Abstract
Coronavirus disease 2019 (COVID-19) and diabetes outcomes (CORONADO) trial revealed that 10.6% of patients with diabetes mellitus hospitalized for COVID-19 (COVID-19) die within 7 days. Several studies from New York, Italy, and China confirm that patients with diabetes are at a much higher risk for mortality due to COVID-19. Besides respiratory illness, COVID-19 increases cardiac injury and diabetic ketoacidosis. In the absence of specific guidelines for the prevention and treatment of COVID-19 for patients with diabetes, they remain at higher risk and are more susceptible to COVID-19. Furthermore, there is a scarcity of basic knowledge on how diabetes affects pathogenesis of severe acute respiratory coronavirus (SARS-CoV-2) infection. In patients with diabetes, impaired glucose use alters metabolic and consequently biological processes instigating pathological remodeling, which has detrimental effects on cardiovascular systems. A majority of biological processes are regulated by noncoding microRNAs (miRNAs), which have emerged as a promising therapeutic candidate for several diseases. In consideration of the higher risk of mortality in patients with diabetes and COVID-19, novel diagnostic test and treatment strategy are urgently warranted in post-COVID-19 era. Here, we describe potential roles of miRNA as a biomarker and therapeutic candidate, especially for heart failure, in patients with diabetes and COVID-19.
Keywords: biomarker, cardiovascular disease, heart failure, noncoding RNA, therapeutic candidate, SARS-CoV-2
INTRODUCTION
Diabetes mellitus (hereafter diabetes) increases severity and mortality of coronavirus disease 2019 (COVID-19), and intriguingly, COVID-19 instigates onset of diabetic phenotypes, mainly ketoacidosis and insulin resistance (24, 44). The relationship of diabetes and COVID-19 is intertwined; however, both increase the risk of heart failure (2, 39). Nevertheless, very little is known about diagnosis and treatment of patients with diabetes and COVID-19, especially for heart failure.
The noncoding regulatory microRNAs (miRNAs) are a promising therapeutic candidate for cardiovascular diseases (32). They are differentially expressed in the diabetic heart (9). The pathophysiology of diabetes-induced heart failure is unique, and it relates to a metabolic disorder (27, 45). Because severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) perturbs energy metabolism (35, 42), it may exacerbate cardiac metabolic remodeling leading to heart failure. How heart failure happens in patients with diabetes and COVID-19 is poorly understood.
miRNAs could be a potential biomarker and therapeutic target for patients with COVID-19. Differential circulating levels of miRNAs could be a potential biomarker for cardiovascular diseases (55). Similarly, they could be a potential biomarker for severity of COVID-19 in patients with and without diabetes. miRNA targets genes and restricts their expression (3). Thus, miRNA could prevent SARS-CoV-2 infection by targeting its protein expressing genes. The single-stranded RNA genome of SARS-CoV-2 has 29,891 nucleotides (GenBank: MN975262.1), which encode for the structural and nonstructural proteins. More studies are needed to understand how miRNAs regulate SARS-CoV-2 gene expression in the host cells to control its amplifications. Furthermore, miRNAs can regulate immune cells and thereby improve immunity of patients with COVID-19. Thus, manipulating endogenous miRNA to improve immunity and decrease the risk of SARS-CoV-2 infection could be another avenue for further investigation.
BACKGROUND ON COVID-19
COVID-19 is caused by SARS-CoV-2. It was first discovered in Wuhan, China, and rapidly spread over the world (16). Currently, >612,829 people have died by COVID-19 worldwide (https://www.worldometers.info/coronavirus/coronavirus-death-toll/). The burden of mortality has been unfairly heavy on elderly (>60 yr) people with comorbidities such as diabetes, hypertension, respiratory disease, cardiovascular disease, and chronic kidney and lung disease (43). Moreover, these populations are more susceptible to SARS-CoV-2 infection and the resultant development of acute respiratory distress syndrome (ARDS) compared with general population (58). The receptor-binding domain of spike glycoprotein of SARS-CoV-2 binds to angiotensin-converting enzyme-2 (ACE2) receptors on cell surface, followed by furin cleavage of S1/S2 domain and priming by the serine proteinase TMPRSS2, facilitating virus entry into host cells (31). The coexpression of ACE2 and TMPRSS2 is a major determinant of permissiveness of a cell to SARS-CoV-2 infection. The binding affinity of SARS-CoV-2 envelope spikes to the cellular ACE2 receptor is 10–20 times higher as compared with that of SARS-CoV-1, probably explaining the high transmission rates and infectivity of SARS-CoV-2 in humans (52).
