Abstract
This study evaluated whether a history of lifetime methamphetamine (MA) use disorder increases risk for poor sleep quality in people with or without HIV infection (HIV+/HIV−). Participants (n=313) were stratified into four groups based on HIV status and lifetime MA use disorder diagnosis [HIV+/MA+ (n=84); HIV+/MA− (n=141); HIV−/MA+ (n=16); and HIV−/MA− (n=72)] and compared on global sleep outcomes using the Pittsburgh Sleep Quality Index (PSQI). Significant differences on global sleep were observed between HIV+/MA+ and HIV+/MA− groups, but not between the HIV− groups. Follow-up multiple regression analyses within the HIV+ subgroups examined global sleep scores as a function of MA status and clinical covariates, including those related to HIV disease and demographics. HIV+ individuals with a history of MA use disorder evidenced significantly poorer sleep quality and were more likely to be classified as problematic sleepers than those without a lifetime disorder. This was independent of depressed mood, body mass index, and viral suppression while on treatment. Poorer reported sleep quality among HIV+/MA+ was associated also with multiple adverse functional outcomes, including greater objective cognitive impairment, unemployment, clinical ratings of functional impairment, and self-reported cognitive difficulties, decreased independence in activities of daily living, and poorer overall life quality. Interventions to avoid or curtail MA use in HIV+ individuals may help protect sleep quality and improve functioning.
Keywords: AIDS, substance use, stimulant, depression
INTRODUCTION
Human immunodeficiency virus (HIV) infection is often accompanied by chronic fatigue and disrupted sleep patterns (1). A considerable number of people with HIV (PWH) report sleep disturbance, with some studies estimating the prevalence of symptoms to be up to five times that of the general population (2, 3). PWH face a variety of unique social (e.g., stigmatization, lack of social support), physical (e.g., inflammatory, metabolic, and cardiovascular effects), and socioeconomic stressors (e.g., loss of medical insurance, unemployment), which may influence sleep disturbance (4, 5). Prolonged poor sleep quality among PWH is associated with anxiety, depression, loss of productivity, interference with employment, physiologic stress, and poorer quality of life (6, 7).
Methamphetamine (MA), a central nervous system stimulant, is among the most commonly used addictive drugs, with 35 million users per year worldwide (8). PWH and individuals at-risk for HIV transmission, such as men who have sex with men, have a particularly high prevalence of MA use (9). A recent study on MA-dependent gay and bisexual men reported the prevlance of HIV infection to be 63% (11). Among PWH, MA is linked to accelerated viral replication, more rapid progression to AIDS, reduced effectiveness of antiretroviral therapy (ART), and increased immune suppression (12–15). Global neuropsychological impairment and dependence on basic and instrumental activities of daily living are more common among PWH who also use MA (16, 17) than among those who do not, with an additive effect of HIV and MA on neuronal injury and glial activation (18). Despite these negative effects, perceived benefits, such as sexual enhancement and relief of negative psychosocial symptoms, continue to drive MA use among PWH (19).
MA functions by stimulating monoamine release (dopamine, norepinephrine, and serotonin), and facilitates hyperactivity, euphoria, feelings of increased mental and physical capacity, and riskier sexual behavior (19, 20). Among the general population, prolonged MA use can have detrimental effects on alertness, mood, cognition, and activity levels (21–23). MA use also has been associated with poor sleep quality, increased sleep latency, and daytime sleepiness (24, 25). Cessation of MA is often accompanied by withdrawal symptoms such as anxiety, depression, and craving (26) that can further contribute to poor sleep quality. The adverse effects of MA also contribute to functional decline (21), such as unemployment (27), which also may exacerbate sleep disturbance. Among MA-using PWH, poorer adherence and missing ART doses after MA use have been reported, in part due to disrupted sleep-wake cycles (28). Taken together, acute and chronic MA use can have multiple direct and indirect effects on sleep quality.
Few studies have examined the combined associations of MA use and HIV on sleep disturbance. This study evaluates effects of lifetime MA use on self-reported sleep quality among participants with or without HIV infection. The hypothesis was that lifetime MA use disorder would be associated with poorer sleep quality, particularly among PWH, and that this would relate to poor outcomes, including poorer cognition, reduced independence in activities of daily living, unemployment, and poorer life quality.
METHODS
Participants
Participants included 225 HIV-seropositive (HIV+) and 88 HIV-seronegative (HIV−) adults (mean age = 55.8 years) enrolled in NIH-funded research studies at the UC San Diego’s HIV Neurobehavioral Research Center (HNRC). All participants completed a standard, self-report evaluation of sleep quality as well as comprehensive neurobehavioral and neuromedical assessments. Exclusion criteria were: 1) sleep apnea or restless leg syndrome; 2) disruptions to sleep due to temporary circumstances (i.e., recent surgery); 3) history of comorbid neurological illness or injury that would affect cognitive functioning (e.g., seizure disorder, head injury with loss of consciousness for longer than 30 minutes, stroke, central nervous system neuroplasm); 4) history of psychotic disorder; 5) alcohol dependence within a year; and 6) low premorbid verbal IQ as estimated by a Wide Range Achievement Test-4 (WRAT4) score less than 80. The study protocol was approved by the UC San Diego Institutional Review Board and each participant provided written, informed consent.
Neuropsychiatric Assessment
The Composite International Diagnostic Interview (CIDI) (29) was administered to diagnose participants for current and lifetime substance use and mood disorders (e.g., Major Depressive Disorder, MDD), as defined by the Fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). For the initial analyses, participants were stratified into four groups based on HIV status and lifetime MA use disorder diagnosis: HIV+/MA+ (n=84); HIV+/MA− (n=141); HIV−/MA+ (n=16); and HIV−/MA− (n=72). Current depressive symptoms were assessed using the Beck Depression Inventory, Second Edition (BDI-II) (30). Item 16 on the BDI-II, which assesses change in sleep pattern in the last two weeks, was excluded from the BDI-II total score to avoid collinearity with our outcome of interest, perceived sleep quality.
Subjective Sleep Assessment
All participants completed the Pittsburgh Sleep Quality Index (PSQI), a self-report questionnaire that assesses perceptions of average sleep quality and disturbances over the past 30 days (31). The PSQI is a widely used and well-validated measure of subjective sleep quality in adults (32, 33). The PSQI has 19-items that assesses seven components of sleep, including quality, latency, duration, efficiency, disturbances, use of medications to aid sleeping, and daytime sleepiness. Component scores range from 0 to 3 (higher is worse). Items were summed to generate a continuous global sleep score ranging from 0 to 21. Global scores > 5 indicate problematic sleep (31). For purposes of the present study, the continuous global PSQI score and dichotomous sleep quality classification (problematic vs. non-problematic sleep) were used as outcome variables.
