Summary
Autoimmune hepatitis is associated with varied clinical presentations and natural history, as well as somewhat unpredictable treatment responses. Understanding how to stratify patients who require further escalation of therapy will help clinicians manage these patients. The presentation of acute severe autoimmune hepatitis (AS-AIH) is relatively uncommon, although its prevalence is potentially greater than currently perceived. Previous studies consist of small retrospective single-centre series and are not directly comparable due to the diversity of presentations, disease definitions and non-standardised treatment regimens. We define AS-AIH as those who present acutely with AIH and are icteric with an international normalised ratio ≥1.5 and no evidence of hepatic encephalopathy. Those with hepatic encephalopathy should be defined as having AS-AIH with acute liver failure. In this review, we provide a structured practical approach for diagnosing and managing this unique group of patients.
Keywords: Autoimmune hepatitis, Acute severe presentation, Acute liver failure, Corticosteroids, Liver transplantation
Abbreviations: ACLF, acute-on-chronic liver failure; AIH, autoimmune hepatitis; ALF, acute liver failure; ALI, acute liver injury; ALT, alanine aminotransferase; ANA, anti-nuclear antibody; anti-LC-1, anti-liver cytosol-1; anti-LKM, anti-liver kidney microsomal; AS-AIH, acute severe autoimmune hepatitis; anti-SLA/LP, anti-soluble liver antigen/liver pancreas; ASMA, anti-smooth muscle antibody; AST, aspartate aminotransferase; AUROC, analysis of area under the receiver operator characteristic curve; CT, computed tomography; DILI, drug-induced liver injury; EBV, Epstein-Barr virus; HE, hepatic encephalopathy; HLA, human leukocyte antigen; IAIHG, International Autoimmune Hepatitis Group; INR, international normalised ratio; LT, liver transplantation; MELD, model for end-stage liver disease; MELD-Na, model for end-stage liver disease-sodium; MHN, massive hepatic necrosis; NAC, N-acetylcysteine; PT, prothrombin time; UKELD, United Kingdom end-stage liver disease; USALF, United States Acute Liver Failure
Key points.
-
•
In the absence of pre-existing liver disease, AS-AIH is defined as an acute presentation of AIH characterised by jaundice and coagulopathy (INR ≥1.5) with no evidence of encephalopathy
-
•
A proportion of these patients develop ALF with HE ± multi-organ failure
-
•
Hypergammaglobulinaemia and autoantibody positivity are useful in the diagnosis of AS-AIH but may not be present early in the disease course
-
•
The typical histological features of AIH are not always present in AS-AIH, however, more prominent features may include centri-lobular haemorrhagic necrosis, massive hepatic necrosis and central perivenulitis
-
•
Based on these atypical findings, a more specific scoring system is required to aid the diagnosis of AS-AIH and autoimmune-ALF
-
•
Corticosteroids in AS-AIH are the mainstay of treatment and are highly effective
-
•
Carefully selected individuals with low grade HE may also benefit from corticosteroid therapy
-
•
Better prognostic markers are required to allow stratification of patients based on corticosteroid response
-
•
Assessment of corticosteroid response with change in bilirubin, INR and MELD-Na/UKELD scores should occur at 7 days, and potentially earlier if required
-
•
Patients with features of advanced liver failure need to be considered for urgent liver transplantation, obviating the need for a trial of corticosteroids
Background
Autoimmune hepatitis (AIH) is a rare, immune-mediated, inflammatory condition of the liver that is characterised by circulating autoantibodies, hypergammaglobulinaemia and distinctive features on liver biopsy.1 It typically responds to immunosuppression. However, if the disease is left untreated, cirrhosis, liver failure, transplantation and death can ensue.2
Several factors contribute to diagnostic uncertainty in AIH: its wide spectrum of presentations, absence of pathognomonic features and variability in treatment response. Recognising these issues and having an open mind when approaching a patient with acute hepatitis, cirrhosis or acute liver failure (ALF) will ensure this important treatable condition is not overlooked. As mentioned, the clinical presentation of AIH is heterogenous and can vary from asymptomatic disease to liver failure requiring liver transplantation (LT) (Fig. 1).
Fig. 1.
The spectrum of presentations in AIH.
AIH, autoimmune hepatitis.
Definitions
The most widely accepted definition of ALF includes evidence of coagulopathy and any degree of hepatic encephalopathy (HE) within 26 weeks of the onset of illness in a patient without pre-existing cirrhosis.3 Over time, several definitions of ALF have developed.[4], [5], [6], [7] ALF can be further sub-categorised into length of illness, e.g. “hyperacute” (<7 days), “acute” (7–21/28 days) and “subacute” (>21 days and <24/26 weeks).3,8 Exact time frames vary between guidelines.
In those with an acute presentation (≤26 weeks) of AIH and no evidence of pre-existing liver disease, we have previously proposed the following categorisation3,9:
-
•
Acute-icteric AIH: icteric with no evidence of coagulopathy or encephalopathy
-
•
AS-AIH: icteric and coagulopathic (international normalised ratio [INR] ≥1.5), but no evidence of encephalopathy
-
•
AS-AIH with ALF: icteric, coagulopathic (INR ≥1.5) and encephalopathic
In the context of AS-AIH without ALF, the time frame of <26 weeks indicates the time between the development of jaundice and coagulopathy. Studies with well-characterised cohorts of patients with AS-AIH ± ALF have previously excluded pre-existing liver disease based on the absence of histological lesions of severe fibrosis or cirrhosis.[10], [11], [12], [13] Therefore, confirmation of AS-AIH may only be possible after biopsy or explant review.
Stratification of patients into the above categories may influence management and prognostication. We estimate that 50–60% of AS-AIH cases progress to ALF, although the true figure is unknown due to the lack of prospective data with well-characterised cohorts.10,11
According to guidelines, patients with acute presentations of AIH, Wilson's disease, HBV and Budd-Chiari syndrome can be included in the definition of ALF, despite the possibility of underlying chronic liver disease.3,8 This is somewhat controversial and better understanding of pathological processes, clinical course, and outcomes are required if these conditions are to be included in the definition of AS-AIH.
Acute-on-chronic liver failure and AIH
Acute-on-chronic liver failure (ACLF) is a clinical syndrome of acute hepatic decompensation with superimposed acute injury observed in patients with pre-existing cirrhosis characterised by the presence of extrahepatic organ failure(s).14,15 Precipitating factors include heavy alcohol intake, viral hepatitis, drugs, AIH, ischaemic hepatitis, infection/sepsis and haemorrhage.14,15 Removal or treatment of the precipitating factor is an important aspect of management, alongside appropriate organ support. ACLF is associated with high mortality, costly intensive care admissions and the requirement for LT.16,17
Clinically, autoimmune-ALF can be difficult to distinguish from ACLF.18 However, the histological features are distinct.18,19 Lymphoid aggregates and perivenulitis are less common in AIH-ACLF, whilst advanced fibrosis (F3/F4), ductular reactions, and large areas of parenchymal collapse with lymphoplasmacytic inflammation are more common.18,19 An Asian-Pacific study of 82 patients with AIH-ACLF demonstrated a survival benefit in those that received corticosteroids.18 However, patients with model for end-stage liver disease (MELD) scores >27 and HE (≥F3) demonstrated poor corticosteroid response, potentially allowing for early stratification of patients.18
Epidemiology
The epidemiology of acute AIH is not well-described. Interestingly, Alaskans have a high prevalence of disease (43 per 100,000) with a significant proportion (35%) presenting acutely with jaundice.20 Black patients with AIH are more likely to present with liver failure (70% in 1 study).21 These findings were corroborated in a multicentre European study which showed that black patients with AIH are more likely to present at a younger age and have a greater risk of LT and liver-related mortality, despite similar corticosteroid response rates to the non-black group.22
Some studies have found no difference between the prevalence of human leukocyte antigen (HLA) DR3 and HLA DR4 in acute AIH and chronic AIH groups.13,23 The absence of HLA B8 and the presence of HLA DR7 may be risk factors for AIH-induced ALF (vs. chronic hepatitis).24 Furthermore, polymorphisms in HLA DRB1∗03 may predict corticosteroid failure.25
Aetiology
The exact mechanisms behind the development of AIH are not fully understood. Simplistically, in a genetically predisposed individual who has been exposed to an environmental trigger, a complex immunological process based on T lymphocyte-mediated cell destruction, imbalance in the regulation of immune cells, loss of tolerance and a defective immune response can instigate damage to the liver.
Certain viruses (hepatitis A, B, C and measles) have been implicated in the pathogenesis of AIH and are believed to trigger ongoing immune-mediated hepatic inflammation, even after resolution of infection.[26], [27], [28], [29], [30], [31], [32], [33], [34] In a single-centre study (n = 52) investigating the potential triggers of AIH-ALF, a trigger was found in 50% of cases.35 These were predominantly drug-related (58%), however, a viral cause was found in 31% of cases. These included Epstein-Barr virus (EBV), cytomegalovirus and HEV.35
Drug-induced liver injury
Drug-induced liver injury (DILI) can be associated with elevated liver enzymes, hypergammaglobulinaemia, autoantibody positivity and histological features of AIH. Drugs associated with autoimmune DILI are listed in Box 1.
Box 1.
Potential drugs associated with autoimmune DILI.

|
∗Severity of damage with these drugs suggests a more severe pathological process and phenotype of disease. DILI, drug-induced liver injury.
Although not categorised in this manner in other guidelines,36,37 the EASL guidelines on AIH describe 3 potential relationships between AIH and DILI1,38,39:
-
•
AIH with superimposed DILI: pre-existent chronic AIH (±advanced fibrosis) with additional DILI insult.
-
•
DILI-induced AIH: drug intake triggers an immune process which leads to the development of chronic AIH; patients may present with serological and/or histological markers of idiopathic AIH; long-term immunosuppression is usually required.
-
•
Immune-mediated DILI: drug intake triggers AIH (limited time course); patients may present with serological and/or histological markers of idiopathic AIH; spontaneous remission after drug cessation and steroid withdrawal.
There is no international consensus on this categorisation. However, understanding these concepts is helpful, e.g. immunosuppression can be successfully withdrawn in patients with immune-mediated DILI. This may not be possible in cases of DILI-induced AIH, which can be difficult to distinguish clinically, serologically and histologically from idiopathic AIH. Despite the overlapping features between AIH and DILI, a liver biopsy can be useful. Portal inflammation, absence of fibrosis, portal neutrophils/plasma cells and intracellular cholestasis are more suggestive of DILI than idiopathic AIH.40 In specific situations, genetic testing may also be diagnostically useful as the carriage of certain HLA alleles makes one diagnosis more likely than another.37
Immune checkpoint inhibitors
These agents restore/enhance T-cell responses and re-establish anti-tumour mechanisms. Unwanted side effects include immune-related hepatotoxicity (ranging from asymptomatic transaminitis to ALF) which can develop soon after treatment initiation, later in the treatment course or occasionally after drug cessation.41,42 In particular, cytotoxic T lymphocyte associated protein-4 (CTLA-4) inhibitors (e.g. ipilimumab) are more hepatotoxic than programmed cell death ligand 1 (PD-L1) agents (e.g. nivolumab).41
Checkpoint inhibitor-induced hepatitis is typically seronegative. From a histological perspective, non-specific features of portal and peri-portal inflammation and hepatocellular necrosis with infiltrating lymphocytes, plasma cells and eosinophils can be observed, similar to acute viral hepatitis and AIH.43,44 Anti-CTLA-4 drugs cause a granulomatous hepatitis with severe lobular necrosis and inflammatory activity, fibrin deposits and central vein endothelitis. Anti-PD-1/PD-L1 agents lead to a more heterogeneous phenotype characterised by active hepatitis with sporadic/confluent necrosis and mild/moderate peri-portal activity with no granulomatous inflammation.45
If discontinuation of the causative agent does not induce spontaneous resolution, corticosteroids are usually effective.[43], [44], [45], [46], [47], [48], [49], [50] Some cases are poorly responsive to corticosteroids and require additional immunosuppression.
