Abstract
In 2017, the American Heart Association (AHA) and American College of Cardiology (ACC) redefined stage 1 hypertension to systolic blood pressure (BP) 130–139 mmHg or diastolic BP 80–89 mmHg; however, the degree to which microvascular endothelial dysfunction is evident in adults with stage 1 hypertension remains equivocal. We tested the hypotheses that cutaneous microvascular endothelial dysfunction would be present in adults with stage 1 hypertension (HTN1) compared with normotensive adults (NTN; BP <120/<80 mmHg) but would be less severe compared with adults with stage 2 hypertension (HTN2; systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg) and that this graded impairment would be mediated by reductions in nitric oxide (NO)-dependent dilation. This retrospective analysis included 20 NTN (5 men; 45–64 yr; BP 94–114/60–70 mmHg), 22 HTN1 (11 men; 40–74 yr; BP 110–134/70–88 mmHg), and 44 HTN2 (27 men; 40–74 yr; BP 128–180/80–110 mmHg). BP and nocturnal dipping status were also assessed using 24-h ambulatory BP monitoring. Red cell flux (laser Doppler flowmetry) was measured during intradermal microdialysis perfusion of acetylcholine (ACh; 10−10 to 10−1M) alone and concurrently with the nonspecific nitric oxide (NO) synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 15 mM). ACh-induced dilation was impaired in HTN2 (P < 0.01), but not in HTN1 (P = 0.85), compared with NTN. Furthermore, reductions in NO-dependent dilation were evident in HTN2 (P < 0.01) but not in HTN1 (P = 0.76). Regardless of BP, endothelium-dependent dilation was impaired in nondippers (nighttime drop in systolic BP <10%) compared with dippers (nighttime drop in systolic BP ≥10%, P < 0.05). In conclusion, functional impairments in NO-mediated endothelium-dependent dilation were not evident in HTN1. However, regardless of BP classification, the lack of a nocturnal dip in BP was associated with blunted endothelium-dependent dilation.
NEW & NOTEWORTHY This is the first study to pharmacologically assess the mechanistic regulation of endothelial function in adults with hypertension, classified according to the 2017 clinical guidelines set for by the American Heart Association (AHA) and American College of Cardiology (ACC). Compared with that in normotensive adults, nitric oxide-mediated endothelium-dependent dilation is impaired in adults with stage 2, but not stage 1, hypertension. Adults lacking a nighttime dip in blood pressure demonstrated reductions in endothelium-dependent dilation.
Keywords: blood pressure, endothelium-dependent dilation, intradermal microdialysis, laser-Doppler flowmetry, nitric oxide
INTRODCUTION
Hypertension is a primary risk factor for cardiovascular disease, which remains the leading cause of morbidity and mortality worldwide (6). In 2017, the American Heart Association (AHA) and American College of Cardiology (ACC) released guidelines for the clinical management of high blood pressure (BP) that redefined BP classifications to normal (systolic BP <120 and diastolic BP <80 mmHg), elevated (systolic BP 120–129 and diastolic BP <80 mmHg), stage 1 hypertension (systolic BP 130–139 or diastolic BP 80–89 mmHg) or stage 2 hypertension (systolic BP ≥140 or diastolic BP ≥90 mmHg) and extensively revised treatment recommendations (53). These guidelines lowered the BP threshold classification for hypertension from the definition of ≥140/≥90 mmHg set by the 7th Joint National Committee (JNC7) in 2003 (7). This revision substantially increased the prevalence of hypertension among U.S. adults to ~45% (36). Epidemiological studies provide strong clinical evidence that lowering the BP categories for defining hypertension is important for further reducing cardiovascular risk (19, 25, 26). Interestingly, the new classification schema has received varied responses from clinical societies (54). Given the potential population impact, it is important to consider whether adults with AHA/ACC-defined stage 1 hypertension (HTN1) demonstrate deleterious alterations in the myriad physiological processes generally associated with chronically elevated BP, e.g., vascular dysfunction, increased sympathetic outflow, target organ damage (15, 16, 33).
In this regard, microvascular endothelial dysfunction, now generally considered to be an early manifestation of the atherosclerotic disease process that precedes clinically apparent target organ damage (13), is a hallmark feature of hypertension (29). Hypertension-associated endothelial dysfunction is characterized by a reduction in nitric oxide (NO) bioavailability (9, 16, 21, 43). Studies from our laboratory (9, 16, 43) and others (11, 18, 32, 39) demonstrate significant impairments in cutaneous microvascular endothelium-dependent dilation, largely mediated by reductions in NO-dependent mechanisms, in naïve-to-treatment hypertensive adults defined according to the JNC7 criteria (9, 16, 21, 43). However, the evidence for endothelial dysfunction in conduit and resistance arteries in JNC7-defined prehypertensive adults (systolic BP 120–139 mmHg or diastolic BP 80–89 mmHg) is equivocal (5, 14, 42, 55). Furthermore, the extent to which reductions in NO-mediated endothelium-dependent dilation are evident in the microvasculature of adults meeting the AHA/ACC threshold for defining stage 1 hypertension has not been elucidated using direct targeted pharmacological approaches.
The AHA/ACC definitions of hypertension are based on repeated measurements of seated BP, assessed on at least two different occasions. It is increasingly apparent that such BP measurements may not accurately reflect true BP values (e.g., white coat or masked hypertension) and also do not consider the diurnal variation in BP (35, 50). Twenty-four-hour ambulatory BP monitoring (ABPM) is a better predictor of cardiovascular morbidity and mortality compared with seated clinic BP measurements (20, 45, 50). ABPM additionally provides an assessment of the diurnal BP profile, which is clinically relevant given the increased cardiovascular risk associated with the lack of a nighttime dip in BP (7, 28, 37, 50, 57). Despite its utility in more precisely quantifying an individual’s true BP profile, to date, no studies have assessed the relation between ABPM-derived BP parameters and microvascular endothelial dysfunction.
The primary aim of this study was to assess cutaneous microvascular endothelial function in healthy middle-aged HTN1 classified according to the 2017 AHA/ACC guidelines for seated BP. We pharmacologically assessed endothelial function in the cutaneous microcirculation, which is thought to be a validated bioassay for systemic microvascular dysfunction and has been used to examine the mechanisms underlying microvascular dysfunction in multiple pathologies (8, 12, 23, 29, 51). We hypothesized that endothelium-dependent dilation, as well as the NO-mediated component, would be blunted in HTN1 compared with age-matched normotensive adults (NTN) but graded in severity compared with adults with stage 2 hypertension (HTN2). Given the aforementioned clinical importance of the diurnal variation in BP, as an exploratory analysis, we also examined endothelium-dependent dilation in relation to the diurnal variation in BP obtained via 24-h ABPM. We hypothesized that nondippers would have reductions in cutaneous endothelium-dependent microvascular function compared with dippers.
