Abstract
Background
New influenza vaccine formulations are designed to improve vaccine effectiveness and protect those most vulnerable to infection. High dose trivalent inactivated influenza vaccine (HD-IIV3), licensed for ages ≥65 years, produces greater antibody responses and efficacy in clinical trials, but post-licensure vaccine effectiveness (VE) compared to standard dose (SD-IIV3/4) vaccine remains an open question.
Methods
Using a test-negative, case control design and propensity analyses to adjust for confounding, US Influenza VE Network data from the 2015–2016 through 2018–2019 seasons were analyzed to determine relative VE (rVE) between HD-IIV3 and SD-IIV3/4 among outpatients ≥65 years old presenting with acute respiratory illness. Influenza vaccination status was derived from electronic medical records and immunization registries.
Results
Among 3,861 enrollees, 2,993 (78%) were vaccinated; 1,573 (53%) received HD-IIV3 and 1,420 (47%) received SD-IIV3/4. HD-IIV3 recipients differed from SD-IIV3/4 recipients by race, previous vaccination, number of outpatient visits in the previous year and timing of vaccination, and were balanced in the propensity model except the timing of vaccination. Compared with no vaccination, significant protection against any influenza A was observed from both HD-IIV3 (VE=29%; 95%CI=10%, 44%) and SD-IIV3/4 (VE=24%; 95%CI=5%, 39%); rVE=18% (95%CI=0%, 33%, SD as referent). When stratified by virus type, against A/H1N1, HD-IIV3 VE was 30% (95%CI= −7%, 54%), SD-IIV3/4 VE was 40% (95%CI=10%, 61%), and rVE=−32; (95%CI= −94, 11); Against A/H3N2, HD-IIV3 VE was 31% (95%CI=9%, 47%), SD-IIV3/4 VE was 19% (95%CI= −5%, 37%), and rVE=27; (95% CI=9, 42).
Conclusions
Among adults ≥65 years of age, recipients of standard and high dose influenza vaccines differed significantly in their characteristics. After adjusting for these differences, high dose vaccine offered more protection against A/H3N2 and borderline significant protection against all influenza A requiring outpatient care during the 2015–2018 influenza.
Keywords: High dose influenza vaccine, standard dose influenza vaccine, older adults, vaccine effectiveness
Introduction
It is well established that older adults bear a disproportionate share of the influenza burden each year in the United States. Although their risk of influenza infection is lower than that of children [1, 2], the risks for an outpatient visit, hospitalization, and death among those ≥65 years (older adults) are higher than any other age group [3, 4]. Furthermore, influenza may have long-term effects such as functional decline and decreased independence among those who experience complications, are hospitalized or are frail [5, 6]. While influenza vaccine is the best means of preventing influenza infection, modest vaccine effectiveness (VE), less-than-optimal population vaccination coverage, underlying high-risk conditions, and age-related loss of immune response to influenza vaccine[7, 8] have contributed to influenza morbidity and mortality of older adults. Efforts to better protect older adults have included the development of new vaccine formulations. A high-dose, trivalent inactivated influenza vaccine (HD-IIV3) was licensed in 2009 and introduced in 2010 for use among adults ≥65 years old [9, 10].
There is a growing body of evidence that HD-IIV3 is effective for preventing influenza and influenza-related hospitalizations in older adults and more effective than SD vaccine. In a large randomized controlled trial conducted in the 2011–2012 and 2012–2013 seasons, HD-IIV3 exhibited significant relative immunogenicity and efficacy compared to the standard dose influenza vaccine (SD-IIV) against all influenza, influenza A and the A/H3N2 strain, but not against the A/H1N1 strain or influenza B [11]. Other randomized controlled trials, though few in number, have reported significant relative VE (rVE) for HD-IIV3 against laboratory confirmed influenza. However, the superiority of effectiveness of HD-IIV3 has not been consistently demonstrated over several seasons, against different subtypes of the virus and for population subgroups [12]. Retrospective studies of large administrative data sets including Medicare and Veterans’ Health Administration, have reported greater protection from influenza-related hospitalization [13–16], and laboratory confirmed influenza [17]. A test-negative case control study has reported favorable but non-significant rVE for HD-IIV3 against laboratory confirmed influenza in hospitalized patients [18].
The overall objective of the US Influenza Vaccine Effectiveness (Flu VE) Network is to evaluate VE against influenza infections serious enough to warrant an outpatient visit among those vaccinated versus the unvaccinated, using an observational study design. Depending on the study year, US Flu VE Network data have shown variable VE results for outpatients ≥65 years old across all vaccine types; VE was significant at 42% [19] in 2015–2016 when H1N1pdm09 and B-lineage viruses predominated; VE was nonsignificant at 20% in 2016–2017 [20]; nonsignificant at 17% in 2017–2018 [21]; and nonsignificant at 12% in 2018–2019 [22].
The purpose of this study was to determine the rVE of the HD-IIV3 compared with SD-IIV3/4 in preventing ambulatory medically attended, laboratory confirmed infections from influenza A viruses among persons 65 years of age and older in the 2015–2016 through 2018–2019 influenza seasons in the US Flu VE Network. This study used a test-negative, case control design and propensity score estimates with boosted regression to account for potential differences between HD-IIV3 and SD-IIV3/4 recipients.
Methods
Detailed methods for the test-negative design for the 5 site (Michigan, Pennsylvania, Texas, Washington and Wisconsin) US Flu VE Network study have been previously published [23, 24] and will be briefly described below. Human subjects’ protection was ensured by the Institutional Review Boards of each participating site and the Centers for Disease Control and Prevention (CDC).
