Abstract
Background
Snakebite is an often-neglected event with a high rate of mortality and is concentrated in poor areas. We aimed to assess the economic impact and health effects of the implementation of interventions for snakebites through a systematic review of the scientific literature.
Methods
Thirty search strategies were conducted in seven databases, applying PRISMA’s identification, screening, selection, and inclusion phases. The reproducibility of the selection of studies and the extraction of information were guaranteed. The methodological quality was evaluated using the Consolidated Health Economic Evaluation Reporting Standards. Qualitative synthesis and meta-analysis were performed for determining the average cost-effectiveness (ACE) for each death and disability-adjusted life years (DALY) avoided.
Results
Six cost-effectiveness studies were included for the supply of antivenom (AV), taken as outcomes on days of hospitalization or in ICU, death and DALYs avoided. All studies only included institutional costs, and majority of them did not specify the analytical model or economic evaluation parameters and did not perform uncertainty analyses. The management protocol standardization with interdisciplinary attention improves ACE of AV. Cost-effectiveness ratio (CER) of treatment with AV was USD 1253 (constant value for the year 2017, adjusted by purchasing power parity) for each death avoided and USD 51 per DALY avoided.
Conclusion
High cost-effectiveness of the AV treatment for snakebites was evidenced, which shows that the allocation of resources for this event should be a healthcare priority in addition to implementation of strategies that improve the access to, opportunity, and quality of hospital and pre-hospital care and reduce the cost of AV.
Keywords: snake envenoming, DALYs, antivenom, cost-effectiveness
Background
Snakebites are considered a particularly serious public health problem in the world’s tropical and subtropical regions, both because of the number of new cases per year and because of the number of deaths and sequelae they cause. In fact, the exact number of cases of snakebites is unknown, the World Health Organization estimate that 5.4 million people are bitten each year with up to 2.7 million envenoming, with 81,000 to 138 000 annual deaths by snake bites and around three times as many amputations.1–3 Snakebites do not present consistent epidemiological data and the burden of suffering it causes to humans remains unacknowledged.4 Therefore, in 2009, the World Health Organization declared that ophidic accident is a neglected disease.4
Envenoming affects the poorest populations in Latin America, Asia, sub-Saharan Africa, and some areas in Oceania, making it a disease of the poor.5,6 Late consultation with a health center as well as misdiagnosis delay initiation of treatment.7,8 Other factors such as low availability, accessibility, and affordability of the antivenom (AV) and poor access to treatment and rehabilitation services further increase the problem, as described in the resolution of the 71st World Health Assembly.9
In addition to the serious clinical and epidemiological impact and the impact on public health in general, this type of envenoming negatively affects the economic and social development of the affected populations. In this context, the study by the Harrison group based on information from 138 countries showed serious macroeconomic and social impacts attributable to this event, with strong correlations between mortality induced by envenoming and poverty, low human development index, low per capita government expenditure on health, a high proportion of the labor force involved in agriculture, and low gross domestic product per capita.10
The foregoing also demonstrates the concentration of the highest mortality rates because of this event in poor countries or areas where availability of finances to bear the costs associated with managing snakebites are less likely. Therefore, it is crucial to know the interventions that have the best relationship between health benefits and costs as a basis for efficient allocation of the scarce resources of the health sector in most endemic countries as well as the prioritization of interventions that maximize health benefits and social development.
In this context, some authors have investigated the effectiveness of some technologies on health and the cost of their provision, but with considerable heterogeneity in the groups studied and the outcomes evaluated and with publications based on patients and controls, modeling, measuring DALYs, death, and hospitalization.11,12
Therefore, this study was conducted with the objective of estimating the economic impact and health effects of the implementation of interventions for snakebite through a systematic review of the scientific literature. The advantages of this type of study include performing a structured, explicit, systematic, exhaustive, and reproducible search of studies referring to the same research question; greater possibilities of extrapolation of results; better accuracy in the estimates; greater statistical power of comparison; synthesis of research needs; and generation of key results to guide decision-making in health matters and guide consensus groups and panels of experts or committees with regulatory responsibilities.13
Methods
Type of Study
Systematic review.