IMPACT OF DIABETES ON SEVERITY AND MORTALITY OF COVID-19
A meta-analyses of 33 studies with 16,003 patients’ data sets revealed that diabetes increases severity and mortality (2-fold) in patients with COVID-19 (24). In a Wuhan hospital, out of 193 patients with severe COVID-19, 48 (24.9%) were diabetic and their mortality rate was higher (81.3%, 39 out of 48 patients with diabetes) compared with patients without diabetes (47.6%, 69 out of 145 patients without diabetes). Moreover, severity of COVID-19 was also higher in patients with diabetes (56). Other studies in China also support higher mortality risk in patients with diabetes (19, 50, 54). Further studies showed that 33.9% of 86,499 patients with severe COVID-19 in Italy and 33.8% of 5,700 patients with severe COVID-19 in New York City had diabetes (14, 41). There are several studies demonstrating that diabetes is a comorbidity in patients with severe COVID-19 (Table 1). It has been suggested that arrhythmia and sudden cardiac arrest are associated with heart failure in patients with COVID-19 (15). However, the cause of heart failure in diabetic COVID-19 remains unclear. Recent COVID-19 and diabetes outcomes (CORONADO) trial (NCT04324736) in 53 French centers revealed increased vascular complications in >40% patients with diabetes. The primary purpose of this study was to evaluate combined mechanical ventilation and/or death within 7 days, which was found to be independently and positively associated with diabetes (6). Thus, diabetes is a significant comorbid condition in patients with COVID-19 that worsens the outcome of the disease (10).
Table 1.
Selected recent peer-reviewed primary research publications implicating diabetes as a comorbidity in patients with COVID-19
| Title | Authors | Publication Date | DOI/PMID |
|---|---|---|---|
| Risk and predictors of in-hospital mortality from COVID-19 in patients with diabetes and cardiovascular disease | Hadith et al. | 2020 Jul 6 | 10.1186/s13098-020-00565-9 |
| Clinical and CT features of the COVID-19 infection: comparison among four different age groups | Li et al. | 2020 Jul 13 | 10.1007/s41999-020-00356-5 |
| The Relationship between Diabetes Mellitus and COVID-19 Prognosis: A Retrospective Cohort Study in Wuhan, China | Shang et al. | 2020 Jul 9 | 10.1016/j.amjmed.2020.05.033 |
| Health-related concerns and precautions during the COVID-19 pandemic: A comparison of Canadians with and without underlying health conditions | Ramage-Morin et al. | 2020 Jul 2 | 10.25318/82-003-x202000500001-eng |
| The impact of type 2 diabetes and its management on the prognosis of patients with severe COVID-19 | Xu et al. | 2020 Jul 8 | 10.1111/1753-0407.13084 |
| Clinical analysis of risk factors for patients with severe COVID-19 with type 2 diabetes | Zhang et al. | 2020 Jun 29 | 10.1016/j.jdiacomp.2020.107666 |
| Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy | Zangrillo et al. | 2020 Apr 23 | PMID: 32353223 |
| Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis | Kumar et al. | 2020 July–August | 10.1016/j.dsx.2020.04.044 |
| New-Onset Diabetes in Covid-19 | Rubino et al. | 2020 Jun 12 | 10.1056/NEJMc2018688 |
| Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China | Huang et al. | 2020 Feb 15 | 10.1016/S0140-6736(20)30183-5 |
| Cardiovascular disease and COVID-19 | Manish Bansal | 2020 May–Jun | 10.1016/j.dsx.2020.03.013 |
| Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China | Wang et al. | 2020 Feb 7 | 10.1001/jama.2020.1585 |
| Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. | Yan et al. | 2020 Apr 27 | 10.1136/bmjdrc-2020-001343 |
| Prevalence and impact of diabetes among people infected with SARS-CoV-2 | Fadini et al. | 2020 Mar 28 | PMID: 32222956 |
| COVID-19 infection in Italian people with diabetes: Lessons learned for our future (an experience to be used) | Gentile et al. | 2020 Apr | 10.1016/j.diabres.2020.108137 |
| Prevention and management of COVID-19 among patients with diabetes: an appraisal of the literature | Katulanda et al. | 2020 Aug | 10.1007/s00125-020-05164-x |
| Diabetes is a risk factor for the progression and prognosis of COVID-19 | Guo et al. | 2020 Mar | 10.1002/dmrr.3319 |
This list is not comprehensive, and hypothetical models, individual case reports, and data mining reports have been excluded from this list. Updated through 2020 Jul 20.
