Abstract
Purpose
Treatment with CDK4/6 inhibitors and endocrine therapy (CDK4/6i + ET) is a standard for patients with advanced hormone receptor–positive, HER2-negative (HR + HER2–) breast cancer (BC). However, real-world data on the implementation of therapy usage, efficacy, and toxicity have not yet been reported.
Methods
The PRAEGNANT registry was used to identify advanced HR + HER2– BC patients (n = 1136). The use of chemotherapy, ET, everolimus + ET, and CDK4/6i + ET was analyzed for first-line, second-line, and third-line therapy. Progression-free survival (PFS) and overall survival (OS) were also compared between patients treated with CDK4/6i + ET and ET monotherapy. Also toxicity was assessed.
Results
CDK4/6i + ET use increased from 38.5% to 62.7% in the first 2 years after CDK4/6i treatment became available (November 2016). Chemotherapy and ET monotherapy use decreased from 2015 to 2018 from 42.2% to 27.2% and from 53% to 9.5%, respectively. In this early analysis no statistically significant differences were found comparing CDK4/6i + ET and ET monotherapy patients with regard to PFS and OS. Leukopenia was was seen in 11.3% of patients under CDK4/6i + ET and 0.5% under ET monotherapy.
Conclusions
In clinical practice, CDK4/6i + ET has been rapidly implemented. A group of patients with a more unfavorable prognosis was possibly treated in the real-world setting than in the reported randomized clinical trials. The available data suggest that longer follow-up times and a larger sample size are required in order to identify differences in survival outcomes. Studies should be supported that investigate whether chemotherapy can be avoided or delayed in this patient population by using CDK4/6i + ET.
Keywords: Advanced breast cancer, Metastatic, Chemotherapy, Endocrine therapy, CDK4/6, Ribociclib, Palbociclib, Abemaciclib
Highlights
-
•
CDK4/6i + ET use increased from 39% to 63% after becoming available.
-
•
Chemotherapy and ET monotherapy use decreased from 42% to 27% and 53%–10%.
-
•
There was no difference between CDK4/6i + ET and ET monotherapy regarding PFS and OS.
1. Introduction
For patients with hormone receptor–positive, HER2-negative (HR+/HER2–) advanced breast cancer (ABC) combination therapies with CDK4/6 inhibitors and endocrine treatment (CDK4/6i + ET) have improved the progression-free survival (PFS) in several therapy lines and in premenopausal and postmenopausal patients in comparison with ET monotherapies (ETmono), as shown in several randomized controlled trials (RCTs; summarized in Refs. [1]). The median PFS increased in all of these studies, with hazard ratios of 0.5–0.6 in favor of the CDK4/6i combination therapies. In three of these trials also an improved overall survival (OS) could be shown [[2], [3], [4]] and in another study a trend to an improved OS was seen [5].
The data for CDK4/6i + ET are from prospective RCTs that excluded patients who either required chemotherapy or had certain co-morbidities. Only the studies Paloma-3 and Monaleesa-7 allowed chemotherapies before study entry [2,6]. Furthermore treatment in the clinical trials was restricted to first and second line endocrine treatment. Few data are available on the usage of CDK4/6i + ET in routine clinical practice. In general, combination treatment with CDK4/6i + ET competes on the one hand with ETmono, possibly for patients with an extremely good prognosis; and on the other with chemotherapy if a rapid treatment response is needed. It has been shown from real-world registries that patients who are treated with ETmono in the first-line metastatic setting usually have a clearly more favorable prognosis than patients who are treated with chemotherapy [7,8]. Chemotherapy has been reported to be administered in 20–40% of HR+/HER– metastatic BC patients as a first-line therapy [[10], [11], [8], [9]].
Real-world data has been increasingly used to address several clinical questions. Results from those analyses are highly dependent on the quality of the data that is used [12]. With regard to CDK4/6i therapies, there have been reports from an extended access program in heavily pretreated patients [13], from a retrospective anaylsis within a U.S. hospital system [14], and a retrospective market research anaylsis [15]. In addition to those analyses our registry captures all patients prospectively and might be able to provide data, how CDK4/6i were introduced in the treatment of ABC as well as prospectively captured survival data.
The aims of this analysis were therefore to describe treatment patterns and outcomes with CDK4/6i + ET in routine clinical practice around the period it was introduced, and to report early efficacy and safety data in comparison with patients receiving ETmono.
2. Patients and methods
2.1. Patients
PRAEGNANT (NCT02338167 [16]) is an ongoing, prospective BC registry with documentation procedures similar to those for clinical trials. Patients can be included at any time point or therapy line during their disease. Within the PRAEGNANT registry, therapy lines are counted regardless of their nature (i.e. whether the therapy line was a chemotherapy, an endocrine therapy or another anti-cancer treatment). All of the patients have provided written informed consent and the study has been approved by the relevant ethics committees.
From July 2014 to May 2019, 3144 patients with ABC were registered at 54 study sites. For the current analysis, patients were excluded in the following hierarchical order: unknown hormone receptor status (n = 111), unknown HER2 status (n = 146), male patients (n = 34), and missing treatment information (n = 37). Of the remaining 2816 patients, 1803 had HR + HER– tumors (Fig. S1). The following groups were defined from among these patients:
-
•
A treatment pattern analysis was performed to describe the distribution of therapies (chemotherapy vs. ETmono vs. everolimus + ET vs. CDK4/6i + ET) for each year before and after the introduction of CDK4/6i in Germany (November 2016). This information was available for 840 patients in first-line treatment, 698 patients in second-line treatment, and 541 patients in third-line treatment. Most of the patients who were not included in this analysis had started the corresponding treatments before November 2014.
