Abstract
Skeletal muscle is a highly plastic tissue capable of remodeling in response to a range of physiological stimuli, including nutrients and exercise. Historically, the lysosome has been considered an essentially catabolic organelle contributing to autophagy, phagocytosis, and exo-/endocytosis in skeletal muscle. However, recent evidence has emerged of several anabolic roles for the lysosome, including the requirement for autophagy in skeletal muscle mass maintenance, the discovery of the lysosome as an intracellular signaling hub for mechanistic target of rapamycin complex 1 (mTORC1) activation, and the importance of transcription factor EB/lysosomal biogenesis-related signaling in the regulation of mTORC1-mediated protein synthesis. We, therefore, propose that the lysosome is an understudied organelle with the potential to underpin the skeletal muscle adaptive response to anabolic stimuli. Within this review, we describe the molecular regulation of lysosome biogenesis and detail the emerging anabolic roles of the lysosome in skeletal muscle with particular emphasis on how these roles may mediate adaptations to chronic resistance exercise. Furthermore, given the well-established role of amino acids to support muscle protein remodeling, we describe how dietary proteins “labeled” with stable isotopes could provide a complementary research tool to better understand how lysosomal biogenesis, autophagy regulation, and/or mTORC1-lysosomal repositioning can mediate the intracellular usage of dietary amino acids in response to anabolic stimuli. Finally, we provide avenues for future research with the aim of elucidating how the regulation of this important organelle could mediate skeletal muscle anabolism.
Keywords: lysosomal biogenesis, lysosome, mTORC1, resistance exercise, TFEB
INTRODUCTION
Skeletal muscle is a highly plastic tissue that responds to a range of physiological stimuli, especially feeding (i.e., amino acid ingestion) and exercise (37). Skeletal muscle mass and quality are regulated by dynamic fluctuations in protein turnover, which is underpinned by changes in muscle protein synthesis and breakdown rates. Considerable research has focused on the cellular regulation of muscle protein synthesis as it represents the primary regulated variable of protein turnover in healthy adults in response to dietary amino consumption and, especially, muscle contraction (3). However, we posit that the lysosome is an underappreciated organelle with the potential to underpin the skeletal muscle adaptative response by impacting both muscle protein synthesis and breakdown.
Lysosomes are highly motile membrane-bound organelles containing ~60 types of hydrolytic enzymes capable of breaking down proteins, DNA, lipids, and glycogen (32). Accordingly, these organelles are colloquially called “the garbage can of the cell” and sit as the end point of several pathways, including endocytosis, phagocytosis, and autophagy (32). Lysosomal hydrolases require an acidic pH (∼4.5) to function optimally, a microenvironment that is produced and maintained by the vacuolar ATPase (v-ATPase), a membrane-spanning proton pump (38). Within skeletal muscle, lysosomes are predominantly involved in the removal of damaged/dysfunctional cytoplasmic proteins and organelles, as large myofibrillar structures cannot be engulfed by lysosomes (15). The removal of these proteins and organelles occurs principally via the autophagy system, which occurs in three distinct forms and in both selective and nonselective fashions (14). The intricacies of these three forms (i.e., macro-, micro-, and chaperone-mediated autophagy) is outside the scope of the current mini-review, but readers are referred to Parzych and Klionsky (43) for a comprehensive overview. Although originally believed to be a purely catabolic organelle, recent evidence suggests a role for the lysosome in several anabolic cellular events such as nutrient sensing, regulation of protein synthesis, and cell growth. The purpose of this review is to summarize these novel roles and to provide hypotheses on how the dynamic regulation of the lysosomal pool may aid adaptations to anabolic stimuli.
REGULATION OF LYSOSOMAL BIOGENESIS/AUTOPHAGY
At the molecular level, lysosomal biogenesis and autophagy are regulated primarily by the microphthalmia/transcription factor E family of basic helix-loop-helix-leucine-zipper transcription factors (MiT/TFE; Ref. 48). Of the 4 transcription factors within this family, transcription factor EB (TFEB) has been purported as the “master regulator of lysosomal biogenesis” (48). TFEB exerts its effects through direct binding to a 10-base E-box-like palindromic sequence in the promoter region of target genes (48), coined the coordinated lysosomal expression and regulation (CLEAR) motif (42), thereby initiating transcription of such genes. Within this CLEAR network are many lysosomal membrane proteins, hydrolases, transporters, and proton pumps (v-ATPase) as well as genes associated with autophagosome production, autophagosome-lysosome fusion, and lysosomal exocytosis (42). Thus overexpression of TFEB in vitro is sufficient to expand the lysosomal pool and increases a cell’s capacity to degrade long-lived proteins/organelles (49). Conversely, reductions in TFEB expression attenuate the expression of lysosomal genes (49), displaying the essential role of this transcription factor in the regulation of lysosomal pool size and activity.