Notably, patients with diabetes even with mild symptoms are more vulnerable to COVID-19 (21). One of the potential underlying mechanisms for increased risk is insulin inactivity that impairs glucose uptake causing metabolic derangement, which in turn increases cellular adaptive stress. In addition, increased circulating levels of glucose cause O-linked attachment of glycan moiety to proteins affecting their functional activity and induce adaptive modifications that instigate pathological remodeling in patients with diabetes (8, 40). Diabetes also increases inflammation and thrombotic tendency, which is exacerbated by SARS-CoV-2 infection (17, 49). Thus, diabetes increases thrombosis and cellular stress leading to heart failure, which is exacerbated by lung dysfunction due to COVID-19 infection (Fig. 1). Hypertension and diabetes are comorbid conditions in patients with COVID-19 (48). Patients with diabetes develop hypertension; however, an independent contribution of hypertension on severity of COVID-19 in patients with diabetes has not been demonstrated.
Fig. 1.
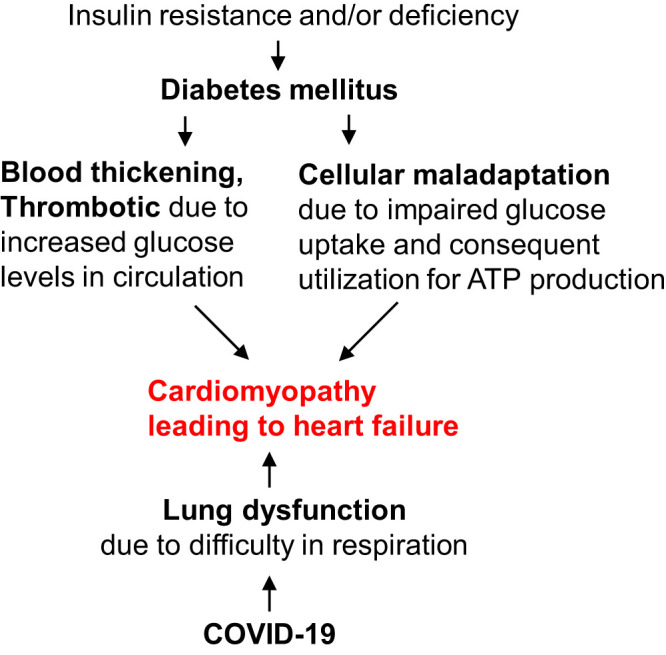
Diabetes and coronavirus disease-19 (COVID-19) double the detrimental effects on the heart. Diabetes mellitus (DM) is caused by insulin resistance (type-2 DM) or deficiency (type-1 DM) or both at the advanced stage of the disease. High-glucose levels in the blood increases its viscosity and risk of thrombosis. Reduced glucose uptake at cellular levels causes metabolic remodeling due to impaired glucose oxidation. Moreover, increased fatty acid oxidation causes maladaptive changes and cellular stress that result in cell death instigating pathological cardiac remodeling leading to heart failure. In COVID-19 disease, severe acute respiratory coronavirus (SARS-CoV-2) infection damages lungs and compromises their function that reduces oxygen supply to the heart and ultimately causes heart failure.
Diabetes does not increase the risk of SAR-CoV-2 infection (10). Therefore, the major effect of diabetes is on pathological remodeling leading to death in patients with COVID-19. Notably, the risk of heart failure in patients with diabetes is not decreased by glycemic control (7). Thus, controlling only glucose levels may not be adequate to decrease the risk of heart failure in patients with diabetes and COVID-19. Future studies focusing on the characterization of health failure and molecular mechanisms, especially underlying heart failure in patients with COVID-19, could provide insights for developing specific therapeutic candidates.
Low abundance of host-cell miRNAs is associated with increasing severity and mortality by COVID-19 in aged individuals (12). Aging increases the risk of diabetes. However, the role of miRNAs in patients with diabetes and COVID-19 is poorly understood.