-
•
For efficacy analyses, all possible therapy sequences allowing comparison between CDK4/6i + ET and ETmono were defined for each of the first three treatment lines (Table S1). In this context, 659 additional patients were excluded (reasons: treatment in clinical trials with CDK4/6i, inconsistent therapy documentation, not matching a treatment scenario). Treatment comparisons were performed separately for each therapy line. Patients who were not being treated with CDK4/6i were also able to take part in the relevant subsequent treatment line analysis. Patients receiving everolimus were excluded from the therapy line in which it was given.
-
•
For a safety analysis, only selected study sites were included in the analysis that passed the quality control for this feature (41 out of 54 sites). The final patient population for this comparison consisted of 141 patients with CDK4/6i + ET and 184 patients with ETmono.
2.2. Materials
The data were collected by trained staff and documented in an electronic case report form [16]. Data are monitored using automated plausibility checks and on-site monitoring. Data not usually documented as part of routine clinical work are collected prospectively using structured paper questionnaires (summarized in Table S2). Toxicity was documented in the same way in which a clinical trial would report an adverse event or severe adverse event, using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAEv4.0).
The definitions of hormone receptors, HER2 status, and grading that were used have been described previously [11]. Briefly, if a biomarker assessment of the metastatic site was available, this receptor status was used for this analysis. Otherwise, the latest biomarker results were used. If any information was missing, all patients treated with ET in the metastatic setting were assumed to be HR+ and all patients who had ever received anti-HER2 therapy were assumed to be HER2+.
2.3. Statistics
Primary study aim was to compare endocrine therapies (CDK4/6i + ET versus endocrine monotherapy) with regard to progression-free survival in three different scenarios. Progression-free survival was defined from the date of therapy begin to the earliest date to disease progression (distant-metastasis, local recurrence, or death from any cause) or the last date known to be progression-free. It was censored at 3 years, and it was left-truncated for time to enter the study if the entry was after therapy begin. Survival rates with 95% confidence intervals (CIs) and median survival time were estimated for both ET groups of interest using the Kaplan-Meier product limit method. The 95% CI of median survival time was computed using the method of Brookmeyer and Crowley [17].
Unadjusted hazard ratios (HRs) for CDK4/6i + ET versus ET monotherapy, one for each scenario, were obtained from simple Cox regression models. Adjusted HRs for CDK4/6i + ET versus ET monotherapy were estimated using a multivariable Cox regression model with predictors that might have an influence either on survival or on therapy choice of the physician: age (continuous), time from primary diagnosis to metastasis (continuous), tumor grade (G1/G2 versus G3), ECOG (ordinal), endoctrine therapy (categorical; AI, FUL, other), metastasis pattern (categorical; brain, visceral, bone only, others), number of previous chemotherapies, and number of comorbidities.
As sensitivity analysis, a backward stepwise variable selection which kept the predictor endocrine therapy was performed to get a second set of adjusted HRs that regarded fewer but possibly only the most influential predictors. Patients with missing survival information or missing endocrine therapy information were excluded. Missing values at other predictors were imputed, and continuous predictors were used as natural cubic spline functions, as done in Salmen et al. [18]. The proportional hazards assumptions were checked using the Grambsch–Therneau method.
Further sensitivity analyses were performed. Survival analyses similar to the main analysis were carried out in patients which were treated after November 2016, the date when CDK4/6i was approved. Survival analyses were performed once more in CDK4/6i + ET patients treated after November 2016 and monotherapy patients treated before November 2016 to address the problem of unobserved decision rules for or against a specific antihormone therapy when both therapies of interest were available at the same time. Since the number of patients and thus the number of events is smaller here than in the main analyses, the HRs were adjusted only for the selected predictors of the main analysis.
Secondary study aim was to explore the effect of these antihormone therapies on overall survival. Analyses similar to the progression-free survival analyses were carried out. Due to the small number of events (i.e., deaths), HRs were adjusted only for age and grade, and sensitivities analyses with restricted study population were carried out without adjustment.
All of the tests were two-sided, and a P value < 0.05 was regarded as statistically significant. Calculations were carried out using the R system for statistical computing (version 3.4.1; R Development Core Team, Vienna, Austria, 2017).
