Abstract
Obesity is influenced by genetics and diet and has wide ranging comorbidities, including anxiety and depressive disorders. Outbred heterogeneous stock (HS) rats are used for fine-genetic mapping of complex traits and may be useful for understanding gene by diet interactions. In this study, HS rats were fed diets containing 60% kcal from fat (high-fat diet, HFD) or 10% kcal from fat (low-fat diet, LFD) and tested for metabolic (study 1) and behavioral (study 2) outcomes. In study 1, we measured glucose tolerance, fasting glucose and insulin, fat pad weights and despair-like behavior in the forced swim test (FST). In study 2, we assessed anxiety-like (elevated plus maze, EPM; open field test, OFT) and despair-like/coping (splash test, SpT; and FST) behaviors. Body weight and food intake were measured weekly in both studies. We found negative effects of HFD on metabolic outcomes, including increased body weight and fat pad weights, decreased glucose tolerance, and increased fasting insulin. We also found negative effects of HFD on despair-like/coping and anxiety-like behaviors. These include increased immobility in the FST, decreased open arm time in the EPM, and increased movement and rest episodes and decreased rearing in the OFT. The diet-induced changes in EPM and OFT were independent of overall locomotion. Additionally, diet-induced changes in OFT behaviors were independent of adiposity, while adiposity was a confounding factor for EPM and FST behavior. This work establishes the HS as a model to study gene by diet interactions affecting metabolic and behavioral health.
Keywords: anxiety, behavior, depression model, obesity, outbred
INTRODUCTION
Obesity is a public health crisis affecting 39.8% of United States adults (16), with prevalence continuing to rise around the world (15a). Obesity is connected to a wide range of comorbidities, including depression and anxiety (15a, 49). Studies demonstrate a bidirectional association such that those with obesity have a 55% higher risk of developing depression and those with depression have a 58% higher risk of becoming obese (33). In addition, studies have consistently found associations between Western diets (high in fat, refined grains, and red meat) and poor metabolic health (47) as well as depression and anxiety in humans (28, 31). Similarly, rodents fed a high-fat diet (HFD) develop anxiety and depressive-like behavior and metabolic complications (1, 3, 58). These studies demonstrate a strong link between obesity, diet, and depression/anxiety, but the underlying mechanisms remain unclear.
Shared genetics may be one feature that links obesity with depression and anxiety. Both obesity and mental health are influenced by genetic background, and human genome-wide association studies (GWAS) have identified some shared genetic risk between obesity and depression, although few shared genetic factors have been identified (36). Human GWAS have identified over 500 loci associated with body mass index (BMI) (32, 46, 64) and just over 50 loci have been linked to depression phenotypes (37). Interestingly, some depression loci with strong association signals are in close proximity or overlapping with BMI/obesity-associated genes (37). For example, studies have shown interactions between a variant of the obesity gene FTO and risk of depression (44, 48), such that those with the FTO risk variation and depression have higher BMI than those with the FTO risk variant but without depression. In addition, Adcy3 has been identified for both obesity and depression in human GWAS (59, 63), and this is supported by work in animal models (8, 26).
Because of the complex interplay between environment and genetics for both obesity and depression/anxiety, development of an animal model in which to study the shared genetics underlying both diseases would be highly valuable. We propose the use of outbred heterogeneous stock (HS) rats, an outbred animal model with high genetic diversity that more closely mimics the range of phenotypes found in the human population than inbred rodent strains. The HS rat is a population-based rodent model created through mating of eight inbred strains (19, 62), followed by maintaining the colony in a way that minimizes inbreeding (45). The result is a mosaic of the founder strains with each rat being genetically and phenotypically distinct. The HS rat has been utilized for genetic fine-mapping to identify loci and underlying genes for insulin resistance and glucose tolerance, adiposity, and multiple behavioral measures (20, 25, 26, 43, 55, 57). In the current study, we sought to determine if HS rats could be used as a model to investigate the link between HFD, obesity, and depression/anxiety.
METHODS
HS Rats
The HS rat colony was established in 1984 by the National Institutes of Health by breeding together eight inbred founder strains, ACI/N, BN/SsN, BUF/N, F344/N, M520/N, MR/N, WKY/N, and WN/N, and maintaining the colony in a way that minimizes inbreeding (19). The HS colony has been maintained at the Medical College of Wisconsin since 2006 with a colony set up at Wake Forest School of Medicine (WFSM) in 2017. The animals in the current study come from the WFSM colony, which has been named NMcwiWFsm:HS (Rat Genome Database number 13673907). The colony is maintained by using 64 breeder pairs in a way that takes into account family relationships using a kinship coefficient to ensure that only distantly related animals are mated. For this study, male rats were weaned at 3 wk of age and placed two per cage (nonsibling cage mates) for the remainder of the study. To maximize genetic diversity, we used no more than two males per diet from each breeder pair. Animals were housed on a 12 h light-dark cycle (0700–1900) and room temperature averaged 74°F ± 2.1 (standard deviation, range: 70–79°F). All protocols were approved by the Institutional Animal Care and Use Committee at WFSM.
Diet
HFD and matched low-fat control diet (LFD) was purchased from Research Diets (New Brunswick, NJ; high fat chow, 5.21 kcal/g, 60% fat, 20% carbohydrate, 20% protein, cat. D12492; low fat chow, 3.82 kcal/g, 10% fat, 70% carbohydrate, 20% protein cat. no D12450J). Diet was provided ad lib and weighed weekly to measure food intake per cage.
Study Design
This work comprises two separate studies. The first study was designed to assess the role of HFD on metabolic measures, and the second study was designed to assess the role of HFD on behavioral measures. We included a single behavioral measure in study 1 and, based on these results, designed the second study to further explore these behavioral differences. Because they were conducted at separate times, there are several differences between the two studies including output measures, number of animals per diet, age at which animals started on HFD or LFD, age at euthanasia, and season. Similarities between the studies include collecting the following measures: body weight, food intake, fat pad weights, and passive-coping/despair-like behavior in the forced swim test (FST).
Study 1: Impact of HFD on Metabolic Health and Coping Response to Stress/Behavioral Despair
Study design.
Male HS rats were fed HFD (n = 48) or LFD (n = 24) at 6 wk of age and weighed weekly throughout the study. Fewer rats were run in the LFD condition because previous work in our laboratory has assessed metabolic phenotypes in over 700 HS rats (26) and several hundred rats in the FST (20) on normal chow, which is similar in fat content to the LFD diet used in the current study (28.7% protein, 58.2% carbohydrates, 13.1% fat and 20% protein, 70% carbohydrates, 10% fat, respectively). Intraperitoneal glucose tolerance test (IPGTT) was administered after 8 wk of diet consumption as described below. To determine if HFD altered passive-coping/despair-like behavior, the FST was administered after 11 wk of diet consumption as described below. Rats were euthanized after 12 wk of diet consumption (Fig. 1A).
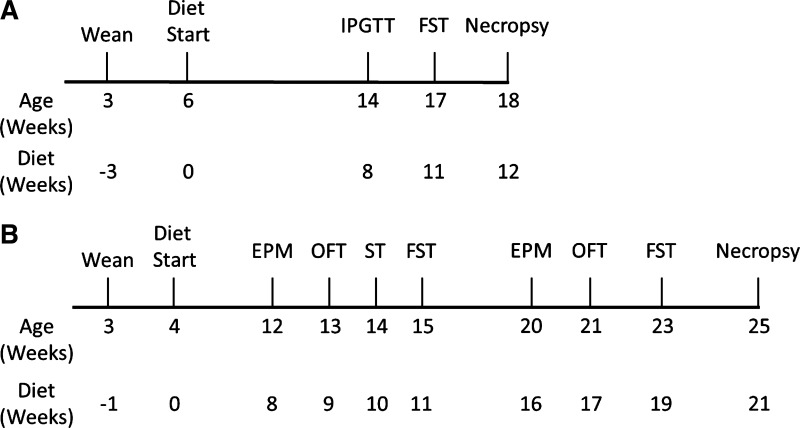
Fig. 1.
Study timelines. A: in study 1, heterogeneous stock (HS) rats were weaned at 3 wk of age and placed on high-fat diet (HFD) or low-fat diet (LFD) at 6 wk of age. After 8 wk of diet consumption, intraperitoneal glucose tolerance test (IPGTT) assayed glucose tolerance. Forced swim test (FST) was performed after 11 wk of diet consumption. Necropsy was performed after 12 wk of diet consumption. B: in study 2, HS rats were weaned at 3 wk of age and began HFD or LFD at 4 wk of age. Behavioral tests began after 8 wk of diet consumption and administered on a weekly basis for 4 wk. Behavioral tests, except splash test (ST), were repeated after 16 wk of diet consumption. Necropsy was performed after 21 wk of diet consumption. EPM, elevated plus maze; OFT, open field test.