MICRORNA IN TESTING AND TREATING DIABETIC COVID-19
Background of miRNA.
miRNAs are a class of noncoding, endogenous, tiny regulatory RNAs that modulate gene expression either by mRNA degradation or translational repression. They are biosynthesized as a double-stranded primary miRNA in the nucleus and matured as a single-stranded RNA in cytoplasm, where they bind mostly at the 3′-untranslated region (3′-UTR) to control the majority of biological functions (3, 32). More than 2,000 miRNAs are present in our body, and more than 800 miRNAs are expressed in the heart (11, 26). Differentially expressed cardiac miRNAs are potential therapeutic candidates for heart failure with diabetes (22, 38). Several miRNAs are associated with SARS-CoV-2 infection. However, as of this date, only five studies have described the role of specific miRNAs in SARS-CoV-2 infection and pathogenesis (Table 2).
Table 2.
List of recent publications discussing the role of microRNAs in SARS-CoV-2 infection
| Title | Authors | Publication Date | DOI |
|---|---|---|---|
| Computational analysis of microRNA-mediated interactions in SARS-CoV-2 infection | Demirici et al. | 2020 Jun 5 | 10.7717/peerj.9369 |
| COVID-19 Virulence in Aged Patients Might Be Impacted by the Host Cellular MicroRNAs Abundance/Profile | Fulzele et al. | 29 Apr 2020 | 10.14336/AD.2020.0428 |
| What is the potential function of microRNAs as biomarkers and therapeutic targets in COVID-19? | Guterres et al. | 2020 Jun 8 | 10.1016/j.meegid.2020.104417 |
| The Prediction of miRNAs in SARS-CoV-2 Genomes: hsa-miR Databases Identify 7 Key miRs Linked to Host Responses and Virus Pathogenicity-Related KEGG Pathways Significant for Comorbidities | Arisan et al. | 2020 Jun 4 | 10.3390/v12060614 |
| The impact of MicroRNAs (miRNAs) on the genotype of coronaviruses | Canatan et al. | 2020 May 11 | 10.23750/abm.v91i2.9534 |
MicroRNA as a biomarker.
miRNAs are excreted in circulation in microvesicles and exosomes. Differential levels of circulating miRNAs are a promising biomarker for heart failure (13, 46, 53). In diabetic conditions, the subclinical changes in the heart are difficult to diagnose with regular cardiovascular evaluations, and miRNA has great potential to serve as a diagnostic biomarker (25). In patients with diabetes and COVID-19, the mild symptoms have detrimental effects, and circulating miRNA profiling could offer an important strategy to determine disease severity. Furthermore, the subcategory of miRNA profiling could provide important information on severity of organ damage and dysfunction, including heart failure. Therefore, circulating miRNA profiling could be a new strategy to assess whether a patient with diabetes and with mild COVID-19 symptoms needs urgent attention/hospitalization. Thus, this miRNA testing strategy could prevent severe/detrimental consequences in patients with diabetes and with mild COVID-19 symptoms.
MicroRNA as a therapeutic target.
Cellular/tissue levels of miRNAs alter in different disease, which is reviewed in Abdellatif (1). By targeting several genes in a biological network, an miRNA can trigger one or more biological processes and thereby maintains cellular homeostasis. Thus, manipulating the endogenous miRNA levels (increasing the downregulated miRNA by miRNA mimic and decreasing the upregulated miRNA by anti-miR treatment) has potential to ameliorate cardiovascular disease at least in preclinical models (32). Because of promising preclinical results, >900 clinical trials have been conducted on miRNAs (www.ClinicalTrial.Gov).
miRNAs could play crucial roles in preventing infection of SARS-CoV-2 by modulating gene expression levels and improving host immune system. An extensive review on miRNAs targeting SARS-CoV-2 genome, their target genes, and associated biological pathways has recently published (12). We postulate that miRNA could target virus genes and modulate their expression by degrading mRNA and inhibiting their translation. It has been shown that small viral RNAs contribute to SARS-CoV-2 pathogenesis (34), and miRNAs that regulate viral RNA could provide a molecular basis for SARS-CoV-2 infection (18, 28). Virus in COVID-19 disease belongs to the betacoronavirus family, and probably miRNAs are involved in regulating their replication and pathogenesis (4). Thus, by reducing the viral replication, miRNA could serve as potential therapeutic candidate to prevent the progression from mild to severe COVID-19 disease in patients with diabetes.
