Keywords: colitis, gut-brain, microbiota, neurogenesis, neuroinflammation
Abstract
Inflammatory bowel diseases (IBDs) are chronic intestinal diseases, frequently associated with comorbid psychological and cognitive deficits. These neuropsychiatric effects include anxiety, depression, and memory impairments that can be seen both during active disease and following remission and are more frequently seen in pediatric patients. The mechanism(s) through which these extraintestinal deficits develop remain unknown, and the study of these phenomenon is hampered by a lack of murine pediatric IBD models. Herein we describe microbiota-gut-brain (MGB) axis deficits following induction of colitis in a pediatric setting. Acute colitis was induced by administration of 2% dextran sodium sulfate (DSS) for 5 days starting at weaning [postnatal day (P)21] causing reduced weight gain, colonic shortening, and colonic inflammation by 8 days post-DSS (P29), which were mostly resolved in adult (P56) mice. Despite resolution of acute disease, cognitive deficits (novel object recognition task) and anxiety-like behavior (light/dark box) were identified in the absence of changes in exploratory behavior (open field test) in P56 mice previously treated with DSS at weaning. Behavioral deficits were found in conjunction with neuroinflammation, decreased neurogenesis, and altered expression of pattern recognition receptor genes in the hippocampus. Additionally, persistent alterations in the gut microbiota composition were observed at P56, including reduced butyrate-producing species. Taken together, these results describe for the first time the presence of MGB axis deficits following induction of colitis at weaning, which persist in adulthood.
NEW & NOTEWORTHY Here we describe long-lasting impacts on the microbiota-gut-brain (MGB) axis following administration of low-dose dextran sodium sulfate (DSS) to weaning mice (P21), including gut dysbiosis, colonic inflammation, and brain/behavioral deficits in adulthood (P56). Early-life DSS leads to acute colonic inflammation, similar to adult mice; however, it results in long-lasting deficits in the MGB axis in adulthood (P56), in contrast to the transient deficits seen in adult DSS. This model highlights the unique features of pediatric inflammatory bowel disease.
INTRODUCTION
Inflammatory bowel diseases (IBD), comprising Crohn’s disease (CD) and ulcerative colitis (UC), are chronic relapsing and remitting diseases of the intestinal tract with an unknown etiology affecting over 3 million Americans (17). Although the clinical manifestations of disease are unique, both UC and CD are characterized by a dysregulated immune response to the commensal microbiota, often in a genetically susceptible individual, with over 163 IBD-associated genes identified to date (69). Inflammation is postulated to be triggered by an unknown environmental exposure, creating a state of chronic intestinal inflammation and dysbiosis of the gut microbiota. The incidence of IBD during childhood and adolescence has been increasing in recent years, ranging from 5–25% of newly diagnosed cases (33, 62). These pediatric patients with IBD have a high risk of developing concurrent extraintestinal comorbidities, including anxiety, depression, and mild cognitive deficits, compared with children with other chronic physical illnesses (11, 25, 70), having a strong negative impact on their quality of life (14, 48). Additionally, there is an increasing appreciation that pediatric patients often have increased disease severity, coupled with an increased risk in the development of extraintestinal manifestations of disease compared with adults (14, 33). Moreover, patients with IBD suffering from depression and psychiatric abnormalities typically present with more severe illness and poor-health-related quality of life compared with patients without these comorbidities (34, 69, 70). This association is thought to occur as a result of alterations in signaling of the microbiota-gut-brain (MGB) axis, although the exact pathways remain incompletely characterized (4, 60).
Early life is a critical time of growth and development, with microbial colonization, maturation of the gastrointestinal (GI) tract and immune system, and establishment of neural processes in the brain occurring simultaneously (42, 67). Given that the microbiota is quite plastic during early neonatal life, with weaning triggering the establishment of a more mature, adult-like microbiota, a shift in the composition of the microbiota during early life due to chronic inflammation is thought to lead to persistent dysbiosis into adulthood (30, 43, 71). Therefore, we hypothesized that inflammation induced at weaning could detrimentally impact normal establishment of the MGB axis, leading to behavioral deficits persisting into adulthood.
Numerous mouse models of IBD have been developed, with each able to replicate many, but not all, aspects of human disease. The dextran sodium sulfate (DSS) model of chemically induced colitis in mice is a robust model of acute or chronic colonic inflammation depending on administration strategies, with similar disease features seen in UC patients, including weight loss, diarrhea, mucosal ulcers, shortened colon, and dysbiosis, as well as altered mood and behavior when administered in adult mice (3, 22). In neonates, DSS administration results in both ileal and colonic disease, representing a more necrotizing enterocolitis-like model (32). In young rats [postnatal day (P)21 or P28], DSS administration caused a reduction in body weight gain, colonic shortening, increased disease activity index scores, poor nutritional status, altered splenic lymphocyte populations, and dysbiosis of the colonic microbiota at P35 (28, 38). Although DSS-induced colitis does not completely mimic disease seen in UC patients, this model allows the study of certain important aspects of disease that genetic models cannot duplicate, including during disease remission.
Despite advances in IBD research using preclinical animal models, the lack of availability of a pediatric model has significantly hindered the understanding of why pediatric patients are more susceptible to extraintestinal manifestations than adults and other additional unique disease features of these patients. Here we describe for the first time long-lasting impairments in the MGB axis following induction of colitis at weaning via administration of low-dose DSS (P21), with dysbiosis, colonic inflammation, and brain/behavioral deficits found in adulthood (P56).
MATERIALS AND METHODS
Mice
Male and female C57BL/6 mice (Jackson Laboratories; bred in-house) were used. Mice were weaned at P21 and housed by sex in cages lined with chip bedding with access to food and water ad libitum throughout the duration of the study. Animals were kept in a specific pathogen-free vivarium with a 12-h light-dark cycle (lights on at 7:00 am). N numbers for each assay were based on individual power calculations [α = 0.05; power (1 − β) = 0.8] for all experimental groups. Male and female mice were evenly distributed and randomly assigned to each group, with data subsequently combined for analysis following no evidence of sexual dimorphism. Mice were euthanized by asphyxiation with CO2 followed by cervical dislocation or anesthesia with isoflurane followed by perfusion with PBS and fixative [4% paraformaldehyde (PFA); for immunofluorescence studies]. All procedures and protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the University of California (UC), Davis (Protocol No. 20072).
Behavioral Testing
Behavioral testing was performed between 9:00 am and 6:00 pm. Preliminary studies (performed by M. Swedek and M. G. Gareau), coupled with published protocols (16), confirmed that performing these tests alone or together in the same mouse or completing testing in the morning or afternoon does not affect outcome (data not shown).
Light/dark box test.
To assess anxiety-like behavior in mice, the light/dark (L/D) box was used as described previously (10, 30). Briefly, mice were placed in a novel arena with a light (2/3) and a dark (1/3) compartment for 10 min (lighting in light box was 675 ± 27 lux), and behavior was video recorded and analyzed using tracking software (XT 8.5, Noldus, Leesburg, VA) for quantification of time spent in the light box and transitions between the light and dark compartment. Increased time spent in the light portion of the box is indicative of decreased anxiety-like behavior. The number of transitions between the light and dark compartments was used as a secondary parameter for assessment of exploratory behavior.
Novel object recognition task.
The novel object recognition (NOR) task was performed as previously described (30, 68). Briefly, mice were habituated to the novel arena for 10 min. Following habituation, mice underwent the NOR task consisting of three phases: 5-min exposure to two identical objects [training phase; stainless steel star (8-cm diameter × 2.5-cm height = 20 cm2)], 45 min of rest and recovery (resting phase); and finally, 5 min of exposure to a novel object [stainless steel cylinder (4-cm diameter × 5-cm length = 20 cm2)] versus the familiar object (testing phase). Videos were analyzed for the number of times of direct contact with the objects, including any contact with nose or paw or an approach within 2 cm, using Ethovision (Noldus) and verified manually. Absence of object bias was confirmed during preliminary studies (data not shown). Results were expressed as an exploration ratio of preference for the novel object to the familiar object during the testing phase. An exploration ratio of greater than 50% indicated that the mouse investigated the new object more than the familiar object, confirming intact memory (30). Mice were excluded from analysis if they did not pass the training phase [i.e., they displayed preference for an object (exploration ratio greater than 60% or less than 40%)] or did not approach the objects during the testing phase a combined minimum of 10 times.
Open field test.
The open field test (OFT) was performed to measure exploratory behavior, general activity, and anxiety-like behavior. Behavior was recorded for 10 min in the novel arena during acclimation period for the NOR task. Locomotor activity was determined by quantification of total distance traveled, whereas time spent in the inner zone (12 × 12 cm), and frequency of inner zone entries were used as a secondary measurement to assess anxiety-like behavior. Lighting in arena was 625 ± 25 lux. All parameters were analyzed automatically by a digital tracking system (Ethovision).
Histology
Distal colon segments were collected, cleared of luminal contents, fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin. Tissues were sectioned by microtome (5 µm) and transferred onto positively charged glass slides and processed for hematoxylin-eosin staining. Slides were visualized using a light microscope (Nikon Eclipse E600 microscope using a Nikon Digital Sight DS-U3).