Neuromedical Assessment
All participants underwent a standardized medical history interview, neuromedical examination, and blood and urine collection. HIV serological status was confirmed via ELISA and Western blot test, and HIV RNA levels were measured in plasma by rtPCR (Amplicor, Roche Diagnostics, Indianapolis, IN; lower limit of quantitation [LLQ] of 50 copies/ml). Current CD4+ T-cell count was measured in blood by clinical flow cytometry. Additional HIV disease and treatment variables included nadir CD4+ T-cell count, AIDS diagnosis, estimated duration of HIV disease, and current ART regimen. MA use characteristics (use within the last 30 days, days since last use, age of first use, total days used, total quantity used) were self-reported. Comorbid medical conditions and current medication use were determined by self-report and medical chart review.
Neurobehavioral Assessment
All participants completed a comprehensive and validated neurocognitive assessment across seven neurocognitive domains commonly affected by HIV and MA use (17, 34); these include verbal fluency, executive functioning, speed of information processing, learning and memory (two domains – learning and recall), working memory/attention, and motor. Using established normative standards, test scores were adjusted for known influences (i.e., age, education, sex, and race/ethnicity) on neurocognitive performance (35–37). Deficit scores were calculated for each domain and averaged across the test battery to derive a global deficit score (GDS) ranging from 0 (normal) to 5 (severe) (38).
Dependence in instrumental activities of daily living (IADL) was determined using a revised version of the Lawton and Brody ADL questionnaire (39, 40), in which participants rated current degree of independence as compared to prior best level of independence across 13 IADL domains. Participants were classified as IADL “dependent” if they endorsed requiring increased assistance in at least 2 IADL domains. Employment status and symptoms of cognitive difficulties in daily life were determined via the Patient’s Assessment of Own Functioning Inventory (PAOFI) (41). The Karnofsky Performance Status Scale is a clinician-administered assessment of disease-related functional impairment with a range from 0 to 100 with standard intervals of 10 (lower is worse) (42). Self-reported physical and mental health quality of life were assessed using the Medical Outcomes Study Short-Form Survey (MOS SF-36). Physical and mental health composite scores were calculated via validated summary score formulas derived from an obliquely rotated factor solution (43).
Statistical Analyses
Group differences on demographics, neuropsychiatric and neuromedical characteristics, HIV disease and treatment parameters, MA use history, and global sleep outcomes were tested using analysis of variance (ANOVA), Kruskal-Wallis tests, Chi-square statistics, or Fisher’s Exact test (FET). Two-tailed t-tests were used to compare groups on HIV disease and methamphetamine use characteristics. Follow-up pairwise comparisons were conducted using Tukey’s Honest Significant Difference (HSD) or Wilcoxon tests for continuous outcomes, or Bonferroni-corrections for categorical outcomes. Cohen’s d measured effect size for pairwise comparisons of means. Based on the pattern of univariable group differences in global sleep health and the small sample size of the HIV−/MA+ group, multiple linear regression examined global sleep scores as a function of MA status and clinical covariates (including those that are specific to HIV) specifically within PWH. Covariates included clinical variables from Table I with univariable associations with the primary independent variable [MA status (i.e., HIV+/MA+ vs. HIV+/MA−)] as well as associations with the primary dependent variable (global PSQI) with p values < 0.10. Variables sex and sexual orientation were included based on theoretical evidence (44, 45). Additionally, HIV disease and treatment covariates were included to determine if HIV-specific factors attenuated the effects of MA status on global sleep in PWH. Stepwise regression models used backward selection based on Akaike Information Criterion (AIC) to select the optimal model.
Table I.
Participant characteristics by HIV serostatus and lifetime methamphetamine (MA) use disorder (n=313)
| HIV+ | HIV− | Test statistic | p-value | Pairwise | |||
|---|---|---|---|---|---|---|---|
| MA+ 1 (N=84) | MA− 2 (N=141) | MA+ 3 (N=16) | MA− 4 (N=72) | ||||
| Demographics | |||||||
| Age (years), mean (SD) | 53.7 (8.8) | 57.7 (11.3) | 49.9 (10.7) | 56.0 (14.6) | H(3)=17.78 | <.001 | 2>1,3 |
| Sex (male), N (%) | 72 (85) | 120 (85) | 11 (68) | 42 (58) | χ(3)2=24.29 | <.001 | 1,2>4 |
| Race/Ethnicity (White), N (%) | 50 (59) | 91 (64) | 7 (44) | 51 (70) | χ(3)2=8.96 | .71 | -- |
| Education (years), mean (SD) | 13.4 (2.5) | 14.6 (2.6) | 13.4 (2.6) | 14.8 (2.3) | F(3,309)=5.77 | <.001 | 1<2,4 |
| Premorbid verbal IQ estimatea, mean (SD) | 102.1 (12.8) | 103.7 (15.0) | 99.6 (11.6) | 106.5 (15.0) | F(3,306)=1.69 | .17 | -- |
| Sexual orientation (homosexual), N (%) | 53 (66) | 88 (69) | 2 (13) | 15 (21) | χ(3)2=78.89 | <.001 | 1>3,4; 2>3,4 |
| Psychiatric and Substance Use | |||||||
| BDI-II, median [IQR]b | 8 [3, 16] | 5.5 [1, 10] | 2.5 [0, 6] | 3 [0, 8] | H(3)=20.61 | <.001 | 1>2,3,4; 2>4 |
| LT MDD, N (%) | 60 (71) | 84 (60) | 4 (25) | 25 (33) | χ(3)2=29.82 | <.001 | 1>3,4; 2>4 |
| LT Alcohol Diagnosis, N (%) | 61 (73) | 61 (43) | 9 (56) | 18 (25) | χ(3)2=37.37 | <.001 | 1>2,4; 2,3>4 |
| Current Alcohol Diagnosis, N (%) | 1 (1) | 2 (2) | 0 (0) | 0 (0) | FET | .83 | -- |
| LT Cannabis Diagnosis, N (%) | 42 (50) | 17 (12) | 4 (25) | 3 (4) | χ(3)2=45.07 | <.001 | 1>2,4; 3>4 |
| Current Cannabis Diagnosis, N (%) | 4 (5) | 2 (2) | 0 (0) | 0 (0) | FET | .15 | -- |
| LT Cocaine Diagnosis, N (%) | 46 (55) | 27 (19) | 8 (50) | 10 (14) | χ(3)2=61.65 | <.001 | 1>2,4; 3>4 |
