Abstract
It has been argued that exercise-induced muscle fatigue and tendon vibration can alter proprioceptive estimates of limb position. While exercise-induced muscle fatigue may also affect central efferent processes related to limb position sense, tendon vibration specifically targets peripheral afferent signals. It is unclear, however, whether either of these perturbations (i.e., muscle fatigue or tendon vibration) can alter the multisensory weighting processes preceding goal-directed movements. The current study sought to specifically explore visual-proprioceptive weighting before or after eccentric exercise-induced antagonist muscle fatigue (experiment 1) versus with or without intertrial simultaneous agonist-antagonist tendon vibration (experiment 2). To assess sensory weighting, a visual-proprioceptive mismatch between the participant’s actual initial starting position and the associated visual cursor position was employed. This method provides an estimate of the participant’s reliance on the proprioceptive or visual starting limb position for their aiming movements. Although there was clear evidence of muscle fatigue, there was no systematic alteration of proprioceptive weighting after eccentric exercise and no relationship between sensory weighting and the level of fatigue. On the other hand, participants’ reliance on their actual (proprioceptive) limb position was systematically reduced when exposed to agonist-antagonist tendon vibration before each aiming movement. These findings provide seminal evidence that intertrial tendon vibration, but not exercise-induced fatigue, can alter the reliability of proprioceptive estimates and the relative contributions of visual and proprioceptive information for goal-directed movement.
NEW & NOTEWORTHY Previous work has used muscle fatigue or tendon vibration to perturb proprioceptive limb position estimates. This study sought to determine whether exercise-induced muscle fatigue versus intertrial tendon vibration can alter multisensory weighting for upper limb-aiming movements. By introducing a discrepancy between participants’ actual proprioceptive and visual finger position, this study provides seminal evidence for the reduction of proprioceptive-to-visual weighting using intertrial tendon vibration but no evidence for a systematic reduction following exercise-induced fatigue.
Keywords: action, multisensory, muscle fatigue, proprioception, tendon vibration, visual
INTRODUCTION
Toward the end of a sporting performance, where muscles are in a fatigued state, mistakes or injuries may result from errors in the perception of our limb’s position and/or the proper planning of our movements. Indeed, exercise-induced muscle fatigue can alter both our motor and sensory processes (see Monjo et al. 2015; Proske and Gandevia 2012 for reviews). For example, much of the literature has investigated how muscle fatigue and damage affects proprioception, which includes our sense of limb position and movement (see Proske 2019 for review). Also, muscle tendon vibration has been used to alter afferent proprioceptive information by specifically stimulating the muscle spindles (Aman et al. 2015; Goodwin et al. 1972). For the planning and control of goal-directed movement, afferent proprioceptive information is combined with afferent information from other sensory modalities (i.e., vision) to create estimates of the limb position before and during the movement (e.g., Elliott et al. 2017; Ernst and Bülthoff 2004; Sober and Sabes 2005). However, the relative reliance on information from different sensory modalities is malleable (i.e., sensory weighting: Ernst and Banks 2002; Sarlegna and Sainburg 2007). The purpose of the current study was to investigate how exercise-induced fatigue versus tendon vibration affects the relative reliance on proprioceptive information for the control of goal-directed action, when combined with visual information.
Both peripheral (afferent) and central (efferent) information may contribute to our sense of limb position (e.g., Fuentes and Bastian 2010; Proske and Gandevia 2012; cf. Darling et al. 2018). Afferent information from the muscle spindles appears to be the main contributor to estimates of limb position (see Proske and Gandevia 2012 for review), although the role of afferent information from joint (e.g., Macefield et al. 1990) and skin receptors (e.g., Collins et al. 2005) has been implicated as well. Afferent activity from primary and secondary endings of the muscle spindles change in response to both the velocity of the muscle stretch and the length of the muscle, respectively (e.g., Matthews 1964). Thus muscle spindle firing from muscles surrounding a joint can inform the position and movement of our limb in space, even without contributions from other modalities (e.g., vision). In addition to the incoming afferent information, an efferent motor command in isolation (e.g., Gandevia et al. 2006; Walsh et al. 2010) or in combination with afferent information (e.g., Smith et al. 2009) has been shown to contribute to limb position and movement sense. For example, when asked to flex or extend their immobilized limb, participants’ perceived limb positions were displaced in the direction of and corresponding to magnitude of their effort, with (Gandevia et al. 2006) or without an artificially induced phantom limb (Smith et al. 2009). Thus a perturbation to either the efferent or afferent processes may alter limb position estimates.
Proprioceptive acuity can also be altered after a bout of intense exercise resulting in muscle fatigue (e.g., Allen and Proske 2006; Allen et al. 2010; Tsay et al. 2012; Walsh et al. 2004, 2006; Zabihhosseinian et al. 2015). However, the mechanism by which proprioceptive errors arise is likely centrally as opposed to peripherally driven (Proske 2019). When limb-matching tasks were performed, a bias in limb position persists as long as there is a reduced capacity of the muscle to produce force (Tsay et al. 2012). However, biases in the same direction (e.g., extension) have been shown regardless of the muscle group fatigued (e.g., flexors or extensors: Allen and Proske 2006; Allen et al. 2010). For example, participants matched their fatigued limb position with their unfatigued limb in a more extended position after both elbow flexor and elbow extensor fatigue (Allen et al. 2010). Therefore, the authors suggest that biases in matching performance could not be accounted for by changes in muscle spindle firing in the fatigued limb. This was corroborated by work suggesting that the discharge rate of the muscle spindles is not systematically affected by intense exercise (Gregory et al. 2004) and that proprioceptive biases can extend to the opposite, unexercised limb (Sadler and Cressman 2019). It is also important to consider that the relationship between the motor command and resulting muscle force differs in a fatigued compared with an unfatigued state (Monjo et al. 2015). The increased sense effort in the fatigued muscle may account for 1) errors in force matching between limbs (e.g., Proske et al. 2004); 2) increased proprioceptive biases in the vertical plane (e.g., Walsh et al. 2006); and 3) proprioceptive biases during an active (e.g., Grose 2017) but not a passively controlled movement task (e.g., Chua et al. 2018). Although muscle fatigue may alter the central processes related to limb position estimates, it is also possible to directly affect the peripheral processes related to proprioception.
Proprioceptive acuity can be altered by mechanically vibrating the tendon of a muscle (e.g., Capaday and Cooke 1981; Cordo et al. 1995; Goodwin et al. 1972; Inglis and Frank 1990). Tendon vibration can increase the firing rate of the muscle spindle afferents (Burke et al. 1976; Roll and Vedel 1982) and lead to the perception of muscle lengthening in stationary limbs (e.g., Goodwin et al. 1972). Tendon vibration can also lead to target undershooting, when vibrating the lengthening muscle during a voluntary movement task without vision (e.g., Capaday and Cooke 1981; Cordo et al. 1995; Grose 2017). Critically for the current study, spindle discharge rates are also affected after the vibration has stopped (i.e., vibration aftereffects; Ribot-Ciscar et al. 1998). For example, when the limb was stationary, discharge rates of primary muscle spindle afferents were decreased for up to 40 s in 73% of the fibers and increased for up to 30 s in 13.5% of the fibers following the cessation of 30 s of tendon vibration (Ribot-Ciscar et al. 1998). Thus, while there was an overall reduction in the afferent firing, there was also an increased variability of spindle afferent firing. In addition, the sensitivity of afferent firing to muscle stretch was reduced for at least 3 s and up to 14 s postvibration (Ribot-Ciscar et al. 1998). Therefore, the postvibration firing rates likely resulted in a weaker and more variable proprioceptive signal compared with before vibration (Goodman et al. 2018; see below). When goal-directed movements were employed, using tendon vibration aftereffects to alter proprioceptive processing avoids tonic vibration reflexes in the vibrated muscle and any associated relaxation of antagonist muscles (Eklund and Hagbarth 1966). Experimentally, this can be achieved by employing tendon vibration between each trial. Indeed, intertrial, dual-muscle vibration (i.e., elbow flexors and extensors) led to an increased variability of limb matching (Goodman 2015) and increased trajectory variability during goal-directed movements (Goodman and Tremblay 2018). Thus muscle tendon vibration, which can directly affect the peripheral afferent information, can increase the variability of the proprioceptive estimate of limb position.
