Abstract
Proper measurement of the QT interval on the 12-lead body-surface ECG is challenging in daily practice. Even more difficult is its correct estimation in the presence of repolarization abnormalities, arrhythmias or bundle-branch blocks (BBB). The QT interval results from two parts of the ECG: (1) the QRS complex, describing the excitation of the ventricles and (2) the JT interval, describing the repolarisation of the ventricles. Prolongation of the QRS width – like in the presence of BBB – entails prolongation of the QT interval, making the estimation of the true repolarisation time challenging. The US recommendations for the standardization and interpretation of the ECG suggest focusing on the JT interval in presence of BBB. However, in clinical practice physicians have become more familiar with the interpretation of QT-interval measurements than with the interpretation of the JT Interval.
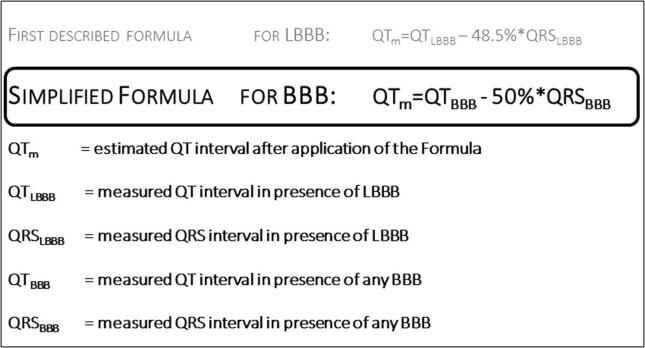
In the last decade, a simple formula for the estimation of the “modified QT interval” in the presence of left or right BBB has been developed and evaluated. In this formula, the modified QT interval is calculated by subtracting 50% of the length of the BBB-QRS from the measured QT interval (QTm = QTBBB − 50% QRSBBB). Subsequently, rate-correction formula should be applied as usual. In this review, we discuss the determination of the QT-interval in the presence of BBB and summarize the origin and application of the modified QT-interval formula.
Keywords: QT formula, Left bundle branch block, Right bundle branch block, QT interval, JT interval, Long QT
1. Introduction
From 2007 to 2009, the American Heart Association (AHA), the American College of Cardiology Foundation (ACCF) and the Heart Rhythm Society (HRS) published a series of recommendations for the standardization and interpretation of the ECG [1], [2], [3], [4], [5]. The fourth section of this series deals with the ST segment, the T wave, the U wave and the QT interval [1]. The QT interval describes the depolarization and repolarization of the ventricles (normal values in women <460 ms, in men <450 ms) [1]. QT-interval prolongation can result from alterations in both components: Depolarization disorders lead to the broadening of the QRS complex, whereas repolarization disorders lead to the prolongation of the JT time. The importance of QT prolongation due to repolarization disorders has been shown in several large studies [6], [7].
2. Arrhythmogenic risk in the setting of QT-interval prolongation
During the last two decades different mechanisms of arrhythmogenesis have been investigated. In the setting of prolonged repolarization inheritable arrhythmogenic disorders due to gene mutations were identified as an important cause of arrhythmogenesis. In these disorders, loss-of-function mutations in repolarizing K+ channels, e.g., long-QT syndrome type 2 (LQT2) due to the rapid delayed rectifier (IKr) channel malfunction, or gain-of-function mutations in depolarizing sodium (Na+) or calcium (Ca2+) channels lead to excessive repolarization prolongation. This repolarization prolongation can result in triggered activity by promoting the genesis of early afterdepolarizations and is often spatially heterogeneous, providing a substrate for conduction block and reentrant arrhythmias. Various cardiovascular and non-cardiovascular drugs can also cause relevant QT prolongation, also [8]. This increase in QT time, in turn, is associated with an increased risk of arrhythmia. In this context, Torsade de pointes tachycardia in particular can occur, particularly in the presence of additional risk factors such as bradycardia or hypokalemia [9].
Although many alternative measures have been suggested (e.g., T-peak-Tend; T-wave right slope; beat-to-beat variability of QT intervals) and the correlation between QT-interval and arrhythmogenesis is imperfect, the QT interval remains the most commonly used indicator of arrhythmogenic risk [10].
