Abstract
We have tested the feasibility of thermal grills, a harmless method to induce pain. The thermal grills consist of interlaced tubes that are set at cool or warm temperatures, creating a painful “illusion” (no tissue injury is caused) in the brain when the cool and warm stimuli are presented collectively. Advancement in objective pain assessment research is limited because the gold standard, the self-reporting pain scale, is highly subjective and only works for alert and cooperative patients. However, the main difficulty for pain studies is the potential harm caused to participants. We have recruited 23 subjects in whom we induced electric pulses and thermal grill (TG) stimulation. The TG effectively induced three different levels of pain, as evidenced by the visual analog scale (VAS) provided by the subjects after each stimulus. Furthermore, objective physiological measurements based on electrodermal activity showed a significant increase in levels as stimulation level increased. We found that VAS was highly correlated with the TG stimulation level. The TG stimulation safely elicited pain levels up to 9 out of 10. The TG stimulation allows for extending studies of pain to ranges of pain in which other stimuli are harmful.
Keywords: electric pulses, electrodermal activity, pain, thermal grill, visual analog scale
INTRODUCTION
Chronic pain is considered a disease in its own right (57) that afflicts 1 in 3 adults in the United States (44, 66). It causes an economic burden of $560–$635 billion annually, which is more than heart disease, cancer, and diabetes (18). Most often, chronic pain in patients is managed by prescription opioids. However, long-term use of opioids comes with severe side effects of addiction and tolerance that underlie the current epidemic of prescription opioid abuse in the United States (9, 53), costing about $500 billion in medical, economic, social, and criminal ramifications per year (55). Developing new treatments for chronic pain, especially nonopioid alternatives, is hindered by the lack of an objective metric of pain. Currently, the gold standard to quantify pain intensity in humans is the numerical rating scale (NRS) or the visual analog scale (VAS) reported by the human subject (17a), i.e., a subjective score usually between 0 and 10, with 0 for no pain and 10 for the worst pain possible. These methods are highly subjective and only work for alert and cooperative patients. For these reasons, there is a growing interest in pain studies aiming to develop objective measurements of pain. Although several approaches to study pain exist, the main difficulty for pain studies is the potential harm and tissue injuries caused to participants (19).
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage (69). In pain studies, quantification of pain levels depends greatly on the repeatability and effectiveness of stimulations that evoke pain (59). Traditionally, subjects are stimulated by the application of pressure to body regions like the nail bed (1) or area adjacent to a surgical incision site (60), heat or cold temperature to regions of the skin (23, 67), and electric shocks (19, 23). For all the above stimuli to cause high-intensity pain, potential damage and harm to the stimulated regions can occur in participants.
Alternatively, different levels of pain can potentially be elicited in human subjects with noninjurious stimuli by implementing Thunberg’s thermal grill (TG) illusion (10). A thermal grill consists of interlaced tubes that are set at cool (e.g., 18°C) or warm (e.g., 40–45°C) water temperatures without causing tissue injury. However, it creates a painful “illusion” in the brain when the cool and warm stimuli are presented collectively (10, 13, 21, 28). We thus hypothesize that the thermal grill is an effective stimulus in pain studies to produce repeatable yet harmless painful stimuli. Additionally, patients with various chronic pain conditions reportedly showed enhanced sympathetic neural activities and reduced parasympathetic neural activities as compared with healthy controls, including headache (26, 43), inflammatory joint disease (51), (52), and irritable bowel syndrome (30). The above anatomic and pain assessing evidence collectively indicate autonomic nervous system (ANS) activities as an objective assessment of pain level with the potential of being a biomarker for patients with chronic pain conditions.
In this study, we validated the effectiveness of the thermal grills to elicit different levels of pain by both subject self-reported VAS and an objective metric of sympathetic neural activities from recordings of electrodermal activities (EDA). To do this, we evoked high-intensity pain in human volunteers by using safe and noninjurious stimuli via a thermal grill. Additionally, we validated the intensity of pain by both subjective measures of subject-reported pain scores and an objective metric of sympathetic neural activities from EDA recordings.
MATERIALS AND METHODS
Subjects
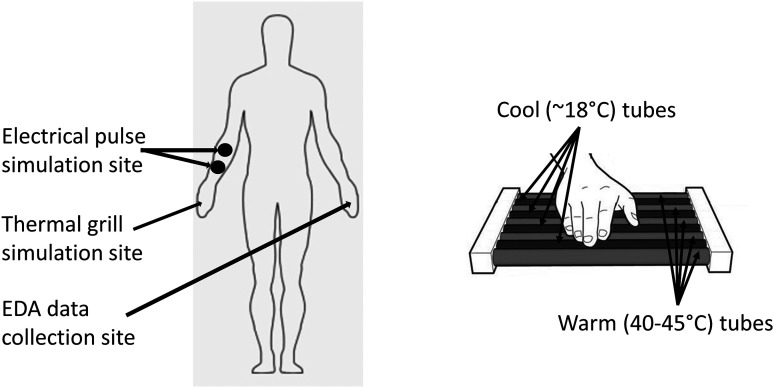
All human study protocols were approved by the University of Connecticut Institutional Review Board. All volunteers were provided information of the protocols, and they provided written informed consent to participate in the study. Twenty-three healthy volunteers (11 males and 12 females) of ages ranging from 19 to 34 yr old (24.5 ± 4.8; means ± SD) were enrolled in this study. To reduce the presence and variability of confounding autonomic reactions, the study was conducted in a quiet and dimly lighted room (ambient temperature, 26–27°C) and participants were asked to avoid caffeine and alcohol during the 24 h preceding the test and instructed to fast for at least 3 h before every test. Of the 23 enrolled subjects, 16 volunteered to undergo both the electrical pulse (EP) stimulation and the thermal grill (TG) stimulation after receiving detailed explanations on the protocols. EP stimulation is selected as reference as it is a commonly used technique to induce different levels of pain (19, 23). Seven subjects participated only in the TG tests and chose not to receive the EP stimulation. As illustrated in Fig. 1, EP was applied to the skin on the subject’s right arm and TG was applied to the subject’s entire glabrous skin of the right palm. Throughout the stimulus protocols, EDA data were collected from the subject’s left hand’s finger skin and processed post hoc in both time and frequency domains to assess and quantify the level of sympathetic activities.
Fig. 1.
Stimulation sites and electrodermal activity (EDA) data collection site (left). Magnified view of thermal grill stimulation on subjects’ entire glabrous skin of the right palm (right).