Making the diagnosis
The absence of pathognomonic features makes the diagnosis of AS-AIH challenging and one of exclusion. Furthermore, the potential complications of corticosteroid therapy necessitate thorough exclusion of alternative causes of acute severe hepatitis, including viral hepatitis, toxins, DILI, ischaemia and metabolic disorders. A full comprehensive history and blood work enables the exclusion of most of these conditions. Most studies on AS-AIH (±ALF) have not specified the exact method of excluding these aetiologies.10,11,13,23,24,51,52 International guidelines suggest the following virological screening in cases of ALF3,8:
-
•
Anti-HAV IgM
-
•
Hepatitis B surface antigen and anti-HBV core IgM (HBV DNA/delta if positive)
-
•
Anti-HCV, HCV RNA
-
•
Anti-HEV IgM
-
•
Anti-herpes simplex virus IgM, anti-varicella zoster virus IgM, EBV, cytomegalovirus and parvovirus
Additionally, EASL recommends a combination of serology and nucleic acid amplification techniques to diagnose HEV infection.53 Cross-reactivity between HEV and liver autoantibodies raises the additional diagnostic dilemma of falsely positive HEV results.53,54
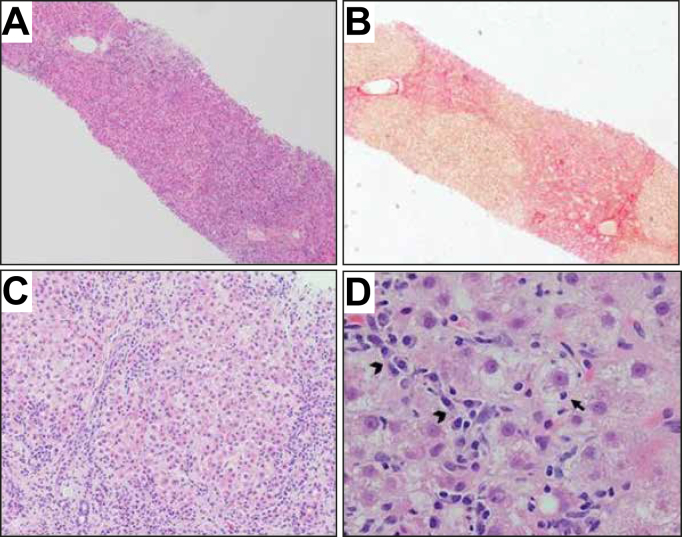
There is increasing recognition that AIH is an important cause of ALF of unknown origin (potentially up to 62% of cases).7,[55], [56], [57], [58], [59], [60] Biopsy provides the opportunity to look for features of AIH, as well as to exclude other aetiologies (Fig. 4).
Fig. 4.
Autoimmune-like acute hepatitis in a patient on nitrofurantoin.
(A) Severe liver damage with areas of recent bridging necrosis and post-necrotic collapse (H&E, 40×). (B) The hepatic parenchyma shows nodular configuration secondary to post-necrosis collapse, with no obvious established fibrosis or very early collagen deposition (Picrosirius red, 40×). (C) Lobular disarray with multiple necro-inflammatory foci and reactive changes of the liver cell plates with regenerative rosettes (H&E, 200×). (D) Detail of an inflammatory area with clusters of plasma cells (arrowheads), hepatocyte reactive changes with cell ballooning and focal emperipolesis (arrow) (H&E, 400×).
Another important aspect of diagnosis is clinical follow-up, which enables an assessment of developing chronicity. In previous studies on AS-AIH, the follow-up period and re-assessment of diagnosis of those not progressing to ALF is not always specified.
Clinical presentation
The lack of previous studies in well-defined cohorts of patients with AS-AIH makes direct comparison between studies difficult. Anorexia, jaundice, ascites and HE are more likely to be present in patients with acute severe forms of AIH (regardless of underlying cirrhosis/fibrosis status).61,62 Bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-globulins, INR and MELD scores have also been shown to be greater in acute and severe presentations of AIH, but not specifically AS-AIH (±ALF).61,62
Yeoman et al. studied a cohort of 32 patients with AS-AIH, defined as an acute presentation of AIH and an INR ≥1.5 at any time without histological evidence of cirrhosis. Compared to 15 patients with an acute exacerbation of chronic AIH, the median bilirubin was greater in the AS-AIH group compared to the acute exacerbation group (463 vs. 269 μmol/L respectively, p = 0.015). Additionally, globulin levels were significantly lower in the AS-AIH group compared to the acute exacerbation group (39 vs. 47 g/dl, respectively, p = 0.02). MELD and United Kingdom end-stage liver disease (UKELD, a modified version of the MELD-Na model) scores were higher in the AS-AIH group compared to the acute exacerbation group (29 vs. 23 [p = 0.005] and 62 vs. 58 [p = 0.006], respectively).10
Serology
Autoantibodies are diagnostically useful in the acute presentations of AIH:
-
-
Type 1 AIH: Anti-nuclear antibody (ANA); anti-smooth muscle antibody (ASMA); anti-soluble liver antigen/liver pancreas antibodies (anti-SLA/LP)
-
-
Type 2 AIH: Anti-liver kidney microsomal-1 and 3 antibodies (anti-LKM 1 and 3); anti-liver cytosol-1 antibody (anti-LC-1)
Autoantibodies are believed to develop during the course of disease and may potentially disappear during treatment, although they are not routinely used to assess treatment response.63,64 Seronegativity in the early stages of an acute presentation of AIH is common (≈10–40%).10,24,51,65 ANA is undetected or present in low titres in 30–40% of patients with severe or fulminant forms of AIH.65,66 Additionally, gamma-globulins may be normal in 25–47% of cases.10,65,66
The absence of autoantibodies does not preclude a diagnosis of AS-AIH. This is relevant when using the International Autoimmune Hepatitis Group (IAIHG) diagnostic scoring systems. Yeoman et al. reported that 88% of their AS-AIH cohort tested positive for ANA, ASMA and/or LKM (including low titres). However, once the IAIHG recommendations of titre strengths were applied, only 66% had ‘positive’ autoantibodies.10 If clinical suspicion is high, autoantibody results should not delay biopsy (or corticosteroid initiation). In cases of seronegativity, it is worth requesting an extended autoantibody screen (including anti-SLA/LP) as the conventional panel usually includes ANA, ASMA and anti-LKM. We also recommend repeating serology several months after disease onset.
Autoantibody testing can yield false positive results. For example, 20–30% of patients with non-alcoholic fatty liver disease or non-alcoholic steatohepatitis can have positive ANA antibodies.[67], [68], [69] ANA, ASMA and LKM antibodies can also be positive in patients with HCV infection.70 Autoantibody results should therefore be interpreted with caution.
Imaging
Ultrasound is useful in the first instance, especially in ruling out vascular conditions. We also advocate the use of computed tomography (CT), although there is little evidence to support this recommendation. However, the identification of a ‘collapsing’ liver may indicate a subacute/acute pathology. CT can also reveal heterogenous hypo-attenuated regions within the liver in 65% of patients with autoimmune-ALF.71 Liver volume is significantly reduced in non-acetaminophen-induced acute liver injury (ALI)/ALF, when compared to acetaminophen-induced ALI/ALF.72 Not only is CT valuable for volumetric analysis, it also allows for the assessment of features of chronicity (e.g. portal hypertension), as well as potential radiological contraindications to LT such as inadequate vasculature and malignancy. Further studies are required to elucidate its true value.
Histology
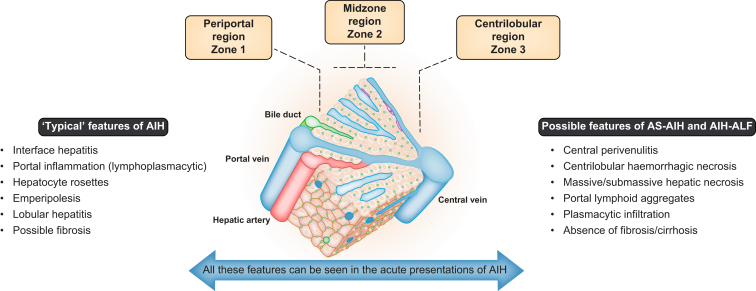
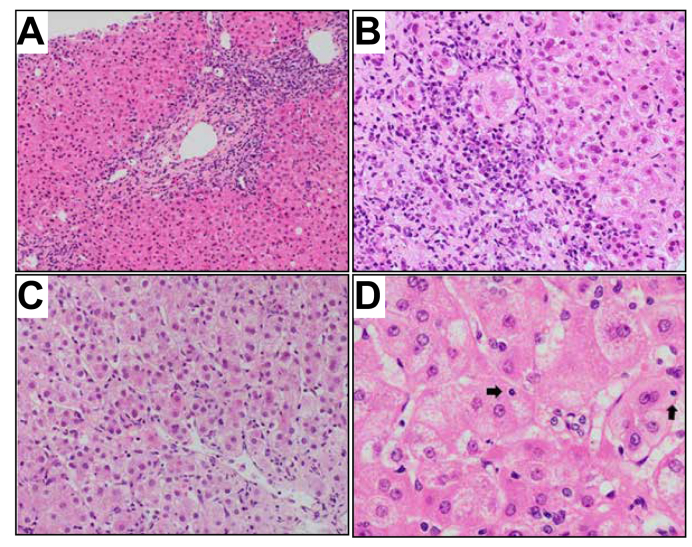
The typical histological features of AIH (Fig. 2 and 3) may not be present early in the disease course or during an acute de novo presentation.73,74 Despite this, a liver biopsy is crucial in making a diagnosis of AS-AIH (±ALF) and should be sought at the earliest opportunity.40 Appropriate histological interpretation requires useful background clinical information such as the time frame from onset of symptoms and potential viral/drug exposures.
Fig. 2.
Schematic diagram of a hepatic lobule and the potential histological changes in acute presentations of AIH.
AIH, autoimmune hepatitis; AIH-ALF, autoimmune hepatitis-acute liver failure; AS-AIH, acute severe autoimmune hepatitis.
Fig. 3.
Typical histological features of autoimmune hepatitis.
(A) Portal inflammation with interface activity (H&E, 100×). (B) Detail of the interface between portal tract and hepatic parenchyma, with moderate activity associated with conspicuous plasma cell component (H&E, 200×). (C) Lobular hepatitis with disarray of the liver cell plates. Diffuse necro-inflammatory activity and regenerative hepatocellular resetting (H&E, 200×). (D) Occasional emperipolesis activity. Intracytoplasmic lymphocytes are spotted in some hepatocytes (arrows) (H&E, 400×).
