Abstract
Interleukin-10 (IL-10) is an anti-inflammatory cytokine that induces nitric oxide (NO) production. IL-10 supplementation has been previously shown to lower blood pressure (BP) in male hypertensive mice, but the effect of exogenous IL-10 in hypertensive female rodents has not been studied. For the present study, we hypothesized that chronic infusion of IL-10 in hypertensive rats would lower BP concomitant with an increase in renal NO synthase (NOS) activity. Male and female spontaneously hypertensive rats (SHRs; 12 wk old) were randomized to receive IL-10 infusion by subcutaneous minipump (3.5 µg·kg−1·day−1) or serve as sham controls (n = 4–6 rats per treatment per sex). BP was measured by tail cuff before and after 2 wk of treatment. Renal T cells and IL-10 were measured by flow cytometry, and NOS activity was determined by conversion of radiolabeled arginine to radiolabeled citrulline. Female SHRs had greater IL-10+ renal cells than male SHRs and greater expression of the IL-10 receptor at baseline. BP did not change in female SHRs treated with IL-10, but BP significantly decreased following IL-10 infusion in male SHRs. Contrary to our hypothesis, NOS enzymatic activity decreased with IL-10 treatment in the renal inner medulla and cortex of both sexes. Renal regulatory T cells also decreased in both sexes after IL-10 treatment. In conclusion, despite male SHRs having less IL-10 and IL-10 receptor expression in the kidney compared with female SHRs, exogenous IL-10 selectively decreased BP only in male SHRs. Furthermore, our data suggest that exogenous IL-10-induced decreases in BP in male SHRs are not dependent on upregulating renal NOS activity.
Keywords: hypertension, nitric oxide synthase, spontaneously hypertensive rats, T cells
INTRODUCTION
Hypertension is a complex disease that currently affects ~80 million adults in the United States (12). Despite the tremendous impact of hypertension on overall morbidity and mortality, efforts to control blood pressure (BP) remain relatively poor, with only ~50% of patients being treated for hypertension attaining adequate BP control (5, 12). Although both sexes develop hypertension, young women are protected from hypertension relative to age-matched men. A critical barrier to improving BP control rates is the lack of knowledge regarding the molecular mechanisms regulating BP in either sex.
Studies over the past decade have confirmed an important role for the immune system in the development of hypertension. Specifically, studies have reported an antihypertensive role for the anti-inflammatory cytokine interleukin-10 (IL-10). Male IL-10−/− knockout mice have a greater increase in BP in response to angiotensin II treatment compared with wild-type mice (11). In the same study (11), subcutaneous IL-10 infusion via osmotic minipumps in wild-type male mice was reported to blunt the rise in BP in response to angiotensin II. IL-10 induces the production of nitric oxide (NO), a potent vasodilator in the vasculature (3). Consistent with this, male IL-10−/− knockout mice have significantly reduced vascular relaxation and increased superoxide production in response to angiotensin II treatment compared with wild-type controls (6). These studies support a protective role for IL-10 in male hypertensive animal models, but studies have not yet directly explored potential sex differences in IL-10 in models of experimental hypertension.
Despite the lack of hypertension studies in female IL-10−/− mice or hypertensive female rodents receiving exogenous IL-10 treatment, there are studies in the literature that support an important role for IL-10 in BP control in female rodents. Female rats with mechanically induced preeclampsia, a form of pregnancy-induced hypertension, treated with exogenous IL-10 infused intraperitoneally via osmotic minipumps exhibit a significant reduction in BP (8). Furthermore, previous studies from our laboratory have reported a sex difference in IL-10 levels in both the spontaneously hypertensive rat (SHR) and angiotensin II models of hypertension, with female subjects having higher levels of renal IL-10 and lower BP compared with male subjects (20, 21). We have also previously reported a sex difference in the abundance of renal regulatory T cells (Tregs; anti-inflammatory T cells that secrete IL-10) in the angiotensin II and SHR models of hypertension (20, 21). Therefore, we propose that IL-10 contributes to the lower BP observed in female rats and that female rats will also respond to exogenous IL-10 treatment as has previously been shown in male hypertensive models. The present study was designed to test the hypothesis that treatment with IL-10 in SHRs will lower BP concomitant with an increase in renal NO synthase (NOS) activity.
METHODS
Animals.
Male and female SHRs (Envigo, Indianapolis, IN) were studied. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved and monitored by the Augusta University Institutional Animal Care and Use Committee. Animals were housed in a controlled environment with constant temperature and humidity along with a fixed 12:12-h light-dark cycle. All rats had access to standard rat chow and water ad libitum.
In initial experiments, 12-wk-old SHRs were used to assess baseline differences in IL-10+ cells (n = 5 male and 7 female rats) and IL-10 receptor expression (n = 6 male and 6 female rats) in the kidney.
In separate 11-wk-old male and female SHRs, tail cuff plethysmography (IITC Life Sciences, Woodland Hills, CA) was used to measure systolic BP (SBP) as previously described (18). Briefly, animals were trained to receive tail cuff measurements in three sessions before the collection of baseline measurements. Rats were placed in a restrainer in a warming chamber and allowed to acclimate for 10 min before SBP measurements. For each rat, the reported SBP is the average of 5 readings/session.
After baseline tail cuff measurements, rats were randomized to receive either IL-10 treatment (n = 6 male and 6 female rats) or sham surgery (n = 4 male and 6 female rats). IL-10 treatment was delivered by subcutaneous osmotic minipump (model 2002, Alzet, Cupertino, CA) at a dose of 3.5 µg·kg−1·day−1 for 2 wk. Osmotic minipumps (3 cm long × 0.7 cm wide) were placed subcutaneously between the shoulder blades of the rat. Rats that received the sham surgery had a small subcutaneous pouch formed but did not receive an osmotic minipump. Final tail cuff SBP measurements were recorded at the end of the 2-wk treatment period.
