Abstract
Iris dabashanensis C.A.Wilson, sp. nov. and I. probstii C.A.Wilson, sp. nov. from China are described and illustrated. Both species occur on grassy slopes in mountainous regions of south-central China. The former is known from the Daba Mountains in rocky, calcareous soils associated with shrubs or mixed conifer and hardwood forests, while the latter is known from a region of karst terrain beside rice fields or under pine woods in Guizhou Province. Molecular data resolves both species in series Chinenses in a subclade that also includes I. odaesanensis, while morphologically they are similar to I. henryi. These newly described species are two of four members of series Chinenses that occur in south-central China.
Keywords: Daba Mountains, Guizhou Province, karst terrain, phylogeny
Introduction
Iris series Chinenses G.H.M. Lawr. comprises several Asian species that have short rhizomes, stolons, and small, open flowers where petals and sepals are horizontal. Dykes (1913) included four species in his informal group (the Chinese group), Lawrence (1953) circumscribed the series to include five species, Mathew (1989) recognized six species, and BIS (1997) recognized seven species in the series (Table 1). The two new species are from south-central China where I. henryi Baker and I. proantha Diels also occur, although none of their distributions overlap. Iris minutoaurea Makino, I. odaesanensis Y.N. Lee, and I. rossii Baker occur in northeastern China and adjacent regions in Korea and/or Japan and I. koreana Nakai is endemic to Korea. A species often included in the series, I. speculatrix Hance, is widespread in central and southern China. A phylogenetic study of the genus (Wilson 2011) that included five species from series Chinenses resolved the series as monophyletic, however I. speculatrix was not sampled. Guo and Wilson (2013) resolved I. speculatrix outside of series Chinenses in their study on crested species from several lineages in Iris. Tillie et al. (2001) included I. speculatrix as well as two series Chinenses species, I. minutoaurea and I. rossii, in their preliminary molecular phylogenetic study on Iris. Tillie et al. (2001) resolved I. minutoaurea and I. rossii as sister species and I. speculatrix outside of series Chinenses. Mavrodiev et al. (2014) suggested at least 23 genera should be recognized in Iris s.l., including a genus representing series Chinenses species. Crespo et al. (2015) formalized the splitting of Iris and circumscribed species from series Chinenses in the genus Zhaoanthus M.B. Crespo, Mart-Azorín & Mavrodiev. Iris speculatrix was not included in analyses of Mavrodiev et al. (2014) but based on several shared morphological characters was included in Zhaoanthus by Crespo et al. (2015).
Table 1.
Taxonomic treatments of Iris series Chinenses. Mathew (1989) included I. grijsi and I. speculatrix in the addendum. Section Lophiris and subsection Evansia circumscribe the same species at different ranks. Dykes (1913) and Lawrence (1953) used the illegitimate name I. minuta Franch. & Sav. for I. minutoaurea.
| Species | Dykes (1913) | Lawrence (1953) | Mathew (1989) | BIS (1997) |
|---|---|---|---|---|
| I. cavaleriei H.Lév. | Synonym of I. grijsi | Not included | Not included | Not included |
| I. gracilipes Pamp. | Not yet described | Not included | Not included | Synonym of I. henryi |
| I. grijsi Maxim. | Chinese group | Series Chinenses | Synonym of I. speculatrix | Synonym of I. speculatrix |
| I. henryi Baker | Chinese group | Series Chinenses | Series Chinenses | Series Chinenses |
| I. koreana Nakai | Not yet described | Series Chinenses | Series Chinenses | Series Chinenses |
| I. minutoaurea Makino | Chinese group | Series Chinenses | Series Chinenses | Series Chinenses |
| I. odaesanensis Y.N.Lee | Not yet described | Not yet described | Series Chinenses | Series Chinenses |
| I. proantha Diels | Not yet described | Not included | Sect. Lophiris Tausch | Series Chinenses |
| I. rossii Baker | Chinese group | Series Chinenses | Series Chinenses | Series Chinenses |
| I. speculatrix Hance | Section Evansia (Alef.) Baker | Subsect. Evansia Benth. | Series Chinenses | Series Chinenses |
Based on molecular studies (Tillie et al. 2001; Guo and Wilson 2013) and its morphology, including larger flowers and rhizomes, I. speculatrix is not considered a member of series Chinenses in this study. However, two taxa that are synonymized with I. speculatrix have not been recently examined. The first is I. grijsi Maxim. that was described based on a plant from Fujian Province in southeastern China. Maximowicz (1880) described I. grijsi as similar to I. ruthenica Ker Gawler, another species not resolved in series Chinenses (Wilson 2011; fig. 1), and gave several characters that distinguish it from I. ruthenica, including the presence of reduced leaves at the base of the flowering stem, a thicker rhizome, and inflorescences with three versus one flower. Collections assigned to I. grijsi are from central or eastern China. The second is I. cavaleriei H. Lév. from Guizhou Province (using the historic name, Kouy-Tchéou Province), the same province where one of the new species occurs. Léveillé (1905) noted the presence of narrow, short “interior” leaves and short vertical rhizomes in his recognition of the new species. Dykes (1913) interpreted the specimen of I. cavaleriei as having one elongated stem and a second flowering stem that was not yet visible, thus reducing the number of subtending smaller leaves, yet considered the taxon a synonym of I. grijsi.
In 2003, Darrell Probst collected one of the new species, I. probstii, at two sites in its native habitat in Guizhou Province and considered its flowers different from other species known from China. He sent materials of these plants to the author for further study. During this study it became apparent that the type specimen for I. henryi that was collected by Augustine Henry in 1885 and its description by Baker (1892) do not match the description of I. henryi in the Flora of China (Zhao et al. 2000) and most of the specimens identified as I. henryi that were studied by the author in Chinese herbaria in 2010. In addition, specimens at UC that were collected in 2001 by Darrell Probst in the Daba Mountains of northeastern Sichuan Province as I. henryi fit the description in Zhao et al. (2000) and are morphologically distinct from the type and description of I. henryi.
This study was undertaken to access the status of these two new species. The first goal was to determine morphological similarities and differences among these two new species, I. dabashanensis and I. probstii, and other species in series Chinenses. A second goal was to determine if species synonymized in I. speculatrix (I. cavaleriei and I. grijsi) are morphologically similar to I. speculatrix and distinct from each of the two new species. A third goal was to study materials of I. henryi and its illegitimate synonym, I. gracilipes Pamp., to determine if these two taxa are morphologically similar. Iris gracilipes Pamp. is illegitimate because the name was previously used by Asa Gray for a different species of Iris that is in section Lophiris Tausch (Gray 1858). A fourth goal was to use DNA sequence data to determine if I. dabashanensis and I. probstii are resolved in series Chinenses. A final goal was to comprehensively examine collections from southern China to identify previously collected specimens that represent these two new species.
