Keywords: microRNA, pancreatic acinar cells, posttranscriptional regulation, SLC19A2, thiamin uptake, THTR-1
Abstract
The water-soluble vitamin B1 (thiamin) plays essential roles in normal metabolism and function of all human/mammalian cells, including the pancreatic acinar cells (PACs). PACs obtain thiamin from their surrounding circulation via transport across the plasma membrane, a process that is mediated by thiamin transporter (THTR)-1 and THTR-2. We have previously characterized different aspects of thiamin uptake by mouse and human primary PACs, but little is known about posttranscriptional regulation of the uptake event. We addressed this by focusing on the predominant thiamin transporter THTR-1 (encoded by SLC19A2 gene) in PACs. Transfecting pmirGLO-SLC19A2 3′-untranslated region (UTR) into mouse-derived PAC 266-6 cells leads to a significant reduction in luciferase activity compared with cells transfected with empty vector. Subjecting the SLC19A2 3′-UTR to different in silico algorithms identified multiple putative microRNA binding sites in this region. Focusing on miR-200a-3p (since it is highly expressed in mouse and human pancreas), we found that transfecting PAC 266-6 and human primary PACs (hPACs) with mimic miR-200a-3p leads to a significant inhibition of THTR-1 expression (both protein and mRNA levels) and in thiamin uptake. In contrast, transfection by miR-200a-3p inhibitor leads to an increase in THTR-1 expression and thiamin uptake. Additionally, truncating the region carrying miR-200a-3p binding site in SLC19A2 3′-UTR and mutating the binding site lead to abrogation in the inhibitory effect of this microRNA on luciferase activity in PAC 266-6. These results demonstrate that expression of THTR-1 and thiamin uptake in PACs is subject to posttranscriptional regulation by microRNAs.
NEW & NOTEWORTHY The findings of this study show, for the first time, that the membrane transporter of vitamin B1, i.e., thiamin transporter-1 (THTR-1), is subject to regulation by microRNAs (specifically miR-200a-3p) in mouse and human primary pancreatic acinar cells (PACs). The results also show that this posttranscriptional regulation has functional consequences on the ability of PACs to take in the essential micronutrient thiamin.
INTRODUCTION
Vitamin B1 (thiamin; also referred to as the “energy vitamin”), a member of the water-soluble family of vitamins, is essential for normal human physiology and health. Thiamin (mainly in the form of thiamin pyrophosphate) acts as a cofactor for multiple enzymes that catalyze critical cellular metabolic reactions related to oxidative energy metabolism, ATP production, and reduction of oxidative stress (4, 12, 23, 49). Deficiency of thiamin at the cellular level “weakens the cell” via reducing its energy level, impairing the function/structure of its mitochondria, and induction of oxidative stress (1, 5, 7, 13). The consequences of these deleterious effects of thiamin deficiency then manifest themselves in the form of clinical abnormalities that include neurological and cardiovascular disorders (4, 8, 14, 49). The incidence of systemic thiamin deficiency and suboptimal levels is not uncommon and occurs in conditions like chronic alcoholism, sepsis, and diabetes mellitus (16, 33, 35, 49).
The pancreas is a vital organ with both exocrine and endocrine functions. Diseases/factors that affect this complex organ lead to significant morbidity and mortality. Cells of this organ are highly metabolically active, and thus they require a constant supply of essential micronutrients, like thiamin, to maintain their health and function. Indeed, the predominant cell type in this organ, i.e., pancreatic acinar cells (PACs), maintains high levels of thiamin (29), and deficiency of this vitamin negatively impacts their exocrine function (29, 38). Like other human (and mammalian) cells, PACs lack the ability to synthesize thiamin endogenously and thus must obtain the vitamin from circulation via transport across the plasma membrane. Using a variety of physiological, cellular, and molecular approaches (including appropriate knockout and transgenic mouse models) and human primary PACs (hPACs), we have previously shown that thiamin uptake by PACs is via a specific carrier-mediated mechanism that involves thiamin transporter (THTR)-1 and THTR-2 (products of the SLC19A2 and SLC19A3 genes, respectively) (45). We have also investigated the effect of common external/environmental factors that are known to negatively impact pancreatic physiology/health, e.g., chronic exposure to alcohol and to components of cigarette smoke (40–44) as well as that of the bacterial flagellin on thiamin uptake by PACs (39), and delineated the cellular/molecular mechanisms involved in the inhibitory effects they cause. Little, however, is currently known about how the thiamin uptake process of PACs is regulated, especially at the posttranscriptional and posttranslational levels. We address these issues in the current investigation and examine possible involvement of microRNAs in posttranscriptional regulation of THTR-1 expression and thiamin uptake in mouse and human PACs.
MicroRNAs are a class of small (∼20–22 nucleotides), noncoding, single-stranded RNAs that are conserved across species. They regulate gene expression at the posttranscriptional level by base pairing to complementary sequences (mainly) present in the 3′‐untranslated regions (3′-UTR) of the target mRNAs. This leads to degradation of the target mRNAs and/or suppression of their translation (9, 24). Expression of microRNAs is tissue specific (21), and although they constitute only 1–3% of the human genome, they control/regulate the expression of ∼30% of all coding genes. Because a single microRNA can act on multiple downstream target mRNAs, microRNAs can exert widespread regulatory functions on many important cellular events/pathways, including membrane transport processes (3, 20, 26, 34, 53). Dysregulation of microRNAs has been implicated in a variety of pathological conditions, including immune/inflammatory disorders (2, 10, 27, 31, 46). In addition, a variety of conditions, e.g., chronic and acute pancreatitis, appear to be associated with altered microRNAs expression (6, 11, 36). Moreover, external/environmental (e.g., chronic alcohol exposure; see Ref. 48) and internal (e.g., proinflammatory cytokines; see Ref. 28) factors appear to influence the level of expression of microRNAs in a variety of cells/tissues, which may contribute to the effect of these factors on target tissues/cells (24, 48).
Results of our current investigation showed that expression of THTR-1 in mouse and human PACs as well as the thiamin uptake process that it mediated in these cells are subject to posttranscriptional regulation by specific microRNAs.
MATERIALS AND METHODS
Materials.
The following were obtained from commercial sources: [3H]thiamin (specific activity 12.8 Ci/mmol; radiochemical purity 99%) from Moravek (Brea, CA); mouse-derived pancreatic acinar cell line 266-6 (PAC 266-6) from American Type Tissue Collection (Rockville, MD); anti-THTR-1 (ab123246) and anti-β-actin (sc47778) from Abcam and Santa Cruz Biotechnology, respectively; mimic miR-200a-3p and miR-200a-3p inhibitor from Sigma (St. Louis, MO); and PCR primers from Integrated DNA Technologies (San Diego, CA).
Cloning of 3′-UTR of SLC19A2 (hTHTR-1).
The full-length SLC19A2 3′-UTR (1,952 bp) was PCR amplified using cDNA reverse transcribed from human total RNA (Takara-Bio). The forward and reverse primer sequences used for amplification of SLC19A2 3′-UTR were fused with SacI and XhoI sites, respectively (Table 1). The amplified PCR product was ligated into pGEM-T easy vector and then subsequently su-cloned into SacI and XhoI sites of pmirGLO dual-luciferase miRNA target expression vector (Promega, Madison, WI). The ligated product (pmirGLO-SLC19A2 3′-UTR) was then transformed into competent Escherichia coli (JM109; Promega), and clones were selected on ampicillin agar plates. The cloned full-length pmirGLO-SLC19A2 3′-UTR [wild type (WT)] construct was confirmed by DNA sequencing (Genewiz, La Jolla, CA).
