Abstract
Spasticity is one of the most common symptoms present in humans with spinal cord injury (SCI); however, its clinical assessment remains underdeveloped. The purpose of the study was to examine the contribution of passive muscle stiffness and active spinal reflex mechanisms to clinical outcomes of spasticity after SCI. It is important that passive and active contributions to increased muscle stiffness are distinguished to make appropriate decisions about antispastic treatments and to monitor its effectiveness. To address this question, we combined biomechanical and electrophysiological assessments of ankle plantarflexor muscles bilaterally in individuals with and without chronic SCI. Spasticity was assessed using the Modified Ashworth Scale (MAS) and a self-reported questionnaire. We performed slow and fast dorsiflexion stretches of the ankle joint to measure passive muscle stiffness and reflex-induced torque using a dynamometer and the soleus H reflex using electrical stimulation over the posterior tibial nerve. All SCI participants reported the presence of spasticity. While 96% of them reported higher spasticity on one side compared with the other, the MAS detected differences across sides in only 25% of the them. Passive muscle stiffness and the reflex-induced torque were larger in SCI compared with controls more on one side compared with the other. The soleus stretch reflex, but not the H reflex, was larger in SCI compared with controls and showed differences across sides, with a larger reflex in the side showing a higher reflex-induced torque. MAS scores were not correlated with biomechanical and electrophysiological outcomes. These findings provide evidence for bilateral and asymmetric contributions of passive and active ankle plantar flexors stiffness to spasticity in humans with chronic SCI and highlight a poor agreement between a self-reported questionnaire and the MAS for detecting asymmetries in spasticity across sides.
NEW & NOTEWORTHY Spasticity affects a number of people with spinal cord injury (SCI). Using biomechanical, electrophysiological, and clinical assessments, we found that passive muscle properties and active spinal reflex mechanisms contribute bilaterally and asymmetrically to spasticity in ankle plantarflexor muscles in humans with chronic SCI. A self-reported questionnaire had poor agreement with the Modified Ashworth Scale in detecting asymmetries in spasticity. The nature of these changes might contribute to the poor sensitivity of clinical exams.
Keywords: biomechanical, H reflex, Modified Ashworth Scale, muscle stiffness, stretch reflex, upper motor neuron
INTRODUCTION
Self-reported questionnaires and clinical exams indicate that >60% of people with spinal cord injury (SCI) present symptoms of spasticity (Holtz et al. 2017; Little et al. 1989; Sangari et al. 2019; Sangari and Perez 2019), which is characterized by involuntary muscle activity including spasms, hyperreflexia, and clonus (Dietz 2000; Nielsen et al. 2007). In fact, the definition of spasticity has been broadened and challenged over the years, opening the consideration of including symptoms of upper motor neuron lesion when referring to spasticity (Pandyan et al. 2005). Clinical exams commonly used to assess spasticity in individuals with SCI include the Modified Ashworth Scale (MAS; Bohannon and Smith 1987) and the Tardieu Scale (Ansari et al. 2013) both based on subjective quantifications, of which the validity and reliability have been questioned (Akpinar et al. 2017; Alhusaini et al. 2010; Alibiglou et al. 2008; Blackburn et al. 2002; Fleuren et al. 2010; Haas et al. 1996).
Modeling studies proposed that changes in passive muscle properties and spinal reflex activity can contribute to the increased spasticity detected clinically after SCI (Mirbagheri et al. 2001). In agreement, evidence showed that whole muscle and single fiber preparations were stiffer in spinal cord injured subjects who had spasticity compared with control subjects (Olsson et al. 2006). Individuals with SCI also show increased passive muscle length-tension curves and lesser muscle-tendon unit length compared with control subjects (Diong et al. 2012). However, while some studies detected differences in passive muscle stiffness using a dynamometer between humans with SCI showing spasticity and control subjects (Firoozbakhsh et al. 1993) others have not (Grippo et al. 2011; Lorentzen et al. 2010; Perell et al. 1996). Controversy also exists regarding the magnitude of changes in reflex torque in spastic SCI participants (Grippo et al. 2011; Perell et al. 1996; Woolacott and Burne 2006). Electrophysiological and biomechanical studies have found evidence for increased reflex activity in individuals with SCI with spasticity compared with control subjects (Dimitrijevíc and Nathan 1967; Grippo et al. 2011; Thompson et al. 2019) and others have not (Perell et al. 1996; Sangari et al. 2019; Schindler-Ivens and Shields 2004; Woolacott and Burne 2006). These studies together highlight that the relationship between spasticity and passive muscle stiffness and active reflex mechanisms is still unclear. Distinguishing passive and active contributions to increased muscle stiffness can have consequences for spasticity treatment decisions. For example, proper delivery and dosing of antispastic treatments such as baclofen and/or botulinum injections are usually based on outcomes from clinical exams (Ianieri et al. 2018). Note that most spinal cord injuries in humans result in bilateral anatomical damage (Bunge et al. 1993; Wrigley et al. 2009). Both MRI and microscopic examinations revealed that such lesions are bilateral and asymmetric often with damage to one side of the spinal cord more than the other (Puckett et al. 1997; Quencer et al. 1992). Asymmetries have been also reported in the distribution of dorsal root collateral sprouting after spinal cord lesions in animals (Murray and Goldberger 1974) and in physiological (Bunday et al. 2013; Bunday and Perez 2012; Hall et al. 2010) and behavioral (Bunday and Perez 2012) outcomes in humans with SCI. We hypothesized that passive muscle properties and active spinal reflex mechanisms increase bilaterally and asymmetrically in humans with chronic SCI compared with control subjects.
To test our hypothesis, we combined biomechanical and electrophysiological assessments of ankle plantarflexor muscles bilaterally in humans with and without chronic SCI. We quantified passive muscle properties, reflex-induced torque, and reflex activity. These outcomes were related to MAS and self-reported questionnaire scores. We found that both passive and active ankle plantar flexors stiffness contributed to clinically identified spasticity in ankle plantarflexor muscles after chronic SCI and argue that MAS scores are largely influenced by nonneural variables.
MATERIALS AND METHODS
Subjects.
Twenty-four individuals with SCI (42.2 ± 14.3 yr, 3 women) and 20 control subjects (35.8 ± 11.0 yr, 9 women) participated in the study. All participants gave informed consent to the experimental procedures, which were approved by the local ethics committee at the University of Miami [Institutional Review Board (IRB) Protocol No. 20170222]. SCI subjects were chronic (≥1 yr) and classified using the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) as having a C2-T12 SCI. Seven out of the 24 subjects were categorized by the American Spinal Cord Injury Impairment Scale (AIS) as AIS A and 1 was AIS B. The remaining 16 subjects were classified as AIS C or D, with AIS A being the most severe injury and AIS E being the least severe injury. Fifteen SCI individuals were under antispastic medication (baclofen and/or tizanidine and/or gabapentin and/or clonazepam; Table 1). These participants were asked to stop antispastic medication on the day of testing (at least 12 h since last dosage). Spasticity was assessed using the Modified Ashworth Scale (MAS) and a self-reported questionnaire. Calf circumference was smaller in SCI compared with control participants (P = 0.04), but no differences were found between sides (SCI: more spastic = 35.5 ± 5.1 cm, less spastic = 35.4 ± 5.1 cm, P = 0.9; controls: left = 37.4 ± 3.3 cm, right = 37.4 ± 3.1 cm, P = 0.8). In all subjects, the self-reported questionnaire was completed first followed by MAS measurements. Later on, in the same session, H reflexes were tested at rest followed by testing of passive muscle stiffness and the reflex-induced torque. To avoid interference between examinations, at least 10 min of rest were given in between all major measurements and at least 30 s in between the stretches.
Table 1.