Histologically, acute forms of AIH predominantly affect the centrilobular zones.7 Centrilobular necrosis in the acute presentations of AIH may reflect the stage preceding portal involvement.65,[75], [76], [77] It is also a manifestation of disease severity.62,78 However, it is not specific to an acute presentation and can be found in chronic AIH or acute-on-chronic disease.62,[78], [79], [80] Therefore, centrilobular necrosis may only be a useful feature in diagnosing new acute-onset disease if it is present in the absence of portal involvement.81,82
In a Japanese study looking at the histological features of patients with severe fulminant forms of AIH, 19/22 (86%) cases showed features of an acute hepatitis with centrizonal, sub-massive and massive necrosis.66 Massive hepatic necrosis (MHN) is common (42–45%) in patients with AS-AIH and AIH-ALF, however, it can also be present in virally induced ALF and other aetiologies.7,66 Another important feature in acute AIH is central perivenulitis, which is characterised by lymphoplasmacytic infiltration surrounding the central vein. This can be found in association with centrilobular necrosis, lymphoid aggregates and plasma cell-enriched infiltration.7,66,78 As a result, the United States Acute Liver Failure (USALF) Study Group proposed the following criteria for autoimmune-ALF7:
-
•
Central perivenulitis
-
•
Lymphoid aggregates
-
•
Plasma cell enrichment
-
•
Type 4∗ or 5∗∗ MHN
(∗Type 4 MHN = pan-lobular necrosis, prominent centrilobular necro-inflammation and haemorrhage; ∗∗Type 5 MHN = classical peri-portal AIH in conjunction with superimposed changes of massive necrosis ± centrilobular necro-inflammation)
Yeoman et al. assessed these criteria in their cohort of patients with AS-AIH and found that 15/22 (68%) cases had central venulitis, 16/22 (72%) had lymphoid aggregates and 17/22 (77%) had plasma cell enrichment. The criteria were less sensitive when assessing the MHN component; a type 4/5 pattern was only observed in 5/22 (23%) of cases.10 However, type 5 MHN was more prevalent in a smaller study and potentially correlated with corticosteroid response.11
There are no pathognomonic histological features to aid the diagnosis of AS-AIH (±ALF). However, the presence of lobular hepatitis (consisting of diffuse necro-inflammation), scavenger macrophages and associated changes of cobblestone/regenerative hepatocytes, with central perivenulitis ± the classical features of AIH (portal inflammation, interface activity and emperipolesis) are highly indicative of the diagnosis.80
Assessment of fibrosis can differentiate between acute and chronic AIH. The challenges associated with this assessment are well recognised by histopathologists, particularly when distinguishing true fibrosis from post-necrotic collapse. Special stains to assess the liver architecture and connective tissue composition are of great importance to avoid overestimation of fibrosis. Recommendations from our institution include using a combination of reticulin (to assess liver structure), a stain for type 1 collagen (e.g. Masson's trichrome or Picrosirius red) and a stain for elastic fibres (e.g. orcein) in addition to assessment of copper-associated protein.[83], [84], [85], [86] Identification of elastic fibres in areas with septal appearance is helpful in establishing old fibrosis, as this is not observed in recent post-necrosis collapse which mimics cirrhosis.87,88
Utility of scoring systems
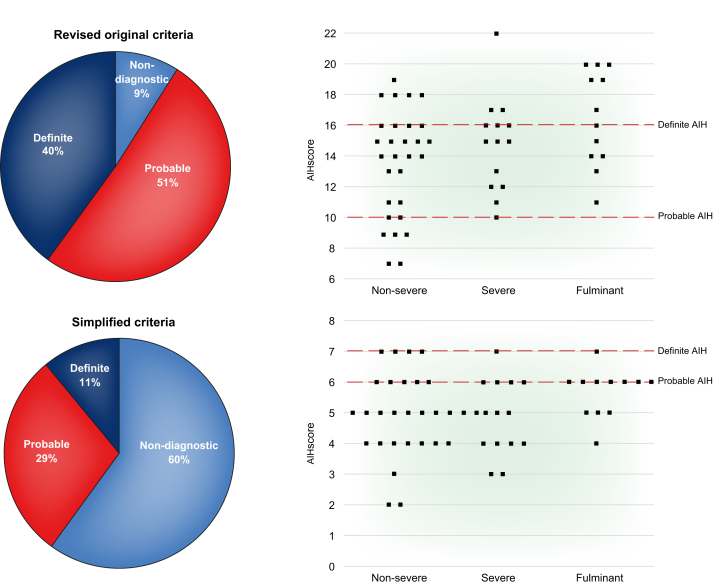
The widely used IAIHG diagnostic criteria have not been validated for use in AS-AIH. Fujiwara et al. showed that the revised original IAIHG criteria may be superior to the simplified version in diagnosing patients with acute-onset AIH, as demonstrated in Fig. 6.89 A subgroup analysis by Yeoman et al. of 70 patients with non-acetaminophen-induced ALF showed that only 24% of patients met the criteria for definite/probable AIH based on the simplified criteria, whilst 40% met the criteria based on the revised criteria.90 These scoring systems may preclude the diagnosis of acute AIH and prevent the initiation of corticosteroids. One could argue that the IAIHG scoring systems should not be applied to any case of AS-AIH, which highlights the unmet need for new diagnostic criteria for AS-AIH.
Fig. 6.
Comparing the diagnostic sensitivity of the IAIHG criteria in acute-onset AIH.
AIH, autoimmune hepatitis; IAIHG, International Autoimmune Hepatitis Group.
Reproduced from Fujiwara et al.89
Balitzer et al. proposed a modified histology-based scoring system including the assessment of inflammatory activity, plasma cell quantification and evaluation of copper and CK7 staining. With this scoring system, 17% of patients were upgraded into a diagnosis of probable/definite AIH, who would have otherwise been classified as non-AIH using the simplified criteria.91
Approach to treatment: Corticosteroids
Corticosteroid responsiveness is a defining/diagnostic feature of AIH. Although older series have reported response rates approaching 80–90% in patients with chronic AIH, newer data suggests that earlier studies overestimated biochemical remission rates, which are more likely to be in the region of 60–80% at 6 months.[92], [93], [94], [95], [96], [97], [98], [99] In the acute severe presentation, corticosteroid response rates are more variable, i.e. 36–100%.10,12,100 Potential reasons for this include diagnostic inaccuracy in the early stages of disease which may delay treatment to the point of irreversibility once hepatic failure and hepatocyte necrosis have occurred.
When reviewing the data on corticosteroid response in milder disease, corticosteroid therapy is clearly favourable. Yeoman et al. reported outcomes of 72 icteric patients with AIH, no HE and a median INR of 1.39. Half the patients had cirrhosis. There were 59 (82%) corticosteroid responders and 13 (18%) non-responders. The interval between presentation to corticosteroid initiation made no difference to response.101
Similarly, Zachou et al. reported on corticosteroid response in 34 patients with newly diagnosed acute hepatitis (onset <24 weeks), INR ≥1.5 and bilirubin ≥4 mg/dl without signs of HE or histological lesions of chronic liver disease.23 All patients received intravenous corticosteroids. Induction regimens included prednisolone (1.5 mg/kg/day) or 3 days of Methylprednisolone (1 g/day) with a subsequent prednisolone dose of 63.2 ± 13.7 mg/day. Overall, 33/34 patients responded to treatment and no patient required LT. Response rates in this study were attributed to lower MELD scores at presentation, earlier initiation of corticosteroids and higher intravenous doses.
Yeoman et al. reported on corticosteroid responsiveness in patients with AS-AIH.10 Twenty-three of 32 patients were treated with corticosteroids, whilst the decision to undergo LT without corticosteroid treatment was made in 9 patients. Of the 23 patients who received corticosteroids, 5 (22%) had grade I/II HE. By our definitions, the latter had AIH-ALF. In the corticosteroid cohort, 10/23 (43%) patients responded to corticosteroid therapy, 2/23 (9%) responded to additional immunosuppression (tacrolimus/mycophenolate mofetil) and 10/23 (43%) underwent LT. Of the 5 patients with low grade HE prior to corticosteroid initiation, HE was reversed in 2 patients with corticosteroids. In replies responding to this study, 2 groups described outcomes in 17 and 14 patients with AS-AIH and corroborated these findings, with corticosteroid response rates of 60–64%.11,102
The largest study to date (reported in abstract form) is a multicentre French study with a well-characterised cohort of 121 patients with AS-AIH. Inclusion criteria consisted of an acute presentation with an INR of ≥1.5 and/or bilirubin ≥200 μmol/L, histological features in keeping with AIH and an IAIHG score of definite/probable, and no previous history of hepatic disease. A total of 110 patients received corticosteroids, whilst 11 did not. HE was present in all 11 non-treated patients and in 8/110 corticosteroid-treated patients. Amongst the corticosteroid-treated patients, 67 (61%) responded, while 43 patients (39%) did not respond and either underwent LT or died. The strongest predictor of treatment failure was the presence of encephalopathy, with only 1/8 patients with HE responding to corticosteroids.103
A few small studies have reported no survival benefit when using corticosteroids in AS-AIH or autoimmune-ALF. However, as summarised in Table 1, these studies included more severe cohorts of AS-AIH with features of ALF, potentially at the point of irreversibility, thus making corticosteroid therapy futile. In patients with fulminant AIH, corticosteroid therapy may only be effective in 8–41% of patients.12,51,104,105
Table 1.
A summary of corticosteroid responses from previous studies on acute severe presentations of AIH – the ‘sicker’ cohorts had poorer response to corticosteroids.
| Study | No. of patients | Definition of AS-AIH or AIH-ALF | Bilirubin of whole cohort | INR/PT of whole cohort | MELD of whole cohort | Corticosteroid therapy n (%) | Corticosteroid dose | HE prior to corticosteroid therapy n (%) | Outcome in corticosteroid cohort n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Zachou et al. 2019 | 34 |
|
Median 173 μmol/L (54–619) |
Median INR 1.52 (1.5-2.27) |
Median 18 (12–24) |
34/34 (100%) |
Induction regimens: Prednisolone 1.5 mg/kg/day (intravenous) or 3 days of Methylprednisolone 1 g/day with subsequent Prednisolone dose of 63.2 ±13.7 mg/day (intravenous) |
0 |
|
| Anastasiou et al. 2018 | 32 |
|
Median 275 μmol/L (188) |
Median INR 1.7 (0.6) |
Median 22 (5) |
32/32 (100%) |
Average Prednisolone dose 153.9 mg/day (60–500 mg) (oral or intravenous) |
?All (100%) |
|
| de Martin et al. 2017 | 121 |
|
Median 252 μmol/L (188-376) |
Median INR 1.9 (1.5-2.8) |
Median 25 (21-28) |
110/121 (91%) |
Corticosteroid dose of 1 mg/kg/day (unspecified route) | 8/110 (7%) |
|
| Moenne-Loccoz et al. 2016 | 17 |
|
Median 429 μmol/L (106–797) |
Median INR 2.3 (1.5-5.5) |
Median 26 (20–51) |
15/17 (88%) |
Majority Prednisolone, 3 treated with Methylprednisolone Initial median dose of 60 mg/day (40-100 mg) (unspecified route) |
?1 or 2/15 (7 or 13%) |
|
| Fujiwara et al. 2016 | 20 |
|
Mean 304 μmol/L (SD ± 160) |
Mean PT 29% (SD ± 13) |
Mean 27 ± 7 |
19/20 (25%) |
Prednisolone 40-60 mg/day (unspecified route) or Methylprednisolone 500-1000 mg/day (unspecified route) |
14/19 (74%) |
Severe hepatitis:
|
| Yeoman et al. 2014 | 32 |
|
Median 463 μmol/L (55-1,208) |
Median INR 2.2 (1.5-3.5) |
Median 29 (22-40) |
23/32 (72%) |
Prednisolone 20-40 mg/day (oral) or Hydrocortisone 100 mg TDS (intravenous) |
5/23 (21%) grade 1-2 |
|
| Mendizibal et al. 2015 | 40 |
|
Median 361 μmol/L ±133 |
Median INR 2.92 ± 1.45 |
Median 29 ± 5 |
17/40 (43%) |
Meprednisone 40-60 mg/day (unspecified route) |
4 /17 (24%) ≥ grade 3 |
|
| Karkhanis et al. 2014 | 66 |
|
Mean 397 μmol/L (SD ± 156) |
Mean INR 3.33 (SD 3.17) |
Mean 30.9 (SD 9.5) |
25/66 (38%) |
Mean prednisone dose 42.5 mg/day (unspecified route) | 25/25 (100%) |
|
| Ichai et al. 2007 | 16 |
|
Median 425 μmol/L (278-850) |
Median INR 5.36 (1.7-12.2) |
Median 37 (24-47) |
12/16 (75%) |
Mean prednisone dose 1.3 mg/kg/day (intravenous) | 8/12 (67%) grade 1: 1 grade 2: 2 grade 3: 3 grade 4: 2 |
|
? not clear in study report.
AIH, autoimmune hepatitis; ALF, acute liver failure; ALI, acute liver injury; AS-AIH, acute severe autoimmune hepatitis; HE, hepatic encephalopathy; IAIHG, International Autoimmune Hepatitis Group; INR, international normalised ratio; LT, liver transplantation; MELD, model for end-stage liver disease; PT, prothrombin time; ULN, upper limit of normal.
Part of the controversy surrounding corticosteroid use in AS-AIH is based on a French report of 16 patients who developed acute hepatitis with features of ALF/HE within 2 weeks (fulminant) or 2–12 weeks (sub-fulminant) of jaundice onset.12 In this cohort, 10/16 patients experienced high grade HE. Notably, the median INR was 5.36 (1.7–12.2). Histologically, 6/16 patients had sub-massive hepatic necrosis and 10/16 patients had MHN on biopsy. Overall, 12/16 received corticosteroids. One patient had mild disease (bilirubin 400 μmol/L, INR 1.7) and responded to corticosteroids within 3 days. One patient had been on dual immunosuppression (corticosteroids/cyclosporin) prior to presentation and subsequently died of disseminated aspergillosis. The remaining 10 patients on corticosteroids underwent LT. In retrospect, corticosteroid failure was predictable based on the severity of disease in this cohort.