3. Results
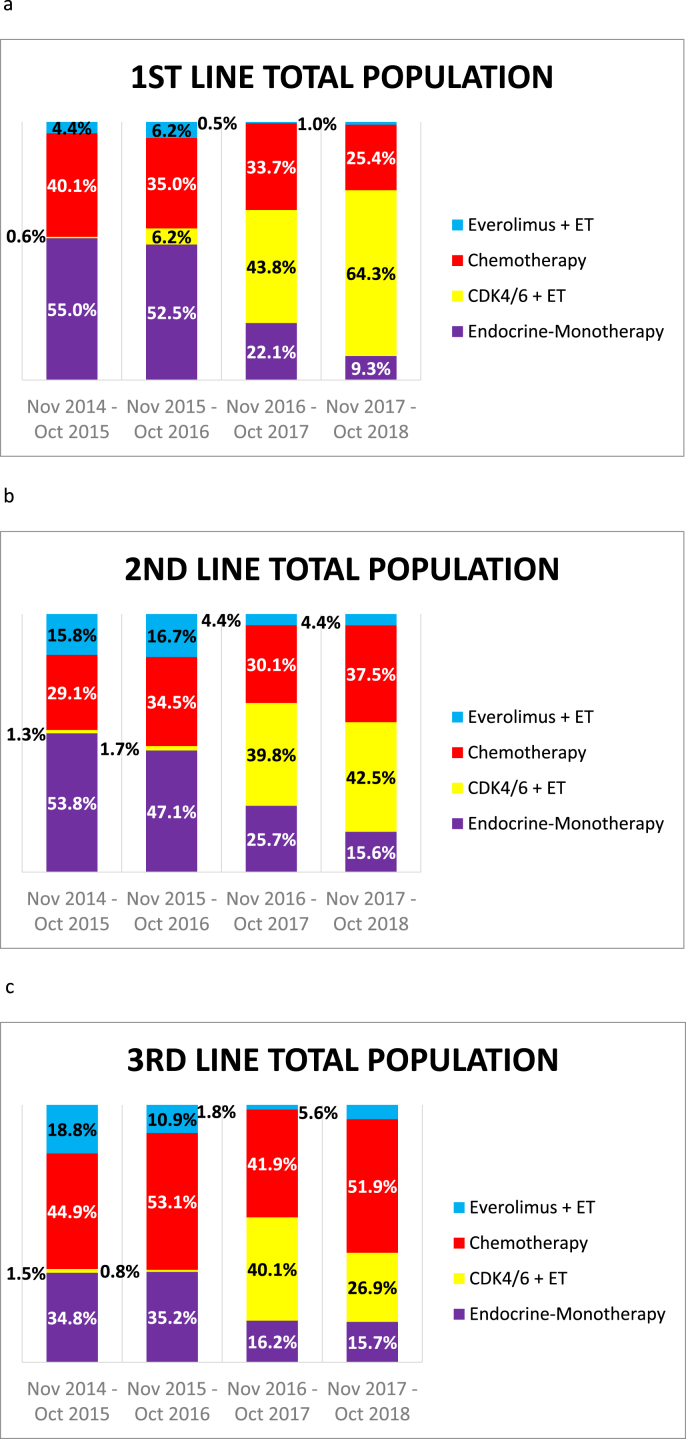
Treatment patterns in the first to third lines are shown in Fig. 1. After CDK4/6i became available in Germany (November 2016), its usage in first-line treatment increased from 44% to 64% during the next 2 years. CDK4/6i usage in the second line remained around the same, at approximately 40%. In the third line, after an initial peak (≈40%), the percentage of patients receiving CDK4/6i decreased to 27%. The use of chemotherapy in first-line treatment decreased considerably over the years, and ETmono was drastically reduced. There were no differences in treatment patterns between large cancer centers, smaller centers, and oncology community practices (Figs. S2–S3).
Fig. 1.
Therapy use among HER2-negative, hormone receptor–positive patients treated (a) in the first-line setting (n = 840), (b) in the second-line setting (n = 698), and (c) in the third-line setting (n = 541). CDK4/6i, CDK4/6 inhibitors; ET, endocrine therapy.
Efficacy analyses were conducted in three settings: first-line, second-line, and third-line (Fig. S1). In all of the therapy lines, patients treated with CDK4/6i + ET were approximately 2–3 years younger. In the first line, they had a slightly higher grading and in the second and third lines generally a lower grading. Also it should be noted that during the observation period of our study patients treated in the first line setting had a better performance status (ECOG) compared to patients treated with ETmono. In later therapy lines there were no major differences concerning performance status. The most common combination partners for CDK4/6i in the first line were aromatase inhibitors (66.7%), while the frequency of fulvestrant as the combination partner increased from first to third line from 33.3% to 68.3% (Table S3).
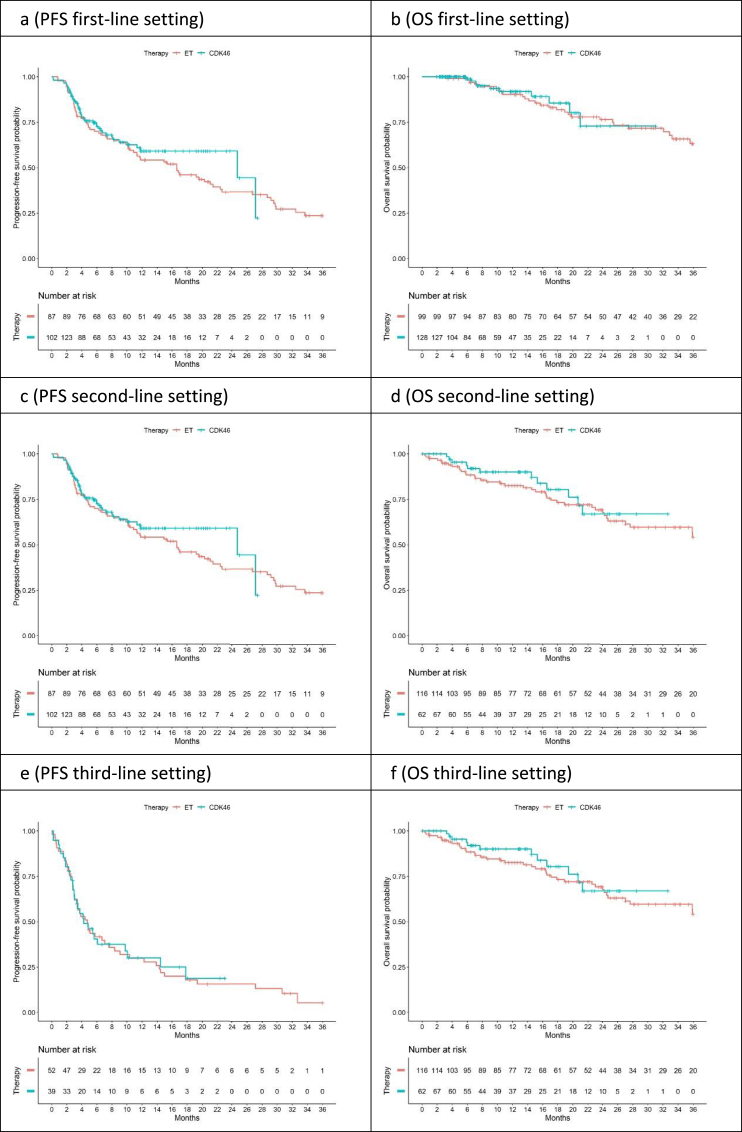
The median observation times for patients without progression were 11.8, 11.9, and 9.0 months in the first-line, second-line, and third-line analyses, respectively. In the first line, the median PFS was 16.6 months (95%CI, 10.9–22.6) for ETmono, while the median PFS was for patients treated with CDK4/6i + ET was 24.7 months (95%CI, 11.9-NA [upper 95% CI not reached]). In the second line, the median PFS figures were 8.7 months for ETmono(95%CI, 6.0–11.5) and 7.8 months for patients with CDK4/6i + ET therapy (95%CI, 5.8–15.4). In the third line, the corresponding figures were 4.7 months (95%CI, 3.4–8.3) and 4.2 months (95%CI, 3.0–14.5). For OS, most of the median survival times were not yet reached. Survival estimates are summarized in Table S4 and Kaplan–Meier curves are shown in Fig. 2.
Fig. 2.
Progression-free survival (PFS) and overall survival (OS) for the total group analyzed. CDK4/6i, CDK4/6 inhibitors; ET, endocrine therapy; OS, overall survival; PFS, progression-free survival.
