Abstract
The Lung Screen Uptake Trial tested a novel invitation strategy to improve uptake and reduce socioeconomic and smoking-related inequalities in lung cancer screening (LCS) participation. It provides one of the first UK-based ‘real-world’ LCS cohorts. Of 2012 invited, 1058 (52.6%) attended a ‘lung health check’. 768/996 (77.1%) in the present analysis underwent a low-dose CT scan. 92 (11.9%) and 33 (4.3%) participants had indeterminate pulmonary nodules requiring 3-month and 12-month surveillance, respectively; 36 lung cancers (4.7%) were diagnosed (median follow-up: 1044 days). 72.2% of lung cancers were stage I/II and 79.4% of non-small cell lung cancer had curative-intent treatment.
Keywords: lung cancer, imaging/CT MRI etc
Introduction
Lung cancer screening (LCS) by low-dose CT (LDCT) has been repeatedly shown in clinical trials to reduce lung cancer mortality.1–3 The benefits of screening may be underestimated in these trials due to participants being younger, of higher socioeconomic position and disproportionately former rather than current smokers compared with the high-risk target population. The risk profile of the population enrolled determines the prevalence and stage of lung cancers, the false positive rate and the mortality benefit. Screening the highest risk quintiles can optimise the benefit-harm ratio while making LCS more equitable, efficient and cost-effective.4 5
Data from a prior UK-based ‘real world’ screening pilot in Manchester has shown compelling results with high levels of attendance by those from lower socioeconomic quintiles and radical treatment rates.6 Here we report the nodule and cancer outcomes from the Lung Screen Uptake Trial (LSUT).
Methods
The LSUT methods and primary attendance results have been described previously7 8 and more detail is included in the online supplementary appendix. LSUT was a randomised controlled trial evaluating the impact of ‘targeted, stepped and low burden’ invitation materials on attendance of a ‘lung health check’ (LHC) appointment. Individuals aged 60 to 75 years, who had been recorded as ‘current smokers’ within the seven preceding years were sent an invitation letter from their usual general practice doctor inviting them to an LHC. Those attending were invited to participate in the study, and those meeting any of the following criteria were offered a single LDCT on the same day (or later if preferred): ≥30 pack-years and if a former smoker had quit ≤15 years ago, or a lung cancer risk of ≥1.51% or ≥2.5% as determined by the Prostate, Lung, Colorectal and Ovarian study or the Liverpool Lung Project models, respectively.
thoraxjnl-2020-214703supp001.pdf (204.8KB, pdf)
Self-reported demographics, smoking, family and medical history were recorded prospectively. Hand-held pre-bronchodilator spirometry, height, weight and blood pressure were recorded. LDCT findings were evaluated and managed in accordance with the British Thoracic Society (BTS) 2015 guidelines for pulmonary nodules9 and the National Institute for Health and Care Excellence (NICE) guidelines for the diagnosis and management of lung cancer.10 Staging was carried out according to the 7th edition TNM (tumour, node, metastases) classification system.
In the present study, we report the outcomes relating to LDCT scans with an indeterminate pulmonary nodule or suspected lung cancer. Other incidental finding outcomes have been reported elsewhere.11 12 Study participants with complete smoking and lung cancer risk data were included. Descriptive statistics were used to present the data pertaining to pulmonary nodules and lung cancer outcomes.
