Abstract
Although increased predisposition to cardiac fibrosis and cardiac dysfunction has been demonstrated in the perinatally nicotine-exposed heart, the underlying mechanisms remain unclear. With the use of a well-established rat model and cultured primary neonatal rat cardiac fibroblasts, the effect of perinatal nicotine exposure on offspring heart extracellular matrix deposition and the likely underlying mechanisms were investigated. Perinatal nicotine exposure resulted in increased collagen type I (COL1A1) and III (COL3A1) deposition along with a decrease in miR-29 family and an increase in long noncoding RNA myocardial infarction-associated transcript (MIAT) levels in offspring heart. Nicotine treatment of isolated primary neonatal rat cardiac fibroblasts suggested that these effects were mediated via nicotinic acetylcholine receptors including α7 and the induced collagens accumulation was reversed by a gain-of function of miR-29 family. Knockdown of MIAT resulted in increased miR-29 family and decreased COL1A1 and COL3A1 levels, suggesting nicotine-mediated MIAT induction as the underlying mechanism for nicotine-induced collagen deposition. Luciferase reporter assay and RNA immunoprecipitation studies showed an intense physical interaction between MIAT, miR-29 family, and argonaute 2, corroborating the mechanistic link between perinatal nicotine exposure and increased extracellular matrix deposition. Overall, perinatal nicotine exposure resulted in lower miR-29 family levels in offspring heart, while it elevated cardiac MIAT and collagen type I and III levels. These findings provide mechanistic basis for cardiac dysfunction in perinatal nicotine-exposed offspring and offer multiple novel potential therapeutic targets.
NEW & NOTEWORTHY Using an established rat model and cultured primary neonatal cardiac fibroblasts, we show that nicotine mediated MIAT induction as the underlying mechanism for the excessive cardiac collagen deposition. These observations provide mechanistic basis for the increased predisposition to cardiac dysfunction following perinatal cigarette/nicotine exposure and offer novel potential therapeutic targets.
Keywords: cardiac fibrosis, collagens, MIAT, miR-29, smoking in pregnancy
INTRODUCTION
Despite awareness of adverse effects to the fetus, as well as to themselves, ~10% of pregnant women smoke, resulting in more than 400,000 smoke-exposed infants born yearly (29, 68). This is likely to be an underestimate since at least 20% of pregnant smokers lie about their habit (73). Probably, an even larger number of women and children are exposed to secondhand and thirdhand smoke (53, 62, 77). Overall, smoking during pregnancy is the largest preventable cause of prematurity, low birth weight, intrauterine growth restriction, and perinatal mortality (22, 65). Nicotine appears to be a major component of cigarette smoke that causes perinatal morbidity (41, 50, 51).
Although pulmonary consequences of early life smoke/nicotine exposure are well established and well studied, emerging evidence from epidemiological and experimental studies suggests a variety of adverse cardiovascular outcomes (2, 9), including the development of coronary ischemia phenotype (25, 32, 43, 69, 86), atherosclerosis (85), hypertension (24, 25, 87), and cardiac arrhythmias (63) in the exposed offspring. Arterial stiffness (34) and myocardial fibrosis (26, 95) seen following perinatal cigarette smoke/nicotine exposure likely explain many of these pathologies; however, the underlying molecular mechanisms remain incompletely understood. Accumulation of matrix proteins such as the collagens (36), a key pathology in myocardial fibrosis, has been observed following prenatal nicotine exposure, which gets exacerbated with further postnatal nicotine exposure (12). Since nicotine-induced fibrosis has been shown to be mediated by nicotine’s effects on fibroblasts in many organs (30), here we examine the effect of perinatal nicotine exposure on cardiac fibroblasts.
Recent studies from our laboratory and those of others have reported that miR-29 family (miR-29a, miR-29b, and miR-29c) is downregulated in several fibrosis-related diseases and has been targeted to modulate many fibrosis-promoting genes (14, 16, 17, 21, 40). Specifically, in cardiovascular conditions, such as myocardial infarction and streptozotocin-induced diabetic cardiomyopathy, miR-29 family was found to be repressed in association with cardiac fibrosis and increased extracellular matrix (ECM) deposition (38, 59, 78, 97, 99). MiR-29 has also been implicated in pathological hypertrophy, fibrosis, and overall cardiac dysfunction in a model of cardiac pressure overload (66). In the present study, we determined the expression and the functional role of miR-29 family in offspring heart following perinatal nicotine exposure, which has not been previously reported.
Similar to miRNAs, increasing evidence indicates that lncRNAs, which are >200 nucleotides in length and lack protein-coding activity, are expressed in a cell- and tissue-specific manner. Through their interactions with miRNAs and mRNAs, lncRNAs post-transcriptionally regulate many protein coding genes (5, 10, 31). In addition to their regulatory function in normal cellular activities, their altered expression has been identified to affect many pathological conditions including cardiovascular diseases and cardiac remodeling (72). One such lncRNA, named myocardial infarction-associated transcript (MIAT), also known as retinal noncoding RNA 2 (RNCR2), has been documented to be involved in myocardial infarction among other biological and pathological processes (20, 45, 67). MIAT exerts it effects, in part, by competing with endogenous RNA to regulate the biological functions of miR-150–5p, miR-93, and miR-24 (28, 44, 60, 93, 100). It has been specifically shown to correlate with two cardiovascular risk factors, i.e., hypertension and smoking (79). Nonetheless, MIAT’s role in perinatal smoke/nicotine-induced cardiac phenotype has not been examined. Using a rat model, here we report the effect of perinatal nicotine exposure on cardiac ECM deposition and whether miR-29 family and MIAT play a causal role in mediating this effect.
MATERIALS AND METHODS
Animal model.
Time-mated, first time-pregnant Sprague-Dawley rat dams (200–250 g body wt; Charles River Laboratories, Inc., Hollister, CA) were housed at a constant temperature and humidity with a 12-h:12-h light-dark cycle. With the use of a well-published protocol, the animals received either placebo (diluent) or nicotine (1 mg/kg sc) in 100-µL volumes daily from the 6th day of gestation until term (day 22) to postnatal day (PND) 21 (37, 61, 64). This dose of nicotine has been used by others and us to mimic daily nicotine exposure to the fetus of the moderately heavy pregnant smoker, i.e., ~1 mg·kg body wt−1·day−1 (37, 61, 64). The control and nicotine-treated dams were pair-fed in accordance with the previous day’s consumption by the nicotine-treated group and allowed free access to water. After spontaneous delivery at term, the pups breastfed ad libitum. At PND 1 and 21 (~age 5 in human years), the pups were euthanized and hearts collected for primary cardiac fibroblast isolation, Western blot and qRT-PCR analyses, and immunofluorescence staining, as needed. Important to point out at the outset was that this study was restricted only to males. All animal procedures were performed following the National Institutes of Health’s “Guidelines for the Care and Use of Laboratory Animals” and approved by the Institutional Animal Care and Use Committee at the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
Reagents and primary cardiac fibroblast cells isolation.
Nicotine bitartrate, α-bungarotoxin, and mecamylamine were acquired from Sigma-Aldrich (St. Louis, MO). Cardiac fibroblasts were isolated from PND 1 hearts following the previously described methods (48). Briefly, hearts were cut into minute pieces, washed twice with phosphate-buffered saline (PBS) containing 1% antibiotic-antimycotic solution, and digested in Hanks’s balanced salt solution containing 100 μg/mL trypsin and 70 U/mL collagenase. Cells were then plated onto a tissue culture plate for 1.5 h for differential adherence. Cardiac fibroblasts attached preferentially, whereas media containing unattached cardiomyocytes was removed. Attached cells, containing mainly cardiac fibroblasts, were cultured in DMEM supplemented with 10% fetal bovine serum until confluence with a change of media every 2 to 3 days. Passage 1–3 cells were used for all experiments. Experiments were performed at least three times from separate fibroblast isolations. All supplies for isolation and cell culture were purchased from Sigma-Aldrich (St. Louis, MO) and Fisher Scientific (Atlanta, GA).
Immunoblotting.