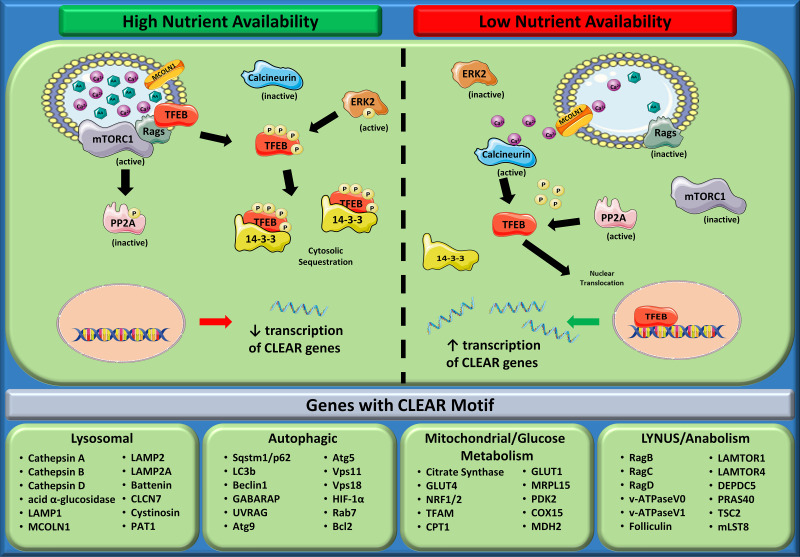
TFEB activity is regulated by its nuclear content, where it can bind to CLEAR motifs on DNA and initiate transcription. When nutrients are replete, TFEB is observed in the cytosol, where it colocalizes with 14-3-3 proteins (46). This spatial modulation of TFEB is coordinated by the phosphorylation of TFEB by the mechanistic target of rapamycin complex 1 (mTORC1) or extracellular regulated kinase 2 (ERK2) on various serine residues. TFEB is then sequestered in the cytosol and localizes with 14-3-3 proteins (40, 46, 50, 54). Under nutrient-deprived conditions, TFEB colocalizes with nuclei, binds to CLEAR elements, and enhances transcription of its target genes (49, 50). This is believed to occur through lysosomal efflux of calcium, which subsequently activates the phosphatase calcineurin to dephosphorylate TFEB (37). Accordingly, silencing of mucolipin-1 (MCOLN1), the lysosomal calcium channel, reduces calcineurin activity and TFEB dephosphorylation, preventing TFEB-regulated gene upregulation during starvation (37). Recent evidence has also suggested that protein phosphatase 2A can also dephosphorylate TFEB (34). Intriguingly, this phosphatase can also be phosphorylated and inhibited by mTORC1 (24), suggesting mTORC1’s inhibitory effects on TFEB may be twofold, via direct phosphorylation and inhibition of dephosphorylation. Moreover, mTORC1-dependent TFEB phosphorylation has been shown to occur at the lysosome (35), adding the fascinating notion that TFEB inhibition occurs at the organelle that it coordinates the expansion of. This regulation of TFEB-mediated gene expression is depicted in Fig. 1.
Fig. 1.
Regulation of transcription factor EB (TFEB)-mediated gene expression of coordinated lysosomal expression and regulation (CLEAR) genes. During nutrient-replete conditions, TFEB is recruited to the lysosomal surface by Rag proteins. Here, TFEB becomes phosphorylated (P) on multiple serine residues by mechanistic target of rapamycin complex 1 (mTORC1) and ERK2 (Ser122, Ser138, Ser142, and Ser211) and becomes sequestered in the cytosol in association with 14-3-3 proteins. Lysosomal efflux of calcium is low in these conditions, resulting in low calcineurin phosphatase activity and maintaining the phosphorylation status of TFEB. mTORC1 also phosphorylates and inhibits protein phosphatase 2A (PP2A), a 2nd phosphatase that could reduce TFEB phosphorylation. Collectively, these signaling events result in reduced nuclear content of TFEB and, therefore, lower transcription of CLEAR genes. Alternatively, when nutrient levels are low, the lysosomal calcium channel mucolipin-1 (MCOLN1) becomes more active, increasing calcium efflux. Elevated cytosolic calcium then activates calcineurin to remove phosphate groups from TFEB. Simultaneously, mTORC1 activity decreases, alleviating its inhibition on PP2A and increasing its phosphatase activity toward TFEB. The reduction in TFEB phosphorylation removes TFEB from its association with 14-3-3 proteins and allows TFEB to enter the nucleus. Here, TFEB binds to CLEAR motifs in promoter regions of genes and initiates transcription. Below the schematic are a subset of genes associated with a variety of cellular processes that contain CLEAR motifs and are thereby regulated by TFEB. Atg7, atrogene 7; LAMP2, lysosomal associated membrane protein 2; LC3b, light chain 3B; LYNUS, lysosomal nutrient sensing; v-ATPase, vacuolar ATPase. [Figure was created using images assembled from Servier Medical Art (https://smart.servier.com/) under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).]
The importance of TFEB has also been reported in skeletal muscle. With muscle-specific overexpression and knockout models of TFEB, Mansueto et al. (33) demonstrated that, in addition to lysosomal content and autophagy, TFEB also regulates mitochondrial biogenesis and glucose metabolism. Moreover, in response to an acute bout of endurance exercise, TFEB-overexpressing mice displayed greater mitochondrial-related gene expression (33). In physiological settings, acute endurance exercise elicits increased TFEB nuclear content (11, 33) and transcriptional activity (11) in wild-type rodent skeletal muscle, a process dependent on calcineurin phosphatase activity. Furthermore, electrically stimulated muscle contractions, a model used to investigate effects of chronic contractile activity, 6 h/day for 7 days, produced higher lysosomal membrane and hydrolase protein content as well as that of MCOLN1 and TFEB (27). Combined, these data suggest that TFEB is an important regulator in skeletal muscle and is responsive to muscle contraction and that the lysosomal pool is plastic and can adapt to chronic exercise/contraction. As little research currently exists regarding lysosomal contributions to human skeletal muscle anabolism, the following section will describe the potential mechanisms by which lysosomal biogenesis may contribute to such adaptations and establish future directions in this novel research area.
