Abstract
A single bout of aerobic exercise improves executive function; however, the mechanism for the improvement remains unclear. One proposal asserts that an exercise-mediated increase in cerebral blood flow (CBF) enhances the efficiency of executive-related cortical structures. To examine this, participants completed separate 10-min sessions of moderate- to heavy-intensity aerobic exercise, a hypercapnic environment (i.e., 5% CO2), and a nonexercise and nonhypercapnic control condition. The hypercapnic condition was included because it produces an increase in CBF independent of metabolic demands. An estimate of CBF was achieved via transcranial Doppler ultrasound and near-infrared spectroscopy that provided measures of middle cerebral artery blood velocity (BV) and deoxygenated hemoglobin (HHb), respectively. Exercise intensity was adjusted to match participant-specific changes in BV and HHb associated with the hypercapnic condition. Executive function was assessed before and after each session via antisaccades (i.e., saccade mirror-symmetrical to a target) because the task is mediated via the same executive networks that demonstrate task-dependent modulation following single and chronic bouts of aerobic exercise. Results showed that hypercapnic and exercise conditions were associated with comparable BV and HHb changes, whereas the control condition did not produce a change in either metric. In terms of antisaccade performance, the exercise and hypercapnic, but not control, conditions demonstrated improved postcondition reaction times (RT), and the magnitude of the hypercapnic and exercise-based increase in estimated CBF was reliably related to the postcondition improvement in RT. Accordingly, results evince that an increase in CBF represents a candidate mechanism for a postexercise improvement in executive function.
NEW & NOTEWORTHY Single-bout aerobic exercise “boosts” executive function, and increased cerebral blood flow (CBF) has been proposed as a mechanism for the benefit. In this study, participants completed 10 min of aerobic exercise and 10 min of inhaling a hypercapnic gas, a manipulation known to increase CBF independently of metabolic demands. Both exercise and hypercapnic conditions improved executive function for at least 20 min. Accordingly, an increase in CBF is a candidate mechanism for the postexercise improvement in executive function.
Keywords: antisaccade, near-infrared spectroscopy, transcranial Doppler ultrasound
INTRODUCTION
Executive function represents a cognitive construct including the core components of inhibitory control, working memory, and cognitive flexibility—processes essential for successful activities of daily living (Diamond 2013). An accumulating literature demonstrates that a single bout of aerobic and/or resistance training provides a transient (i.e., <60 min) “boost” to executive function (for meta-analyses, see Chang et al. 2012; Lambourne and Tomporowski 2010; Ludyga et al. 2016). One explanation for this benefit is an exercise-based increase in regional cerebral blood flow (CBF) leading to improved efficiency within the frontoparietal networks mediating executive function (e.g., Voss et al. 2010). This proposal is indirectly supported by animal and human research reporting that moderate intensity exercise provides a 20–50% steady-state increase in CBF (González-Alonso et al. 2004; Ogoh and Ainslie 2009; Seifert and Secher 2011) that persists for up to 5 min following exercise cessation (Ide et al. 1999) and is observed within frontoparietal structures (Byun et al. 2014; Colcombe et al. 2004; Moriarty et al. 2019). The exercise-based change in CBF may render mechanical and temperature-based changes to the brain’s neural and glial networks that alter the gain of local cortical circuits to improve information processing (Moore and Cao 2008). Moreover, the link between CBF and executive function is supported by evidence that age- and disease-related disruption to CBF impairs executive function (Bertsch et al. 2009).
To our knowledge, no research has examined a benefit to executive function following a transient increase in CBF independent of an exercise manipulation. One method, independent of exercise, known to increase CBF is the inhalation of hypercapnic gas, which leads to a rapid (i.e., <6 s) cerebrovascular vasodilation in response to elevated CO2 and reduced pH (Ainslie and Duffin 2009; Hoiland et al. 2019). A hypercapnic environment increases CBF diffusely across the cerebral cortex and includes a specific increase in frontoparietal executive structures (Mehren et al. 2019). Accordingly, the present study examined executive function before and immediately after a 10-min hypercapnic interval. Estimates of CBF were determined via the combination of blood velocity (BV) through the middle cerebral artery (MCA) and deoxygenated hemoglobin (HHb) as measured by transcranial Doppler ultrasound (TCD) and near-infrared spectroscopy (NIRS), respectively. Notably, TCD changes in BV during a hypercapnic environment robustly correlate with direct measures of CBF (i.e., Xenon tracing of the MCA) and is considered a valid and noninvasive proxy for a direct measure of CBF (see Bishop et al. 1986). As well, changes in NIRS-derived HHb have been linked to changes in oxygen (O2) delivery, assuming O2 uptake is unchanged (e.g., Madsen and Secher 1999), and is indicative of a vascular response. In addition to the hypercapnic manipulation, executive function was examined before and after separate sessions involving 10 min of moderate- to heavy-intensity aerobic exercise (via cycle ergometer) and 10 min of a nonexercise and nonhypercapnic control (i.e., participants sat and rested on the cycle ergometer). In the aerobic exercise session, participant-specific exercise intensities were determined from the end-tidal carbon dioxide () in the hypercapnic condition, a measure providing an indication of CBF responsiveness (Ainslie and Duffin 2009; McSwain et al. 2010; Regan et al. 2014). The control condition was used to determine whether a putative pre- to postimprovement in executive function reflects a change in CBF or relates to a practice-based performance benefit on the antisaccade task (see details below).
Pre- and postcondition executive function was examined via the antisaccade task. Antisaccades involve a goal-directed eye movement (i.e., saccade) mirror-symmetrical to the location of an exogenously presented target stimulus (i.e., 180° spatial transformation) and result in longer reaction times (RT) (Hallett 1978) and less accurate and more variable endpoints (Gillen and Heath 2014a) than counterparts directed to a veridical target location (i.e., prosaccades). Extensive evidence has tied the antisaccade behavioral “costs” to the two-component executive demands of inhibiting a prepotent prosaccade (i.e., response suppression) and inverting a target’s coordinates (i.e., vector inversion) (for review, see Munoz and Everling 2004). The task therefore engages each core component of executive function (i.e., inhibitory control, working memory, and cognitive flexibility). Moreover, antisaccades are mediated via the same frontoparietal (i.e., executive) networks that show increased task-dependent activity following single (Hiura et al. 2010; Seifert and Secher 2011; Verburgh et al. 2014)- and chronic-bout exercise manipulations (Colcombe et al. 2004; Voss et al. 2010). In addition, recent work by our group has shown that 10 min of aerobic exercise completed across a continuum of metabolically sustainable intensities [i.e., 80% of lactate threshold to 50% of the difference between lactate threshold and peak oxygen consumption ()] engenders a reliable decrease in antisaccade (but not prosaccade) RTs immediately and up to 60 min postexercise (Dirk et al. 2020; Heath et al. 2016, 2017, 2018; Petrella et al. 2019; Samani and Heath 2018), and is a result attributed to an exercise-based improvement in executive control. Accordingly, the hands- and language-free nature of antisaccades coupled with the task’s known neuroanatomical substrates provides the requisite resolution to identify subtle changes to executive function (see also Heath et al. 2017; Kaufman et al. 2012; Peltsch et al. 2014).
In terms of research predictions, if the postexercise benefit to executive function is, in part, attributed to an increase in CBF, then the exercise and hypercapnic conditions should demonstrate a postcondition reduction in antisaccade RTs. In contrast, if increased CBF is an epiphenomenon associated with improved executive function, then the exercise, but not hypercapnia, condition should selectively demonstrate a postcondition decrease in antisaccade RT.
METHODS
Participants
Prior to data collection, participants read a letter of information and provided informed written consent via a protocol approved by the Health Sciences Research Ethics Board, University of Western Ontario (no. 113156). This study conformed to the ethical standards set by the most recent iteration of the Declaration of Helsinki with the exception that participants were not registered in a database.