Table 1.
Sequence of the primers used in current investigation
| Primer Name | Forward and Reverse Primer Sequences (5′-3′) |
|---|---|
| Primers used for cloning SLC19A2 3′-UTR in pmirGLO vector | |
| SLC19A2 3′-UTR | ATTGAGCTCTATACTGCTGAAGGGCTTC; CAGCTCGAGTTTTGATTAAAAAGAGAAAATATAC |
| Real-time PCR primers | |
| hTHTR-1 | GCCAGACCGTCTCCTTGTA; TAGAGAGGGCCCACCACAC |
| hβ-actin | CATCCTGCGTCTGGACCT; TAATGTCACGCACGATTTCC |
| mTHTR-1 | GTTCCTCACGCCCTACCTTC; GCATGAACCACGTCACAATC |
| mβ-actin | ATCCTCTTCCTCCCTGGA; TTCATGGATGCCACAGGA |
| Primers used for site-directed mutagenesis | |
| GGTATTAACTTTTTTAGAATGACACAGCTGAAAATTATCAATAATAGTGTGTCAAGATTGCAAAGTCGACA; | |
| TGTCGACTTTGCAATCTTGACACACTATTATTGATAATTTTCAGCTGTGTCATTCTAAAAAAGTTAATACC |
Restriction sites are underlined, and mutated sites are in boldfaced italics; hTHTR-1, human thiamin transporter-1; hβ-actin, human β-actin; mβ-actin, mouse β-actin; mTHTR-1, mouse thiamin transporter-1; UTR, untranslated region.
Generation of truncated SLC19A2 3′- UTR (1293-DEL) construct.
Truncation construct was generated according to the manufacturer’s instruction. Briefly, the full-length WT (pmirGLO-SLC19A2 3′-UTR) was digested with XbaI site (located upstream of the predicted miRNA-200a-3p interacting site) to release a 659-bp region from its 3′-end. The restriction digestion mix was loaded onto agarose gel, and the SLC19A2 3′-UTR (1293-DEL) fragment was then eluted, followed by ligation into pmirGLO dual-luciferase miRNA target expression vector (Promega, Madison, WI), using a rapid DNA ligation kit (Roche Applied Science, Branchburg, NJ). The sequence of the truncated construct was verified by DNA sequencing (Genewiz).
In silico identification of putative miRNAs that target SLC19A2 3′-UTR.
Two widely used prediction algorithms TargetScan (http://www.targetscan.org/) and miRDB (http://mirdb.org/miRDB/) were used to identify putative miRNAs that target the SLC19A2 3′-UTRs of both human and mouse (Table 2). Six putative miRNAs were identified by these programs (Table 3).
Table 2.
miRNAs predicted to target SLC19A2 by 3 different miRNA target prediction algorithms
| Algorithms | miRNAs Predicted Targeting Human SLC19A2 | miRNAs Predicted Targeting Mouse Slc19a2 |
|---|---|---|
| TargetScan | 100 | 128 |
| miRDB | 139 | 96 |
Table 3.
Context score and context score percentile based on TargetScan 7.1 program for predicted miR-200a-3p for human and mouse SlC19A2 3′-UTR
| Organism | miRNA | Base-Pairing Type | Context Score | Context Score Percentile | Conserved Branch Length | Binding Position in SLC19A2 3′-UTR |
|---|---|---|---|---|---|---|
| Human | hsa-miR-200a-3p | 8 mer | −0.32 | 98 | 1.6 | 1337–1344 |
| Mouse | mmu-miR-200a-3p | 8 mer | −0.12 | 83 | 0.3 | 436–443 |
UTR, untranslated region.
Site-directed mutagenesis in SLC19A2 3′-UTR.
We used the Quick-change XL site-directed mutagenesis kit (Agilent, La Jolla, CA) to introduce mutations in the miR-200a-3p-binding site of the SLC19A2 3′-UTR, following the manufacturer’s instructions. The sequences of the mutant primers used in the site-directed mutagenesis are listed in Table 1. The site-specific mutations were verified by DNA sequencing (Genewiz).
Culturing and transfection of mouse PAC 266-6 and thiamin uptake.
The mouse PAC 266-6 were cultured in complete DMEM growth medium supplemented with 10% FBS as well as streptomycin and penicillin in a 5% CO2 atmosphere at 37°C (39). Transient transfection of confluent PAC 266-6 cells was done using 100 nM mimic miR-200a-3p or miR-200a-3p inhibitor with appropriate negative controls for 48 h, using RNAiMax transfection reagent to attain maximum transfection efficiency (Invitrogen, Carlsbad, CA). Thiamin uptake was carried out by incubating the transfected cells for 10 min in Krebs-Ringer buffer containing either [3H]thiamin or unlabeled thiamin (39). Uptake was terminated by adding 1 mL of ice-cold KR buffer and washing the cells, followed by their digesting (using 1 mL of 1 N NaOH, followed by neutralization with 100 μL of 10 N HCl), and counting of the radioactivity in a liquid scintillation counter (LS6500; Beckman Coulter, Brea, CA). Protein content of cell digests was determined using a Bio-Rad DC protein assay kit (Bio-Rad, Carlsbad, CA).
Transduction of hPACs using adenoviral vector and thiamin uptake.
hPACs were from cadaveric pancreatic tissues of organ donors [kindly provided to us by the Islet Cell Laboratory of the University of Louisville (Louisville, KY) and that of the City of Hope (Duarte, CA)]. Isolated hPACs (received between 24 and 48 h postisolation) were cultured in Ham’s F-12K growth medium (supplemented with 10% FCS, 5% BSA, 10 ng/mL epidermal growth factor, and 0.1 mg/mL soybean trypsin inhibitor), as described by us/others previously (37, 44). The study protocols performed in hPACs were approved by the institutional review boards of the University of Louisville, Louisville, KY; the City of Hope, Duarte, CA; and the University of California, Irvine, CA (Institutional Review Board: 2017-1593). These cells were then used in the physiological and molecular studies; when transduction was needed, we used adenoviral vector system and validated the transduction efficiency by transducing recombinant adenoviral vector expressing GFP (Ad-GFP) after 16 h by fluorescent microscopy. The recombinant adenoviral constructs, Ad-200a-3p carrying the miR-200a-3p sequence (accession no. MIMAT0000682) or Ad-control (Applied Biological Materials Inc., Richmond, BC, Canada), were transduced into hPACs for 48 h, by following the manufacturer’s instructions. Posttransduced hPACs (Ad-200a-3p or Ad-control), washed with PBS, were treated with trypsin (to detach them from the cell culture flasks), and then the resulting pellet was suspended in KR buffer. Cells were then incubated with [3H]thiamin for 10 min, and the uptake reaction was terminated by adding 1 mL of ice-cold buffer, followed by rapid filtration, as described by us previously (39, 44). Protein concentrations were assessed using a Bio-Rad DC protein assay kit.
Transient transfection of mouse PAC 266-6 and luciferase assay.