EMERGING ROLES OF THE LYSOSOME AS AN ANABOLIC ORGANELLE
Autophagy is Required for Muscle Mass Maintenance
Lysosomes are the end point of the autophagic pathway and are required for autophagosomal degradation (32). Recent evidence, however, has begun to delineate an anabolic role for autophagy within skeletal muscle. This notion was first observed in seminal work of the Sandri laboratory (36) through the generation of muscle-specific atrogene 7 knockout mice (Atg7 mKO), which exhibit a lack of microtubule-associated protein light chain II (LC3) lipidation and excessive p62 accumulation indicative of impaired autophagosome-lysosome fusion. Here, Atg7 mKO animals displayed significant reductions in skeletal muscle size and force production compared with wild-type (WT) littermates. Moreover, following denervation-induced atrophy, Atg7 mKO mice remarkably lost more muscle mass than their WT counterparts (36). Therefore, autophagy may be essential for the maintenance of skeletal muscle mass and function in both the basal and atrophic states.
Although the importance of autophagy in muscle mass maintenance in homeostatic and atrophic conditions has been well characterized (36), the contribution of autophagy to skeletal muscle hypertrophy has not been investigated comprehensively through loss/gain of function models. Results of animal studies are equivocal with some suggesting hypertrophy is accompanied by increased content of autophagy markers (29), whereas others suggest hypertrophy may elicit reductions in such markers (29). In humans, resistance exercise-induced hypertrophy seems to be accompanied by increased autophagosome content (18) and markers of chaperone-assisted selective autophagy (CASA; Ref. 53), suggesting increased capacity for protein turnover in trained skeletal muscle. Intriguingly, acute resistance exercise is consistently shown to reduce autophagosome clearance, shown via increased p62 protein content (18, 55) and reduced LC3 lipidation (10, 13, 18), for ≤48 h after exercise. Conversely, acute resistance exercise enhanced the removal of CASA substrates (53), suggesting different forms of autophagy may be regulated independently. Collectively, these could suggest that macroautophagy is inhibited by acute resistance exercise, whereas CASA is augmented, although markers of both processes are increased following resistance exercise training. It is important to state here that static measurements of autophagic markers may not truly represent alterations in autophagy flux itself (26). In fact, the optimal method to measure autophagy flux is through the use of an autophagy inhibitor with comparison with a “control” noninhibited condition (7). As these methods are clearly not achievable in human skeletal muscle, only tentative conclusions can be made from in vivo human data. Nevertheless, it is plausible that an increased capacity for autophagy in response to chronic resistance training may contribute to skeletal muscle hypertrophic adaptations, however conclusive, would require currently underdeveloped methodology.
mTORC1-Lysosomal Trafficking is Important for Skeletal Muscle Anabolism
Rodent and in vitro models demonstrate that the lysosome is the site of mTORC1 activation (25, 28, 47) in light of the large concentrations of amino acids within the lysosomal lumen and presence of direct mTORC1 activators [i.e., Ras homolog enriched in brain (Rheb) and phosphatidic acid] on lysosomal membranes (47). As such, an elevation in amino acid availability following nutrient starvation in vitro (47) or following eccentric contractions in rodents (25) elicits mTORC1 translocation to the lysosomal membrane, which is vital for enhancements in mTORC1 kinase activity (47). Interestingly, these findings are at odds with those observed in human skeletal muscle, where mTORC1-lysosomal association remains unchanged in response to protein ingestion following endurance or resistance exercise (2, 20, 51). These contradictory findings are thought to be attributed to fundamental differences in nutrient availability distinguishing in vitro versus in vivo human models (21, 28), as in vitro models commonly deprive cells of all amino acids causing mTORC1-lysosome dissociation (28, 47). Conversely, the continuous flux in protein turnover in human skeletal muscle is thought to provide sufficient intracellular amino acids to sustain mTORC1 colocalization with the lysosome in postabsorptive states (21, 28). One scenario where mTORC1 colocalization with the lysosome does increase, however, is in response to the synergistic effect of whole egg protein consumption after resistance exercise (2). This suggests nonprotein dietary factors (e.g., cholesterol found in yolk) may influence the acute postexercise regulation of mTORC1 activity in human skeletal muscle. Importantly, this increase in colocalization was associated with mTORC1 kinase activity and myofibrillar protein synthesis rates (2), highlighting a potential regulatory role for this mechanism in augmenting mRNA translation. We have also observed greater lysosomal associated membrane protein 2 immunofluorescent staining intensity following resistance exercise in human skeletal muscle (20), which may indicate an acute expansion of the lysosomal pool. Collectively, these data suggest that, in the absence of nonprotein dietary factors, anabolic stimuli of amino acid ingestion and/or exercise have little effect on mTORC1 colocalization with the lysosome. However, the lysosome may still regulate mTORC1 activity in human skeletal muscle through its highly motile nature.
