Structured Abstract
Background:
There is poor understanding of the comparative effectiveness of lobar and sublobar resections for limited stage small cell lung cancer (SCLC). We analyzed the National Cancer Database (NCDB) to examine the outcomes of patients undergoing wedge resection (WR), segmentectomy (SR), and lobectomy (LB) for limited stage SCLC.
Methods:
Patients with cT1-2N0M0 SCLC (2004-2015) who underwent definitive surgery were identified and stratified by extent of resection: WR, SR, or LB. The primary outcome was overall survival (OS) and secondary outcomes were margin-positive resection (>R0) and pathologic nodal upstaging.
Results:
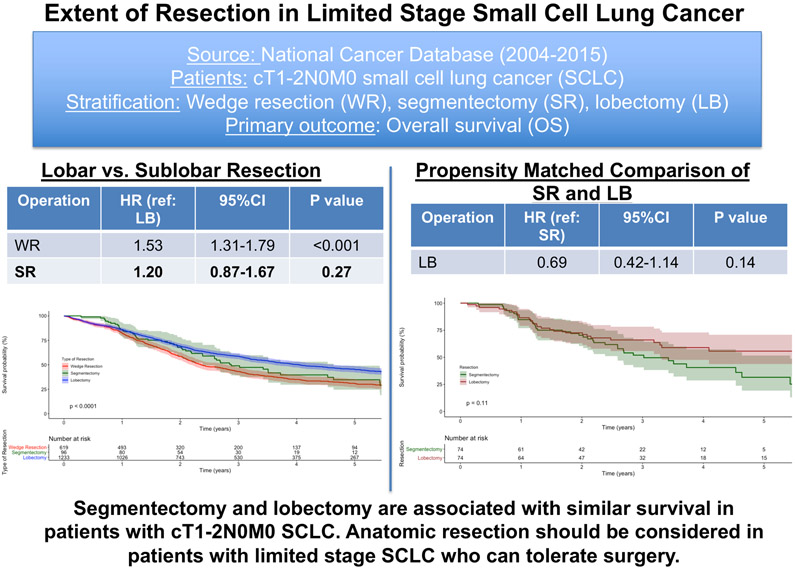
A total 1948 patients met study criteria: 619 (32%) underwent WR, 96 (5%) SR, and 1233 (63%) LB. Patients receiving LB were more likely to be younger, have fewer comorbidities, and be privately insured. The unadjusted five-year OS of WR, SR, and LB patients was 31% (95%CI 27-35), 35% (95%CI 25-49), and 45% (95%CI 42-49), respectively. In a multivariable Cox model, WR was associated with worse OS (HR 1.53; 95%CI 1.31-1.79) and SR similar OS (HR 1.20; 95%CI 0.87-1.67) compared to LB. SR was associated with similar survival compared to LB in a propensity score-matched multivariable analysis as well. WR was also associated with higher odds of >R0 resection compared to LB.
Conclusions:
In this study, patients with limited stage SCLC undergoing wedge resection experienced worse survival compared to those undergoing lobectomy; survival was similar between segmentectomy and lobectomy.
Graphical abstract.
In this National Cancer Database study, patients receiving segmentectomy (SR) and lobectomy (LB) experienced similar outcomes even after propensity score-matching. Patients receiving a wedge resection (WR) had worse survival compared to those receiving lobectomy. Our study suggests that anatomic resection should be considered for patients with limited stage small cell lung cancer. HR = hazard ratio; 95%CI = 95% confidence interval
Introduction
The National Comprehensive Cancer Network (NCCN) guidelines recommend consideration of surgery for patients with limited stage small cell lung cancer (SCLC)1. Two prospective trials have examined the role of surgery in SCLC. The Medical Research Council trial2 randomized 144 patients with resectable SCLC to surgery or definitive radiation and found that unadjusted mean survival was lower for patients in the surgery arm. Similarly, Lad and colleagues3 randomized 146 patients with limited SCLC who demonstrated a response to initial chemotherapy to undergo surgery compared to no surgery and found similar median survival in the two groups. As a result, surgery has not been the mainstay treatment for even limited SCLC. However, these trials have several limitations. Both trials randomized few patients and analyzed patients in an intention-to-treat manner and many patients assigned to surgery did not undergo resection. For instance, 52% of patients randomized to surgery in the Medical Research Council trial either received no surgery or a thoracotomy without resection. Similarly, 17% of patients in the trial reported by Lad and colleagues did not undergo a resection. Both trials are also decades old. With the advent of platinum-based chemotherapy and improved staging and surgical techniques, the multimodal treatment of lung cancer has progressed significantly in the ensuing decades. While contemporary observational studies have demonstrated that patients with limited stage and even locally advanced SCLC undergoing surgery experience good outcomes compared to those undergoing chemoradiation, surgery remains underused4-13.
One of the primary concerns about surgery remains the perioperative morbidity and mortality associated with lobectomy, which remains the standard of care in surgical treatment of lung cancer. Sublobar resection, including wedge resection and segmental resection, has been described as an option for patients with poor pulmonary reserve or performance status in non-small cell lung cancer (NSCLC), with early perioperative data from the CALGB/Alliance 14053 trial demonstrating similar perioperative mortality and morbidity in patients treated with lobar and sublobar resection14-16. However, there are no prospective data and few observational studies comparing lobar and sublobar resection in limited stage SCLC17-20. The decision to offer surgery and the type of surgery to offer is further complicated by the timing of diagnosis of SCLC. SCLC is rarely diagnosed following a core needle biopsy and histological confirmation is often made during or after surgery, when a patient may have been committed to a particular initial treatment.
