Abstract
Introduction
The impact of reduction of systolic blood pressure or body weight on reduction of cardiovascular events during the treatment with glucagon-like peptide 1 receptor agonists (GLP-1RAs) or sodium-glucose cotransporter 2 inhibitors (SGLT2is) for type 2 diabetes is unclear.
Methods
We searched Embase and PubMed. We performed meta-analysis using hazard ratio (HR) and 95% confidence interval (CI) as effect size stratified by drug class on six endpoints of interest, which were major adverse cardiovascular events (MACE), hospitalization for heart failure (HHF), cardiovascular death (CVD), myocardial infarction (MI), stroke, and all-cause death (ACD). We performed meta-regression to assess the difference between GLP-1RAs and SGLT2is, and the impact of reduction of systolic blood pressure or body weight on reduction of cardiovascular events.
Results
We included 11 randomized trials. Compared with placebo, SGLT2is reduced HHF by 32% (HR 0.68, 95% CI 0.60–0.76) whereas GLP-1RAs reduced HHF by only 9% (HR 0.91, 95% CI 0.83–0.99). The benefit from SGLT2is on HHF was significantly greater than that from GLP-1RAs (Psubgroup = 0.004). GLP-1RAs reduced stroke by 16% (HR 0.84, 95% CI 0.76–0.93) whereas SGLT2is did not reduce stroke (HR 0.96, 95% CI 0.82–1.12). GLP-1RAs and SGLT2is similarly reduced MACE by 12%, CVD by 15%, MI by 9%, and ACD by 13%. The effects of systolic blood pressure reduction and body weight reduction on the logarithms of HRs of GLP-1RAs or SGLT2is vs. placebo as for reducing six endpoints of interest were not statistically significant (β ranged from − 0.145 to 0.269, and P ranged from 0.211 to 0.941).
Conclusions
GLP-1RAs and SGLT2is lead to similar benefits on MACE, CVD, MI, and ACD in adults with type 2 diabetes. The benefit from SGLT2is on HHF is greater than that from GLP-1RAs, while GLP-1RAs vs. placebo significantly reduce stroke whereas SGLT2is do not. The two drug classes reduce cardiovascular events independent of reductions of systolic blood pressure and body weight.
Electronic supplementary material
The online version of this article (10.1007/s13300-020-00912-z) contains supplementary material, which is available to authorized users.
Keywords: Cardiovascular events, GLP-1RAs, SGLT2is, Type 2 diabetes
Key Summary Points
| Why carry out this study? |
| The impact of reduction of systolic blood pressure or body weight on reduction of cardiovascular events during the treatment with glucagon-like peptide 1 receptor agonists (GLP-1RAs) or sodium-glucose cotransporter 2 inhibitors (SGLT2is) for type 2 diabetes is unclear. |
| What was learned from the study? |
| GLP-1RAs and SGLT2is reduce cardiovascular events independent of reduction of systolic blood pressure. |
| GLP-1RAs and SGLT2is reduce cardiovascular events independent of reduction of body weight. |
| The benefit from SGLT2is on hospitalization for heart failure is greater than that from GLP-1RAs. |
| GLP-1RAs vs. placebo significantly reduce stroke whereas SGLT2is do not. |
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to 10.6084/m9.figshare.12789854.
Introduction
As two classes of new antihyperglycemic agents, glucagon-like peptide 1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter 2 inhibitors (SGLT2is) can both produce a glucose-lowering effect and prevent cardiovascular events and death in adults with type 2 diabetes. Giugliano et al. [1] reported the impact of reduction of glycated hemoglobin (HbA1c) level on reduction of cardiovascular events during the treatment with GLP-1RAs or SGLT2is for type 2 diabetes. However, the impact of reductions of systolic blood pressure and body weight on reduction of cardiovascular events exhibited by the two drug classes is unclear.
On the other hand, Zelniker et al.’s meta-analysis [2] explored the differences between GLP-1RAs and SGLT2is in reducing different cardiovascular endpoints. However, that study failed to include the three large cardiovascular outcome trials of REWIND [3], PIONEER 6 [4], and CREDENCE [5], whereas the three trials provided the new evidences of GLP-1RAs and SGLT2is as for preventing cardiovascular and mortality endpoints in patients with type 2 diabetes.
