Abstract
The outcomes of refractive surgical procedures to improve uncorrected vision in patients—including photorefractive keratectomy (PRK), laser in-situ keratomileusis (LASIK), Small Incision Lenticule Extraction (SMILE) and corneal inlay procedures—is in large part determined by the corneal wound healing response after surgery. The wound healing response varies depending on the type of surgery, the level of intended correction of refractive error, the post-operative inflammatory response, generation of opacity producing myofibroblasts and likely poorly understood genetic factors. This article details what is known about these specific wound healing responses that include apoptosis of keratocytes and myofibroblasts, mitosis of corneal fibroblasts and myofibroblast precursors, the development of myofibroblasts from keratocyte-derived corneal fibroblasts and bone marrow-derived fibrocytes, deposition of disordered extracellular matrix by myofibroblasts, healing of the epithelial injury, and regeneration of the epithelial basement membrane. Problems with epithelial and stromal cellular viability and function that are altered by corneal inlays are also discussed. A better understanding of the wound healing response in refractive surgical procedures is likely to lead to better treatments to improve outcomes, limit complications of keratorefractive surgical procedures, and improve the safety and and efficiency of refractive surgical procedures.
Keywords: Cornea, wound healing, LASIK, PRK, PTK, small incision lenticule extraction (SMILE), radial keratotomy, laser thermal keratoplasty (LTK), thermal conductive keratoplasty (CK), corneal inlays, apoptosis, myofibroblasts, corneal fibroblasts, corneal scar, corneal haze, mitomycin C
This article details the wound healing responses to corneal refractive surgery procedures that are most commonly performed, including photorefractive keratectomy (PRK), phototherapeutic keratectomy (PTK), laser-assisted in situ keratomileusis (LASIK) and small incision lenticule extraction (SMILE), but also includes incisional procedures such as radial keratotomy (RK), as well as contact laser thermal keratoplasty (LTK), thermal conductive keratoplasty (CK), and corneal inlay procedures.
1. Photorefractive keratectomy (PRK) and phototherapeutic keratectomy (PTK)
PRK remains a popular method for the correction of myopia or hyperopia—with or without astigmatism. The first step of PRK is removal of the central corneal epithelium, which initiates the wound healing response, and the different methods used to remove the epithelium used by surgeons are not equivalent with regards to the ensuing wound healing responses. The original method used for epithelial removal, which is still utilized by many surgeons, was use of a scalpel blade (for example, a #64 scalpel Beaver blade). An approximately 9 mm zone of central epithelium is removed, along with the epithelial basement membrane (EBM), by firmly scraping back and forth with the blade. This epithelial removal initiates the keratocyte apoptosis/necrosis response in the anterior corneal stroma that is also impacted by the subsequent stromal ablation with the excimer laser. Another method used by some surgeons is a rotating motor-driven brush instrument. These instruments are efficient at breaking down and removing the epithelium. However, during the Autonomous Ladar Vision excimer laser PRK trials in the mid-1990s, the author was consulted when a large number of sterile subepithelial infiltrates were noted in the corneas of patients in the first few days after PRK. Over 1.5% of corneas were developing the infiltrates (https://www.accessdata.fda.gov/cdrh_docs/pdf/P970043B.pdf). A rotating motor-driven brush instrument was being used in all of these PRK procedures. The author suggested using a blade to remove the epithelium, hypothesizing the rotating brush so efficiently broke apart all corneal epithelial cells that larger amounts of interleukin-1α were being released into the stroma and triggering surviving keratocytes to produce more chemokines that draw inflammatory cells into the cornea from the limbal blood vessels. When this change was made in the trials, no additional corneas developed subepithelial infiltrates. If the surgeon prefers to use the brush, then an increased frequency of topical corticosteroids could be used if subepithelial corneal infiltrates are noted. Another popular method for epithelial removal is to use a well to expose the central 9 mm of epithelium to 20 to 50% ethanol for 20 to 30 seconds before removal of the ethanol, irrigation of the surface with balanced salt solution and removal of the epithelium with a blade (Carones et al., 1999). However, the use of alcohol results in apoptosis and necrosis of a larger number of anterior keratocytes and rabbit eyes that had epithelial scrape PRK had greater keratocyte density in the anterior stroma at 24 hours after surgery than eyes that had 50% ethanol-assisted epithelial scrape during PRK (Helena et al., 1997). Since keratocyte-produced EBM components contribute to EBM regeneration (Hassell et al., 1992; Santhanam et al., 2015; Torricelli et al., 2015; Santhanam et al., 2017; Saikia et al., 2018) that terminates the anterior stromal late haze myofibroblast-development response by decreasing activated TGFβ penetration into the stroma (Torricelli et al., 2013a,b; Marino et al., 2017), we hypothesized that ethanol-assisted removal of the epithelium can increase PRK-induced stromal fibrosis (late haze), including “breakthrough haze” where fibrosis occurs despite treatment with mitomycin C after PRK (Medeiros et al., 2018b). However, two clinical studies found no difference between ethanol-assisted and mechanical scrape epithelial removal (Carones et al., 1999; Ghoreishi et al., 2010). Transepithelial PRK, where the excimer laser itself is used to remove the central epithelium prior to ablation of the stroma, is another approach to PRK. A study in rabbits found decreased keratocyte apoptosis after transepithelial PRK compared to epithelial-scrape PRK, suggesting a decreased overall wound healing response and decreased fibrosis with transepithelial PRK (Kim et al., 1998). However, a study comparing mechanical epithelial scrape, 20% ethanol-assisted epithelial scrape and excimer laser transepithelial PRK found no difference in stromal opacity, post-operative pain or refractive outcomes between the three groups (Lee et al., 2005). Zarei-Ghanavati and coworkers (2019) found that although the epithelial defect size and epithelial healing time were lower in transepithelial PRK, the postoperative pain, photophobia, and vision fluctuation were significantly less in PRK with scrape epithelial removal in the first few days after surgery. These authors found there was no statistically significant difference in clinical results between the two methods after one week. Similarly, two other studies comparing transepithelial PRK to mechanical epithelial removal PRK found little difference in stromal haze between the two methods (Luger et al., 2012; Antonios et al., 2017).
Anterior stromal keratocyte apoptosis, and subsequent combined stromal cell apoptosis and necrosis of a mixture of keratocytes, corneal fibroblasts, and bone marrow-derived cells in the anterior stroma, is greater with higher levels of correction of myopia than lower levels of correction of myopia with mechanical scrape-PRK in rabbit corneas (Fig. 1, Fig. 2) (Wilson, 2002; Mohan et al., 2003). Similarly, the ensuing proliferation of stromal cells (Fig. 3, Fig. 4) and the development of alpha-smooth muscle actin+ myofibroblasts is higher in rabbit corneas that have high (greater than −6 diopters) mechanical scrape-PRK (Fig. 5, Fig. 6) compared to low (less than −6 diopters) mechanical scrape-PRK where myofibroblasts rarely develop (Wilson, 2002; Mohan et al., 2003). Large numbers of monocytes, polymorphonuclear cells, fibrocytes and other bone marrow-derived cells invade the cornea by 24hr after surgery and persist for over 1 week. A similar influx of cells is noted after irregular PTK in mice (Lassance et al., 2018).
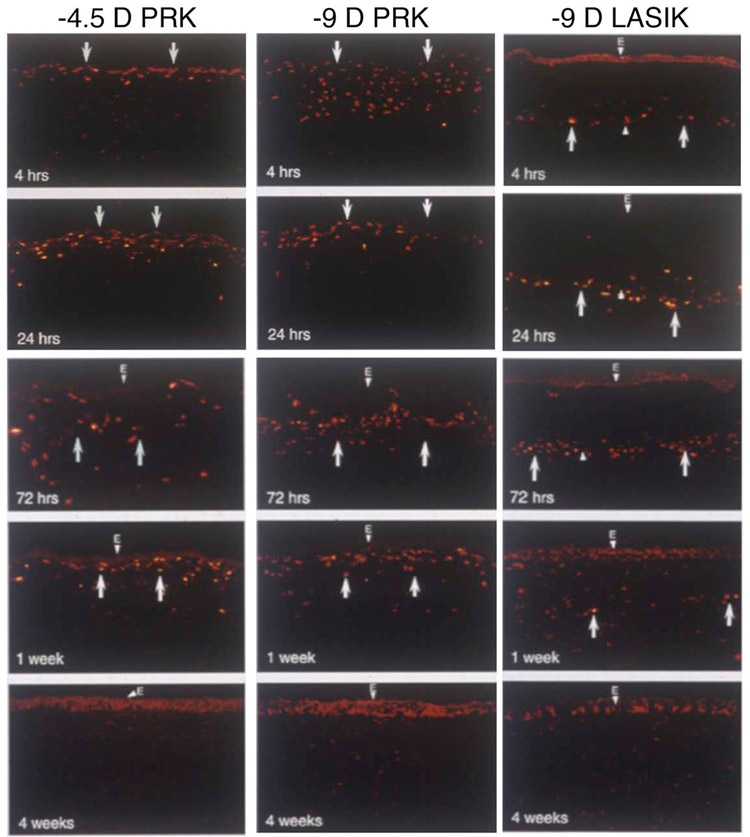
Fig. 1.
TUNEL assay to detect apoptosis in rabbit corneas at different time points after −4.5D PRK, −9D PRK and −9D LASIK. Note that corneas that have −9D PRK have greater keratocyte apoptosis (arrows), especially at the 4 hour time point. The apoptosis response persists for at least one week and includes bone marrow-derived cells that infiltrate the cornea after surgery. After −9D LASIK, the keratocyte apoptosis response is less than −9D PRK and is localized deeper in the corneal stroma along the interface and away from the overlying epithelium. e indicates epithelium stained with DAPI. Mag. 200X. Reprinted with permission from Mohan et al. Exp. Eye Res. 2003;76:71-87.
