Abstract
Introduction
This analysis evaluated the efficacy and safety of dulaglutide in Chinese patients with type 2 diabetes (T2D) aged ≥ 60 and < 60 years.
Methods
This post hoc analysis included patients with T2D enrolled in two phase 3 clinical trials AWARD-CHN1 (NCT01644500) and AWARD-CHN2 (NCT01648582) of dulaglutide 0.75 and 1.5 mg. Patients were categorized into two groups (≥ 60 and < 60 years). Efficacy outcomes (change in glycated hemoglobin [HbA1c], fasting blood glucose [FBG], and weight; percentage of patients achieving HbA1c target [< 7.0%]) and safety outcomes (incidence of hypoglycemia and gastrointestinal treatment-emergent adverse events [GI TEAEs]) at 26 weeks were evaluated for each age group in both trials.
Results
A total of 766 patients (≥ 60 years, n = 222; < 60 years, n = 544) were included in the study. A similar reduction of HbA1c was observed in both age groups: AWARD-CHN1, 1.5 mg (least squares mean [LSM] 95% confidence interval [CI] ≥ 60 years: − 1.45% [− 1.69, − 1.21%] and < 60 years: − 1.43% [− 1.59, − 1.28%]) and 0.75 mg (≥ 60 years: − 1.29% [− 1.53, − 1.05%] and < 60 years: − 1.18% [− 1.33, − 1.03%]); AWARD-CHN2, 1.5 mg (≥ 60 years: − 1.60% [− 1.83, − 1.36%] and < 60 years: − 1.64% [− 1.80, − 1.49%]) and 0.75 mg (≥ 60 years: − 1.31% [− 1.55, − 1.08%] and < 60 years: − 1.33% [− 1.48, − 1.17%]). Dulaglutide showed a reduction in HbA1c as early as 4 weeks after initiation of treatment, which was maintained over 26 weeks in both age groups. The percentage of patients achieving HbA1c target < 7.0% at 26 weeks was also similar in both age groups. Incidence of hypoglycemia and GI TEAEs was low in each age group.
Conclusion
Treatment with once-weekly dulaglutide improved glycemic control in patients with T2D aged ≥ 60 years and < 60 years and was well tolerated in older patients, suggesting it can be considered a safe and effective treatment option for use in older patients with T2D.
Trial registration
AWARD-CHN1 (NCT01644500) and AWARD-CHN2 (NCT01648582).
Electronic supplementary material
The online version of this article (10.1007/s13300-020-00910-1) contains supplementary material, which is available to authorized users.
Keywords: Age, Dulaglutide, GLP-1 receptor agonist, Subgroup analysis, Type 2 diabetes, β-cell function
Key Summary Points
| Why carry out this study? |
| Type 2 diabetes (T2D) is increasing at an alarming rate in geriatric populations, and treatment in these patients is complex because of comorbidities and functional impairments, and a high risk of adverse events such as recurring hypoglycemia. |
| Glucagon-like peptide 1 (GLP-1) receptor agonists (RAs) are considered a good therapy for the elderly patients with diabetes owing to its low risk of hypoglycemia. |
| The present study is a post hoc analysis of AWARD-CHN1 and AWARD-CHN2 to evaluate efficacy and safety of once-weekly dulaglutide (1.5 and 0.75 mg) in patients with T2D aged ≥ 60 and < 60 years. |
| What was learned from the study? |
| The study indicated a similar change in HbA1c, FBG, and weight reduction and increased proportion of patients achieving HbA1c target (< 7%) in Chinese patients in both age groups (≥ 60 and < 60 years). Incidence of GI TEAEs and hypoglycemia were low. |
| The overall findings from the present study demonstrate improvement of glycemic control and tolerability of once-weekly dulaglutide in patients aged ≥ 60 and < 60 years. |
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to 10.6084/m9.figshare.12801050.
Introduction
Diabetes mellitus has been rising at an alarming rate worldwide with the prevalence in adults (20–79 years) at 9.3% (463 million) in 2019, and it is estimated that it will be approximately 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045 [1], of which type 2 diabetes (T2D) accounts for 90–95% [2]. In 2019, China had the highest number of adults with diabetes (116.4 million) and is anticipated to remain so in 2030 and 2045 [3].
