Abstract
Background
Currently, hepatocellular carcinoma (HCC) patients with refractory ascites (RA) have a very poor prognosis, and there are no effective treatments recommended by the guidelines. A treatment strategy that utilizes a transjugular intrahepatic portosystemic shunt (TIPS) combined with subsequent antitumor treatment is explored in this study for its feasibility and clinical value.
Methods
One month after TIPS, the ascites grade and Child-Pugh scores and stages were reassessed to compare changes in the preoperative indicators.
Results
A total of 68 patients from 3 centers were enrolled. After TIPS, the following results were obtained: a complete response (CR), partial response (PR), or absent RA response (AR) of 38 [55.9%], 21 [30.9%], and 9 [13.2%], respectively. The control of RA was 86.8%. The median Child–Pugh scores prior to TIPS and one month after TIPS were 8 (IQR 7–9) and 7 (IQR 6–8), respectively. The down, unchanged, and elevated Child–Pugh stages were 26 [38.2%], 36 [53.0%], and 6 [8.8%], respectively. The postoperative Child–Pugh scores were significantly lower than the preoperative (p < 0.001). 92.6% (63/61) of the patients received subsequent anti-tumor treatment opportunities. The median overall survival (OS) was 8.7 (range, 0.4–49.6) months. The lower postoperative Child-Pugh stage(p = 0.001), downward change of the Child-Pugh stage(p = 0.027), and downward change of the Child-Pugh score (p = 0.002) were independent protected prognostic factors for OS.
Conclusion
As a minimally invasive method, TIPS can effectively control ascites and improve Child–Pugh scores and stages. TIPS combined with subsequent anti-tumor therapy is a feasible and effective management for HCC patients with RA.
Abbreviations: HCC, Hepatocellular carcinoma; RA, Refractory ascites; QoL, Quality of life; LVP, Large volume paracentesis; TIPS, Transjugular intrahepatic portosystemic shunt; ECOG, East Coast Oncology Group; CDUS, Color doppler ultrasonography; CR, Complete response; PR, Partial response; AR, Absent response; TACE, Transarterial chemoembolization; MWA, Microwave ablation; OS, Overall survival; AFP, Alpha-fetoprotein; PVTT, Portal vein tumor thrombosis; MELD, Model of endstage liver disease; IQR, Interquartile range
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide [1]. A total of 80% of patients with HCC have associated liver cirrhosis, which causes progressively increased portal hypertension. This leads to a series of severe clinical complications related to portal hypertension [2,3]. Refractory ascites (RA) is one of the most common complications of HCC with cirrhosis [4,5]. RA is closely related to poor prognosis [6]. The median overall survival (OS) of RA in end-stage liver disease is only six months, and this is even worse in HCC patients [7,8].
RA causes most patients with HCC to lose the opportunity for further tumor treatment due to poor liver function restrictions, and current treatment options are limited [9]. Most patients can only tolerate palliative symptomatic treatment, and the most accepted palliative intervention is large volume paracentesis (LVP) combined with supplementation of albumin [8]. RA is formed by persistent portal hypertension and secondary hemodynamic changes, and LVP only acts downstream of the pathophysiological cascade, so it cannot produce survival benefits and seriously affects patient quality of life (QOL) [6,10,11]. Therefore, treatment to relieve portal hypertension is necessary, which provides possible opportunities to further treat tumors and may bring survival benefits.
In recent years, the transjugular intrahepatic portosystemic shunt (TIPS) has shown obvious advantages as a minimally invasive modality for managing portal hypertension-related complications [12]. As a result of technological advances and the perfect performance of the stent, TIPS is expanding its indications for portal hypertension-related complications [13,14]. TIPS has been a common management model for RA for end-stage liver disease [ 6,15]. Previous small sample studies have also shown that in HCC patients with RA, this treatment may be a feasible palliative management model, with significant benefits for improving symptoms and QOL [16,17].
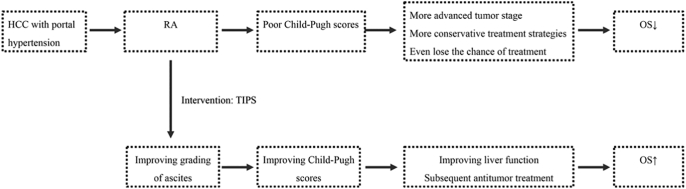
In view of the current HCC with RA treatment dilemma, Fig. 1 shows a proposed treatment strategy that uses TIPS to downgrade ascites and improve Child-Pugh scores. It is proposed that with this strategy, patients will obtain more effective subsequent anti-tumor treatment and achieve a survival benefit. Therefore, the purpose of this study is to evaluate the feasibility and clinical value of TIPS combined with subsequent antitumor treatment in HCC patients with RA.
Fig. 1.
Treatment strategy for hepatocellular carcinoma (HCC) patients with refractory ascites (RA).
Transjugular intrahepatic portosystemic shunt(TIPS) is used to intervene in RA of HCC patients to obtain a downward grading of the ascites. The control of ascites can improve a patient's Child–Pugh score and Child–Pugh stage. Therefore, the patient may obtain subsequent anti-tumor therapy and have enhanced liver function to tolerate the therapy. Finally, HCC patients with RA can obtain a survival benefit.
Materials and methods
Study population
From May 16, 2016 to January 17, 2020, 68 HCC patients with portal hypertension-related RA from 3 centers of interventional radiology were enrolled in this study. All of the patients received TIPS combined with sequential antitumor therapy. Informed consent was signed by all of the patients prior to their treatment with TIPS. The ethics committee of the Sun Yat-sen University Cancer Center approved this study and waived the requirement for patient consent for this retrospective analysis.
Inclusion and exclusion criteria
Patients could be considered for inclusion if they were 18–70 years, met the diagnostic criteria for HCC and RA, had a tumor volume less than 70% of the liver volume, and the East Coast Oncology Group (ECOG) performance status was ≤2. Excluded patients had primary cholangiocarcinoma, multiple hepatic cysts, refractory biliary and pancreatic obstruction, liver failure, or severe cardiopulmonary dysfunction.
