Abstract
The function of site-specific phosphorylation of nucleophosmin (NPM), an essential Bax chaperone, in stress-induced cell death is unknown. We hypothesized that NPM threonine 95 (T95) phosphorylation both signals and promotes cell death. In resting cells, NPM exclusively resides in the nucleus and T95 is nonphosphorylated. In contrast, phosphorylated T95 NPM (pNPM T95) accumulates in the cytosol after metabolic stress, in multiple human cancer cell lines following γ-radiation, and in postischemic human kidney tissue. Based on the T95 phosphorylation consensus sequence, we hypothesized that glycogen synthase kinase-3β (GSK-3β) regulates cytosolic NPM translocation by phosphorylating T95 NPM. In a cell-free system, GSK-3β phosphorylated a synthetic NPM peptide containing T95. In vitro, bidirectional manipulation of GSK-3β activity substantially altered T95 phosphorylation, cytosolic NPM translocation, and cell survival during stress, mechanistically linking these lethal events. Furthermore, GSK-3β inhibition in vivo decreased cytosolic pNPM T95 accumulation in kidney tissue after experimental ischemia. In patients with acute kidney injury, both cytosolic NPM accumulation in proximal tubule cells and NPM-rich intratubular casts were detected in frozen renal biopsy tissue. These observations show, for the first time, that GSK-3β promotes cell death partly by phosphorylating NPM at T95, to promote cytosolic NPM accumulation. T95 NPM is also a rational therapeutic target to ameliorate ischemic renal cell injury and may be a universal injury marker in mammalian cells.
Keywords: apoptosis, glycogen synthase kinase, ischemia, mass spectrometry
INTRODUCTION
Unlike its portrayal as random, chaotic, and unavoidable, cell death is predictable, organized, and regulated (6, 19, 25). This biological reality opens the cell death door to innovative diagnostic and therapeutic tools. Despite the myriad of signals and stressors that precipitate cell death, the regulated death pathways themselves are highly conserved and potentially amenable to intervention. In fact, several proteins critical to cell survival paradoxically participate in its execution. Recently, we and others have identified nucleophosmin (NPM), a nucleolar protein essential for mammalian cell survival, as a critical Bax chaperone responsible for trafficking Bax to the outer mitochondrial membrane during fatal cellular insults (24, 29, 31). Bax, the quintessential “bad actor” of the B cell lymphoma 2 protein family, contributes to several forms of cell death, including apoptosis, regulated necrosis, necroptosis, and membrane pore transition-regulated cell death (13, 21). The recent detection of virtually identical stress-induced differential NPM phosphorylation in murine and human cells, tissue, and urine samples (5) stimulated our hypothesis that site-specific NPM phosphorylation at threonine 95 (T95) mediates its toxicity during stress, is a useful diagnostic tool for monitoring tissue injury, and is a potential therapeutic target for promoting cell survival during stress.
Substantial evidence shows that NPM translocates from the nucleolar region to the cytosol before causing mitochondrial injury and cell death. In fact, cytosolic NPM accumulation appears to be a major predictor of cell death in diverse experimental (29, 31) and pathological settings (3). Although increasing nuclear NPM content is well tolerated, cytosolic NPM accumulation exacerbates stress-induced cell death (29). In fact, oncologists currently exploit intracellular NPM location to prognosticate survival in patients with acute myelogenous leukemia (AML). In these patients, NPM is one of the most highly mutated proteins, and these mutations result in dichotomous NPM accumulation in either the nucleus or cytosol. Mutations that increase cytosolic NPM accumulation render patients more susceptible to chemotherapeutic agents or radiation and portend a favorable prognosis, whereas mutations that restrict NPM to the nucleus increase cause resistance to therapy and associate with a poorer outcome (3, 12). In patients with AML, NPM translocation is regulated, at least in part, by site-specific phosphorylation of T95, ideally located in a key regulatory domain (32). We recently identified NPM T95 phosphorylation in murine and human cells, kidney tissue, and urine by mass spectrometry after physiological and experimental stress (31) but did not investigate its specific role in cell death. Here, we characterized the functional role of T95 NPM phosphorylation during cell death and identifed a stress kinase responsible for this untoward event.
To test this hypothesis, we assessed NPM T95 phosphorylation during stress in renal tissue and cells as well as multiple human cancer cell lines and identified a stress kinase that regulates NPM T95 phosphorylation. We discovered that T95 phosphorylation and NPM translocation are regulated by glycogen synthase 3 kinase (GSK-3β) and that interfering with GSK-3β activity regulates NPM translocation in the cytosol, a critical cell death event. We also discovered that phosphorylated (p)NPM T95 is a novel marker of injury that can be used in vitro and in vitro in human cells and tissue and that interfering with T95 NPM phosphorylation during stress is a rational target for manipulating cell and organ survival.
METHODS
Animals
All animals were maintained under guidance and policies of the Boston University Animal Core facility using National Institutes of Health (NIH) and Institutional Animal Care and Use Committee guidelines. Male and female B6 mice were purchased from Jackson Laboratory (Bar Harbor, ME) and were housed and bred at the Boston University Animal Core facility under license number AN-15024. Animals were handled in a manner consistent with the NIH Guide for the Care and Use of Laboratory Animals under Institutional Animal Care and Use Committee approval no. AN-15024.
Cell Culture
Murine and human primary proximal tubule epithelial cells were harvested as previously described and cultured in renal epithelial cell basal medium (catalog no. PCS-400-030, American Type Culture Collection) supplemented with the Renal Epithelial Cell Growth Kit (PCS-400-040, American Type Culture Collection) and 1% penicillin-streptomycin at 37°C with ambient 5% CO2.
Metabolic Stress
In vitro.
Exposure to metabolic inhibitors is an established model of ischemic stress that reduces ATP content to <10% of baseline values until recovery is initiated (14, 15). To achieve ATP depletion, cells were washed three times in glucose-free DMEM (Invitrogen, Carlsbad, CA) followed by incubation in glucose-free DMEM containing sodium cyanide (5 mM) and 2-deoxyglucose (5 mM) for the indicated times (14). Recovery was initiated by replacing the stress medium described above with complete primary cell culture medium containing glucose and substrates that support the rapid restoration of cell ATP (28).
In vivo.
Mice were subjected to bilateral renal pedicle clamping after being anesthetized with 2,2,2-tribromoethanol. A midline laparotomy and nontraumatic vascular clamps were placed on both renal pedicles for 26 min, an insult that produces severe reversible acute kidney injury (AKI) (5, 29, 30). The clamp was then removed, and reperfusion was confirmed by visual inspection of the kidneys. Sham ischemia was performed by encircling each renal pedicle with a nonocclusive ligature. The first postischemic urine was collected by gentle compression of the inferior abdominal wall.
Immunoblot Analysis
Multiple samples of tissue sections (5 µm) were obtained from each of the biopsy resections of renal patients. Immunohistochemistry using mouse anti-NPM antibody (catalog no. B0556, Sigma, St. Louis, MO) was performed on deparaffinized tissue sections using a routine protocol according to the Immunohistochemistry Protocol for Fixing and Sectioning Paraffin-embedded Tissues (R&D Systems, Minneapolis, MN). Before incubation with the primary antibody (1:500 dilution, as suggested by the manufacturer), tissue sections were subjected to heat-induced epitope retrieval by incubation in 0.01 M EDTA solution (pH 8.0) for 10 min in a pressure steamer followed by 20-min cooldown and treatment with 3% hydrogen peroxide before antibody application. Alexa Fluor 488 goat anti-mouse antibody (catalog no. A11029, ThermoFisher Scientific) was used to detect the primary antibody. ProLong Diamond Antifade Mountant (catalog no. P36961, ThermoFisher Scientific) and glass coverslips were added to each sample, and the results were assessed using a fluorescence microscope.
