Abstract
Erysipelothrix rhusiopathiae is a zoonotic gram positive coccobacillus. It is rarely found in humans as an occupational pathogen that mainly infects animal handlers. There are three forms of human infection: localized erysipeloid, diffuse cutaneous form and lastly, bacteremia that could progress to infective endocarditis. We present a case of a 59-year-old male who was found to have E. rhusiopathiae bacteremia that was diagnosed as aortic valve endocarditis with severe aortic regurgitation. The patient was treated with ampicillin-sulbactam then transitioned to six weeks of intravenous ampicillin. This report summarizes a rare organism that causes a serious human infection and discusses its epidemiology, clinical presentation, diagnosis and treatment options.
Keywords: Erysipelothrix, Endocarditis, Bacteremia, Zoonotic, Aortic regurgitation
Introduction
Erysipelothrix rhusiopathiae is a zoonotic pathogen that can infect humans, mostly in animal handlers. It commonly causes superficial skin infection but can progress to bacteremia and endocarditis. E. rhusiopathiae endocarditis has a high mortality therefore prompt diagnosis and treatment is paramount. A 59-year-old male presented to our hospital with a finding of positive blood culture on his previous emergency room visit for an ulceration of the right great toe. The pathogen was identified as Erysipelothrix rhusiopathiae and it presented in the subsequent blood cultures during hospitalization. Transesophageal echocardiogram showed aortic valve endocarditis with bulky calcifications extending into the annulus. The patient received intravenous ampicillin-sulbactam and achieved sterilization on day 5. His regimen was switched to intravenous ampicillin for a total of 6 weeks.
Case presentation
A 59-year-old male with no reported past medical history presented to the Emergency Department (ED) in December 2018 because he was contacted by the ED staff regarding a positive blood culture collected one day prior. During that visit, the patient had complaints of an ulceration of his right great toe, generalized malaise and myalgia. He had worked as a construction worker but had been unable to work due to his fatigue. His ulcer had been progressively worsening since March 2018 and he reported tremendous discomfort in the past few days. The ulcer was described as an ulceration of the right medial hallux, with hyperkeratotic border. The wound base was fibrogranular with a size of 2.3cm × 2.4cm x 1.0 cm, without erythema, edema, purulent drainage, or fluctuance. Probe-to-bone test was negative. Two sets of blood cultures were obtained, and the patient was given one dose of intravenous cefazolin 1000 milligrams. Podiatry performed a bedside debridement of hyperkeratotic tissue down to healthy tissue, and the patient was discharged home with amoxicillin-clavulanic acid 875 milligrams orally twice daily.
On the next day, the patient was contacted by the staff because his preliminary result of blood culture from the previous day grew gram positive coccobacilli in aerobic bottle and gram positive rods in anaerobic bottle. His only pertinent physical examination finding was the right medial non-infectious hallux that was debrided by Podiatry yesterday. He at that time denied fever or chills, cough, headache, chest pain, shortness of breath, abdominal pain, or urinary symptoms. Another two sets of blood cultures were collected and he was given one dose of piperacillin-tazobactam 4.5 g and one dose of vancomycin 1250 milligrams empirically in the ED. Infectious Diseases was consulted and the initial impression was to stop all the antimicrobials until culture results were finalized. The initial blood cultures both grew Erysipelothrix rhusiopathiae 48 h later. Upon further questioning, the patient had no domestic pets at home and did not have close contact with animals at work. However, he interacted with a stray cat near his house multiple times in the past months.
