Abstract
The epithelial sodium channel (ENaC) regulates blood pressure by fine-tuning distal nephron sodium reabsorption. Our previous work has shown that ENaC gating is regulated by anionic phospholipid phosphates, including phosphatidylinositol 4,5-bisphosphate (PIP2). The PIP2-dependent regulation of ENaC is mediated by the myristoylated alanine-rich protein kinase C substrate-like protein-1 (MLP-1). MLP-1 binds to and is a reversible source of PIP2 at the plasma membrane. We examined MLP-1 regulation of ENaC in distal convoluted tubule clonal cell line DCT-15 cells. Wild-type MLP-1 runs at an apparent molecular mass of 52 kDa despite having a predicted molecular mass of 21 kDa. Native MLP-1 consists of several distinct structural elements: an effector domain that is highly positively charged, sequesters PIP2, contains serines that are the target of PKC, and controls MLP-1 association with the membrane; a myristoylation domain that promotes association with the membrane; and a multiple homology 2 domain of previously unknown function. To further examine MLP-1 in DCT-15 cells, we constructed several MLP-1 mutants: WT, a full-length wild-type protein; S3A, three substitutions in the effector domain to prevent phosphorylation; S3D mimicked constitutive phosphorylation by replacing three serines with aspartates; and GA replaced the myristoylation site glycine with alanine, so GA could not be myristoylated. Each mutant was tagged with either NH2-terminal 3XFLAG or COOH-terminal mCherry or V5. Transfection with MLP mutants modified ENaC activity in DCT-15 cells: activity was highest in S3A and lowest in S3D, and the activity after transfection with either construct was significantly different from WT. In Western blots, when transfected with 3XFLAG-tagged MLP-1 mutants, the expression of the full length of MLP-1 at 52 kDa increased in mutant S3A-MLP-1-transfected DCT-15 cells and decreased in S3D-MLP-1-transfected DCT-15 cells. Several lower molecular mass bands were also detected that correspond to potential presumptive calpain cleavage products. Confocal imaging shows that the different mutants localize in different subcellular compartments consistent with their preferred location in the membrane or in the cytosol. Activation of protein kinase C increases phosphorylation of endogenous MLP-1 and reduces ENaC activity. Our results suggest a complicated role for proteolytic processing in MLP-1 regulation of ENaC.
Keywords: DCT cells, ENaC, MARCKS-like protein-1, MARCKSl1, PIP2
INTRODUCTION
High blood pressure, or hypertension, is a very common disease and the leading cause of death and disability in the United States: 76.4 million US adults have hypertension. The vast majority have essential hypertension, of which there is no known single etiology. There are several physiological causes of high blood pressure, but one significant cause is constitutively elevated reabsorption of sodium by principal cells in the connecting tubule and collecting duct of the mammalian nephron. Much of this excess sodium reabsorption is through amiloride-sensitive epithelial sodium channels (ENaC; Ref. 46). Monogenetic mutations of ENaC underscore the importance of ENaC in controlling blood pressure (46). However, subtle dysregulation of ENaC may also contribute to essential hypertension (45), and since ENaC can be its cause, a thorough understanding of the regulation of ENaC may lead to the prevention and treatment of hypertension.
Probably because monogenetic disorders often alter ENaC trafficking, examination of ENaC has focused on regulation of channel density in the apical membrane of renal principal cells. However, ENaC is an ion channel, so regulating the open probability (Po) may be just as important. Both Liddle syndrome and pseudohypoaldosteronism type 1 (PHA-1) are conventionally described as changes in channel density (an increase and decrease, respectively); however, examination of single ENaC channels in these two syndromes shows that the open probability of ENaC also changes to increase channel activity in Liddle (6) and decrease it in PHA-1 (26). Aldosterone increases sodium transport and is often thought to do so by increasing subunit transcription and translation. Frindt and Palmer, in isolated rat tubules (15), have shown this to be true, but the increase in ENaC density accounts for <25% of the increase in transtubular sodium current, implying that the remaining 75% is due to an increase in single-channel Po.
We and others have shown that ENaC Po is increased by cytosolic phosphatidylinositol 4,5-bisphosphate (PIP2; Refs. 31, 39, 43, 44, 55, 64). The implication of these electrophysiological experiments is that PIP2 must interact with one or more of the ENaC subunits. We and others have shown that PIP2 binds to β- and γ-, but not α-, ENaC subunits at two binding sites for PIP2 at the NH2-terminal domain of β- and γ-ENaC (1, 31, 42, 44, 51, 64). Several reports have shown that the positive charges within the amino terminus of ENaC interact with the anionic phospholipids like PIP2 in the inner leaflet of the plasma membrane to regulate ENaC activity (21, 30, 44, 65). PIP2 is required for normal ENaC activity, and PIP2 reversibly binds ENaC subunits. On the other hand, when phospholipase C is activated to hydrolyze PIP2, it leaves no PIP2 available for binding to ENaC and Po falls close to 0, implying that the Po of ENaC without PIP2 bound is 0.
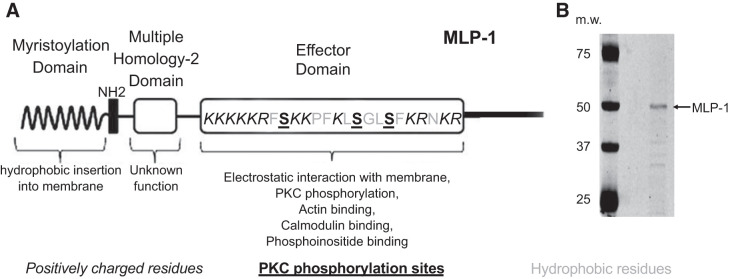
PIP2 is necessary to open ENaC. However, there is a conceptual problem with a simple model of ENaC and PIP2 associating by simple lateral diffusion in the membrane. ENaC is a rare protein (only a few functional channels per square micrometer in the apical membrane). PIP2 is also a rare molecule constituting of <1 in 1,000 lipid molecules. Since PIP2 association is necessary for ENaC activity, a calculation of the likelihood of random diffusional interaction would predict channel openings only every 10 min when in fact the channels open every 1 or 2 s in a typical patch. Therefore, we hypothesized that there must be an adaptor protein that could bind and sequester PIPs and associate with ENaC to increase the local PIP2 concentration near ENaC enough to account for the anonymously high opening rate. Ideally, such a protein should be associated with apical membrane lipid domains and be capable of binding and sequestering PIPs with an affinity that would also allow the PIPs to bind reversibly to and activate ENaC. One such protein is myristoylated alanine-rich C kinase substrate (MARCKS) or its closely related kidney isoform, MARCKS-like protein 1 (MLP-1). MARCKS and MLP-1 consist of a myristoylated amino-terminal domain and a basic effector domain for which positive charge will electrostatically bind anionic lipids (like PIP2) and cause MLP-1 to associate with PIP2-rich lipid domains (Fig. 1). The basic effector domain also contains PKC phosphorylation sites, cytoskeletal binding sites, and calcium/calmodulin binding sites (19). MLP-1 reversibly associates with the inner leaflet of PIP2-rich lipid domains through hydrophobic and electrostatic interactions of its myristoyl group and basic effector domain, respectively (23). MLP-1 cross-links to actin, but the binding is regulated by PKC phosphorylation and calmodulin binding (19, 32, 33). PKC phosphorylates serine residues within the basic effector domain of MLP-1. The negative charges associated with serine phosphorylation neutralize the basic charges, which leads to the translocation of MLP-1 from the membrane to the cytoplasm. Several proteins, including GAP43, CAP23, and MARCKS, have been found to sequester PIPs (28), but only MLP-1 is present in sodium-transporting epithelial tissue. Twenty-six-kilodalton MLP-1 runs at 52 kDa probably because of its rod-shaped structure, unusual concentration of positive charge, and ability to bind SDS molecules.
Fig. 1.
A: schematic diagram of myristoylated alanine-rich protein kinase C substrate (MARCKS)-like protein-1 (MLP-1). Part of the MARCKS family of proteins, MLP-1 is the predominant MARCKS isoform in mouse kidney. MLP-1 consists of a myristoylation domain and a basic effector domain for which positive charge will electrostatically bind phosphatidylinositol 4,5-bisphosphate (PIP2) and cause MARCKS or MLP-1 to associate with PIP2-rich lipid domains. The effector domain has multiple sites for PIP2 interaction (note positively charged residues) and sites for phosphorylation (note serine residues). MLP-1 functions as a reversible source of PIP2 at the membrane. The ability of MLP-1 to function as a PIP2-sequestering protein at the membrane is dependent on hydrophobic interactions between the myristoylation domain and the membrane and electrostatic forces between the effector domain and anionic lipids in the membrane. B: MLP-1 has a predicted molecular mass of ~21 kDa but migrates slowly and close to 52 kDa on SDS-PAGE gels probably due to its unusual positive charge. m.w., Molecular weight (i.e., molecular mass, in kilodaltons).
This paper will examine the association of MLP-1 and ENaC and the structural elements in ENaC and MLP-1 that allow the interaction. We will also examine the ability of MLP-1 to activate ENaC and whether MLP-1 can be regulated by phosphorylation or proteolytic cleavage.
METHODS
Cell culture.
mDCT were originally established from primary cultures of mixed cortical thick ascending limb and distal convoluted tubule cells isolated from male mice (CD-1; Charles River, Wilmington, MA; Ref. 54). mDCT-15 cells are a clonal cell line derived from the original mixed population with high levels of thiazide- and amiloride-sensitive sodium transport (25). mDCT-15 cells (passages 20–30) were plated on plastic cell culture dishes or 24-mm Transwell permeable supports (Corning) and grown in growth medium containing a 50:50 mix of DMEM and F-12 (Sigma), 5% fetal bovine serum (GIBCO), and 1% penicillin-streptomycin at 37°C and 5% CO2. COS-7 cells were cultured in the growth medium with DMEM supplemented with 1% penicillin-streptomycin, 2 mM l-glutamine, and 10% fetal bovine serum (GIBCO) at 37°C and 5% CO2.
Transfection.
All DNA constructs were transfected into mDCT-15 cells and COS-7 cells when the cells were 50–70% confluent using the Xfect Transfection Reagent kit (Takara) following the manufacturer’s suggested protocol. The cells were used for single-channel patch-clamp recordings, checkmate mammalian two-hybrid assay, or protein extraction 48 h after transfection. Western blot and fluorescence analysis were used to examine the efficiency of transfection.
Animals.
In one experiment (Fig. 10), we used 4 C57BL/6 mice (2 males and 2 females) obtained from Jackson to isolate cortical collecting duct, after which we used patch-clamp methods to record single channels from principal cells. The use of these animals was approved by Emory University’s institutional animal care and use committee.
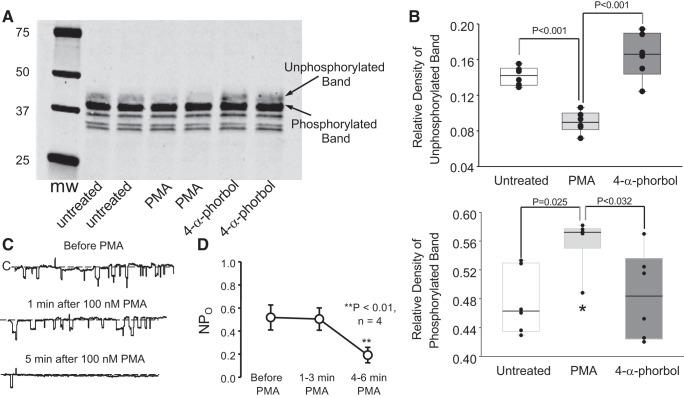
Fig. 10.
Phorbol esters increase myristoylated alanine-rich protein kinase C substrate-like protein-1 (MLP-1) phosphorylation and inhibit epithelial sodium channel (ENaC). Endogenous MLP-1 is a target for protein kinase C phosphorylation. We applied phorbol myristoyl acetate to activate protein kinase C and prepared cell lysates. Western blots (A) of the lysates showed a strong band at 37 kDa, which is characteristic of MLP-1. Just above it is the nonphosphorylated band, which is expected to migrate more slowly on the SDS gel. PMA reduces the density of the unphosphorylated band (B, top; P < 0.001, 1-way analysis of variance on ranks; n = 6) and increases the density of the phosphorylated band (B, bottom; *P < 0.025, 1-way analysis of variance on ranks; n = 6). As control (C), we also applied an inactive phorbol, which does not change the relative density of the phosphorylated and nonphosphorylated bands. We also used single-channel methods (see methods) to examine principal cells in isolated, split-open collecting ducts bathed in the same saline that we used to obtain A and B. As expected, addition of PMA rapidly reduced ENaC channel activity, defined as the number of channels within the patch (N) times the channel open probability (Po) (C and D). **P < 0.01, Kruskal–Wallis 1-way analysis of variance on ranks; 4 patches on 4 principal cells from 4 mice of any sex; n = 4. mw, Molecular weight (i.e., molecular mass, in kilodaltons).