Immunoblotting was performed following previously described methods (15, 18). Briefly, samples were suspended in RIPA buffer containing 1 mM EDTA and EGTA (Boston BioProducts, Ashland, MA) supplemented with 1 mM PMSF and a complete protease inhibitor mixture (Roche Diagnostics, Indianapolis, IN), sonicated, and centrifuged at 4°C for 10 min at 14,000 rpm. The concentration of protein was determined using the BCA protein assay kit (Thermo Scientific Pierce, Rockford, IL). Equal aliquots (50 mg) of total protein for each sample were denatured with SDS-PAGE sample buffer and separated by electrophoresis on an SDS polyacrylamide gel. After the samples were transferred to a nitrocellulose membrane, the membrane was blocked with TBS-Tween plus 5% milk and probed with the following primary antibodies: COL1A1 (Fitzgerald Industries Intl., Acton, MA), COL3A1 (Proteintech Group, Inc., Chicago, Illinois) and argonaute 2 (Ago2) (Millipore, Burlington, MA). The membranes were washed with TBS containing 0.1%Tween-20 wash buffer after each antibody incubation cycle. SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific Pierce) was used for detection, and photographic emulsion was used to identify the protein bands, which were subsequently quantified by densitometry. The membranes were also stripped and probed with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (Santa Cruz Biotechnology, Santa Cruz, CA) serving as the loading control. The densities of the specific protein bands were quantified with a scanning densitometer (Bio-Rad GS-800, Hercules, CA), and the results are shown as box-and-whisker plot.
Immunofluorescence double staining.
For tissue immunofluorescence staining for the relevant proteins, hearts were fixed with 4% paraformaldehyde in PBS and then subsequently transferred to PBS containing 30% sucrose (wt/vol) until equilibrated at 4°C. After fixation, 5-µm paraffin sections were treated three times with Histo-Clear (National Diagnostics, Atlanta, GA) for 5 min and then rehydrated by a sequential ethanol wash. After sections were blocked with PBS-5% normal goat serum-0.2% Triton X-100, tissue sections were incubated with primary antibody (α-sarcomeric actin; 1:200, Santa Cruz Biotechnology) overnight at 4°C in a humidified chamber. After several PBS washes at room temperature, the tissues were incubated with the appropriate Alexa Fluor secondary antibody (1:200; goat anti-mouse Alexa Fluor 488 green; Invitrogen, Carlsbad, CA) for 30 min in a humidified, darkened chamber. For collagen staining, tissue sections were blocked again with 5% goat serum and then incubated with primary antibodies (COL1A1; 1:200, Fitzgerald Industries Intl., and COL3A1; 1:200, Proteintech Group, Inc.) for 1 h at room temperature. After several PBS washes, the tissues sections were incubated with the appropriate Alexa Fluor secondary antibody (1:200; goat anti-rabbit Alexa Fluor 568 Red; Invitrogen) for 30 min in a humidified, darkened chamber. After the final wash in PBS, the sections were mounted with ProLong Gold antifade reagent with DAPI (Invitrogen). Control sections were incubated with the secondary antibody, but the primary antibodies were replaced with the dilution reagent (Dako, Carpinteria, CA). Immunostained sections were examined using excitation/emission wavelengths for detecting the stated fluorescent stain: 490 nm/525 nm for Alexa Fluor 488-conjugated secondary antibodies (green), 578 nm/603 nm for Alexa Fluor 568-conjugated secondary antibody (red), and 350 nm/470 nm for DAPI (blue) under a microscope (Axioskop 40; Carl Zeiss Microimaging, LLC, Thornwood, NY) at 40× magnification. Images were processed by reducing autofluorescence background and potential nonspecific binding of secondary antibodies based on control sections with the same settings applied to every image and were superimposed using Adobe Photoshop version 7.0.
RNA isolation and qRT-PCR analysis.
Total RNA was extracted from primary cardiac fibroblasts using TRIzol (Thermo Fisher Scientific, Waltham, MA), and the quantity and quality were determined (ND-1000 Spectrophotometer, NanoDrop Technologies, Wilmington, DE) as previously described (13). RNA sample of 1 μg each was reverse transcribed using random primers. MiR-29 family primer design and PCR conditions have been previously described (11). Quantitative RT-PCR was carried out using SYBR green expression master mixes (Applied Biosystems, Carlsbad, CA). Reactions were incubated for 10 min at 95°C followed by 40 cycles for 15 s at 95°C and 1 min at 60°C. Levels of mRNA and miRNA were quantified using the Invitrogen StepOne System and normalized to peptidylprolyl isomerase A (PPIA) and RNU6B, respectively. All reactions were run in triplicate, and relative expression was determined using the comparative cycle threshold method (2−ΔΔCT), as recommended by the supplier (Applied Biosystems). Abundance values were expressed as fold changes compared with the corresponding control group. The primer sequences used were as follows:
MIAT (sense, 5′-TCATCTGTCTCAGTGGTACCTT-3′; antisense, 5′-AGGTTCAATCCCTGTGTTGTG-3′), COL1A1 (sense, 5′-CAAGATGGTGGCCGTTACTAC-3′; antisense, 5′-GCTGCGGATGTTCTCAATCT-3′), COL3A1 (sense, 5′-CTGAACTCAAGAGCGGAGAATAC-3′; antisense, 5′-CAGTCATGGGACTGGCATTTA-3′), PPIA (sense, 5′-GGCTATAAGGGTTCCTCCTTTC-3′; antisense, 5′-TTGCCACCAGTGCCATTA-3′), miR-29a (sense, 5′-CGCAGTAGCACCATCTGA-3′; antisense, 5′-TCCAGTTTTTTTTTTTTTTTAACCGA-3′), miR-29b (sense, 5′-CAGTAGCACCATTTGAAATCAG-3′; antisense, 5′-GGTCCAGTTTTTTTTTTTTTTTAACAC-3′), miR-29c (sense, 5′-CAGTAGCACCATTTGAAATCG-3′; antisense, 5′-GGTCCAGTTTTTTTTTTTTTTTAACC-3′), and RNU6B (sense, 5′-ATTGGAACGATACAGAGAAGATTAG-3′; antisense, 5′-AATATGGAACGCTTCACGAAT-3′).
siRNA transfection.
Cultured primary cardiac fibroblasts at 60–70% confluence were transfected with 50 nM of siRNA negative control (siNC) or siRNA against MIAT (5′-CCAACAAUGCCCAGAGAAA-3′) for 48–96 h using PureFection transfection reagent (System Biosciences, Inc., Mountain View, CA) according to the manufacturer’s protocol.
Gain of function of miR-29 family.
Cultured primary cardiac fibroblasts were seeded at a cell density of 3.5 × 104/well in six-well plates and at subconfluence transfected with 50 nM of pre-miR-29 family (miR-29a, -b, -c) or pre-miR negative control (NC) (Applied Biosystems) for 72 h using PureFection transfection reagent (System Biosciences, Inc) according to the manufacturer's protocol.
Reporter plasmid construction.
Recombinant luciferase reporter plasmid pEZX-MT01 (MIAT) was constructed by insertion of EcoRI/Xho1-digested PCR-amplified fragment of MIAT (+2074/+2439) covering the miR-29 family binding sites into the downstream of the luciferase reporter pEZX-MT01 (GeneCopoeia, Rockville, MD). The fragment of MIAT was amplified using primers with the following sequences: forward primer, 5′-CGGAATTCCATGGGAGTATTCAGCCAGAG-3′; and reverse primer, 5′-CCGCTCGAGCACCCTGGACACAGAGAAAG-3′.
Dual-luciferase reporter assay.
Fetal primary cardiac fibroblasts were seeded in six-well plates and, at 70–80% subconfluence, were transfected with 50 nM pre-miR-29 family (miR-29a, -b, -c) or pre-miR negative control (NC) using the PureFection transfection reagent. At the same time, the cells were cotransfected with a luciferase reporter plasmid (1 μg/well) pEZX-MT01 (control) or pEZX-MT01 (MIAT). Firefly and Renilla luciferase activities were measured after 48 h of transfection using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Firefly luciferase activity was normalized to Renilla luciferase activity, and the level of induction was reported as box-and-whisker plot of four sets of independent experiments and compared with a ratio in cells transfected with negative control independently set as 1.
RNA immunoprecipitation.
RNA immunoprecipitation (RIP) assay was performed using an EZ-Magna RIP RNA Binding Protein Immunoprecipitation Kit according to the protocol of the manufacturer (Millipore). Briefly, primary cardiac fibroblasts were lysed in RIP lysis buffer after 96 h of treatment with vehicle control or nicotine, following incubation with RIP buffer containing magnetic bead-bound anti-Ago2 antibody (Millipore) or negative control mouse immunoglobulin G (IgG; Millipore). The samples were next incubated with proteinase K to digest protein, and the immunoprecipitated RNA was isolated and subjected to qRT-PCR for expression analysis.
Statistical analysis.
Data are presented as box-and-whisker plots and were analyzed using Prism version 8 (GraphPad Software, San Diego, CA). Data set normality was determined by the Kolmogrove-Smirnoff test. Comparisons involving two groups were made using unpaired Student’s t test. Statistical significance was established at P < 0.05.