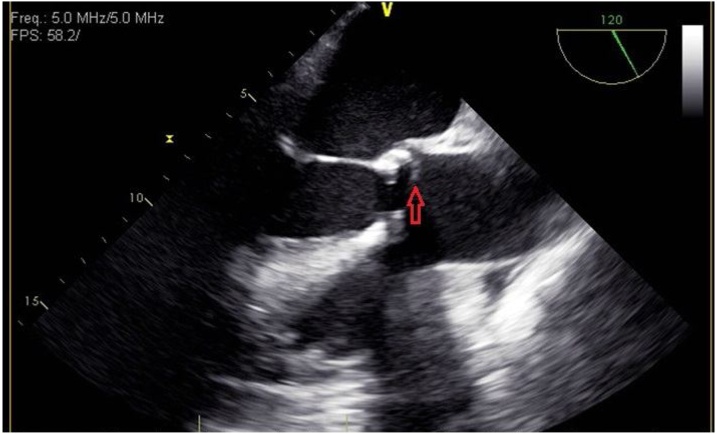
His antimicrobials were switched to ampicillin-sulbactam 3 g every 6 h. Blood cultures collected on hospital day 2, 3, and 4 were all positive for E. rhusiopathiae. Transthoracic echocardiogram on day 5 showed severely calcified aortic valves with probable vegetation on the aortic side and on the non-coronary cusp on the ventricular side and severe aortic stenosis. Transesophageal echocardiogram later was performed and showed aortic valve endocarditis with bulky calcifications extending into the annulus, but no clear aortic root abscess was visualized (Fig. 1). Ampicillin-sulbactam was switched to ampicillin 2 g intravenously every 4 h. Blood cultures had been negative since day 5. MR right foot without contrast was performed but there were no signs of osteomyelitis, abscess, or septic arthritis. Patient then received a PICC line to complete a total of six weeks of intravenous ampicillin 2 g every 6 h. It was recommended to repeat another transesophageal echocardiogram before stopping antimicrobials and another blood culture after completing the course. Unfortunately the patient was lost to follow up.
Fig. 1.
Midesophageal long axis view of transesophageal echocardiogram. The red arrow indicates vegetation on the aortic valve.
Discussion
Erysipelothrix rhusiopathiae is a thin, pleomorphic, nonsporulating gram-positive bacillus organism and a zoonotic pathogen [1]. It is most commonly found in a variety of wild and domestic animals, including swine, sheep, fish, dogs, rodents, and turkeys. Besides infecting animals, E. rhusiopathiae can also cause infection in humans from either direct inoculation of bacteria on the injured skin or a previous contaminated injury. Therefore, most infection happens among the animal handlers such as butchers, fishermen, and farmers. Kobayashi also reported one case of bacteremia following a cat bite [2]. Domestic swine is considered the major reservoir but infection is more common in fish handlers due to skin abrasions from fish bones or fins. E. rhusiopathiae can live for 12 days in direct sunlight, but can be killed by moist heat at 55 Fahrenheit for 15 min. There is no documented human-to-human transmission [3].
The clinical manifestations of human E. rhusiopathiae infection closely resemble those seen in swine [1]. They are categorized into three forms: erysipeloid, diffuse cutaneous form, and septicemia. Erysipeloid is a localized cutaneous infection similar to cellulitis, caused by bacteria invading skin tissue through an open lesion and commonly found in hands and fingers. It was unclear whether the patient’s foot ulcer was a form of erysipeloid lesion, but it could provide a portal of entry for the bacteria. In a case series done by Gorby, erysipeloid lesions only appeared in 40 % of patients with endocarditis [4]. They are typically self-limiting in 3–4 weeks without any therapy. Diffuse cutaneous form is rarer compared to the localized lesions and it manifests as a larger infected area with central clearing and bullous lesions that sometimes appear at a distant site. Other symptoms including fever and joint pain were reported and its clinical course is longer lasting [1,2]. There is no proposed treatment for this form of infection due to its rare occurrence. Antimicrobials could potentially shorten the disease duration and symptomatic treatment such as warm compresses can alleviate swelling and pain.