Sixteen (7 women, aged 19–25 yr) undergraduate and graduate students from the School of Kinesiology, University of Western Ontario, volunteered for this study and reported normal or corrected-to-normal vision, self-reported right-hand dominance, and no history of smoking and/or cardiorespiratory, metabolic, musculoskeletal, neurological (including concussion), or neuropsychiatric disorder. The sample size used was determined a priori and was based on an effect size associated with previous work by our group (α = 0.05, power = 0.95) (Samani and Heath 2018; dz = 1.14). Participants reported that they did not take any medication that may affect metabolic, cardiac, respiratory or hemodynamic responses to exercise. All participants obtained a full score on the 2019 Physical Activity Readiness Questionnaire (PAR-Q+) and completed the Godin Leisure-Time Exercise Questionnaire (GLTEQ). The average score on the GLETQ was 65 (SD = 25; range = 28–104) and therefore indicated that all participants were recreationally active.
Experimental Overview
Four experimental conditions were used with each completed on a different day separated by at least 24 h. In one condition, an incremental ramp test to volitional exhaustion was used to determine peak oxygen consumption (). A second condition (i.e., hypercapnic) entailed a 10-min exposure to a hypercapnic environment via the inhalation of a gas mixture containing a higher-than-atmospheric concentration of CO2 (i.e., 5% CO2, 21% O2, 74% N2). In a third condition (i.e., exercise), participants completed a 10-min bout of aerobic exercise (via cycle ergometer) at an intensity (i.e., moderate to heavy) producing an increase in CBF matched to the hypercapnic condition (see details below). Notably, the group respiratory exchange ratio (RER) was 1.05 (SD = 0.06; range = 0.95–1.23) and therefore supports this intensity classification. In a fourth condition (i.e., control), participants sat on the cycle ergometer for 10 min without exercise or being exposed to a hypercapnic environment (i.e., the condition was isocapnic: 0.03% CO2, 21% O2, 78.97% N2 was inhaled). The hypercapnic condition was always completed before the exercise condition so that exercise intensity in the latter could be matched to the increase in CBF associated with the hypercapnic state. The duration of the hypercapnic and exercise conditions was based on work demonstrating that 10 min of aerobic exercise provides a reliable and large magnitude benefit to post exercise executive function (Johnson et al. 2016; Samani and Heath 2018).
Apparatus and Procedures
For the hypercapnic, exercise, and control conditions, transcranial Doppler ultrasound (TCD) (Neurovision 500M, Neurovision TOC2M; Multigon Industries, Elmsford, CA) and near-infrared spectroscopy (NIRS) (Oxiplex TS, model 92505; ISS, Champaign, IL) probes were used to measure 1) blood velocity through the middle cerebral artery (BV), 2) absolute cerebral deoxygenated hemoglobin (HHb), and 3) total hemoglobin concentrations (THC), measures that provide a valid proxy for a direct measure of CBF (Bishop et al. 1986; Madsen and Secher 1999). NIRS and TCD probes were placed on the frons and the left anterior temporal window, respectively, and secured via headband. The TCD probe was coated in an aqueous ultrasound gel (Aquasonic Clear, Parker Laboratories Inc., Fairfield, NJ). Heart rate (HR) was continuously measured via a heart rate monitor (Garmin Premium heart rate monitor; Garmin Ltd., Olathe, KS) and watch (Garmin Vivoactive GPS watch; Garmin Ltd., Olathe, KS). Blood pressure (BP) was taken at regular intervals (i.e., 3, 6, 9, 13, 16, and 19 min) (see below for session-specific timelines) via a manual sphygmomanometer (Welch Allyn FlexiPort reusable blood pressure cuff; Welch Allyn Inc., Skaneateles Falls, NY) secured to participants’ left upper arm.
V̇o2peak Condition
The condition was used to determine participants’ maximal O2 consumption. We did not include a confirmation ride (i.e., ) given that participants were undergraduate or graduate students in kinesiology (see above) and were all familiar with volitional tests to exhaustion (Chidnok et al. 2013). This is important to note because is a reliable measure of maximal O2 consumption for individuals familiar with maximal exercise testing (Poole and Jones 2017). For this test, participants exercised on a cycle ergometer (Velotron; RacerMate, Seattle, WA) with power output independent of pedal cadence, and the cadence set at 70 rpm. This condition involved an incremental ramp test to volitional exhaustion with a work rate increment of 15 Watts (W) per minute (average time to exhaustion: 16 min, SD = 4). Strong verbal encouragement was given to facilitate peak effort. Participants wore a nose clip to prevent breathing from the nose and a rubber mouthpiece, similar to breathing through a snorkel, for breath-by-breath gas exchange analysis of O2 uptake and CO2 output. Air flow and volumes were measured via a bidirectional turbine of 100-mL dead space (VMM 110; Alpha Technologies, Laguna Hills, CA) and pneumotach (model 4813; Hans Rudolph, Shawnee, KS). Fractional concentrations of O2, CO2, and diatomic nitrogen (N2) at the mouth were measured using mass spectrometry (AMIS 2000; Innovision ApS, Glamsbjerg, Denmark). To provide a profile of each breath, a peak-detection algorithm was used to determine end-tidal CO2 () and O2 () pressures with inspired and expired gas volumes and durations time aligned at a sampling rate of 100 Hz.
Hypercapnia Condition
As per the condition, participants sat on a cycle ergometer and ventilated through a modified breathing apparatus that consisted of a mouthpiece attached to a Douglas bag containing hypercapnic gas. The Douglas bag was placed on its side to ensure a constant flow. A two-way valve controlled the nature of the gas concentration (i.e., isocapnic or hypercapnic). When participants were comfortably seated on the ergometer with the mouthpiece appropriately fitted, they were instructed to breathe normally and were provided isocapnic gas for a period of 10 min to establish a resting baseline. Following the 10-min baseline, the two-way valve was flipped and hypercapnic gas was introduced via the Douglas bag and continued for the 10-min intervention, after which the mouthpiece was removed.
Exercise Condition
Participants sat on the cycle ergometer for 4 min to achieve a resting baseline and then pedaled for 6 min at 25 W (i.e., light intensity) to achieve a physiological “steady-state” baseline. Following the steady state, a step transition was introduced to an intensity corresponding to participants’ elevated steady-state during the hypercapnic condition. Specifically, participants’ values during the hypercapnic condition were compared with time point-specific during the task. The delay of the metabolic response (i.e., the time between ramp increment onset and response) and the time spent at baseline during the condition (i.e., 6 min) were subtracted from the total participant-specific ramp time (i.e., 16 min, SD = 4). This time was then used to obtain participant-specific work rates (see Keir et al. 2016, 2018). In particular, the programing for the cycle ergometer was altered to transition in a stepwise fashion from 25 W to a participant-specific wattage that ranged between 65 and 150 W. As per the condition, power output was independent of pedal cadence, and cadence was set at 70 rpm.
Control Condition
Participants sat on the cycle ergometer for a time period equivalent to the hypercapnic and exercise conditions (i.e., 20 min) without being exposed to a hypercapnic or exercise intervention. During this time participants were able to watch a movie of their choice or browse their mobile device. As in the above three conditions, participants wore a nose clip and breathed through a rubber mouthpiece.
Oculomotor Assessment
Participants completed an oculomotor assessment (with which they were not familiarized) before and after the hypercapnic, exercise, and control conditions. For each assessment, participants sat on a height-adjustable chair in front of a table on which an LCD monitor (60 Hz, 8-ms response rate, 1280 × 960 pixels; Dell 3007WFP, Round Rock, TX) was located 550 mm from the table’s front edge. Participants placed their head in a head-chin rest, and the gaze location of their left eye was tracked via a video-based eye tracking system (EyeLink 1000 Plus; SR Research, Ottawa, ON, Canada) sampling at 1,000 Hz. Prior to data collection, a nine-point calibration and validation of the viewing space was completed (i.e., <1° of error). All experimental events were controlled via MATLAB (R2018a; The MathWorks, Natick, MA) and the Psychophysics Toolbox extension (v. 3.0) (Brainard 1997; Kleiner et al. 2007) including the Eyelink Toolbox (Cornelissen et al. 2002). The lights in the experimental suite were extinguished during data collection.