Participants
Since the 2011–2012 influenza season, the US Flu VE Network has enrolled participants seeking outpatient medical care for an acute respiratory illness (ARI) with cough. For this analysis, only adults ≥65 years of age who were primarily community-dwelling, were included because the HD-IIV3 is only licensed for this age group. The influenza outbreak period was unique to each site and was defined as the time between the week of illness onset for the first influenza positive case and the week of illness onset for the last influenza-positive case enrolled. After confirmation of local influenza circulation each year, eligible patients presenting for outpatient medical care with cough, and symptom onset ≤7 days prior were enrolled. Eligibility criteria included date of birth before August 1, 1950, August 1, 1951, August 1, 1952, and August 1, 1953 for the 2015–2016, 2016–2017, 2017–2018 and 2018–2019 seasons, respectively; not taking influenza antiviral medication in the previous 7 days and not previously enrolled within 14 days. It is possible that enrollees participated in more than one influenza season, but this information was not tracked.
After obtaining informed consent, participants were interviewed to collect demographic data, general and current health status, symptom and illness severity information and influenza vaccination status. Participants provided nasal and throat swabs for confirmation of influenza using real time reverse transcription polymerase chain reaction (RT-PCR) assays. Presence of high-risk medical conditions, as defined by a medical record of a pre-specified (based on ACIP’s high-risk conditions) International Classification of Diseases, 10th Edition, Clinical Modification [25] code assigned to a medical encounter in the preceding 12 months was extracted from electronic medical records (EMR).
Northern Hemisphere Influenza Vaccine Composition
Recommended influenza A vaccine strains during the study period included A/California/H1N1)pdm09 (2015–2016 and 2016–2017), A/Michigan/45/2015pdm09 (2017–2018 and 2018–2019), A/Switzerland/H3N2 (2015–2016), A/HongKong/H3N2 (2016–2017 and 2017–2018) and A/Singapore/H3N2 (2018–2019.
Vaccination Status
Electronic immunization records (EIR) that included electronic medical records and state immunization registries were used to determine vaccination status and type of vaccine received.
Statistical Analysis
Participants excluded from the analytic dataset were those with inconclusive and unrepeatable RT-PCR results, influenza test-negative controls with illness onset dates outside each site’s influenza outbreak period, vaccination 0–13 days prior to illness onset, and vaccinated but of unknown type.
The primary outcome for this study was confirmed influenza infection for influenza A only, because HD did not contain both B lineages during the timeframe of this study. The enrollment period for each season was the time between the first and last positive influenza case in each site. Therefore, controls enrolled outside these dates were not included in analyses. Descriptive statistics for each group (HD-IIV3 versus SD-IIV3/4) were summarized as means and standard deviations for continuous or frequencies and percentages for categorical variables. Differences between the vaccination groups for baseline characteristics were compared with t-test (or nonparametric equivalent, Wilcoxon rank sum test) for continuous characteristics and with a Chi-square test (or Fisher’s exact test) for categorical characteristics.
The exposure variable of interest was vaccine received (HD-IIV3, SD-IIV3/4 or not vaccinated). Logistic regression models were used to calculate odds ratios (ORs) comparing influenza-positive and influenza-negative subjects; VE was estimated as 100% x [1 - OR]). For calculating effectiveness of SD-IIV3/4 and HD-IIV3 compared with no vaccination, models were adjusted a priori for age, sex, race/Hispanic ethnicity, network site, season, presence of one or more high-risk conditions (versus none), days from illness onset to specimen collection (0–2 days, 3–4 days, 5–7 days) and calendar time in 2-week intervals.
To calculate relative VE of SD-IIV3/4 and HD-IIV3, four regression models were conducted using SD-IIV3/4 as the reference: 1) adjusted rVE using a priori variables of age, race/Hispanic ethnicity, sex, site, season, interval from onset to enrollment (0–2 days, 3–4 days, 5–7 days), any prior high risk condition, and calendar time in 2-week intervals; 2) adjusted rVE using a priori variables and instrumental variables of vaccination in the previous season and timing of vaccination (week in the season when vaccinated from July 1); 3) adjusted rVE using propensity weights that were based on model 2 variables and 3-way interactions, and 4) adjusted rVE using inverse probability propensity weights. To determine the instrumental variables, measures of risk that were potentially related to receipt of HD-IIV3 such as, number of high-risk conditions, health care utilization – inpatient and outpatient in the 12 months before enrollment and health care utilization – hospitalization within 30 days after enrollment, were compared between the two vaccine groups using chi-square tests. Demographic and instrumental variables were tested as possible effect modifiers and confounders (Supplemental Tables 1 and 2, respectively). The variables bi-week and time since vaccination were highly correlated. Bi-week was used in the initial VE analyses but was eliminated as an instrumental variable from the propensity analysis in favor of time since vaccination.
Propensity score analysis was used to address the selection bias that potentially confounds the effect of vaccination status. A propensity score weighted logistic regression with influenza status as the dependent variable was used to estimate the effect of vaccine type (HD-IIV3 versus SD-IIV3/4) on outcome. Balance tables and plots were used to assess the quality of the propensity scores and to evaluate common support. A value under 0.25 was indicative of good balance [26–29] (Supplemental Table 3).
All analyses were two-sided and the alpha level was set to 0.05. VE and rVE point estimates including overall and subgroup analyses stratified by season and virus type, were considered statistically significant when confidence intervals did not overlap. All analyses were conducted using SAS, version 9.4 statistical software (SAS Institute Inc., Cary, NC).