Urinary measurements.
Rats were placed in 24-h metabolic cages at baseline, after 1 wk of treatment, and after 2 wk of treatment to collect 24-h urine samples. Body weight, food intake, and water intake were also recorded at each time point to assess if the treatment impacted any of these metabolic parameters. Urinary protein excretion was determined by standard Bradford assay (Bio-Rad, Hercules, CA).
Plasma IL-10 measurements.
Terminal plasma samples were collected from the abdominal aorta at the end of the 2-wk treatment period. Plasma IL-10 was assessed via commercially available IL-10 Quantikine ELISA (catalog no. R1000, R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Western blot analysis.
Kidneys were snap frozen, and 50 mg of the renal cortex were isolated and homogenized using a glass homogenizer in 50 mM Tris buffer (containing 0.1 mM EDTA, 0.1 mM EGTA, 0.1% β-mercaptoethanol, and 10% glycerol at pH to 7.4) in the presence of protease inhibitors (0.0.1 M PMSF, 2 mM leupeptin, 0.1% aprotinin, and 2 mM pepstatin A). The whole homogenate was used in standard Western blot analysis using 8% SDS-polyacrylamide gels run on a Bio-Rad Powerpac 300 at ~120 V. Proteins were then transferred onto polyvinylidene difluoride membranes using a Bio-Rad Powerpac 200 at 100 V for 1 h. Membranes were then blocked in a 1:1 mixture of LI-COR blocking buffer and 1× Tris-buffered saline for 1 h. Protein expression was determined using two-color immunoblots using primary antibodies to the IL-10 receptor α-subunit (catalog no. AF-474, Novus Biologicals, molecular weight: 64 kDa, 50 μg protein/well) and β-actin (A1978, Sigma, St. Louis, MO, 1:10,000). Protein concentrations were determined by standard Bradford assay (Bio-Rad) using BSA as the standard. β-Actin was used to verify equal protein loading, and data are reported normalized to β-actin.
Flow cytometry.
Kidneys were collected after 2 wk of treatment for flow cytometric analysis of the T cell profile as previously described (19). Briefly, whole kidneys were processed into single cell suspensions by mincing the kidney followed by mechanically passing the pieces through 100-µm cell strainers (Fisher Scientific) and then centrifuged (1,500 rpm, 10 min at 4°C). Cells were treated with ammonium-Cl−-K+ lysis buffer (catalog no. A1049201, ThermoFisher), washed, and centrifuged (1,500 rpm, 10 min at 4°C). Cells were stained with cell surface antibodies for CD3 (catalog no. 46-0030-82, Thermo Scientific) and CD4 (catalog no. 554837, Thermo Scientific) for 15 min on ice in the dark followed by a wash. Cells were then fixed and permeabilized (Fix/Perm concentrate, eBioScience, San Diego, CA) and then stained with intracellular antibodies for IL-10 (catalog no. NB100-63026AF647, Novus Biologicals), FoxP3 (catalog no. 17-5773-82, Thermo Scientific), or RAR-related orphan receptor-γ (RORγ; catalog no. IC6006P, R&D Systems) to identify IL-10+ cells (IL-10+), Tregs (CD3+CD4+FoxP3+), and T helper (Th)17 cells (CD3+CD4+RORγ+), respectively. Cells were run on a four-color flow cytometer (FACS Calibur, BD Biosciences) with 20,000 events of total kidney cells recorded per sample. Data were analyzed using Cell Quest software. Single color controls were used to set compensation, and gating was set to exclude all dead cells and debris from the total renal cells.
NOS activity.
NOS activity was assessed by measuring the conversion of radiolabeled arginine to radiolabeled citrulline in the renal cortex and renal inner medulla as previously described (16, 17). Briefly, renal cortex samples were homogenized, and total NOS protein was partially purified from the supernatant fraction using 2′,5′-ADP Sepharose as previously described (14). The partially purified cortex samples and homogenized inner medulla aliquots were incubated with [3H]arginine in the presence of 1 mM NADPH, 30 nM calmodulin, 3 µM tetrahydrobiopterin, 2 mM CaCl2, 1 µM FAD, and 1 µM flavin mononucleotide for a final volume of 50 µL. Additional aliquots were incubated with the nonselective NOS inhibitor Nω-nitro-l-arginine (l-NNA; 1 mM). After a 30-min incubation at room temperature, the reaction was terminated with HEPES buffer (pH 5.5), the reactions were run on 1-mL Dowex AG 50WX-8 columns (Na form, Bio-Rad), and [3H]citrulline was eluted with water. The eluted radioactivity was then quantitated by liquid scintillation counting (Beckman 6500, Beckman-Coulter Instruments, Indianapolis, IN).
Total NOS activity was defined as the [3H]arginine to [3H]citrulline conversion that was inhibited by l-NNA. Therefore, NOS activity was calculated using the following formula: total NOS activity = (picomoles citrilline in the absence of l-NNA) − (picomoles citrilline in the presence of l-NNA). NOS activity was expressed as picomoles of NOS activity per 30 min per mg protein in the kidney tissue.
Statistical analysis.
All data are expressed as means ± SE. For all comparisons, P < 0.05 was considered statistically significant. Renal IL-10 and IL-10 receptor expression data were analyzed by Student’s t test. Urinary protein excretion and BP data were analyzed by repeated-measures ANOVA. Plasma IL-10 levels, NOS activity, and flow cytometry data were analyzed by two-way ANOVA. Analyses were performed using GraphPad Prism version 7.0 software (GraphPad Prism Software, La Jolla, CA).
RESULTS
Metabolic parameters.