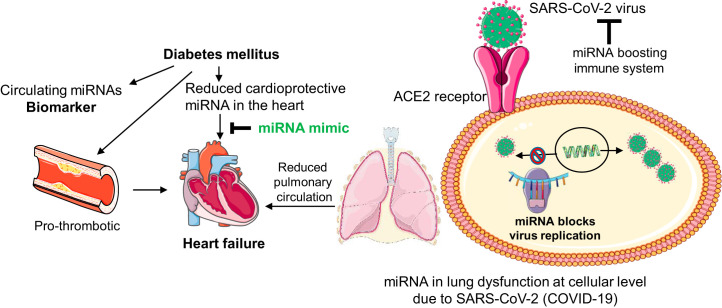
miRNA in patients with diabetes and COVID-19 could also optimize immune metabolism in the diabetic heart to ameliorate cardiomyopathy that leads to heart failure (33). Targeting inflammasome formation and cell death are other potential avenues for miRNA therapeutics that can be used to treat patients with diabetes and COVID-19 (57). Thus, miRNA can act at various levels to suppress virus infection and mitigate its pathological remodeling. It could serve as a biomarker for patients with diabetes and COVID-19 (Fig. 2).
Fig. 2.
MicroRNA (miRNA) as a biomarker and therapeutic candidate for patients with diabetes and coronavirus disease-19 (COVID-19). In diabetes, differential expression of circulating miRNAs could be a potential biomarker for severity of COVID-19 disease with and without cardiac dysfunction. Diabetes also increases thrombosis and reduces cardioprotective miRNAs such as miR-133a in the heart. Increasing the levels of cardioprotective miRNAs by miRNA mimic treatment mitigates diabetic heart failure. In COVID-19 infection, the severe acute respiratory coronavirus (SARS-CoV-2) virus enters lung cells by angiotensin-converting enzyme 2 (ACE2). They replicate inside the host cell to make more viruses. miRNA can restrict viral replication and boost immune system and thereby prevent lung deterioration and consequently improve cardiovascular outcomes. Thus, miRNA could be a potential therapeutic target and biomarker for patients with diabetes and COVID-19.
In the heart, miRNAs involved in regulation of ACE2 expression, arrhythmia, and sudden cardiac arrest are of great interest for patients with COVID-19 due to their potential roles in SARS-CoV-2 protein expression and heart failure. It is found that miR-15b-5p decreased with age in coronary artery disease and that miR-30e-3p decreased with age in myocardial injury. Both of these miRNAs are predicted to target SARS-CoV-2 genome (12, 51, 59). Lipotoxicity and metabolic remodeling play a significant role in diabetic heart failure (27, 29). miRNAs involved in these pathways may have important roles in patients with diabetes and COVID-19. Our studies suggest that increasing the cardiac levels of miR-133a in the diabetic heart reduces cardiac lipid accumulation (20). Thus, miR-133a could regulate lipotoxicity and metabolic remodeling in the diabetic heart. Furthermore, it targets angiotensinogen and thus could be involved in regulation of ACE2 receptor function in congestive heart failure condition (47). It also modulates electrical repolarization caused due to pressure overload in the heart (30) and thus may be involved in controlling arrhythmia. miR-133a is one of the most abundant miRNAs in the human heart (26), which is downregulated in human diabetic and nondiabetic heart failure (5, 37). It is essential for adult heart function, and loss of miR-133a causes cardiac hypertrophy and dysfunction (5). Notably, overexpression of miR-133a does not have any detrimental effects; rather, it protects the heart against cardiac fibrosis after pressure overload (30) and against impaired contractility caused by diabetes (38). Thus, miR-133a is a promising candidate for investigating its role in heart failure in patients with diabetes and COVID-19. Besides, miR-133a, miR-1, miR-208, miR-328, miR-21, miR-212, and miR-590 are involved in arrhythmia. The details of miRNA targets and their roles in cardiac conduction and arrhythmia have been recently elaborated (23).
SUMMARY AND FUTURE DIRECTIONS
Several studies from different parts of the world have confirmed that diabetes increases severity and mortality of COVID-19, plausibly by increasing inflammation and compromising immunity (36). The absence of specific guidelines to manage and treat patients with diabetes and COVID-19 and their increased mortality risk warrant new approaches to diagnose, manage, and treat the detrimental combination of diabetes and COVID-19 disease. We propose that investigating miRNAs in patients with diabetes and COVID-19 as a biomarker and a therapeutic candidate could open a new platform to assess mild to severely ill patients with diabetes and COVID-19 and may pave a way to prevent the progression of viral infection via restricting viral genome amplification and boosting immune system. Thus, miRNA could be a promising diagnostic biomarker and therapeutic target for patients with diabetes and COVID-19.