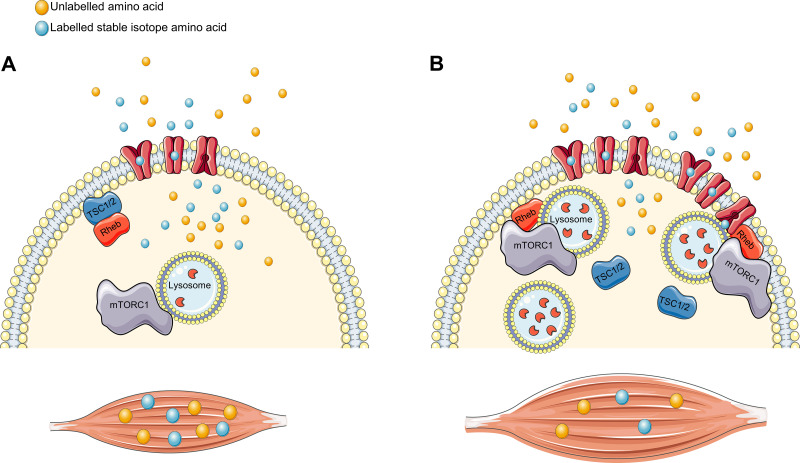
The main site of protein synthesis in human skeletal muscle is believed to be close to the sarcolemma, where the majority of rRNA is located (23) and where newly synthesized proteins are observed in vivo (16). Seminal in vitro studies have reported that the anterograde movement of lysosomal-associated mTORC1, toward the cell periphery, is essential for mTORC1 activation in response to nutrients (28). Accordingly, in human skeletal muscle, we have demonstrated that in response to protein feeding at rest, mTORC1 translocates toward the sarcolemma within 1 h (1, 51) and is sustained for ≤3 h after resistance exercise (51). Protein feeding and resistance exercise also promote the translocation of the lysosome toward the sarcolemma (20, 51), suggesting that mTORC1 and lysosomal translocation are occurring simultaneously. Importantly, these events coincide with tuberous sclerosis complex 2 protein (TSC2) dissociation from the mTORC1 activator Rheb, which subsequently relieves the former’s inhibitory effect on mTORC1 kinase activity (25). These cellular processes are observed to occur in close proximity to the sarcolemma in human skeletal muscle in response to protein feeding after endurance (1) and resistance exercise (2, 51) when mTORC1 activity is enhanced. Therefore, these acute data suggest that the acute mTORC1-Rheb-lysosomal translocation toward and TSC2 migration away from the sarcolemma may be key events in the process of mTORC1 activation in response to acute feeding and exercise in human skeletal muscle. As the lysosome, and in particular its highly motile nature, is essential for mTORC1 activation acutely, it is conceivable that an alteration in these intracellular mechanisms may contribute to the hypertrophic adaptations commonly observed with training. The potential effect of chronic resistance exercise on these mechanisms is depicted in Fig. 2.
Fig. 2.
Schematic illustration integrating mechanistic target of rapamycin complex 1 (mTORC1)-lysosomal trafficking with “labeled” food in human skeletal muscle in response to acute and chronic resistance exercise. A: in the untrained state, mTORC1 and the lysosome are associated and reside in the sarcoplasm. Tuberous sclerosis complex 1/2 (TSC1/2) is near the sarcolemma and associated with Ras homolog enriched in brain (Rheb). This results in suboptimal mTORC1 activity and increases dietary amino acid incorporation to facilitate postexercise skeletal muscle remodeling. B: after resistance training, an enhanced colocalization of the mTOR-Rheb-lysosomal protein complex and translocation to the sarcolemma (and ostensibly near amino acid transporters, capillaries, and ribosomes) with a concomitant disassociation of TSC2 from the sarcolemma is observed. This “primed” activation of mTORC1 may result in a reduced reliance of dietary amino acids to facilitate exercise recovery in the trained state. An increase in lysosomal number and capacity (i.e., enzyme content) in response to resistance training may also contribute to hypertrophic adaptations. This could result in an enhanced capacity for protein synthesis by providing more “sites” of mTORC1 activation and, theoretically, a greater autophagic capacity, further contributing to a “refinement” in protein turnover. [Figure was created using images assembled from Servier Medical Art (https://smart.servier.com/) under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).]
TFEB Mediates mTORC1 Activation
The transcription factor TFEB has myriad target genes spanning a range of cellular processes, including lysosomal biogenesis, autophagy, mitochondrial biogenesis, and glucose transport (33, 42, 48, 49). Intriguingly, recent evidence has discovered that several genes involved in lysosomal nutrient sensing (LYNUS) contain a CLEAR motif, meaning they can be targeted by MiT/TFE transcription factors (9). Accordingly, overexpression of TFEB in HeLa cells results in an upregulation in mRNA transcription of a series of LYNUS genes, including the Rag GTPases, components of the ragulator complex and v-ATPase subunits (9). Moreover, when TFEB is silenced, mTORC1 activation, both in vitro and in vivo, in response to nutrients was drastically blunted, effects that were seen to be primarily mediated via TFEB’s regulation of RagD (9). Importantly, these effects were also apparent in muscle-specific TFEB knockout mice fed leucine following acute exercise and extended to the level of global protein synthesis (9). Therefore, these data suggest an intriguing paradoxical relationship whereby mTORC1 will inhibit TFEB transcriptional activity acutely, whereas TFEB-mediated expression of LYNUS genes is required for optimal mTORC1 activation and protein synthesis in response to nutrients/exercise.