Diagnosis and definitions
The diagnostic criteria for HCC was based on the European Association for the Study of the Liver [17]. A color doppler ultrasonography (CDUS) was necessary to evaluate the status of portal blood flow [18]. The definition of refractory ascites was according to the International Ascites Club: (a) intensive diuretics (spironolactone 400 mg/d combined with furosemide 160 mg/d) and sodium-restricted diet (<90 mmoVd) for at least 1 week have no response. (b) Lack of response to diuretic therapy. (c) Early recurrence of ascites within 4 weeks. (d) Diuretic-induced complications. The grading of ascites was divided into mild ascites, moderate ascites, and large or gross ascites [6].
The diagnosis of Portal vein tumor thrombosis(PVTT) was based on enhanced CT or MR. PVTT was found to be accompanied by HCC or invaded by intrahepatic tumors. In this study, all the enrolled cases involved more than the second-level branch of the portal vein.
The responses to TIPS follow. A complete response (CR) meant no clinically obvious ascites or no requirement for diuretics and sodium limitation. A partial response (PR) meant there existed clinically obvious ascites and no need for further paracentesis. An absent response (AR) indicated the development of large amounts of ascites after TIPS [19].
Shunt dysfunction was defined as follows: A maximum flow velocity within the shunt of ≤60 cm/s or >180 cm/s or within the portal vein of ≤30 cm/s, recurrent ascites, or variceal bleeding. Suspected dysfunction was diagnosed using portography and an evaluation of the portosystemic pressure gradient (PSG) of ≥15 mmHg [18].
TIPS procedure
The primary process was similar to previous reports [18,19]. In brief, a needle punctured the portal vein through the transjugular approach. After a successful puncture, the parenchymal tract was dilated, and covered stents were introduced in all patients(Viatorr; W. L. Gore & Associates, Inc., Flagstaff, AZ, USA). Bare metal stents (Bard Murray Hill, NJ, USA) were implanted in 11 (16.2%) patients to ensure that the shunt tract was adequately functioning. The specifications of the covered stents were 8 mm × 60 mm, 8 mm × 80 mm, and 8 mm × 100 mm. All of the diameters of the bare stents were 8 mm, and the lengths were 80 mm and 100 mm. The portal vein pressure was measured before and after shunt creation. After the insertion of TIPS, all of the patients received a diuretic treatment and a salt-limited diet until the ascites disappeared.
Subsequent antitumor therapy
When the symptoms of portal hypertension were controlled, the benefits of liver function a result of the improvement of ascites. Patients were reassessed using the Child-Pugh scores and stages, and individualized anti-tumor treatment was selected. HCC was treated primarily using transarterial chemoembolization (TACE), microwave ablation (MWA), systemic therapy, TACE combined with MWA, or TACE combined with systemic therapy at least one month after TIPS. Finally, a total of 63 (92.6%) patients received sequential antitumor treatment.
TACE was conducted for patients with a Child–Pugh stage of A/B and rich blood flow in the tumor based on preoperative imaging. A total of 31 (45.6%) patients received TACE, 10 (14.7%) received TACE alone, and 21 (30.9%) received TACE combined with MWA. MWA alone was administered in 3 (4.4%) patients due to the appropriate tumor diameter and stage. A total of 32 (47.1%) patients received systemic therapy (Sorafenib, Lenvatinib, or Apatinib). A total of 10 (14.7%) patients received TACE combined with systemic therapy.
Follow up
The major follow-up outcomes were OS, change in Child–Pugh stage, change in Child–Pugh scores, and the response of TIPS and complications. OS was defined as the interval from TIPS to death or lost to follow-up. The median follow-up time was 9.6 (range, 0.5–49.6) months. Laboratory tests, such as blood count, liver and kidney function, coagulation function, and AFP tests, were conducted at 1 and 2 weeks and every month after TIPS and at any time when symptoms recurred. Abdominal computerized tomography/magnetic resonance imaging (CT/MRI) and color Doppler ultrasound (CDUS) were performed at 1, 2, and 3 months, and then every two months after TIPS.
Statistical analysis
The statistical analysis was conducted using SPSS 26.0 (IBM, Chicago). Significant differences were considered at p < 0.05. Pearsonχ2 was used to compare the qualitative data. Quantitative data before and after TIPS were evaluated using paired t-tests. The OS in the different subgroups was analyzed using a Kaplan-Meier analysis (log-rank test). A stratified Cox proportional hazard regression model was used to explore the independent prognostic factors that were related to OS. Finally, three levels of research variables were included in this model. Liver function factors: preoperative Child-Pugh stage (A,B,C), postoperative Child-Pugh stage (A,B,C), change in Child-Pugh stage (down, unchanged, elevated), and change in Child-Pugh score (down, unchanged/elevated). Ascites factors: postoperative grading of ascites (0/1, 2/3) and response to TIPS (complete response, partial response, absent response). Tumor factors: BCLC classification (A/B, C/D), PVTT(yes, no), number of tumors (single, multiple), intrahepatic local treatment (yes, no), AFP (<400, ≥400), model for end stage liver disease (MELD) (<15, ≥15), and age (< 60, ≥60).
Results
Patient characteristics
The patient characteristics are presented in Table 1. The median age was 58 (46–63) years. The most common baseline characteristics were male (61 [89.7%]), hepatitis B (66 [97.1%]), with PVTT (45[66.2%]), multiple tumors (45 [66.2%]), an AFP < 400 ng/mL (48[70.6%]), outside of Milan(53 [77.9%]), maximum tumor diameter <5 cm 39(57.4%), and a MELD <15 (53 [77.9%]). The distribution in the BCLC classification of A, B, C, and D was 10 (14.7%), 10 (14.7%), 36 (52.9%), and 12 (17.6%), respectively. In addition, patients had symptomatic portal hypertension performed with RA (68 [100%]), variceal bleeding (13 [19.1%]), and gastrointestinal symptoms (2 [2.9%]).
