Abstract
Preeclampsia is a major complication of pregnancy manifested as hypertension and often intrauterine growth restriction, but the underlying pathophysiological mechanisms are unclear. Predisposing genetic and environmental factors cause placental maladaptations leading to defective placentation, apoptosis of invasive cytotrophoblasts, inadequate expansive remodeling of the spiral arteries, reduced uteroplacental perfusion pressure, and placental ischemia. Placental ischemia promotes the release of bioactive factors into the maternal circulation, causing an imbalance between antiangiogenic soluble fms-like tyrosine kinase-1 and soluble endoglin and proangiogenic vascular endothelial growth factor, placental growth factor, and transforming growth factor-β. Placental ischemia also stimulates the release of proinflammatory cytokines, hypoxia-inducible factor, reactive oxygen species, and angiotensin type 1 receptor agonistic autoantibodies. These circulating factors target the vascular endothelium, causing generalized endotheliosis in systemic, renal, cerebral, and hepatic vessels, leading to decreases in endothelium-derived vasodilators such as nitric oxide, prostacyclin, and hyperpolarization factor and increases in vasoconstrictors such as endothelin-1 and thromboxane A2. The bioactive factors also target vascular smooth muscle and enhance the mechanisms of vascular contraction, including cytosolic Ca2+, protein kinase C, and Rho-kinase. The bioactive factors could also target matrix metalloproteinases and the extracellular matrix, causing inadequate vascular remodeling, increased arterial stiffening, and further increases in vascular resistance and hypertension. As therapeutic options are limited, understanding the underlying vascular mechanisms and molecular targets should help design new tools for the detection and management of hypertension in pregnancy and preeclampsia.
Keywords: cytokines, endothelium, hypertension, placental ischemia, vascular smooth muscle
INTRODUCTION
Normal pregnancy is associated with major uteroplacental and hemodynamic changes to meet the nutrient and metabolic demands of the growing fetus. Placental development and trophoblast invasion of uterine spiral arteries maintain adequate uteroplacental perfusion pressure. Increases in maternal plasma volume and cardiac output are paralleled with systemic vasodilation and decreased vascular resistance, leading to little decrease in blood pressure (BP) (177). These pregnancy-related changes require substantial uteroplacental and vascular remodeling and redistribution of blood flow in different maternal tissues and organs. Physiological and hormonal adaptations in endothelial mediators and vascular contraction pathways play a role in the regulation of systemic and uteroplacental blood flow during pregnancy. Also, matrix metalloproteinases (MMPs) play a role in the degradation of extracellular matrix (ECM) proteins and the uteroplacental and vascular remodeling during pregnancy.
In contrast with the slight decrease in BP during normal pregnancy, some women may show hypertension in pregnancy (HTN-Preg) in one of four forms: chronic HTN that predates pregnancy, preeclampsia (PE)-eclampsia, chronic HTN with superimposed PE, and nonproteinuric gestational HTN (164). PE affects 5–8% of pregnancies in the United States and ~8 million pregnancies worldwide (183). PE is characterized by new-onset HTN-Preg at or after 20 wk of gestation and frequently near term, with or without proteinuria (22, 142). PE could progress to eclampsia, with severe HTN, cerebral edema, seizures, and maternal death, accounting for 15–20% of maternal mortality (141). PE is also often associated with fetal intrauterine growth restriction (IUGR), accounting for 10–15% of preterm pregnancy and small-for-gestational-age birth weight (153). PE may also be a part of hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome named by Dr. Louis Weinstein in 1982 (196).
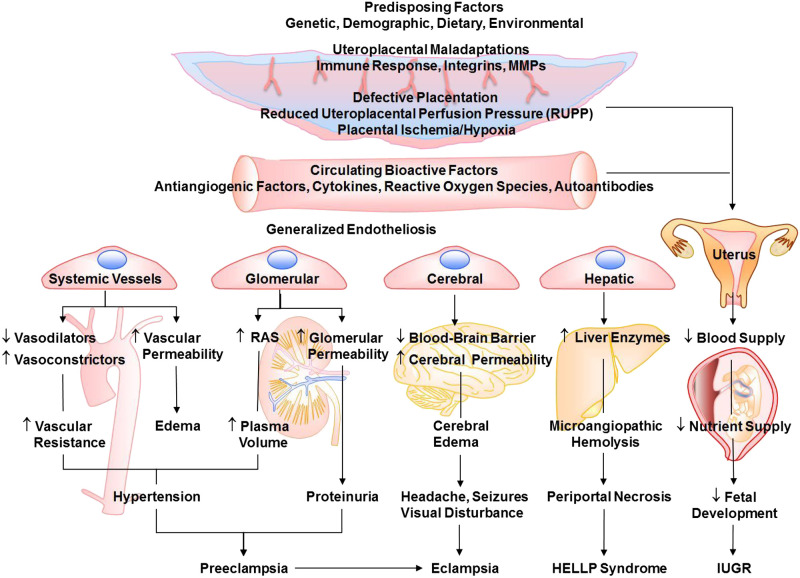
Although PE is a major cause of maternal and fetal morbidity and mortality, its etiology and pathophysiology are not fully understood. Certain genetic, demographic, dietary, and environmental risk factors are believed to cause placental maladaptations, defective placentation, and reduced uteroplacental perfusion pressure (RUPP) (Fig. 1). Inadequate placentation and trophoblast invasion of the spiral arteries and uterine wall result in placental ischemia/hypoxia. Although delineating the mechanisms causing defective placentation in early pregnancy and contributing to human preeclampsia has been challenging, studies in animal models have helped in understanding the events that follow the initial placental ischemia. Surgical induction of RUPP and placental ischemia during late pregnancy in sheep, dog, rabbit, rat, and mouse show some of the characteristics of PE, including HTN-Preg, decreased renal plasma flow, and IUGR (54, 212). Also, Preg rats exposed to extended periods of gestational hypoxia show PE-like characteristics (211). Studies in these models of HTN-Preg have suggested that placental ischemia/hypoxia causes the release of bioactive factors such as antiangiogenic factors, proinflammatory cytokines, hypoxia-inducible factor (HIF), reactive oxygen species (ROS), and angiotensin II (ANG II) type 1 receptor (AT1R) agonistic autoantibodies (AT1AA). These circulating factors target the vascular endothelium, causing endothelial dysfunction and changes in endothelium-derived relaxing and contracting factors. Bioactive factors also target vascular smooth muscle (VSM) and affect the vascular contraction mechanisms, leading to increased vasoconstriction. The bioactive factors could also target MMPs and ECM, leading to inadequate vascular remodeling. If these changes occur in systemic vessels, they cause generalized vascular dysfunction and HTN, whereas changes in renal glomeruli cause glomerular endotheliosis, increased glomerular permeability, and proteinuria. Changes in cerebral vessels cause cerebral edema and seizures, whereas changes in hepatic vessels lead to HELLP syndrome. Changes in uteroplacental remodeling and placental vascularization decrease placental blood flow and lead to IUGR (Fig. 1) (164).
Fig. 1.
Risk factors, mechanisms, and manifestations of preeclampsia-related disorders. Predisposing risk factors cause placental maladaptations, placental ischemia, and the release of bioactive factors. Circulating bioactive factors cause generalized endotheliosis in systemic vessels leading to increased vascular resistance and hypertension, renal vessels leading to increased plasma volume and proteinuria, cerebral vessels leading to cerebral edema and neurological manifestations, and hepatic vessels leading to hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome. Placental maladaptations and bioactive factors also reduce uteroplacental blood flow and lead to intrauterine growth restriction (IUGR). MMPs, matrix metalloproteinases; RAS, renin-angiotensin system.
In this review, we will use data from reports in PubMed and Medline as well as data from our laboratory to first discuss the predisposing factors and uteroplacental maladaptations that lead to RUPP and placental ischemia. We will follow with discussion of how placental ischemia causes the release of various bioactive and circulating factors. We will then discuss the various molecular targets in the endothelium, VSM, and ECM that could lead to vascular dysfunction, decreased vascular relaxation, increased vasoconstriction, inadequate vascular remodeling, and HTN-Preg. Throughout the review, we will briefly introduce the factors involved and their status during normal pregnancy, followed by description of the changes observed in human PE and experimental HTN-Preg. Lastly, we will discuss how identifying the vascular mechanisms and molecular targets could help design new approaches for the detection and management of PE.
PREDISPOSING GENETIC, DEMOGRAPHIC, DIETARY, AND ENVIRONMENTAL FACTORS IN PE
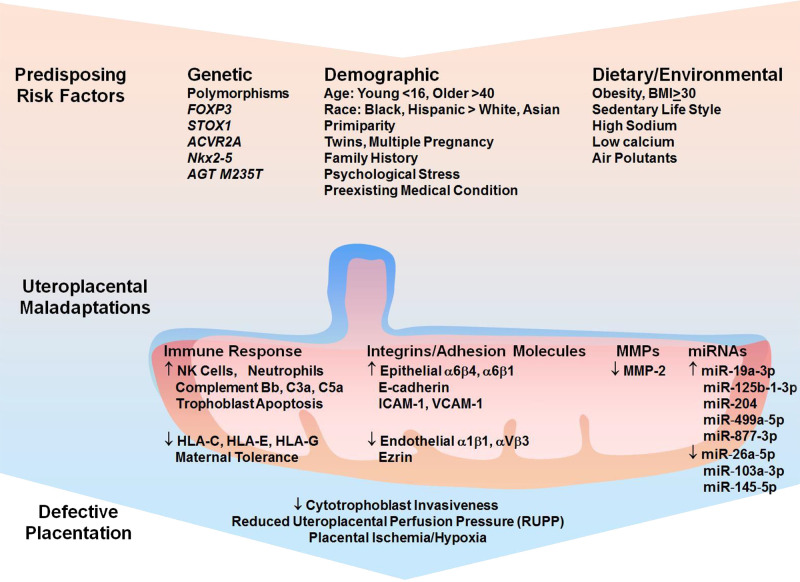
Several gene mutations have been associated with PE (Fig. 2), and increased decidual mRNA expression levels of candidate maternal PE susceptibility genes have been associated with clinical severity of the condition (207). In an earlier study, 31 of 36 placental genes were found to be downregulated in PE (48). In a more recent review of candidate genes and pathways in PE, 250 differentially expressed genes were identified from three profile data sets, of which 228 were upregulated and 22 were downregulated in PE placenta compared with normal placenta. The report also identified LEP, NRIP1, SASH1, and ZADHHC8P1 as candidate genes in PE (119). FOXP3 gene plays a role in the regulation of regulatory T cells and the immune response during pregnancy, and its downregulation could reduce maternal tolerance and predispose to PE (149). ACVR2A and STOX1 are considered susceptibility genes for PE. STOX1 Y153H polymorphism has been detected in several generations of women who developed early-onset and severe PE and has been linked to inadequate trophoblast invasion and IUGR (184). In support, female wild-type mice crossed with male transgenic mice overexpressing human STOX1 show PE characteristics including HTN and proteinuria (40). In Japanese subjects, the TT genotype of the angiotensinogen (AGT) M235T polymorphism and the Glu298Asp variant of endothelial nitric oxide synthase (NOS3) gene are independently associated with HTN-Preg (88). Studies have also demonstrated increased decidual mRNA expression levels of ACVR1, INHBB, ERAP1, ERAP2, LNPEP, COL4A1, and COL4A2 in severe PE. Also, increased mRNA expression levels of several genes (INHA, INHBB, COL4A1, and COL4A2) has been correlated with earlier onset of PE and earlier delivery of the fetus (207). Readers are encouraged to further search for additional genes related to PE from reports in Embase, Scopus, Ovid-Medline, Gene Expression Omnibus (GEO), and Cochrane Library.
Fig. 2.
Risk factors, uteroplacental maladaptations, and placental ischemia. Predisposing genetic polymorphisms, demographic characteristics, obesity, sedentary lifestyle, high-sodium low-calcium diet, and environmental pollutants could cause maladaptations in the uteroplacental immune response, integrins and adhesion molecules, matrix metalloproteinases (MMPs), and microRNAs. The uteroplacental maladaptations in turn lead to defective placentation and placental ischemia/hypoxia. BMI, body mass index; HLA-C, -E, and -G, histocompatibility complex molecules; NK, natural killer.
Age, ethnic background, maternal lifestyle, body weight, preexisting medical condition, history of PE, primiparity, and twin or multiple pregnancy contribute to the risk for PE. Very young (<16 yr) or older (>40 yr) women are more prone to PE. Studies in Finland and India have shown that older women are at higher risk of developing PE (78, 95). Race also plays a role in PE. A study on pregnant women from New York has shown that the incidence of PE is greater in Blacks (2.9%) and Hispanics (2.6%) than in nonHispanic Whites (1.3%) and Asians (1.2%) (154). Also, in placentae from women with early-onset and severe PE, the Nkx2–5 gene is expressed in a racially disparate fashion (Caucasians > African Americans) (152). The incidence of PE is also directly proportionate to the prepregnancy body weight, starting at 3% in women with normal body mass index (BMI, 18.5–24.9) but rising to 7% in overweight women with BMI 30–34.9 and to 13% in obese women with BMI ~50 (171). High total energy and dietary salt intake or lower magnesium and calcium during pregnancy have been related to HTN-Preg (17, 160). Other studies have suggested a linkage between reduced serum levels of magnesium, calcium, vitamin D, and zinc and PE (203). Exposure to environmental contaminants, fine particulate matter, and nitrogen dioxide may also increase the risk of PE (138). Preexisting medical and psychological conditions including heart disease, chronic respiratory disorders, diabetes, kidney disease, systemic lupus erythematosus, reproductive tract surgery, history of antepartum hemorrhage, and mental stress also increase the risk for PE. The role of genetic, demographic, dietary, and environmental factors in predisposing to defective placentation and PE could vary in different geographical and world regions and needs to be thoroughly examined.
UTEROPLACENTAL MALADAPTATIONS TRIGGERING DEFECTIVE PLACENTATION IN PE
The predisposing genetic, demographic, dietary, and environmental factors are thought to trigger uteroplacental maladaptations and lead to defective placentation and decreased placental development and uteroplacental vascularization. Uteroplacental maladaptations could involve the uteroplacental immune response, integrins, and MMPs (Fig. 2).
Uteroplacental Immune Response and Defective Placentation in PE
Although pregnancy is a physiological process, the fetus could pose a challenge to maternal tolerance and the immune response. In healthy pregnancy, the maternal systems need to tolerate the semiallogenic fetus, and the fetus must be protected from rejection by the maternal immune response. In PE, the maternal immune response is altered and proinflammatory cytokines are increased. The potential changes in the immune response in PE have prompted investigation of the incidence and risk factors of PE in human immuodeficiency virus (HIV)-infected women, who usually have a suppressed immune response. A prospective study in South Africa was designed to determine how common PE and gestational hypertension are in HIV-infected women. HIV-negative pregnant women and HIV-positive pregnant women receiving mono- or triple highly active antiretroviral therapy (HAART) for prevention of vertical transmission or maternal care were recruited and observed for the development of HTN-Preg. The South Africa study showed fewer cases of PE and gestational hypertension in the HIV-positive group than in the HIV-negative group, suggesting that PE and gestational hypertension are less common in HIV-infected women being managed with mono- or triple antiretroviral therapy (61). On the other hand, a study of HIV-infected pregnant women in Botswana found that before HAART, viral load greater than 100,000 copies per milliliter was associated with PE and that pre-HAART placental growth factor (PlGF) level was lower and soluble fms-like tyrosine kinase-1 (sFlt-1) was higher in women who developed PE versus those who did not (144). These observations make it important to further examine the changes in the immune response and their effects on the incidence of PE in HIV-infected pregnant women.
In normal pregnancy, cytotrophoblasts express major histocompatibility complex molecules such as HLA-C, HLA-E, and HLA-G, which interact with their respective inhibitory receptors KIR, CD 94/NKGs, and ILT-2 on natural killer (NK) cells, thus inhibiting NK cells and preventing them from attacking placental and fetal tissues (Fig. 2) (178). In PE, a decrease in HLA-C/KIR interaction would lead to increased activity of NK cells and attack of placental and fetal tissues (67). Also, whereas healthy pregnancy is associated with moderate activation of the complement system, HTN-Preg is associated with an increase in the complement activation products Bb, C3a, and C5a (101). PE women also show more neutrophils adherent to the endothelium of subcutaneous vessels, which may contribute to maternal endothelial dysfunction (96). In the RUPP rat model of placental ischemia, inhibition of complement activation or depletion of neutrophils decreases BP, supporting a role of innate immune response in HTN-Preg (101, 150).
