Summary
A major hurdle in the treatment of cancer is chemoresistance induced under hypoxia that is characteristic of tumor microenvironment. Triptolide, a potent inhibitor of eukaryotic transcription, possesses potent antitumor activity. However, its clinical potential has been limited by toxicity and water solubility. To address those limitations of triptolide, we designed and synthesized glucose-triptolide conjugates (glutriptolides) and demonstrated their antitumor activity in vitro and in vivo. Herein, we identified a lead, glutriptolide-2 with an altered linker structure. Glutriptolide-2 possessed improved stability in human serum, greater selectivity toward cancer over normal cells, and increased potency against cancer cells. Glutriptolide-2 exhibits sustained antitumor activity, prolonging survival in a prostate cancer metastasis animal model. Importantly, we found that glutriptolide-2 was more potent against cancer cells under hypoxia than normoxia. Together, this work provides an attractive glutriptolide drug lead and suggests a viable strategy to overcome chemoresistance through conjugation of cytotoxic agents to glucose.
Subject Areas: Medical Substance, Inorganic Chemistry, Medical Biochemistry, Cancer
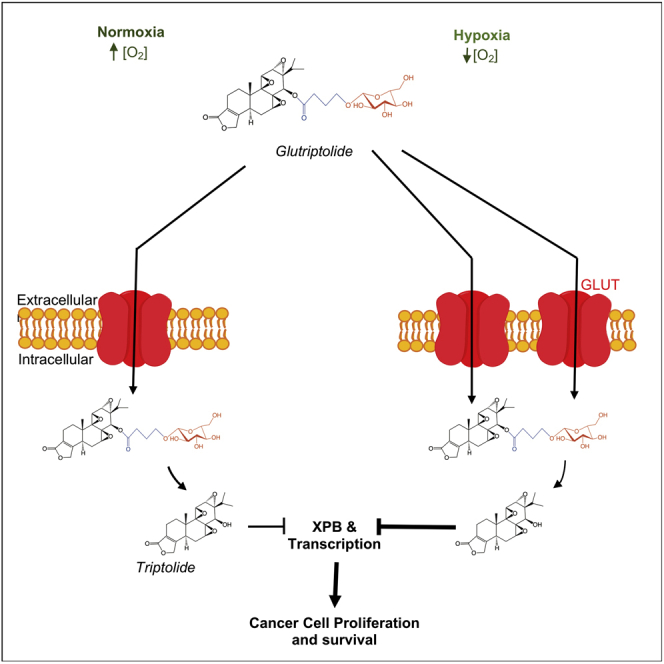
Graphical Abstract
Highlights
-
•
A second-generation glucose-triptolide conjugate (glutriptolide-2) was developed
-
•
Glutriptolide-2 exhibits selective toxicity to cancer cells over normal cells
-
•
Glutriptolide-2 possesses sustained antitumor activity in vivo
-
•
Glutriptolide-2 shows greater potency against cancer cells under hypoxia
Medical Substance; Inorganic Chemistry; Medical Biochemistry; Cancer
Introduction
Despite its fundamental role in cell proliferation and survival, there exist far fewer inhibitors of eukaryotic transcription in comparison to those of translation (Bensaude, 2011; Bhat et al., 2015; McClary et al., 2017). Triptolide ((1S,2S,4S,5S,7R,8R,9S,11S,13S)-8-hydroxy-1-methyl-7-propan-2-yl-3,6,10,16 tetraoxaheptacyclo[11.7.0.02,4.02,9.05,7.09,11.014,18] icos-14(18)-en-17-one), an active ingredient from the traditional Chinese medicinal plant Thunder God Vine (also known as Lei Gong Teng), has emerged as one of the few specific inhibitors of eukaryotic transcription mediated by RNA polymerase II (RNAPII) (Titov et al., 2011; Wang et al., 2011). Known for its potent immunosuppressive and antiinflammatory activity, extracts of Thunder God Vine with enriched triptolide have been used as a powerful immunosuppressant for treating a wide variety of autoimmune disorders for centuries (Lipsky and Tao, 1997; Zhao, 1765). Triptolide also exhibits potent antiproliferative activity in almost all cancer cell lines tested to date. The molecular mechanism underlying the antiproliferative activity of triptolide has been investigated for decades. Although a number of putative triptolide-binding proteins have been reported, most cannot account for its antiproliferative and pro-apoptotic activity (Corson et al., 2011; Leuenroth et al., 2007; Lu et al., 2014; Soundararajan et al., 2009; Westerheide et al., 2006). The identification and validation of the XPB subunit of the general transcription factor TFIIH as the physiological target of triptolide offered a plausible molecular explanation for the broad anticancer activity of triptolide (He et al., 2015; Smurnyy et al., 2014; Titov et al., 2011).
Triptolide forms a covalent adduct with Cys342 in the active site of XPB, leading to the inhibition of the DNA-dependent ATPase activity of XPB, effectively blocking transcriptional initiation by RNAPII (He et al., 2015). We have shown that mutation of Cys342 to a threonine residue in a single remaining allele of the XPB gene produces a viable, albeit slow-growing, HEK293T cells that became nearly completely resistant to triptolide. In addition to the Cys342 residue, a number of other residues in both XPB and its regulatory subunit p52 seem to play important roles in the interaction between TFIIH and triptolide, as their mutations also caused resistance, albeit to different degrees, to triptolide among the mutant-expressing cell lines (Smurnyy et al., 2014). The effect of triptolide on transcription did not seem to be caused solely by the inhibition of the ATPase activity of TFIIH, as the binding of triptolide to XPB subsequently causes degradation of the catalytic subunit of RNAPII, exacerbating the inhibitory effect of triptolide on RNAPII-mediated transcription (Titov et al., 2011; Wang et al., 2011). Recent work has implicated the CDK7 kinase as part of the pathway leading to the ubiquitylation and proteasome-mediated degradation of RNAPII induced by triptolide (Manzo et al., 2012). The precise mechanism by which triptolide triggers the degradation of the RPB1 subunit of RNAPII, however, still remains to be completely elucidated. Thus, triptolide inhibits eukaryotic transcription by a unique two-step mechanism, inhibition of XPB to prevent RNAPII-mediated transcription initiation followed by degradation of RNAPII itself.
Extensive efforts have been made to develop triptolide and its analogs as immunosuppressive and anticancer drugs in the past few decades. One of the major hurdles is the general toxicity of triptolide, most likely attributed to its inhibition of transcription. Another is its limited water solubility. To date, two derivatives of triptolide remain in clinical development. One analog, (5R)-5-hydroxytriptolide, is undergoing clinical trial as an immunosuppressant (Wang et al., 2012; Zhou et al., 2005). The other, Minnelide, a phosphorylated form of triptolide with increased solubility, is undergoing human trials for treating pancreatic and other types of cancer (Chugh et al., 2012). Given the mechanism-based toxicity of triptolide, it is difficult to separate the antitumor activity and intrinsic toxicity of triptolide with existing triptolide analogs, calling for a radically different approach to addressing the problem. Recently, we designed a different class of triptolide analogs by conjugating it to glucose in hopes to target glucose-addicted tumor cells over normal cells. Moreover, the high water solubility of glucose would significantly increase the solubility of the resultant glucose-triptolide conjugates (refer to hereafter as glutriptolides). One of the lead compounds from our first-generation glutriptolide (glutriptolide-1) indeed exhibited higher solubility and tumor cell selectivity over triptolide and was shown to possess sustained antitumor activity in vivo (He et al., 2016). Unfortunately, an obligate degradation intermediate, triptolide-succinate (also known as F60008), has undergone early human clinical study and was found to be lethal to two patients (Kitzen et al., 2009). In addition, glutriptolide-1 suffers from instability in human serum, ruling it out as a viable drug candidate.