Visual stimuli were presented on a black screen (0.1 cd/cm2) and included a midline-located red fixation cross (1°: 50 cd/m2) presented at participants’ eye level and targets (i.e., open white circle; 2.5° in diameter: 127 cd/m2) presented 15° (i.e., proximal target) and 20° (i.e., distal target) to the left and right of fixation and in the same horizontal plane. Fixation onset signaled participants to direct their gaze to its location. Once a stable gaze was achieved (i.e., ± 1.5° for 450 ms), a uniformly distributed randomized foreperiod (1,000–2,000 ms) was introduced, after which the fixation disappeared and a target appeared 200 ms thereafter (i.e., gap paradigm). Target onset cued participants to saccade mirror-symmetrically to the target location (i.e., antisaccade) as “quickly and accurately as possible.” For each oculomotor assessment, 20 trials to each target location (i.e., left and right visual field) and eccentricity (i.e., proximal and distal) were randomly presented (i.e., 80 total trials).
Data Reduction, Dependent Variables, and Statistical Analyses
In terms of ventilatory and NIRS variables, data points 3 SD from a participant-specific mean were removed (Lamarra et al. 1987). Furthermore, data were linearly interpolated on a second-by-second basis, time-aligned to the onset of an experimental session, and averaged into 5-s time bins (Keir et al. 2015). For TCD, data corrupted by signal aliasing and/or signal loss (e.g., a sudden head shift) were omitted (see Terslev et al. 2017).
For the oculomotor task, trials involving a signal loss (e.g., an eye blink) were omitted. Trials involving anticipatory responses (i.e., RTs < 50 ms; see Wenban-Smith and Findlay 1991) or RTs > 2.5 SD of a participant- and task-specific mean were excluded, as were trials with amplitudes <2° or >2.5 SD of a participant- and task-specific mean (Gillen and Heath 2014b). Less than 10% of trials for any participant were omitted. Furthermore, trials involving a direction error (i.e., a prosaccade instead of an instructed antisaccade) were not included in the analysis of RT or amplitude because they are associated with planning mechanisms distinct from their directionally correct counterparts (DeSimone et al. 2014).
Dependent variables for physiological measures included O2 consumption (), CO2 output (), ventilation (), , THC, HHb, and mean blood velocity (BV). Mean values were determined via the last minute of baseline for hypercapnic and exercise conditions and for the last minute of each intervention (i.e., steady state). For the control condition, physiological measures were computed along a timeline that matched the hypercapnic and exercise conditions. Physiological dependent variables were analyzed via 3 (condition: hypercapnia, exercise, control) by 2 (time: baseline, steady state) fully repeated measures ANOVA (α = 0.05).
Oculomotor dependent variables included RT (i.e., time from response cueing to saccade onset), interquartile range of RT (IQR of RT), the percentage of directional errors (i.e., the completion of a prosaccade instead of an instructed antisaccade), saccade gain (i.e., saccade amplitude/veridical target location), and gain variability (i.e., within-participant variability of saccade gain). For RT, we computed median values given the skewness of their distribution (0.94 < g1 < 1.4; mean = 1.11), whereas mean values were used for saccade gain (0.48 < g1 < 0.69; mean = 0.56). Oculomotor dependent variables were examined via 3 (condition: hypercapnia, exercise, control) by 2 (time: pre-, post-) fully repeated measures ANOVA (α = 0.05).
RESULTS
Ventilatory and Hemodynamic Measures
Ventilatory variables.
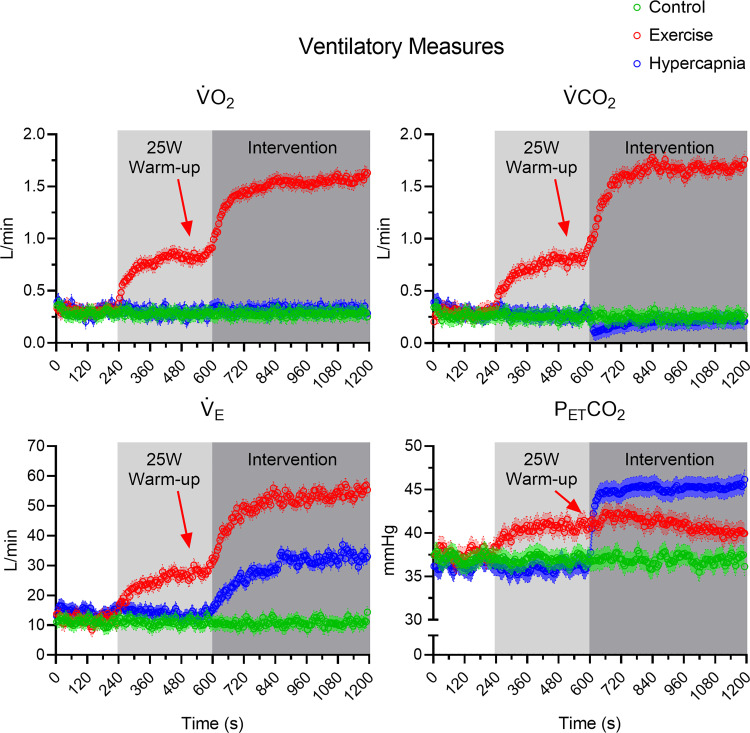
, , and produced main effects for condition (all F1,15 > 15.68, P values < 0.001, all η2 > 0.51), time (all F1,15 > 31.97, P values < 0.001, all η2 > 0.68), and their interactions (all F1,15 > 34.63, P values < 0.001, all η2 > 0.70). Figure 1 shows that for the exercise condition, ventilatory variables increased from baseline to steady state (all t15 greater than −2.40, P values < 0.03, all dz greater than −0.60). For the hypercapnic condition, and increased from baseline to steady state (all t15 greater than −6.87, P values < 0.001, all dz greater than −1.72), whereas baseline and steady-state values did not reliably differ (t15 = −0.25, P = 0.80, dz = −0.06), and decreased from baseline to steady state (t15 = 3.07, P = 0.01, dz = 0.77).1 For the control condition, ventilatory measures did not reliably vary from baseline to steady state (all t15 < 0.39, P values > 0.15, all dz < 0.10) (see Table 1).
Fig. 1.
Group ventilatory data for control, exercise, and hypercapnia conditions presented as 5-s intervals with associated 95% between-participant confidence interval bands. Light and dark gray rectangles depict the durations of an exercise warm-up and intervention (i.e., exercise and hypercapnia), respectively. Red arrows indicate that the 25-W warm-up relates solely to the exercise manipulation. As well, we note that the group average respiratory exchange ratio was 1.05 (SD = 0.06; range = 0.95–1.23) and thus demonstrates that participants exercised at a moderate to heavy intensity. , end-tidal CO2; , CO2 output; , ventilation; , O2 consumption.
Table 1.
Mean baseline, warm-up, and steady-state ventilatory and hemodynamic data for control, exercise, and hypercapnia conditions
| Control |
Exercise |
Hypercapnia |
|||||
|---|---|---|---|---|---|---|---|
| Baseline | Steady state | Baseline | Warm-up | Steady state | Baseline | Steady state | |
| , L/min | 0.29 (0.07) | 0.29 (0.05) | 0.33 (0.09) | 0.83 (0.21) | 1.50* (0.38) | 0.31 (0.11) | 0.32 (0.10) |
| , L/min | 0.25 (0.05) | 0.26 (0.05) | 0.30 (0.08) | 0.79 (0.22) | 1.57* (0.42) | 0.27 (0.08) | 0.23* (0.09) |
| RER | 0.86 (0.10) | 0.90 (0.14) | 0.91 (0.08) | 0.95 (0.09) | 1.05* (0.06) | 0.87 (0.18) | 0.72* (0.20) |
| , L/min | 10.13 (2.43) | 10.99 (2.55) | 12.08 (2.28) | 27.49 (7.28) | 52.92* (12.44) | 14.57 (3.83) | 32.70* (6.32) |
| , mmHg | 36.75 (4.51) | 36.90 (4.12) | 37.01 (4.84) | 40.00 (3.71) | 39.08* (5.22) | 36.58 (6.70) | 45.40* (3.08) |
| THC, mm | 45.86 (21.60) | 45.74 (21.69) | 48.81 (29.77) | 49.12 (27.36) | 47.52 (30.43) | 51.74 (24.13) | 54.19* (24.39) |
| HHb, mm | 10.56 (4.39) | 9.77 (4.23) | 10.21 (6.44) | 10.20 (5.80) | 7.75* (6.37) | 11.36 (5.84) | 9.11* (4.83) |
| BV, cm/s | 60.87 (11.30) | 59.84 (11.82) | 62.82 (10.68) | 69.63 (10.77) | 70.27* (12.63) | 57.50 (11.00) | 78.24* (15.99) |
Group mean (SD) data for O2 consumption (), CO2 output (), respiratory exchange ratio (RER), ventilation (), end-tidal CO2 (), total hemoglobin concentration (THC), deoxygenated hemoglobin (HHb), and blood velocity (BV). Baseline corresponds to 540–600 s during the control and hypercapnia conditions and 180–240 s during exercise. Warm-up corresponds to 540–600 s during the exercise condition. Steady state corresponds to 1,140–1,200 s during each condition.