RESULTS
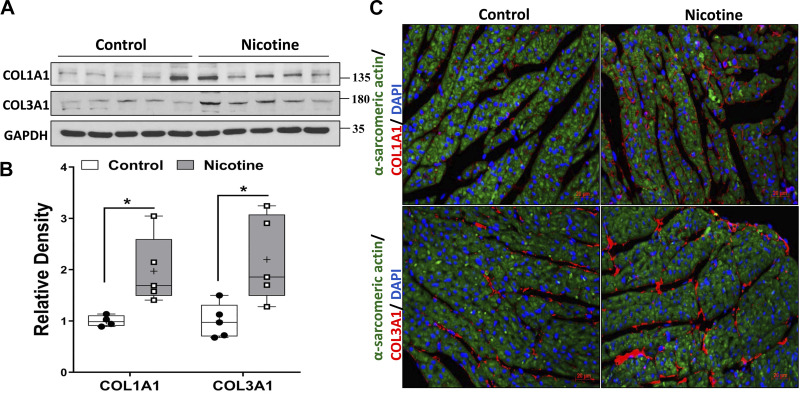
In the perinatal nicotine exposure model studied, as shown in Fig. 1, the expression of ECM proteins COL1A1 and COL3A1 increased in offspring heart at PND21. However, there were no differences in the expression of two cardiac hypertrophy markers atrial natriuretic factor (ANF) and myosin heavy chain 7 (MYH7) between the control and nicotine-exposed groups (data not shown); therefore, in subsequent studies, we focused on the molecular mechanism underlying increased cardiac ECM deposition in the perinatally nicotine-exposed hearts.
Fig. 1.
A: representative protein expression of COL1A1 and COL3A1 in offspring heart at postnatal day (PND) 21 from control and perinatal nicotine exposure groups (N = 8; all male) with their relative band densities shown in B. The results were analyzed using unpaired t test and are presented as box-and-whisker plot with P values (*P < 0.05) indicated by the corresponding lines. Thin horizontal lines within boxes are medians, cross symbols are mean values, and whiskers indicate maximum and minimum values. C: expression of α-sarcomeric actin (shown in green staining) and COL1A1 and COL3A1 (shown in red staining) in offspring heart at PND21 from control and perinatal nicotine exposure group are determined by immunohistochemistry. Nuclear counterstaining was stained with DAPI (blue), and representative immunohistochemistry images are shown (magnification 40×, N = 3).
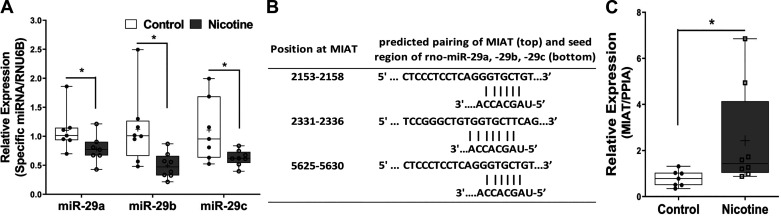
Since in other systems our laboratory has demonstrated miR-29 family to functionally regulate COL1A1 and COL3A1 (14, 16, 17), we initially determined the effect of perinatal nicotine exposure on miR-29 family expression and found it to be decreased in hearts of the perinatally nicotine-exposed PND21 pups (Fig. 2A). We next searched for the possible mechanism underlying nicotine’s modulation of miR-29 expression. Since the long intergenic noncoding RNA MIAT has been recently identified to be a cardiovascular risk factor in smokers (79) and since its sequence pairing analysis with that of miR-29 family revealed complementary base pairing within the seed regions (Fig. 2B), we next determined MIAT expression in control and perinatally nicotine-exposed hearts and found it to be significantly increased in PND21 perinatally nicotine-exposed hearts (Fig. 2C).
Fig. 2.
A: expression of miR-29 family in the offspring heart at postnatal day (PND) 21 (N = 8; all male) from control group and perinatal nicotine exposure group. B: sequence alignments with the coordinated positions of miR-29 family with myocardial infarction-associated transcript (MIAT). C: expression of MIAT in the offspring heart at PND21 (N = 8; all male). Results are presented as box-and-whisker plot and were analyzed using unpaired t test (*P < 0.05). Thin horizontal lines within boxes are medians, cross symbols are mean values, and whiskers indicate maximum and minimum values. PPIA, peptidylprolyl isomerase A.
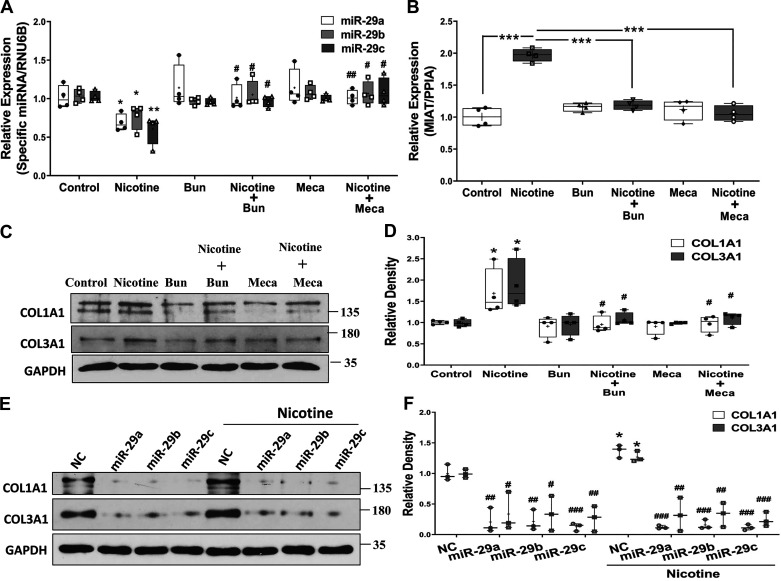
Nicotine’s effect on MIAT and miR-29 family was next determined in cultured primary rat cardiac fibroblasts. As shown in Fig. 3, nicotine (10−9 M) treatment for 96 h resulted in suppressed expression of miR-29a, miR-29b, and miR-29c (Fig. 3A) but increased expression of MIAT (Fig. 3B). Interestingly, this effect was blocked by pretreatment with α-bungarotoxin (10−7 M, a nAChRα7 inhibitor) and mecamylamine (10−5 M, a nonselective nAChR inhibitor). Moreover, the nicotine-induced increase in COL1A1 and COL3A1 protein levels was blocked by the treatment of both nAChR inhibitors and gain of function of miR-29 family (Fig. 3, C–F).
Fig. 3.
Box-and-whisker plot showing nicotine (10−9 M) treatment of cultured primary neonatal rat cardiac fibroblasts for 96 h resulted in suppressed expression of miR-29 family (A; N = 4; *P < 0.05 and **P < 0.01 vs. control; #P < 0.05 and ##P < 0.01 vs. nicotine) and induced myocardial infarction-associated transcript (MIAT) expression (B; N = 4; ***P < 0.001 indicated by the corresponding lines). PPIA, peptidylprolyl isomerase A. C: representative Western blots of COL1A1 and COL3A1 proteins following nicotine treatment for 7 days and relative density histograms are shown in D (N = 4; *P < 0.05 vs. control; #P < 0.05 vs. nicotine), and this effect was blocked by nAChRα7 inhibitor [α-bungarotoxin (Bun); 10−7 M] and a nonselective nAChR inhibitor [mecamylamine (Meca); 10−5 M] pretreatments. E: protein expression of COL1A1 and COL3A1 was determined after treatment of nicotine (10−9 M) for 7 days and transfection of pre-miR-29 family (miR-29a, -b, -c) or scrambled oligonucleotides (NC) for the last 72 h in the primary neonatal rat cardiac fibroblasts. GAPDH was used as loading control. Result is representative of 3 sets of independent experiments. F: box-and-whisker plot present the relative band densities and were analyzed using unpaired t test (*P < 0.05 vs. NC of the control group; #P < 0.05; ##P < 0.01; ###P < 0.01 vs. NC of the corresponding group). Thin horizontal lines within boxes are medians, cross symbols are mean values, and whiskers indicate maximum and minimum values.