Construction of MLP-1 expression vectors.
Mutant MLP-1 constructs (see Table 1) were generous gifts from Dr. Sumiko Watanabe (University of Tokyo, Tokyo, Japan; Ref. 66). The mutant constructs were then subcloned into p3XFLAG-CMV-10 Expression Vector (Sigma) and pmCherry-N1 vector (Clontech) separately. The green fluorescent protein (GFP)-tagged PIP2 reporter PLCδ1 construct was obtained from Addgene. pBIND, pACT, and pG5-Luciferase were purchased from Promega. All pBIND and pACT constructs used in Luciferase assay were generated by inserting PCR-amplified NH2-terminal, COOH-terminal, and multiple-homology 2 (MH-2) domains of MLP-1 into pBIND vector and NH2-terminal and COOH-terminal rat α-, β-, and γ-ENaC DNA sequence into pACT vectors following the manufacturer’s protocol (Promega). All mutant plasmids were confirmed by DNA sequencing (Thermo Fisher Scientific).
Table 1.
Description of MLP-1 constructs
| Description | Name | Mutations | Phenotype |
|---|---|---|---|
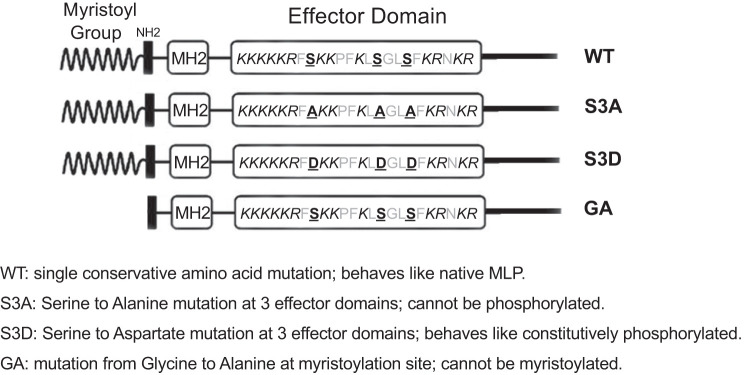
| Wild-type protein | WT | None | Normal function |
| Three protein kinase C phosphorylation target serines in the effector domain changed to alanines | S3A | S93A, S101A, S104A | Cannot be phosphorylated by PKC |
| Three protein kinase C phosphorylation target serines in the effector domain changed to aspartic acid | S3D | S93D, S101D, S104D | Acts like constitutively phosphorylated |
| Myristoylation site changed to alanine | GA | G2A | Cannot be myristoylated |
MLP-1, myristoylated alanine-rich protein kinase C substrate-like protein-1.
SDS-PAGE, Western blotting, and densitometric analysis.
Cells were washed twice with ice-cold 1× PBS before lysing in mammalian protein extraction reagent (Thermo Fisher Scientific) containing EDTA, phosphatase, and protease inhibitors (Thermo Fisher Scientific). The cell lysate was homogenized and then sonicated on ice for 10 s at a frequency of 30 kHz. Cell lysates were centrifuged at 15,000 rpm for 10 min at 4°C, and the supernatant was collected. The BCA protein assay (Thermo Fisher Scientific) was performed following the manufacturer’s instructions to measure protein concentration. All of the samples were prepared in 4× Laemmli sample buffer (Bio-Rad) containing 5% 2-mercaptoethanol followed by heating the samples at 95°C for 5 min. Thirty micrograms of total protein were loaded in each lane of 4–20% Mini-PROTEAN TGX precast gels (Bio-Rad) using Tris glycine/SDS buffer in a Mini-PROTEAN Tetra Vertical Electrophoresis Cell (Bio-Rad). The separated proteins were then transferred onto 0.2-μm nitrocellulose membranes using the Trans-Blot Turbo Transfer System (Bio-Rad). The membranes were blocked in 5% nonfat dry milk in 1× Tris-buffered saline with 0.5% Tween 20 (TBST) for 1 h at room temperature followed by incubating overnight at 4°C in the following primary antibodies: COOH-terminal MLP (1:5,000; cat. no. ab184546; Abcam), NH2-terminal MLP (1:1,000; cat. no. GTX56062; GeneTex), flag (1:2,000; RRID:AB_262044; cat. no. F1804; Sigma-Aldrich), mCherry (1:5,000; cat. no. TA150126; OriGene), V5 (1:5,000; RRID:AB_2556564; cat. no. R960-25; Thermo Fisher Scientific), α-ENaC (1:2,000; RRID:AB_10640131; cat. no. SPC-403; StressMarq Biosciences), β-ENaC (1:2,000; RRID:AB_10644173; cat. no. SPC-404; StressMarq Biosciences), and γ-ENaC (1:1,000; RRID:AB_2825485; cat. no. SPC-405D-DY405; StressMarq Biosciences). The membranes were also probed for β-actin antibody (RRID:AB_2242334; cat. no. 3700; Cell Signaling Technology) or GAPDH antibody (RRID:AB_2756824; cat. no. 97166; Cell Signaling Technology) at a dilution of 1:3,000 for 2 h at room temperature as a loading control. The membranes were washed three times with 1× TBST for 5-min intervals and then incubated with appropriate species-specific IRDye secondary antibodies (RRID:AB_2651127, cat. no. 925-32211, LI-COR Biosciences; RRID:AB_2721181, cat. no. 925-68071, LI-COR Biosciences; RRID:AB_10953628, cat. no. 926-68072, LI-COR Biosciences; RRID:AB_10956736, cat. no. 926-68074, LI-COR Biosciences) at a dilution of 1:10,000 in blocking buffer on a shaker at room temperature for 1 h. After four washes with 1× TBST for 5-min intervals, the membranes were imaged with the Odyssey imaging system (LI-COR Biosciences). The anti-MLP-1 antibodies detect the same molecular mass bands as anti-epitope antibodies for V5, 3XFLAG, and mCherry when cells are transfected with epitope-tagged constructs.
Single-channel patch-clamp studies.
Patch pipettes were fabricated from filamented borosilicate glass capillaries (TW150F; World Precision Instruments) with a two-stage vertical puller (PP2; Narishige, Tokyo, Japan) with a resistance of 6–10 MΩ. mDCT-15 cells were transfected, treated with 1 µM aldosterone, and cultured on 24-mm permeable Transwell supports (Corning). Cells were visualized with Hoffman modulation optics (Modulation Optics) on a Nikon Diaphot. Negative pressure was applied to achieve a cell-attached patch with a seal resistance of 10–20 GΩ after making contact between the pipette tip and the cell surface. The extracellular bath solution consisted of a saline solution (150 mM NaCl, 5 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 5 mM glucose, and 10 mM HEPES, adjusted to pH 7.4). The patch pipette solution consisted of a saline solution (140 mM LiCl, 2 mM MgCl2, and 10 mM HEPES, adjusted to pH 7.4). The cell-attached patch configuration was used for all single-channel experiments on mDCT-15, and voltages are given as the negative of the patch pipette potential. ENaC activity within a patch was calculated using pCLAMP 10 software (Molecular Devices) as a product of the number of functional channels and the open probability. In Fig. 10, to demonstrate the effect of phorbol esters on ENaC activity, we used an isolated, split-open collecting duct preparation. Mice were euthanized by overdose of pentobarbital followed by cervical dislocation, and the kidneys were immediately removed. Cortical collecting ducts were dissected and put in ice-cold physiological saline solution containing, in mM, 140 NaCl, 5 KCl, 1 CaCl2, and 10 HEPES titrated to pH 7.4 with NaOH, as described previously (7). Isolated collecting ducts were allowed to settle onto 5 × 5-mm coverglass coated with poly-l-lysine. Coverglass-containing tubules were placed in a perfusion chamber mounted on an inverted Nikon Eclipse TE2000 microscope. To gain access to the apical membrane of principal cells, tubules were split open with sharpened micropipettes. Isolated, split-open tubules were used for patch-clamp analysis within 2 h after isolation.
Live cell immunofluorescence imaging.
mDCT-15 cells were seeded on 24-mm Transwell permeable supports (Corning) in growth medium. Both a GFP-tagged PLCδ1 construct and one of cherry-tagged MLP-1 S3A, S3D, wild-type, or GA constructs were transfected into mDCT-15 cells following standard Xfect transfection protocol; fresh growth medium was added 24 h after transfection. Forty-eight hours after transfection, a Leica SP8M two-photon microscope was used to examine the fluorescence and capture images of xyz-stacks.
Mammalian two-hybrid assay.
The mammalian two-hybrid assay was carried out using a Dual-Luciferase assay. COS-7 cells were seeded into 96-well plates before transfection. The DNA constructs pACT-COOH (or NH2)-α-ENaC, pACT-COOH (or NH2)-β-ENaC, pACT-COOH (or NH2)-γ-ENaC, pBIND-COOH (or NH2)-MLP-1, pBIND-MH-2-MLP-1, and pG5-Luciferase were prepared in equimolar amounts. Another set of cells was cotransfected with the pBIND-blank and pACT-blank with or without pG5-Luciferase vector as a negative control and pBIND-ID and pACT-MyoD as a positive control. The transfection was done using Xfect Transfection Reagent kit (Takara). Forty-eight hours after transfection, the firefly luminescence and the Renilla luminescence were measured by using Gen5 software in a luminometer (BioTek Synergy 2) following the manufacturer's protocol of Dual-Glo Luciferase Assay System (Promega). For each well, a firefly-to-Renilla ratio was calculated and normalized based on the pG5-Luciferase basal control.
Statistical analysis.
All data are presented as means ± SE and the number of samples (n). The standard deviation can be calculated as × SE. One-way ANOVA or Kruskal–Wallis one-way analysis of variance on ranks was used to compare multiple groups with Holm–Šídák or Dunn posttests. The P value of <0.05 was considered statistically significant. All calculations were performed using SigmaPlot 14.0 software (Systat Software).
RESULTS
MLP-1 mutations modify ENaC open probability but not channel density.
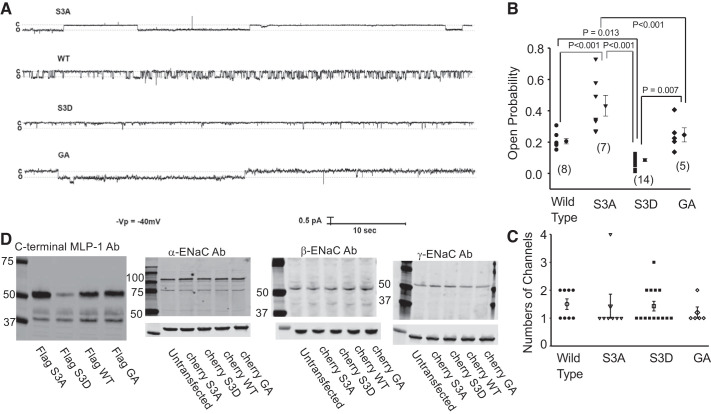
We know that MARCKS protein could regulate ENaC in amphibian cells (1); we hypothesized that MLP-1 was also involved in regulation of ENaC. This would be important since MLP-1 is the major isoform in mammalian kidney. To show the involvement of MLP-1, we used four constructs (described in Table 1 and Fig. 2). The construct that could not be phosphorylated (designated S3A) and, therefore, the construct that presumably remained associated with the membrane increased ENaC open probability compared with wild-type MLP-1 (Fig. 3, A and B; S3A = 0.432 ± 0.0661, n = 7, vs. WT = 0.206 ± 0.0171, n = 8; P < 0.001). The construct that mimicked phosphorylated MLP-1 and was presumably cytosolic had an open probability significantly less than wild type (S3D = 0.0857 ± 0.00941, n = 14, vs. WT = 0.206 ± 0.0171, n = 8; P < 0.013), and the construct that could not be myristoylated had an open probability near wild type (GA = 0.246 ± 0.0448, n = 5, vs. WT = 0.206 ± 0.0171, n = 8; P = 0.448). S3A open probability was larger than GA (P = 0.007) and S3D (P < 0.001), and GA was larger than S3D (P = 0.007). Despite changes in open probability, channel density measured as channels per patch appeared unchanged (Fig. 3C). We also confirmed that there was no change in ENaC protein expression using Western blots for the three ENaC subunits in the presence of each of the MLP-1 constructs (Fig. 3D). An examination of Fig. 3A suggests that the reason the channel’s open probability in cells transfected with S3A, S3D, and wild-type constructs changed was because the mean open time (and possibly the mean closed time) also changed. In fact, the mean open time of channels from S3A-transfected cells (539 ± 147 ms, n = 9) was significantly greater than that of wild-type channels (182 ± 35.8 ms, n = 18; P = 0.012) and that of S3D channels (81.9 ± 16.9 ms, n = 18; P = 0.001). It was not different from GA channels (293 ± 219 ms, n = 6; P = 0.321). Wild-type and S3D channels were not significantly different (P = 0.471). The mean closed time of S3A channels (711 ± 162 ms, n = 9) is much smaller than that of wild-type, S3D, or GA channels (2,730 ± 1,260, 2,330 ± 801, and 2,530 ± 1,910 ms, respectively) but not statistically different.
