1. Introduction
Inhibitory synaptic circuits within the spinal dorsal horn (DH) mature slowly after birth, as evidenced by a delayed emergence of glycinergic signaling and the disappearance of GABAergic depolarizations [6; 25; 50]. These developmental alterations likely contribute to the low mechanical thresholds, enlarged receptive fields, and prolonged afterdischarges that characterize immature DH neurons in vivo [16; 41; 43; 84], and may also explain the exaggerated tactile sensitivity in neonatal rodents and humans [3; 4; 29; 56; 86].
GABAergic DH interneurons can be neurochemically classified into multiple subpopulations [81; 82] which regulate distinct, yet overlapping, somatosensory modalities. For example, the ablation of mature interneurons from the dynorphin (DYN) or parvalbumin lineages produces mechanical allodynia [28; 60] while ablation of spinal neurons from the neuropeptide Y lineage evokes chronic itch [13]. Notably, although the enhanced mechanical sensitivity seen in neonates could reflect the immaturity of spinal DYN and/or parvalbumin circuits, little is known about the functional development of identified subtypes of DH GABAergic interneurons. Furthermore, since the above ablations were exclusively performed in adults, the subtypes of spinal interneurons that regulate nociceptive and pruriceptive processing in neonates have yet to be identified. The ongoing plasticity of spinal inhibitory networks after birth also raises the possibility that the precise somatosensory modalities regulated by a given class of GABAergic interneuron depend on age, which would have important implications for the design of strategies to alleviate chronic pain and itch in the pediatric population.
Despite recent insights into the functional organization of the DH, surprisingly little is known about which subtypes of inhibitory interneurons synapse onto PNs and thereby directly restrict the output of the spinal nociceptive network. Interneurons expressing nNOS synapse onto giant lamina I PNs that mostly fail to exhibit NK1 receptor immunoreactivity [33; 63], but these constitute a small fraction of all PNs since ~90% of lamina I PNs express NK1Rs [20; 75]. Recent studies showed that DYN interneurons directly inhibit spinoparabrachial neurons [37]. Although the prevalence of DYN neurons in the superficial laminae [14] suggests they are well-positioned to dominate the inhibition of PNs, the relative contribution of DYN inputs to the overall GABAergic/glycinergic innervation of PNs remains unclear. More importantly, nothing is known about which interneurons inhibit PNs during early life, or how the efficacy of these key synapses changes with age, thereby preventing a complete understanding of the factors regulating ascending nociceptive transmission at various stages of postnatal development.
The present study is the first to identify developmental alterations in the intrinsic membrane properties and functional connectivity of spinal DYN interneurons, which includes a progressive increase in the efficacy of their inhibitory synapses onto lamina I spinoparabrachial neurons. Notably, spinal DYN neurons appear to shape the processing of certain sensory modalities in an age-dependent manner, as the chemogenetic silencing of this population exacerbates acute non-histaminergic itch only in adults while mechanical sensitivity was enhanced at all ages. These findings yield valuable new insight into the neuronal circuit mechanisms underlying the processing of pain and itch within the developing spinal cord.
2. Materials and Methods
2.1. Animals
All animals in this study were treated in accordance to welfare guidelines outlined by the Institutional Animal Care and Use Committee at the University of Cincinnati.
To identify DH interneurons derived from the DYN lineage, we crossed Pdyn-IRES-Cre mice expressing Cre recombinase under control of the prodynorphin (Pdyn) gene promoter (DYNCre; The Jackson Laboratory; Bar Harbor, ME; Stock No: 027958) with Ai9 mice that express tdTomato (tdTOM) from the Rosa26 locus in the presence of Cre recombinase (Jackson; Stock No: 007909). The offspring will be referred to as DYNtdTOM. To investigate the functional properties of DYN synapses onto projection neurons (PNs), we crossed DYNCre mice with Ai32 mice (Jackson; Stock No: 012569) that express channelrhodopsin-2 (ChR2/EYFP) from the Rosa26 locus after exposure to Cre recombinase. The resulting offspring will be referred to as DYNChR2. To visualize DYN presynaptic terminals apposed to projection neurons, we crossed DYNCre mice with Ai34D mice (Jackson; Stock No: 012570) expressing a Cre recombinase-dependent synaptophysin-tdTomato fusion protein. The resulting offspring will be referred to as DYNSYP-tdTOM.
To express Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) predominantly in inhibitory DYN interneurons in the spinal cord and hindbrain, we crossed three lines of mice: (1) DYNCre mice; (2) mice expressing flpO recombinase (flp) under control of the Lbx1 promoter [13]; and (3) mice expressing a “double stop (ds-)” inhibitory DREADD receptor (ds-hM4Di; Jackson Stock No: 029040) that requires both Cre and flp to initiate expression. Importantly, while Lbx1 expression is not restricted to inhibitory neurons in the DH, a prior study has clearly demonstrated that the ablation of neurons expressing both DYNCre and Lbx1flp evokes a dramatic loss of spinal DYN-lineage neurons expressing the inhibitory markers GAD67 and GlyT2 but does not significantly decrease the number of DYN neurons expressing VGLUT2, which is present in virtually all excitatory neurons in the DH [18], thereby suggesting that excitatory DYN neurons may originate from Lbx1-negative spinal neurons [28]. Therefore, we expect that the intersection of Lbx1 and Pdyn gene expression occurs at least predominantly within inhibitory neurons in the spinal cord and hindbrain, thereby sparing DYN neurons in other regions of the CNS and the dorsal root ganglia. This mouse line will be referred to as Lbx1:DYNds-hM4Di. Since heterozygous Lbx1flp animals were used for breeding, littermates lacking the Lbx1-flpO allele were used as controls for all behavioral experiments.
In order to capture the postnatal development of the somatosensory system, we used both male and female mice from three age ranges: postnatal days (P)6–7, P21–22, and P49–63. Female mice were not evaluated for estrus cycle stage during these experiments.
2.2. Identification of the neurotransmitter phenotype of developing spinal DYNtdTOM interneurons
DYNtdTOM mice were sacrificed and transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (PB). The lumbar enlargement of the spinal cord was removed and placed in 30% sucrose (in 0.1M PB) overnight. The tissue was frozen and 40 μm sections were cut using a cryostat (Leica; Wetzlar, Germany). Free-floating sections were washed in phosphate-buffered saline (PBS) and placed in a blocking buffer containing 10% normal donkey serum and 0.3% Triton X-100. The sections were then incubated in a primary antibody against the transcription factor Pax2 (1:500 dilution; ThermoFisher #716000; RRID: AB_2533990) overnight at 4°C. Pax2 was used as a marker of inhibitory neurons since it is continuously expressed throughout development and is present in both GABAergic and glycinergic neurons [39; 52; 62]. Sections were washed again in PBS and incubated in a species-specific secondary antibody conjugated to AlexaFluor 488 (1:500 dilution; ThermoFisher #A-11008; RRID: AB_143165) for one hour at room temperature.
The spinal cord dorsal horn of each section was imaged on a BX63 upright fluorescent microscope (Olympus; Center Valley, PA) at 20X or 40X, and z-stack images were taken with a separation of 1 μm. The total number of both tdTOM- and tdTOM/Pax2-expressing cells in a section were counted using CellSens Dimension Desktop software (Olympus).
2.3. Retrograde labeling of lamina I projection neurons (PNs)
To identify PNs for histological examination, the retrograde tracer Cholera Toxin B subunit (CTB) was unilaterally injected into the parabrachial nucleus, since this brain region is the target of the majority of lamina I PNs [20]. Five or more days prior to tissue harvest, mice were anesthetized with ketamine (90 mg/kg) and xylazine (P2: 1 mg/kg; P17 and P40–61: 10 mg/kg). Mice were placed in a stereotaxic injection apparatus (World Precision Instruments; Sarasota, FL, USA) and the head secured with non-rupture ear bars (World Precision Instruments). At P2, the parabrachial nucleus was targeted using the following coordinates in relation to lambda (cm): −0.19 rostrocaudal, −0.1 mediolateral, and −0.31 dorsoventral based on an atlas of the mouse brain [59]. A small puncture was made through the scalp and skull with a 27½-gauge needle before injecting (35 nL at an infusion rate of 30 nL/min) the retrograde tracer CTB (1%, Sigma-Aldrich; St. Louis, MO, USA) with a Hamilton syringe (62RN; 2.5 μL; 33-gauge needle; Hamilton Company, Reno, Nevada, USA). A similar procedure was followed for older animals, except an incision was made through the scalp to expose bregma and a small hole was made in the skull with an OmniDrill35 (World Precision Instruments), then 75 nL of CTB was injected at an infusion rate of 35 nL/min. The coordinates (cm in relation to bregma) for P17 animals were −0.42–0.45 rostrocaudal, −0.11 mediolateral, and −0.43 dorsoventral; while the coordinates for P40–61 animals were −0.47–0.49 rostrocaudal, −0.12 mediolateral, and −0.40–0.42 dorsoventral. In adolescent or adult animals, the skin was closed using Vetbond (3M; Maplewood, MN, USA) or Gluture (Zoetis Inc.; Kalamazoo, MI, USA). All animals recovered on a heating pad and were returned to the home cage upon recovery.
For electrophysiological studies, PNs were labeled using the same technique as described above using the retrograde tracer DiI, which was injected at least two days before preparing spinal cord slices for patch clamp recording. At P2–3, an infusion rate of 30 nL/min with a total volume of 70 nL was used, while 150 nL of DiI was injected at P17–19 and P40–61 with an infusion rate of 35 nL/min.
2.4. Characterization of putative DYN contacts onto spinal PNs
In order to visualize presynaptic terminals originating from dynorphin-lineage neurons, DYNSYP-tdTOM mice were deeply anesthetized using sodium pentobarbital (Fatal-Plus, Vortech Pharmaceuticals; Dearborn, MI, USA), and transcardially perfused with 0.1 M PB. Spinal cord lumbar enlargements were removed and post-fixed in paraformaldehyde (4%) in PB for at least two hours. The tissue was then placed in 30% sucrose in PB for a minimum of 12 hours or until the tissue no longer floated in the solution. The spinal cord was then frozen (-20°C) and cut into coronal sections at 40 μm on a cryostat. Spinal cords were processed as on-slide or free-floating sections as previously described [53]. Primary antibodies against CTB (1:2000 dilution; List Biological Catalog #703, RRID: AB_10013220) and VGAT (1:2000 dilution; Synaptic Systems GmbH Catalog #131 002, RRID: AB_88787) were used. Sections were then incubated in species-specific secondary antibodies conjugated to AlexaFluor 488 (1:500 dilution; ThermoFisher #A-11008; RRID: AB_143165) and AlexaFluor 647 (1:500 dilution; ThermoFisher #A-31573; RRID: AB_2536183) for one hour at room temperature. Images were captured on a Nikon A1 LUNA inverted confocal microscope under a 100 × oil-immersion lens. Z-stack images were obtained by sequentially scanning using an interval of 0.5 μm with a resolution of 1024 pixels. The surface area of each cell was calculated by measuring the diameter and length of the PN dendrites and soma (in the region where boutons were counted) and approximating the dendrites to be cylindrical (A=2πrh+2πr2), and the soma to have an ellipsoid shape () as described previously [53; 83]. After a z-series of images captured the majority of the neuron and its dendritic arbor, boutons containing tdTOM (corresponding to the presynaptic terminals of DYN-lineage neurons) and/or VGAT (corresponding to inhibitory terminals) were counted in each image if the terminals were directly apposed to the dendrites or soma of the CTB-labeled PN. The density of tdTOM+, VGAT+, and tdTOM+/VGAT+ boutons were calculated as the number of contacts per 1000 μm2 area of the PN in order to account for any differences in neuronal size.
2.5. Evaluation of DYN expression in PNs
To determine whether the spinal population of DYN-lineage neurons includes lamina I PNs, DYNtdTOM mice were injected with the retrograde tracer CTB as described above. After 1 week, mice were euthanized and transcardially perfused with paraformaldehyde (4% in 0.1 M PB). Spinal cord lumbar enlargements were removed and post-fixed for an additional 4 hours then cryoprotected overnight in 30% sucrose in 0.1 M PB. Frozen sections (20 μm) were cut in the horizontal plane on a Leica 1860 cryostat and mounted directly on slides. Immunostaining for CTB was performed as described above; endogenous tdTomato expression was visible without additional antibody staining. Z-stack images of the stained sections were obtained at 20X magnification as an extended focal image projection in CellSens Dimension Desktop (Olympus).
2.6. In vitro spinal cord preparations
Following the induction of a deep anesthetic state with sodium pentobarbital (Fatal-Plus), mice were perfused through the heart with sucrose-substituted artificial cerebrospinal fluid (dissection solution; containing in mM: 250 sucrose, 2.5 KCl, 25 NaHCO3, 1.0 NaH2PO4, 6 MgCl2, 0.5 CaCl2, and 25 glucose) that was continuously bubbled with 95% O2 / 5% CO2 and chilled on ice. The vertebral column was isolated and quickly submerged in ice-cold dissection solution. The spinal cord was removed from the vertebral column and then stripped of the dura mater and dorsal roots. The L3-L5 segments of the spinal cord were immersed in low-melting point agarose (3% in dissection solution; Invitrogen; Carlsbad, CA, USA) and parasagittal slices (250–400 μm) were cut using a vibrating microtome (7000smz-2; Campden Instruments; Lafayette, IN, USA).
For the intact spinal cord preparation with nerves attached, mice were euthanized and perfused in the same manner described in the previous section. The skin and muscle on the hindlimb were removed to isolate the sciatic nerve, which was then severed before the distal trifurcation into the sural, peroneal, and tibial nerves. The spinal cord (along with the sciatic nerve, L3–L4 dorsal root ganglia and dorsal roots) was removed from the vertebral column, stripped of the dura and pia mater, and cleared of extraneous dorsal roots and dorsal root ganglia. Spinal cords were left intact, and the sciatic nerve and/or the dorsal roots were left attached to the spinal cord. Slices or intact cords recovered at room temperature for 15–20 minutes in a solution [80] containing (in mM): 92 NMDG, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 5 Na ascorbate, 2 thiourea, 3 Na pyruvate, 10 MgSO4, and 0.5 CaCl2 (pH 7.3–7.4 with HCl). After recovery, slices were incubated in artificial CSF (composition in mM: 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.0 NaH2PO4, 1.0 MgCl2, 2.0 CaCl2, and 25 glucose) at room temperature until recording. To determine the primary afferent input type, the sciatic nerve was stimulated via a suction electrode using a constant current stimulator (Master-8; A.M.P.I.; Jerusalem, Israel) with a stimulus isolation unit (ISO-Flex; A.M.P.I.). For experiments involving primary afferent-evoked action potential (AP) firing, only the dorsal root (L3 or L4) was stimulated.
2.7. Recording of compound action potentials (CAPs) from primary afferents
We estimated the stimulation parameters for each class of primary afferent fiber for each animal when possible in order to account for variation between animals and recording conditions (e.g. age, sex, suction electrode fit, etc.). At the end of each recording session, the dorsal root was cut near its entry zone and the sciatic nerve and dorsal root were placed in suction electrodes for stimulation and recording, respectively. CAPs were generated via incremental stimulation of the sciatic nerve (0–1 mA, 100 μs duration) as described above and recorded from the dorsal root. Fiber types were identified based on conduction velocity and threshold, and these parameters used to classify afferent-evoked synaptic responses recorded earlier that day.
2.8. Patch clamp recording from spinal DYN interneurons
After incubation, intact spinal cords or slices were transferred to a submersion chamber (RC-22; Warner Instruments, Hamden, CT, USA), secured with an anchor, placed on the stage of an upright microscope (BX51WI; Olympus), and perfused with room temperature artificial CSF during recordings. DYN-lineage interneurons were visualized by tdTomato fluorescence, infrared illumination, and/or oblique infrared LED illumination as previously described [69; 79]. Patch pipettes were pulled (P-97; Sutter Instruments; Novato, CA, USA) from thin walled single-filament borosilicate glass (1.5 mm diameter, World Precision Instruments). The resistance of the patch pipettes ranged from 3–7 MΩ, and a seal resistance of ≥1 GΩ was obtained before entering whole cell mode. A Multiclamp 700B amplifier (Molecular Devices; Sunnyvale, CA, USA) and a Digidata 1440A (with pClamp 10.7 software; Molecular Devices) were used to acquire recordings. Liquid junction potentials (-14 mV) were calculated using JPCalc software (P. Barry, University of New South Wales, Sydney, Australia; modified for Molecular Devices) and used to adjust membrane potentials. A 4 kHz low-pass Bessel filter and a 10 kHz sampling rate were used for all recordings.
