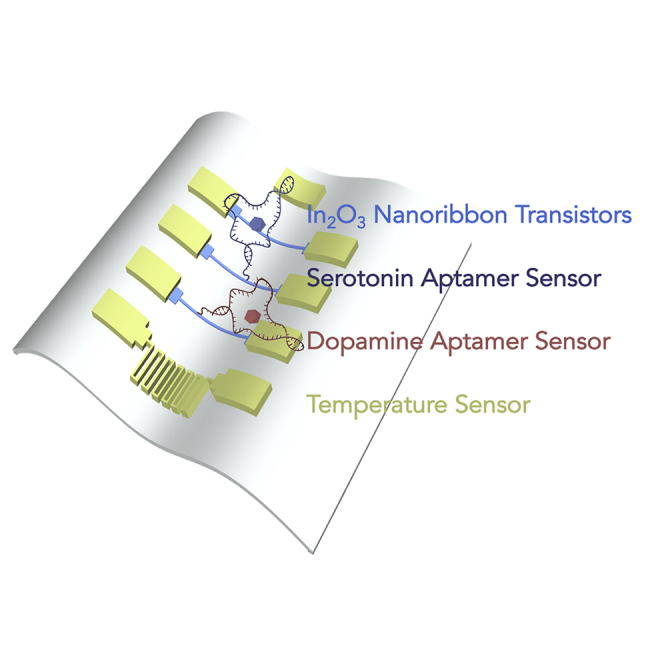
Summary
Flexible sensors are essential for advancing implantable and wearable bioelectronics toward monitoring chemical signals within and on the body. Developing biosensors for monitoring multiple neurotransmitters in real time represents a key in vivo application that will increase understanding of information encoded in brain neurochemical fluxes. Here, arrays of devices having multiple In2O3 nanoribbon field-effect transistors (FETs) were fabricated on 1.4-μm-thick polyethylene terephthalate (PET) substrates using shadow mask patterning techniques. Thin PET-FET devices withstood crumpling and bending such that stable transistor performance with high mobility was maintained over >100 bending cycles. Real-time detection of the small-molecule neurotransmitters serotonin and dopamine was achieved by immobilizing recently identified high-affinity nucleic-acid aptamers on individual In2O3 nanoribbon devices. Limits of detection were 10 fM for serotonin and dopamine with detection ranges spanning eight orders of magnitude. Simultaneous sensing of temperature, pH, serotonin, and dopamine enabled integration of physiological and neurochemical data from individual bioelectronic devices.
Subject Areas: Nanoscience, Bioelectronics, Nanotechnology Fabrication
Graphical Abstract
Highlights
-
•
We fabricated flexible In2O3 nanoribbon transistors using cleanroom-free processes
-
•
Flexible In2O3 transistors withstood crumpling and bending with stable performance
-
•
Flexible aptamer biosensors detect neurotransmitters in real time
-
•
Multiplexed sensors monitor temperature, pH, serotonin, and dopamine simultaneously
Nanoscience; Bioelectronics; Nanotechnology Fabrication
Introduction
Electronic devices that extend or create new capabilities for elucidating complex functions in brains (Alivisatos et al., 2013a, 2013b; Weiss, 2013), skin (Gao et al., 2016; Liu et al., 2018b; Kim et al., 2019; Rogers et al., 2019), and other biological systems (Biteen et al., 2016) are of substantial interest. Advances have been made in the areas of soft (Kim et al., 2010; Minev et al., 2015; Wang et al., 2018) and minimally invasive bioelectronics (Rim et al., 2015; Luan et al., 2017; Zhou et al., 2017). Wearable electronics enable sensor systems for monitoring vital signs from skin and biomarkers in sweat, such as ions, glucose, lactase, and cortisol (Kim et al., 2016; Heo et al., 2018; Nyein et al., 2018; Chung et al., 2019; He et al., 2019, 2020; Liu et al., 2020; Torrente-Rodriguez et al., 2020; Yang et al., 2020; Zhao et al., 2020). Efforts have been made toward bioelectronics for implantable devices, including liquid-metal- and hydrogel-based systems (Yu et al., 2016; Fang et al., 2017; Liu et al., 2019; Wen et al., 2019). Soft materials yield readily to pressure and, thus, more closely comply with the pliable and, in some cases, stretchable nature of biological tissues. Fabrication of electronic devices on substrates having low Young's moduli, i.e., greater elasticity, compared with rigid substrates reduces immunological responses after brain implantation (Gunasekera et al., 2015; Kozai et al., 2015). Soft bioelectronics with capabilities to detect multiple neurotransmitters simultaneously and in real time are needed to investigate chemical information processing in brains (Andrews, 2013), particularly in the context of chronic neural recordings (Du et al., 2017).
We have developed aptamer-field-effect transistor (FET) biosensors that detect small molecules, including serotonin, dopamine, glucose, and phenylalanine under high-ionic strength conditions (Nakatsuka et al., 2018b; Cheung et al., 2019). Aptamers are rare, single-stranded nucleic acid sequences isolated from oligonucleotide combinatorial libraries that recognize specific targets (Hamaguchi et al., 2001; Willner et al., 2007; Nakatsuka et al., 2018a). Aptamers can be functionalized to semiconducting materials used in FETs, e.g., organic (Hammock et al., 2013), carbon-based (So et al., 2008; Sorgenfrei et al., 2011), and metal oxides (Kim et al., 2015). Target-induced rearrangement of negatively charged DNA aptamer backbones results in changes in surface potentials near semiconductor channels to gate FET transconductance (Nakatsuka et al., 2018b). This sensing mechanism is label-free and highly sensitive in physiological environments, e.g., brain tissue, dilute serum.
Metal oxides, such as In2O3, have several advantages over other channel materials for FET-based sensors. Similar to Si devices, which can be fabricated with high scalability and uniformity, metal-oxide thin-film transistors have been widely used in industry, e.g., touch screens, displays, solar cells, owing to their electronic performance and large-area uniformity. The main advantages of metal oxides over silicon in wearable sensor applications are the ease of fabrication and compatibility with flexible substrates (Kim, et al., 2015; Rim, et al., 2015; Liu et al., 2018a; Nakatsuka et al., 2018b; Zhao et al., 2018; Cheung, et al., 2019).
Recently, a number of types of flexible electronics, including displays (Lewis et al., 2004; Park et al., 2009), photovoltaics (Fan et al., 2009), and wearable biosensors (Rim, et al., 2015; Liu et al., 2018a), have been fabricated using metal oxides in their structures. The In2O3 FETs are well suited to wearable or implantable sensing applications versus a variety of other metal oxides, such as indium-gallium-zinc oxide (IGZO) and ZnO, as the latter are unstable under physiological conditions (Aroonyadet et al., 2015; Jin et al., 2015). For example, ZnO nanoribbon FETs dissolve completely after 14 h of exposure to phosphate buffer saline (PBS). Top-down-fabricated In2O3 nanoribbon biosensors, in addition to being stable in physiological solutions, have fast response times, wide detection ranges, low limits of detection, high uniformity, and the capability to be integrated with microfluidics and microprocessors (Aroonyadet, et al., 2015; Liu et al., 2016).