Materials and methods
Morphological observations
Living specimens and vouchers of the two new species were examined and compared with each other. They were also compared to other species using descriptions, herbarium specimens, and images of herbarium specimens, focusing on species that occur in southern China and I. odaesanensis, a species resolved with molecular data in the same subclade as the two new species (Fig. 1). Herbarium sheets (BM, E, KUN, GH, P, PE, UC) and type descriptions of I. cavaleriei, I. gracilipes Pamp., I. grijsi, I. henryi, I. odaesanensis (type not seen), I. proantha, and I. speculatrix were compared to the new species. Iris specimens at KUN, PE, and UC were studied and photographed in May 2010 and E in May 2014. In July 2018 and October 2019 images of specimens were accessed and studied on the Natural History Museum data portal (BM; http://data.nhm.ac.uk), Harvard University Herbaria (A, ECON, GH; https://kiki.huh.harvard.edu/databases/image_search.php), and Muséum National d’Histoire naturelle vascular plant collection (P; https://science.mnhn.fr/institution/mnhn/collection/p/item/search). Images of specimens on the Chinese Virtual Herbarium (CVH; http://www.cvh.ac.cn/en) were accessed in September and October 2018 and August 2019 to study collections not previously seen, including a specimen of I. odaesanensis that was collected at the type locality and deposited in KUN after the visit in 2010. Collections of I. gracilipes Pamp. were not unearthed in these searches. In September 2019 materials of I. gracilipes Pamp. were requested from the herbarium at the Natural History Museum in Florence, Italy (Herbarium Universitatis Florentinae; FI) and six images were received, including the type. For this study, herbarium sheets or images of herbarium sheets of I. cavaleriei (1), I. gracilipes Pamp. (6), I. grijsi (23), I. henryi (54), I. odaesanensis (1), I. proantha (47), and I. speculatrix (322) were studied.
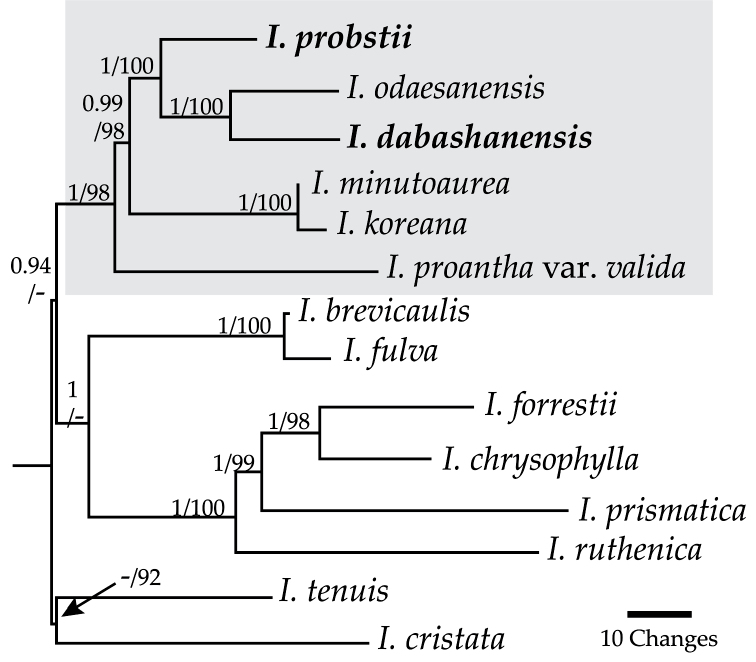
Figure 1.
Phylogenetic tree showing resolution of I. dabashanensis and I. probstii (bold) in series Chinenses (grey box). Posterior probability/bootstrap values shown above branches.
Phylogenetic analyses
Genomic DNA of the new species, I. probstii, was isolated from silica-dried leaf materials using protocols modified from the CTAB method of Doyle and Doyle (1987). Modifications of this procedure included RNase treatment and an ethanol precipitation with ammonium acetate following the initial isopropanol precipitation. Plastid sequence data of the protein-coding matK gene, about 1,100 bp of the flanking trnK introns, and the intergenic spacer region trnL–F were generated and added to previously gathered data (Wilson 2009, 2011; Guo and Wilson 2013). The new species, I. dabashanensis, was included in previous studies (Wilson 2011; Guo and Wilson 2013) but at the time was considered I. henryi. DNAs were amplified in 25 ml reactions using GoTaq Flexi DNA polymerase (Promega, Madison, Wisconsin, U.S.A.) following the manufacturer’s instructions, except that 1–2 ml of glycerol was added to the mix. Reaction conditions were: 97 °C for 1 min; 40 cycles of 97 °C for 10 s, 48 °C for 1 min, 72 °C for 20 s; 72 °C for 4 min. The matK gene and flanking trnK introns were amplified together in two reactions while trnL–F was amplified in a single reaction. Amplification and sequencing primers are given in Wilson et al. (2016). Amplicons were purified using the UltraClean PCR clean-up kit (Mo Bio, Carlsbad, California, U.S.A.). Purified PCR products were processed using a BigDye Terminating (Applied Biosystems, Foster City, California, U.S.A.) cycle sequencing reaction following the manufacturer’s instructions except that 5% DMSO was added to the reaction mix. Cycle sequencing products were purified using filter plates packed with Sephadex (Amersham Biosciences, Piscataway, New Jersey, U.S.A.) and run on an Applied Biosystems 3130 automated sequencer. Molecular data was gathered by the author at the Rancho Santa Ana Botanic Garden, Claremont, California, U.S.A.
Geneious 9.1.4 (Biomatters Ltd., Auckland, New Zealand) was used to assemble the generated sequence reads into loci, concatenate loci by species, and align the final dataset. Equivocal nucleotide sites were coded according to the IUPAC (IUB) codes. Maximum likelihood (ML; Felsenstein 1981) and ML bootstrap (Felsenstein 1985) analyses were performed using RAxML-HPC v. 8 (Stamatakis 2014). Five hundred replicates for bootstrap and two for ML were performed. Bayesian inference (BI) was performed on the dataset using MrBayes v. 3.2.6 (Ronquist et al. 2012). Five replicates were performed for BI with the number of substitution rates set to six, the number of nucleotide frequencies set to four, the gamma distribution was approximated using four rate categories, and a proportion of the nucleotide sites was allowed to be invariable. The BI analyses were set to run for two million generations, each with four chains, and were sampled every 1000 generations. The final average standard deviations of split frequencies was < 0.005 and the potential scale reduction factor (PSRF) was 1.0 for all node and branch parameters indicating convergence. Estimated sample size (ESS) for each parameter was > 440. All phylogenetic analyses were performed on XSEDE accessed through the CIPRES portal (https://www.phylo.org). The resulting ML tree was exported from the CIPRES portal and modified in Adobe Illustrator vers. 15.0.0 (Adobe Inc., San Jose, California, U.S.A.) to include posterior probabilities and identify the series Chinenses clade.
