Highlights
-
•
A GingerAle meta-analysis of 25 voxel-based morphometry studies with N = 7,612.
-
•
Greater body mass index is associated with reduced orbitofrontal cortex gray matter volume.
-
•
Age effects of the orbitofrontal cortex (OFC) finding are explored.
-
•
An exploratory network coactivation analysis with the OFC using Neurosynth is reported.
-
•
The Human Connectome Project is used to explore the network coactivation analysis result.
Abbreviations: ALE, Activation likelihood estimation; AAL, Automated Anatomical Labelling atlas; Fmri, functional magnetic resonance imaging
Keywords: Gray matter volume, Overweight, Obesity, Body mass index, VBM, BMI
Abstract
Neural models of obesity vary in their focus upon prefrontal and striatal differences. Animal and human studies suggest that differential functioning of the orbitofrontal cortex is associated with obesity. However, meta-analyses of functional neuroimaging studies have not found a clear relationship between the orbitofrontal cortex and obesity. Meta-analyses of structural imaging studies of obesity have shown mixed findings with regards to an association with reduced orbitofrontal cortex gray matter volume. To clarify these findings, we conducted a meta-analysis of 25 voxel-based morphometry studies, and found that greater body mass index is associated with decreased gray matter volume in the right orbitofrontal cortex (Brodmanns’ areas 10 and 11), where family-wise corrected p < .05, N = 7,612. Use of the right orbitofrontal cortex as a seed in a Neurosynth Network Coactivation analysis showed that this region is associated with activity in the left frontal medial cortex, left temporal lobe, right precuneus cortex, posterior division of the left middle temporal gyrus, and right frontal pole. When Neurosynth Network Coactivation results were submitted as regions of interest in the Human Connectome Project data, we found that greater body mass index was associated with greater activity in left frontal medial cortex response to the Gambling Task, where p < .05, although this did not survive Bonferroni-correction. Our findings highlight the importance of the orbitofrontal cortex structure and functioning in neural models of obesity. Exploratory analyses suggest more studies are needed that examine the functional significance of reduced orbitofrontal cortex gray matter volume in obesity, and the effect of age and weight changes on this relationship using longitudinal designs.
1. Introduction
Obesity is a major health risk factor for morbidity and mortality. Understanding the brain regions associated with increased body mass index may promote effective interventions. Two meta-analyses of structural neuroimaging data have focused on the relation between gray matter volumes and body mass index, with conflicting findings. One meta-analysis (García-García et al., 2019) showed an association between overweight/obese status (World Health Organization, 1995) with lower gray matter volume in the right medial prefrontal cortex (ventral subdivision), left pre-central gyrus, bilateral cerebellum lobules VIIb and Crus II, left temporal pole, and left inferior parietal cortex, validating these findings with a voxel-based morphometry analysis of the Human Connectome Project dataset (Van Essen et al., 2013). Another meta-analysis (Herrmann et al., 2019) of 10 studies, associated obese status (World Health Organization, 1995) with smaller gray matter volumes in the left inferior frontal gyrus, left-middle frontal gyrus, and right inferior frontal gyrus (including insula). Obesity was also associated with reduced gray matter volume in regions outside of the prefrontal cortex - the left middle temporal cortex, left precentral gyrus, and left cerebellum and superior temporal gyrus (Brodmann’s area 34), including the amygdala and the lenticular nucleus. Greater gray matter volume in certain regions were also associated with obesity.
Cognitive neuroscience models of weight gain are critical to place structural neuroimaging findings into a meaningful framework. As for other maladaptive behaviors (Diehl et al., 2018), models of obesity vary in their emphasis upon executive functioning relative to reward functioning; with some focusing upon prefrontal differences (Lavagnino et al., 2016), others on striatal differences (Everitt, 2014), and others a combination of these (Rolls, 2007aa, Rolls, 2019bb, Rolls, 2019cc). For instance, models of obesity that attribute overeating to failures in inhibitory control when making choices about immediate versus longer-term rewards are supported by studies showing that weight loss maintenance outcomes are predicted by dorsolateral prefrontal cortex activity during delay discounting tasks (Weygandt et al., 2013, Weygandt et al., 2015, Stojek and MacKillop, 2017). One meta-analysis has shown that problems with inhibition and working memory differentiate groups in the overweight / obese range from those in the healthy weight range (Yang et al., 2018); while another shows that weight loss is associated with improvements in executive functioning (Veronese et al., 2017). By contrast, animal models of addiction have informed the conceptualization that habitual overeating leading to weight gain and obesity marks the progressive recruitment of the dorsal striatum (Everitt, 2014, Everitt and Robbins, 2013, Everitt and Robbins, 2016, Smith and Robbins, 2013, Wang et al., 2020). The primary goal of this study was to evaluate these models of obesity in a meta-analysis of structural imaging data in younger and older adults who range from the healthy to obese weight range.