P < 0.05, steady-state value is reliably different from baseline value.1
Hemodynamic variables.
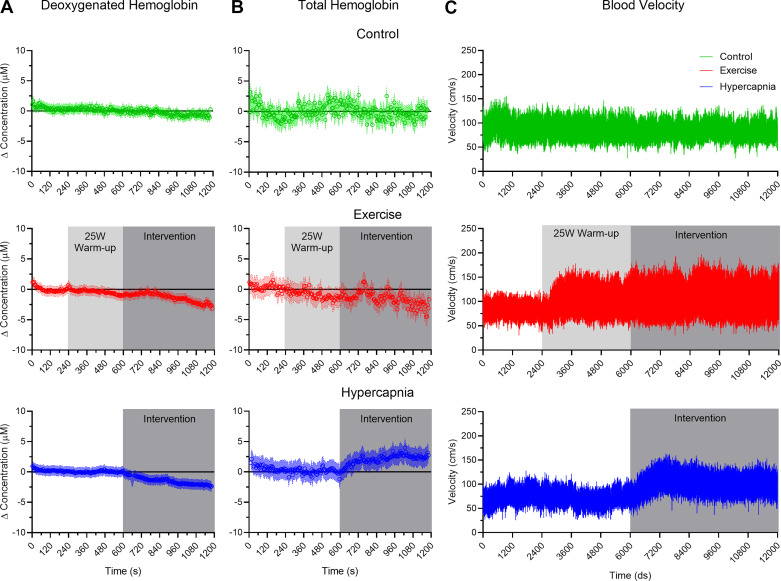
HHb produced a main effect of time (F1,15 = 42.71, P < 0.001, η2 = 0.74) and a condition by time interaction (F1,15 = 8.96, P = 0.001, η2 = 0.37). Figure 2A demonstrates that hypercapnia and exercise conditions produced a significant decrease in HHb from baseline to steady-state (all t15 > 4.02, P values < 0.001, all dz > 1.01), whereas control condition baseline and steady-state values did not reliably vary (t15 = 0.87, P = 0.40, dz = 0.22). In terms of THC, a condition by time interaction (F1,15 = 3.55, P = 0.04, η2 = 0.19) indicated that THC for exercise and control conditions did not reliably vary from baseline to steady state (all t15 < 0.82, P values > 0.42, all dz < 0.21), whereas for the hypercapnia conditions values increased from baseline to steady-state (t15 = 3.70, P = 0.002, dz = −0.93; Fig. 2B and see also Table 1).
Fig. 2.
A and B: normalized (i.e., zeroed to baseline) group average data for deoxygenated hemoglobin (HHb) and total hemoglobin (THC), respectively, presented as 5-s intervals with associated 95% between-participant confidence interval bands. C: an exemplar participant’s blood velocity through the middle cerebral artery (BV; cm/s) via transcranial Doppler (TCD). Light and dark gray rectangles depict the durations of an exercise warm-up and intervention (i.e., exercise and hypercapnia), respectively.
BV produced main effects for condition (F1,15 = 16.87, P < 0.001, η2 = 0.53), time (F1,15 = 28.49, P < 0.001, η2 = 0.66), and their interaction (F1,15 = 25.93, P < 0.001, η2 = 0.63). Figure 2C shows that hypercapnic and exercise conditions produced an increase in BV from baseline to steady-state (all t15 greater than −3.83, P values < 0.002, all dz greater than −0.95), whereas values for the control condition did not reliably differ (t15 = 1.04, P = 0.31, dz = 0.26; see also Table 1).
Oculomotor Performance Measures
Reaction time.
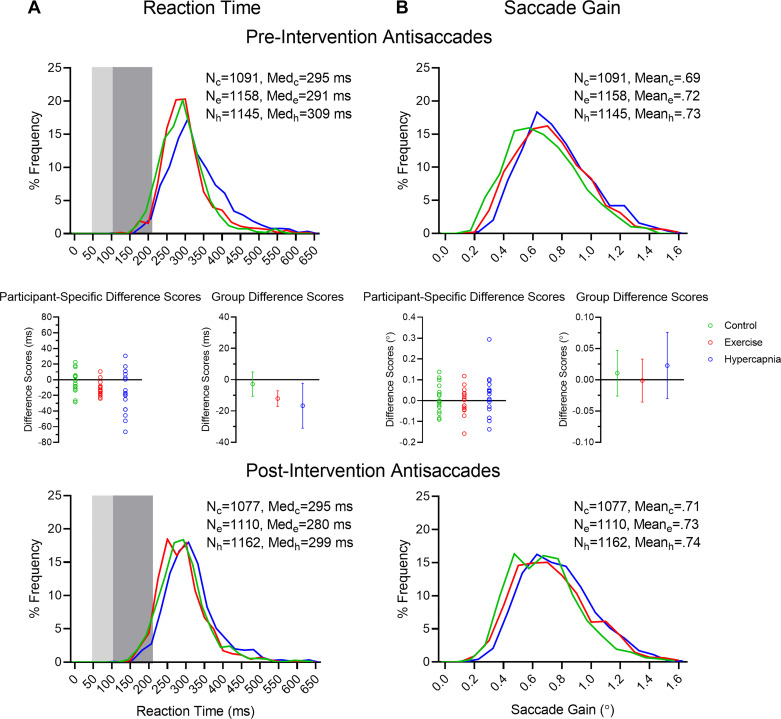
Main effects were observed for condition (F1,15 = 4.06, P = 0.03, η2 = 0.21), time (F1,15 = 9.38, P = 0.01, η2 = 0.39), and their interaction (F1,15 = 3.39, P = 0.047, η2 = 0.18). Figure 3A shows that RTs in the hypercapnic (t15 = 2.46, P = 0.03, dz = 0.61) and exercise (t15 = 5.20, P < 0.001, dz = 1.3) conditions decreased from pre- to postassessments, and the magnitude of this difference did not reliably vary between conditions (t15 = −0.63, P = 0.54, dz = −0.16). In turn, RTs for the control condition did not reliably vary from the pre- to postassessments (t15 = 0.69, P = 0.50, dz = 0.17). IQRs for RT demonstrated a main effect for time (F1,16 = 10.86, P = 0.005, η2 = 0.42); preassessments values (52, SD = 25) were larger than their postassessment counterparts (46, SD = 21).
Fig. 3.
A and B: reaction time (RT) and saccade gain frequency distribution histograms, respectively, for control (c), exercise (e), and hypercapnia (h) conditions pre- (top) and postintervention antisaccades (bottom). Light and dark gray rectangles in A depict anticipatory saccades (i.e., <100 ms) and short-latency saccades (100 < 200 ms), respectively. Total number of trials (N) and median (Med) RT values (A) or mean gain (B) for each condition are indicated. Insets (middle) depict participant-specific median RTs and group mean difference scores (i.e., post- minus precondition) and associated 95% confidence intervals for RT (A) and saccade gain (B).
Directional errors.
Directional errors accounted for 4% of trials and did not elicit any significant main effects or interactions (all F1,15 < 2.10, P values > 0.14, all η2 < 0.13).
Saccade gain and saccade gain variability.
Results for saccade gain and gain variability did not produce any significant main effects or interactions (all F1,15 < 3.10, P values > 0.06, all η2 < 0.17; Fig. 3B).