Distribution and mapping
Collection sites of specimens representing the two new species were located on Google Earth Pro vers. 7.3.2.5776 (Google LLC, Menlo Park, California, U.S.A.) to determine the known range of each species. Maps were produced in QGIS vers. 2.18.13 (QGIS Development Team 1991) using the free vector and raster map data available through Natural Earth (https://www.naturalearthdata.com). Maps were imported into Adobe Illustrator vers. 15.0.0 where species ranges were drawn.
Data resources
The data underpinning the analyses reported in this paper are deposited in the Dryad Digital Repository at: https://doi.org/10.6078/D1C695
Results
Morphological comparisons
The two new species are morphologically most similar to I. henryi but differ in several characters as described below in the new species’ diagnoses. The two new species and I. henryi share several characteristics that distinguish them from I. cavaleriei, I. grijsi, and I. speculatrix, including smaller flowers, slender rhizomes that are also short, and long slender pedicels that result in exerted flowers and ovaries. The two new species and I. henryi also lack reduced basal leaves that are present in I. cavaleriei and I. grijsi. Iris proantha differs morphologically from the new species and I. henryi in several characters, including slightly larger flowers, longer floral tubes, and pedicels that are shorter and wider. Pedicels in I. proantha are more similar to those in I. cavaleriei, I. grijsi, and I. speculatrix than to the new species or I. henryi. Collections from the Daba Mountains and identified as I. henryi were determined to represent the new species, I. dabashanensis. Recent descriptions of I. henryi either fit I. dabashanensis (Zhao et al. 2000) or are broad enough to include this new species as well as I. henryi (Mathew 1989; BIS 1997).
Phylogenetic studies resolved the two new species in series Chinenses in a clade comprised of the new species + I. odaesanensis (Fig. 1). In the description of I. odaesanensis, Lee (1974) considered the species allied to I. koreana but differing in having longer rhizomes and white rather than yellow flowers. Study of the online image of a herbarium sheet at KUN and the species description for I. odaesanensis illustrated differences and similarities with the two new species. The leaves of I. odaesanensis are approximately 1 cm in width, similar to leaves of I. koreana and wider than the 0.1–0.2 and 0.4–0.6 cm leaves present in the new species, I. dabashanensis and I. probstii, respectively. In addition, I. odaesanensis does not occur in south-central China but rather is documented from Korea and is reported from adjacent areas in Jilin Province, northeastern China. The flowers of I. odaesanensis are white with yellow on the center of the sepals; a distinct color and patterning that is not seen in other Asian Iris. Several characters, including short floral tubes, long pedicels, and exerted flowers and ovaries are shared among I. odaesanensis, the two new species, and I. henryi.
Study of specimens and type descriptions revealed morphological differences among I. cavaleriei, I. grijsi, and I. speculatrix. The former two taxa are considered synonyms of I. speculatrix. Each of these three taxa are distinct from the two new species because they lack the long pedicels, and short floral tubes present in I. dabashanensis and I. probstii and have larger flowers and rhizomes than species in series Chinenses. Notes from examination of the I. cavaleriei holotype deposited at the Royal Botanic Garden Edinburgh (E) describes a plant with basal leaves that are 0.3 cm in width with two veins, unequal lower bracts (9 and 12 cm), flower exerted above bracts, ovary hidden by bracts, sepals that are ca. 2.5 cm long x 0.4 wide, and reduced leaves around the base of the flower stem. Photographs taken at E show an ovary enclosed in bracts with the visible portion of the floral tube greater than 1 cm. A more precise description of the floral tube requires re-examination of the type because this measurement was not included in notes taken at E and Léveillé (1905) did not include measurements when he described the species. Images of herbarium sheets of I. grijsi from CVH, KUN, P, and PE, including the type (P), were studied as well as its description. The leaves of I. grijsi appear wider than those of I. cavaleriei and are given as 0.4–1 cm in the description while the bracts are long and narrow, similar to I. cavaleriei. Dykes (1913) gave the bract length of I. grijsi as 2–3 in (ca. 5–7 cm). Neither the flower nor ovary of I. grijsi are exerted above the bracts, although the flower is visible because it extends beyond the shorter of the two bracts due to its floral tube that is ca. 1.2 cm in length. Similar to I. cavaleriei, reduced leaves are present at the plant base. In contrast to these two taxa, I. speculatrix lacks reduced basal leaves, has a shorter floral tube (< 1 cm), and short unequal bracts (ca. 2.5 and 3.5 cm). It also has a flower that is exerted above the bracts and an oblong ovary within or partially exerted above the bracts but visible because the bracts are spreading.
Comparisons between descriptions and herbarium sheets of I. gracilipes Pamp. and I. henryi did not reveal differences between these two species supporting the inclusion of the illegitimately named I. gracilipes Pamp. within I. henryi. However, it is possible that there are differences between these two taxa that are not evident in the known collections of I. gracilipes Pamp. These collections were made by Reverend Père Cipriano Silvestri in the early 1900s in northwestern Hubei Province. The type location of I. henryi is in central Hubei Province from the area of Liantuo (Nanto) west of Ichang (Yichang). Future fieldwork in the region of Silvestri’s collections and the type location of I. henryi is planned to further investigate these taxa. Iris henryi was not located during fieldwork by a colleague in 2019 west of Yichang, leading to concern that the type location may have been lost with development of the Three Gorges Dam on the Yangtze River.
Additional collections of the new species
No additional collections of the new species from Guizhou Province, I. probstii, were identified during study of herbarium specimens and images of specimens, although three specimens from Guizhou Province were examined. One specimen identified as I. speculatrix was collected in 2013 and deposited at the Guizhou Academy of Sciences (HGAS; 123147). Two specimens identified as I. grijsi were collected in 1898 and 1900 and deposited at the Muséum National d’Histoire (P; 01840535 and 0184536, respectively). Study of the image of the former specimen revealed that it fit the description and type of I. speculatrix. Images of the two specimens identified as I. grijsi were also studied and are most similar to other collections of I. grijsi. Specimen 01840535 is particularly informative, although a scale was not included when imaged. This specimen illustrates the short leaves at the base of the plant that are typical for this taxon and the longer bracts when compared to I. speculatrix.
The study of herbarium records revealed 34 additional specimens from the Daba Mountains that are the new species, I. dabashanensis, but were identified as other species. These represent sheets from nine separate collections from the Chongqing Municipality and one from the Shennongjia Forest Region in Hubei Province. Three botanists, Père Paul Guillaume Farges in 1892, Tain Lun Dai in 1958, and Du Wei in 2014 made these collections. Eight of the collections were identified as I. henryi while the 2014 collection was identified as I. proantha. It is not surprising that these collections were mostly identified as I. henryi because I. dabashanensis is similar to this species and in particular has long slender pedicels and flowering stems that are shorter than leaves at flowering, two characters considered distinguishing for I. henryi. A few other collections from near the Daba Mountains may also represent I. dabashanensis but a determination could not be made because flowers were lacking or important characters were not clearly visible.