Mouse PAC 266-6, cultured in 12-well plates, was transiently transfected with 1.5 μg of pmirGLO vector (mock) or pmirGLO-derived SLC19A2 constructs [full-length (WT), truncated (1293-DEL), or mutated (MUT)] using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), by following the manufacturer’s instructions. Forty-eight hours later, the cells were lysed, and luciferase activity was measured using a Dual Luciferase Assay Kit (Promega). For testing the effect of miRNA mimic, PAC 266-6 was transiently transfected (using Lipofectamine 2000) with 1.5 μg of plasmid constructs (WT or MUT) in the presence and absence of 100 nM miR-200a-3p mimic. To examine the effect of the miR-200a-3p inhibitor, PAC 266-6 were transiently transfected (using Lipofectamine 2000) with 1.5 μg of WT or MUT constructs with or without 100 nM of the miR-200a-3p inhibitor. The luciferase activity was calculated as a ratio of firefly luciferase relative to Renilla luciferase luminescence and is expressed as percentage of luciferase activity in the control samples.
Real-time quantitative PCR analysis.
Two micrograms of total RNA (isolated from hPACs and PAC 266-6) were reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad, Carlsbad, CA), and the cDNA samples were used for real-time quantitative PCR with gene-specific primers for human and mouse THTR-1 and β-actin (Table 1). PCR conditions were as described by us previously (44). The level of expression of THTR-1 mRNA was normalized with β-actin. Fold change was quantified using a relative relationship method (22).
Western blot analysis.
Whole cell lysates of hPACs and PAC 266-6 were prepared in radio-immunoprecipitation assay buffer (RIPA) buffer (Sigma) containing protease inhibitor cocktail (Roche, Branchburg, NJ). Soluble protein fractions were separated (by centrifugation at 14,000 rpm for 20 min), followed by determination of protein concentration using the Bio-Rad DC protein assay kit. To examine the expression level of the human and mouse THTR-1, equal amounts of protein (40 μg) were resolved using NuPAGE 4–12% premade gel (Invitrogen), followed by electrotransferring the protein to polyvinylidene difluoride (PVDF) membranes (Fisher Scientific). The membranes were then blocked using Odyssey blocking solution (LI-COR; Bioscience, Lincoln, NE), followed by probing with anti-THTR-1 (1:500) and anti-β-actin (1:3,000). Specificity of the anti-THTR-1 polyclonal antibodies was validated by overexpressing the mouse and human SLC19A2 carrying plasmid construct (MR207986 and RC216515; OriGene Technologies, MD) in PAC 266-6 and human-derived intestinal epithelial Caco-2 cells (see Fig. 3B, graph ii, and Fig. 4C, graph ii), respectively. The immune-reactive bands for human and mouse THTR-1 and β-actin were then detected by using corresponding secondary anti-rabbit IgG IRDye 800 and anti-mouse IRDye-680 (1:30,000) (LI-COR Bioscience). The identified specific bands were then quantified using the Odyssey infrared imaging system (version 3.0; LI-COR Bioscience), and they were normalized relative to β-actin.
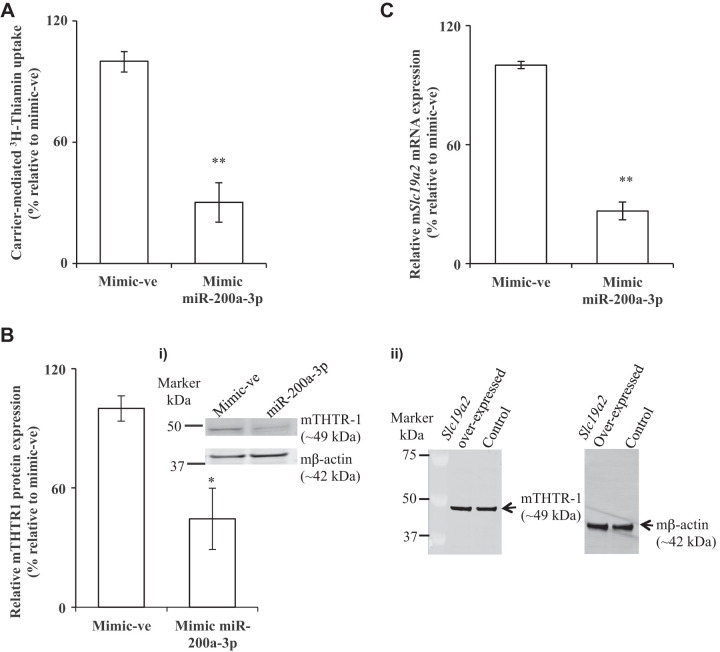
Fig. 3.
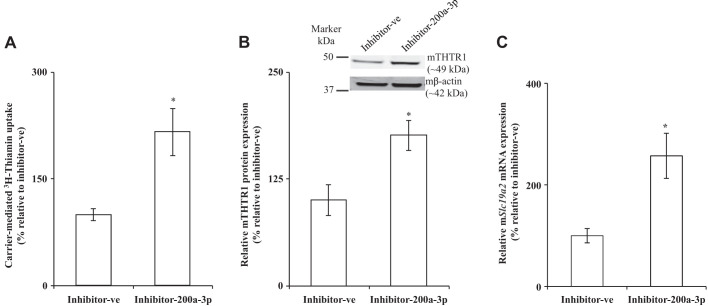
Effect of transfection of PAC 266-6 with miR-200a-3p on thiamin uptake, mouse thiamin transporter-1 (mTHTR-1) protein and mRNA levels. Cells from the pancreatic acinar cell line 266-6 (PAC 266-6) were transfected with negative control mimic or mimic miR-200a-3p for 48 h. A: carrier-mediated thiamin uptake. B: expression of mTHTR-1 protein was determined (graph i); gel image showing the specificity of anti-THTR-1 polyclonal antibodies in PAC-266–6 (graph ii). C: expression of mTHTR-1 mRNA levels were determined. Data are means ± SE of 3–6 independent determinations (**P < 0.01; *P < 0.05).
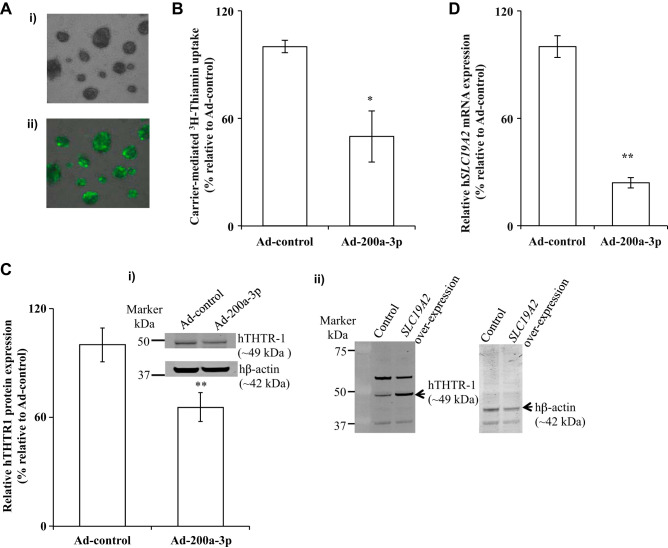
Fig. 4.
Effect of transduction of human primary pancreatic acinar cells (hPACs) with adenoviral miR-200a-3p on thiamin uptake, human thiamin transporter-1 (hTHTR-1) protein, and mRNA. A: bright-field (image i) and green fluorescent microscopic images (image ii) of hPACs transduced with adenovirus carrying green fluorescent protein (GFP). B: hPACs were transduced with miRNAs (Ad-control or Ad-200a-3p, 48 h), and carrier-mediated thiamin uptake was examined. C and D: hTHTR-1 protein in hPAC (graph i) and gel image showing specificity of anti-THTR-1 polyclonal antibodies (image ii) in Caco-2 cells (C) and mRNA (D) expression levels were determined. Data are means ± SE of 3–6 independent determinations (**P < 0.01; *P < 0.05).
