Abstract
The SERPINF1 gene encodes pigment epithelium-derived factor (PEDF), a member of the serpin superfamily with neurotrophic and antiangiogenic properties in the retina. We hypothesized that absence of PEDF would lead to increased stress-associated retinal degeneration in Serpinf1 null mice. Accordingly, using a Serpinf1 null mouse model, we investigated the impact of PEDF absence on retinal morphology, and susceptibility to induced and inherited retinal degeneration. We studied the pattern of Serpinf1 expression in the mouse retina layers. PEDF protein was detected by western blotting. Transmission electron microscopy was performed on mouse retina. Serpinf1 null mice and wild type littermates were injected with NaIO3 (30 mg/kg body weight) intraperitonially. At post-injection day 1, 3, 4, 6 and 8 mice were euthanized, and eyes were enucleated. Serpinf1 null and rd10 double mutant mice were generated and their eyes enucleated at different time points from post-natal day 15 to post-natal day 28. Enucleated eyes were processed for hematoxylin and eosin staining and histopathological evaluations. We found that Serpinf1 was expressed in the retinal pigment epithelium, in the inner nuclear layer and in the ganglion cell layer, but undetectable in the outer nuclear layer of wild type mice. Plasma PEDF protein levels were undetectable in Serpinf1 null animals. RPE atrophy and retinal thinning were observed in NaIO3-treated wild type mice that progressed with time post-injection. NaIO3-treated Serpinf1 null mice showed comparatively better retinal morphology than wild type mice at day 4 post-injection. However, the absence of PEDF in Serpinf1 null x rd10 mice increased the susceptibility to retinal degeneration relative to that of rd10 mice. We concluded that histopathological evaluation of retinas lacking PEDF showed that removal of the Serpinf1 gene may activate PEDF-independent compensatory mechanisms to protect the retina against oxidative stress, while it increases the susceptibility to degenerate the retina in inherited retinal degeneration models.
Keywords: PEDF, Serpinf1, pigment epithelium-derived factor, retinal degeneration, rd10, NaIO3
1. Introduction
The importance of pigment epithelium-derived factor (PEDF) in the maintenance of the retina is evident in several animal models of inherited and light-induced retinal degenerations (Polato and Becerra, 2016). Administration of PEDF protein both in vivo and ex vivo delays the death of photoreceptors (PRs) in models of retinitis pigmentosa, such as rd1, rds and rd10 mice (Cayouette et al., 1999; Hernandez-Pinto et al, 2019; Kenealey et al, 2015). However, the impact of ablation of the Serpinf1 gene, which encodes for PEDF, on retinal degeneration has not been investigated to date. In murine eyes with retinal degeneration, PEDF levels are lower than in their wild type counterparts (Hernandez-Pinto et al., 2019; Wang et al, 2013), nicely modeling the fact that PEDF levels are also decreased in eyes affected by retinal degenerative processes in humans (Holekamp et al., 2002; Mohan et al., 2012; Ogata et al., 2004; Ogata et al., 2002; Wang et al., 2013). The mean levels of PEDF in eyes of patients with retinitis pigmentosa are significantly lower than those in eyes without this disease (Ogata et al., 2004). The vitreous of patients with choroidal neovascularization due to age-related macular degeneration contains lower PEDF levels and lacks the antiangiogenic activity of vitreous from age-matched controls (Holekamp et al., 2002). These observations suggest that loss of PEDF creates a permissive environment for retinal degeneration and ocular neovascularization.
PEDF is a 50-kDa extracellular protein circulating in the blood (Petersen et al., 2003; Wu et al, 1995). In the eye, the retinal pigment epithelium (RPE) expresses the SERPINF1 gene, synthesizes and releases the mature glycosylated PEDF protein from its apical side towards the photoreceptors (Becerra et al., 2004). The secreted glycoprotein deposits in the interphotoreceptor matrix (IPM), where it interacts with glycosaminoglycans, acts on photoreceptor survival and is believed to be responsible for the IPM avascularity (Becerra et al., 2008; Dawson et al., 1999; Wu et al., 1995). Besides its demonstrable neurotrophic action, the multifunctional PEDF protein also exhibits antiangiogenic activity, such as inhibition of retinal and choroidal neovascularization, and consequently has been likened to an ‘ocular guardian’ (Bouck, 2002; Craword et al., 2013). The monomeric protein has separate regions that confer the neurotrophic and antiangiogenic properties to the PEDF polypeptide, each of which interacts with distinct receptors to mediate the individual activities in target cells (Becerra, 2006; Filleur et al., 2009). Synthetic peptides derived from individual PEDF regions mimic the corresponding biological activities of the full-length protein. Peptides from the neurotrophic region of PEDF also prevent death of photoreceptors in the rd1 and rd10 mouse model in vivo and ex vivo, and are considered as promising neuroprotective agents for the clinic (Hernandez-Pinto and Becerra, 2019; Kenealey et al., 2015; Hernandez-Pinto et al., 2019).
Mutations in SERPINF1, the PEDF gene, cause osteogenesis imperfecta (OI) type VI, an autosomal recessive form of OI with moderate to severe bone fragility (Homan et al., 2011). Patients with OI type VI lack circulating PEDF, which is a marker of this disease (Rauch et al., 2012). However, so far, no clinical features affecting the retina have been reported in those patients, and it is not known if retina disorders would develop after insult, injury or with aging in these rare and young patients. As Serpinf1 null mice mimic the principal structural and biochemical features of bone observed in humans with OI type VI they are believed to be a good mouse model for this bone disorder (Bogan et al., 2013).
Considering the role of PEDF in retinal pathophysiology, it is hypothesized that absence of PEDF leads to increased stress-associated retinal degeneration in Serpinf1 null mice. Given that the impact of developmental Serpinf1 gene ablation on retinal degeneration has not yet been investigated, we aimed to study the effects on retina structure in animals in which the Serpinf1 gene is lacking when they are challenged by induced and inherited retinal degeneration. In one experimental model, the systematic delivery of sodium iodate (NaIO3), a stable oxidizing agent, is effective in inducing retinal degeneration associated with regional loss of RPE and recapitulating some of the morphological features of geographic atrophy (Wang et al., 2014). In addition to its application to establishing a retinal degeneration model (Kannan and Hinton, 2014), the selection of NaIO3 as oxidative stressor in this study was based on our previous demonstration that PEDF protects cultured RPE cells from NaIO3 induced cell death (Nadal-Nicolas and Becerra, 2018). In the other system, rd10 mice carry a spontaneous missense mutation in the phosphodiesterase 6B (Pde6b) gene and provides a reliable model for studying autosomal recessive retinitis pigmentosa in humans (Chang et al., 2007). We discuss how lack of PEDF impacts the retina undergoing degeneration in these two models.
2. Material and methods
2.1. Animal models and tissue collection
Serpinf1+/− (C57BL/6J) mice (Doll et al., 2003) were obtained from Dr. Susan Crawford’s laboratory (NorthShore University Health System Research Institute). The mice were confirmed to be rd8 free and used to generate Serpinf1−/− (hereafter referred as Serpinf1 null) and wild type littermate controls Serpinf1+/+ (WT). The C57BL/6J and rd10 mice were from Jackson Laboratories. Homozygous rd10 mice were crossed with Serpinf1 null mice. The resulting double mutant mice were in C57BL/6J and rd8-free background and are referred to as rd10/Serpinf1 null. R91W/Nrl−/− mice were as described (Samardzija et al., 2014).
All mice were maintained in the animal house facility of the National Institutes of Health, and all the experimental procedures were approved by the National Eye Institute Animal Care and Use Committee, and were performed as per guidelines of the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research, USA. All the experimental animals were maintained on normal chow diet and were kept at 280–300 lux light intensity and 12 h light/12 h dark cycle. For the oxidative retinal damage experiments Serpinf1 null mice and their WT littermates were subjected to single intraperitoneal injection of NaIO3 (30 mg/kg body weight). rd10 and rd10/Serpinf1 null mice were euthanized at post-natal day (P)15–28. Left eye (OS) fixed for cryosectioning and right eye (OD) for Hematoxylin and Eosin staining.
2.2. Laser capture and RT-PCR
Retinal nuclear layers were isolated by laser capture microdissection and gene expression analyses were performed with semiquantitative real-time PCR, as described before (Caprara et al., 2014), to test for Serpinf1 and Pnpla2 expression (the genes encoding PEDF and its receptor PEDF-R, respectively). The primers used for reactions for Serpinf1, Pnpla2, β-actin, Mct3, Mwl, Chx10 and Opn4 are shown in Table 1.
Table 1.