To identify glutriptolide analogs with improved pharmacological properties and reduced toxicity, we embarked on the design and synthesis of a series of second-generation glucose-triptolide conjugates by altering the linker structure and accompanying linkage between the linkers and glucose. Screening of these second-generation glutriptolide analogs identified a lead, glutriptolide-2, with a glycosidic linkage between the linker and glucose that upon degradation, would release an alcohol-containing intermediate. Glutriptolide-2 was found to be 4-fold more potent against cancer cells in vitro and exhibited greater selectivity against cancer cells over normal cells than glutriptolide-1. Glutriptolide-2 was also found to have much greater stability in human serum. Unlike triptolide, glutriptolide-2 had little effect on the ATPase activity of TFIIH in vitro. Similar to triptolide, however, glutriptolide-2 inhibited the proliferation of multiple cancer cell lines, induced apoptosis, and caused degradation of the catalytic subunit of RNAPII in an XPB-dependent manner. Using glutriptolide-2 as a probe, we also investigated its effects on cancer cells under hypoxic conditions and found that glutriptolide-2 is more effective against cancer cells under hypoxic than normoxic conditions. In light of the key role of hypoxia in chemoresistance against almost all known anticancer drugs, our finding with glutriptolide-2 raised the exciting possibility of overcoming hypoxia-induced drug resistance through conjugation of drugs to glucose.
Results
Design and Synthesis of Glutriptolide-2 as the Most Potent Inhibitor of Cancer Cell Proliferation among Glucose-Triptolide Conjugates
Glutriptolides can be divided into three structural components: glucose, triptolide, and a linker (Figure 1A). The first-generation glutriptolide-1 (G-1) contained a 4-carbon succinate linker, giving rise to an activation intermediate previously shown to be too toxic to be used in humans (Kitzen et al., 2009). We thus selected a series of alternative linkers to connect glucose and triptolide (Table S1). In brief, these linkers attached at the C2 position of glucose include γ-hydroxybutyric acid (Compound G2-1), addition of two methyl groups to the succinate backbone (compounds G2-2 and G2-3), incorporation of a phenyl group to the succinate backbone (compounds G2-4, G2-5), and elongation of the succinate linker by one carbon (compounds G2-6, G2-7). In addition, we also synthesized two derivatives that contained a C6-substituted glucose with succinate linkers (compounds G2-8, G2-9). We then determined the potency of the newly synthesized glutriptolides in a HEK293T cell proliferation assay (Table S1). As expected, glutriptolides have lower potency than triptolide itself. Among the second-generation glutriptolide, G2-1 is significantly more potent than glutriptolide-1 with an IC50 (71 nM) that is about 13-fold higher than that for triptolide (5.6 nM). The rest of the second-generation glutriptolide analogs were less potent than glutriptolide-1 except for G2-8. But unlike G2-1, G2-8 would release the same toxic triptolide-succinate intermediate upon activation as glutriptolide-1. Thus, the ensuing studies were focused on the characterization of G2-1, named hereafter as glutriptolide-2 (G-2).
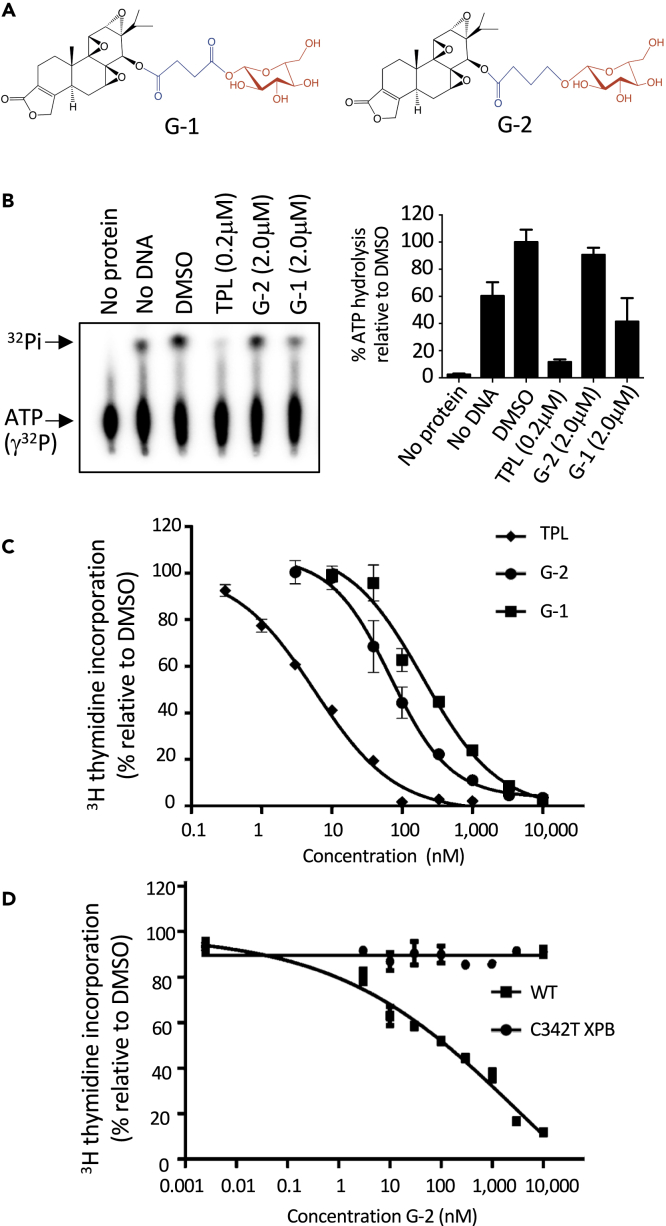
Figure 1.
Glutriptolide-2 Is a Prodrug that Requires XPB Binding for Its Antiproliferative Effect
(A) Chemical structures of glutriptolides 1 (G-1) and 2 (G-2). Structural motifs in glutriptolides are highlighted (black = triptolide, blue = linker, and red = glucose).
(B) G-2 does not inhibit the ATPase activity of TFIIH in vitro, whereas triptolide (TPL) effectively suppresses activity at a 10-fold lower concentration. Data are represented as mean ± SE of released inorganic phosphate (32Pi) relative to DMSO (n = 3).
(C) Treatment with G-2 (circle), G-1 (square), or TPL (diamond) inhibits cell proliferation after 24 h.
(D) The knock-in cell line for XPB expressing only the C342T XPB mutant is resistant to G-2 (circle), whereas inhibition of proliferation is observed in the isogenic cell line expressing WT (square) XPB. Proliferation was measured by 3H thymidine incorporation and plotted using GraphPad prism. Data are represented as mean ± SEM relative to DMSO (n = 3).
G-2 Is a Prodrug that Inhibits Cell Proliferation in an XPB-Dependent Manner
A premise of our original design of glutriptolides is that these conjugates will serve as prodrugs with little inhibitory effect on XPB until they enter cancer cells where the linkers are cleaved by intracellular hydrolytic enzymes to release active triptolide. We thus determined the effect of G-2 on the DNA-dependent ATPase activity of purified TFIIH using γ-[32P]-ATP as a substrate. Upon hydrolysis, the released 32Pi can be separated from the substrate using thin-layer chromatography and visualized with autoradiography. Although the ATPase activity of TFIIH is nearly completely inhibited by 200 nM triptolide, only a small fraction of the activity was affected by 2 μM G-2 (Figure 1B). Although G-2 has negligible effect on the ATPase activity of recombinant TFIIH, it inhibited HEK293T cell proliferation in a dose-dependent manner, being more potent than G-1 (Figure 1C and Table S1). These observations suggest that G-2 is an inactive pro-drug and can be activated inside cells. To determine if the antiproliferative effect of G-2 is mediated through inhibition of XPB, we took advantage of an engineered mutant cell line T7115 that encodes a single allele of C342T XPB mutant, which was previously shown to be resistant to triptolide (Figure S1) (He et al., 2015, 2016). Although the wild-type (WT) 293T cells were inhibited by G-2 in a dose-dependent manner, the isogenic T7115 mutant line is resistant to G-2, suggesting that G-2 works through inhibition of XPB, necessitating the intracellular hydrolytic release of triptolide from G-2 (Figure 1D).