Uteroplacental Integrins and Reduced Trophoblast Invasion in PE
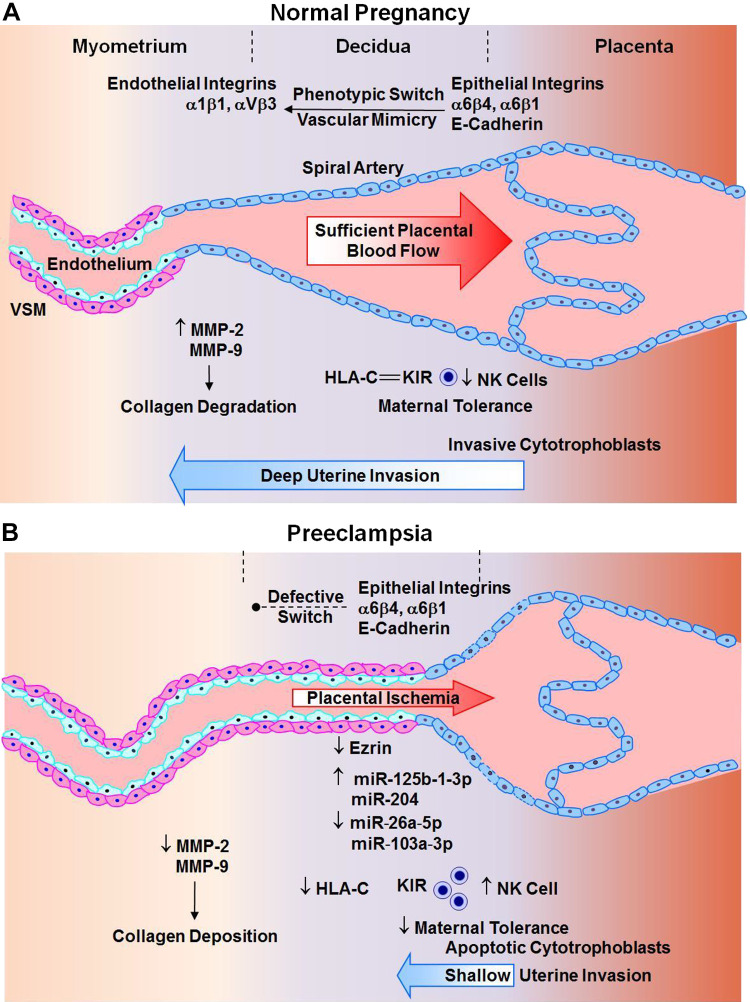
Trophoblast invasion and expansive remodeling of the spiral arteries is partly regulated by integrins and other adhesion molecules (Fig. 3). Cytotrophoblasts initially express epithelial cell adhesion molecules such as integrins α6β4 and α6β1 and E-cadherin. During normal pregnancy, a process termed vascular mimicry or pseudovasculogenesis takes place and cytotrophoblasts become more invasive; instead of expressing epithelial cell adhesion molecules, they express endothelial cell integrins α1β1 and αVβ3 (118). Also, studies in human uterine myometrial microvascular endothelial cells seeded on Matrigel to form endothelial networks then cocultured with trophoblast cells have suggested that appropriate integrins α1β1 modulate trophoblast cell integration into endothelial cellular networks through invasive pathways including galectin-1, tissue inhibitor of metalloproteinase-1 (TIMP-1), plasminogen activator inhibitor-1 (PAI-1), MMP-2, and MMP-9 production (202). These phenotypic changes in integrins and the associated invasive pathways may be impaired during PE. In early placenta, hypoxic stress increases expression of integrin α5 and fibronectin and decreases expression of integrin α1 (74). Abnormal expression of epithelial cell adhesion molecules and apoptosis of cytotrophoblasts cause limited invasion of spiral arteries and placental ischemia (118). Interestingly, studies in human uterine myometrial microvascular endothelial cells cocultured with trophoblast cells have shown that calcium depletion or low calcium inhibits integration of trophoblast cells into endothelial cellular networks, compared with the normal calcium, and is associated with increased mRNA expression of integrin α5 and β4 and decreased integrin α1 in trophoblasts (201) This observation may have clinical relevance, as low serum calcium has been associated with PE. Ezrin, an integrin involved in cell adhesion, organization, and migration, is downregulated in syncytiotrophoblasts from PE women, which could lead to reduced cytotrophoblast invasiveness and decreased placental vascularization (Fig. 3). The decreased trophoblast invasion and replacement of vascular cells also cause retention of VSM in the spiral arteries, further promoting vasoconstriction and placental ischemia.
Fig. 3.
Defective placentation in preeclampsia. A: during normal pregnancy, phenotypic switch from epithelial to endothelial integrins, increased matrix metalloproteinase (MMP) expression/activity, and interaction of histocompatibility complex molecule HLA-C with its receptor rectifying K+ channel (KIR) and subsequent decrease in natural killer (NK) cell activity cause enhanced trophoblast invasion of spiral arteries deep into the decidua, collagen degradation, and uteroplacental remodeling and increased maternal tolerance, leading to sufficient placental vascularization and blood flow. B: in preeclampsia, defective phenotypic switch of epithelial integrins, decreased MMPs, and increased NK cells cause cytotrophoblast apoptosis, shallow invasion of spiral arteries in the superficial decidua, increased collagen deposition, growth-restrictive remodeling, and maternal intolerance, leading to defective placentation and placental ischemia. VSM, vascular smooth muscle.
Endothelial intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) promote leukocyte migration and adhesion to the endothelium. During normal pregnancy, endothelial ICAM-1 and VCAM-1 are downregulated, thus minimizing leukocyte adhesion to endothelial cells and maintaining patency and blood flow in the spiral arteries. In PE, plasma levels of soluble ICAM-1 (sICAM-1) and sVCAM-1 are increased, indicating increased endothelial ICAM-1 and VCAM-1, which in turn increase leukocyte adhesion to endothelial cells and decrease blood flow in the spiral arteries (47).
Uteroplacental MMPs and Abnormal Placental Vascularization in PE
MMPs are zinc-dependent proteases that degrade ECM proteins and play a role in endometrial tissue remodeling during the menstrual cycle and estrous cycle and the uterine remodeling during pregnancy (182). MMP-2 is the main MMP in the umbilical cord, and serum MMP-9 level is elevated during normal pregnancy (120). MMP-2 is abundantly expressed in invading extravillous trophoblasts (73), and epidermal growth factor–mediated trophoblast invasion is associated with increased expression of MMP-9 (146). MMP-2 and MMP-9 are also increased in the uterus of pregnant rats (37). The increases in MMPs during pregnancy may be caused by estrogen and progesterone. In support, estrogen increases the release of MMP-2 from human VSM cells (198), and estrogen and progesterone enhance the expression of MMP-2 and MMP-9 in the uterus of virgin rats (37).
The role of MMPs in trophoblast invasion of the decidua is supported by reports that suppression of MMP-9 inhibits the invasive capability of first trimester trophoblasts (208). Also, MMP-9 knockout mice show a phenotype mimicking PE, likely due to impaired trophoblast invasion (140). Some studies have shown polymorphisms in MMP-2 and MMP-9 genes and decreased MMP-9 in PE placenta (133). Other reports have shown no significant association of MMP-9 rs3918242 polymorphism and PE (15). Also, measurements of plasma MMP levels have not been consistent in PE, with some studies showing an increase in MMP-2 (42) and others showing a decrease in MMP-9 (120). We have shown a decrease in MMP-2 and MMP-9 and an increase in collagen content in the uterus of RUPP versus normal pregnant rats (99). Because MMPs facilitate cell growth and migration by promoting proteolysis of ECM, the decreased MMP-2 and MMP-9 and increased collagen deposition in RUPP tissues could impede cell growth, proliferation, and migration and thus interfere with uteroplacental invasion and vascularization (99). MMP-1 is also expressed in cytotrophoblasts and syncytiotrophoblasts of the placenta and decidua and may play a role in trophoblast invasion. Although some studies have shown reduced levels of MMP-1 in umbilical cord blood, placenta, and decidua of PE versus normal pregnant women (38), other studies suggest a role of MMP-1 in the pathogenesis of PE (131). These observations make it important to further measure MMPs and their modulators in the plasma, placenta, and other tissues of PE women and animal models of HTN-Preg.
MMP activity could be influenced by tissue inhibitors of metalloproteases (TIMPs) and other MMP modulators. TIMP-2 or specific MMP-2 blocking antibody inhibits cytotrophoblast invasion (73). Also, extracellular MMP inducer (EMMPRIN) stimulates the production of MMP-1, MMP-2, MMP-3, and MMP-9 in blood vessels and other tissues (70). We have shown increases in EMMPRIN levels in the uterus and aorta of pregnant rats and in virgin rats treated with estrogen and progesterone compared with nontreated tissues of virgin rats. The sex hormone-induced increases in uterine and aortic MMP-2 and MMP-9 were blocked by EMMPRIN neutralizing antibody, supporting a role of EMMPRIN in the increases in uterine and vascular MMPs during pregnancy (37). Trophoblast invasion and MMPs may also be regulated by microRNAs. In PE, increased placental expression of miR-125b-1-3p may reduce the expression of S1PR1, a G protein-coupled receptor that facilitates invasion of human trophoblasts (98). Increased expression of placental miR-517a/b and miR-517c may also contribute to the decreased invasiveness of extravillous trophoblasts and the placental hypoxia associated with PE (12). In PE, increased miR-204 could target the expression of MMP-9 and in turn decrease trophoblast invasion of spiral arteries (208).
PLACENTAL ISCHEMIA/HYPOXIA IN PE
The differences in placental development, trophoblast invasive capacity of spiral arteries, and placental vascularization lead to marked differences in uteroplacental blood flow during normal pregnancy and PE. In early healthy pregnancy, the increases in vasculogenesis, angiogenesis, and trophoblast invasion and remodeling of spiral arteries contribute to progressive development of the placenta and placental vascularization. During the first trimester, the placental extravillous trophoblasts invade the maternal decidua deep into one-third of the myometrium (Fig. 3). The trophoblasts progressively invade the spiral arteries, replace the lining endothelial cells and VSM, and substitute the elastic tissue with fibrinoid material. The gradual dilation of the spiral arteries transforms them into high-capacity low-resistance vessels that maintain adequate blood supply to the growing fetus.
Because PE remits after delivery of the baby and the placenta, inadequate placental development has been implicated as a major culprit in the disorder. The predisposing risk factors and ensuing placental maladaptations are believed to cause shallow trophoblast invasion, poor expansive remodeling of the spiral arteries, and defective placentation and vascularization (Fig. 3), leading to RUPP and placental ischemia/hypoxia.
CIRCULATING BIOACTIVE FACTORS IN PE
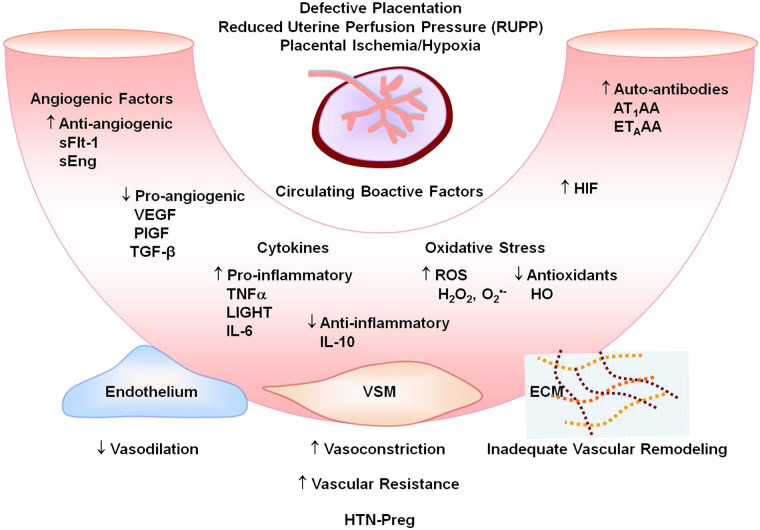
Placental ischemia is believed to trigger the release of antiangiogenic sFlt-1 and soluble endoglin (sEng) and increase their plasma levels relative to the proangiogenic vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) (143). Placental ischemia could also increase the release of proinflammatory cytokines such as TNFα and IL-6, as well as HIF, ROS, and AT1AA (Fig. 4) (94). The release of these bioactive factors locally in the vicinity of the placenta could target uteroplacental MMPs, causing further reduction in placental development and vascularization and further progression of placental ischemia and IUGR. Also, the release of these bioactive factors systemically into the maternal circulation could target the endothelium, VSM and vascular MMPs, and ECM, leading to generalized vasoconstriction and HTN-Preg.
Fig. 4.
Circulating bioactive factors in preeclampsia. Defective placentation and placental ischemia lead to increased antiangiogenic soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng) and decreased proangiogenic VEGF, placental growth factor (PlGF) and transforming growth factor-β (TGF-β); increased proinflammatory cytokines and decreased anti-inflammatory cytokines; increased reactive oxygen species (ROS) and decreased antioxidants such as hemeoxygenase (HO); and increased hypoxia-inducible factor (HIF), angiotensin II type 1 receptor agonistic autoantibodies (AT1AA), and endothelin receptor type A (ETAR) agonistic autoantibodies (ETAAA). Circulating factors could target the endothelium leading to increased vasodilation, vascular smooth muscle (VSM) leading to increased vasoconstriction, and extracellular matrix (ECM) leading to inadequate vascular remodeling, increased vascular resistance, and hypertension in pregnancy (HTN-Preg) and preeclampsia.
Proangiogenic and Antiangiogenic Factors
Vascular endothelial growth factor.
VEGF regulates endothelial cell proliferation, angiogenesis, and vascular permeability. In endothelial cells, VEGF increases cytosolic free Ca2+ concentration ([Ca2+]c), endothelial nitric oxide synthase (eNOS) activity, and prostacyclin (PGI2) (65). Some studies show an increase in circulating VEGF in PE (71). This could be explained by the possibility that the severe vasoconstriction in PE would increase vascular shear stress and, in turn, increase endothelial release of VEGF. Other studies have shown decreased circulating levels of free VEGF in PE (115). Plasma VEGF levels are also decreased in the RUPP rat model of HTN-Preg (54). The differences in the results may be related to the levels of free versus bound VEGF.
Changes in angiogenic factors could contribute to renal injury in PE. Also, although preexisting renal disease such as glomerulonephritis could play a role in the development of PE, PE could mediate glomerular injury and affect the course of glomerulonephritis, suggesting a bidirectional association between the two conditions (39). For example, glomerulonephritis would compromise renal function, make the kidney less responsive to the physiological changes that occur during pregnancy, and cause maternal vascular inflammation, thereby increasing the risk for PE. On the other hand, during PE, placental ischemia promotes the release of antiangiogenic factors, causing an imbalance between pro- and antiangiogenic factors that could result in kidney injury, and may reveal a silent preexisting glomerulonephritis or induce the development of the disease. A decrease in VEGF could also play a role in the glomerular endotheliosis and proteinuria in PE. VEGF is synthesized constitutively by podocytes in the glomerulus, where it maintains endothelial cell health and promotes the formation of fenestrae. In mice, infusion of VEGF antibodies leads to glomerular endotheliosis and proteinuria (43). Also, mice lacking one VEGF allele in renal podocytes develop a renal pathology similar to that in PE. Importantly, infusion of VEGF ameliorates the renal lesions, glomerulonephritis, and thrombotic microangiopathy in rats, suggesting potential benefits of proangiogenic factors in the glomerular endotheliosis associated with HTN-Preg (86).
Placental growth factor.
PlGF is a proangiogenic factor that binds to VEGFR-1 and enhances the angiogenic effects of VEGF. PlGF has only one-tenth the affinity of VEGF for VEGFR-1, but its levels are ~40 times higher than those of VEGF during normal pregnancy. Plasma PlGF levels markedly increase to ~353 pg/mL during gestational weeks 21 and 22, rising steadily to ~574 pg/mL after gestational weeks 29 and 30 (89). Circulating PlGF levels decrease in PE, and the decrease is more apparent in early than in late PE (112). Circulating levels of PlGF are also decreased in RUPP rats (54).
PIGF promotes endothelial cell growth, placental vasculogenesis, and vasodilation of uterine vessels (164). PlGF also promotes vasodilation of mesenteric resistance arteries of pregnant rats via VEGFR-1 and endothelium-derived hyperpolarizing factor (EDHF)-mediated activation of small conductance Ca2+-activated K+ channels (SKCa) (111). A decrease in the levels of PlGF may decrease the vasodilator responses in PE.
Soluble fms-like tyrosine kinase-1.
sFlt-1 (sVEGFR-1) is an antiangiogenic factor expressed as an alternatively spliced variant of VEGFR-1 that lacks both the transmembrane and cytoplasmic domains. sFlt-1 binds VEGF and PlGF and blocks their angiogenic effects on VEGFR. sFlt-1 may also form a heterodimer with the surface membrane VEGFR-1 and inhibit its postreceptor signaling actions (25). sFlt-1 levels are ~1.5 ng/mL in normal pregnant women, remain largely stable, and show an increase after gestational week 36 (164). PE women show imbalance between sFlt-1, VEGF, and PlGF (112, 115). The sFlt-1 gene is located on chromosome 13q12, and in women with trisomy 13, an extra copy of the sFlt-1 gene is associated with increased circulating sFlt-1, decreased PlGF, and increased risk of PE (77). Studies have shown higher circulating levels of sFlt-1 in early and late PE (112). Serum sFlt-1 is also higher in women with previous PE (~0.5 ng/mL) than in women with previous normal pregnancy (~0.3 ng/mL), and the increases can be detected even 6 mo after delivery (181). sFlt-1 levels are also greater in villous explants from PE compared with normal pregnant women (115).