Given the needs for implantable and wearable bioelectronics and the advantageous sensing capabilities of aptamer-FET biosensors under biologically relevant conditions, we aim to advance aptamer-functionalized In2O3 FETs in soft, flexible formats. Previously, we fabricated sol-gel processed thin-film In2O3 FETs on flexible polyimide for pH and glucose sensing (Rim, et al., 2015). The fabrication process involved spin-coating and high-temperature annealing (>350°C). We have also fabricated In2O3 nanoribbon FETs via sputtering at room temperature on thick (∼5 μm) polyethylene terephthalate (PET) for wearable applications in enzymatic glucose sensing (Liu et al., 2018a). Here, we advance flexible substrates by fabricating arrays of In2O3 nanoribbon FETs on thin (1.4 μm) PET. These new devices exhibited uniform transistor performance, small batch-to-batch variation, and stable performance in high-ionic-strength solutions, i.e., undiluted physiological buffered saline (PBS) and artificial cerebrospinal fluid (aCSF), in a format with robust mechanical flexibility. Thin-film PET-FETs were used to detect serotonin and dopamine over wide concentration ranges, including those occurring in the brain extracellular space (Mathews et al., 2004; Yang et al., 2013, 2015), in real time, and in a multiplexed format that included temperature and pH sensing.
Results
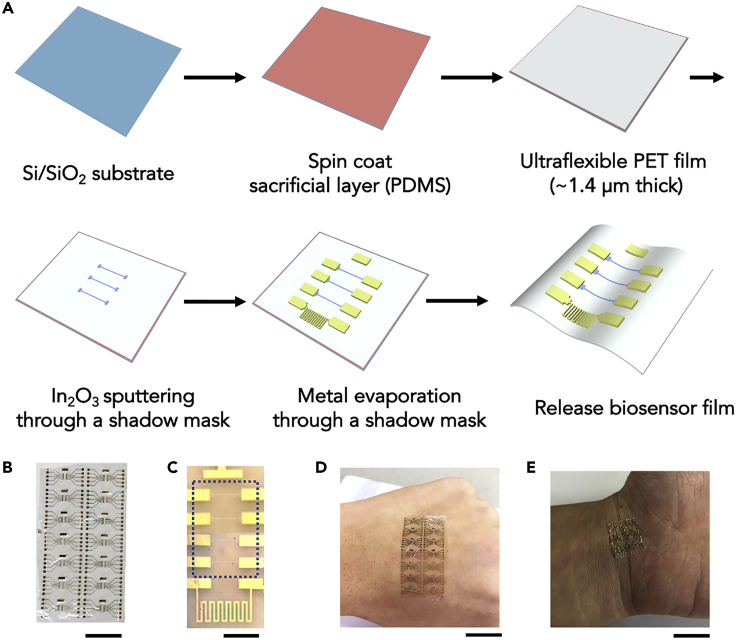
The fabrication process for device arrays on flexible thin-film PET substrates is illustrated in Figure 1A and described in detail in Transparent Methods. Briefly, each 3-inch Si/SiO2 substrate was spin coated with a 20-μm polydimethylsiloxane (PDMS) adhesion layer. Next, 1.4-μm PET films were adhered to PDMS via van der Waals interactions. Polyethylene terephthalate is a common thermoplastic polymer resin used to make single-use food and liquid containers and polyester clothing fiber. Here, PET films were used as received at room temperature. The current approach has potential for highly scalable and uniform roll-to-roll fabrication.
Figure 1.
Fabrication of Flexible In2O3 Nanoribbon Biosensors
(A) Schematic of the fabrication process. Each Si/SiO2 substrate was coated with a polydimethylsiloxane (PDMS) adhesion layer. Next, a 1.4-μm polyethylene terephthalate (PET) film was laminated over the PDMS. The In2O3 nanoribbons were patterned by sputtering on the PET layer through a shadow mask. A 1-nm Ti adhesion layer followed by a 50-nm Au layer were deposited through a different shadow mask to pattern source, drain, gate, and temperature sensor electrodes. The biosensor film was then delaminated from the rigid carrier wafer.
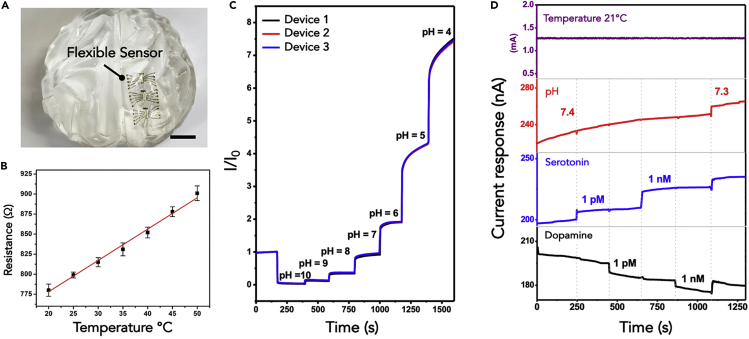
(B) Photograph of as-fabricated device array. Scale bar is 1 cm. Each array has 14 devices with four field-effect transistors (FETs) per device.
(C) Optical microscope image of a single device showing the Au common-gate electrode, four In2O3 nanoribbon FETs (dotted blue box), and a Au resistive temperature sensor (from top to bottom). The low contrast of the In2O3 nanoribbons is due to their transparency. Scale bar is 500 μm.
(D) Flexibility of a sensor array is illustrated by conformal attachment to human skin. Scale bar is 2 cm.
(E) Biosensor film wrinkled during human body movement. Scale bar is 1 cm.
See also Figure S1.
A shadow mask was used to pattern 16-nm-thick In2O3 nanoribbons on PET layers using radio frequency (RF) sputtering. A different shadow mask was then aligned to pattern source, drain, and gate electrodes and temperature sensors each composed of underlying 1-nm Ti layers and overlaying 50-nm Au films. The use of two aligned shadow mask steps eliminated the need for photolithography. The entire fabrication process was completed without the need for a cleanroom. Moreover, this fabrication strategy eliminated photoresist contamination and chemical exposure (Aroonyadet, et al., 2015; Liu, et al., 2016; Liu et al., 2018a). We have used thin metal films on PET for flexible electronics; these films showed high stability and reproducibility (Cao et al., 2016; Liu et al., 2018a). Each patterned PET biosensor film was delaminated from its sacrificial PDMS layer and the FET arrays were used for measurements.
Multiple transistor devices in arrays were fabricated on each 5 cm × 5 cm PET film. Each array consisted of 14 devices (Figure 1B). The total thickness of each array was ∼1.5 μm and the total weight was ∼2.5 mg. Figure 1C shows an individual device containing a Au common gate electrode, four In2O3 nanoribbon FETs, and a temperature sensor. Each In2O3 nanoribbon was 16 nm thick, 25 μm wide, and 500 μm long (Figure S1). Device arrays were conformally applied to human skin (Figure 1D), illustrating the potential for wearable electronics for healthcare and personal monitoring applications. Devices could be bent and wrinkled when worn on skin (Figure 1E) When used as implantable devices, biosensors may be wrinkled or bent during the insertion process.
