Abstract
Acute kidney injury is a common clinical disorder and one of the major causes of morbidity and mortality in the postoperative period. In this study, the safety and efficacy of autologous mitochondrial transplantation by intra-arterial injection for renal protection in a swine model of bilateral renal ischemia-reperfusion injury were investigated. Female Yorkshire pigs underwent percutaneous bilateral temporary occlusion of the renal arteries with balloon catheters. Following 60 min of ischemia, the balloon catheters were deflated and animals received either autologous mitochondria suspended in vehicle or vehicle alone, delivered as a single bolus to the renal arteries. The injected mitochondria were rapidly taken up by the kidney and were distributed throughout the tubular epithelium of the cortex and medulla. There were no safety-related issues detected with mitochondrial transplantation. Following 24 h of reperfusion, estimated glomerular filtration rate and urine output were significantly increased while serum creatinine and blood urea nitrogen were significantly decreased in swine that received mitochondria compared with those that received vehicle. Gross anatomy, histopathological analysis, acute tubular necrosis scoring, and transmission electron microscopy showed that the renal cortex of the vehicle-treated group had extensive coagulative necrosis of primarily proximal tubules, while the mitochondrial transplanted kidney showed only patchy mild acute tubular injury. Renal cortex IL-6 expression was significantly increased in vehicle-treated kidneys compared with the kidneys that received mitochondrial transplantation. These results demonstrate that mitochondrial transplantation by intra-arterial injection provides renal protection from ischemia-reperfusion injury, significantly enhancing renal function and reducing renal damage.
Keywords: acute kidney injury, ischemia-reperfusion injury, mitochondrial transplantation, renal
INTRODUCTION
Acute kidney injury (AKI) is a common clinical disorder and one of the major causes of morbidity and mortality in the postoperative period (43). Current evidence indicates that the incidence of AKI is increasing rapidly, particularly among hospitalized patients with acute illness and those undergoing major surgery. Epidemiological studies have suggested that AKI occurs in more than 20–50% of all hospitalized adults worldwide and has a mortality rate of more than 50% in patients in the intensive care unit (6, 43).
Mitochondrial content and oxygen consumption rate in the kidney are second highest only to those in the heart. As mitochondrial function and viability are reliant upon a steady supply of oxygen delivered by the blood, interruption or cessation of oxygen delivery and the resultant tissue ischemia severely compromise mitochondrial energy production, resulting in renal dysfunction or failure (3, 5, 20).
Current therapy for AKI remains limited to preventive strategies, such as fluid management and dialysis; however, there is a growing interest in exploring therapeutic strategies targeting mitochondrial dysfunction in renal ischemia-reperfusion injury (IRI) for AKI treatment (3, 48, 49).
Recently, we have pioneered a novel therapy for IRI using mitochondrial transplantation. This therapy consists of the replacement of the native mitochondria damaged by IRI through the injection of viable, respiration-competent mitochondria isolated from nonischemic tissue obtained from the patient’s own body (4, 8–12, 18, 23, 30–42, 45, 46). Mitochondrial transplantation has been clinically demonstrated to enhance postischemic cardiac viability and function (11, 12, 32). We have validated the safety and efficacy of mitochondrial transplantation in small- and large-animal models and in human pediatric cardiac patients (4, 8–12, 18, 23, 30–42, 45, 46).
In the present study, we investigated the safety and efficacy of mitochondrial transplantation in providing renal protection from IRI in the clinically relevant porcine model.
MATERIALS AND METHODS
Animal Care and Bio Safety
This investigation was conducted in accordance with the National Institutes of Health’s guidelines on animal care and use and was approved by the Boston Children’s Hospital’s Animal Care and Use Committee (Protocol 18-06-3715). All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals (https://www.nap.edu/catalog/12910/guide-for-the-care-and-use-of-laboratory-animals-eighth).
Experimental Models
Female Yorkshire pigs (n = 34, 40–60 kg) were sedated with intramuscular telazol (4–6 mg/kg) and xylazine (1.1–2.2 mg/kg) and intubated. General anesthesia was induced with isoflurane (3% induction, 0.5–2.0% maintenance). Normothermia was maintained using an air-heated pad.
The left femoral artery and right carotid artery were cannulated with a 6-F angiography sheath using the Seldinger technique (over-wire) or a direct cut with exposure of the vessels to allow for continuous mean arterial pressure (MAP) monitoring and vascular access to the abdominal aorta. Femoral or jugular venous lines were placed for central venous pressure (CVP) monitoring. A Foley urinary catheter was placed into the bladder through the urethra to collect urinary samples.
The abdominal aorta was angiographically identified. Selective catheterization of the renal arteries was performed using a 6-F multipurpose guide catheter and a guidewire (Merit Medical Systems, South Jordan, UT) followed by renal artery injection of iodinated contrast medium (Optiray 350 Ioversol 74%, Guerbet, Villepinte, France). Angiography was acquired at the end of equilibrium.
A 15-cm midline laparotomy incision was made from the xyphoid down to the mid- pelvis. A Bookwalter retractor or a straight lateral retractor was used to mobilize the abdominal skin. The lateral peritoneal attachments were dissected off the abdominal wall to expose the right and left retroperitoneum. The inferior vena cava, abdominal aorta, and bilateral renal arteries were identified. Bilateral renal arteries were bluntly dissected. An ultrasonic transit time flow probe (Transonic Systems, Ithaca, NY) was placed circumferentially and secured around each renal artery and continuously recorded through a T403 Multi-Channel Research Console (Transonic Systems) and analyzed using LabChart 7 Acquisition Software (ADInstruments, Sydney, NSW, Australia). Hemodynamic parameters were continuously monitored via MAP and CVP. Glomerular filtration rate (GFR) and renal blood flow (RBF) were monitored throughout the procedure.
Safety and Uptake of Mitochondrial Transplantation
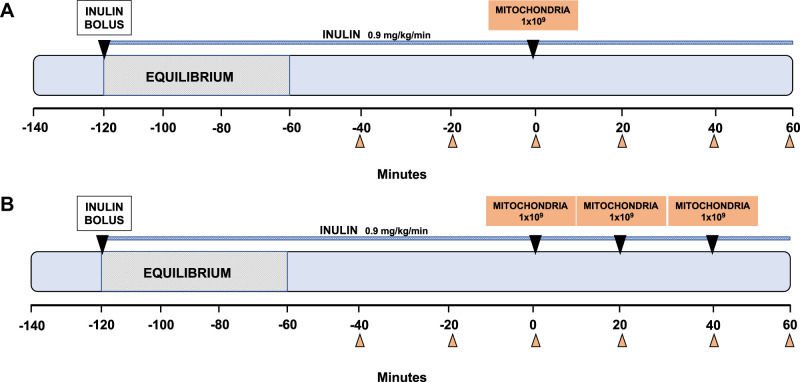
To determine the uptake and safety of autologous mitochondria by intra-arterial delivery in the kidneys, experiments were performed in the nonischemic kidney. The experimental outline is shown in Fig. 1.
Fig. 1.
Description of experimental model: intra-arterial injection of autologous mitochondria in the nonischemic kidney. Female Yorkshire pigs (40–60 kg) were sedated and intubated. The left femoral artery and right carotid artery were cannulated with a 6-F angiography sheath. Femoral or jugular venous lines and a Foley urinary catheter were also placed. Once the renal arteries were angiographically identified, animals were divided into different experimental groups. A small piece of muscle was surgically extracted from the sternocleidomastoid muscle using a 6-mm biopsy punch (~0.01 g) and used for mitochondrial isolation, which was performed 30 min before the injection of mitochondria. Single injections were delivered as a bolus antegrade into each renal artery (1 × 109 in 6 mL respiration buffer, n = 10; A). Serial injections of respiration buffer containing mitochondria (3 injections of 1 × 109 in 6 mL respiration buffer for each injection, n = 10) were delivered every 20 min (B). Following each injection, 5 mL of saline flush were injected as a flush. Before the injections, a 15-cm midline laparotomy incision was made, and bilateral renal arteries were identified and bluntly dissected. An ultrasonic transit time flow was placed circumferentially around each renal artery. Sixty minutes after the first injection of autologous mitochondria, the animal was subsequently euthanized. Blood and urine samples were collected every 20 min.