| Current Cocaine Diagnosis, N (%) | 0 | 0 | 0 | 0 | -- | -- | -- |
| Positive urine toxicology screen, N (%) | 15 (19) | 17 (13) | 2 (15) | 5 (7) | χ(3)2=4.41 | .22 | -- |
| Ever Intravenous drug use, N (%) | 41 (49) | 10 (7) | 6 (38) | 2 (3) | FET | <.001 | 1>2,4; 3>2,4 |
| HIV disease characteristics | |||||||
| AIDS Diagnosis, N (%) | 63 (76) | 89 (64) | -- | -- | χ(1)2=3.14 | .07 | -- |
| Estimated years of infection, mean (SD) | 20.0 (8.5) | 20.3 (9.2) | -- | -- | t(1)=.26 | .80 | -- |
| ART (on), N (%) | 81 (96) | 136 (98) | -- | -- | χ(1)2=.40 | .53 | -- |
| Nadir CD4 (cells/uL), median [IQR] | 149.0 [107.7, 190.4] | 208.8 [174.8, 242.7] | -- | -- | t(1)=2.18 | .03 | 1<2 |
| Current CD4 (cells/uL), median [IQR] | 600.8 [538.3, 663.4] | 634.2 [587.5, 680.9] | -- | -- | t(1)=.86 | .39 | -- |
| Plasma HIV RNA (undetectable), N (%) | 77 (86.5) | 133 (94.3) | -- | -- | χ(1)2=1.12 | .30 | -- |
| Methamphetamine use characteristicsc | |||||||
| Use in the last 30 days, N (%) | 11 (13) | 5 (4) | 1 (6) | 1 (1) | FET | .009 | 1>2,3,4 |
| Days since last use, median [IQR] | 1452 [152, 2584] | -- | 2191 [183, 5479] | -- | t(1)=1.22 | .22 | -- |
| Age of first use, mean (SD) | 25.9 (11.7) | -- | 24.6 (10.2) | -- | t(1)=.72 | .47 | -- |
| Total days used, median [IQR] | 1639 [451, 3593] | -- | 1614 [313, 4385] | -- | t(1)=.24 | .81 | -- |
| Total quantity (grams), median [IQR] | 917 [157, 2946] | -- | 645 [31, 3806] | -- | t(1)=.15 | .87 | -- |
| Density of use (grams/day), median [IQR] | .63 [.26, 1.38] | -- | .35 [.14, 1.62] | -- | t(1)=.68 | .50 | -- |
| Comorbid Medical Conditions | |||||||
| Hypertension, N (%) | 44 (55) | 84 (61) | 2 (12) | 27 (39) | χ(3)2=19.63 | <.001 | 1>3,4; 2>4 |
| Hyperlipidemia, N (%) | 39 (49) | 82 (60) | 4 (25) | 24 (35) | χ(3)2=15.71 | .001 | 2>4 |
| Diabetes, N (%) | 14 (17) | 30 (22) | 1 (6) | 12 (17) | χ(3)2=2.68 | .44 | -- |
| Body mass index, mean (SD) | 28.3 (6.0) | 27.4 (5.7) | 30.9 (8.4) | 28.9 (7.9) | H(3)=3.28 | .35 | -- |
| Hepatitis C, N (%) | 26 (32) | 24 (17) | 1 (6) | 7 (10) | χ(3)2=14.82 | .002 | 1>2,3,4 |
| Medications | |||||||
| Total medications, mean (SD) | 6.8 (4.9) | 7.7 (5.2) | 3.0 (2.1) | 3.8 (2.7) | H(3)=31.27 | <.001 | 1>3,4; 2>3 |
| Number of sedative medications, mean (SD) | .5 (.9) | .4 (.7) | .1 (.3) | .1 (.5) | H(3)=23.03 | <.001 | 1>4 |
| Number of ART medications, mean (SD) | 3.6 (1.0) | 3.6 (1.0) | -- | -- | t(1)=.34 | .73 | -- |
| Neurobehavioral Function | |||||||
| GDS, mean (SD) | .7 (.6) | .7 (.6) | .4 (.4) | .6 (.7) | F(3,306)=.93 | .42 | -- |
| IADL Dependence, N (%) | 23 (28) | 30 (22) | 1 (7) | 4 (6) | χ(3)2=22.53 | <.001 | 1>4 |
| PAOFI unemployment, N (%) | 52 (63) | 97 (69) | 10 (62) | 39 (55) | χ(3)2=4.59 | .205 | -- |
| Karnofsky, mean (SD) | 92.4 (1.6) | 93.1 (1.2) | 99.2 (3.8) | 96.7 (1.7) | H(3)=5.58 | .13 | -- |
| SF-36 Physical Health, mean (SD) | 45.9 (14.3) | 46.9 (12.7) | 52.7 (8.6) | 52.0 (10.9) | F(3,275)=3.51 | .016 | 1<3,4; 2<4 |
| SF-36 Mental Health, mean (SD) | 48.5 (11.5) | 51.5 (10.3) | 54.2 (13.3) | 55.9 (8.0) | H(3)=16.63 | <.001 | 1<3,4; 2<4 |
WRAT4=Wide Range Achievement Test-Fourth Edition; GDS=global deficit score; LT=lifetime; MDD=Major Depressive Disorder; ART=antiretroviral therapy; FET=Fisher’s Exact test; PAOFI=Patients Assessment of Own Functioning Inventory; SF-36 = Physical and Mental Health subcomponents of the Medical Outcomes Study Short-Form Health Survey (MOS SF-36), composite scores were calculated via summary scores from obliquely rotated factor solution (Farivar et al., 2007), higher scores represent better health-related quality of life;
BDI-II=Beck Depression Inventory-Second Edition, does not include sleep item;
Other than use in the last 30 days, methamphetamine use characteristics only describes lifetime MA use disorder groups;
Pairwise comparisons were conducted using Tukey’s Honest Significant Difference (HSD) or Wilcoxon tests for continuous outcomes or Bonferroni-corrections for categorical outcomes
To determine potential co-occuring neurobehavioral functional impairments associated with poor sleep quality within the dual-risk HIV+/MA+ group, additional nominal logistic regression models based on AIC were run to examine the association between problematic sleep membership (global PSQI score<5) and neurobehavioral outcomes (i.e., GDS, IADL dependence, PAOFI unemployment, Karnofsky, and MOS SF-36 physical and mental health composites). Covariates were selected based on univariable associations with global PSQI and did not include HIV or methamphetamine characteristics.
RESULTS
Participant Characteristics
Demographics and clinical characteristics are presented in Table I. Age differed modestly, but significantly across groups (H(3)=17.78, p<.001), with HIV+/MA− individuals (M= 57.7, SD= 11.3) being older than HIV+/MA+ (M= 53.7, SD= 8.8) and HIV−/MA+ individuals (M= 49.9, SD= 10.7). The HIV+ groups had a greater proportion of men (χ(3)2=24.29, p<.001) and individuals who identified as homosexual (χ(3)2=78.89, p<.001) than the HIV−/MA− group, and the HIV+/MA+ group had fewer years of education than the HIV+/MA− and HIV−/MA− groups (F(3,309)=5.77,p< .001). In general, the HIV+/MA+ group reported more current depressive symptoms (BDI-II; H(3)=20.61, p<.001) and were more likely to meet criteria for lifetime Major Depressive Disorder (MDD; χ(3)2=29.82, p<.001). This group also had higher rates of lifetime non-MA substance use disorders (i.e., alcohol, χ(3)2=37.37, p<.001; cannabis, χ(3)2=45.07, p<.001; cocaine, χ(3)2=61.65, p<.001) than the other groups. HIV+ individuals had higher prevalence of hypertension (χ(3)2=19.63, p<.001) and hyperlipidemia (χ(3)2=15.71, p=.001) than HIV− individuals, and individuals within the HIV+/MA+ group had a higher prevalence of hepatitis C co-infection (χ(3)2=14.82, p=.002) than the other three groups.