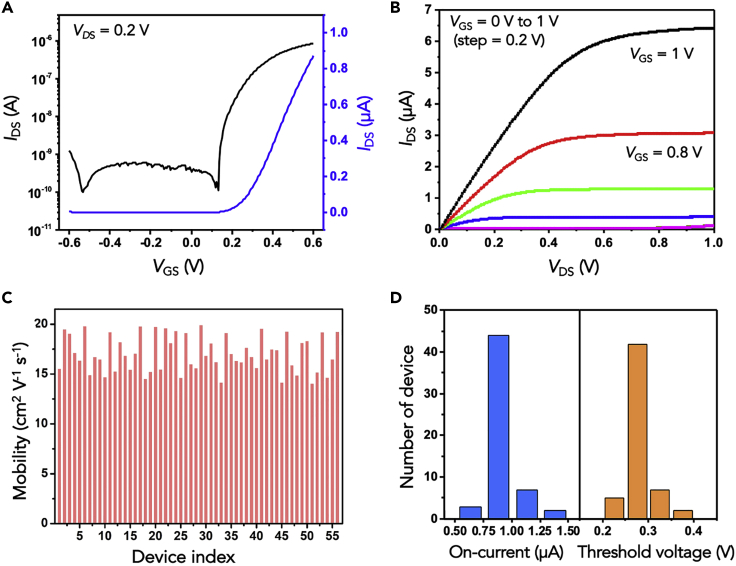
The flexible In2O3 nanoribbon FET devices exhibited uniform and stable electronic properties under high-ionic-strength conditions. Figure 2A shows the transfer characteristics of a representative In2O3 nanoribbon transistor controlled using a common Au gate electrode. Black and blue curves represent drain current-gate voltage (IDS-VGS) characteristics in logarithmic and linear scales, respectively, and indicate gate modulation of drain current in phosphate buffered saline (PBS) with a current on/off ratio of ∼103. The corresponding output characteristics demonstrated that the devices exhibited FET behavior typically associated with drain voltage modulation (Figure 2B). The gate leakage current was negligible compared with the drain-source current. As an example, the gate leakage current was less than 2 nA at VDS = 0.2 V (Figure S2). All transistors in a representative array were characterized. Nearly 100% were functional (55/56) with only one transistor showing significantly lower mobility compared with the other transistors.
Figure 2.
Electronic Performance of Flexible In2O3 Nanoribbon Field-Effect Transistors
(A) Representative transfer characteristics of an In2O3 nanoribbon transistor with L = 500 μm, W = 25 μm, and H = 16 μm in phosphate buffered saline. The ID (drain current) is shown in a logarithmic scale (left, black trace) and a linear scale (right, blue trace); VGS is the gate-source voltage. The applied drain-source voltage VDS was 0.2 V.
(B) Output curves in the linear and saturation regimes. In this plot, IDS is a function of VDS with VGS from 0 to 1 V in 0.2-V steps.
(C) Plot of the charge-carrier mobilities of 56 transistors in an array.
(D) Histograms showing on-currents and threshold voltages from the same 56 transistors. The average mobility was 17.8 ± 1.8 cm2 V−1 s−1. The average on-current was 0.86 ± 0.1 μA. The average threshold voltage was 0.27 ± 0.02 V.
See also Figure S2.
The charge-carrier mobilities of 56 transistors in a single array were measured and calculated (details in Transparent Methods) and showed ∼10% variability (Figure 2C), with an average mobility of 17.8 ± 1.8 cm2 V−1 s−1. Figure 2D shows a histogram of the on-state currents and threshold voltages of the 56 devices. Both showed narrow distributions. Notably, In2O3 nanoribbon transistors exhibited uniform and stable electronic performance in a physiological environment (all data in Figure 2 were collected in undiluted PBS) at relatively low voltages (<1 V). These characteristics are favorable for implantable neural recording devices and on-skin electronics.
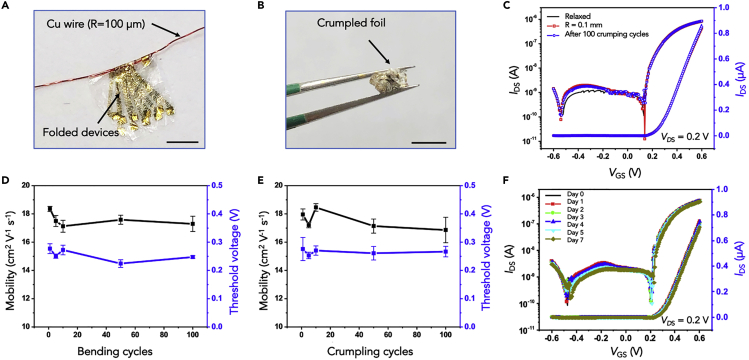
Mechanical flexibility of In2O3 nanoribbon FETs was evaluated in experiments that involved bending and crumpling. Figure 3A shows a photograph of a PET sheet patterned with In2O3 FETs tightly wrapped around a copper wire having a radius of 100 μm. Under these conditions, a tensile strain of ∼0.75% (calculations in Transparent Methods) was applied to the In2O3 nanoribbons with the direction of strain parallel to the current flow. In another test, a 5 cm × 5 cm PET sheet of In2O3 FET devices was tightly crumpled for ∼5 s (Figure 3B), then flattened for electronic measurements.
Figure 3.
Stability of Flexible In2O3 Nanoribbon Sensors
(A) Photograph of flexible thin-film (1.4-μm) PET In2O3 sensor arrays wrapped around a copper wire with a radius of 100 μm. Scale bar is 5 mm.
(B) Photograph of a crumpled In2O3 biosensor film (original size: 5 cm × 5 cm). Scale bar is 0.5 cm.
(C–E) (C) Transfer characteristics of a representative In2O3 transistor in a relaxed state, bent around a copper wire with a radius of ~0.1 mm, and after crumpling, respectively. Mobilities (left) and threshold voltages (right) obtained in a relaxed state (D) after different numbers of bending cycles and (E) after various numbers of crumpling cycles.
(F) Transfer characteristics of an In2O3 transistor measured immediately after fabrication (Day 0) and after immersion in undiluted PBS for 1, 2, 3, 4, 5, and 7 day(s). Error bars in (D) and (E) are standard deviations of N = 15 devices.