Cox regression analyses were performed in order to take imbalances in patient characteristics into account. With regard to PFS, adjusted hazard ratios for the first, second, and third lines were 0.87 (95%CI: 0.58–1.32), 0.84 (95%CI:0.57–1.24), and 0.83 (95%CI:0.51–1.35), respectively (Table 1a). The hazard ratios for OS are shown in Table 1b.
Table 1a.
Cox regression analyses for progression-free survival in the overall analysis group, showing hazard ratios for CDK4/6i + ET versus ET.
| Setting | Unadjusted analysis |
First adjusted analysis a |
Second adjusted analysis b |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| First line | 0.81 (0.54, 1.22) | 0.32 | 0.87 (0.56, 1.34) | 0.52 | 0.87 (0.58, 1.32) | 0.52 |
| Second line | 1.04 (0.72, 1.50) | 0.84 | 0.90 (0.60, 1.35) | 0.61 | 0.84 (0.57, 1.24) | 0.39 |
| Third line | 0.96 (0.60, 1.54) | 0.86 | 0.85 (0.50, 1.43) | 0.53 | 0.83 (0.51, 1.35) | 0.45 |
CDK4/6i, CDK4/6 inhibitor; CI, confidence interval; ET, endocrine therapy; HR, hazard ratio.
HRs were adjusted for age, time from primary diagnosis to metastasis, ECOG, grade, anti-endoctrine therapy, metastasis pattern, number of previous chemotherapies (at Setting 2 and 3), number of comorbidities.
HRs were obtained after variable selection process. At setting 1 the predictor anti-endocrine therapy were selected. At setting 2 age, metastasis pattern and number of previous chemotherapies were selected. At Setting 3, time from primary diagnosis to metastasis and number of comorbidities were selected.
Table 1b.
Cox regression analyses for overall survival in the total analysis population, showing hazard ratios for CDK4/6i + ET versus ET.
| Setting | Unadjusted analysis |
Adjusted analysis a |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| First line | 0.91 (0.42, 1.98) | 0.82 | 0.98 (0.45, 2.13) | 0.95 |
| Second line | 0.80 (0.41, 1.55) | 0.50 | 0.83 (0.43, 1.63) | 0.59 |
| Third line | 1.57 (0.75, 3.29) | 0.23 | 1.69 (0.79, 3.62) | 0.18 |
CDK4/6i, CDK4/6 inhibitor; CI, confidence interval; ET, endocrine therapy; HR, hazard ratio.
HRs are adjusted for age and grade.
In addition to adjustment of the comparison, two sensitivity analyses were conducted (see statistics section). The results of these analyses are shown in Tables S5–S8 and Figs. S4–S7. The results were similar to those of the primary analysis. However, the sensitivity analyses showed a high degree of variability with regard to some results — e.g., in the unadjusted analysis for the third-line metastatic setting, the hazard ratio varied between 0.96 in the total population (Table 2a), 0.67 in sensitivity analysis 1 (Table S5a), and 1.11 in sensitivity analysis 2 (Table S7a). None of the hazard ratios achieved statistical significance.
Table 2.