Table 1.
Patient characteristics.
| Characteristics | N (%)/median (IQRa) |
|---|---|
| Gender | |
| Male | 61(89.7%) |
| Female | 7(10.3%) |
| Age | 58 (46–63) |
| <60 | 37(54.4%) |
| ≥60 | 31(45.6%) |
| Aetiology of liver disease | |
| Hepatitis B | 66(97.1%) |
| Hepatitis C | 2(2.9%) |
| ECOG statusa | |
| 0 | 20(29.4%) |
| 1 | 35(51.5%) |
| 2 | 13(19.1%) |
| Inside of Milan | 15(22.1%) |
| Outside of Milan | 53(77.9%) |
| Maximum tumor diameter | |
| <5 cm | 39(57.4%) |
| ≥5 cm | 29(42.65) |
| BCLC classificationa | |
| A | 10(14.7%) |
| B | 10(14.7%) |
| C | 36(52.9%) |
| D | 12(17.6%) |
| PVTTa | |
| No | 23(33.8%) |
| Yes | 45(66.2%) |
| Number of tumors | |
| Single | 23(33.8%) |
| Multiple | 45(66.2%) |
| MELDa | 12.0 (10.0–14.0) |
| <15 | 53(77.9%) |
| ≥15 | 14(20.6%) |
| Symptomatic portal hypertension | |
| Refractory ascites | 68(100.0%) |
| Variceal bleeding | 13(19.1%) |
| Gastrointestinal symptoms | 2(2.9%) |
| Laboratory tests | |
| Platelets [109/L] | 87 (62–133) |
| INRa | 1.31 (1.16–1.49) |
| AST [U/L]a | 50.80 (37.80–69.5) |
| ALT [U/L]a | 38.90 (25.53–51.93) |
| Albumin [g/dL] | 34.30 (32.15–37.30) |
| Creatinine [mg/dL] | 73.60(61.98–91.98) |
| Bilirubin [mg/dL] | 30.50(22.28–40.63) |
| AFP [ng/mL]a | |
| <400 | 48(70.6%) |
| ≥400 | 20(29.4%) |
ECOG, Eastern Cooperative Oncology Group; BCLC, Barcelona Clinic Liver Cancer; PVTT, portal vein tumor thrombosis; MELD, model of endstage liver disease; INR, international normalised ratio; AST, aspartate aminotransferase; ALT, alanine transaminase; AFP, alpha fetoprotein; IQR, interquartile range.
Efficacy of TIPS
After TIPS, the mean PSG was lowered from 28.3 to 12.9 mmHg, and all of the patients received successful TIPS treatment. As shown in Table 2, before TIPS, all of the patients had grade 2 ascites (19 [27.9%]) and grade 3 ascites (49 [72.1%]). One month after TIPS insertion, grades 0, 1, 2, and 3 were (15 [22.1%]), (32 [47.0%]), (16 [23.5%]), and (5 [7.4%]), respectively. Postoperative grading of ascites was significantly lower than preoperative (p < 0.001). A complete response (CR), partial response (PR), and absent response (AR) of RA were obtained in (38 [55.9%]), (21 [30.9%]), and (9 [13.2%]), respectively. The control (proportion of CR and PR) for RA was 86.8%.
Table 2.
Changes in the Child–Pugh scores, Child–Pugh stages, and ascites.
| Variable | Before TIPS | One month after TIPS | P |
|---|---|---|---|
| Child–Pugh stage | 0.007b | ||
| A | 7(10.3%) | 22(32.4%) | |
| B | 49(72.1%) | 37(54.4%) | |
| C | 12(17.6%) | 9(13.2%) | |
| Change of Child–Pugh stage | |||
| Down | 26(38.2%) | ||
| Unchanged | 36(53.0%) | ||
| Elevated | 6(8.8%) | ||
| Child–Pugh scores (IQR)a | 8 (7–9) | 7(6–8) | |
| Change of Child–Pugh scores | <0.001c | ||
| Down | 53(77.9%) | ||
| Unchanged | 5(7.4%) | ||
| Elevated | 10(14.7%) | ||
| Grading of ascitesa | <0.001b | ||
| 0 | 15(22.1%) | ||
| 1 | 32(47.0%) | ||
| 2 | 19(27.9%) | 16(23.5%) | |
| 3 | 49(72.1%) | 5(7.4%) | |
| Responsea | |||
| CR | 38(55.9%) | ||
| PR | 21(30.9%) | ||
| AR | 9(13.2%) |
IQR, interquartile range; according to the criteria of the International Ascites Club: 0, no ascites; 1, mild ascites; 2, moderate ascites; and 3, large or gross ascites; The response to TIPS: complete response (CR), no clinically detectable ascites with or without diuretics and sodium limitation; partial response (PR), clinically detectable ascites without the requirement for further paracentesis; or absent response (AR), development of large amounts of ascites after TIPS.
Chi-square test.
Paired t-test.
Changes in the Child–Pugh stages and Child–Pugh scores
As shown in Table 2, prior to TIPS, the distribution of the Child–Pugh stage A, B, and C were (7 [10.3%]), (49 [72.1%]), and (12 [17.6%]), respectively. One month after TIPS insertion, the distribution of the Child–Pugh A, B, and C scores was (22 [32.4%]), (37 [54.4%]), and (9 [13.2%]), respectively. The down, unchanged, and elevated Child–Pugh stages were (26 [38.2%]), (36 [53.0%]), and (6 [8.8%]), respectively. The postoperative Child–Pugh stages were significantly lower than the preoperative (p = 0.007). Similarly, the median Child–Pugh scores prior to TIPS and one month after TIPS were 8 (IQR 7–9) and 7 (IQR 6–8), respectively. The down, unchanged, and elevated Child–Pugh scores were (53 [77.9%]), (5 [7.4%]), and (10 [14.7%]), respectively. The postoperative Child–Pugh scores were significantly lower than the preoperative scores (p < 0.001).