The aim of this study was to compare the outcomes of lobar and sublobar resection in patients undergoing definitive surgery for limited stage SCLC. We hypothesized that lobectomy would be associated with improved overall survival compared to wedge and segmental resection in this patient population.
Methods
Data Source and Patient Selection
This study was deemed exempt by the Duke University Institutional Review Board. The NCDB is a collaborative effort of the American Cancer Society and the American College of Surgeons. It contains data about 80% of cancers diagnosed annually in the United States across 1500 centers21. Data are entered by certified, independent tumor registrars who use standardized guidelines.
The NCDB was queried for patients with American Joint Commission on Cancer (AJCC) 8th Edition clinical stage T1-2N0M0 SCLC who underwent wedge resection (WR), segmental resection (SR), or lobectomy (LB) between 2004 and 2015. Patients receiving a bilobectomy or bronchial sleeve resection were classified as LB. Patients who received non-surgical treatment, other types of surgery including unknown description of surgery, who received neoadjuvant chemotherapy or radiation, or who had missing survival information were all excluded (Figure 1).
Figure 1.
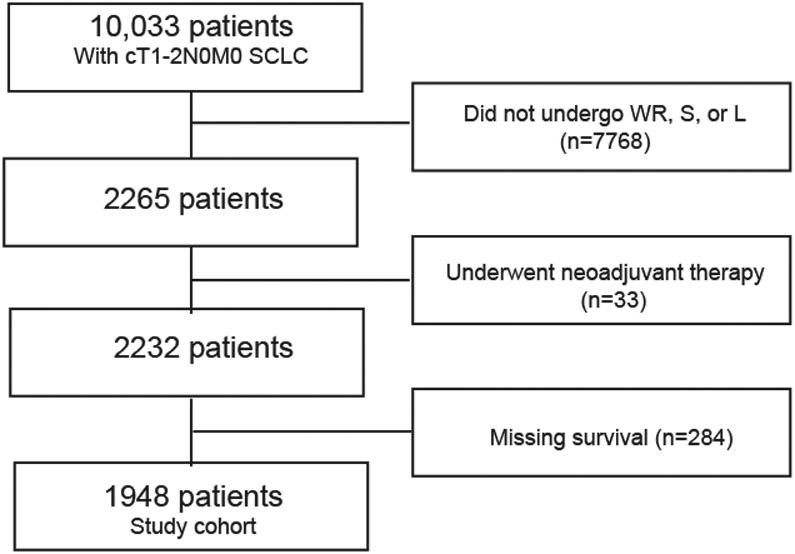
Patient selection scheme for the study
Study Design
Patients were stratified by type of resection: WR, SR, or LB. Background characteristics between the groups were compared using the Wilcoxon rank sum and Pearson’s chi-squared tests for continuous and categorical measures, respectively. The primary outcome was overall survival (OS), which was estimated using Kaplan-Meier and multivariable Cox Proportional Hazards methods. Variables included in the multivariable Cox model were selected a priori based on availability in the NCDB, prior literature demonstrating their association with survival, and from clinical experience: age, sex, race, year of diagnosis, Charlson-Deyo Comorbidity Index (CDCC) score, insurance status, treatment at an academic center, and tumor size. The proportional hazards assumption for the variables and overall model was checked with visual and quantitative representations of Schoenfeld residuals. In every model, the proportional hazards assumption was met; the p value of the Schoenfeld residual for the covariates and the overall model was <0.05.
The secondary outcome measures were odds of a margin-positive resection and odds of pathologic nodal upstaging. These dichotomized outcomes were modelled with multivariable logistic regression using the same variables described above. Only patients with nodes assessed in surgery were included in the logistic regression for pathologic nodal upstaging.
Several additional analyses were performed. (1) Because of the imbalances in preoperative characteristics between patients receiving SR and LB, a 1:1 propensity score-matched analysis was performed to compare these groups. A nearest neighbor algorithm was used and a caliper width of 0.0622. The variables used for matching were determined a priori to be of prognostic significance: age, sex, race, CDCC score, insurance status, treatment at an academic center, and tumor size. Covariate balance was checked using standardized mean differences (Supplemental Table 2 and Supplemental Figure 1). Because only 70 events were observed in the propensity score-matched cohort, a limited multivariable Cox model was performed with variables chosen that most impacted the effect estimate of the type of surgery variable and most improved the precision of the confidence interval23. (2) While only patients who received ‘definitive surgery’ according to the NCDB were included in the study, we performed a subgroup analysis including only WR patients who underwent an intraoperative lymph node assessment as well, hypothesizing that these patients were treated with curative intent24. (3) There was substantial variation in pathologic nodal upstaging between the groups (Table 1). Because the NCDB does not contain information on how patients were clinically staged, including mediastinal nodal evaluation, we performed a subgroup analysis including only patients with proven, pathologic node-negative disease who had at least one node assessed in surgery. (4) The NCCN guidelines recommend adjuvant chemotherapy with or without radiation for patients with clinically limited stage SCLC who undergo resection. Because a minority of patients underwent guideline-concordant adjuvant chemotherapy and there were significant differences between the groups, we performed an analysis examining only patients who received a resection and adjuvant chemotherapy with or without radiation. Survival was computed from the initiation of adjuvant chemotherapy for this analysis. (5) Because WR was associated with significantly worse OS compared to LB, we wondered if it was still superior to definitive concurrent chemoradiation. To test this, we performed an analysis comparing patients who received WR without neoadjuvant therapy with those who received primary concurrent chemoradiation. Because we believed the burden of proof lay with surgery, we applied liberal inclusion criteria for surgery patients and restrictive inclusion criteria for chemoradiation patients. Patients were only included in the definitive chemoradiation group if they started chemotherapy and radiation within 30 days of each other, if they received at least 45.0 Gy of radiation altogether, and if they were not refused surgery after being deemed medically unfit. Patients receiving SR and LB were also included in this analysis for comparison. (6) Given the similar outcomes between patients who underwent SR and LB, we tested the question of whether there is a tumor size threshold at which LB is associated with improved survival compared to SR. We performed a multivariable Cox regression in patients who underwent either SR or LB using the same variables as reflected in Table 2 and included an interaction term between tumor size and type of surgery.