An aging population contributes to the increased prevalence of diabetes, with a prevalence at 20.2% in over 60-year-olds in China [4]. Aging is also associated with many comorbidities such as cognitive dysfunction, neuropathy, renal impairment, and high-risk adverse events such as recurring hypoglycemia. The utility of some therapies, including insulin and sulfonylureas, is limited in geriatric populations by the risk of hypoglycemia [5, 6].
Glucagon-like peptide 1 (GLP-1) receptor agonists (RAs) enhance insulin secretion and suppress glucagon secretion in a glucose-dependent manner [7] and impart low risk of hypoglycemia [8] and could be considered for the management of glycemic control in older people with T2D with either previous cardiovascular disease or cardiovascular risk factors [9]. Dulaglutide, a once-weekly GLP-1RA approved for treatment of T2D in China, has been reported to be beneficial in Chinese patients with T2D in two phase 3 trials (AWARD-CHN1 and AWARD-CHN2) [10, 11].
The present post hoc analysis aimed to investigate the efficacy and safety of dulaglutide in Chinese patients with T2D aged ≥ 60 years and < 60 years and to provide evidence for the older population with T2DM when using dulaglutide in clinical practice for Chinese patients.
Methods
Study Design and Patients
The post hoc analysis of AWARD-CHN1 (NCT01644500) and AWARD-CHN2 (NCT01648582) only included the Chinese sub-population which was stratified by baseline age. The study design of AWARD-CHN studies was published in detail previously [12, 13]. AWARD-CHN1 was a double-blind, multicenter, randomized, phase 3 study to compare the efficacy and safety of dulaglutide with glimepiride monotherapy, and AWARD-CHN2 was an open-label, multicenter, randomized, non-inferiority phase 3 study to compare the efficacy and safety of dulaglutide with insulin glargine add-on to metformin and/or sulfonylurea.
Patients in each trial were categorized into two groups based on age at baseline, ≥ 60 and < 60 years, following Chinese Diabetes Society guidance that considers geriatrics as 60 years and older [14]. With the development of diabetes, lower β-cell function advances with aging, so patients were also categorized into tertiles based on baseline β-cell function [Updated Homeostasis Model Assessment of β-cell function (HOMA2-%B)].
This study is a post hoc analysis of primary publications [12, 13]. Both studies were initiated after approval of the study protocol by the ethics review board at each site including the master ethics review board at Ruijin Hospital Affiliate to Shanghai Jiao Tong University, and written informed consent was obtained from all patients. All procedures followed ethical standards, and the study was conducted in accordance with 1964 declaration of Helsinki and its amendments, Good Clinical Practice guidelines, and applicable local laws and regulations.
Study Outcomes
Efficacy outcomes included change in glycated hemoglobin (HbA1c), fasting blood glucose (FBG), and body weight from baseline to week 26; percentage of patients achieving < 7% HbA1c at week 26; and change in HbA1c over time.
Safety outcomes included the incidence of hypoglycemia and gastrointestinal treatment-emergent adverse events (GI TEAEs), which were the most common TEAEs observed in AWARD-CHN1 and AWARD-CHN2 [10, 11].
Statistical Analysis
Baseline data were compared with post-treatment data at week 26. The efficacy analysis was conducted on the modified intention-to-treat population. This included all randomized patients that received at least one dose of the study drug and had one baseline HbA1c measurement along with at least one post-baseline HbA1c measurement. Changes in HbA1c, FBG, and weight from baseline to 26 weeks were estimated by least-squares mean (LSM) and the corresponding 95% confidence interval (CI) using analysis of covariance (ANCOVA). Counts and percentages were used to summarize categorical data whereas mean, standard deviation, and median were used to summarize continuous data. Safety was assessed in terms of incidence of hypoglycemia and GI TEAEs in the safety population who received at least one dose of the study drug. SAS version 9.3 was used to perform all analyses.