Fig. 2.

Quantitative analysis of TUNEL+ apoptotic stromal cells at each time point for −4.5D PRK, −9D PRK and −9D LASIK, with 8 corneas in each group at each time point. Note that from 4 to 72 hours stromal cell apoptosis was significantly greater in the −9D PRK group. The −9D LASIK group had lower stromal cell apoptosis from 4 to 72 hours than the −9D PRK group and was also lower than the −4.5D PRK group at the 4 hour and 24 hour time points. Modified with permission from Mohan et al. Exp. Eye Res. 2003;76:71-87.
Fig. 3.
Stromal cell mitosis detected with immunohistochemistry for the mitosis marker Ki-67 at different time points after −4.5D PRK, −9D PRK and −9D LASIK. Note there is much greater stromal cell mitosis (arrows) in both PRK groups compared to the LASIK group. Epithelial cells normally undergo mitosis as a part of their life cycle. Mag. 200X. Reprinted with permission from Mohan et al. Exp. Eye Res. 2003;76:71-87.
Fig. 4.
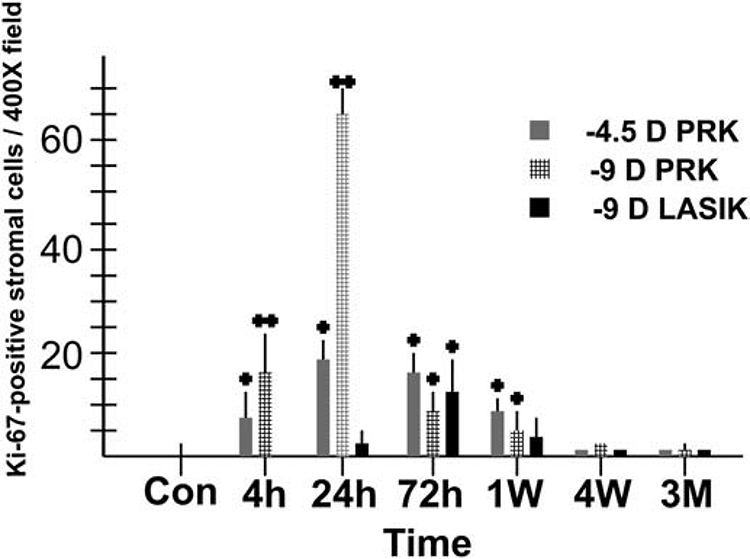
Quantitative analysis of Ki-67+ mitotic stromal cells at each time point for −4.5D PRK, −9D PRK and −9D LASIK, with 8 corneas in each group at each time point. Note that stromal cell mitosis is much greater in the −9D PRK group, especially at 24 hours after surgery. Stromal cell mitosis remained significantly higher in the PRK groups compared to the LASIK group at one week after surgery. Modified with permission from Mohan et al. Exp. Eye Res. 2003;76:71-87.
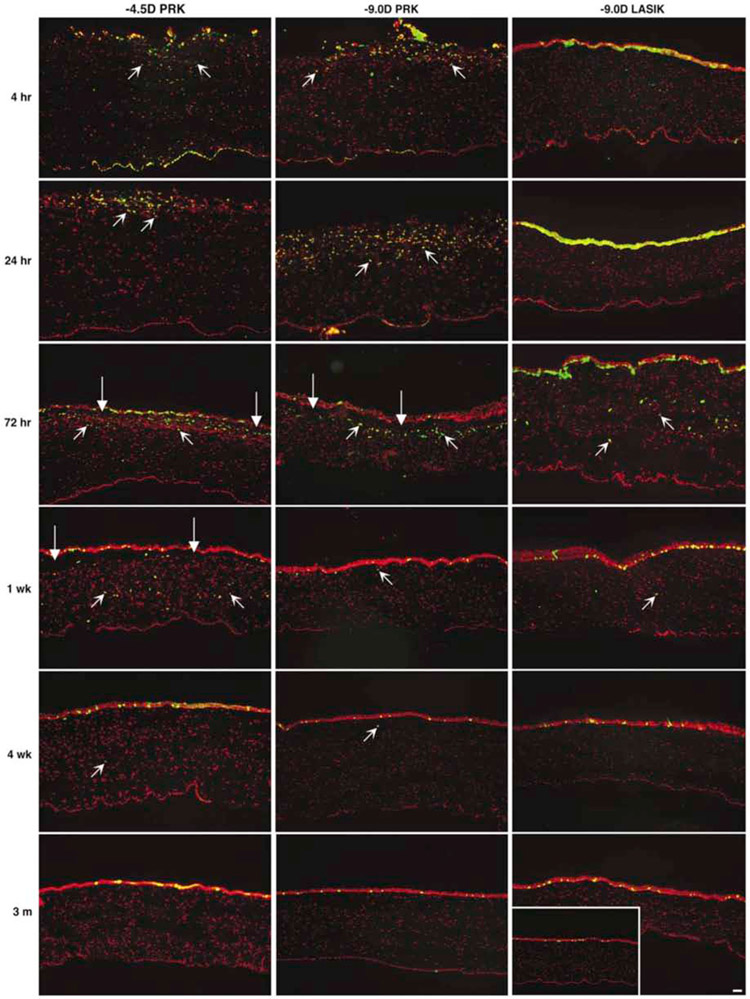
Fig. 5.
Subepithelial myofibroblasts detected with immunohistochemistry for alpha smooth muscle actin (SMA, green, arrows) after −4.5D, −9D PRK or −9D LASIK in rabbits. A. Very few, if any, SMA+ cells are detected in an unwounded rabbit cornea. B. Few SMA+ cells are detected in a cornea that had −4.5D PRK at one month after surgery. C. Similarly few SMA+ cells are detected at one week after −9D LASIK (insert shows the entry point of the microkeratome blade (arrowhead). At one month after −9D PRK (not shown) SMA+ myofibroblasts are only found at the peripheral edge of the LASIK flap where the epithelial basement membrane was damaged by the LASIK microkeratome. D. At one week after −9D PRK, only a single SMA+ myofibroblasts in detected in the subepithelial stroma. E. By one month after −9D PRK, numerous SMA+ myofibroblasts are present in the anterior stroma. F. At three months after −9D PRK, the SMA+ myofibroblasts have decreased. By 6 months after −9D PRK (not shown) few if any SMA+ myofibroblasts can be detected in the stroma. red is propidium iodide staining of nuclei. Mag. 200x Reprinted with permission from Mohan et al. Exp. Eye Res. 2003;76:71-87.
Fig. 6.
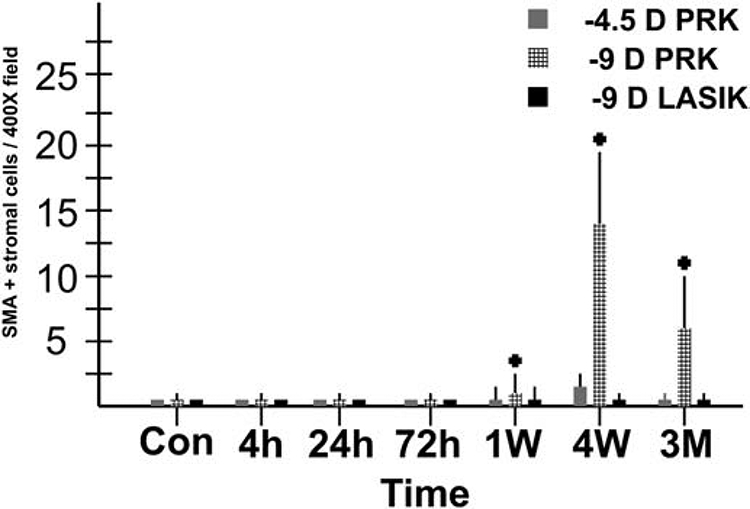
Quantitative analysis of alpha-smooth muscle actin+ myofibroblasts at each time point for −4.5D PRK, −9D PRK and −9D LASIK, with 8 corneas in each group at each time point. Note myofibroblasts were much greater in the −9D PRK group, with only a few myofibroblasts detected in corneas that had −4.5D PRK and none detected in the central cornea of eyes that had −9D LASIK. Modified with permission from Mohan et al. Exp. Eye Res. 2003;76:71-87.
Whether or not the EBM regenerates in a timely manner—typically within 2 to 3 weeks—is the major determinate of regenerative healing of the corneal stroma with mild opacity due to transient corneal fibroblast and fibrocyte progeny or fibrotic healing due to the development of persistent mature myofibroblasts (Torricelli et al., 2013a,b; Marino et al., 2017). Mature myofibroblasts are themselves opaque due to downregulation of corneal crystallins and they produce large amounts of disordered extracellular matrix (Jester et al., 1999; Wilson, 2012). Two factors that have been recognized as major determinates of EBM regeneration after PRK or PTK are stromal surface irregularity that mechanically impedes the assembly (Netto et al., 2006a) and high keratocyte apoptosis and necrosis since keratocytes and corneal fibroblasts derived from keratocytes contribute EBM components to the regenerating EBM, in cooperation with the epithelium (Hassell et al., 1992; Santhanam et al., 2015; Torricelli et al., 2015; Santhanam et al., 2017; Saikia et al., 2018). Although high hyperopia and/or astigmatism hasn’t been studied in animal models, fibrosis of the cornea is also more likely to occur after high PRK corrections for high hyperopia or astigmatism based on the incidence of “late haze” (fibrosis) development, even if the fibrosis develops when mitomycin C is used with PRK (“breakthrough haze”) (Kaiserman et al., 2017). After PRK for hyperopia corneal opacity from fibrosis tends to be most pronounced in the midperiphery of the cornea.