Statistical analyses.
Data on carrier-mediated uptake of thiamin by PAC 266-6 and hPACs are presented as means ± SE from at least six to nine independent determinations and are expressed as percentage relative to simultaneously performed appropriate controls. Data on Western blotting, RT-qPCR, and luciferase assays are expressed as means ± SE of at least three to six separate determinations. Student’s t test, using Microsoft Excel, was used for statistical analysis, with a P value of < 0.05 being considered statistically significant.
RESULTS
The 3′-UTR of the SLC19A2 is a target for posttranscriptional regulation by miRNAs.
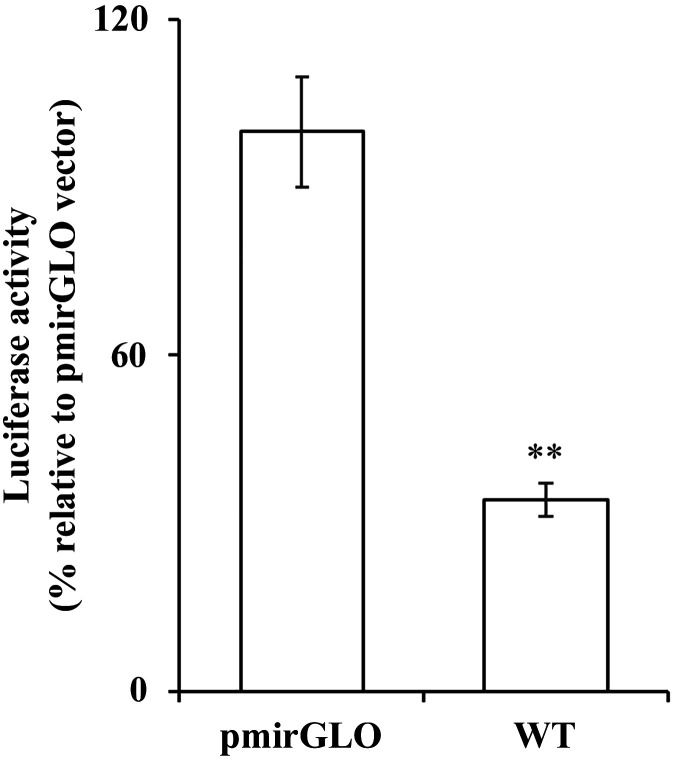
In this study, we aimed at determining whether the 3′-UTR of SLC19A2 is a target for miRNAs. Thus, we generated a reporter construct in which the full-length 3′-UTR of the hSLC19A2 (1,958 bp; WT) was fused to the firefly luciferase gene in the pmirGLO dual luciferase (miRNA target expression) vector and then transfected the reporter construct into PAC 266-6. The result showed a significant (P < 0.01) decrease in luciferase activity in cells transfected with the WT construct compared with cells transfected with the pmirGlo vector alone (Fig. 1). This result suggests that the 3′-UTR of SLC19A2 is a potential target of posttranscriptional regulation by miRNA(s).
Fig. 1.
Determination of luciferase activity of pmirGLO-SLC19A2 3′-untranslated region (UTR) [wild type (WT)] in the pancreatic acinar cell line 266-6 (PAC 266-6). PAC 266-6 were transiently transfected with either pmirGLO vector or the WT constructs for 48 h. Luciferase activity was measured and normalized with Renilla luciferase. Data were expressed as %relative to control (pmirGLO vector). Data are means ± SE of 3–6 independent determinations (**P < 0.01).
Identification of putative miRNAs that target 3′-UTR of SLC19A2.
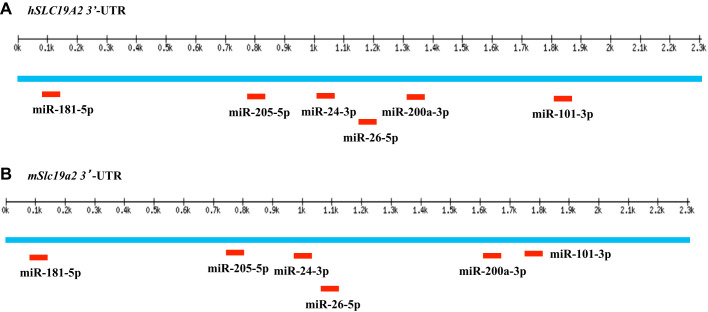
Because SLC19A2 appears to be a potential target for miRNAs, we subjected the 3′-UTR of SLC19A2 to two widely used in silico analyses (see materials and methods) to predict potential miRNAs that interact with the SLC19A2 3′-UTR. Our analysis led to the identification of six putative (miR-181–5p, miR-205-5p, miR-24-3p, miR-26–5p, miR-200a-3p, and miR-101-3p) potential miRNAs that are common to both human and mouse SLC19A2. Our focus on examining the role of the putative microRNA (miR-200a-3p) in posttranscriptional regulation of THTR-1 expression and thiamin uptake by PACs was due to the fact that 1) this microRNA is conserved in mice and humans (as well as certain other mammals like rats, rabbits, and chimpanzees), 2) it has a high context score and percentile, and 3) it has high level of expression in the pancreas (11, 47). Based on these findings, we decided to focus on examining the role of miR-200a-3p in posttranscriptional regulation of SLC19A2 in PACs. Figure 2, A and B, shows the putative binding sites of miR-200a-3p in the 3′-UTRs of human and mouse SLC19A2, respectively.
Fig. 2.
Prediction and localization of miRNA sites in human and mouse SLC19A2 3′-untranslated regions (UTR). miRNA binding sites in human (A) and mouse (B) SLC19A2 3′-UTR regions as predicted by Targetscan. This figure is based on http://www.targetscan.org.
Effect of miR-200a-3p mimic on physiology and molecular biology of thiamin uptake by PAC 266-6 and hPACs.
To determine whether the miR-200a-3p regulates mSlc19a2 (as predicted by the above-described bioinformatics analysis), we examined the effect of miR-200a-3p mimic on thiamin uptake physiology and molecular biology using PAC 266-6 (our choice of these cells was based on a previous observation showing that they express miR-200a-3p at high levels; see Ref. 11). We first confirmed the role of miR-200a-3p on thiamin uptake by transfecting miR-200a-3p mimic (100 nM) into PAC 266-6. The initial rate of thiamin uptake was examined (48 h posttransfection), with results showing a significant (P < 0.01) inhibition in the substrate uptake by cells transfected with miR-200a-3p mimic compared with control cells (i.e., those transfected with negative control mimic) (Fig. 3A). This inhibition was associated with a significant decrease in mTHTR-1 expression at both the protein and mRNA levels (P < 0.05 and 0.01, respectively) compared with controls (Fig. 3, B and C). These results suggest that miR-200a-3p interacts with mSlc19a2 3′-UTR in PAC 266-6 and that this interaction has physiological consequence.