Placental ischemia/hypoxia may trigger the production of sFlt-1. During placental hypoxia, HIF-1 binds to the promoter region of flt-1 gene, leading to upregulation of sFlt-1 (115). sFlt-1 e15a, a splice variant of sFlt-1 and the most abundant form released by the placenta, binds VEGF and in turn decreases endothelial cell migration, invasion, and tube formation. sFlt-1 e15a is expressed in syncytiotrophoblasts, and its serum levels are 10-fold higher in PE than in normal pregnant women (136).
The circulating ratio of sFlt-1 to PlGF (sFlt-1/PlGF ratio) is higher in PE than in normal pregnant women from second trimester onward and may serve as a predictor of early PE (112). Circulating sFlt-1 levels and the sFlt-1/PlGF ratio are also higher in twin than singleton pregnancies, likely due to the greater placental mass in twin pregnancies (46). The proportionate increases in sFlt-1 and sFlt-1/PlGF ratio in twin versus singleton pregnancies support the concept that the placenta is a major source of these factors. Extracorporeal removal of circulating sFlt-1 from PE patients decreases sFlt-1/PlGF ratio, improves symptoms, and prolongs pregnancy (176), further supporting a role of sFlt-1 in PE.
RUPP rats show increases in plasma and placental levels of sFlt-1 and sFlt-1/PlGF ratio (54). Infusion of sFlt-1 or adenoviral overexpression of sFlt-1 in pregnant rats decreases plasma VEGF levels and increases BP, proteinuria, and glomerular endotheliosis (100). Treatment of endothelial cells with PE plasma decreases angiogenesis, and removal of sFlt-1 or treatment with VEGF or a sFlt-1 antibody restores angiogenesis (1).
In human placental explants, VEGF through an action on VEGFR-2 stimulates the production of sFlt-1, which in turn antagonizes the actions of VEGF (45). This feedback modulation of VEGF by sFlt-1 may represent a protective mechanism that controls VEGF levels and prevents damage to the placenta and fetus by excess VEGF during normal pregnancy (45). This VEGF-sFlt-1 feedback mechanism may be altered in PE.
A recent study has investigated the roles of key prosurvival pathways, epidermal growth factor receptor (EGFR) signaling, and the mitochondria as upstream mechanisms regulating the release of sFlt-1 in PE. The study found that EGFR mRNA and protein were increased in PE placental tissue relative to normotensive controls. Inhibiting the EGFR signaling cascade using siRNA or small molecule inhibitors reduced sFlt-1 release from primary cytotrophoblasts. Inhibiting the mitochondrial electron transport chain or activating the downstream energy-sensing molecules AMP-activated kinase (AMPK), sirtuin 1 (SIRT1), and PPAR-γ co-activator 1 also reduced sFlt-1 secretion from cytotrophoblasts. Treating pregnant mice with EGFR inhibitor gefitinib or mitochondrial function disruptor resveratrol reduced circulating sFlt-1. Treating primary cytotrophoblasts with drugs that reduce sFlt-1 secretion either reduced EGFR signaling (esomeprazole and statins) or perturbed mitochondrial function (esomeprazole, resveratrol, and metformin). Targeting both pathways simultaneously produced additive reductions in sFlt-1 secretion. These data suggest EGFR and the mitochondria as two key survival pathways that appear to be overactive in PE and involved in the regulation of placental sFlt-1 secretion (64).
Soluble endoglin.
Transforming growth factor-β1 (TGF-β1) binds to TGF receptor and induces endothelial cell proliferation and migration. Endoglin (Eng) is a coreceptor for TGF-β1 and TGF-β3 that is highly expressed on endothelial cells and syncytiotrophoblasts (103). sEng is an antiangiogenic protein that binds TGF-β1 and prevents it from binding to its natural receptor, thereby preventing its postreceptor vasodilator and angiogenic effects (76)
Serum levels of sEng are barely detectable in nonpregnant and normal pregnant women but increase 3-, 5-, and 10-fold in women with mild PE, severe PE, and HELLP syndrome, respectively (188). Serum levels of sEng are increased in early and late PE (97). In the RUPP rat model of HTN-Preg, sEng levels are increased in the serum and placenta and serum TGF-β levels are decreased (55). sEng may act in concert with sFlt-1 to aggravate vascular permeability, HTN, proteinuria, and IUGR (188). In support, pregnant rats infused with both sEng and sFlt-1 show HELLP syndrome-like characteristics (122).
Cytokines, TNFα, and Interleukins
Circulating levels of TNFα are greater in PE than in normal pregnant women (121). LIGHT, or TNF superfamily member 14, is also increased in PE (194). The plasma levels and CD4+T cell production of TNFα are increased in RUPP versus normal pregnant rats (92). Infusion of TNFα in late pregnant rats causes HTN and proteinuria (4). Similarly, infusion of LIGHT in pregnant mice increases BP, proteinuria, and the expression of sFlt-1 and endothelin-1 (ET-1) (194). Interestingly, blockade of TNFα with the TNFα decoy receptor etanercept reduces BP in RUPP rats (92). TNF-α increases vascular permeability, fibroblast proliferation, and lymphocyte activation and promotes the production of interleukin-6 (IL-6) and IL-8. TNFα downregulates eNOS and mitochondrial biogenesis, leading to mitochondrial dysfunction and oxidative stress (157). TNFα also alters the expression of adhesion molecules in placental vessels (92).
IL-6 is another cytokine that is elevated in PE (121). RUPP rats show increased plasma levels and high CD4+T cell production of IL-6 (191). Chronic infusion of IL-6 in pregnant rats causes HTN, proteinuria, enhanced vascular contraction, and reduced endothelium-dependent relaxation (90). IL-6 promotes dimerization of the surface receptor GP-130 on endothelial cells and disrupts the tight junctions in endothelial cells, leading to vascular dysfunction and increased vascular permeability (102).
IL-10 is an anti-inflammatory cytokine. IL-10 levels are reduced in the plasma and placenta of PE women and RUPP rats (31, 139). Also, exposure of placental trophoblasts to hypoxia increases proinflammatory cytokines and decreases IL-10 (155).
Monocytes and macrophages are the main reservoirs of cytokines. Monocytes produce more TNFα and IL-6 when treated with plasma from PE than normal pregnant women (148). IL-10 may regulate the monocyte response during normal pregnancy by controlling TNFα and IL-1β gene expression (113), and the IL-10-mediated regulatory effects may be lost in PE. Interestingly, uric acid stimulates cytokine release from monocytes, and as hyperuricemia is often observed in PE women, monocytes from PE women produce more TNFα and IL-1β than monocytes from normal pregnant women (113).
Hypoxia-Inducible Factor
HIF is a transcriptional factor that plays a role in the physiologic responses to hypoxia. HIF-1 is a heterodimer consisting of an oxygen-regulated HIF-1α and HIF-2α subunits and a constitutively expressed HIF-1β subunit. In addition to hypoxia, HIF-1α can be induced by nonhypoxic stimuli such as TNFα (18). HIF-1 regulates directly or indirectly more than 2% of human genes, including VEGF, TGF-β3, and NOS (164).
HIF expression increases during pregnancy, likely due to increased estrogen and progesterone. Estrogen stimulates uterine HIF-2α, and progesterone upregulates uterine HIF-1α expression (36). Circulating HIF-1α levels are further increased in PE versus normal pregnant women (3). HIF-1α may contribute to the pathogenesis of PE by upregulating sFlt-1 and sEng, increasing big ET-1 mRNA expression, reducing trophoblast invasiveness, and inducing ANG II-converting enzyme (ACE) expression and ANG II production (164). In support, HIF-1α increases sFlt-1 in human villous trophoblasts (72). Placental levels of HIF-1α are elevated in RUPP rats (55), and downregulation of HIF-1α by siRNA reverses the increases in BP, proteinuria, renal damage, and serum levels of sFlt-1 in HTN-Preg mice (72).
Reactive Oxygen Species
Pregnancy represents a state of oxidative stress caused by increased maternal metabolism and placental metabolic activity. During normal pregnancy, the increased placental production of ROS is counterbalanced by antioxidants (164). In PE, placental ischemia/hypoxia favors oxidative stress (157), and the levels of antioxidants may not be sufficient to counterbalance the increased ROS production (127).
Neutrophils and monocytes are major sources of ROS in PE. Neutrophils from PE women produce more hydrogen peroxide (H2O2) and superoxide (O2·−) and cause more endothelial cell damage than neutrophils from normal pregnant women (179). Neutrophils also produce nitric oxide (NO), which can protect cells from the damaging effects of O2·− during normal pregnancy. However, in PE, excess O2·− scavenge the NO produced by neutrophils to form peroxynitrite (ONOO–), thus reducing NO bioavailability and causing endothelial cell damage (179). NADPH oxidase is a membrane-bound enzyme that catalyzes the one-electron reduction of oxygen to O2·−. NADPH oxidase isoform NOX1 is overexpressed in the placenta of PE women (35). In human umbilical vein endothelial cells (HUVECs), treatment with PE serum increases mRNA expression of the NADPH oxidase subunit gp91phox and augments O2·− production (114) Treatment of HUVECs with PE serum also causes overexpression of inducible nitric oxide synthase (iNOS) (114), which produces excess NO and in turn increases ROS and promotes endothelial cell injury. In first-trimester villous trophoblasts, excessive oxidative stress affects the expression of miRNAs involved in angiogenesis, apoptosis, immune response, and inflammation, thereby contributing to the pathogenesis of PE (34). Other markers of oxidative stress and lipid peroxidation such as malondialdehyde and prostaglandin F2α are increased in serum of PE women at gestational weeks 10–14, initiating progressive oxidative damage in the placenta even before overt PE (52). Plasma levels of the oxidative stress marker 8-isoprostane and total aortic and placental levels of ROS are higher in RUPP than in normal pregnant rats (10). In the RUPP rat, treatment with iNOS inhibitors decreases BP, aortic levels of ROS, and NADPH-dependent production of ROS (10).
A recent study examined potential association between adverse pregnancy outcome and imbalance in angiogenic regulators and oxidative stress biomarkers in PE. The study showed elevated levels of sFlt-1, sFlt-1/PlGF, and 8-epi-PGF2α and reduced levels of PlGF, PlGF/sFlt-1, and total antioxidant capacity among participants with PE coexisting with intrauterine fetal death, placental abruptio, placental previa, HELLP syndrome, and IUGR compared with PE without adverse outcomes. These observations suggest that imbalance in the levels of not only angiogenic regulators but also oxidative stress biomarkers correlates with adverse pregnancy outcomes in PE women (180). Antioxidant levels were also reduced in women who were later diagnosed with early PE (27). PE is associated with decreased expression of antioxidant enzymes such as hemeoxygenase-1 (HO-1), HO-2, copper/zinc superoxide dismutase, glutathione peroxidase, and catalase. HO is the rate-limiting enzyme responsible for degradation of heme to biliverdin, free iron, and carbon monoxide in the endoplasmic reticulum. Biliverdin is rapidly reduced by the cytosolic enzyme biliverdin reductase to bilirubin, an antioxidant. HO-1 via its products inhibits oxidative stress, inflammation, and apoptosis (41). Subjects with human HO-1 deficiency show oxidative stress, endothelial cell injury, and elevation of von Willebrand factor (204). ROS/antioxidant imbalance leads to lipid peroxidation, increased thromboxane A2 (TXA2), and loss of glutathione peroxidase activity in the placenta (151). In PE women, decreased plasma levels of the antioxidant ascorbate are associated with reduced brachial artery flow-mediated dilation, and administration of ascorbic acid improves flow-mediated dilation, supporting a relation between oxidative stress and endothelial dysfunction in PE (24). Also, placental levels of HO-1 are reduced in RUPP compared with normal pregnant rats (55), supporting a role of oxidative stress in HTN-Preg.
ANG II and AT1 Receptor Agonistic Autoantibodies
The renin-angiotensin system (RAS) and ANG II regulate water and electrolyte homeostasis and BP. Normal pregnancy is associated with increased blood volume with little change in BP. In normal pregnant women, plasma renin activity and substrate and plasma aldosterone are elevated as early as gestational week 6, and progressive increases are noted in renin substrate and aldosterone during the course of pregnancy (195). ANG II activates not only AT1R in VSM to promote vasoconstriction and vascular growth and inflammation, but also endothelial AT2R to increase eNOS activity and NO production, PGI2, and vasodilation. Therefore, whereas normal pregnancy is associated with increased renin and ANG II, the pressor effects of ANG II are decreased likely due to decreased sensitivity of AT1R and/or increased activity of AT2R. In a study assessing the pressor response and sensitivity to ANG II infusion throughout pregnancy, among 192 primigravid women studied, 120 remained normotensive and 72 were initially normotensive but ultimately developed HTN-Preg (51). In both groups, vascular resistance to infused ANG II (> 8 ng·kg−1·min−1 required to elicit a 20-mmHg pressor response in diastolic BP) was demonstrated as early as the tenth week of pregnancy. The dose of ANG II required to elicit a 20-mmHg pressor response in diastolic BP was progressively less by the 22nd week of pregnancy and markedly lower at gestational weeks 23–26 in women who ultimately developed HTN-Preg than in women who remained normotensive (51), suggesting an increase in the sensitivity to ANG II before clinically overt HTN-Preg. ANG II levels and AT1R mRNA expression are also increased in chorionic villi and placenta of PE versus normal pregnant women (11).
AT1AA is a bioactive factor that promotes vasoconstriction and VSM growth via AT1R. Serum levels of AT1AA are elevated in PE (199). AT1AA has been linked to increased BP, sFlt-1, ROS, cellular Ca2+, activation of coagulation factors and thrombosis, vascular damage in the adrenal glands, and reduced aldosterone secretion in PE (199). AT1AA also promotes collagen-induced platelet aggregation, which contributes to the hypercoagulability in PE (14). In cultured trophoblasts, stimulation of AT1R with IgG isolated from PE women causes increases in sFlt-1 (210). In HUVECs, treatment with AT1AA isolated from PE women induces the release of the cell death and necrosis marker lactate dehydrogenase (205). Also, in HUVECs, AT1AA induces the activity of caspase-3 and caspase-8 and promotes endothelial cell apoptosis (205). Circulating levels of AT1AA are increased in RUPP versus normal pregnant rats (93). Infusion of AT1AA in pregnant mice causes PE-like manifestations, including increased BP, proteinuria, and plasma sFlt-1 levels (168). Also, infusion of AT1AA in pregnant rats increases ET-1 levels in the placenta and renal cortex (91). Acetylcholine (ACh)-induced vasodilation is reduced in the renal interlobar arteries of pregnant rats infused with AT1AA and reversed by an endothelin receptor type A (ETAR) antagonist, suggesting an interplay between ANG II and ET-1 in the setting of endothelial dysfunction and HTN-Preg (137). Plasma levels of AT1AA are increased in pregnant rats infused with TNFα, suggesting cytokine-mediated induction of AT1AA (93).
Extracellular Vesicles in PE
Extracellular vesicles (EVs) and exosomes are lipid-bilayer structures that are released from red blood cells, fibroblasts, endothelial cells, and trophoblasts into the extracellular environment. EVs contain proteins, miRNAs, growth and apoptotic factors, and other components that induce cell-to-cell communication and signaling throughout the body. Released EVs modify the activity of adjacent cells or travel to regions distal to the site of release in several body fluids. In PE, impaired placental development and placental apoptosis and necrosis cause the release of micro- and nanovesicles. A recent study showed altered expression/activity of the renal sodium transporters sodium potassium chloride cotransporter 2 (NKCC2), sodium chloride cotransporter (NCC), and epithelial sodium channel (ENaC) in urinary extracellular vesicles or exosomes in women with PE. The study showed an increase in phosphorylation of the activating S130 site in NKCC2, the drug target for frusemide; decrease in phosphorylation of the activating T60 site in NCC, the drug target for thiazide diuretics; and increased expression of the larger forms of the α-subunit of ENaC, the drug target for amiloride, in women with PE compared with normal pregnant women. The increase in the activity of NKCC2 and ENaC and decrease in NCC are predicted to increase sodium reabsorption and contribute to hypertension in PE (69). EVs originating from placental explants have been shown to promote cytokine production and endothelial dysfunction and may be involved in the pathogenesis of PE (44).