Rats were placed in metabolic cages at baseline, after 1 wk of treatment, and after 2 wk of treatment at the end of the study to determine if IL-10 treatment had any effect on metabolic parameters compared with controls (Table 1). IL-10 treatment had no effect on body weight, water intake, food intake, or urine output at any time point.
Table 1.
Metabolic data for male and female spontaneously hypertensive rats with and without IL-10 treatment
| Male |
Female |
Two-Way ANOVA P Values |
|||
|---|---|---|---|---|---|
| Control (n = 4) |
IL-10 (n = 6) |
Control (n = 6) |
IL-10 (n = 6) |
||
| Baseline | |||||
| Body weight, g | 288.50 ± 6.62 | 289.67 ± 5.21 | 179.83 ± 4.67 | 181.00 ± 6.78 | Interaction: P > 0.99 Treatment: P = 0.84 Sex: P < 0.01* |
| Water intake, mL | 33.67 ± 2.97 | 33.05 ± 1.52 | 37.18 ± 7.07 | 26.98 ± 2.86 | Interaction: P = 0.26 Treatment: P = 0.21 Sex: P = 0.76 |
| Food intake, g | 20.08 ± 2.42 | 19.03 ± 1.44 | 11.58 ± 0.93 | 10.28 ± 0.81 | Interaction: P = 0.94 Treatment: P = 0.45 Sex: P < 0.01* |
| Urine output, mL | 14.93 ± 0.92 | 14.10 ± 1.05 | 8.60 ± 2.25 | 9.85 ± 1.42 | Interaction: P = 0.50 Treatment: P = 0.89 Sex: P < 0.01* |
| After 1 wk of treatment | |||||
| Body weight, g | 299.5 ± 6.82 | 301.25 ± 4.34 | 187.42 ± 5.05 | 183.83 ± 4.04 | Interaction: P = 0.61 Treatment: P = 0.86 Sex: P < 0.01* |
| Water intake, mL | 53.97 ± 2.54 | 52.73 ± 4.44 | 45.27 ± 3.11 | 39.7 ± 3.37 | Interaction: P = 0.54 Treatment: P = 0.33 Sex: P < 0.01* |
| Food intake, g | 27.48 ± 1.18 | 24.67 ± 1.36 | 17.42 ± 1.26 | 18.28 ± 1.72 | Interaction: P = 0.20 Treatment: P = 0.49 Sex: P < 0.01* |
| Urine output, mL | 25.68 ± 2.74 | 27.70 ± 3.53 | 17.82 ± 1.94 | 15.30 ± 1.21 | Interaction: P = 0.38 Treatment: P = 0.92 Sex: P < 0.01* |
| After 2 wk of treatment | |||||
| Body weight, g | 321.25 ± 8.21 | 311.50 ± 5.40 | 188.67 ± 4.31 | 187.33 ± 4.72 | Interaction: P = 0.48 Treatment: P = 0.36 Sex: P < 0.01* |
| Water intake, mL | 46.40 ± 6.98 | 45.32 ± 4.83 | 41.70 ± 3.82 | 33.28 ± 3.87 | Interaction: P = 0.48 Treatment: P = 0.36 Sex: P = 0.12 |
| Food intake, g | 25.45 ± 1.05 | 24.73 ± 0.80 | 17.57 ± 1.52 | 16.78 ± 1.26 | Interaction: P = 0.98 Treatment: P = 0.56 Sex: P < 0.01* |
| Urine output, mL | 16.25 ± 1.87 | 16.33 ± 2.57 | 15.42 ± 2.33 | 14.62 ± 1.56 | Interaction: P = 0.85 Treatment: P = 0.88 Sex: P = 0.58 |
Values are means ± SE. Metabolic data at baseline, after 1 wk of treatment, and after 2 wk of treatment in male and female control and interleukin-10 (IL-10)-treated spontaneously hypertensive rats are shown. Rats were placed in metabolic cages for 24 h at three time points to collect metabolic data, including body weight, water intake, food intake, and urine output. Data were analyzed by two-way ANOVA at each time point and are presented as the effect of treatment, effect of sex, and interaction of sex and treatment.
P < 0.05.
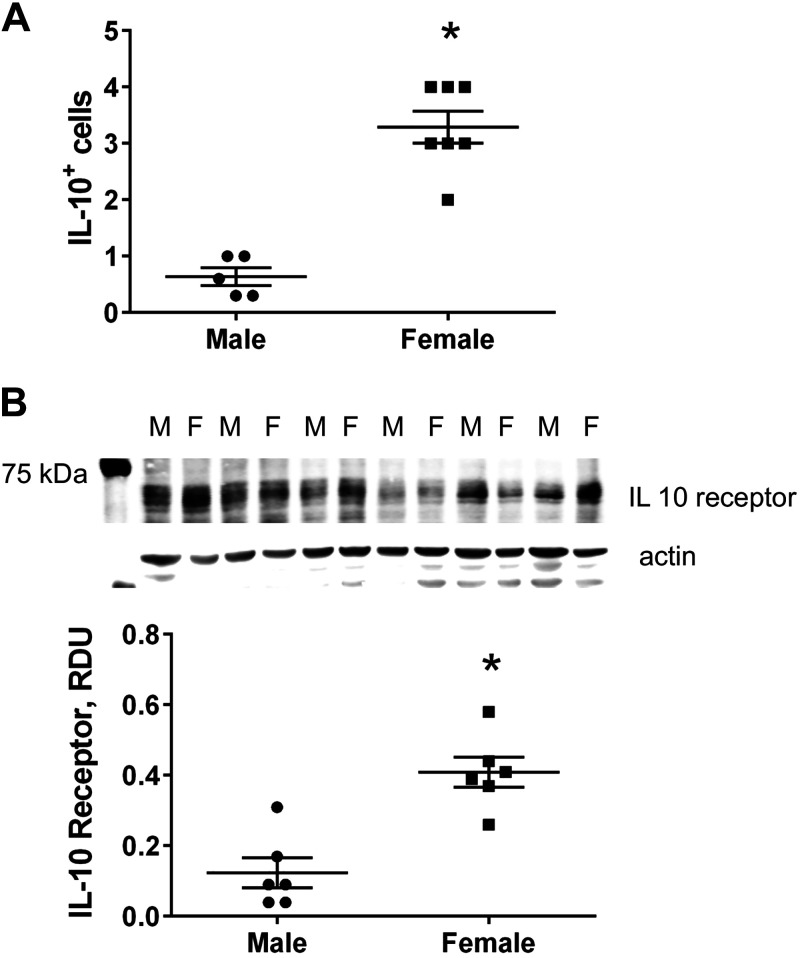
Female SHRs have more IL-10+ cells and greater IL-10 receptor expression in the kidney compared with male SHRs.