The electronic performance of In2O3 FETs while bent and after crumpling was measured in PBS. Typical transfer curves of representative devices in the relaxed state, bent with a radius curvature of ∼0.1 mm, and after 100 crumpling cycles are shown in Figure 3C. Figures 3D and 3E show mobilities and threshold voltages averaged over 15 devices after 5, 10, 50, and 100 bending or crumpling cycles, respectively. After 100 bending cycles, the change in average mobility was 5.6% (from 18.4 to 17.3 cm2 V−1 s−1); the threshold voltage showed only a small variation from 0.23 to 0.27 V. Similar results were observed in repeated crumpling tests. The change in mobility was 6.1% after 100 crumpling cycles, and the threshold voltage remained around 0.26 V. Together, these findings illustrate that the In2O3 nanoribbon FET devices fabricated on thin-film PET are suitable for flexible electronics with reliable performance even under extreme bending and crumpling cycles. Bending events are common for both wearable bioelectronics and implantable devices. For example, bending occurs in and on soft tissue where devices need to adapt to movement.
Figure 3F shows transfer curves for FET devices before and after immersion in PBS for 1, 2, 3, 4, 5, or 7 day(s). Based on data in logarithmic and linear scales, there were no significant changes in FET performance. These findings substantiate the long-term stability of In2O3 transistors in high-ionic-strength solutions and indicate that these sensors are robust for at least 1 week when exposed to physiological fluids.
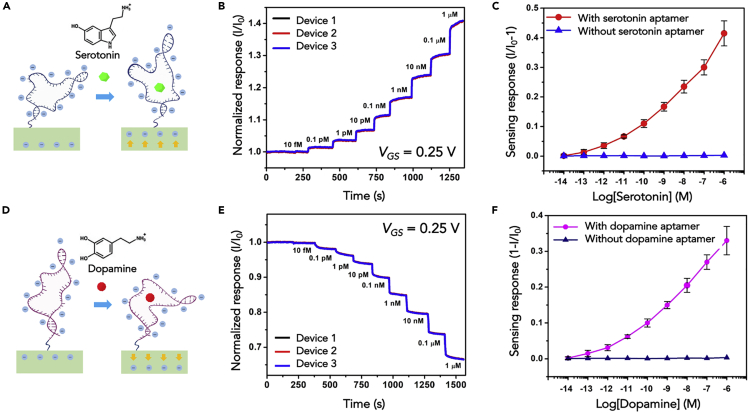
A portion of the negatively charged backbone of the serotonin aptamer used herein is hypothesized to move away from In2O3 channel surfaces upon target capture (Figure 4A) (Nakatsuka et al., 2018b). Thus, the electrostatic repulsion between the electrons in an n-type semiconductor channel and negatively charged aptamers decreases and channel conductance increases in response to aptamer-target association. Results for serotonin detection using In2O3 nanoribbon FETs functionalized with serotonin aptamers are shown in Figure 4B. Details of the surface functionalization and aptamer sequences are in Transparent Methods.
Figure 4.
Characterization of Serotonin- and Dopamine-Aptamer-Functionalized Sensors
(A) Some aptamers, such as the aptamer used here to recognize serotonin, reorient a portion of their backbones away from semiconductor channels upon target binding, thereby increasing transconductance for n-type semiconductors.
(B) Normalized (I/I0; unitless) real-time sensing results from three In2O3 nanoribbon biosensors functionalized with the serotonin aptamer. Devices showed responses to serotonin (in undiluted artificial cerebrospinal fluid) at concentrations ranging from 10 fM to 1 μM.
(C) Relationship between serotonin concentration and current responses from nine different devices. Results from unfunctionalized devices lacking aptamers are plotted for comparison.
(D) Other aptamers, such as the dopamine aptamer used here, reorient a portion of their negatively charged oligonucleotide backbones closer to field-effect transistors upon target recognition to deplete channels electrostatically.
(E) Normalized (I/I0; unitless) real-time sensing results from three In2O3 nanoribbon biosensors functionalized with the dopamine aptamer. Devices showed responses to dopamine (in undiluted artificial cerebrospinal fluid) at concentrations ranging from 10 fM to 1 μM.
(F) Relationship between dopamine concentration and current responses from nine different devices. Results from devices lacking the dopamine aptamer are also plotted. All devices were operated with VDS = 0.2 V and VGS = 0.25 V. Errors bars in (C) and (F) are standard deviations for N = 9 devices (from three separate substrates where each substrate had three devices) and are too small to be visualized in some cases.
See also Figures S3–S5.
To evaluate sensor reliability in a high-ionic-strength buffer that approximates the brain extracellular fluid, serotonin was dissolved in artificial cerebrospinal fluid (aCSF). Devices were operated at a 0.25-V gate bias applied using the Au common gate electrode. Entire devices, including the channels, were submerged in aCSF to obtain baseline currents. After changing the solution to 10 fM serotonin in aCSF, the sensing signal increased by ∼1% after stabilization (∼150 s). As the solutions over the FETs were changed to include higher serotonin concentrations ranging from 0.1 pM to 1 μM sequentially, stepwise IDS increases were observed (Figure 4B).
To test reproducibility of detection, we conducted sensing with nine different devices. The relationship between serotonin concentration and saturated current response is shown in Figure 4C. We performed the same sensing procedure on control devices, i.e., those with surface functionalization sans aptamers (Figure S3). The latter results are plotted in Figure 4C for comparison and showed minimal signal compared with that of FETs functionalized with aptamers. A device functionalized with a scrambled serotonin aptamer sequence also showed minimal response to serotonin indicating target selectivity (Figure S4).
In contrast to the serotonin aptamer, a portion of the negatively charged backbone of the dopamine aptamer used here is hypothesized to move closer to n-type semiconductor channels upon dopamine binding, thus increasing electrostatic repulsion and decreasing the In2O3 transconductance (Figure 4D) (Nakatsuka et al., 2018b). The results of real-time dopamine sensing are shown in Figure 4E, and a summary of the relationship between dopamine concentrations and the corresponding FET responses from nine different devices is plotted in Figure 4F. The limit of detection for dopamine sensing was also on the order of 10 fM. Data were collected every 2 s and indicated that aptamer-FETs have ∼5-s response times (Figure S5). Our determination of response time was limited by the semiconductor analyzer and software, thus measured response times are an upper limit.
We fabricated flexible sensing devices with the capability to monitor temperature, pH, serotonin, and dopamine simultaneously in real time. We performed sensing while devices were conformally applied to the surface of an “artificial brain,” a brain replica made from PDMS, as a demonstration of working on/in irregular tissue surfaces (Figure 5A). The sensors developed here could be used as implanted or surface brain sensors or as wearable biosensors on the skin.
Figure 5.
Multiplexed Biosensing
(A) Photograph of three sensor devices attached conformally to a polydimethylsiloxane brain mimic. Scale bar is 1 cm.
(B) Changes in resistance with respect to temperature in artificial cerebrospinal fluid (aCSF). Error bars are standard deviations for N = 5 devices. For linear regression (y = ax + b), resistance = (3.9×temperature) + 700, where temperature is in degrees Celsius; R2 = 0.99.
(C) Real-time pH sensing at unfunctionalized In2O3 nanoribbons in three different devices exposed to buffer solutions with pH values from 10 to 4.