IPGTT.
IPGTT was run as previously described (57). In brief, rats were fasted for 16 h (1730–0930) before being tested. Blood glucose levels were measured after fasting, and rats were then given glucose at 1g/kg body wt via intraperitoneal injection. Blood glucose was measured at 15, 30, 60, and 90 min after glucose injection. Area under the curve (AUC) was determined by applying the following equation: t*(x+y)/2, where t is the elapsed time in minutes, x is the initial glucose concentration (mg/dL), and y is the final glucose concentration, to each time period (15, 30, 60, and 90 min). We then summed the results to determine each rat’s IPGTT AUC.
FST.
Rats were placed in a tank of water (25°C ± 2, diameter 28.8 cm, height 49.8 cm, water depth 39 cm) for 15 min on day 1 and 5 min on day 2 as previously described (53). Video recording of day 2 was used to manually score movements made by the rat at 5 s intervals: immobility, swimming, climbing, and diving. Passive coping (11) and/or depressive-like behavior is associated with increased immobility (41, 50, 58).
Study 2: Impact of HFD on Coping Response to Stress/Despair- and Anxiety-like Behaviors
Study design.
To investigate if FST immobility is a result of physical limitations imposed by increased fat pad size or an effect of diet on emotional behavior, we conducted a second study to further characterize emotional behavior, including anxiety-like behavior, by using the elevated plus maze (EPM) and open field test (OFT), as well as despair-like behavior via splash test (SpT) and FST. At 4 wk of age, male HS rats were placed on HFD (n = 32) or LFD (n = 32). Body weight and food intake per cage were measured weekly. After 8 wk of diet consumption and again after 16 wk of diet consumption, rats were sequentially tested in the EPM, OFT, SpT, and FST. One test was administered per week, and the order of tests was specifically chosen to minimize influence of test order (34). Rats were euthanized after 21 wk of diet consumption (Fig. 1B).
EPM.
Rats were placed at the junction of two elevated, perpendicular arms, one axis with walls and one axis without walls (axis length 111.8 cm, wall height 74.0 cm, floor to apparatus 45.7 cm). Time spent in closed or open arms was measured over 5 min. Anxiety-like behavior is associated with increased time in the closed arms (18, 40).
OFT.
Rats were placed in an empty arena (40.8 × 40.8 × 30.1 cm) and allowed to explore for 30 min. Behavior was monitored via infrared beam break and collected for analysis using Omnitech tracking software (Omnitech Electronics Inc. Columbus, OH). Data collected were analyzed for total distance, movement, and rest episodes, rearing episodes, center time, and stereotypic activity. Anxiety-like behavior is associated with decreased time spent in the arena center and hyperlocomotion (17, 23).
SpT.
Rats were placed individually within their home cage and sprayed with a 10% sucrose solution on their dorsal coat. Latency to groom and time spent grooming were measured over 5 min. Higher latency and shorter total grooming time is reported as depressive-like behavior based on a reduced interest in self-care (13, 39).
FST.
The FST was performed as described in study 1.
Necropsy
For study 1, animals were fasted 16 h and euthanized by decapitation. Fasting serum was collected and stored at −80 for determination of fasting insulin levels, which was determined by Alpco Insulin ELISA (Salem, NH). For study 2, animals were not fasted before euthanasia. For both studies, multiple tissues were collected and weighed including the following fat pads: retroperitoneal (retro), epididymal (epi), and omental.
Statistical Analysis
All phenotypes were transformed to closely reflect a normal distribution within each diet group before analysis. To evaluate the effect of diet on behavioral tests (EPM, OFT, SpT, and FST) and metabolic outcomes (body weight, fat pad weights, glucose AUC, and fasting glucose and insulin), we used lme4 (4) to build linear mixed-effects models of the form: Phenotype ~ Diet + random effects + error, in which “Diet” is a fixed effect and random effects include litter size (to account for wean weight) and sibling status (to account for the complex family relationships of the HS). For behavioral measures that showed a significant effect of diet according to the above model, we ran an additional model that included body weight (measured the week of testing), retro fat pad weight (taken at time of euthanasia), and starting body weight (measured at time of diet start) as additional fixed effects covariates to rule out an effect of body weight and adiposity on these measures.
Correlations within and between metabolic and behavioral outcomes were analyzed via Pearson correlation with Benjamini-Hochberg correction for multiple comparisons at a 0.05 false discovery rate. Individual body weight and food intake per cage over time were analyzed in Prism (GraphPad Software, San Diego, CA) via repeated-measures ANOVA.
RESULTS
HFD Induces Negative Metabolic Outcomes in HS Rats
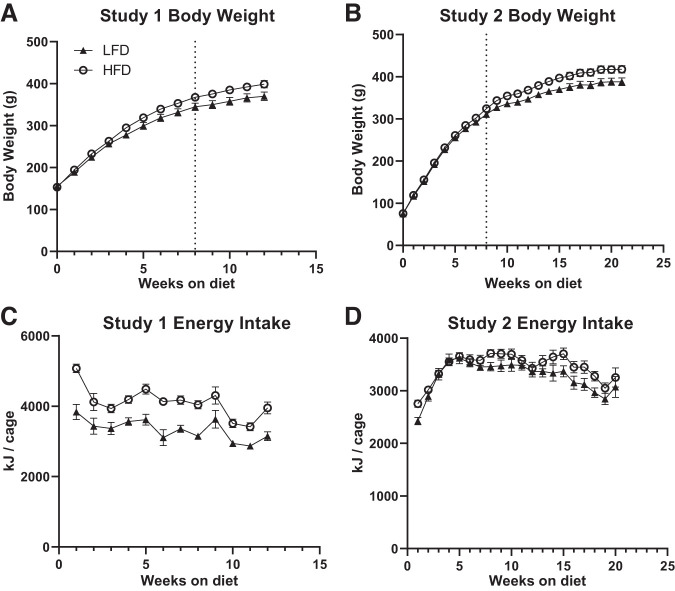
In both studies 1 and 2, there was a significant interaction between diet and time for body weight, with rats fed HFD showing higher body weight over time relative to rats fed LFD (Fig. 2, A and B study 1: F(1, 70) = 5.686, P < 0.0001; study 2 F(1, 70) = 5.414, P < 0.0001). Not surprisingly, rats fed HFD also showed a significant increase in weekly weight gain over time in studies 1 and 2 (F(1, 70) = 8.974, P = 0.0038, F(1, 62) = 5.83, P = 0.02, respectively, data not shown). Animals fed HFD consumed significantly less food over time in both studies 1 and 2 (F(1, 33) = 15.84, P < 0.0005 and F(1, 28) = 58.33, P < 0.0001, respectively, data not shown). That said, animals fed HFD obtained more energy per week compared with animals fed LFD in study 1, although this difference was not statistically significant in study 2 (Fig. 2, C and D, F(1, 33) = 27.81, P < 0.0001 and F(1, 28) = 2.638, P > 0.05, respectively). Interestingly, there was a significant interaction between diet and time for food consumption in study 2, but not study 1 (F(19, 532) = 2.602, P < 0.0005 and F(11, 363) = 0.7198, P > 0.05 respectively). This difference may partially explain the lack of effect of diet on energy consumption in study 2 and is likely explained by the difference in diet start time, with animals in study 2 starting the diet during adolescence. There was, however, no interaction between diet and time for energy consumed in either study 1 or 2 (F(11, 363) = 1.106, P > 0.05 and F(19, 532) = 1.300, P > 0.05).
Fig. 2.
Body weight and energy intake in studies 1 and 2. A, B: body weight was measured weekly in rats from diet start. Rats fed high-fat diet (HFD) showed a significantly higher body weight over time compared with rats fed low-fat diet (LFD). C, D: in study 1, but not study 2, rats on HFD had higher average daily energy intake compared with rats on LFD. Dotted line indicates beginning of testing, IPGTT in study 1 and EPM in study 2. Full statistics are reported in results.