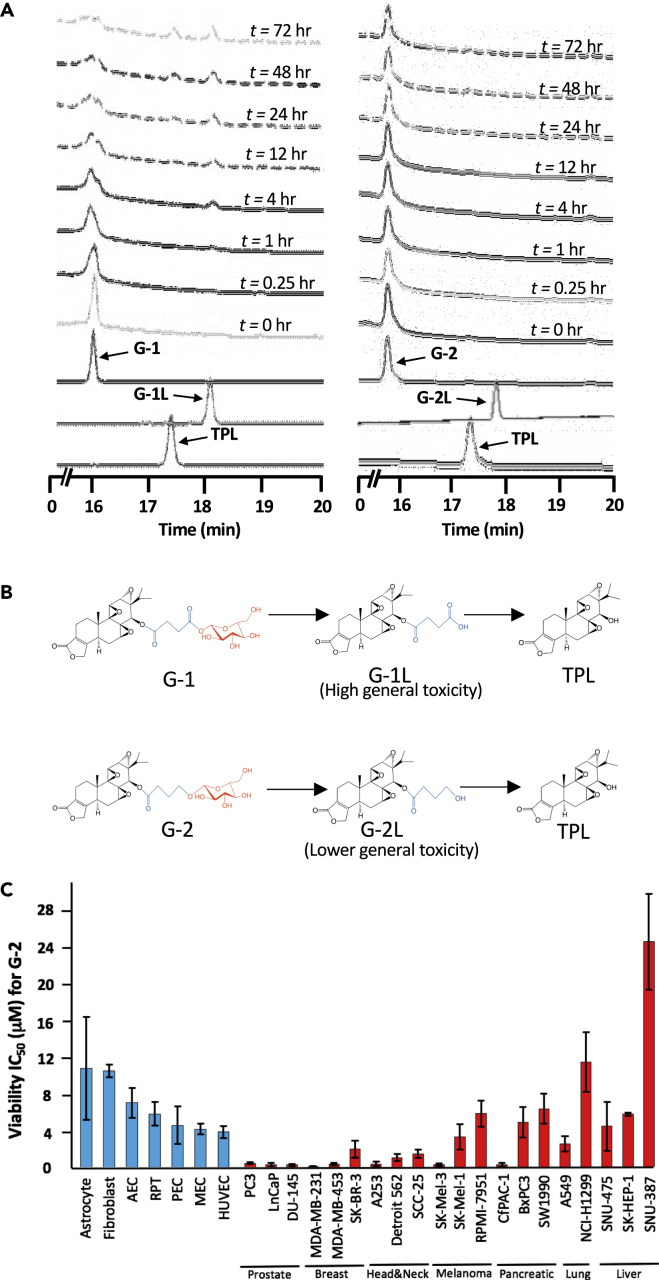
G-2 has Greater Stability in Human Serum and Higher Selectivity for Cancer Cells over Normal Cells Than G-1
For glutriptolides to achieve selectivity toward glucose transporter (GLUT)-overexpressing cancer cells over their normal counterparts, it is imperative that they have sufficiently long half-lives in serum to reduce the amount of free triptolide released in blood prior to their entry into tumor cells. We determined the stability of G-1 and G-2 by incubating them with human serum and detecting the release of free triptolide. Although G-1 underwent degradation to produce the triptolide-succinate intermediate by 4 h with appreciable amount of free triptolide generated by 48 h (Figures 2A and 2B), G-2 remained largely intact after incubation in human serum for up to 72 h (Figure 2A). These results suggest that G-2 is considerably more stable than G-1 in human serum.
Figure 2.
Glutriptolide-2 Possesses Increased Stability in Human Serum and Lower General Toxicity toward Nonmalignant, Primary Cells Relative to G-1
(A) Hydrolysis of G-1 and G-2 at different incubation times in human serum as monitored by tandem HPLC-MS. Chromatograms were taken at A218.
(B) Chemical structures of G-1 and G-2 with hydrolysis intermediates G-1L and G-2L that subsequently releases triptolide (TPL). Structural motifs in glutriptolides are highlighted (black = triptolide, blue = linker, and red = glucose).
(C) Primary cell viability as measured by XTT assay exhibits reduced sensitivity to G-2 in comparison to multiple cancer cell lines. Liver, lung, melanoma, and pancreatic cancer cell lines respond poorly to G-2 treatment. HUVEC = Human Umbilical Vascular Endothelial Cell, MEC = Mammary Epithelial Cell, PEC = Prostate Epithelial Cell, RPT = Renal Proximal Tubule, AEC = Airway Epithelial Cell. Data are represented as mean ± SEM viability relative to DMSO (n = 3–7).
To compare the selectivity of G-2 and G-1 for cancer cells, we determined their IC50 values for inhibition of cell viability using a panel of normal primary cells, including human umbilical vascular endothelial cell (HUVEC), mammary epithelial cell (MEC), prostate epithelial cell (PEC), renal proximal tubule (RPT), airway epithelial cell (AEC), fibroblasts, and astrocytes. The IC50 values of G-2 ranged from 4 μM to 10.9 μM for the primary cells, which is significantly higher than those for cancer cell lines that ranged from 0.26 μM to 6.5 μM (with the exception of a liver cell line SNU-387 and lung cell line NCI- H1299) (Figure 2C, Table S2). This represents a significant improvement over G-1 that had lower IC50 values for each primary cell type and comparable IC50 values for most cancer cell lines (Figure S2 and Table S2). We also note that cancer cell lines seem to segregate in their sensitivity to G-2 and G-1 according to tissue or organ origin. With the limited number of cancer cell lines tested, prostate and breast cancer cells appear to be more sensitive than liver and lung cancer cells (Figure 2C, Table S2).
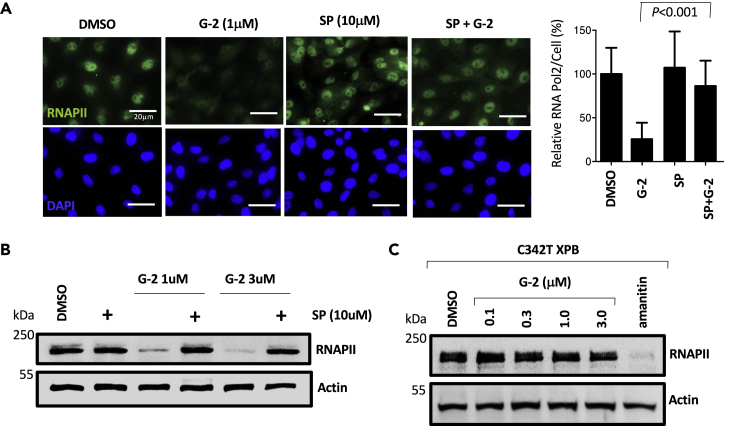
G-2 Causes Degradation of the Catalytic RPB1 Subunit of RNAPII through Interaction with XPB
We and others have previously shown that triptolide induced the degradation of the catalytic RPB1 subunit of RNAPII (Manzo et al., 2012; Titov et al., 2011; Wang et al., 2011), which is one of the hallmark cellular effects of triptolide. Using immunostaining, we observed that G-2 also caused the degradation of RPB1 in HeLa cells (Figure 3A). Aside from triptolide, a known steroidal drug spironolactone (SP) has been reported to bind XPB (Elinoff et al., 2018). Unlike triptolide, however, SP induces proteasome-mediated degradation of XPB without overt cellular toxicity. At 10 μM, SP caused degradation of the majority of XPB (Figure S3A) but had no effect on the stability of RPB1 (Figure 3A). To determine whether depletion of XPB by SP antagonizes the degradation of RPB1 by triptolide released from glutriptolide, we treated cells with a combination of 1 μM G-2 and 10 μM SP. Co-treatment with SP rescued RPB1 from degradation induced by G-2. Similar results were obtained using western blot analysis to detect endogenous levels of RPB1 protein (Figure 3B). To further confirm that RPB1 degradation induced by G-2 required binding of released triptolide to XPB, we determined the level of RPB1 upon treatment of both WT and C342T mutant cell lines. Although degradation of RPB1 was observed in the presence of G-2 in WT cells (Figure 3B), RPB1 level remained stable even when the concentration of G-2 reached 3 μM in the C342T XPB mutant cell line (Figure 3C). This result corroborates with observations made with SP and triptolide (Figure S3B), suggesting that the degradation of RPB1 induced by G-2 requires the covalent binding of released triptolide from G-2 to XPB.