PRK with some brands of excimer lasers was associated with greater clinically significant late haze fibrosis, for example the Summit Technology (Hersh et al., 1996; Hersh et al., 1997; Vestergaard et al., 2013) and LADARVision 6000 (Lin et al., 2004) excimer lasers. The latter laser system was recalled by the Food and Drug Administration on February 21, 2007 due to surface irregularity central islands and peninsulas in patients who had either PRK or LASIK. As a user of both of these laser systems, the author noted at the time that mitomycin C treatment had to be applied at the time of PRK with either excimer laser, even for corrections as low as −1 diopter, or an incidence of late haze as high as 10 to 15% would be noted (Wilson SE, unpublished data, 2006). Thus, the surface irregularity after PRK—which central islands and peninsulas create with these two laser systems—increased the risk of anterior stromal fibrosis (late haze)—likely because that surface irregularity impeded normal EBM regeneration (Torricelli et al., 2013a,b; Marino et al., 2017; Netto et al., 2006a).
The primary biological effect of mitomycin C in inhibiting corneal stromal fibrosis (late haze) is due to its profound effect in reducing the mitosis of stromal cells, including corneal fibroblasts and fibrocytes that can develop in the corneal stroma after PRK (Fig. 7) (Netto et al., 2006b). The later study suggested that 0.002% mitomycin C might be as effective as 0.02% mitomycin C, but clinical studies in humans found that this concentration resulted in more clinically significant late haze (Thornton et al., 2005). The duration of treatment necessary for mitomycin C efficacy in preventing fibrosis has been studied and it was found that treatment with 0.02% mitomycin C for 12 seconds had equal efficacy in preventing haze after PRK to treatments for 60 or 120 seconds (Virasch et al., 2010). Many surgeons have adopted an exposure time of 30 seconds for routine treatments. (Netto et al., 2006b). Cornea fibrosis occasionally occurs in corneas after PRK with mitomycin C treatment and is termed “breakthrough haze” (Kaiserman et al., 2017). The author has noted that when breakthrough late haze has less tendency to resolve spontaneously at one to two years after surgery than haze that develops in corneas not treated with mitomycin C (Wilson SE, unpublished data, 2017). Our working hypothesis is that the mitomycin C produces long term effects on the phenotype of the anterior keratocytes that reduces their capacity to contribute to eventual regeneration of the EBM necessary for the resolution of stromal fibrosis.
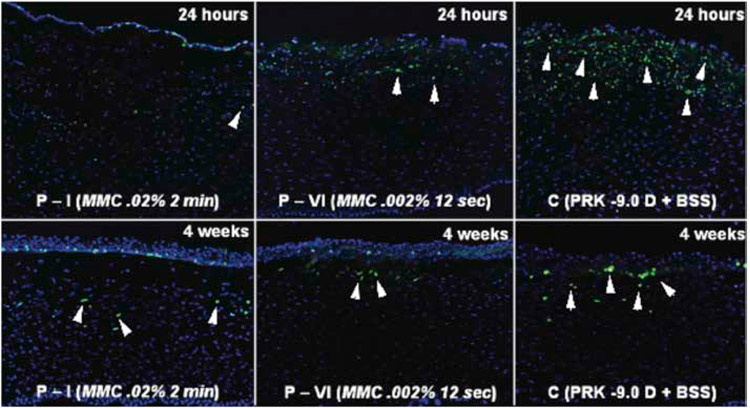
Fig. 7.
Immunohistochemistry for the mitosis marker Ki-67 (green) with DAPI straining (blue) of all cell nuclei in rabbit corneas that had −9.0 D PRK for myopia with mitomycin C or vehicle balanced salt solution (BSS) application at the time of surgery and studied at 24 hours or 4 weeks later. Note there was marked cell proliferation (arrowheads) in the anterior stroma in the BSS group (C) at 24 hours after PRK, with some stromal cell mitosis continuing at 4 weeks after surgery. Markedly reduced proliferation of anterior stromal cells was found at 24 hours and at four weeks after PRK surgery in corneas treated with 0.02% mitomycin C for two minutes. If rabbit corneas were treated with 0.002% mitomycin C for 12 seconds, there were more anterior stromal cells undergoing mitosis at 24 hours and four weeks after PRK than with 0.02% mitomycin C, but less than in the BSS group. Mag. 200X. Reprinted with permission from Netto, et al. J. Ref. Surgery, 2006;22:562-574.
The central cornea nerves are injured by PRK and the nerves suffer retrograde axonal cell death such that the damage extends beyond the excimer laser ablation (Medeiros et al., 2018a). Regeneration of the nerves takes at least six months and innervation may not return to completely normal (Medeiros et al., 2018a). If fibrosis develops, then reinnervation may be even more prolonged since myofibroblasts inhibit corneal nerve regeneration (Jeon et al., 2018; Hindman et al., 2019).
A variant of PRK, laser subepithelial keratomileusis or LASEK, in which an epithelium-only flap on the cornea is produced with 20% ethanol exposure for 15 to 20 seconds (Nakamura et al., 2001) still is used by some refractive surgeons. The concept relies on intact epithelium and basement membrane, and there is variability in the viability of epithelial cells with this method (Gabler et al., 2002). However, a prospective, randomized contralateral eye study that included 167 patients found that late haze fibrosis was more common after PRK without mitomycin C and LASEK, than PRK with mitomycin C (Sia et al., 2014). Few other wound healing studies have been applied to LASEK. Epi-LASIK is another variant in which a blunt blade on a microkeratome devise is used to attempt to lift the epithelium with intact EBM. However, intrusions of the blade into the stroma with resulting stromal fibrosis have been noted with this method. In addition, extensive death of basal epithelial cells was noted with epi-LASIK (Tanioka et al., 2007), indicating that the wound healing effect would not be different from PRK.
The wound healing response after PTK for recurrent erosions is similar to PRK because after epithelial debridement the stromal surface is typically smooth and only a few pulses of PTK need to be applied to the stromal surface to ensure any residual duplicated epithelial basement membrane is removed (Wilson et al., 2017). PTK to remove corneal scarring, whether it is due to trauma, infection, surgical complication, or dystrophy is different because the stromal surface is nearly always irregular (Wilson et al., 2017). Stromal surface irregularity is one of the primary factors leading to corneal fibrosis (Netto et al., 2006a) because the irregularity mechanically inhibits regeneration of the epithelial basement membrane which facilitates epithelial TGFb1 and TGFb2 penetration into the stroma to drive the development of fibrosis-generating myofibroblasts from keratocyte and fibrocyte precursors (Lassance et al., 2018). In mice, for example, generation of surface irregularity by performing PTK over a fine mesh is an optimal method to generate superficial stromal myofibroblasts and fibrosis (Lassance et al., 2018). Thus, when PTK is performed to remove anterior stromal scars, a primary goal should be to also smooth the stromal surface. This is accomplished by performing transepithelial PTK to use the epithelium as a partial masking agent, and then, after some or all of the stromal scar is removed, using masking-smoothing PTK to further smooth the stromal surface (Wilson et al., 2017). Finally, mitomycin C treatment is usually recommended to further inhibit myofibroblast development (Netto et al., 2006b; Wilson et al., 2017). Using this approach, the wound healing response after PTK for scar removal becomes similar to PRK for high myopia where myofibroblasts and fibrosis rarely develop if topical mitomycin C is used.
2. Laser-assisted in situ keratomileusis (LASIK)
Normal LASIK surgery with a microkeratome results in less central corneal keratocyte apoptosis and proliferation than PRK, and rarely is associated with stromal fibrosis (Fig. 1 to Fig. 6), except at the flap edge, where the EBM is damaged and is commonly demarcated by fibrosis (Fig. 8) (Wilson, 2002; Mohan et al., 2003; Medeiros et al., 2018b). Also, the location of the keratocyte apoptosis and early postoperative inflammation in LASIK is different from PRK since these tend to occur just anterior and posterior to the lamellar cut in the stroma (Fig. 1), and is further from the overlying epithelium than in PRK. The apoptosis of keratocytes is thought to be triggered by IL-1 on the blade that passes through the epithelium before cutting through the stroma (Wilson, 2002; Mohan et al., 2003). When there is damage to the central corneal epithelium during cutting of the flap with a microkeratome, excessive inflammation tends to enter the central cornea from the limbal blood vessels through the interface between the flap and the underlying stromal bed to produce diffuse lamellar keratitis (DLK) (Smith and Maloney, 1998; Wilson and Ambrósio Jr., 2002).
Fig. 8.