Antisaccade Difference Scores in Exercise and Hypercapnia Conditions Correlate with Hemodynamic Changes
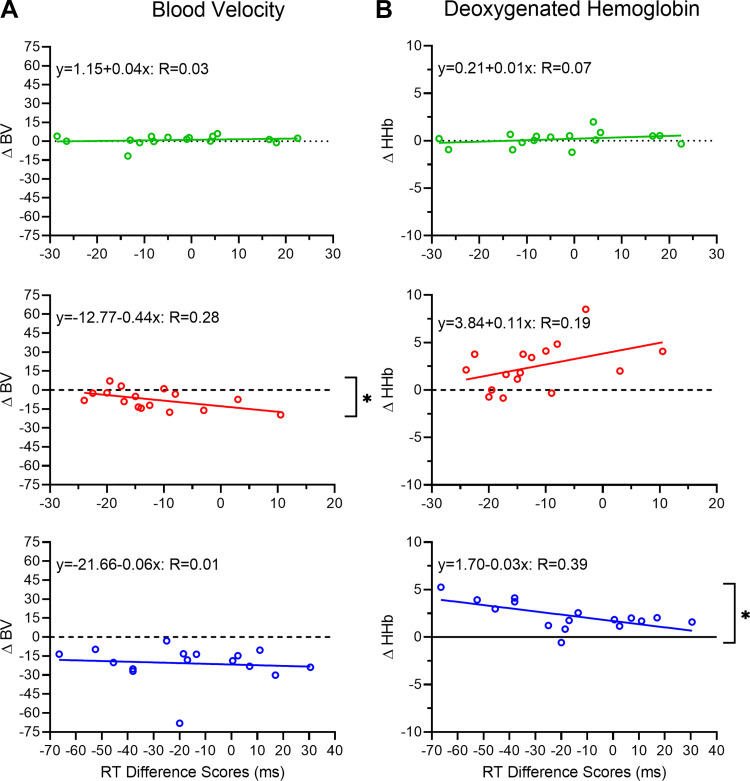
We sought to determine whether the magnitude of a postexercise improvement in antisaccade RT was related to the magnitude of the baseline to steady-state change in hemodynamic responses in the hypercapnic and exercise conditions. Accordingly, we computed participant-specific antisaccade difference scores (i.e., post- minus precondition oculomotor assessment) and correlated those values to BV and HHb difference scores (i.e., baseline minus steady state). Figure 4 demonstrates that for the hypercapnic condition, RT and HHb difference scores were related (r15 = 0.63, P = 0.01), whereas BV difference scores did not relate to RT difference scores (r15 = 0.10, P = 0.75). For the exercise condition, RT and BV difference scores were related (r15 = 0.53, P = 0.04), whereas RT and HHb difference scores were not reliably related (r15 = −0.43, P = 0.10). For the control condition, RT difference scores were not related to HHb (r15 = 0.11, P = 0.69) or BV (r15 = −0.17, P = 0.53) difference scores.
Fig. 4.
A and B: participant-specific blood velocity (BV) and deoxygenated hemoglobin (HHb) difference scores (i.e., baseline minus steady state), respectively, correlated with their respective reaction time (RT) difference scores (i.e., post- minus precondition oculomotor assessment) as a function of control (top), exercise (middle), and hypercapnia (bottom) conditions. Linear regression lines and associated regression equations (and proportion of explained variance) are presented. *P < 0.05, significant correlations.
DISCUSSION
This study examined the effect of aerobic exercise and hypercapnia on postcondition executive function. In outlining our findings, we first discuss the physiological changes associated with our interventions and then address whether our exercise and hypercapnia conditions differentially influenced a postmanipulation benefit to executive function.
Ventilatory and Hemodynamic Data: Increased CBF during Exercise and Hypercapnia
As expected, the control condition did not show a change in ventilatory variables. Furthermore, the exercise condition produced an increase in baseline to steady-state , , and , whereas the hypercapnic condition decreased and and increased from baseline to steady state. The findings for the exercise condition are well documented and reflect a change in venous and muscular CO2 concentrations and oxidative phosphorylation (Coggan et al. 1993; Smith and Ainslie 2017; Thompson 2010; see also Stowe et al. 1975); that is, the change in ventilation variables underscores an adaptive response to the increased metabolic demands of exercise. In turn, findings for the hypercapnia condition indicate an increase in vascular CO2 concentration (Ainslie and Duffin 2009; Coggan et al. 1993). These findings accord a wealth of evidence that a hypercapnic increase in and represents a chemoreceptor-induced change in ventilation (Duffin, 2005). Importantly, the increases in during exercise and hypercapnia are the result of distinct CO2 sources that similarly stimulate central and peripheral chemoreceptors to increase (Ainslie and Duffin 2009; McBryde et al. 2017; Smith and Ainslie 2017).
As hypothesized, the control condition did not alter BV, HHb, or THC. For the exercise and hypercapnia conditions, BV increased and HHb decreased from baseline to steady state, whereas only the latter condition showed a baseline-to-steady-state increase in THC. For the exercise and hypercapnia conditions, the respective changes in the above hemodynamic measures reflect an increase in volumetric flow due to CO2 (for review see Hoiland et al. 2019). The increase in THC, in combination with a decrease in HHb during hypercapnia, reflects an increase in O2 delivery and is a vascular response that may be associated with increased arterial plasma CO2 (Ainslie and Duffin 2009; Hoiland et al. 2019; Robbins et al. 1990; see also Kety and Schmidt 1948; Wasserman and Patterson 1961). Thus, results indicate that exercise and hypercapnic conditions increased CBF due to an increase in arterial CO2 () concentrations.
Increased CBF Improves Executive Function
The exercise and hypercapnic conditions produced a postcondition reduction in antisaccade RTs. These results cannot be attributed to a practice-related performance benefit given that control condition RTs did not change from pre- to postcondition assessments. Moreover, although antisaccade directional errors can show a test-to-retest performance benefit, RTs for such actions are immutable to practice effects (Dyckman and McDowell 2005; see also Ettinger et al. 2003). Indeed, previous work by our group has shown that extensive antisaccade practice (i.e., >500 trials) does not modulate RT (Gillen and Heath 2014a, 2014b; Heath et al. 2015; Weiler et al. 2015; see also Klein and Berg 2001), and Samani and Heath (2018) showed that antisaccade RTs are reduced following an exercise manipulation, but not a condition wherein participants sat and read a magazine for an equivalent period as an exercise manipulation (e.g., Heath and Shukla 2020; Samani and Heath 2018). Put more directly, previous results have shown that the change in antisaccade RT is exercise specific. Moreover, directional errors, gains, and gain variability in exercise and hypercapnic conditions did not vary from pre- to postcondition assessments. This demonstrates that RT changes are independent of a speed-accuracy trade-off (Fitts 1954). In other words, participants did not decrease planning times at the cost of decreased endpoint accuracy. Results for the exercise condition directly support previous work by our group involving a spectrum of exercise durations and intensities (e.g., Heath and Shukla 2020; Heath et al. 2018; Petrella et al. 2019; Samani and Heath 2018), and is a result attributed to a postexercise improvement in executive function. Furthermore, our exercise findings correspond to work reporting that a single bout of exercise elicits an improvement across a number of executive function tasks (e.g., Stroop task, Tower of London, Ericksen flanker; for review see Chang et al. 2014). For the hypercapnic condition, the postcondition decrease in RT demonstrates that executive function is improved independent of an exercise-based increase in metabolic demands. In particular, the combined ventilatory, hemodynamic, and behavioral findings suggest that the improved executive function associated with the hypercapnic condition is related to enhanced CBF.
The exercise and hypercapnia RT difference scores (i.e., postcondition minus precondition) correlated with baseline to steady-state changes in BV and HHb, respectively. The RT-BV correlation during exercise indicates that the exercise-specific vascular response relates to the magnitude of a postexercise improvement in executive function. These results are in line with literature demonstrating a link between BV and improved executive function during exercise. For example, Lucas et al. (2012) found that BV was strongly correlated with executive function, whereas the link with oxygenation was not as robust (see also Fox and Raichle 1986; Stevens et al. 2018). In contrast, recent work has shown a relationship between oxygenation and executive function (Hamasaki et al. 2018; Ji et al. 2019), and the RT-HHb correlation in our hypercapnia condition suggests that improved executive function is related to a CBF-mediated increase in O2 delivery. This finding is aligned with Ji et al.’s (2019) report that a functional NIRS (fNIRS) measure of increased oxygenation during exercise was correlated with improved performance on the executive-mediated Stroop interference task. Similarly, age-related decreases in executive function (via the Stroop task) have been associated with a decrease in cerebral oxygenation hemodynamics (Hamasaki et al. 2018). Accordingly, the literature does not provide equivocal evidence that increased CBF and/or oxygenation account for improved executive function.