Densitometry
Selected digitized immunoblot image band densities were quantified using NIH ImageJ Software.
Dot-Blot Analysis
Because relatively low NPM concentrations in urine are insufficient for routine immunoblot because of volume limitations of sample loading, a semiquantity dot-blot assay was performed using a standard method and protocol (https://www.abcam.com/ps/pdf/protocols/dot%20blot%20protocol.pdf). The immunoblot and dot-blot assays were both performed using identical anti-NPM antibodies.
Immunohistochemistry
NPM was visualized as previously reported (29), and the protocol was performed according to the manufacturer's protocol (catalog no. B0556, Sigma-Aldrich). Deidentified human kidney biopsy tissue was stained and assessed by an observer blinded to the clinical status of each patient or the results of hematoxylin and eosin staining.
GSK-3β Inhibition
Both molecular and pharmacological maneuvers were used to inhibit GSK-3β kinase activity. RNA inhibition was achieved with GSK-3β siRNA (catalog no. AM16708, ThermoFisher Scientific). siRNA transfection was performed with HiPerFect transfection reagent (Qiagen) following the manufacturer’s instructions, and the knockdown effect was detected after 48-h transfection. 4-Benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD-8; catalog no. T8325, Sigma-Aldrich) inhibits GSK-3β by binding to its active site without altering its structure. TDZD-8 dissolved in DMSO was used to selectively inhibit GSK-3β in vitro (2–10 μM) and in vivo (4 mg/kg body wt iv). The effect of these maneuvers on GSK-3β was assessed by immunoblot analysis.
Antibodies
The antibodies used in this study were as follows: NPM (catalog no. B0556, Sigma), pNPM T95 (catalog no. 3517, Cell Signaling Technology, Beverly, MA), GSK3-β (catalog no. 9315, Cell Signaling Technology), β-actin (catalog no. 3700, Cell Signaling Technology), and GAPDH (catalog no. 5174, Cell Signaling Technology).
GSK3β Constructs, Lentivirus Production, and Cell Transfection
V5-tagged GSK-3β wild-type, constitutively active (S9A), or constitutively inactive (K85R) constructs were constructed as described in our previous work (30). All constructs were confirmed by DNA sequencing before cotransfection with pPACKG1 and pPACKF1 lentivector packaging plasmids (catalog no. LV100A-1, SBI, Mountain View, CA) into human embryonic kidney (HEK)-293NT cells to generate lenti-pseudoviral particles. The resultant pseudoviruses were harvested and purified, and titers were determined according to the manufacturer’s instructions. GSK-3β-containing lentivirus was introduced into primary renal epithelial cells (30) by multiplicities of infection (of 5−9) diluted in Opti-MEM (Invitrogen, Carlsbad, CA). After 16 h of incubation with virus-infected medium, a complete culture medium was substituted for an additional 24-h incubation period. Constructs were rendered either active (S9A) or inactive (S9X) using site-specific mutation as previously reported by our laboratory (30).
NPM T95 Phosphorylation by GSK-3β Kinase in a Cell-Free Assay
The V5-tagged constitutively active (S9A) or inactive (K85R) GSK-3β kinase proteins were produced by lentiviral plasmids in HEK-293NT cells (see GSK-3β Constructs, Lentivirus Production, and Cell Transfection). The kinase proteins were purified from transformed 293NT cell lysis using V5-tag affinity gel (catalog no. A7345, Sigma-Aldrich). Kinase activity was assessed by measuring ADP formation that is directly proportional to enzyme phosphotransferase activity as fluorometrically measured using a universal kinase assay kit (catalog no. ab138879, Abcam, Cambridge, MA). In 96-well plates, equal amounts of immunoprecipitation products were reconstitution by an ADP sensor buffer to which an NPM peptide (77–1,022 amino acids, ATLKMSVQPTVSLGGFEITPPVVLRL) containing T95 served as the sole substrate (final substrate concentrationL 100 nM). The reaction mixture was incubated at 37°C temperature while fluorescence intensity at excitation/emission of 540/590 nm (cutoff: 570 nm) was continually monitored using a fluorescence plate reader. Background fluorescence in wells containing only the kinase buffer and substrate without kinase was used as the “baseline.” Casein kinase 2 with its substrate (RRRADDSDDDDD, catalog no. P6010, NEB) was used as a positive control.
Flow Cytometry for Annexin V and Propidium Iodide Staining
Aliquots of 2 × 105 cells harvested by centrifugation were stained according to the manufacturer’s instructions (Annexin V-FITC Apoptosis Detection Kit, catalog no. ab14085, Abcam). Cells were resuspended in 500 µL of 1× binding buffer to which 5 µL each of annexin V-FITC and propidium iodide (PI) stock solution were added. The mixture was incubated in the dark for 5 min at room temperature. Annexin V-FITC binding (excitation: 488 nm and emission: 350 nm) and PI signals were measured in a flow cytometer.
Mass Spectrometry
Using a method recently described by our laboratory (29), NPM purified by immunoprecipitation was obtained from renal cortical homogenates of two kidneys harvested from a single donor rejected for transplantation (kindly provided by Dr. Laurence Beck); one kidney was well perfused with a functional pump (control), whereas the contralateral kidney appeared ischemic because of overt perfusion pump failure (ischemia). Enzyme digestion with glutamic acid and trypsin as well as trypsin alone was performed to optimize NPM fragmentation. Tandem MS spectra obtained by fragmenting a peptide by collision-induced dissociation were acquired using a capillary LC/MS/MS system that consisted of a Surveyor HPLC pump, a Surveyor Micro AS autosampler, and an LTQ linear ion trap mass spectrometer. Detection and mapping of phosphorylation sites were achieved by database searching of tandem mass spectra of proteolytic peptides with specified phosphate modification (P = +80 Da) on serine, threonine, and tyrosine residues against the current mouse and human protein databases. A detailed LC/MS/MS approach for phosphoproteomics has been previously described (2, 22, 23). Mass spectrometry was performed in fresh murine kidney tissue homogenates before and after experimental ischemia, human kidneys rejected for transplantation, as well as primary human and murine cells subjected to metabolic stress. Three replicate samples of purified NPM harvested from kidney tissue were subjected to mass spectrometry to assess differential phosphorylation after stress.
Three-Dimensional Ribbon Diagram Modeling of the Three-Dimensional NPM Structure
Ribbon diagram modeling of the three-dimensional NPM structure was generated with the Swiss-Model *-83 (wissmodel.expasy.org) using the established alignment and amino acid sequence of human NPM (gene ID: AAP36411) that is 93.2% identical with murine NPM (gene ID: NP_032748). The five differential phosphorylation sites identified by mass spectrometry in human and murine tissue and renal cell samples were located and labeled.
Statistical Analysis
Data were analyzed using Excel (Microsoft, Redmond, WA) or SigmaPlot software (Systat Software, San Jose, CA). Directional differences in relative immunoblot density and mitochondrial fragmentation were measured by one-tailed ANOVA. Statistical analysis was otherwise performed using a two-tailed unpaired Student’s t test incorporating Bonferroni’s correction for more than two comparisons. Significance was determined as P < 0.05.
