Abstract
Respiratory dysfunction is one of the most debilitating effects of spinal cord injury (SCI) impacting the quality of life of patients and caregivers. In addition, breathing difficulties impact the rehabilitation routine a patient may potentially undergo. Transcutaneous electrical spinal cord neuromodulation (TESCoN) is a novel approach to reactivate and retrain spinal circuits after paralysis. We demonstrate that acute and chronic TESCoN therapy over the cervical spinal cord positively impacts the breathing and coughing ability in a patient with chronic tetraplegia. ln addition, we show that the improved breathing and coughing ability are not only observed in the presence of TESCoN but persisted for a few days after TESCoN was stopped.
NEW & NOTEWORTHY Noninvasive spinal neuromodulation improves breathing and coughing in a patient with severe and complete tetraplegia.
Keywords: breathing, coughing, noninvasive spinal cord stimulation, spinal cord injury
INTRODUCTION
Impaired respiratory performance is one of the most debilitating effects of spinal cord injury (SCI) (Winslow and Rozovsky 2003). Two typical phenotypes of impaired respiratory function are observed. High cervical injuries usually results in dependence on mechanical respiratory support with positive pressure ventilation. While lower cervical injuries or higher thoracic injuries leave the phrenic nerve and diaphragmatic function intact, patients with these injuries still experience significant respiratory insufficiency and associated comorbidities. Although the diaphragm accounts for most of inspiratory performance, the accessory inspiratory muscles contribute ~35% to 40% of vital capacity in healthy individuals (Agostoni et al. 1964). Thus even lesions at thoracic segments can cause moderate to severe respiratory insufficiency such as dysfunctional cough (Agostoni et al. 1964). Thus impaired respiratory integrity and dysfunctional cough constitute a significant ventilatory impairment, resulting in decreased respiratory performance and a continuously anxious sense of well-being, but also are important contributors to respiratory morbidity and mortality in this population.
The use of electrical stimulation to induce respiratory function traces its roots to the 18th century (Carter et al. 1987). Leopold Caldani demonstrated that phrenic nerve stimulation evoked contraction of the diaphragm and air movement in animals (DiMarco and Kowalski 2013). Innovation of surgical techniques and optimization of stimulation protocols resulted in the development of the phrenic nerve pacemaker (PNP) with over 1,000 patients being implanted by the 1990s. More recently, a novel technique known as the diaphragm motor point pacing (DMPP) was developed and successfully tested (DiMarco et al. 2002). However, most of the current neurostimulation modalities have been developed specifically for individuals with lesions above the C4 level (Gonzalez-Bermejo et al. 2015). As a result, patients with injuries that leave the phrenic nerve intact but result in significant respiratory compromise nonetheless are not candidates for current neurostimulation therapies. Thus patients with low cervical and/or high thoracic injuries can breathe independently because of intact diaphragmatic function but have significantly reduced capacities, peak expiratory flow, and coughing strengths due to paralysis of intercostal and abdominal muscles.
The conceptual foundation for the present study evolved from a series of studies demonstrating that epidural spinal cord stimulation and training could facilitate multiple motor and autonomic functions after chronic complete paralysis (Angeli et al. 2014; Grahn et al. 2017; Harkema et al. 2011). During these studies we also found that after several treatment sessions, each patient also reported improved trunk, cardiovascular, bladder, and respiratory function. Furthermore, several preclinical studies have reported changes in respiratory function with varying sites, frequencies, and intensities of spinal epidural stimulation (DiMarco and Kowalski 2009; Kowalski et al. 2013).
While epidural spinal stimulation is promising, it requires a highly invasive neurosurgical procedure which is expectedly associated with significant morbidity. More recently, we have developed, tested, and patented a novel method to noninvasively neuromodulate spinal networks to more functional physiological states. Transcutaneous electrical spinal cord neuromodulation (TESCoN) delivers a sufficient electrical signal noninvasively to activate the neural structures of the spinal cord without significant cutaneous discomfort (Gerasimenko et al. 2015b, 2015c, 2016). To date, we have demonstrated that this modality can enable recovery of voluntary movement (Gad et al. 2017; Gerasimenko et al. 2015c) of upper (Gad et al. 2018a; Inanici et al. 2018) and lower limbs (Gad et al. 2019a; Gerasimenko et al. 2015a), improved trunk function (Rath et al. 2018), and self-assisted standing (Sayenko et al. 2019). In addition to motor function, TESCoN can lead to significant improvements in autonomic functions after SCI, such as cardiovascular (Phillips et al. 2018) and lower urinary tract function (Gad et al. 2018b; Kreydin et al. 2020).
Thus, based on the effects on motor and other autonomic systems from preclinical models (DiMarco and Kowalski 2009) and clinical studies (Harkema et al. 2011; Lu et al. 2016) using epidural spinal stimulation and our clinical work using transcutaneous electrical spinal stimulation (Freyvert et al. 2018; Gad et al. 2018a; Inanici et al. 2018), we hypothesize that transcutaneous electrical neuromodulation of cervical spinal neural networks can facilitate the activation of intercostal and diaphragmatic muscles, thus increasing inspiratory and expiratory capacities and improving coughing ability. The objective of this case study was to assess the impact of immediate and short-term noninvasive cervical TESCoN on respiratory and cardiovascular function.
METHODS
Patient recruitment.
This study was approved by the Institutional Review Board of Rancho Research Institute, the research arm of Rancho Los Amigos National Rehabilitation Center, Downey, CA. The research participant signed an informed consent form before the start of the study and consented to the data being used in future publications and presentations. The patient is a 39-yr-old man who sustained an ASIA A C5 injury in a diving accident 9 yr before study initiation. The patient reported impaired respiratory function and decreased ability to cough and to clear phlegm, especially in the supine position.