Mitochondrial isolation.
The sternocleidomastoid muscle was located and dissected through the neck incision used for carotid/jugular cannulation. Two small pieces were surgically extracted using a 6-mm biopsy punch (~0.01 g, Miltex, York, PA) and used for mitochondrial isolation, as previously described (40, 41). The isolated mitochondria were suspended in vehicle [250 mM sucrose, 10 mM K+-HEPES (pH 7.2) and 0.5 mM K+-EGTA (pH 8.0)]. Isolation of the mitochondria was performed 30 min before mitochondrial transplantation.
Mitochondrial transplantation.
Mitochondria were delivered antegrade into each renal artery through the 6-F catheter. Mitochondria were delivered either as a single bolus injection of 1 × 109 mitochondria in 6 mL vehicle (n = 10) or as serial injections with three injections of 1 × 109 in 6 mL vehicle each injection (n = 10) delivered every 20 min. Following each injection, 5 mL of saline flush were injected as a flush. Animals were allowed to recover for 60 min and were then euthanized (Fig. 1).
Sampling and GFR calculation.
Serum and urine samplings and renal output measurements were performed every 20 min throughout the experiment (Fig. 1). GFR was obtained by calculating inulin clearance. In brief, a loading dose of 50 mg/kg inulin (Bio Physics Assay Laboratory, Worcester, MA) was injected intravenously followed by continuous infusion of inulin at 0.9 mg·min−1·kg−1. Sixty minutes were allowed as an equilibration period. After equilibrium, three samples of 10 mL arterial blood and three samples of 10 mL urine were collected every 20 min throughout the experiment. GFR was then calculated as the average of the three inulin clearance samples using the following formula: GFR = (urinary inulin × V)/plasma inulin, where V is the urine flow rate (in mL/min).
Uptake and biodistribution of transplanted mitochondria.
To determine mitochondrial uptake and biodistribution, autologous mitochondria were labeled with 18F-rhodamine-6G (18F-R6G). The radiotracer was synthesized as previously described (18, 19). The radiolabeled mitochondria were then administered through a single injection of 1 × 109 in each kidney as previously described (19, 33, 46). Whole body positron emission tomography (PET) and computerized tomography (CT) were then performed on Biograph mCT PET/CT systems (Siemens Medical Solutions) after euthanasia of the animal at the end of 10 min of perfusion (n = 1), a timeframe long enough for the blood to recirculate through the whole body ~10 times, as previously described (19, 33, 46).
Cellular uptake of mitochondria was evaluated by intravascular injection of mitochondria isolated from human cardiac fibroblasts. The use of human mitochondria allows for immunohistochemical (IHC) differentiation of transplanted mitochondria from native swine cardiac mitochondria based on antibody specificity (30, 33, 46). Isolated human mitochondria (1 × 109) in 10 mL vehicle were delivered to the left kidney. The right kidney was injected with 10 mL vehicle. The animal was euthanized 10 min following injection of mitochondria or vehicle, and the kidneys were dissected and sectioned. Transplanted mitochondria were detected by IHC and immunofluorescent staining as previously described (19, 30, 33, 46).
Efficacy of Intra-Arterial Autologous Mitochondrial Transplantation
Efficacy of intra-arterial autologous mitochondrial transplantation for renal IRI was evaluated using a bilateral renal artery occlusion model for temporary IRI. After establishment of equilibrium for 60 min, heparin sodium (300 UI/kg) was given via the central venous line to achieve an activated coagulation time (ACT) of >350 s. ACT was monitored every hour, and an additional heparin dose was administered to maintain ACT > 350 s.
Renal ischemia was achieved by balloon catheter occlusion of the renal arteries identified by angiography (5 mL contrast medium diluted in 5 mL normal saline was used). The balloon catheter (6-F, Cordis, Hialeah, FL) was introduced to the proximal portion of the renal artery and inflated so as to totally occlude the blood flow to the kidneys for 60 min. Confirmation of the occlusion was acquired by injection of iodinated contrast medium (5 mL contrast medium diluted in 5 mL normal saline) in the aorta and by checking for any opacification of the vessel of the kidneys. Flattening of the pulse waveform on the tip of the balloon catheter was also used to confirm occlusion. Hemodynamic parameters were continuously monitored via MAP and CVP.
Following 60 min of occlusion, the balloons were deflated and carefully removed. Angiography was performed to confirm renal artery patency and establishment of renal reperfusion (5 mL contrast medium diluted in 5 mL normal saline). Animals were randomly assigned to receive either a single injection of vehicle (vehicle, 10 mL, n = 6) or vehicle containing mitochondria (mitochondria, 1 × 109 in 10 mL vehicle, n = 6) immediately at reperfusion after a period of 60 min of bilateral warm ischemia (Fig. 2). Following each injection, 5 mL of saline flush were injected as a flush. Mitochondria were isolated from the sternocleidomastoid muscle as previously described (40, 41). The isolated mitochondria were suspended in vehicle [250 mM sucrose, 10 mM K+-HEPES (pH 7.2), and 0.5 mM K+-EGTA (pH 8.0)]. Isolation of the mitochondria was performed 30 min before mitochondrial transplantation.
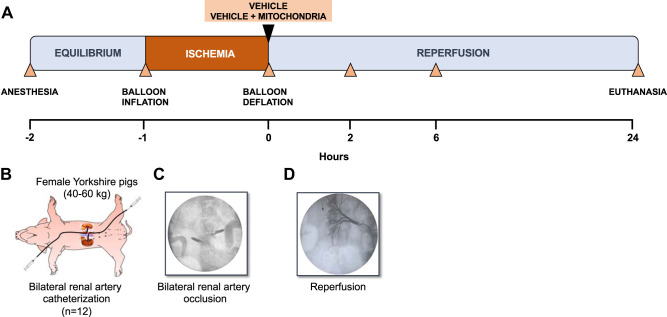
Fig. 2.
Description of experimental model: intra-arterial injection of autologous mitochondria or vehicle after renal ischemia-reperfusion injury. A: description of the experimental model. B: female Yorkshire pigs (40–60 kg) were sedated and intubated. The left or right femoral artery and the carotid artery were cannulated with a 6-F angiography sheath. Femoral or jugular venous lines and a Foley urinary catheter were also placed. Selective catheterization of the renal arteries was performed using a 6-F multipurpose guide catheter. Occlusion of the renal arteries was performed by a 6-F balloon catheter inflated in the proximal portion of the renal artery, totally occluding the blood flow to the kidneys for 60 min. C: confirmation of the occlusion was acquired by injection of iodinated contrast medium in the aorta and by checking for any opacification of the vessel of the kidneys. A small piece of muscle was surgically extracted from the sternocleidomastoid muscle using a 6-mm biopsy punch (~0.01 g) and used for mitochondrial isolation, which was performed 30 min before the injection of mitochondria. Following 60 min of occlusion, the balloons were deflated and carefully removed. D: angiography was performed to confirm renal artery patency and establishment of renal reperfusion. To evaluate efficacy of intra-arterial delivery of mitochondria after ischemia-reperfusion injury, a group of animals (n = 12) received either a single injection of vehicle (10 mL, n = 6) or vehicle solution containing mitochondria (1 × 109 in 10 mL, n = 6) immediately at reperfusion after a period of 60 min of bilateral warm ischemia. Following each injection, 5 mL of saline flush were injected as a flush. Animals were then allowed to reperfuse the kidneys under physiological conditions for the next 24 h and were subsequently euthanized. Blood and urine samples were collected right before and after bilateral renal ischemia at 2, 6, and 24 h after occlusion.