Results
Patients Characteristics
A total of 766 patients [age ≥ 60 years, n = 106 (AWARD-CHN1) and n = 116 (AWARD-CHN2); age < 60 years, n = 264 (AWARD-CHN1) and n = 280 (AWARD-CHN2)] were included in the study. Of these 766 patients, 384 received 1.5 mg and 382 received 0.75 mg once-weekly dulaglutide. Baseline characteristics of patients stratified by age group with dulaglutide 1.5 mg or 0.75 mg in the two trials is shown in Table 1 and baseline characteristics of patients stratified by HOMA2-%B are given in Table S1 in the supplementary material.
Table 1.
Demographics and baseline characteristics stratified by age ≥ 60 and < 60 years
| Demographics | AWARD-CHN1 | AWARD-CHN2 | ||||||
|---|---|---|---|---|---|---|---|---|
| 1.5 mg | 0.75 mg | 1.5 mg | 0.75 mg | |||||
| Age ≥ 60 years (n = 54) | Age < 60 years (n = 130) | Age ≥ 60 years (n = 52) | Age < 60 years (n = 134) | Age ≥ 60 years (n = 59) | Age < 60 years (n = 141) | Age ≥ 60 years (n = 57) | Age < 60 years (n = 139) | |
| Gender, n (%) | ||||||||
| Male | 30 (55.6) | 79 (60.8) | 22 (42.3) | 85 (63.4) | 31 (52.5) | 86 (61.0) | 37 (64.9) | 83 (59.7) |
| Female | 24 (44.4) | 51 (39.2) | 30 (57.7) | 49 (36.6) | 28 (47.5) | 55 (39.0) | 20 (35.1) | 56 (40.3) |
| Age, years (mean ± SD) | 64.1 ± 3.2 | 48.1 ± 8.6 | 64.8 ± 3.4 | 49.5 ± 8.1 | 65.6 ± 3.9 | 49.9 ± 8.0 | 65.8 ± 4.5 | 49.3 ± 7.3 |
| Diabetes duration, years (mean ± SD) | 6.2 ± 6.1 | 3.1 ± 3.6 | 5.2 ± 5.5 | 2.5 ± 2.9 | 9.7 ± 4.9 | 6.9 ± 4.2 | 10.5 ± 5.7 | 6.7 ± 4.8 |
| BMI, kg/m2 (mean ± SD) | 25.4 ± 2.7 | 25.6 ± 3.4 | 26.1 ± 3.6 | 26.0 ± 3.3 | 25.3 ± 2.8 | 26.1 ± 3.3 | 25.7 ± 2.9 | 26.5 ± 3.4 |
| Weight, kg (mean ± SD) | 67.6 ± 10.1 | 70.5 ± 10.9 | 68.3 ± 11.6 | 71.7 ± 12.1 | 68.0 ± 9.7 | 73.6 ± 12.7 | 71.5 ± 10.3 | 73.9 ± 12.6 |
| HbA1c, % (mean ± SD) | 7.9 ± 0.8 | 8.0 ± 1.0 | 8.0 ± 1.0 | 8.1 ± 1.0 | 8.2 ± 1.3 | 8.5 ± 1.1 | 8.3 ± 1.1 | 8.3 ± 1.1 |
| FBG, mmol/L (mean ± SD) | 9.4 ± 2.3 | 9.5 ± 2.7 | 9.0 ± 2.2 | 9.4 ± 2.6 | 8.9 ± 2.2 | 9.9 ± 2.7 | 9.7 ± 2.2 | 9.7 ± 2.3 |
| HOMA2-%B, mean (range), % | 36.9 (7.5–89.9) | 39.2 (7.1–135.7) | 40.8 (10.5–93.6) | 41.3 (6.0–119.1) | 42.7 (5.4–152.6) | 34. 4 (7.5–100.9) | 35.9 (11.2–98.9) | 37.1 (7.3–136.9) |
| OAM regimen, n (%) | ||||||||