Question Population Intervention Comparator Outcome Time Horizon Resources (PICO-RT)
Population: People who suffered the event.
Intervention: AV administration.
Comparator: Modifications or complements to the intervention, such as the use of adjuvants or clinical support.
Outcome: Of the outcomes included in the systematized studies, Disability-Adjusted Life Years (DALYs) avoided, deaths avoided, and reduction in the days of hospitalization were predominant. Some studies included changes in clinical examinations, amputation, and healing.
Resources: The studies mainly included direct costs of treatment and healthcare personnel.
Time horizon: There was a high heterogeneity, from the days of clinical management to risk simulations of death to several years.
Protocol for Searching and Selecting Studies According to PRISMA Guidelines
Identification: A search was conducted in PubMed, Ovid, ScienceDirect and SciELO multi-disciplinary databases; in the Google Scholar open search engine; and in the databases specialized in health economics, Health Technology Assessment and NHS Economic Evaluation Database. Each of the terms snakebite, snake bite, envenoming, Snake Envenomation, and Snake Envenoming were used in combination with the Boolean operator (AND, and) with the terms economic evaluation, cost, cost-effectiveness, cost-utility, cost-benefit, and cost-minimization as a total of 30 search strategies in each database.
Screening: Inclusion criteria of the presence of the terms in the title, abstract, or keywords; being an original study; and being conducted in human subjects were applied, which allowed revisions, editorials, books, and studies on animals, plans, or in-vitro models to be excluded.
Some search syntaxes include the following: i) in PubMed (snakebite [Title/Abstract]) AND economic evaluation [Title/Abstract]; ii) in Ovid, envenoming cost {Including Limited Related Terms}; iii) in Science-Direct, Title, abstract, keywords: Snake Envenomation; iv) in SciELO (ab: (Snake Envenoming cost-effectiveness)); v) in Google Scholar, allintitle: snakebite cost benefit; vi) in Health Technology Assessment Database, Snakebite Envenomation.m_titl.; and vii) in NHS Economic Evaluation, Snake Envenomation.mp. search as a Keyword.
Eligibility: Those studies that did not conduct economic evaluations, such as clinical characterizations, case studies, tests evaluation, and observational epidemiological studies, were excluded; publications in languages other than Spanish, English, or Portuguese were also excluded.
Inclusion: The studies were analyzed with qualitative synthesis of the variables author, title, year, country, population studied, intervention applied, comparison alternatives, costs, health outcomes, and core result.
Reproducibility and Evaluation of Methodological Quality
The search and selection of studies as well as the extraction of variables were performed by two researchers to guarantee the reproducibility of the results. For determining the methodological quality, the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guide was applied.
Analysis of Information
The study variables were analyzed with frequencies in the qualitative synthesis. For the cost-effectiveness studies with DALYs as an outcome, quantitative synthesis was performed where the costs were deflated to USD 2018 and for comparison, the costs were adjusted through the purchasing power parity (PPP) of the World Bank International Comparison Program database, which allows for the estimation of the costs needed for a country to purchase the same products in a market or country of reference (in this case the United States). In this way, a quantitative synthesis of costs was made with DALYs avoided with the intervention of the health problem analyzed.
Results
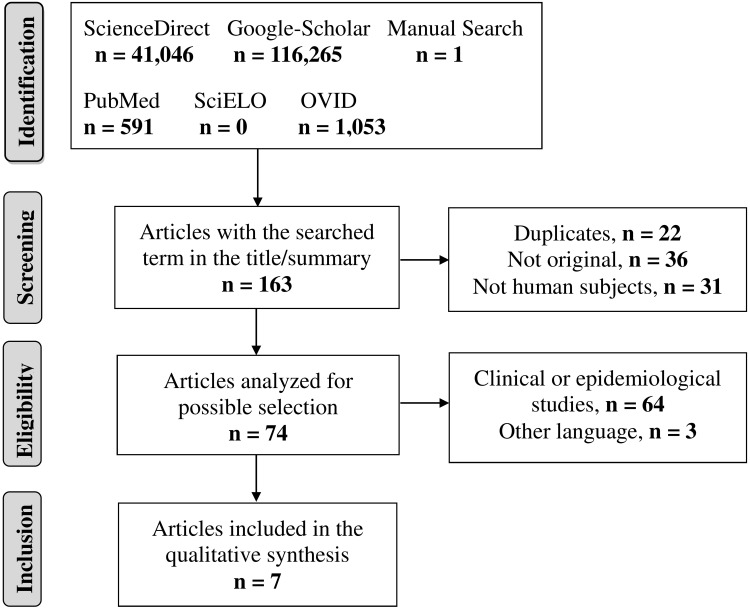
More than 1,00,000 publications were identified using the search terms and 162 of them were screened. Of these 162 publications, 31 were conducted in animals or plants; 23 were narrative reviews; and 13 were editorials, posters, or books, and after the eligibility phase, only six complied with the protocol (Figure 1). Based on an update of July 2020, a study published in the same year (2020) was added.