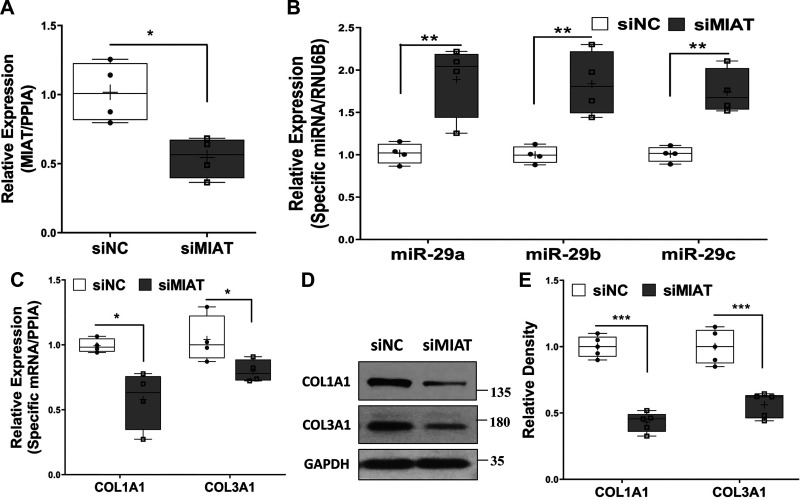
Transfection of cultured primary cardiac fibroblasts with MIAT-specific siRNA (Fig. 4A) resulted in increased expression of miR-29a, miR-29b, and miR-29c (Fig. 4B), while it suppressed COL1A1 and COL3A1 mRNA and protein levels (Fig. 4, C–E), further corroborating MIAT’s modulation of miR-29 family and its downstream targets.
Fig. 4.
A–C: qRT-PCR analysis of myocardial infarction-associated transcript (MIAT; A), miR-29 family (B), or expression of COL1A1 and COL3A1 (C) in rat neonatal primary cardiac fibroblasts after transfection of siRNA negative control (siNC) or MIAT siRNA oligonucleotides (siMIAT) for 48 h (A and B; N = 4) or 72 h (C; N = 4). PPIA, peptidylprolyl isomerase A. D: representative of Western blots of COL1A1 and COL3A1 proteins after 96-h transfection of siNC or siMIAT in rat primary neonatal cardiac fibroblasts with the relative density histograms (E; N = 5). Results were analyzed using unpaired t test and are presented as box-and-whisker plot with P values (*P < 0.05; **P < 0.01, and ***P < 0.001) indicated by the corresponding lines. Thin horizontal lines within boxes are medians, cross symbols are mean values, and whiskers indicate maximum and minimum values.
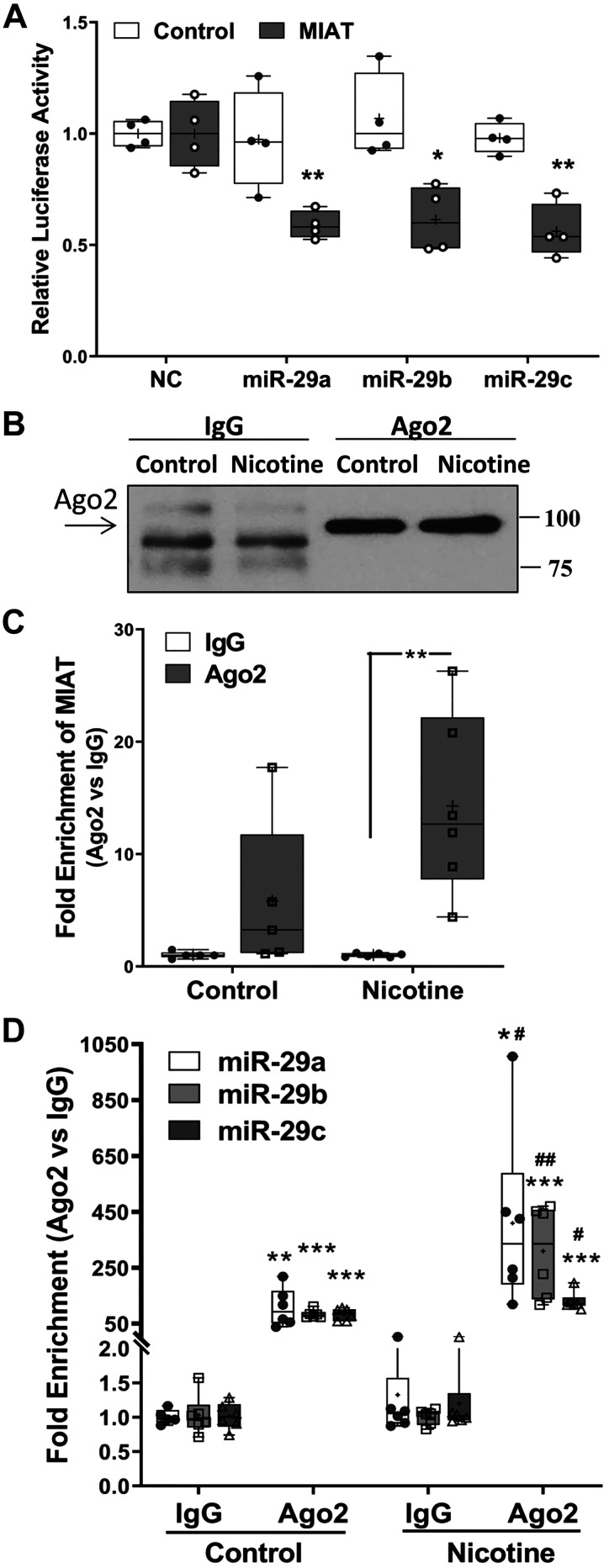
Furthermore, the results of dual-luciferase reporter assay confirmed that MIAT could target miR-29 family in cultured primary rat cardiac fibroblasts (Fig. 5A). To confirm physical association between MIAT and miR-29 family, RNA immunoprecipitation assay with Ago2 antibody was conducted. Argonaute proteins, especially Ago2, have endonuclease activity and play a central role in RNA-induced silencing complex (RISC)-mediated gene silencing via either RNA degradation or translation inhibition (39, 81). As shown in Fig. 5, B–D, both MIAT and miR-29 family were detected to interact with Ago2; however, the interaction was much more intense in the nicotine-treated group (Fig. 5, C and D), indicating a sponge effect of MIAT on miR-29 family regulation following nicotine treatment of primary rat cardiac fibroblasts.
Fig. 5.
A: box-and-whisker plot showing the relative luciferase activity in isolated neonatal cardiac fibroblasts transfected with firefly and Renilla luciferase reporter pEZX-MT01 (control) or pEZX-MT01 [myocardial infarction-associated transcript (MIAT)]. Cells were also cotransfected with pre-miR-29 family (miR-29a, -b, -c; 50 nM) or scramble oligonucleotides (NC). Ratio of firefly to Renilla was determined after 48 h and reported as relative luciferase activity compared with NC, which was independently set as 1. The results were analyzed using unpaired t test, and similar results were obtained from 4 sets of independent experiment. *P < 0.05 and **P < 0.01 vs. NC. B–D: RNA immunoprecipitation with argonaute 2 (Ago2) antibody was conducted to confirm the association between MIAT and miR-29 family using cell lysates from neonatal cardiac fibroblasts following nicotine (10−9 M) treatment for 96 h. B: representative image from 10% volume of the beads suspension after immunoprecipitation was applied to Western blotting for Ago2 antibody efficiency test. The expression of MIAT (C) and miR-29 family (D) from purified RNA was determined by qRT-PCR. The results are presented as box-and-whisker plot from 6 sets of independent experiments and were analyzed using unpaired t test. *P < 0.05; **P < 0.01, and ***P < 0.001 vs. IgG; #P < 0.05 and ##P < 0.01 vs. Ago2 of the control group. Thin horizontal lines within boxes are medians, cross symbols are means values, and whiskers indicate maximum and minimum values.
DISCUSSION
Increasing evidence suggests that intrauterine adverse environment, e.g., exposure to maternal smoke/nicotine, is a significant risk factor in the development of adverse cardiovascular outcomes (7, 9). In the present study, we for the first time demonstrate that perinatal nicotine exposure resulted in increased accumulation of ECM proteins COL1A1 and COL3A1 accompanying lower miR-29 family, but elevated MIAT levels in offspring hearts. Using cultured primary rat cardiac fibroblasts, we determined that this effect was likely mediated via nAChRs including nAChRα7. Furthermore, MIAT knockdown by siRNA transfection of cardiac fibroblasts resulted in increased miR-29 family expression, while it decreased COL1A1 and COL3A1 mRNA and protein levels. Furthermore, nicotine-mediated increase in MIAT, miR-29 family, and Ago2 interaction reinforced it to be the likely epigenetic mechanism mediating abnormal ECM cardiac deposition in perinatal nicotine-exposed offspring.