Single-cell recording studies in monkeys and human functional magnetic resonance imaging (fMRI) studies suggest the orbitofrontal region may play a role in both of the aforementioned models, as it is associated with monitoring the reward value of food (Rolls, 2019bb, Rolls et al., 1990). fMRI meta-analyses show that the orbitofrontal cortex is important in response to food visual (van der Laan et al., 2011) cues, food taste cues (Veldhuizen et al., 2011, Yeung Andy Wai et al., 2017, Chen and Zeffiro, 2020) combined food visual and taste cues (Huerta et al., 2014, Devoto et al., 2018, Tang et al., 2012), and monetary reward (Oldham et al., 2018) cues. Large-scale connectivity meta-analyses reveal the orbitofrontal cortex to be a hub in the executive control, default mode, and salience networks (Alves et al., 2019, Riedel et al., 2018, de la Vega et al., 2016, Smith and Delgado, 2017). However, to date, human fMRI meta-analyses have not identified a relation between orbitofrontal cortex activity and obesity.
The current meta-analysis sought to clarify the findings of previous meta-analyses of structural imaging studies of obesity using the GingerALE (Eickhoff et al., 2009, Eickhoff et al., 2017) program, where models conducted assume an isotropic kernel. Whole-brain voxel-based morphometry studies were included in the primary analysis to confirm differences between individuals in the overweight and obese body mass index range relative to those in the healthy body mass index range (Centers of Disease Control, 2020). Large-scale connectivity meta-analyses have shown that brain regions do not typically act in isolation but rather in a network with other regions (Alves et al., 2019, Riedel et al., 2018, de la Vega et al., 2016, van den Heuvel and Sporns, 2013, Morawetz et al., 2020, Chase et al., 2020). Given this, we next explored the brain regions that are typically coactive with the areas associated with body mass index that were identified in our primary meta-analysis. To do this we used a network coactivation analysis of Neurosynth’s database of neuroimaging studies, which incorporates different imaging modalities and tasks. Finally, we used the coactive regions identified in the Neurosynth analysis as regions of interest in an analysis of the Human Connectome Project dataset. Specifically, we explored whether greater body mass index was associated with neural activity during reward or working memory tasks (Van Essen et al., 2013). The exploratory analysis of the Human Connectome Project dataset was conducted for three reasons. First, to better understand the relationship between differential gray matter structure in obesity and the functioning of associated regions by using an independent dataset. Second, to further validate our primary confirmatory finding. Third, we wanted to see if we could replicate and extend the findings of García-García et al. (2019) who used the structural data from the Human Connectome Project dataset to validate their structural meta-analysis conducted with the Anisotropic Effect-Size Seed-Based d Mapping program (Radua and Mataix-Cols, 2012, Radua and Mataix-Cols, 2012, Radua et al., 2012, Radua et al., 2014) with greater body mass index. This exploratory analysis assessed the functional significance of our structural findings and their implications for neural models of obesity, which differentially emphasize reward and executive functioning.
2. Material and methods
2.1. Study eligibility criteria
Gray matter volume studies were selected by searching Pubmed and Web of Science databases prior to 5th January 2019 using specific search terms listed in Supplementary Methods. Additional studies were found by examining previous reviews and meta-analyses. Inclusion criteria: studies were in 1) a peer reviewed journal, 2) in English, 3) stated the use of whole-brain (not a region of interest analysis) gray matter or white matter findings using voxel-based morphometry methods, 4) used Montreal Neurological Institute coordinates or Talairach space, 5) reported T, Z, r or p value describing group differences between individuals in the overweight or obese range relative to individuals in the healthy weight range (Centers of Disease Control, 2020), or the association between regions of gray matter volume and body mass index, and 6) included adults aged 18 to 60 years. We excluded contrasts of samples in the Underweight body mass index range (Centers of Disease Control, 2020), which includes individuals in the Anorexia Nervosa body mass index range.