HIV Disease and Methamphetamine Use Characteristics
Within the HIV+ group, MA+ individuals had significantly lower nadir CD4 cell count than MA− individuals (t(1)=2.18, p= .03). However, there were no other notable differences between MA groups on HIV disease and treatment characteristics. Overall, current MA use (within the last 30 days) was not common in our sample (n= 18), although the HIV+/MA+ group reported significantly more MA use within the last 30 days than the other three groups. Within people who met criteria for lifetime MA use disorder, there were no differences between HIV+ and HIV− on all other MA use characteristics (p’s≥ .18).
Self-reported Sleep Quality
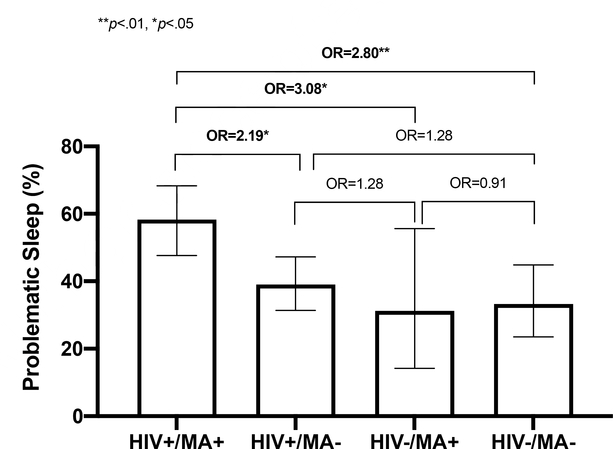
PSQI scores by HIV/MA group are reported in Table II. Groups differed significantly on global PSQI scores (F(3, 309)= 3.63, p= .001) such that HIV+/MA+ individuals endorsed significantly more problematic sleep (global scores) than HIV+/MA− (d= 0.39, p= .005)and HIV−/MA− individuals (d= 0.46, p= .004). Groups also differed significantly by prevalence of problematic sleep status (global scores > 5; χ2(3)= 12.63, p= .006) such that the HIV+/MA+ group had a higher proportion of individuals classified as problematic sleepers compared to the other three groups (ORs≥ 2.19, ps≤ .004; Figure 1). No significant differences were observed among the three remaining groups on global PSQI or problematic sleep classification. The pattern of individual item responses on the PSQI indicated that HIV+/MA+ individuals reported not only poorest overall sleep quality, but also latency of sleep onset, highest prevalence of medication use for sleep, and highest prevalence of sleep disruptions due to bad dreams and other unspecified causes.
Table II.
Perceived sleep quality on the Pittsburgh Sleep Quality Index (PSQI) by HIV serostatus and lifetime methamphetamine (MA) use disorder
| HIV+ | HIV− | Test statistic | p-value | Pairwise | |||
|---|---|---|---|---|---|---|---|
| MA+ 1 (N=84) | MA− 2 (N=141) | MA+ 3 (N=16) | MA− 4 (N=72) | ||||
| Global PSQI | 7.2 (4.4) | 5.6 (4.1) | 5.4 (4.2) | 5.3 (4.5) | F(3,309)=3.63 | .001 | 1>2,4 |
| Problematic Sleep (Global > 5), N (%) | 49 (58) | 55 (39) | 5 (31) | 24 (33) | χ(3)2=12.63 | .006 | 1>2,3,4 |
| Minutes to fall asleep each night | 27.3 (18.9) | 22.5 (19.6) | 23.7 (22.0) | 18.9 (16.5) | F(3,309)=2.59 | .05 | 1>4 |
| Hours of actual sleep per night | 7.3 (1.7) | 7.2 (2.9) | 7.1 (1.2) | 7.1 (1.7) | F(3,309)=.12 | .95 | -- |
| Cannot get to sleep within 30 min | 1.4 (1.1) | 1.1 (1.1) | .8 (1.0) | 1.1 (1.1) | F(3,309)=2.24 | .08 | -- |
| Wake up middle of night or early morning | 2.2 (1.0) | 1.9 (1.1) | 1.9 (.9) | 1.8 (1.3) | H(3)=3.16 | .37 | -- |
| Use bathroom | 2.3 (1.0) | 2.2 (1.0) | 2.2 (1.1) | 2.0 (1.2) | F(3,309)=.88 | .45 | -- |
| Cannot breathe comfortably | .5 (1.0) | .4 (.8) | .2 (.4) | .4 (.8) | F(3,309)=1.40 | .24 | -- |
| Cough or snore loudly | .7 (1.0) | .6 (1.1) | .5 (.9) | .5 (.9) | F(3,309)=.65 | .59 | -- |
| Feel too cold | .6 (.9) | .5 (.8) | .6 (.8) | .6 (.9) | F(3,309)=.60 | .62 | -- |
| Feel too hot | 1.0 (1.2) | .7 (1.0) | .5 (.9) | .8 (1.0) | F(3,309)=2.05 | .11 | -- |
| Have bad dreams | .8 (.9) | .4 (.8) | .6 (.7) | .5 (.8) | F(3,309)=4.81 | .003 | 1>2,4 |
| Have pain | 1.2 (1.2) | 1.0 (1.2) | .8 (1.2) | .8 (1.1) | F(3,309)=1.36 | .26 | -- |
| Other reasons trouble sleeping | .9 (1.2) | .4 (.9) | .7 (1.2) | .4 (.9) | F(3,309)=3.83 | .01 | 1>2 |
| Taken medication to help sleep | 1.2 (1.3) | 1.1 (1.3) | .7 (1.3) | .5 (1.1) | H(3)=14.32 | .003 | 1,2>4 |
| Trouble staying awake during the day | .4 (.7) | .4 (.8) | .3 (.6) | .2 (.5) | H(3)=7.23 | .06 | -- |
| Trouble keep enthusiasm | 1.1 (1.0) | .8 (.9) | .6 (.8) | .7 (.8) | F(3,309)=2.87 | .04 | -- |
| Overall rating of sleep quality | 1.2 (.8) | .9 (.8) | .9 (.5) | .9 (.6) | H(3)=8.03 | .05 | 1>2 |
Data are presented at mean (SD) unless otherwise noted; Global PSQI score range from 0–21; other than minutes to fall asleep each night and hours of actual sleep per night, subitems on the PSQI range from 0–3 (0=not during the past month, 1=less than once a week, 2=once or twice a week, and 3=three or more times a week); ratings for overall rating of sleep quality are classified as 0=very good, 1=fairly good, 2=fairly bad, and 3=very bad
Figure 1.
Problematic sleep (a global PSQI score> 5) grouped by HIV status and lifetime methamphetamine use disorder. The HIV+/MA+ group had higher rates of problematic sleep when compared to the other three groups. Differences among HIV+/MA−, HIV−/MA+, and HIV−/MA- were not significant (p’s> .45). Data is presented as mean proportions and 95% confidence intervals. OR = odds ratio. *p< .05, **p< .01.