Although proprioception has been addressed in isolation thus far, it is important to consider the use of proprioception in combination with other sensory modalities. Indeed, this better reflects daily activities in which we use multiple sources of sensory information to perceive and act in the world. When combining and integrating multiple sensory signals, the weight or reliance associated with each signal is inversely related to the signal’s variance (Ernst and Bülthoff 2004). Models of optimal integration suggest that the central nervous system has access to the variance of each sensory estimate, which in the current study corresponds to visual and proprioceptive estimates (Ernst and Bülthoff 2004). The proprioceptive estimate of limb position is related to muscle spindle afferent firing and can be measured behaviorally with perceptual limb localization tasks. Of the two perturbations to proprioception discussed thus far (i.e., muscle fatigue and tendon vibration), only tendon vibration directly affects the firing of muscle spindle afferents (Gregory et al. 2004; Ribot-Ciscar et al. 1998) and can be associated with an increased proprioceptive perceptual variability in isolation (Goodman 2015). Therefore, it is possible that only tendon vibration but not muscle fatigue can affect the sensory weight or reliance on proprioception in a multisensory task. This is an important contrast that has not been previously explored in the literature and can help differentiate the mechanisms by which exercise-induced fatigue and tendon vibration alter proprioceptive processes. To measure sensory weighting in multisensory contexts, the modalities can be experimentally put in conflict with one another (e.g., Alais and Burr 2004; Ernst and Banks 2002). For a goal-directed movement, the initial actual position of the limb (i.e., proprioceptive signal) can be dissociated from the initial visual position of the limb (e.g., Bagesteiro et al. 2006; Rossetti et al. 1995; Sarlegna and Sainburg 2007; Sober and Sabes 2005). The movement trajectories can be used to measure the participants’ relative reliance on their initial proprioceptive or visual estimates of the limb position and can be altered depending on the context of the task (e.g., target modality: Sarlegna and Sainburg 2007). It is not known, however, how muscle fatigue or tendon vibration can directly affect sensory weighting processes during goal-directed movement.
The current study sought to contrast the effect of exercise-induced muscle fatigue versus intertrial tendon vibration on proprioceptive and visual weighting for the control of upper limb aiming movements. To measure sensory weighting, participants performed targeted movements in the dark with or without an initial mismatch between their actual proprioceptive and visual finger position. In experiment 1, participants performed the aiming movements before and after an eccentric exercise protocol, resulting in muscle fatigue to the antagonist (i.e., lengthening) muscle group. The antagonist muscle was specifically chosen because muscle spindles firing from the lengthening muscle contribute to position sense during movement (e.g., Inglis and Frank 1990). Fatiguing the antagonist muscle group was also employed to minimize changes in the movement trajectory that may have arisen from fatiguing the prime movers for the task (i.e., elbow extensors). Therefore, no changes in movement kinematics were expected after the exercise session. In addition, it was hypothesized that exercise-induced muscle fatigue would not reduce the peripheral proprioceptive afferent information (i.e., muscle spindle firing) and this would not reduce the reliability of the proprioceptive relative to the visual signal. As a result, the relative proprioceptive-to-visual weight was not expected to be reduced after the exercise protocol. In experiment 2, participants were exposed to agonist-antagonist muscle vibration between trials (e.g., Goodman and Tremblay 2018). Tendon vibration to both muscle groups was chosen to minimize biases in estimates of joint angles (e.g., Goodwin et al. 1972). It was hypothesized that intertrial agonist-antagonist tendon vibration would reduce the strength and reliability of the proprioceptive signal relative to the visual signal. As a result of the reduced firing from the proprioceptive afferents (e.g., Ribot-Ciscar et al. 1998), participants were expected to perform aiming movements with larger amplitude in the vibration compared with no vibration trials (e.g., Goodman and Tremblay 2018). Critically and as a result of the reduced strength and reliability of the proprioceptive signal, the relative proprioceptive-to-visual weighting was expected to be reduced when exposed to intertrial vibration compared with no vibration trials.
METHODS
Participants.
Nineteen participants (5 men, mean age: 25 yr old) completed experiment 1 and twelve different participants (2 men, mean age: 23 yr old) completed experiment 2. All participants were from the University of Toronto community and had normal or corrected-to-normal vision with no self-reported history of neurological impairment. Participants in experiment 1 were additionally cleared to complete physical activity using the Physical Activity Readiness Questionnaire for Everyone (PAR-Q+; Warburton et al. 2011). All participants were deemed to be right-handed, as determined through a handedness inventory questionnaire adapted from Oldfield (1971). Four participants from experiment 1 and two participants from experiment 2 were excluded from statistical analyses. In experiment 1, one participant displayed an increase in strength after the exercise protocol and the other three participants experienced difficulties with at least one aspect of the aiming task, which resulted in elimination of over 40% of the trials in either the pre- or postexercise phase. In experiment 2, two participants could not finish the experiment as one or both tendon vibrators malfunctioned during collection. Therefore, 15 participants (5 men, mean age: 25 yr old) in experiment 1 and ten participants in experiment 2 (2 men, mean age: 23 yr old) were included in the statistical analyses. The protocol was approved by the University of Toronto Ethics Review Board, and each participant provided written consent to participate. The protocols in experiments 1 and 2 took ~90 min to complete and participants were compensated $20 and $15, respectively.
Materials and apparatus.
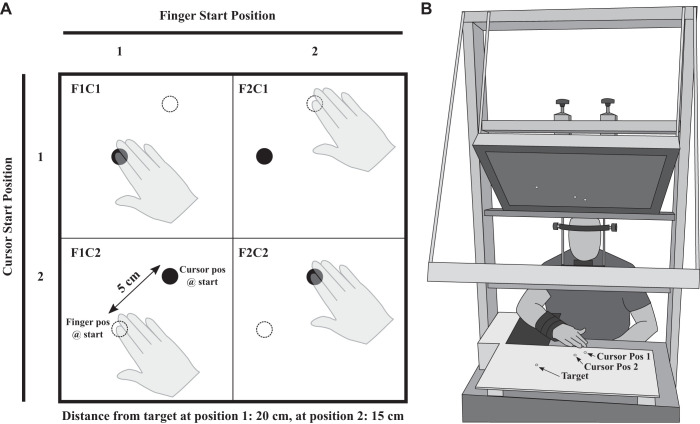
To perform the upper limb-aiming movements, participants were seated at a table including a half-silvered mirror setup (see Fig. 1). With the lights in the room extinguished and with their limb underneath the half-silvered mirror, participants could not see their limb. Above the mirror, an LCD screen (60 Hz) was used to present stimuli (e.g., starting positions, targets, and cursor), which were generated using Psychtoolbox (Brainard 1997; Kleiner et al. 2007; Pelli 1997) through a custom MATLAB script (version R2010a: The MathWorks, Natick, MA). There was one target and two start positions. The target was 20 and 15 cm away from the first and second start position, respectively (see Fig. 1). Both the target and the start positions were green filled circles with a 2-cm diameter. A white filled circular cursor representing a participant’s finger position also had a 2-cm diameter. The mirror was angled such that the visual stimuli appeared aligned with and in the plane of the aiming surface (i.e., horizontal plane).
Fig. 1.
A: the 4 aiming conditions. The cursor is depicted with a black circle, and the limb was not visible. The dotted white circle is a placeholder for the other start position. The aligned conditions are depicted in the top left and bottom right corners in which the starting cursor and starting finger position were in the same location (i.e., F1C1 and F2C2). The misaligned conditions are depicted in the bottom left and top right corners (i.e., F1C2 and F2C1), in which the starting finger position was displaced from the starting cursor position which represented their finger. Note that participants did not have cursor feedback during the movement. B: experimental setup. When the participant’s arm was underneath the half-silvered mirror setup, the participant did not have vision of the limb. The black square underneath the participants arm was Velcro and allowed for a custom positioning of the elbow rest. The 2 cursor starting positions and the target positions are displayed. Note that only 1 cursor starting position was displayed for each trial.
To gather limb position data before the trial and to update the cursor position representing the location of the index finger, an infrared emitting diode (IRED) was sampled at 500 Hz (Optotrak Certus, Northern Digital, Inc., Waterloo, Canada) and was taped to the dorsal surface of a participant’s index fingertip. The IRED position was gathered, and the cursor position was updated until the participant reached the start position. To gather limb position data during a trial, the IRED position was recorded for 1.5 s. An auditory beep from a piezoelectric buzzer (2,900 Hz: Sonalert SC416; Mallory, Inc., Indianapolis, IN) cued participants to begin their movement. A custom MATLAB script was used to control the Optotrak and the piezoelectric buzzer via a data acquisition board (PCI-6024e, National Instruments, Inc., Austin, TX).
There were several steps taken to primarily involve movements around the elbow joint, while limiting movements in other joints. To limit head movements, participants were required to place their chin onto a chin rest. To limit abduction and/or adduction movements at the wrist joint, all participants were required to wear a wrist brace (model 225ZZ: Life Brand, Toronto, ON) throughout the duration of the upper limb-aiming movement task. To limit any movements of the shoulder, participants placed their elbow onto a slightly raised platform and made forearm movements that involved the elbow joint. The position of this platform was adjustable to ensure that each individual participant was in a comfortable position when completing their movements.