Oligonucleotide primers used for semiquantitative real-time PCR in this study.
| Gene name | Primers | Length of amplimer |
|---|---|---|
| Serpinf1 | GAGAAGAACCTCAAGAGTGC | 143 bps; |
| ATCTCCTGAAGGTCTACTCG | ||
| Pnpla2 | GAGCTTCGCGTCACCAAC | 104 bps |
| CACATCTCTCGGAGGACCAT | ||
| Actb | CAACGGCTCCGGCATGTGC | |
| CTCTTGCTCTGGGCCTCG | 75 bps | |
| Slc16a8 (Mct3) | CTCTTGCTCTGGGCCTCG | |
| CTTCGGAGTTTCCTCACCAG | ||
| Opn1mw (Mwl)* | CTCTGCTACCTCCAAGTGTGG | 154 bps |
| AAGTATAGGGTCCCCAGCAGA | ||
| Vsx2 (Chx10) | CCAGAAGACAGGATACAGGTG | 111 bps |
| GGCTCCATAGAGACCATACT | ||
| Opn4 | CCAGCTTCACAACCAGTCCT | 111 bps |
| CAGCCTGATGTGCAGATGTC | ||
2.3. Genotyping
Genomic DNA was isolated from tail clips. For identification of Serpinf1−/− mice, PCR reactions were performed as described before (Doll et al, 2003) with primer sequences as follows: Exon 3-Forward (Serpinf1 WT), 5’-TTGTGGAGTCAGAGAGCACACA-3’ (nucleotide number range: 14513 – 14534); Exon 3-Reverse (Serpinf1 WT), 5’-TAGGAGGCCACTTAAGGAACCA-3’ (nucleotide number range: 15003 – 14982); LacZ Forward (for Serpinf1−/−), 5’-CAGCGACTGATCCACCCAGTCC-3’; LacZ Reverse (for Serpinf1−/−), 5’-CACGAGCATCATCCTCTGCATG-3’. The expected amplicon sizes were a 490 bp product for the wild type allele and an ~650 bp for the knockout allele (see Fig. 2A). The following conditions were used for the PCR reactions: initial denaturation (95°C, 15 min); 30 cycles of denaturation (95°C, 60 s), annealing (temperature 60°C, 30 s) and elongation (72°C, 60 s); and final extension (72°C, 5 min).
Fig. 2.
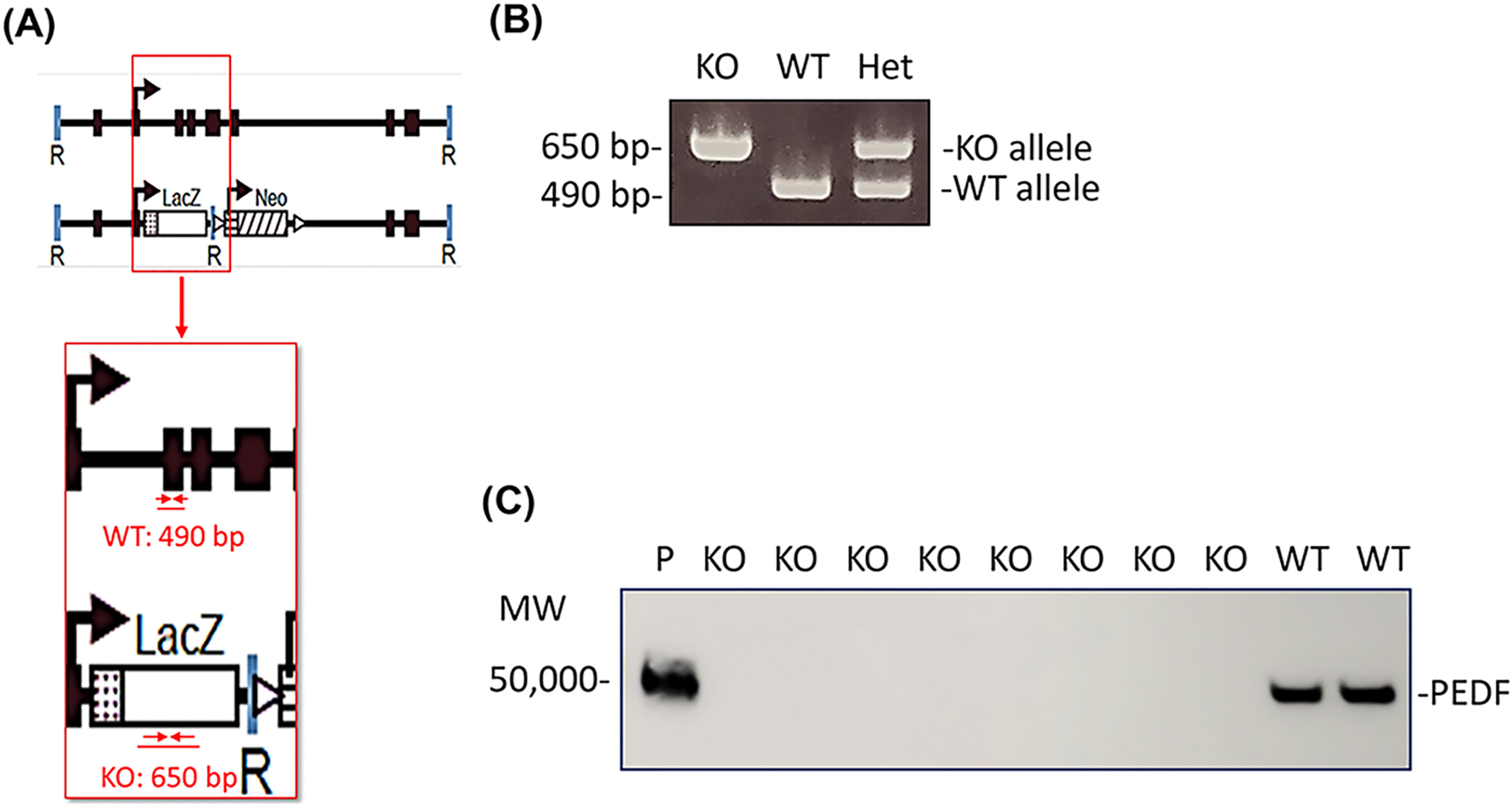
(A) Serpinf1 null mice (KO), organization illustrating location of primers for genotyping and expected product size (adapted from Doll et al. 2003). (B) Detection of Serpinf1 gene by genotyping (C) Plasma PEDF protein levels –Western blotting vs anti-PEDF antibody.
2.4. PEDF protein detection in plasma
Plasma was isolated from blood and proteins were subjected to cation exchange batch chromatography using S-Sepharose fast flow (GE). Plasma (50 μl) was diluted 10-fold with buffer S (20 mM Na-phosphate, pH 6.4 ± 0.2, 1 mM DTT, 5% glycerol) containing 50 mM NaCl. About 50 μl of S-Sepharose resin was equilibrated with 5-resin-volumes of buffer S containing 50 mM NaCl in an Eppendorf tube. The mixture was incubated on ice for 10 min with gentle swirling to allow the proteins to bind the resin. The flow-through containing protein that did not bind to the resin, such as albumin, was collected. The resin was washed with 10-resin-volumes of buffer S with 50 mM NaCl and bound PEDF was eluted from the resin with 2-resin volumes of buffer S containing 500 mM NaCl.
Proteins in the eluate were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Blots were blocked with 1% BSA in TBST (50 mM Tris Cl pH 7.5, 150 mM NaCl, 0.1% Tween 20) for 1 h at room temperature and then probed with an antibody against human PEDF (Rabbit polyclonal human PEDF antibody, Bioproduct MD/Xpressbio #AB-PEDF) diluted 1:100,000 in 1% BSA in TBST at 4°C for 16 h. The membrane was washed for 15 min with TBST, repeated three times and then incubated with secondary antibody (Digital anti-rabbit-HRP, Kindlebio, #R1006) diluted 1:1000 for 60 min at room temperature. The membrane was washed three times with TBST for 15 min each. Blots were developed with Digital ECL mixture (diluted 1:10) and visualized using the KwikQuant imaging system (Kindlebio).
2.5. Transmission electron microscopy (TEM)
Mouse eyes were enucleated and doubly fixed in PBS-buffered glutaraldehyde (2.5% at pH 7.4) and PBS-buffered osmium tetroxide (0.5%) and embedded in epoxy resin. Sections (90 nm thick) were generated and assembled on 200-mesh copper grids, dried for 24 h, and double-stained with uranyl acetate and lead citrate. Sections were photographed with a JEOL JM-1010 electron microscope.