Results
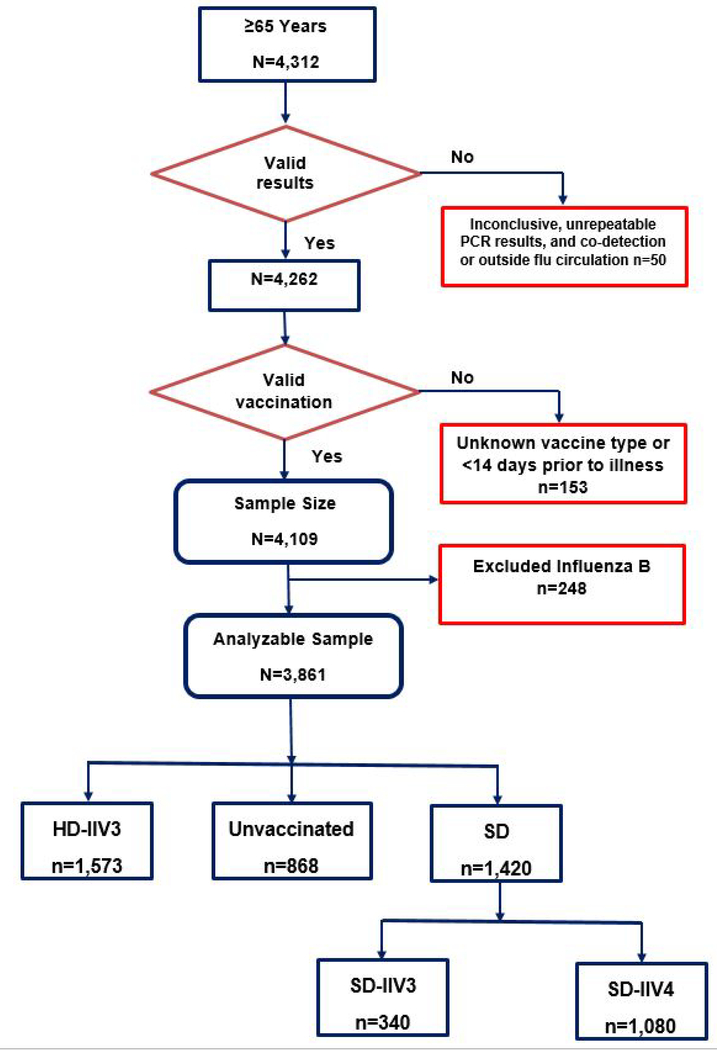
Of the 4,312 enrollees ≥65 years at enrollment, 50 were excluded because they were enrolled outside influenza virus circulation period or did not have conclusive PCR results and 153 were excluded because they were vaccinated <14 days before illness onset (Figure 1). Because this study focused on influenza A viruses only, 248 participants with influenza B virus infection were excluded from analysis, leaving 3,861 enrollees, of whom 1,573 (40%) received HD-IIV3, 1,420 (37%) received SD-IIV3/IIV4, and 868 (22%) were unvaccinated.
Figure 1:
Flow Chart
Table 1 shows the demographic characteristics of the enrollees divided into unvaccinated, HD-IIV3 recipients and SD-IIV3/4 recipients. Compared with enrollees vaccinated with SD-IIV3/4, those who were vaccinated with HD-IIV3 were less likely to be white (84.9% vs 90.1%; P<0.001), less likely to be hospitalized post enrollment (2.2% vs 3.9%; P=0.004), more likely to wait longer to seek medical care after onset of symptoms (21% versus 27% enrolled 0–2 days after onset; P<0.001), more likely to have been vaccinated in the prior season (89% versus 81%; P<0.001), and to have had more outpatient visits in the previous year (6.0 ± 7.0 versus 5.5±6.6; P=0.020). Other significant differences across vaccination groups included days between vaccination and illness onset (120 for HD-IIV3 versus 114 for SD-IIV3/4; P<0.001) and week of vaccination with HD-IIV3 recipients receiving vaccine 2 weeks earlier than SD-IIV3/4 recipients (P<0.001).
Table 1.
Baseline characteristics by vaccination with trivalent/quadrivalent standard dose influenza vaccine (SD-IIV3/4) or trivalent high dose influenza vaccine (HD-IIV3)
| Vaccination Status | |||
|---|---|---|---|
| Characteristics | Unvaccinated N=868 | SD-IIV3/4 n=1,420 | HD-IIV3 n=1,573 |
| Age on Sept.1, years, mean (SD) | 71.8 (6.3) | 73.3 (7) | 73.6 (6.9) |
| Female sex, n (%) | 535 (61.6) | 872 (61.4) | 980 (62.3) |
| Race, n (%) | |||
| White, non-Hispanic | 730 (84.6) | 1272 (90.1) | 1332 (84.9) |
| Black, non-Hispanic | 47 (3.9) | 36 (2.6) | 69 (4.4) |
| Other race, non-Hispanic | 52 (6.0) | 54 (3.8) | 130 (8.3) |
| Hispanic, any race | 34 (3.9) | 49 (3.5) | 37 (2.4) |
| Non-Smoker, n (%), ref.=ever smoked | 563 (91.8) | 1353 (95.0) | 1514 (96.7) |
| Season, n (%) | |||
| 2015–2016 | 186 (21.4) | 339 (23.9) | 260 (16.5) |
| 2016–2017 | 215 (24.8) | 308 (21.7) | 376 (23.9) |
| 2017–2018 | 214 (24.6) | 363 (25.6) | 387 (24.6) |
| 2018–2019 | 253 (29.2) | 410 (28.6) | 550 (35.0) |
| Site, n (%) | |||
| Michigan | |||
| 2015–2016 | 13 (11.5) | 30 (21.4) | 30 (10.2) |
| 2016–2017 | 24 (21.2) | 27 (19.3) | 44 (14.9) |
| 2017–2018 | 31 (27.4) | 31 (22.1) | 80 (27.1) |
| 2018–2019 | 45 (39.8) | 52 (37.2) | 141 (47.8) |
| Pennsylvania | |||
| 2015–2016 | 40 (24.8) | 46 (21.5) | 38 (21.2) |
| 2016–2017 | 35 (21.7) | 35 (16.4) | 59 (33.0) |
| 2017–2018 | 40 (24.8) | 30 (14.0) | 62 (34.6) |
| 2018–2019 | 46 (28.6) | 103 (48.1) | 20 (11.2) |
| Texas | |||
| 2015–2016 | 30 (15.7) | 62 (25.5) | 20 (18.4) |
| 2016–2017 | 45 (23.6) | 62 (25.5) | 26 (23.8) |
| 2017–2018 | 51 (26.7) | 82 (33.8) | 16 (14.7) |
| 2018–2019 | 65 (34.0) | 37 (15.2) | 47 (43.1) |
| Washington | |||
| 2015–2016 | 67 (30.7) | 88 (32.7) | 165 (18.4) |
| 2016–2017 | 52 (23.9) | 47 (17.5) | 235 (26.2) |
| 2017–2018 | 36 (16.5) | 53 (19.7) | 202 (22.5) |
| 2018–2019 | 63 (28.9) | 81 (30.1) | 295 (32.9) |
| Wisconsin | |||
| 2015–2016 | 36 (19.5) | 113 (20.4) | 7 (7.5) |
| 2016–2017 | 59 (31.9) | 137 (24.7) | 12 (12.9) |
| 2017–2018 | 56 (30.3) | 167 (30.2) | 27 (29.0) |
| 2018–2019 | 34 (18.4) | 137 (24.7) | 47 (50.6) |
| Interval from onset to enrollment, n (%) | |||