Devices for Recording Electrodermal Activities
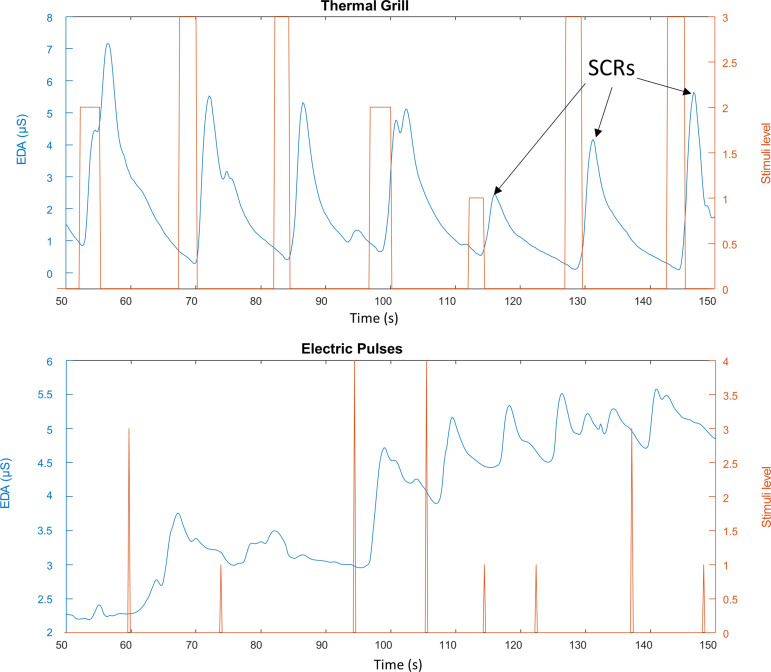
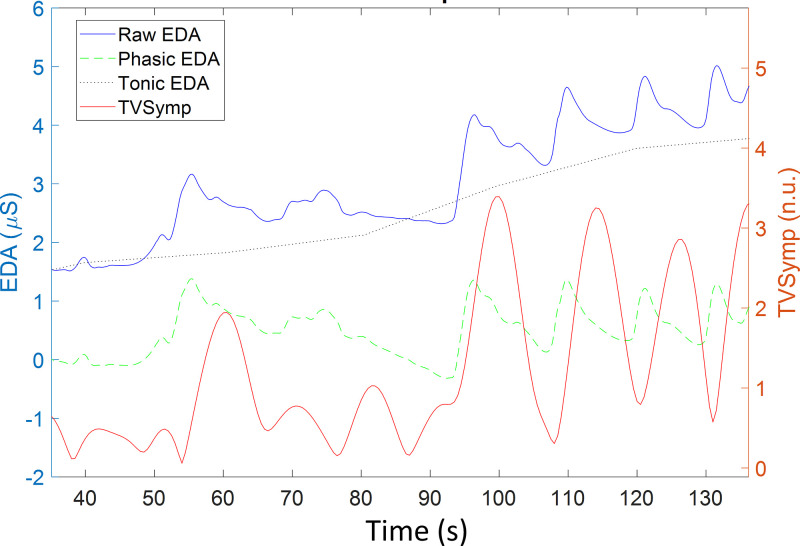
EDA is a marker of sympathetic autonomic control (11), and because of its sensitivity and simplicity (6, 45, 46), it has been recently used to assess subjects’ responses to pain stimulation (1, 11, 14, 60, 64, 71). A galvanic skin response device (GSR MP160) and amplifier (BIONAMADIX 2CH Amp) were used to collect EDA data from the subject’s finger skin during the EP and TG test protocols. The EDA signals were recorded at 500 Hz and stored by using the BIONAMADIX amplifier, MP160 device, and the AcqKnowledge software (BIOPAC Systems, I). EDA electrodes were placed on the index and middle fingers of the subject’s left hand (Fig. 1). EDA is technically a conductance measurement; hence, it does not require a reference electrode. Figure 2 shows a segment of typical EDA recordings from a human subject undergoing TG (top) and EP (bottom) stimulation. Stimuli are shown in red, and the stimulation level for each stimulus is indicated in the right y-axis of the figure.
Fig. 2.
Typical electrodermal activity (EDA) recordings from a human subject undergoing pain stimulation. Top: thermal grill stimulation. The width of each rectangle represents the time the subject sustained their hand on the specific grill. Bottom: electric pulse (EP) stimulation. Each spike represents a 10-ms EP stimulus. SCRs, skin conductance responses.
Protocols for Electrical Pulse and Thermal Grill Stimulations
Subjects either underwent the EP stimulation protocol followed by the TG stimulation protocol or received only the TG stimulation protocol. Each protocol contains stimulus tests of varying intensities. Before each stimulus test, the subject was asked to stay still resting in a chair for at least 5 min to reach hemodynamic stabilization. Detailed descriptions of both EP and TG protocols are as follows. Each subject practiced both tests during the calibration stages (described below) and before undergoing each test.
Electrical pulse stimulation.
EP stimulation was performed with a programmable stimulus isolation adapter STMISOC (BIOPAC Systems), which allows software-controlled adjustment of stimulus pulse width, repetition, and setup of arbitrary pulse stimulus sequence. Electrical pulses were delivered through two disposable Ag/AgCl electrodes located 5 cm apart on the right forearm, following Application Note 257, Safe Use of Electrical Stimulators (4a).
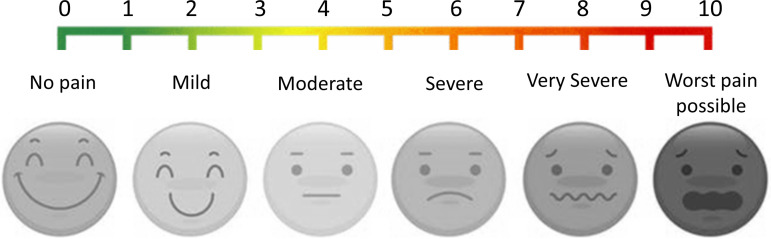
For each subject, we first determined the customized maximum stimulation level that evoked a subject-reported pain level of 7 out of 10, i.e., above severe pain level. The maximum stimulation level was determined by adjusting the electrical pulse amplitude while fixing the pulse width to be 10 ms. The pulse amplitude at maximum stimulation level was termed Smax. Then, we set the four stimulating intensities with pulse amplitude at 25%, 50%, 75%, and 100% of Smax, i.e., S25, S50, S75, and Smax, respectively. This represents four different levels of stimulation using EP. This calibration process lasted ~5 min for each subject. We then used a custom-built computer program to randomly generate a sequence of electrical pulses (10-ms pulse width) with varying amplitudes of S25, S50, S75, and Smax. Subjects were provided with a VAS, as shown in Fig. 3, and asked to report the pain level (0 to 10) verbally right after each stimulus. Pain level was noted as 0 if subject did not report any pain after the stimulus. The EP stimulation test lasted ~30 min. In Fig. 2 (bottom), each spike represents a 10-ms EP stimulus.
Fig. 3.
Visual analog scale (VAS) used in this study.
Thermal grill stimulation.