Experimental design.
The study was divided into three phases: phase I, 1-wk baseline assessment; phase II, 2-wk course of TESCoN therapy; and phase III, 1-wk posttherapy follow-up (Fig. 1A). The patient was provided with an electronic spirometer (MIR Smart One) (Nordlund et al. 2018), wrist-mounted blood pressure and heart rate monitor (Omron) (Kario et al. 2020), and oxygen saturation monitor to take home. The patient was asked to monitor his respiratory function (peak expiratory airflow during expiration and forced expiratory volume in 1 s, FEV1), blood pressure, heart rate, and oxygen saturation every hour between 10:00 AM and 10:00 PM, or as often as possible. The patient was also instructed to maintain his usual lifestyle, including dietary regime, exercise, and physical therapy programs.
Fig. 1.
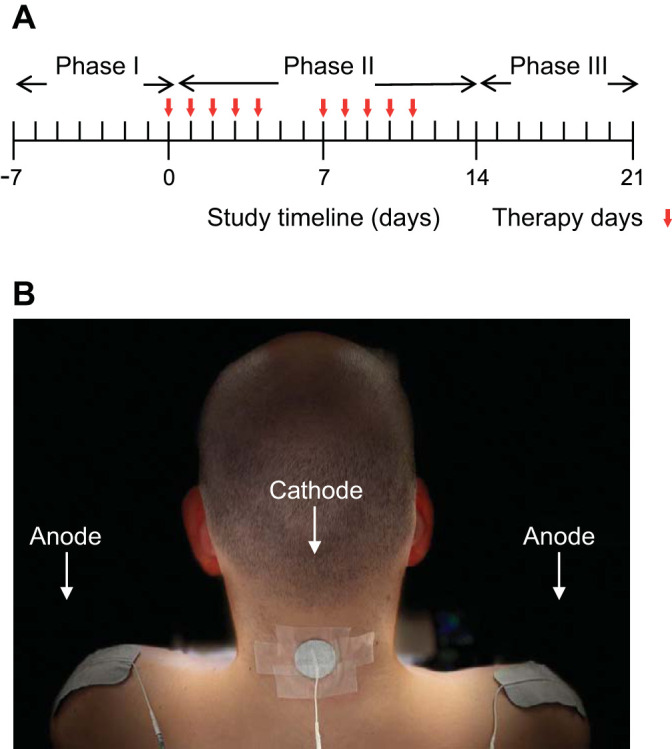
A: daily timeline for 3-wk experiment without and with therapy. B: experimental setup demonstrating position of the cathode at C5 and two anodes over the shoulder.
Electromyography.
During all assessment sessions, surface electromyography (EMG) was recorded from the diaphragm, rectus abdominus, and internal oblique muscles (Chien et al. 2008) via surface EMG electrodes (LabChart and PowerLab; ADInstruments). Data were recorded and sampled at a frequency of 10 kHz and were analyzed using LabChart software. The diaphragm electrodes were attached between the seventh and eighth ribs along the midclavicular line. The rectus abdominus electrodes were attached 2 cm lateral to the umbilicus, and the internal oblique electrodes were attached 4 cm lateral to midline along the iliac crest.
TESCoN therapy.
Spinal stimulation was delivered using a proprietary TESCoN device (spineX, Inc) (Gad et al. 2019b) only in the clinic. The stimulation waveform consisted of two alternating pulses of opposite polarities separated by a 1-μs delay to form a delayed biphasic waveform. The pulses consisted of a high-frequency biphasic carrier pulse (10 kHz) combined with a low-frequency (30 Hz) burst pulse, each with a pulse width of 1 ms. Stimulation was applied using an adhesive electrode between C3–4, C5–6, or T1–2 serving as the cathode and two adhesive electrodes over bilateral shoulders as the anode (Fig. 1B). Dose-response curves were constructed for the inspiratory capacities and forced expiratory volume in 1 s (FEV1), including site and intensity of stimulation. The stimulation site with the lowest intensity that generated the greatest functional respiratory response was selected (Fig. 2, A and B). During the course of the therapy (phase II), TESCoN was delivered for 60 min/day, 5 days/wk for 2 wk.
Fig. 2.