METHODS
Participants.
The Institutional Review Board at The Pennsylvania State University approved all experimental procedures and protocols. A Food and Drug Administration Investigational Drug Number was obtained for all protocols (INDs 105,572; 120,058; 128,891). Verbal and written consent were voluntarily obtained from all subjects before participation and according to guidelines set forth by the Declaration of Helsinki. Data were retrospectively analyzed from 90 adults who have participated in previous (10, 16, 43) and ongoing studies in our laboratory (clinical trial registry: NCT03179163 and NCT03376555). All subjects underwent a complete medical screening, including a physical examination and a blood chemistry analysis (Quest Diagnostics, Pittsburgh, PA). Subjects were recreationally active, did not use tobacco products, and were not taking over-the-counter or prescription medications with primary or secondary cardiovascular effects (e.g., antihypertensives, statins, anticoagulants, antidepressants, etc.) at the time of testing. All participants were free from any overt cardiovascular disease, aside from elevated BP, or any evidence or diagnosis of associated comorbidities, including renal, pulmonary, neurological, or dermatological disease. Women taking or who had recently taken hormone replacement therapy were excluded. All women were postmenopausal during time of testing (self-report).
At the laboratory screening visit, resting BP measurements were obtained by a trained research nurse using brachial auscultation, in accordance with standard AHA guidelines (38, 53). Participants were seated, with both feet flat on the floor, and their right arm supported at heart level. A total of four manual BP readings were obtained and averaged for BP classification. BP measurements were taken twice, separated by a minimum of 5 min, on 2 different days separated by a minimum of 24 h (38, 53). Consistent with the 2017 AHA/ACC guidelines, participants were classified based on their resting seated BP (NTN: systolic BP <120 mmHg and diastolic BP <80 mmHg; HTN1: systolic BP 130–139 mmHg or diastolic BP 80–89 mmHg; HTN2: systolic BP >140 mmHg or diastolic BP ≥90 mmHg) (53). Four participants were excluded from analyses on the basis of seated BP in the elevated range (systolic BP 120–129 mmHg and diastolic BP <80 mmHg). Because the studies comprising this retrospective analysis were conducted over the course of multiple years, several subjects (n = 18) participated in more than one study. In these instances, participants were classified into the appropriate group (NTN, HTN1, or HTN2) based on their resting seated BP obtained at the laboratory screening visit for each unique study. In confirmatory statistical analyses, when randomizing the selection of only one visit per participant, the results and interpretation did not change. Thus, to increase statistical power, data from all individual experimental visits are presented.
Twenty-four-hour ABPM (n = 75; Ambulo 2400; Mortara Instrument, Inc., Milwaukee, WI) was used to assess the BP profile of daily living. Ambulatory measures were obtained every 30 min when awake and every 60 min when asleep. Due to nocturnal dipping, the average BP values obtained using 24-h ABPM are generally lower than those obtained in a clinic; thus, the consensus values for defining hypertension are also lower (HTN1: 24-h systolic BP 125–129 mmHg or diastolic BP 75–79 mmHg; HTN2: 24-h systolic BP ≥130 mmHg or diastolic BP ≥80 mmHg) (22). Participants were further classified as nighttime dippers (a nighttime drop in systolic BP ≥10% of systolic BP when awake) or nondippers (a nighttime drop in systolic BP <10% of systolic BP when awake) (7, 28, 37, 50, 57).
Assessment of cutaneous microvascular endothelial function.
Before each experimental session, participants were instructed to abstain from caffeine and alcohol for 12 h and strenuous physical activity for 24 h. Intradermal microdialysis fibers (CMA Linear 31 probe, 55 kDa, Harvard Apparatus, Holliston, MA; MD 2000, 30 kDa, Bioanalytical Systems, West Lafayette, IN) were inserted into the ventral forearm skin for the local delivery of pharmacological agents, as previously described (10, 16, 43). Cutaneous red blood cell flux was continuously measured directly over each microdialysis site with an integrated laser Doppler flowmeter probe placed in a local heating unit (VP12 and VHP2; Moor Instruments, Wilmington, DE) set to a thermoneutral 33°C to locally clamp skin temperature. Automated brachial BP (Cardiocap; GE Healthcare, Milwaukee, WI; Connex Spot Monitor, Welch Allyn, Skaneateles Falls, NY) was measured every 5 min throughout the protocol.
After microdialysis fiber insertion, 60–90 min were allowed for hyperemia resolution. After baseline measurements, progressively increasing concentrations of the endothelium-dependent agonist acetylcholine (ACh; 10−10 to 10−1 M; USP, Rockville, MD) were perfused sequentially for 5 min at a rate of 2 µL/min (Bee Hive controller and Baby Bee microinfusion pumps; BASi, West Lafayette, IN). In a subset of individuals (n = 64), a second microdialysis fiber was concurrently perfused with a nonspecific NO synthase inhibitor [NG-nitro-l-arginine methyl ester (l-NAME); 15 mM; Calbiochem, EMD Millipore, Billerica, MA]. At the conclusion of the ACh dose-response protocol, 28 mM sodium nitroprusside (USP) was perfused and the local temperature was increased to 43°C to elicit maximal cutaneous vasodilation (10, 16, 43).
Data and statistical analysis.
Intradermal microdialysis data collection procedures have been standardized in our laboratory (10, 16, 43). Data were recorded at 40 Hz and stored for offline analysis (Windaq software and Dataq data acquisition system, Dataq Instruments, Akron, OH; Powerlab and LabChart, ADInstruments, Bella Vista, NSW, Australia). Average values for red cell flux (perfusion units) and cutaneous vascular conductance (CVC; flux/mean arterial pressure) were obtained during 5 min of baseline, during the last minute of each ACh dose, and at maximal CVC. The nitric oxide contribution was calculated as the difference between the area under the curves of the control site and the l-NAME sites (1, 17).
A post hoc power analysis (power = 0.80, α = 0.05) confirmed a sample size of n = 14 per group was needed to determine a meaningful difference of 10% in endothelium-dependent vasodilation between groups. Significance was set a priori at α = 0.05. To account for the lack of independence between observations for subjects who participated in more than one study, and to account for the repeated-measures nature of the experimental protocols, all data were analyzed using a linear mixed effects model (SAS v. 9.4; Cary, NC). When appropriate, post hoc Bonferroni corrections were applied to correct for multiple comparisons. For within group comparisons, a two-way repeated measures ANOVA was used (SAS). Additional ANCOVA analyses were conducted to examine the influence of body mass index (BMI), LDL cholesterol, and sex on endothelium-dependent vasodilation. Results are presented as means ± SD.