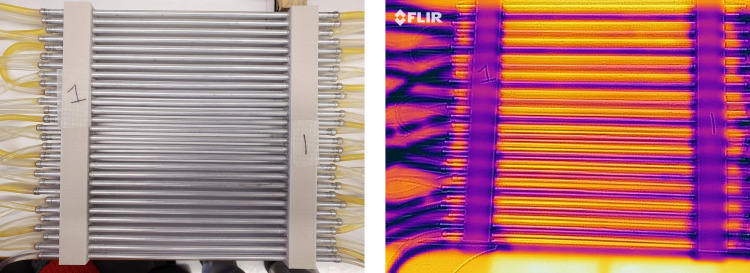
TG stimulation of the hand’s glabrous skin was delivered by a set of three custom-built thermal grills, which consist of parallel aluminum tubes (1/8 inch outer diameter) spaced at 5 mm between the central axes. The grill tubes were perfused with water from either an ice-cold water reservoir (4°C) or a feedback-controlled warm water bath (Isotemp GPD 2S, Fisher Scientific). The cool and warm grill tubes were spatially alternate with one another. The temperature of the warm grill tubes was controlled by adjusting the water bath temperature. To adjust the temperature of the cool grill bars, we perfused the ice-cold water through a warm water jacket, the temperature of which is controlled by another water bath (Isotemp GPD 2S). The temperature pattern of the thermal grill was validated by an infrared imaging camera with 0.1°C detection resolution (FLIR One, FLIR Systems, Wilsonville, OR). Figure 4 shows an example of the infrared images obtained. To appropriately set the stimulus intensity of the thermal grills for each tested subject, we first maintained the cool grill temperature to be ~18°C while fine-adjusting the warm grill temperature to be between 35 and 47°C so that the subject reported a pain score of 5–6 out of 10, i.e., the threshold of severe pain. That temperature was labeled as Tm and set for the second thermal grill (TG2). We then set the warm grill temperature in the first thermal grill (TG1) to be 2°C below Tm and the warm grill temperature in the third thermal grill (TG3) to be 2°C above Tm. To avoid injury, we ensured that the maximum grill temperature was below 47°C. Thus, TG1, TG2, and TG3 were set to induce three different levels of pain that generally fall into the moderate, severe, and very severe levels in the VAS in Fig. 3. The calibration of the TG stimulation test lasted ~10 min. The TG stimulus protocol was set to include 21 stimulus tests. In each test, the subject was asked to fully press his/her right palm on one of the three TGs and maintain contact for at least 3 s but no more than 10 s; the subject was allowed to lift up the palm at any time when feeling unbearable pain sensation. The 21 stimulus tests consisted of a randomized sequence of 7 stimuli from each of the three TGs. The interstimulus interval was set to be 1 min. The TG stimulation test lasted ~30 min. Subjects were provided with a VAS, as shown in Fig. 3, and asked to report the pain level (0 to 10) verbally right after each stimulus. The width of each rectangle in Fig. 2 (top) represents the time the subject sustained their hand on the specific grill.
Fig. 4.
Photos of a thermal grill using a regular camera (left) and infrared camera used to validate the temperature pattern (right).
EDA Signal Processing
As shown in Fig. 5, the blue trace is a typical EDA signal recorded from the finger skin of a subject undergoing TG or EP stimulation, i.e., the raw EDA. The raw EDA signal can be decomposed into tonic and phasic components (6), as indicated by the green and gray dashed lines in Fig. 5, respectively. The tonic component reflects the slow shifts in the skin conductance amplitude. The phasic component reveals the rapid transients visible on the raw EDA signal, i.e., the skin conductance responses (SCRs) that are caused by the rapid reaction of the sympathetic nervous system to a certain external or internal triggering event (12). The event-related SCRs are those transient events noticeable in the EDA signal after delivery of each stimulus (Fig. 2). Measuring the SCRs was routinely implemented in the literature to quantify subjects’ sympathetic responses to a specific stimulus (6).
Fig. 5.
Electrodermal activity (EDA) signal decomposition. Raw EDA, phasic EDA, and tonic EDA are in microsiemens (µS). Time-varying spectral index of EDA (TVSymp) is in normalized units (n.u.).
First, we used the feature extraction scheme based on a nonnegative sparse deconvolution algorithm (SparsEDA) (22) for decomposing the EDA data into tonic and phasic components. SparsEDA is based on nonnegative sparse deconvolution and multiscale modeling of SCRs. SparsEDA algorithm is reportedly faster, more efficient, and more interpretable than previously developed algorithms for tonic/phasic decomposition of EDA. As illustrated by the red trace in Fig. 5, we implemented the time-varying spectral index of EDA (TVSymp) (47). TVSymp incorporates the spectral components of EDA within the range 0.08–0.24 Hz. Given the nonstationarity nature of EDA signals, time-varying spectral analysis is more suitable to analyze the EDA than the fast Fourier transform. TVSymp was recently found to be highly sensitive to orthostatic, cognitive, and physical stress, exhibiting a higher between-subject consistency compared with tonic and phasic components of EDA. The mean levels of the phasic and tonic components of EDA, as well as the TVSymp in a time window of 5 s, were measured before and after each EP or TG stimulus. The subject-reported pain level was considered to be 0 for prestimulus measurements. The TVSymp signal during each EP or TG stimulus was linked to the subject-reported pain level right after each stimulus.
Statistics
Normality of the VAS and measures of EDA were tested for the different pain levels of EP and TG stimulation using the Kolmogorov-Smirnov test (35, 36, 68). Repeated measures analysis was performed to test the difference in the VAS and measures of the EDA at different TG or EP stimulus levels. In normally distributed data, two-way analysis of variance (ANOVA) was performed to test for significant differences between measures. If nonnormality was found in a specific index, we used the Dunn’s test (8). The Bonferroni method was used for correction of multiple comparisons.
We computed the Spearman’s correlation coefficient (r) between VAS self-reported pain and stimulation level for EP and TG stimulation to evaluate the effectiveness of both stimulation modalities to induce pain to the subjects. We also computed the correlation coefficient between VAS self-reported pain and indices of EDA. Correlation analysis was done for each subject, and the results were put together for further analysis. The t test was used to assess the statistical significance of the correlation coefficient (the null hypothesis was that the correlation coefficient was zero) (58).
RESULTS
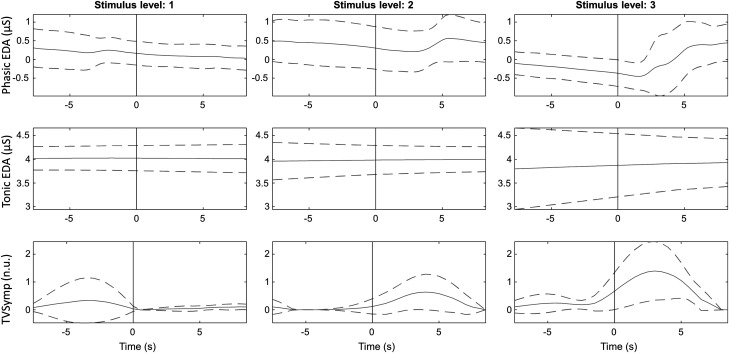
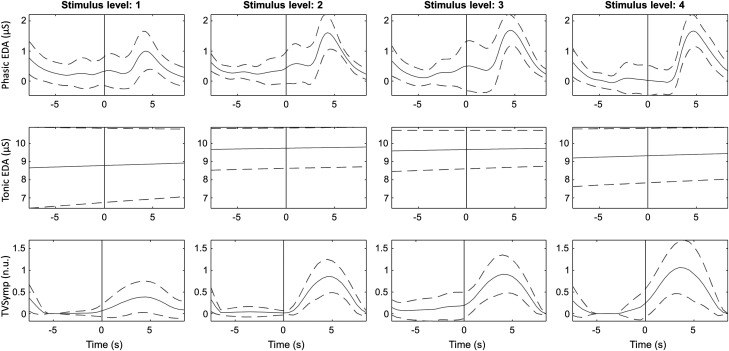
Displayed in Fig. 6 are typical EDA indices (phasic, tonic, and TVSymp) recorded from one subject undergoing TG stimulation at three different intensity levels. The dashed lines indicate the standard deviation. The onset of stimulation is marked with a gray vertical line. Displayed in Fig. 7 is a similar plot recorded from the same subject undergoing EP stimulation at four different intensity levels. It is apparent that the phasic EDA and TVSymp values increase with ascending stimulus levels, albeit more apparent with the latter analysis approach.