Figure 1.
Search flowchart and selection of studies.
All the studies corresponded to cost-effectiveness evaluations; they were published between 2012 and 2018 in different countries (with the exception of the Magalhães et al study which was a partial economic evaluation, based on costs of illness care published in 2020), with patients who suffered the event and were treated in a hospital or with the modeling of all cases in the country under study. The intervention in all studies consisted of the administration of AV, with some variations or additions, such as comparison of administration protocols, use of imported AV, and with adjuvants or supportive care. Regarding the outcomes, the most frequent was DALYs avoided (Table 1).
Table 1.
Description of the Studies According to Year, Country, Population, and Study Alternatives
| Author | Year | Country | Population | Interventions | Outcome |
|---|---|---|---|---|---|
| Weant et al11 | 2012 | United States | 75 patients (30 treated and 45 controls) | Standardized use of AV | Days of hospitalization and in the ICU |
| Qureshi et al14 | 2013 | Pakistan | 80 patients (40 per arm) | AV Pakistan AV India |
Coagulation control and adverse reactions |
| Habib et al12 | 2015 | Nigeria | Total modeling of country cases | AV availability or not | Deaths and DALYs avoided |
| Hamza et al15 | 2016 | 16 African countries | Total modeling of cases by country | AV availability or not | Deaths and DALYs avoided |
| Kasturiratne wet al16 | 2017 | Sri Lanka | 49,819 patients without envenoming and 30,458 patients with envenoming | Case management with AV | Hospitalization and DALYs |
| Herzel et al17 | 2018 | United States | 100 patients | AV + Care AV + Adjuvant + Care |
DALYs |
| Magalhães et al18 | 2020 | Brazil | 11,503 cases and 56 deaths | AV + Care | Cost to the health system, due to premature death and attributed to the loss of productivity |
Abbreviation: AV, antivenom.
With regards to costs, most studies evaluated the costs inherent to the intervention based on the provision of AV and the care of the event. In the study by Habib et al, the costs of diagnostic aids and the cost of feeding and transportation to the hospital were added.12 In the study by Kasturiratne et al, high economic losses were evidenced because of out-of-pocket expenditures, losses of income attributable to the