When evaluating primary afferent-evoked excitatory postsynaptic currents (EPSCs), lamina I DYNtdTOM cells were recorded in an intact spinal cord preparation using a patch electrode solution containing the following (in mM): 130 Cs-gluconate, 10 CsCl, 10 HEPES, 11 EGTA, 1 CaCl2, and 2 MgATP, pH 7.2 (295–305 mOsm). The sciatic nerve was stimulated via a suction electrode at increasing intensities (10 μA-100 μA in 10 μA increments, 100 μA-500 μA in 50 μA increments; 500 μA, 1 mA; 100 μs duration; 0.1 Hz) as described above. Stimulus threshold was classified as the minimum intensity required to evoke a PSC in 50% of the trials. EPSCs were recorded from a holding potential of −70 mV, and primary afferent input was classified as being mediated by Aβ, Aδ, high- or low-threshold C fibers using a combination of onset latency and stimulus threshold as described above. It should be noted that this method of classifying sensory input likely underestimates the true conduction velocity of the stimulated primary afferent fibers [53]. EPSCs were classified as monosynaptic based on their ability to follow repetitive stimulation (Aβ: 10 Hz; Aδ: 2 Hz; C: 1 Hz) without failures and minimal variability in onset latency (≤ 2 ms) [85].
Current clamp recordings used an intracellular solution containing the following (in mM): 130 K-gluconate, 10 KCl, 10 HEPES, 10 Na-phosphocreatine, 0.3 Na2GTP, and 2 MgATP, pH 7.2 (295–305 mOsm). To evaluate primary afferent-evoked firing in DYN interneurons, the L3 or L4 dorsal root was stimulated via a suction electrode at increasing intensities (10 μA-100 μA in 10 μA increments, 100 μA-500 μA in 50 μA increments, 500 μA, 1 mA; 100 μs duration; 0.1 Hz) and the number of action potentials (APs) was measured. The intrinsic excitability of lamina I and II DYN interneurons was measured in a slice preparation. Resting membrane potentials were measured over a time period of ~60–120 s. To record rheobase, AP threshold, AP amplitude and AP half-width, intracellular current was injected (80 ms duration; in 5 pA steps) from the resting membrane potential until an AP was observed, and the resulting traces were analyzed using Clampfit 10.7. Rheobase is presented as the minimum current intensity required to elicit an AP, while AP threshold was calculated as the minimum membrane potential required for spike initiation. AP amplitude was analyzed as difference between the peak membrane potential and spike threshold. AP half-width was measured as the time from the peak amplitude to half the threshold value (in mV) during the repolarization phase. Cell capacitance was measured in DYNtdTOM cells in voltage clamp mode using the pClamp membrane test (33.3–200 Hz, −10 mV).
To measure membrane resistance, repetitive firing pattern, instantaneous firing frequency and discharge duration, DYNtdTOM neurons were injected with current (800 ms duration; from −20 to 170 pA in 10 pA steps) from their resting membrane potential. Firing patterns were characterized as single, phasic, tonic, or delayed [68]. Membrane resistance was calculated using the hyperpolarization produced by a −20 pA current injection. Interspike interval and instantaneous firing frequency were determined using MiniAnalysis 6.0.7 (Synaptosoft; Decatur, GA, USA) to measure the interval between each spike at all intensities where APs were observed. Discharge duration was measured as the time elapsed between the first and last APs during the current injection. If the cell only fired one AP during the current pulse, the single AP duration was used for discharge duration, and the event frequency for the total current injection (1 AP/800 ms) was used as a measure of the evoked firing frequency.
2.9. Optogenetic activation of inhibitory DYN synapses onto PNs
Spinal cord slices were prepared from DYNChR2 mice as described above, and DYN interneurons were activated via 470 nm light exposure delivered via a LED system (CoolLED; Andover, UK). DiI-labeled PNs in lamina I were voltage-clamped at 0 mV using a Cs Gluc-based intracellular solution as described above. Antagonists for AMPA (NBQX, 10 μM) and NMDA receptors (AP-5, 20 μM) were added to the bath during recording to isolate IPSCs. To measure the amplitude and coefficient of variation (CoV) of DYN-mediated IPSCs, 10 ms light pulses were delivered at 0.1 Hz. The mean peak amplitude of the DYN-evoked IPSC was measured over 30 trials, while the CoV was measured as the standard deviation/mean peak amplitude recorded over the same 30 trials. CoV is known to be inversely related to the probability of neurotransmitter release and/or the number of release sites [24]. The paired pulse ratio (PPR), which is inversely related to neurotransmitter release probability [26], was measured via the ratio of IPSC2/IPSC1 in response to identical optogenetic stimuli delivered 75 ms apart. The relative contribution of GABAARs and glycine receptors to the observed IPSCs was determined via the sequential bath application of gabazine (10 μM) and strychnine (0.5 μM), respectively.
2.10. Behavioral measures of mechanical sensitivity
Lbx1:DYNds-hM4Di mice (or flp-negative littermate controls) were allowed to habituate for one hour (or 15 minutes at P6–7) in clear acrylic containers elevated on a mesh floor (or a small closed-bottom container on a heating pad for P6–7 mice). Mechanical thresholds were measured using the simplified up-down method (SUDO) with von Frey hair filaments [11] by an experimenter blinded to genotype. In the SUDO assay, filaments #3–10 (beginning with filament 7) were used for mice aged P21 or older, while filaments #2–9 were used at P6–7 (starting with filament 6). The filament was applied five times, with at least a five-minute interval between presentations. After baseline measurements were taken, mice were given intraperitoneal (i.p.) injections of clozapine dihydrochloride (CLZ; Hello Bio; Princeton, NJ) at a dose of 0.1 mg/kg in sterile saline, which has been previously demonstrated to exert behavioral effects selectively in hM4Di-expressing mice, while higher doses (1 mg/kg) also influenced behavior in control mice [34]. Measurements of mechanical sensitivity began 20–25 minutes after CLZ injection, as CLZ has been shown to evoke behavioral effects in DREADD-expressing animals as soon as 10 min after intraperitoneal injection [34; 91]. Importantly, CLZ levels in the CNS reportedly peak at ~15 minutes after intraperitoneal administration and remain stable for at least 30 minutes after injection [42].
2.11. Assays of thermal sensitivity
Heat sensitivity was tested using the Hargreaves apparatus (Model 390; series 8; IITC Life Science Inc., Woodland Hills, CA) by an experimenter blinded to genotype. Briefly, Lbx1:DYNds-hM4Di mice were enclosed in clear, acrylic containers on a raised glass plate. Following a habituation period, a 4×6 mm beam of light (25% of maximum intensity) was directed at the plantar surface of the left hindpaw and the time to paw withdrawal was measured (in seconds), with a preset cut-off of 20 seconds. Three trials were recorded per animal, with an interval of at least five minutes between each test, both before and 20–25 min after the i.p. injection of CLZ at a dose of 0.1 mg/kg [34].
Sensitivity to noxious cold was assessed using the cold plantar assay as previously described [17]. Briefly, Lbx1:DYNds-hM4Di mice were placed in clear acrylic containers elevated on a glass plate and allowed to habituate as described above. Dry ice was pulverized and packed into a 1 mL syringe with a cut-off top. The syringe tip was pressed onto the glass underneath the footpad of the left hindpaw and the latency to paw withdrawal was recorded (in seconds). The test was repeated three times per animal, with a minimum interval of five minutes separating stimuli presentations, before and 20–25 min after i.p. injection of CLZ at a dose of 0.1 mg/kg [34].
2.12. Behavioral measure of itch sensitivity
Lbx1:DYNds-hM4Di mice were placed in clear acrylic containers surrounded by mirrors (at the same time each day) and pretreated with an intraperitoneal injection of CLZ (0.1 mg/kg). After 20–25 minutes, an intradermal chloroquine injection (10 μg/μL in sterile saline, at a dose of 1 μL/gram body weight) was administered into the nape of the neck [28; 46], and the total time spent scratching the nape with the hindpaw (see Supplemental Digital Content 1, which demonstrates scratching behavior in a P6 mouse after intradermal chloroquine injection) was quantified over a 30 minute period by an experimenter blinded to genotype. While the number of scratch bouts can also be used to quantify itch sensitivity, we observed a large variability in the length of a scratch bout even within an individual mouse. Therefore, to avoid scoring a brief (~1 sec) scratching bout equally to a prolonged (7–10 sec) scratching event, we chose to measure the total time spent scratching in an attempt to more accurately reflect the overall level of itch-related behavior.
2.13. Experimental design and statistical analysis
Power analyses were performed with the parameters α = 0.05 and β = 0.2 on preliminary datasets to determine the sample size required for each experimental design. Each dataset was tested for normality (D’Agostino & Pearson test) to determine if a nonparametric test or data transformation was necessary. Although the majority of the below experiments were not adequately powered to detect sex differences, developmental changes in select intrinsic membrane properties were compared in male vs. female mice. All data are presented as mean ± standard error of the mean (SEM). All statistical analyses were carried out in Prism version 8.2.1 (GraphPad Software; La Jolla, CA).
To determine the percentage of DYN interneurons in the developing DH that are inhibitory (Fig. 1), three male mice were used at each age and 5–6 spinal cord sections were taken from each animal for analysis. The number of cells expressing both tdTOM and Pax2 were expressed as a percentage of the total number of tdTOM cells, and data were analyzed with a one-way ANOVA followed by a Sidak’s multiple comparisons posthoc test. Three adult female DYNtdTOM mice were used to investigate the overlap between PNs and DYN neurons (Fig. 1).
Figure 1: DYN-lineage interneurons in the spinal dorsal horn (DH) are primarily inhibitory throughout development.
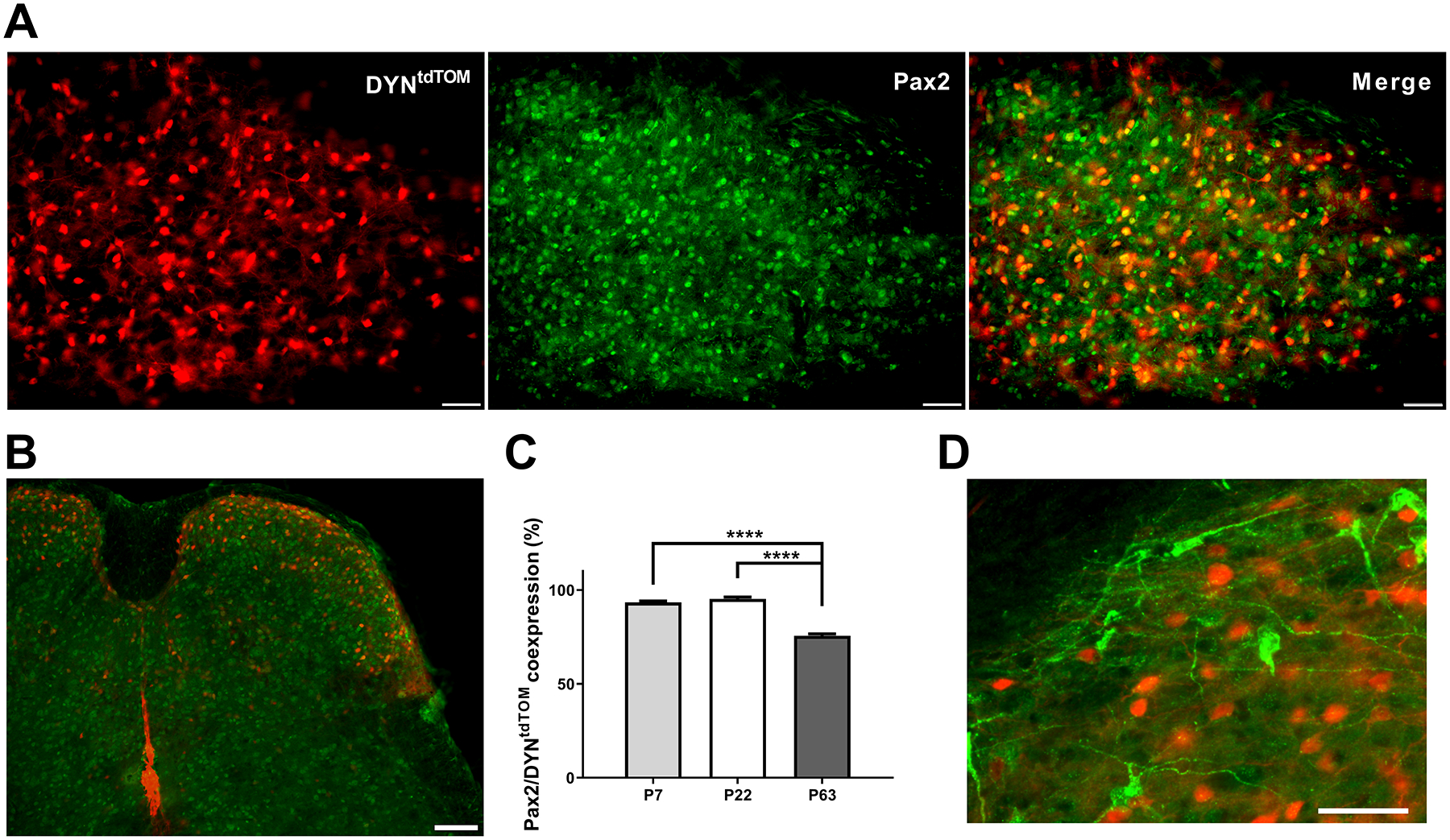
A) Images depicting tdTomato-labeled neurons in DYNtdTOM mice (left), immunostaining for the transcription factor Pax2 as a marker of inhibitory interneurons (middle), and the merged image used for quantification (right; scale bars = 50 μm). B) Image depicting DYN-tdTomato neurons and Pax2-immunoreactive neurons distributed throughout the DH (scale bar = 100 μm). C) Quantification of inhibitory DYN interneurons throughout development reveals a decline in Pax2-expressing DYN interneurons in adulthood (F(2, 50) = 142.5, p < 0.0001, one-way ANOVA; p < 0.0001; Sidak’s multiple comparisons test), although the majority of DYN interneurons are inhibitory regardless of age. D) Image demonstrating the lack of overlap between DYN-tdTomato neurons (red) and PNs retrogradely labeled from the parabrachial nucleus (green), as ~98.5% of adult PNs had never expressed DYN (scale bar = 50 μm).
The CAP measurements described in Fig. 2 were obtained from 4–7 nerves per age group (one nerve per mouse) and analyzed using the Kruskal-Wallis test. The classification of primary afferent input to DYN interneurons (Fig. 3) was determined using recordings from 13–15 neurons in each age group, while the measurements of amplitude and area-under-the-curve (AUC) of afferent-evoked EPSCs (Fig. 4) used recordings from 8 neurons per group and data were analyzed using Repeated Measures (RM) two-way ANOVA (factors: age × stimulation intensity as the repeated measure). These experiments were conducted using two females and two males at P6–7; three females and two males at P21–22; and three females and three males at P49–63. Primary afferent-evoked AP firing (Fig. 5) was examined in separate experiments which sampled 10 neurons per group (using two males and two females at each age), and data were analyzed with a RM two-way ANOVA (factors: age × stimulation intensity as the repeated measure).
Figure 2: Developmental changes in A-fiber conduction within the mouse sciatic nerve.