Fig. 2.
Schematic diagram of myristoylated alanine-rich protein kinase C substrate-like protein-1 (MLP-1) mutants. The S3A mutant replaced the 3 phosphorylatable serines to alanines so that the construct cannot be phosphorylated. The S3D mutant replaces each serine with an aspartate to mimic the charge associated with phosphorylation. The GA mutant replaces the glycine at the myristoylation site with an alanine so construct cannot be myristoylated. MH2, multiple-homology 2 domain; WT, wild type.
Fig. 3.
Myristoylated alanine-rich protein kinase C substrate-like protein-1 (MLP-1) mutations alter epithelial sodium channel (ENaC) activity. Distal convoluted tubule cell line mDCT-15 cells were transfected, treated with 1 μM aldosterone, and cultured on 24-mm permeable supports. The cell-attached patch configuration was used for all single-channel experiments 48 h after transfection of different mutants (see Fig. 2). A shows the representative recordings of each group. Unsurprisingly, S3A-transfected cells had the highest open probability, whereas S3D had the lowest. All differences in open probability between variants except the 1 between wild type (WT) and GA are statistically significant. In B is the open probability for all variants of MLP-transfected mDCT-15 cells with individual values and means ± SE. Numbers in parentheses represent the number of experiments. C shows that the expression levels of ENaC measured as the number of channels per patch are unchanged regardless of the construct. Channels per patch are discrete numbers, so bar graphs are more appropriate than dot plots. D shows expression of MLP-1 constructs (S3A, S3D, WT, and GA) in the 1st panel. The 2nd through 4th panels are Western blots of α-, β-, and γ-ENaC that show that expression of each of the ENaC subunits is unchanged regardless of the MLP-1 construct (representative of 4 similar blots). Molecular mass is in kilodaltons. Ab, antibody; -Vp, negative of the patch pipette potential. “c” and “o” to the left of single channel records indicate ENaC closed and open levels.
The different MLP-1 constructs have differing levels of expression.
Despite there being no measurable change in ENaC subunit density, the different MLP-1 constructs did show different amounts of protein expression even under rigidly controlled transfection conditions and with controls for protein loading in the Western blots (Fig. 3D, lane 1). There are suggestions in the literature that MLP-1 can be proteolytically cleaved and that some of the cleavage products are subject to rapid degradation (38, 49, 50). We hypothesized that S3D, the MLP-1 construct that stayed in the cytosol, would be more subject to proteolytic degradation and, therefore, its expression would be reduced in cell lysates as seen in Fig. 3D.
Recombinant MLP-1 constructs are cleaved.
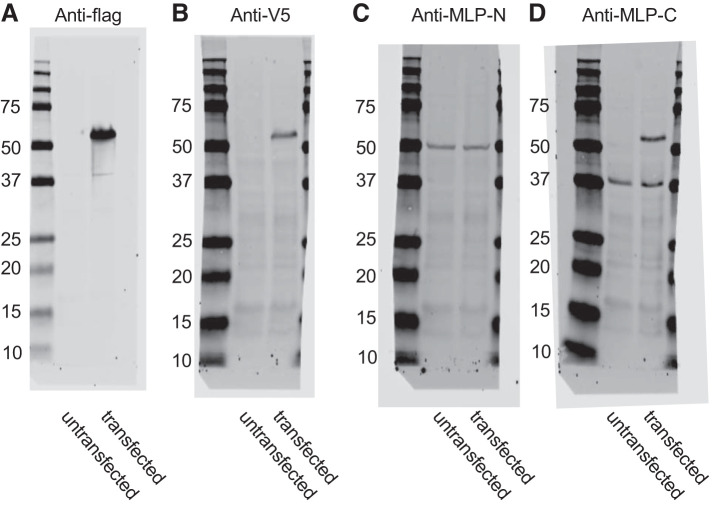
This question was difficult to address since our original antibodies only recognized the COOH-terminal region of MLP-1 so we had no way to identify any NH2-terminal fragment. To address this issue, we modified our constructs by adding epitope tags to the COOH and NH2 termini: either FLAG or V5 (NH2 or COOH terminus, respectively) or mCherry tag (at various positions; Fig. 4). We also found an antibody that recognizes an NH2-terminal epitope. These figures all show that the 52-kDa full-length proteins are recognized by the appropriate epitope tag antibodies (Fig. 4, A and B) but that the antibody to the NH2 terminus of endogenous MLP-1 detects a slightly lower molecular mass band at 50 kDa in both transfected and untransfected cells (Fig. 4C) and the antibody to the COOH terminus of endogenous MLP-1 detects the same full-length band as anti-flag and anti-V5 but also two bands at 37 kDa (Fig. 4D), which we presume is a cleaved product. Since we had verified the specificity of the antibody, we presumed that the bands could represent degradation products of the full-length protein.
Fig. 4.
Western blots from lysates from untransfected DCT-15 cells (a distal convoluted tubule clonal cell line) or cells transfected with a full-length wild-type myristoylated alanine-rich protein kinase C substrate-like protein-1 (MLP-1) with an NH2 (N)-terminal 3XFLAG tag and a COOH (C)-terminal V5 tag. Blots were probed with anti-flag, anti-V5, an MLP-1 antibody to an NH2-terminal epitope, or an MLP-1 antibody to a COOH-terminal epitope. Molecular mass is in kilodaltons.
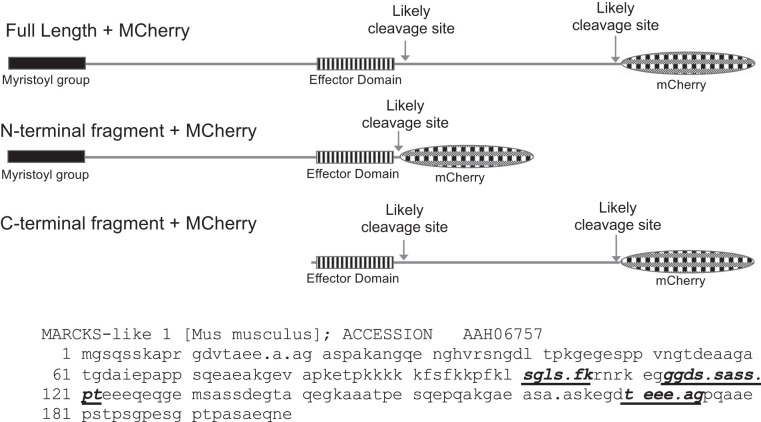
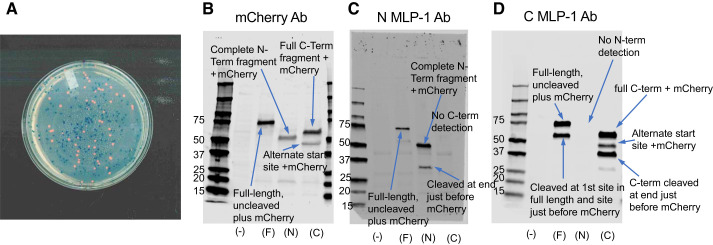
Although the results in Fig. 4D suggest the possibility of MLP-1 cleavage, we wanted to develop further experiments to characterize the cleavage. Several previous reports have shown that MARCKS protein could be cleaved by the calcium-dependent protease calpain (13, 27), and at least one report shows that MLP-1 can be cleaved by calpain-2 (38). When we used a calpain cleavage-predicting algorithm (57) to identify calpain cleavage sites in mouse MLP-1 (acc. no. AAH06757), we found five highly probable cleavage sites (Table 2). Of these, two were very close to the NH2 terminus and two were very close to the COOH terminus. One was close to the center of the protein and the effector domain. To further investigate the cleavage, we produced several additional constructs: all were SP6-driven expression vectors that could also be expressed in a cell-free wheat germ expression system (Fig. 5). We produced a full-length, COOH-terminal, mCherry-tagged MLP-1; an NH2-terminal fragment (amino acids 2–118) with a COOH-terminal mCherry-tag; and a COOH-terminal fragment (amino acids 112–200) with a COOH-terminal mCherry-tag. Since each construct contained a 28-kDa mCherry tag, the constructs when expressed have characteristic sizes (Fig. 5). The expression of the mCherry-containing constructs was easy to detect since when expressed in bacteria, they produced characteristically pink colonies in contrast to the blue of the colonies that contained no insert (Fig. 6A). The expression patterns we observe that were detected with an mCherry antibody or antibodies to the NH2-terminal or COOH-terminal epitope of MLP-1 are consistent with two cleavage sites, one near the middle of the molecule and one just before the COOH terminus of MLP-1 that cleaves off the mCherry epitope. Each antibody, as expected, detected a full-length protein of approximately 75–78 kDa (Fig. 6, B–D).
Table 2.
Likely calpain cleavage sites in MLP-1
| Rank | Position | Site | NH2-Fragment, kDa* | COOH-Fragment, kDa | Frequency Score | Similarity Score | Similarity Sequence | Average Score | Specificity |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 170–175 | TEEE.AG | 17.7 | 2.5 | 14.493 | 50 | TPEQAA | 724.638 | >99% |
| 2 | 14–19 | TAEE.AA | 1.7 | 18.5 | 9.11 | 63.333 | TPEQAA | 576.95 | >99% |
| 3 | 160–165 | EASA.AS | 16.6 | 3.6 | 7.652 | 62.963 | EKSAAT | 481.804 | >99% |
| 4 | 15–20 | AEEA.AG | 1.8 | 18.4 | 9.662 | 34.375 | AENTAH | 332.126 | >99% |
| 5 | 113–118 | GGDS.SA | 11.9 | 8.3 | 5.533 | 58.065 | GGDEAI | 321.252 | >99% |
MLP-1, myristoylated alanine-rich protein kinase C substrate-like protein-1.
If a fragment contains the effector domain, the fragment will run anomalously high on a gel (~2.5× predicted weight).
Fig. 5.
Schematic diagram of myristoylated alanine-rich protein kinase C substrate (MARCKS)-like protein-1 (MLP-1) cleavage sites. Three MLP-1 constructs: the top is a full-length construct with likely cleavage sites marked (see Table 2); the center is the NH2-terminal fragment; and the bottom is the COOH-terminal fragment. All of the fragments contain a COOH-terminal mCherry epitope and an intact effector domain. Below: MLP-1 sequence showing high-probability calpain cleavage sites as bold, italic, underlined residues. Periods mark 2 other calpain cleavage sites (Table 2) that would produce very short products near the NH2- or COOH-terminal ends of MLP-1. The initial methionine is a signal peptide that is cleaved early in protein maturation. The full-length construct is residues 2–200 + mCherry (29 kDa; NH2-terminal fragment is residues 2–118 + mCherry; COOH-terminal fragment is residues 112–200 + mCherry). Residue 131 is an alternative start site in the COOH-terminal fragment.
Fig. 6.
