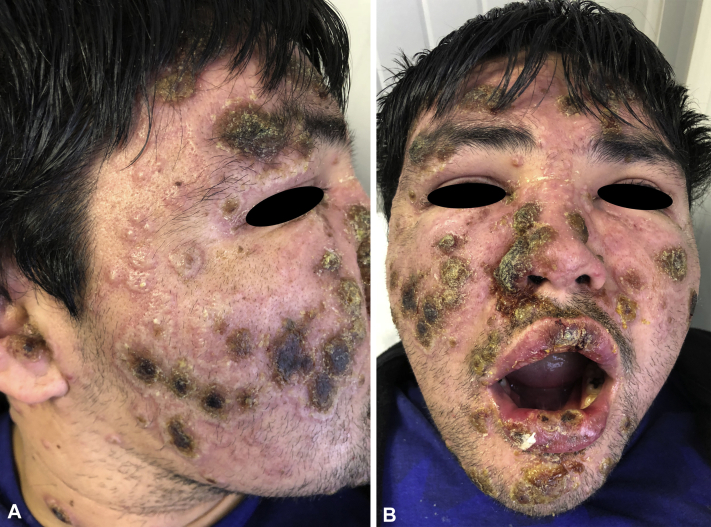
His skin examination revealed noticeable periocular swelling and multiple painful erythematous plaques with crusts and pustules over his face and neck (Fig 1, A). Some lesions were in the upper and lower lips, without lesions in the oral mucosa (Fig 1, B).
Fig 1.
A, Side view of the patient at baseline with erythematous tender plaques with pustules and crusts on their surface. B, Frontal view of the patient showing similar lesions on the upper and lower lips.
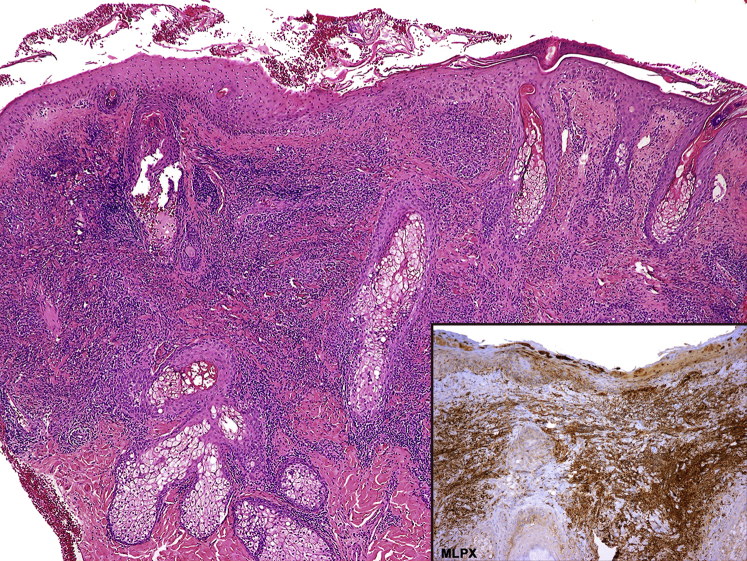
Important laboratory findings were leukocytosis (14.5 × 109/L), neutrophilia (11.2 × 109/L), thrombocytosis (444 × 109/L), elevated C-reactive protein level (403 mg/L), and a high erythrocyte sedimentation rate (105 mm/h). A 4-mm punch biopsy was obtained from an erythematous plaque from his right cheek. Histologic examination revealed a dense perivascular and interstitial neutrophilic dermic infiltrate (Fig 2). Results for direct immunofluorescence studies and stains for microorganisms were negative. For the evaluation of a possible associated malignancy, blood smear and chest radiography were performed, without pathologic findings. Because a drug reaction was suspected, lamotrigine was suspended. Three days after drug withdrawal and without systemic corticosteroids, lesions began to fade (Fig 3) and levetiracetam was started instead. After 12 months of follow-up, the patient remains without recurrences and his complete blood cell counts normalized.
Fig 2.
Section of skin biopsy showing focal hyperparakeratosis, subcorneal neutrophilic pustules, and marked edema of the papillary dermis associated with a dense perivascular and interstitial neutrophilic dermic infiltrate, without vasculitis. The inflammatory infiltrate is positive for myeloperoxidase stain (inset). (Hematoxylin-eosin stain.)
Fig 3.
Patient 1 week after lamotrigine withdrawal. Plaques are less erythematous, with some crusts and desquamation on their surface. No pustules or new lesions are observed.