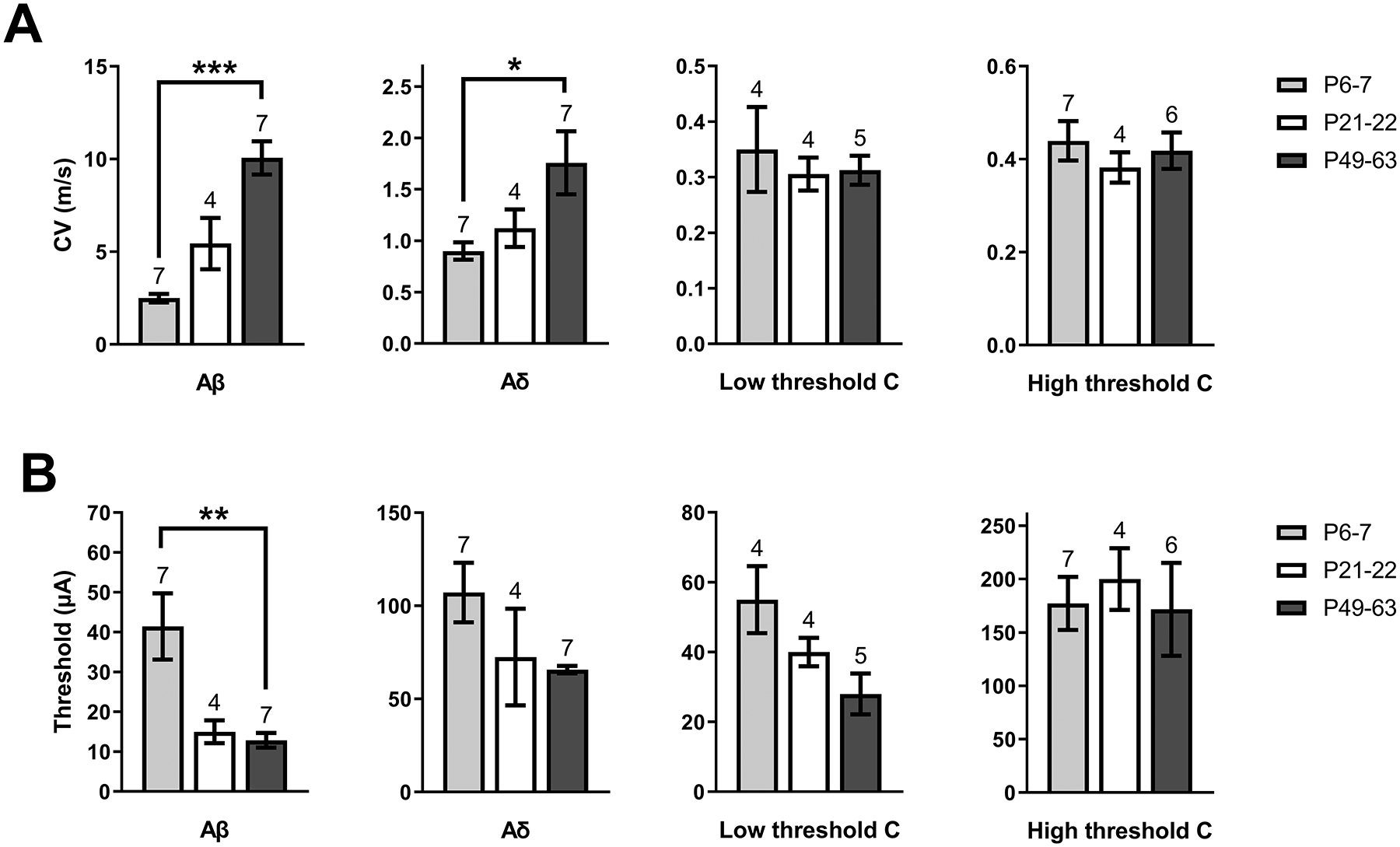
A) Mean conduction velocity (CV) of primary afferent types at each age sampled, showing a significantly lower CV in Aβ (H = 13, p < 0.0001; Kruskal-Wallis test) and Aδ fibers (H = 6.15, p = 0.039; Kruskal-Wallis test) during early life compared with adulthood (*p < 0.05, ***p < 0.001; Dunn’s multiple comparisons test; n indicated above bars = number of nerves for all panels). B) Plot of mean stimulus intensity required to elicit a CAP from each fiber type throughout development. The stimulus thresholds of Aβ fibers at P6–7 were higher than in P49–63 mice (H = 10.98, p = 0.0009, Kruskal-Wallis test; **p < 0.01; Dunn’s multiple comparisons test), suggesting lower Aβ fiber excitability in early life.
Figure 3: A-fiber input to DYN interneurons increases throughout postnatal development.
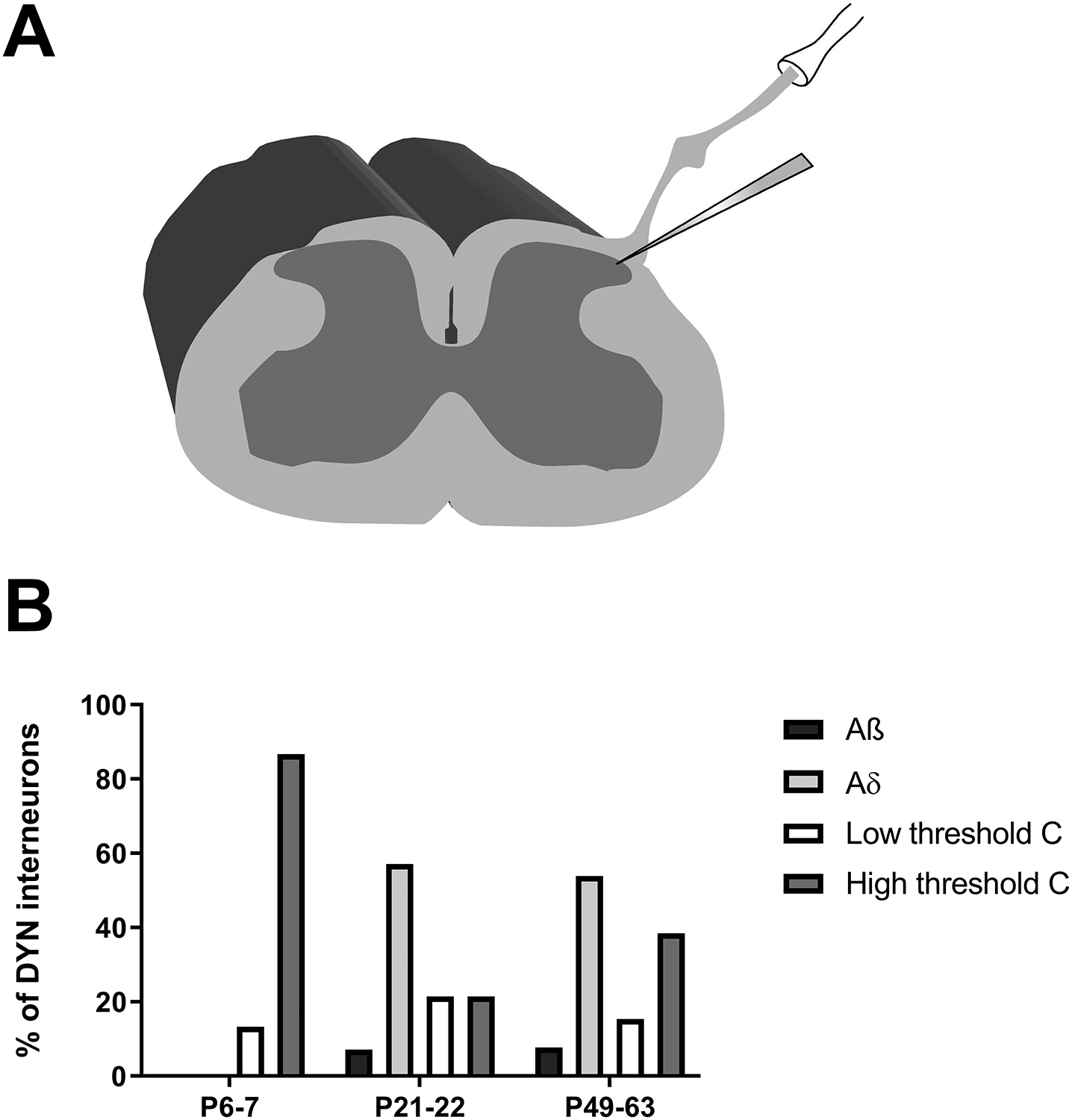
A) Schematic illustrating the use of patch clamp recordings in the intact spinal cord preparation while stimulating the sciatic nerve with attached dorsal roots (L3 and L4). B) Plot of the distribution of monosynaptic primary afferent input to lamina I DYN interneurons throughout development. Data are presented as the percentage of sampled DYN neurons at a given age that received each type of monosynaptic input (note that some neurons received more than one type of direct input). At P6–7, cells receive low- or high-threshold C fiber input, while direct A fiber input was only recorded in cells from P21–22 and P49–63 mice (n = 13–15 neurons per age group).
Figure 4: Age-dependent strengthening of primary afferent-evoked glutamatergic drive to spinal DYN interneurons.
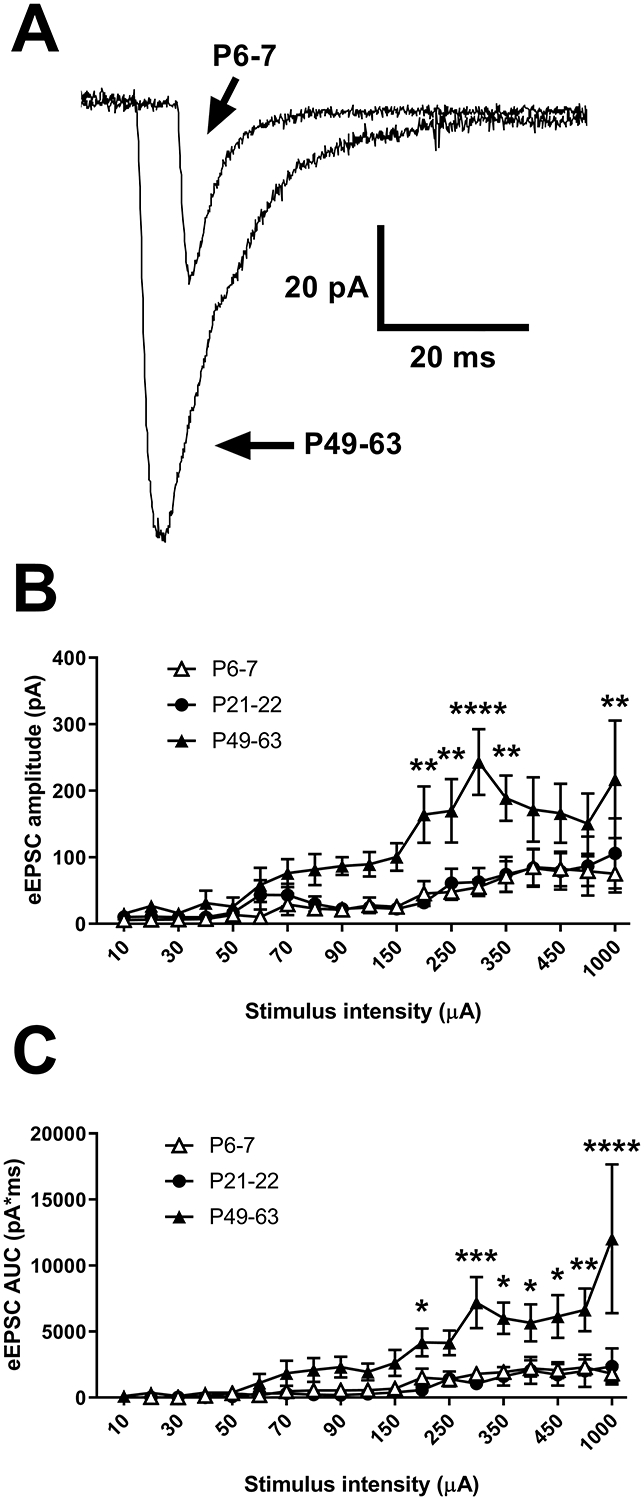
A) Representative recordings of EPSCs evoked via sciatic nerve stimulation in DYN interneurons showing a greater amplitude of EPSCs in adults compared to P6–7. B) There was a significant interaction between age and stimulus intensity (F(36, 378) = 1.68, p = 0.01; RM two-way ANOVA) when measuring the amplitude of afferent-evoked EPSCs (eEPSCs) in DYN interneurons (n = 8 neurons at each age; **p < 0.01, ****p < 0.0001 compared to P6–7 and P21–22; Sidak’s multiple comparisons test). C) There was also a significant interaction between age and stimulus intensity (F(36, 378) = 2.46, p < 0.0001; RM two-way ANOVA) in the area under the curve (AUC) of eEPSCs in DYN interneurons (n = 8 neurons in each group; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared to P6–7 and P21–22; Sidak’s multiple comparisons test).
Figure 5: Immature DYN interneurons exhibit persistent afterdischarge in response to sensory input.
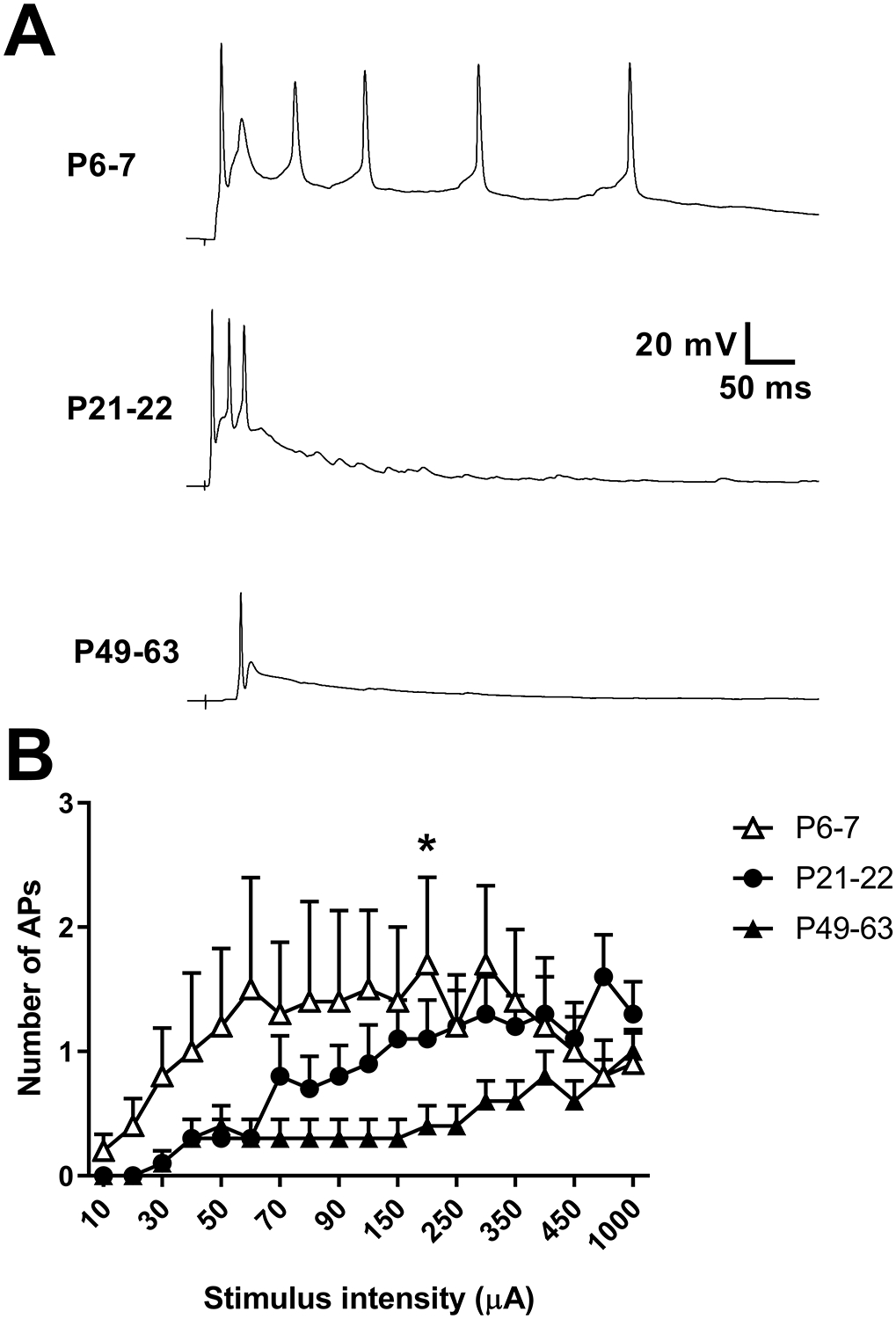
A) Examples of current clamp traces from cells at each indicated age range illustrating a progressive decrease in AP firing evoked by dorsal root stimulation throughout development. B) There was a significant interaction between age and stimulus intensity (F(36,486) = 1.81, p = 0.0032; RM two-way ANOVA) in the number of spikes evoked by primary afferent stimulation (n = 10 in each group; *p = 0.038 compared to P49–63; Sidak’s multiple comparisons test).
Instantaneous firing frequency (Fig. 6; n = 20 neurons per group) and AP discharge duration (Fig. 6; n = 20 neurons per group) were analyzed with RM two-way ANOVA (factors: age × stimulus intensity as the repeated measure). In addition, a one-way ANOVA was used to analyze AP amplitude, AP threshold, and rheobase in DYN interneurons, while a Kruskal-Wallis test was used to compare AP half-width and membrane resistance between age groups due to the data lacking a normal distribution (Fig. 7; n = 20 neurons per group). Sex differences in AP amplitude, AP threshold, AP half-width (transformed via a log function) and membrane resistance (transformed via a log function) were analyzed with a two-way ANOVA (factors: age × sex). The data for all intrinsic excitability measures were obtained from the following mice at each age range: P6–7: two females and two males; P21–22: two females and four males; P49–63: two females and two males.
Figure 6: Intrinsic repetitive firing increases in spinal DYN interneurons after adolescence.