Immunofluorescence
Brains were collected following anesthesia with isoflurane (5%) and transcardial perfusion with 4% PFA. Brains were postfixed overnight in PFA at 4°C, and cryoprotected in 30% (wt/vol) sucrose in 0.1% sodium azide for 4 days. Samples were subsequently embedded in optimal cutting temperature (OCT) medium in ice-cold isopentane and stored at −20°C. Tissues were serially sectioned [20 µm-thick coronal sections for doublecortin (DCX)/Ki67 and 40 µm-thick coronal sections for Iba-1] in 1:6 series (120 µm apart) through the entire hippocampus using a cryostat (Leica Microsystem, Germany) and stored at −20°C until use. The same series of dorsal hippocampus sections was selected for each animal for staining. Tissue sections were processed for immunofluorescence following standard procedures as previously described (49). Briefly, slides were stained with primary antibodies: anti-mouse DCX (AB2253, Millipore, Burlington, MA), anti-mouse Ki67 (LS-C141898, Lifespan Biosciences, Seattle, WA), and anti-mouse Iba-1 (019-19741 Wako) overnight. Appropriate secondary antibodies were selected based on host and fluorophore [Alexa 647 goat anti-rabbit (ab150155, Abcam, Cambridge, UK) and Alexa 555 goat anti-guinea pig (A21435, Invitrogen, Carlsbad, CA)], with DAPI used as a nuclear stain. Finally, sections were mounted using Prolong diamond (Invitrogen) and imaged using a Leica SP8 STED 3X confocal microscope. Immunofluorescent Z-stack images were collected using a ×20 objective with a 1.04-µm step size for neurogenesis (DCX/Ki67) evaluation and a ×63 objective with a 0.3-µm step size for neuroinflammation (Iba-1). Cell quantification and calculation were performed using the entire dorsal hippocampus region per slide for DCX/Ki67 or by using systematic random sampling for Iba-1 in both hemispheres of each section in the dentate gyrus (DG) region. The dorsal hippocampus was defined as AP −0.94 to −2.30 according to the Paxinos and Franklin atlas of the mouse brain (53a).
Neurogenesis.
Cell quantification for DCX/Ki67 and calculation of the volume of the DG were performed using the image processing software package Imaris x64_8.2.1. The ratio between the cell numbers and the DG volumes is expressed as an average of counts from both the left and right hemispheres per animal and presented as cells/micrometer 3 × 10−6.
Neuroinflammation.
Iba-1 staining was used as a marker for neuroinflammation to characterize hippocampal microglia activation by morphological analysis. Three-dimensional reconstructions were created using modified open source 3DMorph Matlab script (78a) and Imaris software to assess microglia numbers, dendrite length, number of branch points, number of terminal points, and cell volume. Microglia were characterized in the same region of the DG in 10 sections for sham and 16 sections for DSS in both hemispheres and presented as cells/high-power field. Random sections were used for morphological analysis.
Quantitative PCR
RNA (hippocampus and colon) was isolated using TRIzol extraction (Invitrogen), treated with DNase 1 (Invitrogen) following the manufacturer’s instructions and stored at −80°C. RNA was purified using a double 2.5-M LiCl precipitation that precipitates and binds contaminants, including DSS. RNA quantity and quality were confirmed by Nanodrop. cDNA synthesis was performed using 1 μg of total RNA (iSCRIPT cDNA Synthesis kit, BioRad), and quantitative PCR (qPCR) was performed using Power SYBR master mix (QuantStudio 6 Flex; Applied Biosystem). β-Actin was used as a housekeeping gene, and data were analyzed using the 2−ΔΔCT method and presented as fold change normalized to sham. All primer sequences used in this study are shown in Table 1.
Table 1.
Primer sequences
| Primer | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Bactin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
| Iba1 | GGATTTGCAG GGAGGAAAAG | TGGGATCATC GAGGAATTG |
| Ifnγ | GCCACGGCACAGTCATTGA | TGCTGATGGCCTGATTGTCTT |
| Ikb | GAAGCCGCTGACCATGGAA | GATCACAGCCAAGTGGAGTGGA |
| Il10 | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG |
| Il12 | TGGTTTGCCATCGTTTTGCTG | ACAGGTGAGGTTCACTGTTTCT |
| Il17ar | TAACTCCCTTGGCGCAAAA | CTTTCCCTCCGCATTGACAC |
| Il1β | CTGTGACTCATGGGATGATGATG | CGGAGCCTGTAGTGCAGTTG |
| Il22 | ATGAGTTTTTCCCTTATGGGGAC | GCTGGAAGTTGGACACCTCAA |
| Il23 | ATGCTGGATTGCAGAGCAGTA | ACGGGGCACATTATTTTTAGTCT |
| Il23R | TTCAGATGGGCATGAATGTTTCT | CCAAATCCGAGCTGTTGTTCTAT |
| Il6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| Nod1 | TCCCTTGCCTGTGAGCAGAAAGTA | GTGGGTATGTGCCATGCTTTGCTT |
| Nod2 | CACACATGGCCTTTGGTTCCAGT | AAAGAGCTGCAGTTGAGGGAGGAA |
| Nos2 | GTTCTCAGCCCAACAATACAAGA | GTGGACGGGTCGATGTCAC |
| Pparγ | TCCTGTAAAAGCCCGGAGTAT | GCTCTGGTAGGGGCAGTGA |
| RegIIIγ | TTCCTGTCCTCCATGATCAAAA | CATCCACCTCTGTTGGGTTCA |
| Tlr2 | GCAAACGCTGTTCTGCTCAG | AGGCGTCTCCCTCTATTGTATT |
| Tlr4 | AGCTCCTGACCTTGGTCTTG | CGCAGGGGAACTCAATGAGG |
| Tlr6 | TGAGCCAAGACAGAAAACCCA | GGGACATGAGTAAGGTTCCTGTT |
| Tlr9 | ATGGTTCTCCGTCGAAGGACT | GAGGCTTCAGCTCACAGGG |
| Tnfα | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
Nos, nitric oxide synthase; Pparγ, peroxisome proliferator-activated receptor γ; Tlr, Toll-like receptor.
Microbiota Analysis
Total bacterial DNA was extracted from fecal pellets collected from the colon at P56 using the PowerFecal extraction kit (Qiagen), and samples were processed by the UC Davis Host-Microbe Systems Biology Core and analyzed by the UC Davis Mouse Metabolic Phenotyping Core. Sample libraries were prepared and analyzed by barcoded amplicon sequencing. In brief, the purified DNA was amplified on the V4 region of the 16S rRNA genes via PCR using the following primers: F319 (5′-ACTCCTACGGGAGGCAGCAGT-3′) and R806 (5′-GGACTACNVGGGTWTCTAAT-3′).
High-throughput sequencing was performed with Illumina MiSeq paired end 250 bp run. The data derived from sequencing were processed using QIIME2 for 16S-based microbiota analyses (8). Demultiplexed paired end sequences that already had barcodes and adapters removed were analyzed using QIIME 2 version 2019.1.0 (8). For quality filtering and feature [operational taxonomic unit (OTU)] prediction, we used DADA2 (13). Forward reads were truncated to 270 nucleotides (nts) and reverse reads to 220 nts. Representative sequences were aligned using MAFFT (40). A phylogenetic tree of the aligned sequences was made using FastTree 2 (55). OTUs/features were taxonomically classified using a pretrained Naive Bayes taxonomy classifier. The classifier was trained using the Silva 132 97% OTUs (57) for the 319F-806R region. Tables of taxonomic counts and percentage (relative frequency) were generated. Diversity analyses were run on the resulting OTU/feature.biom tables to provide both phylogenetic and nonphylogenetic metrics of α-and β-diversity (47). Additional data analysis [partial least squares discriminant analysis (PLS-DA)] and statistics were performed with R. Raw sequences have been uploaded to the NCBI SRA (Sequence Read Archive) under accession PRJNA631781.
Study Design
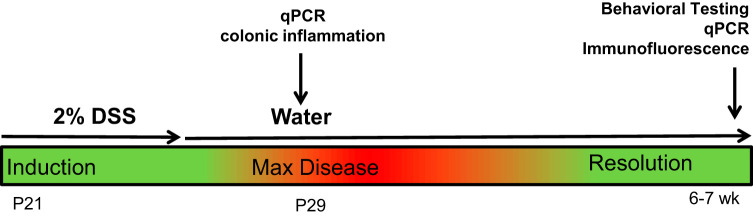
Given that weaning represents a significant developmental shift in feeding patterns that results in changes in the composition of the microbiota, we chose it as our starting point for induction of colonic inflammation. At weaning (P21), litters were sexed and split into sham and DSS treatment groups. Although litters were not culled to a standardized number to avoid additional stress, mice from age-matched litters were combined at weaning to ensure 3–4 mice were caged together per treatment to reduce any potential litter effects. Mice were provided 2% DSS (molecular weight 36,000–50,000; MP Biomedical, Solon, OH) in the drinking water ad libitum for 5 days, which was then replaced with normal drinking water for the remainder of the study (Fig. 1). Water consumption was monitored daily. Sham control mice were given normal drinking water throughout the study. All mice were weighed daily from P21–P29, and weekly thereafter. A subset of animals was euthanized at 8 days post-DSS to collect colon samples (TRIzol and formalin) to assess disease activity scores and quantify acute inflammation. At 6–8 wk of age, mice were tested for behavior and euthanized and tissue samples collected. Behavioral studies were performed by two investigators (P. Stokes and J. A. Sladek) to ensure reproducibility, and spot-checking of the video analysis was performed (E. Salvo) to ensure integrity of the data.