Spinal cord injury participants
| Participants | Age, yr | Sex | AIS | Level | Time Postinjury, yr | Medication |
|---|---|---|---|---|---|---|
| 1 | 51 | M | A | T12 | 9 | BAC |
| 2 | 36 | M | C | C5 | 4 | BAC |
| 3 | 45 | M | A | T5 | 18 | None |
| 4 | 60 | M | C | T5 | 43 | None |
| 5 | 47 | M | B | T9 | 4 | BAC |
| 6 | 38 | M | D | C2 | 13 | None |
| 7 | 30 | M | A | C5 | 12 | CLO |
| 8 | 69 | M | D | C5 | 9 | BAC |
| 9 | 53 | M | A | T4 | 3 | BAC |
| 10 | 66 | M | D | C3 | 13 | BAC |
| 11 | 42 | M | C | C7 | 7 | BAC |
| 12 | 37 | M | C | C4 | 17 | TIZ |
| 13 | 24 | M | A | C5 | 4 | BAC |
| 14 | 44 | M | C | C4 | 11 | None |
| 15 | 61 | M | D | C5 | 17 | BAC |
| 16 | 43 | M | D | C4 | 13 | BAC |
| 17 | 26 | M | A | T7 | 4 | None |
| 18 | 55 | M | C | T12 | 2 | None |
| 19 | 29 | M | C | C4 | 3 | BAC |
| 20 | 21 | F | D | C4 | 3 | None |
| 21 | 21 | M | C | T11 | 1 | None |
| 22 | 42 | F | D | C1 | 27 | BAC, GAB |
| 23 | 22 | M | A | T5 | 1 | BAC |
| 24 | 52 | F | C | C2 | 25 | None |
AIS, American Spinal Injury Association Impairment Scale; BAC, baclofen; CLO, clonazepam; F, female subjects; GAB, gabapentin; M, male subjects; TIZ, tizanidine.
Electromyographic recordings.
Electromyography (EMG) was recorded from both soleus muscles in control subjects and participants with SCI through bipolar surface electrodes (interelectrode distance: 2 cm) placed over the belly of each muscle below the gastrocnemius muscles (AG-AgCl, 10-mm diameter). EMG signals were amplified, filtered (20–1,000 Hz), and sampled at 2 kHz for both online detection (3rd-order Butterworth, 5–150 Hz band pass filtered and rectified) and offline analysis (CED 1401 with signal software, Cambridge Electronic Design, Cambridge, UK).
Passive muscle stiffness and reflex-induced torque.
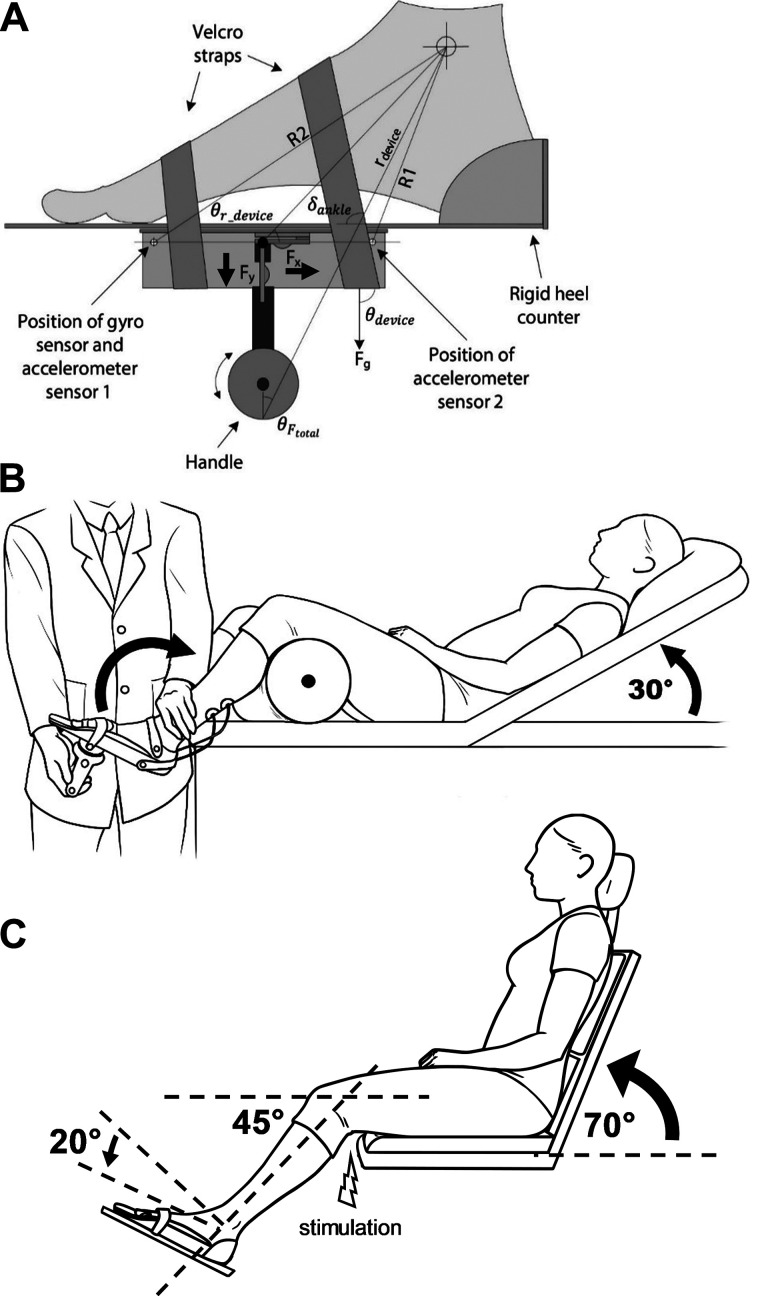
We measured passive and reflex-mediated torque at the ankle joint bilaterally using a portable spasticity assessment device (PSAD; Yamaguchi et al. 2018). The device functions as a dynamometer and integrates measurements of force, joint movement, and reflex-mediated muscle activity (Fig. 1A). During testing, the device was fitted along the axis of the dorsi/plantar flexion of the ankle joint on the foot sole and the tested foot was fixed using a custom-made foot plate and padded Velcro. During testing, subjects were lying in a semi-supine position with the trunk at an angle of 30° of flexion on an examination bed in a relaxed position with the knee stabilized in the popliteal fossa by a tumble forms roll (Fig. 1B). Custom software was used to measure in real time the velocity of the stretches when the experimenter moved the ankle joint at either slow velocity (<20°/s; passive stretch) or as fast as possible (>300°/s; active stretch) by applying force to the foot through a handle on the device. Previous results showed that <20°/s was sufficiently slow not to evoke any stretch reflex activity (Lorentzen et al. 2010) and ankle torques increase linearly with manual perturbations into dorsiflexion at velocities of >300°/s (Lamontagne et al. 1998) in participants with SCI. Visual feedback of the velocity of each stretch was continuously provided to the experimenter on a tablet screen. The dynamometer was equipped with two load cells that measure the force applied along the axis of the handle and perpendicular to it. The position of the device relative to gravity (θdevice) and the angular displacement (δankle) of the device during dorsiflexion was measured by two accelerometers and a gyroscope placed in the base of the device. At the beginning of testing, active range of motion (ROM) was measured in both ankle joints. Later, stretches were performed from a starting resting position for the ankle joint to the end position where no further dorsiflexion was possible. First, five slow stretches were recorded on each side separated by at least 30 s. EMG activity and velocity outcomes were provided to the experimenter after each trial in real time. Trials in which EMG activity in the soleus muscle was >5 µV during the 20-s stretch were automatically excluded from analysis and repeated. An exponential function was fitted to the remaining torque data using a fitting routine in Matlab with passive ROM as the independent variable. The three trials that showed the highest r2 values for the exponential fit were selected for analysis. This function was later used for calculation of passive joint torque during fast trials. Passive muscle stiffness was calculated by measuring changes in ankle torque in three overlapping windows (10–50%, 20–60%, and 30–70%) during the passive ROM. A linear curve fit was made for each of these three overlapping windows using the raw data and reported at 30%, 40%, and 50% of the passive ROM as previously described (Yamaguchi et al. 2018). Passive ROM was larger in control compared with SCI participants (P < 0.01) but no differences were found between sides (controls: left = 63.9 ± 11.1°, right = 65.5 ± 12.5°, P = 0.6; SCI: more spastic = 49.5 ± 12.4°, less spastic = 50.7 ± 14.0°, P = 0.7). Second, five fast stretches were recorded on each side separated by at least 30 s. Previous studies showed that the reflex contribution to total stiffness began ~50 ms after onset of stretch and peaked ~150–300 ms (Bar-On et al. 2013; Sinkjaer et al. 1988; Willerslev-Olsen et al. 2014). Therefore, the peak reflex torque was measured 100 to 200 ms after the peak of the stretch reflex measured as the highest peak amplitude of the rectified EMG in a time window from 30 to 100 ms after the beginning of the stretch (Lorentzen et al. 2010). The amplitude of the stretch reflex was normalized to the soleus maximal motor response (M-max; see details in Soleus H reflex). The peak total torque exerted by the rater on the ankle joint was calculated after removing the gravitational torque of the apparatus and foot (Yamaguchi et al. 2018). An exponential fit obtained from the passive trials was used to subtract the passive ankle joint torque for the corresponding joint position from the peak total torque, yielding a measure of peak reflex torque. Data from the PSAD were sampled at 512 Hz and transferred to a PC via Bluetooth for further analysis using custom-built software.