VASCULAR DYSFUNCTION IN HTN-PREG AND PE
Normal pregnancy is associated with vasodilation of the maternal uterine, renal, and systemic vessels and reduction in the mechanisms of vascular contraction, partly due to increased plasma levels of estrogen and progesterone. Estrogen causes relaxation of uterine artery (161) and promotes endothelium-dependent vascular relaxation by increasing the release of NO, PGI2, and EDHF. Estrogen also inhibits the mechanisms of VSM contraction, including [Ca2+]c and protein kinase C (PKC) (81). Progesterone inhibits contraction of blood vessels and causes vasodilation by mechanisms similar to estrogen (126). In contrast with the systemic vasodilation during normal pregnancy, PE is associated with vascular dysfunction manifested as endothelial cell dysfunction, increased mechanisms of VSM contraction, and inadequate ECM metabolism and vascular remodeling.
ENDOTHELIAL CELL DYSFUNCTION IN PE
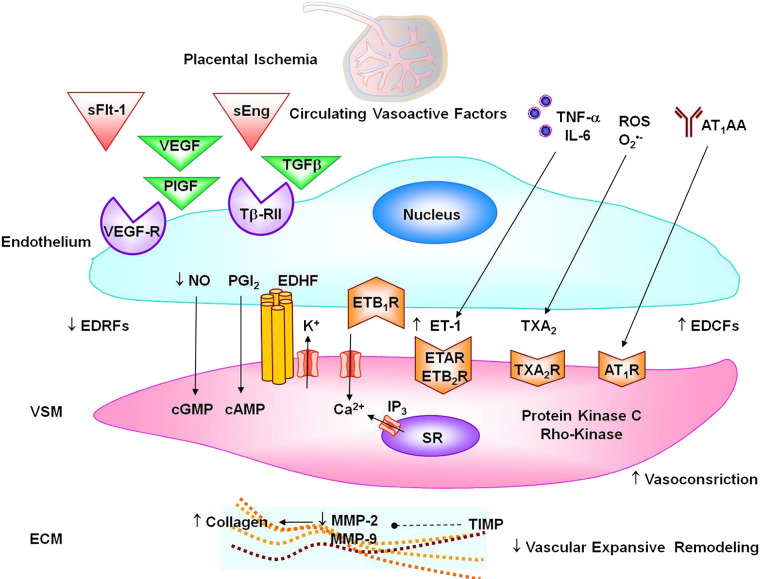
Adaptation of the maternal vasculature to pregnancy is of critical importance to enhance the capacity for blood flow in the uteroplacental circulation to meet the demands of the developing fetus (19). Normal and functional endothelium ensures healthy gestation and a favorable prognosis for the mother and fetus. Brachial artery flow-mediated dilation increases as gestation progresses (169). Endothelium-dependent bradykinin-induced relaxation is increased in subcutaneous arteries from pregnant versus nonpregnant women (87). Failure of the maternal vasculature to adequately adapt to pregnancy can lead to hypertensive disorders of pregnancy including PE (19). Women with PE show systemic endothelial cell dysfunction (164). Brachial artery flow-mediated dilation and radial artery vasodilation are less in PE than normal pregnant women (59). Bradykinin-induced relaxation is decreased in subcutaneous arteries of PE versus normal pregnant women (87). Circulating endothelial cells and other markers of endothelial injury such as soluble VCAM-1, E-selectin, and endocan are increased in PE (181). On the other hand, circulating endothelial progenitor cells are decreased in PE (156). Also, ACh is less potent in inducing relaxation in blood vessels of RUPP versus normal pregnant rats (116). Endothelial dysfunction could manifest as decreased release of vasodilator substances such as nitric oxide (NO), prostacyclin (PGI2), and endothelium-derived hyperpolarizing factor (EDHF) or increased release of contracting factors such as endothelin-1 (ET-1) and thromboxane A2 (TXA2) (Fig. 5).
Fig. 5.
Vascular targets in preeclampsia. Circulating soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng) prevent VEGF, placental growth factor (PlGF), and transforming growth factor-β (TGF-β) from interacting with their natural endothelial cell receptors VEGFR and Tβ-RII, leading to decreased endothelium-derived relaxing factors (EDRFs) nitric oxide (NO), prostacyclin (PGI2), endothelium-derived hyperpolarizing factor (EDHF), and endothelial ETB1R; decreased vascular smooth muscle (VSM) cyclic guanosine monophosphate (cGMP), cAMP, and hyperpolarization; and increased VSM Ca2+ and contraction. Circulating cytokines and reactive oxygen species (ROS) cause increases in endothelium-derived contacting factors (EDCFs) endothelin-1 (ET-1) and thromboxane A2 (TXA2) and stimulation of VSM endothelin receptor type A (ETAR), ETB2R, and TXA2R. Angiotensin II type 1 receptor agonistic autoantibodies (AT1AA) agonistic autoantibodies activate VSM angiotensin II type 1 receptor (AT1R). Activation of vasoconstrictor receptors in VSM causes stimulation of Ca2+ influx through Ca2+ channels and Ca2+ release from the sarcoplasmic reticulum (SR) and activation of protein kinase C and Rho-kinase, leading to further increases in VSM contraction. Vasoactive factors could also decrease matrix metalloproteinase (MMP)-2 and MMP-9 in extracellular matrix (ECM) either directly or indirectly through increasing tissue inhibitor of metalloproteases (TIMP), leading to increased collagen deposition and reduced vascular expansive remodeling.
Changes in Nitric Oxide in PE
NO is a potent vasodilator and relaxant of VSM. NO diffuses into VSM and increases cyclic guanosine monophosphate (cGMP), which decreases VSM [Ca2+]c and causes VSM relaxation. Nitrites are NO metabolites that show increases in serum of pregnant women (163). Plasma levels and urinary excretion of cGMP, a second messenger of NO, are also increased in normal pregnancy. NOS expression/activity increase in human uterine artery and the placenta with gestational age (128). Also, urinary nitrite levels, mRNA expression of eNOS, iNOS and neuronal NOS (nNOS), and protein level of activated phospho-eNOS are increased in normal pregnant versus virgin rats (7).
Polymorphisms in eNOS gene could lead to endothelial dysfunction in PE. Endothelial dysfunction is associated with decreased synthesis/bioavailability of NO. Studies have shown increases (130) or decreases (159) in plasma nitrite levels and no difference in urinary nitrite levels in PE versus normal pregnant women (159). This may not be solely related to dietary nitrate intake, as a study that carefully controlled dietary nitrate/nitrite intake did not show decreased NO production in PE women (30). Also, the plasma and urinary levels of cGMP are not different in PE versus normal pregnant women (159). The lack of change in whole body NO despite the increase in BP and the renal damage in PE suggests tissue-specific changes in NOS expression. Whereas some studies showed a decrease in eNOS expression in the umbilical cord (16), other studies showed increases in eNOS mRNA expression in placenta of PE versus normal pregnant women (170).
In mid-to-late pregnant rats, NOS blockade with Nω-nitro-l-arginine methyl ester (l-NAME) causes increases in BP, renal vasoconstriction, proteinuria, thrombocytopenia, and IUGR (84). However, although plasma nitrite levels are lower in RUPP than in normal pregnant rats (9), no differences are observed in urinary nitrite levels (5). Also, no changes in circulating levels of the NOS substrate l-arginine are observed in RUPP versus normal pregnant rats (6). Vascular function studies have shown reduction in ACh-induced relaxation, eNOS expression, and NO production in blood vessels of RUPP versus normal pregnant rats, supporting reduced NO synthesis in the vasculature (116).
In addition to its vascular effects, NO plays a role in the regulation of renal tubular function and immune modulation and as an antioxidant, and dysregulation of these NO functions may contribute to the pathophysiological mechanisms of HTN-Preg and PE.
Changes in Prostacyclin in PE
PGI2 is a potent vasodilator and inhibitor of platelet aggregation produced from the metabolism of arachidonic acid by cyclooxygenase 2 (COX)-2 and COX-1. During normal pregnancy, the levels of 6-keto-PGF1α, a stable metabolite of PGI2, are increased in fetoplacental tissues (110). Plasma and urinary levels of PGF1α are decreased in severe PE, suggesting diminished overall synthesis of PGI2 (110). Endothelial PGI2 production is also decreased in PE (174). Although PGI2 release from apical and basal trophoblasts is not different in PE versus normal pregnant women, the release of TXA2, another COX product, from basal trophoblasts is increased and may cause placental vasoconstriction in PE (209).
Hydrogen Sulfide
In addition to the contribution of NO and PGI2 to the vascular relaxation responses, some arteries produce hydrogen sulfide (H2S) as a vasodilator substance. Reduction in plasma levels of H2S has been reported in PE. Also, cystathionine-γ-lyase, the primary H2S-synthesizing enzyme in the vasculature, is reduced in PE (193).
Endothelium-Derived Hyperpolarizing Factorin PE
EDHF is a relaxing factor with specialized role in the control of small resistance vessels, local organ blood flow, and BP. Although the nature of EDHF is unclear, it often presents as K+ efflux from endothelial cells through intermediate (IKCa) and small conductance (SKCa) Ca2+-activated K+ channels causing hyperpolarization of endothelial cells. Endothelial cell hyperpolarization then spreads via myoendothelial gap junctions (MEGJs) and connexins to cause VSM hyperpolarization and reduce Ca2+ influx through voltage-dependent Ca2+ channels. The opening of endothelial cell IKCa and SKCa could also cause accumulation of K+ in the myoendothelial interface and in turn cause VSM hyperpolarization by activating the inwardly rectifying K+ channel (KIR) and Na+/K+-ATPase (28). EDHF could also be released from endothelial cells as a diffusible factor such as the cytochrome P450 (CYP450) product epoxyeicosatrienoic acid (EET) and H2O2 (166).
In small subcutaneous arteries of normal pregnant women, EDHF is responsible for ~50% of bradykinin-induced relaxation, acting with NO to maintain proper vascular tone (105). Connexins 37, 40, and 43 in the gap junction are partly involved in EDHF-mediated vascular response during normal pregnancy (63). Increased activity of endothelial IKCa and SKCa channels and the delayed rectifier type of voltage-sensitive K+ channels (Kv) also promote EDHF-mediated dilation in uterine arteries of pregnant rats (50, 57). Studies in mice have shown enhanced bradykinin-induced vasodilation in pregnant wild-type mice but not knockout mice lacking pregnane X receptor, a nuclear receptor that induces the expression of CYP450. Also, treatment with CYP450 inhibitor decreases the vasodilatory response to bradykinin in wild-type but not the knockout mice, supporting that CYP450 metabolites such as EET play a role in vascular relaxation during pregnancy (60).
Whereas MEGJs are the main pathway of EDHF-mediated relaxation in subcutaneous arteries of normal pregnant women, in women with PE, MEGJs (in combination with H2O2 or CYP450 epoxygenase metabolites of arachidonic acid) mediate EDHF-induced vasodilation. The changes in the role of MEGJs may be caused by morphological changes in the vascular wall during PE (105). Small myometrial arteries from PE women also show reduced vasodilation and decreased contribution of EDHF due to physical disruption of MEGJs (104).
Endothelin-1 in PE
ET-1 is a major endothelium-derived vasoconstrictor. In PE, circulating cytokines and AT1AA stimulate endothelial cells to produce ET-1 (53). In HUVECs, PE serum produces greater amounts of ET-1 than normal pregnant serum (158). Some studies have shown elevated plasma ET-1 levels during late PE (175), suggesting that ET-1 may be involved in the progression rather than the initiation of PE. However, in most studies, serum ET-1 levels do not differ in PE versus normal pregnant women and high levels of ET-1 are observed mainly in severe PE and HELLP syndrome (82). Of note, ET-1 is released in a paracrine fashion from endothelial cells toward VSMCs, and the increases in ET-1 levels in PE may be localized. Studies have shown a four- to eight-fold increase in ET-1 levels in umbilical cord cells and renal tissues in later stages of PE (175). In perfused placentas under hypoxia, both the maternal and fetal side produce high levels of ET-1 (75). Also, preproET levels are increased in renal cortex and medulla of RUPP rats (8). It is possible that in severe PE and HELLP syndrome, ET-1 production is so augmented that it loses its paracrine directionality and increases circulating ET-1. In support, rat models of severe PE and HELLP syndrome show increased plasma ET-1 levels (122).
ET-1 may play a role in the pathogenesis of PE by inducing apoptosis of trophoblasts and increasing antiangiogenic and oxidant substances (53). ET-1 activates endothelin receptor type A (ETAR) and type B (ETBR). ET-1 activation of VSM ETAR increases [Ca2+]c protein kinase C activity and VSM contraction. VSM ETAR and ET-1-induced vasoconstriction are reduced in blood vessels of late-pregnant rats (134). Also, treatment with ETAR antagonist reduces BP in RUPP rats (8). Of note, among Brazilian women with PE, 52% of the patients with severe PE showed increases in ETAR agonistic autoantibodies (ETAAA), which could target ETAR and increase vasoconstriction (187).
ET-1 also activates ETBR in endothelial cells and stimulates the release of NO, PGI2, and EDHF and in turn promotes renal vasodilation and hyperfiltration in pregnant rats (29). ETBR expression is reduced in endothelial and renal cells of RUPP rats. Also, ETBR-mediated NO production is less in blood vessels of RUPP versus normal pregnant rats, supporting that downregulation of endothelial ETBR plays a role in HTN-Preg (116).
Thromboxane A2 in PE
TXA2 stimulates platelet aggregation, vasoconstriction, and VSM cell proliferation. TXA2 production is increased and could contribute to HTN and increased platelet aggregation in PE. Imbalance between urinary TXB2 metabolites, markers of TXA2 synthesis, and PGI2 predates clinical symptoms of PE (20). Antiplatelet agents such as low-dose aspirin and other thromboxane modulators have been considered for management of PE. Although women at high risk for PE showed no benefit of low-dose aspirin as a preventive measure (164), ozagrel, a thromboxane modulator, reduces HTN-Preg and proteinuria (162), making it important to further examine the role of TXA2 in PE.
VASCULAR SMOOTH MUSCLE DYSFUNCTION IN PE
VSM Ca2+ in PE
Ca2+ is a major determinant of VSM contraction. During normal pregnancy, increased KCa channel activity decreases uterine artery tonicity and [Ca2+]c, leading to increases in uteroplacental blood flow. In PE, KCa channel activity is suppressed, leading to increased uterine artery [Ca2+]c, vasoconstriction, and reduced fetal blood flow (200). Myometrial vessels from normal pregnant and PE women may show similar vasoconstriction responses to phenylephrine and ANG II (197). However, basal and agonist-stimulated [Ca2+]c are increased in renal arterial VSM of pregnant rats treated with l-NAME versus nontreated pregnant rats (124). ANG II- and caffeine-induced transient contraction and [Ca2+]c in Ca2+-free solution are similar, but KCl-induced maintained [Ca2+]c in a Ca2+-containing medium is greater in VSM of RUPP versus normal pregnant rats, supporting a role of Ca2+ entry from the extracellular in the increased vasoconstriction in HTN-Preg (125).
Protein Kinase C in PE
PKC increases myofilament force sensitivity to Ca2+ and enhances VSM contraction. PKC activity and contraction are reduced in uterine artery of late pregnant ewes and the aorta of late pregnant rats (80, 109). Also, the expression of α-PKC, δ-PKC, and ζ-PKC isoforms is increased in aortic VSM of l-NAME-treated versus nontreated pregnant rats (80). Increased BKCa channel activity inhibits PKC-mediated contraction in ovine uterine arteries during pregnancy, and gestational hypoxia may upregulate PKC and inhibit BKCa (200). In cultured rat cardiomyocytes, treatment with IgG from PE women enhances AT1R-mediated response, and these effects are ameliorated with the PKC inhibitor calphostin C, suggesting a role of PKC in the production and effects of AT1AA (192). Cicletanine is an antihypertensive drug that could lower BP through inhibition of PKC.
VSM Rho-Kinase in PE
Rho is a family of small GTP-binding proteins that are involved in cell migration, cytoskeletal reorganization, and VSM contraction. RhoA binds to GTP and activates Rho-kinase (ROCK). ROCK has two isoforms, ROCK-1 (ROCK-I, ROKβ) and ROCK-2 (ROCK-II, ROKα), which contribute to the formation of placental microvilli during pregnancy. In PE, placental villi show abnormal expression of ROCK-II and apoptosis of the syncytium (13). ROCK increases Ca2+ sensitivity of the contractile proteins in small subcutaneous arteries of PE women (185). Also, ANG II via AT1R increases RhoA/ROCK activity in l-NAME-treated hypertensive rats (83). ROCK may stimulate IL-17 to phosphorylate the inhibitory eNOS Thr495 residue, thus decreasing NO production in PE (129). However, some studies show decreased ROCK expression in umbilical arteries of PE women (49), making it important to further examine the role of ROCK in the vascular changes in PE.