(D) Simultaneous sensing of temperature, pH, serotonin, and dopamine in aCSF (pH = 7.4). Two adjacent devices were simultaneously exposed to solutions of 1 pM serotonin, 1 pM dopamine, 1 nM serotonin, and 1 nM dopamine, sequentially. Only transistors functionalized with the respective target-specific aptamers responded to the corresponding targets. All sensors responded to a pH change in aCSF (7.4–7.3). The devices were operated at VDS = 0.2 V and VGS = 0.25 V.
Local temperature change monitoring provides useful intraparenchymal and superficial information. For example, internal brain temperature is an indicator of neural functional activity (Wang et al., 2014). Brain temperature is regionally specific and fluctuates by as much at 4 °C under normal activation states. Clinical studies indicate a relationship between brain temperature changes and cerebral injury (Nielsen et al., 2013). Moreover, therapeutics, e.g., anesthesia, drugs of abuse, cause large and sometimes devastating changes in brain temperature, in the case of the latter (Kiyatkin, 2018). Skin temperature is also considered clinically informative, where it has been linked to a variety of diseases and skin injuries (Sprigle et al., 2001; Webb et al., 2013). Therefore, sensing devices with integrated temperature sensors provide information to supplement target molecule monitoring.
Thermistors fabricated from Au wires have been used as temperature indicators in wearable sensor systems (Gao, et al., 2016). Here, we adapted a similar design and integrated fabrication within the shadow mask designs. Figure 5B shows the resistance of a representative Au thermistor in PBS ranging from 20°C to 50°C with 5°C increments. The measured resistances were highly correlated with changes in temperature at a sensitivity of ∼4 Ω/°C.
Field-effect transistor sensors are sensitive to pH changes, i.e., [H+], because sensing mechanisms involve detecting changes in charge close to semiconductor surfaces (Aroonyadet, et al., 2015; Rim, et al., 2015; Liu, et al., 2016). Fluctuations in brain pH occur as a result of changes in CO2 levels, i.e., carbonic acid, which increase in conjunction with neural activity, neurotransmitter release, and oxygen consumption (Meunier et al., 2018). Sensing pH is useful in wearable bioelectronics as sweat pH values also vary owing to changes in carbonic acid levels (Gao, et al., 2016). Monitoring pH in sweat can be indicative of variations in electrolyte concentrations, which are biomarkers of disease and metabolic activity (Patterson et al., 2000; Burry et al., 2001; Sonner et al., 2015; Lee et al., 2017). Here, unfunctionalized In2O3 nanoribbons responded to pH in buffer solutions ranging from pH 10 to pH 4 (Figure 5C). Devices showed increases in conductance when the pH of solutions decreased, corresponding to a positive gating effect at n-type In2O3 transistors (Aroonyadet, et al., 2015; Rim, et al., 2015; Liu, et al., 2016). We note that, in addition to unfunctionalized FETs, devices functionalized with aptamers can be pH sensitive (Idili et al., 2014; Porchetta et al., 2015; Gordon et al., 2018). As pH decreases, negatively charged oligonucleotide backbones become associated with larger numbers of protons changing local charge near semiconductor surfaces.
Including pH sensors in our devices enabled discriminating target-specific changes in transconductance at aptamer-functionalized sensors from those associated with local pH changes. Three devices from adjacent groups on the same substrate array were used for multiplexed sensing. One device was functionalized with the serotonin aptamer to work as a serotonin sensor. Another device next to the serotonin sensor was protected by a PDMS mask during the aptamer functionalization. After that, the PDMS mask was removed, and this device consisting of unfunctionalized FETs was used as a pH sensor. A third device from an adjacent group of devices was functionalized with the dopamine aptamer and was used as a dopamine sensor. Lastly, a patterned gold wire thermistor on the same substrate was used as a temperature sensor.
After obtaining a stable baseline current in aCSF (pH 7.4), solutions above FETs were sequentially replaced with aCSF containing 1 pM serotonin, 1 pM dopamine, 1 nM serotonin, or 1 nM dopamine. The two aptamer-functionalized devices detected and differentiated physiologically relevant concentrations of serotonin and dopamine, simultaneously (Figure 5D). No current changes occurred at the pH-sensing FETs in response to the addition of serotonin or dopamine. In contrast, when aCSF (pH 7.3) sans neurotransmitters was introduced at t = 1,100 s, all three devices showed responses to a 0.1 pH unit change. For physiological sensing applications where pH and neurotransmitter concentrations change simultaneously, neurotransmitter-specific FET responses can be distinguished by subtracting responses occurring at pH sensors.
The directions of change for the IDS-t curves (Figures 4B, 4E, and 5D) are consistent with our hypotheses regarding the different types of conformational changes for these particular serotonin and dopamine aptamers and provide complementary and straightforward evidence of aptamer sensing mechanisms (Nakatsuka et al., 2018b). These serotonin and dopamine aptamers have been previously characterized for selectivity in fluorescence and FET sensing formats ( Nakatsuka et al., 2018b). Both aptamers showed high selectivity for their targets versus structurally similar precursors and metabolites found at high concentrations in the brain extracellular space. We also previously investigated serotonin-aptamer FET sensor stability in brain tissue (Nakatsuka et al., 2018b). Although baseline sensor responses changed after tissue exposure, calibrated responses, which correct for baseline drift, were stable and sensitive over a large range of serotonin concentrations even after 12 h of brain tissue exposure. Moreover, we have used glucose- and phenylalanine-aptamer FETs to sense their respective targets at physiological concentrations in serum diluted with Ringer's buffer, which mimics the ionic composition of plasma (Nakatsuka et al., 2018b; Cheung, et al., 2019).
Discussion
We have demonstrated flexible conformal sensor devices for multiplexed and selective detection of neurotransmitters and other key physiological parameters. Devices displayed excellent flexibility when mechanically deformed and long-term stability in high-ionic-strength solutions. Real-time sensing of neurotransmitters at low, physiologically relevant concentrations was demonstrated. These sensors can be combined with integrated circuits for device operation and signal processing for wearable or implantable applications. To overcome spatial limitations for functionalization with different aptamers, an electrochemical approach to addressing different FETs in the same device can be applied for future studies (Curreli et al., 2005). In sum, the current findings illustrate multiplexed, temporally resolved FET sensing of targets over large physiologically relevant concentration ranges, under high-ionic-strength conditions, and in a robust, flexible format. In conjunction with previous demonstrations of selectivity and sensing in biological tissues, small-molecule physiological sensors are expected to advance our understanding of the brain and other biological systems.
Limitations of the Study
Experiments to investigate interference by ascorbic acid and uric acid were not included in this work and require further investigation.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Professor Chongwu Zhou (chongwuz@usc.edu)
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate/analyze datasets/code.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
C.Zhao, A.M.A., and P.S.W. acknowledge support from the National Institute on Drug Abuse (DA045550). C.Zhou acknowledges support from King Abdulaziz City for Science and Technology (KACST) via The Center of Excellence for Nanotechnologies (CEGN). The authors thank Dr. Kevin M. Cheung for helpful feedback on the manuscript.