| Metformin only | NA | 24 (40.7) | 62 (44.0) | 17 (29.8) | 66 (47.8) | |||
| Sulfonylurea only | NA | 10 (16.9) | 18 (12.8) | 11 (19.3) | 16 (11.6) | |||
| Metformin and sulfonylurea | NA | 25 (42.4) | 61 (43.3) | 29 (50.9) | 56 (40.6) | |||
BMI body mass index, FBG fasting blood glucose, HbA1c glycosylated hemoglobin A1c, HOMA2-%B Updated Homeostasis Model Assessment of β-cell function, NA not applicable, OAM oral antihyperglycemia medication
Efficacy Outcomes
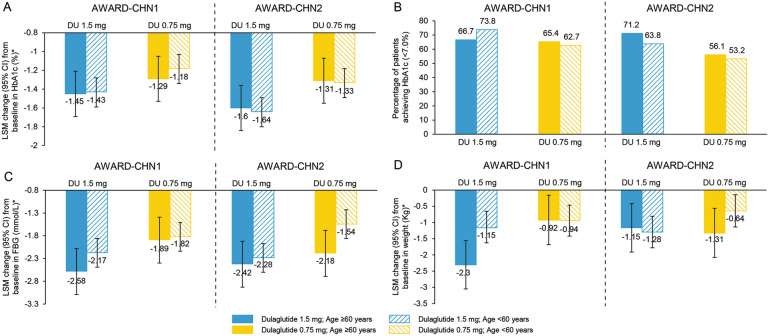
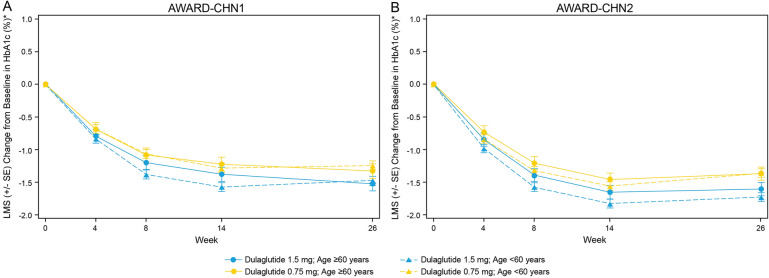
Patients in both age groups, ≥ 60 and < 60 years, experienced significant reduction in HbA1c from baseline to week 26 with 1.5 mg dulaglutide (AWARD-CHN1, ≥ 60 years: LSM [95% CI] − 1.45% [− 1.69, − 1.21%] and < 60 years: − 1.43% [− 1.59, − 1.28%]; P < 0.001 in both groups; AWARD-CHN2, ≥ 60 years − 1.60% [− 1.83, − 1.36%] and < 60 years: − 1.64% [− 1.80, − 1.49%]; P < 0.001 in both groups) and 0.75 mg dulaglutide treatment (AWARD-CHN1, ≥ 60 years: − 1.29% [− 1.53, − 1.05%] and < 60 years: − 1.18% [− 1.33, − 1.03%]; P < 0.001 in both groups; AWARD-CHN2: ≥ 60 years: − 1.31% [− 1.55, − 1.08%] and < 60 years: − 1.33% [− 1.48, − 1.17%]; P < 0.001 in both groups; Fig. 1a). LSM change in HbA1c from baseline showed a rapid reduction in HbA1c as early as 4 weeks of initiation of dulaglutide treatment that was maintained over time up to 26 weeks. A similar reduction in HbA1c was observed in patients aged ≥ 60 and < 60 years with both 1.5 and 0.75 mg at different time points in AWARD-CHN1 and AWARD-CHN2 (Fig. 2). At 26 weeks, the percentage of patients attaining the HbA1c target of < 7% was similar in both age groups (AWARD-CHN1, 1.5 mg [≥ 60 years: 66.7% and < 60 years: 73.8%] and 0.75 mg dulaglutide [≥ 60 years: 65.4% and < 60 years: 62.7%]; AWARD-CHN2, 1.5 mg [≥ 60 years: 71.2% and < 60 years: 63.8%] and 0.75 mg dulaglutide [≥ 60 years: 56.1% and < 60 years: 53.2%]; Fig. 1b).
Fig. 1.
Efficacy outcomes of dulaglutide in patients aged ≥ 60 and < 60 years. a Change in HbA1c from baseline to week 26; b percentage of patients achieving HbA1c target (< 7.0%) at week 26; c change in FBG from baseline to week 26; d change in weight from baseline to week 26
Fig. 2.