Phylogenetics
The aligned, combined dataset was 3,551 bp with 398 (11%) variable and 153 (4%) potentially parsimony informative characters. The matK/trnK marker was more informative than trnL–F because it contributed 2,696 bp with 322 variable and 114 potentially parsimony informative characters. The resulting models from RAxML and MrBayes analyses differed only slightly in the three substitution rate and gamma distribution values (the numbers given are based on RAxML). Both assumed unequal base frequencies, three substitution rates (1.0000, 2.8325, 0.1513), a gamma distribution of 0.5726 and 0.3177 invariable sites. The single ML tree from RAxML and the consensus tree from MrBayes each had a log likelihood = -7,779 and shared the same topology (Fig. 1). Posterior probability and bootstrap values are given in Figure 1.
The two new species were resolved with high support in a clade with I. odaesanensis. As described above, these three species share several morphological characters, including long slender pedicels and short floral tubes. They were resolved with high support within the series Chinenses clade and share several characters with other species in the series, including small, horizontally spreading flowers where petals are not upright, an overall small and delicate stature, short rhizomes, and an Asian distribution. All taxa in series Chinenses, except I. henryi, the nominative form of I. proantha, and I. rossii, were included in this study. Further studies to resolve relationships within the series should include these three taxa and also I. grijsi, I. cavaleriei, and I. speculatrix. The former two taxa are considered synonyms of I. speculatrix but in this study were shown as morphologically distinct. The type locality for I. henryi is not precise and the species has not been relocated in the area of its initial discovery.
Other findings
The study also resulted in the discovery of two type collections and one correction to a species description citation. The type of I. speculatrix (C. Ford 18465) was discovered in BM collections stored as a type of I. henryi and the type of I. grijsi (de Grijs 8583) in P collections but not designated as a type. The description of I. cavaleriei is typically cited as Liliac. & C. Chine 18 1905, but previous searches for this journal were not successful. During this study the author searched journals where Augustin Abel Hector Léveillé published species descriptions, focusing on journals where he published infrequently. The correct citation is given in the references (Léveillé 1905). In this publication Léveillé lists Iridaceae known from China and provides a description of I. cavaleriei sp. nov. citing the collection of Julien Cavalerie from Kouy-Tcheou (Guizhou) Province on May 15, 1900.
Taxonomy
Iris dabashanensis
C.A.Wilson sp. nov.
70F7C51E-1F0F-5B01-B935-E283C1C62D96
urn:lsid:ipni.org:names:77211598-1
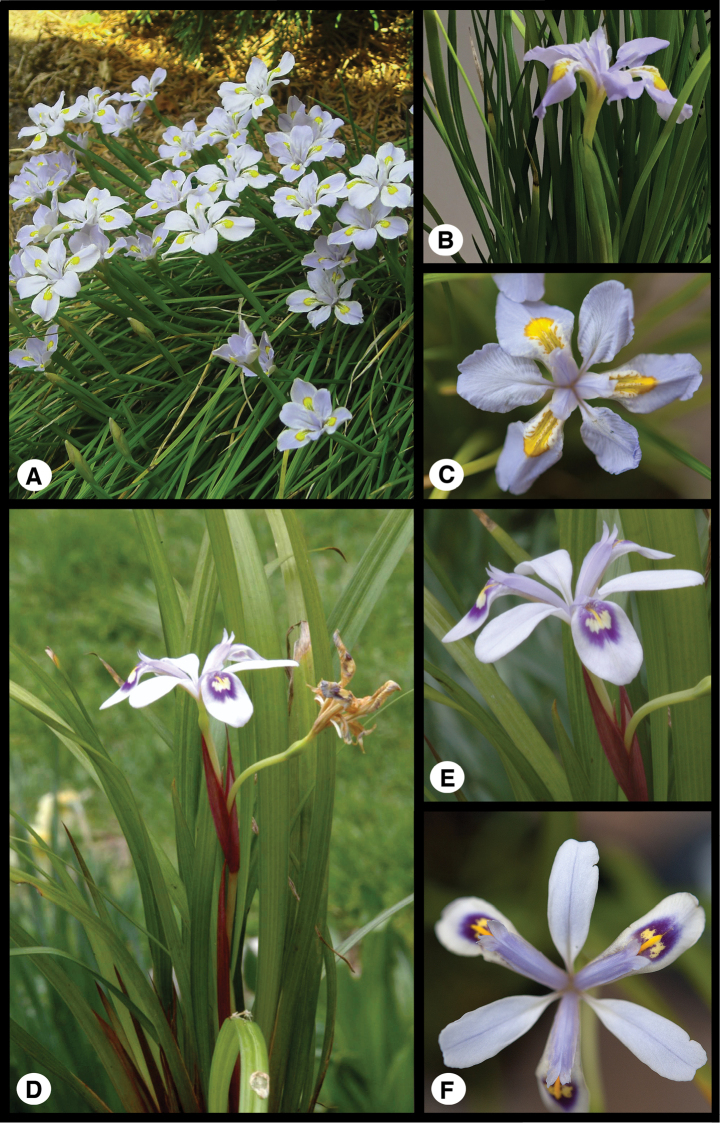
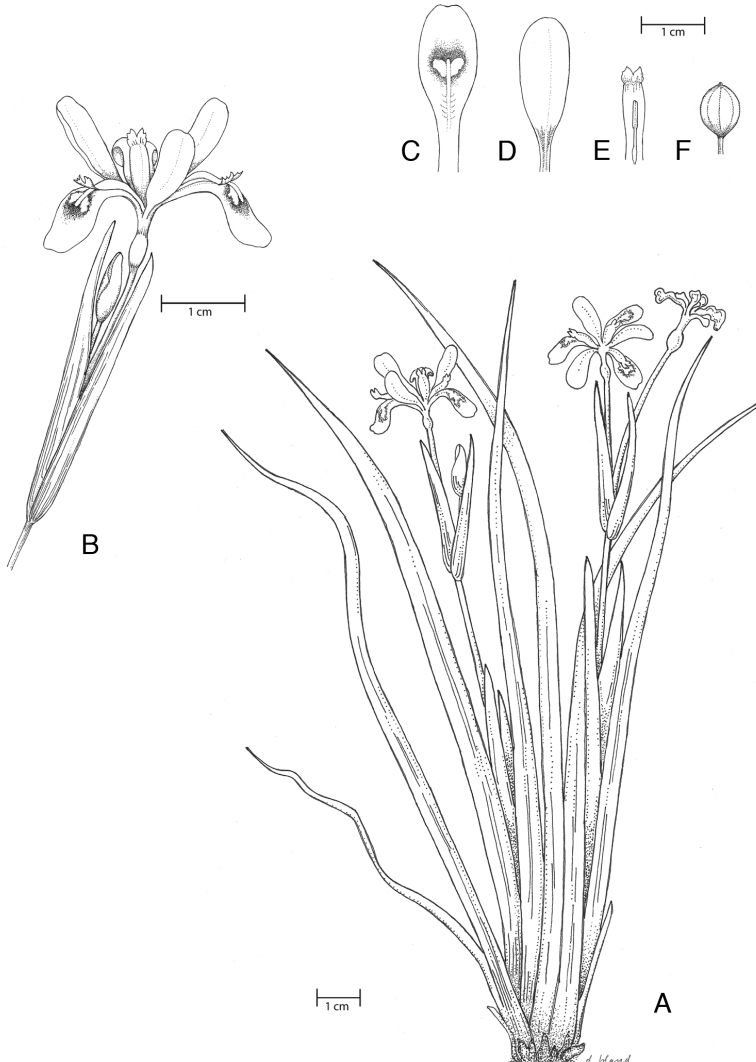
Figure 2.