Fig. 1.
Study design. DSS, dextran sodium sulfate; postnatal day 21 (P21); postnatal day 30 (P29), qPCR, quantitative PCR.
On the day of behavioral testing, mice were transferred to the behavioral testing room and allowed to acclimate for 30 min in their home cage. After acclimatization, mice were subjected to the behavioral testing protocol consisting of the L/D box followed by the OFT and finally the NOR task. Following completion of behavioral tests, mice were euthanized, and tissues were collected for two sets of animals in two parallel experiments.
Immunofluorescent imaging: Mice exposed to behavioral testing protocol were allowed to rest undisturbed in their home-cages for 1 h, followed by perfusion (4% PFA) and neurogenesis/neuroinflammation imaging in the hippocampus (59).
qPCR: Mice exposed to behavioral testing protocol were euthanized by CO2 inhalation followed by cervical dislocation, and colon and brain tissues were collected in TRIzol to measure relative gene expression.
Statistical Analysis
Results are expressed as means ± SE. Normally distributed parametric data (Shapiro–Wilk) were analyzed by unpaired Student’s t test with Welch’s correction where appropriate using Prism 8 GraphPad (San Diego, CA). Non-normally distributed nonparametric data were analyzed by Mann–Whitney test. A P value of less than 0.05 was considered statistically significant. A statistical summary is included for P29 (Table 2) and P56 (Table 3) data.
Table 2.
Postnatal day 29 statistics
| P29 | T Value | F Value | Test |
|---|---|---|---|
| Weight | N/AP | N/AP | Mann–Whitney |
| Colon length | 7.519 | 3.724 | t test |
| PCR (Colon) | |||
| Ifnγ | 3.924 | 3.475 | t test |
| Il10 | 3.549 | 24.25 | Welch’s correction |
| Il12 | 5.662 | 28.43 | Welch’s correction |
| Il1β | 3.495 | 5207 | Welch’s correction |
| Il6 | 2.379 | 81.28 | Welch’s correction |
| Tnfα | 4.293 | 1.75 | t test |
| Ikb | 1.159 | 1.12 | t test |
| Il17a | 6.058 | 20540 | Welch’s correction |
| Il23 | 3.019 | 2.361 | t test |
| Tlr2 | 6.569 | 10.56 | Welch’s correction |
| Tlr4 | 1.914 | 5.942 | t test |
| Tlr6 | 4.174 | 282.9 | Welch’s correction |
| Tlr9 | 5.582 | 6.484 | t test |
| Nod1 | 0.8244 | 4.431 | t test |
| Nod2 | 3.388 | 31.48 | Welch’s correction |
| Pparγ | 4.991 | 6.267 | Welch’s correction |
| iNos2 | N/AP | N/AP | Mann–Whitney |
| RegIIIγ | N/AP | N/AP | Mann–Whitney |
N/AP, not applicable; Nos, nitric oxide synthase; P29, postnatal day 29; Pparγ, peroxisome proliferator-activated receptor γ; Tlr, Toll-like receptor.
Table 3.
Postnatal day 56 statistics
| P56 | T Value | F Value | Test |
|---|---|---|---|
| Weight | 1.202 | 1.332 | t test |
| Colon length | 0.4528 | 1.388 | t test |
| Behavior | |||
| NOR | 3.074 | 1.015 | t test |
| L/D time | N/AP | N/AP | Mann–Whitney |
| L/D transitions | 1.95 | 1.8 | t test |
| OFT distance | 0.4901 | 3.3 | Welch’s correction |
| OFT time | 0.32 | 7.132 | t test |
| OFT frequency | 1.452 | 2.727 | t test |
| PCR (Colon) | |||
| Ifnγ | N/AP | N/AP | Mann–Whitney |
| Il10 | 2.377 | 9.981 | t test |
| Il12 | 2.069 | 1.423 | t test |
| Il1β | 3.14 | 3.197 | t test |
| Il6 | N/AP | N/AP | Mann–Whitney |
| Tnfα | 1.746 | 2.473 | t test |
| Ikb | 2.125 | 1.701 | t test |
| Il17a | N/AP | N/AP | t test |
| Il23 | 3.6 | 7.63 | t test |
| Tlr2 | 2.614 | 1.437 | t test |
| Tlr4 | N/AP | N/AP | Mann–Whitney |
| Tlr6 | 4.297 | 2.736 | t test |
| Tlr9 | 2.452 | 1.104 | t test |
| Nod1 | 4.392 | 2.367 | t test |
| Nod2 | N/AP | N/AP | Mann–Whitney |
| Pparγ | 0.05572 | 3.864 | t test |
| iNos2 | N/AP | N/AP | Mann–Whitney |
| RegIIIγ | N/AP | N/AP | Mann–Whitney |
| PCR (HC) | |||
| Nod1 | 4.006 | 1.284 | t test |
| Nod2 | 3.307 | 1.019 | t test |
| Tlr2 | 2.488 | 1.385 | t test |
| Tlr4 | 3.374 | 2.121 | t test |
| Il17ar | 2.805 | 9.363 | Welch’s correction |
| Il23R | 0.6676 | 1.763 | t test |
| Il22 | N/AP | N/AP | Mann–Whitney |
| Iba1 | 4.498 | 1.256 | t test |
| Il1β | 2.7 | 2.942 | t test |
| iNos2 | 2.594 | 2.291 | t test |
| Microglia | |||
| Process length | 3.34 | 10.55 | Welch’s correction |
| Segments | 3.009 | 7.944 | t test |
| Terminal points | 3 | 7.931 | Welch’s correction |
| Branch points | 3.017 | 7.946 | Welch’s correction |
| Process volume | 2.37 | 3.664 | t test |
| Cell counts | N/AP | N/AP | Mann–Whitney |
| Cell proliferation | |||
| DCX | 2.993 | 1.7 | t test |
| Ki67 | 0.8353 | 3.52 | t test |
DCX; doublecortin; L/D, light/dark box test; N/AP, not applicable; NOR, novel object recognition; Nos, nitric oxide synthase; OFT, open field test; Pparγ, peroxisome proliferator-activated receptor-γ; Tlr, Toll-like receptor.
RESULTS
DSS Treatment in Early Adolescent Mice Results in Acute Colitis, which is Partially Resolved at Adulthood
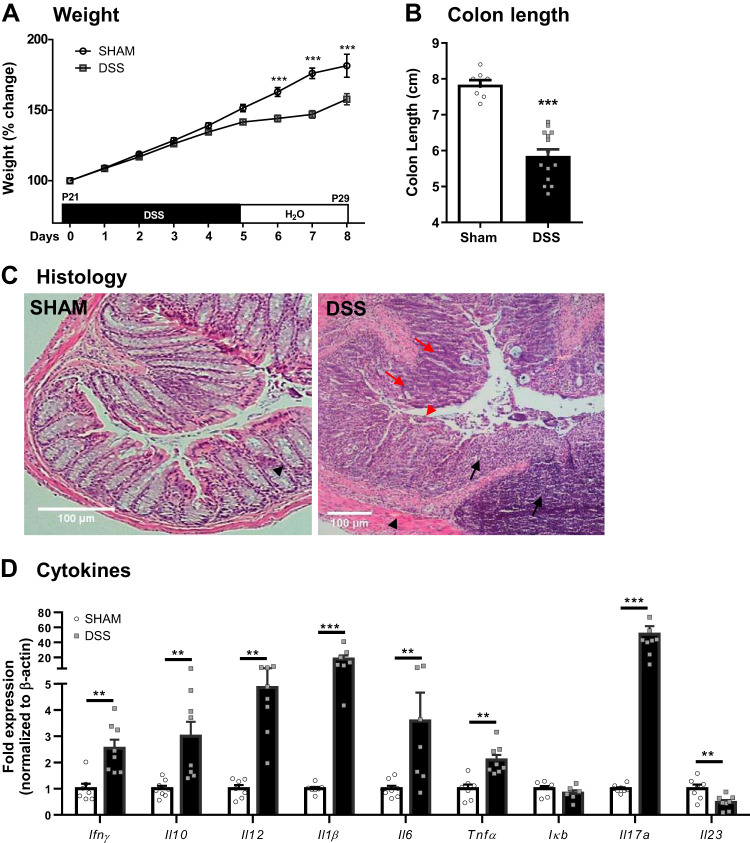
Administration of DSS (2% wt/vol) in weaned mice (P21) resulted in “failure to thrive” as demonstrated by a decrease in weight gain at 8 days post-DSS (Fig. 2A) compared with sham controls, seen similarly in both males and females (data not shown). Correspondingly, colon lengths were significantly reduced (Fig. 2B) and colonic histological analysis demonstrated signs of inflammation, as evidenced by epithelial damage, loss of crypt architecture, goblet cell depletion, muscle thickening, and infiltration of inflammatory cells at 8 days post-DSS, which were absent in sham-treated control mice (Fig. 2C). Additionally, expression of the proinflammatory genes Ifnγ, Il12, Il1β, Il6, and Tnfα as well as anti-inflammatory Il10 expression were increased in distal colonic tissues of DSS-treated mice, confirming the presence of inflammation. Upregulation of Il17 expression, coupled with downregulation of Il23 at P29, suggested initiation of repair mechanisms.
Fig. 2.