Fig. 1.
Experimental setup. A: illustration of the handheld device used in the current study. It is a dynamometer instrumented with 2 load cells (Fx and Fy) that measures the force applied in the plane along the axis of the handle and the plane perpendicular to it. Two built-in accelerometer sensors measure the position of the device in relation to gravity (θdevice) and the angular displacement (δdevice) of the dorsiflexion of the ankle joint. B: participants were lying in supine position with the trunk at an angle of 30° of flexion on an examination bed in a relaxed position with the knee stabilized at the popliteal fossa by a tumble form roll. C: soleus H-reflex testing was completed when participants were seated in a custom armchair with the tested leg placed on a custom platform with the hip (70°) and knee (45°) flexed and the ankle restrained by straps in 20° of plantarflexion. Electrical stimulation with the cathode positioned over the posterior tibial nerve in the popliteal fossa were used to elicit the soleus H reflex.
Soleus H reflex.
Testing was completed when subjects were seated in a custom armchair with the test leg placed on a custom platform with the hip (70°) and knee (45°) flexed and the ankle restrained by straps in 20° of plantarflexion (Fig. 1C). The soleus H reflex was elicited every 5 s by using electrical stimulation with the cathode positioned over the posterior tibial nerve in the popliteal fossa using a constant-current stimulator (1-ms rectangular electrical stimulus, 0.25 Hz; model DS7A, Digitimer, Hertfordshire, UK). The anode electrode was positioned above the patella. The reflex response was measured as peak-to-peak amplitude of the nonrectified reflex response recorded from the soleus muscle. The stimulus intensity was increased in steps of 0.05 mA starting below H-reflex threshold and increasing up to supramaximal intensity to measure the M-max. To ensure that M-max values were reached, the stimulus intensity was increased until a plateau was observed in the M-max. Stimulation and recording were done in both sides. Twenty reflexes were averaged at a stimulus intensity needed to get the maximal H reflex (H-max).
MAS.
This is a clinical scale that measures resistance encountered during manual passive muscle stretching using a five-point ordinal scale (0 = no increase in tone; 1/+1 = slight increase in tone with a catch and release or minimal resistance at the end or less than half of the range of movement, respectively; 2 = more marked increased tone through most of the range of movement but affected parts easily moved; 3 = considerable increase in tone and passive movement difficulty; and 4 = affected parts rigid (Bohannon and Smith 1987). During testing, subjects were in the same position used to test passive muscle stiffness and the reflex-induced torque (Fig. 1B). The same rater performed all MAS assessments on plantarflexor muscles bilaterally and was blinded to the results from biomechanical and physiological outcomes.
Self-reported questionnaire.
Individuals with SCI were asked to rate their spasticity on each leg using a four-point spasticity severity scale (0 = none; 1 = mild; 2 = moderate; and 3 = severe; Lechner et al. 2006) and then to compare the severity of the spasticity between sides by answering the following questions: 1) how would you rate the severity of your spasticity in your legs in general; and 2) which side would you rate with more severe spasticity? Because MAS scores were different across sides in 25% of participants (Fig. 2, A and B) but the self-reported questionnaire detected differences across sides in 96% of the participants (Fig. 2C), the results from the questionnaire were used to determine the more and less spastic side in SCI participants.
Fig. 2.
Clinical measurements. A: the graph shows Modified Ashworth Scale (MAS) scores in the left and right side of spinal cord injury (SCI) participants. The abscissa shows each participant’s MAS values (range from 0 to 4), and the ordinate shows each participant. B: MAS distribution shows that scores were higher in one side compared with the other one in 25% of the SCI participants. C: whereas, using a self-reported questionnaire, 96% of the SCI participants reported a higher degree of spasticity in one side compared with the other one.
Data analyses.
Normal distribution was tested by the Shapiro-Wilk’s test and homogeneity of variances by the Levene’s test. Sphericity was tested using Mauchly’s test. When sphericity was not met, the Greenhouse-Geisser correction was used. A mixed-model ANOVA was used to examine the effect of between subject factor GROUP (controls and SCI) and within subject factor SIDE (left and right for controls, more spastic and less spastic for SCI) on passive muscle stiffness, peak reflex torque, peak total torque, H-max, and M-max. Holm-Sidak post hoc analysis was used to test for significant comparisons. The χ2-test was used for comparing the sensitivity between the MAS and the self-reported questionnaire. The Wilcoxon signed-rank test was used to compare MAS scores across sides. A mixed-model ANOVA was also used to compare calf circumference and ROM across sides and between groups. Correlations between clinical assessments of spasticity and passive muscle stiffness and reflex-induced torque measurements were done using Pearson’s linear correlation coefficient as needed. Statistical analysis was conducted using SigmaPlot (Systat Software, Inc., San Jose, CA), and the significance was set at P < 0.05. Group data are presented as means ± SD in the text.
RESULTS
MAS and self-reported questionnaire.
MAS scores showed that spasticity was present in all SCI participants in at least one of the sides (Fig. 2A). We found that MAS scores were similar across sides (left side, MAS = 3.0 and right side, MAS = 3.0, P = 0.9). Note that 18 of the SCI participants (75%) showed the same MAS score across sides and only 6 of them (25%) showed different scores across sides (Fig. 2B). Within the group with similar MAS scores across sides, 61% of the participants had a MAS = 4, 28% of them had MAS = 3, and 11% of them had a MAS = 1. The self-reported questionnaire revealed that 1 (4%) of the SCI participants reported similar spasticity across sides whereas 23 (96%) of the participants reported that they had more spasticity in one of the legs compared with the other one (Fig. 2C). Note that the self-reported questionnaire was more sensitive to detect changes in the magnitude of spasticity across sides compared with the MAS (χ2 = 24.7, P < 0.01). Therefore, for all the following outcome measurements, the more spastic and less spastic side were determined based on the self-reported questionnaire.
Passive muscle stiffness and reflex-induced torque.
Figure 3A shows raw torque data in representative participants. Note that during a slow stretch a small torque was generated in the control subject and it was similar across sides. However, larger torques were generated in the SCI participant with larger values in one ankle joint compared with the other. Also note that no EMG activity was present during the slow stretches. A mixed model ANOVA showed an effect of GROUP (F1,42 = 14.9, P < 0.01) and SIDE (F1,42 = 5.5, P = 0.02) but not in their interaction (F1,42 = 2.3, P = 0.1) on passive muscle stiffness. We found that passive muscle stiffness was increased in SCI (more spastic = 3.3 ± 2.3 Nm/rad; less spastic = 2.4 ± 1.5 Nm/rad) compared with control (left = 1.4 ± 0.5 Nm/rad; right = 1.3 ± 0.5 Nm/rad, P < 0.01) subjects. Post hoc analysis showed no differences in passive muscle stiffness between the left and right side in controls (P = 0.9) but a difference was found between the more and less spastic side of SCI participants (P = 0.04; Fig. 3, B and C). Passive muscle stiffness was higher in the most spastic compared with the less spastic side in 17/24 (71%) of SCI participants. We also found that passive muscle stiffness was higher in both the more (P < 0.01) and less (P = 0.01) spastic side of SCI participants compared with control subjects. Note that at the individual level, passive muscle stiffness was higher (defined as numbers higher than the maximum value found in the our control participants; Lorentzen et al. 2010) in the more (16/24; mean MAS = 3.0, range from 0 to 4) and less (10/24; mean MAS = 2.7, range from 0 to 4; Fig. 3D) spastic side of SCI participants while no differences were found in the MAS scores across sides in the same subjects (P = 1.0). No correlation was found between passive muscle stiffness and MAS scores (more spastic side: r = 0.1, P = 0.6; less spastic side: r = 0.1, P = 0.6) and between passive muscle stiffness and M-max values (more spastic side: r = −0.2, P = 0.3; less spastic side: r = −0.09, P = 0.6).