To confirm and extend our findings with the PAC 266-6 cells into the human scenario, we used freshly isolated primary hPACs and examined the effect of miR-200a-3p mimic on thiamin uptake physiology and molecular biology. Here, we used an adenoviral-mediated DNA transduction approach to deliver the miR-200a-3p. First, we verified the efficiency of the adenoviral-mediated DNA transduction in hPACs by transducing Ad-GFP. Assessment of transduction efficiency was examined 16 h posttransduction using fluorescent microscopic images of bright field and green fluorescence (Fig. 4A) of the transduced cells. The transduction efficiency of adenoviral vector containing GFP was found to be >95%. To determine the effect of miR-200a-3p in the regulation of SLC19A2, we transduced Ad-200a-3p into hPACs and then examined thiamin uptake physiology and molecular biology. A significant (P < 0.05) inhibition in initial rate of carrier-mediated thiamin uptake by hPACs transduced with the Ad-200a-3p was observed compared with uptake by cells transduced with the Ad-control (Fig. 4B). This decrease in the thiamin uptake was also associated with a significant (P < 0.01 for both) reduction in the expression of hTHTR-1 at the protein and mRNA levels (Fig. 4, C and D). These results demonstrate a role for miR-200a-3p mimic in posttranscriptional regulation of SLC19A2 expression and function in hPACs. The similarity of the findings of our studies between PAC 266-6 and hPACs justifies the use of the former model to further investigate the role of miR-200a-3p in posttranscriptional regulation of SLC19A2.
Effect of inhibiting miR-200a-3p on thiamin uptake physiology and molecular biology in PAC 266-6.
To further confirm the role of miR-200a-3p in regulating mSlc19a2, we examined the effect of transfecting PAC 266-6 with a miR-200a-3p inhibitor (100 nM; 48 h) on thiamin uptake and expression of mTHTR-1. The miR-200a-3p inhibitor is an antagomir of ∼22 nucleotides designed to inhibit miR-200a-3p and has been used in similar studies previously (17, 32). The result showed a significant (P < 0.05) increase in thiamin uptake in cells transfected with miR-200a-3p inhibitor compared with those treated with the negative control inhibitor (Fig. 5A). The increase in the vitamin uptake was associated with a significant increase in expression levels of mTHTR-1 protein and mRNA (P < 0.05 for both) compared with cells transfected with negative control inhibitor (Fig. 5, B and C). These findings further support a role for miR-200a-3p in regulating the function and expression of mSlc19a2 in PAC 266-6.
Fig. 5.
Effect of miR-200a-3p inhibitor on thiamin uptake, mouse thiamin transporter-1 (mTHTR-1) protein, and mRNA. Cells from the pancreatic acinar cell line 266-6 (PAC 266-6) were transfected with negative control inhibitor or miR-200a-3p inhibitor for 48 h; a carrier-mediated thiamin uptake (A), mTHTR-1 protein (B), and mRNA (C) levels were determined. Data are means ± SE of 3–6 independent determinations (*P < 0.05).
Effect of truncating and mutating the putative miR-200a-3p-binding site in 3′-UTR region of SLC19A2 on luciferase activity in PAC 266-6.
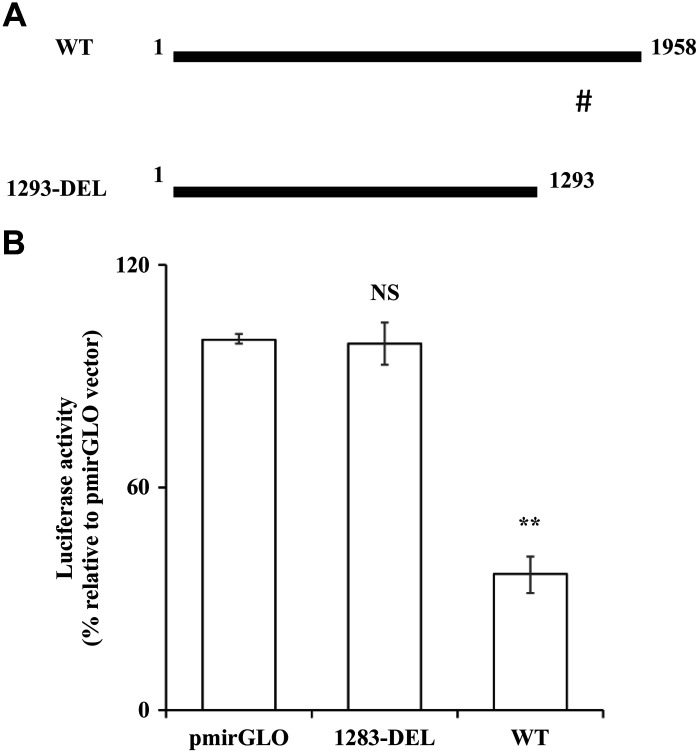
To further confirm that miR-200a-3p exerts its effect on SLC19A2 expression via interaction with specific putative targeting sequence in SLC19A2 3′-UTR, we truncated a 659-bp region from the 3′-UTR of SLC19A2 that harbors the putative miR-200a-3p site (see materials and methods and Fig. 6A), followed by transfection of the remaining SLC19A2 3′-UTR (1293-DEL) into PAC 266-6. The result showed an abrogation of the inhibition in luciferase activity in cells transfected with the 1293-DEL construct compared with those transfected with the WT construct (Fig. 6B). These findings point to the importance of the region between 1,293 and 1,952 bp of the 3′-UTR of SLC19A2 (which contains the putative miR-200a-3p-interacting site) in mediating the effect of miR-200a-3p on SLC19A2 expression and function.
Fig. 6.
Effect of truncation of SLC19A2 3′-untranslated region (UTR) on luciferase activity in the pancreatic acinar cell line 266-6 (PAC 266-6). A: schematic representation of SLC19A2 3′-UTR region shows wild-type (WT) and truncated (1293-DEL) (#miR-200a-3p-binding site). B: PAC 266-6 was transfected with WT and 1293-DEL constructs, and luciferase activity was determined after 48 h. Data are means ± SE of 3–6 independent determinations (**P < 0.01). NS, not significant.
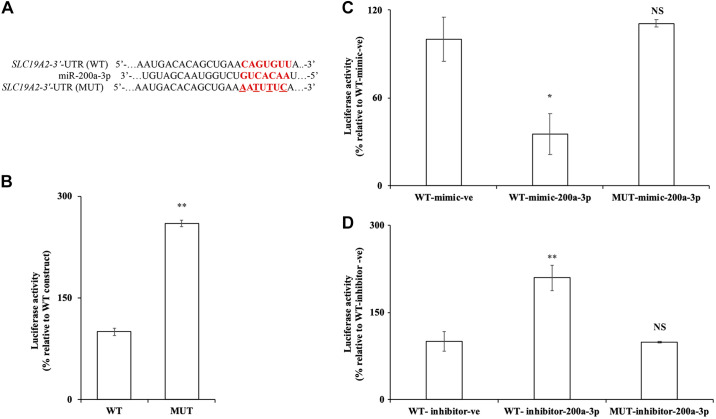
To further validate that miR-200a-3p exerts its effect on SLC19A2 expression via direct interaction with the putative target sequence in SLC19A2 3′-UTR, the seed sequence (1343–1349) of the putative miR-200a-3p-binding site was mutated using site-directed mutagenesis (Fig. 7A). The generated mutant SLC19A2 3′-UTR (MUT) was then transfected into PAC 266-6, and luciferase activity was measured and compared with activity in cells transfected with the WT. The result showed a significant (P < 0.01) increase in luciferase activity in cells transfected with MUT compared with WT (Fig. 7B). These results suggest that the predicted miR-200a-3p-binding site is important for the regulation of SLC19A2 3′-UTR.
Fig. 7.