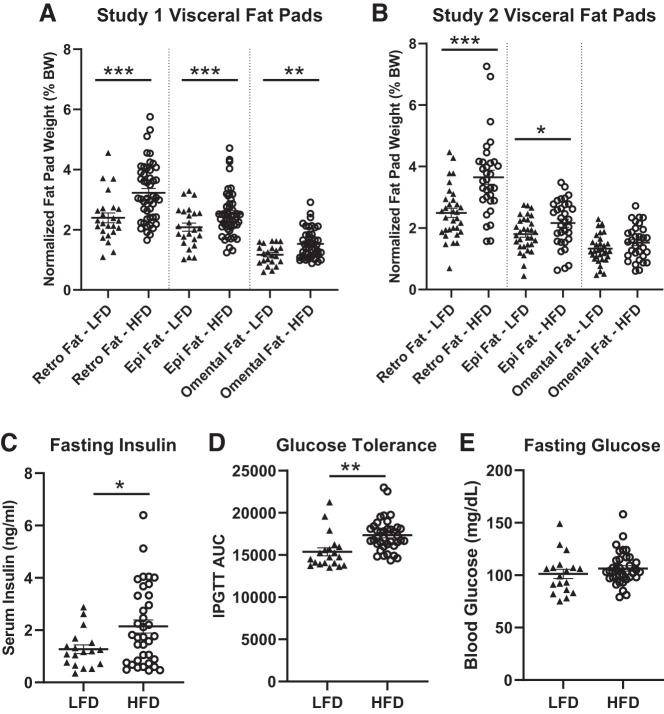
In addition to increased body weight, animals on HFD showed increased retro fat and epi fat in both studies 1 and 2 (Fig. 3, A and B, retro fat: F(1, 70) = 23.557, P < 0.0001, and F(1, 62) = 24.674, P < 0.0001, respectively; epi fat: F(1, 70) = 20.503, P < 0.0002, and F(1, 62) = 5.686, P < 0.05, respectively). Omental fat was significantly higher in rats fed HFD only in study 1 (F(1, 66) = 9.154, P < 0.004 and F(1, 62) = 2.5187, P > 0.05, respectively). In study 1, rats fed HFD exhibited increased fasting insulin (Fig. 3C, F(1, 52) = 5.727, P < 0.05) and reduced glucose tolerance (Fig. 3D, F(1, 56) = 10.851, P < 0.01), with no difference in fasting glucose levels (Fig. 3E, F(1, 52) = 1.6711, P = 0.2033) relative to rats fed LFD. As expected, we see large variation in response to diet for all metabolic traits.
Fig. 3.
Negative metabolic outcomes in rats fed high-fat diet (HFD) compared with low-fat diet (LFD). A, B: rats on HFD had significantly increased fat pad sizes compared with rats on LFD in both studies, except study 2 omental fat. C–E: in study 1, rats fed HFD had significantly increased fasting insulin and decreased glucose tolerance compared with rats fed LFD. Fasting glucose levels were not different between diet groups. Means ± SE are shown. ▲ and ○ represent individual rats on LFD or HFD, respectively; *P < 0.05, **P < 0.01, ***P < 0.001.
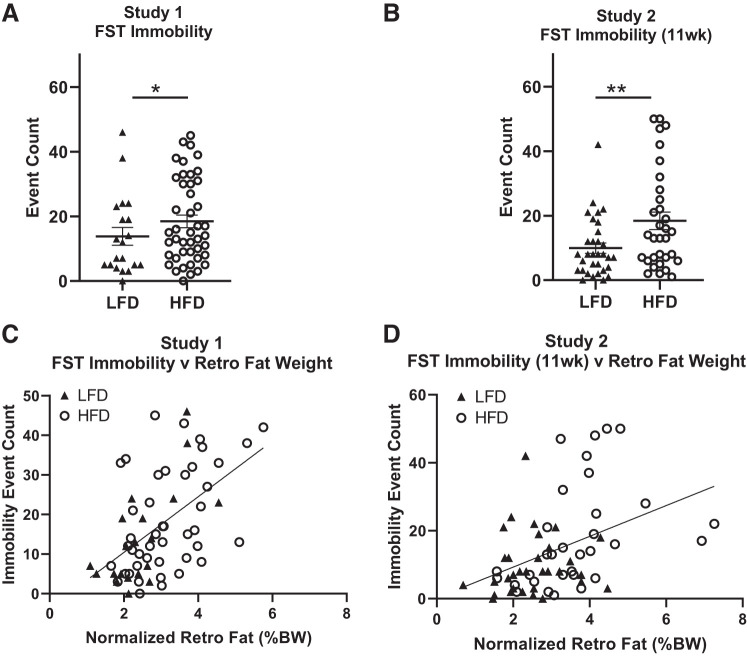
HFD Negatively Impacts Behavior in the FST after 11 Weeks, but not 19 Weeks, of Diet Consumption
In both studies 1 and 2, rats fed HFD showed increased immobility in the FST compared with rats fed LFD (Fig. 4, A and B; F(1, 62) = 6.6399, P = 0.0159, and F(1, 62) = 8.1089, P = 0.0065, respectively). When body weight and fat pad weight were included in the statistical model, however, we found that the effect of diet disappeared, with the effect being driven mainly by fat pad weights (study 1 diet: F(1, 62) = 0.0064, P = 0.9368, study 1 retro fat: F(1, 62) = 15.7877, P = 0.0002; study 2 diet: F(1, 62) = 0.5563, P = 0.4591, study 2 retro fat: F(1, 62) = 15.0994, P = 0.0003). There is large variation in immobility in the HS rats, which confirms previous findings in adolescent HS rats (20). In both studies 1 and 2, a strong positive correlation was found between FST immobility and retro fat pad weight (Fig. 4, C and D; r = 0.5399, P < 0.05, and r = 0.4161, P < 0.05, respectively), as well as epi fat pad weight (r = 0.4653, P < 0.05, and r = 0.5314, P < 0.05, respectively, data not shown). Although the correlation is driven mainly by animals on HFD in study 2 (see Supplementary Table S1; Supplementary information is available at https://doi.org/10.6084/m9.figshare.11897823), the effect is seen in animals on both diets in study 1. This discrepancy indicates that more data are needed to determine if the correlation is driven by diet. FST immobility significantly correlated with omental fat pad weight (r = 0.5885, P < 0.05, and r = 0.3623, P > 0.05, respectively, data not shown) and body weight (r = 0.2885, P < 0.05, and r = 0.1260, P > 0.05; respectively, data not shown) only in study 1.
Fig. 4.
High-fat diet (HFD) induces despair-like phenotypes in the forced swim test (FST). A, B: in both study 1 and study 2, FST immobility increased in rats fed HFD compared with low-fat diet (LFD). C, D: a strong positive correlation was found between FST immobility and retroperitoneal fat pad size in study 1 and study 2 (r = 0.5399, P < 0.05 and r = 0.4161, P < 0.05, respectively). Means ± SE are shown. ▲ and ○ represent individual rats on LFD or HFD, respectively; *P < 0.05, **P < 0.01.
After 19 wk of diet consumption (study 2), although there was a trend toward increased immobility, the effect was no longer statistically significant (F(1, 52) = 3.1484, P > 0.05, data not shown). Mean levels of immobility increased in rats fed both LFD and HFD after the second FST, as expected (5). Some rats (7 HFD and 3 LFD) were excluded from immobility, scoring at 19 wk because of the rat supporting itself on the tank walls.
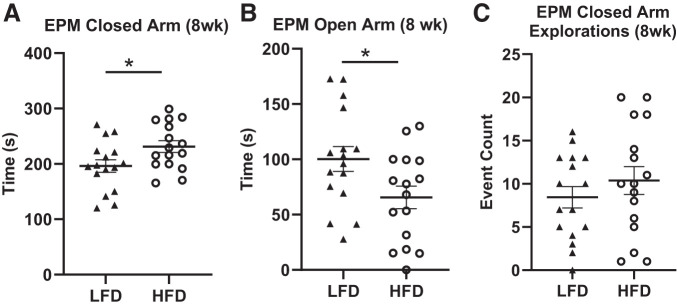
HFD Increases Anxiety-like Behavior in the EPM
We found that rats fed HFD showed significantly increased time spent in the closed arms (Fig. 5A; F(1, 30) = 4.8081, P = 0.0386) and decreased time spent in the open arms (Fig. 5B; F(1, 30) = 4.9417, P = 0.03635) compared with rats fed LFD. When body weight and fat pad weight were included in the model, however, we found that the effect of diet disappeared, indicating a confounding effect of adiposity on the EPM traits. Similar to metabolic measures, there was large variation in these measures in response to diet. Locomotion was assessed in the EPM via explorations (beam break with or without full entry into the arm) and entries (beam break with full entry into the arm) of the closed and open arms. There were no significant differences in closed arm explorations or entries (F(1, 30) = 0.8834, P = 0.3566 (Fig. 5C) and F(1, 30) = 0.0003, P = 0.9867, respectively) or open arm explorations or entries (F(1, 30) = 0.2999, P = 0.5886, and F(1, 30) = 2.5059, P = 0.1286, data not shown) between rats fed HFD vs. LFD, indicating that diet did not affect overall locomotion in the EPM. Rats were tested on two separate mazes. Results reported here are for rats on only one of the mazes because output from the second maze was untrustworthy.
Fig. 5.