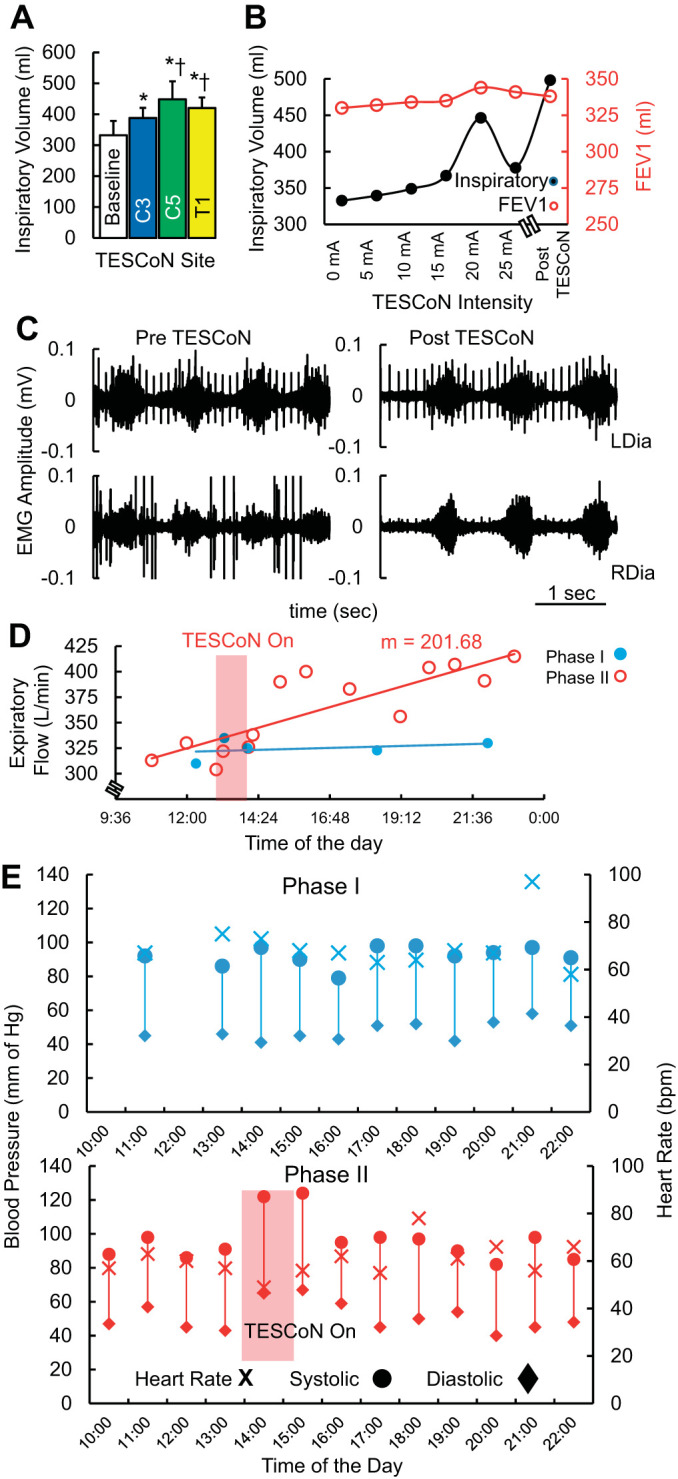
Acute effect of transcutaneous electrical spinal cord neuromodulation (TESCoN) on respiratory and cardiovascular function at the start of phase II (day 0). A: average inspiratory volume (n = 5 cycles) without stimulation (baseline) and with TESCoN at 20 mA at C3–4, C5–6, and T1–2 vertebral levels. B: inspiratory and expiratory volume (FEV1) when stimulated at intensities from 0 mA to 25 mA at C5 and (~75 min) after the first stimulation (Post TESCoN). C: diaphragm electromyography (EMG) during normal breathing before and after 75 min of assessment on the first day of phase II (day 0). D: peak expiration flow over the course of 15 h within a single day during phase I (day −1 in blue) and during phase II (day 1 in red). The 1-h TESCoN therapy occurred during the time window marked by the red box. m, Slope of the linear regression. E: blood pressure and heart rate over the course of 15 hours within a single day during phase I (day −1 in blue) and during phase II (day 1 in red). The 1-h TESCoN therapy occurred during the time window marked by the red box. bpm, beats/min; LDia, left diaphragm; RDia, right diaphragm.
Assessments.
Breathing and coughing assessments were performed in the clinic without and with TESCoN at the start and end of each of the three phases of the study while varying the location (C3, C5, and T1) and intensity (0–25 mA) of stimulation. A minimum 3- to 5-min gap was maintained between each bout of stimulation. In addition, the sites and intensities were randomized to avoid bias. The assessment consisted of 1) ability to inspire using an incentive spirometer (maximum inspiratory volume), 2) peak expiratory airflow during expiration and forced expiratory volume in 1 s (FEV1), measured with an electronic spirometer, and 3) ability to cough, measured by a sound meter (Lee et al. 2017). During all functional assessments of coughing and breathing, EMG from the diaphragm and pelvic floor muscles were recorded. EMG data were filtered using a 60-Hz notch filter and a Butterworth bandpass filter of 10–1000 Hz and were filtered and rectified to analyze area under the curve during breathing and coughing tasks to calculate the mean ± SD integrated EMG (iEMG). A minimum of five cycles were averaged to calculate the mean ± SD. In addition, the patient was instructed to monitor his respiratory function (peak expiratory airflow during expiration and forced expiratory volume in 1 s, FEV1) using the electronic spirometer (MIR Smart One spirometer), blood pressure (wrist-worn sphygmomanometer; Omron), heart rate, and oxygen saturation every hour between 10:00 AM and 10:00 PM, or as often as possible. Standard metrics generated by the spirometer included peak expiratory airflow and forced expiratory volume in 1 s (FEV1) and were captured on a smartphone. All assessments were done with the patient sitting upright in his wheelchair.
Statistical analysis.
All data are reported as actual values or as means ± SD. Mann–Whitney U test was used to compare mean data between different phases of the study or mean data between TESCoN Off and TESCoN On. Linear regression analysis and slope comparisons were performed on multiple recordings within each phase to compare responses from different phases. The criteria level for the determination of a statistical difference was set at P < 0.05 for all comparisons.
RESULTS
During phase I, the patient was asked to measure respiratory and cardiovascular function every hour (from 10:00 AM to 10:00 PM or as often as possible) for 1 wk while maintaining a usual lifestyle including exercise, eating, and sleeping cycles. No major fluctuations in responses of respiratory or cardiovascular parameters were observed. On the first day of phase II, functional assessments were performed without and with TESCoN delivered at C3–4, C5–6, or T1–2 at an intensity of 20 mA (Fig. 2A). The strongest response was observed at C5. Furthermore, with increasing intensity of stimulation at C5–6 from 0 mA to 25 mA, the strongest inspiratory (black) and expiratory (red) response to was observed at 20 mA. Further increases in intensity reduced both inspiratory and expiratory capacities. After ~75 min of assessment including several bouts of TESCoN, inspiratory capacity had increased to its highest level, whereas the recorded expiratory capacity was marginally lower (Fig. 2B). Minimal change in EMG amplitudes of the right and left diaphragm were observed before and after the 75-min assessment session, but a marked increase in cycle duration was observed (Fig. 2C). This increased cycle duration translated to a decrease in breathing rate from ~10 cycles/min to ~8 cycles/min during maximal breathing cycles (Supplemental Video S1; see https://figshare.com/s/2687f22930e3a0ee21c0). The expiratory flow remained high after the assessment session for the rest of the day (slope P < 0.05 compared with phase I) as compared with a representative day from phase I (blue) (mean phase I = 311 ± 33 L/min, mean phase II = 369 ± 39 L/min, P < 0.05; Fig. 2D). Arterial blood pressure increased during TESCoN therapy but returned to a lower baseline shortly after stimulation was stopped.