Change in HbA1c from baseline to week 26 over time stratified by age ≥ 60 and < 60 years. a AWARD-CHN1; b AWARD-CHN2
A comparable statistically significant decrease in FBG from baseline to week 26 was observed in both the age groups (P < 0.001): AWARD-CHN1, ≥ 60 years (1.5 mg LSM [95% CI] − 2.58 mmol/L [− 3.09, − 2.08 mmol/L] and 0.75 mg: − 1.89 mmol/L [− 2.41, − 1.37 mmol/L]) and < 60 years (1.5 mg: − 2.17 mmol/L [− 2.49, − 1.86 mmol/L] and 0.75 mg: − 1.82 mmol/L [− 2.14, − 1.51]); AWARD-CHN2, ≥ 60 years: (1.5 mg: − 2.42 mmol/L [− 2.95, − 1.88 mmol/L] and 0.75 mg: − 2.18 mmol/L [− 2.72, − 1.64 mmol/L] and < 60 years (1.5 mg: − 2.28 mmol/L [− 2.63, − 1.93 mmol/L] and 0.75 mg: − 1.54 mmol/L [− 1.90, − 1.19 mmol/L]; Fig. 1c). In addition, a statistically significant reduction in body weight was observed from baseline to week 26 in patients of both the age groups with two doses of dulaglutide in AWARD-CHN1 and AWARD-CHN2 (P < 0.05; Fig. 1d).
Further analysis of efficacy in patients stratified by baseline β-cell function tertile showed that the reductions of HbA1c and FBG were significant in each of the low, middle, and high β-cell function group (P < 0.05). The percentage of patients achieving HbA1c target of < 7% was 53.2–56.1% even in the low β-cell function group with 1.5 mg dulaglutide in the two trials. Weight reduction in each group was also observed and is shown in Fig. S1 of the supplementary material. A similar reduction in HbA1c was observed from baseline at week 4, 8, 14, and 26 that was maintained over time in low, middle, and high β-cell function groups (Fig. S2 of the supplementary material).
Safety Outcomes
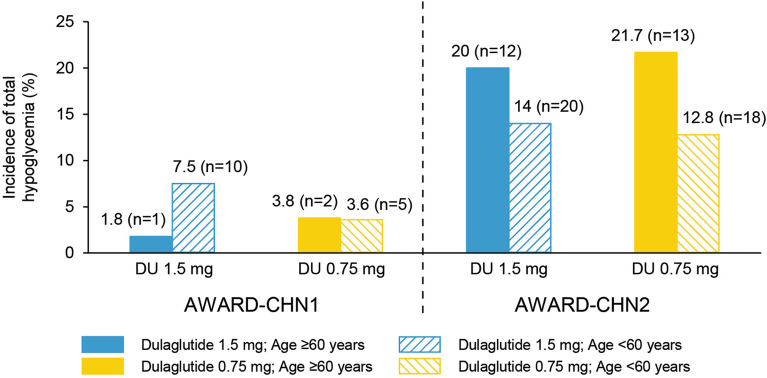
The incidence of total hypoglycemia was as follows: AWARD-CHN1, 1.5 mg (≥ 60 years: 1.8% and < 60 years: 7.5%) and 0.75 mg dulaglutide (≥ 60 years: 3.8% and < 60 years: 3.6%); AWARD-CHN2, 1.5 mg (≥ 60 years: 20.0% and < 60 years: 14.0%) and 0.75 mg dulaglutide (≥ 60 years: 21.7% and < 60 years: 12.8%; Fig. 3). No severe hypoglycemia events were observed in both trials.
Fig. 3.