| 0–2 days | 212 (24.4) | 377 (26.6) | 328 (20.9) |
| 3–4 days | 316 (36.4) | 530 (37.3) | 568 (36.1) |
| 5–7 days | 340 (39.2) | 513 (36.1) | 677 (43.1) |
| One or more high-risk condition, n (%) | 672 (77.4) | 1250 (88.0) | 1371 (87.2) |
| Received previous year’s influenza vaccine, n (%) | 240 (27.6) | 1147 (80.8) | 1395 (88.7) |
| Hospitalized within last year, n (%)2 | 641 (77.0) | 1157 (88.5) | 1368 (87.4) |
| Hospitalization within 30 days after enrollment, n (%) | 31 (3.6) | 56 (3.9) | 34 (2.2) |
| Outpatient visits within last year, mean (SD) 3 | 3.9 (5.6) | 5.5 (6.6) | 6.0 (7.0) |
| High-risk conditions, mean (SD) | 2.3 (2.0) | 2.9 (2.0) | 2.8 (2.0) |
| Days from vaccination to enrollment | --- | 114 (43.1) | 120 (41.3) |
| Timing of vaccination (weeks), mean (SD) 4 | --- | 16.2 (4.1) | 13.0 (3.1) |
P value is for the comparison of SD-IIV3/4 and HD-IIV3
Prior health care utilization
Any outpatient medical encounter in past 12 months based on high risk codes
Timing of vaccination: week in the season when vaccinated beginning July 1.
The distribution of SD-IIV3/4 and HD-IIV3 differed across seasons and differed by site as shown in Table 1, with the proportion of HD-IIV3 vaccine increasing each season overall. Over all seasons, the Wisconsin site had the lowest use of HD-IIV3 and the Washington site had the highest use of HD-IIV3. The use of HD-IIV3 increased steadily over time in Michigan, but use varied over four seasons in the other sites.
Unadjusted and adjusted vaccine effectiveness estimates for influenza A are shown in Table 2. Compared with unvaccinated enrollees, adjusted VE for SD-IIV3/4 over all four seasons was significant against any influenza A virus (24%; 95%CI=5%, 39%); and against influenza A/H1N1 (40%; 95% CI=10%, 61%) and insignificant against A/H3N2 (19%; 95%CI=−5%, 37%). Adjusted HD-IIV3 VE was significant against any influenza A over all four seasons (29%; 95%CI=10%, 44%) and against A/H3N2 viruses (31%; 95%CI=9%, 47%), while VE against A/H1N1 viruses was not significant (30%; 95%CI=−7, 54%).
Table 2.
Vaccine effectiveness (VE) for trivalent/quadrivalent standard dose influenza vaccine (SD-IIV3/4) and for trivalent high dose influenza vaccine (HD-IIV3) for four seasons (2015–2018) against any influenza A, A/H1N1 and A/H3N2 viruses.
| Outcome | SD-IIV3/4 (N = 1420) versus unvaccinated (N = 868) |
HD-IIV3 (N = 1573) versus unvaccinated (N = 868) |
||
|---|---|---|---|---|
| Unadjusted % VE (95% CI) | Adjusteda% VE (95% CI) | Unadjusted % VE (95% CI) | Adjusteda % VE (95% CI) | |
| 2015–2016 | ||||
| All influenza A | 49 (7, 72) | 53 (11, 75) | 56 (14, 77) | 52 (−7, 78) |
| 2016–2017 | ||||
| All influenza A | 14 (−27, 42) | 25 (−14, 51) | 16 (−23, 42) | 29 (−12, 55) |
| Influenza A/H3N2 | 17 (−24, 44) | 27 (−11, 53) | 21 (−15, 46) | 32 (−7, 57) |
| 2017–2018 | ||||
| All influenza A | 5 (−38, 35) | 5 (−45, 38) | 27 (−6, 50) | 13 (−36, 45) |
| Influenza A/H3N2 | 5 (−39, 36) | 2 (−52, 37) | 31 (−2, 54) | 13 (−40, 46) |
| 2018–2019 | ||||
| All influenza A | 10 (−31, 39) | 26 (−14, 52) | 26 (−6, 49) | 29 (−11, 54) |
| Influenza A/H1N1 | 19 (−35, 51) | 47 (3, 71) | 31 (−12, 58) | 26 (−35, 59) |
| Influenza A/H3N2 | 1 (−64, 40) | 5 (−67, 46) | 21 (−29, 52) | 27 (−31, 59) |
| 2015–2018 | ||||
| All influenza A | 17 (−2, 32) | 24 (5, 39)b | 25 (8, 38) | 29 (10, 44)b |
| Influenza A/H1N1 | 31 (−1, 52) | 40 (10, 61)c | 30 (−1, 51) | 30 (−7, 54)b |
| Influenza A/H3N2 | 14 (−8, 31) | 19 (−5, 37)b | 25 (6, 40) | 31 (9, 47)b |
Adjusted for a priori variables age, race/ethnicity, sex, clinical site, interval from onset to enrollment, any prior high-risk condition, and bi-week (indicator variable of 2-week blocks of calendar time).