VASCULAR MMPS, EXTRACELLULAR MATRIX, AND REMODELING IN PE
ECM is an integral component of the vascular wall, and MMPs play a role in angiogenesis, ECM metabolism, and vascular remodeling. MMPs degrade collagen, gelatin, and other protein substrates (189). We have shown a decrease in MMP-2 and MMP-9 and an increase in collagen in the aorta of RUPP versus normal pregnant rats (99). The decreased MMPs and increased vascular collagen could increase the blood vessel rigidity and decrease its plasticity and thus contribute to increased vascular resistance and HTN-Preg. Collagen has 18 types and different subtypes. MMP-2 can degrade collagen I, II, III, IV, V, VII, X, and XI, whereas MMP-9 can degrade collagen IV, V, VII, X, XIV (189), and the changes in various collagen subtypes in HTN-Preg should be further examined.
Although MMPs are largely known for their proteolytic effects on ECM, we and others have identified novel MMP actions that could affect membrane receptors, cell signaling, and vascular function (26, 131, 147). Prolonged increases in intravascular pressure and wall tension cause increases in MMP-2 and MMP-9 expression (147). Also, MMP-2 and MMP-9 cause relaxation of rat aorta (26). Thus, during normal pregnancy, plasma volume expansion could lead to increased MMP-2 and MMP-9, vasodilation, and decreased BP. The decrease in vascular MMP-2 and MMP-9 may explain the decreased ACh-induced relaxation in blood vessels of RUPP rats (116).
MMPs break big-ET-1 into different endothelins with different affinities for ETAR and ETBR. In omental vessels from pregnant women, MMP-1 causes vasoconstriction and enhances reactivity to ANG II via an endothelium-dependent protease-activated receptor, ET-1 release, and activation of VSM ETAR (131). MMP-2 and MMP-9 could degrade big-ET to ET1–32, which preferentially stimulates endothelial ETBR and promotes relaxation. ETBR is upregulated in normal pregnant rats and downregulated in RUPP rats, and infusion of the ETBR antagonist BQ788 increases BP in pregnant rats (116). Also, MMP-2 induces vascular relaxation via hyperpolarization and activation of K+ channels (147), and the decrease in MMP-2 and MMP-9 mediated relaxation may contribute to the increased vascular contraction and BP in RUPP rats.
PREDICTION AND MANAGEMENT OF PE
PE manifests in late gestation, and identifying women at risk early during pregnancy should allow prompt management and improve the maternal and perinatal outcome. Clinical history of PE, HTN in a previous pregnancy, or a preexisting medical condition such as HTN, obesity, or autoimmune disease would help identify women at increased risk of PE, and additional laboratory tests and biomarkers would confirm the diagnosis. Thrombocytopenia is a common finding in PE and usually progresses with severity of the disorder (167). Doppler screening and detection of early diastolic bilateral uterine artery notching in the waveform could be predictors of PE. A decrease in brachial artery flow-mediated vasodilation is also an early indicator of endothelial dysfunction in PE (21).
Measurements of plasma VEGF, PlGF, sFlt-1, and sEng may help early detection of PE. In PE, circulating levels of sFlt-1 are increased more than one month before the onset of clinical symptoms, and PlGF is decreased in women who subsequently develop PE from the end of the first trimester (66). Also, sFlt-1/PlGF ratio is increased in PE, and its overall diagnostic accuracy is higher in early than in late PE (112).
Abnormal maternal immunological response and changes in monocytes and NK cells, proinflammatory cytokines, and AT1AA could help in predicting PE in early pregnancy. The alternate complement pathway is upregulated, and plasma levels of factor B-derived Bb fragment are elevated in PE women (164, 165). Plasma obtained at gestational weeks 11–13 showed high TNFα levels in women who later developed PE (62). Elevated plasma TNFα levels in association with changes in uterine artery Doppler at gestational weeks 11–13 have a 100% sensitivity in predicting PE (58). Elevated serum level of placental glycoprotein pregnancy-associated plasma protein-A (PAPP-A) and placental protein 13 (PP13), together with abnormal Doppler ultrasound of the uterine artery, also have a high predictive value in PE (132),
Amniocentesis and amniotic fluid analysis may be useful in predicting PE. Insulin-like factor 3 (INSL3), which is produced from the fetal testis to promote transabdominal testicular descent, is elevated in amniotic fluid of women with a male fetus who later develop PE (164). The amniotic fluid levels of inhibin A, a glycoprotein produced by syncytiotrophoblast, are elevated in women who later develop severe PE (85). Also, the level of sFlt-1 is elevated in the amniotic fluid of PE women (173).
Microarray analysis could be used to screen the placental transcriptome for upregulated and downregulated genes in PE. Cell-free mRNA levels of plasminogen activator inhibitor-1 (PAI-1), tissue-type plasminogen activator (t-PA), VEGFR-1 (Flt-1), endoglin, placenta-specific protein 1 and P-selectin are increased in plasma of women who later developed PE (145). MicroRNAs (miRNAs, miRs) are small single‐stranded and noncoding molecules that can negatively regulate gene expression and play a role in different pathological conditions, including PE (107). For instance, miR-206 interacts with several genes involved in PE and is elevated at gestational week 28 in plasma and placenta from women who later developed PE (2). Upregulation of miR‐499a‐5p, which is associated with cardiovascular and cerebrovascular diseases, could be found in placentas of patients with PE and IUGR, whereas downregulation of miR‐26a‐5p, miR‐103a‐3p, and miR‐145‐5p could be found in early-onset PE and IUGR (68). Other studies showed eight upregulated miRNAs, including miR-19a-3p, miR-877-3p, miR-148a-3p, miR-212-5p, miR-1825, miR-210-3p, miR-940, and miR-134-5p, and two downregulated miRNAs, miR-3609 and miR-145-5p, in the placenta of women with PE (106). Some studies have suggested that increased expression of placental miR-517a/b and miR-517c may contribute to decreased invasiveness of extravillous trophoblasts and placental hypoxia in PE (12). However, other studies have shown downregulation of miR‐517a, miR‐517b, and miR‐141 in cotyledons isolated from the placenta of women with PE (33); therefore, the predictive value of miRNAs in PE is not universally accepted.
In search for other PE biomarkers, studies have shown that pregnant mice deficient in catechol-O-methyltransferase (COMT) show a PE-like phenotype. COMT deficiency leads to a decrease in 2-methoxyestradiol (2-ME), a natural metabolite of estradiol, which is normally elevated during the third trimester of normal pregnancy. Administration of 2-ME ameliorated the PE-like features in pregnant Comt–/–mice and suppressed placental hypoxia, HIF-1α expression, and sFlt-1. Plasma levels of COMT and 2-ME are decreased in PE women and may be used as biomarkers for PE (79). Despite the large number of proposed biomarkers of PE, a single biomarker may not have a high predictive value alone, and a cluster of biomarkers may be more useful in predicting PE.
Currently, inducing delivery of the fetus and placenta are the most effective measures for PE. Prenatal care, bed rest, and anti-HTN drugs are important in the management of PE. International guidelines recommend one anti-HTN drug such as methyldopa, β-blocker (labetalol, propranolol), or Ca2+ channel blocker (nifedipine) (108). Angiotensin receptor blockers (ARBs) and ACE inhibitors are contraindicated due to their teratogenic effects. If PE worsens to eclampsia, maintaining airway patency should prevent fluid aspiration, and anticonvulsants such as Mg2+ sulfate infusion should control seizures (117).
Research studies have shown that infusion of PlGF or VEGF reduces BP in RUPP rats (56, 172). VEGF could improve the angiogenic imbalance in PE but may enhance vascular permeability (186). Modulators of PlGF could be more promising in PE. Low molecular weight heparin increases circulating PlGF levels during the third trimester (206).
Complement inhibitors such as eculizumab, an anti-C5 antibody, have been suggested to normalize laboratory values and prolong pregnancy in women with PE/HELLP syndrome, but they may increase susceptibility to infection (23). TNFα antagonists such as etanercept decrease BP, increase eNOS expression, and decrease ET-1 levels in RUPP rats (92). IL-17 soluble receptor C inhibits IL-17, prevents the recruitment of host defense cells, suppresses the inflammatory response, decreases AT1AA and ROS, and ameliorates HTN and pup and placental weight in RUPP rats (32).
Another potential approach in PE is to target the downstream vascular mediators affected by cytoactive factors. Sildenafil promotes fetal growth in women with severe early-onset IUGR (190). Sildenafil citrate dilates myometrial artery and restores endothelial cell integrity in placental vessels of the l-NAME-treated pregnant mouse (123). Modulating MMP expression/activity could promote vasodilation and reduce BP. Doxycycline is an MMP inhibitor that has been shown to alleviate HTN and vascular dysfunction in HTN-Preg rats (135). Novel approaches to directly or indirectly correct mediators of vascular dysfunction should provide new strategies in the management of PE.
PERSPECTIVES
Normal pregnancy is associated with uteroplacental and vascular remodeling to adapt for the growing fetus. In PE, genetic and environmental risk factors cause abnormal maternal immune response, apoptosis of trophoblast cells, defective placentation, shallow trophoblast invasion, and inadequate spiral artery remodeling, leading to RUPP and placental ischemia/hypoxia. Ischemic/hypoxic placenta causes the release of bioactive factors such as sFlt-1, sEng, TNFα, IL-6, HIF, ROS, and AT1AA. Bioactive factors could target endothelial cells, causing endothelial dysfunction, decreased vasodilators, and increased ET-1, or VSM cells, causing increases in Ca2+, PKC, and ROCK and leading to increased vasoconstriction. Bioactive factors could also target MMPs and ECM and cause inadequate vascular remodeling, leading to increased vascular rigidity and HTN-Preg, or inadequate uteroplacental remodeling, leading to growth-restrictive remodeling and IUGR. The levels of these bioactive factors and vascular mediators vary during the course of pregnancy, in early versus late, and in mild versus severe PE. Further understanding of the interaction between bioactive factors, vascular mechanisms, and molecular targets should help design more efficient measures for early detection and management of PE.
GRANTS
This work was supported by BRI Fund to Sustain Research Excellence from Brigham Research Institute and a grant from National Heart, Lung, and Blood Institute (R56HL147889-01). Dr. H. Qu was a visiting scholar from Department of Obstetrics, Yuhuangding Hospital Affiliated to Qingdao University Yantai, Shandong, P.R. China, and a recipient of a fellowship from China Scholarship Council.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.A.K. conceived and designed research; R.A.K. prepared figures; R.A.K. drafted manuscript; H.Q. and R.A.K. edited and revised manuscript; H.Q. and R.A.K. approved final version of manuscript.
REFERENCES
- 1.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res 95: 884–891, 2004. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 2.Akehurst C, Small HY, Sharafetdinova L, Forrest R, Beattie W, Brown CE, Robinson SW, McClure JD, Work LM, Carty DM, McBride MW, Freeman DJ, Delles C. Differential expression of microRNA-206 and its target genes in preeclampsia. J Hypertens 33: 2068–2074, 2015. doi: 10.1097/HJH.0000000000000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akhilesh M, Mahalingam V, Nalliah S, Ali RM, Ganesalingam M, Haleagrahara N. Hypoxia-inducible factor-1α as a predictive marker in pre-eclampsia. Biomed Rep 1: 257–258, 2013. doi: 10.3892/br.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-α-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens 15: 170–175, 2002. doi: 10.1016/S0895-7061(01)02255-5. [DOI] [PubMed] [Google Scholar]
- 5.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2001. doi: 10.1161/01.HYP.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 6.Alexander BT, Llinas MT, Kruckeberg WC, Granger JP. l-Arginine attenuates hypertension in pregnant rats with reduced uterine perfusion pressure. Hypertension 43: 832–836, 2004. doi: 10.1161/01.HYP.0000119192.32360.a9. [DOI] [PubMed] [Google Scholar]
- 7.Alexander BT, Miller MT, Kassab S, Novak J, Reckelhoff JF, Kruckeberg WC, Granger JP. Differential expression of renal nitric oxide synthase isoforms during pregnancy in rats. Hypertension 33: 435–439, 1999. doi: 10.1161/01.HYP.33.1.435. [DOI] [PubMed] [Google Scholar]
- 8.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension 37: 485–489, 2001. doi: 10.1161/01.HYP.37.2.485. [DOI] [PubMed] [Google Scholar]
- 9.Amaral LM, Cornelius DC, Harmon A, Moseley J, Martin JN Jr, LaMarca B. 17-Hydroxyprogesterone caproate significantly improves clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension 65: 225–231, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amaral LM, Pinheiro LC, Guimaraes DA, Palei AC, Sertório JT, Portella RL, Tanus-Santos JE. Antihypertensive effects of inducible nitric oxide synthase inhibition in experimental pre-eclampsia. J Cell Mol Med 17: 1300–1307, 2013. doi: 10.1111/jcmm.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anton L, Merrill DC, Neves LA, Stovall K, Gallagher PE, Diz DI, Moorefield C, Gruver C, Ferrario CM, Brosnihan KB. Activation of local chorionic villi angiotensin II levels but not angiotensin (1-7) in preeclampsia. Hypertension 51: 1066–1072, 2008. doi: 10.1161/HYPERTENSIONAHA.107.103861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anton L, Olarerin-George AO, Hogenesch JB, Elovitz MA. Placental expression of miR-517a/b and miR-517c contributes to trophoblast dysfunction and preeclampsia. PLoS One 10: e0122707, 2015. doi: 10.1371/journal.pone.0122707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ark M, Yilmaz N, Yazici G, Kubat H, Aktaş S. Rho-associated protein kinase II (rock II) expression in normal and preeclamptic human placentas. Placenta 26: 81–84, 2005. doi: 10.1016/j.placenta.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Bai K, Wang K, Li X, Wang J, Zhang J, Song L, Wang J, Zhang S, Lau WB, Ma X, Liu H. Autoantibody against angiotensin AT1 receptor from preeclamptic patients enhances collagen-induced human platelet aggregation. Acta Biochim Biophys Sin (Shanghai) 45: 749–755, 2013. doi: 10.1093/abbs/gmt059. [DOI] [PubMed] [Google Scholar]
- 15.Barišić A, Dević Pavlić S, Ostojić S, Pereza N. Matrix metalloproteinase and tissue inhibitors of metalloproteinases gene polymorphisms in disorders that influence fertility and pregnancy complications: A systematic review and meta-analysis. Gene 647: 48–60, 2018. doi: 10.1016/j.gene.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Bhavina K, Radhika J, Pandian SS. VEGF and eNOS expression in umbilical cord from pregnancy complicated by hypertensive disorder with different severity. BioMed Res Int 2014: 982159, 2014. doi: 10.1155/2014/982159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birukov A, Andersen LB, Herse F, Rakova N, Kitlen G, Kyhl HB, Golic M, Haase N, Kräker K, Müller DN, Jørgensen JS, Andersen MS, Dechend R, Jensen BL. Aldosterone, salt, and potassium intakes as predictors of pregnancy outcome, including preeclampsia. Hypertension 74: 391–398, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12924. [DOI] [PubMed] [Google Scholar]
- 18.Bobek G, Surmon L, Mirabito KM, Makris A, Hennessy A. Placental regulation of inflammation and hypoxia after TNF-α infusion in mice. Am J Reprod Immunol 74: 407–418, 2015. doi: 10.1111/aji.12417. [DOI] [PubMed] [Google Scholar]
- 19.Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol 232: R27–R44, 2017. doi: 10.1530/JOE-16-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowen RS, Zhang Y, Gu Y, Lewis DF, Wang Y. Increased phospholipase A2 and thromboxane but not prostacyclin production by placental trophoblast cells from normal and preeclamptic pregnancies cultured under hypoxia condition. Placenta 26: 402–409, 2005. doi: 10.1016/j.placenta.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Brandão AH, Félix LR, do Carmo Patrício E, Leite HV, Cabral AC. Difference of endothelial function during pregnancies as a method to predict preeclampsia. Arch Gynecol Obstet 290: 471–477, 2014. doi: 10.1007/s00404-014-3243-3. [DOI] [PubMed] [Google Scholar]