event, and expenses by relatives because of patient care.16 In the study by Magalhães et al, prehospitalization ambulatory care, outpatient care for those who were not hospitalized, postdischarge consultation, treatment (antivenom therapy), hospitalization, costs from the patient’s perspective, and costs from society’s perspective (loss of productivity due to premature death and morbidity) were analyzed.18
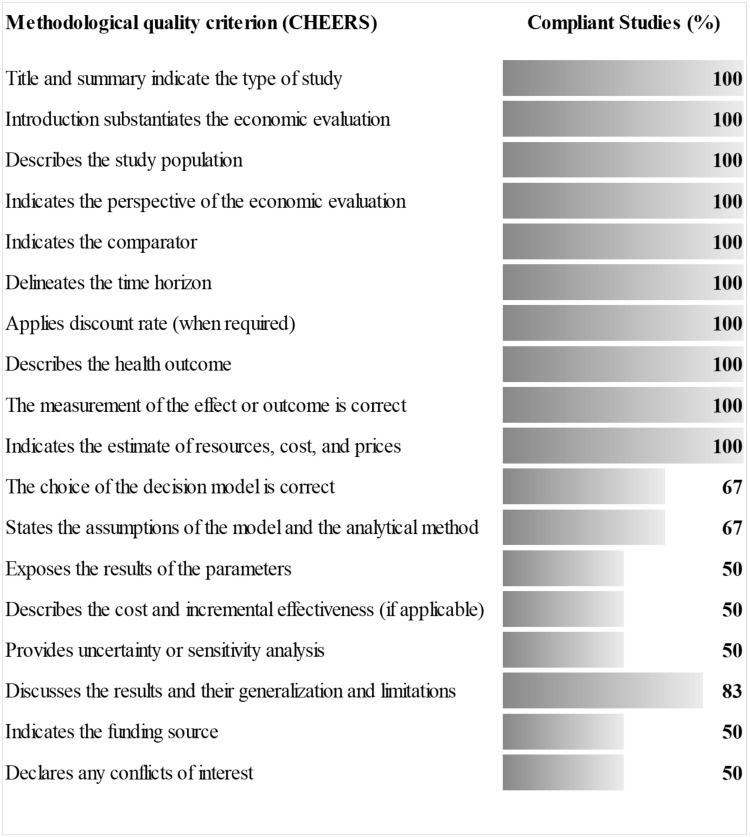
Four studies presented excellent methodological quality with over 80% of criteria fulfilled; the study by Weant et al and Qureshi et al fulfilled 56% and 61% of the items, respectively.11,14 The methodological criteria less applicable to the studies were those related to the analytical model, evaluation assumptions and parameters, and the uncertainty analysis (Figure 2). In the study by Magalhães most of the criteria of the guide CHEERS did not apply because it was not a complete economic evaluation; however, of the quality criteria applicable to the partial evaluations this study fulfilled 90%.18
Figure 2.
Evaluation of the methodological quality of the studies.
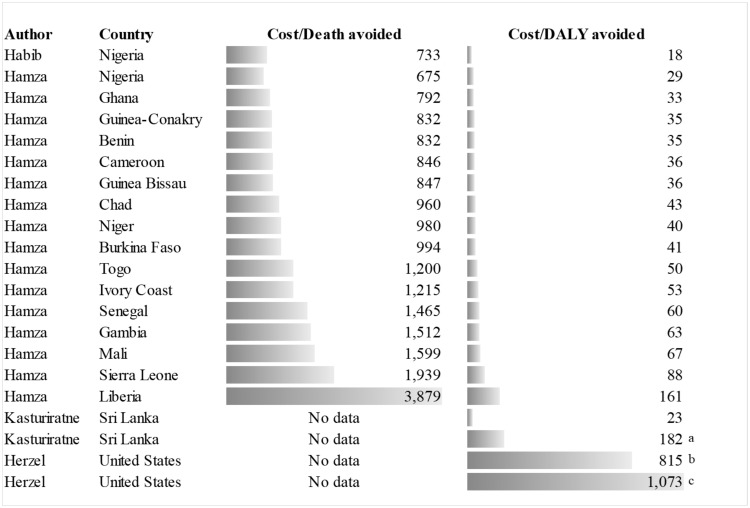
The average cost-effectiveness (ACE) of the interventions showed that the standardization of the interdisciplinary care management protocol improves cost-effectiveness by allowing optimal use of the resource used to manage the event,11 and that importing AV affects ACE as it increases the costs of the intervention.14 In this regard, ACE for patients treated with a standardized protocol was USD 2178 (constant value for 2017, adjusted by PPP) compared with that for those treated with the non-standardized one (USD 4121),11 or the cost for a correct control of coagulation was USD 13 when using local AV versus USD 27 when using imported AV.14
ACE of treatment with AV was USD 1253 (constant value of 2017, adjusted by PPP) for each death avoided, with variations between studies or countries that fluctuated between USD 733 and USD 3879, while the cost per DALY avoided was USD 51 with a range between USD 18 and USD 161, which amounts to USD 1073 when adjuvants and support care are included (Figure 3).
Figure 3.
Average cost-effectiveness analysis of antivenom treatment for deaths and DALYs avoided. “a” indicates AV + Envenoming management. “b” indicates AV + Support. “c” indicates AV + Adjuvant.