Table 1.
Background characteristics of patients. IQR = inter-quartile range; CDCC= Charlson-Deyo Comorbidity Index
| Wedge Resection (n=619) (%) |
Segment (n=96) (%) |
Lobectomy (n=1233) (%) |
p value |
|
|---|---|---|---|---|
| Age (years) (median, IQR) | 70 (64-76) | 70(64-75) | 67(61-73) | <0.001 |
| Sex (female) | 339(55) | 59(62) | 681(55) | 0.46 |
| Race | 0.06 | |||
| White | 578(94) | 85(90) | 1119(91) | |
| Black | 29(5) | 8(8) | 77(6) | |
| Other | 5(1) | 2(2) | 30(2) | |
| Year of diagnosis, median (inter-quartile range) | 2007(2003-2010) | 2007(2004-2009) | 2008(2003-2010) | 0.22 |
| CDCC Score | 0.05 | |||
| 0 | 201(43) | 26(33) | 457(47) | |
| 1 | 183(39) | 37(47) | 375(39) | |
| 2+ | 87(19) | 15(19) | 139(14) | |
| Insurance status | 0.04 | |||
| Private | 152(25) | 29(31) | 383(32) | |
| Government | 453(74) | 66(70) | 821(67) | |
| None | 5(1) | 0(0) | 13(1) | |
| Facility location | 0.29 | |||
| Metro | 466(79) | 76(80) | 930(79) | |
| Urban | 116(20) | 15(16) | 205(18) | |
| Rural | 10(2) | 4(4) | 36(3) | |
| Academic center | 194(31) | 43(45) | 418(34) | 0.03 |
| Tumor size (mm) (median, IQR) | 17(12-23) | 19(14-25) | 21(15-30) | <0.001 |
| Lymph nodes assessed in surgery | 268(43) | 62(65) | 1153(94) | <0.001 |
| Number of nodes examined (median, IQR) | 0(0-3) | 2(0-6) | 8(4-14) | <0.001 |
| Pathologic stage | 0.001 | |||
| IA | 251(78) | 45(70) | 720(65) | |
| IB | 18(6) | 5(8) | 124(11) | |
| IIA | 5(2) | 2(3) | 54(5) | |
| IIB | 24(7) | 6(9) | 131(12) | |
| IIIA | 22(7) | 6(9) | 62(6) | |
| IIIB | 0(0) | 0(0) | 0(0) | |
| IIIC | 0(0) | 0(0) | 0(0) | |
| IV | 1(0) | 0(0) | 10(1) | |
| Pathologic N status | <0.001 | |||
| Unknown | 289(47) | 29(30) | 102(8) | |
| N0 | 283(46) | 56(58) | 933(76) | |
| N1 | 24(4) | 7(7) | 144(12) | |
| N2 | 23(4) | 4(4) | 54(4) | |
| N3 | 0(0) | 0(0) | 0(0) | |
| Positive margins | 62(11) | 8(9) | 45(4) | <0.001 |
| Pathologic nodal upstaging | 403(65) | 44(46) | 291(24) | <0.001 |
| 30-day readmission | 19(4) | 1(1) | 35(4) | 0.48 |
| Postoperative length of stay (days) (median, IQR) | 4(3-7) | 4(3-7) | 6(4-9) | <0.001 |
| Postoperative 90-day mortality | 34(5) | 1(1) | 64(5) | 0.07 |
| Adjuvant chemotherapy | 214(35) | 26(27) | 471(38) | 0.05 |
| Adjuvant thoracic radiation | 178(29) | 17(18) | 281(23) | 0.005 |
| Cranial radiation | 36(6) | 3(3) | 132(11) | <0.001 |
Table 2.
Multivariable Cox Proportional Hazards model for factors associated with survival
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Variable | Hazard Ratio | Lower | Upper | p-value |
| Age (per year) | 1.02 | 1.01 | 1.03 | <0.001 |
| Female sex (reference: male) | 0.84 | 0.73 | 0.97 | 0.02 |
| Race (reference: White) | ||||
| Black | 1.00 | 0.73 | 1.35 | 0.98 |
| Year of diagnosis (per year) | 1.00 | 0.97 | 1.04 | 0.94 |
| Charlson-Deyo Comorbidity Index (reference: 0) | ||||
| 1 | 1.00 | 0.85 | 1.17 | 0.98 |
| 2+ | 1.34 | 1.10 | 1.63 | 0.003 |
| Insurance status (reference: private) | ||||
| Government | 1.19 | 0.99 | 1.42 | 0.07 |
| None | 0.38 | 0.12 | 1.19 | 0.10 |
| Academic center | 1.00 | 0.86 | 1.17 | 0.97 |
| Tumor size (per 10 mm) | 1.16 | 1.08 | 1.25 | <0.001 |
| Type of surgery (reference: lobectomy) | ||||
| Wedge resection | 1.53 | 1.31 | 1.79 | <0.001 |
| Segmentectomy | 1.20 | 0.87 | 1.67 | 0.27 |
| N=1948 | ||||
Missing data were handled with complete case analysis in regression. A comparison of baseline characteristics and outcomes between patients with complete data and missing covariate or survival data is presented in Supplemental Table 1. A two-sided p value less than or equal to 0.05 was considered significant. All analyses were performed using R version 3.5.1 (Vienna, Austria).