Images A–CI. dabashanensisD–FI probstii. A Habit B inflorescence C flower D habit E inflorescence F flower (Photos B–F C. author; A Mark McDonough).
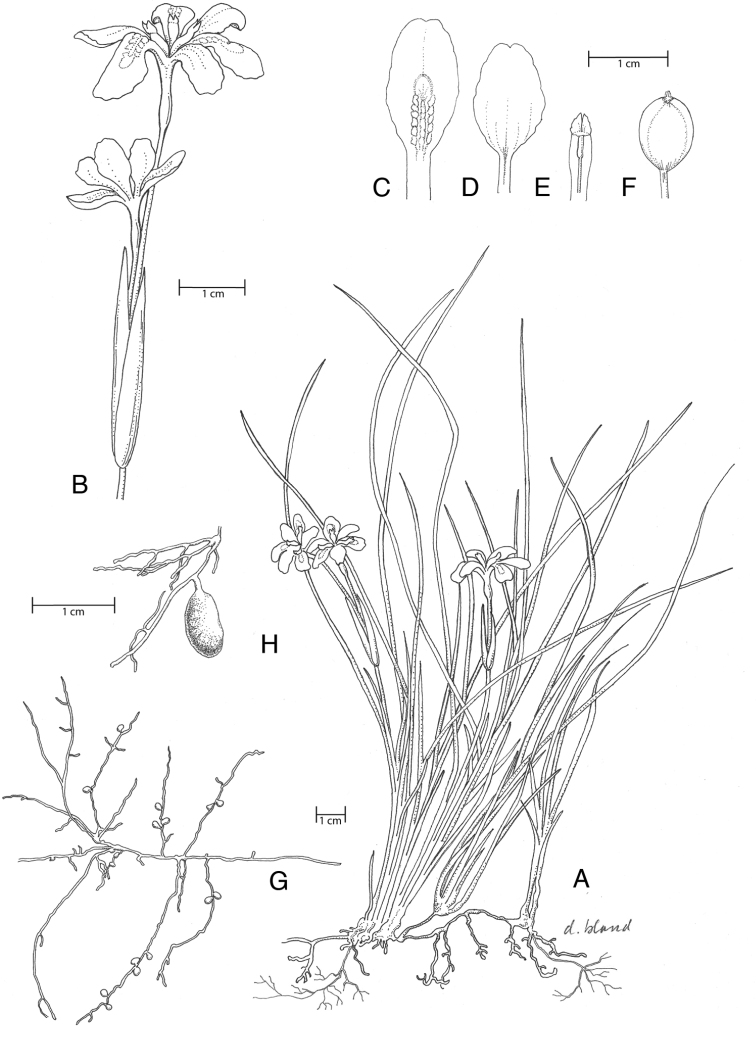
Figure 3.
Illustration of I. dabashanensis. A Habit B inflorescence C sepal D petal E style branch, anther F fruit G stolon, roots, tubers H root tuber (Source: A–ED. Probst CPC3.5.01.3 (UC); F photograph by Marty Schafer and Jan Sacks; G, H photograph by D. Probst, photographs available from author).
Diagnosis.
Morphologically similar to I. henryi the new species differs in having narrower leaves (0.9–2 mm versus 4–4.5 mm), shorter bracts (2–4 cm versus 4–7 cm), and shorter style branches (1 cm versus 1.8 cm).
Type.
China. Sichuan Province: About 5 km N of Wanyuan, Wanyuan County, 1,140 m, 32°11.720'N, 108°05.310'E, 3, May 2001 (fl), voucher from cultivated material, D. Probst CPC3.5.01.3 (holotype: UC!; isotypes: PE!, E!).
Description.
Small evergreen herbaceous plant with aerial flowering stems less than basal leaves. Bracts, cauline leaves, base of basal leaves ± tinged with red. Rhizomes short and branched with fibrous leaf remains. Stolons present. Roots slender, branched, with tubers to 6 mm. Basal leaves distichous, linear with acute apex, bright green, slightly glossy, 28–44 cm long, 0.9–2 (3) mm wide, 1–2 veins; crowded in clumps. Flowering stem ca. 8–18 cm with 1 or 2 cauline leaves, with upper cauline leaf extending well above mid stem. Inflorescence with two opposite bracts (spathes) subtending 2 (3) flowers, terminal flower opening first with a single bract subtending each subsequent flower; lower bracts ± equal, 2.0–4 cm long, 0.4–0.5 cm wide; pedicels long (2.5–5 cm) and slender. Flowers light violet, open, ca. 3 cm diameter, radial with petals slightly angled upward, sepals light violet, horizontal and recurved distally; floral tube 0.2–0.5 cm; sepals ovate, shallow apical notch, narrowed at base, 2–3 cm long, 0.6–1.1 cm wide, obvious median ridge cream-colored with violet spots, a lateral ridge on either side of the median, yellow patch between lateral ridges and extending beyond ridges; petals light violet, rotund, apical notch, 1.6–2.1 cm long, ca. 1.0 cm wide, clawed in lower 1/4; stamens cream, ca. 0.8 cm long, anthers = filaments; style branches petaloid, light violet, ca. 1 cm long, 0.3 cm wide, bi-lobed distally, lobes ca. 0.4 cm long, stigma broad, slightly rounded. Capsule rounded, ca. 1 cm long. Seed light to medium brown, ca. 0.4 cm, with conspicuous white appendage. Flowering: April to May.