Over the course of phase II, the highest and the lowest expiratory flow continued to increase compared with phase I values (Fig. 3A). However, these values showed a downward trend once TESCoN therapy was discontinued during phase III. The increased respiratory capacity corresponded to a significant increase in integrated EMG activity in the diaphragm that persisted even in the absence of TESCoN (Fig. 3B). Phase III blood pressure and heart rate were similar to baseline (phase I) (Fig. 3C). No changes in oxygen saturation were noted throughout the study.
Fig. 3.
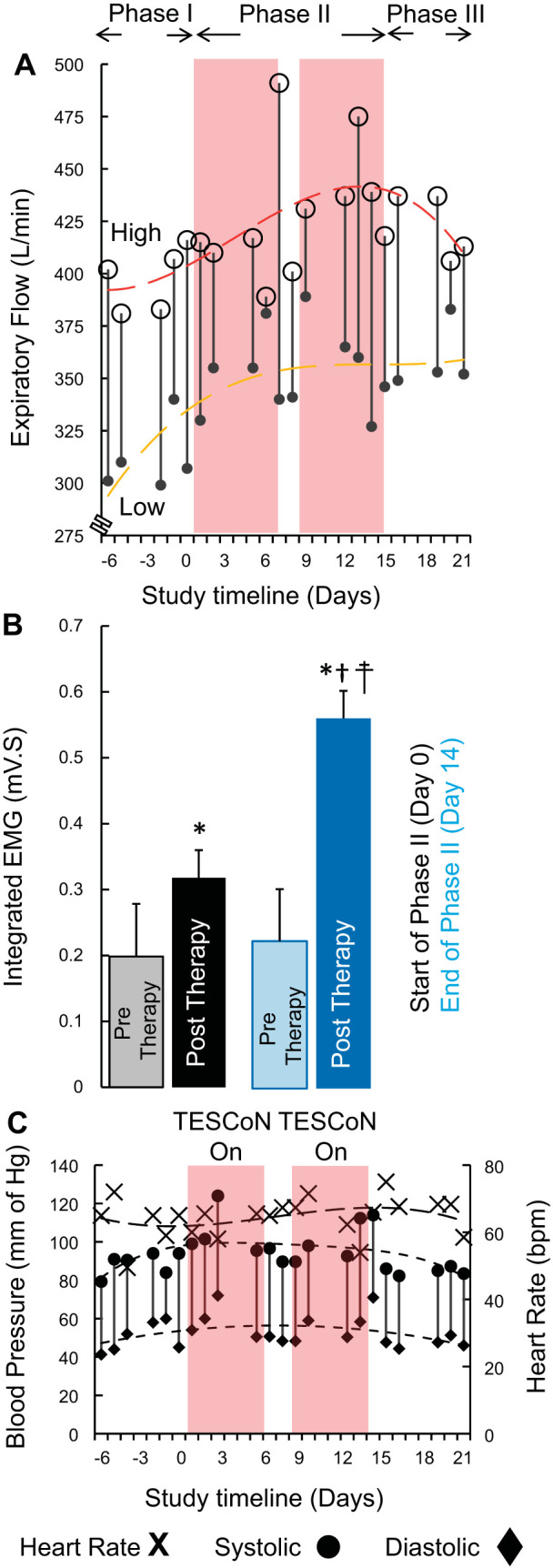
A: highest (open circles) and lowest (solid circle) peak expiratory flow (n = 3 cycles from hourly recordings between 10:00 AM and 10:00 PM) each day during phases I, II, and III. The red and yellow trendlines fit the highest and lowest measures for each day. B: mean (±SD) integrated electromyogram (EMG) during inspiration immediately before the start of phase II (day 0) and immediately after the end of phase II (day 14). Pretherapy recordings were made before initiation of transcutaneous electrical spinal cord neuromodulation (TESCoN). Posttherapy recordings were made after 60 min of TESCoN therapy. C: blood pressure (vertical lines) representing systolic (circle) and diastolic (diamond) data and heart rate (×) recorded during phases I, II, and III. *P < 0.05, statistically different from pretherapy (day 0). †P < 0.05, statistically different from pretherapy (day 14). ‡P < 0.05, statistically different from posttherapy (day 0). Mann–Whitney U test was used to compare mean data (n = 5 cycles) between pretherapy and posttherapy and between day 0 and day 14.