Fig. 6.
Ensemble average of tonic electrodermal activity (EDA), phasic EDA, and time-varying spectral index of EDA (TVSymp) for a given subject for the different levels of thermal grill stimulation. Solid and dashed lines represent the mean and standard deviation, respectively. Onset of stimulation is marked with a gray vertical line. n.u., normalized units.
Fig. 7.
Ensemble average of tonic electrodermal activity (EDA), phasic EDA, and time-varying spectral index of EDA (TVSymp) for a given subject for the different levels of electric pulse stimulation. Solid and dashed lines represent the mean and standard deviation, respectively. Onset of stimulation is marked with a gray vertical line. n.u., normalized units.
The EDA signals within a 5-s window before and right after the stimulus onset were processed for tonic, phasic, and TVSymp indices and are summarized in Table 1 (n = 16 subjects for EP tests, n = 23 subjects for TG tests), which also includes the subject self-reported VAS scores. Statistically significant differences are marked as superscript numbers. For instance, the first line of the table shows that VAS was different in thermal grills between level 1 and level 0 (as superscript), between level 2 and levels 0 and 1, and between level 3 and levels 0, 1, and 2. As anticipated, the self-reported VAS scores were significantly different at varying stimulus intensity levels for both EP and TG modalities; the only exception is the absence of different VAS scores between the levels 3 and 4 of EP stimulation. The tonic EDA did not show any statistically significant differences between all levels of stimulation for EP and TG. The phasic EDA index showed moderate differences at varying stimulus intensity. In TG tests, the phasic EDA values were different between level 2 and levels 0 and 1 and between level 4 and level 0. In EP tests, the values of phasic EDA were different between levels 3 and level 1 and between level 4 and levels 0–4. The TVSymp index is overall different at different levels of stimulation. For TG tests, TYSymp index is significantly different between level 1 and level 0, between level 2 and levels 0 and 1, and between level 3 and levels 0 and 1. For EP tests, TYSymp index is significantly different between level 1 and level 0, between level 2 and level 0 and 1, between level 3 and levels 0–2, and between level 4 and levels 0–2.
Table 1.
Differences in reported pain and indices of EDA between stimulation levels for electric pulses and thermal grill stimulation
| Level 0 | Level 1 | Level 2 | Level 3 | Level 4 | |
|---|---|---|---|---|---|
| Thermal grills | |||||
| VAS | 0 ± 0 | 3.5 ± 1.90 | 5.9 ± 1.30,1 | 7 ± 1.30,1,2 | |
| Phasic EDA | 0.25 ± 0.65 | 0.33 ± 0.7 | 0.58 ± 0.710,1 | 0.45 ± 0.730 | |
| Tonic EDA | 7.3 ± 2.4 | 7.5 ± 2.2 | 7.3 ± 2.3 | 7.2 ± 2.6 | |
| TVSymp | 0.13 ± 0.18 | 0.26 ± 0.290 | 0.34 ± 0.30,1 | 0.39 ± 0.380,1 | |
| Electric pulses | |||||
| VAS | 0 ± 0 | 1.4 ± 1.10 | 3.1 ± 1.80,1 | 5 ± 20,1,2 | 6.1 ± 1.60,1,2 |
| Phasic EDA | 0.21 ± 0.49 | 0.15 ± 0.47 | 0.18 ± 0.49 | 0.35 ± 0.581 | 0.34 ± 0.470,1,2 |
| Tonic EDA | 5.2 ± 1.6 | 5.1 ± 1.8 | 5.1 ± 1.9 | 5.4 ± 1.4 | 5.4 ± 1.3 |
| TVSymp | 0.097 ± 0.17 | 0.23 ± 0.250 | 0.32 ± 0.260,1 | 0.5 ± 0.380,1,2 | 0.66 ± 0.450,1,2 |
Values are means ± SD. Superscript numbers denote significant differences to the given levels of stimulation. EDA, electrodermal activity; TVSymp, time-varying index of EDA; VAS, visual analog scale.
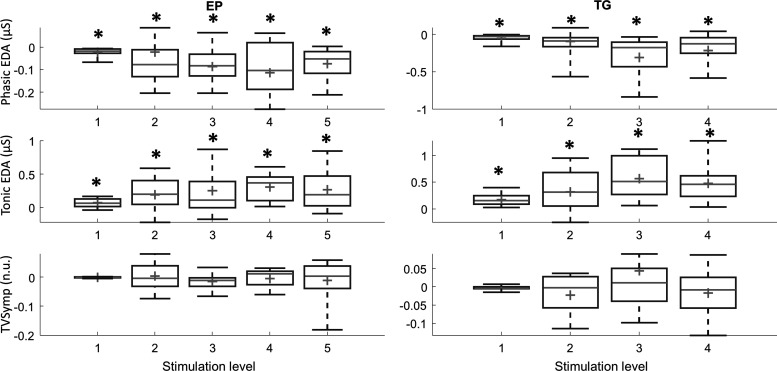
To test the effect of adaptation on measures, we included the stimulation repetition in the unbalanced three-way ANOVA (with Bonferroni correction). The results show that the only measure that was not significantly affected by the repetition of the stimuli for both tests, EP and TG, was TVSymp (P = 0.98 for EP, P = 0.94 for TG). All other measures were affected by stimulus repetition. To further investigate the decline in values on the different measures due to adaptation to stimulation, for each subject we fitted a straight line using the values obtained each time a given stimulation level was applied, using the stimulus number as the abscissa. The slope of such straight lines was analyzed. We found the slope of tonic component values significantly higher than zero, whereas the slope of phasic component values was significantly lower than zero. It means that the tonic component keeps increasing as the experiment goes on, and the amplitude of the phasic component is reduced over time. Again, TVSymp does not show such a decline in value due to adaptation. Figure 8 shows these results. On each box, the central mark is the median, and the edges of the box are the 25th and 75th percentiles.
Fig. 8.
Box plots (median, 25th, and 75th percentiles) of the slopes of phasic electrodermal activity (EDA), tonic EDA, and time-varying spectral index of EDA (TVSymp) to evaluate subjects’ adaptation to electric pulse (EP; left) and thermal grill (TG; right) stimulation. *Statistical significance. Slopes of phasic EDA are significantly lower than zero, and slopes of tonic EDA are significantly higher than zero. n.u., normalized units.