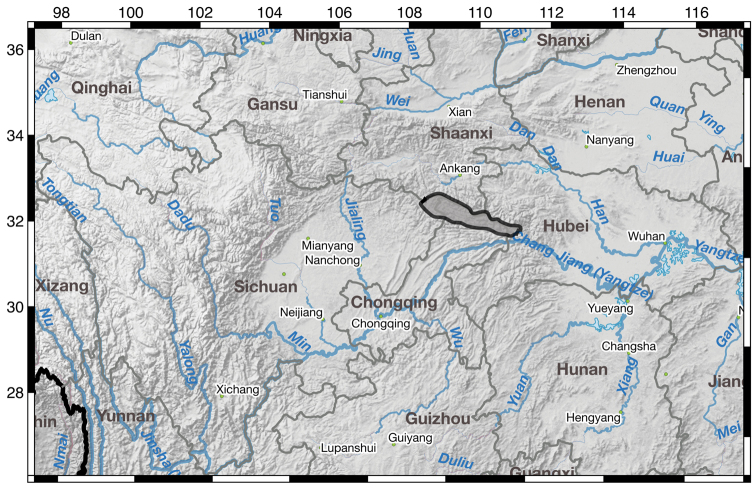
Distribution and ecology.
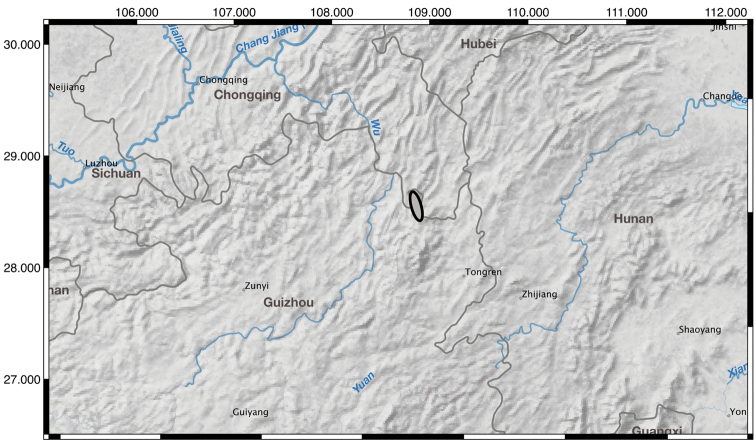
Iris dabashanensis is known from the Daba Mountains in Sichuan and Hubei Provinces and Chongqing Municipality (Fig. 4) on open rocky slopes with calcareous soils associated with shrubs or mixed conifer and hardwood forests.
Figure 4.
Map of known distribution for I. dabashanensis in gray outlined in black.
Etymology.
The new species is named for the Daba Mountains in China where it occurs.
Preliminary conservation status.
Following the criteria and categories of IUCN (2012), Iris dabashanensis is given a preliminary status of Least Concern (LC). One of the known populations is in the Shennongjia Forest Region of Hubei Province. Although the precise location of the I. dabashanensis population is unknown, the Shennongjia National Nature Reserve occurs within the region and has protected status.
Other specimens examined.
China. 4 ⚥ Chongqing Municipality, Chengkou County; 29 April 1958; T.L. Dai leg.; 100265; HNWP 34156, PE 01012365, SZ 00043052, SZ 00043057; · 5 ⚥ Chongqing Municipality: Chengkou County; 1 May 1958; T.L. Dai leg.; 100335; CDBI 0169543, IBK 00251078, SZ 00043056, PE 00034001, PE 01012364; · 4 ⚥ Chongqing Municipality: Liangzhong Hewu ji, Chengkou County; 20 April 1958; T.L. Dai leg.; 100150; PE 00034002, SZ 00043055, HNWP 34209, IBK 00251010; · 1 ⚥ Chongqing Municipality: Liang Yizh, Chengkou County; 2 April 1958; T.L. Dai leg.; 100154; CDBI 0169546; · 6 ⚥ Chongqing Municipality: Yizi Liang Hengyan pengwuji, Chengkou County; 24 May 1958; T.L. Dai leg.; 100583; IBK 00081641, CDBI 0169544, CDBI 0169545, HNWP 34583, SZ 00043061, PE 01012366; · 1 ⚥ Chongqing Municipality: Chengkou County; sin. date; Farges leg.; 101; P 02163317; · 4 ⚥ Chongqing Municipality: Chengkou County; 1892; Farges leg.; 1024; P 02163312, P 02163313, P 02163314, P 02163316; · 11 ⚥; Hubei Province: Shennongjia Forest Region; 27 April 2014; Du Wei leg.; 1407; WH 1933.
Notes.
The date on the specimen T.L. Dai 100154 was 1918 but the collections made at the same time and place had a date of 1958 which is consistent with Dai’s numbering of specimens and other collections.
Iris probstii
C.A.Wilson sp. nov.
839FE4C0-A63B-5D47-8308-F578340B6DEE
urn:lsid:ipni.org:names:77211599-1
Figure 5.
Illustration of I. probstii. A Habit B inflorescence C sepal D petal E style branch, anther F fruit (Sources: A–ED. Probst CPC24.7.03.1 (UC); F photograph by M. Schafer and J. Sacks, photographs available from author).
Diagnosis.
Morphologically similar to I. henryi, the new species differs in having wider sepals (ca. 7 mm versus 4 mm) and style branch lobes that are shorter (ca. 2–3 mm versus 6 mm).
Type.
China. Guizhou Province: about 24 km south of Yanhe on road to Xiushan, 970 m, 28°25.643'N, 108°42.044'E, 24, July 2003 (fl), voucher from cultivated material, D. Probst CPC24.7.03.1 (holotype: UC!; isotypes: PE!, E!).
Description.
Small evergreen herbaceous plant with aerial flowering stems shorter than basal leaves. Bracts, cauline leaves, base of basal leaves ± tinged with red. Rhizomes short (< 4 mm), branched, hidden by leaf remains. Stolons present. Roots highly branched distally with small tubers. Basal leaves distichous, linear with acute apex, bright green, slightly glossy on upper surface, dull green on lower surface, leaf edges obscurely membraneous, 12–40 cm long, 0.4–0.6 cm wide, veins slightly thickened; crowded in clumps. Flowering stem ca. 15 cm with 2 (3) cauline leaves on lower half. Inflorescence with two opposite bracts (spathes) subtending 2 (3) flowers, terminal flower opening first with a single bract subtending each subsequent flower; lower bracts ± unequal, 3.5–6 (7) cm long, 0.4–0.5 cm wide; pedicels long (3–4.5 cm) and slender. Flowers cream with light violet, open, ca. 3 cm diameter, radial with petals slightly angled upward, sepals horizontal and recurved distally; floral tube 0.3–0.4 cm; sepals cream adaxially, light yellow abaxially, narrowly obcordate, ± shallow apical notch, narrowed at base, 2.2–2.9 cm long, 0.6–0.9 wide, obvious yellow-orange median ridge, purple spot beginning at ca. midpoint and extending distally beyond ridge with two bright yellow patches flanking ridge distally; petals cream with light violet tint that is more prominent along median, narrowly spathulate, 1.8–2.2 cm long, 0.6–0.7 cm wide, clawed in lower 1/4; stamens cream, ca. 1 cm long, anthers = filaments; style branches medium violet, 1.2–1.5 cm long, 0.3–0.4 cm wide, bi-lobed distally, light violet lobes ca. 0.2–0.3 cm long with several teeth, stigma broad, slightly rounded. Capsule rounded with short apical beak. Seed light to medium brown, ca. 0.4 cm, with conspicuous white appendage. Flowering: April to May.