The guide catheter, guidewire, and 6-F sheath were removed from the femoral artery. The heparin effect was reversed at the end of ischemia by administration of protamine (3 mg/kg) and assessed by ACT. The wound on the neck was sutured, and the catheters were left in place and secured to allow for blood sampling throughout the study. Sodium chloride (0.9%) was infused at 5 mL·kg−1·h−1 for a total of 2 h following the end of ischemia. Animals were monitored and maintained on mechanical ventilation until waking. Animals were accommodated in a temperature-controlled room with water and food ad libitum. Venous blood sampling and renal functional measurements were performed before ischemia, at the end of ischemia, and 2, 6, and 24 h after reperfusion (Fig. 2). GFR was estimated (eGFR) based on plasma creatinine and body weight (14).
Following a 24-h recovery period, the animal was fully sedated and anesthetized. The renal arteries and veins were ligated, bilateral nephrectomies were performed to collect whole tissue specimens for histological analysis, and the animal was euthanized by exsanguination.
Biomarkers of renal function.
Biomarkers of renal function, i.e., sodium, potassium, calcium, phosphate, glucose, and total proteins, were assessed using a VetScan2 chemistry analyzer (Abaxis, Union City, CA). White blood cell count, platelet count, alanine aminotransferase, amylase, and bilirubin were tested using the VetScan2 chemistry analyzer and a VetScan HM5 (Abaxis).
Histology and transmission electron microscopy.
Tissue samples from the medulla and cortex of each kidney were fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained for hematoxylin and eosin and Masson’s trichrome. Transmission electron microscopy (TEM) was used to analyze structural damage of the mitochondria in the kidneys, as described in previous studies (30, 33, 46). All histological and electron microscopy were performed by a single experienced renal pathologist blinded to the experimental group.
The degree of acute tubular necrosis (ATN) was scored on all (4/animal) trichrome slides, the cortex and medulla separately, by grading the percentage of tubules that displayed cell necrosis, loss of the brush border, cast formation, and tubule dilatation as follows: 0, none; 1, <10%; 2, 11–25%; 3, 26–45%; 4, 46–75%; and 5, >76%.
IL-6.
Expression of IL-6 in the renal cortex was measured as previously described (36, 42).
Statistical Analysis
Continuous variables are expressed as means ± SE. Normality of all continuous variables was tested using the Shapiro-Wilk test and graphically assessed by histograms and Q-Q plots. Longitudinal analysis for between-group comparisons was performed using two-way repeated-measures ANOVA and by fitting mixed-effects linear regression models. To reduce the probability of false positive results (type I error) due to multiple comparisons, the Benjamini and Hochberg's false discovery rate was applied to control family-wise error to α < 0.05. The Mann-Whitney U test was used for between-group comparisons in the case of histopathological indexes. All reported tests are two tailed. Statistical analyses were performed using STATA version 15.1 (Stata, College Station, TX) and GraphPad Prism version 7.00 for Mac OS X (GraphPad Software, La Jolla, CA).
RESULTS
Intra-Arterial Injection of Autologous Mitochondria in the Nonischemic Kidney
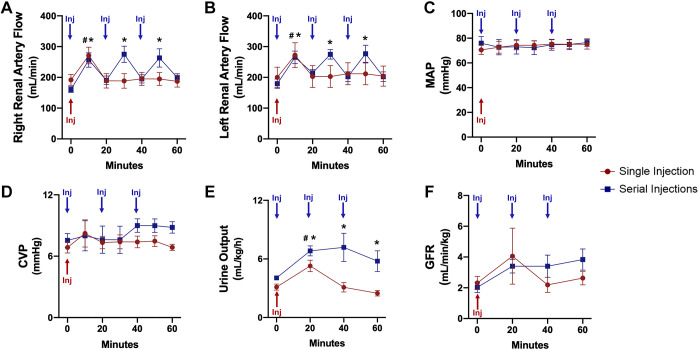
Angiographic images showed patent renal arteries with no detectable lesions or blockages after intra-arterial injection of autologous mitochondria. The delivery was not associated with any adverse hemodynamic effect (no detectable change in ECG, no brady- or tachyarrhythmias, and intra-arterial pressure monitoring) or renal dysfunction. Both single and serial injections of mitochondria led to a transient increase in RBF 60 s postinjection (P < 0.001 for each injection). Mean RBF did not differ between single and serial injections throughout the 120-min period (left RBF: P = 0.741 and right RBF: P = 0.599; Fig. 3).
Fig. 3.
Renal function during intra-arterial injection of autologous mitochondria in the nonischemic kidney. A: right renal artery flow. B: left renal artery flow. C: mean arterial pressure (MAP). D: central vein pressure (CVP). E: urine output. F: glomerular filtration rate (GFR). All results are means ± SE for each group. Blue arrows show the time of injection of mitochondria in the serial injection group; the red arrow represents the time of injection of mitochondria in the single injection group. In A–D, data are shown for the time of injection and 1 min after each injection or the 20-min interval. Data were analyzed by two-way repeated-measures ANOVA with the Benjamini and Hochberg's false discovery rate (n = 10 in both groups). *P < 0.05, designated time point vs. baseline for serial injections; #P < 0.05, designated time point vs. baseline for single injection.
Urine output was significantly increased in the serial injection group compared with the single injection group (P = 0.007). GFR, MAP, and CVP did not differ between the two groups throughout the 120-min period (GFR: P = 0.315, MAP: P = 0.917, and CVP: P = 0.334; Fig. 3).
Uptake and Biodistribution of Transplanted Mitochondria
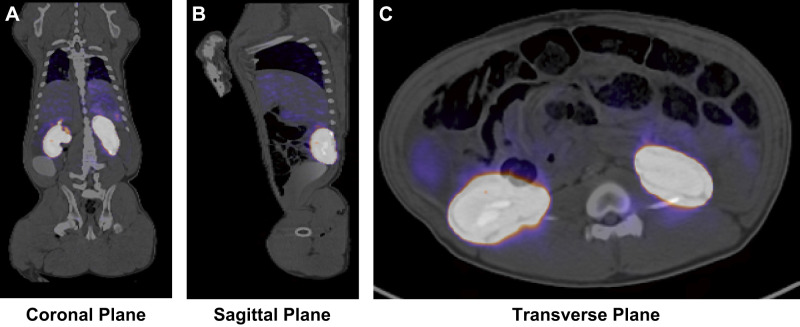
Whole body PET/CT scan images showed that the transplanted 18F-R6G-labeled mitochondria had a widespread biospecific distribution in the kidneys. Radiolabeled mitochondria were not detectable in any other organ or region of the body (Fig. 4).
Fig. 4.
Biodistribution of autologous mitochondria by intra-arterial injection in the nonischemic kidney. 18F-rhodamine-6G-labeled mitochondria were administered through a single injection of 1 × 109 for each kidney. A whole body positron emission tomography and computerized tomography were then performed after euthanization of the animal 10 min following mitochondrial transplantation to obtain coronal (A), sagittal (B), and transverse (C) views.
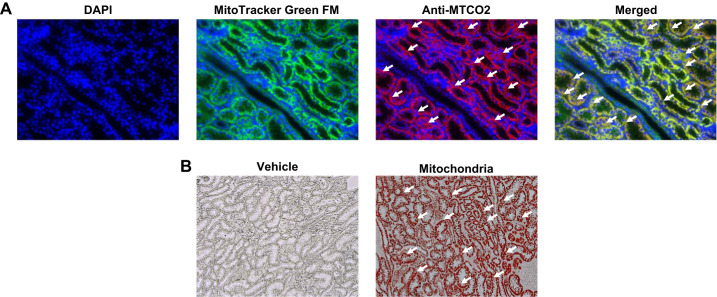
Analysis of mitochondrial uptake revealed that injected human mitochondria were distributed throughout the tubular epithelium of the cortex and medulla (Fig. 5).
Fig. 5.
Renal uptake of autologous mitochondria by intra-arterial injection in the nonischemic kidney. Representative immunofluorescent and immunohistochemical images demonstrating mitochondrial uptake in the kidneys are shown. A: injected kidney sections were fluorescently stained for nuclei using the DNA stain DAPI (blue) and for both human and swine mitochondria with Mito-Tracker Green FM (green) and for human mitochondria only with human mitochondrial MTCO2 antibody (red). Original magnification: ×40. B: representative immunohistochemistry photomicrographs of kidneys that received either vehicle or human mitochondria. Mitochondria were labeled with MTCO2 (red) antibody, which labels human mitochondria. Original magnification: ×20. White arrows indicate examples of transplanted human mitochondria. n = 1.