Initial experiments measured the percentage of IL-10+ cells and IL-10 receptor expression in the kidney of untreated male and female SHRs at 12 wk of age (Fig. 1). Female SHRs had significantly more renal IL-10+ cells than male SHRs as determined by flow cytometric analysis (n = 5–7, P < 0.01; Fig. 1A). Female SHRs also had significantly greater expression of the IL-10 receptor in the kidney compared with male SHRs (n = 6, P < 0.01; Fig. 1B).
Fig. 1.
Sex differences in renal interleukin-10 (IL-10)+ cells and IL-10 receptor expression in 12-wk-old spontaneously hypertensive rats (SHRs). A: total renal IL-10+ cells were determined by flow cytometry in male (M) and female (F) SHRs at 12 wk of age (n = 5–7, *P < 0.05 by Student’s t test). B: IL-10 receptor expression was quantified using Western blot analysis in the renal cortex from male and female SHRs at 12 wk of age (n = 6, *P < 0.05 by Student’s t test). Individual data points are plotted, with bars representing means ± SE. RDU, relative densitometric units.
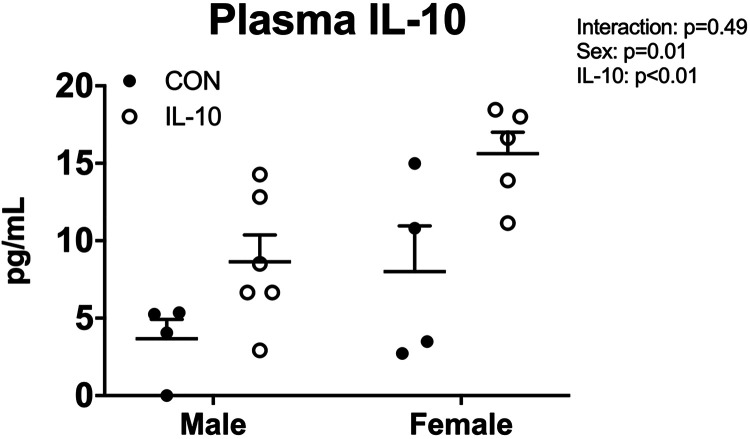
IL-10 treatment increased plasma IL-10 in male and female SHRs.
To ensure that exogenous infusion of IL-10 via osmotic minipumps increased circulating IL-10, plasma IL-10 levels were assessed at the end of the study in control and treated male and female SHRs (Fig. 2). Female SHRs had more IL-10 than male SHRs (n = 4–6, two-way ANOVA sex effect P = 0.01; Fig. 2). Plasma IL-10 was significantly increased in both treated male and female SHRs (n = 4–6, two-way ANOVA treatment effect P < 0.01).
Fig. 2.
Interleukin-10 (IL-10) treatment increases plasma IL-10 in male and female spontaneously hypertensive rats. Plasma IL-10 levels were assessed via ELISA in male and female spontaneously hypertensive rats at the end of the study (n = 4–6, two-way ANOVA). Individual data plots are plotted, with bars representing means ± SE. CON, control.
IL-10 treatment decreased BP in male SHRs but not female SHRs, with no change in urinary protein excretion.
BP and urinary protein excretion were measured to determine the effect of exogenous IL-10 treatment on BP and proteinuria. At baseline, male SHRs had significantly higher SBP than female SHRs (n = 4–6, two-way ANVOA sex effect P < 0.001; Fig. 3A). After 2 wk of treatment, SBP increased in female control and IL-10-treated rats to a similar extent. In contrast, male SHRs exhibited a significant decrease in BP with IL-10 treatment compared with male control SHRs (two-way ANOVA treatment effect P = 0.037). Male SHRs also had significantly higher urinary protein excretion throughout the study compared with female SHRs (n = 4–6, two-way ANOVA sex effect P < 0.01; Fig. 3B), but IL-10 treatment had no effect on urinary protein excretion in either sex.
Fig. 3.