Distribution and ecology.
Iris probstii is currently known from two locations south of Yanhe in Guizhou Province China (Fig. 6) at about 800 to 1,000 m and a third unverified location about 165 km south of Yanhe. This third site, southeast of Tongren, Guizhou, China near Minhezhen (Minhe town), is based on photographs taken by C. Yang in 2016 and uploaded to the Plant Photograph Bank of China (http://ppbc.iplant.cn/tu/6166544). A study of herbarium records from CVH, KUN, and PE did not reveal additional collections but it is likely that this new species occurs in other areas within this region. The species occupies open slopes along edges of rice paddies or under low pine woods in grassy sites associated with rocky, karst soils.
Figure 6.
Map of known distribution for I. probstii in gray outlined in black.
Etymology.
The new species is named in honor of the U.S. horticulturist, Darrell Probst, in recognition of his work to expand our knowledge of species from series Chinenses in their native habitats.
Preliminary conservation status.
Following the criteria and categories of IUCN (2012), Iris probstii is given a preliminary status of Vulnerable (VU D2) due to its restricted area of known occupancy (< 20 km2) and number of locations (< 5). The area of occurrence does not have protected status. If the third location is verified and vouchered the conservation status might change.
Other specimens examined.
China. Guizhou Province: about 55 km south of Yanhe on road to Xiushan, 825 m, 28°19.651'N, 108°41.390'E, 9, January 2002 (fl), D. Probst CPC9.1.02.2 (photo of living plant, UC).
Notes.
D. Probst collected a small rhizome segment at the second site that he grew but did not voucher. An image of the plant was obtained for documentation and deposited in UC. A specimen voucher will be made and also deposited at UC when the plant reflowers.
Discussion
Morphologically these two new species are most similar to I. henryi because they share several characters, including long (> 2.5 cm) slender pedicels, short floral tubes, and narrow leaves (< 0.6 cm). Iris odaesanensis shares the characters of short floral tubes and long pedicels with the two new species and I. henryi but it has leaves that are about 1 cm in width. Molecular data indicates that the two new species are closely related to I. odaesanensis. Unfortunately, because materials of I. henryi were not available for molecular studies it is unknown if all series Chinenses species with short floral tubes and long pedicels are resolved in a clade. These four species can easily be distinguished from each other using several characters. Iris odaesanensis has flowering stems that are almost equal or slightly longer than leaves at flowering while I. dabashanensis, I. henryi, and I. probstii have flowering stems that are substantially shorter than their leaves at flowering. Iris dabashanensis has very narrow (ca. 1–2 mm) leaves while the width of I. henryi and I. probstii leaves are similar at 4–6 mm. Iris dabashanensis also has a median and two lateral ridges while I. henryi, I. odaesanensis, and I. probstii have only a median ridge. Iris probstii has style branches where lobes are 0.2–0.3 cm long which is ca. 1/4 of their total length while lobes in I. dabashanensis and I. henryi are > 0.4 cm and ca. 1/2 of their total length.
Both I. dabashanensis and I. probstii occur in areas that are rocky and considered limestone rich. The border area between Chongqing Municipality and Guizhou Province south of Yanhe, where I. probstii occurs, is considered a karst region with a deep layer of limestone and dolomite and characteristic landforms of caves, sinkholes, outcrops, natural bridges, and gorges. Guizhou Province is one of four provinces that make up the South China Karst, UNESCO World Heritage Site. The Daba Mountains are also rich in limestone and on the northwestern slopes of the Daba Mountains in Shaanxi Province there is a large region of karst. However, the southern slopes of the Daba Mountains above the Sichuan Plateau where I. dabashanensis occurs is not considered a karst region although the soils are calcareous.
An examination of available materials suggests that I. cavaleriei and I. grijsi are similar to each other but morphologically distinct from I. speculatrix. They are also morphologically distinct from the two new species and other series Chinenses species. Previous research utilizing molecular data (Tillie et al. 2001; Guo and Wilson 2013) concluded that I. speculatrix is not within series Chinenses, a finding consistent with the larger flowers and rhizomes of I. speculatrix compared to species in series Chinenses. The larger flowers and rhizomes of I. speculatrix are shared with I. cavaleriei and I. grijsi. The status of I. cavaleriei, which was described after I. grijsi, is not certain although results of this study indicate leaves of I. cavaleriei are narrower than those of I. grijsi and the flower is only partially exerted above the bracts while it is entirely exerted in I. grijsi. These observations are based on only one collection, the holotype, of I. cavaleriei. Examining additional plants from the type locality for I. cavaleriei and/or the identification of additional existing collections is required to determine if the differences observed in the one known collection are consistent within the taxon and if the taxon is distinct from I. grijsi.
It is surprising that during this study of over 450 specimens representing six Iris species with collections in southern China, only three were collected in Guizhou Province. Guizhou Province is a mountainous rural region that is recognized as having high plant diversity (Zhang and Ma 2008; Liu et al. 2018) and endemism (Liu et al. 2018). The karst landscape and ecology is considered an important contributor to the high biodiversity of the Province. Iris may be undercollected within the region indicating that further fieldwork is necessary to more fully understand Iris diversity in the Province and the entire range of I. probstii.
Key to Iris series Chinenses
| 1 | Leaves > 1 cm wide | 2 |
| – | Leaves < 1 cm wide | 3 |
| 2 | Floral tube ca. 1 cm long, flowers yellow | I. koreana |
| – | Floral tube ca. 0.5 cm long, flowers white | I. odaesanensis |
| 3 | Floral tube > 2.0 cm long, pedicels < 2 cm long | 4 |
| – | Floral tube < 1.0 cm long, pedicels > 2 cm long | 6 |
| 4 | Floral tube ca. 6 cm long | I. rossii |
| – | Floral tube 2–3.5 cm long | 5 |
| 5 | Flowers pale violet, floral tube 2.5–3.5 cm long | I. proantha |
| 5a | Flowering stem to bract < 5 cm tall, leaves < 0.4 cm wide | var. proantha |
| – | Flowering stem to bract > 7 cm tall, leaves > 0.5 cm wide | var. valida |
| – | Flowers yellow, floral tube ca. 2 cm | I. minutoaurea |
| 6 | Leaves < 3 mm wide, lateral crests flanking median | I. dabashanensis |
| – | Leaves > 3 mm wide, median crest only | 7 |
| 7 | Style branch lobes 2–3 mm long, sepals ca. 7 mm wide | I. probstii |
| – | Style branch lobes ca. 6 mm long, sepals ca. 4 mm wide | I. henryi |
Supplementary Material
Acknowledgements
I thank Jan Sacks and Marty Schafer for information on seed characteristics, photographs of fruits for both species, and helpful comments on an earlier version of the manuscript, Darrell Probst for photographs of underground structures of I. dabashanensis and information on his collections of these two new species, and X. Zhang for searching for the type locality of I. henryi. I also thank Jaemin Lee who assisted with phylogenetic analyses, Diane Bland who illustrated the new species, reviewers, David Boufford, Manuel Crespo, and Evgeny Mavrodiev, who provided valuable comments, and the associate editor for this paper, Lorenzo Peruzzi. Suggestions by David Boufford were particularly helpful because of his knowledge of the flora and geography of China.