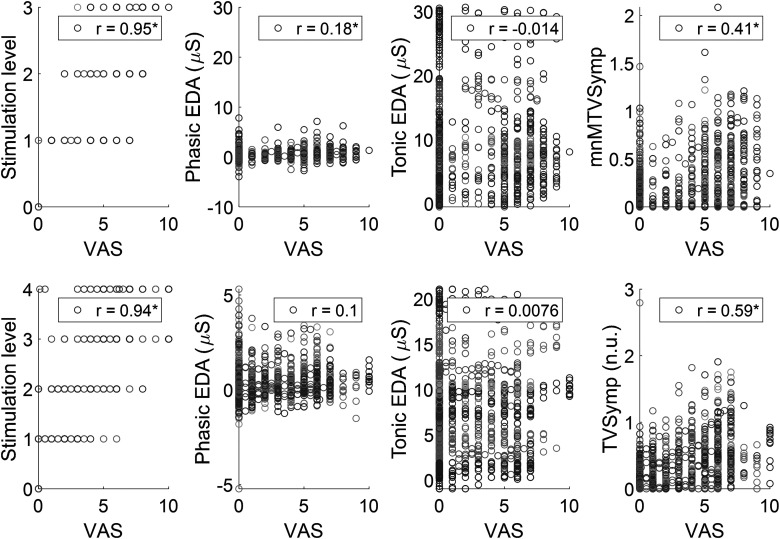
We then analyzed the correlation between the subjective pain level of VAS scores and objective EDA indices (phasic, tonic, and TVSymp), which is summarized in Fig. 9. As anticipated, VAS scores highly correlated with the levels of stimulus intensity for both TG and EP tests (r = 0.95 and 0.94, respectively). Phasic EDA was significantly correlated to VAS for TG tests, although the r value was low (r = 0.18). Tonic EDA was not significantly correlated to VAS for either test. TVSymp was significantly correlated to VAS score for both EP (r = 0.59) and TG (r = 0.41) tests. Among the three indices of EDA, TVSymp exhibited the highest correlation to VAS.
Fig. 9.
Correlation analysis between visual analog scale (VAS) pain reported by subjects and stimulation level and measures of electrodermal activity (EDA) for thermal grill (top) and electric shock (bottom) stimulation. n.u., normalized units; TVSymp, time-varying spectral index of EDA.
DISCUSSION
To the best of our knowledge, this is the first human study that implemented recordings of electrodermal activities (EDA) to quantify pain intensities into severe and very severe range (VAS greater than 7 out of 10), which were safely evoked by thermal grills. EDA is a widely used metric to assess the neural activities of the autonomic nervous system (ANS), which extensively overlaps with the pain neural circuity in brain regions like the periaqueductal gray, amygdala, hypothalamus, anterior cingulate, and insular cortex (3, 4, 37). Noxious pain-evoking stimulus is known to cause autonomic reactions, as has been documented by multiple studies to assess the correlation between the level of ANS activities and stimulus intensity (7, 15, 25, 31, 38, 62). A couple of recent studies assessed the correlation coefficients between the stimulus intensity, subject-reported pain scores (e.g., VAS), and the evoked ANS responses, showing closer correlation of ANS activities to objective stimulus intensities rather than to subjective VAS scores (39, 40). This is consistent with our current finding that TVSymp index has higher correlation with stimulus intensities than with subject-reported VAS for both the TG and EP tests.
The ANS consists of both the sympathetic and parasympathetic nervous systems (SNS, PNS), the activities of which can be estimated by frequency analysis of heart rate variabilities (HRVs). Specifically, the high-frequency (HF) power spectrum is dominated by the PNS activation and can serve as a metric of PNS activities. In contrast, SNS activities cannot be reliably measured by HRV as the low-frequency (LF) power spectrum of HRV is contributed by both SNS and PNS activities (61). Usually, the ratio between the LF and HF power spectrum is used to assess the sympathetic/parasympathetic balance (70). ANS activities measured by the HRV require recordings over a long period of time (at least 5 min) and thus are not suitable to quantify instantaneous changes in pain intensities within seconds (61). In contrast, recordings of EDA quantify the sudomotor reflex contributed exclusively by SNS activity and can capture the change of SNS-altering events within subseconds (47–50). In particular, our recent report of EDA and HRV recordings with six frequency- and time-domain parameters indicates that the TVSymp provides the highest reproducible distinction of ANS activities from baseline in a 5-day study of two stress assays (head-up tilt and Stroop test), more so than the sympathetic activities measured by HRV and the traditional time-domain indices of EDA (46). This is consistent with our current result showing that TVSymp has the highest correlation coefficients with TG and EP stimulus intensities among all three of the analyzed EDA indices. However, there is only modest correlation between the TVSymp and subject-reported VAS score, which is consistent with other recent reports that implemented EDA and machine learning tools to objectively assess pain (23, 32, 33, 60). The fact that the TVSymp index of EDA is elevated by pain but the correlation is rather low reflects the subjective nature of pain that does not quite correlate with stimulus intensity. Nonetheless, the close correlation between TVSymp and stimulus intensities strongly suggests that ANS activities quantified by TVSymp can be a potential nonbiased biomarker for pain intensities.
The thermal grill evokes paradoxical sensations of strong heat and pain via alternating innocuous cold and warm stimuli on the skin, i.e., an illusory painful sensation. The TG-evoked painful sensation depends primarily on the spatial pattern of thermal stimulation, whereas other factors like the number of thermal grill bars, bar diameter, and distance between the bars only have minor influence on the quality and intensity of the illusion (28). The key parameter that determines the TG-evoked pain is the temperature difference between the innocuous cold and warm stimuli. Small differences (10–15°C) evoke heat sensation, whereas larger differences (>20°C) evoke pain (10, 28). The TG illusion was mainly implemented as a behavioral assay for studying the neuroscience of thermal nociceptive circuitry in both the spinal and supraspinal brain regions of the nervous system, indicating contributions from both regions (16, 17). A handful of studies implemented the thermal grill as a noxious stimulus and studied the biological responses via several noninvasive recordings, including scalp electroencephalogram (24), blood pressure and heart rate (54), and magnetic resonance imaging (27, 29). The current study is the first to implement multiple thermal grills to deliver different levels of painful stimuli to human subjects by setting the temperature differences between the cold and warm stimuli. Using the thermal grills, we managed to safely evoke very severe pain in subjects by using warm stimuli below 45°C, a temperature that does not cause tissue injuries. In contrast, previous studies commonly used only one thermal grill and the evoked pain levels were kept low, e.g., a mean VAS of 4 of 10 (24, 54). Recent studies by Patwardhan and colleagues (41, 42) did document a high level of pain evoked by a single thermal grill, VAS beyond 7 of 10, but their studies also only used a single TG. Our current results showed strong correlation between the stimulus intensity of the three TGs and the subject-reported VAS scores, which strongly indicate thermal grills can be used as a safe stimulus modality to evoke severe to very severe pain in human subjects.
Ideally, a high linear correlation is needed to obtain a good quantitative measurement of pain. However, VAS scores reported by subjects are subjective and vary widely among subjects, as some are more tolerant to pain. Given the observed nonlinearity relationship between EDA and pain observed in this study (Fig. 9), which agrees with previous studies (56), a population-based direct quantitative pain measurement based on EDA may not be tenable. However, individualized quantitative pain assessment may be attainable. Moreover, the results show the potentiality of EDA for creating a population-based rank measure of pain rather than a quantitative measurement method. A rank measure of pain would also be suitable for objective pain assessment. Alternatively, machine learning could help to develop a linear measurement of pain using regression techniques.
As for the limitations of the study, we collected all the EDA data from the left hand and did not test the bilateral symmetry with respect to EDA responses. Although it is highly common, not all individuals exhibit bilateral symmetry (34, 63). We have assumed bilateral symmetry of the subjects’ hands, but if some subjects did not exhibit such symmetry, the outcome of the study could be altered. Furthermore, we have chosen the hand for EDA data collection, given our goal was to provide a convenient wearable tool for objective measurement of pain. Hands are an ideal site for wearable devices for practical purposes. However, other sites of the body could have been chosen for EDA data collection, as many other sites can potentially provide sensitive EDA measures (65). Additionally, subjects may have been adapted to pain stimulation affecting the body’s reaction as the experiment duration progressed. Although we noticed some adaptation effect, as evidenced by partial reduction in the amplitude of the EDA and some inconsistency in the EDA responses, we observed that most subjects showed significant reactiveness throughout the entire experiment.