Figure 3.
Glutriptolide-2-Induced RNA Polymerase 2 Degradation is XPB Dependent
(A) Treatment with 1 μM G-2 for 24 h depletes endogenous RNA polymerase II (RNAPII), whereas 10 μM spironolactone (SP) or DMSO by themselves do not affect protein levels in fixed HeLa cells processed for immunocytochemical staining of RPB1 (catalytic subunit of RNAPII) and DAPI (nuclear marker). Pre-treatment of cells with 10 μM spironolactone significantly (P < 0.001) rescues endogenous RNAPII from G-2-induced degradation. Representative images of RPB1 and DAPI staining are shown with quantification of intracellular RPB1 and student's t test analysis. Data are represented as mean ± SE RPB1 levels relative to DMSO (n = 3).
(B) Whole cell lysates of cells treated with G-2, SP, or in combination were subjected to western blot analysis of endogenous RNAPII using antibodies specific for RPB1 showing that G-2 induced RNAPII degradation at 1 or 3 μM is antagonized by 10 μM SP.
(C) Whole cell lysates from isogenic knock-in cells expressing only C342T XPB show that degradation of the catalytic subunit of RNAPII by G-2 as measured by immunoblotting for RPB1 is inhibited in the absence of WT XPB. In contrast, the RPB1-interacting inhibitor α-amanitin induced the degradation of Rpb1 at 1μM in the C342T XPB isogenic cell line. Actin was used as a loading control. Scale bar, 20 μm.
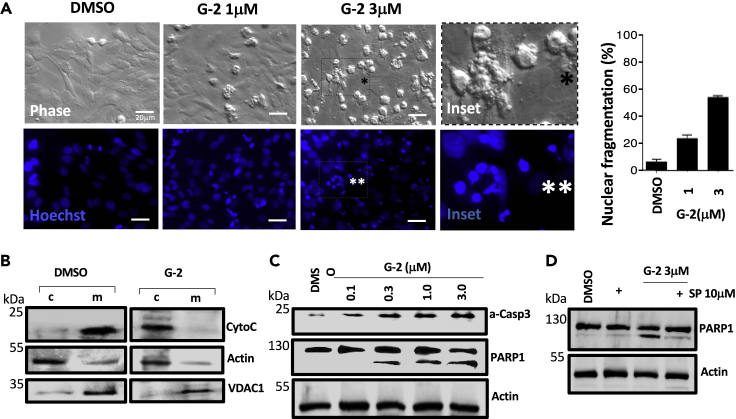
G-2 Induces Apoptosis of Cancer Cells via Activation of the Mitochondria-Mediated Apoptosis Pathway
Triptolide is known to induce apoptosis in a number of cancer cell lines (Zhou et al., 2012). We investigated the cellular effects of G-2 by examining the cellular morphology of HeLa cells upon exposure to G-2. G-2 caused membrane blebbing and nuclear fragmentation indicative of apoptosis (Figure 4A). The percentage of cells with nuclear fragmentation increased from 6% to 23% in the presence of 1 μM G-2 and 53% upon treatment with 3 μM G-2. G-2 induced the release of cytochrome c from the mitochondria into the cytosol, a key step in the activation of the intrinsic apoptotic pathway (Figure 4B). As expected, G-2 activated caspase-3 dose dependently, which was accompanied by cleavage of PARP1 (Figure 4C). Similar to RPB1 degradation, the cleavage of PARP1 requires XPB, as co-treatment with higher concentrations of SP prevented PARP1 cleavage by caspase-3 (Figure 4D). Together, these results suggest that G-2 activated the mitochondria-mediated apoptotic pathway through induction of cytochrome c release and ensuing activation of caspase-3 in HeLa cells.
Figure 4.
Glutriptolide-2 Induces Apoptosis Signaling
(A) Bright phase micrographs show minimal cytopathology with DMSO exposure in contrast to G-2 treatments especially with 3 μM G-2 where numerous cells round up and bleb (inset with black asterisk). Nuclear fragmentation, as detected by cytochemical analysis using Hoechst 33258 stain, in round up HeLa cells is dramatically increased by G-2 treatment (inset with two white asterisks) but not in DMSO. Data are represented as percentage of nuclear fragmented cells relative to total cells ±SE (n = 3).
(B) Cytochrome c release during G-2 treatment was assessed by centrifugal separation of mitochondria followed by western blot analysis using cytochrome-c-specific antibody. Exposure of HeLa cells to 3 μM G-2 triggers the release of cytochrome c from the mitochondria (m) to the cytosol (c). Actin- and VDAC1-specific antibodies were used to ensure the efficiency of cytoplasm and mitochondria fractionation, respectively.
(C) Western blot analysis of whole cell lysates for active caspase 3 (a-Casp3) and PARP1 during G-2 treatment shows a dose-dependent increase in caspase 3 activation. Pronounced PARP1 cleavage by active caspase 3 is also observed with increasing concentrations of G-2.
(D) Degradation of XPB in cells by 10 μM sprironolactone dampens G-2-induced apoptosis signaling as indicated by reduced PARP1 cleavage in whole cell lysates subjected to western blot analysis. Actin was used as loading control. Scale bar, 20 μm.