GRANTS
This work was supported, in part, by National Institutes of Health (NIH) Grants HL-113281 and 1U54GM115458 and the University of Nebraska Medical Center for Heart and Vascular Research (to P.K.M.); National Aeronautics and Space Administration Grant 80NSSC19K1603 and the University of Mississippi Medical Center COVID-19 Fund (to R.T.); and NIH Grant P30MH062261 and Frances E. Lageschulte and Evelyn B. Weese New Frontiers in Medical Research Fund (to S.N.B.).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represents the official views of the grant institutions.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.K.M. conceived and designed research; P.K.M. prepared figures; P.K.M. drafted manuscript; P.K.M., R.T., and S.N.B. edited and revised manuscript; P.K.M., R.T., and S.N.B. approved final version of manuscript.
REFERENCES
- 1.Abdellatif M. Differential expression of microRNAs in different disease states. Circ Res 110: 638–650, 2012. doi: 10.1161/CIRCRESAHA.111.247437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr 14: 247–250, 2020. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233, 2009. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canatan D, De Sanctis V. The impact of MicroRNAs (miRNAs) on the genotype of coronaviruses. Acta Biomed 91: 195–198, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Høydal M, Autore C, Russo MA, Dorn GW 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med 13: 613–618, 2007. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 6.Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, Allix I, Amadou C, Arnault G, Baudoux F, Bauduceau B, Borot S, Bourgeon-Ghittori M, Bourron O, Boutoille D, Cazenave-Roblot F, Chaumeil C, Cosson E, Coudol S, Darmon P, Disse E, Ducet-Boiffard A, Gaborit B, Joubert M, Kerlan V, Laviolle B, Marchand L, Meyer L, Potier L, Prevost G, Riveline JP, Robert R, Saulnier PJ, Sultan A, Thébaut JF, Thivolet C, Tramunt B, Vatier C, Roussel R, Gautier JF, Gourdy P; CORONADO investigators . Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia 63: 1500–1515, 2020. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castagno D, Baird-Gunning J, Jhund PS, Biondi-Zoccai G, MacDonald MR, Petrie MC, Gaita F, McMurray JJ. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: evidence from a 37,229 patient meta-analysis. Am Heart J 162: 938–948, 2011. doi: 10.1016/j.ahj.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 8.Chavali V, Tyagi SC, Mishra PK. Predictors and prevention of diabetic cardiomyopathy. Diabetes Metab Syndr Obes 6: 151–160, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costantino S, Paneni F, Lüscher TF, Cosentino F. MicroRNA profiling unveils hyperglycaemic memory in the diabetic heart. Eur Heart J 37: 572–576, 2016. doi: 10.1093/eurheartj/ehv599. [DOI] [PubMed] [Google Scholar]
- 10.Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest 43: 867–869, 2020. doi: 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedländer MR, Lizano E, Houben AJ, Bezdan D, Báñez-Coronel M, Kudla G, Mateu-Huertas E, Kagerbauer B, González J, Chen KC, LeProust EM, Martí E, Estivill X. Evidence for the biogenesis of more than 1,000 novel human microRNAs. Genome Biol 15: R57, 2014. doi: 10.1186/gb-2014-15-4-r57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fulzele S, Sahay B, Yusufu I, Lee TJ, Sharma A, Kolhe R, Isales CM. COVID-19 Virulence in Aged Patients Might Be Impacted by the Host Cellular MicroRNAs Abundance/Profile. Aging Dis 11: 509–522, 2020. doi: 10.14336/AD.2020.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ge Q, Zhou Y, Lu J, Bai Y, Xie X, Lu Z. miRNA in plasma exosome is stable under different storage conditions. Molecules 19: 1568–1575, 2014. doi: 10.3390/molecules19021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentile S, Strollo F, Ceriello A. COVID-19 infection in Italian people with diabetes: Lessons learned for our future (an experience to be used). Diabetes Res Clin Pract 162: 108137, 2020. doi: 10.1016/j.diabres.2020.108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, Gulick RM, Safford MM. Clinical characteristics of Covid-19 in New York City. N Engl J Med 382: 2372–2374, 2020. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX; China Medical Treatment Expert Group for COVID-19 . Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 55: 2000547, 2020. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, Zhao L, Fan H, Luo S, Hu D. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. e3319, 2020. [published online ahead of print, 2020. March 31]. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasan MM, Akter R, Ullah MS, Abedin MJ, Ullah GM, Hossain MZ. A computational approach for predicting role of human MicroRNAs in MERS-CoV genome. Adv Bioinforma 2014: 967946, 2014. doi: 10.1155/2014/967946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kambis TN, Shahshahan HR, Kar S, Yadav SK, Mishra PK. Transgenic expression of miR-133a in the diabetic Akita heart prevents cardiac remodeling and cardiomyopathy. Front Cardiovasc Med 6: 45, 2019. doi: 10.3389/fcvm.2019.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katulanda P, Dissanayake HA, Ranathunga I, Ratnasamy V, Wijewickrama PS, Yogendranathan N, Gamage KK, de Silva NL, Sumanatilleke M, Somasundaram NP, Matthews DR. Prevention and management of COVID-19 among patients with diabetes: an appraisal of the literature. Diabetologia 63: 1440–1452, 2020. doi: 10.1007/s00125-020-05164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kesherwani V, Shahshahan HR, Mishra PK. Cardiac transcriptome profiling of diabetic Akita mice using microarray and next generation sequencing. PLoS One 12: e0182828, 2017. doi: 10.1371/journal.pone.0182828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim GH. MicroRNA regulation of cardiac conduction and arrhythmias. Transl Res 161: 381–392, 2013. doi: 10.1016/j.trsl.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, Khare S, Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr 14: 535–545, 2020. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.León LE, Rani S, Fernandez M, Larico M, Calligaris SD. Subclinical detection of diabetic cardiomyopathy with microRNAs: challenges and perspectives. J Diabetes Res 2016: 6143129, 2016. doi: 10.1155/2016/6143129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leptidis S, El Azzouzi H, Lok SI, de Weger R, Olieslagers S, Kisters N, Silva GJ, Heymans S, Cuppen E, Berezikov E, De Windt LJ, da Costa Martins P. A deep sequencing approach to uncover the miRNOME in the human heart. PLoS One 8: e57800, 2013. doi: 10.1371/journal.pone.0057800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 90: 207–258, 2010. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 28.Mallick B, Ghosh Z, Chakrabarti J. MicroRNome analysis unravels the molecular basis of SARS infection in bronchoalveolar stem cells. PLoS One 4: e7837, 2009. doi: 10.1371/journal.pone.0007837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marfella R, Amarelli C, Cacciatore F, Balestrieri ML, Mansueto G, D’Onofrio N, Esposito S, Mattucci I, Salerno G, De Feo M, D’Amico M, Golino P, Maiello C, Paolisso G, Napoli C. Lipid accumulation in hearts transplanted from nondiabetic donors to diabetic recipients. J Am Coll Cardiol 75: 1249–1262, 2020. doi: 10.1016/j.jacc.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Matkovich SJ, Wang W, Tu Y, Eschenbacher WH, Dorn LE, Condorelli G, Diwan A, Nerbonne JM, Dorn GW 2nd. MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res 106: 166–175, 2010. doi: 10.1161/CIRCRESAHA.109.202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev 19: 102537, 2020. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra PK, Tyagi N, Kumar M, Tyagi SC. MicroRNAs as a therapeutic target for cardiovascular diseases. J Cell Mol Med 13: 778–789, 2009. doi: 10.1111/j.1582-4934.2009.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishra PK, Ying W, Nandi SS, Bandyopadhyay GK, Patel KK, Mahata SK. Diabetic Cardiomyopathy: an immunometabolic perspective. Front Endocrinol (Lausanne) 8: 72, 2017. doi: 10.3389/fendo.2017.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morales L, Oliveros JC, Fernandez-Delgado R, tenOever BR, Enjuanes L, Sola I. SARS-CoV-encoded small RNAs contribute to infection-associated lung Pathology. Cell Host Microbe 21: 344–355, 2017. doi: 10.1016/j.chom.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mori J, Oudit GY, Lopaschuk GD. SARS-CoV-2 perturbs the renin-angiotensin system and energy metabolism. Am J Physiol Endocrinol Metab 319: E43–E47, 2020. doi: 10.1152/ajpendo.00219.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab 318: E736–E741, 2020. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nandi SS, Duryee MJ, Shahshahan HR, Thiele GM, Anderson DR, Mishra PK. Induction of autophagy markers is associated with attenuation of miR-133a in diabetic heart failure patients undergoing mechanical unloading. Am J Transl Res 7: 683–696, 2015. [PMC free article] [PubMed] [Google Scholar]