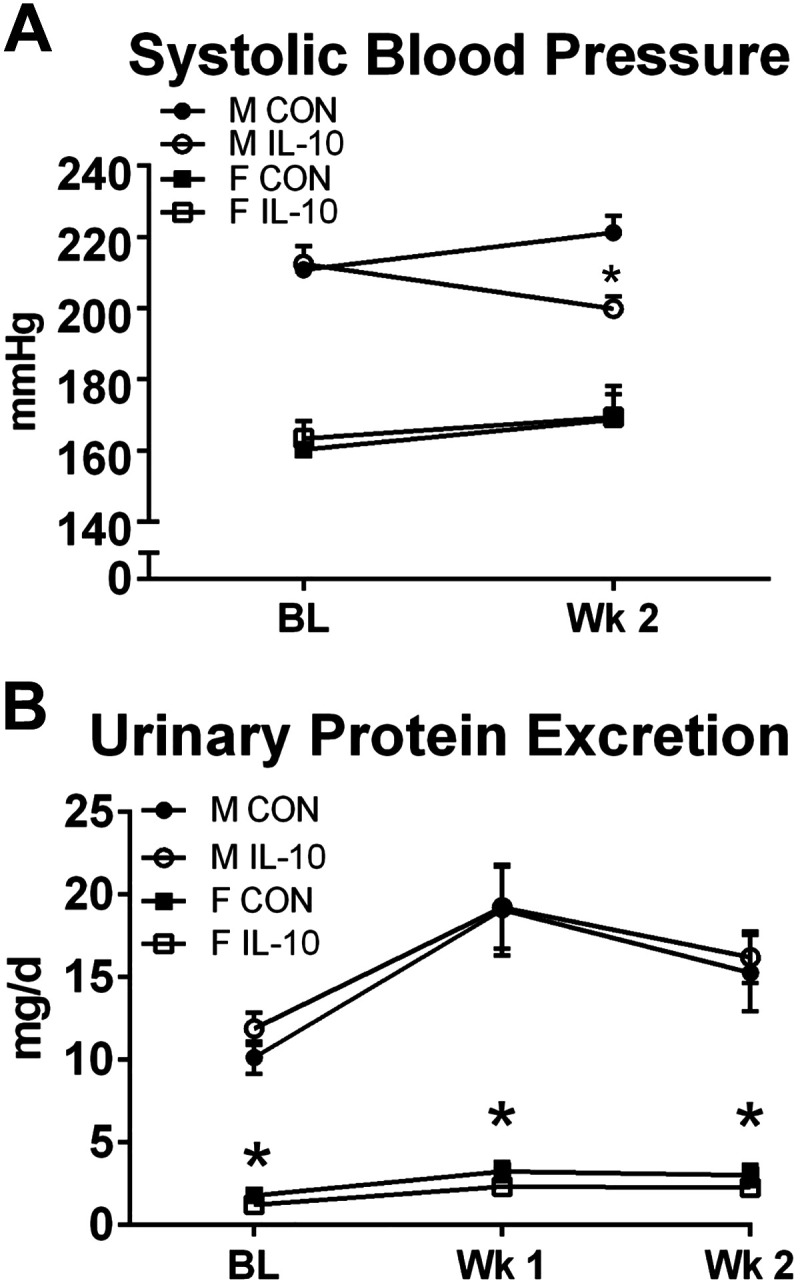
Interleukin-10 (IL-10) treatment decreases systolic blood pressure in male spontaneously hypertensive rats (SHRs) but not female SHRs, with no change in urinary protein excretion. A: systolic blood pressure was measured by tail cuff plethysmography at baseline (BL) and after 2 wk of treatment (Wk 2) in male and female SHRs with IL-10 treatment or surgical sham control (CON; n = 4–6, *P < 0.05 vs. male CON rats by two-way ANOVA). B: urinary protein excretion was determined by Bradford assay in male and female SHRs with IL-10 treatment or surgical sham CON at baseline (BL), after 1 wk of treatment (Wk 1), or after 2 wk of treatment (Wk 2; n = 4–6, *P < 0.05 sex effect by two-way ANOVA). Values are expressed as means ± SE.
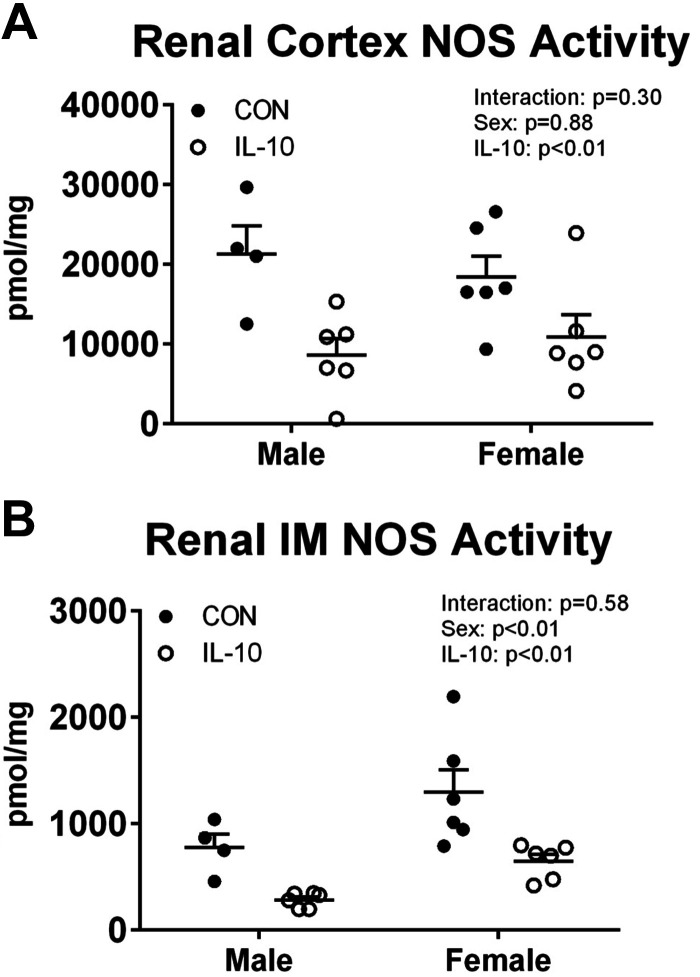
Renal NOS activity decreased with IL-10 treatment in male and female SHRs.