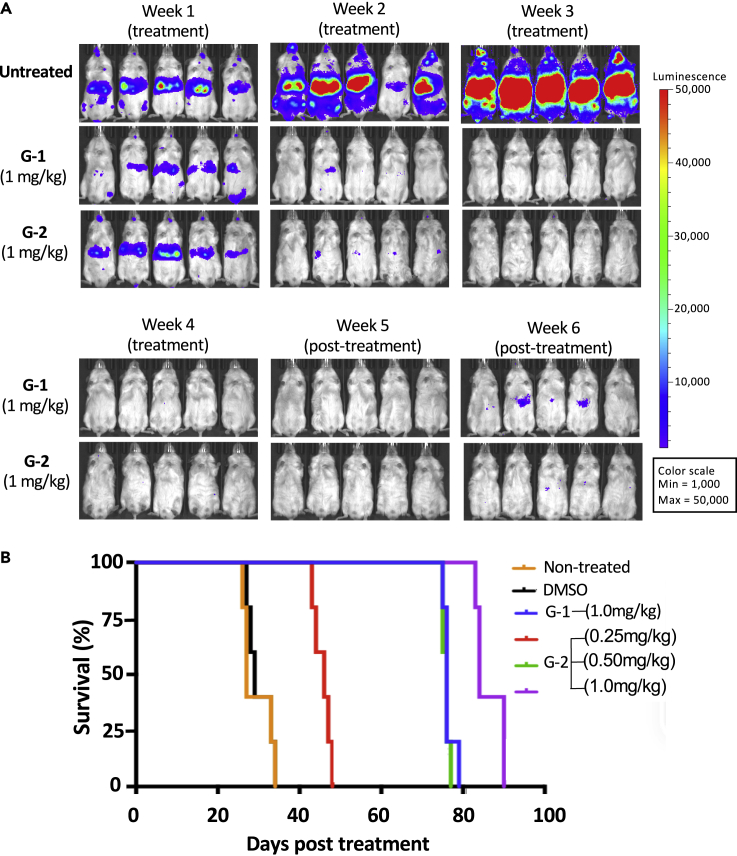
G-2 Showed Sustained Inhibition of Tumor Growth and Prolonged Survival In Vivo
We have previously shown that G-1 exhibited sustained antitumor activity in vivo in an experimental metastatic prostate cancer mouse model (He et al., 2016). Using the same animal model, we assessed the antitumor efficacy of G-2 side by side with G-1. Thus, PC3 prostate cancer cells expressing firefly luciferase as a reporter were injected into animals through the tail vein. Three weeks after tumor cell injection, G-2 and G-1 were administered by intraperitoneal injection once daily at various doses for a total of 30 days. The growth of tumor cells was monitored weekly through bioluminescence imaging. A rapid growth of tumor cells and metastasis to other organs occurred in untreated animals, killing all untreated animals by week 4 (Figure 5A). For animals dosed with 1 mg/kg G-1, tumor cells were cleared by week 2 of treatment and did not return until two weeks after treatment was stopped (Figure 5A). In contrast, animals receiving the same dose of G-2 had undetectable levels of cancer cells two weeks after cessation of treatment, suggesting that G-2 is more effective than G-1 in vivo (Figure 5A). Although G-2 and G-1 were administered for only 30 days, they both significantly prolonged the survival of animals well beyond the four-week treatment window (Figure 5B). Moreover, the prolonged survival upon treatment with G-2 was dose dependent with the longest survival achieved by the highest dose of G-2 (26 days for untreated group versus 86 days for 1 mg/kg G-2 treatment group). Furthermore, chi-square analysis shows that the survival curve for 1 mg/kg G-1 is not significantly different from 0.5 mg/kg G-2 (cannot reject null hypothesis at p = 0.05). In contrast, the survival curve for G-2 at 1 mg/kg is significantly different from G-1 at the same dose (reject null hypothesis at p = 0.001). G-2 given at 0.5 mg/kg led to the same overall survival as G-1 at 1 mg/kg, consistent with the higher potency of G-2 in tumor cell lines than G-1 in vitro.
Figure 5.
Glutriptolide-2 Improves Survival in an In-Vivo Prostate Cancer Model
(A) G-2 and G-1 have similar maximum tolerable dose (MTD) in a metastatic prostate cancer model. After confirmation of tumor growth in NOD/SCID/IL2rnull mice by bioluminescence imaging, daily administration of 1 mg/kg G-1 or G-2 for 30 days was tolerated by animals and able to suppress tumor growth throughout the treatment. Anti-tumor effect by G-1 or G-2 persists 2 weeks posttreatment.
(B) Kaplan-Meier curves showing survival time (days after initiation of treatments [n = 5]) for controls, G-1, or G-2 treatments. Median survival times (days) are as follows: nontreated = 27, DMSO = 29, G-1 (1mg/kg) = 76, G-2 (0.25mg/kg) = 46, G-2 (0.5mg/kg) = 76, G-2 (1mg/kg) = 84.
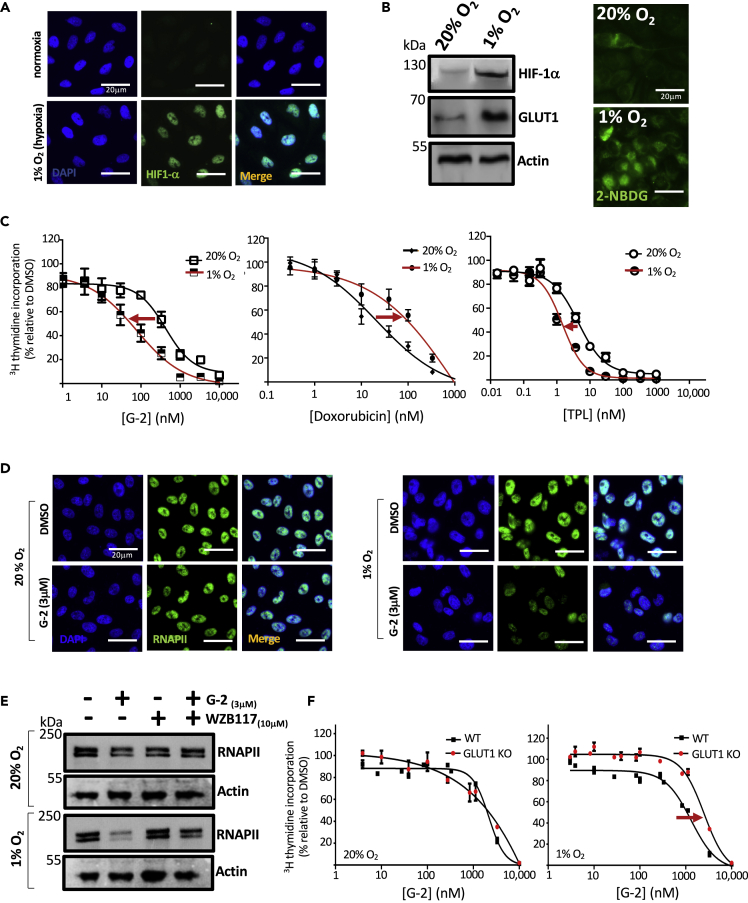
G-2 Is More Effective against Cancer Cells under Hypoxic Than Normoxic Conditions
The tumor microenvironment is hypoxic due to the lack of sufficient blood vessel density in rapidly growing tumors. As such, tumor cells upregulate the expression of HIF-1, which in turn drives the expression of a number of pro-survival and proangiogenic factors including multidrug resistance (MDR) pumps and GLUTs (Semenza, 2003, 2010). The upregulation of MDR and GLUTs under hypoxia renders tumor cells resistant to chemotherapeutic drugs (Tredan et al., 2007). Interestingly, the upregulation of glucose transporters under hypoxia should make cancer cells more susceptible to G-2 due to the presence of the glucose moiety. To test this possibility, we determined the effect of hypoxia on the sensitivity of cancer cells to G-2 using the prostate cancer cell line PC3 because increased HIF-1α has been shown in metastatic prostate biopsies (Semenza, 2010). Thus, PC3 cells were cultured under either hypoxic (1% O2) or normoxic (20% O2) conditions. As expected, HIF-1α is absent under normoxic conditions but is dramatically induced under hypoxia (Figure 6A). Western blot analysis of endogenous HIF-1α revealed a similar increase in HIF-1α with a corresponding increase in GLUT1 levels (Figure 6B). Uptake of the chromogenic glucose analogue 2-NBDG also increased under hypoxia. Importantly, PC3 cells became more sensitive to G-2 under hypoxic conditions with a reduced IC50 of 81 nM from an IC50 of 427 nM under normoxic conditions (Figure 6C), whereas the IC50 for triptolide was modestly reduced from 4.5 nM to 1.5 nM upon switching from normoxia to hypoxia. The same trend of enhanced susceptibility to G-2 under hypoxia was also observed with HeLa and MDA MB231 (Figure S4). In contrast to G-2, the potency of doxorubicin was decreased under hypoxia (Figure 6C). Degradation of RNAPII, an indicator of inhibition of XPB by triptolide (Figure 3), was observed as early as 6 h after treatment with G-2 under hypoxic but not in normoxic conditions (Figure 6D) where G-2 induced degradation of RPB1 also occurs in an XPB-dependent manner (Figure 3). To verify whether this difference in sensitivity was due to the upregulation of GLUT1 levels and function under hypoxic conditions (Figure 6B), we utilized the GLUT1 inhibitor WZB117 (Ojelabi et al., 2016). The addition of the GLUT inhibitor WZB117 abolished the rapid degradation of endogenous RNAPII in PC3 cells by G-2 under hypoxic (1% O2) conditions (Figure 6E), indicating that GLUT1 upregulation during hypoxia contributes to the rapid degradation of endogenous RNAPII by G-2 under hypoxic conditions. To further assess the role of GLUT1 upregulation in hypoxia-induced sensitization to G-2, we examined the effects of G-2 on the proliferation of both WT DLD-1 and its isogenic GLUT1 knockout cell line under normoxic (20% oxygen) and hypoxic (1% oxygen) conditions. The WT DLD-1 cells are more sensitive to G-2 (IC50: 1.3 μM) than the GLUT1 knockout cell line under hypoxic conditions (IC50: 2.5 μM), indicating GLUT1 dependence of hypoxia-induced sensitization to G-2 (Figure 6F). In contrast, no difference in sensitivity was observed between DLD-1 WT and GLUT1 KO under normoxia. Together, these results reveal that in contrast to most existing anticancer agents (Tredan et al., 2007), G-2 is more effective against tumor cells under hypoxia, making it a unique anticancer agent with potentially enhanced antitumor activity in vivo.