Systemic infection such as septicemia and bacteremia is the most serious form of infection. Most cases have identifiable contact with E. rhusiopathiae such as handling animals or seafood and about 36 % of patients had prior or concurrent erysipeloid lesions [3,5,7]. Other risk factors include immunodeficiencies (either from medications or chronic illnesses), diabetes mellitus, chronic kidney disease and history of alcohol abuse [7,8]. However, our patient did not have any predisposing occupational nor medical risks, though he had a chronic right foot ulcer that posted a potential risk for inoculation. E. rhusiopathiae bloodstream infection (BSI) was originally thought to have a very high incidence (close to 90 %) of endocarditis, but Tan et al. believe that it is due to case bias and underreporting of E. rhusiopathiae BSI without IE [7]. Most clinical cases in our literature search are reporting E. rhusiopathiae endocarditis. There are also cases reports regarding E. rhusiopathiae-related septic arthritis, prosthetic joint infection, and osteomyelitis. However none of them was associated with endocarditis [9,10,11]. Recent case reports have similar findings on aortic and mitral valves, sometimes even involving tricuspid valves simultaneously [12,13]. It can cause acute valvular regurgitation that requires surgical valvular replacement/repair. E. rhusiopathiae endocarditis has a high mortality, so immediate medical or surgical treatment is paramount [4,6]. Typical clinical symptoms of E. rhusiopathiae endocarditis are fever, dyspnea, malaise, arthralgia [5]. However, this patient did not exhibit any symptoms and his positive blood cultures were merely incidental findings. Therefore it is important to take careful clinical history and identify potential risk factors including recent animal exposure. Our case is unique as the patient only interacted with a stray cat and he was completely asymptomatic.
Blood culture is the gold standard of diagnosis. E. rhusiopathiae is a slow growing bacteria that is catalase negative, non-motile and not capsulated. In the past, it was frequently misinterpreted as Lactobacillus spp. or Enterococcus spp., but molecular polymerase chain reaction (PCR) significantly improves the accuracy and shortens the duration for speciation [3,10,14].
E. rhusiopathiae differs from most other gram positive organisms due to its intrinsic resistance to vancomycin, one of the initial regimens for gram positive bacteremia. Fidalgo et al. demonstrated that penicillin and ceftriaxone were the recommended treatment with a low MIC [15]. In a case series compiled by Tan et al., most cases were successfully treated with penicillin G and cephalosporins were used in three cases [7]. Venditti et al. tested 10 isolates of E. rhusiopathiae (nine swine isolates and one human isolate) and found that penicillin and imipenem were the most effective antimicrobials. Clindamycin and fluoroquinolone are suitable alternatives [16]. We were able to achieve sterility with ampicillin-sulbactam and then ampicillin for a total of six weeks. As mentioned above, it is crucial to identify E. rhusiopathiae early and tailor an antimicrobial regimen based on its susceptibilities.
Conclusion
Infective endocarditis is a serious bacterial bloodstream infection with high mortality without proper treatment and the key of successful treatment relies on accurate identification of the organism. E. rhusiopathiae is a rare zoonotic pathogen that has intrinsic resistance to vancomycin, a common antimicrobial used for gram positive bacteremia. Clinicians should not underestimate the severity of E. rhusiopathiae BSI as it will cause severe systemic infection such as endocarditis, septic arthritis or osteomyelitis.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Taylor Wang: Conceptualization, Writing - original draft, Writing - review & editing. Danyal Khan: Conceptualization, Writing - original draft, Writing - review & editing. Neville Mobarakai: Conceptualization, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Wang Q., Chang B.J., Riley T.V. Erysipelothrix rhusiopathiae. Vet Microbiol. 