RESULTS
Participant characteristics are presented in Table 1. Resting seated and 24-h AMBP were different between groups (all P < 0.05). Groups were well matched for age and blood biochemistry (Table 1). HTN2 had a significantly higher BMI compared with NTN; however, BMI had no effect on vascular function (all P > 0.05). Other than having high BP, HTN1, and HTN2, all participants were clinically healthy (Table 1).
Table 1.
Participant characteristics
| NTN | HTN1 | HTN2 | |
|---|---|---|---|
| n (M/W) | 20 (5/15) | 22 (11/11) | 44 (27/17) |
| Age, yr | 50 ± 5 (45–64) | 56 ± 8 (40–74) | 55 ± 10 (40–74) |
| BMI, kg/m2 | 25 ± 3 (21–29) | 27 ± 3 (21–33) | 29 ± 4 (19–36)* |
| Seated, mmHg | |||
| SBP | 107 ± 5 (94–114) | 123 ± 7 (110–134)* | 144 ± 8 (128–180)*,† |
| DBP | 68 ± 4 (60–70) | 81 ± 4 (70–88)* | 92 ± 5 (80–110)*,† |
| MAP | 81 ± 3 (71–84) | 95 ± 3 (90–103)* | 109 ± 5 (102–133)*,† |
| 24 h, mmHg | |||
| SBP | 109 ± 6 (103–122) | 119 ± 13 (97–148)* | 133 ± 12 (103–154)*,† |
| DBP | 71 ± 3 (67–75) | 76 ± 7 (67–91)* | 83 ± 7 (67–96)*,† |
| MAP | 83 ± 4 (80–90) | 90 ± 9 (77–110)* | 100 ± 8 (79–113)*,† |
| Daytime, mmHg | |||
| SBP | 109 ± 6 (104–124) | 122 ± 12 (101–149)* | 136 ± 12 (105–159)*,† |
| DBP | 71 ± 3 (67–77) | 78 ± 7 (68–93)* | 85 ± 7 (71–96)*,† |
| MAP | 84 ± 4 (80–92) | 93 ± 8 (80–112)* | 102 ± 8 (82–115)*,† |
| Nighttime, mmHg | |||
| SBP | 99 ± 7 (91–112) | 108 ± 14 (92–146) | 117 ± 14 (92–143)*,† |
| DBP | 65 ± 3 (62–73) | 69 ± 9 (56–89) | 73 ± 8 (60–95)* |
| MAP | 77 ± 4 (72–86) | 81 ± 11 (62–107) | 87 ± 10 (71–111)*,† |
| Cholesterol, mg/dL | |||
| Total | 181 ± 30 (135–251) | 195 ± 25 (134–235) | 198 ± 28 (144–262) |
| HDL-C | 56 ± 20 (29–95) | 59 ± 16 (33–92) | 56 ± 16 (28–98) |
| LDL-C | 106 ± 22 (79–153) | 115 ± 25 (56–159) | 122 ± 25 (76–182) |
| Triglycerides, mg/dL | 100 ± 52 (34–201) | 111 ± 49 (53–232) | 106 ± 59 (32–267) |
| HbA1c, % | 5.4 ± 0.3 (4.9–6.0) | 5.5 ± 0.2 (5.0–6.0) | 5.3 ± 0.3 (4.8–5.8) |
Values are means ± SD (minimum-maximum). M/W, men/women; NTN, normotensives; HTN1, stage 1 hypertensives; HTN2, stage 2 hypertensives; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial blood pressure; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol.
P < 0.05 vs. NTN.
P < 0.05 vs. HTN1.
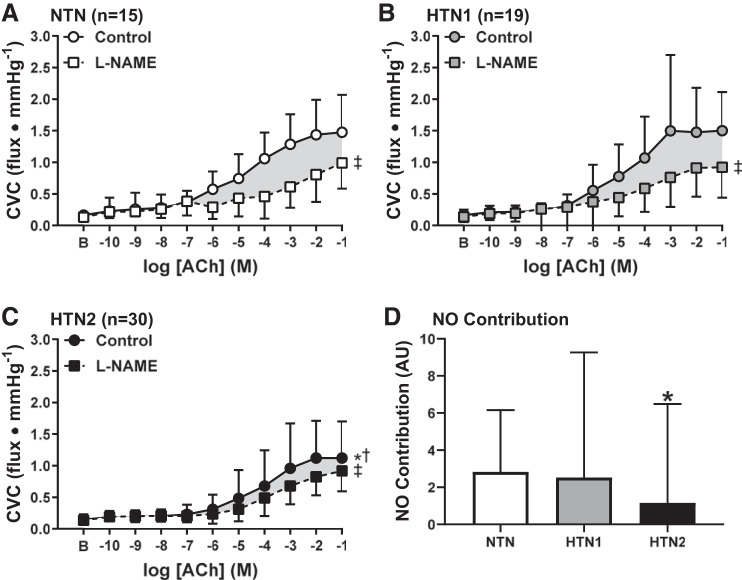
Baseline (NTN: 0.16 ± 0.11, HTN1: 0.16 ± 0.08, HTN2: 0.15 ± 0.08 flux/mmHg, P = 0.76) and maximal CVC (NTN: 1.79 ± 0.65, HTN1: 1.73 ± 0.76, HTN2: 1.60 ± 0.95 flux/mmHg, P = 0.65) was not different between groups. ACh-induced vasodilation was attenuated in HTN2 compared with both HTN1 (P < 0.0001) and NTN (P = 0.016; Fig. 1). However, endothelium-dependent vasodilation was not different between NTN and HTN1 (P = 0.85). NO synthase inhibition blunted ACh-induced vasodilation in all groups (all P ≤ 0.0001; Fig. 2). The magnitude of inhibition was markedly reduced in HTN2, such that a significant reduction in the functional contribution of NO to ACh-induced dilation was evident compared with both NTN and HTN1 (Fig. 2D; both P < 0.05). In the subset of participants who underwent ABPM, 24 adults (34%) demonstrated either “white coat” or “masked hypertension.” Consistent with the cutaneous microvascular function data presented based on seated BP classifications, when these participants were excluded from analysis, reductions in the NO-dependent component of ACh-induced dilation were still only evident in HTN2 (n = 34) compared with both NTN (n = 12) and HTN1 (n = 8; NO contribution at −1 M ACh: NTN = 31.0 ± 12.4%, HTN1 = 37.7 ± 24.5%, HTN2 = 12.6 ± 25.1%, P = 0.02).
Fig. 1.