Validity of the Renal IRI Model: Longitudinal Changes in Renal Function Biomarkers
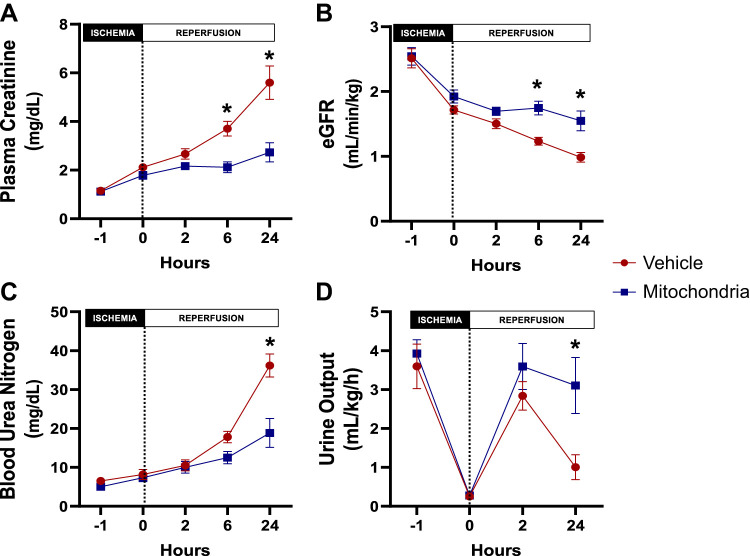
Longitudinal changes of renal function biomarkers in the vehicle-treated group were compared throughout the experiment to establish the validity of the renal IRI model. Following 60 min of bilateral renal artery occlusion, serum creatinine was significantly increased (2.1 ± 0.1 vs. 1.1 ± 0.1 mg/dL, P = 0.011) while eGFR and urine output were significantly decreased (eGFR: 1.7 ± 0.1 vs. 2.5 ± 0.2 mL·min−1·kg−1, P < 0.001, and urine output: 0.2 ± 0.1 vs. 3.6 ± 0.5 mL·kg−1·h−1, P < 0.001) compared with baseline. The increase in serum creatinine remained significant after 2 h (P < 0.001), 6 h (P < 0.001), and 24 h (P < 0.001) of reperfusion compared with baseline. Similarly, blood urea nitrogen (BUN) was significantly increased after 6 h (P < 0.001) and 24 h (P < 0.001) of reperfusion compared with baseline. eGFR and urine output remained significantly decreased at 6 h (P < 0.001) and 24 h (P < 0.001) of reperfusion compared with baseline (Fig. 6).
Fig. 6.
Renal function during intra-arterial injection of autologous mitochondria or vehicle after renal ischemia-reperfusion injury. A: plasma creatinine. B: estimated glomerular filtration rate (eGFR). C: blood urea nitrogen. D: urine output. All results are means ± SE for each group. Injection is denoted by the dotted vertical line. Data were analyzed by two-way repeated-measures ANOVA with the Benjamini and Hochberg's false discovery rate (n = 6 in both groups). *P < 0.05, mitochondria vs. vehicle.
Safety of Intra-Arterial Injection of Autologous Mitochondria After Renal IRI
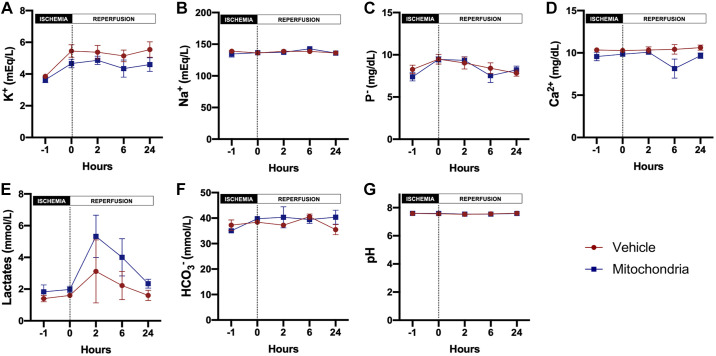
Plasma electrolytes, acid-base status, complete blood count, and biochemical analysis were evaluated for assessment of the safety of intra-arterial injection of autologous mitochondria after renal IRI. Plasma potassium (P = 0.066), sodium (P = 0.826), phosphate (P = 0.694), calcium (P = 0.059), pH (P = 0.733), bicarbonate (P = 0.457), white blood count (P = 0.466), platelets (P = 0.997), glucose (P = 0.218), total protein (P = 0.100), lactate (P = 0.061), alanine transaminase (P = 0.490), amylase (P = 0.587), and total bilirubin (P = 0.651) did not differ between the vehicle- and mitochondria-treated groups at baseline and throughout the 24-h reperfusion period (Fig. 7).
Fig. 7.
Safety assessment of mitochondrial transplantation in renal ischemia-reperfusion injury. A: potassium. B: sodium. C: phosphorus. D: calcium. E: lactates. F: bicarbonates. G: pH. All results are means ± SE for each group. Injection is denoted by the dotted vertical line. Data were analyzed by two-way repeated measures ANOVA with the Benjamini and Hochberg's false discovery rate (n = 6 in both groups).
Efficacy of Intra-Arterial Injection of Autologous Mitochondria After Renal IRI
Serum renal function biomarkers.
There was no difference in serum creatinine, BUN, and eGFR at baseline and following 60 min of bilateral renal artery occlusion between the two groups. Following 24 h of reperfusion, serum creatinine and BUN were significantly increased in the vehicle-treated group compared with the mitochondria-treated group (creatinine: 5.6 ± 0.7 vs. 2.7 ± 0.4 mg/dL, P < 0.001, and BUN: 32.3 ± 4.6 vs. 18.8 ± 3.8 mg/dL, P < 0.001, respectively) while eGFR was significantly decreased in the vehicle-treated group compared with the mitochondria-treated group (1.0 ± 0.1 vs. 1.6 ± 0.2 mL·min−1·kg−1, respectively, P < 0.001; Fig. 6, A–C).
Urine output.
There was no difference in urine output at baseline and following 60 min of bilateral renal artery occlusion between the two groups. Urine output was significantly increased in the mitochondria-treated group compared with the vehicle-treated group following 24-h reperfusion (3.1 ± 0.7 vs. 1.3 ± 0.4 mL·kg−1·h−1, P = 0.007; Fig. 6D).
Pathological findings.