Author Contributions
Data were collected by Q.L., C. Zhao, M.C., Y.L., Z.Z., F.W., and Z.L. and analyzed by Q.L., C. Zhao, A.M.A., and C. Zhou. Figures were prepared by Q.L. and C. Zhao. The manuscript was written by Q.L., C. Zhao, P.S.W., A.M.A., and C. Zhou with assistance from all authors. All authors have read, corrected, and approved the final version of the manuscript.
Declaration of Interests
Stem-loop receptor-based field effector sensor devices for sensing at physiological salt concentration. Co-inventors: A. M. Andrews, P. S. Weiss, N. Nakatsuka, M. N. Stojanović, and K. A. Yang, nonprovisional U.S. and foreign patents filed 2019. PCT/US2019/046891.
Published: September 25, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101469.
Contributor Information
Paul S. Weiss, Email: psw@cnsi.ucla.edu.
Anne M. Andrews, Email: aandrews@mednet.ucla.edu.
Chongwu Zhou, Email: chongwuz@usc.edu.
Supplemental Information
. Transparent Methods and Figures S1–S5
References
- Alivisatos A.P., Andrews A.M., Boyden E.S., Chun M., Church G.M., Deisseroth K., Donoghue J.P., Fraser S.E., Lippincott-Schwartz J., Looger L.L. Nanotools for neuroscience and brain activity mapping. ACS Nano. 2013;7:1850–1866. doi: 10.1021/nn4012847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alivisatos A.P., Chun M., Church G.M., Deisseroth K., Donoghue J.P., Greenspan R.J., McEuen P.L., Roukes M.L., Sejnowski T.J., Weiss P.S. The brain activity map. Science. 2013;339:1284–1285. doi: 10.1126/science.1236939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews A.M. The brain initiative: toward a chemical connectome. ACS Chem. Neurosci. 2013;4:645. doi: 10.1021/cn4001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroonyadet N., Wang X., Song Y., Chen H., Cote R.J., Thompson M.E., Datar R.H., Zhou C. Highly scalable, uniform, and sensitive biosensors based on top-down indium oxide nanoribbons and electronic enzyme-linked immunosorbent assay. Nano Lett. 2015;15:1943–1951. doi: 10.1021/nl5047889. [DOI] [PubMed] [Google Scholar]
- Biteen J.S., Blainey P.C., Cardon Z.G., Chun M., Church G.M., Dorrestein P.C., Fraser S.E., Gilbert J.A., Jansson J.K., Knight R. Tools for the microbiome: nano and beyond. ACS Nano. 2016;10:6–37. doi: 10.1021/acsnano.5b07826. [DOI] [PubMed] [Google Scholar]
- Burry J.S., Coulson H.F., Esser I., Marti V., Melling S.J., Rawlings A.V., Roberts G., Mills A.K. Erroneous gender differences in axillary skin surface/sweat pH. Int. J. Cosmet. Sci. 2001;23:99–107. doi: 10.1046/j.1467-2494.2001.00085.x. [DOI] [PubMed] [Google Scholar]
- Cao X., Cao Y., Zhou C. Imperceptible and ultraflexible p-type transistors and macroelectronics based on carbon nanotubes. ACS Nano. 2016;10:199–206. doi: 10.1021/acsnano.5b02847. [DOI] [PubMed] [Google Scholar]
- Cheung K.M., Yang K.A., Nakatsuka N., Zhao C., Ye M., Jung M.E., Yang H., Weiss P.S., Stojanovic M.N., Andrews A.M. Phenylalanine monitoring via aptamer-field-effect transistor sensors. ACS Sens. 2019;4:3308–3317. doi: 10.1021/acssensors.9b01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H.U., Kim B.H., Lee J.Y., Lee J., Xie Z., Ibler E.M., Lee K., Banks A., Jeong J.Y., Kim J. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science. 2019;363:eaau0780. doi: 10.1126/science.aau0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curreli M., Li C., Sun Y., Lei B., Gundersen M.A., Thompson M.E., Zhou C. Selective functionalization of In2O3 nanowire mat devices for biosensing applications. J. Am. Chem. Soc. 2005;127:6922–6923. doi: 10.1021/ja0503478. [DOI] [PubMed] [Google Scholar]
- Du Z.J., Kolarcik C.L., Kozai T.D.Y., Luebben S.D., Sapp S.A., Zheng X.S., Nabity J.A., Cui X.T. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 2017;53:46–58. doi: 10.1016/j.actbio.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z., Razavi H., Do J.W., Moriwaki A., Ergen O., Chueh Y.L., Leu P.W., Ho J.C., Takahashi T., Reichertz L.A. Three-dimensional nanopillar-array photovoltaics on low-cost and flexible substrates. Nat. Mater. 2009;8:648–653. doi: 10.1038/nmat2493. [DOI] [PubMed] [Google Scholar]
- Fang H., Yu K.J., Gloschat C., Yang Z., Chiang C.H., Zhao J., Won S.M., Xu S., Trumpis M., Zhong Y. Capacitively coupled arrays of multiplexed flexible silicon transistors for long-term cardiac electrophysiology. Nat. Biomed. Eng. 2017;1:0038. doi: 10.1038/s41551-017-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Emaminejad S., Nyein H.Y.Y., Challa S., Chen K., Peck A., Fahad H.M., Ota H., Shiraki H., Kiriya D. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature. 2016;529:509–514. doi: 10.1038/nature16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C.K.L., Eisenstein M., Soh H.T. Direct selection strategy for isolating aptamers with pH-sensitive binding activity. ACS Sens. 2018;3:2574–2580. doi: 10.1021/acssensors.8b00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekera B., Saxena T., Bellamkonda R., Karumbaiah L. Intracortical recording interfaces: current challenges to chronic recording function. ACS Chem. Neurosci. 2015;6:68–83. doi: 10.1021/cn5002864. [DOI] [PubMed] [Google Scholar]
- Hamaguchi N., Ellington A., Stanton M. Aptamer beacons for the direct detection of proteins. Anal. Biochem. 2001;294:126–131. doi: 10.1006/abio.2001.5169. [DOI] [PubMed] [Google Scholar]
- Hammock M.L., Knopfmacher O., Naab B.D., Tok J.B.H., Bao Z.A. Investigation of protein detection parameters using nanofunctionalized organic field-effect transistors. ACS Nano. 2013;7:3970–3980. doi: 10.1021/nn305903q. [DOI] [PubMed] [Google Scholar]
- He X., Xu T., Gu Z., Gao W., Xu L.P., Pan T., Zhang X. Flexible and superwettable bands as a platform toward sweat sampling and sensing. Anal. Chem. 2019;91:4296–4300. doi: 10.1021/acs.analchem.8b05875. [DOI] [PubMed] [Google Scholar]
- He X., Yang S., Pei Q., Song Y., Liu C., Xu T., Zhang X. Integrated smart janus textile bands for self-pumping sweat sampling and analysis. ACS Sens. 2020;5:1548–1554. doi: 10.1021/acssensors.0c00563. [DOI] [PubMed] [Google Scholar]