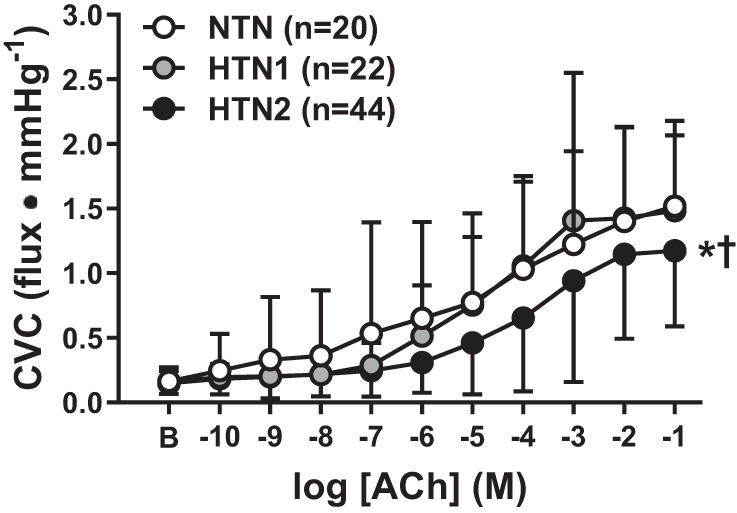
Cutaneous vascular conductance (CVC) in response to increasing concentrations of acetylcholine (ACh) in normotensive adults (NTN; white symbols), adults with stage 1 hypertension (HTN1; gray symbols), and adults with stage 2 hypertension (HTN2; black symbols). Endothelium-dependent dilation was impaired in HTN2, but not HTN1, compared with NTN. Values are means ± SD. Some error bars are not visible because they are smaller than the representative symbol. B, baseline. *P < 0.05 vs. NTN; †P < 0.05 vs. HTN1.
Fig. 2.
Cutaneous vascular conductance (CVC) in response to increasing concentrations of acetylcholine (ACh) alone (control, circles) and during concurrent nitric oxide synthase inhibition [NG-nitro-l-arginine methyl ester (l-NAME), squares] in normotensive adults (NTN; A), adults with stage 1 hypertension (HTN1; B), and adults with stage 2 hypertension (HTN2; C). Nitric oxide (NO) synthase inhibition blunted ACh-induced dilation in all groups. The magnitude of the NO-dependent component of ACh-dilation was reduced in HTN2, but not HTN1, compared with NTN (D). AU, arbitrary units. Values are means ± SD. Some error bars are not visible because they are smaller than the representative symbol. B, baseline. *P < 0.05 vs. NTN; †P < 0.05 vs. HTN1. ‡P < 0.05 vs. control.
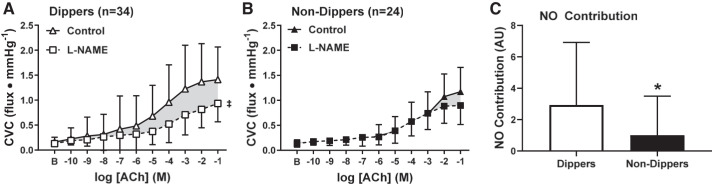
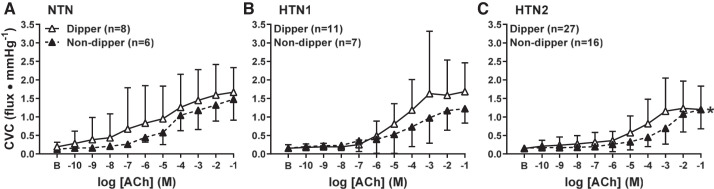
In adults who underwent APBM, 46 participants were classified as nighttime dippers and 29 participants as nondippers. The distribution of the number of adults within each BP classification was not different (P = 0.17) between dippers (NTN: n = 8, 17%; HTN1: n = 11, 24%; HTN2: n = 27, 59%) and nondippers (NTN n = 6, 21%; HTN1: n = 7, 24%; HTN2: n = 16, 55%). Neither baseline (dippers: 0.16 ± 0.09, nondippers: 0.15 ± 0.08 flux/mmHg, P = 0.82) nor maximal CVC (dippers: 1.81 ± 1.0; nondippers: 1.56 ± 0.58 flux/mmHg; P = 0.20) were different between dippers and nondippers. Regardless of BP classification, ACh-induced vasodilation was attenuated in nondippers (P = 0.009; Fig. 3). NO synthase inhibition blunted ACh-induced vasodilation in dippers (P < 0.0001; Fig. 4A) but not in nondippers (P = 0.10, Fig. 4B). Furthermore, the magnitude of inhibition was markedly reduced in nondippers compared with dippers (P = 0.04, Fig. 4C). When taking BP classification into account (Fig. 5), impairments in endothelium-dependent dilation between dippers and nondippers were observed in HTN2 (P = 0.03). However preliminary data collected in NTN and HTN1 participants did not show a difference between dippers and nondippers (NTN: P = 0.99; HTN1: P = 0.59).
Fig. 3.
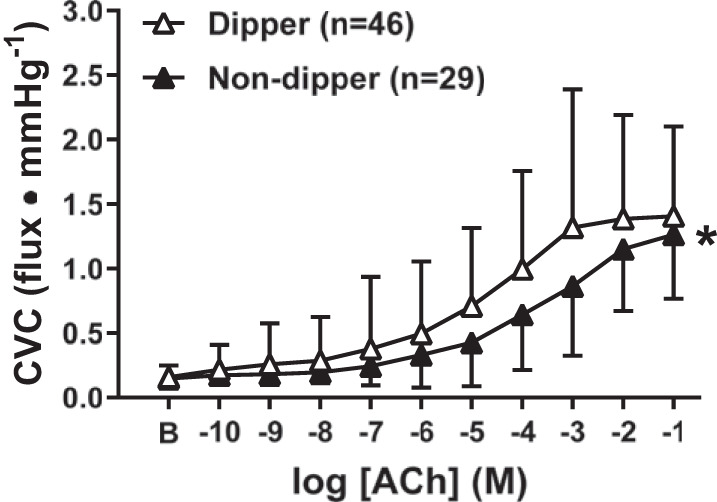
Cutaneous vascular conductance (CVC) in response to increasing concentrations of acetylcholine (ACh) in nighttime dippers (a nighttime drop in systolic blood pressure ≥10% of systolic blood pressure when awake, white symbols) and nondippers (a nighttime drop in systolic blood pressure <10% of systolic blood pressure when awake, black symbols). Endothelium-dependent dilation was blunted in adults lacking a nighttime dip in blood pressure. Values are means ± SD. Some error bars are not visible because they are smaller than the representative symbol. B, baseline. *P < 0.05 vs. dippers.
Fig. 4.