Previous studies using perinatal nicotine exposure in a rat model have demonstrated increased ischemia-reperfusion-induced left ventricular injury and impaired post-ischemic recovery (43, 88). Though increased global DNA methylation and cardiac reactive oxygen species production and an attenuation of protein kinase Cε (PKCε) expression have been proposed in the development of ischemia-sensitive phenotype in perinatal nicotine-exposed offspring hearts (32, 42, 88), there is no information on the accumulation of cardiac ECM proteins, which are important determinants of cardiac contractility and function. Increasing evidence also suggests nicotine’s effects on miRNA regulation (56, 75, 98), especially following perinatal nicotine exposure (33, 47, 84, 92). The present study implicates miR-29 family and its upstream regulator MIAT in mediating perinatal smoke/nicotine-mediated effects on offspring cardiac dysfunction.
Our present findings provide evidence that the increased collagen deposition possibly plays a causal role in perinatal nicotine exposure-induced development of cardiac fibrosis. Collagen proteins type I and III are two major ECM components of heart, and their disorganization is associated with cardiac malfunction (23, 54). In line with our results, Chou et al. (12) has also reported increased cardiac collagen deposition on PND 7 in perinatal nicotine-exposed offspring accompanying cardiomyocyte hypertrophy, elevated β-myosin heavy chain, and higher TGF-β1 expression. Please note that the differences in cardiac hypertrophy between our study (1 mg·kg−1·day−1) and Chou et al.’s study (6 mg·kg−1·day−1) (12) could be due to the dose of perinatal nicotine administered. In addition, in another study, perinatal nicotine-exposed male offspring at PND 90 had increased left ventricle collagen type I deposition along with decreased heart ejection function (95). In atrial biopsies collected from permanent atrial fibrillation patients, augmented stiffness and higher levels of collagen proteins type I and III were observed (19, 26), indicating their detrimental effects on cigarette smoke-mediated cardiac fibrosis. Interestingly, several studies have suggested excess collagen III synthesis to worsen the established cardiac disease (35, 57), further supporting our hypothesis that perinatal nicotine-exposed offspring might be at a higher risk of cardiac dysfunction (3, 58). Several experimental and epidemiological studies suggest that perinatal nicotine exposure could result in myocardial fibrosis and impaired ischemia-reperfusion injury repair in adulthood (43, 95), as well as offspring diabetes and obesity (6, 46), which are additional cardiovascular disease risk factors (55). Collectively, perinatal nicotine/smoke exposure impairs offspring cardiac development and results in cardiac dysfunction.
MicroRNAs, a member of short noncoding RNA with a single strand of 18–25 nucleotides in length, have emerged as important post-transcriptional negative regulators of more than half of protein-coding genes (27, 70). MiRNAs participate in many biological processes including but not limited to hypertrophy, apoptosis, proliferation, inflammation, and stress response (49, 83, 90) and have been shown to regulate endothelial cell function, inflammation, and paracrine cell-to-cell communication within the heart (76). Their altered expression or functions have been associated with a wide range of disorders, including many cardiovascular diseases (8, 52, 82, 91). Circulating miRNAs levels such as those of miR-1, miR-30d, miR-133, miR-208, and miR-499 have been implicated in cardiovascular diseases, and these have been suggested to be of diagnostic and therapeutic potential (80, 89).
In line with our data, miR-29 family has been found to be repressed after myocardial infarction, pathological hypertrophy, and streptozotocin-induced diabetic cardiomyopathy and has been suggested to be associated with cardiac fibrosis through modulation of ECM proteins, matrix metalloproteinases, and TGF-β signaling (1, 38, 59, 78, 97, 99). The levels of miR-29 family members in cardiac fibroblasts are remarkably higher than those in cardiomyocytes (78). However, the functional role of miR-29 family members in cardiac dysfunction is not yet completely clear, since contrary to the previous data in a more recent study, miR-29 family levels were six times higher in cardiomyocytes as compared with cardiac fibroblasts, and these were involved in pathological hypertrophy, fibrosis, and overall cardiac dysfunction in a mouse model of cardiac pressure overload (66). The underlying mechanism involved Wnt activation, resulting in the secretion of profibrotic paracrine proteins that acted on cardiac fibroblasts (66).
In addition to miRNAs, a number of dysregulated lncRNAs have been identified as important cardiac development and vascular disease modifiers (10, 72). Like miRNAs, lncRNAs have significant impact on all aspects of cellular function by regulating gene expression at the transcription, post-transcription, and post-translation levels (4). Generally, interacting with miRNAs, lncRNAs competitively bind miRNAs to act as sponges. Less frequently, lncRNAs can be precursors of miRNAs or act as competitors of miRNAs by binding to shared target sites (94). Interestingly, several lncRNAs have been implicated in many pathologies associated with early life nicotine/smoke exposure, e.g., neuropsychiatric disorders (71, 74). Specifically, supporting our findings, a recent study demonstrated MIAT/miR-29b/Sp1 axis as a modulator of diabetic retinopathy (96). Another study indicated that in gastric cancer, a higher MIAT acts as an endogenous miR-29a sponge (45). Additionally, MIAT has been shown to adversely affect cardiomyocyte hypertrophy and microvascular dysfunction by the sponge activity over miR-93 and miR-150-5p (44, 93). Although we limited our studies only on the interaction of MIAT, miR-29 family, and collagen type I and III, the involvement of other miRNAs is certainly a possibility.
The lack of direct cardiac functional assessment in our model is a potential limitation of our study; however, multiple studies support impaired cardiac function consistent with cardiac fibrosis following gestational and early postnatal nicotine exposures, albeit with somewhat different exposure regimens. Restriction of this study to only males is another limitation. However, since previous studies have differentially demonstrated more predominant cardiovascular effects of perinatal nicotine exposure in males (87, 95), we limited our analysis to only males. Nevertheless, future studies in females and direct comparison with males might be warranted.
In summary, we identify miR-29 family and MIAT dysregulation as a plausible mediator of cardiac dysfunction following perinatal nicotine exposure, warranting further exploration for these to be important potential therapeutic targets against perinatal nicotine/smoke exposure-induced cardiac dysfunction.
GRANTS
This work was supported by National Institutes of Health Grants HL107118, HD071731, HL127237, HL151769, and HD08886) and Tobacco-Related Disease Research Program Grants 23RT-0018, T29IR0737, and 27IP-0050.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.-D.C., O.K., and V.K.R. conceived and designed research; T.-D.C., A.A., C.Y., A.H., and J.L. performed experiments; T.-D.C. and R.S. analyzed data; T.-D.C. and V.K.R. interpreted results of experiments; T.-D.C. prepared figures; T.-D.C. and A.A. drafted manuscript; T.-D.C., A.H., and V.K.R. edited and revised manuscript; T.-D.C., A.A., C.Y., R.S., A.H., J.L., O.K., and V.K.R. approved final version of manuscript.