As stated by the hemo-neural hypothesis, an increase in CBF induces mechanical- and temperature-based changes to the brain’s neural and glial networks that improve the efficiency of local neural circuits (Moore and Cao, 2008) and may serve to enhance resting-state functional connectivity within frontoparietal executive networks (Kelly et al. 2017; Schmitt et al. 2019). Accordingly, we propose that an increase in CBF reflects the postexercise and posthypercapnia improvement in executive function observed here.
Limitations and Future Directions
We recognize that our study is limited by several methodological traits. First, we examined executive function within the first 20 min following the completion of exercise and hypercapnic conditions. As a result, it is unclear whether a hypercapnia-induced increase in CBF and associated improvement in executive function persists along the same timeline as that associated with an exercise intervention (i.e., <60 min) (see Johnson et al. 2016). Second, the change in BV measured by TCD does not quantify vessel diameter. This is a salient consideration because the MCA is capable of dilation and constriction under hyper- and hypocapnic conditions, respectively (Coverdale et al. 2015), and BV measured by TCD underestimates CBF during hypercapnia (Coverdale et al. 2014). Thus, we cannot directly assert that TCD provides a direct measure of CBF (see Hoiland et al. 2019); however, that BV and HHb increased and decreased, respectively, during the exercise and hypercapnic conditions indicates an increase in volumetric flow (see Ainslie and Duffin 2009; Hoiland et al. 2019). Moreover, that THC increased during the hypercapnia condition indicates that more hemoglobin was present under the NIRS probe and may reflect a vasodilatory response to maintain O2 concentrations in the brain (Ainslie and Duffin 2009). In turn, that THC did not change during the exercise condition may reflect that the response to exercise was not as large as the hypercapnic condition and was transient in nature. Notably, the between-condition difference in THC did not alter the goal of matching hypercapnic and exercise conditions for BV and is likely attributed to the larger pressure gradient associated with the exercise intervention.2 As such, we believe that the combined hemodynamic and ventilatory variables evince an exercise and hypercapnic-based increase in CBF that contributed to improved executive function. Third, the current work did not assess menstrual cycle phase for female participants. This may represent a limitation given the oft-reported view that hormonal variations associated with the menstrual cycle are associated with increased variability in cognitive and physiological variables. In accounting for this potential limitation, a purpose-designed study by our group found that the postexercise benefit to executive function does not vary in magnitude across the different stages of the menstrual cycle (Dirk et al. 2020). Moreover, physiological (i.e., O2 uptake, muscle deoxygenation, blood lactate) and performance (i.e., time to fatigue) variables do not reliably vary across the different phases of the menstrual cycle (Lebrun et al. 1995; McCracken et al. 1994; Redman et al. 2003). Thus evidence suggest that the phase of a female participant’s menstrual cycle should not determine their inclusion (or exclusion) in exercise neuroscience research. Last, our work involved healthy young adults completing a 10-min exercise/hypercapnic manipulation. It is therefore unclear whether our results would extend to older and less physically active individuals and whether our findings would be differentially influenced by a longer exercise/hypercapnic bout (i.e., >10 min).
Conclusion
The present findings demonstrate that a 10-min single bout of moderate- to- heavy-intensity aerobic exercise or exposure to a hypercapnic environment increase CBF and improves executive function. We therefore propose that an increase in CBF is a likely candidate mechanism for the well-documented single-bout postexercise benefit to executive function.
GRANTS
This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada and by Faculty Scholar and Major Academic Development Fund Awards from the University of Western Ontario.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.T., G.R.B., J.K.S., and M.H. conceived and designed research; B.T. and J.J.V. performed experiments; B.T., G.R.B., and M.H. analyzed data; B.T., G.R.B., and M.H. interpreted results of experiments; B.T. and M.H. prepared figures; B.T. and M.H. drafted manuscript; G.R.B., J.K.S., and M.H. edited and revised manuscript; B.T. and M.H. approved final version of manuscript.
Footnotes
is expected to decrease at the onset of hypercapnia and is a result demonstrated in Fig. 1 (Nunn 1960). Notably, however, the decrease from baseline was also observed during the steady-state period (i.e., the last 60 s) of the intervention. Although we are unable to offer a direct explanation for such a result, a between-condition comparison of the last minute of the control and hypercapnia conditions indicated that values did not reliably differ (t15 = 1.70, P = 0.11, dz = 0.43), and data windowed from 140 s into the hypercapnic intervention (i.e., at 740 s) to the end of the intervention produced a reliable (and positive) slope (F1,90 = 48.04, P < 0.001, R2 = 0.35; y = 0.15 + 0.00006x). We believe that such results evince a gradual return of to baseline. Moreover, to account for the unexpected decrease in (i.e., 40 mL/min), we computed baseline and steady-state respiratory exchange ratios (RER; /). Results for the hypercapnia condition showed a decreased RER from baseline to steady state (t15 = 2.42, P = 0.03, dz = 0.61) and suggest CO2 storage allowing for the lower at the end of hypercapnic exposure. Additionally, RER for the exercise condition increased from baseline to steady state (t15 = 2.42, P = 0.03, dz = 0.61), whereas control condition values did not differ from baseline (t15 = −1.75, P = 0.100, dz = −0.44).
In support of this view, baseline systolic (SP) and diastolic (DP) blood pressure for exercise (SP: 125 mmHg, SD = 6; DP: 76 mmHg, SD = 4) and hypercapnia (SP: 123 mmHg, SD = 6; DP: 78 mmHg, SD = 3) conditions did not differ (all t15 < 0.95, P values < 0.36, all dz < 0.24). At steady state, SP was greater for the exercise (162 mmHg, SD = 9) than hypercapnia condition (139 mmHg, SD = 8) (t15 = 3.24, P = 0.005, dz = 0.84), whereas DP did not vary between conditions (exercise: 78 mmHg, SD = 6; hypercapnia: 84 mmHg, SD = 6) (t15 = 1.29, P = 0.22, dz = 0.33).