Results
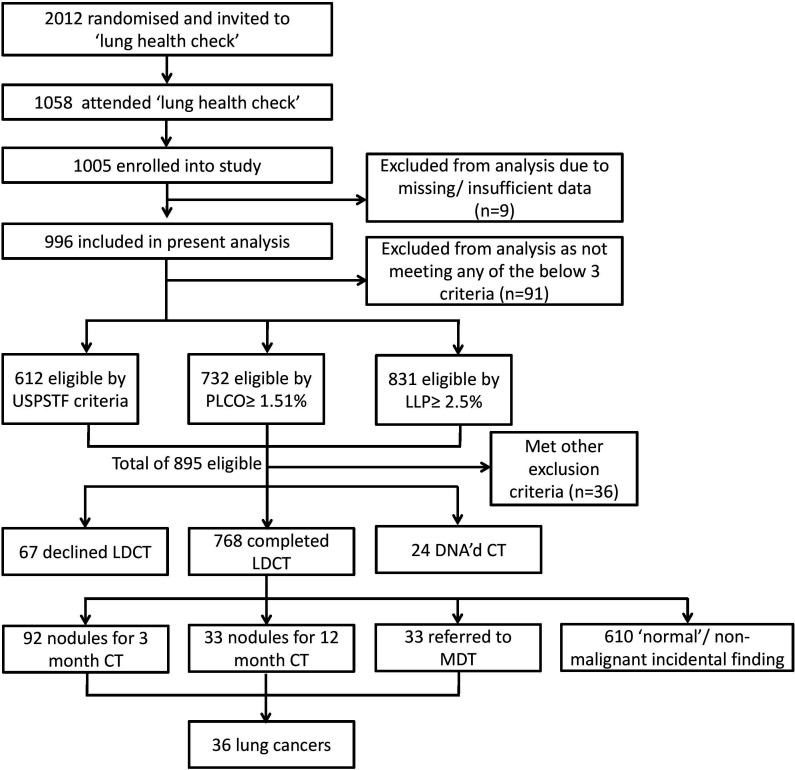
Of the 1058 (52.6%) invitees (n=2012) attending a LHC appointment between November 2015 and July 2017, 996 were included in the present analysis. A total of 895 participants were eligible for LDCT, though 36 were excluded due to prior CT of the chest in the past year, or an inability to lie flat and 91 participants declined or failed to attend the CT. An LDCT examination was completed by 768 (77.1%) of the participants (figure 1). The demographic characteristics of the 996 participants included are presented in table 1.
Figure 1.
Flow chart of invitees and participants demonstrating numbers identified, invited, enrolled, eligible for LDCT and that completed a LDCT examination. DNA, did not attend; LDCT, low-dose CT; LLP, Liverpool Lung Project; MDT, multidisciplinary team; PLCOm2012, Prostate, Lung, Colorectal and Ovarianstudy model 2012; USPSFT, United States Preventive Services Task Force.
Table 1.
Participant characteristics by group (% totals may not sum up due to rounding or missing data)
| Variables | No LDCT n=228 median (IQR) or n (%) |
No lung cancer n=732 median (IQR) or n (%) |
Lung cancers n=36 median (IQR) or n (%) |
All groups n=996 median (IQR) or n (%) |
| Age (in years) | ||||
| 60–63 | 86 (37.7) | 241 (32.9) | 8 (22.2) | 335 (33.6) |
| 64–67 | 72 (31.6) | 238 (32.5) | 11 (30.6) | 321 (32.2) |
| 68–72 | 48 (21.1) | 158 (21.6) | 13 (36.1) | 219 (22.0) |
| 73–76 | 22 (9.7) | 95 (13.0) | 4 (11.1) | 121 (12.2) |
| Gender | ||||
| Female | 109 (47.8) | 317 (43.3) | 23 (63.9) | 449 (45.1) |
| Ethnicity | ||||
| White | 183 (80.3) | 607 (82.9) | 34 (94.4) | 824 (82.7) |
| Black/African/Caribbean | 23 (10.1) | 77 (10.5) | 1 (2.8) | 101 (10.1) |
| Other | 22 (9.7) | 48 (6.6) | 1 (2.8) | 71 (7.1) |
| Highest level of education | ||||
| Left school at or before age 15 | 105 (46.1) | 395 (54.0) | 20 (55.6) | 520 (52.2) |
| GCSEs, O-levels or equivalent | 26 (11.4) | 75 (10.3) | 3 (8.3) | 104 10.4) |
| A-levels or equivalent | 24 (10.5) | 70 (9.6) | 4 (11.1) | 98 (9.8) |
| Further education | 14 (6.1) | 31 (4.2) | 3 (8.3) | 48 (4.8) |
| Bachelor degree | 34 (14.9) | 84 (11.5) | 2 (5.6) | 120 (12.1) |
| Further higher degree | 20 (8.8) | 64 (8.7) | 4 (11.1) | 88 (8.8) |
| Other | 5 (2.2) | 13 (1.8) | 0 (0) | 18 (1.8) |
| Index of Multiple Deprivation quintile | ||||
| 1 (most deprived) | 117 (51.3) | 402 (54.9) | 19 (52.8) | 538 (54.0) |
| 2 | 87 (38.2) | 245 (33.5) | 12 (33.3) | 344 (34.5) |
| 3 | 3 (1.3) | 17 (2.3) | 1 (2.8) | 21 (2.1) |
| 4 | 0 (0) | 2 (0.3) | 0 (0) | 2 (0.2) |
| 5 (least deprived) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Smoking history | ||||