REFERENCES
- 1.Abonnenc M, Nabeebaccus AA, Mayr U, Barallobre-Barreiro J, Dong X, Cuello F, Sur S, Drozdov I, Langley SR, Lu R, Stathopoulou K, Didangelos A, Yin X, Zimmermann W-H, Shah AM, Zampetaki A, Mayr M. Extracellular matrix secretion by cardiac fibroblasts: role of microRNA-29b and microRNA-30c. Circ Res 113: 1138–1147, 2013. doi: 10.1161/CIRCRESAHA.113.302400. [DOI] [PubMed] [Google Scholar]
- 2.Alverson CJ, Strickland MJ, Gilboa SM, Correa A. Maternal smoking and congenital heart defects in the Baltimore-Washington Infant Study. Pediatrics 127: e647–e653, 2011. doi: 10.1542/peds.2010-1399. [DOI] [PubMed] [Google Scholar]
- 3.Barasch E, Gottdiener JS, Aurigemma G, Kitzman DW, Han J, Kop WJ, Tracy RP. Association between elevated fibrosis markers and heart failure in the elderly: the cardiovascular health study. Circ Heart Fail 2: 303–310, 2009. doi: 10.1161/CIRCHEARTFAILURE.108.828343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell 152: 1298–1307, 2013. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol Rev 96: 1297–1325, 2016. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 6.Behl M, Rao D, Aagaard K, Davidson TL, Levin ED, Slotkin TA, Srinivasan S, Wallinga D, White MF, Walker VR, Thayer KA, Holloway AC. Evaluation of the association between maternal smoking, childhood obesity, and metabolic disorders: a national toxicology program workshop review. Environ Health Perspect 121: 170–180, 2013. doi: 10.1289/ehp.1205404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beratis NG, Panagoulias D, Varvarigou A. Increased blood pressure in neonates and infants whose mothers smoked during pregnancy. J Pediatr 128: 806–812, 1996. doi: 10.1016/S0022-3476(96)70333-5. [DOI] [PubMed] [Google Scholar]
- 8.Bernardo BC, Gao XM, Winbanks CE, Boey EJ, Tham YK, Kiriazis H, Gregorevic P, Obad S, Kauppinen S, Du XJ, Lin RC, McMullen JR. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci USA 109: 17615–17620, 2012. doi: 10.1073/pnas.1206432109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blake KV, Gurrin LC, Evans SF, Beilin LJ, Landau LI, Stanley FJ, Newnham JP. Maternal cigarette smoking during pregnancy, low birth weight and subsequent blood pressure in early childhood. Early Hum Dev 57: 137–147, 2000. doi: 10.1016/S0378-3782(99)00064-X. [DOI] [PubMed] [Google Scholar]
- 10.Boon RA, Jaé N, Holdt L, Dimmeler S. Long Noncoding RNAs: From Clinical Genetics to Therapeutic Targets? J Am Coll Cardiol 67: 1214–1226, 2016. doi: 10.1016/j.jacc.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 11.Busk PK. A tool for design of primers for microRNA-specific quantitative RT-qPCR. BMC Bioinformatics 15: 29, 2014. doi: 10.1186/1471-2105-15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou HC, Chen CM. Maternal nicotine exposure during gestation and lactation induces cardiac remodeling in rat offspring. Reprod Toxicol 50: 4–10, 2014. doi: 10.1016/j.reprotox.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Chuang TD, Khorram O. Expression Profiling of lncRNAs, miRNAs, and mRNAs and Their Differential Expression in Leiomyoma Using Next-Generation RNA Sequencing. Reprod Sci 25: 246–255, 2018. doi: 10.1177/1933719117711265. [DOI] [PubMed] [Google Scholar]
- 14.Chuang TD, Khorram O. Mechanisms underlying aberrant expression of miR-29c in uterine leiomyoma. Fertil Steril 105: 236–245, 2016. doi: 10.1016/j.fertnstert.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Chuang TD, Khorram O. Regulation of Cell Cycle Regulatory Proteins by MicroRNAs in Uterine Leiomyoma. Reprod Sci 26: 250–258, 2019. doi: 10.1177/1933719118768692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuang TD, Pearce WJ, Khorram O. miR-29c induction contributes to downregulation of vascular extracellular matrix proteins by glucocorticoids. Am J Physiol Cell Physiol 309: C117–C125, 2015. doi: 10.1152/ajpcell.00254.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuang TD, Sakurai R, Gong M, Khorram O, Rehan VK. Role of miR-29 in mediating offspring lung phenotype in a rodent model of intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 315: R1017–R1026, 2018. doi: 10.1152/ajpregu.00155.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chuang TD, Xie Y, Yan W, Khorram O. Next-generation sequencing reveals differentially expressed small noncoding RNAs in uterine leiomyoma. Fertil Steril 109: 919–929, 2018. doi: 10.1016/j.fertnstert.2018.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collier P, Watson CJ, van Es MH, Phelan D, McGorrian C, Tolan M, Ledwidge MT, McDonald KM, Baugh JA. Getting to the heart of cardiac remodeling; how collagen subtypes may contribute to phenotype. J Mol Cell Cardiol 52: 148–153, 2012. doi: 10.1016/j.yjmcc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Crea F, Venalainen E, Ci X, Cheng H, Pikor L, Parolia A, Xue H, Nur Saidy NR, Lin D, Lam W, Collins C, Wang Y. The role of epigenetics and long noncoding RNA MIAT in neuroendocrine prostate cancer. Epigenomics 8: 721–731, 2016. doi: 10.2217/epi.16.6. [DOI] [PubMed] [Google Scholar]
- 21.Deng Z, He Y, Yang X, Shi H, Shi A, Lu L, He L. MicroRNA-29: A Crucial Player in Fibrotic Disease. Mol Diagn Ther 21: 285–294, 2017. doi: 10.1007/s40291-016-0253-9. [DOI] [PubMed] [Google Scholar]
- 22.Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM. Infant morbidity and mortality attributable to prenatal smoking in the U.S. Am J Prev Med 39: 45–52, 2010. doi: 10.1016/j.amepre.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Eghbali M, Weber KT. Collagen and the myocardium: fibrillar structure, biosynthesis and degradation in relation to hypertrophy and its regression. Mol Cell Biochem 96: 1–14, 1990. doi: 10.1007/BF00228448. [DOI] [PubMed] [Google Scholar]
- 24.Gao Y-J, Holloway AC, Zeng ZH, Lim GE, Petrik JJ, Foster WG, Lee RM. Prenatal exposure to nicotine causes postnatal obesity and altered perivascular adipose tissue function. Obes Res 13: 687–692, 2005. doi: 10.1038/oby.2005.77. [DOI] [PubMed] [Google Scholar]
- 25.Gao YJ, Holloway AC, Su LY, Takemori K, Lu C, Lee RM. Effects of fetal and neonatal exposure to nicotine on blood pressure and perivascular adipose tissue function in adult life. Eur J Pharmacol 590: 264–268, 2008. doi: 10.1016/j.ejphar.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 26.Goette A, Lendeckel U, Kuchenbecker A, Bukowska A, Peters B, Klein HU, Huth C, Röcken C. Cigarette smoking induces atrial fibrosis in humans via nicotine. Heart 93: 1056–1063, 2007. doi: 10.1136/hrt.2005.087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 12: 99–110, 2011. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 28.Ishii N, Ozaki K, Sato H, Mizuno H, Susumu Saito, Takahashi A, Miyamoto Y, Ikegawa S, Kamatani N, Hori M, Satoshi Saito, Nakamura Y, Tanaka T. Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J Hum Genet 51: 1087–1099, 2006. doi: 10.1007/s10038-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 29.Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current Cigarette Smoking Among Adults - United States, 2005-2015. MMWR Morb Mortal Wkly Rep 65: 1205–1211, 2016. doi: 10.15585/mmwr.mm6544a2. [DOI] [PubMed] [Google Scholar]
- 30.Jensen K, Nizamutdinov D, Guerrier M, Afroze S, Dostal D, Glaser S. General mechanisms of nicotine-induced fibrogenesis. FASEB J 26: 4778–4787, 2012. doi: 10.1096/fj.12-206458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang C, Li X, Zhao H, Liu H. Long non-coding RNAs: potential new biomarkers for predicting tumor invasion and metastasis. Mol Cancer 15: 62, 2016. doi: 10.1186/s12943-016-0545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ke J, Dong N, Wang L, Li Y, Dasgupta C, Zhang L, Xiao D. Role of DNA methylation in perinatal nicotine-induced development of heart ischemia-sensitive phenotype in rat offspring. Oncotarget 8: 76865–76880, 2017. doi: 10.18632/oncotarget.20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller RF, Kanlikilicer P, Dragomir A, Fan Y, Akay YM, Akay M. Investigating the effect of perinatal nicotine exposure on dopaminergic neurons in the VTA using miRNA expression profiles. IEEE Trans Nanobioscience 16: 843–849, 2017. doi: 10.1109/TNB.2017.2776841. [DOI] [PubMed] [Google Scholar]