Lifetime Methamphetamine Use Disorder and Sleep in PWH
Multiple regression analysis within PWH examined the independent contribution of MA status on Global PSQI scores while adjusting for clinical covariates and HIV-disease specific factors (Table III). Based on univariable associations with the primary independent variable [MA status (i.e., HIV+/MA+ vs. HIV+/MA−)], the following were included as covariates in AIC-based regression: age, sex, education, BDI-II scores, lifetime alcohol use disorder, lifetime cocaine use disorder, lifetime cannabis use disorder, MA use in the last 30 days, and HCV. In addition, body mass index (BMI) was added to the model based on its association with the primary dependent variable (global sleep; p= .05), along with sexual orientation and HIV-specific covariates (i.e., AIDS diagnosis, plasma HIV RNA, nadir and current CD4+ T-cell count, and estimated duration of infection). The model was significant (F(4, 175)= 14.27, R2= .25, R2(Adj)= .23, p< .001) and contained lifetime MA use disorder, having higher BDI-II scores, higher BMI, and detectable HIV RNA being associated with higher global PSQI scores. In considering the possible contribution of extraneous variables that may be common among participants who reported recent MA use, the regression model was rerun after excluding for those who endorsed MA use within the last 30 days (total n=18). Using AIC selection criteria, lifetime MA use disorder continued to significantly contribute to the variance in sleep quality (b=1.19 compared to b=1.27). Similarly, to focus on a clinically relevant subgroup, the regression model was rerun after excluding participants who were off ART or had HIV RNA levels above 200 copies/ml (n=15). In this virologically suppressed subgroup, lifetime MA dependence again remained associated with global sleep based on AIC selection (data not presented).
Table III.
Multiple regression on global PSQI within PWH (n=225)
| Beta (SE) | 95% CI | p-value | Std. Beta | |
|---|---|---|---|---|
| Lifetime MA use disorder (vs. MA−) | 1.27 (.61) | .06, 2.48 | .039 | .14 |
| BDI-II (higher) | .18 (.03) | .11, .24 | <.001 | .37 |
| BMI (higher) | .10 (.05) | .00, .19 | .042 | .14 |
| HIV Plasma HIV RNA (vs. undetectable) | 2.85 (1.05) | .77, 4.93 | .008 | .18 |
Overall model fit: R2= .25, R2 (Adj)= .23, F(4, 175)= 14.27, p< .001;
Covariates that were not selected based on AIC: age, sex, sexual orientation, education, lifetime alcohol use disorder diagnosis, lifetime cocaine use disorder diagnosis, lifetime cannabis use disorder diagnosis, MA use in the last 30 days, hepatitis C, CD4 count, nadir CD4, estimated duration of HIV infection, and AIDS status
Sleep Quality and Neurobehavioral Function
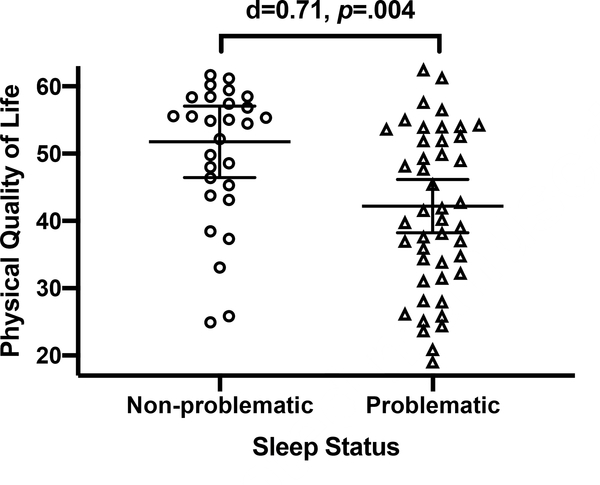
To examine potential co-occuring neurobehavioral functional impairments, in addition to poor sleep, experienced by PWH with a history of MA use disorder, we evaluated relationships between problematic sleep membership (global PSQI score>5) and clinically-relevant neurobehavioral outcomes within the HIV+/MA+ group (n= 84). Problematic sleep quality was associated with decreased self-reported physical (MOS SF-36 Physical Health: d= .71, 95% CI [.23, 1.18], t= 2.98, p= .004) and mental life quality (MOS SF-36 Mental Health: d= .85, 95% CI [.36, 1.33], t=3.58, p= .001), increased odds of IADL dependence (OR= 2.90, 95% CI [1.15, .024], χ2=5.35, p= 0.24), and marginally associated with increased odds of unemployment (OR= 2.35, 95% CI [.94, 5.92], χ2= 3.35, p= .07) and clinician ratings of worse health-related functioning (Karnofsky; d= .46, 95% CI [−.03, .94], t= 1.88, p= .06). Problematic sleep was not associated with neurocognitive impairment (high GDS) (OR= .31, 95% CI [.−.13, .74], t= −1.38, p= .171). Upon adjusting for covariates with univariable associations with the neurobehavioral outcome of interest, problematic sleep significantly explained unique variance in self-reported physical life quality (MOS SF-36 Physical Health), but not other outcomes (R2= .45, F(3, 66)=17.43, p< .001; OR= 7.31, 95% CI [1.60, 13.02], t= 2.56, p= .013). Covariates included in this model include age, sex, BDI-II, hypertension, hyperlipidemia, diabetes, and total number of medications.
DISCUSSION
Rates of MA use are elevated among PWH and are associated with poorer sleep quality in the general population (23, 24) (46). The present study is the first to explore the relationships between past (not current) MA use disorder, HIV disease, and sleep quality. Our results demonstrate that PWH who have a history of prior MA use disorder had significantly poorer sleep quality and were more likely to be classified as problematic sleepers than those without a lifetime disorder. This relationship between lifetime MA use disorder among PWH is robust to MA group differences in biopsychosocial factors and is linked to sleep quality above and beyond the effects of HIV disease severity and other established risk factors for poor sleep. Further, poorer sleep quality among PWH with comorbid lifetime MA use disorder was associated with a number of neurobehavioral functional outcomes, including decreased physical and mental life quality, IADL dependence, unemployment and clinician-rated functional disability.
As expected, lifetime MA use disorder was negatively associated with sleep quality; however, this finding was isolated to PWH and independent of recent MA use. In addition, MA use characteristics (frequency, quantity, duration, and recency) did not differ by HIV serostatus, suggesting sleep among PWH may be specifically related to the effects of non-recent MA use. Prior studies have demonstrated detrimental effects of MA on neurobehavioral health specific to PWH, including neurocognitive impairment (17, 34, 39) and associated everyday life consequences such as unemployment (39, 47, 48) and difficulties performing activities of daily living (39). It is possible that disrupted sleep may mediate the link between MA and functional outcomes, although longitudinal studies are needed to determine causality. Depressive symptoms in the HIV+/MA+ group are also consistent with prior research (49). While depressive symptoms were also associated with global PSQI scores, as expected, this did not attenuate the relationship between MA and global PSQI scores in PWH, suggesting additional mechanisms underlying MA-related sleep disturbance independent of mood.