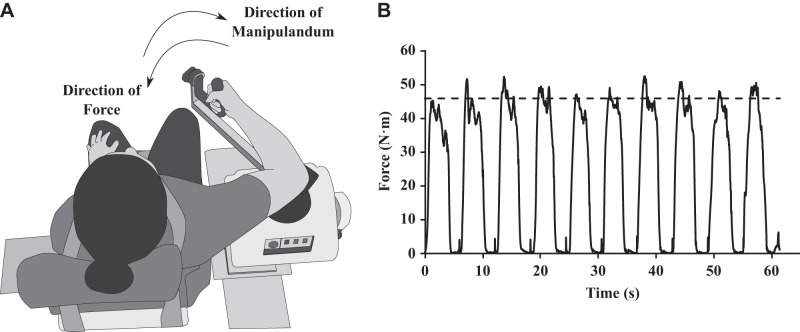
For experiment 1, the exercise protocol was completed using a Biodex System 4 Pro (see Fig. 2A). The system was equipped with an adjustable chair and manipulandum to ensure comfort for each participant. The system measured the torque (N·m) at a sampling frequency of 100 Hz, produced by pulling or pushing against the manipulandum. Each participant’s range of motion was measured, and the manipulandum was restricted to that range to avoid hyperflexion or hyperextension. All movements and measures taken during the exercise protocol were completed in the same plane as the goal-directed movement (i.e., horizontal plane). When the Biodex was set to “isometric mode,” the manipulandum could not be moved and was used to calculate each participant’s maximum voluntary contraction (MVC). For the bout of eccentric exercise, the Biodex was set to “passive mode.” In that case, the manipulandum moved into the direction of elbow extension and the participant pulled against or resisted the movement of the manipulandum (see Fig. 2A). In the “passive mode,” the load was set such that no participant could move the manipulandum into the direction of elbow flexion (i.e., they could not overcome the manipulandum’s force in the extension direction). This ensured that the elbow flexors (i.e., the biceps) were being forcibly lengthened while under tension (i.e., eccentric contraction). Participants were asked to resist the manipulandum or try to pull into the flexion direction at a force corresponding to 65% of their initial MVC. Participants were given visual feedback of their online force output with a visual line indicating the target value (see Fig. 2B and experiment 1 procedure).
Fig. 2.
A: experimental setup used for the exercise session. The participant sat in the Biodex System 4 Pro with their arm in the horizontal plane. During the eccentric contraction, participants were applying force in the flexion direction while the manipulandum was extending their elbow joint. B: online visual feedback available to the participant. This is the force output of 1 set of eccentric contractions from an exemplar participant. During the eccentric contractions, participants were given a target line (dotted line) and asked to reach the goal line with their force. During the protocol, this was continuously updated.
For experiment 2, the main manipulation was intertrial tendon vibration on the agonist and antagonist muscle (i.e., tendons of the elbow flexors and extensors). Such a protocol has been developed in our laboratory and can significantly influence limb trajectories in young healthy adults (see Goodman et al. 2018; Goodman and Tremblay 2018). Two tendon vibrators (Dynatronic VB100, 30 mm in diameter, 75 mm in length, 125 g) were placed on the distal portion of the tendons of the biceps and triceps muscle groups. These areas were manually palpated and located by the experimenter. Athletic polyurethane foam wrap (i.e., 7 cm in width) was used to keep the vibrators in place on the participants right arm. The vibration had an amplitude of 0.5 mm, and the frequency was set at 100 Hz (see Roll and Vedel 1982). Participants had the vibrators on their arm for the entire duration of the experiment.
Experiment 1 procedure.
The experiment was split into three parts: 1) a preexercise upper limb-aiming phase, 2) an eccentric exercise session, and 3) a postexercise upper limb-aiming phase. Participants completed each phase in a repeated-measures design and in that order. The two upper limb-aiming phases were exactly the same, the only difference being the fatigued muscle state of the participant’s biceps muscle group.
The upper limb-aiming phase started with adjusting the height of the chair and the position of the elbow rest to ensure comfort for each participant. Participants performed aiming movements toward a visual target. The direction of the movement was into elbow extension, and therefore, the triceps were considered the agonist and the biceps the antagonist. Participants performed aiming movements from two different start positions (i.e., either 15 cm or 20 cm away from the target). However, the relationship between their actual proprioceptive finger position and their drawn visual cursor position could either be aligned (i.e., veridical) or misaligned. That is, participants performed aiming movements with a combination of two finger positions (F1 or F2) and two cursor positions (C1 and C2; see Fig. 1A). In the aligned conditions (F1C1 and F2C2), both the actual finger positions and the visual representations of their finger positions were the same. In the misaligned conditions (F1C2 and F2C1), the actual finger positions and visual representations of the finger positions did not match. For example, in the F1C2 condition, the finger was 20 cm from the target, but the visual cursor representing the finger location was 15 cm from the target (see Fig. 1A; see Sarlegna and Sainburg 2007). Participants had no prior information regarding the mismatch, and none of the participants were aware of the mismatch during the experiment. This was confirmed after all trials were complete, when the participants were asked if they were aware of any mismatch at any part of the experiment (i.e., “did you notice that your finger was in a different position than the white cursor at any point in the experiment?”). This was also in line with previous work that employed similar methodology with a larger mismatch (i.e., 10 cm; Sarlegna and Sainburg 2007).
The upper limb-aiming phase consisted of calibration, familiarization, and experimental trials. The calibration trials consisted of the participants aligning their visual cursor with the two start positions and then the target position (i.e., 3 trials). The purpose of the calibration trials was to introduce the participants to the movement space and introduce the white visual cursor as a representation of their finger position. The visual cursor and the actual finger position were aligned for all the calibration trials. The familiarization trials consisted of 3 trials of each condition (i.e., 12 trials total), and the experimental trials consisted of 20 trials of each condition (i.e., 80 trials total) for a total of 95 trials.
In both the familiarization and experimental trials, conditions were presented in a pseudorandomized order, with no condition repeating for two trials in a row. At the end of the calibration trials, participants were asked to keep their finger at the target position. The familiarization began with one of the two start positions (i.e., green circle) appearing. Participants were asked to return to the start position, but the cursor representing their finger location was only present when their finger position was within 3 cm of the start position. Therefore, for most of the distance traveling back to a start position, participants did not have cursor feedback. Limiting cursor vision was implemented to reduce any adaptation to, or knowledge of, the misaligned visual-proprioceptive feedback in half of the trials (see Sarlegna and Sainburg 2007). Once the cursor was aligned with the start position (i.e., their white cursor completely covering the green start position), the experimenter started the trial. Once the trial started, the cursor position remained visible and the target appeared. After 300 ms, a 50-ms auditory beep prompted the participants to aim for the target (i.e., “go” signal). The onset of the beep corresponded with the elimination of cursor vision, but the target remained visible. Therefore, cursor vision was only available before the “go” signal. Participants were asked to move to the target “as quickly and as accurately as possible.” The target was extinguished 1,500 ms following the onset of the “go” signal. Between trials, participants remained at the target position and only returned once the new start position appeared. It is important to note that the participant’s finger never rested on or contacted the aiming surface. This included both the time during and between trials. That is, the participant’s arm was slightly elevated during and between the aiming movements. Only during breaks, which were not limited and freely taken by the participants, were participants allowed to take their limb away from the aiming surface for a rest. This was in line with Sarlegna and Sainburg’s (2007) setup and procedures. Also, pilot testing confirmed that any contact with the aiming surface (i.e., at the target or start positions) made the misalignment of the cursor and finger position perceptible. This is likely due to the additional tactile cues, which would be aligned with the proprioceptive cues. The same upper limb-aiming phase, including the randomized order of trials within each participant, was completed after the eccentric exercise session.
After the first aiming session was completed, participants completed the eccentric exercise session. The chair, manipulandum, and range of motion of the Biodex were adjusted for the comfort of the participant. The eccentric exercise session consisted of an initial MVC to test a participant’s baseline strength, followed by a bout of eccentric biceps exercise and then a post MVC to measure the reduction in a participant’s strength. For the initial MVC measurement, the participant’s elbow joint was brought to a 90° angle and the Biodex was set to “isometric mode.” Following an auditory cue, the participants were asked to flex their elbow or bring the manipulandum toward their chest as hard as possible for 2 s and were given visual feedback of their force. The experimenter also provided verbal encouragement to the participants. Participants completed three MVCs with at least 30 s of rest in between each isometric contraction. Each value was recorded, and the largest value from the three attempts was taken as their initial MVC. For the bout of eccentric exercise, the Biodex was set to “passive mode,” taking place at least 60 s after the last MVC. When the manipulandum moved the arm into elbow extension, participants were asked to resist the elbow extension at 65% of their initial MVC value. Participants were given visual feedback of their force with a target line and tried to keep their force as close to the line as possible for the duration of the time the elbow joint was extending (see Fig. 2B). Once the manipulandum got to the maximum range of motion, the manipulandum reversed directions (i.e., elbow flexion). When the manipulandum was moving back toward the participant’s chest, the participant was told to relax. This repeated 10 times for each set with a total of 4 sets. There was 60 s of rest between sets. After the fourth set and another 60 s of rest, the Biodex was set to “isometric mode” again and participants completed three more MVCs to measure their strength reduction. After the exercise session was complete, participants completed the last upper limb-aiming phase (see above).
Experiment 2 procedure.
Participants performed aiming movements under the half-silvered mirror with and without tendon vibration, in a blocked and counterbalanced fashion. That is, half of the participants completed the vibration block first and the no vibration block second. The other half completed the no vibration block first and the vibration block second. Participants completed calibration, familiarization, and experimental trials with the same finger and cursor alignment conditions as experiment 1 (see Fig. 1A). For the experimental trials, there were 15 trials per condition for a grand total of 75 trials. The reduced trial number from experiment 1 was because of the additional time needed for the between-trial tendon vibration and the need to keep the protocol within ~90 min.