Results
Overall Survival
A total of 1948 patients met study criteria: 619 (32%) underwent WR, 96 (5%) SR, and 1233 (63%) LB. Background characteristics of study patients are summarized in Table 1. Patients who underwent lobectomy were more likely to be younger, have fewer comorbidities, be privately insured, have a larger tumor, and a longer hospitalization after surgery compared to patients undergoing sublobar resection. A minority of patients in each group received adjuvant chemotherapy.
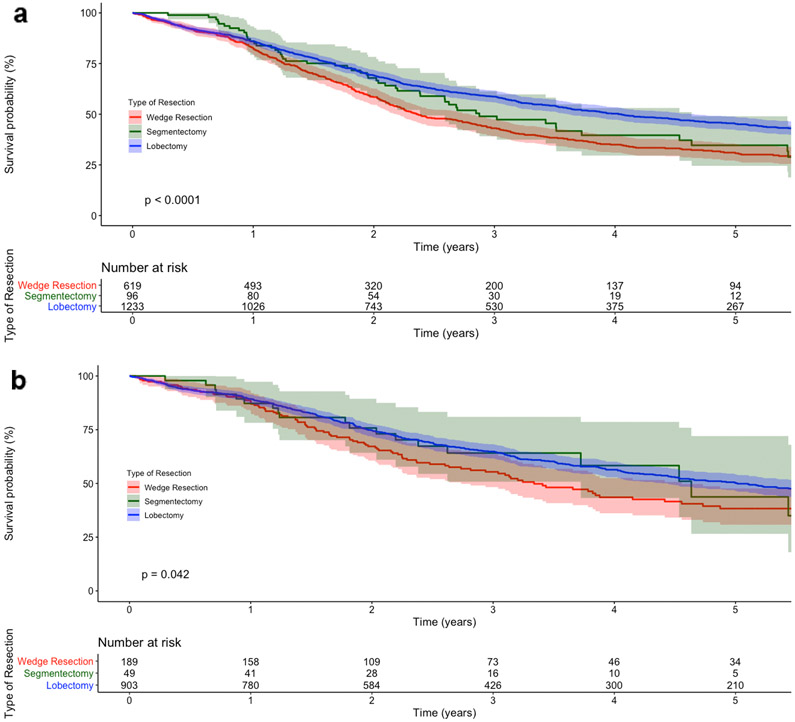
Unadjusted median survival for WR, SR, and LB patients was 28 months (95% confidence interval [CI] 26-34), 35 months (95%CI 29-56), and 49 months (95%CI 43-56), respectively. Five-year OS was 31% (95%CI 27-35), 35% (95%CI 25-49), and 45% (95%CI 42-49) for WR, SR, and LB, respectively (Figure 2a). In multivariable Cox regression, WR patients experienced worse survival compared to LB patients, while SR patients experienced similar survival compared to LB patients (Table 2).
Figure 2.
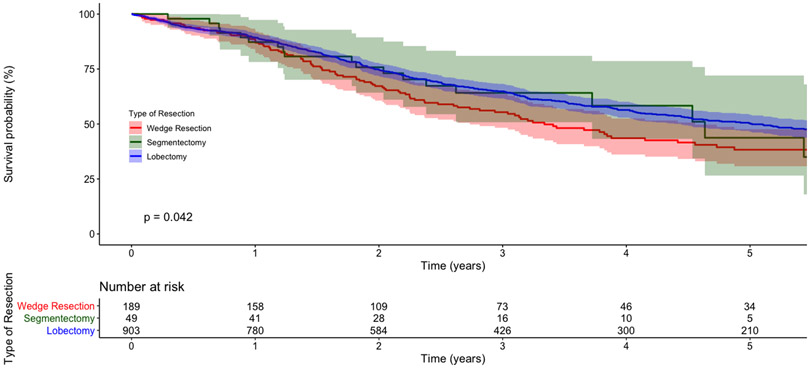
Kaplan-Meier survival curves for (a) patients with cT1-2N0M0 small cell lung cancer (SCLC) who underwent surgery, stratified by type of resection, and (b) a subgroup of patients with pathologic node-negative disease and at least one node assessed, stratified by type of resection. The Y-axis represents the probability of survival and the X-axis the time elapsed from diagnosis in years. The shaded regions demonstrate the bounds of the 95% confidence interval. The p value represents the result of the log-rank test. Numbers at risk are provided below each figure
A total of 74 pairs of patients receiving SR or LB were identified for the propensity score-matched analysis (Supplemental Table 2). SR and LB patients had an unadjusted five-year OS of 32% (95%CI 19-52) and 56% (95%CI 44-71), respectively (Supplemental Figure 2). In a multivariable regression, SR and LB patients had similar survival (Table 3).
Table 3.