One explanation for our findings is the combined, long-term CNS effects of excessive MA use and HIV on brain structures and/or pathways responsible for sleep regulation. While MA’s major mechanism of action is through increased activity of the mesolimbic dopamine system (50), emerging evidence supports that GABA-ergic dysfunction results from abuse of amphetamines (51–53). Projection systems of GABA include the reticular nucleus of the thalamus to the rostral brainstem reticular formation, a structure critical for sleep regulation. Further, GABA also promotes sleep via hypothalamic projections that inhibit serotonergic, noradrenergic, histaminergic, and cholinergic arousal systems (54). Future studies linking GABA to MA use and sleep quality are necessary to establish this theoretical mechanism of action. Also, while the lack of evidence of sleep disturbance in the very small HIV−/MA+ group would not support long-term effects of MA use on CNS mechanisms important for sleep, a much larger subject sample would be needed to draw any confident conclusions about HIV−/MA+ individuals.
Prior literature on the prevalence of sleep disturbance in PWH is variable and comparisons between demographically matched, HIV serostaus groups on sleep quality is lacking. In a meta-analysis of self-reported sleep disturbance in PWH, the overall prevalence was 58% (2). No comparisons have been made with HIV-uninfected individuals from the same population (with many similar lifestyle factors in Table I) to determine whether this prevalence is higher than in this type of comparison group. The current findings suggest HIV status alone may not elicit poor perception of sleep, however, fragmented sleep has been identified in chronic health conditions even without the patient’s perception of poor sleep (55). Consistent with prior literature (56), detectable HIV RNA was associated with poorer perceived sleep quality in our multiple regression analyses, but the specific mechanism for this association (e.g., worse ART adherence) could not be established. Other literature has suggested that HIV infection is linked to objective sleep measurements, including reduced slow wave sleep (57–59) and reduced rapid eye movement latency (60). However, studies have failed to detect similar associations between HIV disease severity and objective sleep measurements (61–63), highlighting the uncertainty to which HIV infection, by itself, may contribute to reductions in sleep quality.
The study has several limitations. First, the data are cross-sectional and cannot determine causality. Lifetime MA use disorder is suspected to precede self-reported poor sleep within the last 30 days, however, such self-reported sleep disturbances may be longstanding and could even have served as a precursor to problematic substance use (64). Thus, future longitudinal evaluations or with increased sample size, the use of structural equation modeling, would be helpful in better determining the timing, duration, and directionality of associations between MA use disorders and sleep. This goes alongside our report of neurobehavioral outcomes associated with problematic sleep within PWH with a history of MA use disorder. While theoretically, sleep should have some influence on function, it is also possible that there is some unique third variable quality within the HIV+/MA+ group that leads to both poor sleep and poor neurobehavioral outcomes. Again, a longitudinal research design or a larger sample size may help in teasing out the directionality of our findings.
Second, the small sample size of the HIV−/MA+ group hinders our ability to detect statistically significant associations between MA use and other findings with the HIV− participants. For example, the difference between HIV+/MA+ and HIV−/MA+ groups on global PSQI was not statistically significant (p= .124), yet the effect size suggests a nontrivial difference (d= .42). While our sample did not demonstrate an interaction between HIV and MA possibily due to this limitation, this relationship may exist. Further, while lifetime MA use disorder independently contributed to sleep quality in PWH, we did not observe a recent MA use effect on sleep. We should note that this too may be due to low power, with very few participants reporting use in the last 30 days. It is also important to highlight the complexity of polysubstance use in the context of a cross-sectional, retrospective study. The MA+ groups had higher rates of all other lifetime substance use disorders (i.e., alcohol., cannabis, and cocaine) than the MA-groups. Despite this, lifetime MA use disorder was retained in the multiple regression model, while the other substances did not. Due to limited data on participants who met criteria for a current substance use disorder or other measurements of current substance use parameters, our finding cannot speak to other potential factors associated with polysubstance use that may explain differences in sleep between MA+ and MA− groups. Future studies to formally investigate polysubstance use in more detail is needed to futher confirm our findings.
In addition, we did not find associations between age, sex, or sexual orientation on sleep quality, which is contrary to well established literature on these topics (44, 45). We suspect that the presence of other clinical risk factors for poor sleep, including those identified in this study (i.e., MA use, depression, BMI, and HIV disease), may be masking the detection of these variables traditionally known to impact sleep quality. There also remains the possibility that other unmeasured factors such as homelessness and/or SES may account for the observed relationship that MA was related to sleep in PWH that should be explored further in future studies. Lastly, the PSQI questionnaire is based on self-report, which is subject to recall and reporting bias. While there is merit in characterizing perceived sleep quality in vulnerable populations, as even the perception of poor sleep can influence mood and physical health (65, 66), subjective measurements are just one facet of sleep quality and the inclusion of objective measurements such as actigraphy would enhance understanding of sleep in PWH and substance using populations. Importantly, the global PSQI score demonstrates strong sensitivity (89.6%) and specificity (86.5%) in distinguishing good from poor sleepers among the general population (31, 67). While the sensitivity in detecting an insomnia diagnosis in PWH remains high (92%), the specificity drops considerably (61%) (68). This suggests that the PSQI may not just be capturing sleep quality in PWH and raises the question as to whether items such as “trouble staying awake during the day” or “trouble keeping enthusiasm” are purely a function of poor sleep or a result of HIV-infection, prescribed medications, and/or associated psychosocial factors. Studies investigating the quality of the PSQI subcomponents in capturing sleep quality within PWH using factor analyses may be a natural next step for future research.
CONCLUSIONS
Findings from this study suggest that self-reported poor sleep quality in PWH is independently associated with lifetime MA use disorder, above and beyond depressed mood and other clinical covariates, and is correlated with clinically-relevant neurobehavioral and life quality outcomes. Understanding the relationship between poor sleep and remote MA use disorder in PWH may promote increased assessment and consequently, treatment for poor sleep in this already vulnerable group, as poor sleep in individuals without HIV and history of MA use disorder has already demonstrated to be predictive of poor emotion regulation (69), cognitive impairment (70), and increased risk-taking behaviors (71). Future studies using a longitudinal design and or a larger sample size is needed to determine whether poor sleep reported in the HIV+/MA+ group is a consequence of remote MA use.
Figure 2.
Self-reported physical quality of life is lower in participants who reported problematic sleep compared to those with non-problematic sleep in comorbid HIV disease and lifetime methamphetamine (MA) use disorder (HIV+/MA+). Lines represent mean values and error bars represent 95% confidence intervals. In HIV+/MA+, individuals classified as problematic sleepers on the Pittsburgh Sleep Quality Index (global score> 5) reported significantly lower physical quality of life than individuals with non-problematic sleep. The association between problematic sleep and lower physical quality of life remained in multivariable models adjusting for age, sex, depressive symptoms, hypertension, hyperlipidemia, diabetes, and total number of medications (b= 7.31, p= .013; total model fit: R2= .45, F(3, 66)=17.43, p< .001).