| Current smoker | 148 (64.9) | 527 (72.0) | 31 (86.1) | 706 (70.9) |
| Years smoked (years) | 42 (33 to 51) | 47 (44 to 51) | 51 (47 to 54) | 47 (42 to 51) |
| Years quit (years) | 0 (0 to 3) | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) |
| Average smoking intensity (cigs/day) | 14 (8 to 20) | 20 (10 to 20) | 20 (10 to 23) | 17 (10 to 20) |
| Pack years | 23 (10 to 41) | 38 (26 to 51) | 46 (26 to 63) | 36 (21 to 50) |
| Lung cancer risk | ||||
| PLCO (% 6-year risk) | 1.40 (0.39 to 5.48) | 3.74 (1.80 to 7.14) | 5.68 (2.96 to 9.27) | 3.43 (1.38 to 6.97) |
| LLP (% 5-year risk) | 3.07 (1.55 to 7.16) | 5.58 (3.79 to 8.75) | 5.5 (4.58 to 9.77) | 5.20 (3.16 to 8.56) |
| Physical measurements | ||||
| FEV1 (l/min) | 2.12 (1.68 to 2.57) | 2.06 (1.64 to 2.56) | 1.74 (1.12 to 2.2) | 2.06 (1.64 to 2.55) |
| FEV1 (% predicted) | 85 (69 to 98) | 82 (66 to 96) | 73 (53 to 89) | 82 (67 to 97) |
| FEV/FVC (%) | 70 (63 to 77) | 69 (61 to 75) | 62 (54 to 69) | 69 (62 to 76) |
| BMI (kg/m2) | 25.8 (22.9 to 29.1) | 26.2 (23 to 29.4) | 23.5 (22.5 to 26) | 26.0 (22.9 to 29.2) |
| WHO Performance Status | ||||
| 0 - asymptomatic | 203 (89.0) | 660 (90.2) | 28 (77.8) | 891 (89.5) |
| 1 - completely ambulatory | 23 (10.1) | 64 (8.7) | 8 (22.2) | 95 (9.5) |
| 2 - <50% of day in chair/ bed | 1 (0.4) | 8 (1.1) | 0 (0) | 9 (0.9) |
| 3 - >50% of day in chair/ bed | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.1) |
| LDCT | ||||
| Follow-up duration since LDCT (days) | n/a | 1007 (851 to 1143) | 1044 (933 to 1153) | 1008 (853 to 1144) |
BMI, body mass index; cigs, cigarettes; CT, Computed Tomography scan; GCSE, General Certificate of Secondary Education; LDCT, low-dose CT; LLP, Liverpool Lung Project; PLCO, Prostate, Lung, Colorectal and Ovarian study; USPSTF, United States Preventive Services Task Force; VATS, Video Assisted Thoracoscopic Surgery.
At the baseline LDCT scan, a total of 125/768 participants had indeterminate pulmonary nodules requiring 3-month (n=92 (11.9%)) or 12-month (n=33 (4.3%)) surveillance and a further 33 (4.3%) were considered to have lesions suspicious for lung cancer that instigated referral to the local multidisciplinary meeting. The remaining 610 participants had a ‘normal’ scan or had non-malignant findings that have been discussed elsewhere.13 14 After a median follow-up of 1044 days, a total of 36 lung cancers (4.7%) were diagnosed. Of these, 17 (51.5% of those referred to the lung cancer clinic) were diagnosed directly following the baseline LDCT and the remainder were diagnosed following further surveillance CT scans of indeterminate nodules in the 3-month (n=16, 17.4% of nodules in this group) or 12-month surveillance groups (n=3, 9.1% of nodules in this group).