RESULTS
Limited Site-Specific NPM Phosphorylation Change Accompanies Stress
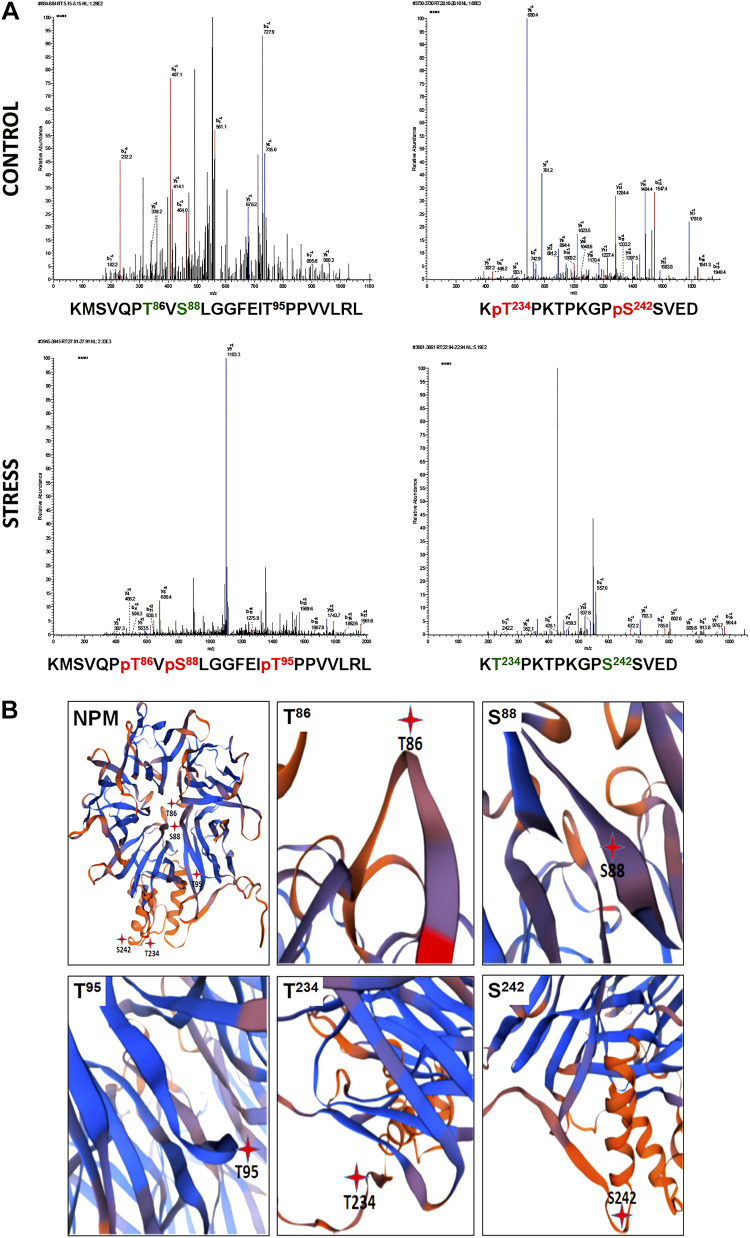
Although NPM contains 40 serine, threonine, and tyrosine residue sites, computer-based modeling predictions suggest that only 19 sites are likely to undergo phosphorylation (Table 1). To assess the actual phosphorylation sites altered by physiological stress, human NPM was isolated from renal cortical homogenates of paired kidneys from a single donor rejected for transplantation; one kidney was well perfused with a functional pump (control), whereas the other kidney appeared grossly ischemic because of overt perfusion pump failure; both cortical homogenates were subjected to mass spectrometry (Fig. 1A). Of the seven NPM sites at which phosphorylation was detected, only five NPM sites were noted to be differentially altered by stress (Table 2). Specifically, two NPM residues at the COOH-terminus were dephosphorylated and three NH2-terminal residues were phosphorylated in human kidney tissue. Of the differentially phosphorylated sites, S88, T95, and T234 were located in domains known to regulate NPM functions, including DNA-binding affinity, oligomerization state, and nuclear export (4). In contrast, the functions of T86 and S242 phosphorylation during stress are presently unknown. Three-dimensional rendering of NPM using an online ribbon diagram analytic tool predicted that 80% (4/5) of the NPM phosphorylation sites differentially altered by stress localize to hinge regions expected to regulate NPM conformation and, hence, its function (Fig. 1B).
Table 1.
Predicted versus detected NPM phosphorylation sites
| Site Number |
|||
|---|---|---|---|
| Phospho Sites | Potential | Predicted | Detected |
| Serines | 26 | 19 | 6 |
| Threonines | 10 | 5 | 3 |
| Tyrosines | 4 | 2 | 0 |
| Total | 40 | 26 | 9 |
The total number of serine, threonine, and tyrosine residues in human and murine nucleophosmin (NPM) is identical. Of the 40 total NPM residues available for phosphorylation, computational analysis predicts that fewer residues will be phosphorylated based on >50% likelihood. In fact, mass spectrometry revealed that only 9 of 26 predicted residues were actually phosphorylated either at baseline or after ischemia in the human kidney.
Fig. 1.
Stress causes differential nucleophosmin (NPM) phosphorylation change at NPM hinge regions. A: mass spectrometry of NPM purified from renal cortical homogenates of two kidneys from a single donor rejected for transplantation; one kidney was well perfused (control), whereas the other kidney appeared ischemic because of overt perfusion pump failure (stress). The unique peptide sequences shown below each tracing represent the specific residues that are either phosphorylated [T234 and S242 (red)] or dephosphorylated [T86, S88, and T95 (green)] during renal ischemia. MAC, mass x; MSMS, mass spectrometry/mass spectrometry. MS tracings were each repeated three times. B: differential NPM phosphorylation sites localize to hinge regions. Three-dimensional ribbon modeling showed that T86, T95, T234, and S242 residues differentially phosphorylated during stress localize to NPM hinge regions and were therefore more likely to regulate NPM conformation.
Table 2.
Ischemia induces differential NPM phosphorylation
| Site No. | Detected Site | Control | Ischemia | Differential Phosphorylation | Function* |
|---|---|---|---|---|---|
| 1 | S4 | + | + | No | Unknown |
| 2 | S43 | + | + | No | Unknown |
| 3 | S70 | + | + | No | Unknown |
| 4 | T86 | − | + | Yes | Unknown |
| 5 | S88 | − | + | Yes | Deoligomerization |
| 6 | T95 | − | + | Yes | Nuclear export |
| 7 | S125 | + | + | No | Unknown |
| 8 | T234 | + | − | Yes | DNA binding |
| 9 | S242 | + | − | Yes | Unknown |
| Total sites | 9 | 6 | 7 | 5 |
Summary of phosphorylation sites detected by mass spectrometry in nucleophosmin (NPM) purified from cortical homogenates harvested from a pair of human cadaver kidneys; one kidney was well perfused, whereas the contralateral kidney appeared grossly ischemic. Of the nine phosphorylation sites detected, only five sites were differentially phosphorylated between the control and ischemic kidneys. Specifically, T86, S88, and T95 were phosphorylated during stress. In contrast, stress caused T232 and S242 dephosphorylation.
Reported function of each phosphorylation site determined in human acute myelogenous leukemia cells is shown. The phosphorylation status of specific NPM residues differentially altered by ischemic stress assessed by mass spectrometry is virtually identical in human kidney tissue and in primary renal cells in vitro (29).