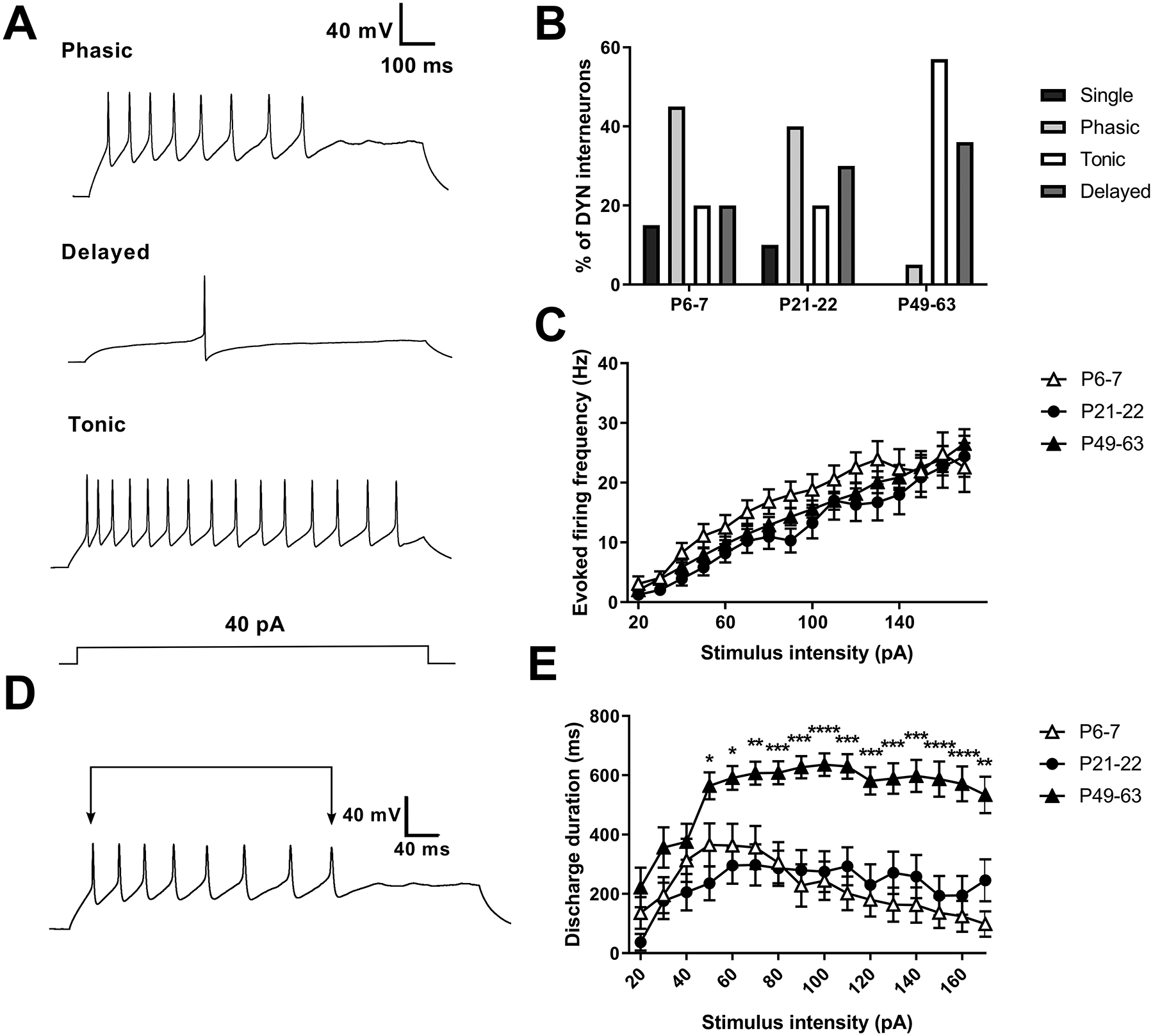
A) Representative firing patterns seen in DYN interneurons, which were classified as phasic (top), delayed (middle), tonic (bottom), and single-spiking (not pictured). B) Plot illustrating the overall distribution of firing patterns as a function of age. The single-spike and phasic firing patterns appear to decrease with age, while tonic and delayed firing patterns increase. C) Analysis of instantaneous firing frequency in developing DYN interneurons revealed a significant effect of stimulus intensity (n = 17–20 per group; F(15, 810) = 89.09, p < 0.0001; RM two-way ANOVA) but no significant effect of age (F(2, 54) = 1.13, p = 0.33) or an interaction between these two factors (F(30, 810) = 1.2, p = 0.21). D) The duration of AP discharge was measured as the time elapsed between the first and last AP during the intracellular current injection. E) Discharge duration was greater at P49–63 compared to younger ages (n = 17–20; F(30,801) = 3.08; p < 0.0001 for interaction between age and stimulus intensity; RM two-way ANOVA; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared to P6–7 or P21–22; Sidak’s posthoc test).
Figure 7: Developmental changes in the intrinsic membrane properties of DYN interneurons within the DH.
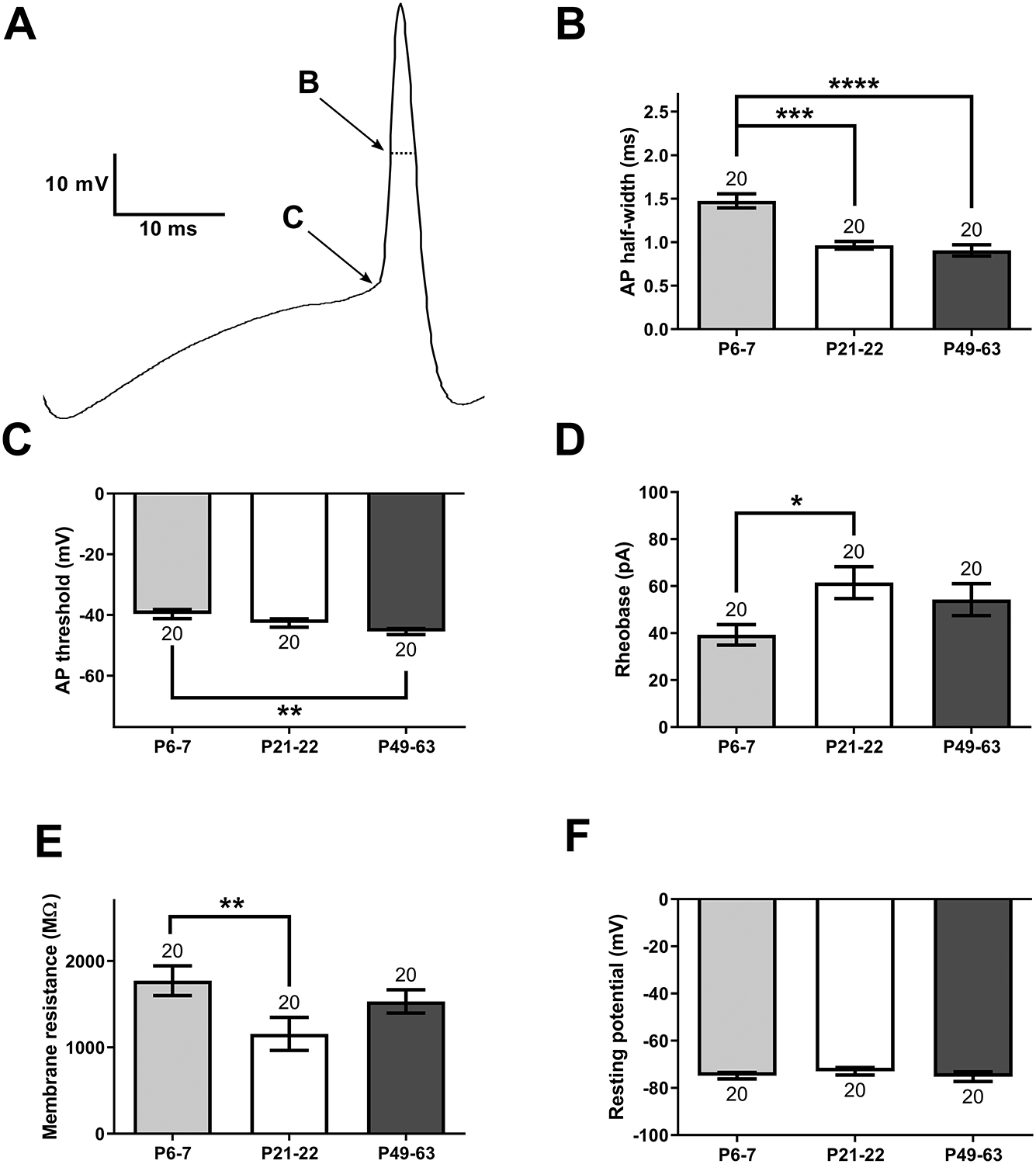
A) Schematic illustrating the measurement of AP half-width (B) and spike threshold (C). B) AP half-width was significantly greater at P6–7 compared with P21–22 (H = 26.62, p < 0.0001; Kruskal-Wallis test; ***p < 0.001; Dunn’s multiple comparisons test) or P49–63 (****p < 0.0001; n indicated above bars = number of neurons for all panels). C) AP threshold in DYN neurons from P6–7 mice was significantly more depolarized compared with adults (F(2, 57) = 4.8, p = 0.012; one-way ANOVA; **p < 0.01; Sidak’s multiple comparisons test). D) DYN interneurons displayed a lower rheobase at P6–7 than at P21–22 (F(2, 57) = 3.46, p = 0.038; one-way ANOVA; *p < 0.05; Sidak’s multiple comparisons test). E) DYN interneurons in early life have a higher membrane resistance than during adolescence (H = 10.28, p = 0.0058; Kruskal-Wallis test; **p < 0.01; Dunn’s multiple comparisons test). F) We found no significant differences in resting membrane potential across the ages tested (F(2, 57) = 0.52, p = 0.6; one-way ANOVA).
For the optogenetic experiments illustrated in Fig. 8, five neurons in each age group (from one female and one male at P6–7, one female and two males at P21–22, and 4 males at P49–63) were sampled to analyze the relative contribution of GABAA and/or glycine receptors to DYN-evoked inhibition of lamina I PNs. The mean amplitude, CoV and PPR of the synaptic input originating from DYN interneurons onto PNs (Fig. 8) were analyzed via a one-way ANOVA (or Kruskal-Wallis test if the data were not normally distributed) with the factor age. The mean IPSC amplitude, CoV and PPR measures were recorded in 14 cells at each age. The IPSC amplitude and CoV were measured in the same cells, which were sampled from three females and two males at P6–7, four females and three males at P21–22, and two females and six males at P49–63. The neurons used for PPR analysis were sampled from three females and two males at P6–7, three females and three males at P21–22, and two females and five males at P49–63.
Figure 8: DYN inhibitory synapses onto lamina I spinoparabrachial neurons strengthen with age.
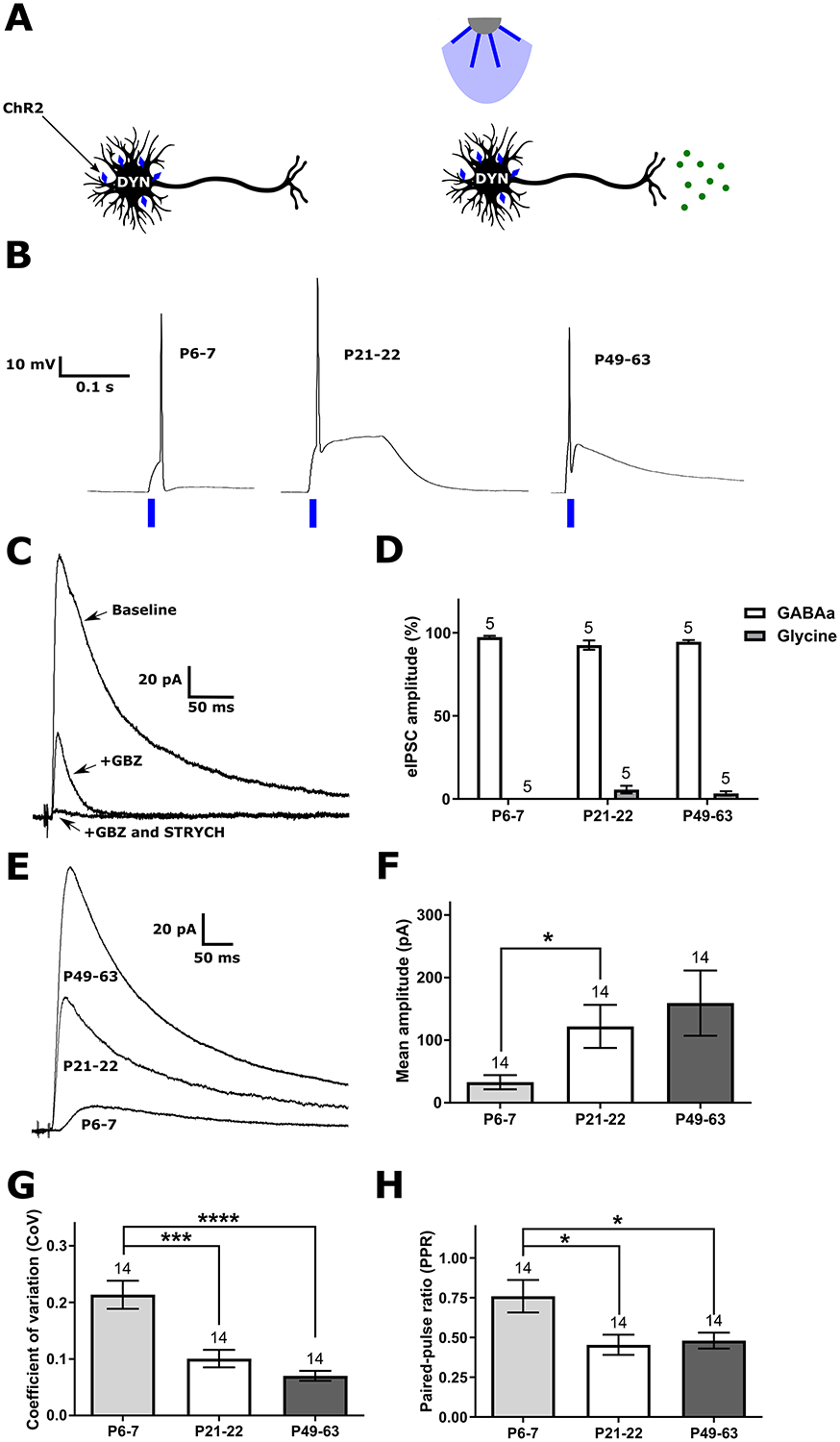
A) Schematic illustrating channelrhodopsin-2 (ChR2) expression in DYN neurons and the activation of this population by blue light. B) Representative traces demonstrating that the chosen light pulse duration (10 ms) elicits a single AP from DYN interneurons at all ages tested. C) Example of monosynaptic IPSCs evoked in a lamina I projection neuron (PN) following optogenetic stimulation of DYN neurons, before and after the sequential bath application of the GABAA receptor antagonist gabazine (GBZ) and the glycine receptor antagonist strychnine (STRYCH). D) Graph of the relative contribution of GABAA and glycine receptors to DYN-evoked inhibition of PNs, showing that virtually all of the input is GABAergic throughout development although a small glycinergic component emerged in adolescence (n indicated above bars = number of neurons in all panels). E) Representative IPSCs illustrating the developmental increase in the amplitude of the DYN-evoked current in PNs. F) The mean peak amplitude of DYN-evoked IPSCs in PNs is lower at P6–7 compared with P21–22 animals (H = 7.99, p = 0.018; Kruskal-Wallis test; *p < 0.05; Dunn’s multiple comparisons test). G) The coefficient of variation (CoV) significantly decreases after the first postnatal week (H = 21.94, p < 0.0001; Kruskal-Wallis test; ***p < 0.001, ****p < 0.0001; Dunn’s multiple comparisons test), indicating a progressive increase in either the probability of GABA release or the number of release sites at inhibitory DYN synapses onto PNs throughout development. H) The paired-pulse ratio (PPR; eIPSC2/eIPSC1) also decreases significantly after P6–7 (F(2, 39) = 5.08, p = 0.012; one-way ANOVA; *p < 0.05; Sidak’s multiple comparisons test), suggesting a reduced probability of GABA release at DYN synapses onto PNs during early life.
Analysis of the density of tdTOM+, VGAT+, and tdTOM/VGAT-expressing boutons that contacted spinoparabrachial neurons (Fig. 9) was performed using 8 cells (4 neurons from each sex) from at least two male and two female mice in each age group (P6–7, P21–22, and P49–63). Data were analyzed via a one-way ANOVA (or Kruskal-Wallis test in the event the data failed normality) with the factor age.
Figure 9: Developmental increase in the innervation of lamina I projection neurons (PNs) by inhibitory DYN neurons.