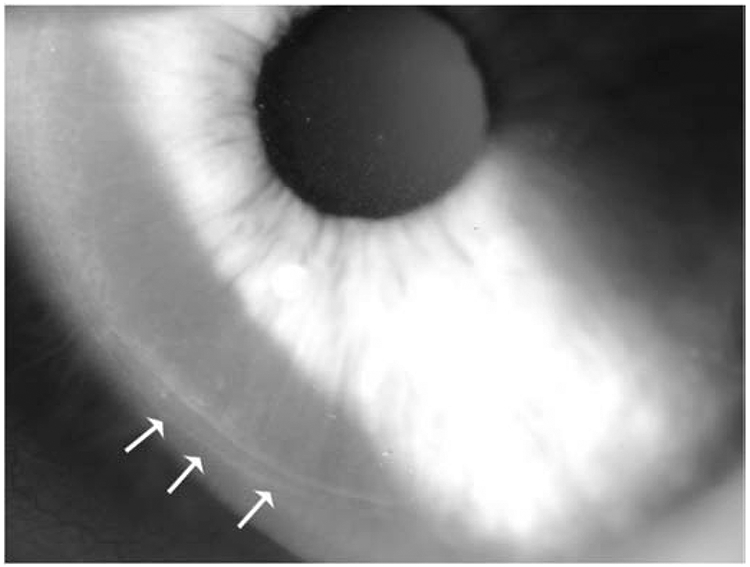
Flap edge fibrosis (arrows) that is commonly noted after LASIK at the slit lamp. Mag. 40X. Reprinted with permission from Medeiros CS et al. Invest. Ophthalmol. Vis. Sci. 2018;59:4044–4053.
The wound healing response to femtosecond laser flap LASIK is potentially different than microkeratome flap LASIK, depending on the laser model and the energy delivered to the stroma to cut the flap. Earlier models of femtosecond lasers, such as the 15 kHz and 30 kHz Intralase lasers, delivered more energy to the stroma and caused more stromal cell death (Netto et al., 2007; de Medeiros et al., 2009). In addition, the mode of cell death is necrosis rather than apoptosis (Netto et al., 2007; de Medeiros et al., 2009), which may trigger more inflammatory cell infiltration along the interface, especially in the periphery (Fig. 9) to cause “flap edge DLK.” As femtosecond lasers improved, the amount of energy needed to cut the flap decreased and the early postoperative interface inflammation decreased. Also, a strong flap edge fibrosis response with the earlier models of femtosecond lasers made flap lifting for retreatment more difficult after a few months. With the latest models of femtosecond lasers, the stromal cell death response is similar to that with microkeratomes (Netto et al., 2007; de Medeiros et al., 2009).Thus, whichever model femtosecond laser is being used for LASIK, stromal cell necrosis, interface inflammation and flap edge fibrosis can be limited by using the lowest energy setting that effectively cuts the LASIK flap (de Medeiros et al., 2009).
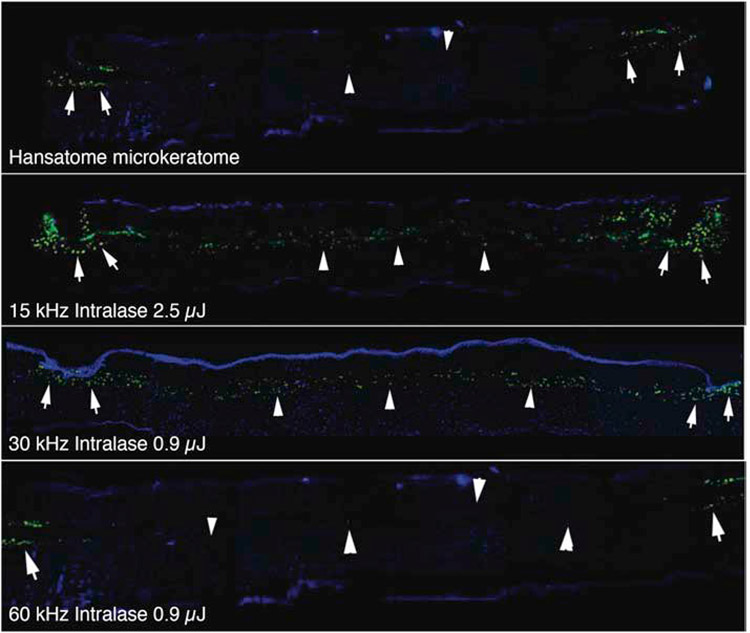
Fig. 9.
Microkeratome compared to femtosecond laser flap inflammation. At 24 hours after flap formation in rabbit corneas, corneas were processed for immunohistochemistry for the monocyte marker CD11b (green). Note that with the microkeratome monocytes were only detected at the edges of the flap (arrows). Arrowheads indicate the interface. The 15 kHz Intralase laser required the 2.5 μJ setting to cut the flap and resulted in much greater inflammation at the flap edge (arrows) and along the interface (arrowheads). This resulted in much more flap edge DLK, and DLK in the central cornea, and necessitated the use of more frequent topical corticosteroids during the early postoperative period.36 Both flap edge and interface inflammation were decreased with the 30 kHz Intralase using the 0.9 μJ energy setting. The 60 kHz Intralase resulted in flap edge inflammation (arrows) similar to the microkeratome and little inflammation along the interface (arrowheads). Mag 200x. Reprinted with permission from Netto et al J. Ref. Surg. 2007;23:667-676.
Whether LASIK is performed with a microkeratome or a femtosecond laser, the overall wound healing response in the cornea is less than it is after PRK for the same level of correction, including stromal cell apoptosis/necrosis, stromal cell mitosis, inflammatory cell infiltration and myofibroblast generation (Wilson, 2002; Mohan et al., 2003). Although the accuracy of correction for the two procedures tends to be similar, there is far less tendency for central stromal late haze (fibrosis) in LASIK than PRK.
A study in rabbits compared flap adhesion at one and two months after LASIK flap generation with a VisuMax laser or a femto-LDV laser and found greater flap adhesion in the VISUMax group, but the difference did not reach statistical significance (Riau et al., 2014). Tissue responses to laser delivery using the two lasers was investigated with immunohistochemistry for fibronectin and the TUNEL assay at four and 24 hours postoperatively and the results suggested less early tissue response with the femto-LDV laser (Riau et al., 2014). Thus, there are differences in the corneal wound healing responses with different lasers used to prepare the flap for LASIK.
3. Small Incision Lenticule Extraction (SMILE)
Few studies of corneal wound healing after SMILE surgery have been reported. One study in human eye bank eyes that had SMILE or LASIK, and were subsequently incubated in organ culture medium, found that keratocyte apoptosis, keratocyte proliferation, and infiltration of immune cells were generally mild and comparable between femtosecond-LASIK and SMILE (Luft et al., 2018). The significance of these results, however, is uncertain since the eyes had been removed from humans at differing times after death and incubated in corneal preservation media prior to surgery, and therefore, there could be no contribution of bone marrow-derived cells from the limbal vasculature to the wound healing response (Lassance et al., 2018). In addition, corneas were placed in organ culture for 72 hours after surgery prior to processing for the analyses.
A study performed in rabbits (Dong et al., 2014) found significantly fewer TUNEL-positive corneal stromal cells after SMILE at 4 hours and 24 hours postoperatively (p<0.01) compared with LASIK (but no TEM was included to distinguish between stromal cell apoptosis and necrosis produced with either surgical method). Immunohistochemistry showed significantly fewer Ki-67-positive cells in the SMILE group than in the femtosecond laser LASIK group at three days and one week after surgery, but there was little Ki-67 expression in the stroma with either surgery at one month. In that study, CD11b-positive cells (monocytes) were significantly fewer in the SMILE group at one day, three days and one week postoperatively. Sun et al. (2019) found the alpha smooth muscle actin (SMA)+ myofibroblasts only at the side cut incision and not in the lamellar interface at 28 days after SMILE in rabbits.
Future studies should examine stromal remodeling at the interface where the two different femtosecond laser cuts are opposed in SMILE surgery to assess whether this correlates with delayed visual recovery sometimes noted in SMILE compared to LASIK (Ivarsen et al., 2014). One study attempted to approach this by analysis of dilated retro-illumination photographs (Ganesh et al., 2018). But further study of the cell biology of stromal remodeling at the interface in SMILE would be informative.
4. Incisional corneal surgeries
Incisional corneal surgeries include radial keratotomy (RK), astigmatic keratotomy (AK), and the incision associated with clear corneal cataract surgery. Other incisional procedures that have been abandoned due to complications such as irregular astigmatism include hexagonal keratotomy and trapezoidal keratotomy.
Early histopathological analyses of RK in owl monkeys that had 8-incision or 16-incision radial keratotomies by Jester and coworkers (1981) found that the loss of initial flattening of the anterior corneal surface was due to corneal wound contracture. Also, they reported high variability in incision depth and 14 to 15% loss of endothelial cells. Subsequent monkey studies showed that the endothelial cell loss that could be associated with RK was not progressive, but an acute response to the incisions or inflammation damaging the endothelial cells (Dunn et al., 1984). MacRae and Rich (1998) reported that endothelial cell loss after RK was not progressive in humans. Within a few years, investigators discovered that epithelium often extended into RK incisions (Fig. 10) and could generate epithelial cysts in the incisions and that these histopathologic changes could result in variability of the refractive result of the surgery (Jester et al., 1983). A subsequent study of human eyes that had RK confirmed epithelial plugs extended an average of 25% corneal thickness in eyes that had the surgery 17 months prior (Ingraham et al., 1985). Importantly, the incisions beneath the epithelial plugs had fibrous scarring. In another study of human eyes that had RK (Deg et al., 1985), documented gaped keratotomy incisions and epithelial plugs, along with Bowman's layer mal-apposition with “slight to moderate” fibroblastic activity at the incision sites. The variable wound healing and epithelial plugs of the RK eyes was likely the cause of diurnal fluctuations in refraction and uncorrected vision persisting years after surgery (Santos et al., 1988). In another study of human eyes after homoplastic lamellar keratoplasty or penetrating keratoplasty confirmed the variability in wound healing of the incisions one to two years after RK surgery (Jester et al., 1992). A histological evaluation of RK in cats (Garana et al., 1992), found that modulation of RK incision-wound gape during healing involved the transformation of corneal keratocytes to a myofibroblast-like cells, and the subsequent formation of intracellular stress fibers composed of f-actin, non-muscle myosin, and alpha-actinin. Melles and coworkers (1995), analyzed ten human eyes that had RK for varying amounts of time prior to death. They found that beneath the typical epithelial plugs, the area of epithelial-stromal interaction commonly had three adjacent morphological zones: 1) a duplicated basement membrane complex, 2) a zone that resembled Bowman's layer, and 3) a zone with collagenous fiber orientation parallel to the plugs. They also noted that scar tissue orientation was transverse at the base of the plug, and sagittal in deeper wound regions. The finding of a zone that resembled Bowman’s layer around the epithelial plugs (Fig. 10) is important in understanding that the generation and maintenance of Bowman’s layer in humans, and other species that have one, are related to ongoing epithelial-stromal interactions modulated by growth factors released from the epithelium and stromal cells (Wilson and Hong, 2000).