Incidence of hypoglycemia [% (n)] through 26 weeks overall stratified by age ≥ 60 years and age < 60 years
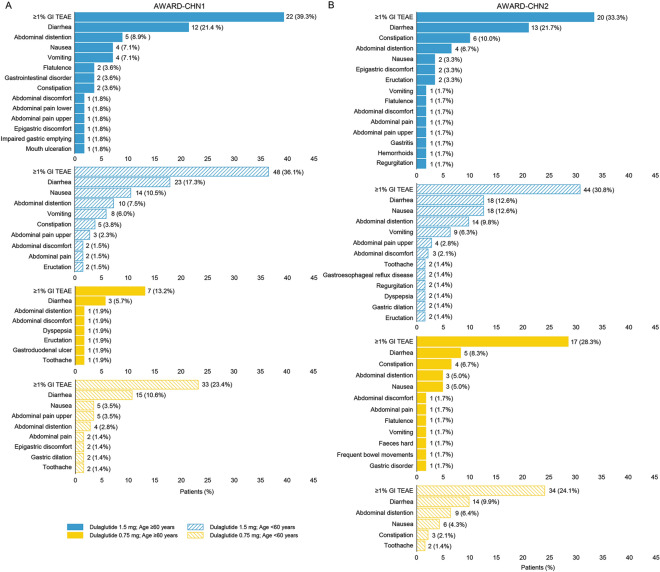
Incidences of GI TEAEs reported in ≥ 1% patients are represented in Fig. 4. Similar incidences of GI TEAEs were reported in both the age groups for 1.5 mg (incidence of ≥ 1% GI TEAE in AWARD-CHN1: 39.3% and 36.1% in patients aged ≥ 60 and < 60 years, respectively; AWARD-CHN2: 33.3% and 30.8% in patients aged ≥ 60 and < 60 years, respectively) and 0.75 mg dulaglutide treatment (incidence of ≥ 1% GI TEAE in AWARD-CHN2: 28.3% and 24.1% in patients aged ≥ 60 years and < 60 years, respectively). The most frequent GI-related adverse event was diarrhea; detailed incidence of GI TEAEs are listed in Fig. 4.
Fig. 4.
Gastrointestinal treatment-emergent adverse events reported in ≥ 1% patients through 26 weeks overall by aged ≥ 60 and < 60 years [n (%)]. a AWARD-CHN1; b AWARD-CHN2
Discussion
In this post hoc analysis of AWARD-CHN1 and AWARD-CHN2 trials, treatment with once-weekly dulaglutide demonstrated clinically relevant HbA1c, FBG, and weight reduction and led to increased proportions of Chinese patients with T2D aged ≥ 60 and < 60 years achieving HbA1c target (< 7%), and the efficacy of glycemic control was not influenced by baseline β-cell function.
In accordance with previous reports reviewing a pooled analysis of six trials, the present study showed similar effectiveness of glycemic control and safety profiles irrespective of baseline age [5]. Other GLP1-RAs such as liraglutide and exenatide also reported effective glycemic control irrespective of baseline age. Bode et al. reported that liraglutide was well tolerated in all patients irrespective of age with low hypoglycemic risk, and suggested it as a suitable option for geriatric patients with comorbidities [15]. Pencek et al. reported the tolerability of exenatide regardless of age in all patients [16]. The results of the present study were in accordance with other GLP-1RAs.
In the AWARD-CHN1 1.5 mg group, weight reduction in patients ≥ 60 years was greater than in patients < 60 years, although in AWARD-CHN2 weight reduction was similar between age groups. While there is not a clear rationale for the observation, dulaglutide was well tolerated in both age groups and weight reduction was within the acceptable range. A pooled analysis of dulaglutide stratified by age reported similar weight loss in both the age groups (< 65 and ≥ 65 years) [5].
A decrease in β-cell function with aging has been reported [17]; however, modulation of insulin secretion from β-cells in a glucose-dependent manner is one of the mechanisms of controlling glycemic levels by GLP-1RAs [18, 19]. The analysis of the effect of baseline β-cell function on dulaglutide efficacy in this study indicates that glycemic control was not influenced by baseline β-cell function, which is consistent with reported studies stating that once-weekly dulaglutide improved glycemic control irrespective of β-cell function [20].