2.6. Electroretinography (ERG)
In dim red light, overnight dark-adapted mice were anesthetized by intraperitoneal (IP) injection of Ketamine (92.5 mg/kg) and xylazine (5.5 mg/kg). Pupils were dilated with a mixture of 1% tropicamide and 0.5 % phenylephrine. A topical anesthetic, Tetracaine (0.5%), was administered before positioning the electrodes on the cornea for recording. ERG was recorded from both eyes by the Espion E2 system with ColorDome (Diagnosys LLC, Lowell, MA, USA). Dark-adapted responses were elicited with increasing light impulses with intensity from 0.0001 to 10 candela-seconds per meter squared (sc cd.s/m2). Light-adapted responses were recorded after 2 min adaptation to a rod-saturating background (20 cd/m2) with light stimulus intensity from 0.3 to 100 sc cd.s/m2. During the recording, the mouse body temperature was maintained at 37°C by placing them on a heating pad. Amplitudes for a-wave were measured from baseline to negative peak, and b-wave amplitudes were measured from a-wave trough to b-wave peak.
2.7. Fundus microscopy
Mice were anaesthetized as above, and the pupils were dilated with 1% tropicamide and 0.5 % phenylephrine solution. Lubricant eye gel (GenTeal) was applied liberally to the eyes to keep them hydrated during the procedure. Micron III Rodent Fundus Imaging System (Phoenix Research Labs, Pleasanton, CA, USA) was employed to take images of ocular fundi at the indicated time points. Mice were kept on heating pad till they regained their consciousness.
2.8. Histological analysis
To analyze retinal morphology with light microscopy, the eyes were enucleated and fixed in 2.5% glutaraldehyde for 20 min and then in 10% neutral buffered formalin at 4 °C for at least 48 h. The fixed eyes were submitted to the NEI Histology Core for dehydration and embedding in polymethyl methacrylate resin, followed by sectioning into 4 μm slices. After mounting on Avantik positive charged slides, the attached tissue slices were used for hematoxylin and eosin (H&E) staining using Meyer’s Hematoxylin and Eosin procedure according to Histopathology Laboratories, Armed Forces Institute of Pathology, Progressive Stain (Luna, 1968). A Zeiss Axio Scan with Axio Observer microscope was used to examine the slides. To generate spider plots, retinal thickness from the nerve fiber layer to the RPE was measured at five different positions from both sides of the optic nerve head (ONH) using ImageJ (software version 1.52; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; NIH).
2.9. Cryosectioning and Immunofluorescence
The left eyes from mice were enucleated and an incision was made in the cornea. The eyes were fixed in 4% paraformaldehyde for 1 h, then cryopreserved by keeping them in 10% and 20% sucrose solution in phosphate-buffered saline (PBS) for 1 hour and overnight respectively. After incubation, eyes were washed in PBS for 10 min and then the cornea, lens and vitreous humor were removed. The eyes were embedded in the mixture of Tissue-Tek OCT compound (Sakura Finek) and 20% sucrose (2:1 ratio) mixture and snap-frozen on dry ice. The blocks were cryosectioned on CM3050S cryostat (Leica Microsystems Inc.) into 10 μm sections and processed for immunostaining. Tissue sections were labeled with primary antibodies anti-S100B (Monoclonal Anti-S100B antibody produced in mouse, Sigma AMAB 91038, diluted 1:300) and anti-GFAP (Rabbit Anti-GFAP antibody, abcam, ab16997, diluted 1:300) and incubated in a humidified chamber overnight at 4°C. Sections were probed with secondary antibodies (goat anti-mouse IgM (heavy chain) cross-adsorbed secondary antibody, Alexa Fluor 555 Invitrogen, #21426, diluted 1:500), and goat anti-rabbit IgG (H+L) cross-adsorbed secondary antibody, Alexa Fluor 488 Invitrogen #A-11008, diluted 1:500, respectively) with incubation at room temperature for 30 min. The nuclei of the cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) stain. Slides were mounted with a coverslip in mounting media (Electron Microscopy Sciences) and then visualized in LSM 700 laser scanning confocal microscope (Carl Zeiss).
3. Results
3.1. Serpinf1 expression is undetectable in ONL.
As the RPE expresses the SERPINF1 gene for PEDF (Becerra et al 2004; Notari et al 2006) it seemed possible that the cells in the layers of the retina may also produce transcripts from this gene. Thus, the spatial expression pattern of Serpinf1 was investigated in the neural retina tissue of mice. Individual layers of retinas of wild type mice (6 weeks old) were isolated by laser capture microdissection. Serpinf1 transcripts were detected in the inner nuclear layer (INL), the ganglion cell layer (GCL), and the RPE, but were undetectable in the ONL (Fig. 1A). Even in mice with only cones, as in the RPE65[R91W] x Nrl−/− mice, Serpinf1 transcripts were not detectable in the ONL, indicating the lack of Serpinf1 expression in both rods and cones photoreceptors. The transcripts for the PEDF receptor, Pnpla2, were detected in all layers, in agreement with the distribution of the Pnpla2/PEDF-R protein in mouse retina previously reported (Hernandez-Pinto et al., 2019). These observations imply that the photoreceptors depend on other cells, such as the RPE, for PEDF supply. Unfortunately, further protein analysis of PEDF in mouse retinas was not conclusive because several commercial antibodies to PEDF gave high background by immunofluorescence.
Fig. 1.
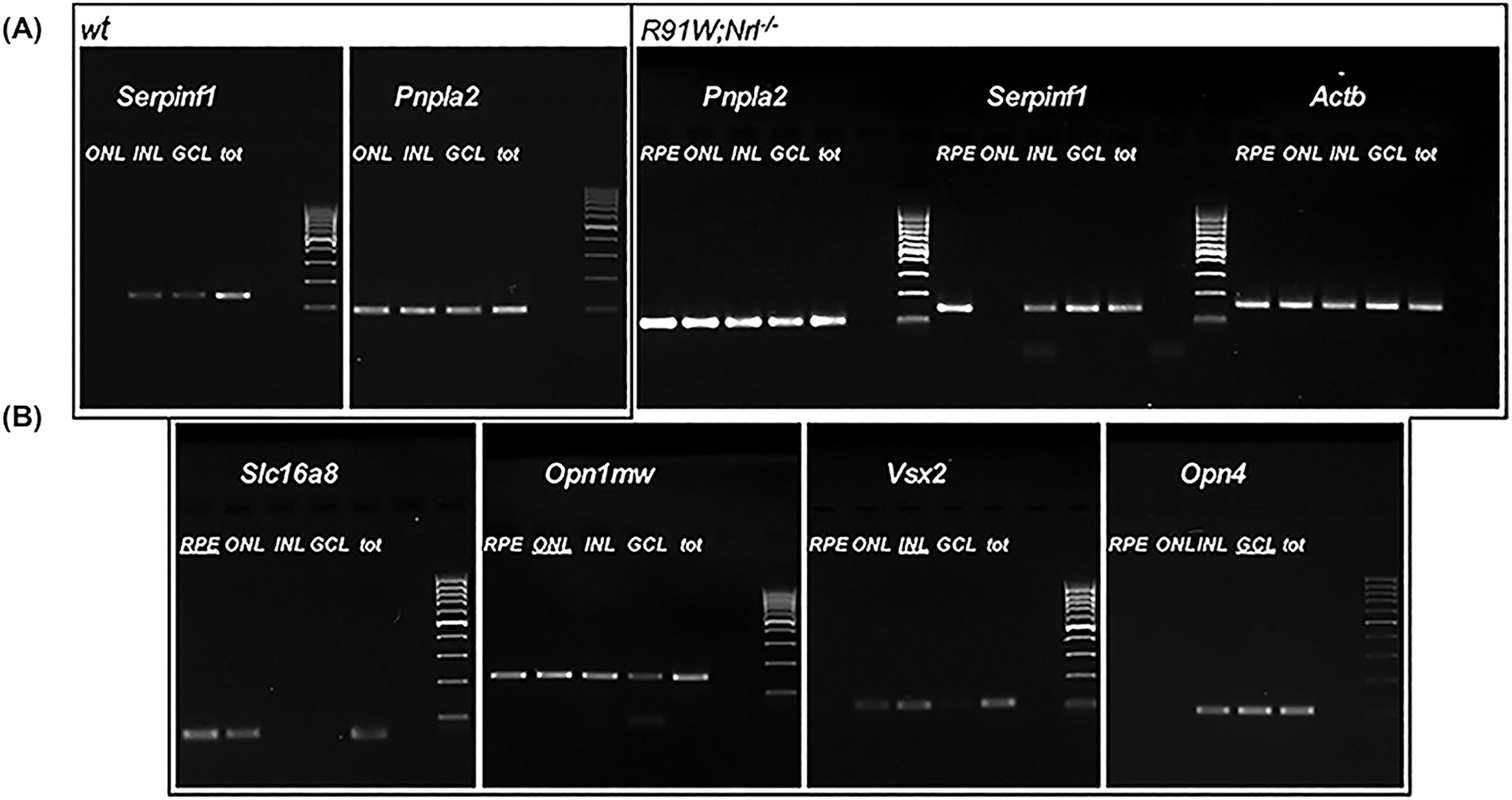
Serpinf1 and Pnpla2 expression in retinal nuclear layers. The RPE, ONL, INL and GCL were isolated from WT and R91W/Nrl−/− using laser capture microdissection. Relative gene expression was determined by semiquantitative real-time PCR in each individual layer. Photos of agarose gels stained with Et-Br are shown and samples of retinal nuclear layers, the gene analyzed, and mouse line are indicated at the top. DNA size markers are M. Expression of Slc16a8 (marker for RPE), Opn1mw (marker for ONL), Vsx2 (marker for INL), and Opn4 (marker for GCL) was determined to monitor contamination between the isolated retinal layers. Actb served as housekeeping gene control. GCL= Ganglion cell layer, INL= Inner nuclear layer, ONL= Outer nuclear layer, RPE= Retinal pigment epithelium.