Grade 3 or 4 adverse events (CTCAE v4.0) grouped by therapy arm, showing numbers and percentages of patients.
| CTCAE term |
ET (n = 184) |
CDK4/6i (n = 141) |
||
|---|---|---|---|---|
| n | % | n | % | |
| Neutrophil count decreased | 0 | 0 | 5 | 3.5 |
| Anemia | 0 | 0 | 3 | 2.1 |
| Abdominal pain | 0 | 0 | 1 | 0.7 |
| Allergic reaction | 1 | 0.5 | 0 | 0 |
| Concentration impairment | 1 | 0.5 | 0 | 0 |
| Diarrhea | 0 | 0 | 1 | 0.7 |
| Dyspnea | 0 | 0 | 1 | 0.7 |
| Edema limbs | 1 | 0.5 | 0 | 0 |
| GGT increased | 0 | 0 | 1 | 0.7 |
| Insomnia | 1 | 0.5 | 0 | 0 |
| Lung infection | 0 | 0 | 1 | 0.7 |
| Lymphedema | 1 | 0.5 | 0 | 0 |
| Mucositis oral | 0 | 0 | 1 | 0.7 |
| Nail discoloration | 0 | 0 | 1 | 0.7 |
| Nausea | 0 | 0 | 1 | 0.7 |
| Non-cardiac chest pain | 0 | 0 | 1 | 0.7 |
| Pain | 1 | 0.5 | 0 | 0 |
| Renal and urinary disorders - Other, specify | 1 | 0.5 | 0 | 0 |
| Surgical and medical procedures - Other, specify | 1 | 0.5 | 0 | 0 |
| Transient ischemic attacks | 0 | 0 | 1 | 0.7 |
| Vomiting | 0 | 0 | 1 | 0.7 |
CDK4/6i, CDK4/6 inhibitors; CTCAE, Common Terminology Criteria for Adverse Events; ET, endocrine therapy.
Fatigue, leukopenia, and nausea were the most frequent side effects (all grades, Table S9). For patients treated with CDK4/6i + ET versus ETmono, fatigue was reported by 11.3% vs. 6.0%, nausea in 11.3% of the CDK4/6i + ET patients and in 2.2% of the ETmono patients and leukopenia or neutropenia was documented in 11.3% vs. 0.5%. Grade 3/4 adverse events were very rare (all <4%) in both therapy groups, with neutropenia being reported in 3.5% of CDK4/6i + ET patients, as the most frequent grade 3/4 adverse event (Table 2).
4. Discussion
This analysis provides information on the introduction of CDK4/6i + ET in the treatment of patients with HR + HER– ABC in the first-line, second-line, and third-line therapy. Particularly in the first line, approximately two-thirds of the patients are now being treated with CDK4/6i + ET. The data also provide early estimates of real-world PFS and OS for patients treated with CDK4/6i + ET and ETmono. In this early analysis with limited power, there were no statistically significant differences between PFS times. Adjusting the analyses for covariates that might explain a prognostic bias did not alter the results relevantly.
With regard to ETmono, the median PFS times in the registry were very similar to those published in the RCTs (summarized in [1]), at 16.6 months in the first line and 8.7 months and 4.7 months in second and third lines, respectively. In the first line setting the median PFS of patients treated with CDK4/6i + ET was about 8 months longer (24.7 months) but most probably due to a small sample size this difference did not become statistically significant. In the second and third line the PFS times of patients treated with CDK4/6i + ET were very similar to those treated with ETmono. Compared with other real-world evidence data our data is similar to analyes in heavily pretreated patients with a median PFS of 4.5 months [13]. Also data of a retrospective study within a U.S. hospital system was mainly similar to our data. That study reported 12.3 and 6.4 months of median PFS in the second and third line of treatment [14]. Concerning the median PFS in the first line setting our analysis had a substantially longer median PFS (24.7 months) than the one reported in the study of the U.S. hospital system with a median PFS of 11.6 months [14].
All of the RCTs in which CDK4/6i + ET and ETmonowere compared favored CDK4/6i + ET, with hazard ratios between 0.5 and 0.6 (summarized in [1]). This provides the highest level of evidence for the conclusion that adding CDK4/6i + ET prolongs the PFS in comparison with ETmono. There were therefore high expectations that a similar effect would be seen in the real-world data. However, a much smaller effect or no effects were seen — a finding that may not be surprising at second glance. A systematic comparison of the treatment effects in 21 oncology RCTs and the corresponding effects in real-world datasets shows that the real-world treatment benefits were 16% lower than in the RCTs with surrogate end points such as PFS [19]. The effects with regard to OS appeared to be similar when RCTs were compared with real-world data. However, in the four RCTs examining BC patients, RCTs appeared to overestimate the effect by up to 46.5% (95%CI, 19.5%–79.8%). This would mean that an assumed hazard ratio of 0.55 in an RCT could translate into a hazard ratio close to 0.81 in real-world registries (see the Methods section in [19]), which was actually a hazard ratio very similar to the hazard ratios observed in our study, although not being statistically significant. The data in the present study thus do not indicate something unexpected, but are within the range of other observations comparing RCTs and real-world evidence.
