Abstract
A number of cell culture approaches have been described for maintenance of primary hepatocytes. Forming hepatocytes into three-dimensional (3-D) spheroids is one well-accepted method for extending epithelial phenotype of these cells. Our laboratory has previously observed enhanced function of two-dimensional (2-D, monolayer) hepatocyte cultures in microfluidic devices due to increased production of several hepato-inductive growth factors, including hepatocyte growth factor (HGF). In the present study, we wanted to test a hypothesis that culturing hepatocyte spheroids (3-D) in microfluidic devices will also result in enhanced phenotype and function. To test this hypothesis, we fabricated devices with small and large volumes. Both types of devices included a microstructured floor containing arrays of pyramidal wells to promote assembly of hepatocytes into spheroids with individual diameters of ~100 µm. The hepatocyte spheroids were found to be more functional, as evidenced by higher level of albumin synthesis, bile acid production, and hepatic enzyme expression, in low-volume compared with large-volume devices. Importantly, high functionality of spheroid cultures correlated with elevated levels of HGF secretion. Although decay of hepatic function (albumin secretion) was observed over the course 3 wk, this behavior could be abrogated by inhibiting TGF-β1 signaling. With TGF-β1 inhibitor, microfluidic hepatocyte spheroid cultures maintained high and stable levels of albumin synthesis over the course of 4 wk. To further highlight utility of this culture platform for liver disease modeling, we carried out alcohol injury experiments in microfluidic devices and tested protective effects of interleukin-22: a potential therapy for alcoholic hepatitis.
Keywords: alcohol injury, hepatocyte spheroids, microfluidic cultures
INTRODUCTION
Hepatocytes are liver parenchymal cells that are responsible for a variety of liver functions, including protein synthesis, urea production, bile acid secretion, and metabolism of xenobiotics (20). After removal from the liver and plating in a culture dish, these highly differentiated epithelial cells lose their polygonal epithelial shape and hepatic function, acquire mesenchymal features, and die within a few days. The dedifferentiation of hepatocytes may be prevented using several approaches, including cocultures with nonparenchymal cells (4, 8, 18), overlaying hepatocytes with collagen gel or other extracellular matrix (ECM) components (3, 9, 10), and forming hepatocyte spheroids (12, 30, 34).
There is increasing interest in placing hepatocytes and other liver cells into microfluidic devices to control hepatic zonation, delivery of metabolites, or injurious agents (6). In fact, standard hepatocyte culture formats known to enhance hepatic function (e.g., cocultures) are being incorporated into microfluidic devices (17, 29). Our laboratory has a long-standing interest in miniaturizing hepatic cultures and characterizing interactions between liver cell compartments in microfluidic devices (16, 36). Recently, we demonstrated that primary hepatocytes cultured on glass or polydimethylsiloxane (PDMS) substrates coated with monomeric collagen type I maintain hepatic function and epithelial phenotype for over 2 wk when confined to microfluidic channels (14). In comparison, hepatocytes cultured under identical conditions in large-volume culture lose phenotype and function within 5–7 days. We determined that confinement to microfluidic channels resulted in upregulation of hepato-inductive signals including hepatocyte growth factor (HGF), epidermal growth factor (EGF), and insulin-like growth factor (IGF). Conversely, expression of transforming growth factor (TGF)-β1 (a signaling molecule known to negatively impact epithelial hepatic phenotype) was diminished in microfluidic cultures compared with standard large-volume cultures. This past study demonstrated that geometry of the cell culture system as well as mass transport play an important yet underappreciated role in accumulation of autocrine signals and maintenance of primary hepatocytes (14).
Having established benefits of small local volume for maintenance of two-dimensional (2-D, monolayer) hepatocyte cultures in the past, we wanted to test a hypothesis that similar enhancement may be achieved in three-dimensional (3-D, spheroid) cultures. Indeed, in this paper, we demonstrate enhanced hepatic function and increased levels of HGF expression in small-volume (2 μL) compared with large-volume (30 μL) cultures of hepatocytes spheroids. We also demonstrate utility of this culture system for testing protective effects of interleukin (IL)-2 in the context of alcohol injury.
MATERIALS AND METHODS
Fabrication of microfluidic devices.
The microfluidic devices were comprised of two layers: 1) the top layer consisted of a flow channel with a culture chamber, and 2) the bottom layer contained an array of 84 inverted pyramidal wells. The top layer master molds were fabricated using standard photolithography techniques. First, the silicon wafers (University Wafer) were cleaned using oxygen plasma. Next, for the small-volume chamber, the wafer was spin-coated using SU-8 2050 negative photoresist (Microchem Corp.) to create a 100-µm thickness film, as recommended by the manufacturer. Followed by a prebake step, the design was micropatterned into the resist by exposing it to UV light through a photomask (CAD/Art Services). After a postbake step, the mold was developed in SU-8 developer and hard-baked for 2 h at 135°C. To fabricate the devices with larger volume, two additional SU-8 layers (1,600 μm in total) were spin-coated and micropatterned with a second photomask of the culture chamber design without flow channels using a mask aligner (UV-KUB 3, Kloé). The master wafer for replicating inverted pyramidal wells was fabricated at the University of Minnesota Nanotechnology Center using isotropic wet etching. The dimensions of the inverted pyramidal wells etched in silicon (Si) were 350 μm in depth and 400 μm2 at the base.
Microfluidic devices were fabricated using standard soft-lithography. A mixture of 10:1 weight ratio of polydimethylsiloxane (PDMS) base to curing agent (Sylgard 184 Silicone Elastomer Kit, Dow Corning) was poured onto the master molds. After degassing for 10 min, the mixture was baked at 70°C for 80 min. Next, the cured PDMS was cast out from the master mold and cut, and the inlet and outlet holes were punched at the most distal part of the flow channel. The top and bottom PDMS layers were aligned and irreversibly bonded using oxygen plasma treatment. For monolayer culture devices, the punched top PDMS layer of a small-volume design was irreversible bonded to a 75 mm × 25 mm glass slide using oxygen plasma bonding. At last, media reservoirs were generated using cloning cylinders (10 mm; Fisher Scientific) attached with uncured PDMS to the inlet and outlet channel’s holes and cured at 70°C for 60 min.