Effect of mutating the miR-200a-3p putative binding site in the SLC19A2 3′-untranslated region (UTR) on luciferase activity in the pancreatic acinar cell line 266-6 (PAC 266-6). A: putative miR-200a-3p-binding site in SLC19A2 3′-UTR [wild-type (WT)] and mutated (MUT; substitution mutation is underlined). B: luciferase activity was measured in PAC 266-6 transfected with MUT construct; the activity was compared with cells transfected with WT after 48 h. C and D: luciferase activity was measured on WT or MUT SLC19A2 3′-UTR in PAC 266-6 cotransfected with mimic/inhibitor of miR-200a-3p compared with negative controls WT mimic/WT inhibitor. All luciferase activities were measured and normalized with Renilla luciferase activities. Data are means ± SE of 3–6 independent determinations (**P < 0.01; *P < 0.05). NS, not significant.
To further examine the direct interaction of miR-200a-3p with SLC19A2 3′-UTR at the predicted seed sequence, the MUT construct was cotransfected with miR-200a-3p mimic in PAC 266-6. As shown in Fig. 7C, the MUT construct when cotransfected with miR-200a-3p mimic showed an increased luciferase activity compared with the WT cotransfected with miR-200a-3p mimic. In other studies, the MUT construct and the WT construct were cotransfected with miR-200a-3p-inhibitor in PAC 266-6. A significant increase (P < 0.01) in the luciferase activity was observed in the cells co-transfected with WT and inhibitor miR-200a-3p, whereas no effect was seen in MUT cotransfected with inhibitor miR-200a-3p (Fig. 7D).
DISCUSSION
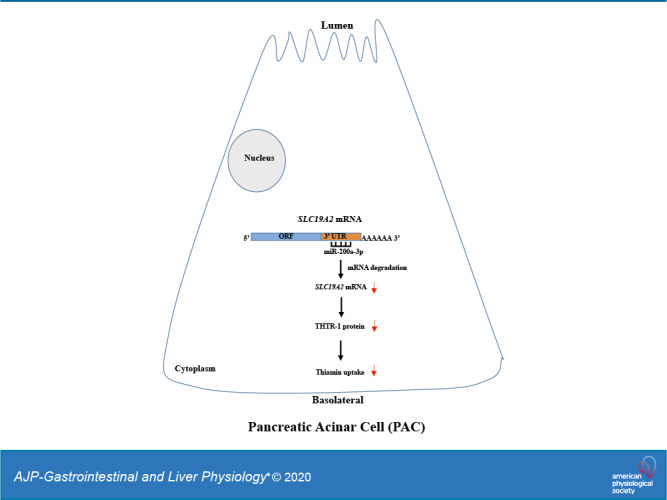
Emerging evidence has shown an important role of miRNAs in pancreatic physiology and pathophysiology (11, 15, 19, 52). PACs obtain their needs of thiamin from circulation via uptake across cell membrane. Studies from our laboratory have characterized the process of thiamin uptake by these cells and showed the involvement of a specialized carrier-mediated process that involves THTR-1 and -2 (45). We have also shown how the process is affected by external/environmental factors and delineated aspects of its transcriptional regulation (18, 39–44, 51). Little, however, is known about posttranscriptional regulation of the uptake process, and thus, we examined this issue using mouse and human PACs, focusing on the role of microRNAs and their effect on expression of the predominant thiamin transporter, i.e., THTR-1. Our findings showed that thiamin uptake process and expression of THTR-1 are targets of posttranscriptional regulation by miRNAs in mouse and human PACs.
Our initial studies have focused on determining whether the SLC19A2 3′-UTR region is a target for posttranscriptional regulation by microRNAs. For this, we transfected pmirGLO-SLC19A2 3′-UTR into PAC 266-6 and observed a significant inhibition in luciferase activity in the transfected cells compared with cells transfected with empty vector. Upon subjecting the SLC19A2 3′-UTR to two widely used bioinformatics in silico programs, six putative miRNAs that have the potential to interact with both mouse and human SLC19A2 3′-UTR regions were identified. One of these potential microRNAs was miR-200a-3p, and we focused our effort on investigating its role in posttranscriptional regulation of THTR-1 expression and thiamin uptake by PACs because 1) it is conserved in mouse and human SLC19A2 3′-UTR, 2) showed a high context score and percentile, and 3) it is highly expressed in the pancreas (11, 47). We found that transfecting PAC 266-6 with mimic of miR-200a-3p led to a significant inhibition in mouse THTR-1 expression (at both the protein and mRNA levels) as well as in thiamin uptake compared with control cells. These results suggest that miR-200a-3p interacts with mSlc19a2 3′-UTR in PAC 266-6 and that this interaction has physiological consequence. Similar results were obtained when freshly isolated primary hPACs were transduced (using adenoviral-mediated DNA transduction approach) with Ad-200a-3p in that significant reductions in the level of expression of human THTR-1 (at the protein and mRNA levels) and in carrier-mediated thiamin uptake were observed.
To confirm the role of miR-200a-3p in regulating the expression of THTR-1 and thiamin uptake by PACs, we used multiple approaches. In the first approach, we utilized miR-200a-3p inhibitor (an antagomir; see Refs. 17 and 32) and observed a significant induction in THTR-1 expression (at both the protein and mRNA levels) as well as in thiamin uptake by PAC 266-6 cells transfected with the inhibitor compared with those treated with negative control inhibitor. In the second approach, we truncated the region in the 3′-UTR of SLC19A2 that contains the putative miR-200a-3p-binding site and found that such a truncation leads to a significant reduction in the level of inhibition in luciferase activity in cells transfected with the deletion construct compared with those transfected with the WT SLC19A2 3′-UTR. In the last approach, we mutated the putative miR-200a-3p-binding site in the SLC19A2 3′-UTR and found that such mutation leads to a significant increase in luciferase activity in cells transfected with the MUT SLC19A2 3′-UTR compared with WT construct.
The miR-200a-3p is a member of the miR-200 family of miRNAs; this family of miRNAs is known to be involved in different functions, including cell proliferation, differentiation, and cancer biology (30, 50). Previous studies have shown that miR-200a is upregulated in pancreatic fibrosis, a common pathological characteristic that leads to chronic pancreatitis (52); it has also been shown to be upregulated in patients with pancreatic cancer (15, 19). Furthermore, exposure to environmental factors like cigarette smoke has been shown to lead to upregulation in level of miR-200a, which has been implicated in the pathogenesis of smoking-related diseases in humans (see Ref. 25 and references therein). It is interesting to mention here that previous studies from our laboratory have shown that cigarette smoke components, like nicotine and NNK, inhibit thiamin uptake by PACs as well as expression of THTR-1 mRNA and protein (41, 44). Although these effects of cigarette smoke components appeared to be mediated, at least in part, via transcriptional mechanisms (41, 44), the current finding of a role of miRNA in posttranscriptional regulation of the vitamin uptake process and expression of THTR-1 adds another potential mechanism through which these environmental factors negatively impact thiamin uptake physiology and molecular biology in PACs.
In summary, the results of the current study show that thiamin uptake by PACs as well as expression of the predominant vitamin uptake system (THTR-1) are subject to posttranscriptional regulation by miRNAs. This mode of regulation may contribute to the effect of external factors and conditions on thiamin uptake by PACs.