2.2. Study selection
2,276 abstracts were identified from the database searches with 15 identified from other reviews or meta-analyses. Of these 2,291 abstracts, 1,317 abstracts were duplicates and discarded. The remaining 974 abstracts were screened, and 255 abstracts were excluded, because they included non-human animals (11/255), were reviews (22/255) or meta-analyses (2/255), were not written in English (2/255), did not assess body mass index (5/255), did not report x, y, z coordinates (3/255), reported conducting a region of interest analysis (1/255), did not report on gray matter results (2/255), involved children under 16 years (14/255), and were clinical studies involving patients (190/255). Of the 719 abstracts remaining, 694 were excluded following review of the full text because they involved non-human animals (8/694), were reviews (29/694), meta-analyses (5/694), had sample sizes ≤ 3 (3/694), were not in English (1/694), did not report body mass indices (215/694), did not report the x, y or z coordinates denoting neural differences between individuals in the overweight/obese and healthy body mass index categories or where there were differences in gray matter volume between individuals in the healthy weight body mass index category relative to the overweight/obese categories (Centers of Disease Control, 2020) (122/694), reported a region of interest analysis and not a whole brain analysis (30/694), did not report gray matter volume findings, for instance reporting diffusion tensor imaging or cortical thickness outcomes (68/694), included children aged ≤16 years in the sample (46/694), used patient samples, e.g., individuals with aphasia or Prader-Willi syndrome, Alzheimer’s disease, Anorexia Nervosa, Bulimia Nervosa, or Diabetes (136/694), or did not report the t, z or r differences or associations of interest (31/694). See Fig. 1 for the Preferred Reporting Items for Systematic Reveiws and Meta-analyses Flow Diagram (Moher et al., 2009).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Flow Diagram for voxel-based morphometry studies of obesity (Moher et al., 2009).
2.3. Study characteristics
These exclusion criteria left 25 articles yielding 28 experiments (separate group findings) for the primary meta-analysis examining the association between increasing body mass index and gray matter volume, see Table 1. See Supplementary Table 1 for further study details.
Table 1.
Experiments examining body mass index and gray matter volume.
| # | Author Date | Sample | Body mass index m (sd) | Female | Age m (sd) | T/Z/ r | P values |
|---|---|---|---|---|---|---|---|
| 1 | Bond 2014 (Bond et al., 2014) | 55 HW | 24.00 (3.90) | 24 | 22.15 (3.65) | r | p FWE < 0.05 |
| 2 | Brooks 2013 (Brooks et al., 2013) | (1) 59 OB (2) 97 HW |
(1) 33.7 (0.4) (2) 22.1 (0.2) |
(1) 58 (2) 54 |
(1) 75 (NA) (2) 75 (NA) |
T | p FWE < 0.05 |
| 3 | Figley 2016 (Figley et al., 2016) | (1) 16 men (2) 16 women |
(1) 26.2 (4.4) (2) 23.5 (4.2) |
16 |
(1) 28.7 (9.7) (2) 30.9 (11.5) |
r |
p FWE < 0.05 |
| 4 | Hayakawa 2018 (Hayakawa et al., 2018) | 523 men | 24.7 (3.1) | 0 | 55.3 (9.7) | r | p FWE < 0.05 |
| 5 | Hayakawa 2018 (Hayakawa et al., 2018) | 269 women | 22.0 (3.3) | 269 | 55.2 (9.9) | r | p FWE < 0.05 |
| 6 | He 2015 (He et al. 2015) | 336 | 20.4 (2.2) | 195 | 20.4 (1.0) | r | p < .01 uncor |
| 7 | Honea 2016 (Honea et al., 2016) | 53/72 dieters a | 35.6 (3.6) | 34 | 40.1 (8.5) | r | p FWE < 0.05 |
| 8 | Horstmann (Horstmann et al., 2011) | 61 HW women | 26.15 (6.64) | 61 | 25.11 (4.43) | Z, r | p < 0.0001 uncor |
| 9 | Horstmann 2011 (Horstmann et al., 2011) | 61 HW men | 27.24 (6.13) | 0 | 25.46 (4.25) | Z, r | p < 0.0002 uncor |
| 10 | Janowitz 2015 (Janowitz et al., 2015) | 758 SHIP-2 sample | 27.40 (4.50) | 408 | 49.80 (9.30) | r | p FWE < 0.05 |
| 11 | Karlsson 2013 (Karlsson et al., 2013) | (1) 23 OB (2) 22 HW |
(1) 43.2 (3.7) (2) 24.0 (2.3) |
(1) 18 (2) 15 |
(1) 47.3 (8.9) (2) 46.5 (9.5) |
r | p FWE < 0.05 |
| 12 | Kennedy (Kennedy et al., 2016) | 137 adolescents | 20.5b | 68 | 14.9 (3.1) | r | p FWE < 0.05 |
| 13 | Kurth 2013 (Kurth et al., 2013) | 115 OW | 25.0 (4.1) | 61 | 45.2 (15.5) | r | p FDR < 0.05 |