For invasive investigations we report the data as a percentage of the total number of lung cancers (table 2). Forty-nine (136%) participants underwent positron emission tomography scan, 10 (27.8%) had endobronchial ultrasound and 5 (13.9%) underwent percutaneous CT-guided lung biopsy. Numbers of diagnostic investigations performed in those without a later diagnosis of cancer are also detailed in table 2. Of note, there were no adverse outcomes from diagnostic investigations in this group. Twenty-one (58.3%) participants had a surgical resection without prior histological confirmation of malignancy (and underwent frozen section at the time of the resection), though some had undergone diagnostic staging examinations prior to surgery. 2 out of 28 (7.1%) lung resections were subsequently found to be benign and this represented 0.3% of participants without lung cancer. There were no deaths within 90 days of surgery.
Table 2.
Investigations rates, and stage, histology and treatments from the baseline LDCT scan
| Number in total cohort (% of total lung cancers, n=36 (*except treatments) |
Number among those without a diagnosis of lung cancer (% of total participants without lung cancer, n=732) |
|
| Diagnostic or staging investigations | ||
| Positron emission tomography (PET) | 49 (136) | 16 (2.2) |
| Percutaneous non-lung biopsy | 5 (13.9) | 0 (0) |
| Other percutaneous biopsy | 6 (16.7) | 1 (0.1) |
| Cervical lymph node FNA | 2 (5.6) | 0 (0) |
| Fibreoptic bronchoscopy | 12 (33.3) | 9 (1.2) |
| Endobronchial ultrasound | 10 (27.8) | 1 (0.1) |
| Endoscopic ultrasound | 1 (2.8) | 0 (0) |
| VATS or open lung biopsy | 21 (58.3) | 2 (0.3) |
| Total: PET or invasive procedures | 29 (4.0) | |
| Histology | ||
| Invasive adenocarcinoma | 16 (44.4) | |
| Minimally invasive adenocarcinoma | 3 (8.3) | |
| Adenocarcinoma in situ | 1 (2.8) | |
| Squamous cell carcinoma | 6 (16.7) | |
| Mixed NSCLC (ie, adenosquamous) | 2 (5.6) | |
| Small cell lung cancer | 2 (5.6) | |
| Multiple or mixed histology (small cell + NSCLC) | 3 (8.3) | |
| Radiological diagnosis | 2 (5.6) | |
| Carcinoid | 1 (2.8) | |
| Stage (TNM 7th edition) | ||
| Stage I & II | 26 (72.2) | |
| Ia | 22 (61.1) | |
| Ib | 1 (2.8) | |
| IIa | 3 (8.3) | |
| IIb | 0 (0) | |
| IIIa | 6 (16.7) | |
| IIIb | 1 (2.8) | |
| IV | 3 (8.3) | |
| Treatments (NSCLC) (*% are of total NSCLC) | ||
| Curative intent | 27 (79.4) | |
| Sub-lobar resection | 11 (32.4) | |
| Lobectomy | 15 (44.1) | |
| SABR | 1 (2.9) | |
| Concurrent chemoradiation | 2 (5.9) | |
| Palliative chemotherapy±radiation | 4 (11.8) | |
| Surveillance | 1 (2.9) | |
| Treatments (SCLC) (*% are of total SCLC) | ||
| Radical chemoradiation | 2 (100) | |
CT, CT scan; DNA, did not attend; FNA, fine needle aspiration; GCSE, General Certificate of Secondary Education; LDCT, low-dose CT; LHC, lung health check; MDT, multidisciplinary team; NSCLC, non-small cell lung cancer; SABR, stereotactic ablative radiotherapy; SCLC, small cell lung cancer; TNM, tumour, node, metastases; UKLS, United Kingdom Lung Cancer Screening Trial; USPSTF, United States Preventive Services Task Force; VATS, video assisted thorascopic surgery.
Twenty-six (72.2%) of all lung cancers were stage I or II and 27 (79.4%) of those with non-small cell lung cancer (NSCLC) had curative-intent treatment (including sublobar resection, lobectomy and stereotactic ablative radiotherapy). Of the two participants with small cell lung cancer, both received concurrent chemoradiation. Ten (27.8%) participants had advanced stage (III or IV) disease, resulting in four (11.8%) of those with NSCLC undergoing palliative chemotherapy or radiotherapy (table 2). Online supplementary table e1 presents details on all 36 lung cancers.