Thus, we included GLP-1RA and SGLT2i cardiovascular outcome trials including the REWIND [3], PIONEER 6 [4], and CREDENCE [5] trials to conduct this meta-analysis, and aimed to assess the differences between the two drug classes in preventing different cardiovascular endpoints and to explore the impact of reductions of systolic blood pressure and body weight on reduction of cardiovascular events exhibited by the two drug classes.
Methods
This meta-analysis is reported on the basis of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [6], and Supplementary Appendix 1 provides the PRISMA checklist for the meta-analysis. This meta-analysis was previously registered in the PROSPERO website with the registration number CRD42020180427.
Search Strategy
We searched the Embase and PubMed databases on April 16, 2020 for correlative randomized trials. Predesigned search strategies used in this meta-analysis are shown in Supplementary Appendix 2 (p 1).
Inclusion and Exclusion Criteria
We included event-driven and cardiovascular outcome randomized controlled trials (RCTs) which compared GLP-1RAs or SGLT2is with other active drugs or placebo as for preventing cardiovascular and mortality endpoints in adults with type 2 diabetes. Six endpoints of interest were major adverse cardiovascular events (MACE), hospitalization for heart failure (HHF), cardiovascular death (CVD), myocardial infarction (MI), stroke, and all-cause death (ACD).
Study Selection, Data Extraction, and Quality Assessment
Study selection after literature search, data extraction from included studies, and risk of bias assessment for original studies according to the Cochrane risk of bias tool [7] were independently completed by two authors. When controversy existed between the two authors, a third author was involved in discussion to come to a consensus. The data extracted from included studies contained type of study, baseline systolic blood pressure and body weight, type and name of intervention and comparator, outcome data, and excess reductions of intervention group in systolic blood pressure and body weight as compared with placebo. Excess reduction in systolic blood pressure was equal to the reduction of systolic blood pressure in the intervention group minus the reduction of systolic blood pressure in the comparator group, and excess reduction in body weight was equal to the reduction of body weight in the intervention group minus the reduction of body weight in the comparator group.
Statistical Analysis
We conducted meta-analysis stratified by drug class (GLP-1RAs or SGLT2is) using a random-effects model to calculate the estimated values of pooled hazard ratios (HRs) and 95% confidence intervals (CIs). I2 statistic [8] was computed to measure statistical heterogeneity, and a value greater than 50% represents substantial heterogeneity. We conducted meta-regression analysis using a random-effects model to evaluate the differences between two drug classes and to evaluate the effects of excess reductions in systolic blood pressure and body weight on the logarithms of HRs of intervention vs. comparator as for reducing six outcomes of interest. Funnel plots were drawn and Egger test was performed to examine publication bias [9]. P values less than 0.05 indicate statistical significance. All statistical analyses were done using Stata (version 15.1).
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
Characteristics of Included Trials
At first we found 1492 records. After primary and secondary screening, we finally included 11 articles [3–5, 10–17] which reported 11 randomized trials (Fig. S1 in Supplementary Appendix 2). Among the included trials, seven [3, 4, 10–14] compared GLP-1RAs with placebo and four [5, 15–17] compared SGLT2is with placebo. All of the 11 trials had low risk of bias (Fig. S2 in Supplementary Appendix 2) and had a total of 94,727 participants which experienced 10,080 MACE in total. Supplementary Appendix 3 provides the data analyzed in this meta-analysis.