Besides the arrhythmogenic aspects, QT interval serves also as a predictor for cardiovascular events. In the multi-ethnic study of Atherosclerosis (MESA-Study), Beinart et al. analyzed the prognostic association between the baseline QT interval and the incidence of cardiovascular events in participants without known cardiac diseases. An increase in the baseline QT interval (for every 10 ms) was associated with increased incidence for heart failure, cardiovascular disease events and stroke [11]. Compared to patients with QT intervals below 500 ms, patients with QT intervals above 500 ms are exposed to a higher short-term mortality risk, independent from the underlying comorbidities, [12]. Additionally, QT-prolongation has been described as a prognostic parameter in Non-ST-Elevation Myocardial Infarction (NSTEMI) patients. Rajvanshi et al. defined the corrected QT Interval above 468 ms as a cut-off value to predict poor prognosis for the occurrence of major adverse cardiac events (MACE) with a sensitivity of 72% and a specificity of 61% [13].
3. Assessing QT-interval in the presence of bundle-branch block
Widening of the QRS complex – in the setting of left bundle-branch block (LBBB) or right bundle-branch block (RBBB) – leads to QT-interval prolongation without significant alterations to the repolarization duration [14]. Therefore, the AHA / ACCF / HRS recommendations for standardized ECG interpretation recommend the use of the JT time to assess the repolarization. However, in daily clinical practice, the measurement of the JT time is not yet common. Moreover, tables for rate adjustment of the JT interval are lacking. However, given the strong dependence of repolarization duration on heart rates, an adequate rate correction seems essential for correct interpretation of potential pathological alterations, especially in the presence of right or left BBB [1].
A bundle brunch block is commonly associated with extended prolonged QTc interval. However, in this setting, the QT interval, which is easily overestimated, is the result of a prolongation of depolarisation and not primarily caused by repolarisation. Interpretation in chronic LBBB with consecutive electrical remodelling is more challenging: unloading of LV areas results in shortening of repolarisation, whilst increased loading in late activated regions may cause lengthening of repolarisation due to downregulation of the slow delayed rectifier channel (IKs).
Besides the suggestion to evaluate the JT interval in patients with LBBB as an alternative risk-stratification method in the presence of LBBB [1], several formulas were developed the last years for avertable estimation of the QT interval [14], [15], [16], [17], [18], [19], [20] (Table 1). A different approach in pacemaker recipients is the subtraction of 50 ms of the QTcLBBB as a rule of thumb. However this application may lead to a relevant overestimation of up to 80 ms [21].
Table 1.
Overview of the different QT-Formulas. *applicability of this formula in RBBB was shown in a following publication [26].**further assessment performed in four following publications [22], [23], [25], [26]. ***further assessment performed in the same publication [20].
| Year | Author | Journal | fixed Formula | additional HR-correction | Applicable in LBBB and RBBB | primary assessment patients [n] | further assessment patients [n] |
|---|---|---|---|---|---|---|---|
| 1973 | Talbot S. | BHJ | LBBB: QTm = QT − 60 ms; QTmc = QT − 70 ms; | Yes/No | No | 95 | – |
| 1973 | Talbot S. | BHJ | RBBB: QTm = QT − 30 ms; QTmc = QT − 40 ms | Yes/No | No | 93 | – |
| 2004 | Rautaharju PM. et al. | Am J Cardiol | QTRR,QRS = QT − 155 × (60/heart rate − 1) − 0.93 X (QRS − 139) + k; [k = –22 ms for men and − 34 ms for women] | No | Yes | 1251 | – |
| 2014 | Bogossian H. et al. | Heart Rhythm | QTm = QT − 48.5%QRS; simplified: QTm = QT − 50% QRS | Yes | Yes* | 60 | 480** |
| 2016 | Tabatabaei P. et al. | Res Cardiovasc Med | QTmc= (0.786 × QT) + (0.305 × CL) − 188.733 | No | No | 101 | – |
| 2017 | Wang B. et al. | JCE | QTm = QT – (0.86 × QRS – 71) | Yes | No | 62 | – |
| 2018 | Yankelson L. et al. | J of Electrocardiol | male: QTmc = QTc-QRS + 95 ms; female: QTmc = QTc-QRS + 88 ms | No | No | 48 | – |
| 2019 | Tang JKK. Et al. | Canad J Cardiol | QTmc = 0.945 × QTc(RBK) − 26 | No | No | 17 | 2610*** |
For easier QT time assessment in the presence of BBB, recent studies have proposed a formula that corrects for prolongations of QT intervals due to QRS widening (due to pacing, LBBB or RBBB) (Fig. 1, Fig. 2) [16].
Fig. 1.