Dextran sodium sulfate (DSS) administration (2%) at weaning causes colonic disease at 8 days post-DSS. Weight gain (A) was measured daily from postnatal day (P)21–P29 (n = 5 litters) and colon length (B) measured at P29 (n = 8–12 mice). Colon histology (C); black arrows indicate cell infiltration, red arrows indicate goblet cell depletion, black arrowhead indicates muscle thickening, and red arrowhead indicates loss of crypt architecture. mRNA expression of cytokines (D) was quantified by quantitative PCR (n = 7–8 mice). **P < 0.01 and ***P < 0.001 by Student’s t test or Mann–Whitney test.
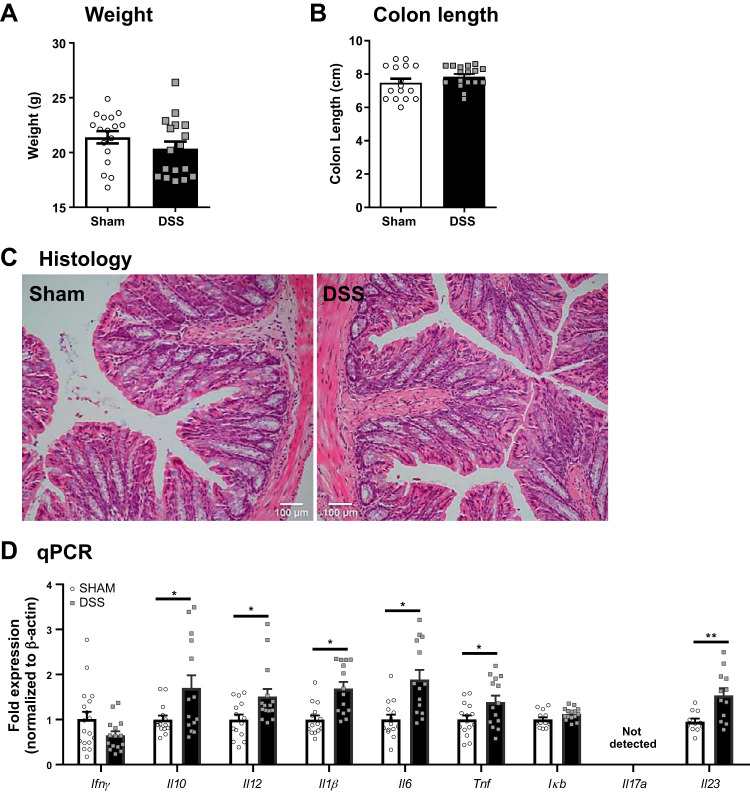
The reduced weight gain in DSS-treated mice (P29) did not lead to persistent stunting of growth and was restored in adult (P56) mice (Fig. 3A). Additionally, colon length and histological damage in DSS-treated mice both improved to levels similar to sham-treated controls (Fig. 3, B and C), suggesting recovery from overt colonic disease. Despite this improvement, colonic proinflammatory gene expression remained significantly different from sham controls at P56, and a shift in the Il17/Il23 expression compared with the expression seen at P29 suggests only partial recovery from disease (Fig. 3D).
Fig. 3.
Colonic disease at weaning after acute dextran sodium sulfate (DSS) administration is partially resolved at adulthood. Weight (A) was measured at postnatal day (P)56 (n = 17 mice). Colon length (B) (n = 17 mice), colon histology (C), and mRNA expression of cytokines by quantitative PCR (qPCR; D) were quantified at P56 (n = 17–18 mice). *P < 0.05 by Student’s t test; **P < 0.01.
DSS-Induced Colitis in Weanling Mice Results in Behavioral Deficits and Hippocampal Alterations in Adulthood
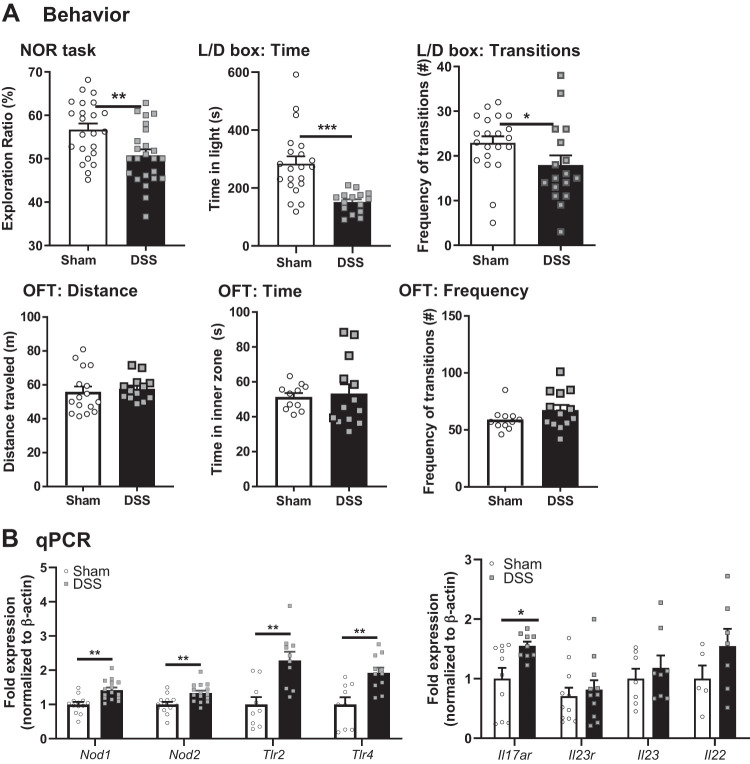
In adult mice, exposure to DSS (3%) causes transient impairments in behavior (both cognitive deficits and anxiety-like behavior) at 8 days post-DSS, but these are restored by 14 days post-DSS (24). To assess the long-term impact of acute pediatric colonic inflammation on anxiety-like behavior and cognitive function in adulthood, behavioral tests were performed at 6–8 wk of age. Mice administered DSS at weaning had a deficit in recognition memory identified in the NOR task, as determined by reduced exploration ratio of a novel versus a known object compared with sham-treated controls (Fig. 4A). This was seen similarly in both males and females; therefore, data were combined.
Fig. 4.
Dextran sodium sulfate (DSS) at weaning causes behavioral deficits and hippocampus gene expression alteration at adulthood. A: behavior: novel object recognition (NOR) task (n = 22–24 mice), light/dark (L/D) box (n = 18–22 mice), and open field test (OFT) (n = 13–16 mice) were performed at postnatal day (P)56. B: hippocampal mRNA expression of pattern-recognition receptors and cytokines was analyzed by quantitative PCR (qPCR) (n = 8–10 mice). *P < 0.05, **P < 0.01, and ***P < 0.001 by Student’s t test or Mann–Whitney test.
DSS-treated mice also presented with anxiety-like behavior, as evidenced by less total time spent in the light zone in the L/D box test compared with sham-treated controls. This was coupled with decreases in the number of transitions between light and dark zone between groups (Fig. 4A). The OFT test was used to determine exploratory behavior and general locomotor activity. Measuring the total distance traveled, there were no differences identified in DSS-treated mice compared with sham-treated controls (Fig. 4A), suggesting overall locomotor activity and wellbeing were similar in both groups. Furthermore, time spent in the inner area of the box and frequency of transitions into the inner area of the box were also similar between groups, suggesting similar exploratory behavior. Additionally, inner zone parameters have also been suggested to reflect anxiety-like behavior. Although these results contrasted with findings from the L/D box, they may simply reflect lighting conditions, which varied slightly between the two tests [625 (OFT) vs. 675 (L/D) lux], or reduced sensitivity of the OFT as a measure of anxiety-like behavior compared with the L/D box (26).
Impaired cognitive function has been associated with proinflammatory conditions within the central nervous system (CNS), including microglia activation that results in the disruption of neuroplasticity (76). To assess a potential role for neuroinflammation in inducing behavioral impairments in DSS-treated mice, we analyzed gene expression of proinflammatory cytokines and pattern-recognition receptors (PRRs) that are known to regulate hippocampal microglia activation (37). Increased expression of PRRs (Nod1, Nod2, Tlr2, and Tlr4) and a receptor for Th17-related cytokines (Il17ra) was observed (Fig. 4B).