Sensitivity analyses were conducted, which showed similar results like the primary analysis. In one of these analyses, the patient population was restricted to patients who started their therapy after the market authorization of palbociclib. This analysis was intended to address cohort effects in the treatment of HR + HER2– BC patients. Another analysis excluded patients with the comparator ETmono after CDK4/6i-availability. This analysis was intended to address the fact that after the drug became available, there had to be a strong reason for not treating a patient with a combination therapy if the RCTs had indicated a benefit with regard to the PFS [19]. This influence of treatment decision-making on the prognosis is supported by studies showing that in patients with HR + HER2– BC, the treatment decision to carry out chemotherapy was an independent prognostic factor [7,8]. It can therefore be assumed that patient selection can have a strong influence on the prognosis in patients with metastatic BC. However, this effect is most probably not caused by insufficient efficacy of chemotherapy, but rather because the patient population selected for chemotherapy has an a priori more unfavorable prognosis that cannot be explained by other commonly documented covariates. This is of specific importance for the present analysis, as the use of chemotherapy has decreased over time. A relevant proportion of these patients must now be treated with CDK4/6i + ET, leading to a poorer prognosis in this group in the real-world data presented here. The dynamic change of therapeutic strategies over time (chemotherapy vs. ETmono vs. CDK4/6i + ET) was clearly documented by our analysis and shows how the change of treatment strategies could have an effect on both side effects and efficacy in the treated patient populations, which was not analyzed in this study due to small sample sizes.
Several ongoing trials are comparing chemotherapy with CDK4/6i + ET (e.g., NCT03355157, NCT03462251). These trials should be supported in order to obtain more information about treatment options for this patient group.
Adverse events were reported much less frequently in this registry than in the RCTs. This is a concern in many registries [20]. However, the most important sign (leukopenia) was detected in the present registry. Nausea was also more frequent in patients receiving CDK4/6i + ET than in those with ET monotherapy, as it was reported in the clinical trials. It is known that registries report side effects less frequently than RCTs [[21], [22], [23]], but detecting the most important safety signals appears feasible, as shown here.
The main limitation of this analysis is its small sample size. The study excluded all patients who were not prospectively included in the registry, in order to provide data of the highest possible quality in a registry setting. This can on one hand ensure a high data quality as required for real-world data analyses [12], however it could also introduce a bias that makes it difficult to generalize the data and make statements concnering the general population of patients who are treated with CDK4/6i. The short follow-up time, with only 2 years between the first patient treated with CDK4/6i in the real-world setting and closure of the database, also only allows a very early analysis. However, real-time monitoring of novel drugs may be necessary in order to detect early signs that a drug is possibly being used in a patient population with a different risk–benefit ratio.
5. Conclusions
In conclusion, this study shows that CDK4/6i + ET has now been implemented as the new standard for first-line treatment of patients with advanced HR + HER– BC. With short observation times, a small sample size, and a small number of events, no statistically significant differences in the progression-free survival were observed when CDK4/6i + ET was compared with ETmonoin the real-world setting. The observed hazard ratios are within the expected range, since real-world effects have consistently been found to show smaller effect sizes. However, a certain amount of caution should be exercised in relation to the patient population being treated in routine clinical practice. Patients who are being considered for chemotherapy could be included in RCTs comparing chemotherapy with CDK4/6i + ET.
Declaration of competing interest