- Heo S.Y., Kim J., Gutruf P., Banks A., Wei P., Pielak R., Balooch G., Shi Y., Araki H., Rollo D. Wireless, battery-free, flexible, miniaturized dosimeters monitor exposure to solar radiation and to light for phototherapy. Sci. Transl. Med. 2018;10:eaau1643. doi: 10.1126/scitranslmed.aau1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idili A., Vallee-Belisle A., Ricci F. Programmable pH-triggered DNA nanoswitches. J. Am. Chem. Soc. 2014;136:5836–5839. doi: 10.1021/ja500619w. [DOI] [PubMed] [Google Scholar]
- Jin S.H., Kang S.K., Cho I.T., Han S.Y., Chung H.U., Lee D.J., Shin J., Baek G.W., Kim T.I., Lee J.H. Water-soluble thin film transistors and circuits based on amorphous indium-gallium-zinc oxide. ACS Appl. Mater. Interfaces. 2015;7:8268–8274. doi: 10.1021/acsami.5b00086. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Viventi J., Amsden J.J., Xiao J., Vigeland L., Kim Y.S., Blanco J.A., Panilaitis B., Frechette E.S., Contreras D. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010;9:511–517. doi: 10.1038/nmat2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Rim Y.S., Chen H.J., Cao H.H., Nakatsuka N., Hinton H.L., Zhao C.Z., Andrews A.M., Yang Y., Weiss P.S. Fabrication of high-performance ultrathin In2O3 film field-effect transistors and biosensors using chemical lift-off lithography. ACS Nano. 2015;9:4572–4582. doi: 10.1021/acsnano.5b01211. [DOI] [PubMed] [Google Scholar]
- Kim J., Jeerapan I., Imani S., Cho T.N., Bandodkar A., Cinti S., Mercier P.P., Wang J. Noninvasive alcohol monitoring using a wearable tattoo-based iontophoretic-biosensing system. ACS Sens. 2016;1:1011–1019. [Google Scholar]
- Kim J., Campbell A.S., de Avila B.E., Wang J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019;37:389–406. doi: 10.1038/s41587-019-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin E.A. Brain temperature: from physiology and pharmacology to neuropathology. Handb. Clin. Neurol. 2018;157:483–504. doi: 10.1016/B978-0-444-64074-1.00030-6. [DOI] [PubMed] [Google Scholar]
- Kozai T.D., Jaquins-Gerstl A.S., Vazquez A.L., Michael A.C., Cui X.T. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem. Neurosci. 2015;6:48–67. doi: 10.1021/cn500256e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Song C., Hong Y.S., Kim M.S., Cho H.R., Kang T., Shin K., Choi S.H., Hyeon T., Kim D.H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017;3:e1601314. doi: 10.1126/sciadv.1601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J., Grego S., Chalamala B., Vick E., Temple D. Highly flexible transparent electrodes for organic light-emitting diode-based displays. Appl. Phys. Lett. 2004;85:3450–3452. [Google Scholar]
- Liu C., Xu T., Wang D., Zhang X. The role of sampling in wearable sweat sensors. Talanta. 2020;212:120801. doi: 10.1016/j.talanta.2020.120801. [DOI] [PubMed] [Google Scholar]
- Liu Q., Aroonyadet N., Song Y., Wang X., Cao X., Liu Y., Cong S., Wu F., Thompson M.E., Zhou C. Highly sensitive and quick detection of acute myocardial infarction biomarkers using In2O3 nanoribbon biosensors fabricated using shadow masks. ACS Nano. 2016;10:10117–10125. doi: 10.1021/acsnano.6b05171. [DOI] [PubMed] [Google Scholar]
- Liu Q., Liu Y., Wu F., Cao X., Li Z., Alharbi M., Abbas A.N., Amer M.R., Zhou C. Highly sensitive and wearable In2O3 nanoribbon transistor biosensors with integrated on-chip gate for glucose monitoring in body fluids. ACS Nano. 2018;12:1170–1178. doi: 10.1021/acsnano.7b06823. [DOI] [PubMed] [Google Scholar]
- Liu Z., Qi D., Leow W.R., Yu J., Xiloyannnis M., Cappello L., Liu Y., Zhu B., Jiang Y., Chen G. 3D-structured stretchable strain sensors for out-of-plane force detection. Adv. Mater. 2018;30:e1707285. doi: 10.1002/adma.201707285. [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu J., Chen S., Lei T., Kim Y., Niu S., Wang H., Wang X., Foudeh A.M., Tok J.B. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 2019;3:58–68. doi: 10.1038/s41551-018-0335-6. [DOI] [PubMed] [Google Scholar]
- Luan L., Wei X.L., Zhao Z.T., Siegel J.J., Potnis O., Tuppen C.A., Lin S.Q., Kazmi S., Fowler R.A., Holloway S. Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration. Sci. Adv. 2017;3:e1601966. doi: 10.1126/sciadv.1601966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews T.A., Fedele D.E., Coppelli F.M., Avila A.M., Murphy D.L., Andrews A.M. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J. Neurosci. Methods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Meunier C.J., Mitchell E.C., Roberts J.G., Toups J.V., McCarty G.S., Sombers L.A. Electrochemical selectivity achieved using a double voltammetric waveform and partial least squares regression: differentiating endogenous hydrogen peroxide fluctuations from shifts in pH. Anal. Chem. 2018;90:1767–1776. doi: 10.1021/acs.analchem.7b03717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minev I.R., Musienko P., Hirsch A., Barraud Q., Wenger N., Moraud E.M., Gandar J., Capogrosso M., Milekovic T., Asboth L. Electronic dura mater for long-term multimodal neural interfaces. Science. 2015;347:159–163. doi: 10.1126/science.1260318. [DOI] [PubMed] [Google Scholar]
- Nakatsuka N., Cao H.H., Deshayes S., Melkonian A.L., Kasko A.M., Weiss P.S., Andrews A.M. Aptamer recognition of multiplexed small-molecule-functionalized substrates. ACS Appl. Mater. Interfaces. 2018;10:23490–23500. doi: 10.1021/acsami.8b02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka N., Yang K.A., Abendroth J.M., Cheung K.M., Xu X., Yang H., Zhao C., Zhu B., Rim Y.S., Yang Y. Aptamer-field-effect transistors overcome Debye length limitations for small-molecule sensing. Science. 2018;362:319–324. doi: 10.1126/science.aao6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen N., Wetterslev J., Cronberg T., Erlinge D., Gasche Y., Hassager C., Horn J., Hovdenes J., Kjaergaard J., Kuiper M. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N. Engl. J. Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- Nyein H.Y.Y., Tai L.C., Ngo Q.P., Chao M., Zhang G.B., Gao W., Bariya M., Bullock J., Kim H., Fahad H.M. A wearable microfluidic sensing patch for dynamic sweat secretion analysis. ACS Sens. 2018;3:944–952. doi: 10.1021/acssensors.7b00961. [DOI] [PubMed] [Google Scholar]