| 14 | Masouleh 2016 (Masouleh et al., 2016) | 617 older adults | 27.5 (4.0) | 258 | 68.7 (4.6) | r | p FDR < 0.05 |
| 15 | Mathar 2016 (Mathar et al., 2016) | (1) 23 HW (2) 19 OB |
(1) 21.8 (1.3) (2) 33.6 (2.3) |
(1) 12 (2) 8 |
(1) 25.2 (3.0) (2) 27.0 (4.1) |
Z | p FWE < 0.05 |
| 16 | Mueller 2015 (Mueller et al., 2015) | 16 OB/OW | 33.6 (5.9) | 9 | 27.2 (6.7) | r | p FWE < 0.05 |
| 17 | Nouwen 2017 (Nouwen et al., 2017) | (1) 20 OB (2) 19 HW |
30.3c | (1) 15 (2) 14 |
(1) 14.9 (2.0) (2) 16.4 (1.7) |
Z | p < .005 uncor |
| 18 | Opel 2015 (Opel et al., 2015) | 141 HW | 25.7 (4.7) | 78 | 37.6 (11.8) | r | p FWE < 0.05 |
| 19 | Opel 2017 (Opel et al., 2017) BiDirect | 347 HW | 26.3 (4.1) | 155 | 51.6 (8.2) | T, r | p FWE < 0.05 |
| 20 | Opel 2017 (Opel et al., 2017) MNC | 330 HW | 24.5 (3.9) | 172 | 39.2 (11.3) | T, r | p FWE < 0.05 |
| 21 | Pannacciulli 2006 (Pannacciulli et al., 2006) | (1) 24 OB (2) 36 HW |
39.4 (4.7) 22.7 (2.2) |
24 | (1) 32.0 (8.0) (2) 33.0 (9.0) |
r | p < .01 uncor |
| 22 | Shott 2015 (Shott et al., 2015) | (1) 18 OB (2) 24 HW |
34.8 (4.4) 21.6 (1.3) |
42 | (1) 28.7 (8.30) (2) 27.4 (6.28) |
T,Z | p FWE < 0.05 |
| 23 | Smucny 2012 (Smucny et al., 2012) | (1) 28 OB-prone d (2) 25 OB-resistant |
26.2 (2.9) 21.0 (2.0) |
26 | (1) 30.3 (3.81) (2) 31.3 (3.45) |
T | p < 0.001 uncor |
| 24 | Taki 2008 (Taki et al., 2008) | 690 men | 23.4 (3.0) | 0 | 44.5 (16.1) | r | p < 0.001 uncor |
| 25 | Tuulari 2016 (Tuulari et al., 2016) | (1) 29 HW (2) 47 OB |
23.2 (2.8) 42.2 (4.0) |
65 | (1) 45.9 (11.8) (2) 44.9 (9.0) |
r | p < .05 uncor |
| 26 | Walther 2010 (Walther et al., 2010) | 95 OW | 28.3 (2.1) | 95 | 69.3 (9.3) | r | p FDR < 0.05 |
| 27 | Weise 2017 (Weise et al., 2017) | 875 HW | 26.6 (5.3) | 489 | 28.8 (3.7) | r | p FWE < 0.05 |
| 28 | Yao 2016 (Yao et al., 2016) | 109 HW | 27.6 (6.1) | 62 | 35.2 (11.2) | r | p < .001 uncor |
Note: # = experiment number; BMI = body mass index, NA = not available; OW = individuals in the BMI range for overweight (Centers of Disease Control, 2020); OB = individuals in the BMI range for obesity (Centers of Disease Control, 2020); HW = individuals in the healthy weight range (Centers of Disease Control, 2020),a Successful dieters; b Reported 59.36 (26.87) %ile which is a healthy BMI given age. Here we calculated the body mass index equivalent using US growth charts (Kuczmarski et al. December 4, 2000 (Revised). c Reported (1) 3.25 (0.78) %ile of 95th percentile and (2) 0.23 (0.96). Here we calculated the mean body mass index of both groups using UK growth charts (Cole et al., 1995) d ‘Obesity prone’ or ‘obesity resistant’ was defined by self-identification, BMI, and personal and family weight history.
26/28 experiments reported that increased body mass index was associated with decreased gray matter volume or that decreased gray matter was associated with an overweight/obese group compared to a healthy weight group (Centers of Disease Control, 2020). 2/28 experiments from one article (Horstmann et al., 2011) reported increased gray matter volume in the overweight/obese group relative to the healthy weight group (Centers of Disease Control, 2020); 4/28 experiments (Pannacciulli et al., 2006, Taki et al., 2008, Weise et al., 2017, Yao et al., 2016) reported both increased and decreased gray matter volume in the overweight group relative to healthy weight group. The number of experiments (6) reporting an association between greater body mass index and larger gray matter volumes was too small to subject to meta-analysis (Eickhoff et al., 2016). The final dataset for our primary analysis examining the association between increasing body mass index and decreased gray matter volume was conducted in 26 experiments. A mass simulation study using the GingerALE program showed that at least 20 studies are necessary to ensure that the final ALE value is not driven by a single study (50% contribution to the ALE score) or two studies (80% contribution to the ALE value) (Eickhoff et al., 2016). 26 experiments were included in the primary confirmatory analysis, suggesting that this analysis was sufficiently powered (Eickhoff et al., 2016).