NOS enzymatic activity was determined in the renal cortex and inner medulla to determine the effect of exogenous IL-10 treatment on renal NOS. A previous study (17) from our laboratory reported that the renal outer medulla has relatively little NOS activity compared with the renal cortex and inner medulla and was therefore not measured in the present study. Control male and female SHRs had similar levels of NOS activity in the renal cortex (n = 4–6, two-way ANOVA sex effect P = 0.91; Fig. 4A). IL-10 treatment significantly decreased NOS activity in the renal cortex to a similar extent in both sexes (two-way ANOVA treatment effect P < 0.01). Control female SHRs had greater NOS activity in the renal inner medulla than control male SHRs (n = 4–6, two-way ANOVA sex effect P < 0.01; Fig. 4B), and IL-10 treatment decreased NOS activity in both sexes (two-way ANOVA treatment effect P < 0.01).
Fig. 4.
Interleukin-10 (IL-10) treatment decreases nitric oxide synthase (NOS) activity in the renal inner medulla and cortex of male and female spontaneously hypertensive rats. Renal NOS enzymatic activity was determined by the conversion of radiolabeled arginine to radiolabeled citrulline in the partially purified renal cortex (A; n = 4–6, two-way ANOVA) or in homogenized renal inner medulla (IM) samples (B; n = 4–6, two-way ANOVA) from male and female spontaneously hypertensive rats with IL-10 treatment or surgical sham control (CON). Individual data points are plotted, with bars representing means ± SE.
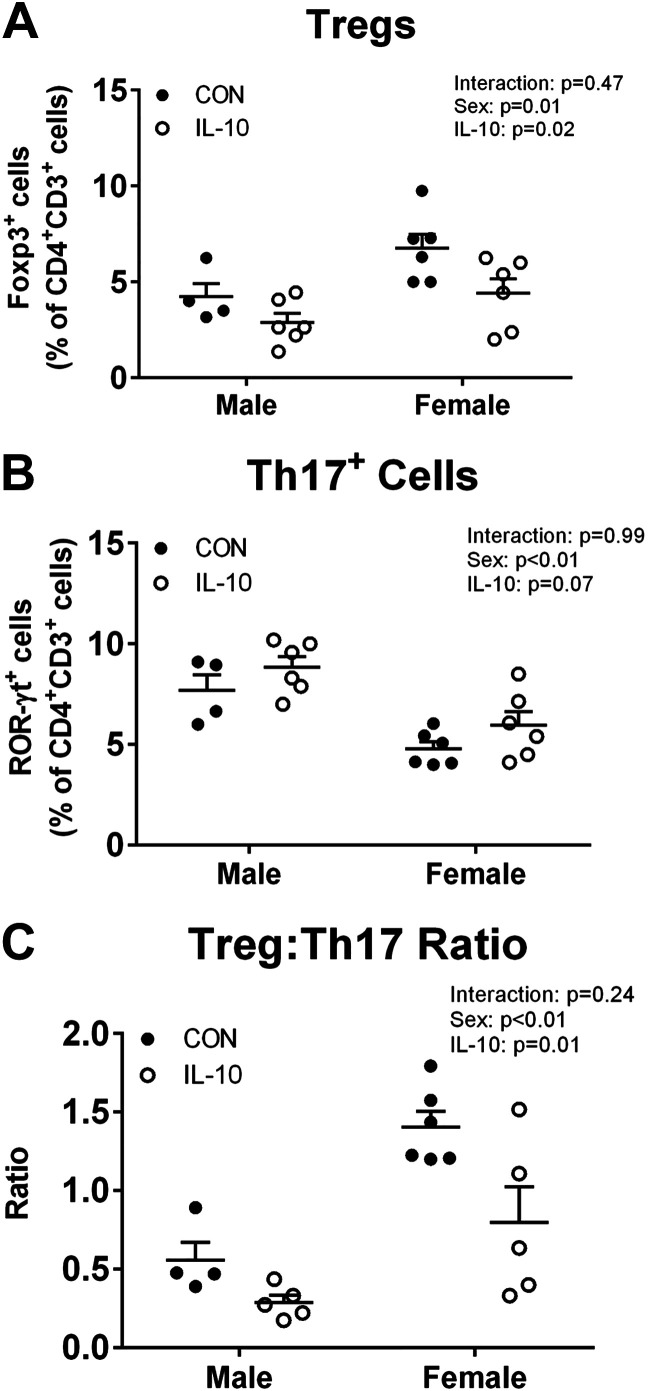
IL-10 treatment deceased renal Tregs in both sexes, leading to a more proinflammatory renal environment.
To determine the effect of exogenous IL-10 on the renal T cell profile, flow cytometry was performed on kidney samples at the end of the 2-wk treatment period. As previously reported by our laboratory, control female SHRs had significantly more renal Tregs (n = 4–6, two-way ANOVA sex effect P = 0.01; Fig. 5A) and fewer renal Th17 cells (n = 4–6, two-way ANOVA sex effect P < 0.01; Fig. 5B) than control male SHRs, resulting in a more anti-inflammatory renal T cell profile (n = 4–6, two-way ANOVA sex effect P = 0.01; Fig. 5C). IL-10 treatment decreased Tregs in both sexes (n = 6, two-way ANOVA treatment effect P = 0.02; Fig. 5A), with no change in Th17 cells (n = 6, two-way ANOVA treatment effect P = 0.07; Fig. 5B). Despite no increase in proinflammatory Th17 cells, this resulted in a significant increase in the proinflammatory T cell profile when assessed by the Treg-to-Th17 ratio (n = 6, two-way ANOVA treatment effect P < 0.01; Fig. 5C).
Fig. 5.