A retrospective analysis of patients with autoimmune, indeterminate and drug-induced ALF by the USALF Study Group included 66 patients with AIH, all of whom presented with coagulopathy and HE. Twenty-five patients received prednisone (median dose 60 mg/day) and were compared with 41 untreated patients. Corticosteroids did not improve overall or spontaneous survival in autoimmune-ALF, although this cohort was not well-characterised (e.g. unknown cirrhosis status and IAIHG scores). Corticosteroid use was associated with increased mortality in patients with higher MELD scores.105
Fujiwara et al. confirm the poor response of corticosteroid therapy in AIH-ALF.52 In their study of 20 patients with AIH-ALF, 19 patients received corticosteroids and 8 (40%) recovered without LT, 4 (20%) underwent LT and 8 (40%) died without LT. In those with severe AIH (n = 5) with no HE, all responded to corticosteroid therapy. In those with fulminant AIH (n = 13), only 2 responded to corticosteroids, while 3 underwent LT and 8 died without LT. In 2 patients with late-onset hepatic failure, 1 patient responded to corticosteroids and 1 required LT.
In summary, careful patient selection is important in ensuring corticosteroid success. If a patient has acute-icteric hepatitis or AS-AIH, there is a reasonable probability that they will respond to a trial of corticosteroids. However, assessment of response is required after the initiation of therapy, to evaluate the need for LT. The development of HE and other features of ALF, make response to corticosteroids less likely. However, some studies have reported reversibility of HE with corticosteroid therapy.10,24,52,103
Predictors of corticosteroid failure at presentation
For patients with AS-AIH and HE, it is crucial to identify those that may respond to corticosteroids and those that would benefit from immediate LT. At present, there is no guidance on risk stratification.
Previous studies have shown that bilirubin and INR levels at presentation may predict corticosteroid response.101,103,106 In a cohort of acute-icteric AIH, Yeoman et al. showed that their corticosteroid failure group had higher median bilirubin (451 vs. 262 μmol/L, p = 0.02) and INR (1.62 vs. 1.33, p = 0.005) values at diagnosis.101 Moenne-Loccoz et al. demonstrated in their cohort of AS-AIH that an INR >2.46 (analysis of area under the receiver operator characteristic curve [AUROC] 0.81; 95% CI 0.55–1.00; p = 0.01) before starting corticosteroid therapy was predictive of failure.11 The interval time from symptom onset to treatment initiation was similar between responders and non-responders. Ichai et al. demonstrated poor corticosteroid response in patients with severe fulminant forms of AIH (median INR 5.36).12
Additionally, the presence of low grade HE and absence of MHN on histology may be associated with corticosteroid response.51,103,106,107 If MHN is present on histology, the presence of type 5 MHN may predict corticosteroid response as this is more indicative of autoimmune aetiology.11,100 Saying that, Yeoman et al. demonstrated that the MHN patterns proposed by the USALF group lacked sensitivity in their cohort.10
Other studies have reported that MELD scores ≤27 or UKELD scores ≤57 may correlate with treatment response.11,51,101 This was corroborated by Moenne-Loccoz et al. who found that an MELD score >28.5 (AUROC 0.85; 95% CI 0.65–1.00; p <0.01) before starting corticosteroid therapy was predictive of failure.11 Conversely, Yeoman et al. demonstrated no significant difference in MELD scores at admission between corticosteroid responders and non-responders.10 An older study from the Mayo Clinic of 214 patients with type 1 AIH (including patients with cirrhosis), demonstrated that the MELD score may be useful for identifying patients likely to fail corticosteroid therapy. They also demonstrated that hyperbilirubinemia and presence of HLA DRB1∗03 may predict corticosteroid failure.108
Assessing response once corticosteroids have started
Transaminase activity
Serum transaminases are not useful markers of response once corticosteroids have been initiated.10,11,51,101,107 One study observed a decrease in serum ALT levels in both responder and non-responder groups. In the latter group, they postulated that the lower production of hepatic enzymes was secondary to disease severity and poor hepatocyte regeneration.11
Bilirubin and INR
Serum bilirubin and INR levels are perhaps better markers of response. In a historical, but important, study on early prognostic predictors in patients with severe chronic active AIH, failure to improve pre-treatment hyperbilirubinaemia after 2 weeks of corticosteroid therapy was invariably associated with early mortality.109
In a subgroup analysis (n = 14) looking at the impact of ethnicity on the natural history of AIH in those that presented with liver failure (INR ≥2 ± HE, including patients with cirrhosis), deteriorating bilirubin and INR levels after 3.7 ± 0.6 days of corticosteroid therapy were associated with poor outcome.21,100
In those with acute (±chronic) liver failure, bilirubin and INR may also be useful markers to assess corticosteroid response. In a small series of AIH-ALF patients, Miyake et al. indicate that the key time to assess the most significant difference in bilirubin levels between survivors and non-survivors was between 8–15 days after corticosteroid initiation.107
Although this is a useful parameter in assessing response, in clinical practice, a quicker time frame for assessment would be more valuable in patients who deteriorate rapidly. Yeoman et al. reported that in their acute-icteric patients with AIH, failure to respond at day 7 after the initiation of corticosteroids should be a cut-off point for withdrawal.101 Similarly, yet to be published data from a multicentre French study report that an improvement in bilirubin at day 7 is associated with a higher likelihood of corticosteroid response.103
In a series (published in abstract form) of 31 patients with fulminant AIH, 25 received corticosteroid therapy. A 20% increase in the prothrombin time (PT) on day 3 after the initiation of corticosteroids was deemed to be a poor prognostic marker.106 Fujiwara et al. have also suggested that PT activity is the best marker of improvement during the first fortnight of treatment, whilst other markers (e.g. bilirubin) can be slower to recover.52
Change in prognostic indices
Specific to acute-icteric AIH, Yeoman et al. showed that failure to improve MELD-Na or UKELD scores within 7 days of corticosteroid therapy indicates a high risk of progressing to ALF. In this study, AUROC values at day 7 identified that changes in bilirubin (AUROC 0.68), creatinine (0.69), MELD (0.79), MELD-Na (0.83) and UKELD (0.83) were the best predictors of treatment failure. Specifically, a fall in UKELD of <2 points predicted treatment failure with a sensitivity of 85% and specificity of 68%.101
In the multicentre French study already described, amongst the corticosteroid-treated patients with AS-AIH, 67/110 (61%) responded with a median decline in MELD of 4 points between days 0 and 7 of therapy. A detectable improvement was also demonstrated at day 3.103 This concept of early treatment response is appealing as it would allow for early stratification of patients into responders and non-responders.
To conclude, there is a need for reliable prognostic predictors to facilitate patient selection for LT. If the point of corticosteroid rescue has passed and there is no improvement in bilirubin, INR/PT or MELD-Na after 7 days of corticosteroid therapy, continuing them may be futile and patients should be assessed for LT immediately. For the sickest cohort (e.g. those with HE), assessment at day 3 of corticosteroid therapy requires further evaluation.
Additional therapies
There is no specific data supporting the use of N-acetylcysteine (NAC) in autoimmune-ALF. However, prompt NAC administration has proven to be an effective therapy for non-acetaminophen-induced ALF, improving transplant-free and post-transplant survival, particularly if used in patients with low grade HE.[110], [111], [112], [113], [114] It has also been reported to reduce HE, multi-organ failure and need for intensive care.
Plasma exchange has reportedly been used for acute presentations of AIH. The presence of confounding factors makes it difficult to prove its independent benefit and true role in influencing transplant-free survival.26,71,[115], [116], [117], [118], [119]
In the event of corticosteroid failure, the role of alternative immunosuppressive agents, e.g. tacrolimus, has not been established. Their use has been reported in some studies, but the numbers are small, and the results are inconclusive.10
Infections secondary to corticosteroids in autoimmune-ALF
ALF itself is associated with increased rates of infectious complications.10,12 In addition, corticosteroid exposure may propagate bacterial septicaemia or fungaemia which subsequently complicates the LT process. Fujiwara et al. reported that in 19 patients with AIH-ALF treated with corticosteroids, the cumulative incidence of infections was 6% at 7 days, 17% at 10 days, 35% at 14 days and 54% at 21 days from the induction of corticosteroids. Furthermore, these rates were increased after the onset of grade 2 HE in patients with fulminant hepatitis and late-onset hepatic failure.120 Whilst on corticosteroids, close monitoring (with bacterial/fungal biomarkers) for sepsis is imperative, with the early instigation of antimicrobials if required. Empirical treatment can be considered, but there is no strong evidence supporting this, in addition to concerns regarding antimicrobial stewardship.
Liver transplantation
With rates of spontaneous recovery as low as 7–15%, LT may be the only effective rescue therapy for some patients with AS-AIH and AIH-ALF.7,121 LT should not be delayed by protracted courses of corticosteroids. In practice, simultaneous transplant assessment should run parallel to a trial of corticosteroids. Assessment of corticosteroid response early in the disease course is crucial in deciding the right time to pursue LT, ideally before septic complications and irreversible brain injury occur. The decision to proceed to urgent listing should be made within a multidisciplinary setting with intensivists, transplant physicians and surgeons.
Outcomes for LT in AS-AIH and AIH-ALF are similar to those of other aetiologies of ALF (1-year survival 80–94%).122,123 Although the frequency of post-transplant rejection (acute/late) is increased in patients with AIH compared to those with non-AIH disease, the frequency of rejection is similar between patients with acute or chronic AIH.[124], [125], [126], [127] Indeed, some reports have potentially shown survival benefit in those who present with autoimmune-ALF as opposed to chronic AIH with cirrhosis.127
Recurrent AIH is common post-LT and can occur in 8–12% of transplanted patients at 1 year and 36–68% at 5 years.124,125,[128], [129], [130] Histological examination of the explanted liver (Fig. 5) and correlation with pre-transplant serology provides useful information to the transplant team. Elevated liver enzymes and immunoglobulins before LT, as well as lymphoplasmacytic infiltration with moderate to severe inflammatory activity in explants, may be associated with a greater probability of recurrent AIH post-transplantation.[124], [125], [126] One study showed no significant difference in post-transplant AIH recurrence between acute and chronic liver failure secondary to AIH.124 Ayata et al. showed that the presence of severe necro-inflammatory activity in the native liver at the time of LT correlated with a higher AIH recurrence rate.126 Conversely, Reich et al. reported that AIH was less likely to recur in patients who initially presented with ALF compared to those who had chronic disease.129 True rates of post-transplant AIH recurrence in autoimmune-ALF are unknown.124,[131], [132], [133]
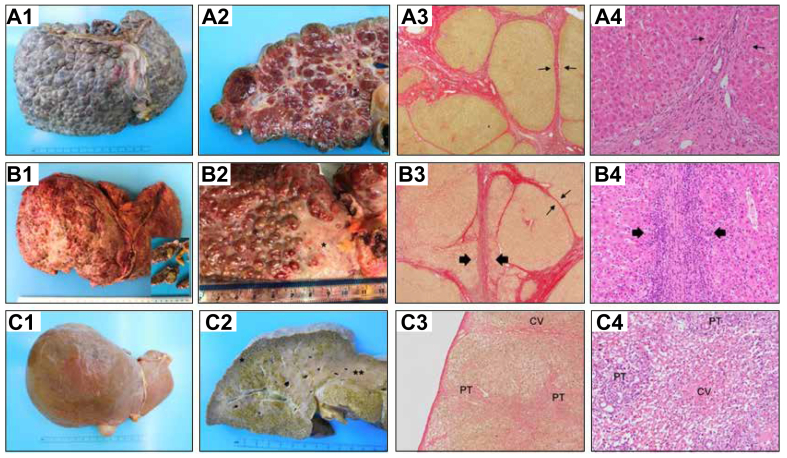
Fig. 5.
Histopathological spectrum of liver damage at the time of transplantation.