Recently, GLP-1RAs have been recommended by the American Diabetes Association (ADA) as preferred over insulin in older adults with T2D at increased risk of hypoglycemia because meta-analyses [21–24] suggest lower risk of hypoglycemia and benefit of weight loss. Generally, few patients (≤ 6 [3.2%] in each dose treatment group) discontinued treatment as a result of AEs in AWARD-CHN1 and AWARD-CHN2 [10, 11]. The incidence of hypoglycemia was low in both AWARD-CHN1 and AWARD-CHN2. It is a little higher in AWARD-CHN2, which may be related to oral antihyperglycemic medication background use (i.e., metformin and/or sulfonylurea) in AWARD-CHN2 patients, suggesting that administration of dulaglutide with metformin and/or sulfonylurea may increase incidence of hypoglycemia in clinical practice in the older population. Dulaglutide was well tolerated in Chinese patients, and the incidence of TEAEs was generally low [10, 11]. GI TEAEs are the most common AEs in patients using GLP-1RAs, and in accordance, this analysis shows that GI adverse events, particularly diarrhea, were the most common TEAEs in the present study [25]. However, the incidence of diarrhea is similar with other GLP-1RAs [26] and is similar regardless of age and dose in this analysis. As per dulaglutide prescribing information, no dose adjustment is required on the basis of age; the results of present study also represent the same.
The present post hoc subgroup analysis has certain limitations of evidence but provides input for clinical practice. The baseline age range was limited to ≥ 60 years; however, the percentage of patients aged ≥ 75 years was small in these two trials. In addition, the duration of this study was limited to 26 weeks, which may not represent the effects of long-term use of dulaglutide.
Conclusion
Our findings indicate that treatment with once-weekly dulaglutide improves glycemic control even in patients aged ≥ 60 years and in lower baseline β-cell function groups. Dulaglutide was well tolerated in elderly patients, suggesting that dulaglutide can be considered a safe and effective treatment option for use in geriatric patients with T2D.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
The study and the journal’s rapid service fee were funded by Eli Lilly (Shanghai, China).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Siying Liu was involved in conception, design, and interpretation of data. Jiankun Zhu was involved in analysis of data and interpretation of data. Jian Kuang and Quanmin Li were involved in acquisition and interpretation of data.
Medical Writing, Editorial, and Other Assistance
The authors would like to acknowledge Dr. Yunjing Zhang (Lilly Suzhou Pharmaceutical Co. Ltd) for medical writing, medical review and project management, and Dr. Satya Lavanya Jakki and Dr. Anuradha Nalli (Indegene Pvt Ltd) for medical writing and editorial support.
Prior Presentation
An abstract and poster of this study were presented at the Chinese Diabetes Society 23rd Scientific Meeting, held on November 20-23, 2019 in Xiamen, China.
Disclosures
Siying Liu and Jiankun Zhu are employees of Eli Lilly and Company. Jian Kuang has received honoraria for attending meetings, consultancy fees, speaker fees for Eli Lilly and Company. Quanmin Li has nothing to disclose.
Compliance with Ethics Guidelines
This study is a post hoc analysis of primary publications [12, 13]. Both studies were initiated after approval of the study protocol by the ethics review board at each site including the master ethics review board at Ruijin Hospital Affiliate to Shanghai Jiao Tong University. All procedures followed were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013, and Good Clinical Practice. Written informed consent was obtained from all patients before participation.
Data Availability
The datasets generated and analyzed during the current study are not broadly publicly available to respect company confidentiality but may be available from the corresponding author on reasonable request.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12801050.
Contributor Information
Siying Liu, Email: liu_si_ying@lilly.com.
Quanmin Li, Email: liqm0806@163.com.
References
- 1.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci. 2014;11:1185–1200. doi: 10.7150/ijms.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IDF Diabetes Atlas, 9th edn. (2019). https://www.idf.org/diabetesatlas.