Publication made possible in part by support from the Berkeley Research Impact Initiative (BRII) sponsored by the UC Berkeley Library.
Citation
Wilson CA (2020) Two new species in Iris series Chinenses (Iridaceae) from south-central China. PhytoKeys 161: 41–60. https://doi.org/10.3897/phytokeys.161.55483
Supplementary materials
Supplementary file for Dryad
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Carol A. Wilson
Data type
Nucleotide dataset, phylogenetic tree
Explanation note
This is a draft file for submission to Dryad as supporting materials.
References
- Baker JG. (1892) Handbook of the Irideae. George Bell & Sons, London, 6 pp 10.5962/bhl.title.15431 [DOI] [Google Scholar]
- BIS (1997) Series Chinenses. In: The Species Group of the British Iris Society (Eds) A Guide to Species Irises, Their Identification and Cultivation.Cambridge University Press, 121–126.
- Crespo MB, Martínez-Azorín M, Mavrodiev EV. (2015) A new comprehensive generic arrangement of the ‘Iris sensu latissimo’ clade (Iridaceae), congruent with morphology and molecular data. Phytotaxa 232(1): 1–78. 10.11646/phytotaxa.232.1.1 [DOI] [Google Scholar]
- Doyle JJ, Doyle JL. (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19: 11–15. [Google Scholar]
- Dykes WR. (1913) The Genus Iris. Dover Publications, Inc, New York, 245 pp 10.5962/bhl.title.116246 [DOI] [Google Scholar]
- Felsenstein J. (1981) Evolutionary trees from DNA sequences: A maximum likelihood approach. Journal of Molecular Evolution 17(6): 368–376. 10.1007/BF01734359 [DOI] [PubMed] [Google Scholar]
- Felsenstein J. (1985) Confidence limits on phylogenies: An approach using the bootstrap. Evolution; International Journal of Organic Evolution 39(4): 783–791. 10.1111/j.1558-5646.1985.tb00420.x [DOI] [PubMed] [Google Scholar]
- Gray A. (1858) Diagnostic characters of new species of phaenogamous plants, collected in Japan by Charles Wright, botanist of the U. S. North Pacific Exploring Expedition. (Published by request of Captain John Rodgers, commander of the expedition.) With observations upon the relations of the Japanese flora to that of North America and of other parts of the northern temperate zone. Memoirs of the American Academy of Arts and Sciences 6: 1–412. 10.2307/25057953 [DOI] [Google Scholar]
- Guo J, Wilson CA. (2013) Molecular phylogeny of crested Iris based on five plastid markers (Iridaceae). Systematic Botany 38(4): 987–995. 10.1600/036364413X674724 [DOI] [Google Scholar]
- IUCN (2012) Red List Categories and Criteria. Version 3.1. Second edition. IUCN, Gland, Switzerland, 32 pp. [Google Scholar]
- Lawrence GHM. (1953) A reclassification of the genus Iris. Gentes Herbarum 8: 346–371. [Google Scholar]
- Lee YN. (1974) New taxa on Korean flora. Korean Journal of Botany 17: 33–35. [Google Scholar]
- Léveillé H. (1905) Liliacées, Amaryllidacées, Iridacées et Hérmodoracées de Chine. Memorie Pontificia Accademia Romana dei Nuovi Lincei 23: 1–346. [Google Scholar]
- Liu B, Zhang M, Bussmann WR, Liu H-M, Liu Y-Y, Peng Y-D, Zu K-L, Zhao Y-M, Liu Z-B, Yu S-X. (2018) Species richness and conservation gap analysis of karst areas: A case study of vascular plants from Guizhou, China. Global Ecology and Conservation 16: e00460 10.1016/j.gecco.2018.e00460 [DOI]
- Mathew B. (1989) The Iris, Reprint with Revisions. B. T. Batsford Ltd, London, 215 pp. [Google Scholar]
- Mavrodiev EV, Martínez-Azorín M, Dranishnikov P, Crespo MB. (2014) At least 23 genera instead of one: The case of Iris L. s.l. (Iridaceae). PLoS ONE 9(8): e106459. 10.1371/journal.pone.0106459 [DOI] [PMC free article] [PubMed]
- Maximowicz CJ. (1880) Iris L. Bulletin de l'Académie impériale des sciences de St.-Pétersbourg series 3, 26: 1–515.
- QGIS Development Team (1991) QGIS geographic information system. Open Source Geospatial Foundation Project. http://qgis.osgeo.org
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61(3): 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. (2014) RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics (Oxford, England) 30(9): 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillie N, Chase MW, Hall T. (2001) Molecular studies in the genus Iris L.: A preliminary study. Annali di Botanica (Roma) 1: 105–112. [Google Scholar]
- Wilson CA. (2009) Phylogenetic relationships among the recognized series in Iris section Limniris. Systematic Botany 34(2): 277–284. 10.1600/036364409788606316 [DOI] [Google Scholar]
- Wilson CA. (2011) Subgeneric classification in Iris re-examined using chloroplast sequence data. Taxon 60(1): 27–35. 10.1002/tax.601004 [DOI] [Google Scholar]
- Wilson CA, Padiernos J, Sapir Y. (2016) The royal irises (Iris subg. Iris sect. Oncocyclus): Plastid and low-copy nuclear data contribute to an understanding of their phylogenetic relationships. Taxon 65(1): 35–46. 10.12705/651.3 [DOI] [Google Scholar]
- Zhang YB, Ma KP. (2008) Geographic distribution patterns and status assessment of threatened plants in China. Biodiversity and Conservation 17(7): 1783–1798. 10.1007/s10531-008-9384-6 [DOI] [Google Scholar]
- Zhao Y, Noltie HJ, Mathew B. (2000) Iridaceae. The Flora of China 24: 297–313. http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=116503 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file for Dryad
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Carol A. Wilson
Data type
Nucleotide dataset, phylogenetic tree
Explanation note
This is a draft file for submission to Dryad as supporting materials.