Multivariable Cox Proportional Hazards model for factors associated with survival in propensity score-matched patients
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Variable | Hazard Ratio | Lower | Upper | p-value |
| Age (per year) | 1.02 | 0.99 | 1.06 | 0.19 |
| Year of diagnosis (per year) | 0.88 | 0.79 | 0.98 | 0.02 |
| Charlson-Deyo Comorbidity Index (reference: 0) | ||||
| 1 | 1.01 | 0.58 | 1.78 | 0.97 |
| 2+ | 1.97 | 1.02 | 3.81 | 0.04 |
| Academic center | 0.91 | 0.54 | 1.51 | 0.70 |
| Tumor size (per 10 mm) | 0.99 | 0.97 | 1.02 | 0.57 |
| Type of surgery (reference: segmentectomy) | ||||
| Lobectomy | 0.69 | 0.42 | 1.14 | 0.14 |
| N=148 | ||||
Secondary Outcomes
The incidence of positive margins in WR, SR, and LB patients was 11% (n=62), 9% (n=8), and 4% (n=45), respectively (Table 1). In a multivariable logistic regression, WR was associated with a greater odds of margin-positive resection compared to LB (Table 4). SR was associated with similar odds of margin-positive resection compared to LB. In a multivariable logistic regression, both WR and SR were associated with similar nodal upstaging compared to LB (Supplemental Table 3).
Table 4.
Multivariable logistic regression model for factors associated with margin-positive resection
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Variable | Odds Ratio | Lower | Upper | p-value |
| Age (per year) | 1.00 | 0.97 | 1.03 | 0.89 |
| Female sex (reference: male) | 1.12 | 0.72 | 1.73 | 0.62 |
| Race (reference: White) | ||||
| Black | 1.61 | 0.71 | 3.69 | 0.26 |
| Year of diagnosis (per year) | 1.01 | 0.92 | 1.10 | 0.86 |
| Charlson-Deyo Comorbidity Index (reference: 0) | ||||
| 1 | 0.90 | 0.57 | 1.44 | 0.67 |
| 2+ | 0.79 | 0.41 | 1.51 | 0.47 |
| Insurance status (reference: private) | ||||
| Government | 0.89 | 0.53 | 1.52 | 0.68 |
| Academic center | 0.91 | 0.57 | 1.45 | 0.71 |
| Tumor size (per 10 mm) | 1.61 | 1.31 | 1.97 | <0.001 |
| Type of surgery (reference: lobectomy) | ||||
| Wedge resection | 4.00 | 2.49 | 6.41 | <0.001 |
| Segmentectomy | 1.98 | 0.74 | 5.30 | 0.17 |
| N=1948 | ||||
Additional Analyses
In the first subgroup analysis, only WR patients who also received a lymph node assessment in surgery were included (n=268; 43%). In a multivariable Cox regression, WR was again associated with worse OS compared to LB (adjusted hazard ratio [HR] 1.34; 95%CI 1.09-1.65; p=0.005). In the second subgroup analysis, only patients with proven, pathologic node-negative disease with at least one node assessed were included: 189 WR, 49 SR, and 903 LB. The five-year unadjusted OS for WR, SR, and LB patients was 38% (95%CI 31-48), 44% (95%CI 27-72), and 50% (95%CI 47-54), respectively (Figure 2b). In multivariable analysis, WR was again associated with worse survival and SR similar survival compared to LB (Table 5).
Table 5.
Multivariable Cox Proportional Hazards model for factors associated with survival in subgroup of patients with pathologic node-negative disease
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Variable | Hazard Ratio | Lower | Upper | p-value |
| Age (per year) | 1.02 | 1.01 | 1.04 | <0.001 |
| Female sex (reference: male) | 0.89 | 0.74 | 1.09 | 0.27 |
| Race (reference: White) | ||||
| Black | 1.10 | 0.74 | 1.64 | 0.62 |
| Year of diagnosis (per year) | 1.00 | 0.96 | 1.05 | 0.96 |
| Charlson-Deyo Comorbidity Index (reference: 0) | ||||
| 1 | 0.99 | 0.80 | 1.23 | 0.91 |
| 2+ | 1.46 | 1.12 | 1.91 | 0.005 |
| Insurance status (reference: private) | ||||
| Government | 1.17 | 0.91 | 1.49 | 0.22 |
| None | 0.89 | 0.28 | 2.87 | 0.85 |
| Academic center | 1.04 | 0.85 | 1.28 | 0.68 |
| Tumor size (per 10 mm) | 1.02 | 1.01 | 1.03 | 0.001 |
| Type of surgery (reference: lobectomy) | ||||
| Wedge resection | 1.37 | 1.07 | 1.75 | 0.01 |
| Segmentectomy | 0.94 | 0.55 | 1.58 | 0.81 |
| N=1141 | ||||
In a third subgroup analysis including 481 patients who received adjuvant chemotherapy with or without radiation (108 WR, 16 SR, and 357 LB), WR was associated with worse survival (HR 1.47; 95%CI 1.05-2.07; p=0.02) and SR similar survival (HR 0.61; 95%CI 0.19-1.96; p=0.41) compared to lobectomy on multivariable analysis. In a fourth analysis comparing 1073 patients who underwent concurrent chemoradiation (CRT) and those who underwent surgery, the unadjusted five-year OS was 27% (95%CI 24-30) for CRT patients and 31% (95%CI 27-35), 35% (95%CI 25-49), and 45% (95%CI 42-49) for WR, SR, and LB patients, respectively (Supplemental Figure 3). WR was not associated with improved survival compared to chemoradiation (HR 0.91; 95%CI 0.78-1.06; p=0.22) in a multivariable Cox model. SR was associated with similar survival compared to chemoradiation (HR 0.73; 95%CI 0.53-1.02; p=0.06) and LB improved survival (HR 0.63; 95%CI 0.55-0.71; p<0.001). In the last subgroup analysis, the interaction between tumor size and receipt of SR or LB was studied. An interaction term between tumor size and extent of resection was nonsignificant (p=0.48) in a multivariable Cox model; this interaction is visualized in Supplemental Figure 4.