Small-volume 3-D culture chamber had dimensions of 7.55 mm × 3.15 mm × 0.1 mm and volume of 4.25 μL. Large-volume 3-D culture chambers had dimensions of 7.55 mm × 3.15 mm × 1.6 mm and a volume of 40 μL. The 2-D microfluidic culture chambers had flat PDMS bottom layers and PDMS flow layers with dimensions of 7.55 mm × 3.15 mm × 0.1 mm and volume of 2.4 μL. Therefore, dimensions of the top (flow) layer were the same for all three microfluidic culture type systems tested here.
Hepatocyte cultures in microfluidic devices.
All animal experiments were performed under the National Institutes of Health (NIH) guidelines for ethical care and use of laboratory animals with the approval of the Institutional Animal Care and Use Committee of the Mayo Clinic, Rochester, Minnesota. Primary rat hepatocytes were isolated from adult female Lewis rats (weighing 90–200 g, Charles River Laboratories, Boston, MA) as described previously (14). Typically, 100–300 million hepatocytes were isolated with viability >90% as determined by Trypan blue exclusion. Primary hepatocytes were cultured in complete media with hormones (CH media) composed with DMEM (Dulbecco’s modified Eagle’s medium; Gibco), supplemented with 1% (vol/vol) penicillin-streptomycin (Invitrogen), 10% FBS (Invitrogen), 0.5 U/mL Insulin (Novolin N), 20 ng/mL EGF (Invitrogen), 7 ng/mL Glucagon (Sigma), and 7.5 μg/mL hydrocortisone sodium succinate (Sigma). Prior to seeding hepatocytes and forming spheroids, microfluidic devices with pyramidal wells were incubated with 1% Pluronic F127 (Sigma) overnight at 4°C followed by washing with PBS.
We wanted to keep the number of cells per device similar between the large- and small-volume culture formats. Given the 9.4-fold difference in volume between the two systems, input concentration for small-volume devices was ~9 times higher than for large-volume devices (2 × 107 cells/mL and 2.2 × 106 cells/mL, respectively). To achieve the target of seeding ~20,000 cells per device or 250 cells per spheroid, we pipetted 50 μL cell suspension into one inlet while keeping the other inlet empty. The difference in hydrostatic pressure between inlets drove cells into the device and into pyramidal microwells. The leftover cells were removed by careful aspiration of media from both inlets, after which 250 μL of CH media were added into each reservoir. Care was taken not to dislodge cells from wells during media exchange process. To achieve this, smaller aliquots (~50 μL) were added to each inlet simultaneously until reaching 250 μL total volume per inlet. Similar protocol was followed for daily exchange of media.
To ensure formation of 2-D hepatocyte monolayer, microfluidic devices with unstructured (flat) PDMS floor devices were incubated with 0.2 mg/mL collagen type I (BD Biosciences) for 1 h at 37°C, then washed three times with 1× PBS and infused with 50-µL suspension of hepatocytes at the density of 4 × 106 cell/mL. Cells were allowed to attach for 2 h at 37°C with 5% CO2. Subsequently, unattached cells were removed by careful aspiration, and fresh media was added in 50-µL increments into each cylinder until the volume of 250 μL was reached.
Alcohol injury in microfluidic devices.
In this set of experiments, we characterized responses of hepatocyte spheroids to alcohol injury in the absence or presence of a putative hepato-protective agent, IL-22. Several culture conditions were tested: hepatocyte spheroids without ethyl alcohol (EtOH), hepatocyte spheroids with EtOH, hepatocyte spheroids with IL-22, and hepatocyte spheroids with EtOH/IL-22. Small-volume (2.4 μL) cell culture systems were used in all of the conditions mentioned above. Alcohol injury was induced by adding 100 mM EtOH into CH media and culturing hepatocyte spheroids for 5 days with daily media exchanges. When testing protective effects of IL-22 during alcohol injury, hepatocyte spheroids were incubated with 10 ng/mL concentration of this cytokine for 24 h before addition of EtOH. IL-22 and EtOH were supplemented daily during media exchanges over the course of 5 days.
We also tested alcohol injury and protective effects of IL-22 using hepatocyte cultures in 12-well plates as described by us previously (23). Briefly, 12-well plates of tissue culture polystyrene (TCPS) were coated with collagen type I using similar protocol described above for 2-D microfluidic cultures. After washing with PBS, hepatocytes were seeded at a density of 300,000 cells/cm2. The cells were incubated for 60 min at 37°C and washed with 1× PBS to remove unbound hepatocytes, and then 1 mL of fresh media was added into each well. Media was changed every 24 h over the course of 4–6 days. IL-22 was added at 10 ng/mL concentration 24 h before commencement of alcohol injury experiments. We used 100 mM EtOH to induce alcohol injury.
Electron microscopy.
Scanning electron microscopy was used for visualizing hepatocyte spheroids. Samples were first exposed to Trump’s fixative (1% glutaraldehyde and 4% formaldehyde in 0.1 M phosphate buffer), then washed for 10 min each in 0.1 M phosphate buffer and distilled water. The spheroids were dehydrated in a graded series of ethanol solutions and critical-point dried (<40°C). The bottom layer of PDMS containing the spheroids was mounted onto an aluminum stub with a carbon sticky tape and sputter coated with gold/palladium. Images were captured on a Hitachi S7400 scanning electron microscope.
Immunofluorescent staining.
Microchambers were washed with 1× PBS and then fixed with 4% paraformaldehyde with 0.1% Triton-X100 (Invitrogen) in PBS (Invitrogen) overnight at 4°C. Cryosections of 8-mm thickness were cut from spheroids. The sections were blocked with BSA (4%) for nonspecific binding and then incubated with primary antibodies overnight at 4°C. Then the sections were incubated with secondary antibody for 1 h at room temperature, followed by mounting with DAPI using the VECTOR M.O.M kit, according to the manufacturer’s instructions (Vector Laboratories), before taking images. The target primary antibodies were: sheep anti-rat albumin (1:100; cat. no. A110-134A, Bethyl Laboratories, Inc.) and rabbit anti-multidrug resistance-associated protein (MRP)-2 (1:100; cat. no. NBP1-42349, Novus Biologics). The secondary antibodies used were: Alexa-488 donkey anti-sheep IgG (cat. no. A11015) and Alexa-546 donkey anti-mouse IgG (cat. no. A110036). Secondary antibodies were purchased from Invitrogen and diluted 1:1,000. Stained cells were visualized and imaged using a fluorescence microscope (IX-83, Olympus).