The circumstances of this pain stimulation study also introduced some potential structural design issues. Unfortunately, not all the subjects agreed to participate in EP stimulation, as some people were reluctant to participate in the experiment using electrical stimulations to induce pain; hence, this may have introduced asymmetry in the data set. Also, subjects that underwent both tests always experienced EP first and TG second, introducing an order effect. As a countermeasure to these potential structural issues, we allowed the subjects to stay still for at least 5 min before each test. Furthermore, the subjects practiced both tests during the calibration stages and before undergoing each test. This helped us allow enough time between the tests for those who underwent EP stimulation.
In this study, we have used SparsEDA algorithm for tonic/phasic decomposition of EDA. However, this selection might not be ideal, as many other techniques are available for tonic/phasic decomposition of EDA, including wavelet analysis (56), continuous decomposition analysis (5), discrete decomposition analysis (5), dynamic causal modeling (2), and convex optimization approach (20), among others. Although the sparse deconvolution and the convex optimization approaches are reportedly faster than other techniques, both tools require setting four or more parameters, making them difficult to generalize. Given that each algorithm provides a very different estimation of the tonic and phasic component, the algorithms must be compared in terms of their ability to quantify adaptation and sensitively capture the sympathetic response to pain. This analysis will be conducted in a subsequent study looking into the development of an objective measurement of pain based on EDA.
Perspectives and Significance
In summary, we have tested the feasibility of safely evoking multilevel pain in human subjects using thermal grills and electrical shock stimuli. The thermal grills and electrical shocks effectively induced three different levels of pain, as evidenced by the self-reported VAS scores right after each stimulus. Furthermore, objective measurements of pain based on time-varying analysis of EDA, termed TVSymp, showed a significant increase with increased level of stimulus intensities for both the TG and EP tests. These findings enable future work with the thermal grills, opening opportunities to study prolonged high-intensity pain and the coping mechanisms. Thermal grills can be potentially implemented in large-scale clinical trials for screening new drugs or treatment methods for managing pain. Further studies are required to validate the tool in larger cohorts of patients with and without pain and evaluate effects of standard pain medicine.
Furthermore, our findings open possibilities for developing objective biomarkers for pain in chronic pain patients. An objective measure of pain can potentially be developed using machine learning algorithms and an index based on EDA, specifically the TVSymp and other measures based on the phasic component of EDA. The convenience of thermal grill stimulation can be extended to more levels of pain, given the ease of fabrication and operation. The modification of the level of stimulation is as easy as controlling the temperature of the hot tubes, which can be safely increased to up to 45°C, the threshold for heat pain (21). Furthermore, recent works have reported new methods for capturing sympathetic responses in chronic pain conditions, such as headaches (43). These novel tools can now be applied in the field to capture pain instances when they naturally occur. In the future, these emergent methods may complement controlled studies.
GRANTS
This work was supported by the National Science Foundation CAREER award 1844762 to B. Feng. This work was also supported by National Institutes of Health Grant R21 DE029563-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.C., B.F., and K.H.C. conceived and designed research; H.F.P.-Q., K.N., C.T., L.C., and T.G. performed experiments; H.F.P.-Q., Y.K., L.B., and K.H.C. analyzed data; H.F.P.-Q., Y.K., X.C., B.F., and K.H.C. interpreted results of experiments; H.F.P.-Q. prepared figures; H.F.P.-Q. drafted manuscript; B.F. and K.H.C. edited and revised manuscript; H.F.P.-Q., Y.K., K.N., C.T., L.B., L.C., T.G., X.C., B.F., and K.H.C. approved final version of manuscript.
REFERENCES
- 1.Aslanidis T, Grosomanidis V, Karakoulas K, Chatzisotiriou A. Electrodermal activity monitoring during painful stimulation in sedated adult intensive care unit patients: a pilot study. Acta Med (Hradec Kralove) 61: 47–52, 2018. doi: 10.14712/18059694.2018.50. [DOI] [PubMed] [Google Scholar]
- 2.Bach DR, Daunizeau J, Kuelzow N, Friston KJ, Dolan RJ. Dynamic causal modeling of spontaneous fluctuations in skin conductance. Psychophysiology 48: 252–257, 2011. doi: 10.1111/j.1469-8986.2010.01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beissner F, Meissner K, Bär KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci 33: 10503–10511, 2013. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benarroch EE. Pain-autonomic interactions: a selective review. Clin Auton Res 11: 343–349, 2001. doi: 10.1007/BF02292765. [DOI] [PubMed] [Google Scholar]
- 4a.BIOPAC Systems, Inc 257 – Safe Use of Electrical Stimulators (Online). https://www.biopac.com/application-note/stimulation-safe-use-of-electrical-stimulators/ [26 December 2019].
- 5.Boucsein W. Electrodermal Activity. New York: Springer Science & Business Media, 2012. [Google Scholar]
- 6.Boucsein W, Fowles DC, Grimnes S, Ben-Shakhar G, Roth WT, Dawson ME, Filion DL; Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures . Publication recommendations for electrodermal measurements. Psychophysiology 49: 1017–1034, 2012. doi: 10.1111/j.1469-8986.2012.01384.x. [DOI] [PubMed] [Google Scholar]
- 7.Bruehl S, Chung OY. Interactions between the cardiovascular and pain regulatory systems: an updated review of mechanisms and possible alterations in chronic pain. Neurosci Biobehav Rev 28: 395–414, 2004. doi: 10.1016/j.neubiorev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Cardillo G. Dunn Test: a Procedure for Multiple, Not Parametric, Comparisons. Natick, MA: MATLAB Central, MathWorks, 2006. [Google Scholar]
- 9.CDC Drug Overdose Deaths in the United States Continue to Increase in 2015 (Online). 2015. [1 April 2020]. https://www.cdc.gov/drugoverdose/epidemic/index.html. [Google Scholar]
- 10.Craig AD, Bushnell MC. The thermal grill illusion: unmasking the burn of cold pain. Science 265: 252–255, 1994. doi: 10.1126/science.8023144. [DOI] [PubMed] [Google Scholar]
- 11.Critchley HD. Electrodermal responses: what happens in the brain. Neuroscientist 8: 132–142, 2002. doi: 10.1177/107385840200800209. [DOI] [PubMed] [Google Scholar]