Stress Increases Both NPM T95 Phosphorylation and Cytosolic NPM Accumulation
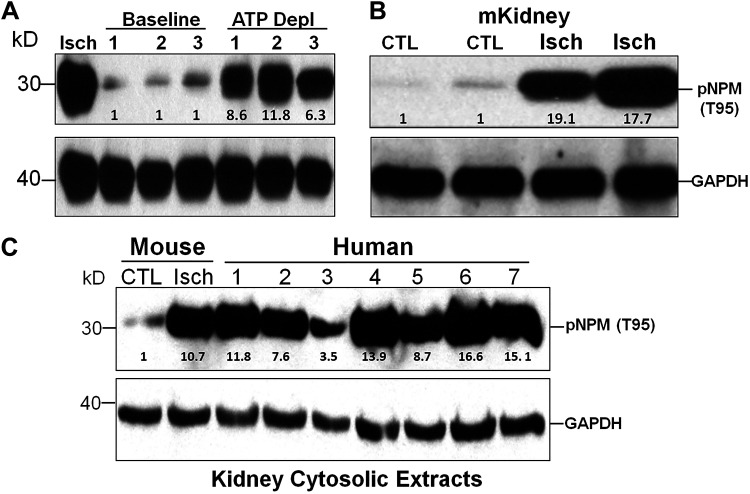
Because kinase consensus sequences for phosphorylation are 100% identical in murine and human cells (29) and because T95 phosphorylation regulates NPM translocation in the cytosol (32) and partly determines the sensitivity of human hematopoietic cancer cells to death caused by chemotherapy and radiation (3, 12), the biological significance of T95 phosphorylation during stress was investigated. Transient exposure to metabolic inhibitors caused a 6- to 12-fold increase in cytosolic pNPM T95 in 3 distinct human cell lines as follows: HepG2 cells, human proximal tubule cells, and 3T3 cells (Fig. 2A). Similarly, almost a 20-fold increase in pNPM T95 accumulation was detected in the cytosolic fraction of murine renal cortical tissues harvested after experimental ischemia in vivo (Fig. 2B) and in human kidneys harvested from seven individual donor kidneys rejected for transplantation that were subjected to variable durations of cold ischemia by nearly 8- to 17-fold (Fig. 2C). NPM was modestly increased (only 3.5-fold) in a single sample harvested from a donated kidney that appeared adequately perfused by a self-contained portable perfusion pump used for organ transportation (Fig. 3C, lane 3, on top).
Fig. 2.
Acute injury increases cytosolic phosphorylated nucleophosmin (pNPM) threonine 95 (T95) in human and murine cells and kidney tissue. A: pNPM T95 content in the cytosolic fraction of human hepatoma (HepG2), human proximal tubule (hPT), and mouse embryo fibroblast NIH-3T3 cells before (baseline) and after metabolic stress (ATP Depl). Lysates harvested from ATP-depleted murine proximal tubule epithelial cells served as a positive control (CTL). B: pNPM T95 content in the cytosolic fraction of renal cortical homogenates harvested from murine (m) kidneys after experimental ischemia (Isch). C: pNPM T95 content in the cytosolic fraction of renal cortical homogenates harvested from seven human kidneys rejected for transplantation (methods). Homogenates of the murine renal cortex before versus after experimental renal ischemia served as negative (baseline) and positive (Isch) controls, respectively. GAPDH was used as a loading control. Immunoblots are representative of three separate experiments.
Fig. 3.
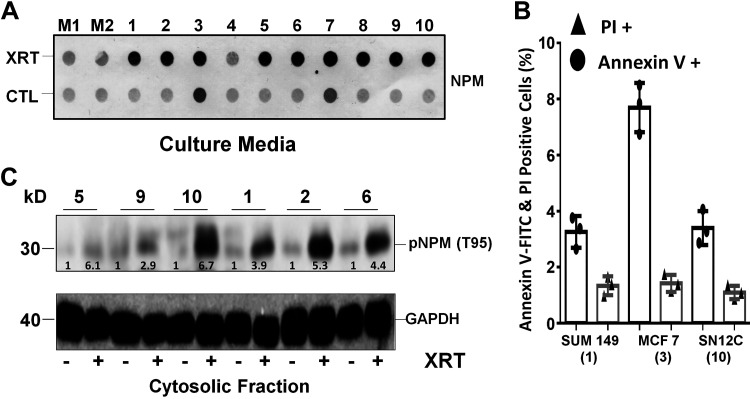
Radiation causes nucleophosmin (NPM) leakage and threonine 95 (T95) phosphorylation in multiple human cancer cell lines. A: increased NPM leakage in the culture medium occurred within 15 min of injury in 9 of 10 different cancer cell lines exposed to 5-Gray γ-radiation (XRT), a dose that kills ~30–50% of cells within 24 h compared with medium alone (M1 and M2). NPM was detected by dot-blot analysis in 200 μL extracellular supernatants harvested from the following human lines: 1) SUM149 breast cancer, 2) Cal27 oral cancer, 3) MCF7 breast cancer, 4) TCP-1009 bone cancer, 5) NCI-H1373 lung cancer, 6) MJ-G11, lymphoma, 7) Hela, cervical cancer, 8) UN-KC-6141 pancreatic cancer, 9) RKO-AS45-1, colon cancer, and 10) SN12C renal cell cancer. The MCF7 breast cancer leaked the most NPM at baseline. B: cell death at baseline (no radiation) in select cancer cell lines measured by annexin V-FITC and propidium iodide (PI) staining showed that MCF7 cells exhibited a high rate of baseline apoptosis but not necrosis compared with SUM 149 or SN12C cells. Numbers in parentheses match cell lines shown in A and C. C: pNPM T95 accumulated in the cytosol of 6 of 7 radiated cancer cell lines detected by immunoblot analysis. CTL, control.
Because NPM is present in all mammalian cells, NPM translocation in the cytosol and extracellular milieu could be a generalized injury marker. To test this novel hypothesis, the leakage of total NPM and pNPM T95 was assessed in 10 distinct cancer cell lines exposed to transient γ-radiation. Within 15 min after exposure to 5-Gray γ-radiation but in the absence of cell detachment or morphological change, marked leakage of total NPM in the medium was detected in 9 of 10 cancer cell lines compared with the respective baseline (Fig. 3A). Only in MCF7 breast cancer cells was NPM readily detected in the medium before radiation treatment. This observation suggested that increased cell turnover, commonly observed in some proliferating cancer cells, might be the primary driver of NPM leakage in this specific cell line. In fact, flow cytometry showed significantly more annexin V-positive MCF7 cells at baseline compared with either renal cancer or SUM 149 cells, another breast cancer cell line (Fig. 3B). This observation shows that NPM leakage detected in MCF7 cells is the result of its intrinsically high proliferation rate and increased apoptosis, as reflected by the selective abundant annexin V staining. In addition to releasing total NPM, radiation exposure also markedly increased cytosolic pNPM T95 by three- to sevenfold in six different human cancer cell lines (Fig. 3C). Overall, our results support the hypothesis that radiation exposure causes NPM translocation in diverse cell types in addition to renal epithelial cells subjected to metabolic stress.