ACKNOWLEDGEMENTS
The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie Beck, M.P.H.;Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Anya Umlauf, M.S., Bin Tang, Ph.D. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Funding: This research was supported by the National Institute on Drug Abuse: grant numbers P50 DA026306, T32 DA031098 (stipend support to NS), and F31 AG064989 (stipend support to RS).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
COMPLIANCE WITH ETHICAL STANDARDS
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (UCSD Human Research Protections Program, HNRC IRB#172092) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Clark JP, 3rd, Sampair CS, Kofuji P, Nath A, Ding JM. HIV protein, transactivator of transcription, alters circadian rhythms through the light entrainment pathway. Am J Physiol Regul Integr Comp Physiol. 2005;289(3):R656–62. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Wu H, Lu C, Guo L, Li P. Self-reported sleep disturbances in HIV-infected people: a meta-analysis of prevalence and moderators. Sleep Med. 2015;16(8):901–7. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. [DOI] [PubMed] [Google Scholar]
- 4.Pellowski JA, Kalichman SC, Matthews KA, Adler N. A pandemic of the poor: social disadvantage and the U.S. HIV epidemic. Am Psychol. 2013;68(4):197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semple SJ, Patterson TL, Temoshok LR, McCutchan JA, Straits-Troster KA, Chandler JL, et al. Identification of psychobiological stressors among HIV-positive women. HIV Neurobehavioral Research Center (HNRC) Group. Women Health. 1993;20(4):15–36. [DOI] [PubMed] [Google Scholar]
- 6.Rondanelli M, Solerte SB, Fioravanti M, Scevola D, Locatelli M, Minoli L, et al. Circadian secretory pattern of growth hormone, insulin-like growth factor type I, cortisol, adrenocorticotropic hormone, thyroid-stimulating hormone, and prolactin during HIV infection. AIDS Res Hum Retroviruses. 1997;13(14):1243–9. [DOI] [PubMed] [Google Scholar]
- 7.Allavena C, Guimard T, Billaud E, De la Tullaye S, Reliquet V, Pineau S, et al. Prevalence and Risk Factors of Sleep Disturbance in a Large HIV-Infected Adult Population. Aids Behav. 2016;20(2):339–44. [DOI] [PubMed] [Google Scholar]
- 8.Passaro RC, Pandhare J, Qian HZ, Dash C. The Complex Interaction Between Methamphetamine Abuse and HIV-1 Pathogenesis. J Neuroimmune Pharmacol. 2015;10(3):477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bateganya M, Colfax G, Shafer LA, Kityo C, Mugyenyi P, Serwadda D, et al. Antiretroviral therapy and sexual behavior: A comparative study between antiretroviral-naive and -experienced patients at an urban HIV/AIDS care and research center in Kampala, Uganda. Aids Patient Care St 2005;19(11):760–8. [DOI] [PubMed] [Google Scholar]
- 10.Shoptaw S Personal communication.
- 11.Passaro RC, Ramsey K, Segura ER, Lake JE, Reback CJ, Clark JL, et al. Speed kills: Associations between methamphetamine use, HIV infection, tobacco use, and accelerated mortality among gay and bisexual men in Los Angeles, CA 20years after methamphetamine dependence treatment. Drug Alcohol Depen. 2019;195:164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Ho WZ. Drugs of abuse and HIV infection/replication: implications for motherfetus transmission. Life Sci. 2011;88(21–22):972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrico AW, Flentje A, Kober K, Lee S, Hunt P, Riley ED, et al. Recent stimulant use and leukocyte gene expression in methamphetamine users with treated HIV infection. Brain Behav Immun. 2018;71:108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I, et al. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188(12):1820–6. [DOI] [PubMed] [Google Scholar]
- 15.Fairbairn N, Kerr T, Milloy MJ, Zhang R, Montaner J, Wood E. Crystal methamphetamine injection predicts slower HIV RNA suppression among injection drug users. Addict Behav. 2011;36(7):762–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackstone K, Iudicello JE, Morgan EE, Weber E, Moore DJ, Franklin DR, et al. Human immunodeficiency virus infection heightens concurrent risk of functional dependence in persons with long-term methamphetamine use. J Addict Med. 2013;7(4):255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10(1):1–14. [DOI] [PubMed] [Google Scholar]
- 18.Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry. 2005;162(2):361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semple SJ, Patterson TL, Grant I. Motivations associated with methamphetamine use among HIV+ men who have sex with men. J Subst Abuse Treat. 2002;22(3):149–56. [DOI] [PubMed] [Google Scholar]
- 20.Kish SJ. Pharmacologic mechanisms of crystal meth. CMAJ. 2008;178(13):1679–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, et al. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry. 2005;58(10):770–8. [DOI] [PubMed] [Google Scholar]
- 22.London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61(1):73–84. [DOI] [PubMed] [Google Scholar]
- 23.Perez AY, Kirkpatrick MG, Gunderson EW, Marrone G, Silver R, Foltin RW, et al. Residual effects of intranasal methamphetamine on sleep, mood, and performance. Drug Alcohol Depend. 2008;94(1–3):258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahoney JJ, 3rd, De La Garza R 2nd, Jackson BJ, Verrico CD, Ho A, Iqbal T, et al. The relationship between sleep and drug use characteristics in participants with cocaine or methamphetamine use disorders. Psychiatry Res 2014;219(2):367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang J, Liao Y, He H, Deng Q, Zhang G, Qi C, et al. Sleeping problems in Chinese illicit drug dependent subjects. BMC Psychiatry. 2015;15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lago JA, Kosten TR. Stimulant withdrawal. Addiction. 1994;89(11):1477–81. [DOI] [PubMed] [Google Scholar]
- 27.Baberg HT, Nelesen RA, Dimsdale JE. Amphetamine use: return of an old scourge in a consultation psychiatry setting. Am J Psychiatry. 1996;153(6):789–93. [DOI] [PubMed] [Google Scholar]
- 28.Reback CJ, Larkins S, Shoptaw S. Methamphetamine abuse as a barrier to HIV medication adherence among gay and bisexual men. Aids Care. 2003;15(6):775–85. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Composite Diagnositic International Interview (CIDI, version 2.1). Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 30.Beck A, Steer R, Brown G. Manual for Beck Depression Inventory II (BDI-II). San Antonio, TX, Psychology Corporation; 1996. [Google Scholar]
- 31.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 32.Rubinstein ML, Selwyn PA. High prevalence of insomnia in an outpatient population with HIV infection. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1998;19(3):260–5. [DOI] [PubMed] [Google Scholar]
- 33.Putnins SI, Griffin ML, Fitzmaurice GM, Dodd DR, Weiss RD. Poor sleep at baseline predicts worse mood outcomes in patients with co-occurring bipolar disorder and substance dependence. J Clin Psychiatry. 2012;73(5):703–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heaton RK, Clifford DB, Franklin DR, Jr., Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, FL: Psychological Assessment Resources, Inc; 2004. [Google Scholar]