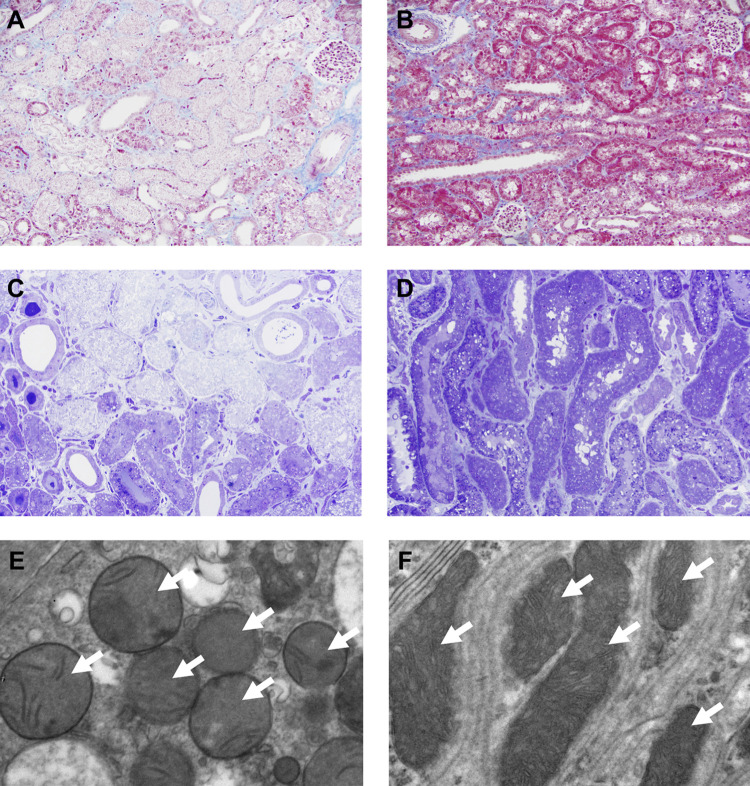
Masson trichrome staining showed that renal cortex of the vehicle-treated group had extensive coagulative necrosis of primarily proximal tubules, following 60 min of ischemia and 24-h reperfusion, while the mitochondria-transplanted kidney showed only patchy mild acute tubular injury (Fig. 8, A and B). Plastic (araldite-epon) embedded sections demonstrated in greater detail the marked difference between the confluent tubular necrosis seen in the vehicle-treated group compared with the more limited and likely sublethal proximal tubular injury in the mitochondria-treated group, consisting primarily of matrix with swelling and degenerative changes in organelles (Fig. 8, C and D). TEM of mitochondrial morphology from the same animals shown in Fig. 8, C and D, revealed dysmorphic mitochondria in the vehicle-treated group (Fig. 8E), with swelling, loss, or distortion of cristae and other alterations in the matrix; in contrast, the mitochondria-treated group (Fig. 8F) displayed mitochondria with normal morphology that are oriented along the basolateral infoldings of an intact proximal tubular cell.
Fig. 8.
Representative light and electron microscopy images after intra-arterial injection of autologous mitochondria or vehicle and ischemia-reperfusion injury. A and B: the renal cortex of the vehicle-treated kidney showed extensive coagulative necrosis of primarily proximal tubules following 60 min of ischemia and 24 h of reperfusion, while the kidney that received mitochondrial transplantation showed only patchy mild acute tubular injury (Masson trichrome). Original magnification: ×20. C and D: these 1-μm plastic (araldite-epon)-embedded sections demonstrate in greater detail the marked difference between the confluent tubular necrosis seen in the vehicle-treated group (C) versus the more limited and likely sublethal proximal tubular injury in the mitochondria-treated group (D), consisting primarily of matrix with swelling and degenerative changes in organelles (toluidine blue). Original magnification: ×40. E and F: transmission electron microscopy of mitochondrial morphology from the same animals shown in C and D revealing dysmorphic mitochondria in the vehicle-treated group (E), with swelling, loss or distortion of cristae, and other alterations in the matrix; in contrast, the mitochondria-treated group (F) displayed mitochondria with normal morphology that were oriented along the basolateral infoldings of an intact proximal tubular cell. Original magnification: ×25,000. White arrows in E and F indicate the mitochondria.
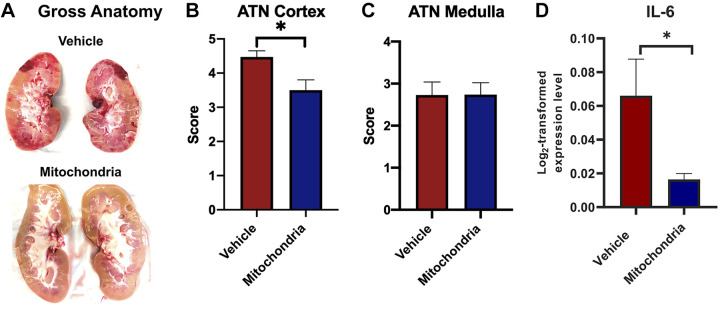
Gross anatomy revealed increased necrosis and hemorrhage in the kidneys that received vehicle, while kidneys injected with mitochondria had a normal gross appearance (Fig. 9A). ATN scoring showed significantly increased injury in the renal cortex of the vehicle-treated group compared with the mitochondria-treated group (4.5 ± 0.3 vs. 3.5 ± 0.2, P = 0.013; Fig. 9B). No difference was noted in medulla injury (P = 0.982; Fig. 9C).
Fig. 9.
Gross kidney anatomy and renal tissue injury at 24 h of reperfusion after intra-arterial injection of autologous mitochondria or vehicle and ischemia-reperfusion injury. A: gross anatomy of the left kidney of the vehicle-treated group, showing pale areas indicative of infarction and red hemorrhagic areas, while the left kidney of the mitochondria-treated group had normal gross morphology following 60 min of bilateral renal artery occlusion and 24 h of reperfusion. Acute tubular necrosis (ATN) score assessment of renal injury showed increased injury in the cortex of the vehicle-treated group compared with the mitochondria-treated group (B), while no difference was noted in the medulla (C). D: IL-6 expression in the renal cortex in the vehicle-treated group compared with the mitochondria-treated group. All results are means ± SE for each group. Data were analyzed by a Mann-Whitney U test (n = 12 in both groups; 2 kidneys/animal were analyzed). *P < 0.05, mitochondria vs. vehicle.
IL-6.
Following 60 min of bilateral renal IRI and 24 h of reperfusion, there was a significant 3.5-fold significant increase in IL-6 expression in the renal cortex in the vehicle-treated group compared with the mitochondria-treated group (P = 0.047; Fig. 8D).
DISCUSSION
Mitochondria play a crucial role in the pathogenesis of AKI and represent a target for therapeutic approaches for AKI treatment. However, almost all the current therapeutic approaches are prophylactic, and this represents an important limitation in their clinical application, since AKI is generally unpredictable (17, 22, 26, 44, 49).
Rather than targeting a single mediator of the pathways leading to mitochondrial damage after IRI, transplantation of autologous mitochondria has been proposed and studied by our group (4, 8–12, 18, 23, 30–42, 45, 46). This technique consists of the functional recovery of native mitochondria damaged by IRI through the injection of viable and respiration-competent autologous mitochondria isolated from the patient's healthy skeletal muscle. The isolation process has been previously validated (21, 40, 41).
In this study, we demonstrated that mitochondrial transplantation by intra-arterial injection is safe and efficacious, enhancing renal function and decreasing renal injury associated with AKI in a clinically relevant large-animal model.
For this study, we used the clinically relevant swine model to allow for translation to humans (17). To ensure renal ischemia, we used a model of temporary bilateral renal IRI (60 min of ischemia). This model was used as it clinically translates to pre-renal AKI that comprises the majority of AKI cases (2, 15, 44). We chose to use bilateral renal artery occlusion over unilateral renal artery occlusion with or without resection of the contralateral kidney since it better mimics the clinical scenario of kidney hypoperfusion perioperatively (21, 28). We confirmed renal ischemia by angiography and monitoring of the pressure of the renal arteries. For our reperfusion phase, we allowed the animals to recover for 24 h to provide a sufficient period of postischemia to assess both the degree of AKI and the efficacy of mitochondrial transplantation.
A percutaneous approach was selected for our AKI experiments to provide clinical relevance and to minimize the impact of a laparotomy on the inflammatory response of the animals and the risk for infection without necessitating the administration of antibiotics (27). Moreover, the same hydration protocol was used for all animals for optimal hemodynamic control and renal perfusion.
We have taken great care to use the same amount of contrast in all animals for visualization of the renal arteries (13.5 ± 1.5 vs. 14.5 ± 1.0 mL in vehicle- and mitochondria-treated groups, respectively) in an attempt to control for confounding effects resulting from contrast-induced nephrotoxicity. The amount of contrast medium was kept at a minimum by diluting one part of contrast medium to one part of normal saline. The total amount of contrast used (0.33 mL/kg) in our study was significantly lower than the estimated safety threshold for humans (3.33 mL/kg) (13, 25).