Analysis of hepatic function and gene expression.
All assays were performed per manufacturer's instructions unless stated otherwise. ELISAs were purchased from Bethyl Laboratories for albumin and R&D System for HGF and TGF-β1. Optical density was measured at 450 nm using UV/vis spectrophotometer (Synergy H1, BioTek Instruments). ELISA values were normalized by the cell number, which was quantified from DNA content using the Picogreen assay (Invitrogen). Bile acid assay kit was purchased from Cell Biolabs, Inc. (San Diego, CA). Cytochrome P450 (CYP)3A4 activity of primary rat hepatocytes was measured using the P450-Glo CYP3A4 Assay (Promega).
Total RNA was extracted from hepatocytes using High Pure RNA Isolation kit (Roche) and dissolved in nuclease-free water, and its concentration was quantified using a NanoDrop One Spectrophotometer (Thermo Fisher Scientific).
Complementary (c) DNA synthesis was performed using the Transcriptor First Strand cDNA Synthesis kit (Roche) according to the manufacturer’s instructions. Gene expression level was quantified using universal SYBR master (Roche). Relative gene expression level was calculated by the comparative Ct method using GAPDH as a housekeeping gene. All target primer sequences were ordered from IDT. Albumin: CAT CCT GAA CCG TCT GTG TG (forward) and TTT CCA AGG ACC CAC TA (reverse), HGF: CTT CTG CCG GTC CTG TTG (forward) and TCT CTT CTG TCC TTC TGC (reverse), TGF-β: CCT GGA AAG GGC TCA ACA C (forward) and CAG TTC TCT GTG GAG CTG A (reverse), CYP1A2: ACC ATC CCC CAC AGT ACA A (forward) and GTT GAC CTG CCA CTG GTT TA (reverse), ADH1: ACC ATC GAG GAC ATA GAA (forward) and GTG GAG CCT GGG GTC AC (reverse), CYP2E1: CCT ACA TGG ATG CTG TGG TG (forward) and CTG GAA ACT CAT GGC TGT CA (reverse), GAPDH: AGA CAG CCG CAT CTT GT (forward) and AGA CAG CCG CAT CTT GT (reverse).
COMSOL modeling of secreted HGF accumulation in culture chambers.
Numerical simulations were performed with COMSOL (Burlington, MA) to model how the reduced headspace affects accumulation of HGF secreted by hepatocyte spheroids. We modeled two culture scenarios being tested experimentally in this study: hepatocyte spheroids residing in pyramidal wells with either 100 or 1,600 µm of headspace above the well with no perfusion. Assuming a constant secretion rate of 0.4 ng·h−1·cell−1 and 118 cells/100 µm spheroid, concentrations in the well were generated every 10 min for 48 h.
Statistical analysis.
The data are presented as the means ± standard deviation with a minimum of 3–6 biological replicates. Data sets were analyzed by Shapiro–Wilk test with an α-value of 0.05 to determine Gaussian distribution. Data sets with a P value higher than the α-value were considered to be normally distributed and analyzed using two-tailed unpaired Welch’s t test. Non-Gaussian data sets were analyzed using two-tailed unpaired Mann–Whitney test. P < 0.05 was deemed statistically significant. All calculations were performed using GraphPad Prism (GraphPad Software, La Jolla, CA).
RESULTS AND DISCUSSION
Seeding and cultivation of hepatocytes in microfluidic devices.
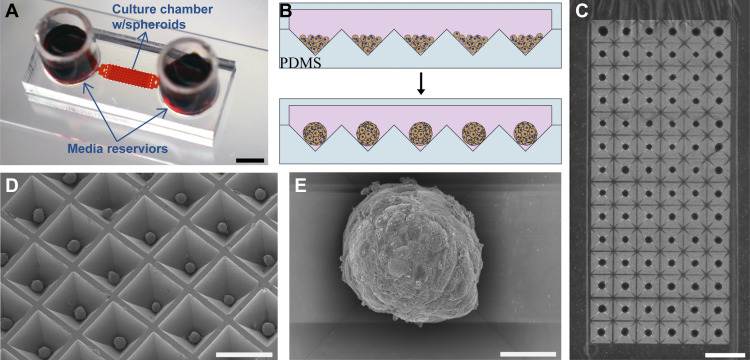
We fabricated microfluidic devices consisting of top and bottom layers composed of poly(dimethyl siloxane) (PDMS) material (Fig. 1A). The top layer contained a cell culture chamber connected to media reservoirs via transport channels. Hepatocyte spheroid cultures were created in two types of devices: small volume (4.25 μL) with 100-μm headspace and large volume (40 μL) with 1,600-μm headspace. Large- and small-volume devices were identical in every way except for the difference in the volume of the cell culture chamber. These devices contained an array of 84 pyramidal wells, each well with 400 × 400 µm2 square opening and 350 µm depth. Figure 1B shows schematically how hepatocytes seeded into a microfluidic device aggregated and formed spheroids. To promote cell-cell interactions and minimize cell adhesion to the surface, microfluidic devices were pretreated with a nonionic surfactant Pluronic. This treatment (described in greater detail in experimental section) ensured robust and reproducible formation of uniformly sized spheroids in devices. This may be appreciated from Fig. 1C, which shows an array of 84 hepatic spheroids formed in the microfluidic device. Close-up images of hepatocyte spheroids may be seen in Fig. 1, D and E. Arrays of hepatocyte spheroids were maintained in microfluidic devices for 3–4 wk. Spheroid formation occurred equally well in large- and small-volume systems, with spheroids forming within 24–48 h of seeding. However, as highlighted in the next section, the hepatic function of small-volume spheroid cultures proved to be more robust.
Fig. 1.
Formation of hepatocyte spheroids in microfluidic chambers. A: typical microfluidic device used for cultivation of hepatocyte spheroid contained two media reservoirs connected to a cell culture chamber via transport channels. Scale bar: 5 mm. Media was exchanged every 24 h in this device. Two types of cell culture chambers for cultivation of spheroids were made: 1) 100-μm headspace [small volume three-dimensional (3-D)] and 2) 1,600-μm headspace (large volume 3-D). B: schematic of hepatocytes aggregating into spheroids inside microfluidic chambers containing pyramidal polydimethylsiloxane (PDMS) microwells. The surfaces of microfluidic chambers were precoated with Pluronic to enhance cell-cell interactions. C: a panel of ×4 magnification images stitched together to show spheroid occupancy in a representative culture chamber. Scale bar: 1 mm. D: scanning electron microscopy (SEM) image of an array of hepatocyte spheroids formed in pyramidal PDMS wells collected at ×50 magnification (scale bar: 50 µm). E: SEM image of an individual hepatocyte spheroid imaged at ×500 magnification (scale bar: 500 µm).