Meta-Analyses
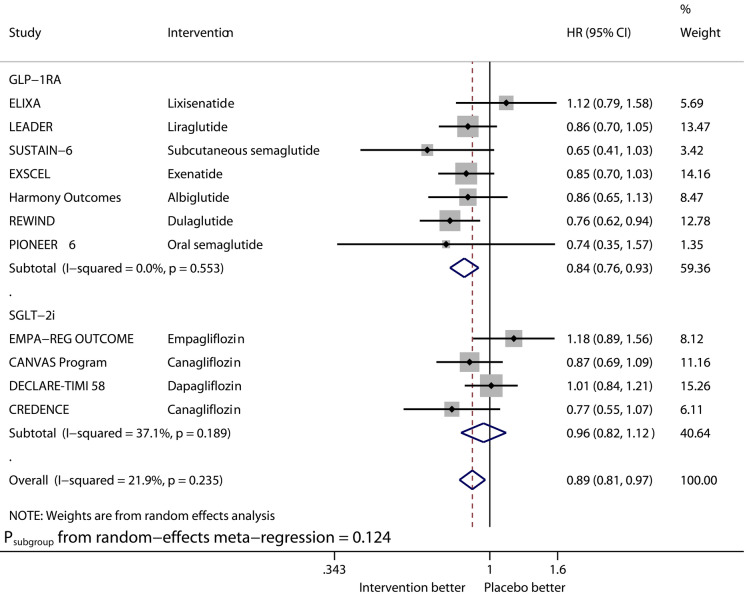
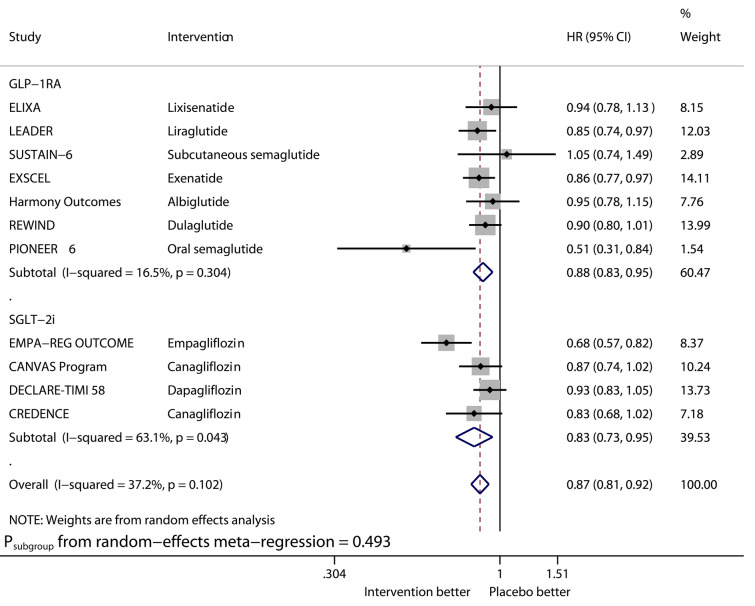
Figures 1–6 show the forest plots of meta-analysis of GLP-1RAs and SGLT2is on the outcomes of MACE (Fig. 1), HHF (Fig. 2), CVD (Fig. 3), MI (Fig. 4), stroke (Fig. 5), and ACD (Fig. 6).
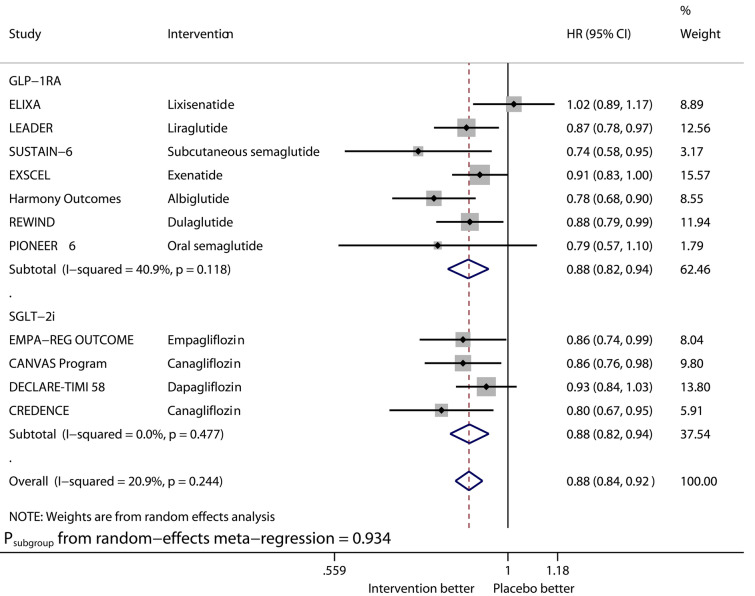
Fig. 1.