GRANTS
This study was supported by grants from the National Institutes of Health (DK-56061, AA-018071, and DK-58057) and the Department of Veterans Affairs (Merit I01BX001142).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.R., K.Y.A., T.Y., P.S., and H.M.S. conceived and designed research; K.R., K.Y.A., T.Y., and P.S. performed experiments; K.R., K.Y.A., T.Y., P.S., and H.M.S. analyzed data; K.R., K.Y.A., T.Y., and P.S. interpreted results of experiments; K.R., K.Y.A., T.Y., and P.S. prepared figures; K.R., K.Y.A., T.Y., and P.S. drafted manuscript; K.R., K.Y.A., T.Y., P.S., and H.M.S. edited and revised manuscript; K.R., K.Y.A., T.Y., P.S., and H.M.S. approved final version of manuscript.
REFERENCES
- 1.Aikawa H, Watanabe IS, Furuse T, Iwasaki Y, Satoyoshi E, Sumi T, Moroji T. Low energy levels in thiamine-deficient encephalopathy. J Neuropathol Exp Neurol 43: 276–287, 1984. doi: 10.1097/00005072-198405000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Ambros V. The functions of animal microRNAs. Nature 431: 350–355, 2004. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 3.Anbazhagan AN, Priyamvada S, Kumar A, Maher DB, Borthakur A, Alrefai WA, Malakooti J, Kwon JH, Dudeja PK. Translational repression of SLC26A3 by miR-494 in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 306: G123–G131, 2014. doi: 10.1152/ajpgi.00222.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berdanier CD. Trace minerals. In: Advanced Nutrition: Micronutrients. New York: CRC, 1998, p. 183–219. [Google Scholar]
- 5.Bettendorff L, Goessens G, Sluse F, Wins P, Bureau M, Laschet J, Grisar T. Thiamine deficiency in cultured neuroblastoma cells: effect on mitochondrial function and peripheral benzodiazepine receptors. J Neurochem 64: 2013–2021, 1995. doi: 10.1046/j.1471-4159.1995.64052013.x. [DOI] [PubMed] [Google Scholar]
- 6.Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, Liu C-G, Bhatt D, Taccioli C, Croce CM. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA 297: 1901–1908, 2007. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 7.Calingasan NY, Chun WJ, Park LC, Uchida K, Gibson GE. Oxidative stress is associated with region-specific neuronal death during thiamine deficiency. J Neuropathol Exp Neurol 58: 946–958, 1999. doi: 10.1097/00005072-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Calingasan NY, Gandy SE, Baker H, Sheu KF, Smith JD, Lamb BT, Gearhart JD, Buxbaum JD, Harper C, Selkoe DJ, Price DL, Sisodia SS, Gibson GE. Novel neuritic clusters with accumulations of amyloid precursor protein and amyloid precursor-like protein 2 immunoreactivity in brain regions damaged by thiamine deficiency. Am J Pathol 149: 1063–1071, 1996. [PMC free article] [PubMed] [Google Scholar]
- 9.Carthew RW. Gene regulation by microRNAs. Curr Opin Genet Dev 16: 203–208, 2006. doi: 10.1016/j.gde.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 10.de Faria O Jr, Moore CS, Kennedy TE, Antel JP, Bar-Or A, Dhaunchak AS. MicroRNA dysregulation in multiple sclerosis. Front Genet 3: 311, 2013. doi: 10.3389/fgene.2012.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixit AK, Sarver AE, Yuan Z, George J, Barlass U, Cheema H, Sareen A, Banerjee S, Dudeja V, Dawra R, Subramanian S, Saluja AK. Comprehensive analysis of microRNA signature of mouse pancreatic acini: overexpression of miR-21-3p in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 311: G974–G980, 2016. doi: 10.1152/ajpgi.00191.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gangolf M, Czerniecki J, Radermecker M, Detry O, Nisolle M, Jouan C, Martin D, Chantraine F, Lakaye B, Wins P, Grisar T, Bettendorff L. Thiamine status in humans and content of phosphorylated thiamine derivatives in biopsies and cultured cells. PLoS One 5: e13616, 2010. doi: 10.1371/journal.pone.0013616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazell AS, Butterworth RF. Update of cell damage mechanisms in thiamine deficiency: focus on oxidative stress, excitotoxicity and inflammation. Alcohol Alcohol 44: 141–147, 2009. doi: 10.1093/alcalc/agn120. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Vazquez AJ, Garcia-Sanchez JA, Moreno-Arriola E, Salvador-Adriano A, Ortega-Cuellar D, Velazquez-Arellano A. Thiamine deprivation produces a liver ATP deficit and metabolic and genomic effects in mice: findings are parallel to those of biotin deficiency and have implications for energy disorders. J Nutrigenet Nutrigenomics 9: 287–299, 2016. doi: 10.1159/000456663. [DOI] [PubMed] [Google Scholar]
- 15.Hong TH, Park IY. MicroRNA expression profiling of diagnostic needle aspirates from surgical pancreatic cancer specimens. Ann Surg Treat Res 87: 290–297, 2014. doi: 10.4174/astr.2014.87.6.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoyumpa AM., Jr Mechanisms of thiamin deficiency in chronic alcoholism. Am J Clin Nutr 33: 2750–2761, 1980. doi: 10.1093/ajcn/33.12.2750. [DOI] [PubMed] [Google Scholar]
- 17.Huang Z, Wang M, Liu L, Peng J, Guo C, Chen X, Huang L, Tan J, Yang G. Transcriptional repression of CYP3A4 by increased miR-200a-3p and miR-150-5p promotes steatosis in vitro. Front Genet 10: 484, 2019. doi: 10.3389/fgene.2019.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huhta H, Helminen O, Kauppila JH, Salo T, Porvari K, Saarnio J, Lehenkari PP, Karttunen TJ. The expression of Toll-like receptors in normal human and murine gastrointestinal organs and the effect of microbiome and cancer. J Histochem Cytochem 64: 470–482, 2016. doi: 10.1369/0022155416656154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karihtala P, Porvari K, Soini Y, Eskelinen M, Juvonen P, Haapasaari K-M. Expression levels of microRNAs miR-93 and miR-200a in pancreatic adenocarcinoma with special reference to differentiation and relapse-free survival. Oncology 96: 164–170, 2019. doi: 10.1159/000494274. [DOI] [PubMed] [Google Scholar]
- 20.Lakhan R, Subramanian VS, Said HM. Role of microRNA-423-5p in posttranscriptional regulation of the intestinal riboflavin transporter-3. Am J Physiol Gastrointest Liver Physiol 313: G589–G598, 2017. doi: 10.1152/ajpgi.00238.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang Y, Ridzon D, Wong L, Chen C. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics 8: 166, 2007. doi: 10.1186/1471-2164-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Singleton CK, Martin PR. Molecular mechanisms of thiamine utilization. Curr Mol Med 1: 197–207, 2001. doi: 10.2174/1566524013363870. [DOI] [PubMed] [Google Scholar]
- 24.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell 148: 1172–1187, 2012. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Momi N, Kaur S, Rachagani S, Ganti AK, Batra SK. Smoking and microRNA dysregulation: a cancerous combination. Trends Mol Med 20: 36–47, 2014. doi: 10.1016/j.molmed.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nabokina SM, Ramos MB, Said HM. Mechanism(s) involved in the colon-specific expression of the thiamine pyrophosphate (TPP) transporter. PLoS One 11: e0149255, 2016. [Erratum in: PLoS One 12: e0186550, 2017.] doi: 10.1371/journal.pone.0149255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakasa T, Nagata Y, Yamasaki K, Ochi M. A mini-review: microRNA in arthritis. Physiol Genomics 43: 566–570, 2011. doi: 10.1152/physiolgenomics.00142.2010. [DOI] [PubMed] [Google Scholar]