Overall survival
The median OS was 8.7 (range, 0.4–49.6) months. The median OS in the BCLC classifications of A, B, C, and D was 14.5 (range, 2.6–49.6), 8.9 (range, 3.8–44), 7.8 (range, 0.5–18.8), and 4.9 (range, 0.4–28.8) months, respectively.
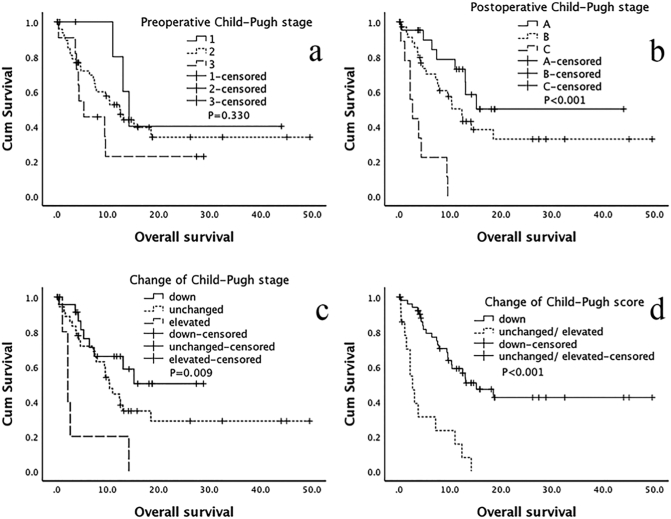
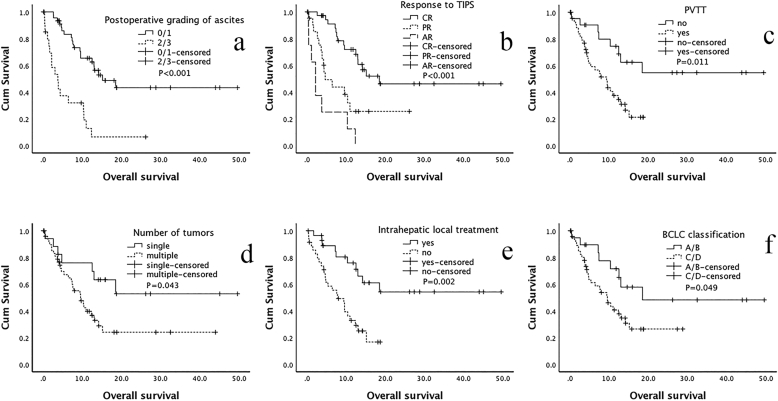
As shown in Table 3 and Fig. 2, Fig. 3, the Univariate Kaplan-Meier analysis showed that the preoperative Child-Pugh stage did not show a significant difference (p = 0.330). However, after TIPS, the lower postoperative Child-Pugh stage [A/C: P < 0.001, HR = 0.109 (95% CI: 0.039–0.301); B/C: P < 0.001, HR = 0.201 (95% CI: 0.086–0.468)], downward change of the Child-Pugh stage [down/elevated: P = 0.002, HR = 0.174 (95% CI: 0.057–0.531); the unchanged/elevated: P = 0.020, HR = 0.308 (95% CI: 0.115–0.829)], and the downward change of Child-Pugh scores [down/(unchanged/elevated): P < 0.001, HR = 0.198 (95% CI: 0.098–0.398)] displayed better OS. Similarly, a good OS was significantly related to lower postoperative grading of ascites [(0/1)/(2/3): P < 0.001, HR = 0.226 (95% CI: 0.115–0.441)] and a better response to TIPS [CR/AR: P < 0.001, HR = 0.120 (95% CI: 0.049–0.296); PR/AR: P = 0.023, HR = 0.359 (95% CI: 0.149–0.868)]. In terms of tumor factors, good OS results were significantly associated with a lower BCLC classification [(A/B)/(C/D): P = 0.049, HR = 0.446 (95% CI: 0.212–1.025)], no PVTT [yes/no: P = 0.011, HR = 0.353 (95% CI: 0.159–0.785)], received intrahepatic local treatment [yes/no: P = 0.002, HR = 0.302 (95% CI: 0.142–0.642)], a single tumor [single/multiple: P = 0.043, HR = 0.421 (95% CI: 0.182–0.971)], and an AFP < 400 [<400/≥400: P = 0.002, HR = 0.341 (95% CI: 0.174–0.672)].
Table 3.
Univariate analysis and Cox proportional hazards regression analysis related to OS.