3.2. Serpinf1 deficiency and retinal morphology and visual function
To explore the effects of PEDF-deficiency in retinal degeneration, we selected the Serpinf1 null mouse (Doll et al., 2003). Genotyping of the Serpinf1 null mouse confirmed that the gene had been ablated from these mice (Fig. 2A–B). Western blotting of plasma proteins from the Serpinf1 null mice further confirmed that these mice are PEDF free (Fig. 2C). Our interest in the effects of PEDF ablation on the retina led us to evaluate its structure by TEM. Figure 3A shows that the retina of Serpinf1 null mice had some degenerative regions in the photoreceptor outer segments (POS). No remarkable differences were seen in the RPE except for regions of irregular basal infolds. Some RPE cells of Serpinf1 null mice had many degenerative vacuoles. This observation prompted us to analyze retinal function. However, b-wave amplitudes and photopic ERGs were not significantly different between Serpinf1 null mice and littermate control mice at 3–6 months of age (Fig. 3). These observations implied that relatively minor structural differences in Serpinf1 null mice were not sufficient to impact retinal function. We proposed to test the influence of PEDF deficiency on the retinal degenerative process in pharmacologically and genetically challenged mice
Fig 3:
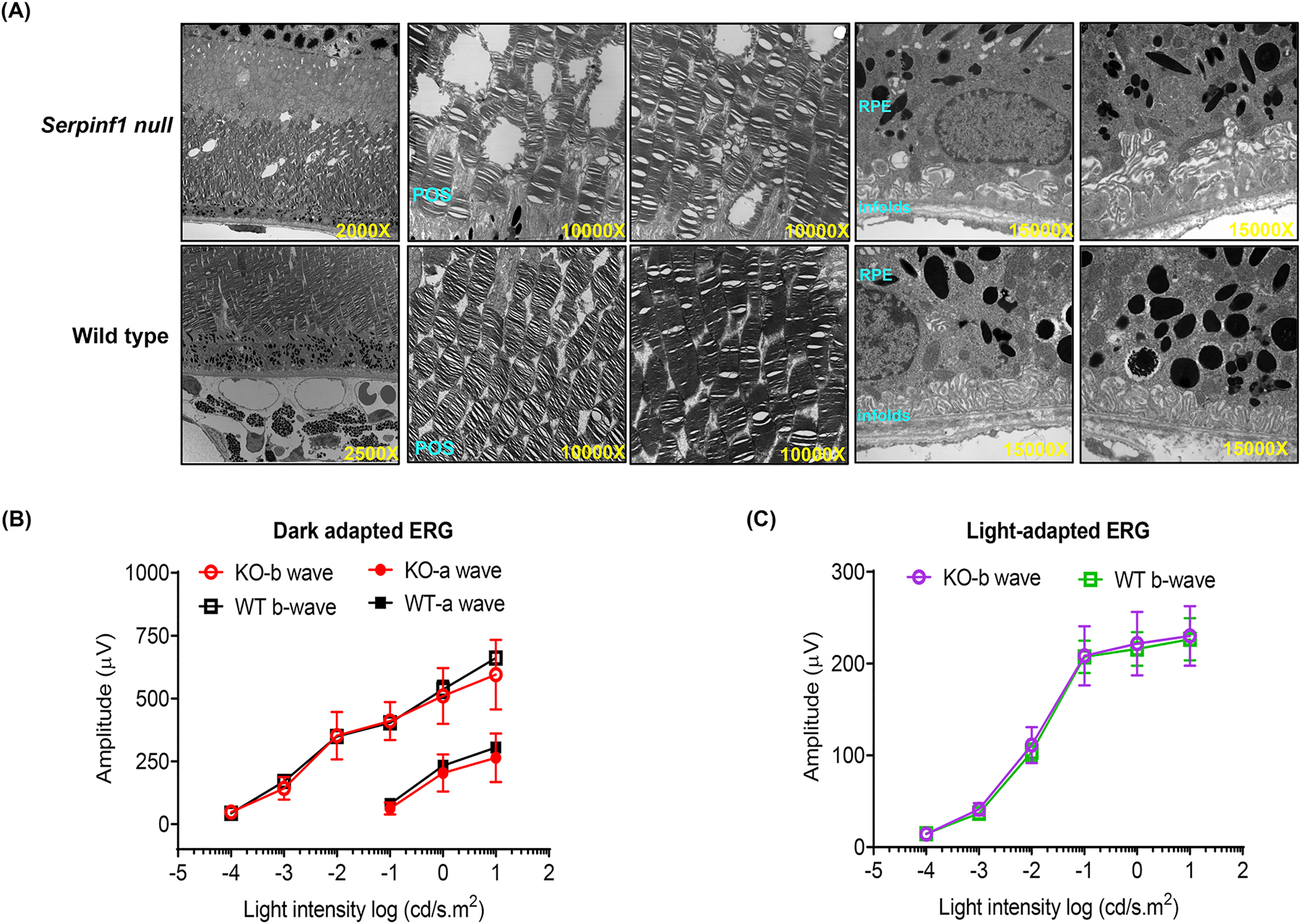
Morphology and visual function of Serpinf1 null (KO) mice and wild type (WT) littermates. (A) Electron microscopic images of KO and WT mice retina sections. Photoreceptor outer segment degeneration is marked by red arrows (B) Electroretinogram (ERG) of dark-adapted WT mice (WT; black squares and lines) and Serpinf1 null (KO; red circles and lines) at 3–6 months of age. Amplitudes of a waves (solid black squares and circles) and b waves (open black squares and circles) are recorded against light stimulus. (C) ERG of light-adapted wild type mice (WT; green squares and lines) and Serpinf1 null mice (KO; purple circles and lines) at 3–6 months of age. (means ± SD, n=5)
3.3. Effects of PEDF absence in retinas of mice exposed to NaIO3
Given that PEDF protects against cellular stress (Brook et al., 2019; Nadal-Nicolas and Becerra, 2018; Tsao et al., 2006; Wang et al., 2019, we set to examine the effects of PEDF absence on NaIO3-induced retinal degeneration. Morphological changes in the retina were evaluated up to 8 days after NaIO3 injections (IP at 30 mg/kg) in wild type (WT) mice of 3–6 months of age. Changes of the RPE layer were observed at day 3 with pigmentary disruption and atrophy that quickly became more widespread with time up to day 8 post injection (Fig. 4A). To confirm the results from the fundus studies, histological examination of retina tissue was performed. RPE distortion was observed in 30 mg/kg-NaIO3-injected mice as early as day 3 (Fig. 4B), as described before (Wang et al. 2014). Cystic spaces in the inner nuclear layer were also observed. The entire retina thinned with time after injection. Disorganization of PRs, undulating ONL pattern and large regions of complete RPE loss, and numerous blood vessels were observed at days 6 and 8 post-injection (Fig. 4B).