Phenotypic and functional analysis of hepatocyte cultures.
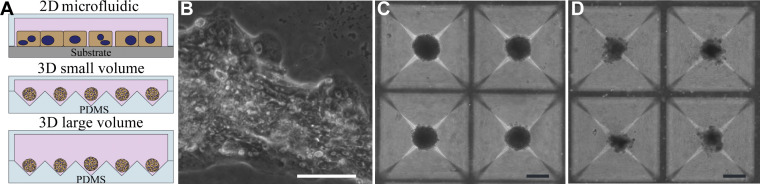
In addition to hepatocyte spheroid cultures described above, we utilized 2-D (monolayer) microfluidic hepatocyte cultures reported by us previously (14). Thus, as shown in Fig. 2A, we compared three types of microfluidic culture systems: 1) small-volume (4.25 μL) spheroid cultures, 2) large-volume (40 μL) spheroid cultures, and 3) 2-D cultures (2.4 μL). We should note that small or large volume refers to the local volume of the cell culture chamber (see Fig. 1A for image of a device) but does not include the volume of media reservoirs. We should also note that hepatocytes were cultured under static conditions for all of the experiments described above. Therefore, when referring to microfluidic devices or systems in this paper, we mean to highlight the use of microliter volumes for cell cultivation. The culture formats used in this study are summarized in Fig. 2A. Although we considered using commercial microtiter plates to form spheroid arrays (e.g., AggreWell plates), we reasoned that keeping the layout of the culture system (e.g., transport channels, media reservoirs) the same, while only changing the headspace and the 3-D versus 2-D format, provided for better comparison of the local volume effects.
Fig. 2.
Morphology of hepatocytes in microfluidic chambers. A: diagram describing three types of culture systems testing in this study: 1) microfluidic monolayer (two-dimensional, 2-D) hepatocyte cultures, 2) microfluidic small-volume three-dimensional (3-D) cultures, and 3) microfluidic large-volume cultures. B–D: representative images of three culture systems at day 14. Note mesenchymal morphology of 2-D cultures (B), disaggregation of spheroids in large-volume cultures (D), and contrast with compact morphology of hepatocyte spheroids in small-volume cultures (C). 2-D format scale bar: 50 µm, 3-D format scale bar: 100 µm. PDMS, polydimethylsiloxane.
Upon seeding, hepatocytes in monolayer cultures exhibited cuboidal morphology typical of epithelial cells, whereas hepatocytes in both small- and large-volume cultures formed compact spheroids over the course of 24–48 h (see Supplemental Fig. S1; all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.12551306.v1). The quality of hepatocytes in 2-D cultures and 3-D large-volume cultures deteriorated over time. By day 14, cells in 2-D microfluidic devices became elongated and mesenchymal, whereas spheroids in large-volume cultures acquired jagged boundaries and began to disaggregate (see Fig. 2B). Conversely, hepatocyte spheroids in small-volume spheroid cultures remained compact with clean and clear boundaries (see Fig. 2B).
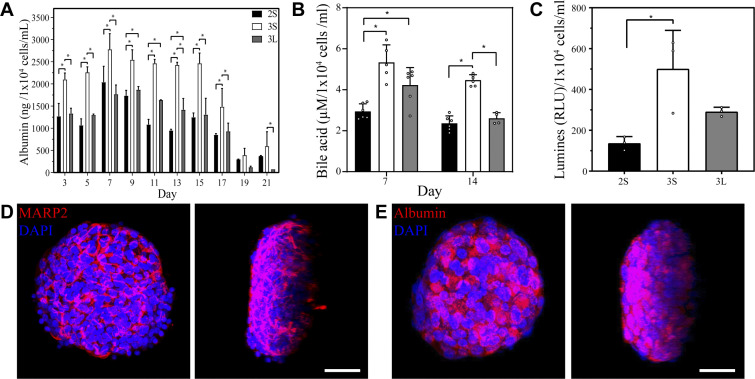
Beyond morphology, we assessed functionality of hepatocytes in large 3-D versus small 3-D versus microfluidic 2-D cultures by ELISA and RT-PCR analyses. As shown in Fig. 3A, albumin synthesis of small-volume spheroid cultures was significantly higher (P < 0.05) than in other conditions from day 5 until day 17, at which point all cultures began to decline. Small-volume 3-D hepatocyte cultures produced ~3,000 ng/1 × 104 cells/mL per day at day 7. Using albumin production at day 7 as a benchmark, our cultures were comparable to or better than some of the seminal reports of primary rat hepatocyte function in micropatterned cocultures (5), spheroid cultures (12, 34), or collagen gel sandwich cultures (9, 10). RT-PCR analysis of albumin gene expression confirmed superiority of small volume spheroid cultures (see Supplemental Fig. S2A). In addition to synthesizing more albumin, hepatocyte spheroids in small volume cultures produced more bile acids at day 14 and had significantly higher cytochrome P450 (CYP)2A1 expression at day 7 (Fig. 3, B and C). Additionally, immunofluorescent staining for multidrug resistant protein (MRP)-2 revealed formation of bile canalicular networks within individual hepatocyte spheroids (Fig. 3D). Immunofluorescent staining for albumin confirmed presence of this protein within spheroids and corroborated ELISA results (Fig. 3E). Taken together, our results highlight that small-volume microfluidic spheroid cultures elicited better level of hepatic function than large-volume 3-D cultures and microfluidic 2-D cultures.
Fig. 3.