Discussion
In this NCDB analysis, we found that lobectomy was associated with significantly improved OS compared to wedge resection and similar survival compared to segmentectomy in patients with cT1-2N0M0 SCLC, including in patients with proven pathologic node-negative disease and those who received adjuvant chemotherapy. Wedge resection was associated with similar survival compared to definitive chemoradiation. Our study suggests that patients with early and limited stage SCLC who can tolerate surgery should undergo anatomic resection.
The findings of our study largely corroborate existing literature. There are no prospective studies comparing the extent of resection in patients with SCLC. The existing observational studies uniformly show that lobar resection is associated with improved survival compared to sublobar resection, although all these studies classify wedge and segmental resection together, in contrast with our study. Combs and colleagues17 used the NCDB to examine the outcomes of surgery in patients with stage I-IIIA SCLC and found that sublobar resection was associated with worse adjusted OS compared to lobar resection (HR 1.39; 95%CI 1.12-1.81). However, this study examined wedge and segmental resection as a single category and included patients with a variety of stages. Three other studies used the SEER registry to examine outcomes of surgery in SCLC. Weksler and colleagues18 analyzed patients with early stage SCLC and found that lobectomy and pneumonectomy were associated with improved unadjusted survival compared to wedge resection. They also found that wedge resection was associated with improved unadjusted survival compared to no resection, though it is unclear what constituted nonsurgical treatment in the latter group. Gu and colleagues19 studied 491 patients who underwent either sublobar or lobar resection for pT1N0M0 SCLC and found similar unadjusted overall and cancer-specific survival between the groups. In an analysis of 548 patients with T1-2N0M0 SCLC, Liu and coworkers20 also found that lobectomy was associated with improved survival compared to sublobar resection (HR 0.54; 95%CI 0.42-0.68); they also found that segmentectomy was associated with better survival than wedge resection (HR 0.47; 95%CI 0.22-0.98).
In contrast with most of the existing literature, our study analyzed wedge and segmental resections as separate entities because of the hypothesis that an anatomic resection may both offer a greater area of resection and offer a better probability of local control by removal of an area with common lymphatic drainage and vascular supply as the primary tumor16. Our finding that segmental resection is associated with similar survival as lobectomy suggests that patients who may tolerate an operation but not a lobectomy may still benefit from a segmentectomy if the tumor is amenable to it. Further, with the propagation of minimally invasive techniques the perioperative outcomes of segementectomy are excellent25. However, our finding that patients undergoing wedge resection and definitive, concurrent chemoradiation experience similar survival suggests that a wedge resection alone is not an acceptable surgical strategy compared to nonoperative management. We did find that segmental resection was also associated with similar survival compared to chemoradiation, but this analysis was significantly limited by the small size of the SR cohort and consequent type II error.
Our findings are framed by important limitations. The most significant limitation is selection bias. We do not know why patients were assigned to a particular type of resection. Because the NCDB does not provide data about preoperative pulmonary function, frailty, or malnutrition, we cannot understand the reasons driving treatment assignment in this cohort. While the NCDB reports the CDCC score for each patient that we used in our multivariable regression, this is a crude measure of overall health. We also do not know when the diagnosis of SCLC was made for patients in the NCDB; the decision to offer surgery and the choice of surgery are contingent on when the patient is known to have SCLC. Similarly, the NCDB does not provide information on how patients were clinically staged. We do not know, for example, how many patients received mediastinal nodal assessment. To mitigate confounding from staging, we performed a subgroup analysis only in patients with proven, pathologic node-negative disease and found that the study’s overall findings held true. The NCDB also does not catalogue specific anatomic information about primary tumors, which would be important in distinguishing anatomic rather than patient fitness related reasons for offering sublobar resection. There is also no information on the type of wedge or segmental resection performed (e.g., single or composite segmentectomy). The description of postoperative outcomes of patients in this study was also limited by the lack of knowledge of specific complications. The NCDB also does not capture postoperative readmission to centers other than the one where patients were treated. Finally, although our study had a significant number of patients receiving wedge or lobar resection, we had a small number of patients who underwent segmentectomy. This likely affected the internal validity of the study and increased the risk of type II error. We attempted several additional analyses comparing SR and LB patients including a propensity score-matched sensitivity analysis to assess the reproducibility of our primary analysis, but these supplemental analyses cannot overcome the intrinsic limitation of the small sample size of patients undergoing SR.
Nonetheless, in this analysis of the NCDB, we found that patients with limited stage SCLC undergoing segmental and lobar resection experienced similar survival. However, patients who underwent wedge resection had worse survival compared to those receiving lobectomy and similar survival compared to those treated with definitive chemoradiation. Lobectomy was also associated with a lower odds of margin-positive resection compared to wedge resection (Figure 3).
Figure 3.