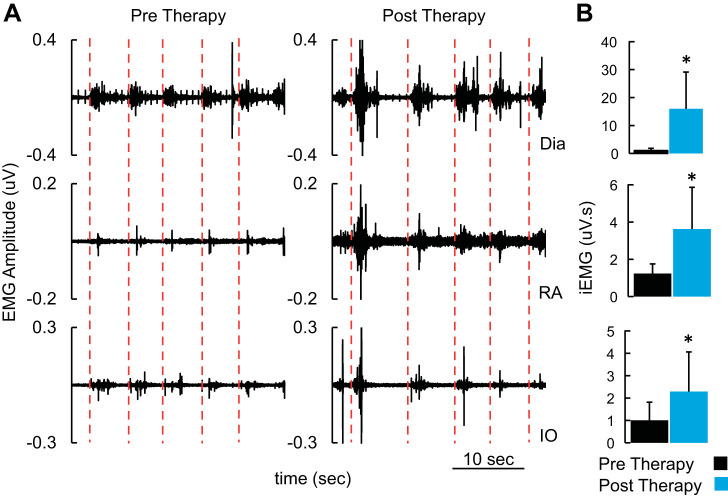
Changes in respiratory function paralleled the improved ability to cough (Fig. 4). Even in the absence of TESCoN, EMG amplitudes and cycle duration of abdominal and diaphragmatic muscles during voluntarily induced coughing were higher at the end of phase II than at baseline. The latency between initiation of cough (red line) and onset of bursts of EMG observed in pelvic floor, abdominal, and diaphragm muscles was shorter at the end of phase II compared with phase I. The strength of cough (average sound generated) increased from 80 dB to 86 dB between start and end of phase II. At the end of phase II, the patient reported a score of 1 (very much better) on the Patient Global Impression of Improvement scale (PGI-I) (Srikrishna et al. 2010). The stimulation was well tolerated by the patient and caused no discomfort. No adverse event was observed during the course of the study.
Fig. 4.
A: electromyogram (EMG) recorded from diaphragm and pelvic floor muscles during voluntary coughing. Note the red lines mark the initiation of the voluntary cough. B: mean (+SD) integrated EMG (iEMG) for each cough cycle shown in A. *P < 0.05, statistically different from pretherapy. Mann–Whitney U test was used to compare mean data (n = 5 cycles) between pretherapy and posttherapy. Dia, diaphragm; IO, internal oblique; RA, rectus abdominus.
DISCUSSION
Within the last decade, a series of studies have shown that chronically implanted epidural spinal electrode arrays can be used to facilitate sensory-motor and autonomic functions of individuals that have been chronically and severely paralyzed by a spinal injury, largely by neuromodulating the excitability states of spinal networks (Angeli et al. 2018; Courtine et al. 2009; Harkema et al. 2011; Hubscher et al. 2018). These studies were followed by the development of a noninvasive transcutaneous spinal stimulation which has resulted in similar effects Gerasimenko et al. 2015c, 2016, 2018). Since we observed functional improvement in several organ systems in individuals with SCI across multiple studies, we hypothesized that transcutaneous spinal cord neuromodulation over the cervical cord would have a positive impact on breathing and coughing.
For individuals that have impaired ventilatory function, access to an effective intervention not requiring any surgery would represent a major advance toward having a more active lifestyle and provide a greater safety net for sustaining metabolic homeostasis and for minimizing airway obstruction because of poor coughing ability. Beyond the functional advantages that could be realized with a noninvasive spinal neuromodulation intervention, the present case study demonstrates the feasibility and application of a patient-friendly technology that could allow the patient to monitor and adjust the neuromodulatory parameters as needed to optimize their ventilator function using an electronic spirometer (MIR Smart One) (Nordlund et al. 2018), wrist-mounted blood pressure and heart rate monitor (Omron) (Kario et al. 2020), and oxygen saturation monitor for home use. In essence, with respiratory and cardiovascular function being monitored noninvasively, a closed-loop system with intermittent objective or patient-reported feedback (and physician guidance as needed) can be provided to the adjust stimulation parameters.
Importance of optimal respiration after paralysis.
Trunk, lumbar, and pelvic stability and ventilatory effectiveness are highly interdependent with the diaphragm, pelvic floor, internal and external obliques, and transverse abdominis muscles. In a normal functional state, none of these muscle groups place the individual in a precarious state unless the total workload duration or the power required for the movement is severely above resting level, but in a tetraplegic state, the muscles that are normally responsible for trunk support become more important for maintaining a critical level of ventilation (Sinderby et al. 1992). This increases the level of metabolic demand on muscles that are not nearly as effective as the diaphragm in sustaining repetitive actions as demanded by breathing. This leaves the individual in a situation where the level of work that can be sustained for rehabilitation and other daily tasks is limited. Thus, in addition to promoting ventilation and oxygenation, improving respiratory function can have downstream effects by reducing the metabolic demands of the accessory respiratory muscles to allow greater rehabilitation capabilities. The data presented herein is the first evidence demonstrating the impact of noninvasive spinal neuromodulation on breathing and coughing acutely and chronically after therapy in a patient with complete tetraplegia. We observed significantly improved breathing and coughing ability after completing 10 treatment sessions within a 14-day period.
These objective changes were reflected in subjective changes noted by the patient such as reduction in the amount of effort needed to breathe and increased levels of energy throughout the day. Furthermore, before the study, the patient required mechanical elevation of his upper torso and head to cough while in the supine. Within 3 days of TESCoN therapy, the patient reported that he was able to cough while in the supine position without truncal elevation for the first time since his injury. This phenomenon was sustained during the 1-wk follow-up period. These data are encouraging in that, with continuing efforts to further refine the treatment parameters and to apply the procedures for more prolonged periods, there is considerable potential in using this intervention to significantly and safety elevate daily ventilatory functions of those individuals with an injury of such severity, especially those that may not qualify for existing neuromodulatory options. In the future, TESCoN may also be used to facilitate ventilator weaning and to lessen or even eliminate ventilator dependence. Given the functional outcomes from the present observations of this single case study, a comprehensive study to better understand the level of functional changes that can occur with refinements in neuromodulatory strategies and identification of patients of different diagnoses are needed.
Other neuromodulatory tools to improve respiratory function in tetraplegics.