- 22.Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S; International Society for the Study of Hypertension in Pregnancy (ISSHP) . The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens 13: 291–310, 2018. doi: 10.1016/j.preghy.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Burwick RM, Feinberg BB. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta 34: 201–203, 2013. doi: 10.1016/j.placenta.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. JAMA 285: 1607–1612, 2001. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- 25.Charnock-Jones DS. Placental hypoxia, endoplasmic reticulum stress and maternal endothelial sensitisation by sFLT1 in pre-eclampsia. J Reprod Immunol 114: 81–85, 2016. doi: 10.1016/j.jri.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chew DK, Conte MS, Khalil RA. Matrix metalloproteinase-specific inhibition of Ca2+ entry mechanisms of vascular contraction. J Vasc Surg 40: 1001–1010, 2004. doi: 10.1016/j.jvs.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 27.Cohen JM, Kramer MS, Platt RW, Basso O, Evans RW, Kahn SR. The association between maternal antioxidant levels in midpregnancy and preeclampsia. Am J Obstet Gynecol 213: 695.e1–695.e13, 2015. doi: 10.1016/j.ajog.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 28.Coleman HA, Tare M, Parkington HC. Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease. Clin Exp Pharmacol Physiol 31: 641–649, 2004. doi: 10.1111/j.1440-1681.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- 29.Conrad KP, Gandley RE, Ogawa T, Nakanishi S, Danielson LA. Endothelin mediates renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. Am J Physiol Renal Physiol 276: F767–F776, 1999. doi: 10.1152/ajprenal.1999.276.5.F767. [DOI] [PubMed] [Google Scholar]
- 30.Conrad KP, Kerchner LJ, Mosher MD. Plasma and 24-h NOx and cGMP during normal pregnancy and preeclampsia in women on a reduced NOx diet. Am J Physiol Renal Physiol 277: F48–F57, 1999. doi: 10.1152/ajprenal.1999.277.1.F48. [DOI] [PubMed] [Google Scholar]
- 31.Cornelius DC, Amaral LM, Harmon A, Wallace K, Thomas AJ, Campbell N, Scott J, Herse F, Haase N, Moseley J, Wallukat G, Dechend R, LaMarca B. An increased population of regulatory T cells improves the pathophysiology of placental ischemia in a rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol 309: R884–R891, 2015. doi: 10.1152/ajpregu.00154.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornelius DC, Hogg JP, Scott J, Wallace K, Herse F, Moseley J, Wallukat G, Dechend R, LaMarca B. Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension 62: 1068–1073, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cronqvist T, Saljé K, Familari M, Guller S, Schneider H, Gardiner C, Sargent IL, Redman CW, Mörgelin M, Åkerström B, Gram M, Hansson SR. Syncytiotrophoblast vesicles show altered micro-RNA and haemoglobin content after ex-vivo perfusion of placentas with haemoglobin to mimic preeclampsia. PLoS One 9: e90020, 2014. doi: 10.1371/journal.pone.0090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cross CE, Tolba MF, Rondelli CM, Xu M, Abdel-Rahman SZ. Oxidative stress alters miRNA and gene expression profiles in villous first trimester trophoblasts. BioMed Res Int 2015: 257090, 2015. doi: 10.1155/2015/257090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui XL, Brockman D, Campos B, Myatt L. Expression of NADPH oxidase isoform 1 (Nox1) in human placenta: involvement in preeclampsia. Placenta 27: 422–431, 2006. doi: 10.1016/j.placenta.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daikoku T, Matsumoto H, Gupta RA, Das SK, Gassmann M, DuBois RN, Dey SK. Expression of hypoxia-inducible factors in the peri-implantation mouse uterus is regulated in a cell-specific and ovarian steroid hormone-dependent manner. Evidence for differential function of HIFs during early pregnancy. J Biol Chem 278: 7683–7691, 2003. doi: 10.1074/jbc.M211390200. [DOI] [PubMed] [Google Scholar]
- 37.Dang Y, Li W, Tran V, Khalil RA. EMMPRIN-mediated induction of uterine and vascular matrix metalloproteinases during pregnancy and in response to estrogen and progesterone. Biochem Pharmacol 86: 734–747, 2013. doi: 10.1016/j.bcp.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng CL, Ling ST, Liu XQ, Zhao YJ, Lv YF. Decreased expression of matrix metalloproteinase-1 in the maternal umbilical serum, trophoblasts and decidua leads to preeclampsia. Exp Ther Med 9: 992–998, 2015. doi: 10.3892/etm.2015.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Leo V, Capaccio F, Gesualdo L. Preeclampsia and glomerulonephritis: a bidirectional association. Curr Hypertens Rep 22: 36, 2020. doi: 10.1007/s11906-020-1033-9. [DOI] [PubMed] [Google Scholar]
- 40.Doridot L, Passet B, Méhats C, Rigourd V, Barbaux S, Ducat A, Mondon F, Vilotte M, Castille J, Breuiller-Fouché M, Daniel N, le Provost F, Bauchet AL, Baudrie V, Hertig A, Buffat C, Simeoni U, Germain G, Vilotte JL, Vaiman D. Preeclampsia-like symptoms induced in mice by fetoplacental expression of STOX1 are reversed by aspirin treatment. Hypertension 61: 662–668, 2013. doi: 10.1161/HYPERTENSIONAHA.111.202994. [DOI] [PubMed] [Google Scholar]
- 41.Dulak J, Deshane J, Jozkowicz A, Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation 117: 231–241, 2008. doi: 10.1161/CIRCULATIONAHA.107.698316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eleuterio NM, Palei AC, Rangel Machado JS, Tanus-Santos JE, Cavalli RC, Sandrim VC. Positive correlations between circulating adiponectin and MMP2 in preeclampsia pregnant. Pregnancy Hypertens 5: 205–208, 2015. doi: 10.1016/j.preghy.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Escudero CA, Herlitz K, Troncoso F, Acurio J, Aguayo C, Roberts JM, Truong G, Duncombe G, Rice G, Salomon C. Role of extracellular vesicles and microRNAs on dysfunctional angiogenesis during preeclamptic pregnancies. Front Physiol 7: 98, 2016. doi: 10.3389/fphys.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan X, Rai A, Kambham N, Sung JF, Singh N, Petitt M, Dhal S, Agrawal R, Sutton RE, Druzin ML, Gambhir SS, Ambati BK, Cross JC, Nayak NR. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J Clin Invest 124: 4941–4952, 2014. doi: 10.1172/JCI76864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faupel-Badger JM, McElrath TF, Lauria M, Houghton LC, Lim KH, Parry S, Cantonwine D, Lai G, Karumanchi SA, Hoover RN, Troisi R. Maternal circulating angiogenic factors in twin and singleton pregnancies. Am J Obstet Gynecol 212: 636.e1–636.e8, 2015. doi: 10.1016/j.ajog.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fei X, Hongxiang Z, Qi C, Daozhen C. Maternal plasma levels of endothelial dysfunction mediators including AM, CGRP, sICAM-1 and tHcy in pre-eclampsia. Adv Clin Exp Med 21: 573–579, 2012. [PubMed] [Google Scholar]
- 48.Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta 30: 15–24, 2009. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friel AM, Sexton DJ, O’reilly MW, Smith TJ, Morrison JJ. Rho A/Rho kinase: human umbilical artery mRNA expression in normal and pre eclamptic pregnancies and functional role in isoprostane-induced vasoconstriction. Reproduction 132: 169–176, 2006. doi: 10.1530/rep.1.01088. [DOI] [PubMed] [Google Scholar]
- 50.Fulep EE, Vedernikov YP, Saade GR, Garfield RE. The role of endothelium-derived hyperpolarizing factor in the regulation of the uterine circulation in pregnant rats. Am J Obstet Gynecol 185: 638–642, 2001. doi: 10.1067/mob.2001.117665. [DOI] [PubMed] [Google Scholar]
- 51.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest 52: 2682–2689, 1973. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Genc H, Uzun H, Benian A, Simsek G, Gelisgen R, Madazli R, Güralp O. Evaluation of oxidative stress markers in first trimester for assessment of preeclampsia risk. Arch Gynecol Obstet 284: 1367–1373, 2011. doi: 10.1007/s00404-011-1865-2. [DOI] [PubMed] [Google Scholar]
- 53.George EM, Granger JP. Endothelin: key mediator of hypertension in preeclampsia. Am J Hypertens 24: 964–969, 2011. doi: 10.1038/ajh.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 50: 1142–1147, 2007. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 55.Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension 53: 399–403, 2009. doi: 10.1161/HYPERTENSIONAHA.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension 55: 380–385, 2010. doi: 10.1161/HYPERTENSIONAHA.109.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gokina NI, Kuzina OY, Vance AM. Augmented EDHF signaling in rat uteroplacental vasculature during late pregnancy. Am J Physiol Heart Circ Physiol 299: H1642–H1652, 2010. doi: 10.1152/ajpheart.00227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomaa MF, Naguib AH, Swedan KH, Abdellatif SS. Serum tumor necrosis factor-α level and uterine artery Doppler indices at 11-13 weeks’ gestation for preeclampsia screening in low-risk pregnancies: a prospective observational study. J Reprod Immunol 109: 31–35, 2015. doi: 10.1016/j.jri.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Guimarães MF, Brandão AH, Rezende CA, Cabral AC, Brum AP, Leite HV, Capuruço CA. Assessment of endothelial function in pregnant women with preeclampsia and gestational diabetes mellitus by flow-mediated dilation of brachial artery. Arch Gynecol Obstet 290: 441–447, 2014. doi: 10.1007/s00404-014-3220-x. [DOI] [PubMed] [Google Scholar]
- 60.Hagedorn KA, Cooke CL, Falck JR, Mitchell BF, Davidge ST. Regulation of vascular tone during pregnancy: a novel role for the pregnane X receptor. Hypertension 49: 328–333, 2007. doi: 10.1161/01.HYP.0000253478.51950.27. [DOI] [PubMed] [Google Scholar]
- 61.Hall D, Gebhardt S, Theron G, Grové D. Pre-eclampsia and gestational hypertension are less common in HIV infected women. Pregnancy Hypertens 4: 91–96, 2014. doi: 10.1016/j.preghy.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 62.Hamai Y, Fujii T, Yamashita T, Nishina H, Kozuma S, Mikami Y, Taketani Y. Evidence for an elevation in serum interleukin-2 and tumor necrosis factor-alpha levels before the clinical manifestations of preeclampsia. Am J Reprod Immunol 38: 89–93, 1997. doi: 10.1111/j.1600-0897.1997.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 63.Hammond S, Mathewson AM, Baker PN, Mayhew TM, Dunn WR. Gap junctions and hydrogen peroxide are involved in endothelium-derived hyperpolarising responses to bradykinin in omental arteries and veins isolated from pregnant women. Eur J Pharmacol 668: 225–232, 2011. doi: 10.1016/j.ejphar.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 64.Hastie R, Brownfoot FC, Pritchard N, Hannan NJ, Cannon P, Nguyen V, Palmer K, Beard S, Tong S, Kaitu’u-Lino TJ. EGFR (epidermal growth factor receptor) signaling and the mitochondria regulate sFlt-1 (soluble FMS-like tyrosine kinase-1) secretion. Hypertension 73: 659–670, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12300. [DOI] [PubMed] [Google Scholar]
- 65.He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem 274: 25130–25135, 1999. doi: 10.1074/jbc.274.35.25130. [DOI] [PubMed] [Google Scholar]
- 66.Herraiz I, Simón E, Gómez-Arriaga PI, Martínez-Moratalla JM, García-Burguillo A, Jiménez EA, Galindo A. Angiogenesis-related biomarkers (sFlt-1/PLGF) in the prediction and diagnosis of placental dysfunction: an approach for clinical integration. Int J Mol Sci 16: 19009–19026, 2015. doi: 10.3390/ijms160819009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hiby SE, Walker JJ, O’shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med 200: 957–965, 2004. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hromadnikova I, Kotlabova K, Hympanova L, Krofta L. Cardiovascular and cerebrovascular disease associated microRNAs are dysregulated in placental tissues affected with gestational hypertension, preeclampsia and intrauterine growth restriction. PLoS One 10: e0138383, 2015. doi: 10.1371/journal.pone.0138383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu CC, Katerelos M, Choy SW, Crossthwaite A, Walker SP, Pell G, Lee M, Cook N, Mount PF, Paizis K, Power DA. Pre-eclampsia is associated with altered expression of the renal sodium transporters NKCC2, NCC and ENaC in urinary extracellular vesicles. PLoS One 13: e0204514, 2018. doi: 10.1371/journal.pone.0204514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huet E, Gabison EE, Mourah S, Menashi S. Role of emmprin/CD147 in tissue remodeling. Connect Tissue Res 49: 175–179, 2008. doi: 10.1080/03008200802151722. [DOI] [PubMed] [Google Scholar]
- 71.Hunter A, Aitkenhead M, Caldwell C, McCracken G, Wilson D, McClure N. Serum levels of vascular endothelial growth factor in preeclamptic and normotensive pregnancy. Hypertension 36: 965–969, 2000. doi: 10.1161/01.HYP.36.6.965. [DOI] [PubMed] [Google Scholar]
- 72.Iriyama T, Wang W, Parchim NF, Song A, Blackwell SC, Sibai BM, Kellems RE, Xia Y. Hypoxia-independent upregulation of placental hypoxia inducible factor-1α gene expression contributes to the pathogenesis of preeclampsia. Hypertension 65: 1307–1315, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Isaka K, Usuda S, Ito H, Sagawa Y, Nakamura H, Nishi H, Suzuki Y, Li YF, Takayama M. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta 24: 53–64, 2003. doi: 10.1053/plac.2002.0867. [DOI] [PubMed] [Google Scholar]
- 74.Iwaki T, Yamamoto K, Matsuura T, Sugimura M, Kobayashi T, Kanayama N. Alteration of integrins under hypoxic stress in early placenta and choriocarcinoma cell line BeWo. Gynecol Obstet Invest 57: 196–203, 2004. doi: 10.1159/000076688. [DOI] [PubMed] [Google Scholar]
- 75.Jain A, Schneider H, Aliyev E, Soydemir F, Baumann M, Surbek D, Hediger M, Brownbill P, Albrecht C. Hypoxic treatment of human dual placental perfusion induces a preeclampsia-like inflammatory response. Lab Invest 94: 873–880, 2014. doi: 10.1038/labinvest.2014.76. [DOI] [PubMed] [Google Scholar]
- 76.Jardim LL, Rios DR, Perucci LO, de Sousa LP, Gomes KB, Dusse LM. Is the imbalance between pro-angiogenic and anti-angiogenic factors associated with preeclampsia? Clin Chim Acta 447: 34–38, 2015. doi: 10.1016/j.cca.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 77.Kakigano A, Mimura K, Kanagawa T, Nakayama M, Kanayama T, Fujita S, Kinugasa-Taniguchi Y, Endo M, Tomimatsu T, Kimura T. Imbalance of angiogenic factors and avascular edematous cystic villi in a trisomy 13 pregnancy: a case report. Placenta 34: 628–630, 2013. doi: 10.1016/j.placenta.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 78.Kanagal DV, Rajesh A, Rao K, Devi UH, Shetty H, Kumari S, Shetty PK. Levels of serum calcium and magnesium in pre-eclamptic and normal pregnancy: a study from coastal India. J Clin Diagn Res 8: OC01–OC04, 2014. doi: 10.7860/JCDR/2014/8872.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J, Strauss JF, Kalluri R. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 453: 1117–1121, 2008. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 80.Kanashiro CA, Cockrell KL, Alexander BT, Granger JP, Khalil RA. Pregnancy-associated reduction in vascular protein kinase C activity rebounds during inhibition of NO synthesis. Am J Physiol Regul Integr Comp Physiol 278: R295–R303, 2000. doi: 10.1152/ajpregu.2000.278.2.R295. [DOI] [PubMed] [Google Scholar]
- 81.Kanashiro CA, Khalil RA. Gender-related distinctions in protein kinase C activity in rat vascular smooth muscle. Am J Physiol Cell Physiol 280: C34–C45, 2001. doi: 10.1152/ajpcell.2001.280.1.C34. [DOI] [PubMed] [Google Scholar]
- 82.Karakus S, Bozoklu Akkar O, Yildiz C, Sancakdar E, Cetin M, Cetin A. Serum levels of ET-1, M30, and angiopoietins-1 and -2 in HELLP syndrome and preeclampsia compared to controls. Arch Gynecol Obstet 293: 351–359, 2016. doi: 10.1007/s00404-015-3803-1. [DOI] [PubMed] [Google Scholar]
- 83.Kataoka C, Egashira K, Inoue S, Takemoto M, Ni W, Koyanagi M, Kitamoto S, Usui M, Kaibuchi K, Shimokawa H, Takeshita A. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension 39: 245–250, 2002. doi: 10.1161/hy0202.103271. [DOI] [PubMed] [Google Scholar]
- 84.Khalil RA, Crews JK, Novak J, Kassab S, Granger JP. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension 31: 1065–1069, 1998. doi: 10.1161/01.HYP.31.5.1065. [DOI] [PubMed] [Google Scholar]