A recent investigation has provided further evidence of this relationship describing a mechanism where TFEB controls the formation of endosomes containing LYNUS proteins in vitro (41). Fusion of these endosomes with lysosomes is required for TSC2 to dissociate from lysosomes to coordinate mTORC1 reactivation following prolonged starvation and mTORC1 activation in standard nutrient conditions (41). A further intriguing aspect of this relationship is evidenced by the finding that Rag GTPase proteins, as well as coordinating mTORC1 recruitment to the lysosome, also recruit TFEB to lysosomal fractions where mTORC1 is able to phosphorylate and inhibit the transcription factor (35). Therefore, TFEB controls the transcription of proteins that coordinate its own inhibition, in addition to mTORC1’s activation. Research regarding the paradoxical relationship between mTORC1 and TFEB in human skeletal muscle is lacking; however, the notion that these two pathways are fundamentally intertwined suggests TFEB transcriptional activity has the potential to influence skeletal muscle adaptations to anabolic stimuli in yet to determined processes.
“LABELED” FOODS AS A TOOL TO DETERMINE DIETARY PROTEIN UTILIZATION: IMPLICATIONS FOR INTRAMUSCULAR PROTEIN TURNOVER
With lysosomes emerging as a central hub in the anabolic response to dietary amino acids at rest and after exercise, it is relevant to highlight that the translocation of mTORC1 to the cell periphery also places it in close proximity to capillaries (51) and amino acid transporters (e.g., L-type amino acid transporter 1; Ref. 19). It is well established that dietary protein-derived amino acids are the prime stimulators of muscle protein synthesis rates after a meal (52), an effect that can persist for ≤24 h after acute exercise (6). Our ability to model the metabolic fate of dietary amino acids into circulation and, ultimately, skeletal muscle has been greatly expanded by the development of food proteins “labeled” with stable isotope amino acids (4). For a greater insight into general stable isotope methodology, the reader is referred to a comprehensive overview by Millward and Smith (39). Seminal work by Boirie et al. (5) using the labeled food approach showed that 40–70% of diet-derived amino acids appear in the circulation at rest with the majority being directed toward protein synthesis, suggesting newly synthesized proteins are preferentially made from “new” dietary amino acids rather than “old” amino acids recycled from intracellular breakdown (4). This would be consistent with observations that dietary amino acids from labeled protein sources are incorporated into new muscle (especially myofibrillar) proteins in proportion to their intake, which sometimes occurs independently of detectable increases in the overall rate of protein synthesis (22). Importantly, acute resistance exercise has been shown to facilitate greater skeletal muscle uptake and utilization of diet-derived amino acids for de novo muscle protein synthesis in untrained men (44), which is in line with previous findings suggesting that resistance exercise “sensitizes” muscle protein synthesis rates to dietary amino acids (6). Therefore, labeled foods could provide an exciting complementary research tool to advance our understanding of how lysosomal biogenesis, autophagy regulation, and/or mTORC1-lysosomal repositioning in response to acute and chronic anabolic stimuli can mediate the intracellular usage of dietary amino acids and hypertrophic adaptations.
FUTURE DIRECTIONS
Several recent investigations have studied how chronic resistance exercise impacts the regulation of several cellular organelles (e.g., ribosomes and mitochondria; Refs. 12, 45). However, no study to date has examined the impact that chronic resistance exercise in humans may have on lysosomal pool size/capacity in skeletal muscle and whether any alterations are accompanied by changes in dietary amino acid utilization. It is possible that an increase in size of the lysosomal pool in response to resistance training may contribute to hypertrophic adaptations as it would provide muscle cells with more “sites” of mTORC1 activation and, theoretically, a greater autophagic capacity (depicted in Fig. 2). Preliminary research from our laboratory suggests that training-induced muscle growth is associated with greater postabsorptive mTORC1-lysosomal colocalization and greater localization of the mTORC1-lysosomal-Rheb complex at the sarcolemma in close proximity to capillaries (S. Abou Sawan, N. Hodson, C. Tinline-Goodfellow, J. Malowany, D. W. West, D. Kumbhare, and D. R. Moore, unpublished observations); these chronic events would align with our acute observations of the capacity of postexercise amino acid ingestion to maintain mTOR-Rheb localization at the sarcolemma that may serve to prime the kinase for future anabolic stimuli (17). We believe these adaptations could allow for a more “efficient” activation of mTORC1 in response to acute anabolic stimuli in the trained state, rather than elevated postabsorptive mTORC1 activity. This could suggest a “refined” exercise-induced stimulation of protein turnover in trained skeletal muscle (8), which, in our hands, appears to be associated with a reduced reliance on dietary amino acids to synthesize new myofibrillar proteins (S. Abou Sawan, N. Hodson, C. Tinline-Goodfellow, J. Malowany, D. W. West, D. Kumbhare, and D. R. Moore, unpublished observations) and may be underpinned, in part, by a “primed” activation of mTORC1. Furthermore, this reduced reliance on dietary amino acids may suggest an increased capacity for intramuscular protein turnover that could be driven by an expansion of the lysosomal pool. Although markers of autophagy do increase in response to resistance training, interpretation of these findings is difficult as it is possible they may not accurately represent autophagic flux (26). As such, a more comprehensive investigation into the effects of chronic resistance exercise training on autophagy in human skeletal muscle is needed. The effect of chronic resistance exercise on lysosomal biogenesis is yet to be investigated in human skeletal muscle, and, as such, elucidating the contribution of lysosomal turnover/biogenesis to skeletal muscle anabolism is vital to fully understand the mechanisms underpinning mTORC1 activation, protein turnover, and, ultimately, skeletal muscle hypertrophy. Therefore, both acute and chronic resistance exercise investigations should incorporate measures of lysosomal pool size (e.g., membrane proteins, transporters, etc.), capacity (e.g., lysosomal hydrolase content), and the associated mechanisms of lysosomal biogenesis (e.g., TFEB content, nuclear localization, and CLEAR/LYNUS gene expression). Moreover, as we have observed alterations in mTORC1-lysosomal-Rheb positioning and dietary protein utilization in trained skeletal muscle, lysosomal measures could greatly benefit from further use of dietary tracers to establish the concordance between these cellular processes. Finally, as certain populations exhibit diminished responses to anabolic stimuli (e.g., older and obese populations), it may be important to determine whether dysregulation of the lysosomal pool may underpin such an “anabolic resistance.”