REFERENCES
- Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296: R1473–R1495, 2009. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Hageman D, Hermes M, Walter C, Khan R, Naumann E. Resting cerebral blood flow, attention, and aging. Brain Res 1267: 77–88, 2009. doi: 10.1016/j.brainres.2009.02.053. [DOI] [PubMed] [Google Scholar]
- Bishop CCR, Powell S, Rutt D, Browse NL. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke 17: 913–915, 1986. doi: 10.1161/01.STR.17.5.913. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis 10: 433–436, 1997. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Byun K, Hyodo K, Suwabe K, Ochi G, Sakairi Y, Kato M, Dan I, Soya H. Positive effect of acute mild exercise on executive function via arousal-related prefrontal activations: an fNIRS study. Neuroimage 98: 336–345, 2014. doi: 10.1016/j.neuroimage.2014.04.067. [DOI] [PubMed] [Google Scholar]
- Chang YK, Chi L, Etnier JL, Wang CC, Chu CH, Zhou C. Effect of acute aerobic exercise on cognitive performance: role of cardiovascular fitness. Psychol Sport Exerc 15: 464–470, 2014. doi: 10.1016/j.psychsport.2014.04.007. [DOI] [Google Scholar]
- Chang YK, Labban JD, Gapin JI, Etnier JL. The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res 1453: 87–101, 2012. doi: 10.1016/j.brainres.2012.02.068. [DOI] [PubMed] [Google Scholar]
- Chidnok W, Dimenna FJ, Bailey SJ, Burnley M, Wilkerson DP, Vanhatalo A, Jones AM. is not altered by self-pacing during incremental exercise. Eur J Appl Physiol 113: 529–539, 2013. doi: 10.1007/s00421-012-2478-6. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Habash DL, Mendenhall LA, Swanson SC, Kien CL. Isotopic estimation of CO2 production during exercise before and after endurance training. J Appl Physiol (1985) 75: 70–75, 1993. doi: 10.1152/jappl.1993.75.1.70. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA 101: 3316–3321, 2004. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen FW, Peters EM, Palmer J. The Eyelink Toolbox: eye tracking with MATLAB and the Psychophysics Toolbox. Behav Res Methods Instrum Comput 34: 613–617, 2002. doi: 10.3758/BF03195489. [DOI] [PubMed] [Google Scholar]
- Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol (1985) 117: 1090–1096, 2014. doi: 10.1152/japplphysiol.00285.2014. [DOI] [PubMed] [Google Scholar]
- Coverdale NS, Lalande S, Perrotta A, Shoemaker JK. Heterogeneous patterns of vasoreactivity in the middle cerebral and internal carotid arteries. Am J Physiol Heart Circ Physiol 308: H1030–H1038, 2015. doi: 10.1152/ajpheart.00761.2014. [DOI] [PubMed] [Google Scholar]
- DeSimone JC, Weiler J, Aber GS, Heath M. The unidirectional prosaccade switch-cost: correct and error antisaccades differentially influence the planning times for subsequent prosaccades. Vision Res 96: 17–24, 2014. doi: 10.1016/j.visres.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu Rev Psychol 64: 135–168, 2013. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirk KL, Belfry GR, Heath M. Exercise and executive function during follicular and luteal menstrual cycle phases. Med Sci Sports Exerc 52: 919–927, 2020. doi: 10.1249/MSS.0000000000002192. [DOI] [PubMed] [Google Scholar]
- Duffin J. Role of acid-base balance in the chemoreflex control of breathing. J Appl Physiol (1985) 99: 2255–2265, 2005. doi: 10.1152/japplphysiol.00640.2005. [DOI] [PubMed] [Google Scholar]
- Dyckman KA, McDowell JE. Behavioral plasticity of antisaccade performance following daily practice. Exp Brain Res 162: 63–69, 2005. doi: 10.1007/s00221-004-2105-9. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Kumari V, Crawford TJ, Davis RE, Sharma T, Corr PJ. Reliability of smooth pursuit, fixation, and saccadic eye movements. Psychophysiology 40: 620–628, 2003. doi: 10.1111/1469-8986.00063. [DOI] [PubMed] [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47: 381–391, 1954. doi: 10.1037/h0055392. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA 83: 1140–1144, 1986. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen C, Heath M. Perceptual averaging governs antisaccade endpoint bias. Exp Brain Res 232: 3201–3210, 2014a. doi: 10.1007/s00221-014-4010-1. [DOI] [PubMed] [Google Scholar]
- Gillen C, Heath M. Target frequency influences antisaccade endpoint bias: evidence for perceptual averaging. Vision Res 105: 151–158, 2014b. doi: 10.1016/j.visres.2014.10.010. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Dalsgaard MK, Osada T, Volianitis S, Dawson EA, Yoshiga CC, Secher NH. Brain and central haemodynamics and oxygenation during maximal exercise in humans. J Physiol 557: 331–342, 2004. doi: 10.1113/jphysiol.2004.060574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res 18: 1279–1296, 1978. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hamasaki A, Akazawa N, Yoshikawa T, Myoenzono K, Tagawa K, Maeda S. Age-related declines in executive function and cerebral oxygenation hemodynamics. Tohoku J Exp Med 245: 245–250, 2018. doi: 10.1620/tjem.245.245. [DOI] [PubMed] [Google Scholar]
- Heath M, Gillen C, Weiler J. The antisaccade task: vector inversion contributes to a statistical summary representation of target eccentricities. J Vis 15: 4, 2015. doi: 10.1167/15.4.4. [DOI] [PubMed] [Google Scholar]
- Heath M, Petrella A, Blazevic J, Lim D, Pelletier A, Belfry GR. A post-exercise facilitation of executive function is independent of aerobically supported metabolic costs. Neuropsychologia 120: 65–74, 2018. doi: 10.1016/j.neuropsychologia.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Heath M, Shellington E, Titheridge S, Gill DP, Petrella RJ. A 24-week multi-modality exercise program improves executive control in older adults with a self-reported cognitive complaint: evidence from the antisaccade task. J Alzheimers Dis 56: 167–183, 2017. doi: 10.3233/JAD-160627. [DOI] [PubMed] [Google Scholar]
- Heath M, Shukla D. A single bout of aerobic exercise provides an immediate “boost” to cognitive flexibility. Front Psychol 11: 1106, 2020. doi: 10.3389/fpsyg.2020.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath M, Weiler J, Gregory MA, Gill DP, Petrella RJ. A six-month cognitive-motor and aerobic exercise program improves executive function in persons with an objective cognitive impairment: a pilot investigation using the antisaccade task. J Alzheimers Dis 54: 923–931, 2016. doi: 10.3233/JAD-160288. [DOI] [PubMed] [Google Scholar]
- Hiura M, Mizuno T, Fujimoto T. Cerebral oxygenation in the frontal lobe cortex during incremental exercise tests: the regional changes influenced by volitional exhaustion. Adv Exp Med Biol 662: 257–263, 2010. doi: 10.1007/978-1-4419-1241-1_37. [DOI] [PubMed] [Google Scholar]
- Hoiland RL, Fisher JA, Ainslie PN. Regulation of the cerebral circulation by arterial carbon dioxide. Compr Physiol 9: 1101–1154, 2019. doi: 10.1002/cphy.c180021. [DOI] [PubMed] [Google Scholar]
- Ide K, Horn A, Secher NH. Cerebral metabolic response to submaximal exercise. J Appl Physiol (1985) 87: 1604–1608, 1999. doi: 10.1152/jappl.1999.87.5.1604. [DOI] [PubMed] [Google Scholar]
- Ji Z, Feng T, Mei L, Li A, Zhang C. Influence of acute combined physical and cognitive exercise on cognitive function: an NIRS study. PeerJ 7: e7418, 2019. doi: 10.7717/peerj.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Addamo PK, Selva Raj I, Borkoles E, Wyckelsma V, Cyarto E, Polman RC. An acute bout of exercise improves the cognitive performance of older adults. J Aging Phys Act 24: 591–598, 2016. doi: 10.1123/japa.2015-0097. [DOI] [PubMed] [Google Scholar]
- Kaufman LD, Pratt J, Levine B, Black SE. Executive deficits detected in mild Alzheimer’s disease using the antisaccade task. Brain Behav 2: 15–21, 2012. doi: 10.1002/brb3.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir DA, Copithorne DB, Hodgson MD, Pogliaghi S, Rice CL, Kowalchuk JM. The slow component of pulmonary O2 uptake accompanies peripheral muscle fatigue during high-intensity exercise. J Appl Physiol (1985) 121: 493–502, 2016. doi: 10.1152/japplphysiol.00249.2016. [DOI] [PubMed] [Google Scholar]
- Keir DA, Fontana FY, Robertson TC, Murias JM, Paterson DH, Kowalchuk JM, Pogliaghi S. Exercise intensity thresholds: Identifying the boundaries of sustainable performance. Med Sci Sports Exerc 47: 1932–1940, 2015. doi: 10.1249/MSS.0000000000000613. [DOI] [PubMed] [Google Scholar]