(A) Chronic autoimmune hepatitis in stage of established cirrhosis, with no inflammatory activity, in a 22-year-old female, diagnosed with autoimmune hepatitis aged 12. (A1) Macroscopy of the explanted liver, with macronodular cirrhosis predominantly. (A2) Macroscopy of a liver section with macro-regenerative nodules up to 15 mm in greatest dimension. (A3) On microscopy, regenerative nodules show well-demarcated outlines, with predominantly thin fibrous septa (arrows) (Picrosirius red, 20×). (A4) No residual significant inflammation is of note at the porto-septal areas. No interface activity is present (arrows), (H&E, 200×). (B) Chronic autoimmune hepatitis in stage of cirrhosis, with persistent inflammatory activity, in a male patient aged 53, diagnosed with AIH 13 years ago. He developed portal hypertension and suffered episodes of variceal bleeding, ascites and encephalopathy. (B1) Macroscopy of the explanted liver (1,299 grams). Of note is the heterogeneous capsular surface, with whitish areas of capsular depression. An incidental hepatocellular carcinoma (inset) was found at the explant, 18 mm, moderately differentiated. (B2) Detail of the liver capsule surface, with micro-macronodular cirrhosis and a large area of fibrous scarring (∗) secondary to previous multiacinar necrosis. B3, Fibrous septa and regenerative nodules show variable appearance. Some of the regenerative nodules show well-defined outline (thin arrows) while blurred interface is observed in many areas (thick arrows) (Picrosirius red, 20×). (B4) Detail of the blurred interface area in image B3, corresponding with marked interface activity (H&E, 200×). (C) Small explanted liver (857 grams) in the clinical context of subacute liver failure secondary to AIH in a 21-year-old male with no underlying chronic liver disease. (B1) Notice the smooth capsular surface in comparison with the liver explants in figure A1 and B1. (C2) Macroscopic appearance of liver sections, showing map-like areas of residual cholestatic (green) parenchyma∗, alternating with areas of parenchymal extinction where massive hepatocellular loss has occurred∗∗. (C3) Microscopy of the Glisson's capsule (thin and smooth) and subcapsular parenchyma (area ∗ of figure C2). The hepatic parenchyma shows vague nodularity secondary to bridging necrosis but no evidence of fibrosis, indicating the acute/subacute damage (Picrosirius red, 20×). (C4) Microscopy of the area of collapse (area ∗∗ of figure C2), with complete hepatocellular loss in areas of multiacinar necrosis and secondary micro-haemorrhage in the centrilobular area, centred by a terminal hepatic venule (CV). Portal tracts (PT) at the periphery of this area show mild inflammatory component and some degree of ductular reaction (H&E, 200×).
Many advocate the use of long-term corticosteroids post-transplantation to prevent rejection and recurrent disease, however, we know that complete corticosteroid withdrawal is possible after transplantation in chronic AIH.2,130,134 Successful corticosteroid withdrawal in AS-AIH (not requiring LT) has also been reported.23
Paediatric disease
There are no studies evaluating AS-AIH as a specific entity in children/adolescents. However, fulminant AIH has been extensively described.[135], [136], [137], [138], [139], [140], [141] During childhood, the mode of AIH presentation can be variable. The differentials of an acute severe hepatitis most commonly include HAV, HBV and Wilson's disease. However, AIH should be suspected and excluded in all children with this presentation.
Type 2 AIH (anti-LKM positivity) is not only more common in children, it is also associated with acute-onset liver failure. Up to 25% of children with a severe acute presentation of AIH have normal IgG at presentation, however, low-titre autoantibodies may still indicate AIH as the aetiology.142,143 Autoantibody positivity should be interpreted with caution, as autoantibodies can be found in other conditions that lead to ALF in children. One study showed that autoantibodies were detected in most children with HAV (63%), as well as AIH (79%). In the same study, gamma-globulins were significantly higher in the HAV group (1.93 ± 0.57 g/dl), but overall lower when compared to the AIH group (2.93 ± 1.2 g/dl).144 Smolka et al. observed significantly lower levels of albumin, higher levels of bilirubin, INR, IgG and more severe histological findings in their acute AIH group compared to their chronic AIH group, although there was no difference in autoantibody positivity between the groups.145 Histological features can be similar to adult AS-AIH and AIH-ALF.135,145
Corticosteroid therapy can be effective in children with ALF secondary to AIH, although LT is still sometimes required.146 Paediatric end-stage liver disease scores on admission may be useful in predicting outcome.147 In a UK-based study, children with fulminant hepatic failure due to AIH were more likely to survive without LT, compared to those with other aetiologies of hepatic failure.138
Conclusions
AS-AIH is a rare disorder, and its true incidence and prevalence are unknown. We have attempted to define this condition, but it requires better characterisation and understanding as an entity. It can pose a diagnostic challenge due to its non-specific symptoms, limitations in serology and confounding drug/viral triggers. It has been demonstrated that an autoimmune process may account for a proportion of patients with indeterminant or seronegative ALF. The typical scoring systems for AIH are obsolete in AS-AIH and autoimmune-ALF. If there is diagnostic uncertainty, an early liver biopsy can be informative, ideally before severe coagulopathy and high grade HE develop. Assessment of liver volume with CT is also valuable and requires better understanding as a prognostic tool.
Based on these results, a trial of corticosteroids is justified with early evaluation of response. The optimal corticosteroid dose and route of administration has yet to be determined, although we suspect in the appropriately selected individual, oral corticosteroids should suffice. However, standardisation of therapy is required universally. To assess response, we recommend evaluating the change in bilirubin and MELD-Na score at 7 days. Earlier assessment may be required if the patient deteriorates in the interim. The presence or development of high grade HE is an indication to proceed to urgent LT. We also need to understand the value of alternative therapies and whether they have a role in the treatment algorithm of AS-AIH.
Due to the rarity of the disease, international collaboration through registries and merging of existing data will be required to answer these important questions.
Financial support
MAH is supported by charitable donations from the Kelly Group and King's College Hospital Charity (Orpin Bequest).
Authors' contributions
MNR: Conceptualization; Visualization; Writing original draft; Writing review & editing. RM: Writing original draft; Writing review & editing; Reviewing histology. MAH: Conceptualization; Supervision; Writing review & editing.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100149.
Supplementary data
References
- 1.Lohse A.W., Chazouillères O., Dalekos G., Drenth J., Heneghan M., Hofer H. EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol. 2015;63(4):971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Mack C.L., Adams D., Assis D.N., Kerkar N., Manns M.P., Mayo M.J. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases. Hepatology. 2019;72(2):671–722. doi: 10.1002/hep.31065. [DOI] [PubMed] [Google Scholar]
- 3.Lee W.M., Stravitz R.T., Larson A.M. Introduction to the revised American Association for the Study of Liver Diseases position paper on acute liver failure 2011. Hepatology. 2012;55(3):965–967. doi: 10.1002/hep.25551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trey C., Davidson C.S. The management of fulminant hepatic failure. Prog Liver Dis. 1970;3:282–298. [PubMed] [Google Scholar]
- 5.Williams R., Schalm S.W., O'Grady J.G. Acute liver failure: redefining the syndromes. Lancet. 1993;342(8866):273–275. doi: 10.1016/0140-6736(93)91818-7. [DOI] [PubMed] [Google Scholar]
- 6.Tandon B.N., Bernauau J., O'Grady J., Gupta S.D., Krisch R.E., Liaw Y.F. Recommendations of the International Association for the Study of the Liver Subcommittee on nomenclature of acute and subacute liver failure. J Gastroenterol Hepatol. 1999;14(5):403–404. doi: 10.1046/j.1440-1746.1999.01905.x. [DOI] [PubMed] [Google Scholar]
- 7.Stravitz R.T., Lefkowitch J.H., Fontana R.J., Gershwin M.E., Leung P.S.C., Sterling R.K. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology. 2011;53(2):517–526. doi: 10.1002/hep.24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66(5):1047–1081. doi: 10.1016/j.jhep.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Rahim M.N., Liberal R., Miquel R., Heaton N.D., Heneghan M.A. Acute severe autoimmune hepatitis: corticosteroids or liver transplantation? Liver Transpl. 2019;25(6):946–959. doi: 10.1002/lt.25451. [DOI] [PubMed] [Google Scholar]
- 10.Yeoman A.D., Westbrook R.H., Zen Y., Bernal W., Al-Chalabi T., Wendon J.A. Prognosis of acute severe autoimmune hepatitis (AS-AIH): the role of corticosteroids in modifying outcome. J Hepatol. 2014;61(4):876–882. doi: 10.1016/j.jhep.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Moenne-Loccoz R., Severac F., Baumert T.F., Habersetzer F. Usefulness of corticosteroids as first-line therapy in patients with acute severe autoimmune hepatitis. J Hepatol. 2016;65(2):444–446. doi: 10.1016/j.jhep.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Ichai P., Duclos-Vallée J.C., Guettier C., Hamida S.B., Antonini T., Delvart V. Usefulness of corticosteroids for the treatment of severe and fulminant forms of autoimmune hepatitis. Liver Transpl. 2007;13(7):996–1003. doi: 10.1002/lt.21036. [DOI] [PubMed] [Google Scholar]
- 13.Abe M., Onji M., Kawai-Ninomiya K., Michitaka K., Matsuura B., Hiasa Y. Clinicopathologic features of the severe form of acute type 1 autoimmune hepatitis. Clin Gastroenterol Hepatol. 2007;5(2):255–258. doi: 10.1016/j.cgh.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Angeli P., Bernardi M., Villanueva C., Francoz C., Mookerjee R.P., Trebicka J. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Jalan R., Gines P., Olson J.C., Mookerjee R.P., Moreau R., Garcia-Tsao G. Acute-on chronic liver failure. J Hepatol. 2012;57(6):1336–1348. doi: 10.1016/j.jhep.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 16.Wlodzimirow K.A., Eslami S., Abu-Hanna A., Nieuwoudt M., Chamuleau R.A.F.M. A systematic review on prognostic indicators of acute on chronic liver failure and their predictive value for mortality. Liver Int. 2013;33(1):40–52. doi: 10.1111/j.1478-3231.2012.02790.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X., Chen P., Gao H., Hao S., Yang M., Zhao H. Bacterial infection and predictors of mortality in patients with autoimmune liver disease-associated acute-on-chronic liver failure. Can J Gastroenterol Hepatol. 2018;2018 doi: 10.1155/2018/5108781. 5108781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anand L., Choudhury A., Bihari C., Sharma B.C., Kumar M., Maiwall R. Flare of autoimmune hepatitis causing acute on chronic liver failure: diagnosis and response to corticosteroid therapy. Hepatology. 2019;70(2):587–596. doi: 10.1002/hep.30205. [DOI] [PubMed] [Google Scholar]