Forest plot of meta-analysis on major adverse cardiovascular events stratified by drug class
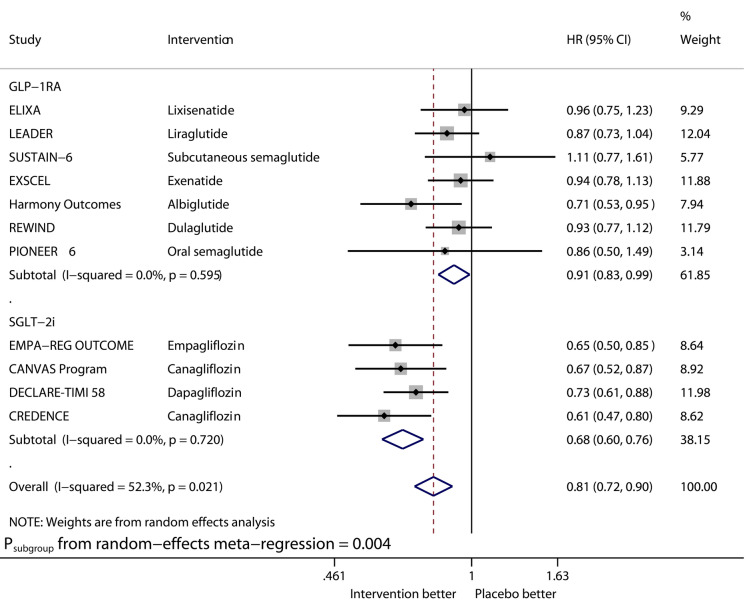
Fig. 2.
Forest plot of meta-analysis on hospitalization for heart failure stratified by drug class
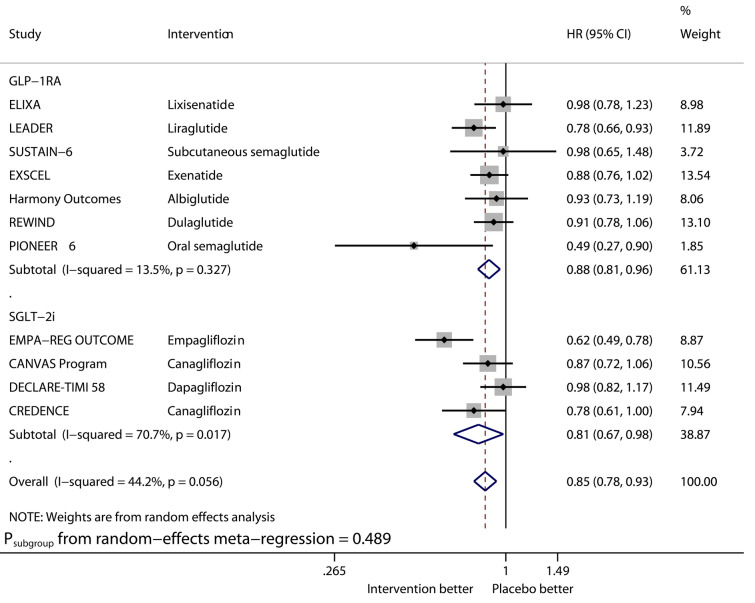
Fig. 3.
Forest plot of meta-analysis on cardiovascular death stratified by drug class
Fig. 4.
Forest plot of meta-analysis on myocardial infarction stratified by drug class
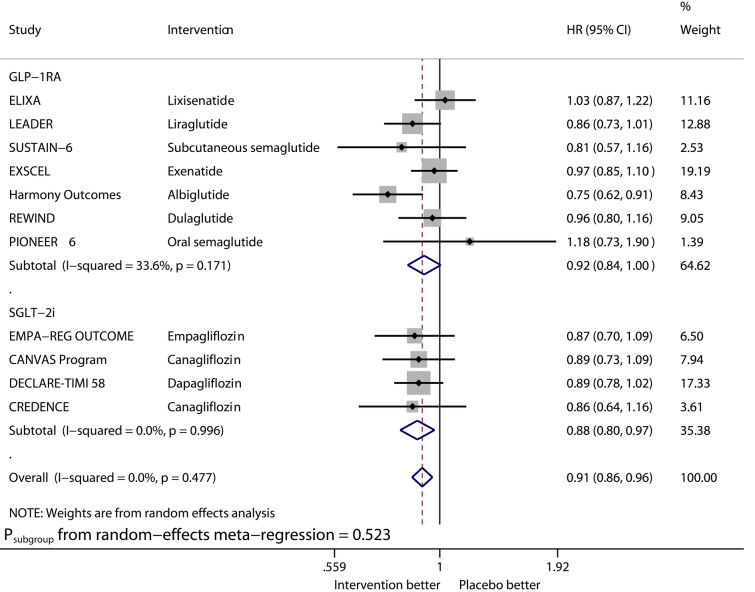
Fig. 5.