CONCLUDING REMARKS
Lysosomes have been historically considered a purely catabolic organelle contributing to autophagy, phagocytosis, and exo-/endocytosis. However, evidence has emerged of several anabolic roles for the lysosome in skeletal muscle, including the requirement for autophagy in skeletal muscle mass maintenance, the discovery of the lysosome as the site of mTORC1 activation, and the importance of TFEB/lysosomal biogenesis-related signaling in mTORC1 activity and protein synthesis. Accordingly, it is plausible that adaptations to the lysosomal pool may contribute to skeletal muscle adaptation, yet this has received relatively little attention. Future work should, therefore, focus on the regulation of lysosomal biogenesis in response to both acute and chronic resistance exercise to fully determine how this organelle contributes to skeletal muscle anabolism.
GRANTS
S. Abou Sawan is supported by a Natural Sciences and Engineering Research Council of Canada Postgraduate Scholarships-Doctoral Scholarship. M. Mazzulla is supported by the Ontario Graduate Scholarship Program. Research by D. R. Moore is supported, in part, by a Natural Sciences and Engineering Research Council Discovery Grant. N. Hodson is supported by a Mitacs Accelerate Postdoctoral Fellowship (IT15730).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A.S. and N.H. prepared figures; S.A.S., M.M., and N.H. drafted manuscript; S.A.S., M.M., D.R.M., and N.H. edited and revised manuscript; S.A.S., M.M., D.R.M., and N.H. approved final version of manuscript.
REFERENCES
- 1.Abou Sawan S, van Vliet S, Parel JT, Beals JW, Mazzulla M, West DWD, Philp A, Li Z, Paluska SA, Burd NA, Moore DR. Translocation and protein complex co-localization of mTOR is associated with postprandial myofibrillar protein synthesis at rest and after endurance exercise. Physiol Rep 6: e13628, 2018. doi: 10.14814/phy2.13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abou Sawan S, van Vliet S, West DWD, Beals JW, Paluska SA, Burd NA, Moore DR. Whole egg, but not egg white, ingestion induces mTOR colocalization with the lysosome after resistance exercise. Am J Physiol Cell Physiol 315: C537–C543, 2018. doi: 10.1152/ajpcell.00225.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atherton PJ, Smith K. Muscle protein synthesis in response to nutrition and exercise. J Physiol 590: 1049–1057, 2012. doi: 10.1113/jphysiol.2011.225003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA 94: 14930–14935, 1997. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boirie Y, Gachon P, Beaufrère B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr 65: 489–495, 1997. doi: 10.1093/ajcn/65.2.489. [DOI] [PubMed] [Google Scholar]
- 6.Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Phillips SM. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr 141: 568–573, 2011. doi: 10.3945/jn.110.135038. [DOI] [PubMed] [Google Scholar]
- 7.Carter HN, Kim Y, Erlich AT, Zarrin-Khat D, Hood DA. Autophagy and mitophagy flux in young and aged skeletal muscle following chronic contractile activity. J Physiol 596: 3567–3584, 2018. doi: 10.1113/JP275998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damas F, Phillips SM, Libardi CA, Vechin FC, Lixandrão ME, Jannig PR, Costa LA, Bacurau AV, Snijders T, Parise G, Tricoli V, Roschel H, Ugrinowitsch C. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J Physiol 594: 5209–5222, 2016. doi: 10.1113/JP272472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Malta C, Siciliano D, Calcagni A, Monfregola J, Punzi S, Pastore N, Eastes AN, Davis O, De Cegli R, Zampelli A, Di Giovannantonio LG, Nusco E, Platt N, Guida A, Ogmundsdottir MH, Lanfrancone L, Perera RM, Zoncu R, Pelicci PG, Settembre C, Ballabio A. Transcriptional activation of RagD GTPase controls mTORC1 and promotes cancer growth. Science 356: 1188–1192, 2017. doi: 10.1126/science.aag2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickinson JM, Reidy PT, Gundermann DM, Borack MS, Walker DK, D’Lugos AC, Volpi E, Rasmussen BB. The impact of postexercise essential amino acid ingestion on the ubiquitin proteasome and autophagosomal-lysosomal systems in skeletal muscle of older men. J Appl Physiol (1985) 122: 620–630, 2017. doi: 10.1152/japplphysiol.00632.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erlich AT, Brownlee DM, Beyfuss K, Hood DA. Exercise induces TFEB expression and activity in skeletal muscle in a PGC-1α-dependent manner. Am J Physiol Cell Physiol 314: C62–C72, 2018. doi: 10.1152/ajpcell.00162.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueiredo VC, McCarthy JJ. Regulation of ribosome biogenesis in skeletal muscle hypertrophy. Physiology (Bethesda) 34: 30–42, 2019. doi: 10.1152/physiol.00034.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Volpi E, Rasmussen BB. Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci 68: 599–607, 2013. doi: 10.1093/gerona/gls209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol 221: 3–12, 2010. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goll DE, Neti G, Mares SW, Thompson VF. Myofibrillar protein turnover: the proteasome and the calpains. J Anim Sci 86, suppl_14: E19–E35, 2008. doi: 10.2527/jas.2007-0395. [DOI] [PubMed] [Google Scholar]