Assessment of hepatic phenotype in microfluidic chambers. A: albumin ELISA from three types of culture systems compared in this study. Statistical significance established using Mann–Whitney test. B: analysis of total bile acids reveals higher levels in three-dimensional (3-D) small-volume cultures (3S) cultures compared with two-dimensional microfluidic cultures (2S) and 3-D large-volume cultures (3L) cultures. Statistical significance established with Mann–Whitney test. C: enzymatic activity of cytochrome P450 1A2 (CYP1A2) assessed at day 14. Statistical significance established by Welch’s t test. D and E: confocal microscopy and 3-D reconstruction images of albumin and multidrug resistance-associated protein 2 (MARP2) immunofluorescence confirm that hepatocyte spheroids in small-volume cultures strongly express these markers of hepatic phenotype at 1 wk of culture. Scale bar: 20 µm. Day 7 of culture. RLU, relative light units. *P < 0.05.
Establishing a connection between HGF/TGF-β1 signaling and hepatic phenotype.
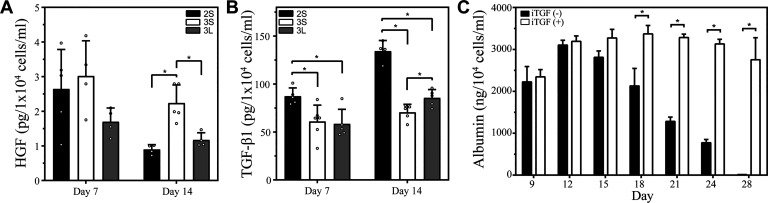
We hypothesized that differences in hepatic function described in the preceding section were, at least in part, due to the accumulation of local hepato-inductive signals and suppression of hepato-disruptive signals. HGF is known to have liver-protective, anti-fibrotic effects and has been explored as a potential therapy for liver diseases (25, 27, 32, 33, 35). Molecular mechanisms underlying anti-fibrotic effects of HGF are not well understood; however, it is generally accepted that HGF signaling antagonizes and suppresses profibrotic effects of TGF-β1. We previously showed this hypothesis to be true for microfluidic 2-D hepatocyte cultures where higher level HGF and lower level of TGF-β1 were observed in small-volume cultures (16). In the present study, HGF and TGF-β1 secretion was analyzed by ELISA and RT-PCR for three culture systems: low volume 3-D, large volume 3-D, and low volume 2-D. As seen from Fig. 4A, small-volume spheroid cultures produced significantly higher levels of HGF compared with large-volume spheroid cultures at day 14 of culture. Conversely, TGF-β1 production was highest in 2-D cultures and lowest in small-volume spheroid cultures after 7 and 14 days. Higher levels of TGF-β1 production in 2-D hepatocyte cultures correlated with the loss of cuboidal epithelial cell morphology, the appearance of elongated/striated cells of mesenchymal appearance (see Fig. 2B) and lower levels of albumin production (see Fig. 3A). RT-PCR analysis of HGF and TGF-β1 expression confirmed trends of higher HGF expression and lower TGF-β1 expression in small-volume spheroid cultures (see Supplemental Fig. S3). In summary, ELISA and RT-PCR supported our hypothesis that phenotype improvement of small-volume spheroid cultures was due, at least in part, to upregulated signaling of endogenous HGF and downregulation of profibrotic TGF-β1.
Fig. 4.
Hepatocyte growth factor (HGF) and TGF-β1 signaling in microfluidic hepatocyte cultures. A: HGF ELISA demonstrates higher levels of this hepato-inductive signal in three-dimensional (3-D) small-volume cultures (3S) compared with small-volume microfluidic (2S) and 3-D large-volume (3L) cultures. Statistical significance determined by Mann–Whitney test. B: TGF-β1 ELISA demonstrating higher levels of this hepato-disruptive signal observed in 3L and 2S cultures where hepatic phenotype was decaying more rapidly. Lowest TGF-β1 levels were observed in 3S cultures where HGF was highest and hepatic phenotype was best maintained. Statistical significance determined by Welch’s t test. C: inhibitor of TGF-β signaling (A83-01, 5 μM) was added into cell culture media on daily basis. This resulted in maintenance of high albumin levels (measured by ELISA) over the course of 28 days. Statistical significance determined by Mann–Whitney test. *P < 0.05.
To provide additional evidence in support of the importance of endogenous signaling in our cultures, microfluidic spheroid cultures were treated with small molecule inhibitor of TGF-β1 signaling (A83-01) starting at day 7. Using albumin secretion as readout, we established 5 µM to be the optimal concentration of TGF-β1 inhibitor (data not shown). Importantly, as seen from Fig. 4C, hepatic cultures treated with TGF-β inhibitor maintained high level of albumin synthesis after 4 wk of cultures, whereas untreated cultures began to decline at day 18 of culture. This result highlights the fact that endogenous signaling is important in our cultures and that cellular phenotype may be further improved by interfering with hepato-disruptive signals like TGF-β1. We should note that similar rescue of hepatic function due to treatment with TGF-β inhibitor was reported by us for 2-D microfluidic hepatocyte cultures previously (14), suggesting that endogenous signaling is important for both 2-D and 3-D microfluidic cultures.
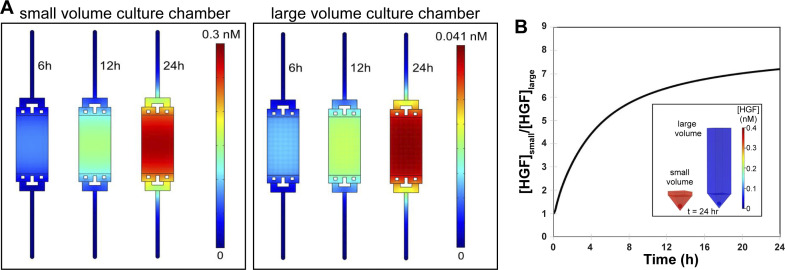
In addition to empirical observations of higher levels of HGF in small-volume 3-D cultures, we performed numerical modeling to compare accumulation of HGF in small- and large-volume spheroid cultures. Parameters used for modeling are listed in Table 1. Figure 5A demonstrates that after 24 h of secretion under static conditions with an HGF secretion rate of 0.4 × 10−6 ng·h−1·cell−1 (28), ~85% and 98% of HGF molecules are expected to be retained in small-volume and large-volume 3-D cultures, respectively. At 24-h time point, local HGF concentrations in the small- and large-volume cultures were predicted to be 0.3 nM and 0.04 nM, respectively.
Table 1.