Forest plot of meta-analysis on stroke stratified by drug class
Fig. 6.
Forest plot of meta-analysis on all-cause death stratified by drug class
Compared with placebo, GLP-1RAs significantly reduced the risk of MACE (HR 0.88, 95% CI 0.82–0.94, I2 40.9%), CVD (HR 0.88, 95% CI 0.81–0.96, I2 13.5%), MI (HR 0.92, 95% CI 0.84–1.00, I2 33.6%), and ACD (HR 0.88, 95% CI 0.83–0.95, I2 16.5%), while SGLT2is significantly reduced that of MACE (HR 0.88, 95% CI 0.82–0.94, I2 0%), CVD (HR 0.81, 95% CI 0.67–0.98, I2 70.7%), MI (HR 0.88, 95% CI 0.80–0.97, I2 0%), and ACD (HR 0.83, 95% CI 0.73–0.95, I2 63.1%). The benefits from the two drug classes on the outcomes of MACE, CVD, MI, and ACD were similar (Psubgroup 0.934, 0.489, 0.523, and 0.493, respectively). Overall, the two drug classes vs. placebo reduced MACE by 12% (HR 0.88, 95% CI 0.84–0.92, I2 20.9%), CVD by 15% (HR 0.85, 95% CI 0.78–0.93, I2 44.2%), MI by 9% (HR 0.91, 95% CI 0.86–0.96, I2 0%), and ACD by 13% (HR 0.87, 95% CI 0.81–0.92, I2 37.2%).
Compared with placebo, GLP-1RAs significantly reduced the risk of HHF (HR 0.91, 95% CI 0.83–0.99, I2 0%), while SGLT2is significantly reduced that of HHF (HR 0.68, 95% CI 0.60–0.76, I2 0%). The benefit from SGLT2is on this outcome was significantly greater than that from GLP-1RAs (Psubgroup = 0.004). GLP-1RAs vs. placebo significantly reduced the risk of stroke (HR 0.84, 95% CI 0.76–0.93, I2 0%), whereas SGLT2is did not significantly reduce that of stroke (HR 0.96, 95% CI 0.82–1.12, I2 37.1%).
Meta-Regression Analyses
Figures S3–S26 (Supplementary Appendix 2) show the results of meta-regression analyses.
The effects of systolic blood pressure reduction on the logarithms of HRs of GLP-1RAs vs. placebo as for reducing MACE, HHF, CVD, MI, stroke, and ACD (Figs. S3–S8) were not statistically significant (β ranged from − 0.069 to 0.048, and P ranged from 0.211 to 0.683).
The effects of body weight reduction on the logarithms of HRs of GLP-1RAs vs. placebo as for reducing MACE, HHF, CVD, MI, stroke, and ACD (Figs. S9–S14) were not statistically significant (β ranged from − 0.053 to 0.059, and P ranged from 0.241 to 0.846).
The effects of systolic blood pressure reduction on the logarithms of HRs of SGLT2is vs. placebo as for reducing MACE, HHF, CVD, MI, stroke, and ACD (Figs. S15–S20) were not statistically significant (β ranged from − 0.145 to 0.116, and P ranged from 0.609 to 0.941).
The effects of body weight reduction on the logarithms of HRs of SGLT2is vs. placebo as for reducing MACE, HHF, CVD, MI, stroke, and ACD (Figs. S21–S26) were not statistically significant (β ranged from 0.032 to 0.269, and P ranged from 0.284 to 0.876).
Publication Bias Detection
The results of funnel plots and Egger test for the six outcomes of interest did not suggest obvious publication bias (Figs. S27–S32 in Supplementary Appendix 2).
Discussion
Main Findings and Comparisons with Prior Studies
In this study, we evaluated the efficacy of GLP-1RAs and SGLT2is on six endpoints of interest (i.e., MACE, HHF, CVD, MI, stroke, and ACD) in adults with type 2 diabetes and assessed the effects of systolic blood pressure reduction and body weight reduction on these six endpoints reduced by GLP-1RAs and SGLT2is. Thus, we produced the key findings as follows.