Fig. 3.
Passive muscle stiffness. A: representative examples showing passive torque traces acquired during a slow stretch in a control (left = light green; right = dark green) and in a spinal cord injury (SCI) (more spastic = dark red; less spastic = orange) participant during 20 s. The ordinate shows the passive torque in Nm. Boxplot charts show the data in both groups. B and C: the abscissa shows the left (light green) and right (dark green) side in control subjects (B) and the more (dark red) and less (orange) spastic side in SCI participants (C). The ordinate shows the passive muscle stiffness in Nm/rad in the graphs. The top and bottom lines of the boxes correspond to the 95% confidence interval and the lines in the boxes correspond to the mean. D: the graph shows individual data from both groups. Dotted lines show the higher value found in control subjects in passive muscle stiffness. The 2 bars extend from the maximum to the minimum value. *P < 0.05.
Figure 4, A and B, shows raw passive ROM and torque data in a control subject and in an individual with SCI during fast stretches. Note here that the reflex-induced torque was larger in the SCI compared with the control subject more on one side compared with the other. Also note that the reflex-induced torque remained similar across sides in the control subject. A mixed model ANOVA showed an effect of GROUP (F1,42 = 34.2, P < 0.01) and SIDE (F1,42 = 7.4, P = 0.01) and in their interaction (F1,42 = 14.5, P < 0.01) on the peak reflex torque. The peak reflex torque was increased in the SCI group (more spastic = 5.2 ± 3.2 Nm; less spastic = 2.9 ± 1.4 Nm) compared with controls (left = 1.3 ± 0.7 Nm; right = 1.6 ± 0.5 Nm, P < 0.01). Post hoc analysis revealed no difference in the peak reflex torque between the left and right side in controls (P = 0.9; Fig. 4C) but there was a difference between the more and less spastic side of SCI participants (P < 0.01; Fig. 4D). Here, the reflex torque was higher in the more spastic compared with the less spastic side in 20/24 (83%) of SCI participants. The peak reflex torque was also higher in the more (P < 0.01) and less (P = 0.01) spastic side of SCI than both sides of the controls. Individual data showed that the peak reflex torque was higher (defined as numbers higher than the maximum value found in the our control participants; Lorentzen et al. 2010) in the majority of SCI participants in the more (19/24; mean MAS = 3.1, range 1 to 4) and less (14/24; mean MAS = 3.1, range 0 to 4; Fig. 4E) spastic side. Note that no differences were found in the MAS scores across sides in the same subjects (P = 1.0). In addition, a mixed model ANOVA showed an effect of GROUP (F1,42 = 23.6, P < 0.01) and SIDE (F1,42 = 6.8, P = 0.01) and in their interaction (F1,42 = 4.2, P = 0.05) on the peak total torque. We found that the peak total torque was increased in the SCI group (more spastic = 9.7 ± 4.3 Nm; less spastic = 7.0 ± 2.6 Nm) compared with controls (left = 5.3 ± 2.1 Nm; right = 5.0 ± 1.6 Nm; P < 0.01). Post hoc analysis revealed no differences in the peak total torque between the left and right sides in control subjects (P = 1.0). However, the peak total torque was higher in the more spastic compared with the less spastic side in 19/24 (79%) of SCI participants. Also, the peak total torque was higher in both sides of SCI participants compared with control subjects (more spastic, P < 0.01 and less spastic, P = 0.01). No correlation was found between the MAS scores and the peak reflex torque in the more (r = 0.2, P = 0.3) and less (r = 0.3, P = 0.2) spastic side. The peak reflex torque and passive muscle stiffness in the more (r = 0.2, P = 0.3) and less (r = 0.08, P = 0.7) spastic side were not correlated. Note that differences found in the reflex-induced torque were more pronounced than the differences observed in passive muscle stiffness between sides (P = 0.01). No correlation was also found between the time postinjury and passive muscle stiffness (more spastic: r = 0.2, P = 0.2; less spastic: r = 0.3, P = 0.1) and the peak reflex torque (more spastic: r = −0.1, P = 0.6; less spastic: r = 0.1, P = 0.5) in both legs.
Fig. 4.
Peak reflex torque. A and B: representative examples showing torque (blue) and range of motion (ROM; red) traces during a fast stretch in a control participant (A) and in an individual with spinal cord injury (SCI) (B). The gray shaded area shows a window of 100–200 ms after the peak of the stretch reflex where the reflex mediated torque was measured. C and D: the abscissa in the boxplot charts show the data in controls (C) and SCI participants (D) as shown on Fig. 3. The ordinate shows the peak reflex torque in Nm for individuals in both groups. E: the graph shows individual data from both groups. Dotted lines show the higher value found in control subjects in the peak reflex torque. *P < 0.05.
Soleus stretch and H reflex.
Figure 5, A and B, illustrates raw traces showing the stretch reflex measured from the soleus EMG in representative participants. Note that the amplitude of stretch reflexes was higher in the SCI compared with the control subject. Also, the stretch reflex was larger on one side compared with the other side in the SCI but not in the control participant. A mixed model ANOVA showed an effect of GROUP (F1,37 = 21.1, P < 0.01) and SIDE (F1,37 = 10.9, P < 0.01) and in their interaction (F1,37 = 11.0, P < 0.01) on the normalized stretch reflex. The soleus stretch reflex was increased in the SCI group (more spastic = 9.3 ± 5.7% of M-max, less spastic = 5.1 ± 3.5% of M-max) compared with controls (left = 3.5 ± 1.6% of M-max, right = 3.2 ± 1.0% of M-max, P < 0.01; Fig. 5C). Post hoc analysis revealed that the stretch reflex was similar between the left and right side in control subjects (P = 0.9; Fig. 5C) but it was larger in the more compared with the less spastic side of SCI participants (15/19, 79%, P < 0.01). We found that stretch reflexes were larger in the more (P = 0.01) and less (P = 0.04; Fig. 5C) spastic side of SCI participants compared with both sides of control subjects. Stretch reflexes were higher in a large number of SCI participants in the more (17/19; mean MAS = 3.3, range from 1 to 4) and less (9/19; mean MAS = 3.5, range from 3 to 4) spastic side while no differences were found in the MAS scores across sides in the same subjects (P = 0.3). The peak reflex torque was positively correlated with stretch reflex size in the more (r = 0.47, P = 0.04; Fig. 5D, left) but not less (r = −0.1, P = 0.7; Fig. 5D, right) spastic side of SCI participants. No correlation was found between the time postinjury and the magnitude of the stretch reflex in both legs (more spastic: r = 0.1, P = 0.6; less spastic: r = 0.07, P = 0.7).
Fig. 5.
Soleus stretch reflex. A and B: electromyographic (EMG) traces showing the soleus stretch reflex during a fast stretch in a control (A) and spinal cord injury (SCI) participant (B). The abscissa in the boxplot charts show the data in both groups as shown above. The ordinate shows the stretch reflex as a %maximal motor response (% of M-max) for individuals in both groups. C: the graphs show a correlation analysis between the peak reflex torque and the stretch reflex. The abscissa shows the magnitude of the peak reflex torque in Nm and the ordinate shows the stretch reflex as a % of M-max. *P < 0.05.