Discussion
The first patient with drug-induced Sweet syndrome was reported by Su and Liu6 in 1986, and the syndrome was associated with trimethoprim-sulfamethoxazole. Ten years later, Walker and Cohen7 proposed 5 major diagnostic criteria for drug-induced Sweet syndrome: an abrupt onset of painful erythematous plaques or nodules; histopathologic evidence of a dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis; pyrexia (temperature >38°C); a temporal relationship between the drug ingestion and clinical presentation, or temporally related recurrence after oral challenge; and a temporally related resolution of lesions after drug withdrawal or treatment with systemic corticosteroids. Our patient fulfilled all these required criteria for a diagnosis of drug-induced Sweet syndrome. Because drug-induced Sweet syndrome is a diagnosis of exclusion, all patients should be screened for malignancy according to age.4 Thrombocytosis, as found in our patient, is present in up to 50% of patients with drug-induced Sweet syndrome and is considered infrequent in classic Sweet syndrome. Also, oral mucosal involvement is present in up to 7% of patients with drug-induced Sweet syndrome, but only in 2% of patients with the classic syndrome.2
Periocular swelling is an uncommon feature of Sweet syndrome but has been reported.8 Epidermal involvement can be present in Sweet syndrome and is observed as subcorneal pustules or neutrophilic spongiotic vesicles.2
Even though pustular drug reaction with eosinophilia and systemic symptoms can have some of these findings, the absence of eosinophilia, lack of internal organ involvement, the presence of a superficial and deep dermic neutrophilic infiltrate, and a rapid response to lamotrigine withdrawal led us to the definitive diagnosis.
To our knowledge, this is the first report of Sweet syndrome induced by lamotrigine. Carbamazepine and diazepam are the only other 2 anticonvulsant drugs reported to date in association with this dermatosis.9,10 Because oral challenge was not necessary, the temporal relationship between disease onset and lamotrigine dosage increase, along with the rapid response to the drug withdrawal, allowed us to consider lamotrigine as the most suspicious etiology in this case. This case serves to alert the clinician of this new possible drug association and highlights the importance of considering lamotrigine as a novel etiology of Sweet syndrome because early drug discontinuation can lead to rapid resolution of the skin lesions.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Sweet R.D. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76(8-9):349–356. doi: 10.1111/j.1365-2133.1964.tb14541.x. [DOI] [PubMed] [Google Scholar]
- 2.Cohen P.R. Sweet's syndrome - a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2(1):1–28. doi: 10.1186/1750-1172-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raza S., Kirkland R.S., Patel A.A., Shortridge J.R., Freter C. Insight into Sweet's syndrome and associated-malignancy: a review of the current literature. Int J Oncol. 2013;42(5):1516–1522. doi: 10.3892/ijo.2013.1874. [DOI] [PubMed] [Google Scholar]
- 4.Nelson C.A., Stephen S., Ashchyan H.J., James W.D., Micheletti R.G., Rosenbach M. Neutrophilic dermatoses. J Am Acad Dermatol. 2018;79(6):987–1006. doi: 10.1016/j.jaad.2017.11.064. [DOI] [PubMed] [Google Scholar]
- 5.Villarreal-Villarreal C.D., Ocampo-Candiani J., Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107(5):369–378. doi: 10.1016/j.ad.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Su W.P., Liu H.N. Diagnostic criteria for Sweet's syndrome. Cutis. 1986;37(3):167–174. [PubMed] [Google Scholar]
- 7.Walker D.C., Cohen P.R. Trimethoprim-sulfamethoxazole-associated acute febrile neutrophilic dermatosis: case report and review of drug-induced Sweet's syndrome. J Am Acad Dermatol. 1996;34(5 II):918–923. doi: 10.1016/s0190-9622(96)90080-8. [DOI] [PubMed] [Google Scholar]
- 8.Anwar S., Hassan S., Al-Fern A., Douglas W.S., Mann B. Bilateral periocular swelling in Sweet's syndrome. Eye (Lond) 2004;18(2):214–216. doi: 10.1038/sj.eye.6700588. [DOI] [PubMed] [Google Scholar]
- 9.Guimera F.J., Garcia-Bustinduy M., Noda A. Diazepam-associated Sweet's syndrome. Int J Dermatol. 2000;39(10):795–800. doi: 10.1046/j.1365-4362.2000.00051.x. [DOI] [PubMed] [Google Scholar]
- 10.Lund J.J., Stratman E.J., Jose D., Xia L., Wilson D., Moizuddin M. Drug-induced bullous Sweet syndrome with multiple autoimmune features. Autoimmune Dis. 2010;1(1):1–5. doi: 10.4061/2010/176749. [DOI] [PMC free article] [PubMed] [Google Scholar]