- 4.Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317:2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boustani MA, Pittman I, Yu M, Thieu VT, Varnado OJ, Juneja R. Similar efficacy and safety of once-weekly dulaglutide in patients with type 2 diabetes aged ≥65 and <65 years. Diabetes Obes Metab. 2016;18:820–828. doi: 10.1111/dom.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morley JE, Abbatecola AM, Woo J. Management of comorbidities in older persons with type 2 diabetes. J Am Med Dir Assoc. 2017;18:639–645. doi: 10.1016/j.jamda.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Nauck MA. Incretin-based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med. 2011;124:S3–18. doi: 10.1016/j.amjmed.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60:2342–2356. doi: 10.1111/jgs.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 10.Shi LX, Liu XM, Shi YQ, et al. Efficacy and safety of dulaglutide monotherapy compared with glimepiride in Chinese patients with type 2 diabetes: post-hoc analyses of a randomized, double-blind, phase III study. J Diabetes Investig. 2020;11:142–150. doi: 10.1111/jdi.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Li L, De Peng Y, et al. Efficacy and safety of dulaglutide versus insulin glargine in Chinese T2DM patients: a subgroup analysis of a randomized trial (AWARD-CHN2) Diabetes Ther. 2019;10:1435–1452. doi: 10.1007/s13300-019-0646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YH, Huang C-N, Cho YM, et al. Efficacy and safety of dulaglutide monotherapy compared with glimepiride in East-Asian patients with type 2 diabetes in a multicentre, double-blind, randomized, parallel-arm, active comparator, phase III trial. Diabetes Obes Metab. 2018;20:2121–2130. doi: 10.1111/dom.13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Nevárez L, Filippova E, et al. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in mainly Asian patients with type 2 diabetes mellitus on metformin and/or a sulphonylurea: a 52-week open-label, randomized phase III trial. Diabetes Obes Metab. 2019;21:234–243. doi: 10.1111/dom.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chinese Diabetes Society China guideline for type 2 diabetes (2017) Chin J Diabetes Melitus. 2018;10:4–67. [Google Scholar]
- 15.Bode BW, Brett J, Falahati A, Pratley RE. Comparison of the efficacy and tolerability profile of liraglutide, a once-daily human GLP-1 analog, in patients with type 2 diabetes ≥65 and <65 years of age: a pooled analysis from phase III studies. Am J Geriatr Pharmacother. 2011;9:423–433. doi: 10.1016/j.amjopharm.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Pencek R, Blickensderfer A, Li Y, Brunell SC, Chen S. Exenatide once weekly for the treatment of type 2 diabetes: effectiveness and tolerability in patient subpopulations: exenatide once weekly for the treatment of type 2 diabetes. Int J Clin Pract. 2012;66:1021–1032. doi: 10.1111/j.1742-1241.2012.03006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Tata V. Age-related impairment of pancreatic beta-cell function: pathophysiological and cellular mechanisms. Front Endocrinol. 2014;5:138. doi: 10.3389/fendo.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Home PD, Shamanna P, Stewart M, et al. Efficacy and tolerability of albiglutide versus placebo or pioglitazone over 1 year in people with type 2 diabetes currently taking metformin and glimepiride: HARMONY 5. Diabetes Obes Metab. 2015;17:179–187. doi: 10.1111/dom.12414. [DOI] [PubMed] [Google Scholar]
- 19.Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015;4:212283. doi: 10.7573/dic.212283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathieu C, Del Prato S, Botros FT, et al. Effect of once weekly dulaglutide by baseline beta-cell function in people with type 2 diabetes in the AWARD programme. Diabetes Obes Metab. 2018;20:2023–2028. doi: 10.1111/dom.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2019. Diabetes Care. 2019;42:S90–102. doi: 10.2337/dc19-S009. [DOI] [PubMed] [Google Scholar]
- 22.Singh S, Wright EE, Kwan AYM, et al. Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab. 2017;19:228–238. doi: 10.1111/dom.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin PA, Nguyen H, Wittbrodt ET, Kim SC. Glucagon-like peptide-1 receptor agonists: a systematic review of comparative effectiveness research. Diabetes Metab Syndr Obes Targets Ther. 2017;10:123–139. doi: 10.2147/DMSO.S130834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abd-El-Aziz MS, Kahle M, Meier JJ, Nauck MA. A meta-analysis comparing clinical effects of short- or long-acting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diabetes Obes Metab. 2017;19:216–227. doi: 10.1111/dom.12804. [DOI] [PubMed] [Google Scholar]
- 25.Gallwitz B, Dagogo-Jack S, Thieu V, et al. Effect of once-weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes Obes Metab. 2018;20:409–418. doi: 10.1111/dom.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab. 2017;19:524–536. doi: 10.1111/dom.12849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are not broadly publicly available to respect company confidentiality but may be available from the corresponding author on reasonable request.