Formula for easy correction of QT interval in presence of LBBB and the simplified formula for BBB.
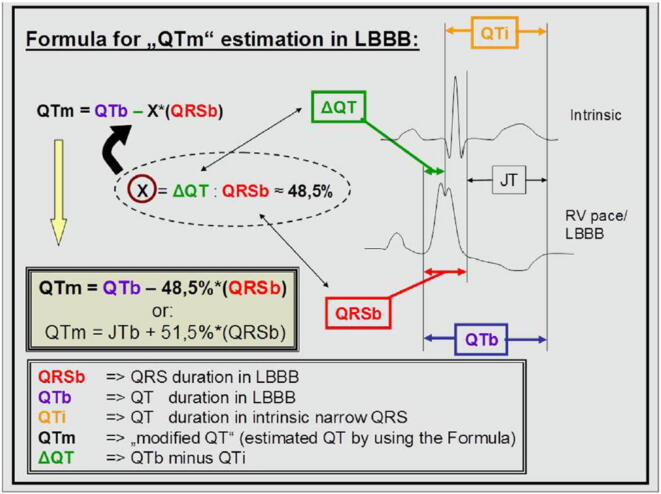
Fig. 2.
Figure from the first Publication in patients with artificial LBBB. (Reprinted from Publication: New formula for evaluation of the QT interval in patients with left bundle branch block. Heart Rhythm, 2014. 11(12): p. 2273–2277; Bogossian, H., et al., with permission from Elsevier.)
3.1. Development of different formulas for modified QT estimation in the presence of LBBB
The correct judgment of the QT interval in the presence of LBBB was focus of several research activities in the past. In 1973, Tablot et al. suggested subtraction of a fixed time interval: QT minus 60 ms in LBBB and minus 30 ms in RBBB or QTc minus 70 ms and minus 40 ms, respectively. As the QRS duration in patients with a BBB varies from 120 ms to more than 200 ms, this formula runs the risk to overestimate or underestimate the modified QT interval in accordance to the QRS width. A more precise formula was developed and described by Rautaharju et al. and covers all kinds of ventricular conduction defects (LBBB, RBBB and intraventricular delay) and the complete heart-rate spectrum (Table 1). However, the complexity of the formula, does not offer a good solution for daily clinical practice. In 2014, a further formula was developed for estimation of the QT interval in presence of LBBB [16] (Fig. 2). In a proof-of-concept study, the alterations of the QRS complex and QT intervals were analyzed during right ventricular (RV) pacing in patients with inherent normal – not widened – QRS complex, who presented for an electrophysiological study and ablation. Thus, the documented LBBB in these patients was due to RV pacing, providing an opportunity to compare QT times and JT times in the presence or absence of simulated LBBB. The results of these comparisons were subsequently translated into a formula for the modified QT interval (QTm). This formula suggested subtracting a particular QRS amount from the measured QT interval (in detail: QTm = QTLBBB – 48.5% of QRSLBBB). Subsequently, this formula was simplified for easier applicability in daily practice (to: QTm = QTLBBB- 50% of QRSLBBB) [16].
In a second step, the formula was analyzed in patients with intrinsic LBBB by comparing ECGs of patients presenting with both a narrow QRS complex and an LBBB [22]. For this purpose, two patient collectives were examined. First, patients with inherent intermittent LBBB and second, patients with peri- or post-procedural LBBB after a trans-venous percutaneous aortic valve implantation (TAVI) [22]. These patients served as their own control groups, since they had both ECGs with a narrow QRS and ECGs with LBBB. In both groups (15 patients each), the acquired mean native QTc intervals and those calculated by the formula during LBBB were not significantly different.
Another patient population with intermittent artificial LBBB are patients with implanted pacemakers. A further step in the analysis of the formula was therefore to check the applicability of the formula in these patients [23]. In total, 163 patients with a cardiac one- or two-chamber pacemaker were included in this prospective, multicentre observational study. Although there was a slight systematic overestimation of the true QT interval by the BBB correction formula, the overall agreement was high [23]. Subsequently, several additional formulas followed and were used in a similar way to the prior assessment methods. Like for the development of the Bogossian-formula in 2014, Tabatabaei et al. (2016) and Wang et al. (2017) used also the method of artificial LBBB during right ventricular pacing in patients who underwent electrophysiological studies [17], [18]. Finally, Yankelson et al. (2018) and Tang et al. (2019) used the second assessment-method of the Bogossian-formula and analyzed patients with intermittent LBBB or with LBBB after trans-aortic valve replacement (TAVR) [19], [20], [22].