Fig. 10.
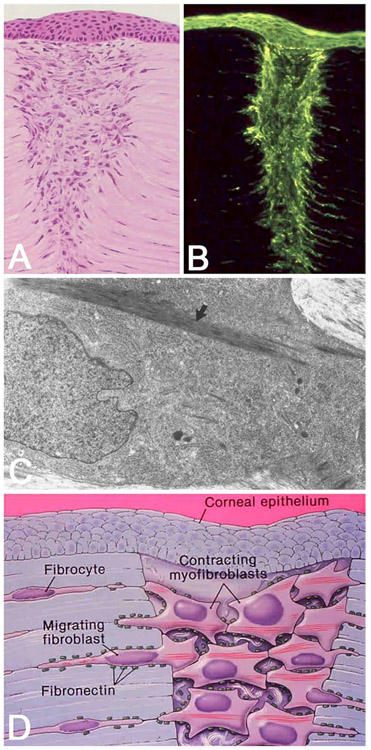
The role of myofibroblasts in corneal wound healing following radial keratotomy (RK), (A, owl monkey and B, cat) and penetrating corneal injury (C) in the rabbit. In histologic sections of the cornea, 14 days after RK, the gaping wound becomes filled with proliferating fibroblastic-like cells that have migrated in from the adjacent corneal stroma (A). These cells when probed for the presence of filamentous actin using the molecular probe, Phalloidin, show intense staining indicating the presence of extensive actin filament arrays within cells immediately adjacent to and within the wound (B). Transmission electron microscopy of these fibroblastic cells shows the presence of microfilaments, actin bundles with electron dense plaques that contain myosin (arrow) indicating the presence of ‘stress fibers’—the electron microscopic marker for myofibroblasts (C). A model for the role of myofibroblasts in corneal wound healing is presented in D, where actinomyosin filament contraction exerts force on newly synthesized collagen and the adjacent wound margin through fibronectin interactions to contract and close the gaping wound. Graciously provided by James V. Jester, PhD, U.C. Irvine.
Petroll and coworkers (1998) did an analysis of f-actin organization in cat RK wounds from 10 to 28 days after surgery using en block staining with fluorescein isothiocyanate (FITC) phalloidin. In these studies, three-dimensional datasets were also collected using laser confocal microscopy at different regions within the wounds. In addition, conventional en face sections were double-labeled using combinations of phalloidin and antibodies to fibronectin and alpha-5 beta-1 integrin. These investigators found that myofibroblast ingrowth started in the posterior portion of the RK wound and progressed anteriorly in the cornea. F-actin was predominantly distributed in long, thick bundles (stress fibers) within the wound at 10 to 14 days and these fibers appeared initially to be randomly oriented anteriorly, but became progressively more aligned with the long axis of the wound posteriorly. By 21 days, the stress fibers were predominantly oriented parallel to the long axis of the wound throughout the RK incision. F-actin, fibronectin and integrin were all coaligned at 14 days and 21 days (Petroll et al., 1998). They concluded that since the majority of RK wound closure occurs between 14 and 28 days after surgery, the parallel alignment of the actin filament-fibronectin-integrin assembly in the RK model leads to wound contraction.
None of these studies took into account bone marrow-derived cells entering into the cornea after the epithelial injuries included in RK, or the potential contributions of fibrocytes to the fibrosis response adjacent to the incisions in RK (Lassance et al., 2018).
AK incisions and clear cornea incisions for phacoemulsification have been less studied with histopathological methodologies or in tissue wound healing studies, but the principles are likely to be similar to RK incisions. A surprising result was seen in rabbits that had non-perforating incisions across the central cornea (Marino et al., 2017). In this species, where myofibroblasts readily develop in the anterior stroma after high correction PRK or at the flap edge in LASIK (Marino et al., 2017), few SMA+ myofibroblasts were noted at one, two or three months after deep linear incisions across the central cornea, including surrounding epithelial plugs (Fig. 11), despite the presence of dense opacity delineating the incisions. There was, however, large amounts of disorganized collagen type 3 not normally found in these corneas localized to the stroma beneath the epithelial plugs (Fig. 11). Since these incisions were similar to RK incisions, except they extended across the central cornea, these experiments suggest there can be interspecies differences in the healing of partial thickness corneal incisions.
Fig. 11.
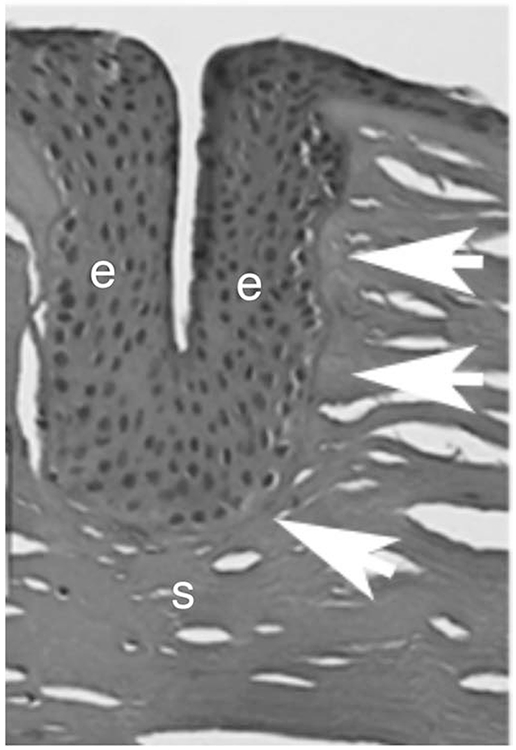
Histopathological evaluation of a human cornea several years after RK. An epithelial plug extends deep into the corneal stroma. Surrounding the epithelial plug there is an intact EBM and acellular areas that appear to represent a Bowman’s-like layer (arrows). e is epithelium. s is stroma. Mag. 25X
5. Laser thermal keratoplasty (LTK) or thermal conductive keratoplasty (CK)
LTK and CK are procedures in which laser (Ayala Espinosa et al., 2000; McDonald, 2005) or radio-frequency thermal (Eggink et al., 1999) energy is applied to the peripheral cornea to cause steepening of the central cornea to correct hyperopia or presbyopia. Eight to 32 circular burns, depending on the intended correction, are placed in the peripheral cornea with either procedure (Ayala Espinosa et al., 2000; McDonald, 2005; Eggink et al., 1999). Both procedures produce burns through the epithelium and into the stroma that denatures collagen and causes necrosis of epithelial cells and keratocytes at the site of the injury (Ayala Espinosa et al., 2000; Eggink et al., 1999). Little detailed histological and cell biological work has been done on the effects of these procedures on the cornea. However, inspection of histological specimens immediately after the LTK procedure in rabbits (Ayala Espinosa et al., 2000) shows the denaturation of the stromal collagen and necrosis of the epithelium and keratocytes at the site of the burns. This results in inflammation at those sites related to bone marrow-derived cells that invade from the limbal blood vessels. The lesions from the laser or radio-frequency burns are initially opaque due to the injury to the stromal collagen and it is likely corneal fibroblasts, and possibly myofibroblasts, develop at that each of the burns. The central corneal steepening produced by all of these procedures regresses over time and, thus, they were approved by the FDA for the temporary correction of hyperopia and presbyopia (Ayala Espinosa et al., 2000; Eggink et al., 1999). It has not been studied in detail, but it is likely that over time keratocytes progressively remove damaged collagen and replace it with new collagen lamellae in the burned area of the stroma and that is associated with the decrease in the opacity and loss of the correction produced by these surgeries over time (Ayala Espinosa et al., 2000; Eggink et al., 1999). For the most part, this instability and regression has led to a marked decrease in utilization of these procedures.
6. Corneal inlays
The use of corneal inlays to correct hyperopia, myopia, presbyopia and astigmatism is attractive because of the tissue additive rather than tissue subtractive nature of the procedures and the potential reversibility of the surgery. However, the issue with these devices over the past 35 years has always been inflammatory cell, keratocyte, and epithelial reactions to the foreign body within the stroma and deprivation of the overlying stroma and epithelium to essential nutritional molecules provided by the aqueous humor that passes through the leaky barrier function of the endothelium-Descemet’s membrane complex and into the stroma. Thus, these inlays precipitate an inflammatory reaction in the stroma (Santhiago et al., 2012), that is often transient, stromal opacity surrounding the inlay (Alió et al., 2004; Mulet et al., 2009; Tan et al., 2013; Jalali et al., 2016; Ong et al., 2018; Fenner et al., 2019), that is attributable to corneal fibroblast generation and deposition of collagenous matrix, release of opaque materials from surrounding stressed keratocytes (Alió et al., 2004; Mulet et al., 2009; Tan et al., 2013; Salamatrad et al., 2015) and sometimes thinning of the overlying epithelium (Kim et al., 2013; Xie et al., 2006). In many cases, these reactions are mild and do not necessitate explantation of the inlay. If the inlay is explanted, most of these corneas appear to return to near normal (Alió et al., 2004; Ong et al., 2018). However, in one study in which ten Kamra corneal inlays were removed, some level of residual stromal haze persisted in the majority of explanted corneas and some patients had a decrease in corrected distance visual acuity (Moshirfar et al., 2019).