- 12.Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Handbook of Psychophysiology, edited by Cacioppo J, Tassinary L, Berntson G. Cambridge, UK: Cambridge University Press, 2007, p. 159–181. [Google Scholar]
- 13.Defrin R, Benstein-Sheraizin A, Bezalel A, Mantzur O, Arendt-Nielsen L. The spatial characteristics of the painful thermal grill illusion. Pain 138: 577–586, 2008. doi: 10.1016/j.pain.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Dubé AA, Duquette M, Roy M, Lepore F, Duncan G, Rainville P. Brain activity associated with the electrodermal reactivity to acute heat pain. Neuroimage 45: 169–180, 2009. doi: 10.1016/j.neuroimage.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Duschek S, Mück I, Reyes Del Paso GA. Relationship between baroreceptor cardiac reflex sensitivity and pain experience in normotensive individuals. Int J Psychophysiol 65: 193–200, 2007. doi: 10.1016/j.ijpsycho.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Fardo F, Beck B, Allen M, Finnerup NB. Beyond labeled lines: a population coding account of the thermal grill illusion. Neurosci Biobehav Rev 108: 472–479, 2020. doi: 10.1016/j.neubiorev.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Fardo F, Finnerup NB, Haggard P. Organization of the Thermal Grill Illusion by Spinal Segments. Ann Neurol 84: 463–472, 2018. doi: 10.1002/ana.25307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Fillingim RB, Loeser JD, Baron R, Edwards RR. Assessment of chronic pain: domains, methods, and mechanisms. J Pain 17: T10–T20, 2016. doi: 10.1016/j.jpain.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain 13: 715–724, 2012. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Gray K, Wegner DM. The sting of intentional pain. Psychol Sci 19: 1260–1262, 2008. doi: 10.1111/j.1467-9280.2008.02208.x. [DOI] [PubMed] [Google Scholar]
- 20.Greco A, Valenza G, Lanata A, Scilingo E, Citi L. cvxEDA: a convex optimization approach to electrodermal activity processing. IEEE Trans Biomed Eng 63: 797–804, 2016. doi: 10.1109/TBME.2015.2474131. [DOI] [PubMed] [Google Scholar]
- 21.Green BG. Temperature perception and nociception. J Neurobiol 61: 13–29, 2004. doi: 10.1002/neu.20081. [DOI] [PubMed] [Google Scholar]
- 22.Hernando-Gallego F, Luengo D, Artes-Rodriguez A. Feature extraction of galvanic skin responses by non-negative sparse deconvolution. [DOI] [PubMed] [Google Scholar]
- 23.Jiang M, Mieronkoski R, Syrjälä E, Anzanpour A, Terävä V, Rahmani AM, Salanterä S, Aantaa R, Hagelberg N, Liljeberg P. Acute pain intensity monitoring with the classification of multiple physiological parameters. J Clin Monit Comput 33: 493–507, 2019. doi: 10.1007/s10877-018-0174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jutzeler CR, Warner FM, Wanek J, Curt A, Kramer JLK. Thermal grill conditioning: Effect on contact heat evoked potentials. Sci Rep 7: 40007, 2017. doi: 10.1038/srep40007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koenig J, Jarczok MN, Ellis RJ, Hillecke TK, Thayer JF. Heart rate variability and experimentally induced pain in healthy adults: a systematic review. Eur J Pain 18: 301–314, 2014. doi: 10.1002/j.1532-2149.2013.00379.x. [DOI] [PubMed] [Google Scholar]
- 26.Koenig J, Williams DP, Kemp AH, Thayer JF. Vagally mediated heart rate variability in headache patients—a systematic review and meta-analysis. Cephalalgia 36: 265–278, 2016. doi: 10.1177/0333102415583989. [DOI] [PubMed] [Google Scholar]
- 27.Leung A, Shukla S, Li E, Duann JR, Yaksh T. Supraspinal characterization of the thermal grill illusion with fMRI. Mol Pain 10: 18, 2014. doi: 10.1186/1744-8069-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Petrini L, Wang L, Defrin R, Arendt-Nielsen L. The importance of stimulus parameters for the experience of the thermal grill illusion. Neurophysiol Clin 39: 275–282, 2009. doi: 10.1016/j.neucli.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Lindstedt F, Johansson B, Martinsen S, Kosek E, Fransson P, Ingvar M. Evidence for thalamic involvement in the thermal grill illusion: an FMRI study. PLoS One 6: e27075, 2011. doi: 10.1371/journal.pone.0027075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q, Wang EM, Yan XJ, Chen SL. Autonomic functioning in irritable bowel syndrome measured by heart rate variability: a meta-analysis. J Dig Dis 14: 638–646, 2013. doi: 10.1111/1751-2980.12092. [DOI] [PubMed] [Google Scholar]
- 31.Loggia ML, Juneau M, Bushnell MC. Autonomic responses to heat pain: Heart rate, skin conductance, and their relation to verbal ratings and stimulus intensity. Pain 152: 592–598, 2011. doi: 10.1016/j.pain.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Martinez D, Picard R.. Multi-task neural networks for personalized pain recognition from physiological signals. 2017 Seventh International Conference on Affective Computing and Intelligent Interaction Workshops and Demos (ACIIW) San Antonio, TX, October 23-26, 2017, p. 181–184. [Google Scholar]
- 33.Lopez-Martinez D, Picard R. Continuous pain intensity estimation from autonomic signals with recurrent neural networks. Conf Proc IEEE Eng Med Biol Soc 2018: 5624–5627, 2018. doi: 10.1109/EMBC.2018.8513575. [DOI] [PubMed] [Google Scholar]
- 34.Macmillan AL, Spalding JM. Human sweating response to electrophoresed acetylcholine: a test of postganglionic sympathetic function. J Neurol Neurosurg Psychiatry 32: 155–160, 1969. doi: 10.1136/jnnp.32.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massey FJ., Jr The Kolmogorov-Smirnov test for goodness of fit. J Am Stat Assoc 46: 68–78, 1951. doi: 10.1080/01621459.1951.10500769. [DOI] [Google Scholar]
- 36.Miller LH. Table of percentage points of Kolmogorov statistics. J Am Stat Assoc 51: 111–121, 1956. doi: 10.1080/01621459.1956.10501314. [DOI] [Google Scholar]
- 37.Nahman-Averbuch H, Coghill RC. Pain-autonomic relationships: implications for experimental design and the search for an “objective marker” for pain. Pain 158: 2064–2065, 2017. doi: 10.1097/j.pain.0000000000001035. [DOI] [PubMed] [Google Scholar]
- 38.Nahman-Averbuch H, Granovsky Y, Sprecher E, Steiner M, Tzuk-Shina T, Pud D, Yarnitsky D. Associations between autonomic dysfunction and pain in chemotherapy-induced polyneuropathy. Eur J Pain 18: 47–55, 2014. doi: 10.1002/j.1532-2149.2013.00349.x. [DOI] [PubMed] [Google Scholar]
- 39.Nickel MM, May ES, Tiemann L, Postorino M, Ta Dinh S, Ploner M. Autonomic responses to tonic pain are more closely related to stimulus intensity than to pain intensity. Pain 158: 2129–2136, 2017. doi: 10.1097/j.pain.0000000000001010. [DOI] [PubMed] [Google Scholar]
- 40.Nickel MM, May ES, Tiemann L, Schmidt P, Postorino M, Ta Dinh S, Gross J, Ploner M. Brain oscillations differentially encode noxious stimulus intensity and pain intensity. Neuroimage 148: 141–147, 2017. doi: 10.1016/j.neuroimage.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patwardhan S, Kawazoe A, Kerr D, Nakatani M, Visell Y.. Too hot, too fast! Using the thermal grill illusion to explore dynamic thermal perception. 2018 IEEE Haptics Symposium (HAPTICS) San Francisco, CA, March 25–28, 2018, p. 145–150. [Google Scholar]