There was no vibration during the familiarization trials in both the no vibration and vibration blocks. To start each trial, participants aligned their cursor with the start position. Once their cursor was aligned, the experimenter started the trial. In the vibration block, both vibrators turned on and the cursor position disappeared. The participant was asked to stay still during the vibration. In the no vibration block, the vibrators did not turn on, but the participant was still asked to stay still. The cursor turned on and the participant had to realign with the start position if any proprioceptive drift occurred (see Wann and Ibrahim 1992). Once the cursor was realigned, the experimenter started the trial. The trial timeline was identical to that of experiment 1.
For the vibration block, the vibration turned on for 60 s before the first experimental trial. This initial 60-s bout of tendon vibration was used to introduce the postvibration alteration of muscle spindle firing (Ribot-Ciscar et al. 1998). Specifically, the activity of most muscle spindles was expected to be reduced for up to 60 s, while the activity of other muscle spindles was expected to be increased or remain stable (Ribot-Ciscar et al. 1998). On the subsequent trials, the vibration was turned on for only 5 s before the start of the aiming movement. This procedure replicates that of Goodman and Tremblay (2018) and was employed to ensure that the vibration aftereffects did not diminish over the course of the procedure or during the movement. Following the vibration in this experiment, participants realigned their cursor and were given only a 300-ms preview period before the “go” signal. The average movement time in this experiment was 558 ms (SD = 28 ms), and therefore, all the movements were completed within the critical window of the tendon vibration aftereffect (see Ribot-Ciscar et al. 1998). In experiment 2, to reduce any fatigue associated with any additional time between the trials and/or associated with the vibration, a mandatory break was implemented every 15 trials. The first trial after every break was paired with 60 s of vibration to reintroduce any aftereffects that may have been washed out. Therefore, 4 trials in the vibration block had 60 s of vibration before the aiming movement and 56 trials had 5 s of vibration. In the no vibration block, the participant had to wait at the start position for either 5 or 60 s to account for any effects of time. Between the vibration and no vibration blocks, there was a mandatory 10-min break to wash out any poststimulation effects of the vibration (Goodman and Tremblay 2018). If the no vibration block was completed first, the break was still employed.
Data analysis.
The main dependent variable was the relative weighting between proprioceptive and visual information for the control of upper limb-aiming movements (see Sarlegna and Sainburg 2007). The mismatch between proprioceptive and visual representations was introduced to calculate sensory weighting using the participant’s movement distance for each condition. For the F1C2 conditions, for example, if the participant used only the proprioceptive information of their finger location (20 cm from the target) to plan and execute their movement, they would reach the target location. However, if the participant used only the visual information of their finger location (15 cm from the target) to plan and execute their movement, they would undershoot the target by 5 cm. To calculate sensory weighting, first the average movement distance for each finger position (e.g., F1C1 and F1C2 conditions were averaged to obtain the mean movement distance when the finger was at F1) and the average movement distance for each cursor position was calculated (e.g., F1C1 and F2C1 conditions were averaged to obtain the mean movement distance when the finger was at C1). To assess the effect of initial proprioceptive finger position on movement distance, the difference of the mean movement distances between F1 and F2 was calculated. To assess the effect of initial visual cursor position on movement distance, the difference of the mean movement distances between C1 and C2 was calculated. Finally, the movement distance differences were normalized to the movement distance difference of the aligned conditions (i.e., movement distance in the F1C1 condition − movement distance in the F2C2 condition): for the normalized effect of finger position: (F1 − F2)/(F1C1 − F2C2); and for the normalized effect of cursor position: (C1 − C2)/(F1C1 − F2C2). The resulting normalized values were considered the proprioceptive and visual weights, with the sum of the weights equaling 1. Within a participant, when proprioceptive weight increased, visual weight decreased by the same value, and vice versa. Sensory weights were calculated for each participant separately.
To understand if the bout of eccentric exercise led to a significant reduction in muscle force, a paired-samples t test was conducted on the highest MVC value before and after the exercise session. To understand changes in sensory weighting, a paired-samples t test was conducted on the proprioceptive weights before and after the exercise session in experiment 1 and for the vibration and no vibration trials for experiment 2. To quantify the relationship between fatigue and sensory weighting, a simple linear regression was performed to predict changes in proprioceptive-to-visual weights based on the level of the force deficit after exercise. To examine the effect of time and practice on sensory weighting in experiment 2, we conducted a paired samples t test between the proprioceptive weights of participants’ first and second block, regardless of whether each block was a vibration or a no vibration block. Movement distance was submitted to a 2 Exercise (Pre and Post) × 2 Finger Position (F1 and F2) × 2 Cursor Position (C1 and C2) repeated measures ANOVA in experiment 1 and a 2 Vibration (Vib and NoVib) × 2 Finger Position (F1 and F2) × 2 Cursor Position (C1 and C2) repeated measures ANOVA in experiment 2. Only when the analyses yielded significant interactions, post hoc comparisons were made using Bonferroni corrected t tests (i.e., corrected alpha = 0.017). For all tests, alpha was set at P = 0.05. Although all tests in the ANOVA model were computed (i.e., main effects, two-way interactions, and three-way interaction), only the significant main effects and interactions were reported.