- 36.Heaton RK, Taylor MJ, Manly J. Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III Clinical interpretation of the WAIS-III and WMS-III. San Diego, CA, US: Academic Press; 2003. p. 181–210. [Google Scholar]
- 37.Norman MA, Moore DJ, Taylor M, Franklin D, Jr., Cysique L, Ake C., et al. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol. 2011;33(7):793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blackstone K, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, et al. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol. 2012;26(6):894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–31. [DOI] [PubMed] [Google Scholar]
- 40.Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS, Grant I. HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology. 2008;22(1):110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chelune GJ, Heaton RK, Lehman RAW. Neuropsychological and personality correlates of patients’ complaints of disability Advances in clinical neuropsychology, Vol 3 New York, NY, US: Plenum Press; 1986. p. 95–126. [Google Scholar]
- 42.Crooks V, Waller S, Smith T, Hahn TJ. The use of the Karnofsky Performance Scale in determining outcomes and risk in geriatric outpatients. J Gerontol. 1991;46(4):M139–44. [DOI] [PubMed] [Google Scholar]
- 43.Farivar SS, Cunningham WE, Hays RD . Correlated physical and mental health summary scores for the SF-36 and SF-12 Health Survey, V.I. Health Qual Life Outcomes. 2007;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dustin DT, Kawachi I, Redline S. The social epidemiology of sleep. New York: Oxford University Press; 2019. pages cm p. [Google Scholar]
- 45.Millar BM, Goedel WC, Duncan DT. Sleep Health Among Sexual and Gender Minorities. The Social Epidemiology of Sleep. 2019:187. [Google Scholar]
- 46.Colfax G, Shoptaw S. The methamphetamine epidemic: implications for HIV prevention and treatment. Curr HIV/AIDS Rep. 2005;2(4):194–9. [DOI] [PubMed] [Google Scholar]
- 47.Heaton RK, Velin RA, McCutchan JA, Gulevich SJ, Atkinson JH, Wallace MR, et al. Neuropsychological impairment in human immunodeficiency virus-infection: implications for employment. HNRC Group. HIV Neurobehavioral Research Center. Psychosom Med. 1994;56(1):8–17. [DOI] [PubMed] [Google Scholar]
- 48.Woods SP, Weber E, Weisz BM, Twamley EW, Grant I, Group HIVNRP. Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabil Psychol. 2011;56(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhatia MS, Munjal S. Prevalence of Depression in People Living with HIV/AIDS Undergoing ART and Factors Associated with it. J Clin Diagn Res. 2014;8(10):WC01–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adinoff B Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry. 2004;12(6):305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiao D, Liu Y, Li X, Liu J, Zhao M. The role of the GABA system in amphetamine-type stimulant use disorders. Front Cell Neurosci. 2015;9:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Padgett CL, Lalive AL, Tan KR, Terunuma M, Munoz MB, Pangalos MN, et al. Methamphetamine-evoked depression of GABA(B) receptor signaling in GABA neurons of the VTA. Neuron. 2012;73(5):978–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamata K, Kameyama T. Effect of chronic administration of methamphetamine on the responsiveness of substantia nigra zona reticulata neurons to GABA or a GABA agonist in rats. Naunyn Schmiedebergs Arch Pharmacol. 1986;334(4):458–62. [DOI] [PubMed] [Google Scholar]
- 54.Blumenfeld H Neuroanatomy through clinical cases. 2 ed. Sunderland, MA: Sinauer Associates Inc.; 2012. [Google Scholar]
- 55.Association AP. Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: 2013. [Google Scholar]
- 56.Downing MJ, Jr., Houang ST, Scheinmann R, Yoon IS, Chiasson MA, Hirshfield S. Engagement in Care, Psychological Distress, and Resilience are Associated with Sleep Quality among HIV-Positive Gay, Bisexual, and Other Men Who Have Sex with Men. Sleep Health. 2016;2(4):322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White JL, Darko DF, Brown SJ, Miller JC, Hayduk R, Kelly T, et al. Early central nervous system response to HIV infection: sleep distortion and cognitive-motor decrements. AIDS. 1995;9(9):1043–50. [DOI] [PubMed] [Google Scholar]
- 58.Norman SE, Chediak AD, Freeman C, Kiel M, Mendez A, Duncan R, et al. Sleep disturbances in men with asymptomatic human immunodeficiency (HIV) infection. Sleep. 1992;15(2):150–5. [DOI] [PubMed] [Google Scholar]
- 59.Norman SE, Chediak AD, Kiel M, Cohn MA. Sleep disturbances in HIV-infected homosexual men. AIDS. 1990;4(8):775–81. [DOI] [PubMed] [Google Scholar]
- 60.Wiegand M, Moller AA, Schreiber W, Krieg JC, Fuchs D, Wachter H, et al. Nocturnal sleep EEG in patients with HIV infection. Eur Arch Psychiatry Clin Neurosci. 1991;240(3):153–8. [DOI] [PubMed] [Google Scholar]
- 61.Ferini-Strambi L, Oldani A, Tirloni G, Zucconi M, Castagna A, Lazzarin A, et al. Slow wave sleep and cyclic alternating pattern (CAP) in HIV-infected asymptomatic men. Sleep. 1995;18(6):446–50. [PubMed] [Google Scholar]
- 62.Jean-Louis G, Weber KM, Aouizerat BE, Levine AM, Maki PM, Liu C, et al. Insomnia symptoms and HIV infection among participants in the Women’s Interagency HIV Study. Sleep. 2012;35(1):131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee KA, Gay C, Portillo CJ, Coggins T, Davis H, Pullinger CR, et al. Symptom experience in HIV-infected adults: a function of demographic and clinical characteristics. J Pain Symptom Manage. 2009;38(6):882–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weissman MM, Greenwald S, Niño-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. General hospital psychiatry. 1997;19(4):245–50. [DOI] [PubMed] [Google Scholar]
- 65.Thomsen DK, Mehlsen MY, Christensen S, Zachariae R. Rumination—relationship with negative mood and sleep quality. Personality and Individual Differences. 2003;34(7):1293–01. [Google Scholar]
- 66.Heitkemper M, Jarrett M, Burr R, Cain K, Landis C, Lentz M, et al. Subjective and objective sleep indices in women with irritable bowel syndrome. Neurogastroenterology & Motility. 2005;17(4):523–30. [DOI] [PubMed] [Google Scholar]
- 67.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737–40. [DOI] [PubMed] [Google Scholar]
- 68.Gutierrez J, Tedaldi EM, Armon C, Patel V, Hart R, Buchacz K. Sleep disturbances in HIV-infected patients associated with depression and high risk of obstructive sleep apnea. SAGE open medicine. 2019;7:2050312119842268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palmer CA, Alfano CA. Sleep and emotion regulation: an organizing, integrative review. Sleep medicine reviews. 2017;31:6–16. [DOI] [PubMed] [Google Scholar]
- 70.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36(7):1027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Brien EM, Mindell JA. Sleep and risk-taking behavior in adolescents. Behavioral sleep medicine. 2005;3(3):113–33. [DOI] [PubMed] [Google Scholar]