GSK-3β Regulates NPM T95 Phosphorylation and NPM Translocation
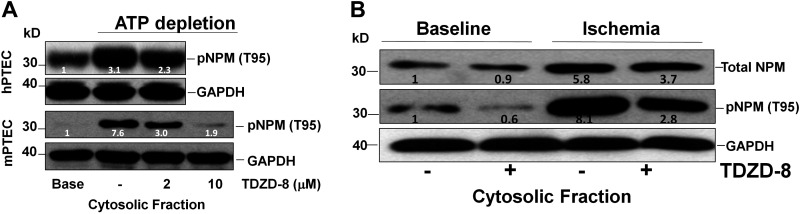
Based on computer modeling (https://services.healthtech.dtu.dk/) with a test score of 0.489 (or about a 50% likelihood), we hypothesized that GSK-3β, a stress kinase activated by renal ischemia (30), phosphorylates NPM T95 during stress. To test this prediction, specific siRNA was used to suppress GSK-3β expression in both primary murine and human renal epithelial cells. Metabolic stress increased cytosolic pNPM T95 content in both cell lines, and TDZD-8, a GSK-3β inhibitor (30), reduced pNPM T95 accumulation during metabolic stress by 40% and 75% using 2 and 10 μM inhibitor doses, respectively (Fig. 4A). Similarly, in vivo ischemia increased total (nearly 9-fold) and pNPM T95 (by 6-fold) in renal cortical homogenates. In contrast, total and pNPM T95 were reduced by 40% and 46%, respectively, in the presence of 10 μM TDZD-8 (Fig. 4B).
Fig. 4.
Pharmacological glycogen synthase kinase-3β (GSK-3β) inhibition decreases phosphorylated nucleophosmin (pNPM) threonine 95 (T95) accumulation in human and murine primary renal cells and kidney cortical homogenates during acute stress. A: immunoblot analysis of steady-state pNPM T95 content in the cytosolic fraction of primary human (h) and murine (m) proximal tubule epithelial cells (PTECs) after metabolic stress (ATP depletion × 70 min) in the presence of 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD-8; 2 or 10 μM, 6-h pretreatment) or vehicle control (−). GAPDH was used as a loading control. B: TDZD-8 (4 mg/kg body wt ip 2 h before ischemia) decreased NPM T95 phosphorylation in the cytosolic fraction of renal cortical homogenates harvested from 8-wk-old male and female mice after ischemia in vivo.
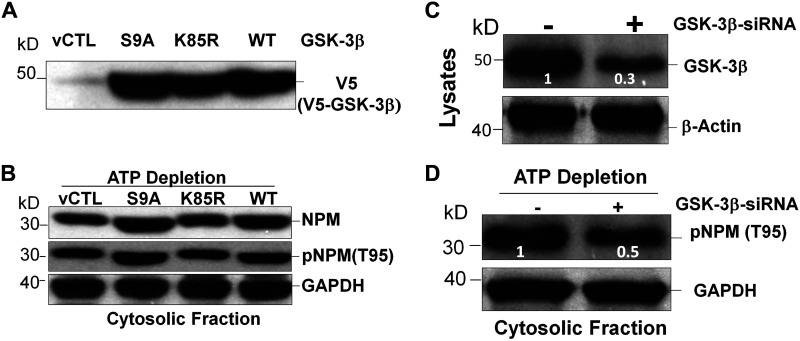
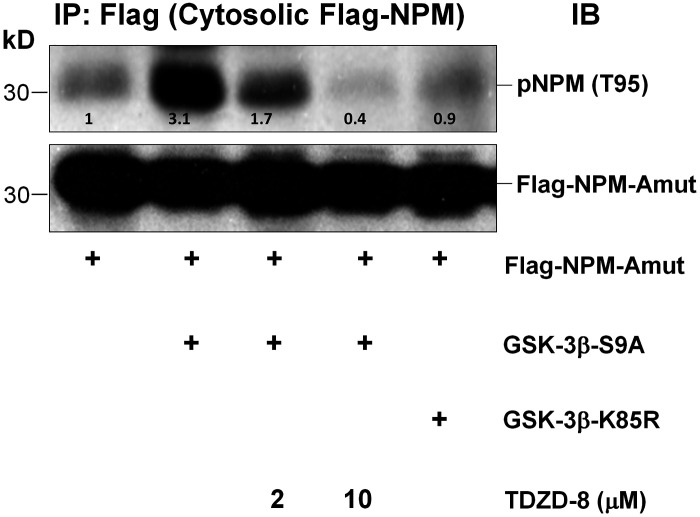
To directly test this hypothesis, equivalent amounts of V5-tagged wild-type constitutively actively active (S9A) or constitutively inactive (K85R) GSK-3β were overexpressed in primary renal cells (Fig. 5A). Compared with either vector control or wild type, active GSK-3β increased cytosolic phosphorylated NPM T95, whereas inactive enzyme reduced it (Fig. 5B). Molecular suppression of GSK-3β using siRNA clearly reduced enzyme expression by nearly 70% (Fig. 5C) and decreased pNPM T95 accumulation by 50% in the cytosol of cells subjected to metabolic stress (Fig. 5D). To evaluate the role of GSK-3β in the absence of stress and avoid stress-induced kinase activation (30), a flag-tagged cytosol-restricted NPM mutant (30) was overexpressed in resting renal cells. The introduction of lentiviral vector containing constitutively active GSK-3β increased pNPM T95 content by threefold in the cytosolic compartment, whereas 2 and 10 μM TDZD-8 reduced cytosolic pNPM T95 content by 50% and 90%, respectively (Fig. 6). In contrast to the active GSK-3β enzyme (Fig. 6, lane 2) expression of a constitutively inactive GSK-3β kinase (lane 6) did not increase pNPM T95 content compared to control (lane 1).
Fig. 5.
Glycogen synthase kinase-3β (GSK-3β) enzyme activity regulates phosphorylated nucleophosmin (pNPM) threonine 95 (T95) accumulation in murine primary proximal tubule epithelial cells (PTECs) during stress. A: S9A (active form), K85R (inactive form), or wild-type (WT) GSK-3β expressed in primary murine PTECs. B: immunoblot analysis of steady-state NPM and pNPM T95 content in the cytosolic fraction of primary murine PTECs after metabolic stress versus vector control (vCTL). C: immunoblot analysis of steady-state GSK-3β expressed content in knockdown GSK-3β by siRNA (+) versus control (−). D: effect of siRNA-mediated GSK-3β knockdown on steady-state pNPM T95 content in the cytosolic fraction of primary murine renal cells. β-Actin or GAPDH served as loading controls.
Fig. 6.
Cytosol-restricted constitutively active glycogen synthase kinase-3β (GSK-3β) enzyme enhances phosphorylated nucleophosmin (pNPM) threonine 95 (T95) phosphorylation in intact cells in the absence of stress. Primary murine proximal tubule epithelial cells (PTECs) were infected with lentiviral vector containing a flag-tagged cytosol-restricted NPM mutant (Flag-NPM-Amut) and either the constitutively active (GSK-3β-S9A) or constitutively inactive (GSK-3β-K85R) enzyme. After pretreatment with 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD-8; 2 or 10 μM), steady-state pNPM T95 content was assessed in immunoprecipitates (IP) harvested with a flag antibody. pNPM T95 specific antibody was used for immunoblot (IB) analysis.