Uncertainty analyses showed the high sensitivity of the cost-effectiveness ratio to AV costs, healthcare, and effectiveness of AV in preventing mortality,12 and to the reduction of the severity of the episode,17 highlighting the relevance of timely care.
Discussion
Snakebites are a cause of high morbidity and mortality, and between 421,000 and 1.2 million bites occur with an outcome of 81,000–138,000 deaths annually.1,5,19 In addition, there are affectations, such as amputations due to necrosis, blindness, kidney failure, and post-traumatic stress.7,20 Despite this wide diversity of health outcomes, only the days of hospitalization or in ICU, coagulation control, deaths, and DALYs were identified in this investigation, which highlights the need to perform cost-effectiveness studies for outcomes, such as amputation, particularly when considering some studies that have reported approximately 4% of patients with this outcome and that the lack of AV can lead to amputation.21,22
Another effect, ie, acute kidney failure, occurred in 12% of cases in Australia.23 This is a frequent effect of this event, given its delayed care and the multiple barriers to initiating treatment, which would result in an increase in the care costs for these complications, increased risk of death, and DALYs as the main combined outcome of morbidity, mortality, and disability used in the systematized studies.
The above shows the importance of the initiation of therapy in a timely manner because it reduces the risk of death, amputations, and other complications or sequelae caused by a lack of AV supply,24,25 or the delay in the initiation of therapy, generally attributed to the long traveling time to the health center,26 and to the conditions of poverty in general.27 For example, in Colombia, 26.1% of accidents do not receive AV treatment;28 in Africa, AV availability is low because of its low production;24 and recently in Latin America, an insufficient production of ampoules to adequately supply the demand of each country has been reported.29
It is important to highlight the fact that in this systematic review, all the interventions focused on AV supply. Currently, there are difficulties in the distribution of AVs because of the reduction in their production and the lack of cold distribution chains, particularly in Africa, which has forced the search for solutions, such as the production of AVs by other laboratories, particularly in Latin America,24,30 although with related efficiency and reactivity problems.31–34 which implies an overload to the cost of AV. This is even more important considering that the studies analyzed showed that the evaluation of the interventions was made against the use of AV, probably because, to date, there are no other interventions available for this event.35,36
Most studies evaluated the costs inherent to the intervention based on the provision of AV and the care of the event; few added the costs related to diagnostic aids and patients’ expenses for food, transportation, out-of-pocket expenses in health, losses of productive days and expenses for patient care,18 which are aspects that would evidence to a greater extent the need to increase the budget for health expenses for this type of event, which in many contexts could constitute a trap to poverty.37–39
In relation to costs, it was also evident that the standardization of the interdisciplinary care management protocol improves cost-effectiveness by allowing optimal use of the resources used to manage the event.11 However, the protocols do not ensure adherence of medical personnel, taking into account that there is no agreement between the clinical signs and symptoms that occur, the identification of the attacking animal, and the classification of the severity of the envenomation, and the use of AV serum and the dose administered.28,40
Conversely, the costs for DALYs were very low compared with that reported for other neglected diseases, such as leishmaniosis, schistosomiasis, dengue, leprosy, rabies, and filariasis,41–43 which proves the relevance of intervention for these diseases by the State, given their high cost-effectiveness, which would add an equity issue to the affected populations, which in most cases is poor.
The cost-effectiveness of AV is high, but it presents problems, such as the high cost of AV during production and low availability in some parts of the world. Likewise, the seriousness of the case, lack of identification of the snake species that caused snakebites, and delay in receiving care make it difficult to address the event properly.7,8,24,30,44 This is consistent with the uncertainty analyses, which show a high sensitivity of the cost-effectiveness ratio to AV costs, healthcare, and effectiveness of AV in preventing mortality,12 and also to the reduction of the severity of the episode,19 highlighting the relevance of its timely attention.
Among the limitations of this study are the low research development in the economic evaluations of this topic; limited number of interventions and outcomes evaluated as well as the low reporting of incremental and uncertainty analyses, which are an axis for decision-making on the allocation of health sector resources.