Parameters used for numerical model
| Parameter | Value |
|---|---|
| Flow condition | Static |
| HGF diffusion coefficient | 8.5 × 10−7 cm2/s |
| Spheroid size | 100 µm |
| No. of cells/spheroid | 118 |
| Secretion rate | 0.45 × 10−6 ng·h−1·cell−1 |
HGF, hepatocyte growth factor.
Fig. 5.
Modeling of hepatocyte growth factor (HGF) secretion in microfluidic hepatocyte cultures. A: COMSOL modeling of HGF accumulation and diffusion at different time points in the microfluidic device. Modeling was based on actual device geometry and cell numbers. B: modeling prediction of HGF accumulation around a single spheroid over 24 h in small- and large-volume cultures.
It is worth noting that the total volume for each of the two microfluidic culture systems modeled in Fig. 5A was similar (~500 μL) and was dominated by the volume of media reservoirs. However, the design of the microfluidic system with the cell culture chamber connected to media reservoirs via long and narrow channels ensured that accumulation of secreted signals was dominated by the difference in the local volume of the culture chambers (4.25 μL for small volume and 40 μL for large volume).
Focusing on HGF accumulation over time at the surface of a single 100-μm hepatocyte spheroid, our modeling predicts HGF concentration to be 4 times higher in the low-volume 3-D culture devices after 12 h (0.17 nM vs. 0.026 nM) and 7.2 times higher after 24 h (0.34 nM vs. 0.047 nM) (see Fig. 5B). The discrepancy in the fold difference of HGF accumulating in small-versus large-volume cultures predicted by modeling (~7-fold) and observed experimentally (~2-fold) may be explained by multiple factors. First, HGF has limited stability, with in vivo half-life reported to be on the order of several minutes and in vitro half-life on the order of 2–3 h (1, 26). This would mean that only a fraction of HGF molecules secreted over the course of 24 h will be present in the detectable form at the time of media collection for ELISA analysis. Additionally, some of the secreted HGF molecules may be bound to c-met receptors or internalized by the cells. Despite these caveats, numerical modeling preformed here highlights the fact that accumulation of endogenous signals like HGF is dependent on the design/geometry of the culture chamber. It also buttresses our experimental observations of increased level of HGF production in small-volume spheroid cultures.
Utility of microfluidic hepatocyte cultures for modeling alcohol injury.
Globally, liver cirrhosis accounts for 1 million deaths every year (2). It is estimated that alcohol consumption causes ~48% of all the cirrhosis-related deaths (21). In the United States, the prevalence of alcoholic liver disease is on the rise (31). Although animal models do exist and are very valuable to the study of liver diseases (8a), these models are too complex to study molecular mechanisms of disease progression or effects of potential therapies. One such therapeutic agent is interleukin (IL)-22, a cytokine produced by T cells and other immune cells in the body (19). Our team has previously optimized conditions leading to alcohol injury in vitro (11, 15, 36) and has demonstrated that treatment with IL-22 protects hepatocytes from alcohol injury (23). Building on this prior work, we wanted to assess utility of our microfluidic hepatocyte spheroid cultures for modeling alcohol injury and testing protective effects of potential therapies such as IL-22.
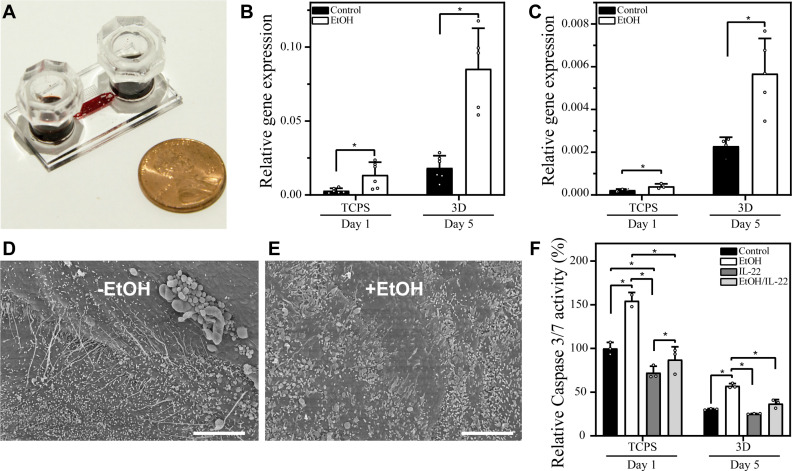
One challenge experienced by researches relying on standard culture systems is rapid evaporation of ethanol from cell culture media. We fabricated PDMS caps that fit snugly over media reservoirs to help minimize changes in ethanol concentration (see image in Fig. 6A). Indeed, ethanol concentration decreased by <10% over the course of 24 h (data not shown). Given that media exchange happened every 24 h, the cells were exposed to stable concentration of ethanol on a daily basis.
Fig. 6.
Alcohol injury in microfluidic hepatocyte spheroid cultures. A: a representative microfluidic cell culture device outfitted with polydimethylsiloxane caps to prevent evaporation of ethanol (EtOH) and culture media. B and C: expression of alcohol dehydrogenase (ADH)1 and cytochrome P450 2E1 (CYP2E1), enzymes responsible for alcohol metabolism, was measured by RT-PCR. These enzymes were expressed at high basal level in microfluidic spheroid (3D) cultures compared with tissue culture polystyrene (TCPS). Induction of these enzymes was observed in the presence of EtOH in microfluidic cultures. Welch’s t test was used to establish statistical significance for both ADH1 and CYP2E1 expression. D and E: scanned electron images showing topography of uninjured spheroids (D) and those exposed to alcohol (E). Scale bar: 5 µm. F: caspase activity assay used to assess apoptosis in standard TCPS cultureware and in small-volume spheroid cultures of hepatocytes exposed to 100 mM EtOH. Treatment with 10 ng/mL IL-22 protects hepatocytes against apoptosis. Mann–Whitney test was used to determine statistical significance. *P < 0.05.
Moving forward, we compared response of hepatocytes to alcohol injury for two cultures: 1) monolayer cultures in standard multiwell plates and 2) 3-D microfluidic cultures. Hepatocyte cultures dedifferentiated rapidly in standard 12-well plates, offering only a short time window of hepatic phenotype stability (~4–5 days total). Because of this, alcohol injury was carried out for 24 h in a multiwell plate. We should note that standard hepatocyte cultures in 12-well plates have been used by us previously to test protective effects of IL-22 during alcohol insult (23) and offered a point of reference for testing microfluidic spheroid cultures. It was not our goal to establish and test the best possible nonmicrofluidic hepatocyte culture system for alcohol injury experiments.