- 16.Goodman CA, Pierre P, Hornberger TA. Imaging of protein synthesis with puromycin. Proc Natl Acad Sci USA 109: E989, 2012. doi: 10.1073/pnas.1202000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannaian SJ, Hodson N, Abou Sawan S, Mazzulla M, Kato H, Matsunaga K, Waskiw-Ford M, Duncan J, Kumbhare DA, Moore DR. Leucine-enriched amino acids maintain peripheral mTOR-Rheb localization independent of myofibrillar protein synthesis and mTORC1 signaling postexercise. J Appl Physiol (1985) 129: 133–143, 2020. doi: 10.1152/japplphysiol.00241.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hentilä J, Ahtiainen JP, Paulsen G, Raastad T, Häkkinen K, Mero AA, Hulmi JJ. Autophagy is induced by resistance exercise in young men, but unfolded protein response is induced regardless of age. Acta Physiol (Oxf) 224: e13069, 2018. doi: 10.1111/apha.13069. [DOI] [PubMed] [Google Scholar]
- 19.Hodson N, Brown T, Joanisse S, Aguirre N, West DWD, Moore DR, Baar K, Breen L, Philp A. Characterisation of L-type amino acid transporter 1 (LAT1) expression in human skeletal muscle by immunofluorescent microscopy. Nutrients 10: 23, 2017. doi: 10.3390/nu10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodson N, McGlory C, Oikawa SY, Jeromson S, Song Z, Rüegg MA, Hamilton DL, Phillips SM, Philp A. Differential localization and anabolic responsiveness of mTOR complexes in human skeletal muscle in response to feeding and exercise. Am J Physiol Cell Physiol 313: C604–C611, 2017. doi: 10.1152/ajpcell.00176.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodson N, Philp A. The importance of mTOR trafficking for human skeletal muscle translational control. Exerc Sport Sci Rev 47: 46–53, 2019. doi: 10.1249/JES.0000000000000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holwerda AM, Paulussen KJM, Overkamp M, Goessens JPB, Kramer IF, Wodzig WKWH, Verdijk LB, van Loon LJC. Dose-dependent increases in whole-body net protein balance and dietary protein-derived amino acid incorporation into myofibrillar protein during recovery from resistance exercise in older men. J Nutr 149: 221–230, 2019. doi: 10.1093/jn/nxy263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horne Z, Hesketh J. Immunological localization of ribosomes in striated rat muscle. Evidence for myofibrillar association and ontological changes in the subsarcolemmal:myofibrillar distribution. Biochem J 268: 231–236, 1990. doi: 10.1042/bj2680231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui L, Rodrik V, Pielak RM, Knirr S, Zheng Y, Foster DA. mTOR-dependent suppression of protein phosphatase 2A is critical for phospholipase D survival signals in human breast cancer cells. J Biol Chem 280: 35829–35835, 2005. doi: 10.1074/jbc.M504192200. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs BL, You JS, Frey JW, Goodman CA, Gundermann DM, Hornberger TA. Eccentric contractions increase the phosphorylation of tuberous sclerosis complex-2 (TSC2) and alter the targeting of TSC2 and the mechanistic target of rapamycin to the lysosome. J Physiol 591: 4611–4620, 2013. doi: 10.1113/jphysiol.2013.256339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ju JS, Varadhachary AS, Miller SE, Weihl CC. Quantitation of “autophagic flux” in mature skeletal muscle. Autophagy 6: 929–935, 2010. doi: 10.4161/auto.6.7.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y, Hood DA. Regulation of the autophagy system during chronic contractile activity-induced muscle adaptations. Physiol Rep 5: e13307, 2017. doi: 10.14814/phy2.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korolchuk VI, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, Jahreiss L, Sarkar S, Futter M, Menzies FM, O’Kane CJ, Deretic V, Rubinsztein DC. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol 13: 453–460, 2011. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon I, Jang Y, Cho JY, Jang YC, Lee Y. Long-term resistance exercise-induced muscular hypertrophy is associated with autophagy modulation in rats. J Physiol Sci 68: 269–280, 2018. doi: 10.1007/s12576-017-0531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol 8: 622–632, 2007. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 33.Mansueto G, Armani A, Viscomi C, D’Orsi L, De Cegli R, Polishchuk EV, Lamperti C, Di Meo I, Romanello V, Marchet S, Saha PK, Zong H, Blaauw B, Solagna F, Tezze C, Grumati P, Bonaldo P, Pessin JE, Zeviani M, Sandri M, Ballabio A. Transcription factor EB controls metabolic flexibility during exercise. Cell Metab 25: 182–196, 2017. doi: 10.1016/j.cmet.