Conclusion
High cost-effectiveness of AV treatment for snakebites was highlighted, which shows that the allocation of resources for this event should be a healthcare priority in addition to the implementation of strategies that improve the access to, the opportunity, and the quality of hospital and pre-hospital care and reduce the cost of AV.
Funding Statement
Universidad Cooperativa de Colombia.
Abbreviations
DALY, disability-adjusted life years; AV, antiVenom, PICO-RT, Population Intervention Comparator Outcome Time horizon Resources; CHEERS, Consolidated Health Economic Evaluation Reporting Standards; PPP, purchasing power parity; ACE, average cost-effectiveness.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure
None of the authors report having any conflict of interest whatsoever for this work.
References
- 1.World Health Organization (WHO). Snakebite envenoming. [Internet] 2019; 2019. Geneva: WHO; Available from: https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming. [Google Scholar]
- 2.Chippaux JP. Estimating the global burden of snakebite can help to improve management. PLoS Med. 2008;5:e221. doi: 10.1371/journal.pmed.0050221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutiérrez JM, Williams D, Fan HW, Warrell DA. Snakebite envenoming from a global perspective: towards an integrated approach. Toxicon. 2010;56:1223–1235. doi: 10.1016/j.toxicon.2009.11.020 [DOI] [PubMed] [Google Scholar]
- 4.Williams D, Gutiérrez JM, Harrison R, et al. The global snake bite initiative: an antidote for snake bite. Lancet. 2010;375:89–91. doi: 10.1016/S0140-6736(09)61159-4 [DOI] [PubMed] [Google Scholar]
- 5.Gutiérrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA. Snakebite envenoming. Nat Rev Dis Primers. 2017;3:17063. doi: 10.1038/nrdp.2017.63 [DOI] [PubMed] [Google Scholar]
- 6.Gutiérrez JM, Theakston RD, Warrell DA. Confronting the neglected problem of snake bite envenoming: the need for a global partnership. PLoS Med. 2006;3:e150. doi: 10.1371/journal.pmed.0030150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otero-Patiño R. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon. 2009;54:998–1011. doi: 10.1016/j.toxicon.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 8.Vaiyapuri S, Vaiyapuri R, Ashokan R, et al. Snakebite and its socio-economic impact on the rural population of Tamil Nadu, India. PLoS One. 2013;8:e80090. doi: 10.1371/journal.pone.0080090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seventy first World Health Assembly. Addressing the burden of snakebite envenoming. Available from: http://www.who.int/neglected_diseases/mediacentre/WHA_71.5_Eng.pdf?ua=1.
- 10.Harrison RA, Hargreaves A, Wagstaff SC, Faragher B, Lalloo DG, White J. Snake envenoming: a disease of poverty. PLoS Negl Trop Dis. 2009;3:e569. doi: 10.1371/journal.pntd.0000569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weant KA, Bowers RC, Reed J, Braun KA, Dodd DM, Baker SN. Safety and cost-effectiveness of a clinical protocol implemented to standardize the use of Crotalidae polyvalent immune Fab antivenom at an academic medical center. Pharmacotherapy. 2012;32:433–440. doi: 10.1002/j.1875-9114.2012.01026.x [DOI] [PubMed] [Google Scholar]
- 12.Habib AG, Lamorde M, Dalhat MM, Habib ZG, Kuznik A, Lalloo DG. Cost-effectiveness of antivenoms for snakebite envenoming in Nigeria. PLoS Negl Trop Dis. 2015;9:e3381. doi: 10.1371/journal.pntd.0003381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardona-Arias JA, Higuita-Gutiérrez LF, Rios-Osorio LA. Revisiones Sistemáticas De La Literatura Científica: La Investigación Teórica Como Principio Para El Desarrollo De La Ciencia Básica y Aplicada. Bogotá: Ediciones Universidad Cooperativa de Colombia; 2016. [Google Scholar]