High-fat diet (HFD) induces anxiety-like phenotypes in the elevated plus maze (EPM). A–C: a significant increase in EPM closed arm time was seen after 8 wk of HFD compared with low-fat diet (LFD) consumption, as well as a significant decrease in EPM open arm time. No significant difference in closed arm explorations, a measure of general locomotion, was found between diet groups. Means ± SE are shown. ▲ and ○ represent individual rats on LFD or HFD, respectively; *P < 0.05.
After 16 wk of diet consumption, although behavioral responses trended in the same direction as those found after 8 wk of diet consumption, closed arm time (F(1, 54) = 2.3556, P > 0.1, data not shown) and open arm time (F(1, 54) = 3.0632, P > 0.05, data not shown) were no longer statistically significant between rats fed HFD vs. LFD. Rat from both mazes were used for the latter analysis.
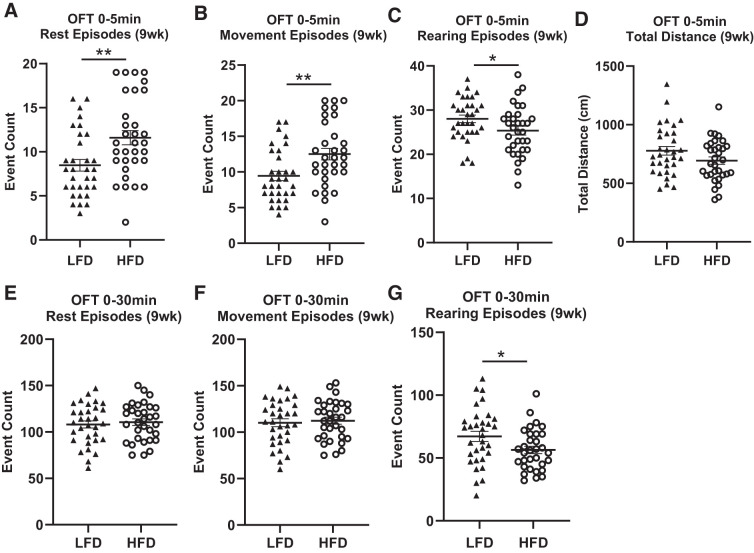
HFD Leads to Anxious Hyperactivity in the OFT
After 9 wk of diet consumption, we found significantly increased episodes of rest (Fig. 6A; F(1, 62) = 9.5121, P = 0.00316) and movement (Fig. 6B; F(1, 62) = 9.3846, P = 0.00335), as well as decreased rearing episodes (Fig. 6C; F(1, 62) = 4.6272, P = 0.0357) after 5 min in the OFT in rats fed HFD compared with those fed LFD, and these measures remained significant after including body weight and fat pad weights as covariates in the statistical model. No effect of diet was seen for center time (F(1, 62) = 0.0758, P > 0.7, data not shown) or total distance traveled (Fig. 6D; F(1, 62) = 2.9148, P > 0.09) after 5 min. We found the effects of diet disappeared after 30 min in the OFT with no differences found between diet for rest episodes (Fig. 6E; F(1, 62) = 0.2545, P > 0.6), movement episodes (Fig. 6F; F(1, 62) = 0.1626, P > 0.6), center time (F(1, 62) = 0.8761, P > 0.3, data not shown), and total distance traveled (F(1, 62) = 1.6021, P > 0.2, data not shown). Similar to after 5 min in the OFT, rearing episodes continued to be significantly lower in rats on HFD relative to LFD after 30 min (Fig. 6G; F(1, 62) = 5.5373, P = 0.02303) and remained significant after including body weight and fat pad weight as fixed factors in the model. These data indicate that the differences in rest and movement episodes during the first 5 min of the test are likely due to anxious hyperactivity and not a result of general locomotion. Again, we see large variation in response to diet.
Fig. 6.
High-fat diet (HFD) induces anxiety-like phenotypes in the OFT. A–C: after 9 wk of diet consumption, rats fed HFD showed a significant increase in rest episodes, movement episodes, and a significant decrease in rearing episodes after 5 min in the open field test (OFT) compared with rats fed low-fat diet (LFD). D: total distance traveled in the OFT was not significantly different between diet groups. E, F: after 30 min, there was no difference between rats fed HFD vs. LFD in rest episodes or movement episodes, but rearing episode count was significantly decreased in rats fed HFD compared with LFD. Means ± SE are shown. ▲ and ○ represent individual rats on LFD or HFD, respectively; *P < 0.05, **P < 0.01.
After 17 wk of diet consumption, rats fed HFD showed reduced ambulatory activity, horizontal activity, and stereotypic behavior after both 5 min (F(1, 62) = 5.377, P = 0.0248; F(1, 62) = 5.8855, P = 0.0192; F(1, 62) = 6.9776, P = 0.0112, respectively, data not shown) and 30 min time period (F(1, 62) = 11.089, P = 0.00169; F(1, 62) = 10.023, P = 0.00271; F(1, 62) = 7.5683, P = 0.00843, respectively, data not shown) relative to rats fed LFD, and these differences remained statistically significant when body weight and fat pad weight were included as covariates in the statistical model. Unlike 9 wk after diet, no significant differences between diet groups were seen for rest, movement, and rearing episodes after 5 min (F(1, 62) = 1.8435, P > 0.05, F(1, 62) = 2.0177, P > 0.05, F(1, 62) = 0.2502, P > 0.05 respectively, data not shown). These data indicate that HFD leads to general lack of movement, as well as decreased exploration after 17 wk of diet consumption.
HFD Rats Show Increased Total Grooming Time in SpT
While no difference was observed between rats fed HFD vs. LFD in latency to begin grooming (Fig. 7A; F(1, 62) = 0.7057, P = 0.405), there was a significant increase in total grooming time by rats fed HFD rats compared with those fed LFD (Fig. 7B; F(1, 62) = 6.1454, P = 0.0168), and this remained statistically significant after including body weight and fat pad weights as covariates in the statistical model.
Fig. 7.
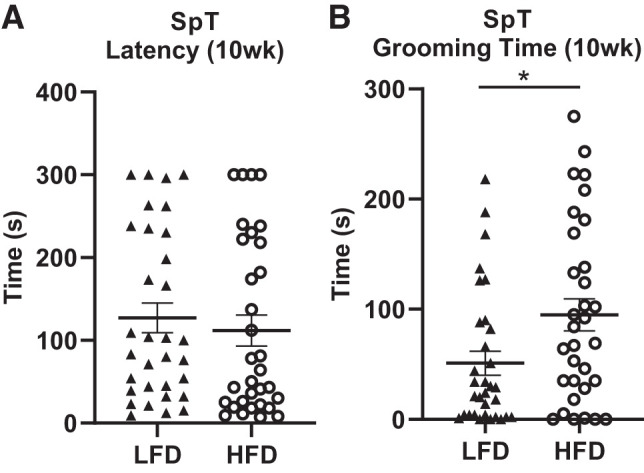
High-fat diet (HFD) increases grooming time in splash test. A: no difference was found between rats fed HFD vs. low-fat diet (LFD) in latency to begin grooming. B: rats fed HFD spent significantly more time grooming in the SpT after 10 wk of diet consumption compared with rats fed LFD. Means ± SE are shown. ▲ and ○ represent individual rats on LFD or HFD, respectively; *P < 0.05.
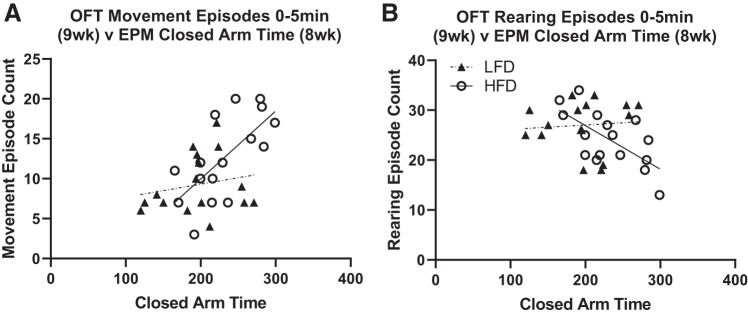
Diet-induced Anxiety-like Measures Correlate between EPM and OFT
We found a significant positive correlation between EPM closed arm time and OFT 0–5 min movement episodes and rest episodes in rats fed HFD (Fig. 8A; r = 0.6785, P < 0.05 and r = 0.6952, P < 0.05, respectively, rest episode data not shown) but not rats fed LFD (Fig. 8A; r = 0.1995, P > 0.05 and r = 0.1783, P > 0.05, respectively, rest episode data not shown). There was also a significant negative correlation between EPM closed arm time and OFT rearing episodes only in rats fed HFD (Fig. 8B; HFD: r = −0.6611, P < 0.05; LFD: r = 0.0788, P > 0.05). Additional behavioral correlations for study 2 are provided in Supplementary Table S1 (https://doi.org/10.6084/m9.figshare.11897823). We did not see correlations between FST measures and OFT, EPM, or SpT measures, suggesting that FST is not measuring anxiety-like behavior as suggested by some groups (2).