- 85.Kim SY, Ryu HM, Yang JH, Kim MY, Ahn HK, Shin JS, Choi JS, Park SY, Kim JM, Lee BY, Kim DJ. Maternal serum and amniotic fluid inhibin A levels in women who subsequently develop severe preeclampsia. J Korean Med Sci 21: 452–456, 2006. doi: 10.3346/jkms.2006.21.3.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim YG, Suga SI, Kang DH, Jefferson JA, Mazzali M, Gordon KL, Matsui K, Breiteneder-Geleff S, Shankland SJ, Hughes J, Kerjaschki D, Schreiner GF, Johnson RJ. Vascular endothelial growth factor accelerates renal recovery in experimental thrombotic microangiopathy. Kidney Int 58: 2390–2399, 2000. doi: 10.1046/j.1523-1755.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- 87.Knock GA, Poston L. Bradykinin-mediated relaxation of isolated maternal resistance arteries in normal pregnancy and preeclampsia. Am J Obstet Gynecol 175: 1668–1674, 1996. doi: 10.1016/S0002-9378(96)70123-0. [DOI] [PubMed] [Google Scholar]
- 88.Kobashi G, Yamada H, Ohta K, Kato E, Ebina Y, Fujimoto S. Endothelial nitric oxide synthase gene (NOS3) variant and hypertension in pregnancy. Am J Med Genet 103: 241–244, 2001. doi: 10.1002/ajmg.1535. [DOI] [PubMed] [Google Scholar]
- 89.Krauss T, Pauer HU, Augustin HG. Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertens Pregnancy 23: 101–111, 2004. doi: 10.1081/PRG-120028286. [DOI] [PubMed] [Google Scholar]
- 90.LaMarca B, Brewer J, Wallace K. IL-6-induced pathophysiology during pre-eclampsia: potential therapeutic role for magnesium sulfate? Int J Interferon Cytokine Mediat Res 2011: 59–64, 2011. doi: 10.2147/IJICMR.S16320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension 54: 905–909, 2009. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-α blockade. Hypertension 52: 1161–1167, 2008. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor α in pregnant rats. Hypertension 52: 1168–1172, 2008. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-α. Hypertension 46: 1022–1025, 2005. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 95.Lamminpää R, Vehviläinen-Julkunen K, Gissler M, Heinonen S. Preeclampsia complicated by advanced maternal age: a registry-based study on primiparous women in Finland 1997-2008. BMC Pregnancy Childbirth 12: 47, 2012. doi: 10.1186/1471-2393-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leik CE, Walsh SW. Neutrophils infiltrate resistance-sized vessels of subcutaneous fat in women with preeclampsia. Hypertension 44: 72–77, 2004. doi: 10.1161/01.HYP.0000130483.83154.37. [DOI] [PubMed] [Google Scholar]
- 97.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA; CPEP Study Group . Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 355: 992–1005, 2006. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 98.Li Q, Pan Z, Wang X, Gao Z, Ren C, Yang W. miR-125b-1-3p inhibits trophoblast cell invasion by targeting sphingosine-1-phosphate receptor 1 in preeclampsia. Biochem Biophys Res Commun 453: 57–63, 2014. doi: 10.1016/j.bbrc.2014.09.059. [DOI] [PubMed] [Google Scholar]
- 99.Li W, Mata KM, Mazzuca MQ, Khalil RA. Altered matrix metalloproteinase-2 and -9 expression/activity links placental ischemia and anti-angiogenic sFlt-1 to uteroplacental and vascular remodeling and collagen deposition in hypertensive pregnancy. Biochem Pharmacol 89: 370–385, 2014. doi: 10.1016/j.bcp.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Z, Zhang Y, Ying Ma J, Kapoun AM, Shao Q, Kerr I, Lam A, O’Young G, Sannajust F, Stathis P, Schreiner G, Karumanchi SA, Protter AA, Pollitt NS. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension 50: 686–692, 2007. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 101.Lillegard KE, Johnson AC, Lojovich SJ, Bauer AJ, Marsh HC, Gilbert JS, Regal JF. Complement activation is critical for placental ischemia-induced hypertension in the rat. Mol Immunol 56: 91–97, 2013. doi: 10.1016/j.molimm.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lockwood CJ, Yen CF, Basar M, Kayisli UA, Martel M, Buhimschi I, Buhimschi C, Huang SJ, Krikun G, Schatz F. Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. Am J Pathol 172: 1571–1579, 2008. doi: 10.2353/ajpath.2008.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Luft FC. Soluble endoglin (sEng) joins the soluble fms-like tyrosine kinase (sFlt) receptor as a pre-eclampsia molecule. Nephrol Dial Transplant 21: 3052–3054, 2006. doi: 10.1093/ndt/gfl439. [DOI] [PubMed] [Google Scholar]
- 104.Luksha L, Luksha N, Kublickas M, Nisell H, Kublickiene K. Diverse mechanisms of endothelium-derived hyperpolarizing factor-mediated dilatation in small myometrial arteries in normal human pregnancy and preeclampsia. Biol Reprod 83: 728–735, 2010. doi: 10.1095/biolreprod.110.084426. [DOI] [PubMed] [Google Scholar]
- 105.Luksha L, Nisell H, Luksha N, Kublickas M, Hultenby K, Kublickiene K. Endothelium-derived hyperpolarizing factor in preeclampsia: heterogeneous contribution, mechanisms, and morphological prerequisites. Am J Physiol Regul Integr Comp Physiol 294: R510–R519, 2008. doi: 10.1152/ajpregu.00458.2007. [DOI] [PubMed] [Google Scholar]
- 106.Luo S, Cao N, Tang Y, Gu W. Identification of key microRNAs and genes in preeclampsia by bioinformatics analysis. PLoS One 12: e0178549, 2017. doi: 10.1371/journal.pone.0178549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lv Y, Lu C, Ji X, Miao Z, Long W, Ding H, Lv M. Roles of microRNAs in preeclampsia. J Cell Physiol 234: 1052–1061, 2019. doi: 10.1002/jcp.27291. [DOI] [PubMed] [Google Scholar]
- 108.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy (HDP) Working Group . Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens 4: 105–145, 2014. doi: 10.1016/j.preghy.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 109.Magness RR, Rosenfeld CR, Carr BR. Protein kinase C in uterine and systemic arteries during ovarian cycle and pregnancy. Am J Physiol Endocrinol Metab 260: E464–E470, 1991. doi: 10.1152/ajpendo.1991.260.3.E464. [DOI] [PubMed] [Google Scholar]
- 110.Majed BH, Khalil RA. Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol Rev 64: 540–582, 2012. doi: 10.1124/pr.111.004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mandalà M, Gokina N, Barron C, Osol G. Endothelial-derived hyperpolarization factor (EDHF) contributes to PlGF-induced dilation of mesenteric resistance arteries from pregnant rats. J Vasc Res 49: 43–49, 2012. doi: 10.1159/000329821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.March MI, Geahchan C, Wenger J, Raghuraman N, Berg A, Haddow H, Mckeon BA, Narcisse R, David JL, Scott J, Thadhani R, Karumanchi SA, Rana S. Circulating angiogenic factors and the risk of adverse outcomes among Haitian women with preeclampsia. PLoS One 10: e0126815, 2015. doi: 10.1371/journal.pone.0126815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Matias ML, Romão M, Weel IC, Ribeiro VR, Nunes PR, Borges VT, Araújo JP Jr, Peraçoli JC, de Oliveira L, Peraçoli MT. Endogenous and uric acid-induced activation of NLRP3 inflammasome in pregnant women with preeclampsia. PLoS One 10: e0129095, 2015. doi: 10.1371/journal.pone.0129095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Matsubara K, Matsubara Y, Hyodo S, Katayama T, Ito M. Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. J Obstet Gynaecol Res 36: 239–247, 2010. doi: 10.1111/j.1447-0756.2009.01128.x. [DOI] [PubMed] [Google Scholar]
- 115.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mazzuca MQ, Li W, Reslan OM, Yu P, Mata KM, Khalil RA. Downregulation of microvascular endothelial type B endothelin receptor is a central vascular mechanism in hypertensive pregnancy. Hypertension 64: 632–643, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McDonald SD, Lutsiv O, Dzaja N, Duley L. A systematic review of maternal and infant outcomes following magnesium sulfate for pre-eclampsia/eclampsia in real-world use. Int J Gynaecol Obstet 118: 90–96, 2012. doi: 10.1016/j.ijgo.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 118.McMaster MT, Zhou Y, Fisher SJ. Abnormal placentation and the syndrome of preeclampsia. Semin Nephrol 24: 540–547, 2004. doi: 10.1016/j.semnephrol.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 119.Mohamad MA, Mohd Manzor NF, Zulkifli NF, Zainal N, Hayati AR, Ahmad Asnawi AW. A review of candidate genes and pathways in preeclampsia—an integrated bioinformatical analysis. Biology (Basel) 9: 62, 2020. doi: 10.3390/biology9040062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Montagnana M, Lippi G, Albiero A, Scevarolli S, Salvagno GL, Franchi M, Guidi GC. Evaluation of metalloproteinases 2 and 9 and their inhibitors in physiologic and pre-eclamptic pregnancy. J Clin Lab Anal 23: 88–92, 2009. doi: 10.1002/jcla.20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moreno-Eutimio MA, Tovar-Rodríguez JM, Vargas-Avila K, Nieto-Velázquez NG, Frías-De-León MG, Sierra-Martinez M, Acosta-Altamirano G. Increased serum levels of inflammatory mediators and low frequency of regulatory T cells in the peripheral blood of preeclamptic Mexican women. BioMed Res Int 2014: 413249, 2014. doi: 10.1155/2014/413249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morris R, Spencer SK, Kyle PB, Williams JM, Harris A, Owens MY, Wallace K. Hypertension in an animal model of HELLP syndrome is associated with activation of endothelin 1. Reprod Sci 23: 42–50, 2016. doi: 10.1177/1933719115592707. [DOI] [PubMed] [Google Scholar]
- 123.Motta C, Grosso C, Zanuzzi C, Molinero D, Picco N, Bellingeri R, Alustiza F, Barbeito C, Vivas A, Romanini MC. Effect of sildenafil on pre-eclampsia-like mouse model induced by L-NAME. Reprod Domest Anim 50: 611–616, 2015. doi: 10.1111/rda.12536. [DOI] [PubMed] [Google Scholar]
- 124.Murphy JG, Fleming JB, Cockrell KL, Granger JP, Khalil RA. [Ca2+]i signaling in renal arterial smooth muscle cells of pregnant rat is enhanced during inhibition of NOS. Am J Physiol Regul Integr Comp Physiol 280: R87–R99, 2001. doi: 10.1152/ajpregu.2001.280.1.R87. [DOI] [PubMed] [Google Scholar]
- 125.Murphy JG, Herrington JN, Granger JP, Khalil RA. Enhanced [Ca2+]i in renal arterial smooth muscle cells of pregnant rats with reduced uterine perfusion pressure. Am J Physiol Heart Circ Physiol 284: H393–H403, 2003. doi: 10.1152/ajpheart.00247.2002. [DOI] [PubMed] [Google Scholar]
- 126.Murphy JG, Khalil RA. Decreased [Ca2+]i during inhibition of coronary smooth muscle contraction by 17beta-estradiol, progesterone, and testosterone. J Pharmacol Exp Ther 291: 44–52, 1999. [PubMed] [Google Scholar]
- 127.Nakamura M, Sekizawa A, Purwosunu Y, Okazaki S, Farina A, Wibowo N, Shimizu H, Okai T. Cellular mRNA expressions of anti-oxidant factors in the blood of preeclamptic women. Prenat Diagn 29: 691–696, 2009. doi: 10.1002/pd.2278. [DOI] [PubMed] [Google Scholar]
- 128.Nelson SH, Steinsland OS, Wang Y, Yallampalli C, Dong YL, Sanchez JM. Increased nitric oxide synthase activity and expression in the human uterine artery during pregnancy. Circ Res 87: 406–411, 2000. doi: 10.1161/01.RES.87.5.406. [DOI] [PubMed] [Google Scholar]
- 129.Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res 97: 696–704, 2013. doi: 10.1093/cvr/cvs422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Noorbakhsh M, Kianpour M, Nematbakhsh M. Serum levels of asymmetric dimethylarginine, vascular endothelial growth factor, and nitric oxide metabolite levels in preeclampsia patients. ISRN Obstet Gynecol 2013: 104213, 2013. doi: 10.1155/2013/104213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nugent WH, Mishra N, Strauss JF III, Walsh SW. Matrix metalloproteinase 1 causes vasoconstriction and enhances vessel reactivity to angiotensin II via protease-activated receptor 1. Reprod Sci 23: 542–548, 2016. doi: 10.1177/1933719115607998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Odibo AO, Zhong Y, Goetzinger KR, Odibo L, Bick JL, Bower CR, Nelson DM. First-trimester placental protein 13, PAPP-A, uterine artery Doppler and maternal characteristics in the prediction of pre-eclampsia. Placenta 32: 598–602, 2011. doi: 10.1016/j.placenta.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Omran OM, Shokry M, Ismail H, Omar G, Rezk M. Expression of matrix metalloproteinases 2 and 9 in human trophoblasts of normal and preeclamptic placentas. Int J Health Sci (Qassim) 5, Suppl 2: 21–23, 2011. [PMC free article] [PubMed] [Google Scholar]
- 134.Ou M, Dang Y, Mazzuca MQ, Basile R, Khalil RA. Adaptive regulation of endothelin receptor type-A and type-B in vascular smooth muscle cells during pregnancy in rats. J Cell Physiol 229: 489–501, 2014. doi: 10.1002/jcp.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Palei AC, Granger JP, Tanus-Santos JE. Matrix metalloproteinases as drug targets in preeclampsia. Curr Drug Targets 14: 325–334, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Palmer KR, Kaitu’u-Lino TJ, Hastie R, Hannan NJ, Ye L, Binder N, Cannon P, Tuohey L, Johns TG, Shub A, Tong S. Placental-specific sFLT-1 e15a protein is increased in preeclampsia, antagonizes vascular endothelial growth factor signaling, and has antiangiogenic activity. Hypertension 66: 1251–1259, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05883. [DOI] [PubMed] [Google Scholar]
- 137.Parrish MR, Ryan MJ, Glover P, Brewer J, Ray L, Dechend R, Martin JN Jr, Lamarca BB. Angiotensin II type 1 autoantibody induced hypertension during pregnancy is associated with renal endothelial dysfunction. Gend Med 8: 184–188, 2011. doi: 10.1016/j.genm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pedersen M, Stayner L, Slama R, Sørensen M, Figueras F, Nieuwenhuijsen MJ, Raaschou-Nielsen O, Dadvand P. Ambient air pollution and pregnancy-induced hypertensive disorders: a systematic review and meta-analysis. Hypertension 64: 494–500, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03545. [DOI] [PubMed] [Google Scholar]
- 139.Peixoto AB, Araujo Júnior E, Ribeiro JU, Rodrigues DB, Castro EC, Caldas TM, Rodrigues Júnior V. Evaluation of inflammatory mediators in the deciduas of pregnant women with pre-eclampsia/eclampsia. J Matern Fetal Neonatal Med 29: 75–79, 2016. doi: 10.3109/14767058.2014.987117. [DOI] [PubMed] [Google Scholar]
- 140.Plaks V, Rinkenberger J, Dai J, Flannery M, Sund M, Kanasaki K, Ni W, Kalluri R, Werb Z. Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proc Natl Acad Sci USA 110: 11109–11114, 2013. doi: 10.1073/pnas.1309561110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Podymow T, August P. Hypertension in pregnancy. Adv Chronic Kidney Dis 14: 178–190, 2007. doi: 10.1053/j.ackd.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 142.Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, McAuliffe F, da Silva Costa F, von Dadelszen P, McIntyre HD, Kihara AB, Di Renzo GC, Romero R, D’Alton M, Berghella V, Nicolaides KH, Hod M. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet 145, Suppl 1: 1–33, 2019. [Erratum in Int J Gynaecol Obstet 146: 390–391, 2019]. doi: 10.1002/ijgo.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 123: 2856–2869, 2011. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Powis KM, McElrath TF, Hughes MD, Ogwu A, Souda S, Datwyler SA, von Widenfelt E, Moyo S, Nádas M, Makhema J, Machakaire E, Lockman S, Essex M, Shapiro RL. High viral load and elevated angiogenic markers associated with increased risk of preeclampsia among women initiating highly active antiretroviral therapy in pregnancy in the Mma Bana study, Botswana. J Acquir Immune Defic Syndr 62: 517–524, 2013. doi: 10.1097/QAI.0b013e318286d77e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Purwosunu Y, Sekizawa A, Okazaki S, Farina A, Wibowo N, Nakamura M, Rizzo N, Saito H, Okai T. Prediction of preeclampsia by analysis of cell-free messenger RNA in maternal plasma. Am J Obstet Gynecol 200: 386.e1–386.e7, 2009. doi: 10.1016/j.ajog.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 146.Qiu Q, Yang M, Tsang BK, Gruslin A. EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction 128: 355–363, 2004. doi: 10.1530/rep.1.00234. [DOI] [PubMed] [Google Scholar]
- 147.Raffetto JD, Ross RL, Khalil RA. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: relevance to varicose vein formation. J Vasc Surg 45: 373–380, 2007. doi: 10.1016/j.jvs.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rahardjo B, Widjajanto E, Sujuti H, Keman K. Different levels of IL-1α, IL-6, TNF-α, NF-κB and PPAR-γ in monocyte cultures exposed by plasma preeclampsia and normotensive pregnancy. Pregnancy Hypertens 4: 187–193, 2014. doi: 10.1016/j.preghy.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 149.Rahimzadeh M, Norouzian M, Arabpour F, Naderi N. Regulatory T-cells and preeclampsia: an overview of literature. Expert Rev Clin Immunol 12: 209–227, 2016. doi: 10.1586/1744666X.2016.1105740. [DOI] [PubMed] [Google Scholar]