The most severe reactions noted have been necrosis of the overlying stroma and/or epithelium, which can occur a decade or more after the original implantation (Taneri et al., 2018; Kirkness et al., 1985), resulting in severe damage to the cornea requiring lamellar keratoplasty or penetrating keratoplasty. Fenestrations in the inlay material or use of more permeable hydrogels or other materials for inlays—that allow passage of nutritional molecules—have been used to try to alleviate these problems.
One study evaluated the early stromal response to the Karma Inlay (Acufocus, Irvine, CA) in rabbits (Santhiago et al., 2012). The inlay group had significantly more stromal cell death than the control group with a pocket but no inlay, at 48 hours after surgery. At 24 hours and 6 weeks after surgery there was no significant difference in stromal cell death between the inlay and control groups. Also, significantly more CD11b+ monocytes (as a marker of inflammation) were noted in the stroma in the inlay group compared to the control group at 24 and 48 hours after surgery. However, no significant difference in CD11b+ monocytes was observed between the control and inlay groups at 6 weeks after surgery. No haze or stromal deposits were noted in the inlay group in this study.
The stromal and epithelial responses appear to be less of an issue with currently available FDA-approved inlays (Jalali et al., 2016; Beer et al., 2019), but longer follow-up is needed since severe changes can occur many years following the implantation of these devices (Taneri et al., 2018; Kirkness et al., 1985).
7. Intrastromal corneal ring segments
Intrastromal corneal ring segments are inlays placed in the midperipheral corneal stroma used to correct low to moderate myopia or that are used in keratoconus to produce a more regular corneal topography to improve spectacle-corrected vision or improve contact lens fitting to postpone the need for corneal transplantation (Ruckhofer et al., 2002; Rabinowitz, 2013). Similar to inlays, these Intacs impact stromal keratocytes and the overlying epithelium, but usually less severely because of the relative smaller block of nutrition to the keratocyte and overlying epithelium. Thus, Intacs often trigger mild inflammation (Levy and Lifshitz, 2010), fibrosis in their channels (Ruckhofer et al., 2002), and lipid deposition in the stroma surrounding the segments(Ruckhofer et al., 2002; Ly et al., 2006). In a histologic study of Intacs removed because of lack of clinical benefit or extrusion, thinning of the epithelium overlying the Intacs was noted and no SMA+ cells were found in fibrosis surrounding the Intacs—indicating myofibroblasts were not involved in producing the channel fibrosis noted in some cases (Samimi et al., 2007).
8. Conclusions
The corneal wound healing response is a major determinant of the outcomes and complications of all refractive surgical procedures. Future research to unravel the factors controlling stromal and epithelial molecular and cellular responses to individual procedures will likely improve outcomes to surgery and help to limit specific complications such as scarring fibrosis after PRK or stromal ischemia after lamellar inlays.
Fig. 12.
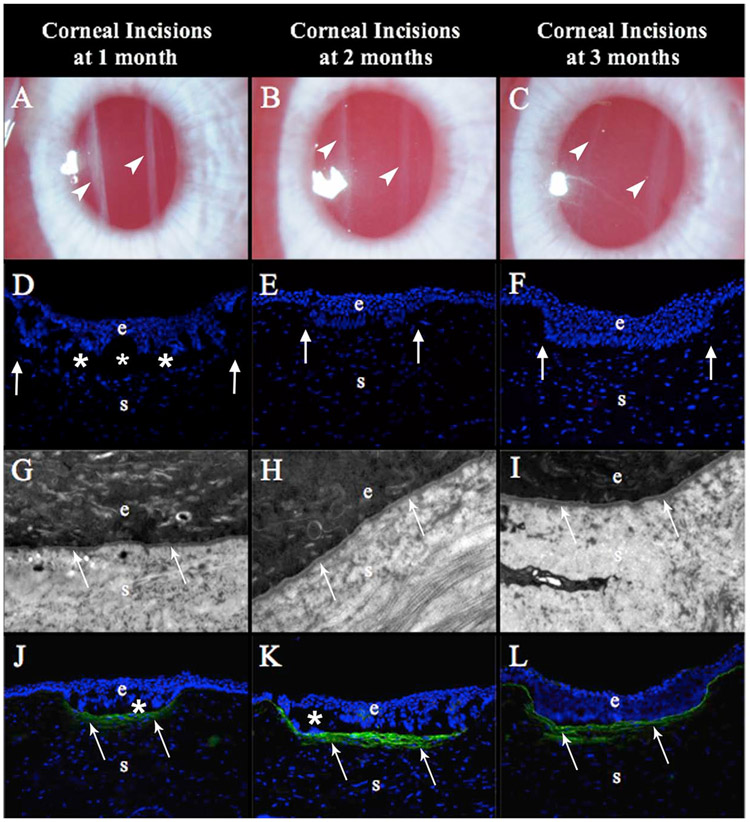
Rabbit corneas at one month, two months and three months after deep non-perforating linear incisions across the central cornea (Marino et al., 2017). At one month (A) the incisions are seen as dense linear opacities, but became progressively fainter at two months (B) and three months (C) after surgery. Surprisingly, no SMA+ myofibroblasts were detected anywhere in these corneas at one month (D), two months (E) or three months (F) after surgery. A single EBM (arrows) surrounded the epithelial plugs in all eyes at one month (G), two months (H) and three months (I) 23,000 mag. Disorganized collagen type 3, that is not normally detected in the corneal stroma, was present beneath the epithelial plugs of all corneal incisions at one month (J), and intensified at 2 months (K) and three months (L) after surgery, adjacent to high densities of cells that were likely corneal fibroblasts since they were SMA- cells. 25X mag. Blue is DAPI staining of all cell nuclei. e is epithelium; s is stroma, and * indicates artifactual separation of the epithelium and stroma that occurs during sectioning of some specimens. Reprinted with permission from Marino GK et al. J. Ref Surg. 2017;33:337-346.
Wound healing is a major determinate of outcomes for corneal refractive surgery
The corneal wound healing response varies with the type of corneal refractive surgery
The risk of scarring fibrosis haze increases with higher levels of correction after PRK
LASIK is rarely associated with haze because the central epithelial basement membrane is preserved
Acknowledgments
Funding
Supported in part by US Public Health Service grants RO1EY10056 (SEW) and P30-EY025585 from the National Eye Institute, National Institutes of Health, Bethesda, MD, Department of Defense grant VR180066, and Research to Prevent Blindness, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Proprietary interest statement
The authors don’t have any commercial or proprietary interests in this study.