- 42.Patwardhan S, Kawazoe A, Kerr D, Nakatani M, Visell Y. Dynamics and perception in the thermal grill illusion. IEEE Trans Haptics 12: 604–614, 2019. doi: 10.1109/TOH.2019.2904226. [DOI] [PubMed] [Google Scholar]
- 43.Pavlidis I, Garza I, Tsiamyrtzis P, Dcosta M, Swanson JW, Krouskop T, Levine JA. Dynamic quantification of migrainous thermal facial patterns—a pilot study. IEEE J Biomed Health Inform 23: 1225–1233, 2019. doi: 10.1109/JBHI.2018.2855670. [DOI] [PubMed] [Google Scholar]
- 44.Portenoy RK, Ugarte C, Fuller I, Haas G. Population-based survey of pain in the United States: differences among white, African American, and Hispanic subjects. J Pain 5: 317–328, 2004. doi: 10.1016/j.jpain.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Posada-Quintero HF, Chon KH. Innovations in electrodermal activity data collection and signal processing: a systematic review. Sensors (Basel) 20: 479, 2020. doi: 10.3390/s20020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Posada-Quintero HF, Dimitrov T, Moutran A, Park S, Chon KH. Analysis of reproducibility of noninvasive measures of sympathetic autonomic control based on electrodermal activity and heart rate variability. IEEE Access 7: 22523–22531, 2019. doi: 10.1109/ACCESS.2019.2899485. [DOI] [Google Scholar]
- 47.Posada-Quintero HF, Florian JP, Orjuela-Cañón ÁD, Chon KH. Highly sensitive index of sympathetic activity based on time-frequency spectral analysis of electrodermal activity. Am J Physiol Regul Integr Comp Physiol 311: R582–R591, 2016. doi: 10.1152/ajpregu.00180.2016. [DOI] [PubMed] [Google Scholar]
- 49.Posada-Quintero HF, Florian JP, Orjuela-Cañón AD, Chon KH. Electrodermal activity is sensitive to cognitive stress under water. Front Physiol 8: 1128, 2018. doi: 10.3389/fphys.2017.01128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Posada-Quintero HF, Reljin N, Mills C, Mills I, Florian JP, VanHeest JL, Chon KH. Time-varying analysis of electrodermal activity during exercise. PLoS One 13: e0198328, 2018. doi: 10.1371/journal.pone.0198328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Provan SA, Olstad DS, Solberg EE, Smedslund G, Dagfinrud H. Evidence of reduced parasympathetic autonomic regulation in inflammatory joint disease: a meta-analyses study. Semin Arthritis Rheum 48: 134–140, 2018. doi: 10.1016/j.semarthrit.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Reneau M. Heart rate variability biofeedback to treat fibromyalgia: an integrative literature review. Pain Manag Nurs 21: 225–232, 2020. doi: 10.1016/j.pmn.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep 65: 1445–1452, 2016. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 54.Scheuren R, Duschek S, Schulz A, Sütterlin S, Anton F. Blood pressure and the perception of illusive pain. Psychophysiology 53: 1282–1291, 2016. doi: 10.1111/psyp.12658. [DOI] [PubMed] [Google Scholar]
- 55.Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician 15, Suppl 3: ES67–ES92, 2012. [PubMed] [Google Scholar]
- 56.Shastri D, Merla A, Tsiamyrtzis P, Pavlidis I. Imaging facial signs of neurophysiological responses. IEEE Trans Biomed Eng 56: 477–484, 2009. doi: 10.1109/TBME.2008.2003265. [DOI] [PubMed] [Google Scholar]
- 57.Siddall PJ, Cousins MJ. Persistent pain as a disease entity: implications for clinical management. Anesth Analg 99: 510–520, 2004. doi: 10.1213/01.ANE.0000133383.17666.3A. [DOI] [PubMed] [Google Scholar]
- 58.Spiegel MR, Stephens LJ. Schaum’s Outline of Statistics (5th ed). New York: McGraw-Hill Education, 2014. [Google Scholar]
- 59.Staahl C, Drewes AM. Experimental human pain models: a review of standardised methods for preclinical testing of analgesics. Basic Clin Pharmacol Toxicol 95: 97–111, 2004. doi: 10.1111/j.1742-7843.2004.950301.x. [DOI] [PubMed] [Google Scholar]
- 60.Susam BT, Akcakaya M, Nezamfar H, Diaz D, Xu X, de Sa VR, Craig KD, Huang JS, Goodwin MS. Automated pain assessment using electrodermal activity data and machine learning. Conf Proc IEEE Eng Med Biol Soc 2018: 372–375, 2018. doi: 10.1109/EMBC.2018.8512389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, Schwartz PJ; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J 17: 354–381, 1996. doi: 10.1093/oxfordjournals.eurheartj.a014868. [DOI] [PubMed] [Google Scholar]
- 62.Treister R, Kliger M, Zuckerman G, Goor Aryeh I, Eisenberg E. Differentiating between heat pain intensities: the combined effect of multiple autonomic parameters. Pain 153: 1807–1814, 2012. doi: 10.1016/j.pain.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 63.Tsiamyrtzis P, Dcosta M, Shastri D, Prasad E, Pavlidis I. Delineating the operational envelope of mobile and conventional EDA sensing on key body locations. Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems San Jose, CA, May 7–12, 2016, p. 5665–5674. [Google Scholar]
- 64.Turner L, Linden W, Marshall C. Electrodermal activity at acupuncture points differentiates patients with current pain from pain-free controls. Appl Psychophysiol Biofeedback 38: 71–80, 2013. doi: 10.1007/s10484-013-9209-6. [DOI] [PubMed] [Google Scholar]
- 65.van Dooren M, Gert-Jan de Vries JJ, Janssen JH. Emotional sweating across the body: comparing 16 different skin conductance measurement locations. Physiol Behav 106: 298–304, 2012. doi: 10.1016/j.physbeh.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 66.Velly AM, Mohit S. Epidemiology of pain and relation to psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 87: 159–167, 2018. doi: 10.1016/j.pnpbp.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 67.Walter S, Gruss S, Ehleiter H, Tan Junwen, Traue HC, Werner P, Al-Hamadi A, Crawcour S, Andrade AO, Moreira da Silva G. The biovid heat pain database data for the advancement and systematic validation of an automated pain recognition system. 2013 IEEE International Conference on Cybernetics (CYBCO) Lausanne, Switzerland, June 13–15, 2013, p. 128–131. [Google Scholar]
- 68.Wang J, Tsang WW, Marsaglia G. Evaluating Kolmogorov’s distribution. J Stat Softw 8: 1–4, 2003. doi: 10.18637/jss.v008.i18. [DOI] [Google Scholar]
- 69.Williams AC, Craig KD. Updating the definition of pain. Pain 157: 2420–2423, 2016. doi: 10.1097/j.pain.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 70.Winchell RJ, Hoyt DB. Spectral analysis of heart rate variability in the ICU: a measure of autonomic function. J Surg Res 63: 11–16, 1996. doi: 10.1006/jsre.1996.0214. [DOI] [PubMed] [Google Scholar]
- 71.Xu X, Susam BT, Nezamfar H, Diaz D, Craig KD, Goodwin MS, Akcakaya M, Huang JS, Virginia RS. Towards automated pain detection in children using facial and electrodermal activity. CEUR Workshop Proc 2142: 208–211, 2018. [PMC free article] [PubMed] [Google Scholar]