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martina JA, Puertollano R. Protein phosphatase 2A stimulates activation of TFEB and TFE3 transcription factors in response to oxidative stress. J Biol Chem 293: 12525–12534, 2018. doi: 10.1074/jbc.RA118.003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martina JA, Puertollano R. Rag GTPases mediate amino acid-dependent recruitment of TFEB and MITF to lysosomes. J Cell Biol 200: 475–491, 2013. doi: 10.1083/jcb.201209135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M. Autophagy is required to maintain muscle mass. Cell Metab 10: 507–515, 2009. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 17: 288–299, 2015. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem 55: 663–700, 1986. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 39.Millward DJ, Smith K. The application of stable-isotope tracers to study human musculoskeletal protein turnover: a tale of bag filling and bag enlargement. J Physiol 597: 1235–1249, 2019. doi: 10.1113/JP275430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Napolitano G, Esposito A, Choi H, Matarese M, Benedetti V, Di Malta C, Monfregola J, Medina DL, Lippincott-Schwartz J, Ballabio A. mTOR-dependent phosphorylation controls TFEB nuclear export. Nat Commun 9: 3312, 2018. doi: 10.1038/s41467-018-05862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nnah IC, Wang B, Saqcena C, Weber GF, Bonder EM, Bagley D, De Cegli R, Napolitano G, Medina DL, Ballabio A, Dobrowolski R. TFEB-driven endocytosis coordinates MTORC1 signaling and autophagy. Autophagy 15: 151–164, 2019. doi: 10.1080/15548627.2018.1511504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmieri M, Impey S, Kang H, di Ronza A, Pelz C, Sardiello M, Ballabio A. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum Mol Genet 20: 3852–3866, 2011. doi: 10.1093/hmg/ddr306. [DOI] [PubMed] [Google Scholar]
- 43.Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal 20: 460–473, 2014. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr 93: 322–331, 2011. doi: 10.3945/ajcn.2010.29649. [DOI] [PubMed] [Google Scholar]
- 45.Porter C, Reidy PT, Bhattarai N, Sidossis LS, Rasmussen BB. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc 47: 1922–1931, 2015. doi: 10.1249/MSS.0000000000000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roczniak-Ferguson A, Petit CS, Froehlich F, Qian S, Ky J, Angarola B, Walther TC, Ferguson SM. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci Signal 5: ra42, 2012. doi: 10.1126/scisignal.2002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290–303, 2010. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A. A gene network regulating lysosomal biogenesis and function. Science 325: 473–477, 2009. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 49.Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science 332: 1429–1433, 2011. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, Sabatini DM, Ballabio A. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J 31: 1095–1108, 2012. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song Z, Moore DR, Hodson N, Ward C, Dent JR, O’Leary MF, Shaw AM, Hamilton DL, Sarkar S, Gangloff YG, Hornberger TA, Spriet LL, Heigenhauser GJ, Philp A. Resistance exercise initiates mechanistic target of rapamycin (mTOR) translocation and protein complex co-localisation in human skeletal muscle. Sci Rep 7: 5028, 2017. doi: 10.1038/s41598-017-05483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Staples AW, Burd NA, West DW, Currie KD, Atherton PJ, Moore DR, Rennie MJ, Macdonald MJ, Baker SK, Phillips SM. Carbohydrate does not augment exercise-induced protein accretion versus protein alone. Med Sci Sports Exerc 43: 1154–1161, 2011. doi: 10.1249/MSS.0b013e31820751cb. [DOI] [PubMed] [Google Scholar]
- 53.Ulbricht A, Gehlert S, Leciejewski B, Schiffer T, Bloch W, Höhfeld J. Induction and adaptation of chaperone-assisted selective autophagy CASA in response to resistance exercise in human skeletal muscle. Autophagy 11: 538–546, 2015. doi: 10.1080/15548627.2015.1017186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vega-Rubin-de-Celis S, Peña-Llopis S, Konda M, Brugarolas J. Multistep regulation of TFEB by MTORC1. Autophagy 13: 464–472, 2017. doi: 10.1080/15548627.2016.1271514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng N, D’Souza RF, Figueiredo VC, Markworth JF, Roberts LA, Peake JM, Mitchell CJ, Cameron-Smith D. Acute resistance exercise induces Sestrin2 phosphorylation and p62 dephosphorylation in human skeletal muscle. Physiol Rep 5: e13526, 2017. doi: 10.14814/phy2.13526. [DOI] [PMC free article] [PubMed] [Google Scholar]