References
- Alió JL, Mulet ME, Zapata LF, Vidal MT, De Rojas V, Javaloy J, 2004. Intracorneal inlay complicated by intrastromal epithelial opacification. Arch. Ophthalmol 122, 1441–6. [DOI] [PubMed] [Google Scholar]
- Antonios R, Abdul Fattah M, Arba Mosquera S, Abiad BH, Sleiman K, Awwad ST, 2017. Single-step transepithelial versus alcohol-assisted photorefractive keratectomy in the treatment of high myopia: a comparative evaluation over 12 months. Br. J. Ophthalmol 101, 1106–1112. [DOI] [PubMed] [Google Scholar]
- Ayala Espinosa MJ, Alió Y Sanz J, Ismail MM, Sánchez Castro P, 2000. [ Experimental corneal histological study after thermokeratoplasty with holmium laser]. Arch. Soc. Esp. Oftalmol 75, 619–25. [PubMed] [Google Scholar]
- Beer SMC, Werner L, Nakano EM, Santos RT, Hirai F, Nitschke EJ, Francesconi Benicio C, Campos MSQ, A 3-year follow-up study of a new corneal inlay: clinical results and outcomes B.r J. Ophthalmol Published Online First: 24 August 2019. doi: 10.1136/bjophthalmol-2019-314314 [DOI] [PubMed] [Google Scholar]
- Carones F, Fiore T, Brancato R, 1999. Mechanical vs. alcohol epithelial removal during photorefractive keratectomy. J. Ref. Surg 15, 556–562. [DOI] [PubMed] [Google Scholar]
- de Medeiros FW, Kaur H, Agrawal V, Chaurasia SC, Hammel J, Dupps WJ Jr., Wilson SE, 2009. Effect of femtosecond laser energy level on corneal stromal cell death and inflammation. J Refract. Surg 25, 869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deg JK, Zavala EY, Binder PS, 1985. Delayed corneal wound healing following radial keratotomy. Ophthalmology 92, 734–40. [DOI] [PubMed] [Google Scholar]
- Dong Z, Zhou X, Wu J, Zhang Z, Li T, Zhou Z, Zhang S, Li G, 2014. Small incision lenticule extraction (SMILE) and femtosecond laser LASIK: comparison of corneal wound healing and inflammation. Br. J. Ophthalmol 98, 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S, Jester JV, Arthur J, Smith RE, 1984. Endothelial cell loss following radial keratotomy in a primate model. Arch. Ophthalmol 102, 1666–70. [DOI] [PubMed] [Google Scholar]
- Eggink CA, Bardak Y, Cuypers MH, Deutman AF, 1999. Treatment of hyperopia with contact Ho: YAG laser thermal keratoplasty. J. Refract. Surg 15, 16–22. [DOI] [PubMed] [Google Scholar]
- Fenner BJ, Liu YC, Koh SK, Gao Y, Deng L, Beuerman RW, Zhou L, Theng JTS, Mehta JS, 2019. Mediators of corneal haze following implantation of presbyopic cornea inlays. Invest. Ophthalmol. Vis. Sci 60, 868–876. [DOI] [PubMed] [Google Scholar]
- Gabler B, Winkler von Mohrenfels C, Dreiss AK, Marshall J, Lohmann CP, 2002. Vitality of epithelial cells after alcohol exposure during laser-assisted subepithelial keratectomy flap preparation. J. Cat. Refract. Surg 28, 1841–6. [DOI] [PubMed] [Google Scholar]
- Ganesh S, Brar S, Pandey R, Pawar A, 2018. Interface healing and its correlation with visual recovery and quality of vision following small incision lenticule extraction. Indian J. Ophthalmol 66, 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garana RM, Petroll WM, Chen WT, Herman IM, Barry P, Andrews P, Cavanagh HD, Jester JV, 1992. Radial keratotomy. II. Role of the myofibroblast in corneal wound contraction. Invest. Ophthalmol. Vis. Sci 33, 3271–82. [PubMed] [Google Scholar]
- Ghoreishi M, Attarzadeh H, Tavakol i M., Moini HA, Zandi A, Masjedi A, Rismanchian A, 2010. Alcohol-assisted versus mechanical epithelium removal in photorefractive keratectomy. J. Ophthalmic Vis. Res; 5, 223–227. [PMC free article] [PubMed] [Google Scholar]
- Hassell JR, Schrecengost PK, Rada JA, SundarRaj N, Sossi G, Thoft RA, 1992. Biosynthesis of stromal matrix proteoglycans and basement membrane components by human corneal fibroblasts. Invest. Ophthalmol. Vis. Sci 33, 547–57. [PubMed] [Google Scholar]
- Helena MC, Filatov VV, Johnston WT, Vidaurri-Leal J, Wilson SE, Talamo JH, 1997. Effects of 50% ethanol and mechanical epithelial debridement on corneal structure before and after excimer photorefractive keratectomy. Cornea 16, 571–9. [PubMed] [Google Scholar]
- Hersh PS, Shah SI, Geiger D, Holladay JT, 1996. Corneal optical irregularity after excimer laser photorefractive keratectomy. The Summit photorefractive keratectomy topography study group. J. Cat. Refract. Surg 22, 197–204. [DOI] [PubMed] [Google Scholar]
- Hersh PS, Stulting RD, Steinert RF, Waring GO 3rd., Thompson KP, O'Connell M, Doney K, Schein OD, 1997. Results of phase III excimer laser photorefractive keratectomy for myopia. The Summit PRK Study Group. Ophthalmology 104, 1535–53. [DOI] [PubMed] [Google Scholar]
- Hindman HB, DeMagistris M, Callan C, McDaniel T, Bubel T, Huxlin KR, 2019. Impact of topical anti-fibrotics on corneal nerve regeneration in vivo. Exp. Eye Res 181, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham HJ, Guber D, Green WR, 1985. Radial keratotomy. Clinicopathologic case report. Arch. Ophthalmol 103, 683–8. [DOI] [PubMed] [Google Scholar]
- Ivarsen A, Asp S, Hjortdal J, 2014. Safety and complications of more than 1500 small-incision lenticule extraction procedures. Ophthalmology 121, 822–8. [DOI] [PubMed] [Google Scholar]
- Jalali S, Aus der Au W, Shaarawy T, 2016. AcuFocus Corneal inlay to correct presbyopia using femto-LASIK. One year results of a prospective cohort study. Klin. Monbl. Augenheilkd 233, 360–4. [DOI] [PubMed] [Google Scholar]
- Jeon KI, Hindman HB, Bubel T, McDaniel T, DeMagistris M, Callan C, Huxlin KR, 2018. Corneal myofibroblasts inhibit regenerating nerves during wound healing. Sci. Rep 8, 12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavanagh HD, Petroll WM, Piatigorsky J, 1999. The cellular basis of corneal transparency: evidence for ‘corneal crystallins'. J. Cell. Sci 112, 613–22. [DOI] [PubMed] [Google Scholar]
- Jester JV, Steel D, Salz J, Miyashiro J, Rife L, Schanzlin DJ, Smith RE, 1981. Radial keratotomy in non-human primate eyes. Am. J. Ophthalmol 92, 153–71. [DOI] [PubMed] [Google Scholar]
- Jester JV, Villaseñor RA, Miyashiro J, 1983. Epithelial inclusion cysts following radial keratotomy. Arch. Ophthalmol 101, 611–5. [DOI] [PubMed] [Google Scholar]
- Jester JV, Villaseñor RA, Schanzlin DJ, Cavanagh HD, 1992. Variations in corneal wound healing after radial keratotomy: possible insights into mechanisms of clinical complications and refractive effects. Cornea. 11, 191–9. [PubMed] [Google Scholar]
- Kaiserman I, Sadi N, Mimouni M, Sela T, Munzer G, Levartovsky S, 2017. Corneal breakthrough haze after photorefractive keratectomy with mitomycin C: Incidence and risk factors. Cornea 36, 961–966. [DOI] [PubMed] [Google Scholar]
- Kim E, Ehrmann K, Choo J, Franz S, Moilanen J, 2013. The effect of inlay implantation on corneal thickness and radius of curvature in rabbit eyes. Cornea 32, e106–12. [DOI] [PubMed] [Google Scholar]
- Kim W-J, Shah S, Wilson SE, 1998. Differences in keratocyte apoptosis following transepithelial and laser-scrape photorefractive keratectomy. J. Refract. Surg 14, 526–33. [DOI] [PubMed] [Google Scholar]
- Kirkness CM, Steele AD, Garner A, 1985. Polysulfone corneal inlays. Adverse reactions: a preliminary report. Trans. Ophthalmol. Soc. U.K 104, 343–50. [PubMed] [Google Scholar]
- Lassance L, Marino GK, Medeiros CS, Thangavadivel S, Wilson SE, 2018. Fibrocyte migration, differentiation and apoptosis during the corneal wound healing response to injury. Exp. Eye Res 170, 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Lee KS, Kim JK, Kim HC, Seo KR, Kim EK, 2005, Epithelial healing and clinical outcomes in excimer laser photorefractive surgery following three epithelial removal techniques: mechanical, alcohol, and excimer laser. Am. J. Ophthalmol 139, 56–63. [DOI] [PubMed] [Google Scholar]
- Levy J, Lifshitz T, 2010. Keratitis after implantation of intrastromal corneal ring segments (Intacs) aided by femtosecond laser for keratoconus correction: case report and description of the literature. Eur. J. Ophthalmol 20, 780–4. [DOI] [PubMed] [Google Scholar]
- Lin N, Yee SB, Mitra S, Chuang AZ, Yee RW, 2004. Prediction of corneal haze using an ablation depth/corneal thickness ratio after laser epithelial keratomileusis. J. Refract. Surg. 20, 797–802. [DOI] [PubMed] [Google Scholar]
- Luft N, Schumann RG, Dirisamer M, Kook D, Siedlecki J, Wertheimer C, Priglinger SG, Mayer WJ, 2018. Wound healing, inflammation, and corneal ultrastructure after SMILE and femtosecond laser-assisted LASIK: A human ex vivo study. J. Refract. Surg 34, 393–399. [DOI] [PubMed] [Google Scholar]
- Luger MH, Ewering T, Arba-Mosquera S, 2012. Consecutive myopia correction with transepithelial versus alcohol-assisted photorefractive keratectomy in contralateral eyes: one-year results. J. Cat. Refract. Surg 38, 1414–1423. [DOI] [PubMed] [Google Scholar]
- Ly LT, McCulley JP, Verity SM, Cavanagh HD, Bowman RW, Petroll WM, 2006. Evaluation of intrastromal lipid deposits after Intacs implantation using in vivo confocal microscopy. Eye Contact Lens 32, 211–5. [DOI] [PubMed] [Google Scholar]
- MacRae SM, Rich LF, 1998. Long-term effects of radial keratotomy on the corneal endothelium. J Refract Surg. 14, 49–52. [DOI] [PubMed] [Google Scholar]