- Keir DA, Paterson DH, Kowalchuk JM, Murias JM. Using ramp-incremental V̇O2 responses for constant-intensity exercise selection. Appl Physiol Nutr Metab 43: 882–892, 2018. doi: 10.1139/apnm-2017-0826. [DOI] [PubMed] [Google Scholar]
- Kelly NA, Wood KH, Allendorfer JB, Ford MP, Bickel CS, Marstrander J, Amara AW, Anthony T, Bamman MM, Skidmore FM. High-intensity exercise acutely increases substanstia nigra and prefrontal brain activity in Parkinson’s disease. Med Sci Monit 23: 6064–6071, 2017. doi: 10.12659/MSM.906179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 27: 484–492, 1948. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Berg P. Four-week test-retest stability of individual differences in the saccadic CNV, two saccadic task parameters, and selected neuropsychological tests. Psychophysiology 38: 704–711, 2001. doi: 10.1111/1469-8986.3840704. [DOI] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D, Ingling A, Murray R, Broussard C. What’s new in Psychtoolbox-3? Perception 36: 1–16, 2007. [Google Scholar]
- Lamarra N, Whipp BJ, Ward SA, Wasserman K. Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J Appl Physiol (1985) 62: 2003–2012, 1987. doi: 10.1152/jappl.1987.62.5.2003. [DOI] [PubMed] [Google Scholar]
- Lambourne K, Tomporowski P. The effect of exercise-induced arousal on cognitive task performance: a meta-regression analysis. Brain Res 1341: 12–24, 2010. doi: 10.1016/j.brainres.2010.03.091. [DOI] [PubMed] [Google Scholar]
- Lebrun CM, McKenzie DC, Prior JC, Taunton JE. Effects of menstrual cycle phase on athletic performance. Med Sci Sports Exerc 27: 437–444, 1995. doi: 10.1249/00005768-199503000-00022. [DOI] [PubMed] [Google Scholar]
- Lucas SJE, Ainslie PN, Murrell CJ, Thomas KN, Franz EA, Cotter JD. Effect of age on exercise-induced alterations in cognitive executive function: relationship to cerebral perfusion. Exp Gerontol 47: 541–551, 2012. doi: 10.1016/j.exger.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Ludyga S, Gerber M, Brand S, Holsboer-Trachsler E, Pühse U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: a meta-analysis. Psychophysiology 53: 1611–1626, 2016. doi: 10.1111/psyp.12736. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol 58: 541–560, 1999. doi: 10.1016/S0301-0082(98)00093-8. [DOI] [PubMed] [Google Scholar]
- McBryde FD, Malpas SC, Paton JFR. Intracranial mechanisms for preserving brain blood flow in health and disease. Acta Physiol (Oxf) 219: 274–287, 2017. doi: 10.1111/apha.12706. [DOI] [PubMed] [Google Scholar]
- McCracken M, Ainsworth B, Hackney AC. Effects of the menstrual cycle phase on the blood lactate responses to exercise. Eur J Appl Physiol Occup Physiol 69: 174–175, 1994. doi: 10.1007/BF00609412. [DOI] [PubMed] [Google Scholar]
- McSwain SD, Hamel DS, Smith PB, Gentile MA, Srinivasan S, Meliones JN, Cheifetz IM. End-tidal and arterial carbon dioxide measurements correlate across all levels of physiologic dead space. Respir Care 55: 288–293, 2010. [PMC free article] [PubMed] [Google Scholar]
- Mehren A, Diaz Luque C, Brandes M, Lam AP, Thiel CM, Philipsen A, Özyurt J. Intensity-dependent effects of acute exercise on executive function. Neural Plast 2019: 8608317, 2019. doi: 10.1155/2019/8608317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CI, Cao R. The hemo-neural hypothesis: on the role of blood flow in information processing. J Neurophysiol 99: 2035–2047, 2008. doi: 10.1152/jn.01366.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty T, Bourbeau K, Bellovary B, Zuhl MN. Exercise intensity influences prefrontal cortex oxygenation during cognitive testing. Behav Sci (Basel) 9: 1–17, 2019. doi: 10.3390/bs9080083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci 5: 218–228, 2004. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Nunn JF. Elimination of carbon dioxide by the lung. Anesthesiology 21: 620–633, 1960. doi: 10.1097/00000542-196011000-00006. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol (1985) 107: 1370–1380, 2009. doi: 10.1152/japplphysiol.00573.2009. [DOI] [PubMed] [Google Scholar]
- Peltsch A, Hemraj A, Garcia A, Munoz DP. Saccade deficits in amnestic mild cognitive impairment resemble mild Alzheimer’s disease. Eur J Neurosci 39: 2000–2013, 2014. doi: 10.1111/ejn.12617. [DOI] [PubMed] [Google Scholar]
- Petrella AFM, Belfry G, Heath M. Older adults elicit a single-bout post-exercise executive benefit across a continuum of aerobically supported metabolic intensities. Brain Res 1712: 197–206, 2019. doi: 10.1016/j.brainres.2019.02.009. [DOI] [PubMed] [Google Scholar]
- Poole DC, Jones AM. Measurement of the maximal oxygen uptake: is no longer acceptable. J Appl Physiol 122: 997–1002, 2017. doi: 10.1152/japplphysiol.01063.2016. [DOI] [PubMed] [Google Scholar]
- Redman LM, Scroop GC, Norman RJ. Impact of menstrual cycle phase on the exercise status of young, sedentary women. Eur J Appl Physiol 90: 505–513, 2003. doi: 10.1007/s00421-003-0889-0. [DOI] [PubMed] [Google Scholar]
- Regan RE, Fisher JA, Duffin J. Factors affecting the determination of cerebrovascular reactivity. Brain Behav 4: 775–788, 2014. doi: 10.1002/brb3.275. doi: 10.1002/brb3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PA, Conway J, Cunningham DA, Khamnei S, Paterson DJ. A comparison of indirect methods for continuous estimation of arterial PCO2 in men. J Appl Physiol (1985) 68: 1727–1731, 1990. doi: 10.1152/jappl.1990.68.4.1727. [DOI] [PubMed] [Google Scholar]
- Samani A, Heath M. Executive-related oculomotor control is improved following a 10-min single-bout of aerobic exercise: evidence from the antisaccade task. Neuropsychologia 108: 73–81, 2018. doi: 10.1016/j.neuropsychologia.2017.11.029. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Upadhyay N, Martin JA, Rojas S, Strüder HK, Boecker H. Modulation of distinct intrinsic resting state brain networks by acute exercise bouts of differing intensity. Brain Plast 5: 39–55, 2019. doi: 10.3233/BPL-190081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert T, Secher NH. Sympathetic influence on cerebral blood flow and metabolism during exercise in humans. Prog Neurobiol 95: 406–426, 2011. doi: 10.1016/j.pneurobio.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Ainslie PN. Regulation of cerebral blood flow and metabolism during exercise. Exp Physiol 102: 1356–1371, 2017. doi: 10.1113/EP086249. [DOI] [PubMed] [Google Scholar]
- Stevens D, Halaki M, Chow CM, O’Dwyer N. The effects of multi-stage exercise with and without concurrent cognitive performance on cardiorespiratory and cerebral haemodynamic responses. Eur J Appl Physiol 118: 2121–2132, 2018. doi: 10.1007/s00421-018-3942-8. [DOI] [PubMed] [Google Scholar]
- Stowe DF, Owen TL, Anderson DK, Haddy FJ, Scott JB. Interaction of O2 and CO2 in sustained exercise hyperemia of canine skeletal muscle. Am J Physiol 229: 28–33, 1975. doi: 10.1152/ajplegacy.1975.229.1.28. [DOI] [PubMed] [Google Scholar]
- Terslev L, Diamantopoulos AP, Døhn UM, Schmidt WA, Torp-Pedersen S. Settings and artefacts relevant for Doppler ultrasound in large vessel vasculitis. Arthritis Res Ther 19: 167, 2017. doi: 10.1186/s13075-017-1374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DL. What is oxygen consumption? ACSMs Health Fit J 14: 4, 2010. [Google Scholar]
- Verburgh L, Königs M, Scherder EJ, Oosterlaan J. Physical exercise and executive functions in preadolescent children, adolescents and young adults: a meta-analysis. Br J Sports Med 48: 973–979, 2014. doi: 10.1136/bjsports-2012-091441. [DOI] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, Kim JS, Morris KS, White SM, Wójcicki TR, Hu L, Szabo A, Klamm E, McAuley E, Kramer AF. Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia 48: 1394–1406, 2010. doi: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman AJ, Patterson JL Jr. The cerebral vascular response to reduction in arterial carbon dioxide tension. J Clin Invest 40: 1297–1303, 1961. doi: 10.1172/JCI104359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler J, Hassall CD, Krigolson OE, Heath M. The unidirectional prosaccade switch-cost: electroencephalographic evidence of task-set inertia in oculomotor control. Behav Brain Res 278: 323–329, 2015. doi: 10.1016/j.bbr.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Wenban-Smith MG, Findlay JM. Express saccades: is there a separate population in humans? Exp Brain Res 87: 218–222, 1991. doi: 10.1007/BF00228523. [DOI] [PubMed] [Google Scholar]