| Variable | Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|---|
| HR6 | 95%CIb | P | HR | 95%CI | P | ||
| Preoperative Child-Pugh stage | 0.330 | ||||||
| A | 0.401 | 0.105–1.524 | 0.180 | ||||
| B | 0.615 | 0.277–1.367 | 0.233 | ||||
| C | |||||||
| Postoperative Child-Pugh stageb | <0.001 | 0.001 | |||||
| A | 0.109 | 0.039–0.301 | <0.001 | 0.023 | 0.003–0.167 | <0.001 | |
| B | 0.201 | 0.086–0.468 | <0.001 | 0.141 | 0.039–0.507 | 0.003 | |
| C | |||||||
| Change of Child-Pugh stageb | 0.009 | 0.027 | |||||
| Down | 0.174 | 0.057–0.531 | 0.002 | 0.542 | 0.198–0.731 | 0.011 | |
| Unchanged | 0.308 | 0.115–0.829 | 0.020 | 0.645 | 0.293–0.879 | 0.036 | |
| Elevated | |||||||
| Change of Child-Pugh scoreb | Down | 0.198 | 0.098–0.398 | <0.001 | 0.085 | 0.018–0.398 | 0.002 |
| Unchanged/elevated | |||||||
| Postoperative grading of ascitesb | 0/1 | 0.226 | 0.115–0.441 | <0.001 | 0.541 | 0.137–2.130 | 0.379 |
| 2/3 | |||||||
| Response to TIPSa | <0.001 | 0.248 | |||||
| CR | 0.120 | 0.049–0.296 | <0.001 | 1.316 | 0.205–8.441 | 0.772 | |
| PR | 0.359 | 0.149–0.868 | 0.023 | 2.434 | 0.623–9.515 | 0.201 | |
| AR | |||||||
| BCLC classificationa | A/B | 0.466 | 0.212–1.025 | 0.049 | 64,709 | 0.001–79,050 | 0.910 |
| C/D | |||||||
| PVTTa | No | 0.353 | 0.159–0.785 | 0.011 | 0.001 | 0.001–3303 | 0.914 |
| Yes | |||||||
| Number of tumors | Single | 0.421 | 0.182–0.971 | 0.043 | 0.822 | 0.225–3.004 | 0.767 |
| Multiple | |||||||
| Intrahepatic local treatmentc | Yes | 0.302 | 0.142–0.642 | 0.002 | 0.680 | 0.181–2.558 | 0.568 |
| No | |||||||
| AFPa | <400 | 0.341 | 0.174–0.672 | 0.002 | 0.277 | 0.113–0.679 | 0.005 |
| ≥400 | |||||||
| MELDa | <15 | 1.701 | 0.662–4.370 | 0.270 | 0.971 | 0.312–3.024 | 0.960 |
| ≥15 | |||||||
| Age | <60 | 1.387 | 0.722–2.666 | 0.326 | 1.394 | 0.606–3.205 | 0.434 |
| ≥60 | |||||||
The p-value with bold indicates a statistically significant difference.
The response to TIPS: complete response (CR), no clinically detectable ascites with or without diuretics and sodium limitation; partial response (PR), clinically detectable ascites without the requirement for further paracentesis; or absent response (AR), development of large amount of ascites after TIPS; BCLC, Barcelona Clinic Liver Cancer; PVTT, portal vein tumor thrombosis; AFP, alpha fetoprotein; MELD, model of end stage liver disease; IQR, interquartile range; CI. Confidence interval.
At one month after TIPS, the grading of ascites, Child-Pugh stage, and Child-Pugh score were reassessed.
Including transarterial chemoembolization and ablation.
Fig. 2.
Overall survival related to the Child–Pugh scores and Child–Pugh stages.
One month after TIPS, the Child-Pugh scores and Child-Pugh stages were reassessed, and the results were used to determine the changes in the Child-Pugh score and Child-Pugh stage. a. Preoperative Child-Pugh stage; b. Postoperative Child-Pugh stage; c. Change in the Child-Pugh stage; d. Change in the Child-Pugh score.
Fig. 3.
Overall survival related with ascites and tumor factors.
One month after TIPS, the gradings of ascites were reassessed, and the results were used to determine the response to TIPS. Complete response (CR), no clinically detectable ascites with or without diuretics and sodium limitation; partial response (PR), clinically detectable ascites without the requirement for further paracentesis; or absent response (AR), development of large amount of ascites after TIPS; a-b is the variable of ascites factors and c-f is the variable of tumor factors. a. Postoperative grading of ascites; b. response to TIPS; c. portal vein tumor thrombosis (PVTT); d. number of tumors; e. intrahepatic local treatment, including transarterial chemoembolization and ablation; and f. BCLC classification.
The Cox proportional hazards regression analysis found that the postoperative Child-Pugh stage [A/C: P < 0.001, HR = 0.023 (95% CI: 0.003–0.167); B/C: P = 0.003, HR = 0.141 (95% CI: 0.03–0.507)], change in the Child-Pugh stage [down/elevated: P = 0.011, HR = 0.542 (95% CI: 0.198–0.731); unchanged/elevated: P = 0.036, HR = 0.645 (95% CI: 0.293–0.879)], change in the Child-Pugh score [down/(unchanged/elevated): P = 0.002, HR = 0.085 (95% CI:0.018–0.398)], and AFP [<400/≥400: P = 0.005, HR = 0.277 (95% CI: 0.113–0.679)] were independent prognostic factors for OS.
Shunt dysfunction
A total of 10 (14.7%) patients were diagnosed with shunt dysfunction used with CDUS during follow-up. The primary patency rates at 30 and 90 days were 95.6% (65/68) and 89.7% (61/68), respectively. Thrombosis occurred in 3 patients within 30 days after the TIPS insertion, and tumor invasion led to shunt dysfunction as diagnosed by CT in 7 patients. Among these patients with shunt dysfunctions, TIPS revision using balloon dilation or additional stent placement were performed in 2 and 5 patients, respectively. All 10 patients received a 75-mg daily oral dose of clopidogrel (Plavix; Bristol-Myers Squibb, Princeton, NJ, USA) after the revision.
Procedure-related complications
The TIPS-related complications are shown in Table 4. Due to puncture injury, small amounts of localized hematomas were found in five (7.4%) patients. One case (1.5%) of biliary bleeding was diagnosed according to persistent melena and CT images. These patients were relieved after symptomatic treatment. Intraabdominal bleeding occurred in 6 (8.8%) patients, and this was found using persistent light red ascites. Most of these cases did not require special intervention, and only 1 (1.5%) developed shock and required emergency intervention to staunch the bleeding. Laboratory tests showed a transient increase in the ALT/AST (30 [44.1%]) and bilirubin (14 [20.6%]). However, most of these indicators did not exceed four times their amount prior to surgery, and patients recovered after receiving symptomatic treatment. Only 1 (1.5%) patient developed acute liver failure and died 11 days after TIPS. Six (8.8%) patients experienced a mild HE (grade I–II), and only three (4.4%) patients experienced a grade III-IV HE, which improved after receiving standard medical treatment.