Adjusted for a priori variables age, race/ethnicity, sex, clinical site, season, interval from onset to enrollment, any prior high-risk condition, and bi-week (indicator variable of 2-week blocks of calendar time).
Season included as continuous variable, because there was no A/H1N1 in 2016–17 (There was no change in VE, when season was excluded from the model).
Table 3 shows the rVE comparing HD-IIV3 with SD-IIV3/4 as the reference vaccine. The first two columns show the number and percent of cases and controls among those who were vaccinated with HD-IIV3 vaccine. The next four columns represent the progression of the comparisons from unadjusted to adjusted rVE calculated using propensity weights and interaction terms. The adjusted rVE in four modeling strategies during the four seasons were not significant for any of the individual seasons, with wide confidence intervals suggesting insufficient sample size. When data from all four seasons were combined, HD-IIV3 was significantly more effective against all influenza A (rVE=18; 95% CI=0, 33) and against A/H3N2 (rVE=27; 95% CI=9, 42), but was not different from SD-IIV3/4 for A/H1N1 (rVE=−32; 95%CI=−94, 11). In all sensitivity analyses that excluded one site at a time to assess potential site-specific selection biases, the rVE of HD-IIV3 was not significant (Supplemental Tables 4 and 5).
Table 3.
Relative vaccine effectiveness (rVE) of trivalent high dose influenza vaccine compared with trivalent/quadrivalent standard dose influenza vaccine.
| MD-IIV3 vaccinees |
Adjusted using a priori variablesa % rVE (95% CI) | Adjusted using a priori and instrumental variablesb % rVE (95% CI) | Adjusted using propensity score weightsc % rVE (95% CI) | Adjusted using Inverse probability weightsc % rVE (95% CI) | ||
|---|---|---|---|---|---|---|
| Cases N (%) | Controls N (%) | |||||
| 2015–2016 | ||||||
| All influenza A | 16 (40) | 241 (43) | 9 (−92, 57) | 3 (−105, 54) | −9 (−158, 54) | −33 (−187, 39) |
| 2016–2017 | ||||||
| All influenza A | 93 (54) | 282 (55) | 8 (−46, 42) | −2 (−65, 37) | 2 (−69, 43) | 11 (−31, 39) |
| Influenza A/H3N2 | 87 (53) | 282 (55) | 10 (−44, 44) | 1 (−61, 39) | 5 (−65, 45) | 15 (−26, 43) |
| 2017–2018 | ||||||
| All influenza A | 90 (47) | 297 (53) | 5 (−45, 38) | 8 (−42, 41) | 6 (−55, 43) | 11 (−33, 40) |
| Influenza A/H3N2 | 77 (46) | 297 (53) | 9 (−41, 42) | 13 (−37, 45) | 7 (−56, 45) | 12 (−34, 42) |
| 2018–2019 | ||||||
| All influenza A | 97 (53) | 452 (58) | −2 (−52, 32) | 13 (−37, 42) | 19 (−27, 48) | 15 (−27, 43) |
| Influenza A/H1N1 | 46 (54) | 452 (58) | −32 (−134, 25) | −23 (−122, 32) | −5 (−98, 44) | −4 (−80, 40) |
| Influenza A/H3N2 | 51 (52) | 452 (58) | 14 (−43, 48) | 30 (−19, 59) | 34 (−17, 63) | 28 (−21, 58) |
| All seasons 2015–2018 | ||||||
| All influenza A | 297 (51) | 1276 (53) | 7 (−16, 26) | 9 (−14, 28) | 10 (−15, 30) | 18 (0, 33) |
| Influenza A/H1N1 | 73 (53) | 1276 (53) | −19 (−80, 21) | −19 (−82, 22) | −14 (−82, 29) | −32 (−94, 11) |
| Influenza A/H3N2 | 218 (50) | 1276 (53) | 14 (−11, 34) | 17 (−8, 36) | 16 (−11, 37) | 27 (9, 42) |
A priori variables = age, race/ethnicity, sex, clinical site, season, interval from onset to enrollment (0–2 days, 3–4 days, 5–7 days), any prior high-risk condition, and bi-week (indicator variable of 2-week blocks of calendar time).
A priori variables plus instrumental variables prior vaccination status, and timing of vaccination. (Excluded a priori variable bi-week because bi-week and timing of vaccination were highly correlated. The relative VEs remain the same when bi-week is included).
Propensity score weights were calculated using a priori variables, instrumental variables and the 120 three way interactions (10 variables, including vaccination status, with three-way interactions). Bi-week is excluded from propensity score modeling.