In this study, we used 2 × 105 mitochondria/g wet wt based on our previous investigations (33, 38, 46). These studies have demonstrated that maximal benefits are obtained at 2 × 105 mitochondria/g wet wt and that additional mitochondria, while not injurious, do not increase efficacy (4, 8, 10, 18, 23, 24, 29–31, 33, 34, 36–38, 46). Our previous results agree with those of Shoffner et al. (47), who demonstrated that only 2–6% of mtDNA need to be wild type to alter oxygen uptake and the devastating effects of the mitochondrial myopathy myoclonic epilepsy and ragged-red fiber (MERRF) disease, and with Chomyn et al. (7), who reported that levels of only 6% wild-type mtDNA are sufficient to modulate the effects of mitochondrial encephalopathy, lactic acidosis, and stroke-like (MELAS) episodes.
We have previously demonstrated both in vitro and in vivo that the transplanted mitochondria are rapidly internalized by actin-dependent endocytosis and remain viable to immediately increase total tissue ATP levels and ATP synthesis and upregulate protein pathways for the mitochondrion and the generation of precursor metabolites for energy and cellular respiration (8, 9, 39). The transplanted mitochondria maintain viability and function for at least 28 days, the limit of our studies (23, 30, 39). These data have recently been replicated in vitro by others (1).
In the present study, we used a single injection of 1 × 109 mitochondria/kidney, which was increased in the serial injections to 3 × 109 mitochondria/kidney. Our results demonstrate that, despite the threefold increase in mitochondria delivered to the kidneys, renal function indexes were not significantly different using either a single or serial injection of mitochondria. Since a single injection requires only a brief time period to perform compared with the longer time for serial injections, we chose to use a single injection strategy in our IRI experiments. This strategy allows for less surgical intervention and is more clinically relevant. These results are in agreement with our previous study in the heart (18). Vehicle alone has never demonstrated a protective benefit; therefore, serial injections of vehicle were not investigated.
The injected mitochondria were rapidly taken up by the kidneys and were not observed to be present in any other organs in the swine. PET/CT imaging using 18F-R6G-labeled mitochondria demonstrated renal-specific global distribution of the transplanted mitochondria. To confirm uptake of the transplanted mitochondria, we used mitochondria isolated from human cardiac fibroblast cell culture. The use of human mitochondria in a swine model allows for IHC differentiation of the transplanted mitochondria from the native swine cardiac mitochondria based on antibody specificity (30, 33, 46).
The specificity of the antibody used for the staining of the human mitochondria has been demonstrated in our previous studies and was also shown here by the lack of binding to the renal tissue of a kidney that received vehicle. Autologous mitochondria were rapidly taken up and integrated into the renal cells. We have previously demonstrated that the mechanism by which mitochondria enter the cells is through actin-dependent endocytosis (9, 39).
The rapid uptake and integration of mitochondria into the kidney are in agreement with our previous results demonstrating the biospecificity of mitochondrial transplantation when delivered either by direct or intra-arterial injection to the end organ (36, 38, 46). This has been shown in the heart but also in the lungs, where mitochondria were delivered either intra-arterially in the pulmonary artery or by nebulizer (36). Our previous study (8) has also demonstrated that the transplanted mitochondria are being uptaken by both ischemic and nonischemic tissue.
Mitochondrial transplantation is safe for use in both the ischemic and nonischemic kidney. No systemic inflammatory response and organ dysfunction, or any change in hemodynamic parameters, were observed. This is in agreement with our previous studies demonstrating that there is no inflammatory response, no direct or indirect, acute or chronic alloreactivity, allorecognition, or damage-associated molecular pattern reaction and no autoimmune response to mitochondrial transplantation (30, 42).
In recent experiments, we have shown that an intra-arterial delivery of autologous mitochondria into the coronary arteries of the heart can induce an increase in coronary blood flow (4, 18, 46). The increase in coronary blood flow is unaffected by inhibition of endothelium-derived pathways of coronary vasodilation and is modulated at least in part by inward rectifier potassium channels (46). In the present study, a transient increase in RBF was observed 60 s after single and serial injections of mitochondria. The increase in RBF was not accompanied by significant hemodynamic effects. While it was possible to maintain an increase in the urine output for a longer period when serial injections were performed, GFR was not different when a serial rather than single injection scheme was opted.
Our results demonstrate that renal functional parameters (eGFR, creatinine, BUN, and urine output) all showed significant improvement in AKI in animals treated with an intra-arterial injection of autologous mitochondria after IRI. Due to hypotensive episodes precipitated by inulin injection, the method was not used. For these reasons, we used a validated formula for estimating eGFR based on plasma creatinine and body weight (14).
The effects of mitochondrial transplantation in alleviating the effects of renal IRI are evident. Histological analysis, performed by a blinded observer, showed extensive coagulative necrosis in vehicle-treated kidneys, while only patchy mild acute tubular injury was found in mitochondria-treated kidneys. The protective effect of mitochondrial transplantation was mostly evident in the cortex since this is the area of the kidney with the highest oxygen demand. Electron microscopy confirmed widespread dysmorphic mitochondria in the vehicle-treated group, while the mitochondrial morphology in the mitochondria-treated group was within normal limits.
The results in our study have recently been demonstrated by Jabbari et al. (21) in a rat model of AKI. These authors used our methodology for mitochondrial isolation in a rat model of renal IRI induced by right nephrectomy and 45-min left kidney ischemia followed by 1-wk reperfusion (21). The authors showed that autologous mitochondrial transplantation attenuated renal tissue injury, restored renal function, and enhanced renal cell repair and proliferation (21). While these experiments were performed in a small-rodent model, the results therein agree with our findings.
Several possible clinical scenarios have been identified as possible applications of mitochondrial transplantation by intra-arterial injection for the prevention of renal IRI: cardiovascular events with consequent reduction of renal perfusion; renal transplants with retrieval of the organ from marginal donors, as in the case of donations from circulatory death; cardiac procedures with unexpected cardiocirculatory arrest times; and catheter-based interventional procedures at high risk of renal ischemia.
Our study has some limitations. We used only female animals in our study. This was done so that we could reduce any possible effects related to urinary catheterization, which is less traumatic in female animals than in male animals. We also used only 24-h recovery, and therefore long-term efficacy studies are needed for verification. We also used young, otherwise healthy, animals, thus eliminating confounding variables that may be related to coexisting diseases. Although uptake experiments were performed in healthy kidneys and experiments regarding the mechanism of action were not extensively performed in the present study, these aspects have been thoroughly studied in previous reports in vitro (9, 39) and in vivo in the heart (8, 10, 30, 33, 37, 46), lung (36), skeletal muscle (38), liver (27), and spinal cord (16) as well as in the kidneys in rodents (21).
With these caveats in mind, our results demonstrate that mitochondrial transplantation by intra-arterial injection is safe and effective as a therapy for renal IRI, significantly reducing histological damage and improving renal function. This new technique has considerable potential to reduce morbidity and mortality in patients with AKI. We suggest the results of the present study provide a basis for clinical translation to humans and will enhance the armamentarium of surgeons and clinicians for the treatment of AKI.
GRANTS
This work was supported by the Richard A. and Susan F. Smith President’s Innovation Award, Michael B. Klein and Family, The Sidman Family Foundation, The Michael B. Rukin Charitable Foundation, The Kenneth C. Griffin Charitable Research Fund, The George and Marie Vergottis Foundation, National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK117183, and The Boston Investment Council.
DISCLAIMERS
The authors attest that they had full freedom to explore the data and analyze the results independently of any sponsor and that they had sole authority to make the final decision to submit the material for publication.
DISCLOSURES
P. J. del Nido and J. D. McCully have patents pending for the isolation and usage of mitochondria and are consultants with CellVita Sciences, Inc. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
I.P.D., A.G., S.M.P., I.E.S., P.J.d.N., and J.D.M. conceived and designed research; I.P.D., A.G., T.D., T.K., A.O., M.Y.S., V.H.W., D.B., B.S., E.R.S., T.R., A.B., and I.E.S. performed experiments; I.P.D., A.G., D.Z., and A.B. analyzed data; I.P.D., A.G., E.R.S., J.A.I., A.B.P., S.M.P., I.E.S., P.J.d.N., and J.D.M. interpreted results of experiments; I.P.D., A.G., and E.R.S. prepared figures; I.P.D. and A.G. drafted manuscript; I.P.D., A.G., T.D., T.K., A.O., M.Y.S., V.H.W., D.B., B.S., E.R.S., J.A.I., A.B.P., D.Z., T.R., A.B., S.M.P., I.E.S., P.J.d.N., and J.D.M. edited and revised manuscript; I.P.D., A.G., T.D., T.K., A.O., M.Y.S., V.H.W., D.B., B.S., E.R.S., J.A.I., A.B.P., D.Z., T.R., A.B., S.M.P., I.E.S., P.J.d.N., and J.D.M. approved final version of manuscript.