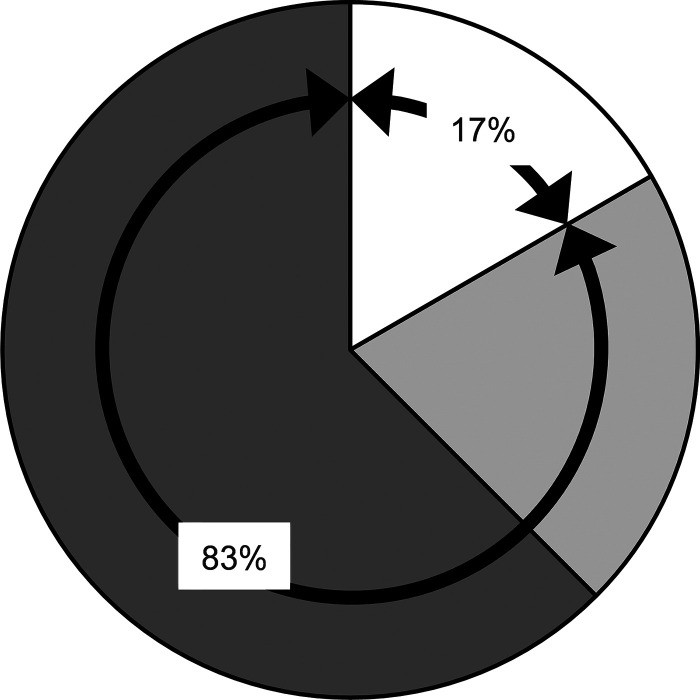
Figure 6 illustrates the proportion of people with SCI who showed increases in passive muscle stiffness, the peak reflex torque and/or in both of them. We found that 15/24 participants showed increases on both or one side in passive muscle stiffness and reflex excitability (i.e., dark gray) and only 4/24 participants showed increases in the peak reflex torque without changes in passive muscle stiffness (i.e., white). The rest of participants (5/24) showed mixed patterns (i.e., light gray) that included increases in passive muscle stiffness on one or both sides without changes in the peak reflex torque, increases in the peak reflex torque on one side, or no increases in the peak reflex torque and passive muscle stiffness on either side. Note that these five participants had MAS scores bilaterally of three and four indicating that even though the peak reflex torque was not increased other “nonneural” variables might have contributed to the high MAS scores. Thus, altogether our results suggest that MAS scores on one or both sides were influenced, at least to some extent, by nonneural variables in 83% of the SCI participants.
Fig. 6.
Distribution of changes in passive muscle stiffness and the peak reflex torque. This figure illustrates the proportion of people with spinal cord injury (SCI) who had increases in passive muscle stiffness, the peak reflex torque, and/or in both of them. Our results suggest that MAS scores on one or in both sides were influenced, at least to some extent, by nonneural variables in 83% of the SCI participants.
Figure 7A illustrates raw traces showing the H reflex measured from the soleus EMG on both sides in representative participants. Note that the amplitude of soleus H reflex was similar in the SCI compared with the control subject and remained similar across sides in both participants. A mixed model ANOVA showed no effect of GROUP (F1,37 = 0.3, P = 0.6) or SIDE (F1,37 = 0.02, P = 0.9) nor in their interaction (F1,37 = 0.02, P = 0.9) on the soleus H-max. We found that the H-max was similar across sides in control (left = 6.0 ± 2.4 mV; right = 5.9 ± 2.1) and SCI (more spastic = 5.7 ± 2.8 mV; less spastic = 5.7 ± 2.3 mV; Fig. 7B) participants. A mixed model ANOVA showed an effect of GROUP (F1,37 = 17.5, P < 0.01) but not SIDE (F1,37 = 0.05, P = 0.8) nor in their interaction (F1,37 = 0.02, P = 0.9) on the M-max. The M-max was reduced in SCI (more spastic = 8.2 ± 2.9 mV; less spastic = 8.0 ± 2.9 mV) compared with control (left = 11.9 ± 4.2 mV; right = 11.6 ± 3.4 mV, P < 0.01; Fig. 7B) participants. Post hoc analysis showed no differences in the M-max between left and right side of the control subjects (P = 0.8) and between the more and less spastic sides of SCI participants (P = 1.0).
Fig. 7.
Soleus H reflex. A: EMG traces showing the soleus H reflex in a control and in a SCI participant. Note that there is no difference in the size of the H reflex across sides in both participants. B: boxplot charts show the data in controls and SCI participants. The abscissa shows maximal H reflex (H-max) and maximal motor response (M-max) in controls (left = light green; right = dark green) and SCI (more spastic = dark red; less spastic = orange) participants. The ordinate shows the maximum response in mV for the H reflex and the M-max. The top and bottom lines of the boxes correspond to the 95% confidence interval and the lines in the boxes correspond to the mean. The 2 bars extend from the maximum to the minimum value. *P < 0.05.
DISCUSSION
With the use of biomechanical and electrophysiological assessments our findings demonstrate that both passive and active ankle plantar flexors stiffness contribute to clinically identified spasticity in humans with chronic SCI. We found that passive muscle stiffness was increased in people with SCI compared with control subjects but more on one side than the other. Similarly, the peak reflex torque was larger in people with SCI compared with controls but more on one side than the other. Notably, the soleus stretch reflex, but not the H reflex, was larger in SCI compared with control participants and showed differences across sides, with a larger stretch reflex present on the side showing higher reflex-induced torques. MAS and biomechanical outcomes were not correlated, and MAS scores on one or both sides were likely influenced by nonneural variables in 83% of the participants. Our comprehensive set of results support the hypothesis that passive muscle properties and active spinal reflex mechanisms contribute bilaterally and asymmetrically to spasticity measured by clinical outcomes in ankle plantarflexor muscles in humans with chronic SCI and highlight the need for more sensitive tools for the evaluation of spasticity following SCI.
Spasticity after SCI.
In humans with SCI, symptoms of spasticity are characterized by involuntary muscle activity including spasms, hyperreflexia, and clonus (Dietz 2000; Nielsen et al. 2007). Here, we measured spasticity using the MAS scale and a self-reported questionnaire. We found that 96% of individuals with SCI reported more pronounced symptoms of spasticity on one side compared with the other using a self-reported questionnaire, whereas only 25% of the participants with SCI showed differences across sides based on the MAS. This is consistent with previous studies showing discrepancies between different manifestations of spasticity described by self-reported questionnaires and clinical examinations (Little et al. 1989). Limitations have been reported in the validity of MAS scores (Akpinar et al. 2017; Fleuren et al. 2010). Evidence also showed that MAS scores have inadequate reliability for measuring ankle plantarflexors muscle spasticity between raters and over time in humans with chronic SCI (Craven and Morris 2010; Haas and Crow 1995). Thus it is possible that the variability and lack of sensitivity of the clinical nominal scale used to measure spasticity contributed to our findings (Craven and Morris 2010).
Modeling (Mirbagheri et al. 2001) and biomechanical (Grippo et al. 2011; Lorentzen et al. 2010; Perell et al. 1996) studies proposed that outcomes of clinical spasticity exams likely reflect a combination of changes in passive muscle properties and active reflex mechanisms (Biering-Sørensen et al. 2006). Thus a dynamometer combined with electrophysiological measurements can provide more sensitive quantifications to examine contributions of passive and active muscle stiffness to spasticity (Lorentzen et al. 2010; Sinkjaer et al. 1993; Yamaguchi et al. 2018). This is supported by our results showing bilateral increases in passive muscle stiffness and reflex-induced torque in SCI compared with control subjects. This is consistent with results showing increases in muscle tension at the whole muscle and single-fiber level in humans with chronic SCI (Olsson et al. 2006). A tendency to increase passive muscle stiffness in ankle plantarflexor muscles was also reported in participants with SCI with a variety of times postinjury compared with controls by using a dynamometer (Lorentzen et al. 2010). Skeletal muscles of people with SCI showed marked atrophy with a switch from oxidative slow-twitch toward glycolytic fast-twitch fibers (Dudley-Javoroski and Shields 2008; Qin et al. 2010). Indeed, muscles of individuals with SCI have a higher percentage of fast fibers expressing myosin heavy chain type IIx compared with controls (Talmadge 2000), and these fast fibers show increases in passive tension after chronic SCI (Olsson et al. 2006). A possibility is that all these changes might have contributed, at least to some extent, to the bilateral increases in passive muscle stiffness observed in our study. However, we have to consider that the severity of muscle weakness and atrophy after SCI may relate to chronic muscle denervation due to motoneuron death (Grumbles and Thomas 2017). This is consistent with our results showing smaller M-max values in SCI participants compared with control subjects. Motoneuron innervation plays a role in determining the fiber type that is expressed within muscles (Lømo and Waerhaug 1985; Waerhaug and Lømo 1994). Thus, if more units switch from type I to type IIx in subjects with SCI, we cannot completely exclude the possibility that these changes might be influenced by changes in motoneurons themselves, and therefore are not purely passive in nature. In addition, we found that passive ROM was decreased in SCI participants compared with control subjects. Because passive ROM can be influenced by both joint and muscle properties, it is possible that changes in passive ROM may also contribute to explain inconsistences in muscle stiffness detected by a dynamometer.