Respiratory support is an important aspect of care after spinal cord injury. In the most severe cases, positive pressure ventilation is required. While it is indispensable in high cervical injuries, positive pressure ventilation is associated with significant morbidity by increasing the risk of pulmonary infections, chronic pulmonary disease, and overall mortality (DeVivo et al. 1999). Some patients with a high cervical injury may be candidates for phrenic nerve stimulation or diaphragm pacing. These interventions markedly improve speech and the ability to eat and drink, increase breathing capabilities, reduce the risk of infection and sepsis, and lower the risk of respiratory morbidity by enabling diaphragmatic activity even after phrenic nerve damage (DiMarco 2009). However, given that SCI patients rely so heavily on accessory respiratory muscles, diaphragmatic stimulation may only be one aspect in optimizing breathing and oxygenation. Thus there exists the potential to improve respiratory parameters in SCI patients not only with a more functional diaphragm but also by increasing the functionality of impaired intercostal and trunk muscles. Patients like this may not require positive pressure ventilation, phrenic nerve stimulation, or diaphragm pacing but, nonetheless, stand to benefit from significant pulmonary rehabilitation to optimize function and reduce pulmonary complications. Unlike other neuromodulatory tools, TESCoN modulates the automaticity that is intrinsic to the spinal cord, enabling diaphragm, trunk, and abdominal muscles to be activated in a functionally coordinated pattern that facilitates respiratory function (Freyvert et al. 2018; Gad et al. 2018a; Phillips et al. 2018). In addition, reactivating these intrinsic networks may trigger spinal learning and bidirectional communication with the higher neural centers in the brain, thereby potentially reducing the long-term reliance on stimulation (Gerasimenko et al. 2015c; Harkema et al. 2011).
High-frequency epidural spinal cord stimulation over the cervical regions has shown potential for evoking respiratory function by recruiting inspiratory intercostal muscles with or without synergistic diaphragm contraction in an animal model (DiMarco and Kowalski 2009). However, implantable spinal cord stimulators present the risk and cost associated with invasive surgical approach as well as the limited scope of improvement in ventilatory function only. TESCoN offers a safer, cheaper, and relatively more effective approach, since multiple organ systems can be targeted simply by moving the electrode along the length of the spinal cord. Evidence to date suggests that the transcutaneous approach can have a greater effect by activation of a broader combination of spinal networks.
Limitations and unique features of the study.
This data set can be viewed conservatively given the nonstandard equipment used and assessments performed by the patient himself in the home environment. We suggest, however, that the data are unique, compelling, and robust because of the exploratory strategy that was adopted for a single patient in a real-life environment. This approach provided a more global perspective on the potential effects of TESCoN on multiple organ systems. While this article describes a unique single case experiment, it is limited in the depth of the characterization of the interactions between the respiratory and cardiovascular systems, particularly given the incidence of autonomic dysreflexia that occurs after an SCI. While a more extensive examination of the therapeutic stimulation parameters is needed, the data presented here lie the foundation for a more comprehensive functional and mechanistic clinical investigation in a larger cohort of tetraplegic patients.
Proposed mechanisms of enhanced ventilatory function with spinal neuromodulation.
Using similar neuromodulatory parameters, we observed improvements in musculoskeletal, bladder, and cardiovascular functions after paralysis (Gad et al. 2018b; Kreydin et al. 2020; Phillips et al. 2018). This suggests that a similar neural mechanism underlies our present and previous observations. An important aspect of both transcutaneous and epidural neuromodulation is the submotor intensity of stimulation (Gad et al. 2013). As observed in each of our studies involving epidural and transcutaneous neuromodulation and as evidenced by the decreased responses above 25 mA in the present study, there is a window within which the current level used is most effective. Some subthreshold current levels are essential for the spinal interneuronal control features to function in an enabling manner as opposed to activating at levels that induce a response and thus bypass the control mechanisms that are intrinsic to spinal networks. Thus these approaches seem to promote spinal networks to an excitatory functional state without directly driving each burst of diaphragmatic or truncal muscular activity. Another clear demonstration of the enabling effect is evident during coughing. When a continuous, tonic stimulation pattern is presented to the spinal segment, the diaphragm and trunk muscles respond in a bursting pattern with a higher amplitude, but not with a tonic pattern. Theoretically, this response also reflects the subthreshold phenomenon by more motor units being activated within each of the motor pools because the initial state of excitabilities were closer to the motor pools’ threshold, thus allowing the voluntary effort to reach greater levels of excitation and above the motor threshold of more units. Multiple electrophysiological studies of epidural and transcutaneous spinal stimulation have demonstrated this phenomenon (Angeli et al. 2014; Gad et al. 2015, 2018a; Gerasimenko et al. 2015c; Lu et al. 2016). The above mechanism can account for the acute effects of neuromodulation, but when this enabling phenomenon is repeated over multiple treatment sessions, there is clearly a trigger that initiates what must be a cascade of adaptive events that leads to functional neural reorganization that is manifested as chronic (adaptive, learned) functions that persist for minutes to days. This activity-dependent response also is obviously well suited with ventilation, theoretically providing a breath-by-breath source of functional guidance for network reorganization, even with intermittent neuromodulation, though the synaptic mechanisms through which these changes in connectivity among neurons occur is largely unknown.
GRANTS
This research was funded in part by Walkabout Foundation, Dana & Albert R. Broccoli Charitable Foundation, Nanette and Burt Forester, including matching by PricewaterhouseCoopers LLP, and National Institute of Neurological Disorders and Stroke Grant 1U01NS113871-01.