- 150.Regal JF, Lillegard KE, Bauer AJ, Elmquist BJ, Loeks-Johnson AC, Gilbert JS. Neutrophil depletion attenuates placental ischemia-induced hypertension in the rat. PLoS One 10: e0132063, 2015. doi: 10.1371/journal.pone.0132063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Reslan OM, Khalil RA. Molecular and vascular targets in the pathogenesis and management of the hypertension associated with preeclampsia. Cardiovasc Hematol Agents Med Chem 8: 204–226, 2010. doi: 10.2174/187152510792481234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rivers ER, Horton AJ, Hawk AF, Favre EG, Senf KM, Nietert PJ, Chang EY, Foley AC, Robinson CJ, Lee KH. Placental Nkx2-5 and target gene expression in early-onset and severe preeclampsia. Hypertens Pregnancy 33: 412–426, 2014. doi: 10.3109/10641955.2014.925564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension 46: 1243–1249, 2005. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 154.Rosenberg TJ, Garbers S, Lipkind H, Chiasson MA. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: differences among 4 racial/ethnic groups. Am J Public Health 95: 1545–1551, 2005. doi: 10.2105/AJPH.2005.065680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Royle C, Lim S, Xu B, Tooher J, Ogle R, Hennessy A. Effect of hypoxia and exogenous IL-10 on the pro-inflammatory cytokine TNF-α and the anti-angiogenic molecule soluble Flt-1 in placental villous explants. Cytokine 47: 56–60, 2009. doi: 10.1016/j.cyto.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 156.Sakashita T, Higashi Y, Soga J, Miyoshi H, Kudo Y. Circulating endothelial progenitor cells and placental abruption in women with preeclampsia. Pregnancy Hypertens 4: 203–208, 2014. doi: 10.1016/j.preghy.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 157.Sánchez-Aranguren LC, Prada CE, Riaño-Medina CE, Lopez M. Endothelial dysfunction and preeclampsia: role of oxidative stress. Front Physiol 5: 372, 2014. doi: 10.3389/fphys.2014.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Scalera F, Schlembach D, Beinder E. Production of vasoactive substances by human umbilical vein endothelial cells after incubation with serum from preeclamptic patients. Eur J Obstet Gynecol Reprod Biol 99: 172–178, 2001. doi: 10.1016/S0301-2115(01)00412-2. [DOI] [PubMed] [Google Scholar]
- 159.Schiessl B, Strasburger C, Bidlingmaier M, Mylonas I, Jeschke U, Kainer F, Friese K. Plasma- and urine concentrations of nitrite/nitrate and cyclic Guanosinemonophosphate in intrauterine growth restricted and preeclamptic pregnancies. Arch Gynecol Obstet 274: 150–154, 2006. doi: 10.1007/s00404-006-0149-8. [DOI] [PubMed] [Google Scholar]
- 160.Schoenaker DA, Soedamah-Muthu SS, Mishra GD. The association between dietary factors and gestational hypertension and pre-eclampsia: a systematic review and meta-analysis of observational studies. BMC Med 12: 157, 2014. doi: 10.1186/s12916-014-0157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Scott PA, Tremblay A, Brochu M, St-Louis J. Vasorelaxant action of 17 -estradiol in rat uterine arteries: role of nitric oxide synthases and estrogen receptors. Am J Physiol Heart Circ Physiol 293: H3713–H3719, 2007. doi: 10.1152/ajpheart.00736.2007. [DOI] [PubMed] [Google Scholar]
- 162.Seki H, Kuromaki K, Takeda S, Kinoshita K, Satoh K. Trial of prophylactic administration of TXA2 synthetase inhibitor, ozagrel hydrochloride, for preeclampsia. Hypertens Pregnancy 18: 157–164, 1999. doi: 10.3109/10641959909023075. [DOI] [PubMed] [Google Scholar]
- 163.Shaamash AH, Elsnosy ED, Makhlouf AM, Zakhari MM, Ibrahim OA, EL-dien HM. Maternal and fetal serum nitric oxide (NO) concentrations in normal pregnancy, pre-eclampsia and eclampsia. Int J Gynaecol Obstet 68: 207–214, 2000. doi: 10.1016/S0020-7292(99)00213-1. [DOI] [PubMed] [Google Scholar]
- 164.Shah DA, Khalil RA. Bioactive factors in uteroplacental and systemic circulation link placental ischemia to generalized vascular dysfunction in hypertensive pregnancy and preeclampsia. Biochem Pharmacol 95: 211–226, 2015. doi: 10.1016/j.bcp.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sheppard SJ, Khalil RA. Risk factors and mediators of the vascular dysfunction associated with hypertension in pregnancy. Cardiovasc Hematol Disord Drug Targets 10: 33–52, 2010. doi: 10.2174/187152910790780096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shimokawa H. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pflugers Arch 459: 915–922, 2010. doi: 10.1007/s00424-010-0790-8. [DOI] [PubMed] [Google Scholar]
- 167.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 365: 785–799, 2005. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 168.Siddiqui AH, Irani RA, Zhang W, Wang W, Blackwell SC, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody-mediated soluble fms-like tyrosine kinase-1 induction contributes to impaired adrenal vasculature and decreased aldosterone production in preeclampsia. Hypertension 61: 472–479, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sierra-Laguado J, Garcia RG, López-Jaramillo P. Flow-mediated dilatation of the brachial artery in pregnancy. Int J Gynaecol Obstet 93: 60–61, 2006. doi: 10.1016/j.ijgo.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 170.Smith-Jackson K, Hentschke MR, Poli-de-Figueiredo CE, Pinheiro da Costa BE, Kurlak LO, Broughton Pipkin F, Czajka A, Mistry HD. Placental expression of eNOS, iNOS and the major protein components of caveolae in women with pre-eclampsia. Placenta 36: 607–610, 2015. doi: 10.1016/j.placenta.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 171.Spradley FT, Palei AC, Granger JP. Increased risk for the development of preeclampsia in obese pregnancies: weighing in on the mechanisms. Am J Physiol Regul Integr Comp Physiol 309: R1326–R1343, 2015. doi: 10.1152/ajpregu.00178.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Spradley FT, Tan AY, Joo WS, Daniels G, Kussie P, Karumanchi SA, Granger JP. Placental growth factor administration abolishes placental ischemia-induced hypertension. Hypertension 67: 740–747, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol 122: 33–39, 2005. doi: 10.1016/j.ejogrb.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 174.Suzuki Y, Hattori T, Kajikuri J, Yamamoto T, Suzumori K, Itoh T. Reduced function of endothelial prostacyclin in human omental resistance arteries in pre-eclampsia. J Physiol 545: 269–277, 2002. doi: 10.1113/jphysiol.2002.022384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Taylor RN, Varma M, Teng NN, Roberts JM. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J Clin Endocrinol Metab 71: 1675–1677, 1990. doi: 10.1210/jcem-71-6-1675. [DOI] [PubMed] [Google Scholar]
- 176.Thadhani R, Hagmann H, Schaarschmidt W, Roth B, Cingoez T, Karumanchi SA, Wenger J, Lucchesi KJ, Tamez H, Lindner T, Fridman A, Thome U, Kribs A, Danner M, Hamacher S, Mallmann P, Stepan H, Benzing T. Removal of soluble Fms-like tyrosine kinase-1 by dextran sulfate apheresis in preeclampsia. J Am Soc Nephrol 27: 903–913, 2016. doi: 10.1681/ASN.2015020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Thornburg KL, Jacobson SL, Giraud GD, Morton MJ. Hemodynamic changes in pregnancy. Semin Perinatol 24: 11–14, 2000. doi: 10.1016/S0146-0005(00)80047-6. [DOI] [PubMed] [Google Scholar]
- 178.Trowsdale J, Moffett A. NK receptor interactions with MHC class I molecules in pregnancy. Semin Immunol 20: 317–320, 2008. doi: 10.1016/j.smim.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 179.Tsukimori K, Fukushima K, Tsushima A, Nakano H. Generation of reactive oxygen species by neutrophils and endothelial cell injury in normal and preeclamptic pregnancies. Hypertension 46: 696–700, 2005. doi: 10.1161/01.HYP.0000184197.11226.71. [DOI] [PubMed] [Google Scholar]
- 180.Turpin CA, Sakyi SA, Owiredu WK, Ephraim RK, Anto EO. Association between adverse pregnancy outcome and imbalance in angiogenic regulators and oxidative stress biomarkers in gestational hypertension and preeclampsia. BMC Pregnancy Childbirth 15: 189, 2015. doi: 10.1186/s12884-015-0624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tuzcu ZB, Asicioglu E, Sunbul M, Ozben B, Arikan H, Koc M. Circulating endothelial cell number and markers of endothelial dysfunction in previously preeclamptic women. Am J Obstet Gynecol 213: 533.e1–533.e7, 2015. doi: 10.1016/j.ajog.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 182.Ulbrich SE, Meyer SU, Zitta K, Hiendleder S, Sinowatz F, Bauersachs S, Büttner M, Fröhlich T, Arnold GJ, Reichenbach HD, Wolf E, Meyer HH. Bovine endometrial metallopeptidases MMP14 and MMP2 and the metallopeptidase inhibitor TIMP2 participate in maternal preparation of pregnancy. Mol Cell Endocrinol 332: 48–57, 2011. doi: 10.1016/j.mce.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 183.Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag 7: 467–474, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.van Dijk M, Oudejans CB. STOX1: key player in trophoblast dysfunction underlying early onset preeclampsia with growth retardation. J Pregnancy 2011: 521826, 2011. doi: 10.1155/2011/521826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.van Wijk MJ, Boer K, van der Meulen ET, Bleker OP, Spaan JA, VanBavel E. Resistance artery smooth muscle function in pregnancy and preeclampsia. Am J Obstet Gynecol 186: 148–154, 2002. doi: 10.1067/mob.2002.119184. [DOI] [PubMed] [Google Scholar]
- 186.Van Wijk MJ, Kublickiene K, Boer K, VanBavel E. Vascular function in preeclampsia. Cardiovasc Res 47: 38–48, 2000. doi: 10.1016/S0008-6363(00)00087-0. [DOI] [PubMed] [Google Scholar]
- 187.Velloso EP, Pimentel RL, Braga JF, Cabral AC, Reis ZS, Bader M, Santos RA, Wallukat G. Identification of a novel agonist-like autoantibody in preeclamptic patients. Am J Hypertens 29: 405–412, 2016. [DOI] [PubMed] [Google Scholar]
- 188.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D’Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 12: 642–649, 2006. [Erratum in Nat Med 12: 862, 2006]. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 189.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92: 827–839, 2003. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 190.von Dadelszen P, Dwinnell S, Magee LA, Carleton BC, Gruslin A, Lee B, Lim KI, Liston RM, Miller SP, Rurak D, Sherlock RL, Skoll MA, Wareing MM, Baker PN; Research into Advanced Fetal Diagnosis and Therapy (RAFT) Group . Sildenafil citrate therapy for severe early-onset intrauterine growth restriction. BJOG 118: 624–628, 2011. doi: 10.1111/j.1471-0528.2010.02879.x. [DOI] [PubMed] [Google Scholar]
- 191.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, Wilson M, Martin JN Jr, LaMarca B. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension 57: 949–955, 2011. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jüpner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 103: 945–952, 1999. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang K, Ahmad S, Cai M, Rennie J, Fujisawa T, Crispi F, Baily J, Miller MR, Cudmore M, Hadoke PW, Wang R, Gratacós E, Buhimschi IA, Buhimschi CS, Ahmed A. Dysregulation of hydrogen sulfide producing enzyme cystathionine γ-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation 127: 2514–2522, 2013. doi: 10.1161/CIRCULATIONAHA.113.001631. [DOI] [PubMed] [Google Scholar]
- 194.Wang W, Parchim NF, Iriyama T, Luo R, Zhao C, Liu C, Irani RA, Zhang W, Ning C, Zhang Y, Blackwell SC, Chen L, Tao L, Hicks MJ, Kellems RE, Xia Y. Excess LIGHT contributes to placental impairment, increased secretion of vasoactive factors, hypertension, and proteinuria in preeclampsia. Hypertension 63: 595–606, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Weinberger MH, Kramer NJ, Petersen LP, Cleary RE, Young PC. Sequential changes in the renin—angiotensin—aldosterone systems and plasma progesterone concentration in normal and abnormal human pregnancy. Perspect Nephrol Hypertens 5: 263–269, 1976. [PubMed] [Google Scholar]
- 196.Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: a severe consequence of hypertension in pregnancy. Am J Obstet Gynecol 142: 159–167, 1982. doi: 10.1016/S0002-9378(16)32330-4. [DOI] [PubMed] [Google Scholar]
- 197.Wimalasundera RC, Thom SA, Regan L, Hughes AD. Effects of vasoactive agents on intracellular calcium and force in myometrial and subcutaneous resistance arteries isolated from preeclamptic, pregnant, and nonpregnant woman. Am J Obstet Gynecol 192: 625–632, 2005. doi: 10.1016/j.ajog.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 198.Wingrove CS, Garr E, Godsland IF, Stevenson JC. 17β-oestradiol enhances release of matrix metalloproteinase-2 from human vascular smooth muscle cells. Biochim Biophys Acta 1406: 169–174, 1998. doi: 10.1016/S0925-4439(97)00097-5. [DOI] [PubMed] [Google Scholar]
- 199.Xia Y, Ramin SM, Kellems RE. Potential roles of angiotensin receptor-activating autoantibody in the pathophysiology of preeclampsia. Hypertension 50: 269–275, 2007. doi: 10.1161/HYPERTENSIONAHA.107.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xiao D, Zhu R, Zhang L. Gestational hypoxia up-regulates protein kinase C and inhibits calcium-activated potassium channels in ovine uterine arteries. Int J Med Sci 11: 886–892, 2014. doi: 10.7150/ijms.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xu B, Makris A, Liu CC, Hennessy A. Calcium deficient placental growth restriction is mediated by an increase in non-invasive integrin α5 and β4 phenotype. Pregnancy Hypertens 19: 138–142, 2020. doi: 10.1016/j.preghy.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 202.Xu B, Shanmugalingam R, Chau K, Makris A, Hennessy A. Galectin-1–related modulation of trophoblast endothelial interactions by integrins α1 and β1. Reprod Sci 27: 1097–1109, 2020. doi: 10.1007/s43032-019-00046-z. [DOI] [PubMed] [Google Scholar]
- 203.Xu H, Shatenstein B, Luo ZC, Wei S, Fraser W. Role of nutrition in the risk of preeclampsia. Nutr Rev 67: 639–657, 2009. doi: 10.1111/j.1753-4887.2009.00249.x. [DOI] [PubMed] [Google Scholar]
- 204.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 103: 129–135, 1999. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yang X, Wang F, Lau WB, Zhang S, Zhang S, Liu H, Ma XL. Autoantibodies isolated from preeclamptic patients induce endothelial dysfunction via interaction with the angiotensin II AT1 receptor. Cardiovasc Toxicol 14: 21–29, 2014. doi: 10.1007/s12012-013-9229-8. [DOI] [PubMed] [Google Scholar]
- 206.Yinon Y, Ben Meir E, Margolis L, Lipitz S, Schiff E, Mazaki-Tovi S, Simchen MJ. Low molecular weight heparin therapy during pregnancy is associated with elevated circulatory levels of placental growth factor. Placenta 36: 121–124, 2015. doi: 10.1016/j.placenta.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 207.Yong HE, Murthi P, Borg A, Kalionis B, Moses EK, Brennecke SP, Keogh RJ. Increased decidual mRNA expression levels of candidate maternal pre-eclampsia susceptibility genes are associated with clinical severity. Placenta 35: 117–124, 2014. doi: 10.1016/j.placenta.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yu Y, Wang L, Liu T, Guan H. MicroRNA-204 suppresses trophoblast-like cell invasion by targeting matrix metalloproteinase-9. Biochem Biophys Res Commun 463: 285–291, 2015. doi: 10.1016/j.bbrc.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 209.Zhao S, Gu Y, Lewis DF, Wang Y. Predominant basal directional release of thromboxane, but not prostacyclin, by placental trophoblasts from normal and preeclamptic pregnancies. Placenta 29: 81–88, 2008. doi: 10.1016/j.placenta.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med 14: 855–862, 2008. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhou J, Xiao D, Hu Y, Wang Z, Paradis A, Mata-Greenwood E, Zhang L. Gestational hypoxia induces preeclampsia-like symptoms via heightened endothelin-1 signaling in pregnant rats. Hypertension 62: 599–607, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhu M, Ren Z, Possomato-Vieira JS, Khalil RA. Restoring placental growth factor-soluble fms-like tyrosine kinase-1 balance reverses vascular hyper-reactivity and hypertension in pregnancy. Am J Physiol Regul Integr Comp Physiol 311: R505–R521, 2016. doi: 10.1152/ajpregu.00137.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]