As discussed in the preceding sections, hepatocyte spheroids remained highly functional in microfluidic devices for over 2 wk. Taking into account stability of hepatocytes, we chose to injure microfluidic spheroids by daily exposure to alcohol over the course of 5 days.
One requirement for an in vitro model of alcohol injury is that hepatocytes retain expression of enzymes responsible for ethanol breakdown. Alcohol dehydrogenase (ADH) is the main enzyme responsible for oxidation of ethanol in the liver; however, CYP2E1 becomes an important contributor at higher levels of ethanol (mM concentrations) (7). RT-PCR analysis revealed that both ADH1 (a gene responsible for a subunit of ADH) and CYP2E1 expression was considerably higher in microfluidic spheroid cultures compared with hepatocytes cultured in a standard 12-well plate (Fig. 6, B and C). More importantly, levels of ADH1 and CYP2E1 expression were 4.2-fold and 2.5-fold higher, respectively, in microfluidic cultures exposed to alcohol compared with untreated microfluidic cultures. This result was consistent with induction of liver enzymes in the presence of substrate (ethanol) reported in vivo and in functional hepatocyte cultures in vitro (22, 24) and further confirmed responsiveness of our microfluidic cultures to alcohol injury.
Alcohol treatment had negative effects on morphology/ultrastructure of hepatocytes (Fig. 6E) and their survival (Fig. 6F). We have previously observed that in vitro, alcohol injury induced hepatic apoptosis via upregulation of caspase 3/7 (23). This observation was confirmed in our microfluidic cultures, which experienced moderate level of apoptosis (30% increase after 5 days of treatment). The feasibility of testing IL-22 therapy in vitro was proven by experiments described in Fig. 6F, which show that pretreatment with 10 ng/mL IL-22 for 12 h before alcohol injury helped prevent apoptosis. Although molecular mechanisms of IL-22 protection of hepatic apoptosis are under investigation, preliminary evidence points to the involvement of X-linked inhibitor-of-apoptosis protein (XIAP) (23).
Conclusions.
This paper tested a hypothesis that placement of hepatocyte spheroids into a small volume of microfluidic devices will result in enhancement of hepatic phenotype and function. We organized primary rat hepatocytes into arrays of spheroids inside microfluidic chambers with small (~4 μL) and large (40 µL) local volume. The 2-D microfluidic cultures represented the third culture format tested in our study. We demonstrated that hepatocytes spheroids were far more functional in small-volume cultures and that improved function correlated with enhanced expression of HGF and downregulation of TGF-β1. Among the three culture conditions tested, hepatic phenotype expression followed this pattern (from highest to lowest): small-volume 3-D cultures > large-volume 3-D cultures ≥ 2-D microfluidic cultures. The fact that 3-D large-volume hepatocyte cultures were better or similar to small-volume 2-D cultures highlights that multiple factors, including improved ECM deposition and polarization associated with 3-D cultures, contribute to hepatic phenotype maintenance. Despite this fact, our study is significant in highlighting that minimizing local volume is important in the context of 3-D cultures. As one application of this culture system, we demonstrated that robust maintenance of hepatic phenotype in our microfluidic cultures may be parlayed into an alcohol injury model. Hepatocytes in our system exhibited moderate levels of apoptosis and only gradual decay in phenotype expression during 5 days of continuous exposure to 100 mM ethanol. Additionally, we demonstrated that pretreatment with IL-22 prevented apoptosis and protected hepatocytes against alcohol injury. Going forward, we see microfluidic hepatocyte spheroid cultures as a valuable tool for modeling liver diseases, including alcoholic and nonalcoholic steatohepatitis.
GRANTS
The authors acknowledge financial support from NH (National Institute of Diabetes and Digestive and Kidney Diseases Grant DK107255).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.H.C., V.H.S., G.S., and A.R. conceived and designed research; J.H.C., L.L., and N.D. performed experiments; J.H.C., J.M.D.H.-V., K.L., and G.S. analyzed data; J.H.C., L.L., and G.S. interpreted results of experiments; J.H.C. and J.M.D.H.-V. prepared figures; J.H.C. drafted manuscript; J.H.C., J.M.D.H.-V., and A.R. edited and revised manuscript; J.H.C., L.L., J.M.D.H.-V., K.L., V.H.S., G.S., and A.R. approved final version of manuscript.