- 14.Qureshi H, Alam SE, Mustufa MA, Nomani NK, Asnani JL, Sharif M. Comparative cost and efficacy trial of Pakistani versus Indian anti snake venom. J Pak Med Assoc. 2013;63:1129–1132. [PubMed] [Google Scholar]
- 15.Hamza M, Idris MA, Maiyaki MB, et al. Cost-effectiveness of antivenoms for snakebite envenoming in 16 countries in West Africa. PLoS Negl Trop Dis. 2016;10:e0004568. doi: 10.1371/journal.pntd.0004568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasturiratne A, Pathmeswaran A, Wickremasinghe AR, et al. The socio-economic burden of snakebite in Sri Lanka. PLoS Negl Trop Dis. 2017;11:e0005647. doi: 10.1371/journal.pntd.0005647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzel BJ, Samuel SP, Bulfone TC, Raj CS, Lewin M, Kahn JG. Snakebite: an exploratory cost-effectiveness analysis of adjunct treatment strategies. Am J Trop Med Hyg. 2018;99:404–412. doi: 10.4269/ajtmh.17-0922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magalhães SFV, Peixoto HM, de Almeida Gonçalves Sachett J, et al. Snakebite envenomation in the Brazilian Amazon: a cost-of-illness study. Trans R Soc Trop Med Hyg. 2020;traa005. doi: 10.1093/trstmh/traa005 [DOI] [PubMed] [Google Scholar]
- 19.Kasturiratne A, Wickremasinghe AR, de Silva N, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams SS, Wijesinghe CA, Jayamanne SF, et al. Delayed psychological morbidity associated with snakebite envenoming. PLoS Negl Trop Dis. 2011;5:e1255. doi: 10.1371/journal.pntd.0001255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao YC, Liu PY, Chiang LC, et al. Naja atra snakebite in Taiwan. Clin Toxicol (Phila). 2018;56:273–280. doi: 10.1080/15563650.2017.1366502 [DOI] [PubMed] [Google Scholar]
- 22.Kallel H, Mayence C, Houcke S, et al. Severe snakebite envenomation in French Guiana: when antivenom is not available. Toxicon. 2018;146:87–90. doi: 10.1016/j.toxicon.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 23.Johnston CI, Ryan NM, Page CB, et al. The Australian Snakebite project, 2005–2015 (ASP-20). The Australian. Med J Aust. 2017;207:119–125. doi: 10.5694/mja17.00094 [DOI] [PubMed] [Google Scholar]
- 24.Gutiérrez JM, Rojas E, Quesada L, et al. Pan-African polyspecific antivenom produced by caprylic acid purification of horse IgG: an alternative to the antivenom crisis in Africa. Trans R Soc Trop Med Hyg. 2005;99:468–475. doi: 10.1016/j.trstmh.2004.09.014 [DOI] [PubMed] [Google Scholar]
- 25.Harrison RA, Casewell NR, Ainsworth SA, Lalloo DG. The time is now: a call for action to translate recent momentum on tackling tropical snakebite into sustained benefit for victims. Trans R Soc Trop Med Hyg. 2019;113(12):835–838. doi: 10.1093/trstmh/try134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox S, Rathuwithana AC, Kasturiratne A, Lalloo DG, de Silva HJ. Underestimation of snakebite mortality by hospital statistics in the Monaragala District of Sri Lanka. Trans R Soc Trop Med Hyg. 2006;100:693–695. doi: 10.1016/j.trstmh.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 27.Molesworth AM, Harrison R, Theakston RD, Lalloo DG. Geographic information system mapping of snakebite incidence in northern Ghana and Nigeria using environmental indicators: a preliminary study. Trans R Soc Trop Med Hyg. 2003;97:188–192. doi: 10.1016/S0035-9203(03)90115-5 [DOI] [PubMed] [Google Scholar]
- 28.Rojas AM. Informe De Evento. Accidente Ofidico, Colombia, 2017. Bogotá: Instituto Nacional de Salud; 2018. [Google Scholar]
- 29.Temprano G, Aprea P, Dokmetjian JC. La producción pública de antivenenos en la Región de las Américas como factor clave en su accesibilidad. Rev Panam Salud Publica. 2017;41:1. doi: 10.26633/RPSP.2017.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laing GD, Renjifo JM, Ruiz F, et al. A new Pan African polyspecific antivenom developed in response to the antivenom crisis in Africa. Toxicon. 2003;42:35–41. doi: 10.1016/S0041-0101(03)00098-9 [DOI] [PubMed] [Google Scholar]