- 28.Prajapati P, Sripada L, Singh K, Bhatelia K, Singh R, Singh R. TNF-α regulates miRNA targeting mitochondrial complex-I and induces cell death in dopaminergic cells. Biochim Biophys Acta 1852: 451–461, 2015. doi: 10.1016/j.bbadis.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Prasannan KG, Sundaresan R, Venkatesan D. Thiamine deficency and protein secretion by pancreatic slices in vitro. Experientia 33: 169–170, 1977. doi: 10.1007/BF02124046. [DOI] [PubMed] [Google Scholar]
- 30.Qiu W, Kassem M. miR-141-3p inhibits human stromal (mesenchymal) stem cell proliferation and differentiation. Biochim Biophys Acta 1843: 2114–2121, 2014. doi: 10.1016/j.bbamcr.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Rebane A, Akdis CA. MicroRNAs in allergy and asthma. Curr Allergy Asthma Rep 14: 424, 2014. doi: 10.1007/s11882-014-0424-x. [DOI] [PubMed] [Google Scholar]
- 32.Saha S, Choudhury J, Ain R. MicroRNA-141-3p and miR-200a-3p regulate insulin-like growth factor 2 during mouse placental development. Mol Cell Endocrinol 414: 186–193, 2015. doi: 10.1016/j.mce.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 33.Saito N, Kimura M, Kuchiba A, Itokawa Y. Blood thiamine levels in outpatients with diabetes mellitus. J Nutr Sci Vitaminol (Tokyo) 33: 421–430, 1987. doi: 10.3177/jnsv.33.421. [DOI] [PubMed] [Google Scholar]
- 34.Sangani R, Periyasamy-Thandavan S, Kolhe R, Bhattacharyya MH, Chutkan N, Hunter M, Isales C, Hamrick M, Hill WD, Fulzele S. MicroRNAs-141 and 200a regulate the SVCT2 transporter in bone marrow stromal cells. Mol Cell Endocrinol 410: 19–26, 2015. doi: 10.1016/j.mce.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sassoon CS, Zhu E, Fang L, Subramanian VS, Said HM. Inhibition of intestinal thiamin transport in rat model of sepsis. Crit Care Med 44: e875–e881, 2016. doi: 10.1097/CCM.0000000000001745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schultz NA, Werner J, Willenbrock H, Roslind A, Giese N, Horn T, Wøjdemann M, Johansen JS. MicroRNA expression profiles associated with pancreatic adenocarcinoma and ampullary adenocarcinoma. Mod Pathol 25: 1609–1622, 2012. doi: 10.1038/modpathol.2012.122. [DOI] [PubMed] [Google Scholar]
- 37.Singh L, Bakshi DK, Vasishta RK, Arora SK, Majumdar S, Wig JD. Primary culture of pancreatic (human) acinar cells. Dig Dis Sci 53: 2569–2575, 2008. doi: 10.1007/s10620-007-0162-1. [DOI] [PubMed] [Google Scholar]
- 38.Singh M. Effect of thiamin deficiency on pancreatic acinar cell function. Am J Clin Nutr 36: 500–504, 1982. doi: 10.1093/ajcn/36.3.500. [DOI] [PubMed] [Google Scholar]
- 39.Srinivasan P, Anandam KY, Ramesh V, Geltz ET, Said HM. Effect of bacterial flagellin on thiamin uptake by human and mouse pancreatic acinar cells: inhibition mediated at the level of transcription of thiamin transporters 1 and 2. Am J Physiol Gastrointest Liver Physiol 316: G735–G743, 2019. doi: 10.1152/ajpgi.00048.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srinivasan P, Nabokina S, Said HM. Chronic alcohol exposure affects pancreatic acinar mitochondrial thiamin pyrophosphate uptake: studies with mouse 266-6 cell line and primary cells. Am J Physiol Gastrointest Liver Physiol 309: G750–G758, 2015. doi: 10.1152/ajpgi.00226.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srinivasan P, Subramanian VS, Said HM. Effect of the cigarette smoke component, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), on physiological and molecular parameters of thiamin uptake by pancreatic acinar cells. PLoS One 8: e78853, 2013. doi: 10.1371/journal.pone.0078853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srinivasan P, Subramanian VS, Said HM. Mechanisms involved in the inhibitory effect of chronic alcohol exposure on pancreatic acinar thiamin uptake. Am J Physiol Gastrointest Liver Physiol 306: G631–G639, 2014. doi: 10.1152/ajpgi.00420.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srinivasan P, Thrower EC, Gorelick FS, Said HM. Inhibition of pancreatic acinar mitochondrial thiamin pyrophosphate uptake by the cigarette smoke component 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Am J Physiol Gastrointest Liver Physiol 310: G874–G883, 2016. doi: 10.1152/ajpgi.00461.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivasan P, Thrower EC, Loganathan G, Balamurugan AN, Subramanian VS, Gorelick FS, Said HM. Chronic nicotine exposure in vivo and in vitro inhibits vitamin B1 (thiamin) uptake by pancreatic acinar cells. PLoS One 10: e0143575, 2015. doi: 10.1371/journal.pone.0143575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramanian VS, Subramanya SB, Said HM. Relative contribution of THTR-1 and THTR-2 in thiamin uptake by pancreatic acinar cells: studies utilizing Slc19a2 and Slc19a3 knockout mouse models. Am J Physiol Gastrointest Liver Physiol 302: G572–G578, 2012. doi: 10.1152/ajpgi.00484.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol 10: 542–552, 2013. doi: 10.1038/nrgastro.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szafranska AE, Davison TS, John J, Cannon T, Sipos B, Maghnouj A, Labourier E, Hahn SA. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene 26: 4442–4452, 2007. doi: 10.1038/sj.onc.1210228. [DOI] [PubMed] [Google Scholar]
- 48.Tang Y, Zhang L, Forsyth CB, Shaikh M, Song S, Keshavarzian A. The role of miR‐212 and iNOS in alcohol‐induced intestinal barrier dysfunction and steatohepatitis. Alcohol Clin Exp Res 39: 1632–1641, 2015. doi: 10.1111/acer.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanphaichitr V. Modern Nutrition in Health and Disease, edited by Shils ME, Olsen JA, and Shike M. New York: Lea and Febiger, 1994, p. 359–375. [Google Scholar]
- 50.Vrba L, Jensen TJ, Garbe JC, Heimark RL, Cress AE, Dickinson S, Stampfer MR, Futscher BW. Role for DNA methylation in the regulation of miR-200c and miR-141 expression in normal and cancer cells. PLoS One 5: e8697, 2010. doi: 10.1371/journal.pone.0008697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao Y, Liu F, Yang J, Zhong M, Zhang E, Li Y, Zhou D, Cao Y, Li W, Yu J, Yang Y, Yan H. Over-activation of TLR5 signaling by high-dose flagellin induces liver injury in mice. Cell Mol Immunol 12: 729–742, 2015. doi: 10.1038/cmi.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu M, Wang G, Zhou H, Cai J, Li P, Zhou M, Lu Y, Jiang X, Huang H, Zhang Y, Gong A. TGF-β1-miR-200a-PTEN induces epithelial-mesenchymal transition and fibrosis of pancreatic stellate cells. Mol Cell Biochem 431: 161–168, 2017. doi: 10.1007/s11010-017-2988-y. [DOI] [PubMed] [Google Scholar]
- 53.Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology 141: 1323–1333, 2011. doi: 10.1053/j.gastro.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]