- 34.Kim JW, Park CG, Hong SJ, Park SM, Rha SW, Seo HS, Oh DJ, Rho YM. Acute and chronic effects of cigarette smoking on arterial stiffness. Blood Press 14: 80–85, 2005. doi: 10.1080/08037050510008896. [DOI] [PubMed] [Google Scholar]
- 35.Klappacher G, Franzen P, Haab D, Mehrabi M, Binder M, Plesch K, Pacher R, Grimm M, Pribill I, Eichler HG, Glogar HD. Measuring extracellular matrix turnover in the serum of patients with idiopathic or ischemic dilated cardiomyopathy and impact on diagnosis and prognosis. Am J Cardiol 75: 913–918, 1995. doi: 10.1016/S0002-9149(99)80686-9. [DOI] [PubMed] [Google Scholar]
- 36.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 71: 549–574, 2014. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krebs M, Sakurai R, Torday JS, Rehan VK. Evidence for in vivo nicotine-induced alveolar interstitial fibroblast-to-myofibroblast transdifferentiation. Exp Lung Res 36: 390–398, 2010. doi: 10.3109/01902141003714023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kriegel AJ, Liu Y, Fang Y, Ding X, Liang M. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics 44: 237–244, 2012. doi: 10.1152/physiolgenomics.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kupferschmidt K. A lethal dose of RNA. Science 341: 732–733, 2013. doi: 10.1126/science.341.6147.732. [DOI] [PubMed] [Google Scholar]
- 40.Kwon JJ, Factora TD, Dey S, Kota J. A systematic review of miR-29 in cancer. Mol Ther Oncolytics 12: 173–194, 2018. doi: 10.1016/j.omto.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol 20: 115–126, 1996. doi: 10.1016/S0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- 42.Lawrence J, Chen M, Xiong F, Xiao D, Zhang H, Buchholz JN, Zhang L. Foetal nicotine exposure causes PKCε gene repression by promoter methylation in rat hearts. Cardiovasc Res 89: 89–97, 2011. doi: 10.1093/cvr/cvq270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawrence J, Xiao D, Xue Q, Rejali M, Yang S, Zhang L. Prenatal nicotine exposure increases heart susceptibility to ischemia/reperfusion injury in adult offspring. J Pharmacol Exp Ther 324: 331–341, 2008. doi: 10.1124/jpet.107.132175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Wang J, Sun L, Zhu S. LncRNA myocardial infarction-associated transcript (MIAT) contributed to cardiac hypertrophy by regulating TLR4 via miR-93. Eur J Pharmacol 818: 508–517, 2018. doi: 10.1016/j.ejphar.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Wang K, Wei Y, Yao Q, Zhang Q, Qu H, Zhu G. lncRNA-MIAT regulates cell biological behaviors in gastric cancer through a mechanism involving the miR-29a-3p/HDAC4 axis. Oncol Rep 38: 3465–3472, 2017. doi: 10.3892/or.2017.6020. [DOI] [PubMed] [Google Scholar]
- 46.Lisboa PC, de Oliveira E, de Moura EG. Obesity and endocrine dysfunction programmed by maternal smoking in pregnancy and lactation. Front Physiol 3: 437–437, 2012. doi: 10.3389/fphys.2012.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu B, Hu X, Li Y, Ke J, Dasgupta C, Huang X, Walayat A, Zhang L, Xiao D. Epigenetic down-regulation of BKCa channel by miR-181a contributes to the fetal and neonatal nicotine-mediated exaggerated coronary vascular tone in adult life. Int J Cardiol 281: 82–89, 2019. doi: 10.1016/j.ijcard.2019.01.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J, Wu LL, Li L, Zhang L, Song ZE. Growth-promoting effect of platelet-derived growth factor on rat cardiac myocytes. Regul Pept 127: 11–18, 2005. doi: 10.1016/j.regpep.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 49.Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, Xiao C, Bezzerides V, Boström P, Che L, Zhang C, Spiegelman BM, Rosenzweig A. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab 21: 584–595, 2015. doi: 10.1016/j.cmet.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luck W, Nau H. Nicotine and cotinine concentrations in serum and milk of nursing smokers. Br J Clin Pharmacol 18: 9–15, 1984. doi: 10.1111/j.1365-2125.1984.tb05014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luck W, Nau H. Nicotine and cotinine concentrations in serum and urine of infants exposed via passive smoking or milk from smoking mothers. J Pediatr 107: 816–820, 1985. doi: 10.1016/S0022-3476(85)80427-3. [DOI] [PubMed] [Google Scholar]
- 52.Lv D, Liu J, Zhao C, Sun Q, Zhou Q, Xu J, Xiao J. Targeting microRNAs in Pathological Hypertrophy and Cardiac Failure. Mini Rev Med Chem 15: 475–478, 2015. doi: 10.2174/1389557515666150324124751. [DOI] [PubMed] [Google Scholar]
- 53.Mahabee-Gittens EM, Matt GE, Hoh E, Quintana PJE, Stone L, Geraci MA, Wullenweber CA, Koutsounadis GN, Ruwe AG, Meyers GT, Zakrajsek MA, Witry JK, Merianos AL. Contribution of thirdhand smoke to overall tobacco smoke exposure in pediatric patients: study protocol. BMC Public Health 19: 491, 2019. doi: 10.1186/s12889-019-6829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCurdy S, Baicu CF, Heymans S, Bradshaw AD. Cardiac extracellular matrix remodeling: fibrillar collagens and secreted protein acidic and rich in cysteine (SPARC). J Mol Cell Cardiol 48: 544–549, 2010. doi: 10.1016/j.yjmcc.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol 63: 250–259, 2014. doi: 10.1016/j.jjcc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ng TK, Carballosa CM, Pelaez D, Wong HK, Choy KW, Pang CP, Cheung HS. Nicotine alters MicroRNA expression and hinders human adult stem cell regenerative potential. Stem Cells Dev 22: 781–790, 2013. doi: 10.1089/scd.2012.0434. [DOI] [PubMed] [Google Scholar]
- 57.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J; Randomized Aldactone Evaluation Study Investigators . The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341: 709–717, 1999. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 58.Plaksej R, Kosmala W, Frantz S, Herrmann S, Niemann M, Störk S, Wachter R, Angermann CE, Ertl G, Bijnens B, Weidemann F. Relation of circulating markers of fibrosis and progression of left and right ventricular dysfunction in hypertensive patients with heart failure. J Hypertens 27: 2483–2491, 2009. doi: 10.1097/HJH.0b013e3283316c4d. [DOI] [PubMed] [Google Scholar]
- 59.Qi H, Liu Y, Li S, Chen Y, Li L, Cao Y, e M, Shi P, Song C, Li B, Sun H. Activation of AMPK attenuated cardiac fibrosis by inhibiting CDK2 via p21/p27 and miR-29 family pathways in rats. Mol Ther Nucleic Acids 8: 277–290, 2017. doi: 10.1016/j.omtn.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qu X, Du Y, Shu Y, Gao M, Sun F, Luo S, Yang T, Zhan L, Yuan Y, Chu W, Pan Z, Wang Z, Yang B, Lu Y. MIAT is a pro-fibrotic long non-coding RNA governing cardiac fibrosis in post-infarct myocardium. Sci Rep 7: 42657, 2017. doi: 10.1038/srep42657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rehan VK, Liu J, Sakurai R, Torday JS. Perinatal nicotine-induced transgenerational asthma. Am J Physiol Lung Cell Mol Physiol 305: L501–L507, 2013. doi: 10.1152/ajplung.00078.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rehan VK, Sakurai R, Torday JS. Thirdhand smoke: a new dimension to the effects of cigarette smoke on the developing lung. Am J Physiol Lung Cell Mol Physiol 301: L1–L8, 2011. doi: 10.1152/ajplung.00393.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rueda-Clausen CF, Morton JS, Davidge ST. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc Res 81: 713–722, 2009. doi: 10.1093/cvr/cvn341. [DOI] [PubMed] [Google Scholar]
- 64.Sakurai R, Liu J, Gong M, Bo J, Rehan VK. Perinatal nicotine exposure induces myogenic differentiation, but not epithelial-mesenchymal transition in rat offspring lung. Pediatr Pulmonol 51: 1142–1150, 2016. doi: 10.1002/ppul.23458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salihu HM, Aliyu MH, Pierre-Louis BJ, Alexander GR. Levels of excess infant deaths attributable to maternal smoking during pregnancy in the United States. Matern Child Health J 7: 219–227, 2003. doi: 10.1023/A:1027319517405. [DOI] [PubMed] [Google Scholar]