Cutaneous vascular conductance (CVC) in response to increasing concentrations of acetylcholine (ACh) alone (control, triangles) and during concurrent nitric oxide synthase inhibition [NG-nitro-l-arginine methyl ester (l-NAME), squares] in dippers (A, open symbols) and nondippers (B, closed symbols). Nitric oxide synthase inhibition blunted ACh-induced dilation in dippers, but not nondippers. Thus, the magnitude of the NO-dependent component of ACh-dilation was reduced in nondippers compared with dippers (C). AU, arbitrary units. Values are means ± SD. Some error bars are not visible because they are smaller than the representative symbol. B, baseline. *P = 0.01 vs. dippers; ‡P < 0.001 vs. control.
Fig. 5.
Cutaneous vascular conductance (CVC) in response to increasing concentrations of acetylcholine (ACh) in normotensive adults (NTN; A), adults with stage 1 hypertension (HTN1; B), and adults with stage 2 hypertension (HTN2; C) adults, classified as nighttime dippers (a nighttime drop in systolic blood pressure ≥10% of systolic blood pressure when awake, white symbols) and nondippers (a nighttime drop in systolic blood pressure <10% of systolic blood pressure when awake, black symbols). Values are means ± SD. Some error bars are not visible because they are smaller than the representative symbol. B, baseline; *P < 0.05 vs. dippers.
DISCUSSION
This retrospective analysis is the first direct mechanistic examination of cutaneous microvascular endothelial function in adults classified according to the revised AHA/ACC definitions of hypertension. Using an in vivo pharmacological approach, we demonstrated that impairments in endothelium-dependent dilation were evident only in HTN2, but not HTN1, compared with NTN. Moreover, NO-mediated dilation was preserved in HTN1. These results were confirmed with ABPM, which is more accurate for determining the true BP profile of daily living (20, 45, 50) and to subsequently exclude adults with either white coat or masked hypertension from analyses. Regardless of resting seated BP, our results demonstrate blunted ACh-induced dilation in adults lacking a nocturnal dip in BP. When accounting for HTN classification, further reductions in endothelium-dependent dilation in nondippers were only observed in HTN2. Considered collectively, these data suggest that cutaneous microvascular endothelial function is preserved in HTN1.
It is clear that cardiovascular risk is increased in adults with chronically elevated BP (6). The BP guidelines set by the AHA/ACC in 2017 lower the threshold for the clinical diagnosis of hypertension (53). With the use of the 2003 JNC7 guidelines, limited studies have demonstrated small but significant reductions in vascular function (reactive hyperemia-induced dilation in conduit and resistance arteries) in adults with prehypertension (5, 14). The JNC7 prehypertension classification encompasses a broader range of BP, such that many of these prehypertensive adults would now be classified as HTN1. With the widespread clinical adoption of updated BP classification, it has become increasingly important to understand the extent to which progressive declines in endothelial function occur in concert with chronic elevations in elevated resting BP. Because microvascular dysfunction is a better predictor of long-term cardiovascular health than large-vessel disease (39, 52), we assessed endothelial function in the cutaneous microcirculation, a validated model for generalized vascular dysfunction in multiple pathologies (2, 8, 23, 29, 39, 51, 52). The overwhelming majority (>90%) of patients with essential hypertension first develop microvascular dysfunction in the microcirculation, before overt target organ damage (29). These data substantiate the utility of investigating cutaneous microvascular endothelial function using direct pharmacological approaches in the present study. As expected, and consistent with previous studies from our laboratory (9, 16, 43) and others (11, 18, 32, 39), HTN2 adults demonstrated significantly blunted ACh-induced endothelium-dependent dilation. However, contrary to our hypothesis, no impairments in endothelium-dependent dilation were observed in HTN1 compared with NTN adults, suggesting a relative preservation of cutaneous microvascular endothelial function in this otherwise healthy middle-aged cohort.
Microvascular dysfunction in hypertension is largely mediated by reductions in NO bioavailability and function (23). Impaired NO-dependent dilation has been consistently observed in hypertensive adults with a BP >140/>90 mmHg (9, 16, 21, 43). However, surprisingly few studies have examined the specific mechanistic mediators of microvascular function in prehypertensive adults, and to our knowledge, no studies have assessed these mechanisms in HTN1 defined by the 2017 AHA/ACC guidelines. Using a targeted pharmacological approach to inhibit vascular NO production, we show marked reductions in NO-mediated dilation in HTN2. We did not detect deficits in the NO-mediated contribution to ACh-induced vasodilation in HTN1, suggesting a maintenance of NO bioavailability and function in the cutaneous regional microcirculation of HTN1. Collectively, these data suggest that studies specifically investigating the mechanistic mediators of hypertension-associated microvascular dysfunction, particularly those attempting to identify novel regulatory pathways or therapeutic targets, should primarily target HTN2 to ensure that functional deficits are apparent and treatment-induced improvements can be detected.
Vascular dysfunction is thought to occur in a similar fashion across multiple tissue beds throughout the body, including the skin. A series of studies has demonstrated a relation between skin blood flow and renal resistance, coronary flow reserve, and brachial artery flow-mediated dilation indicating that functional decrements in the cutaneous microcirculation evolve in parallel to those in these nutritive vascular beds (8, 12, 30, 31, 34, 40, 48). This phenomenon has specifically been shown with essential hypertension. For example, Taddei et al. reported blunted ACh-induced dilation in the forearm (i.e., skeletal muscle circulation) in normotensive offspring of essential hypertensive parents, suggesting that impairments in ACh-induced dilation precede the onset of essential hypertension (47). Based on these data, we were surprised that we did not detect impairments in ACh-induced dilation in adults with HTN1. However, the pathophysiology of essential hypertension is complex and future prospective studies to better understand the temporal development and mechanistic regulation of microvascular dysfunction are warranted.
The AHA/ACC definitions of hypertension are based on seated clinic BP measurements (53). However, seated measurements fail to identify those with white coat or masked hypertension. Reports differ on the prevalence of white coat and masked hypertension, ranging from 9 to 54% of the population (3, 24). Thus, ABPM is an adjunctive methodology that more appropriately reflects an individual’s “true” BP profile in daily living, has ecological validity, and is more precise in predicting cardiovascular events (20, 45, 50). In the present study, based on ABPM, 34% of participants demonstrated either white coat or masked hypertension, which is consistent with population estimates (3, 24). Excluding these participants did not change our primary findings. These data are somewhat surprising, as we expected to observe more severe impairments in NO-mediated endothelial function in HTN2 after removing these individuals from analysis. Instead, adults with white coat or masked hypertension may, in fact, have impaired or maintained cutaneous microvascular function, respectively. However, the current study is underpowered to comprehensively test this hypothesis. Because both white coat and masked hypertension are associated with increased cardiovascular risk (24, 56), using ABPM in addition to resting seated BP to more fully characterize the BP profile of daily living has substantial clinical utility.