Fig 4:
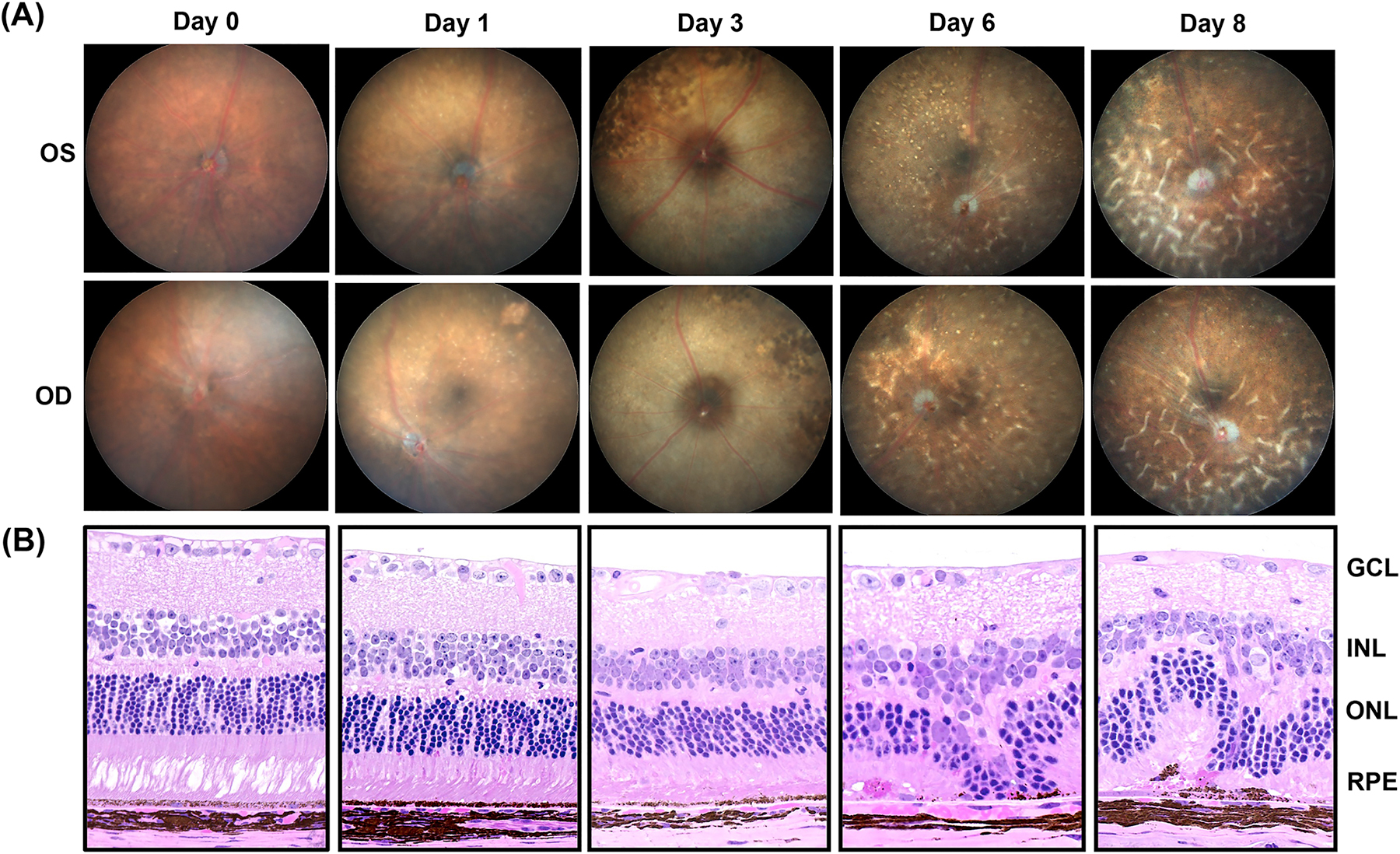
Progression of retinal damage after NaIO3 treatment in C57BL6/J wild type mice. (A) Follow-up of the retinal damage through fundus microscopy of the mice eyes after 0 (Saline treated), 1, 3, 6 and 8 days post-treatment with NaIO3. (B) Photomicrographs of hematoxylin and eosin stained eye sections of NaIO3-treated mice harvested at different time points. Mice were treated with single intraperitoneal injection of NaIO3 at 30mg/kg body weight. OD=Oculus Dextrus (right eye), OS=Oculus Sinister (left eye), GCL= Ganglion cell layer, INL= Inner nuclear layer, ONL= Outer nuclear layer, RPE= Retinal pigment epithelium. (n=3)
Based on the above results, the effects of NaIO3 (30 mg/kg) at day 4 post-injection in Serpinf1 null and littermate control (WT) mice of 3–6 months of age were compared. Day 4 post injection for WT mice was expected to produce RPE and retina disorganization slightly more severe than those shown at day 3 post-injection (Fig. 4B). The retina morphology by H&E staining for WT and Serpinf1 null mice is shown in Figs. 5A–B. RPE distortion and migration of pigmented cells into the OS layer were observed in the WT mice injected with NaIO3, which were not seen in the saline injected ones (Fig. 5A) nor in day 3 (see Fig. 4). Three out of five Serpinf1 null mice also had RPE distortion, while the other two did not seem to be affected and resembled the saline-injected ones (Fig. 5B). The observations suggest that a mechanism to compensate the lack of PEDF might have been induced during development, which warrant further studies. To quantitatively assess the global effect of NaIO3 in ONL of retinas of both experimental groups, the height of ONL was measured at five definite points on both side of the optic nerve head and plotted as spider plot (Fig. 5C). The spider plot showed that the NaIO3 treated Serpinf1 null mice possess thicker ONL on one side of the retina than NaIO3 treated WT littermates, while the other side had no difference between the two mouse lines.
Fig 5:
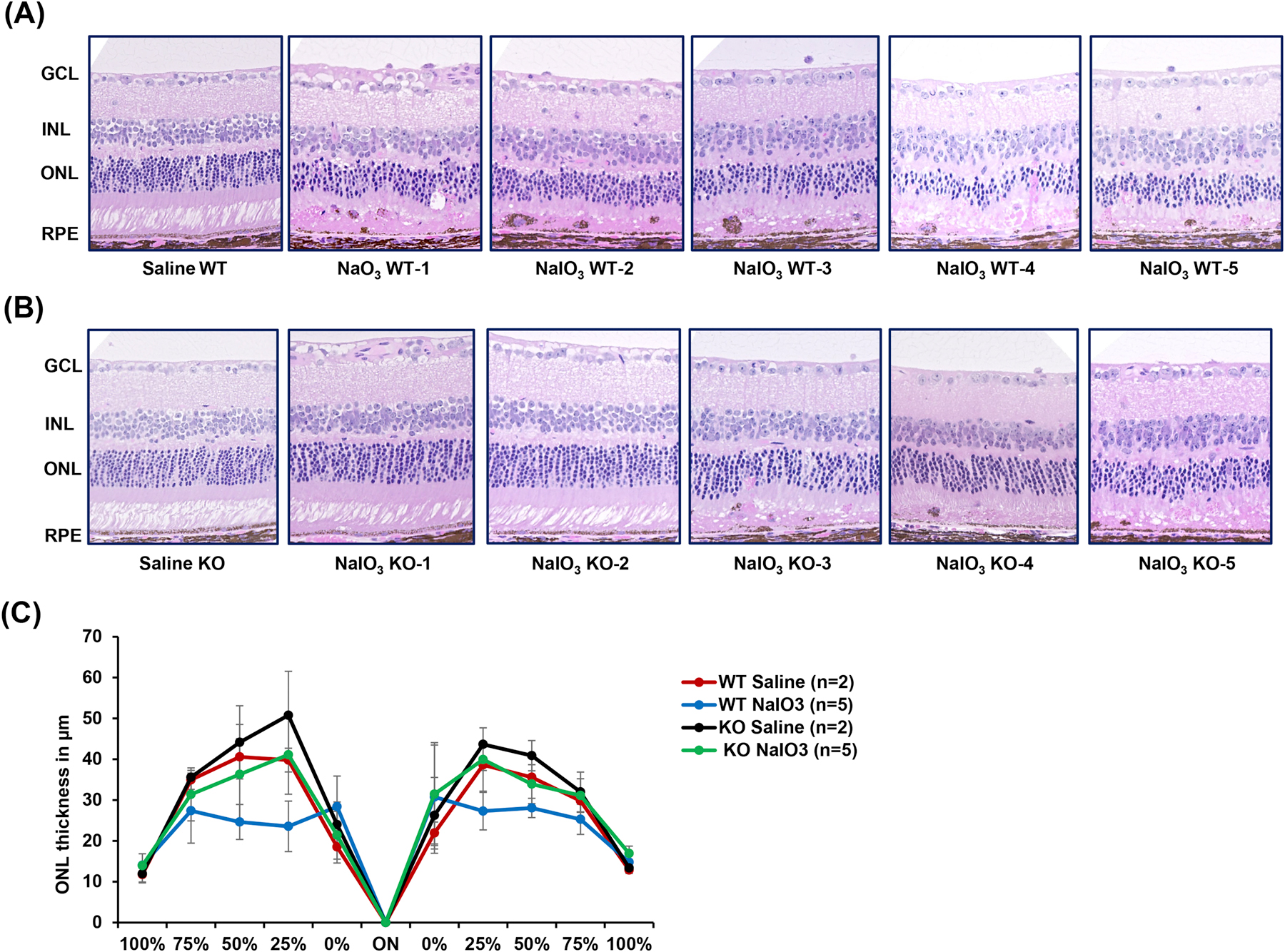
Histology of retinas of WT and Serpinf1 null mice injected with NaIO3. Serpinf1 null (KO) and WT mice were treated with 30mg/kg body weight of NaIO3 (n=5) or saline (n=2). After 4 days post injection, mice were euthanized and eyes enucleated, fixed, sectioned and stained. (A-B) Microphotographs of retina sections of the saline or NaIO3 treated WT (A) and KO (B) mice stained with hematoxylin and eosin. (B) Spider plot analysis illustrating outer nuclear layer (ONL) thickness in NaIO3 (n = 5) and saline (n=2) treated WT and KO mice.