Of the 26 experiments that reported an association between decreased gray matter volume and increased body mass index, 14 experiments included participants <40 years old; 12 experiments included participants ≥40 years; and 2 experiments included both adolescents as well as adults (Kennedy et al., 2016, Nouwen et al., 2017). With respect to race and sex, 4/26 experiments were composed of only Asian samples (Taki et al., 2008, He et al., 2015, Hayakawa et al., 2018), 1/26 only male participants (He et al., 2015), and 2/26 (Shott et al., 2015, Walther et al., 2010), with only female participants. 2/26 experiments reported both group comparisons (e.g., overweight/obese vs. healthy weight) and correlational relationships (e.g., body weight × brain volumes) (Horstmann et al., 2011, Opel et al., 2017).
All results reported in Talaraich space were converted to Montreal Neurological Institute coordinates, and all model results are presented in Montreal Neurological Institute space (Lancaster et al., 2007).
2.4. Primary Coordinate-Based Meta-Analysis to confirm the location of gray matter volume differences given body mass index
To confirm the location of gray matter regions associated with increased body mass index, we used a Coordinate-Based Meta-Analysis technique, an Activation likelihood estimation (ALE) method implemented in GingerALE 2.3.6 (http://www.brainmap.org/ale/) (Eickhoff et al., 2009, Eickhoff et al., 2017). Coordinate-based ALE meta-analyses estimate the spatial convergence of coordinates between experiments relative to a null hypothesis that these experiment foci are uniformly and randomly distributed across the brain. Specifically, our primary confirmatory analysis will show the most probable location where differences in gray matter volume occur given body mass index status relative to the null hypothesis that these experiment foci are uniformly and randomly distributed across the brain. In this comparison, an assumption of ALE methods are that each voxel has the same a priori likelihood of differentiating groups in the overweight body mass index range from those in the healthy body mass index range. Unlike meta-analyses of two-dimensional outcomes, which pool effect sizes across studies to determine the size and direction of the effect, Coordinate-Based ALE meta-analyses evaluate whether the location of the effect is the same across experiments. Unlike meta-analyses of two-dimensional outcomes, Coordinate-Based ALE meta-analyses typically do not report effect size magnitudes, and meta-analyses of neuroimaging studies are unlikely to routinely report effect size magnitudes until unthresholded statistical maps are released as standard practice. Because we do not have the access to unthresholded statistical maps, Coordinate-Based ALE meta-analysis typically include only significant findings (which may be defined in different ways in different studies) of spatial findings of experiment contrasts where the direction of the relationship is the same. For instance, our primary confirmatory meta-analysis only includes experiments with significant findings showing that decreased gray matter volume was associated with overweight status. The hypotheses for our primary meta-analysis are therefore one-sided and this is constrained by the nature of the GingerALE framework. Conducting this primary confirmatory meta-analysis allows for comparison of findings with gray matter volume meta-analyses using other programs (García-García et al., 2019, Herrmann et al., 2019) as well as previous fMRI meta-analyses (Veldhuizen et al., 2011, Huerta et al., 2014). The ALE method creates a likelihood map for each reported peak coordinate by convolving an isotropic kernel with each peak, and then modelling the likelihood of reduced or increased gray matter volume in that area as a normally distributed Gaussian probability distribution (Turkeltaub et al., 2002, Turkeltaub et al., 2012, Eickhoff et al., 2009, Eickhoff et al., 2017). Isotropic kernel values assume Euclidean distances between voxels and peaks. Comparison between the modelled activity map, that is the maximum of all the Gaussian distributions (Turkeltaub et al., 2012) for all the experiment foci, and the ALE null distribution yields a 3 dimensional image for each probability level. A random effects model was used. To correct for multiple comparisons and maintain a cluster-level family-wise error rate correction of 5%, we used a cluster-forming threshold of p = 0.001 with 1,000 random permutations, as recommended (Eickhoff et al., 2016, Muller et al., 2018). This correction for ALE meta-analyses is recommended because a mass simulation study of more than 120,000 meta-analytic datasets from the BrainMap database has shown this correction to lead to less risk of false positives in terms of spatial convergence than other forms of correction, and so represents current best practice (Eickhoff et al., 2016).