Discussion
This study, based on US Flu VE Network data, examined and compared vaccine effectiveness of HD-IIV3 and SD-IIV3/4 among individuals ≥65 years old. Over four seasons combined, SD-IIV3/4 influenza vaccine was effective against all influenza A and against influenza A/H1N1 but was not effective against influenza A/H3N2. HD-IIV3 was also effective against all influenza A and against influenza A/H3N2, when compared with unvaccinated individuals, but not against influenza A/H1N1, consistent with previous research [11]. Using inverse probability weights, the rVE for HD-IIV3 compared with SD-IIV3/4 was borderline significant against all influenza A and significant against A/H3N2, indicating that HD-IIV3 sometimes outperforms SD-IIV3/4 for prevention of ambulatory medically attended influenza.
Using Medicare administrative data sets, rVE for HD-IIV3 in 2012–2013 against probable influenza infection was reported to be 22% [13]. A study using Veterans’ Health Administration data reported that rVE for HD-IIV3 in 2015–2016 against influenza- or pneumococcal-related outpatient visits was 14% and against laboratory-confirmed influenza was 38% [17]. An analysis from the 2010–2011 season [30] used propensity analyses to account for the potential differences between those who received HD-IIV3 and those who received SD-IIV to assess rVE for reducing risk of influenza- or pneumonia-related hospitalization. HD-IIV3 was not more effective than SD-IIV, except in those >85 years old.
The most recent randomized controlled trial comparing high dose and standard dose influenza vaccine found significantly higher relative efficacy for high dose vaccine against all influenza, influenza A and the A/H3N2 strain but was conducted three years before the current study [11]. Influenza epidemiology has changed since then. For example, circulating virus strains continue to change with a major change noted in 2014–2015 [31], vaccine effectiveness has varied by season [19, 20, 22, 32], vaccine manufacturing processes have changed with the introduction of cell-derived and adjuvanted vaccines, and the possible effects of repeated vaccination with the high dose vaccine may affect its subsequent effectiveness [33].
A difference between randomized controlled trials and observational studies is the ability in a randomized trial to match vaccine recipients on demographic and medical/health characteristics. For example, in this study we note the demographic and site differences found between SD-IIV3/4 recipients and HD-IIV3 recipients, including race, enrollment site, and enrollment season. Our data indicate that HD-IIV3 recipients were more often non-white, had more outpatient visits in the preceding year, and were vaccinated earlier in the season. Given the significant price differential of HD-IIV3, which ranged from approximately 2 to 2.75 times the cost of SD-IIV during 2015–2018 [34], selective use of HD-IIV3 for more vulnerable older adults might be expected. These host factors might contribute to differences in VE. Washington state policy for at least two of the study years preferentially recommended high dose vaccine use among sicker individuals, due to limited supplies (personal communication, M Jackson, 2019). Higher cost of HD-IIV3 may also help to explain its low use in other sites, leading to preferential use among the frailest individuals. Finally, use of HD-IIV3 has generally increased over time. More widespread use of the vaccine may eliminate unaccounted for differences among HD-IIV3 and SD-IIV3/4 recipients. However, new influenza vaccine products continue to become licensed and enter the market. A broader array of vaccine products may limit the use of any given vaccine type, thereby reducing the number of recipients. In such case, it may not be possible to use test-negative, case control studies to identify improved vaccine effectiveness of one type of vaccine for a population subgroup despite its effectiveness compared with no vaccination. Additional strategies such as cluster-randomization [35–37] in target groups warrant consideration, particularly when clinical equipoise exists on the comparative protective benefits of emerging vaccine types.
One 2017 review and meta-analysis concluded that there is some, though limited evidence of HD vaccine’s superiority over standard dose vaccine in ambulatory adults over age 65 years, but noted that additional research is needed to broaden our understanding of its relative benefit for population subgroups [12]. This study adds to that body of literature.
Limitations
The validity of observational vaccine effectiveness studies depends upon accurate classification of vaccination status and influenza infection. To address this issue, this study limited vaccination reports to those confirmed in electronic immunization records. Influenza infection was determined through systematic testing by highly specific molecular assays, and participants were enrolled within 7 days of illness onset when viral shedding was highest, decreasing the likelihood of false-negative results. Wide confidence intervals around low VE estimates occur when sample sizes are small. Because of small numbers of SD-IIV3 and SD-IIV4, these vaccine types were combined for analyses. Larger sample sizes may have provided sufficient power to detect significant relative vaccine effectiveness across vaccine types. As with any observational study, we cannot rule out unmeasured confounding as an explanation for our findings despite the use of propensity analyses to adjust for this possibility. However, it is possible that there remained undetected selection biases for which propensity analyses were unable to adjust. In addition, we did not add degree of match to the analyses. Finally, these estimates are limited to the prevention of ambulatory medical visits, rather than more severe illness outcomes, such as hospitalization or death for which high dose vaccine may provide a significant advantage.
Conclusions
Among older adults, we found that SD-IIV3/4 was effective against influenza illness leading to outpatient visits due to any influenza A and influenza A/H1N1, while HD-IIV3 was effective against any influenza A and influenza A/H3N2, compared with unvaccinated individuals. HD-IIV3 was significantly more effective than SD-IIV3/4 against A/H3N2, while the significance for relative VE of HD against all influenza A was borderline. Further research in observational studies of specific vaccine products with larger sample sizes is needed.
Supplementary Material
Highlights.
Relative VE of high dose to standard dose influenza vaccine was measured in an observational study.
High dose and standard dose recipients differed on several demographic and clinical factors.
Both high and standard dose vaccines were effective against influenza compared to no vaccination.
High dose vaccine provided better protection against influenza A requiring outpatient care.