Table 4.
Procedure-related complications.
| Complications | N(%) |
|---|---|
| Localized hematoma | 5(7.4%) |
| Intraabdominal bleeding | 6(8.8%) |
| Bile duct bleeding | 1(1.5%) |
| Tumor rupture | 0(0.0%) |
| ALT/AST increasing† | 30(44.1%) |
| Bilirubin increasing | 14(20.6%) |
| Acute liver failure | 1(1.5%) |
| HE† | |
| I-II | 6(8.8%) |
| III-IV | 3(4.4%) |
Note:1. A LT, alanine transaminase; AST, aspartate aminotransferase; 2. HE. hepatic encephalopathy.
Discussion
This research demonstrated that the proposed treatment strategy that combined TIPS with subsequent antitumor treatment improved the OS of HCC patients with RA by improving the grading of ascites and Child–Pugh scores. Clinically significant portal hypertension is an independent influencing factor in the prognosis of HCC patients [20,21]. RA, one symptom of clinically significant portal hypertension, seriously affects a patient's QOL and the choice of subsequent treatment strategies [6,10,22].
To date, there are no guideline recommendations for the treatment of HCC patients with RA. Therefore, this exploratory study discussed the feasibility and clinical value of TIPS combined with subsequent antitumor therapy for these patients. Previous studies have shown the effectiveness of TIPS in the management of RA [23]. TIPS acts on the initial stage of portal hypertension to relieve RA. It can improve the QOL and improve Child–Pugh scores, in addition to providing the possibility for subsequent antitumor treatment(as shown in Fig. 4). This is also the theoretical basis for the treatment strategy presented in this research. The results of this study also showed that patients that had an opportunity to receive intrahepatic local treatment after TIPS had a higher OS [P = 0.002, HR = 0.302 (95% CI: 0.142–0.642)].
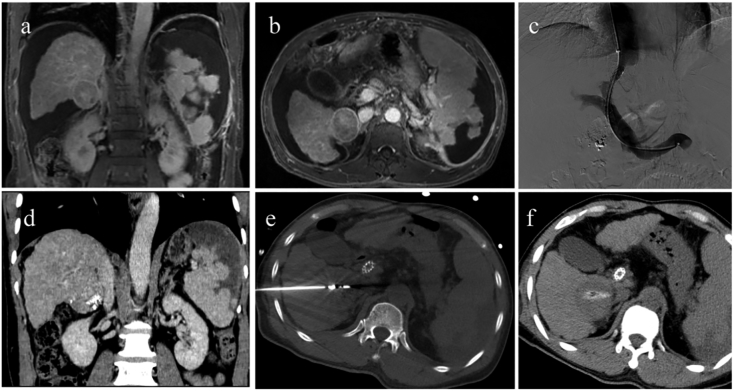
Fig. 4.
Case of TIPS combined with subsequent antitumor treatment.
A 59-year-old male patient, primary hepatocellular carcinoma(HCC) with a diameter of 5 cm. He had experienced a splenic arterial embolization due to hypersplenism. Because of his refractory ascites, the patient only received a transarterial chemoembolization (TACE) within 6 months after diagnosed with HCC. a-b. ascites and tumor before transjugular intrahepatic portosystemic shunt(TIPS). The Child-Pugh score and Child-Pugh stag were 8 and B; c. TIPS was completed. d. One month after TIPS, the ascites completely disappeared. The re-evaluated Child-Pugh score and Child-Pugh stag were 6 and A. e-f. Patients received subsequent microwave ablation because of the improved liver function. Subsequently, the patient experienced multiple ablation and TACE because of his intrahepatic recurrent tumors, and finally died of tumor progression. The overall survival was 18.5 months (from TIPS) but the ascites did not reappear.
The median OS in this study was 8.7 months. The end-stage liver disease and tumor conditions of most of these patients were higher than that of previous studies [6,[23], [24], [25]]. This treatment was able to reduce the ascites burden of patients through TIPS, prevent the continued loss of beneficial substances, such as albumin, and increase the effective blood volume and liver function perfusion. After TIPS, 55.9% of patients achieved CR of ascites, 30.9% of patients achieved PR, and the ascites control rate was as high as 86.8%. Correspondingly, 77.9% of the patients' Child–Pugh scores and 38.2% of the Child–Pugh stages were reduced, which provides for the possibility of subsequent anti-tumor treatment. In this study, 92.6% (63/61) of the patients received subsequent anti-tumor treatment opportunities, and 50% (34/68) of the patients received intrahepatic local treatment opportunities. 11(16.2%) patients with BCLC classification C/D still received intrahepatic local treatment after TIPS due to the improvement of liver function and ascites brought by TIPS. Therefore, TIPS increased the chance of intrahepatic local treatment by at least 16.2% (11/68). Which resulted in a survival benefit.
This study explored the prognosis of HCC patients with RA from three aspects. In terms of liver function, the lower postoperative Child-Pugh stage (p < 0.001), the downward change in the Child-Pugh stage (p = 0.009), and the downward change in the Child-Pugh scores (p < 0.001) indicated better OS. In terms of ascites factors, a good OS was significantly related to lower postoperative grading of ascites (p < 0.001) and a better response to TIPS (p < 0.001). In terms of tumor factors, good OS results were significantly associated with a lower BCLC classification (p = 0.049), no PVTT (p = 0.011), receiving intrahepatic local treatment (p = 0.002), presence of a single tumor (p = 0.043), and an AFP < 400 (p = 0.002). TIPS improved the Child-Pugh score by reducing ascites, and patients could better tolerate subsequent anti-tumor therapy, thereby affecting survival through changes in liver function. The Cox proportional hazards regression analysis also indicated that the lower a postoperative Child-Pugh stage (p < 0.001), a downward change in the Child-Pugh stage (p = 0.027), and a downward change in the Child-Pugh score (p = 0.002) were independent protected prognostic factors for OS. Prognosis of HCC with RA was determined using multiple factors of tumor burden and liver function [23,26]. Liver function should be improved before anti-tumor treatment, and this treatment strategy responds to this demand. Therefore, this treatment strategy is reasonable.