First, the benefits from GLP-1RAs and SGLT2is on the outcomes of MACE, CVD, MI, and ACD were similar. Overall, the two drug classes vs. placebo reduced MACE by 12%, CVD by 15%, MI by 9%, and ACD by 13%. Similarly, the benefits from GLP-1RAs and SGLT2is on these cardiovascular and mortality endpoints were also observed in meta-analyses [18–20] of GLP-1RA trials and those [21, 22] of SGLT2is trials.
Second, the benefit from SGLT2is on HHF was significantly greater than that from GLP-1RAs. SGLT2is vs. placebo reduced HHF by 32% whereas GLP-1RAs reduced HHF by only 9%. Two conventional meta-analyses [18, 21] provided the similar estimated values for the treatment effects of GLP-1RAs [18] and SGLT2is [21] in reducing HHF. Besides, a network meta-analysis [23] validated the superiority of SGLT2is (HR 0.79, 95% CI 0.69–0.90) over GLP-1RAs in reducing HHF.
Third, GLP-1RAs vs. placebo reduced stroke by 16% (HR 0.84, 95% CI 0.76–0.93) whereas SGLT2is did not reduce stroke (HR 0.96, 95% CI 0.82–1.12). Consistent with the results, a meta-analysis [18] demonstrated the benefit of GLP-1RAs on stroke in type 2 diabetes, whereas one other meta-analysis [21] revealed the neutral effect of SGLT2is on stroke.
Fourth, the effects of systolic blood pressure reduction and body weight reduction on the logarithms of HRs of GLP-1RAs or SGLT2is vs. placebo as for reducing the six endpoints of interest were not statistically significant (β ranged from − 0.145 to 0.269, and P ranged from 0.211 to 0.941). This finding suggests that GLP-1RAs and SGLT2is reduce cardiovascular and mortality endpoints independent of reductions of systolic blood pressure and body weight.
In this study we directly revealed the cardiovascular benefits of GLP-1RAs and SGLT2is in patients with type 2 diabetes. Meanwhile, the mechanisms by which the two drug classes exert cardiac protective effects are summarized in two recent review articles [24, 25]. Among all mechanisms mentioned in the two articles [24, 25], an important mechanism is that the two drug classes are able to reduce cardiac afterload [26, 27] and preload [28–30] by various means.
Giugliano et al. [1] reported the relation between reduction of HbA1c and risk of MACE during the treatment with GLP-1RAs or SGLT2is for type 2 diabetes, and Hu et al. [31] revealed the relation between weight reduction and blood pressure reduction. However, our study explored the relation between reductions of systolic blood pressure and body weight and reduction of cardiovascular events during the GLP-1RA treatment and the SGLT2i treatment for type 2 diabetes.
The latest consensus report [32] recommends that GLP-1RAs and SGLT2is should be considered in patients with type 2 diabetes to prevent cardiovascular events independently of baseline HbA1c and individualized HbA1c target. However, that report fails to recommend whether reductions of systolic blood pressure and body weight should be considered when the two drug classes are prescribed for type 2 diabetes. Thus, the fourth finding in our study fills this knowledge gap. It is worth mentioning that other antihyperglycemic agents should be appropriately adjusted when GLP-1RAs and SGLT2is are considered to be used in diabetic patients, especially when HbA1c is the target prior to initiation of the two drug classes.
Strengths and Limitations
This study has two main strengths. First, we performed meta-analysis stratified by drug class, and found the differences between the two drug classes in reducing different endpoints: the benefit from SGLT2is on HHF was significantly greater than that from GLP-1RAs, while GLP-1RAs reduced stroke by 16% whereas SGLT2is did not reduce stroke compared with placebo. Second, all the included RCTs in this meta-analysis had low risk of bias and there was not obvious publication bias found for all endpoints of interest.
This study has two main limitations. First, the relative efficacy of GLP-1RAs and SGLT2is in reducing different cardiovascular outcomes must be assessed in head-to-head trials comparing GLP-1RAs with SGLT2is. Second, we observed substantial heterogeneity in few analyses, which needs to be further investigated.
Conclusions
GLP-1RAs and SGLT2is lead to similar benefits on MACE, CVD, MI, and ACD in adults with type 2 diabetes. The benefit from SGLT2is on HHF is greater than that from GLP-1RAs, while GLP-1RAs vs. placebo significantly reduce stroke whereas SGLT2is do not. The two drug classes reduce cardiovascular events independent of reductions of systolic blood pressure and body weight.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Appendix 1: PRISMA checklist (DOC 63 kb)
Supplementary Appendix 2: Supplementary methods and results (PDF 851 kb)
Supplementary Appendix 3: Data analyzed in the study (XLSX 12 kb)
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Mei Qiu, Liang-Liang Ding, Miao Zhang, Jin-Hao Lin, Xu-Bin Wei and Hua Huang have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12789854.