Interleukin-10 (IL-10) treatment increases the proinflammatory profile of renal T cells in male and female spontaneously hypertensive rats (SHRs). T cell profiles were analyzed by flow cytometry to determine the abundance of FoxP3+ regulatory T cells (Tregs; A; n = 4–6, two-way ANOVA) and RAR-related orphan receptor-γ+ T helper (Th)17 cells (B; n = 4–6, two-way ANOVA) in male and female SHRs with IL-10 treatment or surgical sham control (CON). The ratio of Tregs to Th17 cells was calculated to assess the proinflammatory profile in the kidney in IL-10 treated or surgical sham CON male and female SHRs (C; n = 4–6, two-way ANOVA). Individual data points are plotted, with bars representing means ± SE.
DISCUSSION
Studies over the past decade have established a critical role for the immune system in the control of BP. To further examine the role of the anti-inflammatory cytokine IL-10 on BP control and renal NOS activity, we treated male and female SHRs with exogenous IL-10 for 2 wk. The major findings of the present study are that IL-10 treatment in male and female SHRs resulted in 1) a sex-specific decrease in BP in male, but not female, SHRs; 2) a decrease in renal NOS activity in both sexes; and 3) an increase in the proinflammatory renal T cell profile in both sexes. These data support a sex-specific role for IL-10 in BP control, although the mechanism remains unclear.
In the present study, we report that 2 wk of exogenous IL-10 treatment in male SHRs significantly decreased BP, consistent with multiple studies that have reported the beneficial effects of IL-10 treatment in hypertensive male animal models (11, 13, 15). Male stroke-prone SHRs with sustained increases in IL-10 expression via adeno-associated virus vector have lower BP and reductions in markers of end-organ damage compared with control stroke-prone SHRs (13), and male SHR that received intracerebroventricular IL-10 infusions also had a significant reduction in BP (15). However, the present study is the first to also examine the impact of exogenous IL-10 infusion on BP in a female experimental hypertensive model. There is evidence in the pregnancy literature to suggest that IL-10 is antihypertensive in female subjects as well; IL-10 treatment decreases BP in both mouse (4) and rat (8) models of preeclampsia while also having beneficial effects on the immune cell profile. However, in the present study, we did not observe any effect on BP with exogenous IL-10 treatment in nonpregnant hypertensive female rats.
Although exogenous IL-10 treatment did not have an effect on BP in female SHRs in the present study, there is evidence that sex differences in endogenous IL-10 may contribute to sex differences in BP. Our laboratory has previously reported that hypertensive female SHRs (20) and female Sprague-Dawley rats treated with angiotensin II (21) have higher levels of renal IL-10 than male rats along with significantly lower BP. Studies in the RAG1−/− knockout mouse treated with angiotensin II have also reported an increase in IL-10 with adoptive transfer of female T cells, resulting in an attenuation in BP compared with adoptive transfer of male T cells (10). Therefore, IL-10 levels may already be sufficiently high enough in female subjects that further increases do not alter BP. Although BP in female subjects is not altered by exogenous IL-10 infusion, female subjects may be more dependent on endogenous IL-10 to maintain a lower BP compared with male subjects. To test this hypothesis, future studies will assess the contribution of endogenous IL-10 on BP control by neutralizing IL-10.
To begin to gain mechanistic insight into how IL-10 decreases BP in hypertension, we further assessed renal NOS activity. Experiments were focused on the kidney due to the central role of the kidney in the long-term control of BP. One proposed mechanism by which IL-10 reduces BP in male subjects is by increasing NO. Exogenous IL-10 treatment has been previously reported to increase NO in vitro (9) and prevent angiotensin II-induced vascular dysfunction in vivo (11). However, in the present study, we found a decrease in renal NOS activity in both sexes following treatment with IL-10. A limitation of the present study is that we did not directly measure circulating or renal NO; the citrulline assay reflects the potential of the NOS enzyme to increase NO and is not a reflection of actual NO bioavailability. Additionally, we did not measure vascular NOS. Therefore, we can’t rule out an effect of IL-10 infusion on NOS in additional tissue beds. Regardless, the present study does not support the hypothesis that exogenous IL-10 infusion increases renal NOS activity.
Since we did not find an increase in renal NOS with exogenous IL-10, we further examined the renal T cell profile. IL-10 is secreted by anti-inflammatory Tregs, and Treg adoptive transfer has been reported to lower BP in male mice with angiotensin II-induced hypertension (1). Exogenous IL-10 has previously been shown to increase Tregs in a rodent model of mechanically induced preeclampsia (8), and in the present study, we predicted that IL-10 treatment would increase Tregs in male and female SHRs. However, we report that exogenous IL-10 treatment decreased renal Tregs, with no significant change in renal Th17 cells, resulting in an overall increase in the proinflammatory T cell profile in SHR kidneys of both sexes. Consistent with our finding, there is evidence that IL-10 secreted by T cells can inhibit Th1 cell differentiation, acting as a negative feedback loop to decrease Tregs (7). Female SHRs have a greater abundance of Tregs than male SHRs, and we have previously proposed that Tregs contribute to the sex difference in BP (20). We have also recently published that hypertensive female subjects are more dependent on Tregs for BP control relative to male subjects (2). Therefore, the decrease in renal Tregs with IL-10 treatment may account for the lack of a BP response in female SHRs in the present study.
In conclusion, the present study found that exogenous IL-10 reduces BP in male, but not female, SHRs. Contrary to our hypothesis, this reduction in BP was not due to increases in renal NOS activity or anti-inflammatory Tregs. Additional studies are needed to further elucidate the mechanisms by which exogenous IL-10 results is a sex-specific decrease in BP.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01HL127091 (to J. C. Sullivan) and American Heart Association Grants EIA33410565 (to J. C. Sullivan) and POST34030252 (to E. E. Gillis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.E.G. and J.C.S. conceived and designed research; E.E.G. and J.B.M. performed experiments; E.E.G., J.B.M., and B.B. analyzed data; E.E.G. and J.C.S. interpreted results of experiments; E.E.G. prepared figures; E.E.G. and J.C.S. drafted manuscript; E.E.G., J.B.M., B.B., and J.C.S. edited and revised manuscript; E.E.G., J.B.M., B.B., and J.C.S. approved final version of manuscript.