Our findings revealed that the reflex-induced torque was larger in people with SCI compared with control subjects. This is supported by our results showing larger stretch reflexes on the same side where the reflex-induced torque was increased in people with SCI. This also agrees with evidence showing larger stretch reflexes in humans with SCI compared with control subjects (Dimitrijevíc and Nathan 1967; Mirbagheri et al. 2001; Thompson et al. 2019). It is important to note that some studies found no differences in the magnitude of stretch reflexes between people with and without SCI (Woolacott and Burne 2006) but variations in the degree of spasticity in the population tested in this compared with our study might contribute to these differences. Indeed, in the study by Woolacott and Burne (2006) participants with SCI had lower MAS values compared with either side of our SCI participants. We found similar H reflexes between SCI and controls regardless of the presence of spasticity. This is consistent with findings suggesting that spasticity in people with chronic SCI is not associated with increased excitability of the connections between Ia afferent projections and motoneurons (Sangari et al. 2019; Schindler-Ivens and Shields 2004).
A critical question is why both passive muscle stiffness and active reflex mechanisms increased asymmetrically after SCI? Several spinal and supraspinal pathways are thought to contribute to the development of spasticity (D’Amico et al. 2014; Sangari et al. 2019; Sangari and Perez 2019). Damage to these neuronal pathways can readily contribute to the findings that we observed. Most spinal cord injuries in humans result in bilateral anatomical damage (Bunge et al. 1993; Wrigley et al. 2009). MRI and microscopic examinations revealed that such bilateral lesions are asymmetric with more damage on one side compared with the other side of the spinal cord (Puckett et al. 1997; Quencer et al. 1992). Animal models of SCI showed the existence of axon sprouting and nervous tissue remodeling (Ballermann and Fouad 2006; Bareyre et al. 2004). Such sprouting is present from ventral (unlesioned) to dorsal (lesioned) regions (Weidner et al. 2001) as well as from the contralateral to the ipsilateral side (Rosenzweig et al. 2009, 2010). Also, note that these differences across sides might contribute to explain why some studies reported increases in passive muscles stiffness and reflex torque in spastic subjects with SCI (Dimitrijevíc and Nathan 1967; Diong et al. 2012; Grippo et al. 2011; Mirbagheri et al. 2001; Thompson et al. 2019), while others did not (Lorentzen et al. 2010; Perell et al. 1996; Sangari et al. 2019; Schindler-Ivens and Shields 2004). For example, differences might be more difficult to find when the less spastic side is tested. It is also possible that changes in the use of muscles contributed to the asymmetries found in passive muscle stiffness. Recent evidence showed that spastic muscles of individuals with chronic SCI are weaker compared with muscles without or with lower spasticity (Sangari and Perez 2019). Asymmetries have been reported in voluntary motor output across sides in people with chronic SCI (Calabro and Perez 2016). Lesser use of muscles can, at least to some extent, contribute to the higher percentage of fast fibers expressing myosin heavy chain type IIx compared with control subjects (Talmadge 2000), which are the fibers showing high passive tension after chronic SCI (Olsson et al. 2006). Higher passive muscle stiffness was found on the side showing higher reflex-induced torque, supporting the view that weakness related to higher spasticity (Sangari and Perez 2019) could be a factor contributing to our results. In animals, selective lesions of the corticospinal tract results in asymmetric maladaptive proprioceptive afferent fiber plasticity and asymmetric microglial distribution in the dorsal horn and intermediate zone of the spinal cord, which might contribute to the symptoms of hyperreflexia observed in the tested animals (Tan et al. 2012). Spinal microglia increases after damage of descending motor pathways (Leong et al. 1995), which can affect spinal neuronal excitability, and together with the enhanced sprouting could represent factors contributing to asymmetries in active reflex mechanisms. This is supported by the asymmetric changes that we found in stretch reflexes across sides. Note that asymmetries found in the reflex-induced torque were more pronounced than the asymmetries observed in passive muscle stiffness. Also, note that we did not find any relationship between increases in passive muscle stiffness and increases in the reflex-induced torque; thus the relationship between these outcomes needs further investigation. We ran multiple ANOVA tests in our study. Although we considered that our dependent variables were independent, we cannot completely exclude the possibility of a relationship among the variables tested. Therefore, we have to acknowledge the possibility that this may have increased type 1 error.
Functional considerations.
We want to highlight that this is the first comprehensive study that examined passive muscle stiffness, activity spinal reflex mechanisms, and clinical measurements bilaterally in humans with chronic incomplete SCI. Other studies have assessed some of these outcomes but not altogether in a single study. We argue that this comprehensive assessment is important for the interpretation of findings. Previous studies showed no differences in passive muscle stiffness (Grippo et al. 2011; Lorentzen et al. 2010; Perell et al. 1996) and reflex torque (Woolacott and Burne 2006) or differences in these outcomes (Firoozbakhsh et al. 1993; Grippo et al. 2011) between SCI and control participants. Our results indicate that the lack of changes in passive stiffness between a SCI and a control subject, for example, could be simply related to the fact that the less spastic leg was tested. This is also the first study that examined the extent to which asymmetries in spasticity can be detected by clinical measurements after SCI. A previous study (Okuma et al. 2002) showed asymmetries in spasticity using the MAS in a preselected a group of five individuals with SCI. We found that the MAS detected asymmetries in spasticity across sides in 25% of participants with chronic SCI suggesting that the preselected group tested by Okuma and collaborators is not representative. This is important because most studies determine the more or less spastic side using the MAS (Mirbagheri et al. 2001; Okuma et al. 2002; Thompson et al. 2019). On the other side, we found the self-reported questionnaire detected asymmetries in spasticity across sides in 96% of participants, suggesting that this outcome might be more useful to determine the more or less spastic side. The validity and reliability of the MAS have been questioned (Akpinar et al. 2017; Blackburn et al. 2002; Fleuren et al. 2010; Haas et al. 1996; Platz et al. 2005). We argue that the bilateral and asymmetrical increases in passive muscle stiffness and active spinal reflex mechanisms make it more difficult for the MAS to accurately assess spasticity. This is supported by our results showing that MAS scores in one or both sides were influenced by nonneural variables in 83% of the SCI participants.
It has been proposed that spasticity restricts activities of daily living (Tibbett et al. 2019), affects the quality of life (Andresen et al. 2016; Westerkam et al. 2011), and interferes with physical activity (Holtz et al. 2017) after SCI. However, accurate quantifications of spasticity remain a problem and failure to distinguish between passive and active ankle plantar flexors stiffness can have consequences on assessing the impact of spasticity on different domains. Thus, the clinical and functional impact of spasticity remains questionable without a proper definition and distinction of its underlying pathology. Here, we demonstrate for the first time that passive and active variables contributing to clinically identified spasticity are increased bilaterally and asymmetrically after chronic SCI. This is important knowledge to consider when quantifications of spasticity are made across subjects, before and after interventions, and in a number of other motor control-related areas. Consistent with previous results, we found a decrease in calf circumference in SCI compared with control subjects (Yaeshima et al. 2015). However, because we found no differences in calf circumference across sides, we think that is less likely that this factor contributed to our results. We favor the interpretation that after SCI bilateral damage to neuronal structures contribute to asymmetries in central and peripheral changes reported in our study.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS) Grants R01-NS-090622 and R01-NS-100810-01 (to M. A. Perez); U.S. Department of Veterans Affairs (VA) Grants I01-RX-001807, I01-RX-002848, and I01-RX-002474 (to M. A. Perez); and Craig H. Neilsen Foundation Grant 068477546 (S. Sangari).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.C., S.S., J.L., J.B.N., and M.A.P. conceived and designed research; B.C., S.S., and M.A.P. performed experiments; B.C., S.S., and M.A.P. analyzed data; B.C., S.S., J.L., J.B.N., and M.A.P. interpreted results of experiments; B.C., S.S., and M.A.P. prepared figures; B.C., S.S., J.L., J.B.N., and M.A.P. drafted manuscript; B.C., S.S., J.L., J.B.N., and M.A.P. edited and revised manuscript; B.C., S.S., J.L., J.B.N., and M.A.P. approved final version of manuscript.