ABPM also allows for the characterization of diurnal variations in BP. BP typically follows a circadian rhythm, reaching its lowest values while asleep, termed “nighttime BP dipping.” The lack of at least a 10% dip in nighttime BP is associated with increased risk for the occurrence of a cardiovascular event (7, 28, 37, 50, 57). The present study is the first to assess NO-mediated endothelium-dependent dilation in relation to the nocturnal dip in BP. Our results demonstrate that, regardless of seated BP, endothelium-dependent dilation was significantly blunted in nondippers. When further evaluating the nighttime dip in BP based on BP classification, a statistical difference in ACh-induced dilation between dippers and nondippers was only evident in HTN2, likely owing to the relatively small sample size of NTN and HTN1 included in this ancillary analysis. The mechanistic underpinnings of endothelial dysfunction in adults lacking a nocturnal dip in BP may be related to alterations in sympathetic neural control of BP or salt sensitivity, both of which have been shown to modulate the diurnal variation in BP (4, 41, 44, 49). Future longitudinal studies investigating the time course of the development of HTN2, as well as treatment interventions, and the associated cutaneous microvascular endothelial dysfunction are needed to further characterize these changes.
Limitations.
There were several limitations to our study. First, due to the nature of the retrospective analysis, the number of subjects in each BP classification group, as well as men and women within each group, was not evenly distributed. As with the general public, the majority of these participants were unaware of their hypertensive status when screening for the study. Thus, there is no way to know how long they have been hypertensive. This may potentially introduce variability into the study design and results. In the subset of adults who participated in multiple studies, there was a minimum of 8 mo between study visits. Although we treated these “repeat” participants as independent samples, which may have statistical limitations, in confirmation analyses in which data from only one visit per participant was randomly selected, the results did not change, thus providing further support for our interpretation and overall conclusions. Additionally, although we were not powered to make sex comparisons, in preliminary analyses, we did not detect any differences in endothelium-dependent dilation between sexes (P = 0.18). The existing research pertaining to sex differences in microvascular function and dysfunction is equivocal (27, 46). Studies elucidating the influence of sex as a biological variable in the development of cutaneous microvascular dysfunction in adults with high blood pressure are needed. Finally, the current study did not quantify endothelium-independent dilation. However, studies have demonstrated preserved endothelium-independent dilation in adults with HTN (10), therefore, it is unlikely that this influenced the results of the present investigation.
Perspectives and Conclusions
The cutaneous microcirculation is a unique vascular bed in terms of its neurovascular control. While it is not thought to contribute to the modulation of BP during thermoneutral conditions, it is an easily accessible vascular bed in which to pharmacologically dissect the mechanistic regulation of microvascular function in a manner that is otherwise highly invasive or, oftentimes, not possible to conduct in humans. Alterations in cutaneous microvascular function are detectable before overt target-organ damage becomes apparent (29). Using this model, however, we did not detect cutaneous microvascular dysfunction in HTN1. It may be possible that these adults were very healthy, despite having elevated resting BP, or perhaps there may be other unknown confounding factors contributing to these results. Future studies are warranted to further elucidate the temporal development of HTN-associated microvascular dysfunction. Furthermore, the magnitude of HTN-induced microvascular dysfunction is an important consideration for studies designed to mechanistically examine the mediators of endothelial dysfunction in adults with chronically elevated BP.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-093238 (to L.M.A.); National Dairy Council; and Clinical and Translational Science Institute Grant TL1 TR002016 (to G.A.D.).
DISCLOSURES
L.M.A. serves on the Performance Health Scientific Advisory Committee on the Nutrition Research Scientific Advisory Council.
AUTHOR CONTRIBUTIONS
J.L.G. and L.M.A. conceived and designed research; G.A.D., J.L.G., S.S., and U.A.L. performed experiments; G.A.D., J.L.G., and S.S. analyzed data; G.A.D., J.L.G., U.A.L., and L.M.A. interpreted results of experiments; G.A.D. prepared figures; G.A.D. and J.L.G. drafted manuscript; G.A.D., J.L.G., S.S., U.A.L., and L.M.A. edited and revised manuscript; G.A.D., J.L.G., S.S., U.A.L., and L.M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the time and effort given by the study volunteers. We also acknowledge Sue Slimak and Jane Pierzga for assistance.
REFERENCES
- 1.Alba BK, Greaney JL, Ferguson SB, Alexander LM. Endothelial function is impaired in the cutaneous microcirculation of adults with psoriasis through reductions in nitric oxide-dependent vasodilation. Am J Physiol Heart Circ Physiol 314: H343–H349, 2018. doi: 10.1152/ajpheart.00446.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander LM, Kutz JL, Kenney WL. Tetrahydrobiopterin increases NO-dependent vasodilation in hypercholesterolemic human skin through eNOS-coupling mechanisms. Am J Physiol Regul Integr Comp Physiol 304: R164–R169, 2013. doi: 10.1152/ajpregu.00448.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aung K, Htay T. Relationship between outpatient clinic and ambulatory blood pressure measurements and mortality. Curr Cardiol Rep 21: 28, 2019. doi: 10.1007/s11886-019-1114-z. [DOI] [PubMed] [Google Scholar]
- 4.Bankir L, Bochud M, Maillard M, Bovet P, Gabriel A, Burnier M. Nighttime blood pressure and nocturnal dipping are associated with daytime urinary sodium excretion in African subjects. Hypertension 51: 891–898, 2008. doi: 10.1161/HYPERTENSIONAHA.107.105510. [DOI] [PubMed] [Google Scholar]