- Marino GK, Santhiago MR, Santhanam A, Lassance L, Thangavadivel S, Medeiros CS, Torricelli AAM, Wilson SE, 2017. Regeneration of defective epithelial basement membrane and restoration of corneal transparency. J. Ref. Surg 33, 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros CS, Marino G, Lassance L, Thangavadivel S, Santhiago MR, Wilson SE, 2018a. The impact of photorefractive keratectomy (PRK) and mitomycin C (MMC) on corneal nerves and their regeneration. J. Ref. Surg 34, 790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros CS, Marino GK, Santhiago MR, Wilson SE, 2018b. The corneal basement membranes and stromal fibrosis. Invest. Ophthalmol. Vis. Sci 59, 4044–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melles GR, Binder PS, Moore MN, Anderson JA, 1995. Epithelial-stromal interactions in human keratotomy wound healing. Arch. Ophthalmol 113, 1124–30. [DOI] [PubMed] [Google Scholar]
- Moshirfar M, Skanchy DF, Rosen DB, Heiland MB, Liu HY, Buckner B, Gomez AT, Ronquillo YC, Melton T, Hoopes PCJ, 2019. Me.d Hypothesis Discov. Innov. Ophthalmol 8, 129–133. [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AEK, Choi R, Hong J-W, Lee J-S, Mohan RR, Ambrósio R, Zieske JD, Wilson SE, 2003. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp. Eye Res 76, 71–87. [DOI] [PubMed] [Google Scholar]
- Marino GK, Santhiago MR, Santhanam A, Lassance L, Thangavadivel S, Medeiros CS, Torricelli AAM, Wilson SE, 2017. Regeneration of defective epithelial basement membrane and restoration of corneal transparency after photorefractive keratectomy. J. Ref. Surg 33, 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald MB, 2005. Conductive keratoplasty: a radiofrequency-based technique for the correction of hyperopia. Trans. Am. Ophthalmol. Soc 103, 512–36. [PMC free article] [PubMed] [Google Scholar]
- Mulet ME, Alio JL, Knorz MC, 2009. Hydrogel intracorneal inlays for the correction of hyperopia: outcomes and complications after 5 years of follow-up. Ophthalmology 116, 1455–60. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kurosaka D, Bissen-Miyajima H, Tsubota K, 2001. Intact corneal epithelium is essential for the prevention of stromal haze after laser assisted in situ keratomileusis. Br. J. Ophthalmol 85, 209–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Medeiros FW, Dupps WJ Jr., Sinha S, Krueger RR, Stapleton WM, Rayborn M, Suto C, Wilson SE, 2007. Femtosecond laser and microkeratome corneal flaps: Comparison of stromal wound healing and inflammation. J. Refract. Surg 23, 667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE, 2006a. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp. Eye Res 82, 788–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Gupta PC, Wilson SE, 2006b. Effect of prophylactic and therapeutic mitomycin C on corneal apoptosis, proliferation, haze, and keratocyte density. J. Ref. Surg 22, 562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HS, Chan AS, Yau CW, Mehta JS, 2018. Corneal inlays for presbyopia explanted due to corneal haze. J. Refract. Surg 34, 357–360. [DOI] [PubMed] [Google Scholar]
- Petroll WM, Cavanagh HD, Jester JV, 1998. Assessment of stress fiber orientation during healing of radial keratotomy wounds using confocal microscopy. Scanning 20, 74–82. [DOI] [PubMed] [Google Scholar]
- Rabinowitz YS, 2013. INTACS for keratoconus and ectasia after LASIK. Int. Ophthalmol. Clin 53, 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riau AK, Liu YC, Lwin NC, Ang HP, Tan NY, Yam GH, Tan DT, Mehta JS, 2014. Comparative study of nJ- and μJ-energy level femtosecond lasers: evaluation of flap adhesion strength, stromal bed quality, and tissue responses. Invest. Ophthalmol. Vis. Sci 55, 3186–94. [DOI] [PubMed] [Google Scholar]
- Ruckhofer J, 2002. [Clinical and histological studies on the intrastromal corneal ring segments (ICRS(R), Intacs(R))]. Klin. Monbl. Augenheilkd 219, 557–74. [DOI] [PubMed] [Google Scholar]
- Saikia P, Thangavadivel S, Lassance L, Medeiros CS, Wilson SE, 2018. IL-1 and TGFβ modulation of epithelial basement membrane components perlecan and nidogen production by corneal stromal cells. Invest. Ophth. Vis. Sci 59, 5589–5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamatrad A, Jabbarvand M, Hashemian H, Khodaparast M, Askarizadeh F, 2015. Histological and confocal changes in rabbit cornea produced by an intrastromal inlay made of hexafocon A. Cornea 34, 78–81. [DOI] [PubMed] [Google Scholar]
- Samimi S, Leger F, Touboul D, Colin J, 2007. Histopathological findings after intracorneal ring segment implantation in keratoconic human corneas. J. Cat. Refract. Surg 33, 247–53. [DOI] [PubMed] [Google Scholar]
- Santhanam A, Marino GK, Torricelli AAM, Wilson SE Epithelial basement membrane (EBM) regeneration and changes in EBM component mRNA expression in the anterior stroma after corneal injury. Mol. Vision 23, 39–51. [PMC free article] [PubMed] [Google Scholar]
- Santhanam A, Torricelli AAM, Wu J, Marino GK, Wilson SE, 2015. Differential expression of epithelial basement membrane components nidogens-1 and 2 and perlecan in corneal stromal cells in vitro. Mol. Vision 21, 1318–27. [PMC free article] [PubMed] [Google Scholar]
- Santhiago MR, Barbosa FL, Agrawal V, Binder PS, Christie B, Wilson SE, 2012. Short-term cell death and inflammation after intracorneal inlay implantation in rabbits. J. Refract. Surg 28, 144–9. [DOI] [PubMed] [Google Scholar]
- Santos VR, Waring GO 3rd., Lynn MJ, Schanzlin DJ, Cantillo N, Espinal ME, Garbus J, Justin N, Roszka-Duggan V, 1988. Morning-to-evening change in refraction, corneal curvature, and visual acuity 2 to 4 years after radial keratotomy in the PERK Study. Ophthalmology 95, 1487–93. [DOI] [PubMed] [Google Scholar]
- Sia RK, Ryan DS, Edwards JD, Stutzman RD, Bower KS, 2014. The U.S. Army surface ablation study: comparison of PRK, MMC-PRK, and LASEK in moderate to high myopia. J. Refract. Surg. 30, 256–64. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Maloney RK, 1998. Diffuse lamellar keratitis. A new syndrome in lamellar refractive surgery. Ophthalmology. 105, 1721–6. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang T, Liu M, Zhou Y, Weng S, Yang X, Liu Q, 2019. Early Corneal Wound Healing Response After Small Incision Lenticule Extraction Cornea. 38, 1582–1588. [DOI] [PubMed] [Google Scholar]
- Tan XW, Hartman L, Tan KP, Poh R, Myung D, Zheng LL, Waters D, Noolandi J, Beuerman RW, Frank CW, Ta CN, Tan DT, Mehta JS, 2013. In vivo biocompatibility of two PEG/PAA interpenetrating polymer networks as corneal inlays following deep stromal pocket implantation. J. Mater. Sci .Mater .Med 24, 967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneri S, Kiessler S, Rost A, Schultz T, Dick HB, 2018. Flap melting over corneal inlay for hyperopic correction. J. Refract. Surg 34, 775–778. [DOI] [PubMed] [Google Scholar]
- Tanioka H, Hieda O, Kawasaki S, Nakai Y, Kinoshita S, 2007. Assessment of epithelial integrity and cell viability in epithelial flaps prepared with the epi-LASIK procedure. J. Cat. Refract. Surg 33, 1195–200. [DOI] [PubMed] [Google Scholar]
- Thornton I, Xu M, Krueger RR, 2008. Comparison of standard (0.02%) and low dose (0.002%) mitomycin C in the prevention of corneal haze following surface ablation for myopia. J. Refract. Surg 24, S68–76. [DOI] [PubMed] [Google Scholar]
- Torricelli AAM, Marino GK, Santhanam A, Wu J, Singh A, Wilson SE, 2015. Epithelial basement membrane proteins perlecan and nidogen-2 are up-regulated in stromal cells after epithelial injury in human corneas. Exp. Eye Res 134, 33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AAM, Singh V, Agrawal V, Santhiago MR, Wilson SE, 2013a. Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Invest. Ophth. Vis. Sci 54, 4026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AAM, Singh V, Santhiago MR, Wilson SE, 2013b. The corneal epithelial basement membrane: Structure, function and disease. Invest. Ophth. Vis. Sci 54, 6390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard AH, Hjortdal JØ, Ivarsen A, Work K, Grauslund J, Sjølie AK, 2013. Long-term outcomes of photorefractive keratectomy for low to high myopia: 13 to 19 years of follow-up. J. Refract. Surg 29, 312–9. [DOI] [PubMed] [Google Scholar]
- Virasch VV, Majmudar PA, Epstein RJ, Vaidya NS, Dennis RF, 2010. Reduced application time for prophylactic mitomycin C in photorefractive keratectomy. Ophthalmology 117, 885–9. [DOI] [PubMed] [Google Scholar]
- Wilson SE, 2002. Analysis of the keratocyte apoptosis, keratocyte proliferation, and myofibroblast transformation response after photorefractive keratectomy and laser insitu keratomilieusis. Trans. Amer. Ophthalmol. Soc 100, 411–33. [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, 2012. Corneal myofibroblast biology and pathobiology: Generation, persistence, and transparency. Exp. Eye Res 99, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Ambrósio R Jr., 2002. Sporadic diffuse lamellar keratitis (DLK) following LASIK. Cornea 21, 560–3. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Hong JW, 2000. Bowman's layer structure and function: critical or dispensable to corneal function? A hypothesis. Cornea 19, 417–20 [DOI] [PubMed] [Google Scholar]
- Wilson SE, Marino G, Medeiros C, Santhiago MR, 2017. Phototherapeutic keratectomy (PTK): science and art. J. Ref. Surg 33, 203–210. [DOI] [PubMed] [Google Scholar]
- Xie RZ, Evans MD, Bojarski B, Hughes TC, Chan GY, Nguyen X, Wilkie JS, McLean KM, Vannas A, Sweeney DF, 2006. Two-year preclinical testing of perfluoropolyether polymer as a corneal inlay. Invest. Ophthalmol. Vis. Sci 47, 574–81. [DOI] [PubMed] [Google Scholar]
- Zarei-Ghanavati S, Shandiz JH, Abrishami M, Karimpou r M., 2019. Comparison of mechanical debridement and trans-epithelial myopic photorefractive keratectomy: A contralateral eye study. J. Curr. Ophthalmol 31, 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]