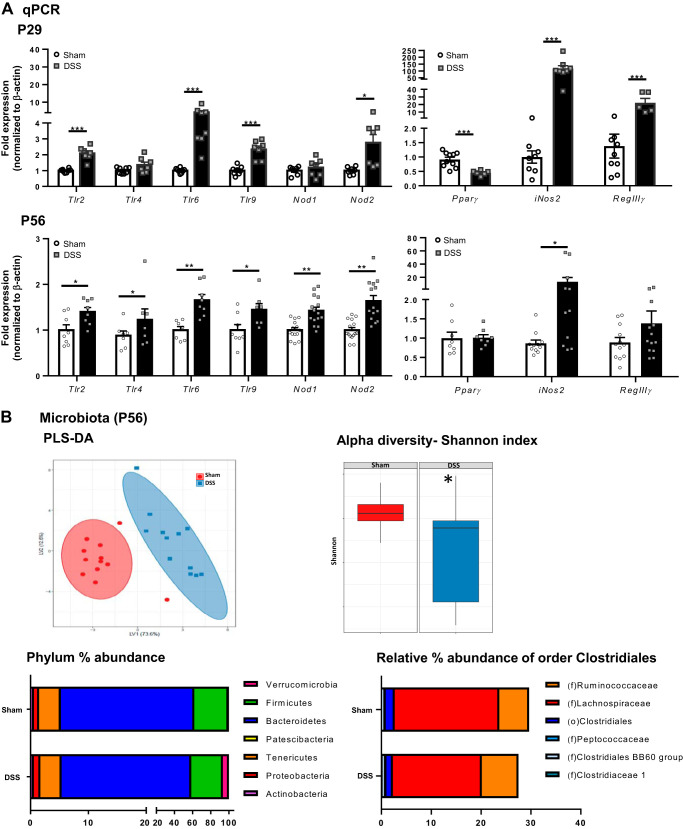
Acute DSS Administration at Weaning Causes Alterations in Colonic Pattern-Recognition Receptor Gene Expression as Well as Changes in Microbiota Composition in Adulthood
Given the critical role of host-microbe interactions in maintaining intestinal homeostasis (53) and the persisting changes in proinflammatory gene expression into adulthood, we assessed gene expression of PRRs at both P29 and in adulthood (P56) following DSS administration. DSS-treated mice express increased levels of expression of PRRs, at both P29 and P56, including Nod2 and Tlr2, Tlr6, and Tlr9, with Tlr4 only increased at P56 (Fig. 5A), suggesting an increased innate immune response following activation by bacterial ligands. Colonic expression of the antimicrobial peptide RegIIIγ was also increased, but only at P29 (Fig. 5A). Interestingly, levels of the peroxisome proliferator-activated receptor-γ (Pparγ) receptor, which helps negatively regulate proinflammatory gene expression (6), were decreased at P29. Expression of inducible nitric oxide synthase (iNOS, Nos2), which produces nitric oxide (NO) in response to proinflammatory cytokines, was substantially increased at both P29 and P56 compared with sham-treated controls. At P56, levels of Pparγ were back to baseline, but Nos2 was still upregulated compared with sham controls (Fig. 5A). This suggests the presence of prolonged alterations in host-microbe interactions that persist into adulthood.
Fig. 5.
Acute dextran sodium sulfate (DSS) administration at weaning causes colonic pattern-recognition receptor gene expression alterations and changes in microbiota composition in adulthood. A: colonic mRNA expression of pattern-recognition receptors, Pparγ, iNos2, and RegIIIγ was analyzed by quantitative PCR (qPCR) at both postnatal day (P)29 (n = 8 mice) and P56 (n = 8–15 mice); *P < 0.05, **P < 0.01, and ***P < 0.001 by Student’s t test or Mann–Whitney test. B: fecal microbiota analysis by 16S Illumina sequencing for partial least squares discriminant analysis (PLS-DA), α-diversity (Shannon index), phylum abundance, and relative abundance of order Clostridiales (n = 12 mice). *P < 0.05 by Kruskal–Wallis pairwise comparison (Shannon diversity).
Given this lasting impairment in expression of critical host-microbe-sensing receptors in the colon in adulthood, the fecal microbiota was sequenced by 16S Illumina sequencing to study microbial composition. The microbiota profiling shows lasting significant differences in the composition of DSS-treated mice compared with sham controls. Partial least squares discriminant analysis (PLS-DA), a model that assesses variation in microbiota between groups, shows taxonomic differences at genus level between DSS and sham controls, which persist into adulthood (Fig. 5B). This dysbiosis was coupled with reduced α-diversity, as showed by the Shannon index, particularly with respect to evenness (Fig. 5B). The most abundant phylotypes in both sham- and DSS-treated mice were members of the phylum Firmicutes, which include the classes Clostridia, Bacilli, and Erysipelotrichia, as well as Bacteroidetes (Fig. 5B), which include the class Bacteroidia and the order Bacteroidales. Strikingly, members of the phylum Verrucomicrobia were increased in the DSS-treated mice and with a low relative abundance in the sham control mice [sham: 0.6 ± 0.15 vs. DSS: 6.73 ± 2.41%; *P < 0.0001; expressed as mean percent abundance of phylum (filtered level taxa)]. Finally, other members of the phylum Proteobacteria were increased in DSS-treated mice [sham: 0.9 ± 0.25 vs. DSS: 1.13 ± 0.16%; mean abundance (%); ***P < 0.001] compared with sham controls.
As Pparγ expressed by colonocytes can respond to butyrate, and not to other short-chain fatty acids (SCFAs), we investigated whether DSS administration at weaning would decrease butyrate-producing bacteria, specifically the class Clostridia, thereby potentially increasing Nos2 expression in colonocytes (12). Adult mice treated with DSS at weaning had a reduced relative abundance of the order Clostridiales (class Clostridia, phylum Firmicutes), specifically Lachnospiraceae [sham: 20.99 ± 0.60 vs. DSS: 17.83 ± 0.97%; mean abundance (%); **P < 0.01, Fig. 5B]. Taken together, these data suggest that pediatric DSS administration causes long-term changes in distal colonic epithelial signaling by changing the gut bacterial community.
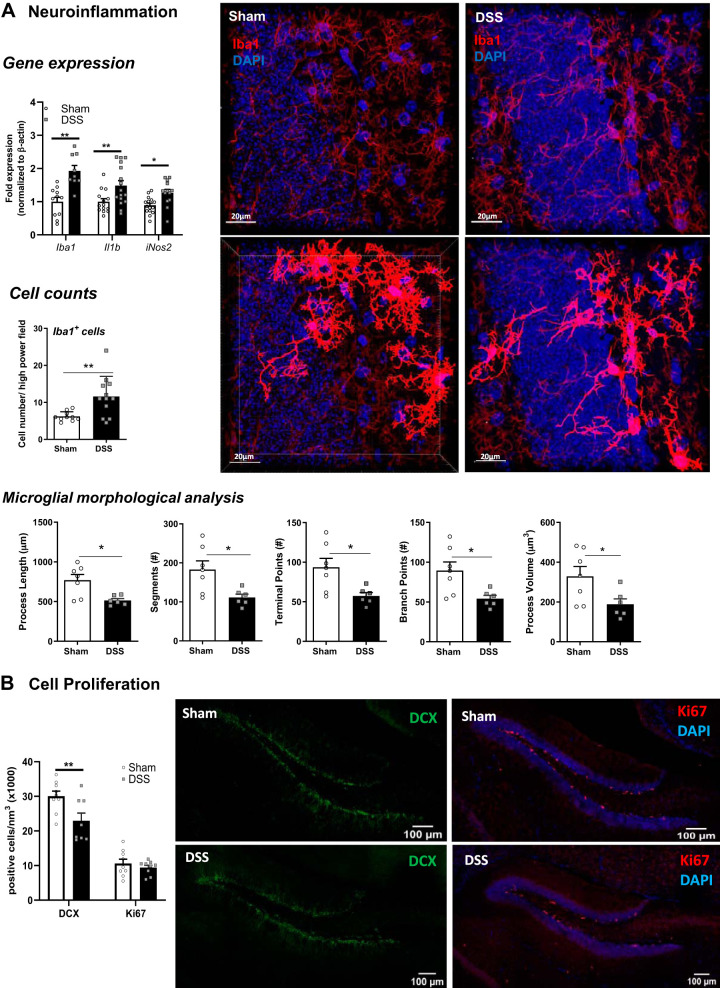
Adult Mice Exposed to DSS at Weaning Display Activation of Microglia and Impaired Neurogenesis in the Hippocampus
Given the alteration in gene expression in the hippocampus for PRR and Th17-related cytokines and the role for the microbiota and SCFAs in microglia development and function, particularly in early life (27, 66), we sought to assess activation of microglia at P56. Hippocampal expression of the microglia specific gene Iba-1 was significantly increased, along with Il1β expression (Fig. 6A). At the protein level, immunostaining for Iba-1 in hippocampal sections revealed evidence of microglia activation, including increased cell numbers, decreased length of processes, and decreased numbers of processes as quantified using Imaris (Fig. 6A). Nos2 expression was also increased in the hippocampus of DSS-treated mice, potentially from activated microglia.
Fig. 6.
Acute dextran sodium sulfate (DSS) administration at weaning causes decreased neural activation, impaired neurogenesis, and neuroinflammation in the hippocampus. Hippocampal mRNA expression of Iba-1, Il1β, and iNos2 was quantified by quantitative PCR (n = 11–14 mice); *P < 0.05 and **P < 0.01 by Student’s t test. A: quantification of Iba-1 positive cells (n = 10–12 mice) along with morphological analysis by immunofluorescence in the dentate gyrus (DG) (n = 6–7 mice). B: neurogenesis was assessed by quantification of doublecortin (DCX)+ and Ki67+ cells using immunofluorescence from the DG (n = 8 mice); **P < 0.01 by Student’s t test or Mann–Whitney test.
Given the association between neuroinflammation and reduced neurogenesis and the critical role for neurogenesis in maintaining cognitive function (31), we quantified hippocampal neurogenesis in adult mice that had received DSS at weaning. Hippocampal expression of brain-derived neurotropic factor (Bdnf), an important neural growth factor, was increased in DSS-treated mice compared with sham-treated controls (Fig. 6B), suggesting an increase in neurogenesis. Immunostaining for the immature neuronal marker DCX was performed to assess neurogenesis, revealing a significant decrease in the number of immature neurons in the hippocampus of DSS-treated mice. This was not coupled with any change in expression of the cell proliferation marker Ki67 (Fig. 6B).