REFERENCES
- 1.Appasamy R, Tanabe M, Murase N, Zarnegar R, Venkataramanan R, Van Thiel DH, Michalopoulos GK. Hepatocyte growth factor, blood clearance, organ uptake, and biliary excretion in normal and partially hepatectomized rats. Lab Invest 68: 270–276, 1993. [PubMed] [Google Scholar]
- 2.Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol 70: 151–171, 2019. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Berthiaume F, Moghe PV, Toner M, Yarmush ML. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuration. FASEB J 10: 1471–1484, 1996. doi: 10.1096/fasebj.10.13.8940293. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J 13: 1883–1900, 1999. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Microfabrication of hepatocyte/fibroblast co-cultures: role of homotypic cell interactions. Biotechnol Prog 14: 378–387, 1998. doi: 10.1021/bp980036j. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol 32: 760–772, 2014. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 7.Cederbaum AI. Alcohol metabolism. Clin Liver Dis 16: 667–685, 2012. doi: 10.1016/j.cld.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clement B, Guguen-Guillouzo C, Campion JP, Glaise D, Bourel M, Guillouzo A. Long-term co-cultures of adult human hepatocytes with rat liver epithelial cells: modulation of albumin secretion and accumulation of extracellular material. Hepatology 4: 373–380, 1984. doi: 10.1002/hep.1840040305. [DOI] [PubMed] [Google Scholar]
- 8a.de la M Hall P, Lieber CS, DeCarli LM, French SW, Lindros KO, Järveläinen H, Bode C, Parlesak A, Bode JC. Models of alcoholic liver disease in rodents: a critical evaluation. Alcohol Clin Exp Res 25, Suppl 5: 254S–261S, 2001. doi: 10.1111/j.1530-0277.2001.tb02405.x. [DOI] [PubMed] [Google Scholar]
- 9.Dunn JCY, Tompkins RG, Yarmush ML. Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J Cell Biol 116: 1043–1053, 1992. doi: 10.1083/jcb.116.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn JCY, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog 7: 237–245, 1991. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 11.Gao W, Zhou P, Ma X, Tschudy-Seney B, Chen J, Magner NL, Revzin A, Nolta JA, Zern MA, Duan Y. Ethanol negatively regulates hepatic differentiation of hESC by inhibition of the MAPK/ERK signaling pathway in vitro. PLoS One 9: e112698, 2014. doi: 10.1371/journal.pone.0112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glicklis R, Merchuk JC, Cohen S. Modeling mass transfer in hepatocyte spheroids via cell viability, spheroid size, and hepatocellular functions. Biotechnol Bioeng 86: 672–680, 2004. doi: 10.1002/bit.20086. [DOI] [PubMed] [Google Scholar]
- 14.Haque A, Gheibi P, Gao Y, Foster E, Son KJ, You J, Stybayeva G, Patel D, Revzin A. Cell biology is different in small volumes: endogenous signals shape phenotype of primary hepatocytes cultured in microfluidic channels. Sci Rep 6: 33980, 2016. doi: 10.1038/srep33980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haque A, Gheibi P, Stybayeva G, Gao Y, Torok N, Revzin A. Ductular reaction-on-a-chip: Microfluidic co-cultures to study stem cell fate selection during liver injury. Sci Rep 6: 36077, 2016. doi: 10.1038/srep36077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones CN, Tuleuova N, Lee JY, Ramanculov E, Reddi AH, Zern MA, Revzin A. Cultivating liver cells on printed arrays of hepatocyte growth factor. Biomaterials 30: 3733–3741, 2009. doi: 10.1016/j.biomaterials.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Kang YBA, Eo J, Bulutoglu B, Yarmush ML, Usta OB. Progressive hypoxia-on-a-chip: an in vitro oxygen gradient model for capturing the effects of hypoxia on primary hepatocytes in health and disease. Biotechnol Bioeng 117: 763–775, 2020. doi: 10.1002/bit.27225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol 26: 120–126, 2008. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 19.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology 52: 1291–1300, 2010. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knobeloch D, Ehnert S, Schyschka L, Büchler P, Schoenberg M, Kleeff J, Thasler WE, Nussler NC, Godoy P, Hengstler J, Nussler AK. Human hepatocytes: isolation, culture, and quality procedures. In: Human Cell Culture Protocols, edited by Mitry RR, Hughes RD. Totowa, NJ: Humana Press, 2012, p. 99–120. [DOI] [PubMed] [Google Scholar]
- 21.Liangpunsakul S, Haber P, McCaughan GW. Alcoholic liver disease in Asia, Europe, and North America. Gastroenterology 150: 1786–1797, 2016. doi: 10.1053/j.gastro.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev 77: 517–544, 1997. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Verma VK, Malhi H, Gores GJ, Kamath PS, Sanyal A, Chalasani N, Gao B, Shah VH. Lipopolysaccharide downregulates macrophage-derived IL-22 to modulate alcohol-induced hepatocyte cell death. Am J Physiol Cell Physiol 313: C305–C313, 2017. doi: 10.1152/ajpcell.00005.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med 44: 723–738, 2008. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem 119: 591–600, 1996. doi: 10.1093/oxfordjournals.jbchem.a021283. [DOI] [PubMed] [Google Scholar]
- 26.Meneghello G, Storm MP, Chaudhuri JB, De Bank PA, Ellis MJ. An investigation into the stability of commercial versus MG63-derived hepatocyte growth factor under flow cultivation conditions. Biotechnol Lett 37: 725–731, 2015. doi: 10.1007/s10529-014-1701-4. [DOI] [PubMed] [Google Scholar]
- 27.Mungunsukh O, Day RM. Transforming growth factor-β1 selectively inhibits hepatocyte growth factor expression via a micro-RNA-199-dependent posttranscriptional mechanism. Mol Biol Cell 24: 2088–2097, 2013. doi: 10.1091/mbc.e13-01-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel D, Haque A, Gao Y, Revzin A. Using reconfigurable microfluidics to study the role of HGF in autocrine and paracrine signaling of hepatocytes. Integr Biol 7: 815–824, 2015. doi: 10.1039/C5IB00105F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prodanov L, Jindal R, Bale SS, Hegde M, McCarty WJ, Golberg I, Bhushan A, Yarmush ML, Usta OB. Long-term maintenance of a microfluidic 3D human liver sinusoid. Biotechnol Bioeng 113: 241–246, 2016. doi: 10.1002/bit.25700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakai Y, Furukawa K, Suzuki M. Immobilization and long-term albumin secretion of hepatocyte spheroids rapidly formed by rotational tissue culture methods. Biotechnol Tech 6: 527–532, 1992. doi: 10.1007/BF02447826. [DOI] [Google Scholar]
- 31.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ 362: k2817, 2018. doi: 10.1136/bmj.k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E, Fujimoto J. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med 5: 226–230, 1999. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- 33.Valdés-Arzate A, Luna A, Bucio L, Licona C, Clemens DL, Souza V, Hernandez E, Kershenobich D, Gutiérrez-Ruiz MC, Gómez-Quiroz LE. Hepatocyte growth factor protects hepatocytes against oxidative injury induced by ethanol metabolism. Free Radic Biol Med 47: 424–430, 2009. doi: 10.1016/j.freeradbiomed.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Wu FJ, Friend JR, Hsiao CC, Zilliox MJ, Ko W-J, Cerra FB, Hu W-S. Efficient assembly of rat hepatocyte spheroids for tissue engineering applications. Biotechnol Bioeng 50: 404–415, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 35.Yasuda H, Imai E, Shiota A, Fujise N, Morinaga T, Higashio K. Antifibrogenic effect of a deletion variant of hepatocyte growth factor on liver fibrosis in rats. Hepatology 24: 636–642, 1996. doi: 10.1002/hep.510240328. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Q, Patel D, Kwa T, Haque A, Matharu Z, Stybayeva G, Gao Y, Diehl AM, Revzin A. Liver injury-on-a-chip: microfluidic co-cultures with integrated biosensors for monitoring liver cell signaling during injury. Lab Chip 15: 4467–4478, 2015. doi: 10.1039/C5LC00874C. [DOI] [PubMed] [Google Scholar]