Fig. 8.
Diet-specific behavioral correlations between anxiety-like measures. A: rats fed high-fat diet (HFD) show a significant positive correlation between elevated plus maze (EPM) closed arm time and open field test (OFT) movement episode count (r = 0.6785, P < 0.05), while rats fed low-fat diet (LFD) did not correlate between these measures (r = 0.1995, P > 0.05). B: a negative correlation was found between EPM closed arm time and OFT rearing episode count in rats fed HFD (r = −0.6611, P < 0.05) but not LFD (r = 0.0788, P > 0.05).
DISCUSSION
This study is the first to demonstrate negative effects of an HFD on both metabolic and behavioral measures in the outbred HS rat, an animal model that enables genetic fine-mapping and gene identification of complex traits. Specifically, we find that HFD leads to poorer metabolic outcomes as well as passive coping/despair and anxiety-like behaviors in the HS rat. We find that diet directly impacts anxious hyperactivity in the OFT, but that adiposity is a confounding factor for behaviors in the EPM and FST, such that we are unable to claim a direct effect of diet on these measures. The high variability in response to HFD for both metabolic and behavioral measures indicates that the genetics of the HS rat population likely interact with diet to influence these outcomes. It is also interesting to note that anxiety measures in OFT and EPM strongly correlate only when rats are fed HFD, indicating that environmental stress, such as HFD, may be necessary to measure trait anxiety in this model. These results demonstrate the role of diet on metabolism and behavior and establish the HS rat as a model to study gene-by-diet interactions affecting metabolic and behavioral health.
As expected, we demonstrate that HFD negatively impacts metabolic health in the HS rat population. Specifically, in both studies 1 and 2, rats fed HFD exhibit increased body weight and visceral fat pad weights relative to rats fed LFD, despite starting the diet at different ages. In study 1, we also show that rats fed HFD exhibit reduced glucose tolerance and increased fasting insulin relative to rats fed LFD. These findings replicate previous work in other animal models (27) and humans (47). Although statistically significant, the increase in average body weight on HFD compared with LFD is not as large as we were expecting based on studies in other rat strains (7). The modest increase in body weight in HS rats fed HFD may be a result of the genetic variability inherent to the HS population. Indeed, we found large variation in metabolic response to HFD, with some rats gaining much more weight than others. It is known that some inbred strains of mice (27) and rats (7, 12, 30) gain significant amount of weight on HFD, while others do not, supporting a strong genetic component in metabolic response to a HFD. In studies using the diversity outbred (DO) mouse, a highly recombinant outbred model similar to the HS (54), a high-fat/cholic acid diet did not significantly affect body weight (51) but did increase cholesterol and insulin (52), fat mass (61), and induce dyslipidemia (9) relative to a low-fat/high-protein diet. Importantly, quantitative trait loci for metabolic traits in the DO have been shown to interact with diet (61), further supporting a role of genetics. It is also possible that diet had a relatively small effect in the current studies due to the rats being housed at room temperature as opposed to thermoneutral temperatures, which have been shown to increase diet-induced weight gain and metabolic pathophysiological phenotypes in rodents (15). Because the HS rats were pair-housed in the current study, we are unable to determine if the weight gain in HS rats is due to an increase in food intake. We did find, however, that although rats fed HFD in study 1 consumed more kcal than those fed the LFD, they ate less food per day, as previously shown in Sprague-Dawley rats (22).
In addition to negative effects on metabolic health, we show that HFD worsens behavioral health in HS rats. Specifically, in both studies 1 and 2, we show that animals fed HFD exhibit increased immobility in the FST compared with rats fed LFD, although this effect is strongly confounded by fat pad weight . We also find that FST immobility positively correlates with retro fat pad weight. Increased immobility in the FST has been interpreted as despair-like behavior due to its response to acute antidepressant treatment (41). More recently, however, several groups have argued that this behavior is more representative of a passive coping response to an acute stress (11, 38). The current work is unable to differentiate between these two interpretations. Previous work has shown a positive correlation between body weight and immobility in the FST (6), as well as an effect of HFD on immobility in the FST (58). After this initial study, however, it was unclear whether the increased immobility in response to HFD was due to physical limitations of the rat or if it represented increased behavioral despair.
To further investigate if the correlation between fat pad weight and FST immobility was due to behavioral/brain changes in response to diet or physical limitations imposed by increased fat pad sizes, we conducted an additional study to include tests for anxiety (EPM and OFT) and an additional test for despair-like behavior (SpT). Similar to work in other models (65), we find that HS rats fed an HFD exhibit anxiety-like behaviors that are independent of overall locomotion. Specifically, rats fed HFD exhibit increased anxiety in the EPM with increased time spent in the closed arms and decreased time in the open arms relative to rats fed LFD. Rats fed HFD also exhibit anxious hyperactivity and decreased exploration in the OFT with increased movement and rest episodes and decreased rearing episodes relative to rats fed LFD, while no changes are seen in overall locomotion. The current studies show an increase, as opposed to decrease, in SpT total grooming time in rats fed HFD relative to LFD, which is in contrast to previous work. A potential explanation for this is that grooming in the SpT is a marker of anxiety in the HS rat as opposed to decreased self-care as previously proposed (24, 35). The diet-induced effects in the OFT and SpT, but not EPM, are independent of body weight and fat pad, indicating a direct effect of diet on these measures. Similar to the HFD-induced changes in metabolic traits, we see high variation in behavioral response to HFD, suggesting underlying genetics in the HS rat population may be interacting with diet to influence behavioral outcomes.
In study 2, we attempted to determine if increased duration of HFD consumption would affect behavioral phenotypes. We found that rats fed HFD for these longer periods had decreased locomotion in the OFT relative to rats fed LFD, but that all other anxiety-like and passive-coping/despair-like behaviors went away at this later time-point. We expect that this is due to repeated testing, as previous work has shown that repeated testing in the same apparatus shifts behavior toward nonexploratory or nonescape phenotypes (5, 14).
Interestingly, we found strong positive correlations between EPM and OFT anxiety measures only in rats on HFD, supporting the interpretation that a behavioral change is occurring due to HFD consumption. Although studies have shown a correlation between OFT and EPM measures for anxiety-like behaviors (21), other groups have found conflicting results within similar tests (14), highlighting the importance of using more than one behavioral test to properly identify a true behavioral response to novel environments (60). The importance of multiple tests for anxiety-like behavior is to ultimately differentiate state anxiety, the transient display of anxiety phenotypes in a given situation, and trait anxiety, the individual likelihood of displaying anxiety phenotypes in general (29). In the current study, we did not find correlations between EPM and OFT measures in the LFD rats, suggesting state anxiety. In contrast, the strong correlations found between tests in animals on the HFD indicate that HFD has induced trait anxiety, which is more stable and less influenced by subtle changes in environment. Demonstration of stable, replicable traits is important for genetic studies, providing further evidence that HS rats will be useful for identification of genes that interact with diet to influence behavior.
Similar to our findings in the HS rat, studies in humans have also shown an association between “Western” diets high in fat and anxiety or depression (28, 31). One meta-analysis indicated that the association between diet and depression is stronger in subjects who were identified as “Asian” and “other” (31), which could be due to variation in the genetics between populations. Human studies have also shown genetic influences in response to diet on metabolic outcomes (42). Unfortunately, conducting well-controlled studies with sufficient power to identify genes that interact specifically with diet to influence behavior or metabolism is challenging in humans. Based on our current results, we propose HS rats as an alternative model for these studies.
The HS rat is a powerful model that allows for genetic fine-mapping to small genomic intervals (56). Coupled with bioinformatics, transcriptomics, and/or protein modeling, causal genes, and even the underlying causal variants, can be identified. Our laboratory and others have used HS rats or mice to identify genes and/or variants underlying diabetic traits (55), adiposity (26), and fear-related behaviors (43). We have also shown that passive coping/despair-like behavior in the FST is heritable in the HS population (20). To date, however, the HS population has not been used to study how environment, specifically HFD, interacts with genetics to alter metabolism or behavior in this population. The current study lays the groundwork for future investigation to identify genes and variants that predispose individuals to increased adiposity and worsened metabolic and behavioral health specifically under HFD conditions. We expect that this future work will lead to identification of novel shared genetic mechanisms between obesity and behavior. Given the high levels of obesity, the preponderance of HFD worldwide and the complex interplay between obesity and behavioral health, having a model to study the underlying genetics of these traits is of extreme importance.