Cleavage of myristoylated alanine-rich protein kinase C substrate-like protein-1 (MLP-1). A: the expression of constructs that contained mCherry produced characteristically pink colonies in contrast to the blue of the colonies that contained no insert. B–D: the expression patterns we observed that were detected with an mCherry antibody (Ab) or antibodies to the NH2-terminal or COOH-terminal epitope of MLP-1 are consistent with 2 cleavage sites, 1 near the middle of the molecule and 1 just before the COOH terminus of MLP-1 that cleaves off the mCherry epitope (Fig. 5). In B–D, (–), F, N, and C are untransfected and transfected with full-length MLP + mCherry, NH2-terminal MLP + mCherry, or COOH-terminal MLP + mCherry, respectively. B: the mCherry Ab also detected the 112-residue NH2-terminal (N-Term) fragment + mCherry at ~51 kDa and the 118-residue COOH-terminal (C-Term) fragment + mCherry at 58 kDa. It also detected a 43-kDa band that is likely produced by an alternative start site corresponding to the methionine at position 131 (a 68-residue fragment + mCherry). C: the NH2-terminal antibody detected the same full-length band and NH2-terminal fragment as mCherry Ab. As expected, it detected nothing in the COOH-terminal lane. It did, however, detect a lower molecular mass band at 32 kDa in the NH2-terminal lane that was not detected by mCherry Ab. This band is consistent with full-length MLP-1 cleaved just before the mCherry tag after the effector domain (Fig. 5). D: the COOH-terminal antibody also detected the same full-length band, COOH-terminal fragment, and alternative start fragment as mCherry Ab. As expected, it detected nothing in the NH2-terminal lane. It did, however, detect a lower molecular mass band at 33 kDa in the COOH-terminal lane that was not detected by mCherry Ab. This band is consistent with the COOH-terminal fragment cleaved just before the mCherry tag (Fig. 5). Molecular mass is in kilodaltons.
The mCherry antibody also detected the 112-residue NH2-terminal fragment + mCherry at ~51 kDa and the 118-residue COOH-terminal fragment + mCherry at 58 kDa. It also detected a 43-kDa band that is likely produced by an alternative start site corresponding to the methionine at position 131 (a 68-residue fragment + mCherry; Fig. 6B).
The NH2-terminal antibody (Fig. 6C) detected the same full-length band and NH2-terminal fragment as the mCherry antibody. As expected, it detected nothing in the COOH-terminal lane. It did, however, detect a lower molecular mass band at 32 kDa in the NH2-terminal lane that was not detected by mCherry antibody. This band is consistent with full-length MLP-1 cleaved just before the mCherry tag after the effector domain (Figs. 5 and 6C).
The COOH-terminal antibody (Fig. 6D) also detected the same full-length band, COOH-terminal fragment, and alternative start fragment as mCherry antibody. As expected, it detected nothing in the NH2-terminal lane. It did, however, detect a lower molecular mass band at 33 kDa in the COOH-terminal lane that was not detected by mCherry antibody. This band is consistent with the COOH-terminal fragment cleaved just before the mCherry tag (Figs. 5 and 6D). There might be additional cleavage sites near the NH2 or COOH terminus consistent with the cleavage algorithm predictor, but the fragments of these cleavages would generally be too small to easily detect by Western blot. The predicted sizes and comparison with the measured sizes from the Western blots are given in Tables 3–5.
Table 3.
Western blot of full-length MLP-1 + mCherry (lane F), NH2-terminal 112-amino-acid fragment + mCherry (lane N), and COOH-terminal 118-amino-acid fragment + mCherry (lane C)
| mCherry Ab |
||
|---|---|---|
| Lane | Mol Mass, kDa | Comparison Between Measured and Calculated Molecular Masses |
| F | 75.5 | Lane F, band 1 (top): MLP-1 weighs 20.17 kDa; runs at 47.8 kDa + mCherry 76.6 kDa. Compare with measured 75.5 kDa. Conclusion: full length + mCherry. Detected by mCherry Ab, NH2-terminal MLP-1 Ab, and COOH-terminal MLP-1 Ab. |
| N | 50.5 | Lane N, band 1: NH2-terminal fragment is 112 residues. Mol mass 11.5 kDa corrected for effector domain (time 2.37) = 27.3 kDa + mCherry = 56.1 kDa. Compare with measured 50.5 kDa. Conclusion: full-length NH2-terminal fragment + mCherry. Detected by mCherry Ab and NH2-terminal MLP-1 Ab but not COOH-terminal MLP-1 Ab. |
| C | 59.0 | Lane C, band 1: COOH-terminal fragment is 118 amino acids. Mol mass 12.2 kDa; corrected for effector domain should run at 28.9 kDa + mCherry = 57.7 kDa. Compare with measured 59.0 kDa. Conclusion: full-length COOH-terminal fragment with mCherry. Detected by COOH-terminal MLP-1 Ab and mCherry Ab. |
| 43.0 | Lane C, band 2: alternative start site protein might run at 6.8 kDa; corrected to 16.1 kDa + mCherry 44.9 kDa. Compare with measured 43.0 kDa. Detected by COOH-terminal MLP-1 Ab and mCherry Ab. | |
Blotted with anti-mCherry (as in Fig. 7). Ab, antibody; MLP-1, myristoylated alanine-rich protein kinase C substrate-like protein-1.
Table 5.
Western blot of full-length MLP-1 + mCherry (lane F), NH2-terminal 112-amino acid fragment + mCherry (lane N), and COOH-terminal 118-amino acid fragment + mCherry (lane C)
| COOH-Terminal MLP Ab | ||
|---|---|---|
| Lane | Mol Mass, kDa | Comparison Between Measured and Calculated Molecular Masses |
| F | 78.9 | Lane F, band 1 (top): MLP-1 weighs 20.17 kDa; runs at 47.8 kDa + mCherry 76.6 kDa. Compare with measured 78.9 kDa. Conclusion: full length + mCherry. Detected by mCherry Ab, NH2-terminal MLP-1 Ab, and COOH-terminal MLP-1 Ab. |
| 56.6 | Lane F, band 2: MLP-1 weighs 20.17 kDa; runs at 47.8 kDa. mCherry cleaved at last site. Compare with 56.6 kDa. Conclusion: full length. Detected by COOH-terminal MLP-1 Ab. | |
| C | 57.4 | Lane C, band 1: COOH-terminal fragment is 118 residues. Mol mass 12.5 kDa corrected for effector domain (time 2.37) = 27.3 kDa + mCherry = 56.1 kDa. Compare with measured 57.4 kDa. Conclusion: full-length COOH-terminal fragment with mCherry. Detected by COOH-terminal MLP-1 Ab and mCherry Ab. |
| 43.2 | Lane C, band 2: alternative start site protein might run at 6.8 kDa; corrected to 16.1 kDa + mCherry 44.9 kDa. Compare with measured 43.2 kDa. Detected by COOH-terminal MLP-1 Ab and mCherry Ab. | |
| 33.2 | Lane C, band 3: full-length COOH terminus is 118 amino acids = 12.2 kDa; corrected for effector domain should run at 28.9 kDa. Compare with measured 33.2 kDa. Conclusion: full length without mCherry. Detected by COOH-terminal MLP-1 Ab. | |
Blotted with anti-COOH-terminal myristoylated alanine-rich protein kinase C substrate-like protein-1 (MLP-1; as in Fig. 7). NH2-terminal fragment not detected. Ab, antibody.
Table 4.
Western blot of full-length MLP-1 + mCherry (lane F), NH2-terminal 112-amino acid fragment + mCherry (lane N), and COOH-terminal 118-amino acid fragment + mCherry (lane C)
| NH2-Terminal MLP Ab |
||
|---|---|---|
| Lane | Mol Mass, kDa | Comparison Between Measured and Calculated Molecular Masses |
| F | 75.2 | Lane F, band 1 (top): MLP-1 weighs 20.17 kDa; runs at 47.8 kDa + mCherry 76.6 kDa. Compare with measured 75.2 kDa. Conclusion: full length + mCherry. Detected by mCherry Ab, NH2-terminal MLP-1 Ab, and COOH-terminal MLP-1 Ab. |
| N | 53.4 | Lane N, band 1: NH2-terminal fragment is 112 residues. Mol mass 11.5 kDa corrected for effector domain (time 2.37) = 27.3 kDa + mCherry = 56.1 kDa. Compare with measured 53.4 kDa. Conclusion: full-length NH2-terminal fragment + mCherry. Detected by mCherry Ab and NH2-terminal MLP-1 Ab but not COOH-terminal MLP-1 Ab. |
| 31.8 | Lane N, band 2: NH2-terminal fragment is 112 residues. Mol mass 11.5 kDa corrected for effector domain (time 2.37) = 27.3 kDa. mCherry is cleaved. Compare with measured 31.8 kDa. Conclusion: full-length NH2-terminal fragment without mCherry. Detected by NH2-terminal MLP-1 Ab but not COOH-terminal MLP-1 Ab or mCherry Ab. | |
Blotted with anti-NH2-terminal myristoylated alanine-rich protein kinase C substrate-like protein-1 (MLP-1; as in Fig. 7). COOH-terminal fragment not detected. Ab, antibody.
MLP-1 colocalizes with PIP2.
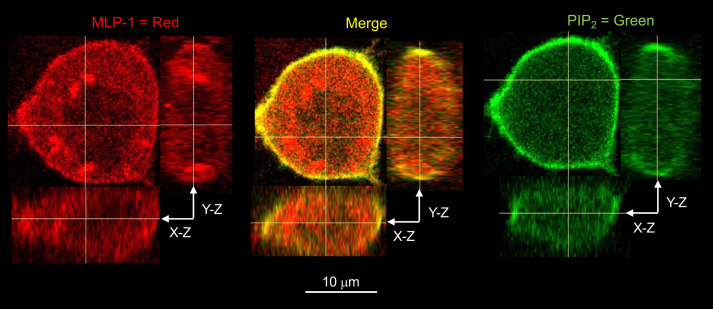
We have proposed that MLP-1 regulates the delivery of inositol lipid phosphates to ENaC and, thereby, increases ENaC open probability (Fig. 3). We and many others have shown that that MLP-1 specifically binds phosphatidylinositol 4,5-bisphosphate (PIP2) at the membranes of epithelial cells (1, 4, 12, 17, 23, 28, 35, 43, 44, 53, 59). We used a PIP2 reporter (GFP-labeled pleckstrin homology domain of PKCδ) and fluorescently labeled anti-MLP-1 to show this colocalization in untransfected DCT-15 cells (Fig. 7).
Fig. 7.
Endogenous myristoylated alanine-rich protein kinase C substrate-like protein-1 (MLP-1) colocalizes with phosphatidylinositol 4,5-bisphosphate (PIP2). We used a PIP2 reporter (green fluorescent protein-labeled pleckstrin homology domain of PKCδ) and fluorescently labeled anti-MLP-1 to show this colocalization in DCT-15 cells (a distal convoluted tubule clonal cell line). On the left, MLP-1 fluorescence is distributed throughout the cell with some concentration at the membrane. There also appears to be some concentration in specific subcellular locations (best seen in the y-z-orthogonal image). On the right, the PIP2 reporter is strongly concentrated in the cell membrane. Signal in other parts of the cell may be the PIP2 degradation product inositol 1,4,5-trisphosphate, which the reporter also detects. In the middle is the merged image that shows that PIP2 and MLP-1 are strongly colocalized at the membrane. This colocalization is visible in the orthogonal views. Colocalization inside of the cell is limited and distinct green and red, but few yellow pixels are visible.
ENaC is associated with MLP-1.
To be most effective, MLP-1 needs to associate with PIP2-rich lipid domains, which it does through its myristoyl modification and association with PIP2, but MLP-1 should also interact directly with ENaC. In fact, it is possible to coimmunoprecipitate β-ENaC with an antibody to MARCKS, a closely related homolog of MLP-1 (1). In addition, fluorescently labeled β-ENaC and MARCKS show significant fluorescence resonance energy transfer (FRET) between β-ENaC and MARCKS implying that the two molecules are in very close association in living cells (1).
Since ENaC is composed of three subunits, the FRET result does not necessarily mean that β-ENaC binds to MARCKS, only that it is close enough for FRET as might be expected if it were part of a trimeric complex with α- and γ-ENaC. It is also possible that MLP-1 and MARCKS do not behave exactly the same. Therefore, we examined direct binding using a Dual-Luciferase mammalian two-hybrid assay in COS-7 cells. We tested the association between MLP-1 and the ENaC subunits (Fig. 8A). The pBIND-ID and pACT-MyoD fusion proteins were used as a positive control to represent maximum binding, whereas the pBIND and pACT empty vectors were used as a negative control. We found strong binding between NH2-terminal MLP-1 and NH2-terminal γ-ENaC relative to the controls. Similarly, there was moderate binding between NH2-terminal MLP-1 and NH2-terminal β-ENaC and between COOH-terminal MLP-1 and NH2-terminal γ-ENaC but not α-ENaC in cells. This is an interesting result since in recent work, Archer et al. (5) described PIP2 binding to the NH2-terminal regions of β- and γ-ENaC.