RESULTS
Experiment 1.
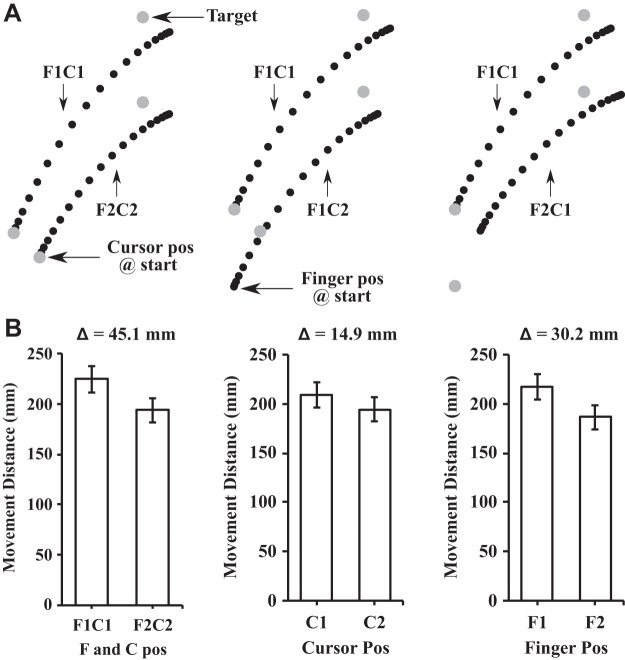
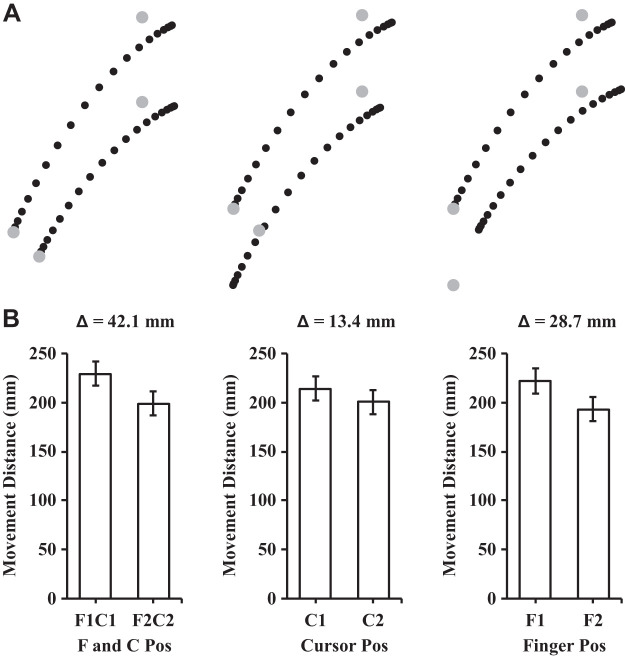
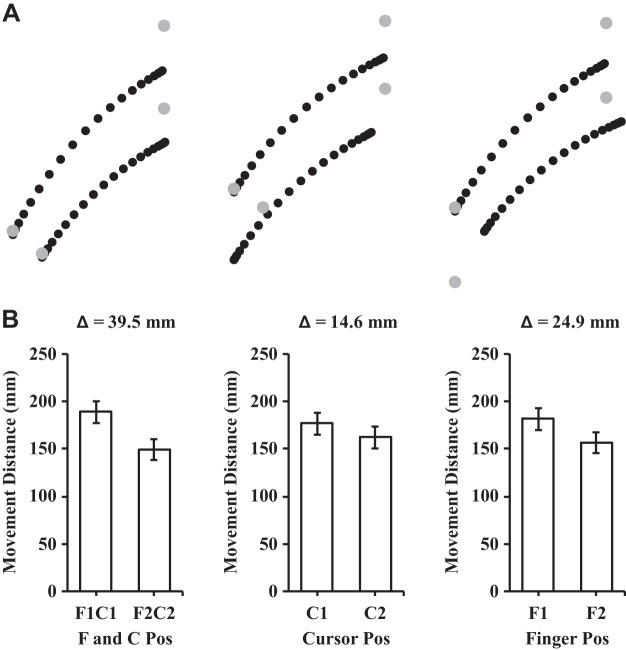
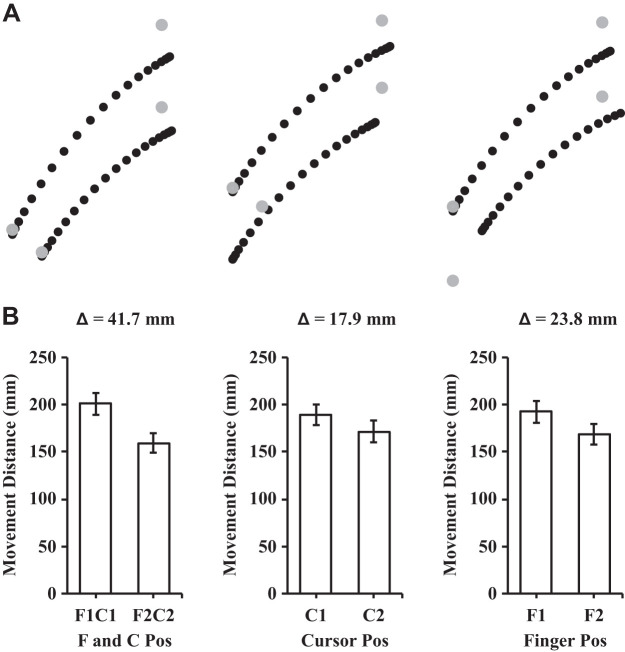
The analysis of the participants’ maximum strength (MVC) before and after the exercise session revealed a significant reduction in their MVC after the exercise session {t(14) = 6.18, P < 0.01, 95% confidence interval (CI) [10.01 N·m, 20.65 N·m]; see Fig. 3A}. The mean reduction in strength was 15.33 N·m (SD = 9.61) which corresponded to 71.1% (SD = 17.5) of the participants’ baseline, preexercise strength. The analysis of proprioceptive weight for the control of movement before and after the exercise session revealed that proprioceptive weight did not differ {t(14) = −0.513, P = 0.62, 95% CI [−0.07, 0.04]; see Fig. 3B}. Figure 4 illustrates the average hand paths (Fig. 4A) and movement distances (Fig. 4B) under aligned and mismatched conditions before the exercise session, and Fig. 5 displays the average hand paths (Fig. 5A) and movement distances (Fig. 5B) after the exercise session. Furthermore, the proprioceptive weight after the exercise session was not correlated with the level of the postexercise force deficit across participants [F(1,13) = 0.02, P = 0.88, R2 = 0.002].
Fig. 3.
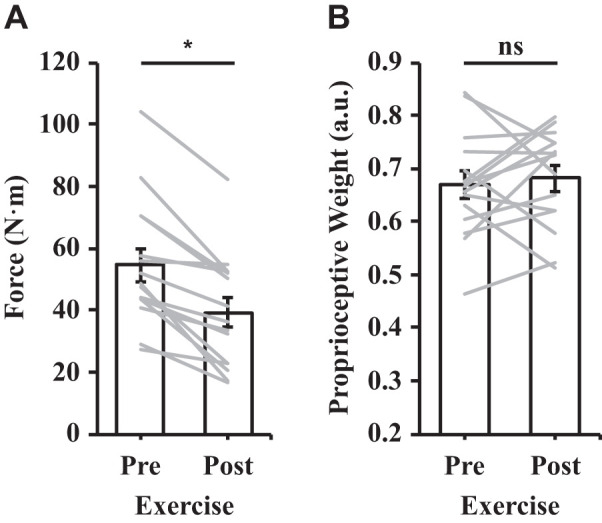
A: mean force before and after the exercise session. B: proprioceptive weight before and after the exercise session; a.u., arbitrary units. See Fig. 4 for the process of calculating proprioceptive weight. Error bars depict means ± SE, and gray lines depict individual data (n = 15). *P < 0.05; ns, not significant.
Fig. 4.
A: mean trajectories for the preexercise aiming conditions. Left: aligned conditions (i.e., F1C1 and F2C2); middle: different cursor positions with the same finger position (i.e., F1C1 and F1C2); right: different finger positions with the same cursor position (i.e., F1C1 and F2C1). Each dot represents the position at every 5% of the movement time. B: mean movement distances in the different finger and cursor positions. The Δ value describes the difference in mean movement distance between the conditions in the associated bar graphs. To calculate proprioceptive weight, the differences in movement distance (Δ = 30.2) between the 2 finger starting positions conditions (i.e., F1 and F2) were collapsed across cursor positions and divided by the difference in movement distance (Δ = 45.1) in the aligned conditions (i.e., F1C1 and F2C2). Error bars depict means ± SE.
Fig. 5.
A: mean trajectories for the postexercise aiming conditions. Left: aligned conditions (i.e., F1C1 and F2C2); middle: different cursor positions with the same finger position (i.e., F1C1 and F1C2); right: different finger positions with the same cursor position (i.e., F1C1 and F2C1). Each dot represents the position at every 5% of the movement time. B: mean movement distances in the different finger and cursor positions. The Δ value describes the difference in mean movement distance between the conditions in the associated bar graphs. Error bars depict means ± SE.
The ANOVA conducted on movement distance yielded a main effect of finger location [F(1,14) = 523.64, P < 0.01, = 0.97], a main effect of cursor location [F(1,14) = 184.26, P < 0.01, = 0.93], and a finger by cursor interaction [F(1,14) = 5.48, P = 0.035, = 0.28]. For the main effects of finger and cursor location, the distance of participants’ aiming movements were larger when their finger or cursor started from the further start positions (i.e., F1 and C1; see Figs. 4B and 5B). For the finger by cursor interaction, we compared the movement distances between C1 and C2 at both finger locations and then compared the amplitude of the differences (i.e., 3 comparisons; corrected alpha = 0.017). The difference in movement distance between C1 and C2 reached significance at both finger locations {i.e., at F1: t(14) = 11.77, P < 0.01, 95% CI [12.70 mm, 18.36 mm]; at F2: t(14) = 12.06, P < 0.01, 95% CI [10.50 mm, 15.05 mm]}. The difference between C1 and C2 was larger at F1 than F2 and approached statistical significance {t(14) = 2.34, P = 0.035, 95% CI [0.23 mm, 5.27 mm]}.
Experiment 2.
The analysis of proprioceptive weight between the vibration conditions revealed that participants exhibited a significantly higher proprioceptive weight in the no vibration than the vibration condition {t(9) = 2.303, P = 0.047, 95% CI [0.001, 0.134]; see Fig. 6}. The analysis of proprioceptive weight between the first and second block of movements revealed no significant differences {t(9) = 0.034, P = 0.973, 95% CI [−0.082, 0.085]}. Figure 7 illustrates the average hand paths (Fig. 7A) and movement distances (Fig. 7B) under aligned and mismatched conditions in the no vibration condition, and Fig. 8 illustrates the average hand paths (Fig. 8A) and movement distances (Fig. 8B) in the vibration condition. In the no vibration condition, changing the finger position had a greater effect on the overall movement distance than in the vibration condition (i.e., notice the larger Δ values in Fig. 7 vs. Fig. 8). This effect is evidenced by the increased contribution of the initial finger position to the overall movement distance in the veridical conditions for the no vibration (64.3%) versus the vibration condition (57.6%).
Fig. 6.
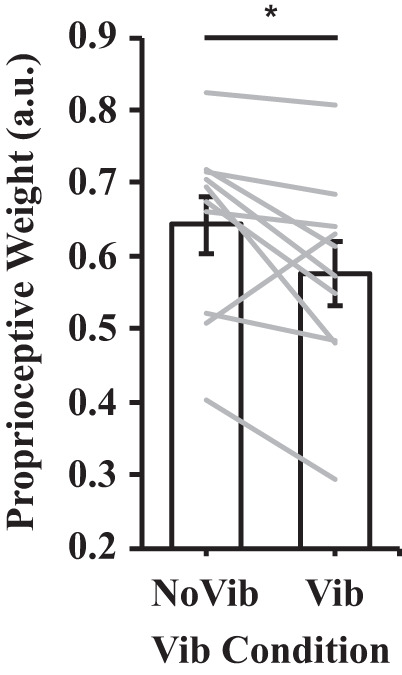
Proprioceptive weight in the no vibration and vibration conditions. See Fig. 4 for the process of calculating proprioceptive weight. Error bars depict means ± SE, and gray lines depict individual data (n = 10). *P < 0.05.
Fig. 7.
A: mean trajectories for the no vibration aiming conditions. Left: aligned conditions (i.e., F1C1 and F2C2); middle: different cursor positions with the same finger position (i.e., F1C1 and F1C2); right: different finger positions with the same cursor position (i.e., F1C1 and F2C1). Each dot represents the position at every 5% of the movement time. B: mean movement distances in the different finger and cursor positions. The Δ value describes the difference in mean movement distance between the conditions in the associated bar graphs. Error bars depict means ± SE.