- 66.Sassi Y, Avramopoulos P, Ramanujam D, Grüter L, Werfel S, Giosele S, Brunner AD, Esfandyari D, Papadopoulou AS, De Strooper B, Hübner N, Kumarswamy R, Thum T, Yin X, Mayr M, Laggerbauer B, Engelhardt S. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nat Commun 8: 1614, 2017. doi: 10.1038/s41467-017-01737-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sattari A, Siddiqui H, Moshiri F, Ngankeu A, Nakamura T, Kipps TJ, Croce CM. Upregulation of long noncoding RNA MIAT in aggressive form of chronic lymphocytic leukemias. Oncotarget 7: 54174–54182, 2016. doi: 10.18632/oncotarget.11099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scherman A, Tolosa JE, McEvoy C. Smoking cessation in pregnancy: a continuing challenge in the United States. Ther Adv Drug Saf 9: 457–474, 2018. doi: 10.1177/2042098618775366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schrör K, Zimmermann KC, Tannhäuser R. Augmented myocardial ischaemia by nicotine–mechanisms and their possible significance. Br J Pharmacol 125: 79–86, 1998. doi: 10.1038/sj.bjp.0702061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature 455: 58–63, 2008. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 71.Semick SA, Collado-Torres L, Markunas CA, Shin JH, Deep-Soboslay A, Tao R, Huestis MA, Bierut LJ, Maher BS, Johnson EO, Hyde TM, Weinberger DR, Hancock DB, Kleinman JE, Jaffe AE. Developmental effects of maternal smoking during pregnancy on the human frontal cortex transcriptome. Mol Psychiatry, 2018. [Online ahead of print.] doi: 10.1038/s41380-018-0223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen S, Jiang H, Bei Y, Xiao J, Li X. Long non-coding RNAs in cardiac remodeling. Cell Physiol Biochem 41: 1830–1837, 2017. doi: 10.1159/000471913. [DOI] [PubMed] [Google Scholar]
- 73.Shipton D, Tappin DM, Vadiveloo T, Crossley JA, Aitken DA, Chalmers J. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ 339: b4347, 2009. doi: 10.1136/bmj.b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silva JP, Lambert G, van Booven D, Wahlestedt C. Epigenomic and metabolic responses of hypothalamic POMC neurons to gestational nicotine exposure in adult offspring. Genome Med 8: 93–93, 2016. doi: 10.1186/s13073-016-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taki FA, Pan X, Zhang B. Chronic nicotine exposure systemically alters microRNA expression profiles during post-embryonic stages in Caenorhabditis elegans. J Cell Physiol 229: 79–89, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tijsen AJ, Pinto YM, Creemers EE. Non-cardiomyocyte microRNAs in heart failure. Cardiovasc Res 93: 573–582, 2012. doi: 10.1093/cvr/cvr344. [DOI] [PubMed] [Google Scholar]
- 77.Tong VT, Dietz PM, Morrow B, D’Angelo DV, Farr SL, Rockhill KM, England LJ; Centers for Disease Control and Prevention (CDC) . Trends in smoking before, during, and after pregnancy–Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000-2010. MMWR Surveill Summ 62: 1–19, 2013. [PubMed] [Google Scholar]
- 78.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA 105: 13027–13032, 2008. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vausort M, Wagner DR, Devaux Y. Long noncoding RNAs in patients with acute myocardial infarction. Circ Res 115: 668–677, 2014. doi: 10.1161/CIRCRESAHA.115.303836. [DOI] [PubMed] [Google Scholar]
- 80.Viereck J, Thum T. Circulating noncoding RNAs as biomarkers of cardiovascular disease and injury. Circ Res 120: 381–399, 2017. doi: 10.1161/CIRCRESAHA.116.308434. [DOI] [PubMed] [Google Scholar]
- 81.Wang B, Li S, Qi HH, Chowdhury D, Shi Y, Novina CD. Distinct passenger strand and mRNA cleavage activities of human Argonaute proteins. Nat Struct Mol Biol 16: 1259–1266, 2009. doi: 10.1038/nsmb.1712. [DOI] [PubMed] [Google Scholar]
- 82.Wang H, Bei Y, Shen S, Huang P, Shi J, Zhang J, Sun Q, Chen Y, Yang Y, Xu T, Kong X, Xiao J. miR-21-3p controls sepsis-associated cardiac dysfunction via regulating SORBS2. J Mol Cell Cardiol 94: 43–53, 2016. doi: 10.1016/j.yjmcc.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 83.Wang H, Bei Y, Shi J, Xiao J, Kong X. Non-coding RNAs in cardiac aging. Cell Physiol Biochem 36: 1679–1687, 2015. doi: 10.1159/000430141. [DOI] [PubMed] [Google Scholar]
- 84.Wang L, Ke J, Li Y, Ma Q, Dasgupta C, Huang X, Zhang L, Xiao D. Inhibition of miRNA-210 reverses nicotine-induced brain hypoxic-ischemic injury in neonatal rats. Int J Biol Sci 13: 76–84, 2017. doi: 10.7150/ijbs.17278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Z, Huang Z, Lu G, Lin L, Ferrari M. Hypoxia during pregnancy in rats leads to early morphological changes of atherosclerosis in adult offspring. Am J Physiol Heart Circ Physiol 296: H1321–H1328, 2009. doi: 10.1152/ajpheart.00440.2008. [DOI] [PubMed] [Google Scholar]
- 86.Winniford MD, Wheelan KR, Kremers MS, Ugolini V, van den Berg E Jr, Niggemann EH, Jansen DE, Hillis LD. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: evidence for adrenergically mediated alterations in coronary artery tone. Circulation 73: 662–667, 1986. doi: 10.1161/01.CIR.73.4.662. [DOI] [PubMed] [Google Scholar]
- 87.Xiao D, Huang X, Lawrence J, Yang S, Zhang L. Fetal and neonatal nicotine exposure differentially regulates vascular contractility in adult male and female offspring. J Pharmacol Exp Ther 320: 654–661, 2007. doi: 10.1124/jpet.106.113332. [DOI] [PubMed] [Google Scholar]
- 88.Xiao D, Wang L, Huang X, Li Y, Dasgupta C, Zhang L. Protective effect of antenatal antioxidant on nicotine-induced heart ischemia-sensitive phenotype in rat offspring. PLoS One 11: e0150557, 2016. doi: 10.1371/journal.pone.0150557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiao J, Gao R, Bei Y, Zhou Q, Zhou Y, Zhang H, Jin M, Wei S, Wang K, Xu X, Yao W, Xu D, Zhou F, Jiang J, Li X, Das S. Circulating miR-30d predicts survival in patients with acute heart failure. Cell Physiol Biochem 41: 865–874, 2017. doi: 10.1159/000459899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xiao J, Liang D, Zhang H, Liu Y, Zhang D, Liu Y, Pan L, Chen X, Doevendans PA, Sun Y, Liang X, Sluijter JP, Chen YH. MicroRNA-204 is required for differentiation of human-derived cardiomyocyte progenitor cells. J Mol Cell Cardiol 53: 751–759, 2012. doi: 10.1016/j.yjmcc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 91.Xiao J, Liang D, Zhang Y, Liu Y, Zhang H, Liu Y, Li L, Liang X, Sun Y, Chen YH. MicroRNA expression signature in atrial fibrillation with mitral stenosis. Physiol Genomics 43: 655–664, 2011. doi: 10.1152/physiolgenomics.00139.2010. [DOI] [PubMed] [Google Scholar]
- 92.Xiao R, Noël A, Perveen Z, Penn AL. In utero exposure to second-hand smoke activates pro-asthmatic and oncogenic miRNAs in adult asthmatic mice. Environ Mol Mutagen 57: 190–199, 2016. doi: 10.1002/em.21998. [DOI] [PubMed] [Google Scholar]
- 93.Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li YJ, Tao ZF, Song YC, Chen Q, Jiang Q. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res 116: 1143–1156, 2015. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 94.Yoon JH, Abdelmohsen K, Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin Cell Dev Biol 34: 9–14, 2014. doi: 10.1016/j.semcdb.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu F, Zheng A, Qian J, Li Y, Wu L, Yang J, Gao X. Prenatal nicotine exposure results in the myocardial fibrosis in the adult male offspring rats. Exp Toxicol Pathol 68: 445–450, 2016. doi: 10.1016/j.etp.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 96.Zhang J, Chen M, Chen J, Lin S, Cai D, Chen C, Chen Z. Long non-coding RNA MIAT acts as a biomarker in diabetic retinopathy by absorbing miR-29b and regulating cell apoptosis. Biosci Rep 37: BSR20170036, 2017. doi: 10.1042/BSR20170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y, Huang XR, Wei LH, Chung AC, Yu CM, Lan HY. miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-beta/Smad3 signaling. Mol Ther 22: 974–985, 2014. doi: 10.1038/mt.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Y, Pan T, Zhong X, Cheng C. Nicotine upregulates microRNA-21 and promotes TGF-β-dependent epithelial-mesenchymal transition of esophageal cancer cells. Tumour Biol 35: 7063–7072, 2014. doi: 10.1007/s13277-014-1968-z. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y, Wang JH, Zhang YY, Wang YZ, Wang J, Zhao Y, Jin XX, Xue GL, Li PH, Sun YL, Huang QH, Song XT, Zhang ZR, Gao X, Yang BF, Du ZM, Pan ZW. Deletion of interleukin-6 alleviated interstitial fibrosis in streptozotocin-induced diabetic cardiomyopathy of mice through affecting TGFβ1 and miR-29 pathways. Sci Rep 6: 23010, 2016. doi: 10.1038/srep23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhu XH, Yuan YX, Rao SL, Wang P. LncRNA MIAT enhances cardiac hypertrophy partly through sponging miR-150. Eur Rev Med Pharmacol Sci 20: 3653–3660, 2016. [PubMed] [Google Scholar]