DISCUSSION
In the present study, we describe for the first time long-lasting impairments in the MGB axis following induction of acute colonic inflammation at weaning. We demonstrate that acute DSS administration in early adolescence causes colitis-like disease associated with long-lasting effects on the MGB axis in adulthood, including impaired recognition memory and anxiety-like behavior in the context of hippocampal neuroinflammation. Our findings demonstrate that colonic inflammation in early life can trigger significant intestinal dysbiosis that persists into adulthood, with alterations in the brain and behavior identified later in life.
MGB axis alterations seen in IBD patients significantly impact the overall quality of life, with pediatric patients being the most at risk for these complications given the developmental overlap between microbial colonization, gut physiology maturation, and establishment of neural processes in the brain (7, 42). Administration of the colitogenic agent DSS for five days (2%) starting at weaning successfully reproduced many of the colonic inflammatory features as seen in adult mice, including shortening of colon, histological damage, and increased proinflammatory cytokine expression in the distal colon, at a lower dose than that typically seen in adult mice (3–5%) (22). Although weight loss was not demonstrated in weanling mice, a significant decrease in weight gain was observed, which is a more physiologically relevant feature of pediatric disease, suggesting that the failure of mice to thrive might be occurring due to colonic inflammation. In adulthood, mice exposed to DSS at weaning had recovered their body weight and histological damage was restored. In contrast, DSS-treated animals had significantly increased levels of proinflammatory cytokines in the colon compared with sham-controls at P56, along with a shift in the gut microbial composition. Taken together, these findings may suggest the presence of persistent residual inflammation and dysbiosis continuing into adulthood, which may represent a more susceptible state to disease relapse as often occurs clinically.
The gut microbiota is important for maintaining intestinal physiology and homeostasis, with tolerance of microbes helping the development of innate and adaptive immune responses (1). Several studies have demonstrated that a proinflammatory environment persisting after an initial insult, such as in DSS-induced colitis (19), promotes ongoing perturbations of the gut microbiota. These perturbations are often characterized by blooms of inflammation-associated bacterial species or a reduction of beneficial microbes, such as SCFA producers, leading to increased intestinal inflammation (5, 21, 29, 72, 75). The 16S Illumina sequencing revealed a significant alteration in the gut microbiota between sham- and DSS-treated mice at adulthood, as shown by PLS-DA, coupled with a significant decrease in overall α-diversity with respect to evenness. Additionally, in line with previous publications (5, 50), we found that the abundance of selected bacterial community members decreases after DSS administration, whereas others are highly increased, such as Verrucomicrobia. These findings suggest a selective effect of the DSS exposure, as Verrucomicrobia metabolize sulfur and degrade mucin (5), with mucolytic bacteria and increased mucolytic activity increased in inflammation (54).
DSS administration has also been found to increase epithelial oxygenation, shifting the dominance of obligate anaerobes, including SCFA-producers, to a dysbiotic expansion of facultative anaerobes, such as Proteobacteria (45, 64). Changes in community structure in SCFA producers have also been reported in patients with IBD and after DSS administration in mice (2, 21, 80). Microbial-SCFA producers maintain homeostasis and sustain epithelial Pparγ signaling in epithelial cells (12). Consistent with this, we observed decreased colonic Pparγ expression following acute DSS-induced inflammation. Although we did not observe dramatic changes in the microbiota, and Pparγ expression was normalized to sham control levels by P56, we observed slight decreases in the abundance of bacterial families that produce SCFA in adult mice exposed to DSS at weaning, including families belonging to Clostridiales and Bacteroidales orders, coupled with a mild increase in Proteobacteria abundance. Together, these findings suggest ongoing residual dysbiosis into adulthood following DSS administration at weaning.
Perturbations of the gut microbiota can also alter immune responses, thus contributing to intestinal inflammation characterized, for example, by an expansion of Th17 cells in patients with IBD (35). In mice exposed to DSS at weaning (P21), we observed increased expression of IL17A at P29 and an upregulation of IL23 in adulthood, suggesting a shift in Th17 cells from repair and protective functions to a colitogenic function that exacerbates disease (74). Taken together, it is reasonable to assume that an imbalance in the composition of the gut microbiota at weaning can foster changes in cytokine expression that can persist much later in life (19). Innate and adaptive immune phenotypes can be programmed via gut microbiota-host interactions and mediated via activation of PRRs on host cells. Moreover, the gut microbiota can modulate the expression of genes involved in inflammatory responses through PRRs (78). Here we report an increase in expression of PRRs in colonic tissue and a proinflammatory immune profile after DSS treatment at weaning that is maintained into adulthood, altogether suggesting the presence of ongoing, persistent inflammation.
It is now well established that both chronic intestinal inflammation and alterations of the intestinal microbiota are associated with changes in cognitive behavior and anxiety-like behavior in animal models (24, 29, 52) and in patients (9, 73). Cognitive dysfunction and alterations in mood have been identified in adolescents with IBD (15, 48, 70), but the mechanisms behind this association remain undefined. Here we show that DSS administration in mice, starting at weaning, results in reduced preference for a novel versus a known object and an increase in time spent in the light box compared with sham-treated controls, 3 to 4 wk post-DSS. These findings suggest the presence of prolonged behavioral deficits following resolution of acute colitis in a pediatric setting, in contrast to the transient cognitive deficits and anxiety-like behavior observed in adult mice acutely treated with DSS (24). The impairments in object recognition and exploratory behaviors observed in DSS-treated mice occurred without reduced locomotor behavior or activity, indicating that these behavioral deficits were not simply due to pain, motor deficits, or residual sickness behavior.
The mammalian hippocampus is critical for maintaining learning and memory (20), and has been implicated in regulating mood and anxiety (39). The dentate gyrus region of the hippocampus continuously generates new neurons during adulthood, which is important for storage of new memories. Interestingly, chronic intestinal inflammation is associated with behavioral disturbances linked to disrupted adult hippocampal neurogenesis (81). Here we report that administration of acute DSS at weaning caused a decrease in neurogenesis, as indicated by decreased expression of the immature neuronal marker DCX in the hippocampus. Altered neurogenesis in DSS-treated mice was seen coupled with increased expression of PRR and Th17-cytokine receptor and neuroinflammation by increased Iba1. Recent evidence implicates Toll-like receptors (TLRs) and Nod-like receptors (NLRs) as mediators of CNS plasticity as they can regulate cognitive function and behavior (56) as well as modulate neurogenesis in the adult mammalian brain (61). Moreover, Il17ar expression in the hippocampus has been suggested to have a role in regulating adult hippocampal neurogenesis (46), with increased Th17 cell infiltration and elevated Th17 proinflammatory cytokines levels associated with impaired neurogenesis in other neurodegenerative diseases, including multiple sclerosis and Alzheimer’s disease (79).
In the CNS, microglia are critical for maintaining homeostasis by regulating neuronal survival and cell death (36, 58, 65). Moreover, the hippocampus harbors one of the highest densities of microglial cells (41), which express IL-1β receptors, a cytokine that has been well characterized to regulate cognition (63). Microglia express several different PRRs that can induce the production of proinflammatory cytokines, including IL-1β, IL-6, and TNFα following binding by pathogen-associated molecular patterns (44). Activated microglia also produce increased amounts of NO following the induction of iNOS expression (51). Given the role of the microbiota on microglia maturation and function (27) and the role of microglia in regulating neurogenesis (65), we assessed hippocampal microglia activation through morphological analysis. We identified increased hippocampal microglia numbers and increased expression of genes associated with activated microglia, including Iba-1, Il1β, and Nos2, in DSS-treated mice. These changes were supported by morphological analysis, which identified increased microglia activation as demonstrated by transformation of microglia from a ramified to an amoeboid morphology (18) in DSS-treated mice compared with sham controls. Although we cannot rule out that some of the Iba1+ cells may represent macrophages, microglia vastly outnumber macrophages in the hippocampus both at baseline and following exposure to stress (77). These findings suggest the presence of neuroinflammation within the hippocampus, mediated by increased microglia activation that may in part lead to decreased neurogenesis and cognitive deficits observed following DSS-induced colitis at weaning.
Taken together, our findings identified long-lasting changes in the MGB axis following induction of colitis at weaning that persist into adulthood. This model replicates both colonic proinflammatory features and behavioral deficits as seen in adult mice exposed to acute DSS; however, the deficits were observed well past the exposure to the colitogenic DSS compound when administered starting at weaning. Changes in the gut microbiota and increased immune activation in early life could reprogram adult immune function and impact neural processes important for mediating cognitive function. This altered gut-brain communication highlights common pathways, including PRR, that can regulate both systems. Future studies will better define the mechanisms involved in regulating the MGB axis in pediatric IBD and identify the potential benefits of therapeutically modulating the microbiota to improve intestinal and extraintestinal manifestations in pediatric IBD patients.