GSK-3β Promotes T95 Phosphorylation in a Cell-Free Assay and Regulates Cell Death
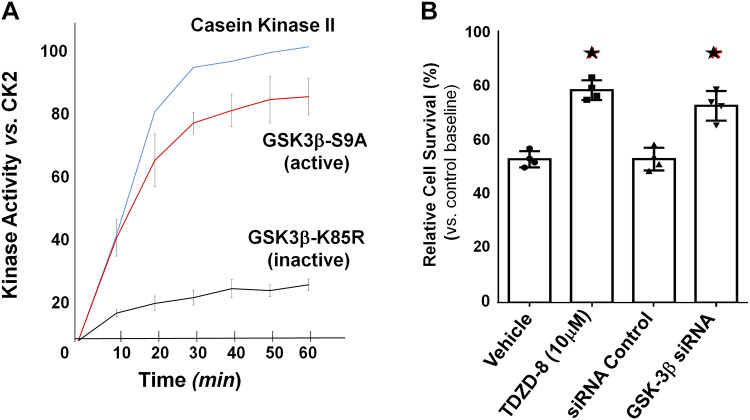
To directly assess pNPM T95 phosphorylation by GSK-3β in the absence of other kinases, a cell-free assay was performed in which flag-tagged GSK-3β harvested from primary renal cells that overexpressed either the active or inactive kinase was incubated with a synthetic peptide substrate containing T95. Compared with constitutively inactive GSK-3β, active GSK-3β rapidly increased enzyme activity (measured as ADP accumulation) similar to casein kinase 2, a positive control, when incubated with the NPM peptide containing T95 (Fig. 7A). To show that GSK-3β is a critical target for regulating cell survival, GSK-3β activity was manipulated in primary renal cells subjected to metabolic stress. Both pharmacological and molecular suppression of GSK-3β with TDZD-8 and siRNA, respectively, significantly improved cell survival after metabolic stress (P < 0.05; Fig. 7B).
Fig. 7.
Glycogen synthase kinase-3β (GSK-3β) promotes phosphorylation of a nucleophosmin (NPM) peptide containing threonine 95 (T95) in a cell-free assay. A: constitutively active (S9A) or inactive (K85R) GSK-3β enzyme purified from 239NT cells was added to specific peptide substrates (100 μM) that included either T95 (NPM amino acids 77–102 with the sequence ATLKMSVQPTVSLGGFEIT95PPVVLRL) or a casein kinase substrate (sequence RRRADDSDDDDD). Only the active kinases casein kinase 2 (CK2) and GSK-3β-S9A showed significant enzyme activity. Casein kinase 2 served as a kinase-positive control and was used to establish 100% kinase activity in this assay. B: both pharmacological [4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD-8); 10 μM] and molecular (GSK-3β siRNA) suppression significantly improved cell survival after transient ATP depletion (70 min with 6-h recovery), as measured by an MTT assay (n = 5 experiments, *P < 0.05).
NPM Detects Acute Cellular Injury in Normal and Cancer Cells
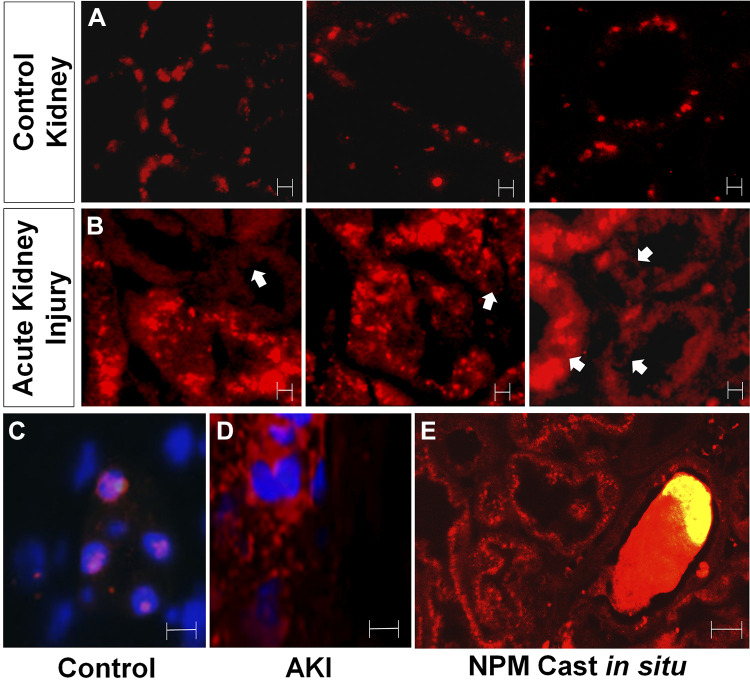
NPM was detected using routine immunofluorescence microscopy to assess NPM as a potential marker of cell injury in human tissue. Because of the fact that the commercially available anti-T95 antibody was not suitable for immunohistochemistry, total NPM was assessed as an acute cell injury marker in human kidney tissue. In three patients with thin basement membrane disease biopsied for microscopic hematuria but without AKI, NPM was detected in a noncytosolic pattern (Fig. 8A). In contrast, NPM localized to the cytosol in 3 of 3 patients (100%) with acute tubular injury caused by diverse insults that included tubular inflammation and ischemia (Fig. 8B). Higher magnification in kidney tissue with nuclear costaining revealed that NPM was exclusively localized to the nucleus in patients with thin basement membrane disease (Fig. 8C). In contrast, NPM was detected in a diffuse cytosolic pattern in a patient with AKI caused by acute tubular injury (Fig. 8D) In some cells with a cytosolic NPM distribution, nuclei appeared relatively devoid of NPM. Remarkably, large casts containing NPM were detected in the intraluminal space (Fig. 8E).
Fig. 8.
Nucleophosmin (NPM) redistribution reflects acute cell injury in human kidney biopsy tissue. A: NPM localizes in a punctate intranuclear pattern in paraffin-fixed human kidney biopsy tissue obtained from three patients with microscopic hematuria caused by thin basement membrane disease but without acute kidney injury (“control”); in control tissue, NPM localized to nuclear regions of proximal tubule cells. Magnification: ×40. B: in contrast, NPM diffusely localized to the cytosol and lumen of kidney tissue in three patients with acute tubular injury (“acute injury”); proximal tubular cells showed NPM distribution in a diffuse cytosolic pattern; arrows show nuclei minimal NPM content. Magnification: ×40. C: high-power view of nuclear NPM staining in a patient with thin basement membrane disease (control) showing NPM-laden nuclei costained with Hoechst dye. D: high-power view of cytosolic NPM staining in a patient with clinical acute kidney injury (AKI) showing distinct distributions for NPM and Hoechst dye. E: an NPM-containing cast trapped inside a collecting tubule lumen. Magnification: ×100. Bars = 5 μm.
DISCUSSION
NPM, a ubiquitous chaperone protein, shuttles between the nucleolar and cytosolic regions to promote ribosomal protein synthesis essential for cell growth and survival (1). Under stress, however, NPM partners with conformationally activated Bax in the cytosol, and the complex moves to the outer mitochondrial membrane to release additional cell death mediators, including cytochrome c and apoptosis-inducing factor (18, 29). Stress-induced differential NPM phosphorylation is highly conserved and has been recently shown to render NPM toxic by promoting cytosolic accumulation and NPM-Bax complex formation (29). Interestingly, two of the five differentially phosphorylated sites detected in murine and human renal tissue or cells during ischemic stress (S88 and T95; see Ref. 31) are identical to those described in patients with AML and mediate sensitivity to radiation and chemotherapy-induced cell death (8). Specifically, S88 phosphorylation regulates NPM deoligomerization and generates 21-kDa NPM monomers able to pass through the nuclear pore (26), whereas T95 appears to regulate cytosolic NPM translocation (32). In contrast, the function of the other three differentially phosphorylated NPM sites is unknown. Based on observations in leukemic cells from patients with AML, we hypothesized that T95 phosphorylation is essential for cytosolic NPM translocation during ischemic stress, that its kinase is amenable to regulation, and that NPM T95 phosphorylation is a potential marker of acute injury in diverse cell types.