2010;140(3-4):405–417. doi: 10.1016/j.vetmic.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi K.I., Kawano T., Mizuno S., Kubo K., Komiya N., Otsu S. Erysipelothrix rhusiopathiae bacteremia following a cat bite. IDCases. 2019;18 doi: 10.1016/j.idcr.2019.e00631. Published 2019 Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reboli A.C., Farrar W.E. Erysipelothrix rhusiopathiae: an occupational pathogen. Clin Microbiol Rev. 1989;2(4):354–359. doi: 10.1128/cmr.2.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorby G.L., Peacock J.E., Jr. Erysipelothrix rhusiopathiae endocarditis: microbiologic, epidemiologic, and clinical features of an occupational disease. Rev Infect Dis. 1988;10(2):317–325. doi: 10.1093/clinids/10.2.317. [DOI] [PubMed] [Google Scholar]
- 5.Borchardt K.A., Sullivan R.W., Blumberg R.S., Gelber R.H., Botch V., Crull S. Erysipelothrix rhusiopathiae endocarditis. West J Med. 1977;127(2):149–151. [PMC free article] [PubMed] [Google Scholar]
- 6.Nassar I.M., de la Llana R., Garrido P., Martinez-Sanz R. Mitro-aortic infective endocarditis produced by Erysipelothrix rhusiopathiae: case report and review of the literature. J Heart Valve Dis. 2005;14(3):320–324. [PubMed] [Google Scholar]
- 7.Tan E.M., Marcelin J.R., Adeel N., Lewis R.J., Enzler M.J., Tosh P.K. Erysipelothrix rhusiopathiae bloodstream infection – a 22-year experience at Mayo Clinic, Minnesota. Zoonoses Public Health. 2017;64(7):e65–e72. doi: 10.1111/zph.12348. [DOI] [PubMed] [Google Scholar]
- 8.Schuster M.G., Brennan P.J., Edelstein P. Persistent bacteremia with Erysipelothrix rhusiopathiae in a hospitalized patient. Clin Infect Dis. 1993;17(4):783–784. doi: 10.1093/clinids/17.4.783. [DOI] [PubMed] [Google Scholar]
- 9.Hocqueloux L., Poisson D.M., Sunder S., Guilbert S., Prazuck T. Septic arthritis caused by Erysipelothrix rhusiopathiae in a prosthetic knee joint. J Clin Microbiol. 2010;48(1):333–335. doi: 10.1128/JCM.01683-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorenz M.L., Bouton T.C., Caliendo A.M. First reported case of vertebral osteomyelitis due to Erysipelothrix rhusiopathiae. IDCases. 2017;11:3–5. doi: 10.1016/j.idcr.2017.11.002. Published 2017 Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alawdah L.S., Campbell J.N., Pollock N., Watnick P.I. Erysipelothrix rhusiopathiae suppurative arthritis in a 12-year-old boy after an unusual fresh water exposure. Pediatr Infect Dis J. 2017;36(4):431–433. doi: 10.1097/INF.0000000000001461. [DOI] [PubMed] [Google Scholar]
- 12.Tomaszuk-Kazberuk A., Kamińska M., Sobkowicz B., Hirnle T., Prokop J., Lewczuk A. Infective endocarditis caused by Erysipelothrix rhusiopathiae involving three native valves. Kardiol Pol. 2011;69(8):827–829. [PubMed] [Google Scholar]
- 13.Yamamoto Y., Shioshita K., Takazono T., Seki M., Izumikawa K., Kakeya H. An autopsy case of Erysipelothrix rhusiopathiae endocarditis. Intern Med. 2008;47(15):1437–1440. doi: 10.2169/internalmedicine.47.1150. [DOI] [PubMed] [Google Scholar]
- 14.Hua P., Liu J., Tao J., Liu J., Yang Y., Yang S. Erysipelothrix rhusiopathiae-induced aortic valve endocarditis: case report and literature review. Int J Clin Exp Med. 2015;8(1):730–736. Published 2015 Jan 15. [PMC free article] [PubMed] [Google Scholar]
- 15.Fidalgo S.G., Longbottom C.J., Rjley T.V. Susceptibility of Erysipelothrix rhusiopathiae to antimicrobial agents and home disinfectants. Pathology. 2002;34(5):462–465. doi: 10.1080/0031302021000009405. [DOI] [PubMed] [Google Scholar]
- 16.Venditti M., Gelfusa V., Tarasi A., Brandimarte C., Serra P. Antimicrobial susceptibilities of Erysipelothrix rhusiopathiae. Antimicrob Agents Chemother. 1990;34(10):2038–2040. doi: 10.1128/aac.34.10.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]