3.2. Applicability of the LBBB formula in heart failure patients
LBBB is a common ECG feature in patients with heart failure due to impaired left-ventricular (LV) function. Hence, the applicability of the formula was also verified for these patients. As patients with severe LV dysfunction typically have extensive structural and electrical remodeling leading to an increased risk of sudden cardiac death [24], this observation has great implications for daily medical practice. LV dysfunction might persist despite optimal drug therapy. In these cases, the implantation of a cardioverter defibrillator (ICD) is recommended. The adequate functioning of the defibrillator lead (placed in the right ventricle) is checked telemetrically during regular follow-ups. Part of the routine examination is the measurement of the lead threshold. During this maneuver, the right ventricle is stimulated, resulting in a typical change of the ECG with artificial LBBB. Again, taking advantage of this setting, the modified QT formula could be assessed for measurement of QT times in the presence and absence of provoked LBBB, for this patient cohort [25].
3.3. Applicability of the LBBB formula in RBBB
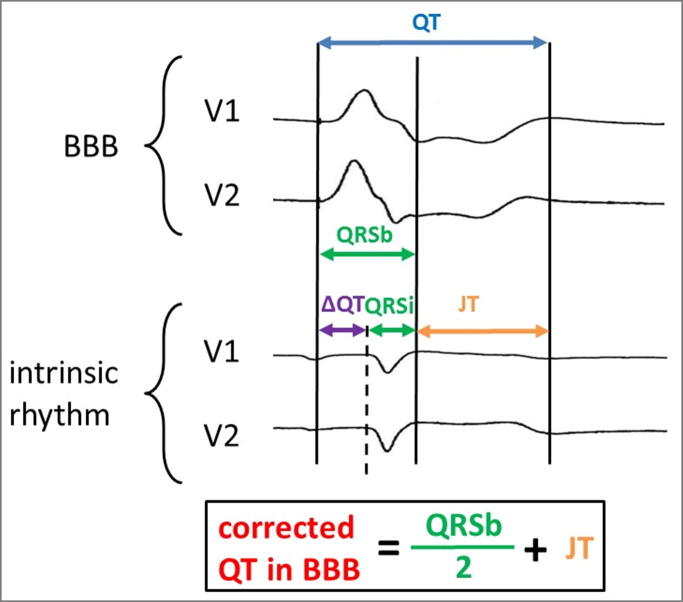
As presented in Table 1, just the formulas by Rautaharju and Bogossian were studied regarding the applicability in the presence of RBBB. In the study by Ratauharju, 593 out of 1251 patients presented a RBBB pattern. Erkapic et al. investigated the applicability of the Bogossian-formula to assess the QT interval in patients with RBBB. Based on a similar method of design as for the development of the LBBB-formula, the appropriate use during RBBB was further investigated [26]. In particular, during left-sided EP procedures an artificial RBBB (with left anterior or left posterior hemiblock) was induced by left ventricular pacing. Again, the same patient collective could serve as control group by comparing their initial ECGs (with narrow QRS complexes) to the generated ECGs during LV pacing (with broadened QRS-complexes in the course of artificial RBBB) (Fig. 3). In this prospective multicenter observational study, 71 patients were included. The mean difference between intrinsic QTc and corrected QTc during RBBB was −3 ± 24 ms and − 6 ± 25 ms for RBBB with left posterior and left anterior hemiblock, respectively [26], thus demonstrating the applicability of the LBBB formula in patients with RBBB.
Fig. 3.
Figure from the last Publication in patients with artificial RBBB. (Reprinted from Publication “How to measure QT-interval in right bundle branch block and bifascicular block”. Clin Cardiol, 2020; Erkapic D et al.)
3.4. Combination of BBB formula and heart rate correction formula
The estimation of the QT interval in the presence of BBB is significantly more complex at extreme heart rates (i.e., heart-rates other than 60 bpm and especially in bradycardia or tachycardia). This was a relevant aspect in the Publications of Rautaharju, Tang and Erkapic [14], [20], [26]. In the Study of Rautahrju et al., rate- and gender-adjusted JT interval was in focus during the formula investigation. Tang et al. focuses for the rate correction on the spline QT formula (QTcRBK) [27], which was earlier described by the same working group.