Figure 6.
Hypoxia Enhances Antiproliferative Effect of Glutriptolide-2
(A) Immunocytochemical analysis of fixed cells using antibodies specific to HIF-1α show that exposure to hypoxia (1% O2) for 24 h stabilizes endogenous HIF-1α compared with normoxia (20% O2) in PC3 cells.
(B) Western blot analysis of whole cell lysates for endogenous HIF-1α shows an increase during hypoxia compared with normoxia, which also corresponds with an increase in glucose transporter 1 (GLUT1).
(C) Hypoxia enhances the antiproliferative effect of G-2 at 48 h posttreatment as measured by 3H thymidine incorporation, whereas co-treatment with doxorubicin and hypoxia reduces drug potency. Triptolide (TPL) shows a modest antiproliferative effect. Data are represented as mean ± SE relative to DMSO (n = 3).
(D) Immunocytochemistry using antibody specific to RPB1 shows that exposure of cells to hypoxia triggers an early onset of RNAPII subunit RPB1 degradation by 3 μM glutriptolide-2 after 6 h.
(E) Whole cell lysates subjected to western blot using anti-RPB1-specific antibody shows that 10 μM glucose transporter 1 inhibitor WZB117 antagonizes the early onset of RNAPII degradation triggered by 3 μM G-2 and hypoxia.
(F) DLD-1 WT cells exposed to hypoxia exhibited enhanced sensitivity to G-2 in comparison to DLD-1 GLUT1 knockout (GLUT1 KO) cells. No difference in sensitivity is observed between DLD-1 WT and GLUT1 KO under normoxia. Data are represented as mean ± SEM relative to DMSO (n = 3). Scale bar, 20 μm.
Discussion
Most cytotoxic anticancer drugs, including those that are currently used in the clinic such as taxol, doxorubicin, and cyclophosphamide exert their antiproliferative and proapoptotic effects on cancer cells by blocking essential cellular protein targets that are shared with normal cells. As such, it is not surprising that those chemotherapeutic agents have severe adverse effects on patients. Transcription mediated by RNAPII is essential for mammalian cell proliferation and growth. As such, it is not surprising that cancer cells are susceptible to triptolide that blocks RNAPII-mediated mRNA synthesis through covalent modification of XPB/TFIIH accompanied by induced degradation of RPB1 catalytic subunit of RNAPII (Titov et al., 2011) in an XPB-dependent manner (Figure S3B). The toxicity of triptolide can also be attributed to the same mechanism given the essential role of RNAPII-mediated transcription in normal cells, making it difficult to reduce the toxicity of triptolide without compromising its antitumor efficacy given the shared molecular mechanism. By conjugating triptolide to glucose, selectivity toward cancer cells was achieved by taking advantage of the higher levels of glucose transporters expressed in fast-growing tumor cells than most normal tissues. In the present study, we have identified a second-generation glutriptolide analog, G-2, that met our expectations with significantly enhanced selectivity for tumor cells over normal cells, improved serum stability, and sustained antitumor activity in a PC-3 tumor model. Importantly, in the course of characterizing G-2, we found that G-2 gained antitumor potency under hypoxia in contrast to conventional cytotoxic drugs in vitro, pointing to an emerging strategy of overcoming drug resistance during cancer treatment.
The best lead of the second-generation glutriptolide called G-2 is superior to the first-generation compound G-1 in a number of ways. First, degradation of G-1 by plasma esterases produces a highly toxic intermediate that was previously proven lethal to two of twenty subjects in a phase 1 clinical trial (Kitzen et al., 2009). Although the mechanistic basis of the toxicity for the G-1 intermediate is still unknown, our data from a limited number of primary human cells indicate a significantly lower toxicity for G-2 in comparison with G-1 in noncancerous cells (Figure S2). The reported toxicity for the G-1 intermediate occurred in two patients receiving the highest dose of therapy, suggesting dose-limiting toxicity (Kitzen et al., 2009). Maximum serum levels of F60008 and triptolide from the lethal case dosed with 18 mg/m2 were 1,361 ng/mL (∼2.96 μM) and 58.5 ng/mL (∼0.16 μM), respectively (Kitzen et al., 2009). Our cell-based viability assays with primary human cells show IC50 values for G-1 ranging from 1.37 to 5.6 μM (Figure S2 and Table S2), which includes the above-reported plasma concentration of F60008 in the lethal case with 18 mg/m2 F60008. We have also shown previously using a smaller panel of primary human cells that the IC50 of triptolide ranges from 0.0042 to 0.0235 μM (He et al., 2016), which is 7- to 38-fold lower than the maximum serum concentration of triptolide from the lethal case. In summary, the toxicities observed with G-1 intermediate F60008 are in part dose dependent as no lethality was observed in 18 of the 20 patients administered with <12 mg/m2 F60008 (Kitzen et al., 2009). By replacing the ester linkage to glucose with a glycosidic bond, the potential intermediate will be an alcohol that is expected to have less toxicity. More importantly, given the much greater stability of G-2 in human serum than G-1, the amount of this alcohol degradation intermediate is expected to be significantly reduced, further reducing the potential toxicity of G-2 (Figure 2A). Second, G-2 exhibited greater stability in human serum than G-1 (Figure 2A). This is likely attributable to the glycosidic linkage between the linker and glucose moieties that requires a different type of hydrolytic enzyme(s) than the corresponding ester bond in G-1. The increase in serum stability makes G-2 a potentially better lead for drug development as it is a prodrug, and premature degradation in serum would release free triptolide that can exert toxicity to normal tissues. Third, G-2 showed lower cytotoxicity to normal cells than to a subset of cancer cells (Figure 2C). It is interesting to note that different types of cancer lines exhibited distinct sensitivity. Among the limited cancer cell lines tested, it appears that prostate, breast, and head and neck cancers are particularly sensitive to G-2. In contrast, melanoma, pancreatic, lung, and liver cancer lines appear to be less sensitive to G-2 with an average IC50 values comparable or even higher than normal cells. Extensive profiling of a large number of cancer cell lines and collections of cultured patient-derived tumor cells will be needed to comprehensively determine whether the selective toxicity of G-2 to certain types of cancer such as those of the prostate holds true.