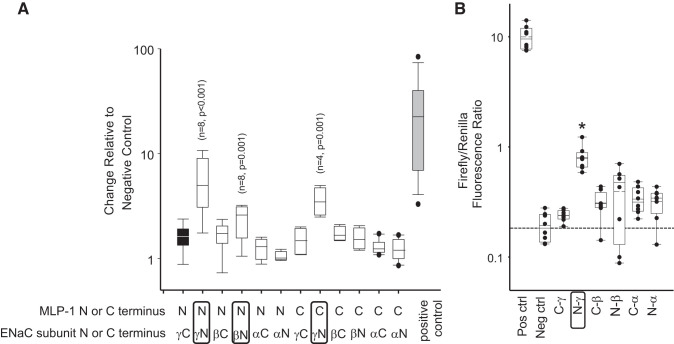
Fig. 8.
Mammalian Dual-Luciferase assay. In A, we found significant binding between NH2 (N)-terminal myristoylated alanine-rich protein kinase C substrate-like protein-1 (MLP-1) and NH2-terminal γ-epithelial sodium channel (ENaC) relative to the negative control (black bar; Kruskal–Wallis 1-way analysis of variance on ranks). Similarly, there was moderate binding between NH2-terminal MLP-1 and NH2-terminal β-ENaC and between COOH (C)-terminal MLP-1 and NH2-terminal γ-ENaC but neither NH2- nor COOH-terminal α-ENaC in cells. In B, we examine binding of the multiple-homology domain 2 (residues 18–62 in MLP-1; Fig. 1) to cytosolic NH2- and COOH-terminal fragments of ENaC. Only NH2-terminal γ-ENaC bound significantly (*P < 0.001, Kruskal–Wallis 1-way analysis of variance on ranks; n = 6). Neg ctrl, negative control; Pos ctrl, positive control.
The specific ENaC binding domain in MLP-1.
We wanted to narrow down ENaC’s binding region in MLP-1. We were particularly interested in the multiple-homology 2 domain, which has generally been considered to be of unknown function. We generated a two-hybrid prey vector from residues 18 to 62 that includes the MH-2 domain and used it in our Luciferase 2-hybrid system with COOH- and NH2-terminal ENaC subunit as bait. In this assay, only the NH2-terminal region of γ-ENaC bound with significantly greater affinity than the negative control (Fig. 8B).
Differential cellular distribution of MLP-1 mutants.
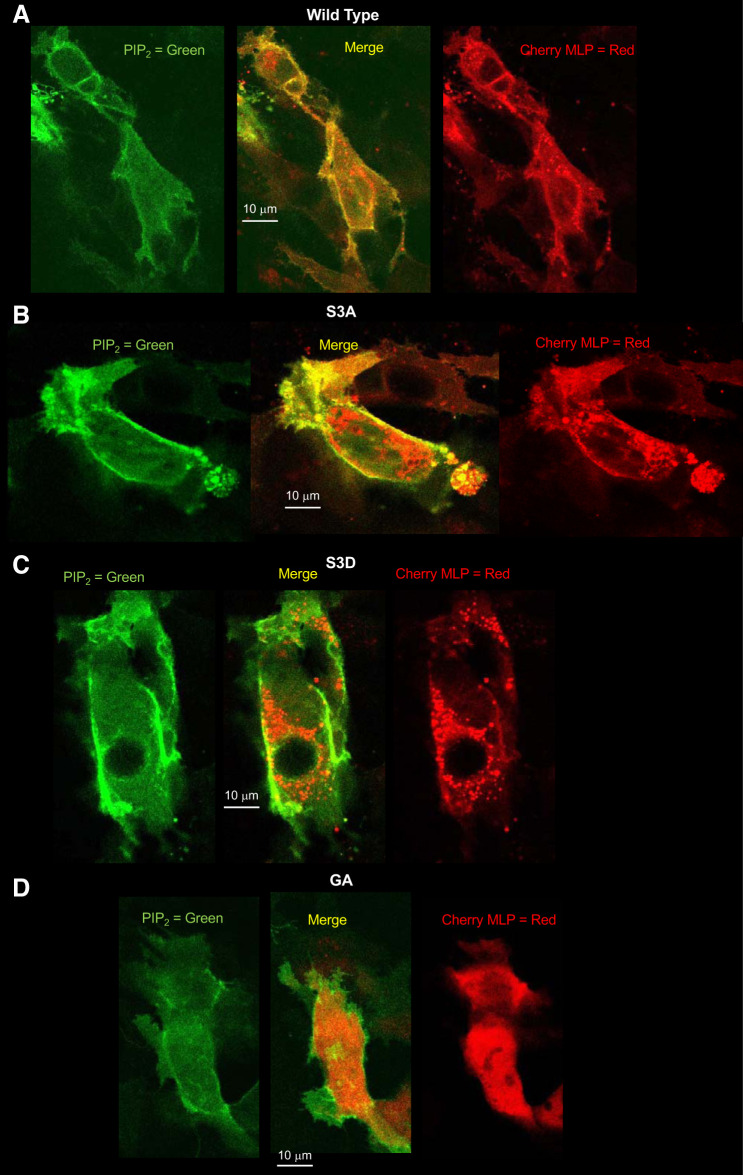
The high density of positive charge in the effector domain of MLP-1 is known to bind PIP2, but the binding can be reversed by phosphorylation of the serine residues in the effector domain. Phosphorylation also destabilizes the association of MLP-1 with the membrane and causes MLP-1 to enter the cytosol. If this scenario is correct, we would expect that the S3A construct, which cannot be phosphorylated, should be predominantly or wholly at the apical membrane. The wild-type construct will distribute between the membrane and the cytosol. S3D, which acts as if it is always phosphorylated, should be mostly in the cytosol. GA, the construct incapable of myristoylation, might not bind the membrane as strongly as wild type even though it is still capable of binding PIP2, so its localization is unclear. To help examine the localization of the constructs, we transfected DCT-15 cells with a PIP2 reporter and each of the MLP-1 constructs and then used multiphoton microscopy to produce z-axis stacks of the transfected cells (Fig. 9, A–D).
Fig. 9.
A–D: differential cellular distribution of myristoylated alanine-rich protein kinase C substrate-like protein-1 (MLP-1) mutants. To examine the cellular localization of the different MLP-1 constructs, we transfected DCT-15 cells (a distal convoluted tubule clonal cell line) with a phosphatidylinositol 4,5-bisphosphate (PIP2) reporter and each of the MLP-1 constructs and then used multiphoton microscopy to produce z-axis stacks of the transfected cells. A: an examination of the merged image of the wild-type construct shows that MLP-1 strongly colocalizes with PIP2 (yellow pixels at the membrane); however, quantitative analysis with ImageJ (Coloc 2 module) shows that only 25% of pixels in the image colocalize. Examination of green pixels shows that 64% colocalize with red pixels, but 62% of red pixels do not colocalize with green pixels. This is consistent with the prediction that most green pixels (PIP2) are at the membrane and many of them are associated with red pixels (wild-type MLP-1), but there are many red pixels in the cytosol. B: for S3A, 58% of all pixels colocalize. Ninety-nine percent of green pixels are associated with red pixels, and 96% of red pixels are associated with green pixels. This is consistent with the S3A mutant being predominantly associated with the membrane with little in the cytosol compared with wild-type MLP-1. C: for S3D, only 13% of red and green pixels are colocalized with 71% of green pixels unassociated with red pixels. This reflects the distribution of most of S3D in the cytosol with only a small fraction associated with the membrane. D: the GA construct has 22% green and red colocalized pixels. This is close to the colocalization of the wild-type construct, implying that the myristoylation does not strongly influence MLP-1 association with the membrane. Images are typical of 3 similar experiments.
An examination of the merged image of the wild-type construct shows that MLP-1 strongly colocalizes with PIP2 (yellow pixels at the membrane); however, quantitative analysis with ImageJ (Coloc 2 module) shows that only 25% of pixels in the image colocalize. Examination of green pixels shows that 64% colocalize with red pixels, but 62% of red pixels do not colocalize with green pixels. This is consistent with the prediction that most green pixels (PIP2) are at the membrane and many of them are associated with red pixels (wild-type MLP-1), but there are many red pixels in the cytosol. For S3A, 58% of all pixels colocalize. Ninety-nine percent of green pixels are associated with red pixels, and 96% of red pixels are associated with green pixels. This is consistent with the S3A mutant being predominantly associated with the membrane with little in the cytosol compared with wild-type MLP-1. For S3D, only 13% of red and green pixels are colocalized with 71% of green pixels unassociated with red pixels. This reflects the distribution of most of S3D in the cytosol with only a small fraction associated with the membrane. The GA construct has 22% green and red colocalized pixels. This is close to the colocalization of the wild-type construct implying that the myristoylation does not strongly influence MLP-1 association with the membrane. This is consistent with one report that myristoylation of MLP-1 does not dramatically alter its association with the membrane; i.e., the PIP2 electrostatic interaction provides stronger membrane stabilization (47).
The quantitative analysis of the various constructs is consistent with our expectations for their behavior of the different constructs. However, there are several qualitative aspects of images that we noted. In every case in which the effector domain was modified, the MLP-1 present in the cytosol appeared to aggregate. This is especially noticeable for S3D, where the cytosolic MLP-1 collects in grapelike structures in the cytosol. Even in the wild type, there appear to be punctate distributions of the cherry fluorescence (visible best in the serial mosaic). Only the GA construct appears to have a more uniform distribution in the cytosol.
Phorbol esters increase MLP-1 phosphorylation and inhibit ENaC.
Many investigators have shown that endogenous MLP-1 is a target for protein kinase C phosphorylation (8, 11, 29, 36, 53, 56). We applied 100 nM phorbol myristoyl acetate to activate protein kinase C and prepared cell lysates. Western blots (Fig. 10A) of the lysates showed a strong band at 37 kDa, which is characteristic of MLP-1 (8). Just above it is the nonphosphorylated band, which is expected to migrate more slowly on the SDS gel (10, 11). PMA reduces the density of the unphosphorylated band and increases the density of the phosphorylated band (Fig. 10B). As control, we also applied an inactive phorbol, which does not change the relative density of the phosphorylated and nonphosphorylated bands. Other investigators have shown that activation of protein kinase C strongly inhibits ENaC (52, 63). We also used single-channel methods to examine principal cells in isolated, split-open collecting ducts bathed in the same saline that we used to obtain Fig. 10, A and B. As expected, addition of PMA rapidly reduced ENaC channel activity. (Fig. 10, C and D).
DISCUSSION
It has been known for a number of years that ENaC could be activated by phosphatidylinositol 4,5-bisphosphate (PIP2) on the cytosolic surface of channels in excised, inside out patches (31, 39, 43, 44, 55, 64). Ma et al. (31) found that the regulation of ENaC by PIP2 and phosphatidylinositol 3,4,5-trisphosphate (PIP3) did not involve a change in surface expression of ENaC and occurred through a mechanism independent of ENaC trafficking; i.e., it was a result of a change in ENaC open probability. This was consistent with the channel open probability changes shown in Fig. 3 without a change in channel density or total ENaC expression in Western blots. The implication of these electrophysiological experiments is that PIP2 must interact with one or more of the ENaC subunits. In fact, anti-PIP2 antibody coimmunoprecipitates β- and γ-, but not α-, ENaC subunits (64). Other anionic lipids also seemed capable of activating ENaC (but to a lesser extent than PIP2; Ref. 31). Subsequently, two binding sites for PIP2 were identified at the NH2 terminus of β- and γ-ENaC (30) and one site for PIP3 on γ-ENaC (21). The same work also showed that all of the early aldosterone-induced increase in sodium transport was due to increases in inositol lipid phosphate binding. Other investigators have also described additional sites for PIP2 and PIP3 binding to the subunits (5, 41–44, 51), but the NH2-terminal sites appear to be the critical sites for PIP2 regulation of ENaC.
MLP-1 promotes ENaC-PIP2 interaction.