During the first description of the Bogossian-formula a rate-correction was not necessary, as the paced QRS complexes were generated just a few beats faster than the intrinsic heart rate [16]. In the subsequent publications, the paced rhythm was significantly higher than the intrinsic heart rate [22], [23], [25], making heart-rate correction indispensable in these cohorts. Initially, the Bazett formula was applied, resulting in a substantial deviation in corrected intervals whenever the heart-rate deviation was high. A regression analysis revealed a linear relationship between the deviation of the intrinsic QTc from the modified QTc and the difference between the intrinsic heart rate and paced heart rate [23]. A fast heart rate resulted in an increasing difference between intrinsic and modified QTc. Recently, in the manuscript evaluating the LBBB-formula in RBBB-QRS-complexes, the authors applied three different heart rate correction formulas (Bazett, Fridericia, and Hodges) after QT-correction (due to QRS width) [26]. This comparison revealed that the smallest deviation was achieved by the Hodges-formula, intermediate deviation by the Fridericia-formula and the greatest deviation by Bazett’s formula.
4. Conclusions and clinical implications
Different QRS-QT-formulas have recently been introduced in order to identify easier patients with LBBB or RBBB at high risk for ventricular arrhythmias due to true prolonged QT duration. None of the formulas has the power to unmask the real QT time behind the BBBs. Incorporating more complex formulas (like that by Rautaharju) in automated ECG analysis programs may help to keep deviations as small as possible and increase comparability in the assessment of QT intervals during BBB. Furthermore, better heart rate corrections (as described by Rabkin and Tang) could lead to further reduce heart rate related deviations.
However, in routine clinical practice, easy formulas (as described by Bogossian) for the estimation of the modified QTc in the presence of LBBB, RBBB or in pacemaker patients with LBBB-like ECG patterns remain reliable tools for a quick appraisal. Additional rate correction should in this case preferably be performed by Hodges’ formula (or alternatively using the Fridericia formula). Bazett’s formula seems to lead to the greatest deviation of the expected values.
5. Limitation
In the presence of BBB, the estimation of the real repolarization time from the ECG remains very challenging. The JT interval seems to be the best representative value for this evaluation. However, in clinical practice the experience with the JT interval is limited. The estimation of the exact and real QT interval in the presence of BBB needs further experimental and clinical investigations.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
Figure 2 reprinted from Publication: New formula for evaluation of the QT interval in patients with left bundle branch block. Heart Rhythm, 2014. 11(12): p. 2273-2277; Bogossian, H., et al., with permission from Elsevier.“
Figure 3 reprinted from open access publication: Erkapic, D., et al., QTc interval evaluation in patients with right bundle branch block or bifascicular blocks. Clin Cardiol, 2020. 2020 May 19. doi: 10.1002/clc.23389, with creative commons license unrestricted use permission.
References
- 1.Rautaharju P.M. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119(10):e241–e250. doi: 10.1161/CIRCULATIONAHA.108.191096. [DOI] [PubMed] [Google Scholar]
- 2.Kligfield P. Recommendations for the standardization and interpretation of the electrocardiogram: part I: The electrocardiogram and its technology: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2007;115(10):1306–1324. doi: 10.1161/CIRCULATIONAHA.106.180200. [DOI] [PubMed] [Google Scholar]
- 3.Mason J.W. Recommendations for the standardization and interpretation of the electrocardiogram: part II: electrocardiography diagnostic statement list a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll Cardiol. 2007;49(10):1128–1135. doi: 10.1016/j.jacc.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Hancock E.W. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2009;53(11):992–1002. doi: 10.1016/j.jacc.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Wagner G.S. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part VI: acute ischemia/infarction: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2009;53(11):1003–1011. doi: 10.1016/j.jacc.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen J.B. Risk prediction of cardiovascular death based on the QTc interval: evaluating age and gender differences in a large primary care population. Eur. Heart J. 2014;35(20):1335–1344. doi: 10.1093/eurheartj/ehu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Priori S.G. Risk stratification in the long-QT syndrome. N Engl. J. Med. 2003;348(19):1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 8.Indraratna P. Measurement and management of QT interval prolongation for general physicians. J. Gen Int. Med. 2020;35(3):865–873. doi: 10.1007/s11606-019-05477-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimizu W. Bradycardia-dependent early afterdepolarizations in a patient with QTU prolongation and torsade de pointes in association with marked bradycardia and hypokalemia. Pacing Clin. Electrophysiol. 1991;14(7):1105–1111. doi: 10.1111/j.1540-8159.1991.tb02841.x. [DOI] [PubMed] [Google Scholar]