Acknowledgements
Mary Kylberg, Jeremy Ray, Deborah Price, Natalie Settele, Jennifer Thomas, Jaime Walkowiak, Madhava Beeram and Alejandro Arroliga from Baylor Scott & White; Philip Iozzi, Leonard Urbanski, Edward Garofolo and David Figucia from University of Pittsburgh
Source of Funding: This investigation was supported at the University of Pittsburgh by the Centers for Disease Control and Prevention through a cooperative agreement (grant U01 IP000467) at Kaiser Permanente Washington Health Research Institute (5U01 IP001037), at Baylor Scott & White Health (1-5U01 IP000473), at Marshfield Clinic (U01IP001038), and at the University of Michigan (1U01P000474). It is subject to the CDC’s Open Access Policy. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. In Pittsburgh, the CTSI infrastructure was also supported by the National Institutes of Health (grant 1UL1 TR001857). This study does not have a clinical trial number because it is an observational study.
Potential conflicts of interest: RKZ has/had a research grant from Sanofi Pasteur, Inc. RKZ and MPN have/had research grant funding from Merck & Co, Inc. MLG has received institutional research grant from AstraZeneca-MedImmune. MLJ reports research funding from Sanofi Pasteur, unrelated to the present work. ASM reports consulting fees from Sanofi, Seqirus and Roche. ETM reports consulting fees from Pfizer. HQM reports research funding from Seqirus unrelated to the present work. The other authors have no conflicts to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Long CE, Hall CB, Cunningham CK, Weiner LB, Alger KP, Gouveia M, Colella CB, Schnabel KC, Barker WH, Influenza surveillance in community-dwelling elderly compared with children, Archives of family medicine 6(5) (1997) 459. [DOI] [PubMed] [Google Scholar]
- [2].Monto AS, Koopman JS, LONGINI JR IM, Tecumseh study of illness. XIII. Influenza infection and disease, 1976–1981, American journal of epidemiology 121(6) (1985) 811–822. [DOI] [PubMed] [Google Scholar]
- [3].Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB, The annual impact of seasonal influenza in the US: measuring disease burden and costs, Vaccine 25(27) (2007) 5086–96. [DOI] [PubMed] [Google Scholar]
- [4].Nichol KL, Influenza Vaccination in the Elderly, Drugs Aging 22(6) (2005) 495–515. [DOI] [PubMed] [Google Scholar]
- [5].Andrew MK, Bowles SK, Pawelec G, Haynes L, Kuchel GA, McNeil SA, McElhaney JE, Influenza vaccination in older adults: recent innovations and practical applications, Drugs Aging 36(1) (2019) 29–37. [DOI] [PubMed] [Google Scholar]
- [6].Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, Burant CJ, Landefeld CS, Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age, Journal of the American Geriatrics Society 51(4) (2003) 451–458. [DOI] [PubMed] [Google Scholar]
- [7].Song JY, Cheong HJ, Hwang IS, Choi WS, Jo YM, Park DW, Cho GJ, Hwang TG, Kim WJ, Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence, Vaccine 28(23) (2010) 3929–3935. [DOI] [PubMed] [Google Scholar]
- [8].Haq K, McElhaney JE, Immunosenescence: influenza vaccination and the elderly, Current opinion in immunology 29 (2014) 38–42. [DOI] [PubMed] [Google Scholar]
- [9].Centers for Disease Control and Prevention, Licensure of a high-dose inactivated influenza vaccine for persons aged >or=65 years (Fluzone High-Dose) and guidance for use - United States, 2010, MMWR. Morbidity and mortality weekly report 59(16) (2010) 485–6. [PubMed] [Google Scholar]
- [10].Centers for Disease Control and Prevention, Licensure of a High-Dose Inactivated Influenza Vaccine for Persons Aged ≥65 Years (Fluzone High-Dose) and Guidance for Use --- United States, 2010, Morbidity and Mortality Weekly Report (MMWR) 59(16) (2010) 485–486. [PubMed] [Google Scholar]
- [11].DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V, Efficacy of high-dose versus standard-dose influenza vaccine in older adults, New England Journal of Medicine 371(7) (2014) 635–645. [DOI] [PubMed] [Google Scholar]
- [12].Wilkinson K, Wei Y, Szwajcer A, Rabbani R, Zarychanski R, Abou-Setta AM, Mahmud SM, Efficacy and safety of high-dose influenza vaccine in elderly adults: a systematic review and meta-analysis, Vaccine 35(21) (2017) 2775–2780. [DOI] [PubMed] [Google Scholar]
- [13].Izurieta HS, Thadani N, Shay DK, Lu Y, Maurer A, Foppa IM, Franks R, Pratt D, Forshee RA, MaCurdy T, Comparative effectiveness of high-dose versus standard-dose influenza vaccines in US residents aged 65 years and older from 2012 to 2013 using Medicare data: a retrospective cohort analysis, The Lancet Infectious diseases 15(3) (2015) 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shay DK, Chillarige Y, Kelman J, Forshee RA, Foppa IM, Wernecke M, Lu Y, Ferdinands JM, Iyengar A, Fry AM, Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014, The Journal of infectious diseases 215(4) (2017) 510–517. [DOI] [PubMed] [Google Scholar]
- [15].Izurieta HS, Chillarige Y, Kelman J, Wei Y, Lu Y, Xu W, Lu M, Pratt D, Chu S, Wernecke M, Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017–2018, The Journal of infectious diseases 220(8) (2019) 1255–1264. [DOI] [PubMed] [Google Scholar]
- [16].Izurieta HS, Chillarige Y, Kelman J, Wei Y, Lu Y, Xu W, Lu M, Pratt D, Wernecke M, MaCurdy T, Relative Effectiveness of Influenza Vaccines Among the United States Elderly, 2018–2019, The Journal of infectious diseases (2020). [DOI] [PubMed] [Google Scholar]
- [17].Young-Xu Y, Van Aalst R, Mahmud SM, Rothman KJ, Snider JT, Westreich D, Mor V, Gravenstein S, Lee JK, Thommes EW, Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients, The Journal of infectious diseases 217(11) (2018) 1718–1727. [DOI] [PubMed] [Google Scholar]