REFERENCES
- Akpinar P, Atici A, Ozkan FU, Aktas I, Kulcu DG, Sarı A, Durmus B. Reliability of the Modified Ashworth Scale and Modified Tardieu Scale in patients with spinal cord injuries. Spinal Cord 55: 944–949, 2017. doi: 10.1038/sc.2017.48. [DOI] [PubMed] [Google Scholar]
- Alhusaini AA, Dean CM, Crosbie J, Shepherd RB, Lewis J. Evaluation of spasticity in children with cerebral palsy using Ashworth and Tardieu Scales compared with laboratory measures. J Child Neurol 25: 1242–1247, 2010. doi: 10.1177/0883073810362266. [DOI] [PubMed] [Google Scholar]
- Alibiglou L, Rymer WZ, Harvey RL, Mirbagheri MM. The relation between Ashworth scores and neuromechanical measurements of spasticity following stroke. J Neuroeng Rehabil 5: 18, 2008. doi: 10.1186/1743-0003-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen SR, Biering-Sørensen F, Hagen EM, Nielsen JF, Bach FW, Finnerup NB. Pain, spasticity and quality of life in individuals with traumatic spinal cord injury in Denmark. Spinal Cord 54: 973–979, 2016. doi: 10.1038/sc.2016.46. [DOI] [PubMed] [Google Scholar]
- Ansari NN, Naghdi S, Hasson S, Rastgoo M, Amini M, Forogh B. Clinical assessment of ankle plantarflexor spasticity in adult patients after stroke: inter-and intra-rater reliability of the Modified Tardieu Scale. Brain Inj 27: 605–612, 2013. doi: 10.3109/02699052.2012.750744. [DOI] [PubMed] [Google Scholar]
- Ballermann M, Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci 23: 1988–1996, 2006. doi: 10.1111/j.1460-9568.2006.04726.x. [DOI] [PubMed] [Google Scholar]
- Bar-On L, Aertbeliën E, Wambacq H, Severijns D, Lambrecht K, Dan B, Huenaerts C, Bruyninckx H, Janssens L, Van Gestel L, Jaspers E, Molenaers G, Desloovere K. A clinical measurement to quantify spasticity in children with cerebral palsy by integration of multidimensional signals. Gait Posture 38: 141–147, 2013. doi: 10.1016/j.gaitpost.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci 7: 269–277, 2004. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Biering-Sørensen F, Nielsen JB, Klinge K. Spasticity-assessment: a review. Spinal Cord 44: 708–722, 2006. doi: 10.1038/sj.sc.3101928. [DOI] [PubMed] [Google Scholar]
- Blackburn M, van Vliet P, Mockett SP. Reliability of measurements obtained with the modified Ashworth scale in the lower extremities of people with stroke. Phys Ther 82: 25–34, 2002. doi: 10.1093/ptj/82.1.25. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 67: 206–207, 1987. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- Bunday KL, Oudega M, Perez MA. Aberrant crossed corticospinal facilitation in muscles distant from a spinal cord injury. PLoS One 8: e76747, 2013. doi: 10.1371/journal.pone.0076747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Perez MA. Impaired crossed facilitation of the corticospinal pathway after cervical spinal cord injury. J Neurophysiol 107: 2901–2911, 2012. doi: 10.1152/jn.00850.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol 59: 75–89, 1993. [PubMed] [Google Scholar]
- Calabro FJ, Perez MA. Bilateral reach-to-grasp movement asymmetries after human spinal cord injury. J Neurophysiol 115: 157–167, 2016. doi: 10.1152/jn.00692.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven BC, Morris AR. Modified Ashworth scale reliability for measurement of lower extremity spasticity among patients with SCI. Spinal Cord 48: 207–213, 2010. doi: 10.1038/sc.2009.107. [DOI] [PubMed] [Google Scholar]
- D’Amico JM, Condliffe EG, Martins KJ, Bennett DJ, Gorassini MA. Recovery of neuronal and network excitability after spinal cord injury and implications for spasticity. Front Integr Nuerosci 8: 36, 2014. doi: 10.3389/fnint.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V. Spastic movement disorder. Spinal Cord 38: 389–393, 2000. doi: 10.1038/sj.sc.3101030. [DOI] [PubMed] [Google Scholar]
- Dimitrijevíc MR, Nathan PW. Studies of spasticity in man. 2. Analysis of stretch reflexes in spasticity. Brain 90: 333–358, 1967. doi: 10.1093/brain/90.2.333. [DOI] [PubMed] [Google Scholar]
- Diong JH, Herbert RD, Harvey LA, Kwah LK, Clarke JL, Hoang PD, Martin JH, Clarke EC, Bilston LE, Gandevia SC. Passive mechanical properties of the gastrocnemius after spinal cord injury. Muscle Nerve 46: 237–245, 2012. doi: 10.1002/mus.23356. [DOI] [PubMed] [Google Scholar]
- Dudley-Javoroski S, Shields RK. Muscle and bone plasticity after spinal cord injury: review of adaptations to disuse and to electrical muscle stimulation. J Rehabil Res Dev 45: 283–296, 2008. doi: 10.1682/JRRD.2007.02.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firoozbakhsh KK, Kunkel CF, Scremin AM, Moneim MS. Isokinetic dynamometric technique for spasticity assessment. Am J Phys Med Rehabil 72: 379–385, 1993. doi: 10.1097/00002060-199312000-00008. [DOI] [PubMed] [Google Scholar]
- Fleuren JF, Voerman GE, Erren-Wolters CV, Snoek GJ, Rietman JS, Hermens HJ, Nene AV. Stop using the Ashworth Scale for the assessment of spasticity. J Neurol Neurosurg Psychiatry 81: 46–52, 2010. doi: 10.1136/jnnp.2009.177071. [DOI] [PubMed] [Google Scholar]
- Grippo A, Carrai R, Hawamdeh Z, Falsini C, Aito S, Pinto F, de Scisciolo G, Pizzi A. Biomechanical and electromyographic assessment of spastic hypertonus in motor complete traumatic spinal cord-injured individuals. Spinal Cord 49: 142–148, 2011. doi: 10.1038/sc.2010.56. [DOI] [PubMed] [Google Scholar]
- Grumbles RM, Thomas CK. Motoneuron death after human spinal cord injury. J Neurotrauma 34: 581–590, 2017. doi: 10.1089/neu.2015.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BM, Bergström E, Jamous A, Bennie A. The inter rater reliability of the original and of the modified Ashworth scale for the assessment of spasticity in patients with spinal cord injury. Spinal Cord 34: 560–564, 1996. doi: 10.1038/sc.1996.100. [DOI] [PubMed] [Google Scholar]
- Haas BM, Crow JL. Towards a clinical measurement of spasticity? Physiotherapy 81: 474–479, 1995. doi: 10.1016/S0031-9406(05)66743-0. [DOI] [Google Scholar]
- Hall BJ, Lally JE, Vukmanic EV, Armstrong JE, Fell JD, Gupta DS, Hubscher CH. Spinal cord injuries containing asymmetrical damage in the ventrolateral funiculus is associated with a higher incidence of at-level allodynia. J Pain 11: 864–875, 2010. doi: 10.1016/j.jpain.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz KA, Lipson R, Noonan VK, Kwon BK, Mills PB. Prevalence and effect of problematic spasticity after traumatic spinal cord injury. Arch Phys Med Rehabil 98: 1132–1138, 2017. doi: 10.1016/j.apmr.2016.09.124. [DOI] [PubMed] [Google Scholar]
- Ianieri G, Marvulli R, Gallo GA, Fiore P, Megna M. “Appropriate Treatment” and therapeutic window in spasticity treatment with incobotulinumtoxinA: from 100 to 1000 units. Toxins (Basel) 10: 140, 2018. doi: 10.3390/toxins10040140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne A, Malouin F, Richards CL, Dumas F. Evaluation of reflex- and nonreflex-induced muscle resistance to stretch in adults with spinal cord injury using hand-held and isokinetic dynamometry. Phys Ther 78: 964–975, 1998. doi: 10.1093/ptj/78.9.964. [DOI] [PubMed] [Google Scholar]
- Lechner HE, Frotzler A, Eser P. Relationship between self- and clinically rated spasticity in spinal cord injury. Arch Phys Med Rehabil 87: 15–19, 2006. doi: 10.1016/j.apmr.2005.07.312. [DOI] [PubMed] [Google Scholar]
- Leong SK, Ling EA, Fan DP. Glial reaction after pyramidotomy in mice and rats. Neurodegeneration 4: 403–413, 1995. doi: 10.1006/neur.1995.0049. [DOI] [PubMed] [Google Scholar]
- Little JW, Micklesen P, Umlauf R, Britell C. Lower extremity manifestations of spasticity in chronic spinal cord injury. Am J Phys Med Rehabil 68: 32–36, 1989. doi: 10.1097/00002060-198902000-00009. [DOI] [PubMed] [Google Scholar]