In this study, we show, for the first time, that ischemic stress in vitro and in vivo causes NPM T95 to accumulate in murine and human cells using mass spectrometry (Fig. 1 and Table 2), and phosphospecific immunodetection techniques revealed that T95 localizes to the cytosol cell fraction after physiological stress in vitro (Fig. 2A) and in vivo. Specifically, cytosolic pNPM T95 accumulation also occurred in murine kidneys after experimental ischemia (Fig. 2B) as well as in 6 of 7 human kidneys (85%) rejected for transplantation that were subjected to prolonged cold ischemia (Fig. 2C). Like most other differentially phosphorylated sites (with the possible exception of the S88 residue), T95 localizes to a key NPM hinge region in which a change in phosphorylation state would predictably cause conformational and functional changes (Fig. 1B). Our computer-based prediction model implicates T95 phosphorylation as a critical regulatory event likely to alter protein conformation during ischemic (31) and nonischemic (e.g., AML, see Ref. 32) stress.
In 9 of 10 cancer human cell lines from diverse tissues, NPM leakage was detected in the medium within minutes after radiation (Fig. 3A). In fact, radiation failed to increase NPM leakage only in bone cancer cells, known to be highly resistant to radiation-induced cell death (10). Despite high proliferation and turnover rates in cancer cells, NPM leakage associated with high degree of baseline apoptosis (annexin V staining) was detected only in a single breast cancer cell line (MCF7) under control conditions (Fig. 3A). In all other cancer cell lines, radiation exposure clearly increased NPM leakage. Furthermore, pNPM T95 was also detected in the cytosolic fraction harvested from these irradiated cancer cell lines (Fig. 3C) in a manner identical to that observed in intact cells and tissue after ischemia, showing that NPM T95 leakage more broadly detects acute injury associated with regulated cell death. During preliminary experiments, metabolic stress increased NPM T95 phosphorylation within 5 min (data not shown), suggesting that NPM T95 is a rapid cell injury signal. Based on these findings, we speculate that NPM T95 might distinguish effective from ineffective chemotherapy and radiation therapy in patients with diverse forms of cancer.
Computational analysis of the T95 phosphorylation consensus sequence suggested that GSK-3β, a stress-activated kinase that promotes cell injury during AKI (30), potentially regulates T95 phosphorylation during renal cell death. In fact, both pharmacological and molecular manipulation of GSK-3β in intact proximal tubule cells (Fig. 5, B−D) and TDZD-8 in the murine kidney during ischemia in vivo (Fig. 4B) altered cytosolic pNPM T95 accumulation in a GSK-3β-dependent manner. In addition, selective manipulation of GSK-3β activity altered T95 phosphorylation in resting renal cells independent of other stress kinases known to accompany ischemia (Fig. 6). In a cell-free system, active GSK-3β alone is sufficient to phosphorylate an NPM peptide that contains the T95 residue (Fig. 7A). We demonstrated that pharmacological and molecular GSK-3β inhibition of NPM T95 phosphorylation significantly improves cell survival during ischemic stress (Fig. 7B), physiologically linking GSK-3β to T95 phosphorylation and cytosolic NPM accumulation during regulated cell death.
During lethal insults and under the influence of GSK-3β, Bax itself undergoes differential phosphorylation at the S184 residue (7). In renal cells and in the intact kidney, pharmacological GSK-3β inhibition promotes survival and significantly improves renal function after experimental ischemia (30). Specifically, S9 phosphorylation exposes the 6A7 epitope required for NPM-Bax interaction (27). The present study strongly suggests that GSK-3β promotes cell death by both conformationally activating Bax and increasing the amount of cytosolic NPM available to complex with it. This hypothesis partially explains recent enthusiasm for targeting GSK-3β in acute leukemia (11). In retrospect, it is also likely that organ protection during experimental ischemia in mice treated with TDZD-8 reported by our laboratory reflects both its inhibitory effect on NPM T95 phosphorylation and its role in Bax conformational activation required for NPM-Bax complex formation. Once activated, Bax (and Bak) have been implicated in promoting nuclear NPM translocation (17). Conservation of GSK-3β as a single stress kinase involved in both Bax activation and NPM translocation required for lethal complex formation illustrates the remarkable efficiency of regulated cell death.
NPM localization and phosphorylation are dramatically altered by stress. Therefore, cytosolic NPM translocation and T95 phosphorylation may be novel markers of cell injury. To test this hypothesis, total NPM distribution was assessed in blinded human kidney tissue obtained from control patients with hematuria caused by thin basement membrane disease in the absence of AKI compared with kidney tissue from patients with AKI. In 3 of 3 cases (100%) of control tissue samples, NPM was restricted to the cytosol (Fig. 8A). In contrast, NPM appeared in a diffuse cytosolic pattern in three patients with AKI (Fig. 8B). High-resolution microscopy in dual-stained cells (NPM and Hoechst) confirmed cytosolic NPM accumulation during AKI. In addition, NPM casts were detected within the tubular lumen in a patient with ischemic tubular injury (Fig. 8E), supporting our recent contention that urinary NPM is a promising AKI diagnostic tool (31). Interestingly, NPM localized only to the nuclear region in epithelial cells in the renal medulla, a region of the kidney that is relatively resistant to ischemic injury (9, 16). Unfortunately, nonspecific binding limits preclude using the commercially available pNPM T95 antibody for immunohistochemistry.
Given its importance in NPM translocation and conformational Bax activation, GSK-3β is an ideal target for simultaneously interfering with both Bax and NPM, the new “kissing cousins of regulated cell death.” Finally, NPM shows promise as a novel marker of acute proximal tubule cell injury in human kidney biopsy tissue with the potential to detect and quantify tubular injury in clinical AKI (20, 33).
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK118267 (to S. C. Borkan) and DK117612 (to C. Igwebuike) and by an award from the Boston University Office of Technology Development (Ignition Award).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.W., M.B., E.S., J.H., C.I., and S.C.B. conceived and designed research; Z.W., M.B., E.S., J.H., and C.I. performed experiments; Z.W., M.B., E.S., J.H., C.I., A.H., and S.C.B. interpreted results of experiments; Z.W., M.B., E.S., J.H., C.I., A.H., and S.C.B. analyzed data; Z.W., M.B., E.S., J.H., C.I., and S.C.B. prepared figures; E.S., C.I., and S.C.B. drafted manuscript; C.I., A.H., and S.C.B. edited and revised manuscript; Z.W., M.B., E.S., J.H., C.I., A.H., and S.C.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank to Dr. Laurence Beck for providing access to human kidneys rejected for transplantation.