GRANTS
This work was funded by a Crohn’s and Colitis Foundation (CCF) Senior Research award (to M. G. Gareau) and National Institutes of Health Grant U24-DK092993 (MMPC, University of California Davis Microbiome and Host Response Core, RRID:SCR_015361). M. Swedek was the recipient of a CCF summer student research fellowship award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.S. and M.G.G. conceived and designed research; E.S., P.S., C.E.K., J.A.S., M.S., G.R., and M.G.G. performed experiments; E.S., P.S., C.E.K., I.B.-M., C.H., T.A.K., J.A.S., M.S., G.R., and M.G.G. analyzed data; E.S., P.S., C.E.K., I.B.-M., C.H., T.A.K., J.A.S., G.R., and M.G.G. interpreted results of experiments; E.S., P.S., K.M.R., G.R., and M.G.G. prepared figures; E.S., P.S., and M.G.G. drafted manuscript; E.S., P.S., C.E.K., G.R., and M.G.G. edited and revised manuscript; E.S., P.S., C.E.K., I.B.-M., C.H., T.A.K., J.A.S., K.M.R., M.S., G.R., and M.G.G. approved final version of manuscript.
REFERENCES
- 1.Alam A, Neish A. Role of gut microbiota in intestinal wound healing and barrier function. Tissue Barriers 6: 1539595, 2018. doi: 10.1080/21688370.2018.1539595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araki Y, Andoh A, Tsujikawa T, Fujiyama Y, Bamba T. Alterations in intestinal microflora, faecal bile acids and short chain fatty acids in dextran sulphate sodium-induced experimental acute colitis in rats. Eur J Gastroenterol Hepatol 13: 107–112, 2001. doi: 10.1097/00042737-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil 23: 1132–1139, 2011. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, Lu J, Khan WI, Corthesy-Theulaz I, Cherbut C, Bergonzelli GE, Collins SM. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 139: 2102–2112.e1, 2010. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 5.Berry D, Kuzyk O, Rauch I, Heider S, Schwab C, Hainzl E, Decker T, Müller M, Strobl B, Schleper C, Urich T, Wagner M, Kenner L, Loy A. Intestinal microbiota signatures associated with inflammation history in mice experiencing recurring colitis. Front Microbiol 6: 1408, 2015. doi: 10.3389/fmicb.2015.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertin B, Dubuquoy L, Colombel JF, Desreumaux P. PPAR-gamma in ulcerative colitis: a novel target for intervention. Curr Drug Targets 14: 1501–1507, 2013. doi: 10.2174/13894501113149990162. [DOI] [PubMed] [Google Scholar]
- 7.Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci 3: 14, 2009. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, , et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37: 852–857, 2019. [Erratum in Nat Biotechnol 37: 1091, 2019]. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 144: 36–49, 2013. doi: 10.1053/j.gastro.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol 463: 55–65, 2003. doi: 10.1016/S0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 11.Burke P, Meyer V, Kocoshis S, Orenstein DM, Chandra R, Nord DJ, Sauer J, Cohen E. Depression and anxiety in pediatric inflammatory bowel disease and cystic fibrosis. J Am Acad Child Adolesc Psychiatry 28: 948–951, 1989. doi: 10.1097/00004583-198911000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y, Litvak Y, Lopez CA, Xu G, Napoli E, Giulivi C, Tsolis RM, Revzin A, Lebrilla CB, Bäumler AJ. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357: 570–575, 2017. doi: 10.1126/science.aam9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13: 581–583, 2016. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castaneda AE, Tuulio-Henriksson A, Aronen ET, Marttunen M, Kolho KL. Cognitive functioning and depressive symptoms in adolescents with inflammatory bowel disease. World J Gastroenterol 19: 1611–1617, 2013. doi: 10.3748/wjg.v19.i10.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lönnqvist J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord 106: 1–27, 2008. doi: 10.1016/j.jad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron 57: 809–818, 2008. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of inflammatory bowel disease among adults aged ≥18 years—United States, 2015. MMWR Morb Mortal Wkly Rep 65: 1166–1169, 2016. doi: 10.15585/mmwr.mm6542a3. [DOI] [PubMed] [Google Scholar]
- 18.Davis BM, Salinas-Navarro M, Cordeiro MF, Moons L, De Groef L. Characterizing microglia activation: a spatial statistics approach to maximize information extraction. Sci Rep 7: 1576, 2017. doi: 10.1038/s41598-017-01747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Fazio L, Cavazza E, Spisni E, Strillacci A, Centanni M, Candela M, Praticò C, Campieri M, Ricci C, Valerii MC. Longitudinal analysis of inflammation and microbiota dynamics in a model of mild chronic dextran sulfate sodium-induced colitis in mice. World J Gastroenterol 20: 2051–2061, 2014. doi: 10.3748/wjg.v20.i8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11: 339–350, 2010. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eeckhaut V, Maes S, Steppe M, Cleynen I, De Preter V, Machiels K, Ducatelle R, Vermeire S, Van Immerseel F. The butyrate producing Clostridium cluster IV genus Butyricicoccus has a decreased abundance in IBD stool samples and a comparative efficacy in TNBS models compared to currently available therapeutics: P-177. Inflamm Bowel Dis 17: S65–S66, 2011. doi: 10.1097/00054725-201112002-00211. [DOI] [Google Scholar]
- 22.Eichele DD, Kharbanda KK. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol 23: 6016–6029, 2017. doi: 10.3748/wjg.v23.i33.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emge JR, Huynh K, Miller EN, Kaur M, Reardon C, Barrett KE, Gareau MG. Modulation of the microbiota-gut-brain axis by probiotics in a murine model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 310: G989–G998, 2016. doi: 10.1152/ajpgi.00086.2016. [DOI] [PubMed] [Google Scholar]
- 25.Engström I. Mental health and psychological functioning in children and adolescents with inflammatory bowel disease: a comparison with children having other chronic illnesses and with healthy children. J Child Psychol Psychiatry 33: 563–582, 1992. doi: 10.1111/j.1469-7610.1992.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 26.Ennaceur A. Tests of unconditioned anxiety - pitfalls and disappointments. Physiol Behav 135: 55–71, 2014. doi: 10.1016/j.physbeh.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 27.Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermöhlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18: 965–977, 2015. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzpatrick LR, Hertzog KL, Quatse AL, Koltun WA, Small JS, Vrana K. Effects of the probiotic formulation VSL#3 on colitis in weanling rats. J Pediatr Gastroenterol Nutr 44: 561–570, 2007. doi: 10.1097/MPG.0b013e31803bda51. [DOI] [PubMed] [Google Scholar]
- 29.Gao X, Cao Q, Cheng Y, Zhao D, Wang Z, Yang H, Wu Q, You L, Wang Y, Lin Y, Li X, Wang Y, Bian JS, Sun D, Kong L, Birnbaumer L, Yang Y. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci USA 115: E2960–E2969, 2018. [Erratum in Proc Natl Acad Sci USA 115: E4542, 2018]. doi: 10.1073/pnas.1720696115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60: 307–317, 2011. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- 31.Gemma C, Bachstetter AD. The role of microglia in adult hippocampal neurogenesis. Front Cell Neurosci 7: 229, 2013. doi: 10.3389/fncel.2013.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginzel M, Feng X, Kuebler JF, Klemann C, Yu Y, von Wasielewski R, Park JK, Hornef MW, Vieten G, Ure BM, Kaussen T, Gosemann JH, Mayer S, Suttkus A, Lacher M. Dextran sodium sulfate (DSS) induces necrotizing enterocolitis-like lesions in neonatal mice. PLoS One 12: e0182732, 2017. doi: 10.1371/journal.pone.0182732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodhand J, Dawson R, Hefferon M, Tshuma N, Swanson G, Wahed M, Croft NM, Lindsay JO. Inflammatory bowel disease in young people: the case for transitional clinics. Inflamm Bowel Dis 16: 947–952, 2010. doi: 10.1002/ibd.21145. [DOI] [PubMed] [Google Scholar]
- 34.Guthrie E, Jackson J, Shaffer J, Thompson D, Tomenson B, Creed F. Psychological disorder and severity of inflammatory bowel disease predict health-related quality of life in ulcerative colitis and Crohn’s disease. Am J Gastroenterol 97: 1994–1999, 2002. doi: 10.1111/j.1572-0241.2002.05842.x. [DOI] [PubMed] [Google Scholar]
- 35.Hedin C, Whelan K, Lindsay JO. Evidence for the use of probiotics and prebiotics in inflammatory bowel disease: a review of clinical trials. Proc Nutr Soc 66: 307–315, 2007. doi: 10.1017/S0029665107005563. [DOI] [PubMed] [Google Scholar]
- 36.Hong S, Dissing-Olesen L, Stevens B. New insights on the role of microglia in synaptic pruning in health and disease. Curr Opin Neurobiol 36: 128–134, 2016. doi: 10.1016/j.conb.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation 12: 114, 2015. doi: 10.1186/s12974-015-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izumi H, Minegishi M, Sato Y, Shimizu T, Sekine K, Takase M. Bifidobacterium breve alters immune function and ameliorates DSS-induced inflammation in weanling rats. Pediatr Res 78: 407–416, 2015. doi: 10.1038/pr.2015.115. [DOI] [PubMed] [Google Scholar]