As mentioned in the introduction, PIP2 is necessary to open ENaC. However, there is a conceptual problem with a simple model of ENaC and PIP2 associating by simple lateral diffusion in the membrane. ENaC is a relatively rare protein (only a few functional channels per square micrometer in the apical membrane). PIP2 is also a rare molecule constituting <1 in 1,000 membrane lipid molecules (22). In Ref. 24, the authors calculated the average time it would take a PIP2 molecule to find an ENaC channel by random lateral diffusion given the know abundance of PIP2 and ENaC and the diffusion constant of PIP2 in the apical membrane as 6.3 × 102 s or approximately once in 10 min. Figure 3 shows that for cells with wild-type MLP-1, the channel opens every few seconds. MLP-1 sequesters PIP2 electrostatically, that is, there is no direct covalent binding (17). This implies interesting differences between PIP2 association with MLP-1 and the binding of a more typical ligand to a specific receptor. First, the effect of MLP-1 charge acts at a distance. The coulombic force exerted by MLP-1 on PIP2 does decrease as the square of the distance between MLP-1 effector domain and PIP2, but the distances within the membrane are relatively small and the membrane dielectric constant is also very small, so the forces can be significant over a large membrane area. In the absence of MLP-1, PIP2 moves around the membrane lipid in a two-dimensional random walk, but the presence of the charged effector domain biases the walk so that PIP2 always moves toward MLP-1, at first slowly and then rapidly until it is in MLP-1’s potential well. This implies that MLP-1 is increasing the local concentration of PIP2 exponentially as predicted by electrostatic theory (35, 59, 60). MLP-1 membrane concentration should be near 10 μM, which is comparable with the concentration of PIP2 (35). Given the lateral mobility of PIP2 in the membrane of ~10−8 cm2/s (20, 58), MLP-1 would rapidly sequester most of the membrane PIP2. Others have measured the amount of sequestered PIP2 associated with the effector domain of MARCKS, which has a very similar charge structure to MLP-1, and found that approximately two-thirds of all of the PIP2 in the membrane is associated with the effector domain (59). This would increase the local PIP2 concentration over a hundredfold. Since our data show that ENaC binds MLP-1 in the MH-2 domain, this increased concentration of PIP2 would be in close proximity to PIP2-binding sites on the cytosolic NH2-terminal regions of ENaC. This binding is presumably also electrostatic since the binding sites contain multiple basic residues (5, 31, 40, 42). The advantage of electrostatic interaction is that PIP2 can continue to remain in close proximity to both ENaC and MLP-1 (17).
The second difference between ligand binding to a receptor and electrostatic binding is that electrostatic binding is not stoichiometric. It is true that the effector domain of MLP-1 does contain three regions of focal positive charge and, on average, each region associates with one PIP2 molecule, but, because the effector domain charge can be felt over a large area, the effector domain can associate with variable numbers of PIP2. Therefore, to discuss a specific binding constant is somewhat misleading. Rather, what is more appropriate is to use the apparent binding constant as a measure of the energy well represented by the effector domain of MLP-1. This is the amount of energy necessary to move one PIP2 from close proximity to MLP-1 to free diffusion in the membrane lipid domain. Nonetheless, the PIP2 binding constant to the effector domain has been variously reported from 10 to 30 μM. If we choose a dissociation constant of ~15 μM, the MLP-1 energy well would be between 6 and 6.5 kcal/mol. If the PIP2 associated with MLP-1 is to activate ENaC, then PIP2 must gain enough energy to climb out MLP-1’s potential well and rapidly associate with ENaC. If association of PIP2 causes ENaC to open, then the rate at which PIP2 leaves MLP-1 should be related to the opening rate of ENaC.
ENaC is associated with MLP-1.
Our experiments support several aspects of this model of MLP-1-ENaC-PIP2 interaction. To be most effective, MLP-1 needs to associate with both PIP2 and ENaC. We and others have previously shown and we, herein, show again (Fig. 7) that MLP-1 associates with PIP2 (1–4, 12, 18, 23, 35, 59, 61), but MLP-1 should also interact directly with ENaC to promote PIP2-ENaC interaction.
We were interested in a recent paper that describes PIP2-binding sites in β- and γ-ENaC (5) that corresponded to the regions of ENaC we found that bound MLP-1 in our two-hybrid assay. In this paper, Archer et al. (5) identified two PIP2-binding sites in the cytosolic NH2-terminal domains with apparent dissociation constants of 5 μM (β-ENaC) and 13 μM (γ-ENaC). This new information coupled with information we already know about MLP-1 and that we have obtained in this paper allows us to make some quantitative predictions about ENaC activity and channel kinetics. The authors suggest that ENaC interaction with PIP2 is also via electrostatic binding. Again, the apparent dissociation constants imply potential energy wells associated with β- and γ-ENaC of 7.2 and 6.7 kcal/mol, respectively. The rate of PIP2 leaving the energy wells should be proportional to the closing rate of ENaC. Determining the rate of PIP2 leaving ENaC or MLP-1 depends on recognizing that the dissociation constant, Kd, is the ratio of the off rate (dissociation) and the on rate (association; Fig. 11). We assumed that the on rate for PIP2 associating with ENaC or MLP-1 was diffusion limited. The diffusion-limited rate is proportional to the diffusion constant. In water, the mean rate of eight diffusion-limited reactions is 10.9 ± 3.1 × 107 mol·L−1·s−1 (14). The diffusion constant of small solutes in water is 10−5 cm2/s. The diffusion constant for PIP2 near MLP-1 is reported to be 0.8 ± 0.2 × 10−8 cm2/s and for the free movement of PIP2 far away from MLP-1 in the plasma membrane is 2.5 ± 0.8 × 10−8 cm2/s, implying that diffusion-limited rate of PIP2 should be between 8.7 × 104 and 27 × 104 mol·L−1·s−1. The off rate for the PIP2 association site on β-ENaC is the product of the dissociation constant and the rate of PIP2 free diffusion; that is between 0.44 and 1.4 s−1. The mean open time, τo, is the reciprocal of the off rate or between 2.3 and 0.71 s. A similar calculation can be made for the γ-ENaC association site and gives values for the off rate between 1.1 and 5.1 s−1 with τo values between 0.91 and 0.20 s.
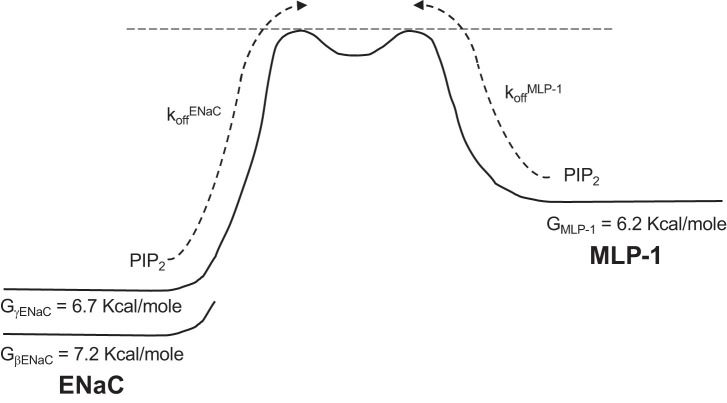
Fig. 11.
Schematic of the energy wells and kinetics for myristoylated alanine-rich protein kinase C substrate-like protein-1 (MLP-1) and epithelial sodium channel (ENaC). This shows theoretical Gibbs Free energy wells (G) for phosphatidylinositol 4,5-bisphosphate (PIP2) associated with β- or γ-ENaC on the left and for MLP-1 on the right. The rates for PIP2 dissociation from ENaC and MLP-1 are indicated by dotted arrows. A complete description of the model is in the main text.
In the reverse direction for transitions of PIP2 from MLP-1 to ENaC, the forward rate will be between 1.3 and 4.0 s−1, implying a mean closed time between 0.35 and 0.77 s. A schematic diagram of the ENaC-MLP-1 interaction is shown in Fig. 11.
The model we describe presumes that MLP-1 is always available to exchange PIP2 with ENaC. This means that the mutant S3A, which is always associated with the membrane, is the most appropriate subject for comparison with our calculations. From our single-channel data, we determined that the mean open and closed time for S3A is 539 ± 147 and 532 ± 89.6 ms, respectively. The open time is within the expected range for γ-ENaC but slightly outside of the expected range for β-ENaC. The closed time is in the middle of the calculated range of our model.
The relationship of the other MLP-1 constructs to our model is less clear since we do not know how much time the modified MLP-1 molecules are associated with the membrane, but we would expect that wild type would have reduced mean open time and increased closed time compared with S3A and that S3D would have very short mean open time and long closed time. In fact, as we expected, the mean open and closed times of wild-type MLP-1 were 182 ± 35.8 and 2,730 ± 1,264 ms, respectively; the mean open and closed times of S3D were 81.9 ± 16.9 and 2,330 ± 803 ms, respectively.
If the mean open time is a measure of the availability of MLP-1 to deliver PIP2 to ENaC, then the mean open time of ENaC provides an estimate of the residency time of MLP-1 at the membrane. S3A is always present, but based on the mean open time of cells transfected with the wild-type construct, it appears that wild-type MLP-1 is only associated with the membrane ~34% of the time. This is actually consistent with reports of constitutive PKC activity in renal cells that has been reported to reduce ENaC activity (7, 63) but that we would suggest reduces MLP-1 residency and, thereby, reduces ENaC open probability. We presume that the very low open probability of S3D is not because S3D never associates with the membrane but rather that there is a very small amount of endogenous MLP-1 that activates some ENaC.
The GA mutant is more of an anomaly. Although one would expect that removal of a myristoyl group would reduce targeting to the membrane, the activity compared with wild type is similar. One paper has reported that demyristoylation of MARCKS does not affect MARCKS association with the membrane (47). The homology at the NH2 terminus and in the effector domain of MLP-1 and MARCKS makes it seem likely that demyristoylation of MLP-1 also does not dramatically affect its membrane association. We hypothesize that the myristoyl group is important for membrane targeting like other myristoylated proteins (37), but once at the membrane, the energy of association with PIP2 and especially the hydrophobic phenylalanines in the effector domain is more important for MLP-1 association than the myristoyl group (17). On the other hand, the channel kinetics of the different mutants is different despite the open probability of GA and wild type being similar. Both the mean open and mean closed times of wild-type MLP-1 are relatively short, whereas the mean open time of GA is longer and the mean closed time is much longer, leading to an open probability similar to wild type. However, we interpret this to mean that when GA is associated with and embedded in the membrane that it is relatively stable; however, when it leaves the membrane, it no longer has the initial stabilization energy of the myristoyl group to re-enter the membrane and, therefore, remains away from the membrane longer (long mean closed time). The overexpression of all of the mutants disguises the importance of ordinary myristoylation in membrane trafficking to the membrane.
The movement of PIP2 between ENaC and MLP-1 can be thought of as an equilibrium reaction:
with an equilibrium constant, kPIP2, with forward rate k1 and a reverse rate, k–1. Of course, the rate constants are the ones we derived above for PIP2 leaving ENaC or leaving MLP-1. Using the rate constant for PIP2 leaving β-ENaC and the rate for PIP2 leaving MLP-1 gives a dissociation constant between 2.8 and 3.0; the γ-ENaC rate constant gives a value between 0.86 and 1.3. The implication of these numbers is that ENaC and MLP-1 share the PIP2 that MLP-1 has captured from the membrane at large almost equally. It also means that ENaC and MLP-1 are close enough together that they share a common charge cloud in which PIP2 moves easily between them. The effector domain of MLP-1 has a linear structure and, due to the strongly hydrophobic phenylalanines in the domain, is embedded in the membrane at the level of phospholipid head groups with the positive charge residues at or near the surface of the membrane (17). This likely leads to an extended structure of the β- and γ- NH2-terminal ENaC domains along the inner surface of the membrane with the positively charged residues associating with PIP2 in the positively charged electrostatic cloud surrounding MLP-1 and ENaC. Although we described the rate of PIP2 leaving β- or γ-ENaC, a more realistic picture might be the NH2-terminal tails leaving PIP2 and moving into or out of close association with MLP-1 and membrane-bound PIP2; that is, moving from the membrane surface to the cytosol, which would coincide with channel opening and closing.
There is some prior evidence for ENaC interaction with MARCKS, a member of the same protein family as MLP-1. Alli et al. (1) showed that ENaC-yellow fluorescent protein and MARCKS-cyan fluorescent protein constructs were close enough to one another to allow fluorescence resonance energy transfer (FRET), implying that MARCKS and ENaC were very close, ~6.5 nm. We were interested in which regions of MLP-1 interacted with ENaC, so we used a Dual-Luciferase system to construct six bait constructs, three from the cytosolic NH2 termini and three from the cytosolic COOH termini of the three ENaC subunits, and two prey constructs from NH2- and COOH-terminal regions of MLP-1. We found a strong interaction between the NH2-terminal region of MLP-1 and the NH2 terminus of γ-ENaC and weaker interactions with the NH2-terminal region of MLP-1 and the NH2 terminus of β-ENaC and COOH-terminal region of MLP-1 and the NH2 terminus of γ-ENaC (Fig. 8A). If the purpose of the interaction is to bring the PIP2-binding regions in the effector domain of MLP-1 into close proximity of the PIP2-binding regions of ENaC, then interactions of the NH2-terminal domains of β- and γ-ENaC with the NH2 terminus of MLP-1 are consistent with the known PIP2-binding regions in ENaC (40, 42–44, 51) and consistent with the newly reported binding regions (5).