- 10.J. Heijman, H.J. Crijns, T-wave right slope provides a new angle in the prediction of drug-induced ventricular arrhythmias: editorial to: “Electrocardiographic Predictors of Torsadogenic Risk During Dofetilide or Sotalol Initiation: Utility of a Novel T Wave Analysis Program” by Sugrue A. et al. Cardiovasc Drugs Ther 29(5) (2015) 411–3. [DOI] [PubMed]
- 11.Beinart R. The QT interval is associated with incident cardiovascular events: the MESA study. J Am Coll Cardiol. 2014;64(20):2111–2119. doi: 10.1016/j.jacc.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbs C. QT prolongation predicts short-term mortality independent of comorbidity. Europace. 2019;21(8):1254–1260. doi: 10.1093/europace/euz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajvanshi S. Correlation of corrected QT interval with quantitative cardiac troponin-I levels and its prognostic role in Non-ST-elevation myocardial infarction. Int. J. Cardiol. 2017;240:55–59. doi: 10.1016/j.ijcard.2017.03.091. [DOI] [PubMed] [Google Scholar]
- 14.Rautaharju P.M. Assessment of prolonged QT and JT intervals in ventricular conduction defects. Am. J. Cardiol. 2004;93(8):1017–1021. doi: 10.1016/j.amjcard.2003.12.055. [DOI] [PubMed] [Google Scholar]
- 15.Talbot S. QT interval in right and left bundle-branch block. Br. Heart J. 1973;35(3):288–291. doi: 10.1136/hrt.35.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogossian H. New formula for evaluation of the QT interval in patients with left bundle branch block. Heart Rhythm. 2014;11(12):2273–2277. doi: 10.1016/j.hrthm.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Tabatabaei P. Assessment of QT and JT intervals in patients with left bundle branch block. Res. Cardiovasc. Med. 2016;5(2):e31528. doi: 10.5812/cardiovascmed.31528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B. A new formula for estimating the true QT interval in left bundle branch block. J. Cardiovasc. Electrophysiol. 2017;28(6):684–689. doi: 10.1111/jce.13203. [DOI] [PubMed] [Google Scholar]
- 19.Yankelson L. New formula for defining “normal” and ”prolonged” QT in patients with bundle branch block. J. Electrocardiol. 2018;51(3):481–486. doi: 10.1016/j.jelectrocard.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 20.Tang J.K.K., Rabkin S.W. Determination of the QT interval in left bundle branch block: development of a novel formula. Can. J. Cardiol. 2019;35(7):855–865. doi: 10.1016/j.cjca.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Chakravarty S. Corrected QT in ventricular paced rhythms: what is the validation for commonly practiced assumptions? Cardiology. 2015;130(4):207–210. doi: 10.1159/000370026. [DOI] [PubMed] [Google Scholar]
- 22.Bogossian H. A new experimentally validated formula to calculate the QT interval in the presence of left bundle branch block holds true in the clinical setting. Ann. Noninvasive Electrocardiol. 2017;22(2) doi: 10.1111/anec.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weipert K.F. Application of the Bogossian formula for evaluation of the QT interval in pacemaker patients with stimulated left bundle branch block. Clin. Res. Cardiol. 2018;107(11):1033–1039. doi: 10.1007/s00392-018-1275-6. [DOI] [PubMed] [Google Scholar]
- 24.Priori S.G. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Europace. 2015;17(11):1601–1687. doi: 10.1093/europace/euv319. [DOI] [PubMed] [Google Scholar]
- 25.Frommeyer G. Applicability of a Novel Formula (Bogossian formula) for Evaluation of the QT-Interval in Heart Failure and Left Bundle Branch Block Due to Right Ventricular Pacing. Pacing. Clin. Electrophysiol. 2017;40(4):409–416. doi: 10.1111/pace.13027. [DOI] [PubMed] [Google Scholar]
- 26.Erkapic D. QTc interval evaluation in patients with right bundle branch block or bifascicular blocks. Clin. Cardiol. 2020 doi: 10.1002/clc.23389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabkin S.W., Szefer E., Thompson D.J.S. A new QT interval correction formulae to adjust for increases in heart rate. JACC Clin. Electrophysiol. 2017;3(7):756–766. doi: 10.1016/j.jacep.2016.12.005. [DOI] [PubMed] [Google Scholar]