3.4. Lack of PEDF accelerated retinal degeneration in the rd10 model
Given that PEDF protects photoreceptors against death in rd10 mice (Hernandez-Pinto et al., 2019), we set to examine the effects of PEDF absence in rd10 mice, which provide a slower retinal degenerative model than the one described above. We compared the morphology of retinas of rd10 and rd10/Serpinf1 null mice at different ages between P15 and P28. Histological examination revealed progressive retinal ONL degeneration in rd10 mice that starts in the central retina at P15 and spreads towards the peripheral retina by P25 of age (Chang et al., 2007). While nuclei count of the ONL mirrored this decline, the thickness of the inner nuclear layer and of the ganglion cell layer were not affected. Comparison of ONL thickness between rd10/Serpinf1 null and rd10 at P15-P18 showed similarities at the same ages (Fig. 6 and data not shown). However, there were significant differences between rd10 and rd10/Serpinf1 null at P21 and P25 (Fig. 6). At P15 the rd10/Serpinf1 null RPE was more swollen than in rd10. At P21, the mice without PEDF had a disorganized ONL with swollen and less pigmented cells in the RPE layer than in rd10. At P25 the rd10/Serpinf1 null retina had other features not observed in rd10 mice that had PEDF, such as ONL with fewer rows, migration of cells into the ONL, swollen and less pigmented cells. We concluded that rd10/Serpinf1 null mice without PEDF showed more intense retinal degeneration than the rd10 mice at P25.
Fig 6:
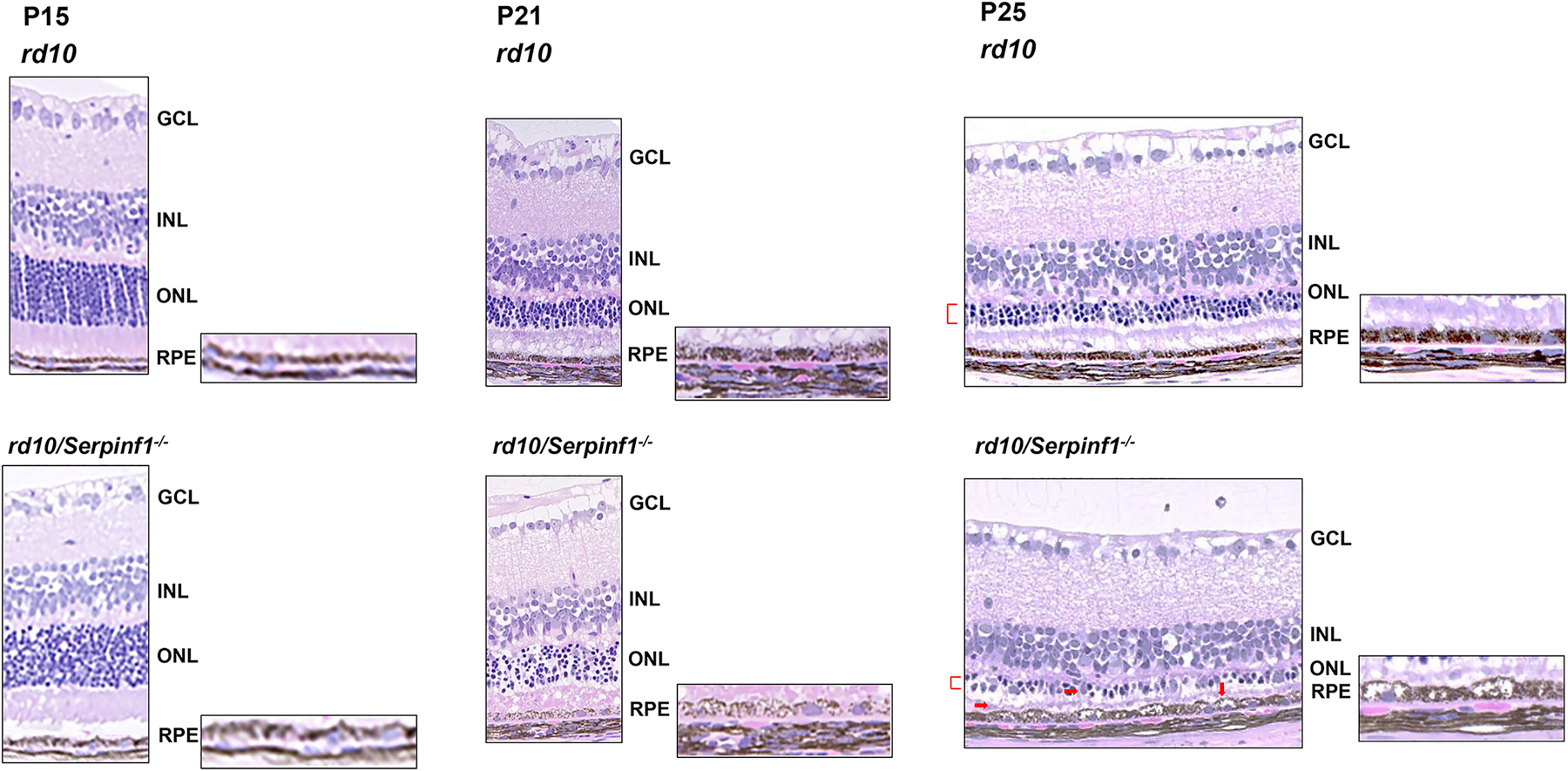
Histology of retinas of rd10 and rd10/Serpinf1 null mice. Eyes of rd10 and rd10/Serpinf1 null mice and mice at P15, P21 and P25 were enucleated, fixed and stained with hematoxylin and eosin. Representative of images retina sections displaying pathologies associated with PEDF deficiency are shown (red markings). GCL = ganglion cell layer, INL=inner nuclear layer, ONL= Outer nuclear layer, RPE= Retinal pigment epithelium. n=4.
4. Discussion
In this study, we have assessed the effects of PEDF deficiency in two models of retinal degeneration. To this end, we examined the Serpinf1 null mouse undergoing retinal degeneration provoked by oxidative stress with NaIO3 and in the inherited rd10 retinal degeneration mouse model, both on aspects of retinal structure. Firstly, we conclude that the absence of Serpinf1 expression in the ONL of WT mice implies that the photoreceptors must rely on other cells, such as their neighboring RPE, for PEDF production and supply to act on survival. Second, in complete absence of PEDF, Serpinf1 null mice have electroretinograms identical to those of wild type mice, despite the subtle distortion in photoreceptor structure observed by TEM. Third, a further challenge on the Serpinf1 null retina, such as NaIO3-induced retinal degeneration, was not enough to exacerbate the injury. However, with a point mutation in the photoreceptor specific Pde6 gene, e.g. rd10 mouse, the lack of PEDF makes the retina more vulnerable to degeneration than its WT counterpart, thus aggravating the injury in photoreceptors, cells in the INL and distorting the RPE. The fact that mice with PEDF (rd10) had less injury relative to that in mice without PEDF (rd10/Serpinf1 null), agrees with the protective effects of PEDF protein and peptides on photoreceptors of rd10 and other models of retinal degeneration, e.g., rd1, RCS, DKO (Hernandez-Pinto et al., 2019; Polato and Becerra, 2016). The findings suggest that oxidative stress may not pose a risk to OI type VI patients, and that retinitis pigmentosa patients with a Pde6b gene mutation and PEDF deficiency may undergo accelerated retinal degeneration and vision loss.
This is the first time that the Serpinf1 null mouse model has been used to study the impact of PEDF deficiency on the retina. Previously, the effects of PEDF absence in the vascular retina investigated using a different PEDF-deficient mouse line (PEDF−/−) showed that mice lacking PEDF underwent enhanced retinal vascularization processes before P21 and were more sensitive to hyperoxia-mediated vessel obliteration (Huang et al., 2008). Their isolated RPE cells showed enhanced proliferation and adhesion properties in culture, but consistently slower migration ability (Farnoodian et al., 2015). They also exhibited significantly higher apoptosis levels compared with WT. In our study, the lack of PEDF results in an increased sensitivity of the RPE cells to oxidative challenge, in agreement with the established fact that PEDF protects against cellular stress (Brook et al., 2019). We and others have shown that PEDF additions to cultured RPE cells prevent death induced by the oxidative agents NaIO3 and H2O2 (Nadal-Nicolas and Becerra, 2018; Tsao et al., 2006; Wang et al., 2019). PEDF also protects the cone-like 661W cells against both photo-oxidative stress and H2O2-induced oxidative injury (Cheng et al., 2014; Krishnamoorthy et al., 1999; Rapp et al., 2014; Comitato et al., 2018).