In conclusion, this work demonstrates that HS rats exhibit worsened metabolic and behavioral health in response to a HFD. Adiposity is a confounding factor for FST and EPM behaviors, suggesting that the diet effect is, in part, due to increased weight gain, and we are unable to unravel if these changes are due to the physical or mental aspects of increased adiposity. In contrast, the effects of diet on behavior in the OFT and SpT are independent of adiposity, suggesting a role of diet on the brain independent of weight gain. The high variability of both metabolic and behavioral measures in response to diet, with some animals showing little or no response to HFD, suggests an interaction between diet and genetics in this population. As such, this work lays the groundwork for a future GWAS to identify genes that create a predisposition to worsened metabolic and behavioral health under HFD conditions.
GRANTS
Funding was provided by the Center for Diabetes, Obesity and Metabolism at Wake Forest School of Medicine Pilot and Feasibility grant and Solberg Woods start-up funds. Support was provided by P50 award AA26117.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.C.S.W. and A.W.D. conceived and designed research; A.W.D., O.S., A.L., N.C., and N.O. performed experiments; A.W.D. analyzed data; A.W.D. and L.C.S.W. interpreted results of experiments; A.W.D. prepared figures; A.W.D. drafted manuscript; O.S., A.L., N.C., N.O., and L.C.S.W. edited and revised manuscript; A.W.D., A.L., N.C., and L.C.S.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jeff Weiner, Ann Chappell, and Eugenia Carter for use of and assistance with behavioral equipment within the Alcohol Center.
REFERENCES
- 1.André C, Dinel AL, Ferreira G, Layé S, Castanon N. Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: focus on brain indoleamine 2,3-dioxygenase activation. Brain Behav Immun 41: 10–21, 2014. doi: 10.1016/j.bbi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Anyan J, Amir S. Too Depressed to Swim or Too Afraid to Stop? A Reinterpretation of the Forced Swim Test as a Measure of Anxiety-Like Behavior. Neuropsychopharmacology 43: 931–933, 2018. doi: 10.1038/npp.2017.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arcego DM, Toniazzo AP, Krolow R, Lampert C, Berlitz C, Dos Santos Garcia E, do Couto Nicola F, Hoppe JB, Gaelzer MM, Klein CP, Lazzaretti C, Dalmaz C. Impact of High-Fat Diet and Early Stress on Depressive-Like Behavior and Hippocampal Plasticity in Adult Male Rats. Mol Neurobiol 55: 2740–2753, 2018. doi: 10.1007/s12035-017-0538-y. [DOI] [PubMed] [Google Scholar]
- 4.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Usinglme4. J Stat Softw 67: 2015. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 5.Bogdanova OV, Kanekar S, D’Anci KE, Renshaw PF. Factors influencing behavior in the forced swim test. Physiol Behav 118: 227–239, 2013. doi: 10.1016/j.physbeh.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenes JC, Rodríguez O, Fornaguera J. Differential effect of environment enrichment and social isolation on depressive-like behavior, spontaneous activity and serotonin and norepinephrine concentration in prefrontal cortex and ventral striatum. Pharmacol Biochem Behav 89: 85–93, 2008. doi: 10.1016/j.pbb.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Buettner R, Schölmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring) 15: 798–808, 2007. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Luo J, Leng Y, Yang Y, Zweifel LS, Palmiter RD, Storm DR. Ablation of Type III Adenylyl Cyclase in Mice Causes Reduced Neuronal Activity, Altered Sleep Pattern, and Depression-like Phenotypes. Biol Psychiatry 80: 836–848, 2016. doi: 10.1016/j.biopsych.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffey AR, Smallwood TL, Albright J, Hua K, Kanke M, Pomp D, Bennett BJ, Sethupathy P. Systems genetics identifies a co-regulated module of liver microRNAs associated with plasma LDL cholesterol in murine diet-induced dyslipidemia. Physiol Genomics 49: 618–629, 2017. doi: 10.1152/physiolgenomics.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem Neurosci 8: 955–960, 2017. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol 299: G440–G448, 2010. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ducottet C, Belzung C. Behaviour in the elevated plus-maze predicts coping after subchronic mild stress in mice. Physiol Behav 81: 417–426, 2004. doi: 10.1016/j.physbeh.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Ennaceur A Tests of unconditioned anxiety - pitfalls and disappointments. Physiol Behav 135: 55–71, 2014. doi: 10.1016/j.physbeh.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 15.Fischer AW, Cannon B, Nedergaard J. Optimal housing temperatures for mice to mimic the thermal environment of humans: An experimental study. Mol Metab 7: 161–170, 2018. doi: 10.1016/j.molmet.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.GBD 2015 Obesity Collaborators; Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med 377: 13–27, 2017. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015-2016. NCHS Data Brief Oct: 1–8, 2017. [PubMed] [Google Scholar]
- 17.Haller J, Aliczki M, Gyimesine Pelczer K. Classical and novel approaches to the preclinical testing of anxiolytics: A critical evaluation. Neurosci Biobehav Rev 37: 2318–2330, 2013. doi: 10.1016/j.neubiorev.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol 327: 1–5, 1984. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- 19.Hansen C, Spuhler K. Development of the National Institutes of Health genetically heterogeneous rat stock. Alcohol Clin Exp Res 8: 477–479, 1984. doi: 10.1111/j.1530-0277.1984.tb05706.x. [DOI] [PubMed] [Google Scholar]
- 20.Holl K, He H, Wedemeyer M, Clopton L, Wert S, Meckes JK, Cheng R, Kastner A, Palmer AA, Redei EE, Solberg Woods LC. Heterogeneous stock rats: a model to study the genetics of despair-like behavior in adolescence. Genes Brain Behav 17: 139–148, 2018. doi: 10.1111/gbb.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu C, Luo Y, Wang H, Kuang S, Liang G, Yang Y, Mai S, Yang J. Re-evaluation of the interrelationships among the behavioral tests in rats exposed to chronic unpredictable mild stress. PLoS One 12: e0185129, 2017. doi: 10.1371/journal.pone.0185129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hume C, Jachs B, Menzies J. Homeostatic responses to palatable food consumption in satiated rats. Obesity (Silver Spring) 24: 2126–2132, 2016. doi: 10.1002/oby.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishikawa J, Ogawa Y, Owada Y, Ishikawa A. Hyperlocomotor activity and stress vulnerability during adulthood induced by social isolation after early weaning are prevented by voluntary running exercise before normal weaning period. Behav Brain Res 264: 197–206, 2014. doi: 10.1016/j.bbr.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Isingrini E, Camus V, Le Guisquet AM, Pingaud M, Devers S, Belzung C. Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: a model of fluoxetine resistance in mice. PLoS One 5: e10404, 2010. doi: 10.1371/journal.pone.0010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johannesson M, Lopez-Aumatell R, Stridh P, Diez M, Tuncel J, Blázquez G, Martinez-Membrives E, Cañete T, Vicens-Costa E, Graham D, Copley RR, Hernandez-Pliego P, Beyeen AD, Ockinger J, Fernández-Santamaría C, Gulko PS, Brenner M, Tobeña A, Guitart-Masip M, Giménez-Llort L, Dominiczak A, Holmdahl R, Gauguier D, Olsson T, Mott R, Valdar W, Redei EE, Fernández-Teruel A, Flint J. A resource for the simultaneous high-resolution mapping of multiple quantitative trait loci in rats: the NIH heterogeneous stock. Genome Res 19: 150–158, 2009. doi: 10.1101/gr.081497.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keele GR, Prokop JW, He H, Holl K, Littrell J, Deal A, Francic S, Cui L, Gatti DM, Broman KW, Tschannen M, Tsaih SW, Zagloul M, Kim Y, Baur B, Fox J, Robinson M, Levy S, Flister MJ, Mott R, Valdar W, Solberg Woods LC. Genetic Fine-Mapping and Identification of Candidate Genes and Variants for Adiposity Traits in Outbred Rats. Obesity (Silver Spring) 26: 213–222, 2018. doi: 10.1002/oby.22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreznar JH, Keller MP, Traeger LL, Rabaglia ME, Schueler KL, Stapleton DS, Zhao W, Vivas EI, Yandell BS, Broman AT, Hagenbuch B, Attie AD, Rey FE. Host Genotype and Gut Microbiome Modulate Insulin Secretion and Diet-Induced Metabolic Phenotypes. Cell Reports 18: 1739–1750, 2017. doi: 10.1016/j.celrep.2017.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang UE, Beglinger C, Schweinfurth N, Walter M, Borgwardt S. Nutritional aspects of depression. Cell Physiol Biochem 37: 1029–1043, 2015. doi: 10.1159/000430229. [DOI] [PubMed] [Google Scholar]