- 5.Beck DT, Casey DP, Martin JS, Emerson BD, Braith RW. Exercise training improves endothelial function in young prehypertensives. Exp Biol Med (Maywood) 238: 433–441, 2013. doi: 10.1177/1535370213477600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UK, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 137: e67–e492, 2018. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee . The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289: 2560–2572, 2003. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 8.Coulon P, Constans J, Gosse P. Impairment of skin blood flow during post-occlusive reactive hyperhemy assessed by laser Doppler flowmetry correlates with renal resistive index. J Hum Hypertens 26: 56–63, 2012. doi: 10.1038/jhh.2010.117. [DOI] [PubMed] [Google Scholar]
- 9.Craighead DH, Smith CJ, Alexander LM. Blood pressure normalization via pharmacotherapy improves cutaneous microvascular function through NO-dependent and NO-independent mechanisms. Microcirculation 24: 2017. doi: 10.1111/micc.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craighead DH, Wang H, Santhanam L, Alexander LM. Acute lysyl oxidase inhibition alters microvascular function in normotensive but not hypertensive men and women. Am J Physiol Heart Circ Physiol 314: H424–H433, 2018. doi: 10.1152/ajpheart.00521.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cupisti A, Rossi M, Placidi S, Fabbri A, Morelli E, Vagheggini G, Meola M, Barsotti G. Responses of the skin microcirculation to acetylcholine in patients with essential hypertension and in normotensive patients with chronic renal failure. Nephron 85: 114–119, 2000. doi: 10.1159/000045643. [DOI] [PubMed] [Google Scholar]
- 12.Debbabi H, Bonnin P, Ducluzeau PH, Lefthériotis G, Levy BI. Noninvasive assessment of endothelial function in the skin microcirculation. Am J Hypertens 23: 541–546, 2010. doi: 10.1038/ajh.2010.10. [DOI] [PubMed] [Google Scholar]
- 13.Dharmashankar K, Widlansky ME. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep 12: 448–455, 2010. doi: 10.1007/s11906-010-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannotti G, Doerries C, Mocharla PS, Mueller MF, Bahlmann FH, Horvath T, Jiang H, Sorrentino SA, Steenken N, Manes C, Marzilli M, Rudolph KL, Luscher TF, Drexler H, Landmesser U. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension 55: 1389–1397, 2010. doi: 10.1161/HYPERTENSIONAHA.109.141614. [DOI] [PubMed] [Google Scholar]
- 15.Greaney JL, Kenney WL, Alexander LM. Neurovascular mechanisms underlying augmented cold-induced reflex cutaneous vasoconstriction in human hypertension. J Physiol 595: 1687–1698, 2017. doi: 10.1113/JP273487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greaney JL, Kutz JL, Shank SW, Jandu S, Santhanam L, Alexander LM. Impaired hydrogen sulfide-mediated vasodilation contributes to microvascular endothelial dysfunction in hypertensive adults. Hypertension 69: 902–909, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greaney JL, Saunders EF, Santhanam L, Alexander LM. Oxidative stress contributes to microvascular endothelial dysfunction in men and women with major depressive disorder. Circ Res 124: 564–574, 2019. doi: 10.1161/CIRCRESAHA.118.313764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gryglewska B, Nęcki M, Cwynar M, Baron T, Grodzicki T. Local heat stress and skin blood flowmotion in subjects with familial predisposition or newly diagnosed hypertension. Blood Press 19: 366–372, 2010. doi: 10.3109/08037051.2010.488053. [DOI] [PubMed] [Google Scholar]
- 19.Guo X, Zhang X, Guo L, Li Z, Zheng L, Yu S, Yang H, Zhou X, Zhang X, Sun Z, Li J, Sun Y. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep 15: 703–716, 2013. doi: 10.1007/s11906-013-0403-y. [DOI] [PubMed] [Google Scholar]
- 20.Hara A, Tanaka K, Ohkubo T, Kondo T, Kikuya M, Metoki H, Hashimoto T, Satoh M, Inoue R, Asayama K, Obara T, Hirose T, Izumi S, Satoh H, Imai Y. Ambulatory versus home versus clinic blood pressure: the association with subclinical cerebrovascular diseases: the Ohasama Study. Hypertension 59: 22–28, 2012. doi: 10.1161/HYPERTENSIONAHA.111.174938. [DOI] [PubMed] [Google Scholar]
- 21.Hayakawa H, Raij L. The link among nitric oxide synthase activity, endothelial function, and aortic and ventricular hypertrophy in hypertension. Hypertension 29: 235–241, 1997. doi: 10.1161/01.hyp.29.1.235. [DOI] [PubMed] [Google Scholar]
- 22.Head GA, Mihailidou AS, Duggan KA, Beilin LJ, Berry N, Brown MA, Bune AJ, Cowley D, Chalmers JP, Howe PR, Hodgson J, Ludbrook J, Mangoni AA, McGrath BP, Nelson MR, Sharman JE, Stowasser M; Ambulatory Blood Pressure Working Group of the High Blood Pressure Research Council of Australia . Definition of ambulatory blood pressure targets for diagnosis and treatment of hypertension in relation to clinic blood pressure: prospective cohort study. BMJ 340: c1104, 2010. doi: 10.1136/bmj.c1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holowatz LA, Thompson-Torgerson CS, and Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol 105: 370–372, 2008. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, Huang W, Mai W, Cai X, An D, Liu Z, Huang H, Zeng J, Hu Y, Xu D. White-coat hypertension is a risk factor for cardiovascular diseases and total mortality. J Hypertens 35: 677–688, 2017. doi: 10.1097/HJH.0000000000001226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y, Su L, Cai X, Mai W, Wang S, Hu Y, Wu Y, Tang H, Xu D. Association of all-cause and cardiovascular mortality with prehypertension: a meta-analysis. Am Heart J 167: 160–168.e1, 2014. doi: 10.1016/j.ahj.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Wang S, Cai X, Mai W, Hu Y, Tang H, Xu D. Prehypertension and incidence of cardiovascular disease: a meta-analysis. BMC Med 11: 177, 2013. doi: 10.1186/1741-7015-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huxley VH, Kemp SS. Sex-specific characteristics of the microcirculation. Adv Exp Med Biol 1065: 307–328, 2018. doi: 10.1007/978-3-319-77932-4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingelsson E, Björklund-Bodegård K, Lind L, Arnlöv J, Sundström J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA 295: 2859–2866, 2006. doi: 10.1001/jama.295.24.2859. [DOI] [PubMed] [Google Scholar]
- 29.Jung F, Pindur G, Ohlmann P, Spitzer G, Sternitzky R, Franke RP, Leithäuser B, Wolf S, Park JW. Microcirculation in hypertensive patients. Biorheology 50: 241–255, 2013. doi: 10.3233/BIR-130645. [DOI] [PubMed] [Google Scholar]
- 30.Kenney WL, Edward F. Adolph Distinguished Lecture: skin-deep insights into vascular aging. J Appl Physiol 123: 1024–1038, 2017. doi: 10.1152/japplphysiol.00589.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan F, Patterson D, Belch JJ, Hirata K, Lang CC. Relationship between peripheral and coronary function using laser Doppler imaging and transthoracic echocardiography. Clin Sci (Lond) 115: 295–300, 2008. doi: 10.1042/CS20070431. [DOI] [PubMed] [Google Scholar]