- Park J.S., Kim T.W., Stryakhilev D., Lee J.S., An S.G., Pyo Y.S., Lee D.B., Mo Y.G., Jin D.U., Chung H.K. Flexible full color organic light-emitting diode display on polyimide plastic substrate driven by amorphous indium gallium zinc oxide thin-film transistors. Appl. Phys. Lett. 2009;95:013503. [Google Scholar]
- Patterson M.J., Galloway S.D., Nimmo M.A. Variations in regional sweat composition in normal human males. Exp. Physiol. 2000;85:869–875. doi: 10.1111/j.1469-445x.2000.02058.x. [DOI] [PubMed] [Google Scholar]
- Porchetta A., Idili A., Vallee-Belisle A., Ricci F. General strategy to introduce pH-induced allostery in DNA-based receptors to achieve controlled release of ligands. Nano Lett. 2015;15:4467–4471. doi: 10.1021/acs.nanolett.5b00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rim Y.S., Bae S.H., Chen H., Yang J.L., Kim J., Andrews A.M., Weiss P.S., Yang Y., Tseng H.R. Printable ultrathin metal oxide semiconductor-based conformal biosensors. ACS Nano. 2015;9:12174–12181. doi: 10.1021/acsnano.5b05325. [DOI] [PubMed] [Google Scholar]
- Rogers J., Bao Z., Lee T.W. Wearable bioelectronics: opportunities for chemistry. Acc. Chem. Res. 2019;52:521–522. doi: 10.1021/acs.accounts.9b00048. [DOI] [PubMed] [Google Scholar]
- So H.M., Park D.W., Jeon E.K., Kim Y.H., Kim B.S., Lee C.K., Choi S.Y., Kim S.C., Chang H., Lee J.O. Detection and titer estimation of Escherichia coli using aptamer-functionalized single-walled carbon-nanotube field-effect transistors. Small. 2008;4:197–201. doi: 10.1002/smll.200700664. [DOI] [PubMed] [Google Scholar]
- Sonner Z., Wilder E., Heikenfeld J., Kasting G., Beyette F., Swaile D., Sherman F., Joyce J., Hagen J., Kelley-Loughnane N. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics. 2015;9:031301. doi: 10.1063/1.4921039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgenfrei S., Chiu C.Y., Gonzalez R.L., Jr., Yu Y.J., Kim P., Nuckolls C., Shepard K.L. Label-free single-molecule detection of DNA-hybridization kinetics with a carbon nanotube field-effect transistor. Nat. Nanotechnol. 2011;6:126–132. doi: 10.1038/nnano.2010.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprigle S., Linden M., McKenna D., Davis K., Riordan B. Clinical skin temperature measurement to predict incipient pressure ulcers. Adv. Skin Wound Care. 2001;14:133–137. doi: 10.1097/00129334-200105000-00010. [DOI] [PubMed] [Google Scholar]
- Torrente-Rodriguez R.M., Tu J., Yang Y., Min J., Wang M., Song Y., Yu Y., Xu C., Ye C., IsHak W.W. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter. 2020;2:921–937. doi: 10.1016/j.matt.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Wang B., Normoyle K.P., Jackson K., Spitler K., Sharrock M.F., Miller C.M., Best C., Llano D., Du R. Brain temperature and its fundamental properties: a review for clinical neuroscientists. Front. Neurosci. 2014;8:307. doi: 10.3389/fnins.2014.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Xu J., Wang W., Wang G.N., Rastak R., Molina-Lopez F., Chung J.W., Niu S., Feig V.R., Lopez J. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature. 2018;555:83–88. doi: 10.1038/nature25494. [DOI] [PubMed] [Google Scholar]
- Webb R.C., Bonifas A.P., Behnaz A., Zhang Y., Yu K.J., Cheng H., Shi M., Bian Z., Liu Z., Kim Y.S. Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat. Mater. 2013;12:938–944. doi: 10.1038/nmat3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P.S. President obama announces the brain initiative. ACS Nano. 2013;7:2873–2874. doi: 10.1021/nn401796f. [DOI] [PubMed] [Google Scholar]
- Wen X., Wang B., Huang S., Liu T.L., Lee M.S., Chung P.S., Chow Y.T., Huang I.W., Monbouquette H.G., Maidment N.T. Flexible, multifunctional neural probe with liquid metal enabled, ultra-large tunable stiffness for deep-brain chemical sensing and agent delivery. Biosens. Bioelectron. 2019;131:37–45. doi: 10.1016/j.bios.2019.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner I., Zayats M. Electronic aptamer-based sensors. Angew. Chem. Int. Ed. 2007;46:6408–6418. doi: 10.1002/anie.200604524. [DOI] [PubMed] [Google Scholar]
- Yang H., Thompson A.B., McIntosh B.J., Altieri S.C., Andrews A.M. Physiologically relevant changes in serotonin resolved by fast microdialysis. ACS Chem. Neurosci. 2013;4:790–798. doi: 10.1021/cn400072f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Sampson M.M., Senturk D., Andrews A.M. Sex- and SERT-mediated differences in stimulated serotonin revealed by fast microdialysis. ACS Chem. Neurosci. 2015;6:1487–1501. doi: 10.1021/acschemneuro.5b00132. [DOI] [PubMed] [Google Scholar]
- Yang Y., Song Y., Bo X., Min J., Pak O.S., Zhu L., Wang M., Tu J., Kogan A., Zhang H. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 2020;38:217–224. doi: 10.1038/s41587-019-0321-x. [DOI] [PubMed] [Google Scholar]
- Yu K.J., Kuzum D., Hwang S.W., Kim B.H., Juul H., Kim N.H., Won S.M., Chiang K., Trumpis M., Richardson A.G. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 2016;15:782–791. doi: 10.1038/nmat4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Xu X., Bae S.H., Yang Q., Liu W., Belling J.N., Cheung K.M., Rim Y.S., Yang Y., Andrews A.M. Large-area, ultrathin metal-oxide semiconductor nanoribbon arrays fabricated by chemical lift-off lithography. Nano Lett. 2018;18:5590–5595. doi: 10.1021/acs.nanolett.8b02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Wang B., Hojaiji H., Wang Z., Lin S., Yeung C., Lin H., Nguyen P., Chiu K., Salahi K. A wearable freestanding electrochemical sensing system. Sci. Adv. 2020;6:eaaz0007. doi: 10.1126/sciadv.aaz0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Hong G., Fu T.M., Yang X., Schuhmann T.G., Viveros R.D., Lieber C.M. Syringe-injectable mesh electronics integrate seamlessly with minimal chronic immune response in the brain. Proc. Natl. Acad. Sci. U S A. 2017;114:5894–5899. doi: 10.1073/pnas.1705509114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
. Transparent Methods and Figures S1–S5
Data Availability Statement
This study did not generate/analyze datasets/code.