REFERENCES
- 1.Ali Pour P, Kenney MC, Kheradvar A. Bioenergetics consequences of mitochondrial transplantation in cardiomyocytes. J Am Heart Assoc 9: e014501, 2020. doi: 10.1161/JAHA.119.014501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amdisen C, Keller AK, Hansen RS, Nørregaard R, Krag SP, Møldrup U, Pedersen M, Jespersen B, Birn H. Testing danegaptide effects on kidney function after ischemia/reperfusion injury in a new porcine two week model. PLoS One 11: e0164109, 2016. doi: 10.1371/journal.pone.0164109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol 13: 629–646, 2017. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blitzer D, Guariento A, Doulamis IP, Shin B, Moskowitzova K, Barbieri GR, Orfany A, Del Nido PJ, McCully JD. Delayed transplantation of autologous mitochondria for cardioprotection in a porcine model. Ann Thorac Surg 109: 711–719, 2020. doi: 10.1016/j.athoracsur.2019.06.075. [DOI] [PubMed] [Google Scholar]
- 5.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003. doi: 10.1097/01.ASN.0000079785.13922.F6. [DOI] [PubMed] [Google Scholar]
- 6.Case J, Khan S, Khalid R, Khan A. Epidemiology of acute kidney injury in the intensive care unit. Crit Care Res Pract 2013: 479730, 2013. doi: 10.1155/2013/479730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chomyn A, Martinuzzi A, Yoneda M, Daga A, Hurko O, Johns D, Lai ST, Nonaka I, Angelini C, Attardi G. MELAS mutation in mtDNA binding site for transcription termination factor causes defects in protein synthesis and in respiration but no change in levels of upstream and downstream mature transcripts. Proc Natl Acad Sci USA 89: 4221–4225, 1992. doi: 10.1073/pnas.89.10.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowan DB, Yao R, Akurathi V, Snay ER, Thedsanamoorthy JK, Zurakowski D, Ericsson M, Friehs I, Wu Y, Levitsky S, Del Nido PJ, Packard AB, McCully JD. Intracoronary delivery of mitochondria to the ischemic heart for cardioprotection. PLoS One 11: e0160889, 2016. doi: 10.1371/journal.pone.0160889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowan DB, Yao R, Thedsanamoorthy JK, Zurakowski D, Del Nido PJ, McCully JD. Transit and integration of extracellular mitochondria in human heart cells. Sci Rep 7: 17450, 2017. doi: 10.1038/s41598-017-17813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doulamis IP, Guariento A, Duignan T, Orfany A, Kido T, Zurakowski D, Del Nido PJ, McCully JD. Mitochondrial transplantation for myocardial protection in diabetic hearts. Eur J Cardiothorac Surg 57: 836–845, 2020. doi: 10.1093/ejcts/ezz326. [DOI] [PubMed] [Google Scholar]
- 11.Emani SM, McCully JD. Mitochondrial transplantation: applications for pediatric patients with congenital heart disease. Transl Pediatr 7: 169–175, 2018. doi: 10.21037/tp.2018.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emani SM, Piekarski BL, Harrild D, Del Nido PJ, McCully JD. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J Thorac Cardiovasc Surg 154: 286–289, 2017. doi: 10.1016/j.jtcvs.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Freeman RV, O’Donnell M, Share D, Meengs WL, Kline-Rogers E, Clark VL, DeFranco AC, Eagle KA, McGinnity JG, Patel K, Maxwell-Eward A, Bondie D, Moscucci M; Blue Cross-Blue Shield of Michigan Cardiovascular Consortium (BMC2) . Nephropathy requiring dialysis after percutaneous coronary intervention and the critical role of an adjusted contrast dose. Am J Cardiol 90: 1068–1073, 2002. doi: 10.1016/S0002-9149(02)02771-6. [DOI] [PubMed] [Google Scholar]
- 14.Gasthuys E, Devreese M, Millecam J, Sys S, Vanderperren K, Delanghe J, Vande Walle J, Heyndrickx M, Croubels S. Postnatal maturation of the glomerular filtration rate in conventional growing piglets as potential juvenile animal model for preclinical pharmaceutical research. Front Pharmacol 8: 431, 2017. doi: 10.3389/fphar.2017.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giraud S, Favreau F, Chatauret N, Thuillier R, Maiga S, Hauet T. Contribution of large pig for renal ischemia-reperfusion and transplantation studies: the preclinical model. J Biomed Biotechnol 2011: 532127, 2011. doi: 10.1155/2011/532127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gollihue JL, Patel SP, Eldahan KC, Cox DH, Donahue RR, Taylor BK, Sullivan PG, Rabchevsky AG. Effects of mitochondrial transplantation on bioenergetics, cellular incorporation, and functional recovery after spinal cord injury. J Neurotrauma 35: 1800–1818, 2018. doi: 10.1089/neu.2017.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossini E, Molinari C, Pollesello P, Bellomo G, Valente G, Mary D, Vacca G, Caimmi P. Levosimendan protection against kidney ischemia/reperfusion injuries in anesthetized pigs. J Pharmacol Exp Ther 342: 376–388, 2012. doi: 10.1124/jpet.112.193961. [DOI] [PubMed] [Google Scholar]
- 18.Guariento A, Blitzer D, Doulamis I, Shin B, Moskowitzova K, Orfany A, Ramirez-Barbieri G, Staffa SJ, Zurakowski D, Del Nido PJ, McCully JD. Preischemic autologous mitochondrial transplantation by intracoronary injection for myocardial protection. J Thorac Cardiovasc Surg 160: e15–e29, 2020. doi: 10.1016/j.jtcvs.2019.06.111. [DOI] [PubMed] [Google Scholar]
- 19.Inkster JAH, Zhang S, Akurathi V, Belanger A, Dubey S, Treves T, Packard AB. New chemical and radiochemical routes to [18F]Rho6G-DEG-F, a delocalized lipophilic cation for myocardial perfusion imaging with PET. MedChemComm 8: 1891–1896, 2017. doi: 10.1039/C7MD00326A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishimoto Y, Inagi R. Mitochondria: a therapeutic target in acute kidney injury. Nephrol Dial Transplant 31: 1062–1069, 2016. doi: 10.1093/ndt/gfv317. [DOI] [PubMed] [Google Scholar]
- 21.Jabbari H, Roushandeh AM, Rostami MK, Razavi-Toosi MT, Shokrgozar MA, Jahanian-Najafabadi A, Kuwahara Y, Roudkenar MH. Mitochondrial transplantation ameliorates ischemia/reperfusion-induced kidney injury in rat. Biochim Biophys Acta Mol Basis Dis 1866: 165809, 2020. doi: 10.1016/j.bbadis.2020.165809. [DOI] [PubMed] [Google Scholar]
- 22.Jesinkey SR, Funk JA, Stallons LJ, Wills LP, Megyesi JK, Beeson CC, Schnellmann RG. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J Am Soc Nephrol 25: 1157–1162, 2014. doi: 10.1681/ASN.2013090952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaza AK, Wamala I, Friehs I, Kuebler JD, Rathod RH, Berra I, Ericsson M, Yao R, Thedsanamoorthy JK, Zurakowski D, Levitsky S, Del Nido PJ, Cowan DB, McCully JD. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J Thorac Cardiovasc Surg 153: 934–943, 2017. doi: 10.1016/j.jtcvs.2016.10.077. [DOI] [PubMed] [Google Scholar]