Fig. 8.
A: mean trajectories for the vibration aiming conditions. Left: aligned conditions (i.e., F1C1 and F2C2); middle: different cursor positions with the same finger position (i.e., F1C1 and F1C2); right: different finger positions with the same cursor position (i.e., F1C1 and F2C1). Each dot represents the position at every 5% of the movement time. B: mean movement distances in the different finger and cursor positions. The Δ value describes the difference in mean movement distance between the conditions in the associated bar graphs. Error bars depict means ± SE.
The ANOVA conducted on movement distance yielded a main effect of vibration [F(1,9) = 7.76, P = 0.021, = 0.46], a main effect of finger position [F(1,9) = 309.41, P < 0.01, = 0.97], and a main effect of cursor location [F(1,9) = 57.76, P < 0.01, = 0.87]. For the main effect of vibration, the distance of participants’ aiming movements were larger in the vibration [mean (M) = 181 mm, SD = 35] than in the no vibration condition (M = 169 mm, SD = 35; see Figs. 7 and 8 for difference in overall movement distance across aiming conditions). For the main effects of finger and cursor location, the distance of participant’s aiming movements were larger when the participant’s finger or cursor started from the further start positions (i.e., F1 and C1; see Figs. 7B and 8B).
DISCUSSION
The goal of this study was to determine whether exercise-induced muscle fatigue and intertrial tendon vibration affected visual-proprioceptive weighting for goal-directed movement. To that end, participants performed aiming movements in the dark with a mismatch between their actual finger position and a visual representation of their finger position. Their movement distance was indicative of the reliance on their visual cursor and/or proprioceptive finger position, which was used to calculate relative visual-proprioceptive weight. In experiment 1, participants performed aiming movements before and after an eccentric exercise protocol, which resulted in significant antagonist muscle fatigue. In experiment 2, participants performed aiming movements with or without dual-muscle tendon vibration between each trial. Of the two perturbations, only dual-muscle intertrial vibration resulted in a systematic reduction in proprioceptive weight for the aiming movements. In other words, participants relied on their actual finger position significantly less during the vibration compared with the no vibration trials, whereas the reliance on their actual finger position did not systematically change after antagonist muscle fatigue.
The eccentric exercise protocol employed in the current study resulted in significant fatigue to the antagonist muscle group (i.e., elbow flexors). After the exercise protocol, the average decrease in the participants’ maximum voluntary contraction strength (M = 29%, SD = 18) was comparable to the decrease in strength in similar studies investigating muscle fatigue and proprioception (e.g., Allen et al. 2010: M = 28% decrease, SD = 3; Walsh et al. 2006: M = 34% decrease, SD = 9). In the current study, however, the exercise prescription (i.e., 4 sets of 10 repetitions with a submaximal load) was the same for each participant and was chosen to reflect a typical resistance training protocol (e.g., Abou Sawan et al. 2018; Song et al. 2017) and one that could be prescribed to a group of athletes, for example. This method differs from other studies that employed a custom exercise prescription for each participant involving repeated sets until a certain level of force deficit is obtained (e.g., Allen et al. 2010). Although the exercise protocol employed in the current study resulted in a high level of between-participant force deficit variability, this also allowed for the quantification of a potential relationship between the level of fatigue and change in sensory weighting in typical exercise regimes.
Intertrial vibration, but not exercise-induced fatigue, led to a change in movement distance. A change in movement distance was not hypothesized after the exercise session as only the antagonist muscle group was subjected to fatiguing exercise as opposed to the prime movers (i.e., elbow extensors). When exposed to tendon vibration before each trial, however, participants performed significantly longer aiming movements (i.e., increased movement distance) compared with trials without tendon vibration. This effect was unlikely the result of any perceptual biases before movement onset. Indeed, tendons of both the agonist and antagonist muscle group were exposed to vibration to avoid any known biases in either the flexion or extension direction that exist when vibrating the tendons of the biceps or triceps only (e.g., Goodwin et al. 1972). The reduced firing of the muscle spindle afferents after the cessation of tendon vibration may explain the increased movement distance in the vibration condition (Ribot-Ciscar et al. 1998). Indeed, after prolonged biceps vibration, participants can experience an illusion of flexion at the elbow that is opposite to the illusion experienced during the vibration (Seizova-Cajic et al. 2007). In the context of the elbow extension movement used in this study, the overall reduced firing rate of the lengthening muscle may have led to the perception that the arm was extending at a slower rate and to a less extended position than it actually was. Therefore, this perceptual bias may account for an increased movement amplitude in which the participants elbows were in a more extended end point position than without tendon vibration (see also Goodman and Tremblay 2018). The increase in movement amplitude after vibration in this study supports a similar mechanism as decreases in movement amplitude when tendon vibration is applied during the movement that increases muscle spindle firing in the lengthening muscle (e.g., Capaday and Cooke 1981; Grose 2017). While changes in movement distance replicate (Goodman and Tremblay 2018) and lend support to previous findings (e.g., Capaday and Cooke 1981; Cordo et al. 1995; Inglis and Frank 1990), the current study extends previous work by quantifying changes in sensory weighting following exercise-induced fatigue and intertrial tendon vibration.
Across both experiments, participants used a combination of both proprioceptive and visual information regarding their initial finger position to control their aiming movements (see Bagesteiro et al. 2006; Rossetti et al. 1995; Sarlegna and Sainburg 2007; Sober and Sabes 2005). However, participants relied more heavily on proprioceptive than on visual information, as reflected in the mean proprioceptive weight for preexercise and no vibration conditions (M = 66%, SD = 11). When considering that participants were aiming for a visual target, the proprioceptive-to-visual weight was high compared with similar studies (e.g., Sarlegna and Sainburg 2007: mean proprioceptive weight = 37%, SD = 11). The discrepancy in sensory weighting can be attributed to differences in the experimental setups. In Sarlegna and Sainburg (2007), the participant’s right arm was supported in the horizontal plane by a frictionless air jet system, which minimizes sensory cues related to gravity and friction. With the setup in the current study, the participant’s right arm was unsupported and their movements pivoted from the elbow resting on a surface. The proprioceptive-to-visual weight in the current study suggests that gravitational cues and/or tactile information from the elbow generated from the movement may have provided additional limb position estimates. Indeed, it has been reported that higher limb-matching variability was associated with conditions in which the limbs were supported (i.e., akin to Sarlegna and Sainburg 2007) compared with conditions in which the limbs were unsupported (i.e., akin to the current study; Walsh et al. 2006). As well, humans can quickly and accurately use tactile information for spatial processing (e.g., Pruszynski et al. 2018). Therefore, proprioceptive estimates of limb position may be more reliable when the limb is unsupported and tactile information from the movement is available. Furthermore, an increased baseline proprioceptive weight can be advantageous for studies perturbing proprioceptive estimates as it avoids floor effects if the baseline proprioceptive weight is low. Thus, although the baseline sensory weights differed from previous work, the current experimental setup was sensitive to changes in proprioceptive-to-visual weighting using intertrial tendon vibration.
Exercise-induced muscle fatigue did not systematically reduce the proprioceptive-to-visual weighting for goal-directed movement. Furthermore, each individual participant’s change in proprioceptive weight from pre- to postexercise was not related to their level of postexercise force deficit. This finding suggests that muscle fatigue of the antagonist muscle group does not reduce the reliability of proprioceptive information. Both neurophysiological and behavioral evidence support the lack of an effect of antagonist muscle fatigue on proprioceptive weighting. Indeed, muscle spindle afferents firing was unaltered following eccentric exercise resulting in muscle damage (e.g., Gregory et al. 2004). In addition, participants overestimated the angle of their limb regardless of the muscle group fatigued (i.e., elbow flexors or extensors; Allen et al. 2010) and limb localization biases can extend to the unfatigued limb without any changes in limb localization bias (Sadler and Cressman 2019). The only studies that displayed an increase in proprioceptive variability employed limb-matching or end point position tasks in the vertical plane (e.g., Tsay et al. 2012; Vafadar et al. 2012). It is also important to note that the limb-matching variability did not immediately increase after exercise but emerged between 2 and 24 h after exercise (Tsay et al. 2012). This may suggest that an increased sense of effort to match the limbs against gravity (i.e., in the vertical plane) after fatigue may have led to an increased limb-matching variability (Tsay et al. 2012). However, other limb-matching studies in the vertical plane do not report a change in limb-matching variability in a loaded compared with an unloaded arm (Allen et al. 2007). Participants in the current study performed all movements, including the exercise protocol, in the horizontal plane. In addition, the antagonist muscle group as opposed to the agonist muscle group was fatigued. Therefore, the current study cannot address whether changes in output variability can lead to changes in sensory weighting. Nevertheless, models of multisensory integration suggest that the weight given to or reliance on sensory estimates is inversely related to the variance of the afferent information (Ernst and Bülthoff 2004). Although muscle fatigue has the potential to alter the central efferent processes related to limb position estimates, the current results suggest that the afferent proprioceptive information was not systematically altered by fatigue and subsequently did not change sensory weighting processes. Future experiments should thus consider passive movement conditions to further isolate afferent processes and explore its relationship with fatigue (e.g., Chua et al. 2018).