REFERENCES
- 1.Box JK, Paquet N, Adams MN, Boucher D, Bolderson E, O’Byrne KJ, Richard DJ. Nucleophosmin: from structure and function to disease development. BMC Mol Biol 17: 19, 2016. doi: 10.1186/s12867-016-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Czernick D, Liu J, Serge D, Salih E. Topographical distribution of phosphorylation sites of phosvitins by mass spectrometry. J Proteomics 83: 76–98, 2013. doi: 10.1016/j.jprot.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, La Starza R, Diverio D, Colombo E, Santucci A, Bigerna B, Pacini R, Pucciarini A, Liso A, Vignetti M, Fazi P, Meani N, Pettirossi V, Saglio G, Mandelli F, Lo-Coco F, Pelicci PG, Martelli MF; GIMEMA Acute Leukemia Working Party . Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med 352: 254–266, 2005. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 4.Fu X, Liang C, Li F, Wang L, Wu X, Lu A, Xiao G, Zhang G. The rules and functions of nucleocytoplasmic shuttling proteins. Int J Mol Sci 19: 1445, 2018. doi: 10.3390/ijms19051445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gall JM, Wang Z, Bonegio RG, Havasi A, Liesa M, Vemula P, Borkan SC. Conditional knockout of proximal tubule mitofusin 2 accelerates recovery and improves survival after renal ischemia. J Am Soc Nephrol 26: 1092–1102, 2015. doi: 10.1681/ASN.2014010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A. Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol 35: 24–32, 2014. doi: 10.1016/j.semcdb.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Gardai SJ, Hildeman DA, Frankel SK, Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL, Henson PM. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem 279: 21085–21095, 2004. doi: 10.1074/jbc.M400063200. [DOI] [PubMed] [Google Scholar]
- 8.Grisendi S, Mecucci C, Falini B, Pandolfi PP. Nucleophosmin and cancer. Nat Rev Cancer 6: 493–505, 2006. doi: 10.1038/nrc1885. [DOI] [PubMed] [Google Scholar]
- 9.Heyman SN, Lieberthal W, Rogiers P, Bonventre JV. Animal models of acute tubular necrosis. Curr Opin Crit Care 8: 526–534, 2002. doi: 10.1097/00075198-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther 6: 2609–2617, 2007. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]
- 11.Kuenzi BM, Remsing Rix LL, Kinose F, Kroeger JL, Lancet JE, Padron E, Rix U. Off-target based drug repurposing opportunities for tivantinib in acute myeloid leukemia. Sci Rep 9: 606, 2019. doi: 10.1038/s41598-018-37174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunchala P, Kuravi S, Jensen R, McGuirk J, Balusu R. When the good go bad: mutant NPM1 in acute myeloid leukemia. Blood Rev 32: 167–183, 2018. doi: 10.1016/j.blre.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB, Bar-Sagi D, Davis RJ. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH2-terminal kinase. Mol Cell Biol 22: 4929–4942, 2002. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li F, Mao HP, Ruchalski KL, Wang YH, Choy W, Schwartz JH, Borkan SC. Heat stress prevents mitochondrial injury in ATP-depleted renal epithelial cells. Am J Physiol Cell Physiol 283: C917–C926, 2002. doi: 10.1152/ajpcell.00517.2001. [DOI] [PubMed] [Google Scholar]
- 15.Lieberthal W, Menza SA, Levine JS. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am J Physiol 274: F315–F327, 1998. doi: 10.1152/ajprenal.1998.274.2.F315. [DOI] [PubMed] [Google Scholar]
- 16.Lieberthal W, Nigam SK. Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am J Physiol 275: F623–F631, 1998. doi: 10.1152/ajprenal.1998.275.5.F623. [DOI] [PubMed] [Google Scholar]
- 17.Lindenboim L, Blacher E, Borner C, Stein R. Regulation of stress-induced nuclear protein redistribution: a new function of Bax and Bak uncoupled from Bcl-x(L). Cell Death Differ 17: 346–359, 2010. doi: 10.1038/cdd.2009.145. [DOI] [PubMed] [Google Scholar]
- 18.Lindenboim L, Borner C, Stein R. Nuclear proteins acting on mitochondria. Biochim Biophys Acta 1813: 584–596, 2011. doi: 10.1016/j.bbamcr.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z. Regulated cell death in AKI. J Am Soc Nephrol 25: 2689–2701, 2014. doi: 10.1681/ASN.2014030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moeckel GW. Pathologic perspectives on acute tubular injury assessment in the kidney biopsy. Semin Nephrol 38: 21–30, 2018. doi: 10.1016/j.semnephrol.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Narita M, Shimizu S, Ito T, Chittenden T, Lutz RJ, Matsuda H, Tsujimoto Y. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc Natl Acad Sci USA 95: 14681–14686, 1998. doi: 10.1073/pnas.95.25.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salih E. Phosphoproteomics by mass spectrometry and classical protein chemistry approaches. Mass Spectrom Rev 24: 828–846, 2005. doi: 10.1002/mas.20042. [DOI] [PubMed] [Google Scholar]
- 23.Salih E, Siqueira WL, Helmerhorst EJ, Oppenheim FG. Large-scale phosphoproteome of human whole saliva using disulfide-thiol interchange covalent chromatography and mass spectrometry. Anal Biochem 407: 19–33, 2010. doi: 10.1016/j.ab.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson J, Finlayson K, Salvo-Chirnside E, MacDonald D, McCulloch J, Kerr L, Sharkey J. Characterisation of the Bax-nucleophosmin interaction: the importance of the Bax C-terminus. Apoptosis 13: 394–403, 2008. doi: 10.1007/s10495-007-0177-2. [DOI] [PubMed] [Google Scholar]
- 25.Tonnus W, Linkermann A. The in vivo evidence for regulated necrosis. Immunol Rev 277: 128–149, 2017. doi: 10.1111/imr.12551. [DOI] [PubMed] [Google Scholar]
- 26.Velimezi G, Liontos M, Vougas K, Roumeliotis T, Bartkova J, Sideridou M, Dereli-Oz A, Kocylowski M, Pateras IS, Evangelou K, Kotsinas A, Orsolic I, Bursac S, Cokaric-Brdovcak M, Zoumpourlis V, Kletsas D, Papafotiou G, Klinakis A, Volarevic S, Gu W, Bartek J, Halazonetis TD, Gorgoulis VG. Functional interplay between the DNA-damage-response kinase ATM and ARF tumour suppressor protein in human cancer. Nat Cell Biol 15: 967–977, 2013. doi: 10.1038/ncb2795. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Sun SY, Khuri F, Curran WJ, Deng X. Mono- or double-site phosphorylation distinctly regulates the proapoptotic function of Bax. PLoS One 5: e13393, 2010. doi: 10.1371/journal.pone.0013393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang YH, Borkan SC. Prior heat stress enhances survival of renal epithelial cells after ATP depletion. Am J Physiol Renal Fluid Electrolyte Physiol 270: F1057–F1065, 1996. doi: 10.1152/ajprenal.1996.270.6.F1057. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Gall JM, Bonegio R, Havasi A, Illanes K, Schwartz JH, Borkan SC. Nucleophosmin, a critical Bax cofactor in ischemia-induced cell death. Mol Cell Biol 33: 1916–1924, 2013. doi: 10.1128/MCB.00015-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Havasi A, Gall J, Bonegio R, Li Z, Mao H, Schwartz JH, Borkan SC. GSK3beta promotes apoptosis after renal ischemic injury. J Am Soc Nephrol 21: 284–294, 2010. doi: 10.1681/ASN.2009080828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Salih E, Igwebuike C, Mulhern R, Bonegio RG, Havasi A, Borkan SC. Nucleophosmin phosphorylation as a diagnostic and therapeutic target for ischemic AKI. J Am Soc Nephrol 30: 50–62, 2019. doi: 10.1681/ASN.2018040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao X, Ji J, Yu LR, Veenstra T, Wang XW. Cell cycle-dependent phosphorylation of nucleophosmin and its potential regulation by peptidyl-prolyl cis/trans isomerase. J Mol Biochem 4: 95–103, 2015. [PMC free article] [PubMed] [Google Scholar]
- 33.Zuk A, Palevsky PM, Fried L, Harrell FE Jr, Khan S, McKay DB, Devey L, Chawla L, de Caestecker M, Kaufman JS, Thompson BT, Agarwal A, Greene T, Okusa MD, Bonventre JV, Dember LM, Liu KD, Humphreys BD, Gossett D, Xie Y, Norton JM, Kimmel PL, Star RA. Overcoming translational barriers in acute kidney injury: a report from an NIDDK workshop. Clin J Am Soc Nephrol 13: 1113–1123, 2018. doi: 10.2215/CJN.06820617. [DOI] [PMC free article] [PubMed] [Google Scholar]