- 39.Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, Zhou P, Ong SK, Wright MA, Zweifel L, Paninski L, Hen R, Kheirbek MA. Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 97: 670–683.e6, 2018. doi: 10.1016/j.neuron.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30: 772–780, 2013. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keller D, Erö C, Markram H. Cell densities in the mouse brain: a systematic review. Front Neuroanat 12: 83, 2018. doi: 10.3389/fnana.2018.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kundu P, Blacher E, Elinav E, Pettersson S. Our gut microbiome: the evolving inner self. Cell 171: 1481–1493, 2017. doi: 10.1016/j.cell.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 43.Lakhan SE, Kirchgessner A. Neuroinflammation in inflammatory bowel disease. J Neuroinflammation 7: 37, 2010. doi: 10.1186/1742-2094-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia 58: 253–263, 2010. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- 45.Litvak Y, Byndloss MX, Tsolis RM, Bäumler AJ. Dysbiotic Proteobacteria expansion: a microbial signature of epithelial dysfunction. Curr Opin Microbiol 39: 1–6, 2017. doi: 10.1016/j.mib.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Liu Q, Xin W, He P, Turner D, Yin J, Gan Y, Shi FD, Wu J. Interleukin-17 inhibits adult hippocampal neurogenesis. Sci Rep 4: 7554, 2014. doi: 10.1038/srep07554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J 5: 169–172, 2011. doi: 10.1038/ismej.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mackner LM, Greenley RN, Szigethy E, Herzer M, Deer K, Hommel KA. Psychosocial issues in pediatric inflammatory bowel disease: report of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 56: 449–458, 2013. doi: 10.1097/MPG.0b013e3182841263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murray K, Godinez DR, Brust-Mascher I, Miller EN, Gareau MG, Reardon C. Neuroanatomy of the spleen: mapping the relationship between sympathetic neurons and lymphocytes. PLoS One 12: e0182416, 2017. doi: 10.1371/journal.pone.0182416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagalingam NA, Kao JY, Young VB. Microbial ecology of the murine gut associated with the development of dextran sodium sulfate-induced colitis. Inflamm Bowel Dis 17: 917–926, 2011. doi: 10.1002/ibd.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nathan C, Calingasan N, Nezezon J, Ding A, Lucia MS, La Perle K, Fuortes M, Lin M, Ehrt S, Kwon NS, Chen J, Vodovotz Y, Kipiani K, Beal MF. Protection from Alzheimer’s-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp Med 202: 1163–1169, 2005. doi: 10.1084/jem.20051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nyuyki KD, Cluny NL, Swain MG, Sharkey KA, Pittman QJ. Altered brain excitability and increased anxiety in mice with experimental colitis: consideration of hyperalgesia and sex differences. Front Behav Neurosci 12: 58, 2018. doi: 10.3389/fnbeh.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohland CL, Jobin C. Microbial activities and intestinal homeostasis: A delicate balance between health and disease. Cell Mol Gastroenterol Hepatol 1: 28–40, 2015. doi: 10.1016/j.jcmgh.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53a.Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates (3rd ed.) Cambridge, MA: Academic Press, 2008. [Google Scholar]
- 54.Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin TH. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol 105: 2420–2428, 2010. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 55.Price MN, Dehal PS, Arkin AP. FastTree 2 – approximately maximum-likelihood trees for large alignments. PLoS One 5: e9490, 2010. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pusceddu MM, Barboza M, Keogh CE, Schneider M, Stokes P, Sladek JA, Kim HJ, Torres-Fuentes C, Goldfild LR, Gillis SE, Brust-Mascher I, Rabasa G, Wong KA, Lebrilla C, Byndloss MX, Maisonneuve C, Bäumler AJ, Philpott DJ, Ferrero RL, Barrett KE, Reardon C, Gareau MG. Nod-like receptors are critical for gut-brain axis signalling in mice. J Physiol 597: 5777–5797, 2019. doi: 10.1113/JP278640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41: D590–D596, 2013. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reemst K, Noctor SC, Lucassen PJ, Hol EM. The indispensable roles of microglia and astrocytes during brain development. Front Hum Neurosci 10: 566, 2016. doi: 10.3389/fnhum.2016.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reichmann F, Painsipp E, Holzer P. Environmental enrichment and gut inflammation modify stress-induced c-Fos expression in the mouse corticolimbic system. PLoS One 8: e54811, 2013. doi: 10.1371/journal.pone.0054811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 6: 306–314, 2009. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol 9: 1081–1088, 2007. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- 62.Ruel J, Ruane D, Mehandru S, Gower-Rousseau C, Colombel JF. IBD across the age spectrum: is it the same disease? Nat Rev Gastroenterol Hepatol 11: 88–98, 2014. doi: 10.1038/nrgastro.2013.240. [DOI] [PubMed] [Google Scholar]
- 63.Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO. A neuromodulatory role of interleukin-1β in the hippocampus. Proc Natl Acad Sci USA 95: 7778–7783, 1998. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 33: 496–503, 2015. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 65.Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7: 483–495, 2010. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silva JP, Navegantes-Lima KC, Oliveira AL, Rodrigues DV, Gaspar SL, Monteiro VV, Moura DP, Monteiro MC. Protective mechanisms of butyrate on inflammatory bowel disease. Curr Pharm Des 24: 4154–4166, 2018. doi: 10.2174/1381612824666181001153605. [DOI] [PubMed] [Google Scholar]
- 67.Sinnema G. The Effects of a Chronic Illness on Somatic Growth and Psychological Development. In: Development in Adolescence: Psychological, Social and Biological Aspects, edited by Everaerd W, Hindley CB, Bot A, van der Werff ten Bosch JJ. Dordrecht: Springer Netherlands, 1983, p. 114–130. [Google Scholar]
- 68.Smith CJ, Emge JR, Berzins K, Lung L, Khamishon R, Shah P, Rodrigues DM, Sousa AJ, Reardon C, Sherman PM, Barrett KE, Gareau MG. Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. Am J Physiol Gastrointest Liver Physiol 307: G793–G802, 2014. doi: 10.1152/ajpgi.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stasi C, Orlandelli E. Role of the brain-gut axis in the pathophysiology of Crohn’s disease. Dig Dis 26: 156–166, 2008. doi: 10.1159/000116774. [DOI] [PubMed] [Google Scholar]
- 70.Szigethy E, Levy-Warren A, Whitton S, Bousvaros A, Gauvreau K, Leichtner AM, Beardslee WR. Depressive symptoms and inflammatory bowel disease in children and adolescents: a cross-sectional study. J Pediatr Gastroenterol Nutr 39: 395–403, 2004. doi: 10.1097/00005176-200410000-00017. [DOI] [PubMed] [Google Scholar]
- 71.Van Hemert S, Marlicz W, Szachta P, Pekelharing E, Ormel G, Łoniewski I, Ostrowska L, Samochowiec J. The role of the gut microbiota in mood and behaviour. Whether psychobiotics can become an alternative in therapy in psychiatry? Eur Psychiatry 33: S26, 2016. doi: 10.1016/j.eurpsy.2016.01.842. [DOI] [Google Scholar]
- 72.Walker AW, Sanderson JD, Churcher C, Parkes GC, Hudspith BN, Rayment N, Brostoff J, Parkhill J, Dougan G, Petrovska L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol 11: 7, 2011. doi: 10.1186/1471-2180-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walker JR, Graff LA, Dutz JP, Bernstein CN. Psychiatric disorders in patients with immune-mediated inflammatory diseases: prevalence, association with disease activity, and overall patient well-being. J Rheumatol Suppl 88: 31–35, 2011. doi: 10.3899/jrheum.110900. [DOI] [PubMed] [Google Scholar]
- 74.Whibley N, Gaffen SL. Gut-busters: IL-17 ain’t afraid of no IL-23. Immunity 43: 620–622, 2015. doi: 10.1016/j.immuni.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z, Järnerot G, Tysk C, Jansson JK, Engstrand L. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 139: 1844–1854.e1, 2010. doi: 10.1053/j.gastro.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 76.Wohleb ES. Neuron-microglia interactions in mental health disorders: “for better, and for worse”. Front Immunol 7: 544, 2016. doi: 10.3389/fimmu.2016.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci 33: 13820–13833, 2013. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiao Y, Yan H, Diao H, Yu B, He J, Yu J, Zheng P, Mao X, Luo Y, Chen D. Early gut microbiota intervention suppresses DSS-induced inflammatory responses by deactivating TLR/NLR signalling in pigs. Sci Rep 7: 3224, 2017. doi: 10.1038/s41598-017-03161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78a.York EM, LeDue JM, Bernier LP, MacVicar BA. 3DMorph automatic analysis of microglial morphology in three dimensions from ex vivo and in vivo imaging. eNeuro 5: ENEURO.0266-18.2018, 2018. doi: 10.1523/ENEURO.0266-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang J, Ke KF, Liu Z, Qiu YH, Peng YP. Th17 cell-mediated neuroinflammation is involved in neurodegeneration of aβ1-42-induced Alzheimer’s disease model rats. PLoS One 8: e75786, 2013. doi: 10.1371/journal.pone.0075786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhuang X, Li T, Li M, Huang S, Qiu Y, Feng R, Zhang S, Chen M, Xiong L, Zeng Z. Systematic review and meta-analysis: short-chain fatty acid characterization in patients with inflammatory bowel disease. Inflamm Bowel Dis 25: 1751–1763, 2019. doi: 10.1093/ibd/izz188. [DOI] [PubMed] [Google Scholar]
- 81.Zonis S, Pechnick RN, Ljubimov VA, Mahgerefteh M, Wawrowsky K, Michelsen KS, Chesnokova V. Chronic intestinal inflammation alters hippocampal neurogenesis. J Neuroinflammation 12: 65, 2015. doi: 10.1186/s12974-015-0281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]