Discussion
This observational cohort study demonstrated that despite the very high risk of lung cancer in the cohort, 75.0% of lung cancers detected were early stage and 79.4% of the patients with NSCLC had treatment with curative intent. Indeterminate pulmonary nodules for 3-month and 12-month surveillance were detected in 11.9% and 4.3% of the participants screened, respectively, and lung cancer was detected in 4.7%.
The rate of indeterminate pulmonary nodules (16.2%) was lower than in NLST (National Lung Screening Trial; 24.2%)1 and NELSON trial (19.2%).15 This may have been in part due to implementation of the 2015 BTS pulmonary nodule guidelines which enables a more conservative approach to nodules smaller than 5 mm.9 The lung cancer prevalence was significantly higher than the majority of LCS trials, which have reported a 1% to 2% prevalence.1 16 17 However other higher-risk LCS cohorts have demonstrated a similar lung cancer prevalence to that seen here.6 18 The proportion of participants with early-stage lung cancer who received treatment with curative intent was slightly lower than observed in UKLS,17 which again may reflect the population screened. The number of invasive tests for those without a diagnosis of lung cancer was low, with only 4% of individuals without cancer having a positron emissiontomography-CT (PET-CT) scan or other invasive tests such as bronchoscopy or percutaneous biopsy.
A strength of this study is that it demonstrates a method of recruiting otherwise underserved populations as evidenced by the low socioeconomic and education levels in the majority of the cohort and as such this study illustrates a pragmatic, ‘real-world’ approach to LCS. It is limited by the small sample size and low number of cancers. We acknowledge that this cohort had particularly high lung cancer risk, however, in light of emerging evidence advocating risk-based selection of LCS-eligible individuals,4 19 we believe the findings reported here are generalisable to the LCS-eligible population.
In conclusion, the rate of indeterminate pulmonary nodules was lower and the rate of lung cancer was higher than previous randomised LCS trials, and one in six individuals with an indeterminate nodule requiring 3-month surveillance LDCT were subsequently diagnosed with lung cancer. From these findings, as well as the impressive early-stage disease and curative intent treatment rates observed, we propose that LCS in a ‘real-world’ setting may be less harmful, more efficient and more cost-effective than has been seen in larger LCS studies.
Footnotes
Twitter: @mamta_ruparel, @LUNGRADIOLOGIST
Contributors: All authors were involved in the design and/or conduct of the study or the interpretation of the results. All authors contributed to and approved the final manuscript.
Funding: This study was part of the Lung Screen Uptake Trial project, which was funded by a National Awareness and Early Diagnosis Initiative (NAEDI) project grant awarded by Cancer Research UK (CRUK) and a consortium of funders (Department of Health (England); Economic and Social Research Council; Health and Social Care R&D Division, Public Health Agency, Northern Ireland; National Institute for Social Care and Health Research, Wales; Scottish Government) (SLQ and SMJ). SMJ is a Wellcome Trust Senior Fellow in Clinical Science (WT107963AIA). SMJ is supported by the Rosetrees Trust, the Roy Castle Lung Cancer Foundation, the Stoneygate Trust, the Welton Trust, the Garfield Weston Trust and the UCLH Charitable Foundation. This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centre’s funding scheme (NN and SMJ). SLQ is supported by a CRUK Postdoctoral Fellowship (C50664/A24460).
Competing interests: SMJ, MR, JLD, CH, ST and HH are supported by funding for a large trial of low dose CT screening, called the ‘SUMMIT Study’ by GRAIL Inc. SLQ and NN collaborate on the SUMMIT Study. SMJ has received honoraria from AstraZeneca, BARD1 Bioscience and Janssen for being an Advisory Board Expert and travel to a US conference. SMJ received grant funding from Owlstone for a separate research study and has a family member who has a financial association with AstraZeneca. MR has received travel funding for a conference from Takeda and an honorarium for planning and speaking at educational meetings from AstraZeneca.
Patient consent for publication: Not required.