In this National Cancer Database study, patients receiving segmentectomy (SR) and lobectomy (LB) experienced similar outcomes even after propensity score-matching. Patients receiving a wedge resection (WR) had worse survival compared to those receiving lobectomy. Our study suggests that anatomic resection should be considered for patients with limited stage small cell lung cancer. HR = hazard ratio; 95%CI = 95% confidence interval
Supplementary Material
Supplemental Figure 4. Graph of the interaction between tumor size and extent of resection in patients undergoing either segmentectomy (SR) or lobectomy (LB) for cT1-2N0M0 small cell lung cancer. The Y-axis demonstrates the hazard ratio of a Cox model adjusted for age, sex, race, insurance status, year of diagnosis, Charlson score, treatment at an academic center, and tumor size. The X-axis shows tumor size as a continuous variable modelled using restricted cubic splines with four pre-specified knots. The survival curves for patients undergoing segmentectomy (bold line) and lobectomy (dashed line) are shown. Grey areas represent the bounds of the 95% confidence interval
Supplemental Figure 3. Kaplan-Meier survival curves for patients with cT1-2N0M0 small cell lung cancer treated with either surgery or definitive, concurrent chemoradiation. The Y-axis represents the probability of survival and the X-axis the time elapsed from diagnosis in years. The shaded regions demonstrate the bounds of the 95% confidence interval. The p value represents the result of the log-rank test. Numbers at risk are provided below each figure
Supplemental Figure 2. Kaplan-Meier survival curves for propensity score-matched patients with cT1-2N0M0 small cell lung cancer treated with segmentectomy (SR) or lobectomy (LB). The Y-axis represents the probability of survival and the X-axis the time elapsed from diagnosis in years. The shaded regions demonstrate the bounds of the 95% confidence interval. The p value represents the result of the log-rank test. Numbers at risk are provided below each figure
Supplemental Figure 1. Balance diagnostics for propensity score-matched patients. (a) Love plot demonstrating the spread of standardized mean differences in matched and unmatched patients and (b) mirror histograms demonstrating the spread of propensity scores between the two treatment groups before and after matching
Central Picture.
Lobectomy has better survival compared to wedge resection in early small cell lung cancer
Central Message.
Segmental and lobar resection are associated with similar survival in patients with cT1-2N0M0 small-cell lung cancer.
Perspective Statement.
In this National Cancer Database study, patients undergoing wedge resection experienced worse survival compared to those receiving segmentectomy or lobectomy for limited stage small-cell lung cancer (SCLC).
Acknowledgements and Funding
The American College of Surgeons is in a Business Associate Agreement that includes a data use agreement with each of its Commission on Cancer accredited hospitals. The data used in the study are derived from a de-identified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the investigators.
Drs. Raman and Voigt were supported by a National Institutes of Health T-32 grant 5T32CA093245 in surgical oncology. Dr. Jawitz was supported by a National Institutes of Health T-32 grant 5T32HL069749 in clinical research.
This work received no direct funding.
Footnotes
We are grateful to Hanghang Wang, M.D., Ph.D., for statistical review of this manuscript.
This paper was presented as a poster at the AATS International Thoracic Surgical Oncology Summit (ITSOS) in New York City in September 2019 and received first prize in the poster competition.
The authors have no relevant conflicts of interest to disclose.
References
- 1.sclc.pdf. https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accessed February 7, 2020.
- 2.Fox W, Scadding JG. MEDICAL RESEARCH COUNCIL COMPARATIVE TRIAL OF SURGERY AND RADIOTHERAPY FOR PRIMARY TREATMENT OF SMALL-CELLED OR OAT-CELLED CARCINOMA OF BRONCHUS: Ten-year Follow-up. The Lancet. 1973;302(7820):63–65. doi: 10.1016/S0140-6736(73)93260-1 [DOI] [PubMed] [Google Scholar]
- 3.Lad T, Piantadosi S, Thomas P, Payne D, Ruckdeschel J, Giaccone G. A Prospective Randomized Trial to Determine the Benefit of Surgical Resection of Residual Disease Following Response of Small Cell Lung Cancer to Combination Chemotherapy. Chest. 1994;106(6, Supplement):320S–323S. doi: 10.1378/chest.106.6_Supplement.320S [DOI] [PubMed] [Google Scholar]
- 4.Ahmed Z, Kujtan L, Kennedy KF, Davis JR, Subramanian J. Disparities in the Management of Patients With Stage I Small Cell Lung Carcinoma (SCLC): A Surveillance, Epidemiology and End Results (SEER) Analysis. Clinical Lung Cancer. 2017;18(5):e315–e325. doi: 10.1016/j.cllc.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 5.Paximadis P, Beebe-Dimmer JL, George J, Schwartz AG, Wozniak A, Gadgeel S. Comparing Treatment Strategies for Stage I Small-cell lung Cancer. Clinical Lung Cancer. 2018;19(5):e559–e565. doi: 10.1016/j.cllc.2018.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takenaka T, Takenoyama M, Inamasu E, et al. Role of surgical resection for patients with limited disease-small cell lung cancer. Lung Cancer. 2015;88(1):52–56. doi: 10.1016/j.lungcan.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 7.Uprety D, Arjyal L, Vallatharasu Y, et al. Utilization of surgery and its impact on survival in early stage small-cell lung cancer patients in the United States. Clinical Lung Cancer. August 2019. doi: 10.1016/j.cllc.2019.07.013 [DOI] [PubMed] [Google Scholar]
- 8.Varlotto JM, Recht A, Flickinger JC, Medford-Davis LN, Dyer A-M, DeCamp MM. Lobectomy leads to optimal survival in early-stage small cell lung cancer: A retrospective analysis. The Journal of Thoracic and Cardiovascular Surgery. 2011;142(3):538–546. doi: 10.1016/j.jtcvs.2010.11.062 [DOI] [PubMed] [Google Scholar]