One region of MLP-1 is the multiple-homology domain (MH-2) in the NH2-terminal region of MLP-1, a region that previously had no known function (8, 29). We developed a prey construct from this region and showed that it only strongly interacted with the NH2 terminus of γ-ENaC (Fig. 8B). This suggests that the MH-2 is critical for MLP-1-ENaC interaction and MLP-1’s delivery of PIP2 to ENaC. Once the MH-2 domain bound to NH2-terminal γ-ENaC, the electrostatic potential of the PIP2 associated with MLP-1 effector domain (59, 60) could alter the conformation of the cytosolic domains of ENaC to promote a channel open state.
Proteolytic cleavage of MLP-1.
There are many reports of proteolytic cleavage of MARCKS by the protease calpain (13, 27, 38), but there have been few reports of calpain cleavage of MLP-1. Nonetheless, the similarity in sequence and properties of MARCKS and MLP-1 made it seem likely that MLP-1 would also be cleaved by calpain. We used a predictive algorithm (57) to determine the most likely cleavage points in the MLP-1 sequence. Table 2 lists and Fig. 5 shows the location of the five highest probability cleavage sites. Of the five, two were near the NH2 terminus, two were near the COOH terminus, and one was near the middle of the sequence just COOH-terminal to the effector domain. We had gone out of our way to tag MLP-1 with COOH- or NH2-terminal epitopes, but the four cleavage sites near the end of the sequence effectively removed these tags, so the remaining molecule could not be detected with anti-epitope antibodies in Western blots. Fortunately, we did have available anti-MLP-1 antibodies for which epitopes were not removed by cleavage. This allowed us to identify cleavage fragments that corresponded to the likely cleavage sites (Fig. 6), explaining some of the lower molecular mass bands in Western blots of MLP-1. The NH2-terminal domains of MARCKS and MLP-1 are highly homologous until the effector domain, after which the sequences begin to diverge with MARCKS being >100 amino acids longer. Both sequences are predicted to have a cleavage site shortly after the effector domain, and Montgomery et al. (38) have reported that the cleavage increases MARCKS’ ability to associate with the membrane and activate ENaC. Such activation may also be true for MLP-1.
MLP-1 association with apical membrane and PIP2 is regulated.
Besides sequestering and acting as a source of PIPs at the cytosolic surface of the apical membrane, MLP-1 can also translocate from its association with the apical membrane to the cytosol, after which it no longer sequesters PIP2 (23, 33, 34). Thus MLP-1-mediated delivery of PIP2 to ENaC can be regulated by controlling MLP-1’s translocation. There are several mechanisms that can promote MLP-1 translocation. This is significant since it shows that there is a new mechanism for altering ENaC activity. The PKC-mediated phosphorylation of serines within the basic effector domain displaces MLP-1 from the cytoplasmic face of the plasma membrane since the extra negative charges reduce the strength of the electrostatic interactions between negative head groups on phospholipids and the cationic residues in the effector domain. If phosphorylation reduces MLP-1 association with the membrane, and, thereby, produces a reduction in the ability of MLP-1 to present PIP2 to ENaC with a concomitant reduction in ENaC activity, then removal of PKC activity should strongly increase ENaC activity by increasing open probability. In isolated, split-open tubules from PKCα knockout mice, the open probability of ENaC is higher in knockout mice than in wild-type mice. This increase in ENaC activity is associated with a substantial increase in the blood pressure of knockout mice (7). In contrast, activation of PKC is known to reduce ENaC open probability (Fig. 10; Refs. 9, 16, 52, 63), but PKC does not inhibit ENaC in excised patches (62), implying that it must be phosphorylating an ENaC regulatory protein, presumably MLP-1. In fact, phorbol activation of PKC increases phosphorylated and decreases unphosphorylated MLP-1 (Fig. 10, A and B). What was surprising to us was that in the Western blot most of the MLP-1 appeared to be phosphorylated, i.e., most of the MLP-1 is in the cytosol and only a small fraction was unphosphorylated and presumably associated with the apical membrane. Our previous work (48) showed that DCT-15 cells had small but measurable amiloride-sensitive currents (2.87 ± 0.237 μA, n = 36), showing that these cells contain functional ENaC that have relatively low open probability.
Summary of a model for MLP-1 regulation of ENaC and sodium transport.
First, to be open, ENaC must be associated with an inositol lipid phosphate (e.g., PIP2). Both PIP2 and ENaC are stabilized in the membrane by MLP-1 associated with the inner lipid leaflet of the plasma membrane. Under appropriate conditions (e.g., PKC activation), MLP-1 is phosphorylated as shown by our results after PKC stimulation, and, thereafter, MLP-1 can move from association with the membrane lipid into the cytosol as shown in our confocal images. MLP-1 translocation from the membrane results in it no longer stabilizing the PIP2-ENaC complex, allowing PIP2 to be hydrolyzed (18, 34) and ENaC to be ubiquitinated and internalized. Besides phosphorylation, MLP-1 association with ENaC can be regulated by proteolysis either positively (38) or negatively (49).
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-110409 to D. C. Eaton and National Institute of Diabetes and Digestive and Kidney Diseases Grant K01 DK-115660 and an American Society of Nephrology Gottschalk Award to B. M. Wynne.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.S., L.W., and D.C.E. conceived and designed research; C.S., Q.Y., A.M., O.A.-K., B.M.W., and D.C.E. performed experiments; C.S., Q.Y., A.M., B.M.W., and D.C.E. analyzed data; C.S., O.A.-K., B.M.W., H.M., and D.C.E. interpreted results of experiments; C.S. and D.C.E. prepared figures; C.S. and D.C.E. drafted manuscript; C.S., B.M.W., H.M., and D.C.E. edited and revised manuscript; C.S., O.A.-K., B.M.W., H.M., L.W., and D.C.E. approved final version of manuscript.
REFERENCES
- 1.Alli AA, Bao HF, Alli AA, Aldrugh Y, Song JZ, Ma HP, Yu L, Al-Khalili O, Eaton DC. Phosphatidylinositol phosphate-dependent regulation of Xenopus ENaC by MARCKS protein. Am J Physiol Renal Physiol 303: F800–F811, 2012. doi: 10.1152/ajprenal.00703.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbuzova A, Murray D, McLaughlin S. MARCKS, membranes, and calmodulin: kinetics of their interaction. Biochim Biophys Acta 1376: 369–379, 1998. doi: 10.1016/S0304-4157(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 3.Arbuzova A, Wang J, Murray D, Jacob J, Cafiso DS, McLaughlin S. Kinetics of interaction of the myristoylated alanine-rich C kinase substrate, membranes, and calmodulin. J Biol Chem 272: 27167–27177, 1997. doi: 10.1074/jbc.272.43.27167. [DOI] [PubMed] [Google Scholar]
- 4.Arbuzova A, Wang L, Wang J, Hangyás-Mihályné G, Murray D, Honig B, McLaughlin S. Membrane binding of peptides containing both basic and aromatic residues. Experimental studies with peptides corresponding to the scaffolding region of caveolin and the effector region of MARCKS. Biochemistry 39: 10330–10339, 2000. doi: 10.1021/bi001039j. [DOI] [PubMed] [Google Scholar]
- 5.Archer CR, Enslow BT, Carver CM, Stockand JD. Phosphatidylinositol 4,5-bisphosphate directly interacts with the β and γ subunits of the sodium channel ENaC. J Biol Chem 295: 7958–7969, 2020. doi: 10.1074/jbc.RA120.012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auberson M, Hoffmann-Pochon N, Vandewalle A, Kellenberger S, Schild L. Epithelial Na+ channel mutants causing Liddle’s syndrome retain ability to respond to aldosterone and vasopressin. Am J Physiol Renal Physiol 285: F459–F471, 2003. doi: 10.1152/ajprenal.00071.2003. [DOI] [PubMed] [Google Scholar]
- 7.Bao HF, Thai TL, Yue Q, Ma HP, Eaton AF, Cai H, Klein JD, Sands JM, Eaton DC. ENaC activity is increased in isolated, split-open cortical collecting ducts from protein kinase Cα knockout mice. Am J Physiol Renal Physiol 306: F309–F320, 2014. doi: 10.1152/ajprenal.00519.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackshear PJ, Verghese GM, Johnson JD, Haupt DM, Stumpo DJ. Characteristics of the F52 protein, a MARCKS homologue. J Biol Chem 267: 13540–13546, 1992. [PubMed] [Google Scholar]
- 9.Booth RE, Stockand JD. Targeted degradation of ENaC in response to PKC activation of the ERK1/2 cascade. Am J Physiol Renal Physiol 284: F938–F947, 2003. doi: 10.1152/ajprenal.00373.2002. [DOI] [PubMed] [Google Scholar]
- 10.Buehl CJ, Deng X, Liu M, McAndrew MJ, Hovde S, Xu X, Kuo MH. Resolving acetylated and phosphorylated proteins by neutral urea Triton-polyacrylamide gel electrophoresis: NUT-PAGE. Biotechniques 57: 72–80, 2014. doi: 10.2144/000114197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang S, Hemmings HC Jr, Aderem A. Stimulus-dependent phosphorylation of MacMARCKS, a protein kinase C substrate, in nerve termini and PC12 cells. J Biol Chem 271: 1174–1178, 1996. doi: 10.1074/jbc.271.2.1174. [DOI] [PubMed] [Google Scholar]
- 12.Denisov G, Wanaski S, Luan P, Glaser M, McLaughlin S. Binding of basic peptides to membranes produces lateral domains enriched in the acidic lipids phosphatidylserine and phosphatidylinositol 4,5-bisphosphate: an electrostatic model and experimental results. Biophys J 74: 731–744, 1998. doi: 10.1016/S0006-3495(98)73998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dulong S, Goudenege S, Vuillier-Devillers K, Manenti S, Poussard S, Cottin P. Myristoylated alanine-rich C kinase substrate (MARCKS) is involved in myoblast fusion through its regulation by protein kinase Calpha and calpain proteolytic cleavage. Biochem J 382: 1015–1023, 2004. doi: 10.1042/BJ20040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fersht A. Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding. New York: W. H. Freeman, 1999, p. 166. [Google Scholar]
- 15.Frindt G, Palmer LG. Acute effects of aldosterone on the epithelial Na channel in rat kidney. Am J Physiol Renal Physiol 308: F572–F578, 2015. doi: 10.1152/ajprenal.00585.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frindt G, Palmer LG, Windhager EE. Feedback regulation of Na channels in rat CCT. IV. Mediation by activation of protein kinase C. Am J Physiol Renal Physiol 270: F371–F376, 1996. doi: 10.1152/ajprenal.1996.270.2.F371. [DOI] [PubMed] [Google Scholar]
- 17.Gambhir A, Hangyás-Mihályné G, Zaitseva I, Cafiso DS, Wang J, Murray D, Pentyala SN, Smith SO, McLaughlin S. Electrostatic sequestration of PIP2 on phospholipid membranes by basic/aromatic regions of proteins. Biophys J 86: 2188–2207, 2004. doi: 10.1016/S0006-3495(04)74278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glaser M, Wanaski S, Buser CA, Boguslavsky V, Rashidzada W, Morris A, Rebecchi M, Scarlata SF, Runnels LW, Prestwich GD, Chen J, Aderem A, Ahn J, McLaughlin S. Myristoylated alanine-rich C kinase substrate (MARCKS) produces reversible inhibition of phospholipase C by sequestering phosphatidylinositol 4,5-bisphosphate in lateral domains. J Biol Chem 271: 26187–26193, 1996. doi: 10.1074/jbc.271.42.26187. [DOI] [PubMed] [Google Scholar]