- Lømo T, Waerhaug O. Motor endplates in fast and slow muscles of the rat: what determines their difference? J Physiol (Paris) 80: 290–297, 1985. [PubMed] [Google Scholar]
- Lorentzen J, Grey MJ, Crone C, Mazevet D, Biering-Sørensen F, Nielsen JB. Distinguishing active from passive components of ankle plantar flexor stiffness in stroke, spinal cord injury and multiple sclerosis. Clin Neurophysiol 121: 1939–1951, 2010. doi: 10.1016/j.clinph.2010.02.167. [DOI] [PubMed] [Google Scholar]
- Mirbagheri MM, Barbeau H, Ladouceur M, Kearney RE. Intrinsic and reflex stiffness in normal and spastic, spinal cord injured subjects. Exp Brain Res 141: 446–459, 2001. doi: 10.1007/s00221-001-0901-z. [DOI] [PubMed] [Google Scholar]
- Murray M, Goldberger ME. Restitution of function and collateral sprouting in the cat spinal cord: the partially hemisected animal. J Comp Neurol 158: 19–36, 1974. doi: 10.1002/cne.901580103. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity–from a basic science point of view. Acta Physiol (Oxf) 189: 171–180, 2007. doi: 10.1111/j.1748-1716.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- Okuma Y, Mizuno Y, Lee RG. Reciprocal Ia inhibition in patients with asymmetric spinal spasticity. Clin Neurophysiol 113: 292–297, 2002. doi: 10.1016/S1388-2457(02)00004-4. [DOI] [PubMed] [Google Scholar]
- Olsson MC, Krüger M, Meyer LH, Ahnlund L, Gransberg L, Linke WA, Larsson L. Fibre type-specific increase in passive muscle tension in spinal cord-injured subjects with spasticity. J Physiol 577: 339–352, 2006. doi: 10.1113/jphysiol.2006.116749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandyan AD, Gregoric M, Barnes MP, Wood D, Van Wijck F, Burridge J, Hermens H, Johnson GR. Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil 27: 2–6, 2005. doi: 10.1080/09638280400014576. [DOI] [PubMed] [Google Scholar]
- Perell K, Scremin A, Scremin O, Kunkel C. Quantifying muscle tone in spinal cord injury patients using isokinetic dynamometric techniques. Paraplegia 34: 46–53, 1996. doi: 10.1038/sc.1996.8. [DOI] [PubMed] [Google Scholar]
- Platz T, Eickhof C, Nuyens G, Vuadens P. Clinical scales for the assessment of spasticity, associated phenomena, and function: a systematic review of the literature. Disabil Rehabil 27: 7–18, 2005. doi: 10.1080/09638280400014634. [DOI] [PubMed] [Google Scholar]
- Puckett WR, Hiester ED, Norenberg MD, Marcillo AE, Bunge RP. The astroglial response to Wallerian degeneration after spinal cord injury in humans. Exp Neurol 148: 424–432, 1997. doi: 10.1006/exnr.1997.6692. [DOI] [PubMed] [Google Scholar]
- Qin W, Bauman WA, Cardozo C. Bone and muscle loss after spinal cord injury: organ interactions. Ann N Y Acad Sci 1211: 66–84, 2010. doi: 10.1111/j.1749-6632.2010.05806.x. [DOI] [PubMed] [Google Scholar]
- Quencer RM, Bunge RP, Egnor M, Green BA, Puckett W, Naidich TP, Post MJ, Norenberg M. Acute traumatic central cord syndrome: MRI-pathological correlations. Neuroradiology 34: 85–94, 1992. doi: 10.1007/BF00588148. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Brock JH, Culbertson MD, Lu P, Moseanko R, Edgerton VR, Havton LA, Tuszynski MH. Extensive spinal decussation and bilateral termination of cervical corticospinal projections in rhesus monkeys. J Comp Neurol 513: 151–163, 2009. doi: 10.1002/cne.21940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Courtine G, Jindrich DL, Brock JH, Ferguson AR, Strand SC, Nout YS, Roy RR, Miller DM, Beattie MS, Havton LA, Bresnahan JC, Edgerton VR, Tuszynski MH. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci 13: 1505–1510, 2010. doi: 10.1038/nn.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangari S, Lundell H, Kirshblum S, Perez MA. Residual descending motor pathways influence spasticity after spinal cord injury. Ann Neurol 86: 28–41, 2019. doi: 10.1002/ana.25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangari S, Perez MA. Imbalanced corticospinal and reticulospinal contributions to spasticity in humans with spinal cord injury. J Neurosci 39: 7872–7881, 2019. doi: 10.1523/JNEUROSCI.1106-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler-Ivens SM, Shields RK. Soleus H-reflex recruitment is not altered in persons with chronic spinal cord injury. Arch Phys Med Rehabil 85: 840–847, 2004. doi: 10.1016/j.apmr.2003.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer T, Toft E, Andreassen S, Hornemann BC. Muscle stiffness in human ankle dorsiflexors: intrinsic and reflex components. J Neurophysiol 60: 1110–1121, 1988. doi: 10.1152/jn.1988.60.3.1110. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Toft E, Larsen K, Andreassen S, Hansen HJ. Non-reflex and reflex mediated ankle joint stiffness in multiple sclerosis patients with spasticity. Muscle Nerve 16: 69–76, 1993. doi: 10.1002/mus.880160112. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ. Myosin heavy chain isoform expression following reduced neuromuscular activity: potential regulatory mechanisms. Muscle Nerve 23: 661–679, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- Tan AM, Chakrabarty S, Kimura H, Martin JH. Selective corticospinal tract injury in the rat induces primary afferent fiber sprouting in the spinal cord and hyperreflexia. J Neurosci 32: 12896–12908, 2012. doi: 10.1523/JNEUROSCI.6451-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Mrachacz-Kersting N, Sinkjær T, Andersen JB. Modulation of soleus stretch reflexes during walking in people with chronic incomplete spinal cord injury. Exp Brain Res 237: 2461–2479, 2019. doi: 10.1007/s00221-019-05603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbett J, Widerström-Noga EG, Thomas CK, Field-Fote EC. Impact of spasticity on transfers and activities of daily living in individuals with spinal cord injury. J Spinal Cord Med 42: 318–327, 2019. doi: 10.1080/10790268.2017.1400727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waerhaug O, Lømo T. Factors causing different properties at neuromuscular junctions in fast and slow rat skeletal muscles. Anat Embryol (Berl) 190: 113–125, 1994. doi: 10.1007/BF00193409. [DOI] [PubMed] [Google Scholar]
- Weidner N, Ner A, Salimi N, Tuszynski MH. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci USA 98: 3513–3518, 2001. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerkam D, Saunders LL, Krause JS. Association of spasticity and life satisfaction after spinal cord injury. Spinal Cord 49: 990–994, 2011. doi: 10.1038/sc.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerslev-Olsen M, Andersen JB, Sinkjaer T, Nielsen JB. Sensory feedback to ankle plantar flexors is not exaggerated during gait in spastic hemiplegic children with cerebral palsy. J Neurophysiol 111: 746–754, 2014. doi: 10.1152/jn.00372.2013. [DOI] [PubMed] [Google Scholar]
- Woolacott AJ, Burne JA. The tonic stretch reflex and spastic hypertonia after spinal cord injury. Exp Brain Res 174: 386–396, 2006. doi: 10.1007/s00221-006-0478-7. [DOI] [PubMed] [Google Scholar]
- Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG, Siddall PJ, Henderson LA. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex 19: 224–232, 2009. doi: 10.1093/cercor/bhn072. [DOI] [PubMed] [Google Scholar]
- Yaeshima K, Negishi D, Yamamoto S, Ogata T, Nakazawa K, Kawashima N. Mechanical and neural changes in plantar-flexor muscles after spinal cord injury in humans. Spinal Cord 53: 526–533, 2015. doi: 10.1038/sc.2015.9. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Hvass Petersen T, Kirk H, Forman C, Svane C, Kofoed-Hansen M, Boesen F, Lorentzen J. Spasticity in adults with cerebral palsy and multiple sclerosis measured by objective clinically applicable technique. Clin Neurophysiol 129: 2010–2021, 2018. doi: 10.1016/j.clinph.2018.07.004. [DOI] [PubMed] [Google Scholar]