DISCLOSURES
V.R.E. holds shareholder interest in NeuroRecovery Technologies and holds certain inventorship rights on intellectual property licensed by The Regents of the University of California to NeuroRecovery Technologies and its subsidiaries. V.R.E. and P.G. hold shareholder interest in spineX Inc. and hold certain inventorship rights on intellectual property licensed by The Regents of the University of California to spineX Inc. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
P.N.G. conceived and designed research; P.N.G., E.K., and H.Z. performed experiments; P.N.G. analyzed data; P.N.G. interpreted results of experiments; P.N.G. prepared figures; P.N.G. and E.K. drafted manuscript; P.N.G., E.K., H.Z., and V.R.E. edited and revised manuscript; P.N.G., E.K., H.Z., and V.R.E. approved final version of manuscript.
REFERENCES
- Agostoni E, Torri G, Mognoni P, Bricchi G. [Time constant and viscous resistance of the thoracic cage and of the abdomen-diaphragm ]. Boll Soc Ital Biol Sper 40, Suppl: 2083–2084, 1964. [PubMed] [Google Scholar]
- Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, Ferreira CK, Harkema SJ. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med 379: 1244–1250, 2018. doi: 10.1056/NEJMoa1803588. [DOI] [PubMed] [Google Scholar]
- Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137: 1394–1409, 2014. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RE, Donovan WH, Halstead L, Wilkerson MA. Comparative study of electrophrenic nerve stimulation and mechanical ventilatory support in traumatic spinal cord injury. Paraplegia 25: 86–91, 1987. doi: 10.1038/sc.1987.16. [DOI] [PubMed] [Google Scholar]
- Chien MY, Wu YT, Chang YJ. Assessment of diaphragm and external intercostals fatigue from surface EMG using cervical magnetic stimulation. Sensors (Basel) 8: 2174–2187, 2008. doi: 10.3390/s8042174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 12: 1333–1342, 2009. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil 80: 1411–1419, 1999. doi: 10.1016/S0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- DiMarco AF. Phrenic nerve stimulation in patients with spinal cord injury. Respir Physiol Neurobiol 169: 200–209, 2009. doi: 10.1016/j.resp.2009.09.008. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. High-frequency spinal cord stimulation of inspiratory muscles in dogs: a new method of inspiratory muscle pacing. J Appl Physiol (1985) 107: 662–669, 2009. doi: 10.1152/japplphysiol.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. Activation of inspiratory muscles via spinal cord stimulation. Respir Physiol Neurobiol 189: 438–449, 2013. doi: 10.1016/j.resp.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Onders RP, Kowalski KE, Miller ME, Ferek S, Mortimer JT. Phrenic nerve pacing in a tetraplegic patient via intramuscular diaphragm electrodes. Am J Respir Crit Care Med 166: 1604–1606, 2002. doi: 10.1164/rccm.200203-175CR. [DOI] [PubMed] [Google Scholar]
- Freyvert Y, Yong NA, Morikawa E, Zdunowski S, Sarino ME, Gerasimenko Y, Edgerton VR, Lu DC. Engaging cervical spinal circuitry with non-invasive spinal stimulation and buspirone to restore hand function in chronic motor complete patients. Sci Rep 8: 15546, 2018. doi: 10.1038/s41598-018-33123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad P, Gerasimenko Y, Edgerton VR. Tetraplegia to overground stepping using non-invasive spinal neuromodulation. 9th Int IEEE/EMBS Conf Neural Eng (NER). 2019: 89–92, 2019a. doi: 10.1109/NER.2019.8717096. [DOI] [Google Scholar]
- Gad P, Choe J, Shah P, Garcia-Alias G, Rath M, Gerasimenko Y, Zhong H, Roy RR, Edgerton VR. Sub-threshold spinal cord stimulation facilitates spontaneous motor activity in spinal rats. J Neuroeng Rehabil 10: 108, 2013. doi: 10.1186/1743-0003-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad P, Edgerton VR, Taccola G, Kreydin EI. Transcutaneous Electrical Spinal Cord Neuromodulator and Uses Thereof. US Patent pending. January 24, 2019b.
- Gad P, Gerasimenko Y, Zdunowski S, Turner A, Sayenko D, Lu DC, Edgerton VR. Weight bearing over-ground stepping in an exoskeleton with non-invasive spinal cord neuromodulation after motor complete paraplegia. Front Neurosci 11: 333, 2017. doi: 10.3389/fnins.2017.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad P, Lee S, Terrafranca N, Zhong H, Turner A, Gerasimenko Y, Edgerton VR. Non-invasive activation of cervical spinal networks after severe paralysis. J Neurotrauma 35: 2145–2158, 2018a. doi: 10.1089/neu.2017.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad P, Roy RR, Choe J, Creagmile J, Zhong H, Gerasimenko Y, Edgerton VR. Electrophysiological biomarkers of neuromodulatory strategies to recover motor function after spinal cord injury. J Neurophysiol 113: 3386–3396, 2015. doi: 10.1152/jn.00918.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad PN, Kreydin E, Zhong H, Latack K, Edgerton VR. Non-invasive neuromodulation of spinal cord restores lower urinary tract function after paralysis. Front Neurosci 12: 432, 2018b. doi: 10.3389/fnins.2018.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko Y, Gad P, Sayenko D, McKinney Z, Gorodnichev R, Puhov A, Moshonkina T, Savochin A, Selionov V, Shigueva T, Tomilovskaya E, Kozlovskaya I, Edgerton VR. Integration of sensory, spinal, and volitional descending inputs in regulation of human locomotion. J Neurophysiol 116: 98–105, 2016. doi: 10.1152/jn.00146.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko Y, Gorodnichev R, Moshonkina T, Sayenko D, Gad P, Reggie Edgerton V. Transcutaneous electrical spinal-cord stimulation in humans. Ann Phys Rehabil Med 58: 225–231, 2015a. doi: 10.1016/j.rehab.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko Y, Gorodnichev R, Puhov A, Moshonkina T, Savochin A, Selionov V, Roy RR, Lu DC, Edgerton VR. Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans. J Neurophysiol 113: 834–842, 2015b. doi: 10.1152/jn.00609.2014. [DOI] [PubMed] [Google Scholar]