One alternative interpretation for the lack of an effect of muscle fatigue on sensory weighting is that muscle fatigue led to alterations of proprioceptive weight for movement that differed across individuals and instead manifested in increases and decreases in weighting from pre- to postexercise. Indeed, an examination of each participant’s change in proprioceptive weight from pre- to postexercise (see Fig. 3B) reveals that eight individual’s proprioceptive weight increased, five decreased, and two did not change (i.e., difference under 1%). Incidentally, the average absolute magnitude of the difference from pre- to postexercise (M = 8%, SD = 5) did not differ from the absolute magnitude of the difference between no vibration and vibration trials [M = 9%, SD = 6; t(23) = 0.50, P = 0.62]. One possible explanation for increased proprioceptive weighting in some individuals may be that the bout of exercise acted as a warm-up between aiming phases. For the lower limb, there is evidence for an increase in proprioceptive acuity after a dynamic warm-up (e.g., Daneshjoo et al. 2012; Walsh 2017). However, the load in warm-ups is typically much lower than that prescribed in the current exercise protocol (i.e., 65% of each individual’s MVC), and there was no relationship between the level of force deficit after exercise and change in proprioceptive weight. Also, we did not collect information about the participant’s level of physical activity. Future research on this topic should perhaps explore individual differences further. Nevertheless, eccentric exercise and muscle fatigue did not yield a systematic increase or reduction in proprioceptive weight for goal-directed movements.
On the other hand, intertrial vibration of the agonist and antagonist muscle tendons reduced proprioceptive weighting for the aiming movements. In the context of the experiment, participants relied less on their actual limb position and more on their visual cursor in mismatch conditions when exposed to intertrial vibration. Both neurophysiological and behavioral evidence suggest that the afferent proprioceptive information may be altered following the cessation of tendon vibration. Indeed, when measuring muscle spindle afferent firing, Ribot-Ciscar et al. (1998) found that the spindle discharge rate in a stationary limb was reduced in 73% and increased in 13.5% of the fibers for up to 40 and 30 s following vibration, respectively. This was compounded by a reduction in sensitivity to muscle stretch for up to 14 s (Ribot-Ciscar et al. 1998). Therefore, in the current study, participants performed movements in the vibration condition while their proprioceptive afferent information from both the lengthening and shortening muscles was altered and likely more variable than without vibration. In addition, behavioral evidence indicated that intertrial agonist-antagonist tendon vibration increased limb-matching variability in the horizontal plane with no associated changes in limb-matching bias (i.e., constant error) compared with no vibration trials (Goodman 2015). Collectively, the muscle spindle afferent firing and limb-matching evidence suggest that the reliability and strength of the proprioceptive signals may be reduced after intertrial dual-muscle vibration. The current study extended these findings by introducing conflicting multisensory cues to understand changes in sensory weighting. Indeed, systematically changing the unisensory variance changes the reliance on those sensory cues during multisensory perceptual tasks (e.g., Alais and Burr 2004; Ernst and Banks 2002). For sensory weighting and action, previous work has manipulated the sensory variance indirectly by manipulating the sensory processes related to sensorimotor transformations (e.g., Sarlegna and Sainburg 2007; Sober and Sabes 2005). In the current study, the central nervous system likely had access to the increased variance of the proprioceptive estimate that resulted in a decreased weight or reliance on the actual proprioceptive finger position during vibration trials (Ernst and Bülthoff 2004). Therefore, this study provides seminal evidence that intertrial agonist-antagonist muscle vibration can be used as a tool to directly alter peripheral proprioceptive afferent information and reduce proprioceptive relative to visual weighting for movement.
It is lastly important to consider the methodological differences between the exercise and vibration manipulations. Specifically, only the antagonist muscle was fatigued in experiment 1, but both the agonist and antagonist muscle groups were subjected to vibration in experiment 2. While different muscle groups were targeted by each manipulation, this was employed to limit any differences in efferent control processes across the experiments, which would have arisen if the agonist was also fatigued in experiment 1. Furthermore, vibration was applied to both muscle groups to avoid any perceptual biases known to occur when vibrating a single muscle group (e.g., Capaday and Cooke 1981; Seizova-Cajic et al. 2007). One other consideration was that vibration was applied on a trial-by-trial basis, while exercise was completed in a single bout between sets of aiming movements. Although there is a difference in the timing of the manipulations, fatigue could not have been employed on a trial-by-trial basis and it is not known how vibration applied in one long bout (e.g., 20 min) would influence muscle spindle firing. Therefore, it is important to consider these limitations when interpreting the results of the current study.
Conclusion.
The results of the current study revealed that sensory weighting processes for movement were altered by intertrial agonist-antagonist tendon vibration but not by a single bout of eccentric exercise that resulted in antagonist muscle fatigue. Specifically, the proprioceptive-to-visual weighting was systematically reduced when exposed to tendon vibration between aiming trials. The discrepancy of the effects of each perturbation on sensory weighting was likely accounted for by the differential mechanisms each perturbation has on limb position estimates. The current results suggest that muscle fatigue primarily affects central efferent processes related to limb position sense, while tendon vibration primarily affects peripheral afferent processes. Thus the current study provides support that the alteration of peripheral afferent processes can affect sensory weighting (Ernst and Bülthoff 2004) and that exercise-induced muscle fatigue does not systematically change the reliance on one’s actual limb position during multisensory movement tasks.
GRANTS
Support for this research was provided by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian Foundation for Innovation (CFI), and the Ontario Research Fund (ORF; all granted to L. Tremblay), a NSERC doctoral scholarship (granted to D.M. Manzone), and the University of Toronto.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.M.M. and L.T. conceived and designed research; D.M.M. performed experiments; D.M.M. analyzed data; D.M.M. and L.T. interpreted results of experiments; D.M.M. prepared figures; D.M.M. drafted manuscript; D.M.M. and L.T. edited and revised manuscript; D.M.M. and L.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Catherine Sabiston and the Mental Health and Physical Activity Research Centre (MPARC) for providing research space and equipment (Biodex System 4 Pro) to perform the exercise protocol. The authors gratefully acknowledge Julia Rudecki for help during data collection and Richard Chen for creating the methodological figures for this paper.
REFERENCES
- Abou Sawan S, van Vliet S, West DW, Beals JW, Paluska SA, Burd NA, Moore DR. Whole egg, but not egg white, ingestion induces mTOR colocalization with the lysosome after resistance exercise. Am J Physiol Cell Physiol 315: C537–C543, 2018. doi: 10.1152/ajpcell.00225.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alais D, Burr D. The ventriloquist effect results from near-optimal bimodal integration. Curr Biol 14: 257–262, 2004. doi: 10.1016/j.cub.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Allen TJ, Ansems GE, Proske U. Effects of muscle conditioning on position sense at the human forearm during loading or fatigue of elbow flexors and the role of the sense of effort. J Physiol 580: 423–434, 2007. doi: 10.1113/jphysiol.2006.125161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TJ, Leung M, Proske U. The effect of fatigue from exercise on human limb position sense. J Physiol 588: 1369–1377, 2010. doi: 10.1113/jphysiol.2010.187732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TJ, Proske U. Effect of muscle fatigue on the sense of limb position and movement. Exp Brain Res 170: 30–38, 2006. doi: 10.1007/s00221-005-0174-z. [DOI] [PubMed] [Google Scholar]
- Aman JE, Elangovan N, Yeh IL, Konczak J. The effectiveness of proprioceptive training for improving motor function: a systematic review. Front Hum Neurosci 8: 1075, 2015. doi: 10.3389/fnhum.2014.01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagesteiro LB, Sarlegna FR, Sainburg RL. Differential influence of vision and proprioception on control of movement distance. Exp Brain Res 171: 358–370, 2006. doi: 10.1007/s00221-005-0272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Löfstedt L, Wallin BG. The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol 261: 673–693, 1976. doi: 10.1113/jphysiol.1976.sp011580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Cooke JD. The effects of muscle vibration on the attainment of intended final position during voluntary human arm movements. Exp Brain Res 42: 228–230, 1981. doi: 10.1007/BF00236912. [DOI] [PubMed] [Google Scholar]