- 19.Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature 356: 618–622, 1992. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- 20.Haugh JM, Codazzi F, Teruel M, Meyer T. Spatial sensing in fibroblasts mediated by 3′ phosphoinositides. J Cell Biol 151: 1269–1280, 2000. doi: 10.1083/jcb.151.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helms MN, Liu L, Liang YY, Al-Khalili O, Vandewalle A, Saxena S, Eaton DC, Ma HP. Phosphatidylinositol 3,4,5-trisphosphate mediates aldosterone stimulation of epithelial sodium channel (ENaC) and interacts with gamma-ENaC. J Biol Chem 280: 40885–40891, 2005. doi: 10.1074/jbc.M509646200. [DOI] [PubMed] [Google Scholar]
- 22.Ingólfsson HI, Melo MN, van Eerden FJ, Arnarez C, Lopez CA, Wassenaar TA, Periole X, de Vries AH, Tieleman DP, Marrink SJ. Lipid organization of the plasma membrane. J Am Chem Soc 136: 14554–14559, 2014. doi: 10.1021/ja507832e. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Blackshear PJ, Johnson JD, McLaughlin S. Phosphorylation reverses the membrane association of peptides that correspond to the basic domains of MARCKS and neuromodulin. Biophys J 67: 227–237, 1994. doi: 10.1016/S0006-3495(94)80473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleyman TR, Eaton DC. Regulating ENaC’s gate. Am J Physiol Cell Physiol 318: C150–C162, 2020. doi: 10.1152/ajpcell.00418.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko B, Mistry AC, Hanson L, Mallick R, Wynne BM, Thai TL, Bailey JL, Klein JD, Hoover RS. Aldosterone acutely stimulates NCC activity via a SPAK-mediated pathway. Am J Physiol Renal Physiol 305: F645–F652, 2013. doi: 10.1152/ajprenal.00053.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kucher V, Boiko N, Pochynyuk O, Stockand JD. Voltage-dependent gating underlies loss of ENaC function in pseudohypoaldosteronism type 1. Biophys J 100: 1930–1939, 2011. doi: 10.1016/j.bpj.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lampe WR, Park J, Fang S, Crews AL, Adler KB. Calpain and MARCKS protein regulation of airway mucin secretion. Pulm Pharmacol Ther 25: 427–431, 2012. doi: 10.1016/j.pupt.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laux T, Fukami K, Thelen M, Golub T, Frey D, Caroni P. GAP43, MARCKS, and CAP23 modulate PI(4,5)p2 at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J Cell Biol 149: 1455–1472, 2000. doi: 10.1083/jcb.149.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Aderem A. MacMARCKS, a novel member of the MARCKS family of protein kinase C substrates. Cell 70: 791–801, 1992. doi: 10.1016/0092-8674(92)90312-Z. [DOI] [PubMed] [Google Scholar]
- 30.Ma HP, Chou CF, Wei SP, Eaton DC. Regulation of the epithelial sodium channel by phosphatidylinositides: experiments, implications, and speculations. Pflugers Arch 455: 169–180, 2007. doi: 10.1007/s00424-007-0294-3. [DOI] [PubMed] [Google Scholar]
- 31.Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC). J Biol Chem 277: 7641–7644, 2002. doi: 10.1074/jbc.C100737200. [DOI] [PubMed] [Google Scholar]
- 32.Martín-Belmonte F, Puertollano R, Millán J, Alonso MA. The MAL proteolipid is necessary for the overall apical delivery of membrane proteins in the polarized epithelial Madin-Darby canine kidney and fischer rat thyroid cell lines. Mol Biol Cell 11: 2033–2045, 2000. doi: 10.1091/mbc.11.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLaughlin S, Aderem A. The myristoyl-electrostatic switch: a modulator of reversible protein-membrane interactions. Trends Biochem Sci 20: 272–276, 1995. doi: 10.1016/S0968-0004(00)89042-8. [DOI] [PubMed] [Google Scholar]
- 34.McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438: 605–611, 2005. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin S, Wang J, Gambhir A, Murray D. PIP2 and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct 31: 151–175, 2002. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 36.McNamara RK, Lenox RH. Distribution of the protein kinase C substrates MARCKS and MRP in the postnatal developing rat brain. J Comp Neurol 397: 337–356, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 37.Meinnel T, Dian C, Giglione C. Myristoylation, an ancient protein modification mirroring eukaryogenesis and evolution. Trends Biochem Sci 45: 619–632, 2020. doi: 10.1016/j.tibs.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery DS, Yu L, Ghazi ZM, Thai TL, Al-Khalili O, Ma HP, Eaton DC, Alli AA. ENaC activity is regulated by calpain-2 proteolysis of MARCKS proteins. Am J Physiol Cell Physiol 313: C42–C53, 2017. doi: 10.1152/ajpcell.00244.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pochynyuk O, Bugaj V, Stockand JD. Physiologic regulation of the epithelial sodium channel by phosphatidylinositides. Curr Opin Nephrol Hypertens 17: 533–540, 2008. doi: 10.1097/MNH.0b013e328308fff3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pochynyuk O, Staruschenko A, Tong Q, Medina J, Stockand JD. Identification of a functional phosphatidylinositol 3,4,5-trisphosphate binding site in the epithelial Na+ channel. J Biol Chem 280: 37565–37571, 2005. doi: 10.1074/jbc.M509071200. [DOI] [PubMed] [Google Scholar]
- 41.Pochynyuk O, Stockand JD, Staruschenko A. Ion channel regulation by Ras, Rho, and Rab small GTPases. Exp Biol Med (Maywood) 232: 1258–1265, 2007. doi: 10.3181/0703-MR-76. [DOI] [PubMed] [Google Scholar]
- 42.Pochynyuk O, Tong Q, Medina J, Vandewalle A, Staruschenko A, Bugaj V, Stockand JD. Molecular determinants of PI(4,5)P2 and PI(3,4,5)P3 regulation of the epithelial Na+ channel. J Gen Physiol 130: 399–413, 2007. doi: 10.1085/jgp.200709800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pochynyuk O, Tong Q, Staruschenko A, Ma HP, Stockand JD. Regulation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. Am J Physiol Renal Physiol 290: F949–F957, 2006. doi: 10.1152/ajprenal.00386.2005. [DOI] [PubMed] [Google Scholar]
- 44.Pochynyuk O, Tong Q, Staruschenko A, Stockand JD. Binding and direct activation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. J Physiol 580: 365–372, 2007. doi: 10.1113/jphysiol.2006.127449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossier BC, Schild L. Epithelial sodium channel: mendelian versus essential hypertension. Hypertension 52: 595–600, 2008. doi: 10.1161/HYPERTENSIONAHA.107.097147. [DOI] [PubMed] [Google Scholar]
- 46.Schild L. The ENaC channel as the primary determinant of two human diseases: Liddle syndrome and pseudohypoaldosteronism. Nephrologie 17: 395–400, 1996. [PubMed] [Google Scholar]
- 47.Schleiff E, Schmitz A, McIlhinney RA, Manenti S, Vergères G. Myristoylation does not modulate the properties of MARCKS-related protein (MRP) in solution. J Biol Chem 271: 26794–26802, 1996. doi: 10.1074/jbc.271.43.26794. [DOI] [PubMed] [Google Scholar]
- 48.Song C, Le J, Yue Q, Mistry M, Wynne BM, Ma HP, Eaton DC. ENaC activity and regulation in renal distal convoluted tubule cells. FASEB J, 1_supplement 33: 824–826, 2019. [Google Scholar]
- 49.Spizz G, Blackshear PJ. Identification and characterization of cathepsin B as the cellular MARCKS cleaving enzyme. J Biol Chem 272: 23833–23842, 1997. doi: 10.1074/jbc.272.38.23833. [DOI] [PubMed] [Google Scholar]
- 50.Spizz G, Blackshear PJ. Protein kinase C-mediated phosphorylation of the myristoylated alanine-rich C-kinase substrate protects it from specific proteolytic cleavage. J Biol Chem 271: 553–562, 1996. doi: 10.1074/jbc.271.1.553. [DOI] [PubMed] [Google Scholar]
- 51.Staruschenko A, Pochynyuk O, Vandewalle A, Bugaj V, Stockand JD. Acute regulation of the epithelial Na+ channel by phosphatidylinositide 3-OH kinase signaling in native collecting duct principal cells. J Am Soc Nephrol 18: 1652–1661, 2007. doi: 10.1681/ASN.2007010020. [DOI] [PubMed] [Google Scholar]
- 52.Stockand JD, Bao HF, Schenck J, Malik B, Middleton P, Schlanger LE, Eaton DC. Differential effects of protein kinase C on the levels of epithelial Na+ channel subunit proteins. J Biol Chem 275: 25760–25765, 2000. doi: 10.1074/jbc.M003615200. [DOI] [PubMed] [Google Scholar]
- 53.Sundaram M, Cook HW, Byers DM. The MARCKS family of phospholipid binding proteins: regulation of phospholipase D and other cellular components. Biochem Cell Biol 82: 191–200, 2004. doi: 10.1139/o03-087. [DOI] [PubMed] [Google Scholar]
- 54.Tenenhouse HS, Roy S, Martel J, Gauthier C. Differential expression, abundance, and regulation of Na+-phosphate cotransporter genes in murine kidney. Am J Physiol Renal Physiol 275: F527–F534, 1998. doi: 10.1152/ajprenal.1998.275.4.F527. [DOI] [PubMed] [Google Scholar]
- 55.Tong Q, Gamper N, Medina JL, Shapiro MS, Stockand JD. Direct activation of the epithelial Na+ channel by phosphatidylinositol 3,4,5-trisphosphate and phosphatidylinositol 3,4-bisphosphate produced by phosphoinositide 3-OH kinase. J Biol Chem 279: 22654–22663, 2004. doi: 10.1074/jbc.M401004200. [DOI] [PubMed] [Google Scholar]
- 56.Verghese GM, Johnson JD, Vasulka C, Haupt DM, Stumpo DJ, Blackshear PJ. Protein kinase C-mediated phosphorylation and calmodulin binding of recombinant myristoylated alanine-rich C kinase substrate (MARCKS) and MARCKS-related protein. J Biol Chem 269: 9361–9367, 1994. [PubMed] [Google Scholar]
- 57.Verspurten J, Gevaert K, Declercq W, Vandenabeele P. SitePredicting the cleavage of proteinase substrates. Trends Biochem Sci 34: 319–323, 2009. doi: 10.1016/j.tibs.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Wagner ML, Tamm LK. Reconstituted syntaxin1a/SNAP25 interacts with negatively charged lipids as measured by lateral diffusion in planar supported bilayers. Biophys J 81: 266–275, 2001. doi: 10.1016/S0006-3495(01)75697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J, Gambhir A, Hangyás-Mihályné G, Murray D, Golebiewska U, McLaughlin S. Lateral sequestration of phosphatidylinositol 4,5-bisphosphate by the basic effector domain of myristoylated alanine-rich C kinase substrate is due to nonspecific electrostatic interactions. J Biol Chem 277: 34401–34412, 2002. doi: 10.1074/jbc.M203954200. [DOI] [PubMed] [Google Scholar]
- 60.Wang J, Gambhir A, McLaughlin S, Murray D. A computational model for the electrostatic sequestration of PI(4,5)P2 by membrane-adsorbed basic peptides. Biophys J 86: 1969–1986, 2004. doi: 10.1016/S0006-3495(04)74260-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J, Richards DA. Segregation of PIP2 and PIP3 into distinct nanoscale regions within the plasma membrane. Biol Open 1: 857–862, 2012. doi: 10.1242/bio.20122071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yue G, Eaton DC. Protein kinase C does not affect epithelial Na+ channel activity in inside-out patches from A6 cells (Abstract). FASEB J 12: A179, 1998. [Google Scholar]
- 63.Yue G, Edinger RS, Bao HF, Johnson JP, Eaton DC. The effect of rapamycin on single ENaC channel activity and phosphorylation in A6 cells. Am J Physiol Cell Physiol 279: C81–C88, 2000. doi: 10.1152/ajpcell.2000.279.1.C81. [DOI] [PubMed] [Google Scholar]
- 64.Yue G, Malik B, Yue G, Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem 277: 11965–11969, 2002. doi: 10.1074/jbc.M108951200. [DOI] [PubMed] [Google Scholar]
- 65.Zhang ZR, Chou CF, Wang J, Liang YY, Ma HP. Anionic phospholipids differentially regulate the epithelial sodium channel (ENaC) by interacting with α, β, and γ ENaC subunits. Pflugers Arch 459: 377–387, 2010. doi: 10.1007/s00424-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao J, Izumi T, Nunomura K, Satoh S, Watanabe S. MARCKS-like protein, a membrane protein identified for its expression in developing neural retina, plays a role in regulating retinal cell proliferation. Biochem J 408: 51–59, 2007. doi: 10.1042/BJ20070826. [DOI] [PMC free article] [PubMed] [Google Scholar]