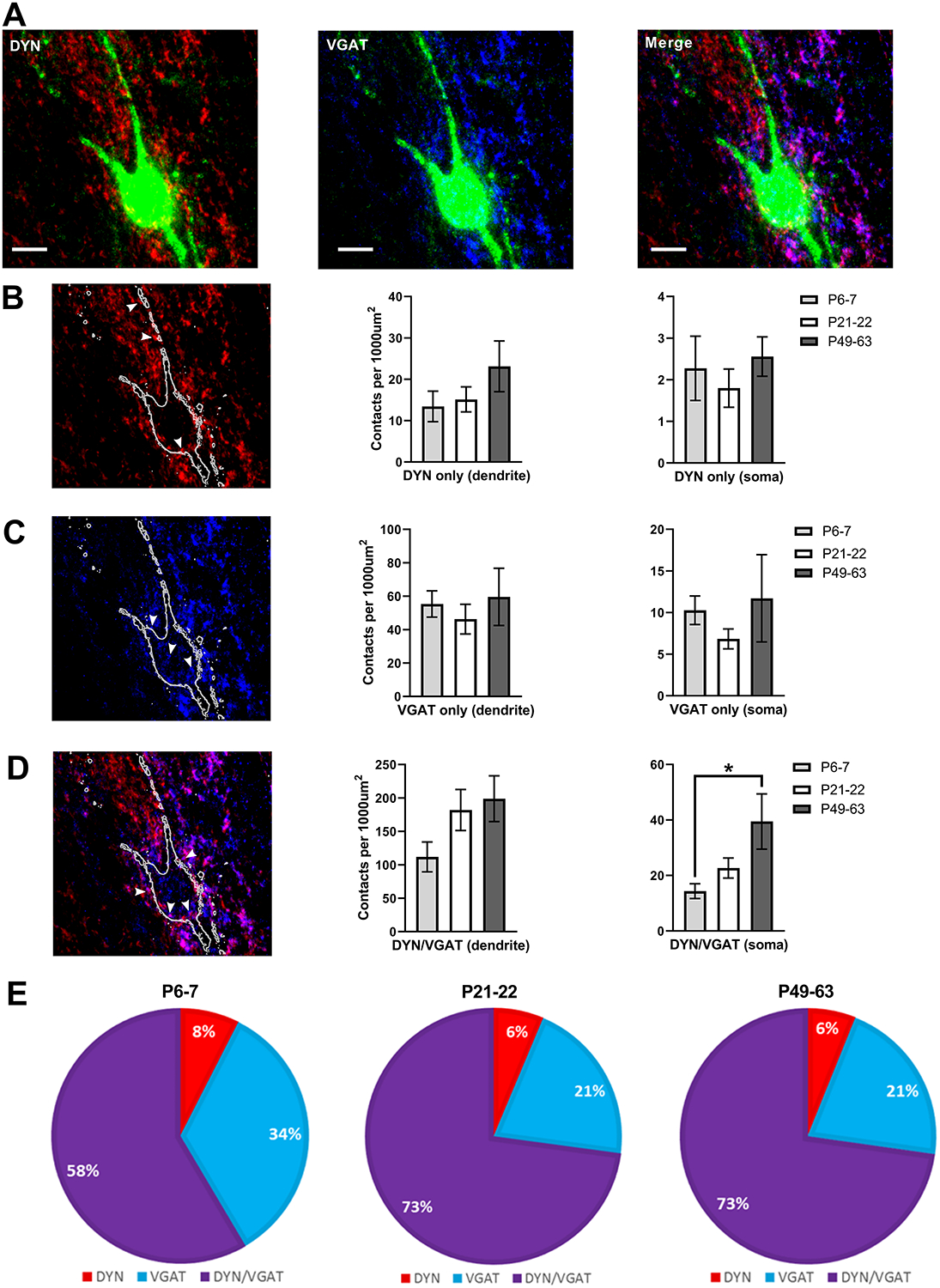
A) Images showing PNs labeled with antibody-based staining for the retrograde tracer CTB (green, all panels), DYN presynaptic terminals genetically labeled via synaptophysin-tdTOM (red, left and right panels), and inhibitory boutons labeled via antibody staining for VGAT (blue, middle and right panels; z-stack projection; scale bar = 10 μm). B) Left: z-stack projection image of presumed excitatory DYN terminals (white arrows) with synaptophysin-tdTOM expression but no VGAT immunoreactivity. Middle and right panels: Plot of the density of boutons in apposition to the dendrites and soma of PNs as a function of age, demonstrating that the density of excitatory DYN terminals onto PNs does not change throughout development (n = 8 neurons in each group). C) Left: Examples of inhibitory (i.e. VGAT+) boutons that do not originate from DYN-lineage neurons (i.e. are tdTOM-negative, white arrows). Middle and right panels: Quantification of DYN-negative inhibitory contacts onto PNs reveals no effect of age (n = 8). D) Left: Representative z-stack projection image of terminals expressing both VGAT and synaptophysin-tdTOM (white arrows), which represent inhibitory presynaptic terminals originating from DYN interneurons. While there was no statistically significant influence of age on the density of DYN/VGAT terminals apposed to PN dendrites (middle panel), there was a developmental increase in the density of inhibitory DYN contacts onto the somata of PNs (right panel; n = 8, H = 7.07, p = 0.03; Kruskal-Wallis test; *p < 0.05; Dunn’s multiple comparisons test). Please note the change in y-axis scale from panels B to D. E) The majority of inhibitory presynaptic terminals contacting PNs originate from DYN-expressing interneurons at all ages tested.
The von Frey measurements of mechanical sensitivity (Fig. 10) were obtained from 14–15 mice at each age (nine females and six males at P6–7, six females and eight males at P21–22, and eight females and seven males at P49–63). Noxious heat (Fig. 11A) was assayed using 14–21 mice per age group (six females and eight males at P6–7, sixteen females and five males at P21–22, and thirteen females and eight males at P49–63). Cold sensitivity (Fig. 11B) was measured in 15–19 mice at each age (seven females and nine males at P6–7, eleven females and four males at P21–22, and nine females and ten males at P49–63). Data were analyzed using a RM three-way ANOVA with the factors: genotype, drug (repeated measure), and age. Mice with baseline measurements that were out of the range of the final dataset (± two standard deviations) were not included in the final analysis. For the assay of acute non-histaminergic itch (Fig. 12), 16–19 mice were used at each age (seven females and nine males at P6–7, and nine females and ten males at P49–63), and data were analyzed using a RM three-way ANOVA with the factors genotype, age, and time (repeated measure). Given the substantial number of posthoc comparisons required in these three-way ANOVA analyses, we used the two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli to control the false discovery rate (FDR) using a q value cut-off of 0.05, and the adjusted p values (i.e. q values) are reported [9].
Figure 10: Spinal DYN interneurons gate mechanical sensation throughout life.
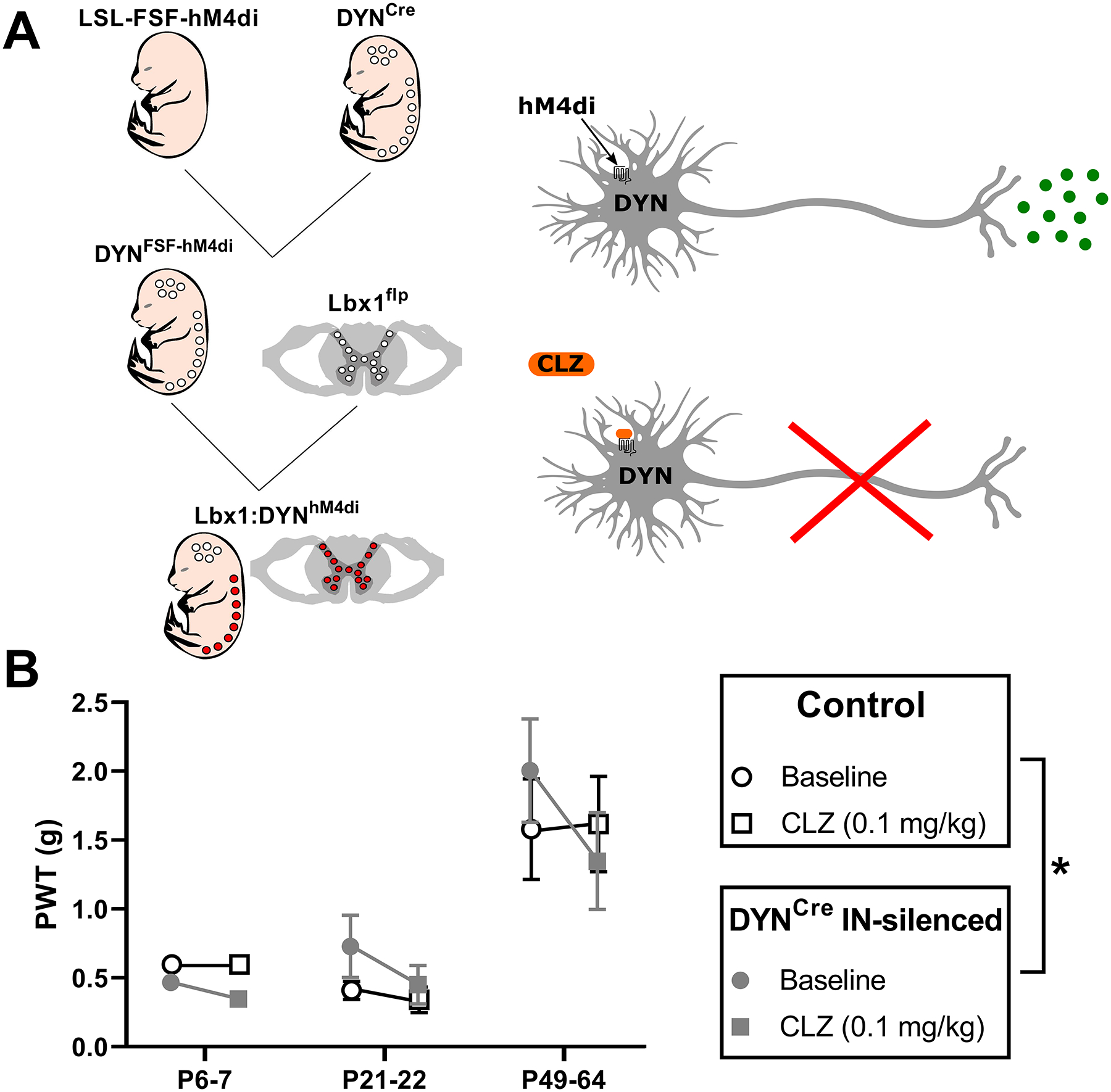
A) Left: Illustration of intersectional genetic strategy to obtain DREADD receptor expression predominantly within inhibitory DYN interneurons in the spinal cord. The inhibitory DREADD (hM4Di) allele is preceded by lox-stop-lox and frt-stop-frt sites, preventing its expression in the absence of both Cre and flp recombinases. Cre expression is restricted to neurons derived from the DYN lineage, while flp is expressed in neurons of the spinal cord and hindbrain via the Lbx1 promoter. Notably, excitatory DYN neurons in the spinal cord are reportedly excluded by this genetic strategy [28]. Right: Neuronal expression of the inhibitory DREADD receptor (hM4Di) allows for controlled silencing via exposure to low doses of clozapine (CLZ). B) Plot of mechanical withdrawal thresholds measured in the hindpaw (PWT) before (circle) and 20–25 min after CLZ administration (square) in flp-negative control (white) and hM4Di-expressing (gray) mice. We found a significant interaction between genotype × drug (F(1, 40) = 5.57, p = 0.023; RM three-way ANOVA), with CLZ administration lowering PWTs in adult DREADD-expressing animals (p = 0.001; FDR), but no statistically significant interaction between age × genotype × drug (F(2, 40) = 1.54, p = 0.23; RM three-way ANOVA).
Figure 11: Developing DYN interneurons do not modulate thermal sensitivity.
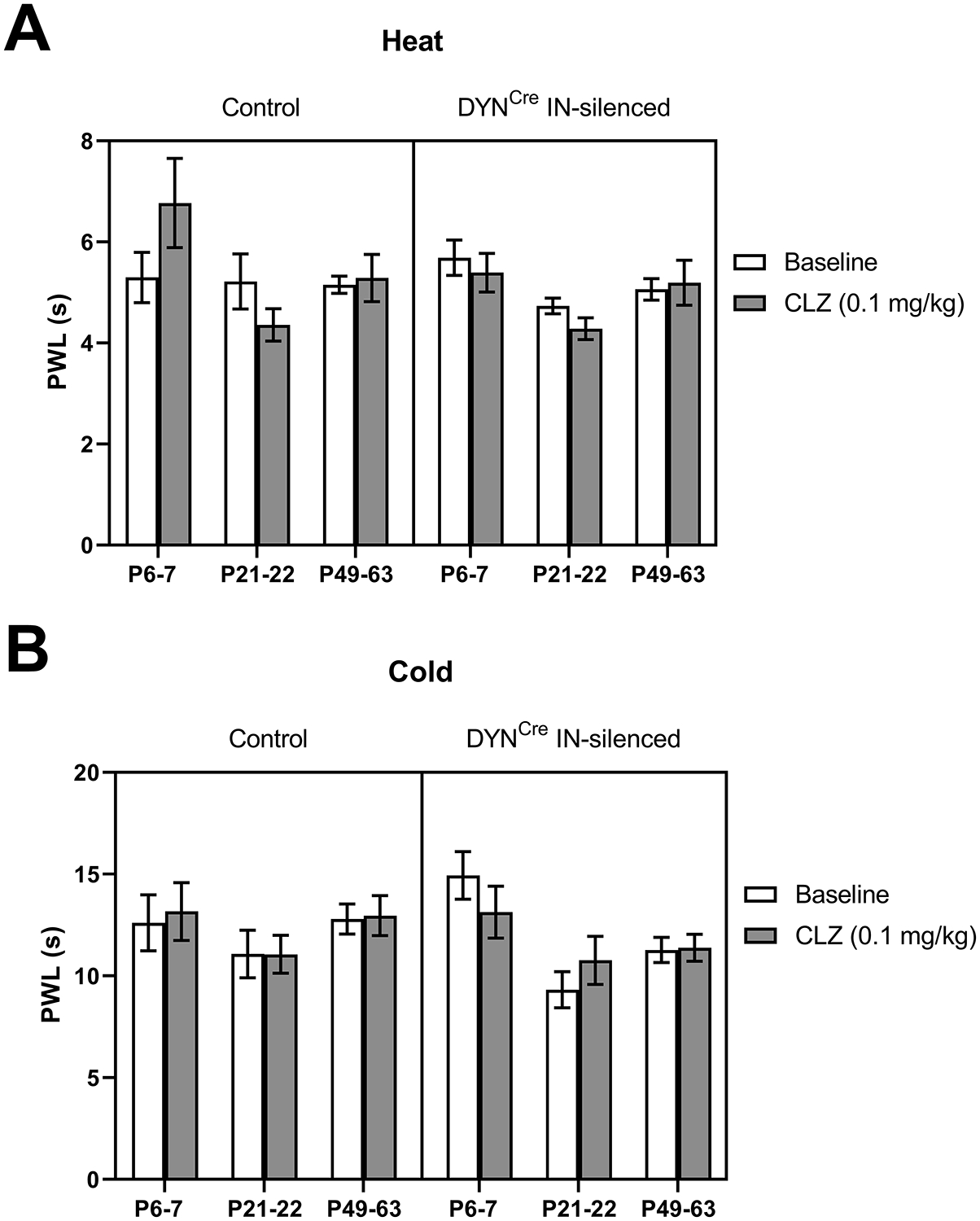
A) Plot of paw withdrawal latencies (PWL) in response to noxious heat before and 20–25 min after CLZ administration. There was no significant interaction between genotype and drug (F(1, 57) = 1.48, p = 0.22; RM three-way ANOVA), suggesting that spinal DYN interneurons do not influence heat sensation. B) Similarly, measurements of the withdrawal thresholds in response to noxious cold revealed no significant interaction between genotype and drug (F(1,61) = 0.21, p = 0.65; RM three-way ANOVA), thereby arguing against a role of inhibitory DYN interneurons in regulating cold sensitivity.
Figure 12: Silencing DYN interneurons in the DH increases acute non-histaminergic itch in adults but not neonates.