- 29.Leal PC, Goes TC, da Silva LCF, Teixeira-Silva F. Trait vs. state anxiety in different threatening situations. Trends Psychiatry Psychother 39: 147–157, 2017. doi: 10.1590/2237-6089-2016-0044. [DOI] [PubMed] [Google Scholar]
- 30.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol 273: R725–R730, 1997. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Lv MR, Wei YJ, Sun L, Zhang JX, Zhang HG, Li B. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res 253: 373–382, 2017. doi: 10.1016/j.psychres.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 32.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Mägi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Zhao JH, Zhao W, Chen J, Fehrmann R, Hedman ÅK, Karjalainen J, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bolton JL, Bragg-Gresham JL, Buyske S, Demirkan A, Deng G, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Goel A, Gong J, Jackson AU, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Mangino M, Leach IM, Medina-Gomez C, Medland SE, Nalls MA, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Shungin D, Stančáková A, Strawbridge RJ, Sung YJ, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Isaacs A, Albrecht E, Ärnlöv J, Arscott GM, Attwood AP, Bandinelli S, Barrett A, Bas IN, Bellis C, Bennett AJ, Berne C, Blagieva R, Blüher M, Böhringer S, Bonnycastle LL, Böttcher Y, Boyd HA, Bruinenberg M, Caspersen IH, Chen YI, Clarke R, Daw EW, de Craen AJM, Delgado G, Dimitriou M, Doney ASF, Eklund N, Estrada K, Eury E, Folkersen L, Fraser RM, Garcia ME, Geller F, Giedraitis V, Gigante B, Go AS, Golay A, Goodall AH, Gordon SD, Gorski M, Grabe HJ, Grallert H, Grammer TB, Gräßler J, Grönberg H, Groves CJ, Gusto G, Haessler J, Hall P, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hengstenberg C, Holmen O, Hottenga JJ, James AL, Jeff JM, Johansson Å, Jolley J, Juliusdottir T, Kinnunen L, Koenig W, Koskenvuo M, Kratzer W, Laitinen J, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindström J, Lo KS, Lobbens S, Lorbeer R, Lu Y, Mach F, Magnusson PKE, Mahajan A, McArdle WL, McLachlan S, Menni C, Merger S, Mihailov E, Milani L, Moayyeri A, Monda KL, Morken MA, Mulas A, Müller G, Müller-Nurasyid M, Musk AW, Nagaraja R, Nöthen MM, Nolte IM, Pilz S, Rayner NW, Renstrom F, Rettig R, Ried JS, Ripke S, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Scott WR, Seufferlein T, Shi J, Smith AV, Smolonska J, Stanton AV, Steinthorsdottir V, Stirrups K, Stringham HM, Sundström J, Swertz MA, Swift AJ, Syvänen AC, Tan ST, Tayo BO, Thorand B, Thorleifsson G, Tyrer JP, Uh HW, Vandenput L, Verhulst FC, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Warren HR, Waterworth D, Weedon MN, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q, LifeLines Cohort Study, Brennan EP, Choi M, Dastani Z, Drong AW, Eriksson P, Franco-Cereceda A, Gådin JR, Gharavi AG, Goddard ME, Handsaker RE, Huang J, Karpe F, Kathiresan S, Keildson S, Kiryluk K, Kubo M, Lee JY, Liang L, Lifton RP, Ma B, McCarroll SA, McKnight AJ, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Okada Y, Perry JRB, Dorajoo R, Reinmaa E, Salem RM, Sandholm N, Scott RA, Stolk L, Takahashi A, Tanaka T, van ’t Hooft FM, Vinkhuyzen AAE, Westra HJ, Zheng W, Zondervan KT, ADIPOGen Consortium, AGEN-BMI Working Group, CARDIOGRAMplusC4D Consortium, CKDGen Consortium, GLGC, ICBP, MAGIC Investigators, MuTHER Consortium, MIGen Consortium, PAGE Consortium, ReproGen Consortium, GENIE Consortium; International Endogene Consortium, Heath AC, Arveiler D, Bakker SJL, Beilby J, Bergman RN, Blangero J, Bovet P, Campbell H, Caulfield MJ, Cesana G, Chakravarti A, Chasman DI, Chines PS, Collins FS, Crawford DC, Cupples LA, Cusi D, Danesh J, de Faire U, den Ruijter HM, Dominiczak AF, Erbel R, Erdmann J, Eriksson JG, Farrall M, Felix SB, Ferrannini E, Ferrières J, Ford I, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gejman PV, Gieger C, Gottesman O, Gudnason V, Gyllensten U, Hall AS, Harris TB, Hattersley AT, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Homuth G, Hovingh GK, Humphries SE, Hunt SC, Hyppönen E, Illig T, Jacobs KB, Jarvelin MR, Jöckel KH, Johansen B, Jousilahti P, Jukema JW, Jula AM, Kaprio J, Kastelein JJP, Keinanen-Kiukaanniemi SM, Kiemeney LA, Knekt P, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Marchand LL, Lehtimäki T, Lyssenko V, Männistö S, Marette A, Matise TC, McKenzie CA, McKnight B, Moll FL, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Madden PAF, Pasterkamp G, Peden JF, Peters A, Postma DS, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Rioux JD, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schunkert H, Schwarz PEH, Sever P, Shuldiner AR, Sinisalo J, Stolk RP, Strauch K, Tönjes A, Trégouët DA, Tremblay A, Tremoli E, Virtamo J, Vohl MC, Völker U, Waeber G, Willemsen G, Witteman JC, Zillikens MC, Adair LS, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bornstein SR, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PIW, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hui J, Hunter DJ, Hveem K, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, März W, Melbye M, Metspalu A, Moebus S, Munroe PB, Njølstad I, Oostra BA, Palmer CNA, Pedersen NL, Perola M, Pérusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sattar N, Schadt EE, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Thorsteinsdottir U, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Walker M, Wallaschofski H, Wareham NJ, Watkins H, Weir DR, Wichmann HE, Wilson JF, Zanen P, Borecki IB, Deloukas P, Fox CS, Heid IM, O’Connell JR, Strachan DP, Stefansson K, van Duijn CM, Abecasis GR, Franke L, Frayling TM, McCarthy MI, Visscher PM, Scherag A, Willer CJ, Boehnke M, Mohlke KL, Lindgren CM, Beckmann JS, Barroso I, North KE, Ingelsson E, Hirschhorn JN, Loos RJF, Speliotes EK. Genetic studies of body mass index yield new insights for obesity biology. Nature 518: 197–206, 2015. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 67: 220–229, 2010. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 34.McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiol Behav 73: 705–717, 2001. doi: 10.1016/S0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- 35.Mendez-David I, Boursier C, Domergue V, Colle R, Falissard B, Corruble E, Gardier AM, Guilloux JP, David DJ. Differential Peripheral Proteomic Biosignature of Fluoxetine Response in a Mouse Model of Anxiety/Depression. Front Cell Neurosci 11: 237, 2017. doi: 10.3389/fncel.2017.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milaneschi Y, Lamers F, Peyrot WJ, Baune BT, Breen G, Dehghan A, Forstner AJ, Grabe HJ, Homuth G, Kan C, Lewis C, Mullins N, Nauck M, Pistis G, Preisig M, Rivera M, Rietschel M, Streit F, Strohmaier J, Teumer A, Van der Auwera S, Wray NR, Boomsma DI, Penninx BWJH; CHARGE Inflammation Working Group and the Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium . Genetic Association of Major Depression With Atypical Features and Obesity-Related Immunometabolic Dysregulations. JAMA Psychiatry 74: 1214–1225, 2017. doi: 10.1001/jamapsychiatry.2017.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry 24: 18–33, 2019. doi: 10.1038/s41380-018-0017-5. [DOI] [PubMed] [Google Scholar]
- 38.Molendijk ML, de Kloet ER. Coping with the forced swim stressor: Current state-of-the-art. Behav Brain Res 364: 1–10, 2019. doi: 10.1016/j.bbr.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Mutlu O, Gumuslu E, Ulak G, Celikyurt IK, Kokturk S, Kır HM, Akar F, Erden F. Effects of fluoxetine, tianeptine and olanzapine on unpredictable chronic mild stress-induced depression-like behavior in mice. Life Sci 91: 1252–1262, 2012. doi: 10.1016/j.lfs.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 40.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14: 149–167, 1985. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 41.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229: 327–336, 1977. [PubMed] [Google Scholar]