- Chua R, Grose G, Manzone D, Eschelmuller G, Peters RM, Carpenter MG, Inglis JT. The Effects of Exercise-Induced Fatigue and Eccentric Muscle Damage on Kinesthesia. Dublin, Ireland: International Society of Electrophysiology and Kinesiology, 2018. [Google Scholar]
- Collins DF, Refshauge KM, Todd G, Gandevia SC. Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol 94: 1699–1706, 2005. doi: 10.1152/jn.00191.2005. [DOI] [PubMed] [Google Scholar]
- Cordo P, Gurfinkel VS, Bevan L, Kerr GK. Proprioceptive consequences of tendon vibration during movement. J Neurophysiol 74: 1675–1688, 1995. doi: 10.1152/jn.1995.74.4.1675. [DOI] [PubMed] [Google Scholar]
- Daneshjoo A, Mokhtar AH, Rahnama N, Yusof A. The effects of comprehensive warm-up programs on proprioception, static and dynamic balance on male soccer players. PLoS One 7: e51568, 2012. doi: 10.1371/journal.pone.0051568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling WG, Wall BM, Coffman CR, Capaday C. Pointing to one’s moving hand: putative internal models do not contribute to proprioceptive acuity. Front Hum Neurosci 12: 177, 2018. doi: 10.3389/fnhum.2018.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund G, Hagbarth KE. Normal variability of tonic vibration reflexes in man. Exp Neurol 16: 80–92, 1966. doi: 10.1016/0014-4886(66)90088-4. [DOI] [PubMed] [Google Scholar]
- Elliott D, Lyons J, Hayes SJ, Burkitt JJ, Roberts JW, Grierson LE, Hansen S, Bennett SJ. The multiple process model of goal-directed reaching revisited. Neurosci Biobehav Rev 72: 95–110, 2017. doi: 10.1016/j.neubiorev.2016.11.016. [DOI] [PubMed] [Google Scholar]
- Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415: 429–433, 2002. doi: 10.1038/415429a. [DOI] [PubMed] [Google Scholar]
- Ernst MO, Bülthoff HH. Merging the senses into a robust percept. Trends Cogn Sci 8: 162–169, 2004. doi: 10.1016/j.tics.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Fuentes CT, Bastian AJ. Where is your arm? Variations in proprioception across space and tasks. J Neurophysiol 103: 164–171, 2010. doi: 10.1152/jn.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Smith JL, Crawford M, Proske U, Taylor JL. Motor commands contribute to human position sense. J Physiol 571: 703–710, 2006. doi: 10.1113/jphysiol.2005.103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. Using Tendon Vibration Between Trials to Alter Proprioceptive Sensitivity and Its Influence on Upper-Limb Control During Voluntary Reaching (Master’s dissertation). Toronto, Ontario, Canada: University of Toronto, 2015. [Google Scholar]
- Goodman R, Crainic VA, Bested SR, Wijeyaratnam DO, de Grosbois J, Tremblay L. Amending ongoing upper-limb reaches: visual and proprioceptive contributions? Multisens Res 31: 455–480, 2018. doi: 10.1163/22134808-00002615. [DOI] [PubMed] [Google Scholar]
- Goodman R, Tremblay L. Using proprioception to control ongoing actions: dominance of vision or altered proprioceptive weighing? Exp Brain Res 236: 1897–1910, 2018. doi: 10.1007/s00221-018-5258-7. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PBC. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain 95: 705–748, 1972. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Responses of muscle spindles following a series of eccentric contractions. Exp Brain Res 157: 234–240, 2004. doi: 10.1007/s00221-004-1838-9. [DOI] [PubMed] [Google Scholar]
- Grose GS. The Effect of Exercise-Induced Fatigue and Eccentric Muscle Damage on Kinaesthesia (Master’s dissertation). Vancouver, British Columbia, Canada: University of British Columbia, 2017. [Google Scholar]
- Inglis JT, Frank JS. The effect of agonist/antagonist muscle vibration on human position sense. Exp Brain Res 81: 573–580, 1990. doi: 10.1007/BF02423506. [DOI] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D. What’s new in Psychtoolbox-3. Perception 36: ECVP Abstract Supplement, 2007. [Google Scholar]
- Macefield G, Gandevia SC, Burke D. Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. J Physiol 429: 113–129, 1990. doi: 10.1113/jphysiol.1990.sp018247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. Muscle spindles and their motor control. Physiol Rev 44: 219–288, 1964. doi: 10.1152/physrev.1964.44.2.219. [DOI] [PubMed] [Google Scholar]
- Monjo F, Terrier R, Forestier N. Muscle fatigue as an investigative tool in motor control: A review with new insights on internal models and posture-movement coordination. Hum Mov Sci 44: 225–233, 2015. doi: 10.1016/j.humov.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997. doi: 10.1163/156856897X00366. [DOI] [PubMed] [Google Scholar]
- Proske U. Exercise, fatigue and proprioception: a retrospective. Exp Brain Res 237: 2447–2459, 2019. doi: 10.1007/s00221-019-05634-8. [DOI] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 92: 1651–1697, 2012. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Proske U, Gregory JE, Morgan DL, Percival P, Weerakkody NS, Canny BJ. Force matching errors following eccentric exercise. Hum Mov Sci 23: 365–378, 2004. doi: 10.1016/j.humov.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Flanagan JR, Johansson RS. Fast and accurate edge orientation processing during object manipulation. eLife 7: e31200, 2018. doi: 10.7554/eLife.31200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Rossi-Durand C, Roll JP. Muscle spindle activity following muscle tendon vibration in man. Neurosci Lett 258: 147–150, 1998. doi: 10.1016/S0304-3940(98)00732-0. [DOI] [PubMed] [Google Scholar]
- Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res 47: 177–190, 1982. doi: 10.1007/BF00239377. [DOI] [PubMed] [Google Scholar]
- Rossetti Y, Desmurget M, Prablanc C. Vectorial coding of movement: vision, proprioception, or both? J Neurophysiol 74: 457–463, 1995. doi: 10.1152/jn.1995.74.1.457. [DOI] [PubMed] [Google Scholar]
- Sadler CM, Cressman EK. Central fatigue mechanisms are responsible for decreases in hand proprioceptive acuity following shoulder muscle fatigue. Hum Mov Sci 66: 220–230, 2019. doi: 10.1016/j.humov.2019.04.016. [DOI] [PubMed] [Google Scholar]
- Sarlegna FR, Sainburg RL. The effect of target modality on visual and proprioceptive contributions to the control of movement distance. Exp Brain Res 176: 267–280, 2007. doi: 10.1007/s00221-006-0613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seizova-Cajic T, Smith JL, Taylor JL, Gandevia SC. Proprioceptive movement illusions due to prolonged stimulation: reversals and aftereffects. PloS One 2: e1037, 2007. doi: 10.1371/journal.pone.0001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Crawford M, Proske U, Taylor JL, Gandevia SC. Signals of motor command bias joint position sense in the presence of feedback from proprioceptors. J Appl Physiol (1985) 106: 950–958, 2009. doi: 10.1152/japplphysiol.91365.2008. [DOI] [PubMed] [Google Scholar]
- Sober SJ, Sabes PN. Flexible strategies for sensory integration during motor planning. Nat Neurosci 8: 490–497, 2005. doi: 10.1038/nn1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Moore DR, Hodson N, Ward C, Dent JR, O’Leary MF, Shaw AM, Hamilton DL, Sarkar S, Gangloff YG, Hornberger TA, Spriet LL, Heigenhauser GJ, Philp A. Resistance exercise initiates mechanistic target of rapamycin (mTOR) translocation and protein complex co-localisation in human skeletal muscle. Sci Rep 7: 5028, 2017. doi: 10.1038/s41598-017-05483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay A, Allen TJ, Leung M, Proske U. The fall in force after exercise disturbs position sense at the human forearm. Exp Brain Res 222: 415–425, 2012. doi: 10.1007/s00221-012-3228-z. [DOI] [PubMed] [Google Scholar]
- Vafadar AK, Côté JN, Archambault PS. The effect of muscle fatigue on position sense in an upper limb multi-joint task. Mot Contr 16: 265–283, 2012. doi: 10.1123/mcj.16.2.265. [DOI] [PubMed] [Google Scholar]
- Walsh GS. Effect of static and dynamic muscle stretching as part of warm up procedures on knee joint proprioception and strength. Hum Mov Sci 55: 189–195, 2017. doi: 10.1016/j.humov.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Walsh LD, Allen TJ, Gandevia SC, Proske U. Effect of eccentric exercise on position sense at the human forearm in different postures. J Appl Physiol (1985) 100: 1109–1116, 2006. doi: 10.1152/japplphysiol.01303.2005. [DOI] [PubMed] [Google Scholar]
- Walsh LD, Gandevia SC, Taylor JL. Illusory movements of a phantom hand grade with the duration and magnitude of motor commands. J Physiol 588: 1269–1280, 2010. doi: 10.1113/jphysiol.2009.183038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh LD, Hesse CW, Morgan DL, Proske U. Human forearm position sense after fatigue of elbow flexor muscles. J Physiol 558: 705–715, 2004. doi: 10.1113/jphysiol.2004.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wann JP, Ibrahim SF. Does limb proprioception drift? Exp Brain Res 91: 162–166, 1992. doi: 10.1007/BF00230024. [DOI] [PubMed] [Google Scholar]
- Warburton DE, Jamnik VK, Bredin SS, Gledhill N. The physical activity readiness questionnaire for everyone (PAR-Q+) and electronic physical activity readiness medical examination (ePARmed-X+). Health Fit J Can 4: 3–17, 2011. [Google Scholar]
- Zabihhosseinian M, Holmes MW, Murphy B. Neck muscle fatigue alters upper limb proprioception. Exp Brain Res 233: 1663–1675, 2015. doi: 10.1007/s00221-015-4240-x. [DOI] [PubMed] [Google Scholar]