- 31.Visser LE, Kyei-Faried S, Belcher DW, Geelhoed DW, van Leeuwen JS, van Roosmalen J. Failure of a new antivenom to treat Echis ocellatus snake bite in rural Ghana: the importance of quality surveillance. Trans R Soc Trop Med Hyg. 2008;102:445–450. doi: 10.1016/j.trstmh.2007.11.006 [DOI] [PubMed] [Google Scholar]
- 32.Herrera M, León G, Segura A, et al. Factors associated with adverse reactions induced by caprylic acid-fractionated whole IgG preparations: comparison between horse, Sheep and camel IgGs. Toxicon. 2005;46:775–781. doi: 10.1016/j.toxicon.2005.08.004 [DOI] [PubMed] [Google Scholar]
- 33.Williams DJ, Gutierrez JM, Calvete JJ, et al. Ending the drought: new strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J Proteomics. 2011;74:1735–1767. doi: 10.1016/j.jprot.2011.05.027 [DOI] [PubMed] [Google Scholar]
- 34.Calvete JJ, Arias AS, Rodríguez Y, et al. Preclinical evaluation of three polyspecific antivenoms against the venom of Echis ocellatus: neutralization of toxic activities and antivenomics. Toxicon. 2016;119:280–288. doi: 10.1016/j.toxicon.2016.06.022 [DOI] [PubMed] [Google Scholar]
- 35.Maria Gutierrez JM, Leon G, Lomonte B, Angulo Y. Antivenoms for snakebite envenomings. Inflamm Allergy Drug Targets. 2011;10:369–380. doi: 10.2174/187152811797200669 [DOI] [PubMed] [Google Scholar]
- 36.Laustsen AH, María Gutiérrez J, Knudsen C, et al. Pros and cons of different therapeutic antibody formats for recombinant antivenom development. Toxicon. 2018;146:151–175. doi: 10.1016/j.toxicon.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 37.Whitehead M, Dahlgren G. Concepts and principles for tackling social inequities in health: levelling up Part 1 In: Studies on Social and Economic Determinants of Population Health. Vol. 2 Copenhagen: WHO Regional Office for Europe; 2007:10–19. [Google Scholar]
- 38.Jo C. Cost-of-illness studies: concepts, scopes, and methods. Clin Mol Hepatol. 2014;20(4):327–337. doi: 10.3350/cmh.2014.20.4.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown GC, Brown MM, Sharma S. Value-based medicine: evidence-based medicine and beyond. Ocul Immunol Inflamm. 2003;11(3):157–170. doi: 10.1076/ocii.11.3.157.17355 [DOI] [PubMed] [Google Scholar]
- 40.Zambrano A. Accidente ofídico como evento de interés en salud pública en Colombia: Aportes al diseño de estrategias de gestión. Bogotá: Universidad Nacional de Colombia; 2012. [Google Scholar]
- 41.Martins-Melo FR, Carneiro M, Ramos AN, Heukelbach J, Ribeiro ALP, Werneck GL. The burden of neglected tropical diseases in Brazil, 1990–2016: a subnational analysis from the global burden of disease study 2016. PLoS Negl Trop Dis. 2018;12:e0006559. doi: 10.1371/journal.pntd.0006559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deribew A, Kebede B, Tessema GA, et al. Mortality and disability-adjusted life-years (DALYs) for common neglected tropical diseases in Ethiopia, 1990–2015: evidence from the global burden of disease Study 2015. Ethiop Med J. 2017;55(Suppl 1):3–14. [PMC free article] [PubMed] [Google Scholar]
- 43.Mitra AK, Mawson AR. Neglected tropical diseases: epidemiology and global burden. Trop Med Infect Dis. 2017;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otero R, León G, Gutiérrez JM, et al. Efficacy and safety of two whole IgG polyvalent antivenoms, refined by caprylic acid fractionation with or without beta-propiolactone, in the treatment of Bothrops asper bites in Colombia. Trans R Soc Trop Med Hyg. 2006;100:1173–1182. doi: 10.1016/j.trstmh.2006.01.006 [DOI] [PubMed] [Google Scholar]