Currently, few studies have reported on the feasibility and efficacy of TIPS in HCC patients [23,[26], [27], [28]], and this study further demonstrated the feasibility and effectiveness of TIPS in HCC patients with RA. First, a technical success of up to 100% was achieved in this study. This proposed treatment performed better than that of previous research, where two patients had portal cavernomas. That approach consisted of a percutaneous transhepatic puncture of the portal vein to increase the technical success [29]. Second, this study had an acceptable complication. Only 1 (1.5%) patient developed intraabdominal bleeding with shock and required emergency intervention to staunch the bleeding. The patient eventually recovered. Liu et al. reported on five cases of tumor rupture caused by intraabdominal bleeding, and two patients died [31]. The patients in this study underwent strict selection and evaluation, and the operation was performed by senior surgeons. These measures ensured the safety of the operation. Furthermore, the incidence of HE was 13.2%. This result is similar to previous research, which indicated that TIPS for HCC patients was able to obtain an acceptable HE [[26], [27], [28], [29], [30]]. Finally, the primary patency rates at 30 and 90 days were 95.6% and 89.7%, respectively. Thrombosis occurred in 3 patients within 30 days after TIPS, and only 7 patients experienced shunt dysfunction that was due to the invasion of a tumor. The shunt dysfunction in this study was lower than that reported by Bettinger et al. [26] Their study included more cases of tumor thrombus and the use of bare stents, which increases the risk of tumors invading the shunt. In this study, a new type of covered stent was used to further reduce the occurrence of shunt dysfunction.
The proportion of complete response of RA was higher than reported by Liu et al. [31]. This may be related to the selection of cases. All of their cases were BCLC classification C/D, and our study included 20 (29.4%) patients in BCLC classification A/B, the earlier stage of patients may have better recovery capabilities.
This study had some limitations. First, the median follow-up time of patients with the BCLC classification of A/B was only 12.5 months, and 50% of the patients were still alive, which seriously reduced the OS of these patients. In addition, the sample size of this study was small, and the number of patients in the different BCLC classifications was too small. Finally, this study was also limited because it is a retrospective study and lack of control group.
Conclusion
As a minimally invasive method, TIPS can effectively control ascites of HCC patients with RA and improve the Child–Pugh scores and stages. TIPS combined with subsequent anti-tumor therapy is a feasible and effective management for HCC patients with RA. This study provided a new alternative treatment strategy for HCC patients with RA.
Guarantor of the article
Fei Gao; Fujun Zhang.
Role of the funding source
This work was supported by Science and Technology Planning Project of Guangdong Province (fund No: 2017B020210004), National Natural Science Foundation (fund No: 81871467) and Sanming Project of Medicine in Shenzhen (fund No:SZSM201612053).
Role of the Funder: The funding had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Ethics approval statement and patient consent statement
The ethics committee of the Sun Yat-sen University Cancer Center approved this study and waived the requirement for patient consent for this retrospective analysis.
CRediT authorship contribution statement
Huzheng Yan: Methodology, Software, Validation, Investigation, Writing - Original Draft; Guobao Wang: Validation, Formal analysis, Writing - Original Draft; Wenliang Zhu: Formal analysis, Data Curation; Kai Feng: Formal analysis, Data Curation; Wenke Zhu: Formal analysis, Data Curation; Xuan Wu: Formal analysis, Data Curation; Zhenkang Qiu: Software, Formal analysis, Data Curation; Guanyu Chen: Software, Investigation; Weiwei Jiang: Formal analysis, Data Curation; Fujun Zhang: Conceptualization, Methodolog, Resources, Writing - Review & Editing, Project administration, Funding acquisition; Fei Gao: Conceptualization, Methodology, Resources, Writing - Review & Editing, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the patients enrolled in this study.
Contributor Information
Huzheng Yan, Email: yanhzh@sysucc.org.cn.
Guobao Wang, Email: wanggb@sysucc.org.cn.
Wenliang Zhu, Email: zhuwl1@sysucc.org.cn.
Zhenkang Qiu, Email: qiuzk@sysucc.org.cn.
Guanyu Chen, Email: chengy@sysucc.org.cn.
Weiwei Jiang, Email: jiangww@sysucc.org.cn.
Fujun Zhang, Email: zhangfj@sysucc.org.cn.
Fei Gao, Email: gaof@sysucc.org.cn.