Aside from its unique anticancer activity in vitro, G-2 also exhibited sustained antitumor activity in a PC3 xenograft model in vivo (Figure 5). Cancer cells failed to re-emerge two weeks after treatment with G-2 ceased but not with G-1. The slower reappearance of cancer cells after treatment with G-2 than G-1 is also consistent with longer survival in G-2-treated animals. G-2 at 0.5 mg/kg was as effective as G-1 at 1 mg/kg in prolonging the survival of xenograft model animals in vivo. The greater serum stability, the lower cytotoxicity toward normal cells compared with a subset of cancer cells, and the increased efficacy toward cancer cells under hypoxia along with sustained antitumor activity of G-2 in vivo render this glutriptolide analogue an interesting example of a promising lead candidate for further development as a type of anticancer drug targeting transcription.
The microenvironment of solid tumors is known to be hypoxic, and hypoxia has been shown to confer resistance in tumor cells against cytotoxic anticancer drugs (Semenza, 2003; Tredan et al., 2007), which is a major hurdle for cancer therapy. As hypoxia is known to upregulate GLUT expression on the cancer cell surface and given that GLUTs confer the tumor cell selectivity of glutriptolides, we investigated the effect of hypoxia on the potency of G-2. The increase in potency of G-2 for inhibition of cancer cell proliferation during hypoxia contrasts the decrease in potency of the broadly used, FDA-approved, anticancer drug doxorubicin (Figures 6C and S4). This feature of G-2 as an anticancer drug candidate offers an additional advantage of being more effective toward cancer cells under hypoxia where other conventional anticancer drugs encounter resistance. It is also interesting to note that unlike doxorubicin, triptolide itself also showed a modest enhancement, rather than reduction, in its inhibitory effect on cancer cell growth under hypoxic conditions. This may be attributed in part to its inhibition of the transcriptional activity of HIF-1 that requires TFIIH and RNAPII. Because hypoxia involves the transcription of genes to adapt the survival of cancer cells to a hypoxic condition, the ability of triptolide to inhibit mammalian transcription initiation can dampen HIF-driven transcription of hypoxia-activated genes that facilitate the proliferation of cancer cells experiencing hypoxia. Treatment of cancer cells under hypoxia with triptolide inhibits the transcription of HIF-1α target genes VEGF, BNIP3, and CAIX, including a hypoxia responsive element (HRE)-driven luciferase reporter (Zhou et al., 2010). Triptolide treatment also reverses hypoxia-induced epithelial-mesenchymal transition (Liu et al., 2014) explaining the observed three-fold enhancement of triptolide's anti-proliferative effect in vitro (Figure 6C). The increased expression of GLUTs in cancer cells during hypoxia further amplifies the impact of transcription inhibition by G-2 on the proliferation of hypoxic cancer cells as seen with the five-fold increase in G-2 IC50 during hypoxia (Figure 6C). The conjugation of triptolide to glucose in G-2 enhances the effect size of triptolide during hypoxia from 0.71 during triptolide treatment alone to 64.89 in G-2-treated hypoxic PC3 cells. The GLUT1 dependence of enhanced G-2-induced anti-proliferation in hypoxic cancer cells is demonstrated in the reduced sensitivity of hypoxic DLD-1 GLUT1 knock out cells compared with parental DLD-1 cells (Figure 6F). Although the enhanced sensitivity to glucose-conjugated triptolide G-2 under hypoxia was also observed in HeLa and triple-negative breast cancer cell line MDA MB231 (Figure S4), this effect is not observed in all the cell lines tested, as the liver cancer cell line HepG2 remains resistant to G-2 under hypoxia. Despite the apparent tissue-specific sensitivity of cancer cells to G-2, our results suggest that conjugation of potent, nonspecific antiproliferative agents to glucose offers a promising strategy for targeting cancer cells in hypoxic conditions such as those in solid tumors. This finding offers an alternative albeit viable strategy to combat hypoxia-induced drug resistance in solid tumors through conjugation of cytotoxic drugs to glucose.
To summarize, triptolide is a key ingredient from a traditional Chinese medicinal plant that has been used for centuries. It possesses potent antitumor activity through irreversible inhibition of the XPB subunit of the general transcription factor TFIIH, effectively blocking transcription initiation. Its potential development as an anticancer drug has been limited by its toxicity and insolubility in water. In an attempt to address these issues, we have designed and synthesized glucose conjugates of triptolide exhibiting lower toxicity and sustained antitumor activity in vivo. However, the previous lead glutriptolide releases a potentially toxic degradation intermediate, rendering it unsuitable as a drug candidate. By using molecular linkers that connect triptolide to glucose, we identified a glutriptolide with enhanced stability in serum and reduced toxicity to normal cells. Importantly, glutriptolide G-2 is more potent against cancer cells under hypoxic conditions likely due to the upregulation of glucose transporters, in contrast to most cytotoxic anticancer drugs to which cancer cells gain resistance under hypoxia. G-2 showed sustained antitumor activity in vivo and significantly prolonged survival of treated animals. These findings suggest that conjugation of cytotoxic drugs to glucose may be a viable strategy to overcome drug resistance in general and that glutriptolide-2 is a promising candidate for further development as a targeted, anticancer pro-drug.
Limitations of the Study
Although conjugation of the cytotoxic natural product triptolide to glucose led to an increase in potency against cancer cells under hypoxia, the finding has been confined to in vitro studies. The observations remain to be verified in vivo. Further investigation of the underlying mechanism involved in enhanced sensitivity of hypoxic tumor cells to glucose-conjugated cytotoxic agents in vivo will help establish glucose conjugation as a viable strategy to enhance cancer cell targeting and overcome drug resistance under hypoxia.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jun O. Liu (joliu@jhu.edu).
Materials Availability
All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Data and Code Availability
No large-scale datasets were generated in this study. Raw data are available from the lead contact upon request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
Financial support: This work was supported by the Flight Attendant Medical Research Institute, a generous gift from Mr. Shengjun Yan and Ms. Hongju Mao (J. O. L.); the Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded in part by Grant Number UL1 TR 001079 and NCI (P30CA006973); and an NIH-funded Pharmacology Training Program at Johns Hopkins School of Medicine (T32 GM008763) (E.D.). We thank Dr. Ronald Schnaar, Mr. Steve Fernandez, and Ms. August Li for training and access to imaging equipment. We also thank Drs. Sarah A. Head, Jennifer Schmitt, Shaima Salman, Yongmie Xie, Wukun Liu, Hanjing Peng, and Zufeng Guo for technical advice and assistance. We are also grateful to Drs. Gregg Semenza and Shaima Salman for helpful suggestions.
Authors Contributions
J.O.L. coordinated and supervised the study. J.O.L., M.G.P., and B.Y. initiated the work. E.D. performed the in vitro XPB/TFIIH ATPase activity assay, cell proliferation assay, metabolic labeling, western blot analyses, immunocytochemical and cytochemical assays, and hypoxia experiments. I.M. performed the in vivo animal studies while I.M. and H.A. performed the in vitro viability experiments. P.X. has synthesized, purified, and characterized the glutriptolide analogues. Q.L.H. performed the serum stability assays and generated the knock-in C342T XPB cell line. J.O.L., E.D., I.M., Q.L.H., and M.G.P. analyzed the data. E.D. and J.O.L. wrote the manuscript with input from all coauthors.
Declaration of Interests
A patent covering glutriptolides has been licensed from Johns Hopkins to Rapafusyn Pharmaceuticals, of which J.O.L. is a cofounder and an equity owner. The potential conflict of interest is being managed by Johns Hopkins University. No potential conflicts of interest were disclosed by the other authors.
Published: September 25, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101536.
Contributor Information
Biao Yu, Email: byu@mail.sioc.ac.cn.
Martin G. Pomper, Email: mpomper@jhmi.edu.
Jun O. Liu, Email: joliu@jhu.edu.