A.S. received honoraria from Roche, AstraZeneca, Aurikamed GmbH, Celgene, ClinSol Research GmbH, Connectmedica Sp.Z o.o., Deutsche Gesellschaft für Senologie GmbH, if-kongress management gmbh, I-MED Institute GmbH, Lilly Deutschland GmbH, Medicultus GmbH, med publico GmbH, MSD Sharp & Dohme GmbH, onkowissen.de GmbH, Pfizer GmbH, Schattauer Verlag GmbH, Promedicis GmbH, Tesaro Bio Germany GmbH, W. Zuckschwerdt Verlag GmbH. J.E. received honoraria from AstraZeneca, Celgene, Novartis, Lilly, Pfizer, Pierre Fabre, Roche and TEVA and travel support from Celgene, Pfizer, TEVA, and Pierre Fabre. D.L. received honoraria from Amgen, AstraZeneca, Celgene, Lilly, Loreal, MSD, Novartis, Pfizer, Tesaro, Teva. M.W.B reports support from Novartis, Merck Sharp & Dohme, AstraZeneca, Roche, Amgen, Eisai, paid to his institution. E.B. received honoraria from Novartis, Celgene, Riemser, Pfizer, Hexal, Amgen, onkowissen.de for consulting, clinical research management or medical education activities. P.A.F. received honoraria from Novartis, Pfizer, Roche, Amgen, Celgene, Daiichi-Sankyo, AstraZeneca, Merck-Sharp & Dohme, Eisai, Puma and Teva. His institution conducts research with funding from Novartis and Biontech. T.N.F. reports personal fees from Roche, personal fees from Novartis, personal fees from Pfizer, personal fees from Daichii Sankyo, personal fees from Lilly, personal fees from MSD, outside the submitted work. P.H. received honoraria, unrestricted educational grants and research funding from Amgen, AstraZeneca, Eli Lilly, MSD, Novartis, Pfizer, and Roche. A.D.H. received honoraria from Teva, GenomicHealth, Celgene, AstraZeneca, Novartis, Pfizer, and Roche. C.H. received honoraria from Amgen, Celgene, Oncovis, Roche, and Pfizer. J.H. received honoraria from Astra Zeneca, MSD, Lilly, travel grants from Novartis and research grants from Novartis, Celgene. E.R. reports personal fees from Roche, Pfizer, Amgen, Novartis, Tesaro, Astra Zeneca, Celgene, Pierre Fabre and Riemer, non-financial support from Olympus GmbH, personal fees from Riemser, outside the submitted work. W.J. received research grants and honoroaria from Novartis, Pfizer, Amgen, Chugai, Roche, Genomic-Health, AstraZeneca, Lilly. H.-C.K. received honoraria from Carl Zeiss meditec, TEVA, Theraclion, Novartis, Amgen, AstraZeneca, Pfizer, Janssen-Cilag, GSK, LIV Pharma, and Genomic Health. C.M.K. received honoraria from Amgen, Axios, Roche, Teva, Novartis, MSD Sharp & Dohme, Mundipharma, NewCo, Pfizer, Riemser, and ZytoService, research grants from Amgen, Axios, Novartis, MSD Sharp & Dohme, NewCo, Pfizer, and ZytoService, and travel support from Amgen, Paxman Inc., PharmaMar, and Pfizer. M.P.L. has participated on advisory boards for AstraZeneca, MSD, Novartis, Pfizer, Eisai, Genomic Health, Tesaro and Roche and has received honoraria for lectures from MSD, Lilly, Roche, Novartis, Pfizer, Genomic Health, AstraZeneca, medac and Eisai. V.M. received speaker honoraria from Amgen, Astra Zeneca, Celgene, Daiichi-Sankyo, Eisai, Pfizer, Novartis, Roche, Teva, Janssen-Cilag and consultancy honoraria from Genomic Health, Hexal, Roche, Pierre Fabre, Amgen, Novartis, MSD, Daiichi-Sankyo, Eisai, Lilly, Tesaro and Nektar. N.N. received consultancy honoraria from Janssen-Cilag and travel support from Novartis. F.O. received speaker and consultancy honoraria from Amgen, AstraZeneca, Bayer, BMS, Boehringer, Celgene, Cellex, Chugai, Gilead, Hexal, Ipsen, Janssen-Cilag, Merck, MSD, Novartis, Riemser, Roche, Tesaro, Teva. H.T. received honoraria from Novartis, Roche, Celgene, TEVA, and Pfizer and travel support. F.-A. T. received honoraria from Astra Zeneca, Genomic Health and Novartis. C.T. received honoraria from Amgen, Astra-Zeneca, Celgene, Novartis, Pfizer, and Roche. M.U. reports support paid to his institution from Abbvie, Amgen GmbH München, Astra Zeneca, BMS, Celgene GmbH München, Daiji Sankyo, Eisai GmbH München, Janssen Cilag, Johnsen&Johnsen, Lilly Deutschland, Lilly Int., MSD Merck, Mundipharma, Myriad Genetics GmbH Zürich, Odonate, Pfizer GmbH Berlin, PUMA Biotechnology, Riemser, Roche Pharma AG, Grenzach Wyhlen, Sanofi Aventis Deutschland GmbH, Sividon Diagnostics Köln, TEVA Pharmaceuticals Ind. Ltd. und. Berlin. P.W. received honoraria from Amgen, AstraZeneca, Merck Sharp & Dohme, Novartis, Pfizer, PharmaMar, Roche, TEVA, Eisai Clovis and Tesaro. She participated in advisory boards of Amgen, AstraZeneca, Merck Sharp & Dohme, Novartis, Pfizer, PharmaMar, Roche, TEVA, Eisai Clovis and Tesaro. Her institution received research grants from Amgen, AstraZeneca, MSD, Novartis, Pfizer, Pharmamar, Clovis and Tesaro. R.W. received honoraria from Agendia, Amgen, Astra Zeneca, Boeringer Ingelheim, Carl Zeiss, Celgene, Daiichi-Sankyo, Esai, Genomic Health, Glaxo Smith Kline, Lilly, MSD, Mundipharma, Nanostring, Novartis, Odonate, Paxman, Palleos, Pfizer, Pierre Fabre, PumaBiotechnolgogy, Riemser, Roche, Sandoz/Hexal, Seattle Genetics, Tesaro Bio, Teva. M.W. received speaker honoraria from AstraZeneca, Celgene, and Novartis. S.Y.B. reports personal fees from Novartis and Pfizer, both outside the submitted work. All remaining authors (J.S., E.K., M.G., L.H., B.V., D.W.) have declared that they do not have any conflicts of interest.