References
- 1.Bray Freddie, Ferlay Jacques, Soerjomataram Isabelle. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;70:313. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ripoll C., Banares R., Rincon D., Catalina M.V., Lo Iacono O., Salcedo M. Influence of hepatic venous pressure gradient on the prediction of survival of patients with cirrhosis in the MELD era. Hepatology. 2005;42:793–801. doi: 10.1002/hep.20871. [DOI] [PubMed] [Google Scholar]
- 3.Boleslawski E., Petrovai G., Truant S., Dharancy S., Duhamel A., Salleron J. Hepatic venous pressure gradient in the assessment of portal hypertension before liver resection in patients with cirrhosis. Br. J. Surg. 2012;99:855–863. doi: 10.1002/bjs.8753. [DOI] [PubMed] [Google Scholar]
- 4.Potts J., Goubet S., Heneghan M., Verma S. Determinants of longterm outcome in severe alcoholic hepatitis. Aliment. Pharmacol. Ther. 2013;38:584–595. doi: 10.1111/apt.12427. [DOI] [PubMed] [Google Scholar]
- 5.D'Amico G., Garcia-Tsao G., Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J. Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Angeli P., Bernardi M., Villanueva Càndid. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. S0168827818319664. [DOI] [PubMed] [Google Scholar]
- 7.Salerno F., Borroni G., Moser P., Badalamenti S., Cassara L., Maggi A. Survival and prognostic factors of cirrhotic patients with ascites: a study of 134 outpatients. Am. J. Gastroenterol. 1993;88:514–519. [PubMed] [Google Scholar]
- 8.EASL EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J. Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Llovet J.M., Di Bisceglie A.M., Bruix J., Kramer B.S., Lencioni R., Zhu A.X. Design and endpoints of clinical trials in hepatocellular carcinoma. J. Natl. Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 10.Day R., Hollywood C., Durrant D., Perkins P. Patient experience of non-malignant ascites and its treatment: a qualitative study. Int. J. Palliat. Nurs. 2015;21:372–379. doi: 10.12968/ijpn.2015.21.8.372. [DOI] [PubMed] [Google Scholar]
- 11.Ripoll C., Groszmann R., Garcia-Tsao G., Grace N., Burroughs A., Planas R. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Christophe Bureau, Dominique Thabut, Frédéric Oberti. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157–163. doi: 10.1053/j.gastro.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Yong Lv, Yang Zhiping, Lei Liu. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4:587–598. doi: 10.1016/S2468-1253(19)30090-1. [DOI] [PubMed] [Google Scholar]
- 14.Yong Lv, Luo Zuo, Zhu Xuan. Identifying optimal candidates for early TIPS among patients with cirrhosis and acute variceal bleeding: a multicentre observational study. Gut. 2019;68:1297–1310. doi: 10.1136/gutjnl-2018-317057. [DOI] [PubMed] [Google Scholar]
- 15.Salerno F., Merli M., Riggio O., Cazzaniga M., Valeriano V., Pozzi M. Randomized controlled study of TIPS vs. paracentesis plus albumin in cirrhosis with severe ascites. Hepatology. 2004;40:629–635. doi: 10.1002/hep.20364. [DOI] [PubMed] [Google Scholar]
- 16.Adriano De Santis, Claudia Iegri, Loreta Kondili. Hepatocellular carcinoma in cirrhotic patients with transjugular intrahepatic portosystemic shunt: a retrospective case-control study. Dig Liver Dis. 2014;46:726–730. doi: 10.1016/j.dld.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 17.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Han G., Qi X., He C. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with symptomatic portal hypertension in liver cirrhosis. J. Hepatol. 2011;54:78–88. doi: 10.1016/j.jhep.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 19.Thalheimer U., Leandro G., Samonakis D.N. TIPS for refractory ascites: a single-centre experience. Gastroenterol. 2009;44:1089–1095. doi: 10.1007/s00535-009-0099-6. [DOI] [PubMed] [Google Scholar]
- 20.Danielle Adebayo, Fong Neong Shuet, Florence Wong. Refractory ascites in liver cirrhosis. Am. J. Gastroenterol. 2019;114:40–47. doi: 10.1038/s41395-018-0185-6. [DOI] [PubMed] [Google Scholar]
- 21.Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: A systematic review and meta-analysisHepatology. 2016;63:349. doi: 10.1002/hep.28351. [DOI] [PubMed] [Google Scholar]
- 22.Bruix J., Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bin Qiu, Kai Li, Dong Xiaoqun. Transjugular intrahepatic portosystemic shunt for portal hypertension in hepatocellular carcinoma with portal vein tumor thrombus. Cardiovasc. Intervent. Radiol. 2017;40:1372–1382. doi: 10.1007/s00270-017-1655-8. [DOI] [PubMed] [Google Scholar]
- 24.Lipika Goyal, Zheng Hui, Abrams Thomas A. A phase II and biomarker study of sorafenib combined with modified FOLFOX in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2019;25:80–89. doi: 10.1158/1078-0432.CCR-18-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verslype C., Rosmorduc O., Rougier P. Hepatocellular carcinoma: ESMO-ESDO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012;23:41–48. doi: 10.1093/annonc/mds225. null: vii41-8. [DOI] [PubMed] [Google Scholar]
- 26.Bettinger D., Knüppel E., Euringer W. Efficacy and safety of transjugular intrahepatic portosystemic shunt (TIPSS) in 40 patients with hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2015;41:126–136. doi: 10.1111/apt.12994. [DOI] [PubMed] [Google Scholar]
- 27.Hur J., Lee K.H., Lee J.H., Yu J.S., Won J.Y., Lee D.Y. Stent-graft for TIPS in a hepatocellular carcinoma patient with main portal vein invasion. AJR Am. J. Roentgenol. 2004;182:1301–1304. doi: 10.2214/ajr.182.5.1821301. [DOI] [PubMed] [Google Scholar]
- 28.Luo Shi-Hua, Jian-Guo Chu, He Huang. Safety and efficacy of transjugular intrahepatic portosystemic shunt combined with palliative treatment in patients with hepatocellular carcinoma. World J Clin Cases. 2019;7:1599–1610. doi: 10.12998/wjcc.v7.i13.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilbao J.I., Elorz M., Vivas I., Martinez-Cuesta A., Bastarrika G., Benito A. Transjugular intrahepatic portosystemic shunt (TIPS) in the treatment of venous symptomatic chronic portal thrombosis in non-cirrhotic patients. Cardiovasc. Intervent. Radiol. 2004;27:474–480. doi: 10.1007/s00270-004-0241-z. [DOI] [PubMed] [Google Scholar]
- 30.Bin Chen, Long Pang, Hao-Bin Chen. TIPS is not associated with a higher risk of developing hcc in cirrhotic patients: a systematic review and meta-analysis. J Clin Transl Hepatol. 2019;7:232–237. doi: 10.14218/JCTH.2019.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lei Liu, Yan Zhao, Xingshun Qi. Transjugular intrahepatic portosystemic shunt for symptomatic portal hypertension in hepatocellular carcinoma with portal vein tumor thrombosis. Hepatol. Res. 2014;44:621–630. doi: 10.1111/hepr.12162. [DOI] [PubMed] [Google Scholar]