Ethics approval: This study is part of the Lung Screen Uptake Trial (LSUT), which was granted ethical approval by the City Road and Hampstead NHS Research Ethics Committee (REC; reference: 15/LO/1186) and was registered with ClinicalTrials.gov (NCT02558101) and the International Standard Registered Clinical/soCial sTudy Number (ISRCTN21774741).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020;382:503–13. 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 3. Pastorino U, Silva M, Sestini S, et al. Prolonged lung cancer screening reduced 10-year mortality in the mild trial: new confirmation of lung cancer screening efficacy. Ann Oncol 2019;30:1162–9. 10.1093/annonc/mdz117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med 2013;369:245–54. 10.1056/NEJMoa1301851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Black WC, Gareen IF, Soneji SS, et al. Cost-effectiveness of CT screening in the National lung screening trial. N Engl J Med Overseas Ed 2014;371:1793–802. 10.1056/NEJMoa1312547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crosbie PA, Balata H, Evison M, et al. Implementing lung cancer screening: baseline results from a community-based 'Lung Health Check' pilot in deprived areas of Manchester. Thorax 2019;74:405–9. 10.1136/thoraxjnl-2017-211377 [DOI] [PubMed] [Google Scholar]
- 7. Quaife SL, Ruparel M, Beeken RJ, et al. The Lung Screen Uptake Trial (LSUT): protocol for a randomised controlled demonstration lung cancer screening pilot testing a targeted invitation strategy for high risk and 'hard-to-reach' patients. BMC Cancer 2016;16:281. 10.1186/s12885-016-2316-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quaife SL, Ruparel M, Dickson JL, et al. Lung screen uptake trial (LSUT): randomized controlled clinical trial testing targeted invitation materials. Am J Respir Crit Care Med 2020;201:965–75. 10.1164/rccm.201905-0946OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guideline Development Group The British thoracic Society guidelines on the investigation and management of pulmonary nodules. Thorax 2015;70:794–8. 10.1136/thoraxjnl-2015-207221 [DOI] [PubMed] [Google Scholar]
- 10. Baldwin DR, White B, Schmidt-Hansen M, et al. Diagnosis and treatment of lung cancer: summary of updated NICE guidance. BMJ 2011;342:d2110. 10.1136/bmj.d2110 [DOI] [PubMed] [Google Scholar]
- 11. Ruparel M, Quaife SL, Dickson JL, et al. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax 2019;74:1140–6. 10.1136/thoraxjnl-2018-212812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruparel M, Quaife SL, Dickson JL. Prevalence, symptom burden and under-diagnosis of COPD in a lung cancer screening cohort, 2019. Available: www.atsjournals.org [Accessed 7 Jun 2019].
- 13. Ruparel M, Quaife SL, Dickson JL, et al. Prevalence, symptom burden, and underdiagnosis of chronic obstructive pulmonary disease in a lung cancer screening cohort. Ann Am Thorac Soc 2020;17:869–78. 10.1513/AnnalsATS.201911-857OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ruparel M, Quaife SL, Dickson JL, et al. Is lung cancer screening an opportunity to reduce cardiovascular mortality? 2019;259:A5896. [Google Scholar]
- 15. Horeweg N, van der Aalst CM, Vliegenthart R, et al. Volumetric computed tomography screening for lung cancer: three rounds of the Nelson trial. Eur Respir J 2013;42:1659–67. 10.1183/09031936.00197712 [DOI] [PubMed] [Google Scholar]
- 16. Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the Nelson trial of low-dose CT screening. Lancet Oncol 2014;15:1332–41. 10.1016/S1470-2045(14)70389-4 [DOI] [PubMed] [Google Scholar]
- 17. Field JK, Duffy SW, Baldwin DR, et al. UK lung cancer RCT pilot screening trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161–70. 10.1136/thoraxjnl-2015-207140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bechtel JJ, Kelley WA, Coons TA, et al. Lung cancer detection in patients with airflow obstruction identified in a primary care outpatient practice. Chest 2005;127:1140–5. 10.1016/S0012-3692(15)34459-7 [DOI] [PubMed] [Google Scholar]
- 19. Ten Haaf K, Tammemägi MC, Bondy SJ, et al. Performance and cost-effectiveness of computed tomography lung cancer screening scenarios in a population-based setting: a Microsimulation modeling analysis in Ontario, Canada. PLoS Med 2017;14:e1002225. 10.1371/journal.pmed.1002225 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2020-214703supp001.pdf (204.8KB, pdf)