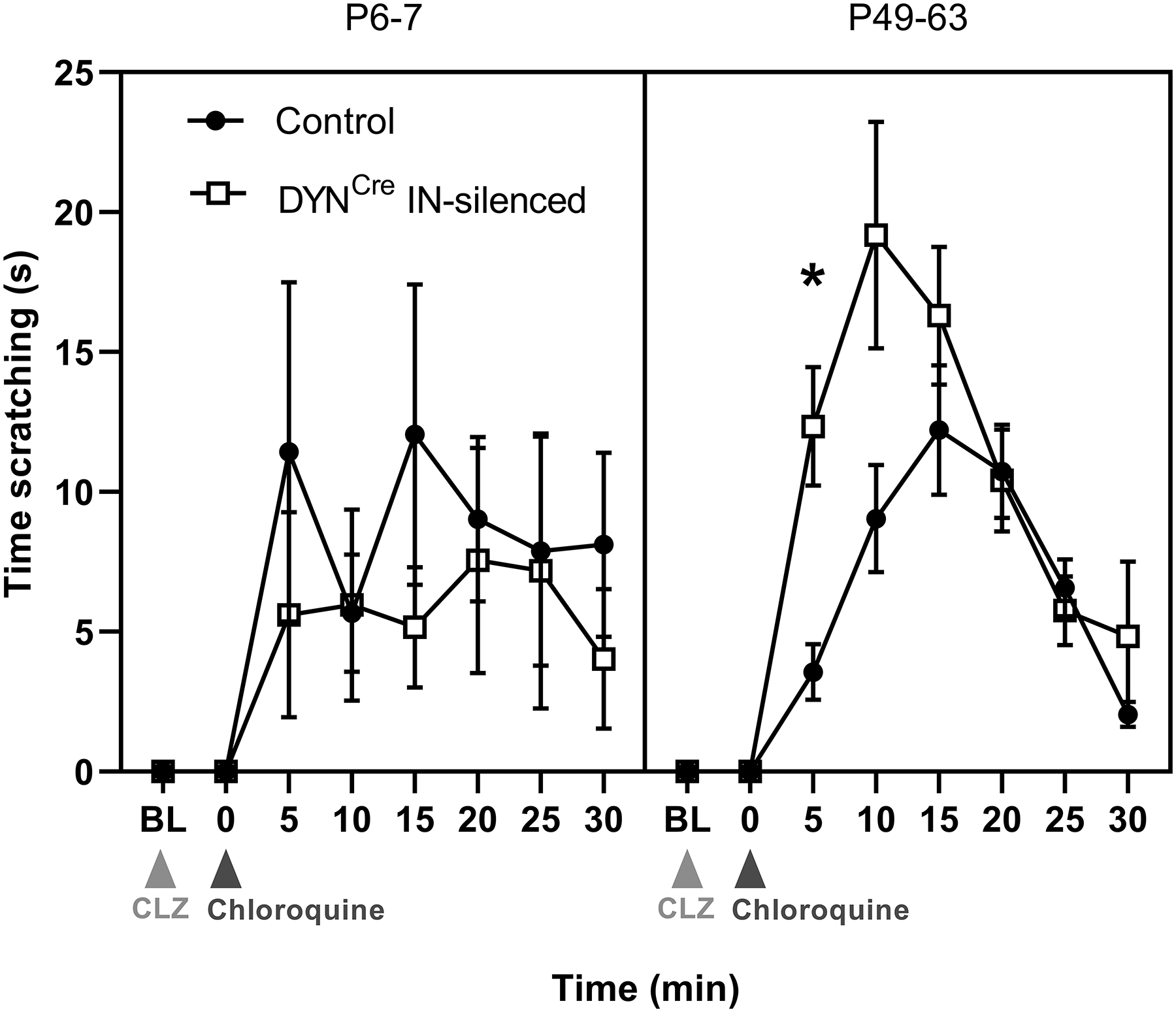
Graph depicting time spent scratching the nape of the neck with the hindpaw after CLZ injection (light gray arrow), both before (Baseline; BL) and after intradermal chloroquine injection (dark gray arrow). Quantification of time spent scratching after chloroquine injection into the nape revealed a significant interaction between genotype and age (F(1, 37) = 5.57, p = 0.035; RM three-way ANOVA, transformed data not shown), suggesting that DYN interneurons reduce itch in adulthood but not during early life (*p = 0.041; FDR; n = 8–14 mice per group).
For all behavioral assays, mice expressing the inhibitory DREADD receptor hM4Di (i.e. DYNCre; Lbx1flp; Rosa26ds-hM4Di) were analyzed alongside littermate controls that did not express flp recombinase. No animals were used for more than one day of testing or given more than one injection of CLZ. The same tester measured responses in all behavioral assays in order to avoid the potential confound of interrater reliability.
3. Results
3.1. DYN expression marks inhibitory DH interneurons throughout development
The DYNtdTOM population within the spinal DH includes all cells that have expressed PdynCre (see Methods) at any stage of development. Prior studies have shown that ~80% of adult DH neurons derived from the Pdyn lineage still express prodynorphin [14; 74], and the majority of these (~67–90%) have been identified as inhibitory [14]. Nonetheless, given the documented age-related changes in GABA expression within the rodent DH [70], along with uncertainty regarding the relative onset of DYN expression within inhibitory vs. excitatory DH neurons, we first sought to determine if PdynCre expression marked inhibitory interneurons throughout postnatal development. We used immunohistochemistry for Pax2, which is required to specify an inhibitory phenotype in the developing DH [23] and exhibits stable expression throughout the lifespan [52]. As expected, Pax2 was widely expressed throughout the gray matter of the spinal cord, while DYNtdTOM neurons were located throughout the dorsal horn but concentrated in the superficial laminae (Fig. 1A, 1B). While there was a significant effect of age on the degree of tdTomato/Pax2 co-localization (F(2, 50) = 142.5, p < 0.0001; one-way ANOVA; Fig. 1C), we found that the majority of DYNtdTOM cells expressed Pax2 at all ages (Fig. 1A, C).
We also investigated the possibility that lamina I projection neurons (PNs) express dynorphin, as it has been previously reported that dynorphin A is expressed in spinoparabrachial neurons [77]. However, we found little overlap between lamina I spinoparabrachial neurons and DYNtdTOM neurons (Fig. 1D), with 98.5% of identified PNs lacking tdTomato expression (522/530 PNs, n = 3 mice). Collectively, these results confirm that DYNtdTOM neurons in the DH predominantly correspond to inhibitory interneurons.
3.2. Age-dependent changes in sensory input to DYN interneurons within the DH
A rigorous examination of primary afferent input to developing DH neurons must account for ongoing changes in the diameter and myelination of sensory fibers during the early postnatal period [49]. Therefore, in order to accurately classify the type of afferent input received by DYN interneurons throughout development, we characterized the conduction velocities (CV) and recruitment thresholds of Aβ, Aδ and C-fibers at different ages using compound action potential (CAP) recordings from the mouse sciatic nerve. The data demonstrate an age-related increase in the CV of both Aβ (H = 13, p < 0.0001; Kruskal-Wallis test; Fig. 2A) and Aδ fibers (H = 6.15, p = 0.039; Kruskal-Wallis test; Fig. 2A). Meanwhile, there were no significant developmental changes in the CV of low- (H = 0.03, p = 0.99) or high-threshold (H = 0.74, p = 0.71) C-fibers (Fig. 2A). Furthermore, as demonstrated in Fig. 2B, although an age-dependent reduction in the stimulus intensity needed to activate Aβ fibers was observed (H = 10.98, p = 0.0009, Kruskal-Wallis test), we did not find any significant developmental changes in Aδ (H = 4.99, p = 0.08), low-threshold C (H = 5.26, p = 0.072), or high-threshold C-fiber thresholds (H = 0.37, p = 0.84).
Prior work has shown that primary afferent synapses onto spinal DH neurons undergo significant reorganization during early life [8; 31]. Therefore, we sought to determine if the type and magnitude of afferent input to DYN interneurons changes during postnatal development. We recorded monosynaptic primary afferent-evoked EPSCs in lamina I DYN interneurons in an intact spinal cord preparation while stimulating the sciatic nerve with both L3 and L4 dorsal roots attached (Fig. 3A), and found that DYN interneurons exclusively received C-fiber input at postnatal days (P) 6–7, with A-fiber connections appearing by P21–22 (Fig. 3B).
The developmental reorganization in the pattern of primary afferent input to spinal DYN interneurons was accompanied by an age-dependent increase in the overall strength of glutamatergic drive to this population (Fig. 4A), as the mean amplitude of the afferent-evoked EPSCs increased significantly after the third week of life (F(36, 378) = 1.68, p = 0.01 for the interaction between age and stimulus intensity; RM two-way ANOVA; Fig. 4B). Similarly, when the area under the total afferent-evoked EPSC was measured to better encompass polysynaptic excitatory connections to DYN neurons, there was a significant interaction between age and stimulus intensity (F(36, 378) = 2.46, p < 0.0001; RM two-way ANOVA; Fig. 4C), as the total glutamatergic input to DYNtdTOM neurons was greater in adulthood (P49–63) than at earlier ages (p < 0.05, Sidak’s multiple comparisons test). Taken together, these results point to an age-dependent increase in the strength of primary afferent input to inhibitory DYN interneurons within the DH.
Despite this weaker afferent-evoked excitatory drive to the DYN population during early life, dorsal root stimulation evoked prolonged, low-frequency firing at P6–7, which was not seen at older ages (Fig. 5A). Overall, we observed a significant interaction between age and stimulus intensity (F(36,486) = 1.81, p = 0.0032; RM two-way ANOVA; Fig. 5B), with neonatal DYN neurons discharging more action potentials (APs) in response to primary afferent input compared to their adult counterparts (p = 0.038; Sidak’s multiple comparisons test; Fig. 5B).
3.3. Intrinsic firing in DYN interneurons increases with postnatal age
To investigate the extent to which the observed developmental alterations in afferent-evoked firing might be explained by age-dependent changes in the intrinsic membrane properties of DYN interneurons, we next examined repetitive AP discharge evoked by intracellular current injection (Fig. 6A). In line with prior work on unidentified mouse DH neurons during early life [87], we found that the single-spike phenotype appeared to decrease throughout development in DYN interneurons, while the prevalence of delayed and tonic firing patterns increased with age (Fig. 6B). In addition, the percentage of DYNtdTOM neurons exhibiting phasic firing decreased markedly after P21–22 (Fig. 6B). Analysis of the instantaneous firing frequency revealed a significant effect of stimulus intensity (F(15, 810) = 89.09, p < 0.0001; RM two-way ANOVA) but no significant effect of age (F(2, 54) = 1.13, p = 0.33) or an interaction between age and stimulus intensity (F(30, 810) = 1.2, p = 0.21; Fig. 6C). Nonetheless, as might be expected from the observed shift in repetitive firing patterns, the overall duration of AP discharge (Fig. 6D) in the DYN population depended on age (F(30,801) = 3.08, p < 0.0001 for the interaction between age and stimulus intensity; RM two-way ANOVA; Fig. 6E) with more prolonged discharge observed in adults compared to P6–7 (p < 0.05; Sidak’s multiple comparisons test). Collectively, these data strongly suggest that the increased AP discharge evoked in neonatal DYN neurons by primary afferent stimulation (Fig. 5) is likely to reflect developmental differences in the integrative properties of the DH synaptic network rather than an enhanced intrinsic ability of immature DYN interneurons to fire repetitively.
Further characterization of the active and passive membrane properties of developing DYN interneurons (Fig. 7A) revealed an age-dependent reduction in AP half-width (H = 26.62, p < 0.0001; Kruskal-Wallis test; Fig. 7B) and spike threshold (F(2,57) = 4.8, p = 0.012; one-way ANOVA; Fig. 7C) without accompanying changes in AP amplitude (F(2, 57) = 1.42, p = 0.25; one-way ANOVA; data not shown). Furthermore, neonatal DYN interneurons exhibited lower rheobase (39.25 ± 4.34 pA) compared to P21–22 (54.25 ± 6.80 pA; F(2, 57) = 3.46, p = 0.038; one-way ANOVA; p < 0.05; Sidak’s multiple comparisons test; Fig. 7D). This could reflect the higher membrane resistance of DYN interneurons at P6–7 (1771 ± 172 MΩ) compared with P21–22 (1156 ± 190 MΩ; H = 10.28, p = 0.0058; Kruskal-Wallis test; p < 0.01; Dunn’s multiple comparisons test; Fig. 7E). Nonetheless, we observed no significant differences in resting membrane potential between age groups (F(2, 57) = 0.52, p = 0.6; one-way ANOVA; Fig. 7F).
We also evaluated potential sex differences in the intrinsic excitability of developing DYN neurons using two-way ANOVAs and found no significant interaction between age and sex in the measurements of AP half-width (F(2, 54) = 0.11, p = 0.9), AP threshold (F(2, 54) = 0.48, p = 0.62), rheobase (F(2, 54) = 2.24, p = 0.12), membrane resistance (F(2, 54) = 1.3, p = 0.28), or resting membrane potential (F(2, 54) = 0.043, p = 0.96; n = 7–13 neurons per group; Fig. S1).
3.4. Inhibitory drive from DYN interneurons onto lamina I PNs strengthens with age
To begin identifying the subpopulations of GABAergic interneurons that directly inhibit ascending spinal PNs at various stages of postnatal development, we used optogenetic stimulation of DYN interneurons expressing channelrhodopsin-2 (DYNChR2; Fig. 8A) to evoke IPSCs in lamina I spinoparabrachial neurons at different ages. First, it was confirmed that a 10 ms stimulation with blue light (470 nm) reliably evoked a single AP in DYN interneurons at all ages examined (Fig. 8B). More importantly, across all ages tested, 92.3% (i.e. 72/78) of the sampled lamina I PNs exhibited monosynaptic IPSCs in response to the optogenetic activation of DYNChR2 neurons, indicating that the majority of lamina I spinoparabrachial PNs in the lumbar enlargement are directly inhibited by this subpopulation of DH interneurons. Sequential bath application of selective antagonists to GABAA and glycine receptors (Fig. 8C) demonstrated that GABAA receptors were responsible for virtually all of the DYN-evoked inhibitory drive onto developing PNs (Fig. 8D). However, a small glycinergic component emerged in adolescence (Fig. 8D), which is concordant with prior research demonstrating that glycinergic synaptic transmission appears in the DH after the first postnatal week [6].
Interestingly, the efficacy of the DYN inhibitory synapses onto PNs depended on postnatal age (H = 7.99, p = 0.018; Kruskal-Wallis test; Fig. 8E), as the mean amplitude of DYN-evoked IPSCs was significantly lower at P6–7 (32.8 ± 11.3 pA) than in adolescence (122.0 ± 34.5 pA; p < 0.05; Dunn’s multiple comparisons test; Fig. 8F). Next, to evaluate the extent to which this reflects developmental changes in presynaptic GABA release from DYN interneurons, we measured the coefficient of variation (CoV) and paired-pulse ratio (PPR) of DYN-evoked IPSCs in lamina I PNs. The CoV decreased throughout development, suggesting a general increase in the number of transmitter release sites and/or the probability of GABA release at inhibitory DYN synapses onto PNs (H = 21.94, p < 0.0001; Kruskal-Wallis test; p < 0.001; Dunn’s multiple comparisons test; Fig. 8G). Furthermore, we also observed a higher PPR during the neonatal period (F(2, 39) = 5.08, p = 0.012; one-way ANOVA; p < 0.05; Sidak’s multiple comparisons test; Fig. 8H), indicating that a lower release probability contributes to the weaker inhibitory drive from DYN interneurons onto PNs in early life.
3.5. Number of putative inhibitory DYN contacts onto PN somata increases with age
The developmental decrease in both CoV and PPR (Fig. 8) makes it difficult to evaluate whether the number of inhibitory DYN inputs to PNs also changes as a function of age. Therefore, we investigated the number of inhibitory presynaptic terminals originating from DYN neurons that contacted the somata and proximal dendrites of lamina I spinoparabrachial neurons retrogradely labeled with CTB (see Methods). DYN terminals were genetically labelled with synaptophysin-tdTomato, while the subset of DYN boutons that were inhibitory were identified via immunohistochemistry using a primary antibody raised against VGAT. As a result, terminals expressing only tdTOM were assumed to originate from excitatory DYN interneurons (Fig. 9A, B, left). There was no significant effect of age on the number of excitatory DYN terminals in apposition to the dendrites (F(2, 21) = 1.33, p = 0.29; one-way ANOVA; Fig. 9B, middle) or soma (H = 1.4, p = 0.5; Kruskal-Wallis test; Fig. 9B, right) of lamina I PNs. Similarly, an analysis of inhibitory contacts originating outside of the DYN population (i.e. VGAT-positive but negative for synaptophysin-tdTomato; Fig. 9A, middle and 9C, left) demonstrated no significant age differences in the density of these appositions on the dendrites (H = 0.56, p = 0.76; Kruskal-Wallis test, Fig. 9C, middle) or cell bodies (H = 2.74, p = 0.25; Kruskal-Wallis test, Fig. 9C, right) of PNs.
Measurements of the density of inhibitory DYN presynaptic terminals (i.e. those expressing both VGAT and synaptophysin-tdTomato; Fig. 9A, right and Fig. 9D, left) suggested that DYN neurons might represent the predominant source of inhibitory input onto lamina I PNs throughout postnatal development (Fig. 9E). While there was no significant effect of age on the number of putative inhibitory DYN contacts at the dendritic level (H = 5.77, p = 0.056; Kruskal-Wallis test, Fig. 9D, middle), there was an age-dependent increase in the number of DYN inhibitory boutons apposed to the somata of PNs (H = 7.07, p = 0.03; Kruskal-Wallis test) due to a lower density at P6–7 (14.36 ± 2.64 contacts/1000 μm2) compared to adulthood (39.45 ± 9.94 contacts/1000 μm2; p < 0.05; Dunn’s multiple comparisons test; Fig. 9D, right). This developmental increase in the somatic innervation of PNs could contribute to the age-related strengthening of DYN inhibitory input to these important output neurons of the DH network (Fig. 8). Notably, the absence of developmental changes in the density of VGAT-only boutons suggests that such plasticity might be selective for the DYN population of inhibitory DH interneurons.