Supplemental Information
References
- Bensaude O. Inhibiting eukaryotic transcription: which compound to choose? How to evaluate its activity? Transcription. 2011;2:103–108. doi: 10.4161/trns.2.3.16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat M., Robichaud N., Hulea L., Sonenberg N., Pelletier J., Topisirovic I. Targeting the translation machinery in cancer. Nat. Rev. Drug Discov. 2015;14:261–278. doi: 10.1038/nrd4505. [DOI] [PubMed] [Google Scholar]
- Chugh R., Sangwan V., Patil S.P., Dudeja V., Dawra R.K., Banerjee S., Schumacher R.J., Blazar B.R., Georg G.I., Vickers S.M. A preclinical evaluation of Minnelide as a therapeutic agent against pancreatic cancer. Sci. Transl. Med. 2012;4:156ra139. doi: 10.1126/scitranslmed.3004334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson T.W., Cavga H., Aberle N., Crews C.M. Triptolide directly inhibits dCTP pyrophosphatase. Chembiochem. 2011;12:1767–1773. doi: 10.1002/cbic.201100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinoff J.M., Chen L.Y., Dougherty E.J., Awad K.S., Wang S., Biancotto A., Siddiqui A.H., Weir N.A., Cai R., Sun J. Spironolactone-induced degradation of the TFIIH core complex XPB subunit suppresses NF-kappaB and AP-1 signalling. Cardiovasc. Res. 2018;114:65–76. doi: 10.1093/cvr/cvx198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q.L., Minn I., Wang Q., Xu P., Head S.A., Datan E., Yu B., Pomper M.G., Liu J.O. Targeted delivery and sustained antitumor activity of triptolide through glucose conjugation. Angew. Chem. Int. Ed. 2016;55:12035–12039. doi: 10.1002/anie.201606121. [DOI] [PubMed] [Google Scholar]
- He Q.L., Titov D.V., Li J., Tan M., Ye Z., Zhao Y., Romo D., Liu J.O. Covalent modification of a cysteine residue in the XPB subunit of the general transcription factor TFIIH through single epoxide cleavage of the transcription inhibitor triptolide. Angew. Chem. Int. Ed. 2015;54:1859–1863. doi: 10.1002/anie.201408817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzen J.J., de Jonge M.J., Lamers C.H., Eskens F.A., van der Biessen D., van Doorn L., Ter Steeg J., Brandely M., Puozzo C., Verweij J. Phase I dose-escalation study of F60008, a novel apoptosis inducer, in Patients with Advanced Solid Tumours. Eur. J. Cancer. 2009;45:1764–1772. doi: 10.1016/j.ejca.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Leuenroth S.J., Okuhara D., Shotwell J.D., Markowitz G.S., Yu Z., Somlo S., Crews C.M. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc. Natl. Acad. Sci. U S A. 2007;104:4389–4394. doi: 10.1073/pnas.0700499104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P.E., Tao X.L. A potential new treatment for rheumatoid arthritis: thunder god vine. Semin. Arthritis Rheum. 1997;26:713–723. doi: 10.1016/s0049-0172(97)80040-6. [DOI] [PubMed] [Google Scholar]
- Liu L., Salnikov A.V., Bauer N., Aleksandrowicz E., Labsch S., Nwaeburu C., Mattern J., Gladkich J., Schemmer P., Werner J. Triptolide reverses hypoxia-induced epithelial-mesenchymal transition and stem-like features in pancreatic cancer by NF-κB downregulation. Int. J. Cancer. 2014;134:2489–2503. doi: 10.1002/ijc.28583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Zhang Y., Li L., Feng X., Ding S., Zheng W., Li J., Shen P. TAB1: a target of triptolide in macrophages. Chem. Biol. 2014;21:246–256. doi: 10.1016/j.chembiol.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Manzo S.G., Zhou Z.L., Wang Y.Q., Marinello J., He J.X., Li Y.C., Ding J., Capranico G., Miao Z.H. Natural product triptolide mediates cancer cell death by triggering CDK7-dependent degradation of RNA polymerase II. Cancer Res. 2012;72:5363–5373. doi: 10.1158/0008-5472.CAN-12-1006. [DOI] [PubMed] [Google Scholar]
- McClary B., Zinshteyn B., Meyer M., Jouanneau M., Pellegrino S., Yusupova G., Schuller A., Reyes J.C.P., Lu J., Guo Z. Inhibition of eukaryotic translation by the antitumor natural product Agelastatin A. Cell Chem. Biol. 2017;24:605–613.e605. doi: 10.1016/j.chembiol.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojelabi O.A., Lloyd K.P., Simon A.H., De Zutter J.K., Carruthers A. WZB117 (2-Fluoro-6-(m-hydroxybenzoyloxy) phenyl m-hydroxybenzoate) inhibits GLUT1-mediated sugar transport by binding reversibly at the exofacial sugar binding site. J. Biol. Chem. 2016;291:26762–26772. doi: 10.1074/jbc.M116.759175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Semenza G.L. HIF-1: upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 2010;20:51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smurnyy Y., Cai M., Wu H., McWhinnie E., Tallarico J.A., Yang Y., Feng Y. DNA sequencing and CRISPR-Cas9 gene editing for target validation in mammalian cells. Nat. Chem. Biol. 2014;10:623–625. doi: 10.1038/nchembio.1550. [DOI] [PubMed] [Google Scholar]
- Soundararajan R., Sayat R., Robertson G.S., Marignani P.A. Triptolide: an inhibitor of a disintegrin and metalloproteinase 10 (ADAM10) in cancer cells. Cancer Biol. Ther. 2009;8:2054–2062. doi: 10.4161/cbt.8.21.9803. [DOI] [PubMed] [Google Scholar]
- Titov D.V., Gilman B., He Q.L., Bhat S., Low W.K., Dang Y., Smeaton M., Demain A.L., Miller P.S., Kugel J.F. XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat. Chem. Biol. 2011;7:182–188. doi: 10.1038/nchembio.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tredan O., Galmarini C.M., Patel K., Tannock I.F. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 2007;99:1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- Wang L., Xu Y., Fu L., Li Y., Lou L. (5R)-5-hydroxytriptolide (LLDT-8), a novel immunosuppressant in clinical trials, exhibits potent antitumor activity via transcription inhibition. Cancer Lett. 2012;324:75–82. doi: 10.1016/j.canlet.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Wang Y., Lu J.J., He L., Yu Q. Triptolide (TPL) inhibits global transcription by inducing proteasome-dependent degradation of RNA polymerase II (Pol II) PLoS One. 2011;6:e23993. doi: 10.1371/journal.pone.0023993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerheide S.D., Kawahara T.L., Orton K., Morimoto R.I. Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J. Biol. Chem. 2006;281:9616–9622. doi: 10.1074/jbc.M512044200. [DOI] [PubMed] [Google Scholar]
- Zhao X.-M. China Zhang's Jie Xing Tang Pulishing House; 1765. Supplement to Materia Medica. [Google Scholar]
- Zhou R., Zhang F., He P.L., Zhou W.L., Wu Q.L., Xu J.Y., Zhou Y., Tang W., Li X.Y., Yang Y.F. (5R)-5-hydroxytriptolide (LLDT-8), a novel triptolide analog mediates immunosuppressive effects in vitro and in vivo. Int. Immunopharmacol. 2005;5:1895–1903. doi: 10.1016/j.intimp.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Luo Z., Yu B., Jiang Y., Chen Y., Feng J., Dai M., Tong L., Li Z., Li Y. Increased accumulation of hypoxia-inducible factor-1α with reduced transcriptional activity mediates the antitumor effect of triptolide. Mol. Cancer. 2010;9:268. doi: 10.1186/1476-4598-9-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.L., Yang Y.X., Ding J., Li Y.C., Miao Z.H. Triptolide: structural modifications, structure-activity relationships, bioactivities, clinical development and mechanisms. Nat. Prod. Rep. 2012;29:457–475. doi: 10.1039/c2np00088a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No large-scale datasets were generated in this study. Raw data are available from the lead contact upon request.