The NaIO3 treatment is known to target RPE cells. However, there is evidence for its effect in retinal neuron injury (Wang et al., 2014). One consideration is that under the dose conditions used in our study (30mg/Kg) cytotoxicity occurs too rapidly to facilitate elucidation of mechanisms leading to photoreceptor damage. Several mechanisms may occur at the same time giving rise to large variability in the severity of retinal degeneration (Wang et al., 2014). We noticed that Serpinf1 null and WT mice displayed similar trend of gliosis in response to NaIO3 treatment (personal observations, Fig S1). Swelling and distortion of the RPE by NaIO3 is likely to affect gene expression in these cells resulting in changes, such as Serpinf1 gene repression, thereby decreasing the availability of neurotrophic factors that protect against degeneration and damage of the retina. This is in agreement with the fact that oxidative stress-induced by H2O2-exposed pericytes decreases their PEDF mRNA levels (Amano et al., 2005). A report described the identification of genes dysregulated in the Serpinf1 null mouse, which included those coding for proteins affecting cell-cell interactions, junction formation and ECM proteins (e.g., ZO-1 (lower), β-catenin (high), 120 (higher), FAK (increased), fibronectin (high), collagen IV (low), TSP1 (low), tenascin C (high), periostin (high), opticin (low)) (Farnoodian et al., 2015). The lack of a more intensified degeneration under oxidative stress when PEDF is deficient suggests that during development a compensatory mechanism independent of PEDF is likely to be activated, thereby minimizing NaIO3-mediated retinal injury in the Serpinf1 null mouse. These implications are unknown and need further study. A model of inducible knock down of Serpinf1 at adult stages may be instrumental to address the role of PEDF in mature retina unbiased by compensatory mechanisms during development.
The findings with rd10/Serpinf1 null mice suggest that PEDF is required to protect photoreceptors in which the Pde6 gene is mutated and impaired, in agreement with the known photoreceptor protection by this factor in the rd10 retinas. In this model, the mechanisms of cell death are specific and better known. Photoreceptor cell death peaks at P25, at a time in which PEDF levels are lower in the retina than in WT mice and the levels of its PEDF-R receptor are also decreased at P25 due to the low number of PRs existing in the retina at that age (Hernandez-Pinto et al., 2019). The lower levels of the PEDF can be compensated by intravitreal injection of the factor prior to reaching P25 for a successful prevention of further death, demonstrating a gain of function effect. Upon binding PEDF-R, PEDF mediates a decrease of intracellular calcium in the degenerating retina, and in turn triggers a cascade of events to ultimately hinder photoreceptor cell death (Comitato et al., 2018). The findings demonstrate loss-of-function in the PEDF-deficient mice, thus highlighting the importance of this factor in limiting photoreceptor degeneration and its blinding consequences.
Supplementary Material
Fig S1: NaIO3 treatment induced gliosis in Serpinf1 null (KO) and Wild type (WT) mice: Representative images of eye cryosections of WT (A, B and C) and KO mice (C, D and E) immuno-stained for GFAP (green) and S100B (red). The nuclei of the cells are counter stained with DAPI (blue). GCL = ganglion cell layer, INL=inner nuclear layer, ONL=outer nuclear layer, RPE= retinal pigment epithelium. (n=3)
Highlights.
The SERPINF1 gene encodes the pigment epithelium-derived factor (PEDF) polypeptide.
Serpinf1 was expressed in RPE, INL and RGC, but undetected in photoreceptors.
Retinas of Serpinf1 null and WT mice had identical ERGs and similar structure.
NaIO3 administration impacted the RPE of WT more than the Serpinf1 null RPE.
Lack of PEDF influenced the magnitude of photoreceptor degeneration in rd10 mice.
Acknowledgements
This work was supported in part by the NIH-NEI intramural research program [Project #EY000306 to SPB]; and Swiss National Science Foundation [SNSF 31003A_173008 to CG]. We thank Megan Kopera for providing rd10 mice, Mercedes Campos and the NEI Histopathology Core for assistance with histology, and the NEI Visual Function Core with assistance with ERGs.
Abbreviations:
- PEDF
pigment epithelium-derived factor
- RPE
retinal pigment epithelium
- IPM
interphotoreceptor matrix
- OI
osteogenesis imperfecta
- NaIO3
sodium iodate
- Pde6b
phosphodiesterase 6B
- WT
wild type
- OS
oculus sinister or left eye
- OD
oculus dextrus or right eye
- H&E
hematoxylin and eosin
- INL
inner nuclear layer
- GCL
ganglion cell layer
- ONL
outer nuclear layer
- TEM
Transmission electron microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano S, Yamagishi S, Inagaki Y, Nakamura K, Takeuchi M, Inoue H, Imaizumi T, 2005. Pigment epithelium-derived factor inhibits oxidative stress-induced apoptosis and dysfunction of cultured retinal pericytes. Microvasc. Res 69, 45–55. 10.1016/j.mvr.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Becerra SP, 2006. Focus on Molecules: Pigment epithelium-derived factor (PEDF). Exp. Eye Res 82, 739–740. 10.1016/j.exer.2005.10.016 [DOI] [PubMed] [Google Scholar]
- Becerra SP, Fariss RN, Wu YQ, Montuenga LM, Wong P, Pfeffer BA, 2004. Pigment epithelium-derived factor in the monkey retinal pigment epithelium and interphotoreceptor matrix: apical secretion and distribution. Exp. Eye Res 78, 223–234. 10.1016/j.exer.2003.10.013 [DOI] [PubMed] [Google Scholar]
- Becerra SP, Perez-Mediavilla LA, Weldon JE, Locatelli-Hoops S, Senanayake P, Notari L, Notario V, Hollyfield JG, 2008. Pigment epithelium-derived factor binds to hyaluronan. Mapping of a hyaluronan binding site. J. Biol. Chem 283, 33310–33320. 10.1074/jbc.M801287200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan R, Riddle RC, Li Z, Kumar S, Nandal A, Faugere MC, Boskey A, Crawford SE, Clemens TL, 2013. A mouse model for human osteogenesis imperfecta type VI. J. Bone Miner. Res 28, 1531–1536. 10.1002/jbmr.1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck N, 2002. PEDF: anti-angiogenic guardian of ocular function. Trends Mol. Med 8, 330–334. 10.1016/s1471-4914(02)02362-6 [DOI] [PubMed] [Google Scholar]
- Brook N, Brook E, Dharmarajan A, Chan A, Dass CR, 2019. The role of pigment epithelium-derived factor in protecting against cellular stress. Free Radic. Res 53, 1166–1180. 10.1080/10715762.2019.1697809 [DOI] [PubMed] [Google Scholar]
- Caprara C, Britschgi C, Samardzija M, Grimm C, 2014. The erythropoietin receptor is not required for the development, function, and aging of rods and cells in the retinal periphery. Mol. Vis 20, 307–324. PMCID: [PMC free article] [PubMed] [Google Scholar]
- Cayouette M, Smith SB, Becerra SP, Gravel C, 1999. Pigment epithelium-derived factor delays the death of photoreceptors in mouse models of inherited retinal degenerations. Neurobiol. Dis 6, 523–532. 10.1006/nbdi.1999.0263 [DOI] [PubMed] [Google Scholar]
- Chang B, Hawes NL, Pardue MT, German AM, Hurd RE, Davisson MT, Nusinowitz S, Rengarajan K, Boyd AP, Sidney SS, Phillips MJ, Stewart RE, Chaudhury R, Nickerson JM, Heckenlively JR, Boatright JH, 2007. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vision Res 47, 624–633. 10.1016/j.visres.2006.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Zhong M, Kawaguchi R, Kassai M, Al-Ubaidi M, Deng J, Ter-Stepanian M, Sun H, 2014. Identification of PLXDC1 and PLXDC2 as the transmembrane receptors for the multifunctional factor PEDF. Elife 3:e05401 10.7554/eLife.05401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comitato A, Subramanian P, Turchiano G, Montanari M, Becerra SP, Marigo V, 2018. Pigment epithelium-derived factor hinders photoreceptor cell death by reducing intracellular calcium in the degenerating retina. Cell Death Dis 9, 560 10.1038/s41419-018-0613-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craword SE, Fitchev P, Veliceasa D, Volpert OV, 2013. The many facets of PEDF in drug discovery and disease: a diamond in the rough or split personality disorder? Expert Opin. Drug Discov 8, 769–792. 10.1517/17460441.2013.794781 [DOI] [PubMed] [Google Scholar]
- Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP, 1999. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science 285, 245–248. 10.1126/science.285.5425.245 [DOI] [PubMed] [Google Scholar]
- Doll JA, Stellmach VM, Bouck NP, Bergh AR, Lee C, Abramson LP, Cornwell ML, Pins MR, Borensztajn J, Crawford SE, 2003. Pigment epithelium-derived factor regulates the vasculature and mass of the prostate and pancreas. Nat. Med 9, 774–780. 10.1038/nm870 [DOI] [PubMed] [Google Scholar]
- Farnoodian M, Kinter JB, Yadranji Aghdam S, Zaitoun I, Sorenson CM, Sheibani N, 2015. Expression of pigment epithelium-derived factor and thrombospondin-1 regulate proliferation and migration of retinal pigment epithelial cells. Physiol. Rep 3 10.14814/phy2.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filleur S, Nelius T, de Riese W, Kennedy RC, 2009. Characterization of PEDF: a multi-functional serpin family protein. J. Cell Biochem 106, 769–775. 10.1002/jcb.22072 [DOI] [PubMed] [Google Scholar]
- Hernandez-Pinto A, Becerra SP, 2019. PEDF peptides in retinal degenerations Royal Society of Chemistry, Cambridge, UK. [Google Scholar]
- Hernandez-Pinto A, Polato F, Subramanian P, Rocha-Munoz A, Vitale S, de la Rosa EJ, Becerra SP 2019. PEDF peptides promote photoreceptor survival in rd10 retina models. Exp. Eye Res 184, 24–29. 10.1016/j.exer.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holekamp NM, Bouck N, Volpert O, 2002. Pigment epithelium-derived factor is deficient in the vitreous of patients with choroidal neovascularization due to age-related macular degeneration. Am. J. Ophthalmol 134, 220–227. 10.1016/s0002-9394(02)01549-0 [DOI] [PubMed] [Google Scholar]
- Homan EP, Rauch F, Grafe I, Lietman C, Doll JA, Dawson B, Bertin T, Napierala D, Morello R, Gibbs R, White L, Miki R, Cohn DH, Crawford S, Travers R, Glorieux FH, Lee B, 2011. Mutations in SERPINF1 cause osteogenesis imperfecta type VI. J. Bone Miner. Res 26, 2798–2803. 10.1002/jbmr.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Wang S, Sorenson CM, Sheibani N, 2008. PEDF-deficient mice exhibit an enhanced rate of retinal vascular expansion and are more sensitive to hyperoxia-mediated vessel obliteration. Exp. Eye Res 87, 226–241. 10.1016/j.exer.2008.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan R, Hinton DR, 2014. Sodium iodate induced retinal degeneration: new insights from an old model. Neural Regen. Res 9, 2044–2045. 10.4103/1673-5374.147927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealey J, Subramanian P, Comitato A, Bullock J, Keehan L, Polato F, D., Marigo V, Becerra SP, 2015. Small Retinoprotective Peptides Reveal a Receptor-binding Region on Pigment Epithelium-derived Factor. J. Biol. Chem 290, 25241–25253. 10.1074/jbc.M115.645846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy RR, Crawford MJ, Chaturvedi MM, Jain SK, Aggarwal BB, Al-Ubaidi MR, Agarwal N, 1999. Photo-oxidative stress down-modulates the activity of nuclear factor-kappaB via involvement of caspase-1, leading to apoptosis of photoreceptor cells. J. Biol. Chem 274, 3734–3743. 10.1074/jbc.274.6.3734 [DOI] [PubMed] [Google Scholar]
- Luna LG, 1968. Manual of histologic staining methods of the Armed Forces Institute of Pathology Second ed. Blakiston Division, McGraw-Hill, New York. [Google Scholar]
- Mohan N, Monickaraj F, Balasubramanyam M, Rema M, Mohan V, 2012. Imbalanced levels of angiogenic and angiostatic factors in vitreous, plasma and postmortem retinal tissue of patients with proliferative diabetic retinopathy. J. Diabetes Complications 26, 435–441. 10.1016/j.jdiacomp.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Nadal-Nicolas FM, Becerra SP, 2018. Pigment Epithelium-derived Factor Protects Retinal Pigment Epithelial Cells Against Cytotoxicity “In Vitro”. Adv. Exp. Med. Biol 1074, 457–464. 10.1007/978-3-319-75402-4_56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N, Matsuoka M, Imaizumi M, Arichi M, Matsumura M, 2004. Decreased levels of pigment epithelium-derived factor in eyes with neuroretinal dystrophic diseases. Am. J. Ophthalmol 137, 1129–1130. 10.1016/j.ajo.2003.11.080 [DOI] [PubMed] [Google Scholar]
- Ogata N, Nishikawa M, Nishimura T, Mitsuma Y, Matsumura M, 2002. Unbalanced vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor in diabetic retinopathy. Am. J. Ophthalmol 134, 348–353. 10.1016/s0002-9394(02)01568-4 [DOI] [PubMed] [Google Scholar]
- Petersen SV, Valnickova Z, Enghild JJ, 2003. Pigment-epithelium-derived factor (PEDF) occurs at a physiologically relevant concentration in human blood: purification and characterization. Biochem. J 374, 199–206. 10.1042/BJ20030313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polato F, Becerra SP, 2016. Pigment Epithelium-Derived Factor, a Protective Factor for Photoreceptors in Vivo. Adv. Exp. Med. Biol 854, 699–706. 10.1007/978-3-319-17121-0_93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp M, Woo G, Al-Ubaidi MR, Becerra SP, Subramanian P, 2014. Pigment epithelium-derived factor protects cone photoreceptor-derived 661W cells from light damage through Akt activation. Adv. Exp. Med. Biol 801, 813–820. 10.1007/978-1-4614-3209-8_102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch F, Husseini A, Roughley P, Glorieux FH, Moffatt P, 2012. Lack of circulating pigment epithelium-derived factor is a marker of osteogenesis imperfecta type VI. J. Clin. Endocrinol 97, E1550–1556. 10.1210/jc.2012-1827 [DOI] [PubMed] [Google Scholar]
- Samardzija M, Caprara C, Heynen SR, Willcox DeParis S, Meneau I, Traber G, Agca C, von Lintig J, C.. 2014. A mouse model for studying cone photoreceptor pathologies. Invest. Ophthalmol. Vis. Sci 55, 5304–5313. 10.1167/iovs.14-14789 [DOI] [PubMed] [Google Scholar]
- Tsao YP, Ho TC, Chen SL, Cheng HC, 2006. Pigment epithelium-derived factor inhibits oxidative stress-induced cell death by activation of extracellular signal-regulated kinases in cultured retinal pigment epithelial cells. Life Sci 79, 545–550. 10.1016/j.lfs.2006.01.041 [DOI] [PubMed] [Google Scholar]
- Wang J, Iacovelli J, Spencer C, Saint-Geniez M, 2014. Direct effect of sodium iodate on neurosensory retina. Invest. Ophthalmol. Vis. Sci 55, 1941–1953. 10.1167/iovs.13-13075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu X, Ren Y, Liu Y, Han S, Zhao J, Gou X, He Y, 2019. PEDF protects human retinal pigment epithelial cells against oxidative stress via upregulation of UCP2 expression. Mol. Med. Rep 19, 59–74. 10.3892/mmr.2018.9645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Subramanian P, Shen D, Tuo J, Becerra SP, Chan CC, 2013. Pigment epithelium-derived factor reduces apoptosis and pro-inflammatory cytokine gene expression in a murine model of focal retinal degeneration. ASN Neuro 5, e00126 10.1042/AN20130028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YQ, Notario V, Chader GJ, Becerra SP, 1995. Identification of pigment epithelium-derived factor in the interphotoreceptor matrix of bovine eyes. Protein Expr. Purif 6, 447–456. 10.1006/prep.1995.1060 [DOI] [PubMed] [Google Scholar]
- Znoiko SL, Rohrer B, Lu K, Lohr HR, Crouch RK, Ma JX, 2005. Downregulation of cone-specific gene expression and degeneration of cone photoreceptors in the Rpe65−/− mouse at early ages. Invest. Ophthalmol. Vis. Sci 46, 1473–9. 10.1167/iovs.04-0653 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1: NaIO3 treatment induced gliosis in Serpinf1 null (KO) and Wild type (WT) mice: Representative images of eye cryosections of WT (A, B and C) and KO mice (C, D and E) immuno-stained for GFAP (green) and S100B (red). The nuclei of the cells are counter stained with DAPI (blue). GCL = ganglion cell layer, INL=inner nuclear layer, ONL=outer nuclear layer, RPE= retinal pigment epithelium. (n=3)