In order to assess the robustness of our primary finding that greater body mass index is associated with reduced gray matter volume in a certain region/s, we assessed the number of experiments that contributed to this primary result (Muller et al., 2018). This yielded the average non-linear contribution of an experiment to the ALE score, calculated from the ratio of ALE-values at the location of the cluster with and without the experiment in question. Jacknifing can also address the issue of how heterogeneous the finding is (location associated with greater body mass index) given the various studies included in the analysis. We repeated the coordinate-based meta-analysis 26 times, removing one experiment from each analysis (so that there were only 25 experiments for each iteration) to examine if the same gray matter location was found to be associated with body mass index. We then noted the number of experiments where the same primary result was yielded.
2.5. Exploratory network coactivation analysis using Neurosynth
The region generated from the GingerALE meta-analysis associated with reduced gray matter volume and increased body mass index was made into a mask. This mask was then used as a seed to examine the correlated network regions from more than 57,792 activations drawn from 1,500 studies in the Neurosynth database (3/28/2018) (Yarkoni et al., 2011). The reverse inference z-score (‘specificity_z’) generated by the network coactivation analysis was thresholded at q < 0.001 and this region was then submitted to Neurosynth for the decoding analysis. The regions found to be associated with this region in the Neurosynth database of studies were thresholded where z ≥ 4.14, and k ≥ 200. The exploratory network coactivation analysis using Neurosynth was two-sided. This analysis was conducted in Python 3.6.4.
2.6. Functional significance of network coactivation results using the Human Connectome Project (Van Essen et al., 2013)
Regions of interest yielded from the Network Coactivation Analysis were made into separate masks in FSL6.0 (Jenkinson et al., 2012). These masks were used to extract activity from (1) the Working Memory task and (2) Gambling Task in the Human Connectome Project dataset (Van Essen et al., 2013), see task, preprocessing and analysis details in Supplementary Methods. Participants from the Human Connectome Project were included if they completed fMRI tasks of interest and genotyping zygosity tests. Exclusions were: monozygotic / dizygotic twins, lifetime major depressive episodes or Alcohol Abuse or Dependence Marijuana Dependence or Nicotine use or panic disorder or agoraphobia (Diagnostic Statistical Manual-IV, (American Psychiatric Association, 2000), a blood alcohol level ≥0.05, or testing positive for Cocaine, Marijuana, Opiates, Amphetamines, or Methamphetamine at the assessment. This resulted in a dataset of 170 participants. Separate multiple regressions were conducted to assess if body mass index was associated with activity in each of these regions of interest for each of these tasks. Because body mass index is associated with head motion (Siegel et al., 2016, Hodgson et al., 2017) and sociodemographic variables (Flegal et al., 1998, Flegal et al., 2002, Mokdad et al., 2003, Hedley et al., 2004, Centers of Disease Control, 2020, Jones-Smith et al., 2011, McLaren, 2007, Kinge et al., 2015) such as age, education, race, ethnicity, sex, and income level, these were used as covariates in each multiple regression model to understand the contribution of body mass index. Exploratory Network Coactivation Hypotheses tested using the Human Connectome Project data were 2-sided.
3. Results
3.1. Primary analysis: Areas associated with increased body mass index and gray matter atrophy
The primary meta-analysis was conducted on the 26 experiments where decreased gray matter volume was associated with increased body mass index. See Supplementary Table 1 for the direction of the relationship between gray matter volume and body mass index for each experiment. Only 6 experiments showed that increases in gray matter volume were associated with greater body mass index. Because this number of experiments was less than the 20 experiment minimum required to provide sufficient power for the results to be reliable as assessed by mass simulation studies using GingerALE, we did not submit these coordinates for analysis (Eickhoff et al., 2016). The 26 experiments showing decreased gray matter volumes yielded a sample of 7,612 participants (2,644 women) with a mean of 293 (sd = 27) participants per experiment (range 16 to 2,344). 211,251 significant foci were reported. The mean age of participants in these 26 experiments was 39.81 (sd = 16.19) and the mean body mass index was 27.03 (sd = 3.96), which is in the overweight range (Centers of Disease Control, 2020). The mean age and the mean body mass index of the samples across the 26 experiments were not significantly correlated (Pearson r = 0.13, p = .53).
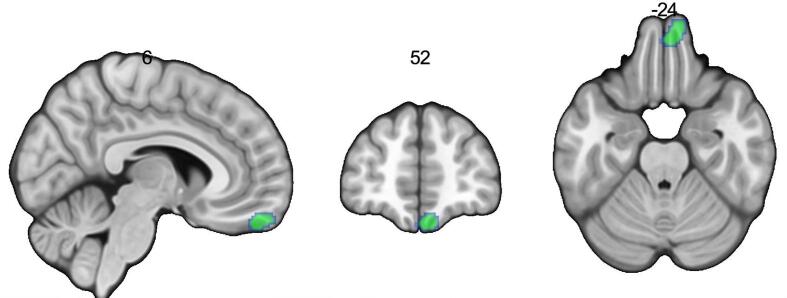
Results of the meta-analysis showed that increased body mass index was associated with decreased gray matter volume in the right frontal pole and right frontal medial cortex (Harvard-Oxford Cortical Structural Atlas), a portion of the ventromedial surface of the frontal pole or the right orbitofrontal cortex, and respectively Brodmann’s areas (BA) 10 and 11, see Table 2, Fig. 2. GingerALE output can be found in Neurovault: https://identifiers.org/neurovault.collection:8703.