- [18].Doyle JD, Beacham L, Martin ET, Talbot HK, Monto A, Gaglani M, Middleton DB, Silveira FP, Zimmerman RK, Alyanak E, Relative and absolute effectiveness of high-dose and standard-dose influenza vaccine against influenza-related hospitalization among older adults—United States, 2015–2017, Clinical Infectious Diseases (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jackson ML, Chung JR, Jackson LA, Phillips CH, Benoit J, Monto AS, Martin ET, Belongia EA, McLean HQ, Gaglani M, Murthy K, Zimmerman RK, Nowalk MP, Fry AM, F. B, Influenza Vaccine Effectiveness in the United States — 2015/16 Season, New England Journal of Medicine 377(6) (2017) 534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Flannery B, Chung JR, Monto AS, Martin ET, Belongia EA, McLean HQ, Gaglani M, Murthy K, Zimmerman RK, Nowalk MP, Influenza vaccine effectiveness in the United States during the 2016–2017 season, Clinical Infectious Diseases 68(11) (2018) 1798–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rolfes MA, Flannery B, Chung JR, O’Halloran A, Garg S, Belongia EA, Gaglani M, Zimmerman RK, Jackson ML, Monto AS, Effects of influenza vaccination in the United States during the 2017–2018 influenza season, Clinical Infectious Diseases (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Flannery B, Kondor RJG, Chung JR, Gaglani M, Reis M, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Monto AS, Spread of antigenically drifted influenza A (H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season, The Journal of infectious diseases 221(1) (2020) 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ohmit SE, Thompson MG, Petrie JG, Thaker SN, Jackson ML, Belongia EA, Zimmerman RK, Gaglani M, Lamerato L, Spencer SM, Jackson L, Meece JK, Nowalk MP, Song J, Zervos M, Cheng P, Rinaldo CR, Clipper L, Shay DK, Piedra P, Monto AS, Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates, Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 58(3) (2014) 319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].McLean HQ, Thompson MG, Sundaram ME, Kieke BA, Gaglani M, Murthy K, Piedra PA, Zimmerman RK, Nowalk MP, Raviotta JM, Jackson ML, Jackson L, Ohmit SE, Petrie JG, Monto AS, Meece JK, Thaker SN, Clippard JR, Spencer SM, Fry AM, Belongia EA, Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type, The Journal of infectious diseases 211(10) (2015) 1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].International Classification of Diseases, Clinical Modification. <http://www.cdc.gov/nchs/icd/icd9cm.htm>, 2015. (accessed February 17.2016).
- [26].Cochran WG, The effectiveness of adjustment by subclassification in removing bias in observational studies, Biometrics (1968) 295–313. [PubMed] [Google Scholar]
- [27].Ho DE, Imai K, King G, Stuart EA, Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference, Political analysis 15(3) (2007) 199–236. [Google Scholar]
- [28].Imai K, King G, Stuart EA, Misunderstandings between experimentalists and observationalists about causal inference, Journal of the royal statistical society: series A (statistics in society) 171(2) (2008) 481–502. [Google Scholar]
- [29].McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF, A tutorial on propensity score estimation for multiple treatments using generalized boosted models, Statistics in medicine 32(19) (2013) 3388–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Richardson DM, Medvedeva EL, Roberts CB, Linkin DR, Comparative effectiveness of high-dose versus standard-dose influenza vaccination in community-dwelling veterans, Clinical infectious diseases 61(2) (2015) 171–176. [DOI] [PubMed] [Google Scholar]
- [31].Flannery B, Zimmerman RK, Gubareva LV, Garten RJ, Chung JR, Nowalk MP, Jackson ML, Jackson LA, Monto AS, Ohmit SE, Belongia EA, McLean HQ, Gaglani M, Piedra PA, Mishin VP, Chesnokov AP, Spencer S, Thaker SN, Barnes JR, Foust A, Sessions W, Xu X, Katz J, Fry AM, Enhanced genetic characterization of influenza A(H3N2) viruses and vaccine effectiveness by genetic group, 2014–2015, The Journal of infectious diseases (2016) In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rolfes MA, Flannery B, Chung JR, O’Halloran A, Garg S, Belongia EA, Gaglani M, Zimmerman RK, Jackson ML, Monto AS, Effects of influenza vaccination in the United States during the 2017–2018 influenza season, Clinical Infectious Diseases 69(11) (2019) 1845–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Skowronski DM, Chambers C, Gilca R, De Serres G, Repeat influenza vaccination and high-dose efficacy, Clinical Infectious Diseases 63(9) (2016) 1265–1266. [DOI] [PubMed] [Google Scholar]
- [34].C.f.M.M. Services, ASP Pricing File. <https://www.cms.gov/apps/ama/license.asp?file=/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/downloads/2015-October-ASP-Pricing-File.zip >, 2015).
- [35].Jewell NP, Dufault S, Cutcher Z, Simmons CP, Anders KL, Analysis of cluster-randomized test-negative designs: cluster-level methods, Biostatistics 20(2) (2019) 332–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hayes R, Moulton L, Introduction. Cluster Randomised Trials, Interdisciplinary Statistics, Chapman and Hall/CRC, Boca Raton, FL, 2009. [Google Scholar]
- [37].Gravenstein S, Davidson HE, Han LF, Ogarek JA, Dahal R, Gozalo PL, Taljaard M, Mor V, Feasibility of a cluster-randomized influenza vaccination trial in US nursing homes: Lessons learned, Human vaccines & immunotherapeutics 14(3) (2018) 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.