- 19.Sarin S.K., Choudhury A., Sharma M.K., Maiwall R., Al Mahtab M., Rahman S. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL): an update. Hepatol Int. 2019;13(4):353–390. doi: 10.1007/s12072-019-09946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurlburt K.J., McMahon B.J., Deubner H., Hsu-Trawinski B., Williams J.L., Kowdley K.V. Prevalence of autoimmune liver disease in Alaska natives. Am J Gastroenterol. 2002;97(9):2402–2407. doi: 10.1111/j.1572-0241.2002.06019.x. [DOI] [PubMed] [Google Scholar]
- 21.Verma S., Torbenson M., Thuluvath P.J. The impact of ethnicity on the natural history of autoimmune hepatitis. Hepatology. 2007;46(6):1828–1835. doi: 10.1002/hep.21884. [DOI] [PubMed] [Google Scholar]
- 22.de Boer Y.S., Gerussi A., van den Brand F.F., Wong G.W., Halliday N., Liberal R. Association between black race and presentation and liver-related outcomes of patients with autoimmune hepatitis. Clin Gastroenterol Hepatol. 2019;17(8):1616–1624. doi: 10.1016/j.cgh.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Zachou K., Arvaniti P., Azariadis K., Lygoura V., Gatselis N.K., Lyberopoulou A. Prompt initiation of high-dose i.v. corticosteroids seems to prevent progression to liver failure in patients with original acute severe autoimmune hepatitis. Hepatol Res. 2019;49(1):96–104. doi: 10.1111/hepr.13252. [DOI] [PubMed] [Google Scholar]
- 24.Anastasiou O.E., Dogan-Cavus B., Kucukoglu O., Baba H., Kahraman A., Gerken G. Corticosteroid therapy improves the outcome of autoimmune hepatitis-induced acute liver failure. Digestion. 2018;98(2):104–111. doi: 10.1159/000487940. [DOI] [PubMed] [Google Scholar]
- 25.Czaja A.J., Cookson S., Constantini P.K., Clare M., Underhill J.A., Donaldson P.T. Cytokine polymorphisms associated with clinical features and treatment outcome in type 1 autoimmune hepatitis. Gastroenterology. 1999;117(3):645–652. doi: 10.1016/s0016-5085(99)70458-0. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka H., Tujioka H., Ueda H., Hamagami H., Kida Y., Ichinose M. Autoimmune hepatitis triggered by acute hepatitis A. World J Gastroenterol. 2005;11(38):6069–6071. doi: 10.3748/wjg.v11.i38.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huppertz H.I., Triechel U., Gassel A.M., Jeschke R., Meyer zum Büschenfelde K.H. Autoimmune hepatitis following hepatitis A virus infection. J Hepatol. 1995;23(2):204–208. doi: 10.1016/0168-8278(95)80336-x. [DOI] [PubMed] [Google Scholar]
- 28.Laskus T., Slusarczyk J. Autoimmune chronic active hepatitis developing after acute type B hepatitis. Dig Dis Sci. 1989;34(8):1294–1297. doi: 10.1007/BF01537282. [DOI] [PubMed] [Google Scholar]
- 29.Vento S., Garofano T., Dolci L., Di Perri G., Concia E., Bassetti D. Identification of hepatitis A virus as a trigger for autoimmune chronic hepatitis type 1 in susceptible individuals. Lancet. 1991;337(8751):1183–1187. doi: 10.1016/0140-6736(91)92858-y. [DOI] [PubMed] [Google Scholar]
- 30.Fainboim L., Cañero Velasco M.C., Marcos C.Y., Ciocca M., Roy A., Theiler G. Protracted, but not acute, hepatitis a virus infection is strongly associated with HLA-DRB1∗1301, a marker for pediatric autoimmune hepatitis. Hepatology. 2001;33(6):1512–1517. doi: 10.1053/jhep.2001.24562. [DOI] [PubMed] [Google Scholar]
- 31.Grünhage F., Spengler U., Fischer H.P., Sauerbruch T. Autoimmune hepatitis - sequel of a relapsing hepatitis A in a 75-year-old woman. Digestion. 2004;70(3):187–191. doi: 10.1159/000082253. [DOI] [PubMed] [Google Scholar]
- 32.Robertson D.A.F., Guy E.C., Zhang S.L., Wright R. Persistent measles virus GENOME IN autoimmune chronic active hepatitis. Lancet. 1987;2(8549):9–11. doi: 10.1016/s0140-6736(87)93051-0. [DOI] [PubMed] [Google Scholar]
- 33.Yeend-Curd-Trimble H., Kelly K., Ghosh I., MacDonald D. Autoimmune liver disease following acute hepatitis A infection. BMJ Case Rep. 2019;12(5):e228433. doi: 10.1136/bcr-2018-228433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leem G., Kim B.K., Shin E.C., Park J.Y. Autoimmune hepatic failure following acute hepatitis a is accompanied by inflammatory conversion of regulatory T cells. Yonsei Med J. 2020;61(1):100–102. doi: 10.3349/ymj.2020.61.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buechter M., Manka P., Heinemann F.M., Lindemann M., Baba H.A., Schlattjan M. Potential triggering factors of acute liver failure as a frst manifestation of autoimmune hepatitis-a single center experience of 52 adult patients. World J Gastroenterol. 2018;24(13):1410–1418. doi: 10.3748/wjg.v24.i13.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chalasani N.P., Hayashi P.H., Bonkovsky H.L., Navarro V.J., Lee W.M., Fontana R.J. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950–966. doi: 10.1038/ajg.2014.131. [DOI] [PubMed] [Google Scholar]
- 37.Andrade R.J., Aithal G.P., Björnsson E.S., Kaplowitz N., Kullak-Ublick G.A., Larrey D. EASL clinical practice guidelines: drug-induced liver injury. J Hepatol. 2019;70(6):1222–1261. doi: 10.1016/j.jhep.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Castiella A., Zapata E., Lucena M.I., Andrade R.J. Drug-induced autoimmune liver disease: a diagnostic dilemma of an increasingly reported disease. World J Hepatol. 2014;6(4):160–168. doi: 10.4254/wjh.v6.i4.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiler-Normann C., Schramm C. Drug induced liver injury and its relationship to autoimmune hepatitis. J Hepatol. 2011;55(4):747–749. doi: 10.1016/j.jhep.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki A., Brunt E.M., Kleiner D.E., Miquel R., Smyrk T.C., Andrade R.J. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis vs. drug-induced liver injury. Hepatology. 2011;54(3):931–939. doi: 10.1002/hep.24481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W., Lie P., Guo M., He J. Risk of hepatotoxicity in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis of published data. Int J Cancer. 2017;141(5):1018–1028. doi: 10.1002/ijc.30678. [DOI] [PubMed] [Google Scholar]
- 42.Huffman B.M., Kottschade L.A., Kamath P.S., Markovic S.N. Hepatotoxicity after immune checkpoint inhibitor therapy in melanoma :natural progression and management. Am J Clin Oncol Cancer Clin Trials. 2018;41(8):760–765. doi: 10.1097/COC.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 43.Kleiner D.E., Berman D. Pathologic changes in ipilimumab-related hepatitis in patients with metastatic melanoma. Dig Dis Sci. 2012;57(8):2233–2240. doi: 10.1007/s10620-012-2140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johncilla M., Misdraji J., Pratt D.S., Agoston A.T., Lauwers G.Y., Srivastava A. Ipilimumab-associated hepatitis: clinicopathologic characterization in a series of 11 cases. Am J Surg Pathol. 2015;39(8):1075–1084. doi: 10.1097/PAS.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 45.De Martin E., Michot J.M., Papouin B., Champiat S., Mateus C., Lambotte O. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68(6):1181–1190. doi: 10.1016/j.jhep.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 46.Suzman D.L., Pelosof L., Rosenberg A., Avigan M.I. Hepatotoxicity of immune checkpoint inhibitors: an evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int. 2018;38(6):976–987. doi: 10.1111/liv.13746. [DOI] [PubMed] [Google Scholar]
- 47.Reddy H.G., Schneider B.J., Tai A.W. Immune checkpoint inhibitor-associated colits and hepatitis. Clin Transl Gastroenterol. 2018;9(11):206. doi: 10.1038/s41424-018-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zen Y., Yeh M.M. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol. 2018;31(6):965–973. doi: 10.1038/s41379-018-0013-y. [DOI] [PubMed] [Google Scholar]
- 49.Nishida N., Kudo M. Liver damage related to immune checkpoint inhibitors. Hepatol Int. 2019;13(3):248–252. doi: 10.1007/s12072-018-9921-7. [DOI] [PubMed] [Google Scholar]
- 50.Reynolds K., Thomas M., Dougan M. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist. 2018;23(9):991–997. doi: 10.1634/theoncologist.2018-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendizabal M., Marciano S., Videla M.G., Anders M., Zerega A., Balderramo D.C. Fulminant presentation of autoimmune hepatitis: clinical features and early predictors of corticosteroid treatment failure. Eur J Gastroenterol Hepatol. 2015;27(6):644–648. doi: 10.1097/MEG.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 52.Fujiwara K., Yasui S., Yonemitsu Y., Arai M., Kanda T., Fukuda Y. Analysis of infectious complications and timing for emergency liver transplantation in autoimmune acute liver failure. J Hepatobiliary Pancreat Sci. 2016;23(4):212–219. doi: 10.1002/jhbp.326. [DOI] [PubMed] [Google Scholar]
- 53.Dalton H.R., Kamar N., Baylis S.A., Moradpour D., Wedemeyer H., Negro F. EASL clinical practice guidelines on hepatitis E virus infection. J Hepatol. 2018;68(6):1256–1271. doi: 10.1016/j.jhep.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 54.Llovet L.P., Gratacós-Ginés J., Ortiz O., Rodriguez-Tajes S., Lens S., Reverter E. Higher seroprevalence of hepatitis E virus in autoimmune hepatitis: role of false-positive antibodies. Liver Int. 2020;40(3):558–564. doi: 10.1111/liv.14332. [DOI] [PubMed] [Google Scholar]
- 55.Chapin C.A., Horslen S.P., Squires J.E., Lin H., Blondet N., Mohammad S. Corticosteroid therapy for indeterminate pediatric acute liver failure and Aplastic anemia with acute hepatitis. J Pediatr. 2019;208:23–29. doi: 10.1016/j.jpeds.2018.12.042. [DOI] [PubMed] [Google Scholar]
- 56.Ganger D.R., Rule J., Rakela J., Bass N., Reuben A., Stravitz R. Acute liver failure of indeterminate etiology: a comprehensive systematic approach by an expert committee to establish causality. Am J Gastroenterol. 2018;113(9):1319. doi: 10.1038/s41395-018-0160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brennan P.N., Donnelly M.C., Simpson K.J. Systematic review: non A-E, seronegative or indeterminate hepatitis; what is this deadly disease? Aliment Pharmacol Ther. 2018;47(8):1079–1091. doi: 10.1111/apt.14566. [DOI] [PubMed] [Google Scholar]
- 58.Di Giorgio A., Bravi M., Bonanomi E., Alessio G., Sonzogni A., Zen Y. Fulminant hepatic failure of autoimmune aetiology in children. J Pediatr Gastroenterol Nutr. 2015;60(2):159–164. doi: 10.1097/MPG.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 59.Bernal W., Ma Y., Smith H.M., Portmann B., Wendon J., Vergani D. The significance of autoantibodies and immunoglobulins in acute liver failure: a cohort study. J Hepatol. 2007;47(5):664–670. doi: 10.1016/j.jhep.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 60.Fujiwara K., Yasui S., Nakano M., Yonemitsu Y., Arai M., Kanda T. Severe and fulminant hepatitis of indeterminate etiology in a Japanese center. Hepatol Res. 2015;45(10):E141–E149. doi: 10.1111/hepr.12483. [DOI] [PubMed] [Google Scholar]
- 61.Shen Y., Lu C., Men R., Liu J., Ye T., Yang L. Clinical and pathological characteristics of autoimmune hepatitis with acute presentation. Can J Gastroenterol Hepatol. 2018;2018 doi: 10.1155/2018/3513206. 3513206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sonthalia N., Rathi P.M., Jain S.S., Surude R.G., Mohite A.R., Pawar S.V. Natural history and treatment outcomes of severe autoimmune hepatitis. J Clin Gastroenterol. 2017;51(6):548–556. doi: 10.1097/MCG.0000000000000805. [DOI] [PubMed] [Google Scholar]