Acknowledgments
The PRAEGNANT network is supported by grants from Pfizer, Hexal, Celgene; Daiichi-Sankyo, Roche, Merrimack, Eisai, and Novartis. These companies did not have any involvement in the study design, in the collection, analysis, or interpretation of the data, in the writing of the report, or in the decision to submit this article for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.08.011.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Fasching P.A., Schneeweiss A., Kolberg H.C., Ettl J., Fehm T.N., Overkamp F. Translational highlights in breast cancer research and treatment: recent developments with clinical impact. Curr Opin Obstet Gynecol. 2019;31:67–75. doi: 10.1097/GCO.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 2.Im S.A., Lu Y.S., Bardia A., Harbeck N., Colleoni M., Franke F. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 3.Sledge G.W., Jr., Toi M., Neven P., Sohn J., Inoue K., Pivot X. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2019;6(1):116–124. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slamon D.J., Neven P., Chia S., Fasching P.A., De Laurentiis M., Im S.A. Overall survival results from the phase 3 MONALEESA-3 study of fulvestrant ± ribociclib in postmenopausal patients with HR+/HER2− advanced breast cancer. Ann Oncol. 2019;30(suppl_5):v851–v934. doi: 10.1093/annonc/mdz394. [DOI] [Google Scholar]
- 5.Turner N.C., Slamon D.J., Ro J., Bondarenko I., Im S.A., Masuda N. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 6.Turner N.C., Ro J., Andre F., Loi S., Verma S., Iwata H. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 7.Huober J., Fasching P.A., Taran F.A., Volz B., Overkamp F., Kolberg H.C. Factors associated with first line chemotherapy use in patients with hormone receptor positive, HER2 negative metastatic breast cancer - data from the PRAEGNANT breast cancer registry. Canc Res. 2018;78 doi: 10.1158/538-7445.SABCS17-P3-11-07. [DOI] [Google Scholar]
- 8.Lobbezoo D.J., van Kampen R.J., Voogd A.C., Dercksen M.W., van den Berkmortel F., Smilde T.J. In real life, one-quarter of patients with hormone receptor-positive metastatic breast cancer receive chemotherapy as initial palliative therapy: a study of the Southeast Netherlands Breast Cancer Consortium. Ann Oncol. 2016;27:256–262. doi: 10.1093/annonc/mdv544. [DOI] [PubMed] [Google Scholar]
- 9.Bonotto M., Gerratana L., Di Maio M., De Angelis C., Cinausero M., Moroso S. Chemotherapy versus endocrine therapy as first-line treatment in patients with luminal-like HER2-negative metastatic breast cancer: a propensity score analysis. Breast. 2017;31:114–120. doi: 10.1016/j.breast.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Andre F., Neven P., Marinsek N., Zhang J., Baladi J.F., Degun R. Disease management patterns for postmenopausal women in Europe with hormone-receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer. Curr Med Res Opin. 2014;30:1007–1016. doi: 10.1185/03007995.2014.887002. [DOI] [PubMed] [Google Scholar]
- 11.Hartkopf A.D., Huober J., Volz B., Nabieva N., Taran F.A., Schwitulla J. Treatment landscape of advanced breast cancer patients with hormone receptor positive HER2 negative tumors - data from the German PRAEGNANT breast cancer registry. Breast. 2018;37:42–51. doi: 10.1016/j.breast.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Booth C.M., Karim S., Mackillop W.J. Real-world data: towards achieving the achievable in cancer care. Nat Rev Clin Oncol. 2019;16:312–325. doi: 10.1038/s41571-019-0167-7. [DOI] [PubMed] [Google Scholar]
- 13.Brufsky A., Mitra D., Davis K.L., Nagar S.P., McRoy L., Cotter M.J. Treatment patterns and outcomes associated with palbociclib plus letrozole for postmenopausal women with HR(+)/HER2(-) advanced breast cancer enrolled in an expanded access program. Clin Breast Canc. 2019;19:317–325 e4. doi: 10.1016/j.clbc.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Varella L., Eziokwu A.S., Jia X., Kruse M., Moore H.C.F., Budd G.T. Real-world clinical outcomes and toxicity in metastatic breast cancer patients treated with palbociclib and endocrine therapy. Breast Canc Res Treat. 2019;176:429–434. doi: 10.1007/s10549-019-05176-1. [DOI] [PubMed] [Google Scholar]
- 15.Taylor-Stokes G., Mitra D., Waller J., Gibson K., Milligan G., Iyer S. Treatment patterns and clinical outcomes among patients receiving palbociclib in combination with an aromatase inhibitor or fulvestrant for HR+/HER2-negative advanced/metastatic breast cancer in real-world settings in the US: results from the IRIS study. Breast. 2019;43:22–27. doi: 10.1016/j.breast.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Fasching P.A., Brucker S.Y., Fehm T.N., Overkamp F., Janni W., Wallwiener M. Biomarkers in patients with metastatic breast cancer and the PRAEGNANT study network. Geburtshilfe Frauenheilkd. 2015;75:41–50. doi: 10.1055/s-0034-1396215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brookmeyer R., Crowley J. A confidence-interval for the median survival-time. Biometrics. 1982;38:29–41. [Google Scholar]
- 18.Salmen J., Neugebauer J., Fasching P.A., Haeberle L., Huober J., Wockel A. Pooled analysis of the prognostic relevance of progesterone receptor status in five German cohort studies. Breast Canc Res Treat. 2014;148:143–151. doi: 10.1007/s10549-014-3130-4. [DOI] [PubMed] [Google Scholar]
- 19.Lakdawalla D.N., Shafrin J., Hou N., Peneva D., Vine S., Park J. Predicting real-world effectiveness of cancer therapies using overall survival and progression-free survival from clinical trials: empirical evidence for the ASCO value framework. Value Health. 2017;20:866–875. doi: 10.1016/j.jval.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Dreyer N.A., Sheth N., Trontell A., Gliklich R.E. Good practices for handling adverse events detected through patient registries. Drug Inf J. 2008;42:421–428. [Google Scholar]
- 21.Lionetti G., Kimura Y., Schanberg L.E., Beukelman T., Wallace C.A., Ilowite N.T. Using registries to identify adverse events in rheumatic diseases. Pediatrics. 2013;132:e1384–e1394. doi: 10.1542/peds.2013-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caixeta A., Campos C.M., Felix C., Chieffo A., Capranzano P., Kawamoto H. Predictors of long-term adverse events after absorb bioresorbable vascular scaffold implantation: a 1,933-patient pooled analysis from international registries. EuroIntervention. 2018;15(7):623–630. doi: 10.4244/EIJ-D-16-00796. [DOI] [PubMed] [Google Scholar]
- 23.Bergqvist D., Bjorck M., Sawe J., Troeng T. Randomized trials or population-based registries. Eur J Vasc Endovasc Surg. 2007;34:253–256. doi: 10.1016/j.ejvs.2007.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.