References
- 1.Giugliano D, Bellastella G, Longo M, et al. Relationship between improvement of glycaemic control and reduction of major cardiovascular events in 15 cardiovascular outcome trials: a meta-analysis with meta-regression. Diabetes Obes Metab. 2020;22(8):1397–1405. doi: 10.1111/dom.14047. [DOI] [PubMed] [Google Scholar]
- 2.Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139(17):2022–2031. doi: 10.1161/CIRCULATIONAHA.118.038868. [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 4.Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 5.Mahaffey KW, Jardine MJ, Bompoint S, et al. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation. 2019;140(9):739–750. doi: 10.1161/CIRCULATIONAHA.119.042007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 11.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 13.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 15.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 16.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 17.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 18.Kristensen SL, Rorth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 19.Marsico F, Paolillo S, Gargiulo P, et al. Effects of glucagon-like peptide-1 receptor agonists on major cardiovascular events in patients with type 2 diabetes mellitus with or without established cardiovascular disease: a meta-analysis of randomized controlled trials. Eur Heart J. 2020. 10.1093/eurheartj/ehaa082. [DOI] [PubMed]
- 20.Pulipati VP, Ravi V, Pulipati P. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Eur J Prev Cardiol. 2020.10.1177/2047487320903638. [DOI] [PubMed]
- 21.Arnott C, Li Q, Kang A, et al. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9(3):e14908. doi: 10.1161/JAHA.119.014908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 23.Fei Y, Tsoi MF, Cheung B. Cardiovascular outcomes in trials of new antidiabetic drug classes: a network meta-analysis. Cardiovasc Diabetol. 2019;18(1):112. doi: 10.1186/s12933-019-0916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilcox T, De Block C, Schwartzbard AZ, Newman JD. Diabetic agents, from metformin to SGLT2 Inhibitors and GLP1 receptor agonists: JACC focus seminar. J Am Coll Cardiol. 2020;75(16):1956–1974. doi: 10.1016/j.jacc.2020.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee M, Petrie MC, McMurray J, Sattar N. How do SGLT2 (sodium-glucose cotransporter 2) inhibitors and GLP-1 (glucagon-like peptide-1) receptor agonists reduce cardiovascular outcomes? Completed and ongoing mechanistic trials. Arterioscler Thromb Vasc Biol. 2020;40(3):506–522. doi: 10.1161/ATVBAHA.119.311904. [DOI] [PubMed] [Google Scholar]
- 26.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Newman JD, Vani AK, Aleman JO, Weintraub HS, Berger JS, Schwartzbard AZ. The changing landscape of diabetes therapy for cardiovascular risk reduction: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(15):1856–1869. doi: 10.1016/j.jacc.2018.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muskiet M, Tonneijck L, Smits MM, et al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol. 2017;13(10):605–628. doi: 10.1038/nrneph.2017.123. [DOI] [PubMed] [Google Scholar]
- 29.Ferrannini G, Rydén L. Sodium-glucose transporter 2 inhibition and cardiovascular events in patients with diabetes: information from clinical trials and observational real-world data. Clin Sci (Lond) 2018;132(18):2003–2012. doi: 10.1042/CS20171374. [DOI] [PubMed] [Google Scholar]
- 30.Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney D. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136(17):1643–1658. doi: 10.1161/CIRCULATIONAHA.117.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu M, Cai X, Yang W, Zhang S, Nie L, Ji L. Effect of hemoglobin A1c reduction or weight reduction on blood pressure in glucagon-like peptide-1 receptor agonist and sodium-glucose cotransporter-2 inhibitor treatment in type 2 diabetes mellitus: a meta-analysis. J Am Heart Assoc. 2020;9(7):e15323. doi: 10.1161/JAHA.119.015323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2020;43(2):487–93. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix 1: PRISMA checklist (DOC 63 kb)
Supplementary Appendix 2: Supplementary methods and results (PDF 851 kb)
Supplementary Appendix 3: Data analyzed in the study (XLSX 12 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.