- 63.Mehendiratta V., Mitroo P., Bombonati A., Navarro V.J., Rossi S., Rubin R. Serologic markers do not predict histologic severity or response to treatment in patients with autoimmune hepatitis. Clin Gastroenterol Hepatol. 2009;7(1):98–103. doi: 10.1016/j.cgh.2008.08.043. [DOI] [PubMed] [Google Scholar]
- 64.Czaja A.J. Performance parameters of the conventional serological markers for autoimmune hepatitis. Dig Dis Sci. 2011;56(2):545–554. doi: 10.1007/s10620-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 65.Fujiwara K., Fukuda Y., Yokosuka O. Precise histological evaluation of liver biopsy specimen is indispensable for diagnosis and treatment of acute-onset autoimmune hepatitis. J Gastroenterol. 2008;43(12):951–958. doi: 10.1007/s00535-008-2254-x. [DOI] [PubMed] [Google Scholar]
- 66.Yasui S., Fujiwara K., Yonemitsu Y., Oda S., Nakano M., Yokosuka O. Clinicopathological features of severe and fulminant forms of autoimmune hepatitis. J Gastroenterol. 2011;46(3):378–390. doi: 10.1007/s00535-010-0316-3. [DOI] [PubMed] [Google Scholar]
- 67.Loria P., Lonardo A., Leonardi F., Fontana C., Carulli L., Verrone A.M. Non-organ-specific autoantibodies in nonalcoholic fatty liver disease: prevalence and correlates. Dig Dis Sci. 2003;48(11):2173–2181. doi: 10.1023/b:ddas.0000004522.36120.08. [DOI] [PubMed] [Google Scholar]
- 68.Adams L.A., Lindor K.D., Angulo P. The prevalence of autoantibodies and autoimmune hepatitis in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2004;99(7):1316–1320. doi: 10.1111/j.1572-0241.2004.30444.x. [DOI] [PubMed] [Google Scholar]
- 69.Yatsuji S., Hashimoto E., Kaneda H., Taniai M., Tokushige K., Shiratori K. Diagnosing autoimmune hepatitis in nonalcoholic fatty liver disease: is the International Autoimmune Hepatitis Group scoring system useful? J Gastroenterol. 2005;40(12):1130–1138. doi: 10.1007/s00535-005-1711-z. [DOI] [PubMed] [Google Scholar]
- 70.Himoto T., Nishioka M. Autoantibodies in liver disease: important clues for the diagnosis, disease activity and prognosis. Autoimmun Highlights. 2013;4(2):39–53. doi: 10.1007/s13317-013-0046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yasui S., Fujiwara K., Okitsu K., Yonemitsu Y., Ito H., Yokosuka O. Importance of computed tomography imaging features for the diagnosis of autoimmune acute liver failure. Hepatol Res. 2012;42(1):42–50. doi: 10.1111/j.1872-034X.2011.00892.x. [DOI] [PubMed] [Google Scholar]
- 72.Zabron A., Quaglia A., Fatourou E., Peddu P., Lewis D., Heneghan M. Clinical and prognostic associations of liver volume determined by computed tomography in acute liver failure. Liver Int. 2018;38(9):1592–1601. doi: 10.1111/liv.13725. [DOI] [PubMed] [Google Scholar]
- 73.Hennes E.M., Zeniya M., Czaja A.J., Parés A., Dalekos G.N., Krawitt E.L. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48(1):169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 74.Tiniakos D.G., Brain J.G., Bury Y.A. Role of histopathology in autoimmune hepatitis. Dig Dis. 2015;33(Suppl 2):53–64. doi: 10.1159/000440747. [DOI] [PubMed] [Google Scholar]
- 75.Singh R., Nair S., Farr G., Mason A., Perrillo R. Acute autoimmune hepatitis presenting with centrizonal liver disease: case report and review of the literature. Am J Gastroenterol. 2002;97(10):2670–2673. doi: 10.1111/j.1572-0241.2002.06052.x. [DOI] [PubMed] [Google Scholar]
- 76.Kessler W.R., Cummings O.W., Eckert G., Chalasani N., Lumeng L., Kwo P.Y. Fulminant hepatic failure as the initial presentation of acute autoimmune hepatitis. Clin Gastroenterol Hepatol. 2004;2(7):625–631. doi: 10.1016/s1542-3565(04)00246-0. [DOI] [PubMed] [Google Scholar]
- 77.Misdraji J., Thiim M., Graeme-Cook F.M. Autoimmune hepatitis with centrilobular necrosis. Am J Surg Pathol. 2004;28(4):471–478. doi: 10.1097/00000478-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 78.Miyake Y., Iwasaki Y., Terada R., Onishi T., Okamoto R., Takaguchi K. Clinical features of Japanese type 1 autoimmune hepatitis patients with zone III necrosis. Hepatol Res. 2007;37(10):801–805. doi: 10.1111/j.1872-034X.2007.00125.x. [DOI] [PubMed] [Google Scholar]
- 79.Okano N., Yamamoto K., Sakaguchi K., Miyake Y., Shimada N., Hakoda T. Clinicopathological features of acute-onset autoimmune hepatitis. Hepatol Res. 2003;25(3):263–270. doi: 10.1016/s1386-6346(02)00274-7. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen Canh H., Harada K., Ouchi H., Sato Y., Tsuneyama K., Kage M. Acute presentation of autoimmune hepatitis: a multicentre study with detailed histological evaluation in a large cohort of patients. J Clin Pathol. 2017;70(11):961–969. doi: 10.1136/jclinpath-2016-204271. [DOI] [PubMed] [Google Scholar]
- 81.Hofer H., Oesterreicher C., Wrba F., Ferenci P., Penner E. Centrilobular necrosis in autoimmune hepatitis: a histological feature associated with acute clinical presentation. J Clin Pathol. 2006;59(3):246–249. doi: 10.1136/jcp.2005.029348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abe K., Kanno Y., Okai K., Katsushima F., Monoe K., Saito H. Centrilobular necrosis in acute presentation of Japanese patients with type 1 autoimmune hepatitis. World J Hepatol. 2012;4(9):262–267. doi: 10.4254/wjh.v4.i9.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vyberg M., Junge J., Horn T. Detection of early zone 3 liver fibrosis in chronic alcoholics a comparison of four connective tissue staining methods. Acta Pathol Microbiol Scand Ser A Pathol. 1987;95(1):11–16. doi: 10.1111/j.1699-0463.1987.tb00003_95a.x. [DOI] [PubMed] [Google Scholar]
- 84.Kazarine A., Gopal A.A., Wiseman P.W. Nonlinear microscopy of common histological stains reveals third harmonic generation harmonophores. Analyst. 2019;144(10):3239–3249. doi: 10.1039/c9an00267g. [DOI] [PubMed] [Google Scholar]
- 85.Iezzoni J.C. Diagnostic histochemistry in hepatic pathology. Semin Diagn Pathol. 2018;35(6):381–389. doi: 10.1053/j.semdp.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 86.Lefkowitch J.H. Special stains in diagnostic liver pathology. Semin Diagn Pathol. 2006;23(3-4):190–198. doi: 10.1053/j.semdp.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 87.Scheuer P.J., Maggi G. Hepatic fibrosis and collapse: histological distinction by orcein staining. Histopathology. 1980;4(5):487–490. doi: 10.1111/j.1365-2559.1980.tb02943.x. [DOI] [PubMed] [Google Scholar]
- 88.Ferrell L.D., Greenberg M.S. Special stains can distinguish hepatic necrosis with regenerative nodules from cirrhosis. Liver Int. 2007;27(5):681–686. doi: 10.1111/j.1478-3231.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 89.Fujiwara K., Yasui S., Tawada A., Fukuda Y., Nakano M., Yokosuka O. Diagnostic value and utility of the simplified International Autoimmune Hepatitis Group criteria in acute-onset autoimmune hepatitis. Liver Int. 2011;31(7):1013–1020. doi: 10.1111/j.1478-3231.2011.02524.x. [DOI] [PubMed] [Google Scholar]
- 90.Yeoman A.D., Westbrook R.H., Al-Chalabi T., Carey I., Heaton N.D., Portmann B.C. Diagnostic value and utility of the simplified International Autoimmune Hepatitis Group (IAIHG) criteria in acute and chronic liver disease. Hepatology. 2009;50(2):538–545. doi: 10.1002/hep.23042. [DOI] [PubMed] [Google Scholar]
- 91.Balitzer D., Shafizadeh N., Peters M.G., Ferrell L.D., Alshak N., Kakar S. Autoimmune hepatitis: review of histologic features included in the simplified criteria proposed by the International Autoimmune Hepatitis Group and proposal for new histologic criteria. Mod Pathol. 2017;30(5):773–783. doi: 10.1038/modpathol.2016.267. [DOI] [PubMed] [Google Scholar]
- 92.Purnak T., Efe C., Kav T., Wahlin S., Ozaslan E. Treatment response and outcome with two different prednisolone regimens in autoimmune hepatitis. Dig Dis Sci. 2017;62(10):2900–2907. doi: 10.1007/s10620-017-4728-2. [DOI] [PubMed] [Google Scholar]
- 93.Summerskill W.H.J., Korman M.G., Ammon H.V., Baggenstoss A.H. Prednisone for chronic active liver disease: dose titration, standard dose, and combination with azathioprine compared. Gut. 1975;16(11):876–883. doi: 10.1136/gut.16.11.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kirk A.P., Jain S., Pocock S., Thomas H.C., Sherlock S. Late results of the Royal Free Hospital prospective controlled trial of prednisolone therapy in hepatitis B surface antigen negative chronic active hepatitis. Gut. 1980;21(1):78–83. doi: 10.1136/gut.21.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kanzler S., Löhr H., Gerken G., Galle P.R., Lohse A.W. Long-term management and prognosis of autoimmune hepatitis (AIH): a single center experience. Z Gastroenterol. 2001;39(5):339–341. doi: 10.1055/s-2001-13708. 344-348. [DOI] [PubMed] [Google Scholar]
- 96.Hartl J., Ehlken H., Sebode M., Peiseler M., Krech T., Zenouzi R. Usefulness of biochemical remission and transient elastography in monitoring disease course in autoimmune hepatitis. J Hepatol. 2018;68(4):754–763. doi: 10.1016/j.jhep.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 97.Pape S., Gevers T.J.G., Belias M., Mustafajev I.F., Vrolijk J.M., van Hoek B. Predniso(lo)ne dosage and chance of remission in patients with autoimmune hepatitis. Clin Gastroenterol Hepatol. 2019;17(10):2068–2075.e2. doi: 10.1016/j.cgh.2018.12.035. [DOI] [PubMed] [Google Scholar]
- 98.Manns M.P., Woynarowski M., Kreisel W., Lurie Y., Rust C., Zuckerman E. Budesonide induces remission more effectively than prednisone in a controlled trial of patients with autoimmune hepatitis. Gastroenterology. 2010;139(4):1198–1206. doi: 10.1053/j.gastro.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 99.Zhang C., Wu S.-S., Dong X.-Q., Wu Z., Zhao H., Wang G.-Q. The Efficacy and safety of different doses of Glucocorticoid for autoimmune hepatitis: a systematic review and meta-analysis. Med. 2019;98(52):e18313. doi: 10.1097/MD.0000000000018313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Verma S., Maheshwari A., Thuluvath P. Liver failure as initial presentation of autoimmune hepatitis: clinical characteristics, predictors of response to steroid therapy, and outcomes. Hepatology. 2009;49(4):1396–1397. doi: 10.1002/hep.22894. [DOI] [PubMed] [Google Scholar]
- 101.Yeoman A.D., Westbrook R.H., Zen Y., Maninchedda P., Portmann B.C., Devlin J. Early predictors of corticosteroid treatment failure in icteric presentations of autoimmune hepatitis. Hepatology. 2011;53(3):926–934. doi: 10.1002/hep.24141. [DOI] [PubMed] [Google Scholar]
- 102.De Martin E., Coilly A., Ichai P., Samuel D., Duclos-Vallée J.C. The role of corticosteroids in acute-severe autoimmune hepatitis is still highly debatable. J Hepatol. 2015;63(4):1041–1042. doi: 10.1016/j.jhep.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 103.De Martin E., Coilly A., Houssel-Debry P., Ollivier-Hourmand I., Heurgue-Berlot A., Artru F. Treatment and prognosis of acute severe autoimmune hepatitis. J Hepatol. 2017 S0168-8278(17)30270-2. [Google Scholar]
- 104.Takikawa Y., Suzuki K. Clinical epidemiology of fulminant hepatitis in Japan. Hepatol Res. 2008;38(Suppl 1):S14–S18. doi: 10.1111/j.1872-034X.2008.00421.x. [DOI] [PubMed] [Google Scholar]