- 42.Rana A, Jain S, Puri N, Kaw M, Sirianni N, Eren D, Mopidevi BR, Kumar A. The transcriptional regulation of the human angiotensinogen gene after high-fat diet is haplotype-dependent: Novel insights into the gene-regulatory networks and implications for human hypertension. PLoS One 12: e0176373, 2017. doi: 10.1371/journal.pone.0176373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rat Genome Sequencing and Mapping Consortium; Baud A, Hermsen R, Guryev V, Stridh P, Graham D, McBride MW, Foroud T, Calderari S, Diez M, Ockinger J, Beyeen AD, Gillett A, Abdelmagid N, Guerreiro-Cacais AO, Jagodic M, Tuncel J, Norin U, Beattie E, Huynh N, Miller WH, Koller DL, Alam I, Falak S, Osborne-Pellegrin M, Martinez-Membrives E, Canete T, Blazquez G, Vicens-Costa E, Mont-Cardona C, Diaz-Moran S, Tobena A, Hummel O, Zelenika D, Saar K, Patone G, Bauerfeind A, Bihoreau MT, Heinig M, Lee YA, Rintisch C, Schulz H, Wheeler DA, Worley KC, Muzny DM, Gibbs RA, Lathrop M, Lansu N, Toonen P, Ruzius FP, de Bruijn E, Hauser H, Adams DJ, Keane T, Atanur SS, Aitman TJ, Flicek P, Malinauskas T, Jones EY, Ekman D, Lopez-Aumatell R, Dominiczak AF, Johannesson M, Holmdahl R, Olsson T, Gauguier D, Hubner N, Fernandez-Teruel A, Cuppen E, Mott R, Mott R, Flint J. Combined sequence-based and genetic mapping analysis of complex traits in outbred rats. Nat Genet 45: 767–775, 2013. doi: 10.1038/ng.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rivera M, Locke AE, Corre T, Czamara D, Wolf C, Ching-Lopez A, Milaneschi Y, Kloiber S, Cohen-Woods S, Rucker J, Aitchison KJ, Bergmann S, Boomsma DI, Craddock N, Gill M, Holsboer F, Hottenga JJ, Korszun A, Kutalik Z, Lucae S, Maier W, Mors O, Müller-Myhsok B, Owen MJ, Penninx BWJH, Preisig M, Rice J, Rietschel M, Tozzi F, Uher R, Vollenweider P, Waeber G, Willemsen G, Craig IW, Farmer AE, Lewis CM, Breen G, McGuffin P. Interaction between the FTO gene, body mass index and depression: meta-analysis of 13701 individuals. Br J Psychiatry 211: 70–76, 2017. doi: 10.1192/bjp.bp.116.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rockman MV, Kruglyak L. Breeding designs for recombinant inbred advanced intercross lines. Genetics 179: 1069–1078, 2008. doi: 10.1534/genetics.107.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rohde K, Keller M, la Cour Poulsen L, Bluher M, Kovacs P, Bottcher Y. Genetics and epigenetics in obesity. Metabolism 92: 37–50, 2019. doi: 10.1016/j.metabol.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Rosenbaum M, Knight R, Leibel RL. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab 26: 493–501, 2015. doi: 10.1016/j.tem.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samaan Z, Anand SS, Zhang X, Desai D, Rivera M, Pare G, Thabane L, Xie C, Gerstein H, Engert JC, Craig I, Cohen-Woods S, Mohan V, Diaz R, Wang X, Liu L, Corre T, Preisig M, Kutalik Z, Bergmann S, Vollenweider P, Waeber G, Yusuf S, Meyre D. The protective effect of the obesity-associated rs9939609 A variant in fat mass- and obesity-associated gene on depression. Mol Psychiatry 18: 1281–1286, 2013. doi: 10.1038/mp.2012.160. [DOI] [PubMed] [Google Scholar]
- 49.Scott KM, McGee MA, Wells JE, Oakley Browne MA. Obesity and mental disorders in the adult general population. J Psychosom Res 64: 97–105, 2008. doi: 10.1016/j.jpsychores.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Sharma S, Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int J Obes 37: 382–389, 2013. doi: 10.1038/ijo.2012.48. [DOI] [PubMed] [Google Scholar]
- 51.Shorter JR, Huang W, Beak JY, Hua K, Gatti DM, de Villena FP, Pomp D, Jensen BC. Quantitative trait mapping in Diversity Outbred mice identifies two genomic regions associated with heart size. Mamm Genome 29: 80–89, 2018. [Erratum in Mamm Genome 30: 42, 2019] doi: 10.1007/s00335-017-9730-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smallwood TL, Gatti DM, Quizon P, Weinstock GM, Jung KC, Zhao L, Hua K, Pomp D, Bennett BJ. High-resolution genetic mapping in the diversity outbred mouse population identifies Apobec1 as a candidate gene for atherosclerosis. G3 (Bethesda) 4: 2353–2363, 2014. doi: 10.1534/g3.114.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solberg LC, Baum AE, Ahmadiyeh N, Shimomura K, Li R, Turek FW, Churchill GA, Takahashi JS, Redei EE. Sex- and lineage-specific inheritance of depression-like behavior in the rat. Mamm Genome 15: 648–662, 2004. doi: 10.1007/s00335-004-2326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Solberg Woods LC QTL mapping in outbred populations: successes and challenges. Physiol Genomics 46: 81–90, 2014. doi: 10.1152/physiolgenomics.00127.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solberg Woods LC, Holl KL, Oreper D, Xie Y, Tsaih SW, Valdar W. Fine-mapping diabetes-related traits, including insulin resistance, in heterogeneous stock rats. Physiol Genomics 44: 1013–1026, 2012. doi: 10.1152/physiolgenomics.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solberg Woods LC, Palmer AA. Using Heterogeneous Stocks for Fine-Mapping Genetically Complex Traits. Methods Mol Biol 2018: 233–247, 2019. doi: 10.1007/978-1-4939-9581-3_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solberg Woods LC, Stelloh C, Regner KR, Schwabe T, Eisenhauer J, Garrett MR. Heterogeneous stock rats: a new model to study the genetics of renal phenotypes. Am J Physiol Renal Physiol 298: F1484–F1491, 2010. doi: 10.1152/ajprenal.00002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soulis G, Papalexi E, Kittas C, Kitraki E. Early impact of a fat-enriched diet on behavioral responses of male and female rats. Behav Neurosci 121: 483–490, 2007. doi: 10.1037/0735-7044.121.3.483. [DOI] [PubMed] [Google Scholar]
- 59.Stergiakouli E, Gaillard R, Tavaré JM, Balthasar N, Loos RJ, Taal HR, Evans DM, Rivadeneira F, St Pourcain B, Uitterlinden AG, Kemp JP, Hofman A, Ring SM, Cole TJ, Jaddoe VW, Davey Smith G, Timpson NJ. Genome-wide association study of height-adjusted BMI in childhood identifies functional variant in ADCY3. Obesity (Silver Spring) 22: 2252–2259, 2014. doi: 10.1002/oby.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sudakov SK, Nazarova GA, Alekseeva EV, Bashkatova VG. Estimation of the level of anxiety in rats: differences in results of open-field test, elevated plus-maze test, and Vogel’s conflict test. Bull Exp Biol Med 155: 295–297, 2013. doi: 10.1007/s10517-013-2136-y. [DOI] [PubMed] [Google Scholar]
- 61.Tyler AL, Ji B, Gatti DM, Munger SC, Churchill GA, Svenson KL, Carter GW. Epistatic Networks Jointly Influence Phenotypes Related to Metabolic Disease and Gene Expression in Diversity Outbred Mice. Genetics 206: 621–639, 2017. doi: 10.1534/genetics.116.198051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valdar W, Flint J, Mott R. Simulating the collaborative cross: power of quantitative trait loci detection and mapping resolution in large sets of recombinant inbred strains of mice. Genetics 172: 1783–1797, 2006. doi: 10.1534/genetics.104.039313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wray NR, Pergadia ML, Blackwood DH, Penninx BW, Gordon SD, Nyholt DR, Ripke S, MacIntyre DJ, McGhee KA, Maclean AW, Smit JH, Hottenga JJ, Willemsen G, Middeldorp CM, de Geus EJ, Lewis CM, McGuffin P, Hickie IB, van den Oord EJ, Liu JZ, Macgregor S, McEvoy BP, Byrne EM, Medland SE, Statham DJ, Henders AK, Heath AC, Montgomery GW, Martin NG, Boomsma DI, Madden PA, Sullivan PF. Genome-wide association study of major depressive disorder: new results, meta-analysis, and lessons learned. Mol Psychiatry 17: 36–48, 2012. doi: 10.1038/mp.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, Frayling TM, Hirschhorn J, Yang J, Visscher PM; GIANT Consortium . Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet 27: 3641–3649, 2018. doi: 10.1093/hmg/ddy271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zemdegs J, Quesseveur G, Jarriault D, Pénicaud L, Fioramonti X, Guiard BP. High-fat diet-induced metabolic disorders impairs 5-HT function and anxiety-like behavior in mice. Br J Pharmacol 173: 2095–2110, 2016. doi: 10.1111/bph.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]