Table 2.
In 26 experiments, increased body mass index is associated with reduced gray matter volume in the right frontal pole (Brodmann’s area 10) and right frontal medial cortex (Brodmann’s area 11) in the primary GingerALE analysis.
| Label | Side | x | y | z | Volume (mm3) | Z | ALE | p |
|---|---|---|---|---|---|---|---|---|
| Frontal pole | R | 10 | 58 | –22 | 1,128 | 4.829 | 0.028648 | 6.85E-07 |
| Frontal medial cortex | R | 6 | 52 | −24 | 5.096 | 0.030985 | 1.74E-07 |
Note: The critical cluster threshold was p FWE < 0.05, with a voxel-wise threshold of p uncorrected < 0.001, after 1,000 permutations. BA = Brodmann’s Area. Montreal Neurological Institute coordinates. Labels use the Harvard-Oxford Cortical Structural Atlas from the FSL program. Brodmann’s regions were labelled using Automated Anatomical Labeling.
Fig. 2.
Increased body mass index is associated with reduced gray matter volume in the right orbitofrontal cortex (Brodmann’s areas 10 and 11) in 26 experiments, in the primary analysis using GingerALE. Note: The critical cluster threshold was p < .05 (corrected for multiple comparisons), with a cluster-forming threshold of p < .001 (uncorrected). This is presented in neurological orientation at these Montreal Neurological Institute coordinates of x = 6, y = 52, z = −24.
Assessment of the robustness of the primary result showed that 9/26 (34.6%) experiments contributed to this result. When the meta-analysis was rerun repeatedly, excluding each experiment one at a time, only one experiment – (Weise et al., 2017) – appeared to have a strong effect on the ALE values, as indicated by the highest score of 26.24, range 0.10 to 26.24, median = 12.74. We found that 26/26 experiments yielded significant findings in the orbitofrontal cortex BA10 or BA11 region, with this finding being partial (BA10 only or BA11 only) in 4/26 experiments.
Ageing is associated with both prefrontal atrophy (Minkova et al., 2017), and weight gain (Flegal et al., 1998, Flegal et al., 2002, Mokdad et al., 2003, Hedley et al., 2004). Given this we were concerned that the association between orbitofrontal cortex gray matter volume reduction and body mass index may have been driven by studies with older samples. We also wanted to further interrogate our primary confirmatory finding. Follow up exploratory meta-analysis were conducted separately with experiments where the mean age <40 years (younger) versus experiments where the mean age was ≥40 years (older). Results showed weaker effects in the older samples, see Supplementary Results. To interrogate these age-based comparisons, we explored the relative percentage contribution of the samples divided evenly into 5 different mean age groups to the primary result showing the association between greater body mass index and gray matter volume. We found that experiments grouped into 5 different age groups contributed similarly to the primary result, suggesting that age did not play a significant role in the association between greater gray matter atrophy and higher body mass index.
3.2. Exploratory network coactivation analysis using Neurosynth
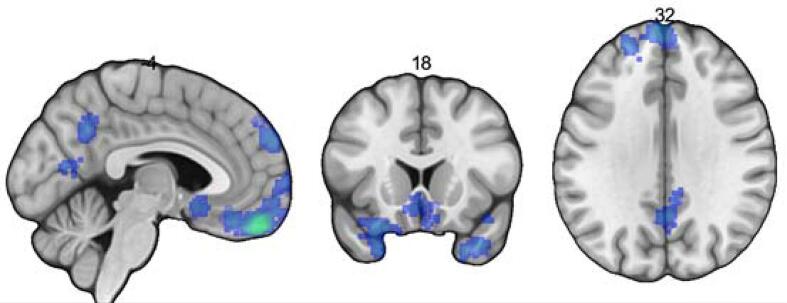
This right orbitofrontal cortex region (BA 10 and BA 11) was used as a seed in a meta-analytic connectivity analysis to identify the brain regions that show corresponding activity in neuroimaging studies in the Neurosynth database. Table 3 and Fig. 3 respectively list and display the network regions coactive with the right orbitofrontal cortex where FDR q < 0.01 and where the number of voxels was 200 or more and using the Harvard-Oxford Cortical Structural Atlas. The regions associated with the right orbitofrontal cortex (BA 10, BA 11) were the: (1) left frontal medial cortex (gyrus rectus in the Automated Anatomical Labelling [AAL] atlas), (2) the left temporal lobe (left middle temporal pole or BA 38 in AAL), (3) right precuneus cortex (right precuneus in AAL), (4) posterior division of the left middle temporal gyrus (left middle temporal gyrus in AAL), the (5) right frontal pole (right frontal superior medial gyrus, BA 10, AAL), and (6) right frontal pole (right superior frontal gyrus, AAL) (Van Essen et al., 2013)
Table 3.