3.6. Spinal DYN interneurons suppress mechanical sensitivity throughout life
The age-related increase in Aβ drive to spinal DYN neurons could suggest a delayed ability of these interneurons to gate mechanical sensitivity, given the known role of Aβ primary afferents in transmitting sensations of touch [1]. This emphasizes the importance of elucidating how developing DYN interneurons shape somatosensory processing at the behavioral level.
As a result, we utilized an intersectional genetic strategy to express a double-stop inhibitory DREADD receptor (ds-hM4Di, which requires both Cre and flp recombinases to be expressed) predominantly within inhibitory DYN interneurons (Lbx1:DYNds-hM4Di; Fig. 10A) of the spinal cord and hindbrain [28]. Littermates that expressed Cre but did not express the flp recombinase (or therefore the hM4Di receptor) were used as controls for all behavioral experiments. In vitro patch clamp recordings confirmed that the bath application of clozapine (CLZ; 25 μM) evoked membrane hyperpolarization in DYN neurons expressing hM4Di but not in adjacent DH neurons lacking DREADD expression (data not shown), as expected from prior studies using the DREADD agonist clozapine N-oxide [64].
Mechanical withdrawal thresholds were measured using von Frey filaments as described previously [11] before and after CLZ administration in order to chemogenetically silence spinal DYN neurons (Fig. 10A). As shown in Fig. 10B, there was a significant interaction between genotype and drug (F(1, 40) = 5.57, p = 0.023; RM three-way ANOVA), thus demonstrating that the chosen dose of CLZ increased mechanical sensitivity via actions at the hM4Di receptor. Although the magnitude of the observed decrease in mechanical thresholds appeared relatively modest compared to prior reports [28], this likely reflects our use of inhibitory DREADDs to manipulate DYN neuronal activity rather than cell ablation. More importantly, although we observed an enhanced mechanical sensitivity in adult DREADD-expressing mice after CLZ (p = 0.001; FDR; Fig. 10B), there was no statistically significant interaction between age × genotype × drug (F(2, 40) = 1.54, p = 0.23; RM three-way ANOVA), thereby suggesting that DYN interneurons gate mechanical sensitivity to a similar degree throughout development in Lbx1:DYNds-hM4Di mice.
3.7. DYN interneurons in the developing DH do not regulate thermal sensitivity
Although prior work has shown that spinal DYN interneurons do not play a role in thermal sensation in adult animals [28], our electrophysiological data suggest that the types of sensory neurons innervating DYN neurons within the DH change throughout life (Fig. 3), thus raising the possibility that these interneurons could gate different sensory modalities early in postnatal development. We investigated this issue by characterizing the effects of silencing the spinal DYN population on the sensitivity of Lbx1:DYNds-hM4Di mice to noxious heat and cold at different ages.
In our assessment of heat sensitivity, we found no significant interaction between genotype and drug (F(1, 57) = 1.48, p = 0.22; RM three-way ANOVA; Fig. 11A), suggesting that dampening the activity of spinal DYN interneurons with CLZ does not affect heat sensation. We also tested cold sensitivity using the cold plantar assay [17] before and after CLZ administration, which revealed no significant interaction between genotype and drug (F(1,61) = 0.21, p = 0.65; RM three-way ANOVA; Fig. 11B). Collectively, these results extend the previous findings in adult mice [28] by suggesting that spinal DYN interneurons fail to modulate thermal sensitivity at any stage of postnatal development.
3.8. Temporary silencing of spinal DYN interneurons enhances non-histaminergic itch after the first postnatal week
Chemogenetic activation of spinal DYN neurons reduces itch responses to pruritogens [38] while the loss of neurons derived from the Bhlhb5 lineage, which includes DYN neurons in the DH, exacerbates both histaminergic and non-histaminergic itch in adult mice [38; 46; 66]. Nonetheless, nothing is known about the subpopulations of DH interneurons that dampen itch during early life.
As a first step towards addressing this issue, we induced acute non-histaminergic itch at different ages via injection of the anti-malaria drug chloroquine into the nape of the neck of Lbx1:DYNds-hM4Di mice (or hM4Di-negative littermate controls) after CLZ administration, and measured the total time spent scratching. The data revealed a significant interaction between genotype and age (F(1,37) = 5.57, p = 0.035; RM three-way ANOVA; Fig. 12), with adult DREADD-expressing mice demonstrating greater scratching in the first five minutes after chloroquine injection compared to flp-negative controls (p = 0.041; FDR; Fig. 12, right). Importantly, no hindpaw scratching directed at the nape of the neck was observed before chloroquine injection in any of the experimental groups (Baseline; BL = 0; Fig. 12). These findings suggest that the ability of spinal DYN interneurons to suppress acute non-histaminergic itch emerges after the first week of life.
4. Discussion
The present results reveal, for the first time, developmental plasticity in the connectivity and function of a genetically identified subpopulation of spinal DH interneurons, as neurons derived from the DYN lineage (which are predominantly inhibitory) exhibit clear age-dependent changes in primary afferent input as well as their intrinsic membrane properties. Furthermore, the data suggest that while DYN synapses inhibit lamina I spinoparabrachial neurons throughout life, the efficacy of these synapses is lower in the neonate, which may reflect both a lower probability of GABA release and a decreased number of inhibitory contacts onto the somata of projection neurons (PNs). Finally, the current findings provide the first evidence that the precise somatosensory modalities governed by a particular GABAergic “microcircuit” within the DH may depend on postnatal age.
A subset of DYN neurons in laminae I-IIo of the adult mouse DH receive monosynaptic Aβ fiber input, which could underlie the ability of these interneurons to dampen mechanical sensitivity [28] given that Aβ afferents are classically associated with conveying innocuous mechanosensation [45]. However, our results point to an absence of direct Aβ inputs to neonatal DYN neurons, as these cells received monosynaptic input almost exclusively from high-threshold C-fibers during the first week of life (Fig. 3). The dominance of monosynaptic C-fiber input during early life was somewhat surprising given that A-fibers are prevalent in the neonatal rodent DH and gradually withdraw over the first weeks of life [8; 31], while C-fiber synaptic inputs gradually strengthen during this period [5; 61]. It should be noted that while our analysis focused on classifying monosynaptic inputs to the DYN population, polysynaptic Aβ input to immature DYN neurons was observed (data not shown) and is undoubtedly important for somatosensory processing in vivo. A main limitation of our chosen in vitro experimental preparation is that the peripheral terminals of the sensory neurons have been lost, thus preventing the determination of whether different functional subclasses of C-fibers (i.e. those responding to mechanical, thermal, chemical, and/or pruriceptive stimuli) [21; 71; 72] drive activity in spinal DYN neurons at various ages. This issue could be addressed via the use of a semi-intact ex vivo spinal cord preparation that preserves the sensory innervation of the skin [36], in vivo calcium imaging approaches [44; 73], or in vitro optogenetic stimulation of genetically-defined sensory afferents [2; 27].
Despite receiving weaker glutamatergic drive from primary afferents (Fig. 4), neonatal DYN neurons displayed protracted afferent-evoked AP discharge compared to later ages (Fig. 5). This observation is highly consistent with in vivo electrophysiological studies showing that immature DH neurons exhibit prolonged afterdischarges that often exceed the initial firing evoked by sensory stimulation [30; 43]. This afterdischarge is likely explained by immature inhibitory signaling onto DYN interneurons originating from other GABAergic neurons within the developing DH network [6; 25; 48; 50] rather than an enhanced intrinsic ability of newborn DYN neurons to fire repetitively, given the prevalent single-spike phenotype seen at P6–7 (Fig. 6). Indeed, GABAergic synaptic inputs to immature DH neurons exhibit smaller amplitudes and a greater susceptibility to short-term depression during repetitive stimulation [41], while inhibitory receptive fields are poorly tuned in neonates [16], which may collectively contribute to the prolonged afferent-evoked AP firing observed in the DYN population during early life. Furthermore, although tonic firing has been considered a hallmark feature of GABAergic interneurons in the mature DH [32; 82; 90], our current results highlight the risk of extrapolating such classification schemes to the neonatal DH, as tonic firing was rarely seen in immature DYN neurons despite their clear inhibitory phenotype (Fig. 1). While intrinsic firing patterns may not rigorously predict the responses of DH neurons to physiologically relevant stimuli [35], these data nonetheless provide further evidence that the voltage-dependent, and/or voltage-independent, ionic conductances expressed by DH neurons change substantially during early postnatal development [10; 87]. Future studies are needed to identify the specific ionic channels which are altered in the developing DYN population. Notably, the magnitude and subcellular location of potassium conductances within a neuron plays a substantial role in governing the resulting firing pattern [7], and age-dependent changes in potassium channel expression have been documented in mouse SDH neurons [87]. In addition, while we did not find a significant interaction between age and sex in the measures of intrinsic membrane excitability in DYN neurons (Fig. S1), additional work is required to conclusively identify the overall influence of sex on the function of DYN circuits within the developing DH.
DYN interneurons act as a critical gate for somatosensory processing in the DH, and provide feedforward inhibition onto excitatory somatostatin-expressing (SOM) interneurons [28] and neurokinin 1 receptor (NK1R)-expressing neurons [55], both of which have been extensively implicated in pain [28; 54; 57]. Spinal DYN neurons also receive monosynaptic inputs from menthol- and capsaicin-sensitive primary afferents [46], and inhibit DH neurons expressing gastrin-related peptide receptor (GRPR) that have been linked to pruriception [38; 40; 58; 78], potentially underlying the ability of menthol and capsaicin to act as counter-stimuli that reduce itch during adulthood [46]. Here we demonstrate that DYN neurons likely serve as a critical source of GABAergic input to lamina I PNs throughout postnatal development (Figs. 8, 9). As a result, given their direct innervation by primary afferents (Fig. 3), DYN interneurons likely participate in a feedforward inhibitory circuit that directly governs the level of ascending nociceptive and pruriceptive signaling to the developing brain. The lower probability of GABA release seen at neonatal DYN synapses onto PNs (Fig. 8), along with a reduced density of inhibitory presynaptic terminals contacting PN somata (Fig. 9), suggests a decreased efficacy of this feedforward pathway during early life. However, it should be noted that ultrastructural analysis and/or immunohistochemical localization of a postsynaptic marker (such as gephyrin) would be needed to accurately quantify the density of DYN synapses onto PNs [19]. In addition, the precise contribution of spinal DYN neurons to the overall level of feedforward inhibition onto ascending PNs [53] remains to be elucidated, and we cannot completely exclude the possibility that the DYN neurons receiving primary afferent input correspond to a separate subpopulation from those synapsing onto lamina I spinoparabrachial neurons.
The delayed maturation of spinal DYN circuits might predict a reduced capability to suppress mechanical pain during the neonatal period. However, we found that inhibitory DYN interneurons gate mechanical sensitivity throughout postnatal development (Fig. 10). This could potentially be explained by the concomitant age-dependent strengthening of both glutamatergic and GABAergic/glycinergic signaling across the DH [5; 6], which may maintain a stable balance of excitation vs. inhibition within the mechanosensory networks of the developing spinal cord. Indeed, despite clear developmental alterations in the properties of spinal inhibitory transmission, GABAAR-mediated inhibition of DH neuronal activity is fully functional from the first days of life [15]. In contrast, we observed that the chemogenetic silencing of spinal DYN interneurons enhanced chloroquine-evoked scratching in adult mice, but not at P6–7 (Fig. 12), suggesting that the DYN population does not modulate acute non-histaminergic itch during early life. It will ultimately be essential to determine the degree to which neonatal DYN interneurons can shape the sensitivity to histaminergic itch, as well as chronic itch under pathological conditions. Unfortunately, while children commonly suffer pathological itch due to conditions such as atopic dermatitis, urticaria or psoriasis [12; 67; 89], pruriceptive processing within the immature nervous system remains poorly understood at a cellular and molecular level.
The circuit mechanisms underlying the differential ability of spinal DYN interneurons to modulate mechanical vs. pruritic sensitivity during the neonatal period are still unclear. It should be noted that our analysis of mechanical sensitivity was restricted to measurements of the nociceptive withdrawal reflex, which remains intact in the absence of supraspinal control [40; 47]. However, itch is abolished after lesion of the anterolateral funiculus [88], and spinal NK1R-expressing neurons, many of which are ascending PNs [20], have been implicated in the propagation of pruritogen-evoked itch [22; 40; 76]. This raises the possibility that developmental shifts in the efficacy of DYN-mediated inhibition of PNs (Fig. 8) is more likely to influence active coping behaviors, such as pruritogen-evoked scratching (Fig. 12), compared to reflexes [40]. Nonetheless, it is also possible that separate groups of DYN interneurons, perhaps residing in different laminae within the DH, are responsible for regulating mechanical pain vs. itch [28; 46], and the DYN population governing itch is slower to mature during the early postnatal period. Recent strategies to transcriptionally profile spinal DYN interneurons [74] could be combined with single-nucleus RNA-seq techniques [51; 65] in order to yield new insight into the potential heterogeneity of DYN neurons within the developing DH.
In conclusion, these studies demonstrate clear developmental changes in the functional organization of inhibitory DYN circuits within the DH, and further suggest that such circuits may exert an age-dependent influence on somatosensory processing within the spinal cord. A more complete understanding of the mechanisms governing nociceptive and pruriceptive signaling in the immature CNS would facilitate the design of age-appropriate interventions with the goal of alleviating pathological pain and itch in children and adolescents.
Supplementary Material
Supplemental Digital Content 1. Video that demonstrates hindpaw scratching directed at the nape of the neck in a P6 mouse after chloroquine injection. mp4
Supplemental Digital Content 2. Supplemental data: Figure S1.pdf
Acknowledgements
This work was funded by NIH grant NS080889 to M.L.B. The authors thank Dr. Jie Li for technical assistance, and Dr. Temugin Berta and Dr. Steve Davidson for feedback on the manuscript. The Lbx1flp mice were a kind gift from Dr. Qiufu Ma at Harvard University. The authors declare that they have no conflicts of interest with any of the work presented in this manuscript.