- 32.Lindstedt IH, Edvinsson ML, Edvinsson L. Reduced responsiveness of cutaneous microcirculation in essential hypertension–a pilot study. Blood Press 15: 275–280, 2006. doi: 10.1080/08037050600996586. [DOI] [PubMed] [Google Scholar]
- 33.Mancusi C, Canciello G, Izzo R, Damiano S, Grimaldi MG, de Luca N, de Simone G, Trimarco B, Losi MA; The Campania Salute Network . Left atrial dilatation: a target organ damage in young to middle-age hypertensive patients. Int J Cardiol 265: 229–233, 2018. doi: 10.1016/j.ijcard.2018.03.120. [DOI] [PubMed] [Google Scholar]
- 34.Matsuda J, Murai T, Kanaji Y, Usui E, Araki M, Niida T, Ichijyo S, Hamaya R, Lee T, Yonetsu T, Isobe M, Kakuta T. Prevalence and clinical significance of discordant changes in fractional and coronary flow reserve after elective percutaneous coronary intervention. J Am Heart Assoc 311: 5, 2016. doi: 10.1161/JAHA.116.004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Millar-Craig MW, Bishop CN, Raftery EB. Circadian variation of blood-pressure. Lancet 1: 795–797, 1978. doi: 10.1016/S0140-6736(78)92998-7. [DOI] [PubMed] [Google Scholar]
- 36.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 137: 109–118, 2018. doi: 10.1161/CIRCULATIONAHA.117.032582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Brien E, Sheridan J, O’Malley K. Dippers and non-dippers. Lancet 332: 397, 1988. doi: 10.1016/S0140-6736(88)92867-X. [DOI] [PubMed] [Google Scholar]
- 38.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 111: 697–716, 2005. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 39.Rossi M, Bradbury A, Magagna A, Pesce M, Taddei S, Stefanovska A. Investigation of skin vasoreactivity and blood flow oscillations in hypertensive patients: effect of short-term antihypertensive treatment. J Hypertens 29: 1569–1576, 2011. doi: 10.1097/HJH.0b013e328348b653. [DOI] [PubMed] [Google Scholar]
- 40.Rossi M, Matteucci E, Pesce M, Consani C, Franzoni F, Santoro G, Giampietro O. Peripheral microvascular dysfunction as an independent predictor of atherosclerotic damage in type 1 diabetes patients: a preliminary study. Clin Hemorheol Microcirc 54: 381–391, 2013. doi: 10.3233/CH-2012-1628. [DOI] [PubMed] [Google Scholar]
- 41.Sherwood A, Steffen PR, Blumenthal JA, Kuhn C, Hinderliter AL. Nighttime blood pressure dipping: the role of the sympathetic nervous system. Am J Hypertens 15: 111–118, 2002. doi: 10.1016/S0895-7061(01)02251-8. [DOI] [PubMed] [Google Scholar]
- 42.Shimbo D, Muntner P, Mann D, Viera AJ, Homma S, Polak JF, Barr RG, Herrington D, Shea S. Endothelial dysfunction and the risk of hypertension: the multi-ethnic study of atherosclerosis. Hypertension 55: 1210–1216, 2010. doi: 10.1161/HYPERTENSIONAHA.109.143123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension 58: 935–942, 2011. doi: 10.1161/HYPERTENSIONAHA.111.178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staessen JA, Birkenhäger W, Bulpitt CJ, Fagard R, Fletcher AE, Lijnen P, Thijs L, Amery A. The relationship between blood pressure and sodium and potassium excretion during the day and at night. J Hypertens 11: 443–447, 1993. doi: 10.1097/00004872-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 45.Staessen JA, Thijs L, Fagard R, O’Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J; Systolic Hypertension in Europe Trial Investigators . Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA 282: 539–546, 1999. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 46.Stanhewicz AE, Wenner MM. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am J Physiol Heart Circ Physiol 315: H1569–H1588, 2018. doi: 10.1152/ajpheart.00396.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taddei S, Virdis A, Mattei P, Ghiadoni L, Sudano I, Salvetti A. Defective L-arginine-nitric oxide pathway in offspring of essential hypertensive patients. Circulation 94: 1298–1303, 1996. doi: 10.1161/01.CIR.94.6.1298. [DOI] [PubMed] [Google Scholar]
- 48.Tona F, Osto E, Famoso G, Previato M, Fedrigo M, Vecchiati A, Perazzolo Marra M, Tellatin S, Bellu R, Tarantini G, Feltrin G, Angelini A, Thiene G, Gerosa G, Iliceto S. Coronary microvascular dysfunction correlates with the new onset of cardiac allograft vasculopathy in heart transplant patients with normal coronary angiography. Am J Transplant 15: 1400–1406, 2015. doi: 10.1111/ajt.13108. [DOI] [PubMed] [Google Scholar]
- 49.Uzu T, Ishikawa K, Fujii T, Nakamura S, Inenaga T, Kimura G. Sodium restriction shifts circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation 96: 1859–1862, 1997. doi: 10.1161/01.CIR.96.6.1859. [DOI] [PubMed] [Google Scholar]
- 50.Verdecchia P. Prognostic value of ambulatory blood pressure: current evidence and clinical implications. Hypertension 35: 844–851, 2000. doi: 10.1161/01.hyp.35.3.844. [DOI] [PubMed] [Google Scholar]
- 51.Walther G, Obert P, Dutheil F, Chapier R, Lesourd B, Naughton G, Courteix D, Vinet A. Metabolic syndrome individuals with and without type 2 diabetes mellitus present generalized vascular dysfunction: cross-sectional study. Arterioscler Thromb Vasc Biol 35: 1022–1029, 2015. doi: 10.1161/ATVBAHA.114.304591. [DOI] [PubMed] [Google Scholar]
- 52.Weis M, Hartmann A, Olbrich HG, Hör G, Zeiher AM. Prognostic significance of coronary flow reserve on left ventricular ejection fraction in cardiac transplant recipients. Transplantation 65: 103–108, 1998. doi: 10.1097/00007890-199801150-00020. [DOI] [PubMed] [Google Scholar]
- 53.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 138: e426–e483, 2018. [DOI] [PubMed] [Google Scholar]
- 54.Wilt TJ, Kansagara D, Qaseem A; Clinical Guidelines Committee of the American College of Physicians . Hypertension limbo: balancing benefits, harms, and patient preferences before we lower the bar on blood pressure. Ann Intern Med 168: 369–370, 2018. doi: 10.7326/M17-3293. [DOI] [PubMed] [Google Scholar]
- 55.Zeng H, Jiang Y, Tang H, Ren Z, Zeng G, Yang Z. Abnormal phosphorylation of Tie2/Akt/eNOS signaling pathway and decreased number or function of circulating endothelial progenitor cells in prehypertensive premenopausal women with diabetes mellitus. BMC Endocr Disord 16: 13, 2016. doi: 10.1186/s12902-016-0093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang DY, Guo QH, An DW, Li Y, Wang JG. A comparative meta-analysis of prospective observational studies on masked hypertension and masked uncontrolled hypertension defined by ambulatory and home blood pressure. J Hypertens 37: 1775–1785, 2019. doi: 10.1097/HJH.0000000000002109. [DOI] [PubMed] [Google Scholar]
- 57.Zweiker R, Eber B, Schumacher M, Toplak H, Klein W. “Non-dipping” related to cardiovascular events in essential hypertensive patients. Acta Med Austriaca 21: 86–89, 1994. [PubMed] [Google Scholar]