- 38.Nandi SS, Zheng H, Sharma NM, Shahshahan HR, Patel KP, Mishra PK. Lack of miR-133a decreases contractility of diabetic hearts: a role for novel cross talk between tyrosine aminotransferase and tyrosine hydroxylase. Diabetes 65: 3075–3090, 2016. doi: 10.2337/db16-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 27: 1879–1884, 2004. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- 40.Qin CX, Sleaby R, Davidoff AJ, Bell JR, De Blasio MJ, Delbridge LM, Chatham JC, Ritchie RH. Insights into the role of maladaptive hexosamine biosynthesis and O-GlcNAcylation in development of diabetic cardiac complications. Pharmacol Res 116: 45–56, 2017. doi: 10.1016/j.phrs.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 41.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, and the Northwell COVID-19 Reasearch Consortium; Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 323: 2052–2059, 2020. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothan HA, Acharya A, Reid SP, Kumar M, Byrareddy SN. Molecular aspects of COVID-19 differential pathogenesis. Pathogens 9: 9, 2020. doi: 10.3390/pathogens9070538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 109: 102433, 2020. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, Mingrone G, Boehm B, Cooper ME, Chai Z, Del Prato S, Ji L, Hopkins D, Herman WH, Khunti K, Mbanya JC, Renard E. New-onset diabetes in Covid-19. N Engl J Med [published online ahead of print, 2020. June 12]. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30: 595–602, 1972. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 46.Shah P, Bristow MR, Port JD. MicroRNAs in heart failure, cardiac transplantation, and myocardial recovery: biomarkers with therapeutic potential. Curr Heart Fail Rep 14: 454–464, 2017. doi: 10.1007/s11897-017-0362-8. [DOI] [PubMed] [Google Scholar]
- 47.Sharma NM, Nandi SS, Zheng H, Mishra PK, Patel KP. A novel role for miR-133a in centrally mediated activation of the renin-angiotensin system in congestive heart failure. Am J Physiol Heart Circ Physiol 312: H968–H979, 2017. doi: 10.1152/ajpheart.00721.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr 14: 303–310, 2020. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, Deftereos S, Tousoulis D. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol 14: 50–59, 2019. doi: 10.15420/ecr.2018.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323: 1061–1069, 2020. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang XT, Wu XD, Lu YX, Sun YH, Zhu HH, Liang JB, He WK, Zeng ZY, Li L. Potential involvement of MiR-30e-3p in myocardial injury induced by coronary microembolization via autophagy activation. Cell Physiol Biochem 44: 1995–2004, 2017. doi: 10.1159/000485905. [DOI] [PubMed] [Google Scholar]
- 52.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367: 1260–1263, 2020. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu T, Chen Y, Du Y, Tao J, Li W, Zhou Z, Yang Z. Circulating exosomal miR-92b-5p is a promising diagnostic biomarker of heart failure with reduced ejection fraction patients hospitalized for acute heart failure. J Thorac Dis 10: 6211–6220, 2018. doi: 10.21037/jtd.2018.10.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center For Disease Control And Prevention. JAMA [published online ahead of print, 2020. February 24]. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 55.Xu J, Zhao J, Evan G, Xiao C, Cheng Y, Xiao J. Circulating microRNAs: novel biomarkers for cardiovascular diseases. J Mol Med (Berl) 90: 865–875, 2012. doi: 10.1007/s00109-011-0840-5. [DOI] [PubMed] [Google Scholar]
- 56.Yan Y, Yang Y, Wang F, Ren H, Zhang S, Shi X, Yu X, Dong K. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care 8: e001343, 2020. doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yap JK, Moriyama M, Iwasaki A. Inflammasomes and pyroptosis as therapeutic targets for COVID-19. J Immunol 205: 307–312, 2020. doi: 10.4049/jimmunol.2000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zangrillo A, Beretta L, Scandroglio AM, Monti G, Fominskiy E, Colombo S, Morselli F, Belletti A, Silvani P, Crivellari M, Monaco F, Azzolini ML, Reineke R, Nardelli P, Sartorelli M, Votta CD, Ruggeri A, Ciceri F, De Cobelli F, Tresoldi M, Dagna L, Rovere-Querini P, Serpa Neto A, Bellomo R, Landoni G; COVID-BioB Study Group . Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc [published online ahead of print, 2020. April 23]. 32353223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu LP, Zhou JP, Zhang JX, Wang JY, Wang ZY, Pan M, Li LF, Li CC, Wang KK, Bai YP, Zhang GG. MiR-15b-5p regulates collateral artery formation by targeting AKT3 (protein kinase B-3). Arterioscler Thromb Vasc Biol 37: 957–968, 2017. doi: 10.1161/ATVBAHA.116.308905. [DOI] [PubMed] [Google Scholar]