- 24.Konari N, Nagaishi K, Kikuchi S, Fujimiya M. Mitochondria transfer from mesenchymal stem cells structurally and functionally repairs renal proximal tubular epithelial cells in diabetic nephropathy in vivo. Sci Rep 9: 5184, 2019. doi: 10.1038/s41598-019-40163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laskey WK, Jenkins C, Selzer F, Marroquin OC, Wilensky RL, Glaser R, Cohen HA, Holmes DR Jr; NHLBI Dynamic Registry Investigators . Volume-to-creatinine clearance ratio: a pharmacokinetically based risk factor for prediction of early creatinine increase after percutaneous coronary intervention. J Am Coll Cardiol 50: 584–590, 2007. doi: 10.1016/j.jacc.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Nagothu KK, Desai V, Lee T, Branham W, Moland C, Megyesi JK, Crew MD, Portilla D. Transgenic expression of proximal tubule peroxisome proliferator-activated receptor-α in mice confers protection during acute kidney injury. Kidney Int 76: 1049–1062, 2009. doi: 10.1038/ki.2009.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Khouri-Farah N, Wu CH, Wu GY. Targeted delivery of mitochondria to the liver in rats. J Gastroenterol Hepatol. In press. doi: 10.1111/jgh.15091. [DOI] [PubMed] [Google Scholar]
- 28.Malagrino PA, Venturini G, Yogi PS, Dariolli R, Padilha K, Kiers B, Gois TC, da Motta-Leal-Filho JM, Takimura CK, Girardi ACC, Carnevale FC, Zeri ACM, Malheiros DMAC, Krieger JE, Pereira AC. Catheter-based induction of renal ischemia/reperfusion in swine: description of an experimental model. Physiol Rep 2: e12150, 2014. doi: 10.14814/phy2.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin JL, Gruszczyk AV, Beach TE, Murphy MP, Saeb-Parsy K. Mitochondrial mechanisms and therapeutics in ischaemia reperfusion injury. Pediatr Nephrol 34: 1167–1174, 2019. doi: 10.1007/s00467-018-3984-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masuzawa A, Black KM, Pacak CA, Ericsson M, Barnett RJ, Drumm C, Seth P, Bloch DB, Levitsky S, Cowan DB, McCully JD. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 304: H966–H982, 2013. doi: 10.1152/ajpheart.00883.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCully JD, Bhasin MK, Daly C, Guerrero MC, Dillon S, Liberman TA, Cowan DB, Mably JD, McGowan FX, Levitsky S. Transcriptomic and proteomic analysis of global ischemia and cardioprotection in the rabbit heart. Physiol Genomics 38: 125–137, 2009. doi: 10.1152/physiolgenomics.00033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCully JD, Cowan DB, Emani SM, Del Nido PJ. Mitochondrial transplantation: from animal models to clinical use in humans. Mitochondrion 34: 127–134, 2017. doi: 10.1016/j.mito.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 33.McCully JD, Cowan DB, Pacak CA, Toumpoulis IK, Dayalan H, Levitsky S. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Physiol Heart Circ Physiol 296: H94–H105, 2009. doi: 10.1152/ajpheart.00567.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCully JD, Levitsky S, Del Nido PJ, Cowan DB. Mitochondrial transplantation for therapeutic use. Clin Transl Med 5: e16, 2016. doi: 10.1186/s40169-016-0095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moskowitzova K, Orfany A, Liu K, Ramirez-Barbieri G, Thedsanamoorthy JK, Yao R, Guariento A, Doulamis IP, Blitzer D, Shin B, Snay ER, Inkster JAH, Iken K, Packard AB, Cowan DB, Visner GA, Del Nido PJ, McCully JD. Mitochondrial transplantation enhances murine lung viability and recovery after ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol 318: L78–L88, 2020. doi: 10.1152/ajplung.00221.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moskowitzova K, Shin B, Liu K, Ramirez-Barbieri G, Guariento A, Blitzer D, Thedsanamoorthy JK, Yao R, Snay ER, Inkster JAH, Orfany A, Zurakowski D, Cowan DB, Packard AB, Visner GA, Del Nido PJ, McCully JD. Mitochondrial transplantation prolongs cold ischemia time in murine heart transplantation. J Heart Lung Transplant 38: 92–99, 2019. doi: 10.1016/j.healun.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orfany A, Arriola CG, Doulamis IP, Guariento A, Ramirez-Barbieri G, Moskowitzova K, Shin B, Blitzer D, Rogers C, Del Nido PJ, McCully JD. Mitochondrial transplantation ameliorates acute limb ischemia. J Vasc Surg 71: 1014–1026, 2020. doi: 10.1016/j.jvs.2019.03.079. [DOI] [PubMed] [Google Scholar]
- 39.Pacak CA, Preble JM, Kondo H, Seibel P, Levitsky S, Del Nido PJ, Cowan DB, McCully JD. Actin-dependent mitochondrial internalization in cardiomyocytes: evidence for rescue of mitochondrial function. Biol Open 4: 622–626, 2015. doi: 10.1242/bio.201511478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preble JM, Kondo H, Levitsky S, McCully JD. Quality control parameters for mitochondria transplant in cardiac tissue. JSM Biochem Mol Biol 2: 1008, 2014. [Google Scholar]
- 41.Preble JM, Pacak CA, Kondo H, MacKay AA, Cowan DB, McCully JD. Rapid isolation and purification of mitochondria for transplantation by tissue dissociation and differential filtration. J Vis Exp 91: e51682, 2014. doi: 10.3791/51682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramirez-Barbieri G, Moskowitzova K, Shin B, Blitzer D, Orfany A, Guariento A, Iken K, Friehs I, Zurakowski D, del Nido PJ, McCully JD. Alloreactivity and allorecognition of syngeneic and allogeneic mitochondria. Mitochondrion 46: 103–115, 2019. doi: 10.1016/j.mito.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10: 193–207, 2014. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 44.Sekijima M, Sahara H, Miki K, Villani V, Ariyoshi Y, Iwanaga T, Tomita Y, Yamada K. Hydrogen sulfide prevents renal ischemia-reperfusion injury in CLAWN miniature swine. J Surg Res 219: 165–172, 2017. doi: 10.1016/j.jss.2017.05.123. [DOI] [PubMed] [Google Scholar]
- 45.Shin B, Cowan DB, Emani SM, Del Nido PJ, McCully JD. Mitochondrial transplantation in myocardial ischemia and reperfusion injury. Adv Exp Med Biol 982: 595–619, 2017. doi: 10.1007/978-3-319-55330-6_31. [DOI] [PubMed] [Google Scholar]
- 46.Shin B, Saeed MY, Esch JJ, Guariento A, Blitzer D, Moskowitzova K, Ramirez-Barbieri G, Orfany A, Thedsanamoorthy JK, Cowan DB, Inkster JA, Snay ER, Staffa SJ, Packard AB, Zurakowski D, Del Nido PJ, McCully JD. A novel biological strategy for myocardial protection by intracoronary delivery of mitochondria: safety and efficacy. JACC Basic Transl Sci 4: 871–888, 2019. doi: 10.1016/j.jacbts.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoffner JM, Lott MT, Lezza AMS, Seibel P, Ballinger SW, Wallace DC. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell 61: 931–937, 1990. doi: 10.1016/0092-8674(90)90059-N. [DOI] [PubMed] [Google Scholar]
- 48.Steichen C, Giraud S, Bon D, Barrou B, Badet L, Salamé E, Kerforne T, Allain G, Roumy J, Jayle C, Hannaert P, Hauet T, Thuillier R. Barriers and advances in kidney preservation. BioMed Res Int 2018: 9206257, 2018. doi: 10.1155/2018/9206257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoon YE, Lee KS, Choi KH, Kim KH, Yang SC, Han WK. Preconditioning strategies for kidney ischemia reperfusion injury: implications of the “time-window” in remote ischemic preconditioning. PLoS One 10: e0124130, 2015. doi: 10.1371/journal.pone.0124130. [DOI] [PMC free article] [PubMed] [Google Scholar]