- 9.Wakeam E, Byrne JP, Darling GE, Varghese TK. Surgical Treatment for Early Small Cell Lung Cancer: Variability in Practice and Impact on Survival. The Annals of Thoracic Surgery. 2017;104(6):1872–1880. doi: 10.1016/j.athoracsur.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 10.Wakeam E, Varghese TK, Leighl NB, Giuliani M, Finlayson SRG, Darling GE. Trends, practice patterns and underuse of surgery in the treatment of early stage small cell lung cancer. Lung Cancer. 2017;109:117–123. doi: 10.1016/j.lungcan.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 11.Wakeam E, Acuna SA, Leighl NB, et al. Surgery Versus Chemotherapy and Radiotherapy For Early and Locally Advanced Small Cell Lung Cancer: A Propensity-Matched Analysis of Survival. Lung Cancer. 2017;109:78–88. doi: 10.1016/j.lungcan.2017.04.021 [DOI] [PubMed] [Google Scholar]
- 12.Yang C-FJ, Chan DY, Speicher PJ, et al. Surgery Versus Optimal Medical Management for N1 Small Cell Lung Cancer. Ann Thorac Surg. 2017;103(6):1767–1772. doi: 10.1016/j.athoracsur.2017.01.043 [DOI] [PubMed] [Google Scholar]
- 13.Yang C-FJ, Chan DY, Shah SA, et al. Long-term Survival After Surgery Compared With Concurrent Chemoradiation for Node-negative Small Cell Lung Cancer. Ann Surg. 2018;268(6):1105–1112. doi: 10.1097/SLA.0000000000002287 [DOI] [PubMed] [Google Scholar]
- 14.Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). The Lancet Respiratory Medicine. 2018;6(12):915–924. doi: 10.1016/S2213-2600(18)30411-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. The Annals of Thoracic Surgery. 1995;60(3):615–623. doi: 10.1016/0003-4975(95)00537-U [DOI] [PubMed] [Google Scholar]
- 16.Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic Segmentectomy in the Treatment of Stage I Non-Small Cell Lung Cancer. The Annals of Thoracic Surgery. 2007;84(3):926–933. doi: 10.1016/j.athoracsur.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 17.Combs SE, Hancock JG, Boffa DJ, Decker RH, Detterbeck FC, Kim AW. Bolstering the Case for Lobectomy in Stages I, II, and IIIA Small-Cell Lung Cancer Using the National Cancer Data Base. Journal of Thoracic Oncology. 2015;10(2):316–323. doi: 10.1097/JTO.0000000000000402 [DOI] [PubMed] [Google Scholar]
- 18.Weksler B, Nason KS, Shende M, Landreneau RJ, Pennathur A. Surgical Resection Should Be Considered for Stage I and II Small Cell Carcinoma of the Lung. The Annals of Thoracic Surgery. 2012;94(3):889–893. doi: 10.1016/j.athoracsur.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 19.Gu C, Huang Z, Dai C, et al. Prognostic Analysis of Limited Resection Versus Lobectomy in Stage IA Small Cell Lung Cancer Patients Based on the Surveillance, Epidemiology, and End Results Registry Database. Front Genet. 2018;9. doi: 10.3389/fgene.2018.00568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Shan L, Shen J, et al. Choice of surgical procedure – lobectomy, segmentectomy, or wedge resection – for patients with stage T1-2N0M0 small cell lung cancer: A population-based study. Thoracic Cancer. 2019;10(4):593–600. doi: 10.1111/1759-7714.12943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. doi: 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. https://gking.harvard.edu/matchit. Accessed April 19, 2019.
- 23.Heinze G, Wallisch C, Dunkler D. Variable selection – A review and recommendations for the practicing statistician. Biom J. 2018;60(3):431–449. doi: 10.1002/bimj.201700067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox ML, Yang C-FJ, Speicher PJ, et al. The role of extent of surgical resection and lymph node assessment for clinical stage I pulmonary lepidic adenocarcinoma: An analysis of 1,991 patients. J Thorac Oncol. 2017;12(4):689–696. doi: 10.1016/j.jtho.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg. 2019;158(3):895–907. doi: 10.1016/j.jtcvs.2019.03.090 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 4. Graph of the interaction between tumor size and extent of resection in patients undergoing either segmentectomy (SR) or lobectomy (LB) for cT1-2N0M0 small cell lung cancer. The Y-axis demonstrates the hazard ratio of a Cox model adjusted for age, sex, race, insurance status, year of diagnosis, Charlson score, treatment at an academic center, and tumor size. The X-axis shows tumor size as a continuous variable modelled using restricted cubic splines with four pre-specified knots. The survival curves for patients undergoing segmentectomy (bold line) and lobectomy (dashed line) are shown. Grey areas represent the bounds of the 95% confidence interval
Supplemental Figure 3. Kaplan-Meier survival curves for patients with cT1-2N0M0 small cell lung cancer treated with either surgery or definitive, concurrent chemoradiation. The Y-axis represents the probability of survival and the X-axis the time elapsed from diagnosis in years. The shaded regions demonstrate the bounds of the 95% confidence interval. The p value represents the result of the log-rank test. Numbers at risk are provided below each figure
Supplemental Figure 2. Kaplan-Meier survival curves for propensity score-matched patients with cT1-2N0M0 small cell lung cancer treated with segmentectomy (SR) or lobectomy (LB). The Y-axis represents the probability of survival and the X-axis the time elapsed from diagnosis in years. The shaded regions demonstrate the bounds of the 95% confidence interval. The p value represents the result of the log-rank test. Numbers at risk are provided below each figure
Supplemental Figure 1. Balance diagnostics for propensity score-matched patients. (a) Love plot demonstrating the spread of standardized mean differences in matched and unmatched patients and (b) mirror histograms demonstrating the spread of propensity scores between the two treatment groups before and after matching