REFERENCES
- 1.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57: 469–476, 2011. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 2.Belanger KM, Crislip GR, Gillis EE, Abdelbary M, Musall JB, Mohamed R, Baban B, Elmarakby A, Brands MW, Sullivan JC. Greater T regulatory cells in females attenuate DOCA-salt-induced increases in blood pressure versus males. Hypertension 75: 1615–1623, 2020. doi: 10.1161/HYPERTENSIONAHA.119.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cattaruzza M, Słodowski W, Stojakovic M, Krzesz R, Hecker M. Interleukin-10 induction of nitric-oxide synthase expression attenuates CD40-mediated interleukin-12 synthesis in human endothelial cells. J Biol Chem 278: 37874–37880, 2003. doi: 10.1074/jbc.M301670200. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee P, Chiasson VL, Seerangan G, Tobin RP, Kopriva SE, Newell-Rogers MK, Mitchell BM. Cotreatment with interleukin 4 and interleukin 10 modulates immune cells and prevents hypertension in pregnant mice. Am J Hypertens 28: 135–142, 2015. doi: 10.1093/ajh/hpu100. [DOI] [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee . The seventh report of the joint national committee on prevention, detection, evaluation and treatment of high blood pressure: the JNC 7 report. JAMA 289: 2560–2572, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension 54: 619–624, 2009. doi: 10.1161/HYPERTENSIONAHA.109.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabryšová L, Nicolson KS, Streeter HB, Verhagen J, Sabatos-Peyton CA, Morgan DJ, Wraith DC. Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10-secreting Th1 cells. J Exp Med 206: 1755–1767, 2009. doi: 10.1084/jem.20082118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harmon A, Cornelius D, Amaral L, Paige A, Herse F, Ibrahim T, Wallukat G, Faulkner J, Moseley J, Dechend R, LaMarca B. IL-10 supplementation increases Tregs and decreases hypertension in the RUPP rat model of preeclampsia. Hypertens Pregnancy 34: 291–306, 2015. doi: 10.3109/10641955.2015.1032054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs F, Chaussabel D, Truyens C, Leclerq V, Carlier Y, Goldman M, Vray B. IL-10 up-regulates nitric oxide (NO) synthesis by lipopolysaccharide (LPS)-activated macrophages: improved control of Trypanosoma cruzi infection. Clin Exp Immunol 113: 59–64, 1998. doi: 10.1046/j.1365-2249.1998.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE, Sandberg K. Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension 64: 573–582, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lima VV, Zemse SM, Chiao CW, Bomfim GF, Tostes RC, Clinton Webb R, Giachini FR. Interleukin-10 limits increased blood pressure and vascular RhoA/Rho-kinase signaling in angiotensin II-infused mice. Life Sci 145: 137–143, 2016. doi: 10.1016/j.lfs.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics−2015 update: a report from the American Heart Association. Circulation 131: e29–e322, 2015. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 13.Nomoto T, Okada T, Shimazaki K, Yoshioka T, Nonaka-Sarukawa M, Ito T, Takeuchi K, Katsura KI, Mizukami H, Kume A, Ookawara S, Ikeda U, Katayama Y, Ozawa K. Systemic delivery of IL-10 by an AAV vector prevents vascular remodeling and end-organ damage in stroke-prone spontaneously hypertensive rat. Gene Ther 16: 383–391, 2009. doi: 10.1038/gt.2008.151. [DOI] [PubMed] [Google Scholar]
- 14.Pollock JS, Nakane M, Förstermann U, Murad F. Particulate and soluble bovine endothelial nitric oxide synthases are structurally similar proteins yet different from soluble brain nitric oxide synthase. J Cardiovasc Pharmacol 20, Suppl 12: S50–S53, 1992. doi: 10.1097/00005344-199204002-00015. [DOI] [PubMed] [Google Scholar]
- 15.Segiet A, Smykiewicz P, Kwiatkowski P, Żera T. Tumour necrosis factor and interleukin 10 in blood pressure regulation in spontaneously hypertensive and normotensive rats. Cytokine 113: 185–194, 2019. doi: 10.1016/j.cyto.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan JC, Giulumian AD, Pollock DM, Fuchs LC, Pollock JS. Functional NOS 1 in the rat mesenteric arterial bed. Am J Physiol Heart Circ Physiol 283: H658–H663, 2002. doi: 10.1152/ajpheart.00073.2002. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan JC, Pardieck JL, Hyndman KA, Pollock JS. Renal NOS activity, expression, and localization in male and female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 298: R61–R69, 2010. doi: 10.1152/ajpregu.00526.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor LE, Gillis EE, Musall JB, Baban B, Sullivan JC. High-fat diet-induced hypertension is associated with a proinflammatory T cell profile in male and female Dahl salt-sensitive rats. Am J Physiol Heart Circ Physiol 315: H1713–H1723, 2018. doi: 10.1152/ajpheart.00389.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am J Physiol Regul Integr Comp Physiol 303: R359–R367, 2012. doi: 10.1152/ajpregu.00246.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension 64: 557–564, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmerman MA, Baban B, Tipton AJ, O’Connor PM, Sullivan JC. Chronic ANG II infusion induces sex-specific increases in renal T cells in Sprague-Dawley rats. Am J Physiol Renal Physiol 308: F706–F712, 2015. doi: 10.1152/ajprenal.00446.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]