- 105.Karkhanis J., Verna E.C., Chang M.S., Stravitz R.T., Schilsky M., Lee W.M. Steroid use in acute liver failure. Hepatology. 2014;59(2):612–621. doi: 10.1002/hep.26678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Villamil A.G., Casciato P., Eduardo M., Bustos D., Giunta D., Ciardullo M. Fulminant autoimmune hepatitis: clinical presentation, outcome and prognostic factors (Abstract 480) Am J Transplant. 2005;5(Suppl 11):278. [Google Scholar]
- 107.Miyake Y., Iwasaki Y., Terada R., Onishi T., Okamoto R., Sakai N. Clinical characteristics of fulminant-type autoimmune hepatitis: an analysis of eleven cases. Aliment Pharmacol Ther. 2006;23(9):1347–1353. doi: 10.1111/j.1365-2036.2006.02894.x. [DOI] [PubMed] [Google Scholar]
- 108.Montano-Loza A.J., Carpenter H.A., Czaja A.J. Features associated with treatment failure in type 1 autoimmune hepatitis and predictive value of the model of end-stage liver disease. Hepatology. 2007;46(4):1138–1145. doi: 10.1002/hep.21787. [DOI] [PubMed] [Google Scholar]
- 109.Czaja A.J., Rakela J., Ludwig J. Features reflective of early prognosis in corticosteroid-treated severe autoimmune chronic active hepatitis. Gastroenterology. 1988;95(2):448–453. doi: 10.1016/0016-5085(88)90503-3. [DOI] [PubMed] [Google Scholar]
- 110.Darweesh S.K., Ibrahim M.F., El-Tahawy M.A. Effect of N-acetylcysteine on mortality and liver transplantation rate in non-acetaminophen-induced acute liver failure: a multicenter study. Clin Drug Investig. 2017;37(5):473–482. doi: 10.1007/s40261-017-0505-4. [DOI] [PubMed] [Google Scholar]
- 111.Hu J., Zhang Q., Ren X., Sun Z., Quan Q. Efficacy and safety of acetylcysteine in “non-acetaminophen” acute liver failure: a meta-analysis of prospective clinical trials. Clin Res Hepatol Gastroenterol. 2015;39(5):594–5999. doi: 10.1016/j.clinre.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 112.Aguila E., Edding S., Cua I. Efficacy and safety of N-acetylcysteine in patients with non-acetaminophen-induced acute liver failure: a meta-analysis. Abstracts. Hepatol Int. 2019;13:1–266. [Google Scholar]
- 113.Lee W.M., Hynan L.S., Rossaro L., Fontana R.J., Stravitz R.T., Larson A.M. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856–864. doi: 10.1053/j.gastro.2009.06.006. 864.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Singh S., Hynan L.S., Lee W.M. Improvements in hepatic serological biomarkers are associated with clinical benefit of intravenous N-acetylcysteine in early stage non-acetaminophen acute liver failure. Dig Dis Sci. 2013;58(5):1397–1402. doi: 10.1007/s10620-012-2512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Larsen F.S., Schmidt L.E., Bernsmeier C., Rasmussen A., Isoniemi H., Patel V.C. High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J Hepatol. 2016;64(1):69–78. doi: 10.1016/j.jhep.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 116.Takahashi A., Ohira H., Onizawa M., Monoe K., Kanno Y., Saito H. A recovery case of acute-onset autoimmune hepatitis presenting as fulminant hepatic failure, who received living donor-liver transplantion. Intern Med. 2006;45(21):1217–1220. doi: 10.2169/internalmedicine.45.1799. [DOI] [PubMed] [Google Scholar]
- 117.Fujiwara K., Yasui S., Tawada A., Okitsu K., Yonemitsu Y., Chiba T. Autoimmune fulminant liver failure in adults: experience in a Japanese center. Hepatol Res. 2011;41(2):133–141. doi: 10.1111/j.1872-034X.2010.00755.x. [DOI] [PubMed] [Google Scholar]
- 118.Nakao M., Nakayama N., Uchida Y., Tomiya T., Ido A., Sakaida I. Nationwide survey for acute liver failure and late-onset hepatic failure in Japan. J Gastroenterol. 2018;53(6):752–769. doi: 10.1007/s00535-017-1394-2. [DOI] [PubMed] [Google Scholar]
- 119.Sato H., Tomita K., Yasue C., Umeda R., Ebinuma H., Ogata S. Pregnant woman with non-comatose autoimmune acute liver failure in the second trimester rescued using medical therapy: a case report. Hepatol Res. 2015;45(3):349–355. doi: 10.1111/hepr.12344. [DOI] [PubMed] [Google Scholar]
- 120.Yasui S., Fujiwara K., Haga Y., Nakamura M., Mikata R., Arai M. Infectious complications, steroid use and timing for emergency liver transplantation in acute liver failure: analysis in a Japanese center. J Hepatobiliary Pancreat Sci. 2016;23(12):756–762. doi: 10.1002/jhbp.399. [DOI] [PubMed] [Google Scholar]
- 121.Reddy K.R., Ellerbe C., Schilsky M., Stravitz R.T., Fontana R.J., Durkalski V. Determinants of outcome among patients with acute liver failure listed for liver transplantation in the United States. Liver Transpl. 2016;22(4):505–515. doi: 10.1002/lt.24347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ikegami T., Taketomi A., Soejima Y., Yoshizumi T., Sanefuji K., Kayashima H. Living donor liver transplantation for acute liver failure: a 10-year experience in a single center. J Am Coll Surg. 2008;206(3):412–418. doi: 10.1016/j.jamcollsurg.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 123.Khalaf H., Mourad W., El-Sheikh Y., Abdo A., Helmy A., Medhat Y. Liver transplantation for autoimmune hepatitis: a single-center experience. Transplant Proc. 2007;39(4):1166–1170. doi: 10.1016/j.transproceed.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 124.Montano-Loza A.J., Mason A.L., Ma M., Bastiampillai R.J., Bain V.G., Tandon P. Risk factors for recurrence of autoimmune hepatitis after liver transplantation. Liver Transpl. 2009;15(10):1254–1261. doi: 10.1002/lt.21796. [DOI] [PubMed] [Google Scholar]
- 125.Montano-Loza A.J., Bhanji R.A., Wasilenko S., Mason A.L. Systematic review: recurrent autoimmune liver diseases after liver transplantation. Aliment Pharmacol Ther. 2017;45(4):485–500. doi: 10.1111/apt.13894. [DOI] [PubMed] [Google Scholar]
- 126.Ayata G., Gordon F.D., Lewis W.D., Pomfret E., Pomposelli J.J., Jenkins R.L. Liver transplantation for autoimmune hepatitis: a long-term pathologic study. Hepatology. 2000;32(2):185–192. doi: 10.1053/jhep.2000.9077. [DOI] [PubMed] [Google Scholar]
- 127.Núñez-Martínez O., De La Cruz G., Salcedo M., Molina J., De Diego A., Ripoll C. Liver transplantation for autoimmune hepatitis: fulminant vs. chronic hepatitis presentation. Transplant Proc. 2003;35(5):1857–1858. doi: 10.1016/s0041-1345(03)00591-8. [DOI] [PubMed] [Google Scholar]
- 128.Kerkar N., Yanni G. “De novo” and “recurrent” autoimmune hepatitis after liver transplantation: a comprehensive review. J Autoimmun. 2016;66:17–24. doi: 10.1016/j.jaut.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 129.Reich D.J., Fiel I., Guarrera J.V., Emre S., Guy S.R., Schwartz M.E. Liver transplantation for autoimmune hepatitis. Hepatology. 2000;32(4 Pt 1):693–700. doi: 10.1053/jhep.2000.16666. [DOI] [PubMed] [Google Scholar]
- 130.Campsen J., Zimmerman M.A., Trotter J.F., Wachs M., Bak T., Steinberg T. Liver transplantation for autoimmune hepatitis and the success of aggressive corticosteroid withdrawal. Liver Transpl. 2008;14(9):1281–1286. doi: 10.1002/lt.21525. [DOI] [PubMed] [Google Scholar]
- 131.Kerkar N., Hadz̃ić N., Davies E.T., Portmann B., Donaldson P.T., Rela M. De-novo autoimmune hepatitis after liver transplantation. Lancet. 1998;351(9100):409–413. doi: 10.1016/S0140-6736(97)06478-7. [DOI] [PubMed] [Google Scholar]
- 132.Czaja A.J. Diagnosis, pathogenesis, and treatment of autoimmune hepatitis after liver transplantation. Dig Dis Sci. 2012;57(9):2248–2266. doi: 10.1007/s10620-012-2179-3. [DOI] [PubMed] [Google Scholar]
- 133.Stirnimann G., Ebadi M., Czaja A.J., Montano-Loza A.J. Recurrent and De Novo Autoimmune Hepatitis. Liver Transpl. 2019;25(1):152–166. doi: 10.1002/lt.25375. [DOI] [PubMed] [Google Scholar]
- 134.Krishnamoorthy T.L., Miezynska-Kurtycz J., Hodson J., Gunson B.K., Neuberger J., Milkiewicz P. Longterm corticosteroid use after liver transplantation for autoimmune hepatitis is safe and associated with a lower incidence of recurrent disease. Liver Transpl. 2016;22(1):34–41. doi: 10.1002/lt.24323. [DOI] [PubMed] [Google Scholar]
- 135.Porta G., da Costa Gayotto L.C., Alvarez F. Anti-liver-kidney microsome antibody-positive autoimmune hepatitis presenting as fulminant liver failure. J Pediatr Gastroenterol Nutr. 1990;11(1):138–140. doi: 10.1097/00005176-199007000-00027. [DOI] [PubMed] [Google Scholar]
- 136.Czaja A.J., Souto E.O., Bittencourt P.L., Cancado E.L.R., Porta G., Goldberg A.C. Clinical distinctions and pathogenic implications of type 1 autoimmune hepatitis in Brazil and the United States. J Hepatol. 2002;37(3):302–308. doi: 10.1016/s0168-8278(02)00182-4. [DOI] [PubMed] [Google Scholar]
- 137.Aydoǧdu S., Özgenç F., Yurtsever S., Akman S.A., Tokat Y., Yaǧci R.V. Our experience with fulminant hepatic failure in Turkish children: etiology and outcome. J Trop Pediatr. 2003;49(6):367–370. doi: 10.1093/tropej/49.6.367. [DOI] [PubMed] [Google Scholar]
- 138.Lee W.S., McKiernan P., Kelly D.A. Etiology, outcome and prognostic indicators of childhood fulminant hepatic failure in the United Kingdom. J Pediatr Gastroenterol Nutr. 2005;40(5):575–581. doi: 10.1097/01.mpg.0000158524.30294.e2. [DOI] [PubMed] [Google Scholar]
- 139.Maggiore G., Porta G., Bernard O., Hadchouel M., Alvarez F., Homberg J.C. Autoimmune hepatitis with initial presentation as acute hepatic failure in young children. J Pediatr. 1990;116(2):280–282. doi: 10.1016/s0022-3476(05)82892-6. [DOI] [PubMed] [Google Scholar]
- 140.Latif N., Mehmood K. Risk factors for fulminant hepatic failure and their relation with outcome in children. J Pak Med Assoc. 2010;60(3):175–178. [PubMed] [Google Scholar]
- 141.Della Corte C., Sartorelli M.R., Sindoni C.D., Girolami E., Giovannelli L., Comparcola D. Autoimmune hepatitis in children: an overview of the disease focusing on current therapies. Eur J Gastroenterol Hepatol. 2012;24(7):739–746. doi: 10.1097/MEG.0b013e328353750c. [DOI] [PubMed] [Google Scholar]
- 142.Mieli-Vergani G., Heller S., Jara P., Vergani D., Chang M.H., Fujisawa T. Autoimmune hepatitis. J Pediatr Gastroenterol Nutr. 2009;49(2):158–164. doi: 10.1097/MPG.0b013e3181a1c265. [DOI] [PubMed] [Google Scholar]
- 143.Mieli-Vergani G., Vergani D. Autoimmune liver diseases in children - what is different from adulthood? Best Pract Res Clin Gastroenterol. 2011;25(6):783–795. doi: 10.1016/j.bpg.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 144.Abdel-Ghaffar T.Y., Sira M.M., Sira A.M., Salem T.A., El-Sharawy A.A., El Naghi S. Serological markers of autoimmunity in children with hepatitis A. Eur J Gastroenterol Hepatol. 2015;27(10):1161–1169. doi: 10.1097/MEG.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 145.Smolka V., Tkachyk O., Ehrmann J., Karaskova E., Zapalka M., Volejnikova J. Acute onset of autoimmune hepatitis in children and adolescents. Hepatobiliary Pancreat Dis Int. 2020;19(1):17–21. doi: 10.1016/j.hbpd.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 146.Gregorio G.V., Portmann B., Reid F., Donaldson P.T., Doherty D.G., McCartney M. Autoimmune hepatitis in childhood: a 20-year experience. Hepatology. 1997;25(3):541–547. doi: 10.1002/hep.510250308. [DOI] [PubMed] [Google Scholar]
- 147.Sanchez M.C., D'Agostino D.E. Pediatric end-stage liver disease score in acute liver failure to assess poor prognosis. J Pediatr Gastroenterol Nutr. 2012;54(2):193–196. doi: 10.1097/MPG.0b013e3182349a04. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.