References
- [1].Abraira VE, Ginty DD. The sensory neurons of touch. Neuron 2013;79(4):618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Albisetti GW, Pagani M, Platonova E, Hösli L, Johannssen HC, Fritschy J-M, Wildner H, Zeilhofer HU. Dorsal horn gastrin-releasing peptide expressing neurons transmit spinal itch but not pain signals. Journal of Neuroscience 2019;39(12):2238–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Andrews K, Fitzgerald M. The cutaneous withdrawal reflex in human neonates: sensitization, receptive fields, and the effects of contralateral stimulation. Pain 1994;56(1):95–101. [DOI] [PubMed] [Google Scholar]
- [4].Andrews K, Fitzgerald M. Cutaneous flexion reflex in human neonates: a quantitative study of threshold and stimulus-response characteristics after single and repeated stimuli. Developmental medicine and child neurology 1999;41(10):696–703. [DOI] [PubMed] [Google Scholar]
- [5].Baccei ML, Bardoni R, Fitzgerald M. Development of nociceptive synaptic inputs to the neonatal rat dorsal horn: glutamate release by capsaicin and menthol. The Journal of physiology 2003;549(1):231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baccei ML, Fitzgerald M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J Neurosci 2004;24(20):4749–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Balachandar A, Prescott SA. Origin of heterogeneous spiking patterns from continuously distributed ion channel densities: a computational study in spinal dorsal horn neurons. The Journal of physiology 2018;596(9):1681–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Beggs S, Torsney C, Drew LJ, Fitzgerald M. The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity‐dependent process. European Journal of Neuroscience 2002;16(7):1249–1258. [DOI] [PubMed] [Google Scholar]
- [9].Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 2006;93(3):491–507. [Google Scholar]
- [10].Blankenship ML, Coyle DE, Baccei ML. Transcriptional expression of voltage-gated Na+ and voltage-independent K+ channels in the developing rat superficial dorsal horn. Neuroscience 2013;231:305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bonin RP, Bories C, De Koninck Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Molecular pain 2014;10(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Boozalis E, Grossberg AL, Püttgen KB, Cohen BA, Kwatra SG. Itching at night: A review on reducing nocturnal pruritus in children. Pediatric dermatology 2018;35(5):560–565. [DOI] [PubMed] [Google Scholar]
- [13].Bourane S, Duan B, Koch SC, Dalet A, Britz O, Garcia-Campmany L, Kim E, Cheng L, Ghosh A, Ma Q, Goulding M. Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science 2015;350(6260):550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Boyle KA, Gutierrez-Mecinas M, Polgár E, Mooney N, O’Connor E, Furuta T, Watanabe M, Todd AJ. A quantitative study of neurochemically defined populations of inhibitory interneurons in the superficial dorsal horn of the mouse spinal cord. Neuroscience 2017;363:120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bremner L, Fitzgerald M, Baccei M. Functional GABAA-receptor–mediated inhibition in the neonatal dorsal horn. Journal of neurophysiology 2006;95(6):3893–3897. [DOI] [PubMed] [Google Scholar]
- [16].Bremner LR, Fitzgerald M. Postnatal tuning of cutaneous inhibitory receptive fields in the rat. The Journal of physiology 2008;586(6):1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Brenner DS, Golden JP, Gereau IV RW. A novel behavioral assay for measuring cold sensation in mice. PloS one 2012;7(6):e39765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brumovsky PR. VGLUTs in peripheral neurons and the spinal cord: time for a review. ISRN neurology 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Burette A, Collman F, Micheva KD, Smith SJ, Weinberg RJ. Knowing a synapse when you see one. Frontiers in neuroanatomy 2015;9:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cameron D, Polgár E, Gutierrez-Mecinas M, Gomez-Lima M, Watanabe M, Todd AJ. The organisation of spinoparabrachial neurons in the mouse. Pain 2015;156(10):2061–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Campero M, Bostock H. Unmyelinated afferents in human skin and their responsiveness to low temperature. Neuroscience letters 2010;470(3):188–192. [DOI] [PubMed] [Google Scholar]
- [22].Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport 2010;21(4):303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cheng L, Arata A, Mizuguchi R, Qian Y, Karunaratne A, Gray PA, Arata S, Shirasawa S, Bouchard M, Luo P, Chen C-L, Busslinger M, Goulding M, Onimaru H, Ma Q. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nature neuroscience 2004;7(5):510–517. [DOI] [PubMed] [Google Scholar]
- [24].Chuhma N, Ohmori H. Postnatal development of phase-locked high-fidelity synaptic transmission in the medial nucleus of the trapezoid body of the rat. Journal of Neuroscience 1998;18(1):512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cordero-Erausquin M, Coull JA, Boudreau D, Rolland M, De Koninck Y. Differential maturation of GABA action and anion reversal potential in spinal lamina I neurons: impact of chloride extrusion capacity. Journal of Neuroscience 2005;25(42):9613–9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron 1997;18(6):995–1008. [DOI] [PubMed] [Google Scholar]
- [27].Draxler P, Honsek SD, Forsthuber L, Hadschieff V, Sandkühler J. VGluT3+ primary afferents play distinct roles in mechanical and cold hypersensitivity depending on pain etiology. Journal of Neuroscience 2014;34(36):12015–12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross SE, Lowell BB, Wang Y, Goulding M, Ma Q. Identification of spinal circuits transmitting and gating mechanical pain. Cell 2014;159(6):1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].FitzgeraId M, Shaw A, MacIntosh N. Postnatal development of the cutaneous flexor reflex: comparative study of preterm infants and newborn rat pups. Developmental Medicine & Child Neurology 1988;30(4):520–526. [DOI] [PubMed] [Google Scholar]
- [30].Fitzgerald M. The post‐natal development of cutaneous afferent fibre input and receptive field organization in the rat dorsal horn. The Journal of physiology 1985;364(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fitzgerald M, Butcher T, Shortland P. Developmental changes in the laminar termination of A fibre cutaneous sensory afferents in the rat spinal cord dorsal horn. Journal of Comparative Neurology 1994;348(2):225–233. [DOI] [PubMed] [Google Scholar]
- [32].François A, Low SA, Sypek EI, Christensen AJ, Sotoudeh C, Beier KT, Ramakrishnan C, Ritola KD, Sharif-Naeini R, Deisseroth K, Delp SL, Malenka RC, Luo L, Hantman AW, Scherrer G. A brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and enkephalins. Neuron 2017;93(4):822–839. e826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ganley RP, Iwagaki N, Del Rio P, Baseer N, Dickie AC, Boyle KA, Polgár E, Watanabe M, Abraira VE, Zimmerman A, Riddell JS, Todd AJ. Inhibitory interneurons that express GFP in the PrP-GFP mouse spinal cord are morphologically heterogeneous, innervated by several classes of primary afferent and include lamina I projection neurons among their postsynaptic targets. Journal of Neuroscience 2015;35(19):7626–7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 2017;357(6350):503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Graham B, Brichta A, Callister R. In vivo responses of mouse superficial dorsal horn neurones to both current injection and peripheral cutaneous stimulation. The Journal of physiology 2004;561(3):749–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hachisuka J, Baumbauer KM, Omori Y, Snyder LM, Koerber HR, Ross SE. Semi-intact ex vivo approach to investigate spinal somatosensory circuits. Elife 2016;5:e22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hachisuka J, Koerber HR, Ross SE. Selective-cold output via a distinct subset of lamina I spinoparabrachial neurons. Pain 2019;Epub head of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Huang J, Polgár E, Solinski HJ, Mishra SK, Tseng P-Y, Iwagaki N, Boyle KA, Dickie AC, Kriegbaum MC, Wildner H, Zeilhofer HU, Watanabe M, Riddell JS, Todd AJ, Hoon MA. Circuit dissection of the role of somatostatin in itch and pain. Nature neuroscience 2018;21(5):707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Huang M, Huang T, Xiang Y, Xie Z, Chen Y, Yan R, Xu J, Cheng L. Ptf1a, Lbx1 and Pax2 coordinate glycinergic and peptidergic transmitter phenotypes in dorsal spinal inhibitory neurons. Developmental biology 2008;322(2):394–405. [DOI] [PubMed] [Google Scholar]
- [40].Huang T, Lin S-H, Malewicz NM, Zhang Y, Zhang Y, Goulding M, LaMotte RH, Ma Q. Identifying the pathways required for coping behaviours associated with sustained pain. Nature 2019;565(7737):86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ingram RA, Fitzgerald M, Baccei ML. Developmental changes in the fidelity and short-term plasticity of GABAergic synapses in the neonatal rat dorsal horn. J Neurophysiol 2008;99(6):3144–3150. [DOI] [PubMed] [Google Scholar]
- [42].Jendryka M, Palchaudhuri M, Ursu D, van der Veen B, Liss B, Kätzel D, Nissen W, Pekcec A. Pharmacokinetic and pharmacodynamic actions of clozapine-N-oxide, clozapine, and compound 21 in DREADD-based chemogenetics in mice. Scientific reports 2019;9(1):4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jennings E, Fitzgerald M. Postnatal changes in responses of rat dorsal horn cells to afferent stimulation: a fibre‐induced sensitization. The Journal of physiology 1998;509(3):859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Johannssen HC, Helmchen F. In vivo Ca2+ imaging of dorsal horn neuronal populations in mouse spinal cord. The Journal of physiology 2010;588(18):3397–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Johnson KO, Yoshioka T, Vega–Bermudez F. Tactile functions of mechanoreceptive afferents innervating the hand. Journal of Clinical Neurophysiology 2000;17(6):539–558. [DOI] [PubMed] [Google Scholar]
- [46].Kardon AP, Polgár E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, Bernard CS, Schwartz ES, Nagase H, Schwarzer C, Watanabe M, Furuta T, Kaneko T, Koerber HR, Todd AJ, Ross SE. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 2014;82(3):573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kauppila T. Spinalization increases the mechanical stimulation-induced withdrawal reflex threshold after a sciatic cut in the rat. Brain research 1997;770(1–2):310–312. [DOI] [PubMed] [Google Scholar]
- [48].Keller AF, Coull JA, Chéry N, Poisbeau P, De Koninck Y. Region-specific developmental specialization of GABA–glycine cosynapses in laminas I–II of the rat spinal dorsal horn. Journal of Neuroscience 2001;21(20):7871–7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kinney HC, Volpe JJ. Myelination Events Volpe’s Neurology of the Newborn: Elsevier, 2018. pp. 176–188. [Google Scholar]
- [50].Koch SC, Tochiki KK, Hirschberg S, Fitzgerald M. C-fiber activity-dependent maturation of glycinergic inhibition in the spinal dorsal horn of the postnatal rat. Proceedings of the National Academy of Sciences 2012;109(30):12201–12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lake BB, Ai R, Kaeser GE, Salathia NS, Yung YC, Liu R, Wildberg A, Gao D, Fung H-L, Chen S, Vijayaraghavan R, Wong J, Chen A, Sheng X, Kaper F, Shen R, Ronaghi M, Fan J-B, Wang W, Chun J, Zhang K. Neuronal subtypes and diversity revealed by single-nucleus RNA sequencing of the human brain. Science 2016;352(6293):1586–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Larsson M. Pax2 is persistently expressed by GABAergic neurons throughout the adult rat dorsal horn. Neuroscience letters 2017;638:96–101. [DOI] [PubMed] [Google Scholar]
- [53].Li J, Kritzer E, Craig PE, Baccei ML. Aberrant synaptic integration in adult lamina I projection neurons following neonatal tissue damage. Journal of Neuroscience 2015;35(6):2438–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science 1997;278(5336):275–279. [DOI] [PubMed] [Google Scholar]
- [55].Marvizón JCG, Chen W, Murphy N. Enkephalins, dynorphins, and β‐endorphin in the rat dorsal horn: An immunofluorescence colocalization study. Journal of Comparative Neurology 2009;517(1):51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].McKelvey R, Berta T, Old E, Ji RR, Fitzgerald M. Neuropathic pain is constitutively suppressed in early life by anti-inflammatory neuroimmune regulation. J Neurosci 2015;35(2):457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science 1999;286(5444):1558–1561. [DOI] [PubMed] [Google Scholar]
- [58].Pagani M, Albisetti GW, Sivakumar N, Wildner H, Santello M, Johannssen HC, Zeilhofer HU. How Gastrin-Releasing Peptide Opens the Spinal Gate for Itch. Neuron 2019;103(1):102–117.e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Paxinos G, Franklin KB. Paxinos and Franklin’s the mouse brain in stereotaxic coordinates: Academic Press, 2012. [Google Scholar]
- [60].Petitjean H, Pawlowski SA, Fraine SL, Sharif B, Hamad D, Fatima T, Berg J, Brown CM, Jan L-Y, Ribeiro-da-Silva AB, Joao M, Basbaum AI, Sharif-Naeini R. Dorsal horn parvalbumin neurons are gate-keepers of touch-evoked pain after nerve injury. Cell reports 2015;13(6):1246–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Pignatelli D, Ribeiro-da-Silva A, Coimbra A. Postnatal maturation of primary afferent terminations in the substantia gelatinosa of the rat spinal cord. An electron microscopic study. Brain research 1989;491(1):33–44. [DOI] [PubMed] [Google Scholar]
- [62].Punnakkal P, von Schoultz C, Haenraets K, Wildner H, Zeilhofer HU. Morphological, biophysical and synaptic properties of glutamatergic neurons of the mouse spinal dorsal horn. The Journal of physiology 2014;592(4):759–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Puskár Z, Polgár E, Todd A. A population of large lamina I projection neurons with selective inhibitory input in rat spinal cord. Neuroscience 2001;102(1):167–176. [DOI] [PubMed] [Google Scholar]
- [64].Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science 2011;333(6042):637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Rosenberg AB, Roco CM, Muscat RA, Kuchina A, Sample P, Yao Z, Graybuck LT, Peeler DJ, Mukherjee S, Chen W, Pun SH, Sellers DL, Tasic B, Seelig G. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 2018;360(6385):176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 2010;65(6):886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Rubini N, Ensina L, Silva E, Sano F, Solé D. Effectiveness and safety of Omalizumab in the treatment of chronic spontaneous urticaria: Systematic review and meta-analysis. Allergologia et immunopathologia 2019;47(6):515–522. [DOI] [PubMed] [Google Scholar]
- [68].Ruscheweyh R, Ikeda H, Heinke B, Sandkühler J. Distinctive membrane and discharge properties of rat spinal lamina I projection neurones in vitro. The Journal of physiology 2004;555(2):527–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Safronov BV, Pinto V, Derkach VA. High-resolution single-cell imaging for functional studies in the whole brain and spinal cord and thick tissue blocks using light-emitting diode illumination. Journal of neuroscience methods 2007;164(2):292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Schaffner AE, Behar T, Nadi S, Barker JL. Quantitative analysis of transient GABA expression in embryonic and early postnatal rat spinal cord neurons. Developmental brain research 1993;72(2):265–276. [DOI] [PubMed] [Google Scholar]
- [71].Schepers RJ, Ringkamp M. Thermoreceptors and thermosensitive afferents. Neuroscience & Biobehavioral Reviews 2009;33(3):205–212. [DOI] [PubMed] [Google Scholar]
- [72].Schmelz M. Itch and pain. Neuroscience & Biobehavioral Reviews 2010;34(2):171–176. [DOI] [PubMed] [Google Scholar]
- [73].Sekiguchi KJ, Shekhtmeyster P, Merten K, Arena A, Cook D, Hoffman E, Ngo A, Nimmerjahn A. Imaging large-scale cellular activity in spinal cord of freely behaving mice. Nature communications 2016;7:11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Serafin EK, Chamessian A, Li J, Zhang X, McGann A, Brewer CL, Berta T, Baccei M. Transcriptional profile of spinal dynorphin-lineage interneurons in the developing mouse. Pain 2019;160(10):2380–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Spike RC, Puskar Z, Andrew D, Todd AJ. A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. Eur J Neurosci 2003;18(9):2433–2448. [DOI] [PubMed] [Google Scholar]
- [76].St John Smith E, Blass GR, Lewin GR, Park TJ. Absence of histamine-induced itch in the African naked mole-rat and “rescue” by Substance P. Molecular pain 2010;6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Standaert DG, Watson SJ, Houghten R, Saper C. Opioid peptide immunoreactivity in spinal and trigeminal dorsal horn neurons projecting to the parabrachial nucleus in the rat. Journal of Neuroscience 1986;6(5):1220–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sun Y-G, Chen Z-F. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 2007;448(7154):700–703. [DOI] [PubMed] [Google Scholar]
- [79].Szűcs P, Pinto V, Safronov BV. Advanced technique of infrared LED imaging of unstained cells and intracellular structures in isolated spinal cord, brainstem, ganglia and cerebellum. Journal of neuroscience methods 2009;177(2):369–380. [DOI] [PubMed] [Google Scholar]
- [80].Ting JT, Daigle TL, Chen Q, Feng G. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics Patch-Clamp Methods and Protocols: Springer, 2014. pp. 221–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Tiong SY, Polgár E, van Kralingen JC, Watanabe M, Todd AJ. Galanin-immunoreactivity identifies a distinct population of inhibitory interneurons in laminae I-III of the rat spinal cord. Molecular pain 2011;7(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Todd AJ. Identifying functional populations among the interneurons in laminae I-III of the spinal dorsal horn. Molecular pain 2017;13:1744806917693003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Todd AJ, Puskár Z, Spike RC, Hughes C, Watt C, Forrest L. Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance p-containing afferents and respond to noxious stimulation. Journal of Neuroscience 2002;22(10):4103–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Torsney C, Fitzgerald M. Spinal dorsal horn cell receptive field size is increased in adult rats following neonatal hindpaw skin injury. The Journal of physiology 2003;550(1):255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. Journal of Neuroscience 2006;26(6):1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Vega-Avelaira D, McKelvey R, Hathway G, Fitzgerald M. The emergence of adolescent onset pain hypersensitivity following neonatal nerve injury. Mol Pain 2012;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Walsh MA, Graham BA, Brichta AM, Callister RJ. Evidence for a critical period in the development of excitability and potassium currents in mouse lumbar superficial dorsal horn neurons. J Neurophysiol 2009;101(4):1800–1812. [DOI] [PubMed] [Google Scholar]
- [88].White JC, Sweet WH. Pain and the neurosurgeon: a forty-year experience: CC Thomas, 1969. [Google Scholar]
- [89].Yang K, Kumamoto E, Furue H, Yoshimura M. Capsaicin facilitates excitatory but not inhibitory synaptic transmission in substantia gelatinosa of the rat spinal cord. Neuroscience letters 1998;255(3):135–138. [DOI] [PubMed] [Google Scholar]
- [90].Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain® 2010;151(2):475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Zhou W, Cheung K, Kyu S, Wang L, Guan Z, Kurien PA, Bickler PE, Jan LY. Activation of orexin system facilitates anesthesia emergence and pain control. Proceedings of the National Academy of Sciences 2018;115(45):E10740–E10747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Video that demonstrates hindpaw scratching directed at the nape of the neck in a P6 mouse after chloroquine injection. mp4
Supplemental Digital Content 2. Supplemental data: Figure S1.pdf