- Gerasimenko Y, Sayenko D, Gad P, Kozesnik J, Moshonkina T, Grishin A, Pukhov A, Moiseev S, Gorodnichev R, Selionov V, Kozlovskaya I, Edgerton VR. Electrical spinal stimulation, and imagining of lower limb movements to modulate brain-spinal connectomes that control locomotor-like behavior. Front Physiol 9: 1196, 2018. doi: 10.3389/fphys.2018.01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko YP, Lu DC, Modaber M, Zdunowski S, Gad P, Sayenko DG, Morikawa E, Haakana P, Ferguson AR, Roy RR, Edgerton VR. Noninvasive reactivation of motor descending control after paralysis. J Neurotrauma 32: 1968–1980, 2015c. doi: 10.1089/neu.2015.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Bermejo J, LLontop C, Similowski T, Morélot-Panzini C. Respiratory neuromodulation in patients with neurological pathologies: for whom and how? Ann Phys Rehabil Med 58: 238–244, 2015. doi: 10.1016/j.rehab.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Grahn PJ, Lavrov IA, Sayenko DG, Van Straaten MG, Gill ML, Strommen JA, Calvert JS, Drubach DI, Beck LA, Linde MB, Thoreson AR, Lopez C, Mendez AA, Gad PN, Gerasimenko YP, Edgerton VR, Zhao KD, Lee KH. Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia. Mayo Clin Proc 92: 544–554, 2017. doi: 10.1016/j.mayocp.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377: 1938–1947, 2011. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubscher CH, Herrity AN, Williams CS, Montgomery LR, Willhite AM, Angeli CA, Harkema SJ. Improvements in bladder, bowel and sexual outcomes following task-specific locomotor training in human spinal cord injury. PLoS One 13: e0190998, 2018. doi: 10.1371/journal.pone.0190998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inanici F, Samejima S, Gad P, Edgerton VR, Hofstetter CP, Moritz CT. Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia. IEEE Trans Neural Syst Rehabil Eng 26: 1272–1278, 2018. doi: 10.1109/TNSRE.2018.2834339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kario K, Shimbo D, Tomitani N, Kanegae H, Schwartz JE, Williams B. The first study comparing a wearable watch-type blood pressure monitor with a conventional ambulatory blood pressure monitor on in-office and out-of-office settings. J Clin Hypertens (Greenwich) 22: 135–141, 2020. doi: 10.1111/jch.13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski KE, Hsieh YH, Dick TE, DiMarco AF. Diaphragm activation via high frequency spinal cord stimulation in a rodent model of spinal cord injury. Exp Neurol 247: 689–693, 2013. doi: 10.1016/j.expneurol.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreydin E, Zhong H, Latack K, Ye S, Edgerton VR, Gad P. Transcutaneous electrical spinal cord neuromodulator (TESCoN) improves symptoms of overactive bladder. Front Syst Neurosci 14: 1, 2020. doi: 10.3389/fnsys.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KK, Matos S, Ward K, Rafferty GF, Moxham J, Evans DH, Birring SS. Sound: a non-invasive measure of cough intensity. BMJ Open Respir Res 4: e000178, 2017. doi: 10.1136/bmjresp-2017-000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DC, Edgerton VR, Modaber M, AuYong N, Morikawa E, Zdunowski S, Sarino ME, Sarrafzadeh M, Nuwer MR, Roy RR, Gerasimenko Y. Engaging cervical spinal cord networks to reenable volitional control of hand function in tetraplegic patients. Neurorehabil Neural Repair 30: 951–962, 2016. doi: 10.1177/1545968316644344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund B, Carleborg A, Ljungberg H. Effect on asthma control using a novel digital self management system: a physician blinded randomized controlled cross-over pilot trial (Abstract). Eur Respir J 52, Suppl 62: PA4434, 2018. doi: 10.1138/13993003.congress-2018.PA4434. [DOI] [PubMed] [Google Scholar]
- Phillips AA, Squair JW, Sayenko DG, Edgerton VR, Gerasimenko Y, Krassioukov AV. An autonomic neuroprosthesis: noninvasive electrical spinal cord stimulation restores autonomic cardiovascular function in individuals with spinal cord injury. J Neurotrauma 35: 446–451, 2018. doi: 10.1089/neu.2017.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath M, Vette AH, Ramasubramaniam S, Li K, Burdick J, Edgerton VR, Gerasimenko YP, Sayenko DG. Trunk stability enabled by noninvasive spinal electrical stimulation after spinal cord injury. J Neurotrauma 35: 2540–2553, 2018. doi: 10.1089/neu.2017.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayenko D, Rath M, Ferguson AR, Burdick J, Havton L, Edgerton VR, Gerasimenko Y. Self-assisted standing enabled by non-invasive spinal stimulation after spinal cord injury. J Neurotrauma, 36: 1435–1450, 2019. doi: 10.1089/neu.2018.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinderby C, Ingvarsson P, Sullivan L, Wickström I, Lindström L. The role of the diaphragm in trunk extension in tetraplegia. Paraplegia 30: 389–395, 1992. doi: 10.1038/sc.1992.88. [DOI] [PubMed] [Google Scholar]
- Srikrishna S, Robinson D, Cardozo L. Validation of the Patient Global Impression of Improvement (PGI-I) for urogenital prolapse. Int Urogynecol J Pelvic Floor Dysfunct 21: 523–528, 2010. doi: 10.1007/s00192-009-1069-5. [DOI] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil 82: 803–814, 2003. doi: 10.1097/01.PHM.0000078184.08835.01. [DOI] [PubMed] [Google Scholar]