Results of the exploratory Network Coactivation analysis of the Neurosynth database using the right orbitofrontal cortex seed.
| Label | Side | Cluster Index | Voxels | Z | x | y | z | |
|---|---|---|---|---|---|---|---|---|
| 1 | Frontal medial cortex | L | 376 | 4347 | 33.1 | −4 | 48 | −28 |
| 2 | Temporal lobe | L | 375 | 715 | 9.1 | −34 | 18 | −36 |
| 3 | Precuneus cortex | R | 374 | 427 | 8.69 | 2 | −56 | 38 |
| 4 | Middle temporal gyrus, posterior division | L | 373 | 382 | 8.33 | −60 | −12 | −24 |
| 5 | Frontal pole | R | 372 | 312 | 8.81 | 6 | 60 | 30 |
| 6 | Frontal pole | R | 371 | 215 | 9.04 | 22 | 50 | 32 |
Note: This lists the findings from the network coactivation analysis of the Neurosynth database and uses the thresholded specificity_z map (where FDR q < 0.01) where the number of voxels was 200 or more. BA = Brodmann Area; x, y, z are in Montreal Neurological Institute coordinates. Labels use the Harvard-Oxford Cortical Structural Atlas in MNI152 space after normalization with FNIRT from the FSL program, reporting the label with the highest probability.
Fig. 3.
Results of the exploratory Network Coactivation analysis of the Neurosynth database using the right orbitofrontal cortex seed. Note: The network regions coactive with the right orbitofrontal cortex seed (BA 10 and BA 11) where FDR q < 0.01 and where the number of voxels was 200 or more. This is presented in neurological orientation.
3.3. Functional significance of network coactivation results using the Human Connectome Project
Next, we evaluated the relation between body mass index and activity of the six co-active regions in cognitive processing that feature prominently in models of obesity (e.g., executive functioning and reward processing). To do this, we used the Working Memory Task and Reward Task from the Human Connectome Project (Van Essen et al., 2013). For each of these two tasks, for each of these 6 regions, body mass index was entered as a predictor in separate multiple regressions. Because age, years of education, self-reported sex, race, ethnicity, and head motion may be associated with body mass index (Flegal et al., 1998, Flegal et al., 2002, Mokdad et al., 2003, Hedley et al., 2004, Centers of Disease Control, 2020, Jones-Smith et al., 2011, McLaren, 2007, Kinge et al., 2015), these were entered as covariates in the model. These exploratory results are reported in Supplementary Results and Supplementary Tables 2A, 2B, and 2C. Body mass index was associated only with reward-related activity of the left medial cortex for the Gambling Task where p = .035, although this was not significant following Bonferroni-correction for the number of tasks, 2-sided hypotheses and 6 regions of interest (p < .002).
4. Discussion
Our primary meta-analysis results show that body mass index in the overweight and obese body mass index range relative to the healthy body mass index range is associated with reduced gray matter volume in the right frontal pole and right frontal medial cortex (Harvard-Oxford Cortical Structural Atlas), part of the ventromedial surface of the frontal pole or the right orbitofrontal cortex. Using AAL, both regions are described as the right medial frontal gyrus, and respectively as BA 10 and BA 11. In a network coactivation analysis of Neurosynth, the right BA10 and right BA11 areas from the primary analysis were found to be coactive with an adjacent region—the posterior division of the left frontal medial cortex (gyrus rectus), other parts of the right frontal pole, as well as the left temporal lobe, left middle temporal gyrus, and right precuneus cortex. Use of the Network Coactivation results in analyses of the Working Memory and Gambling Tasks from the Human Connectome Project (Van Essen et al., 2013) showed that greater body mass index was associated with greater activity in the left frontal medial cortex in response to the Gambling task, although this finding was not significant after Bonferroni-correction.
4.1. The importance of reduced orbitofrontal cortex gray matter in obesity
Our primary meta-analysis finding, supported by that of another group using a different meta-analytic method (García-García et al., 2019), highlights the central role of the orbitofrontal cortex in obesity. Previous human fMRI meta-analyses have not shown that the orbitofrontal cortex plays a central role in obesity – although they have shown its importance in response to food visual (van der Laan et al., 2011) cues, food taste cues (Veldhuizen et al., 2011, Yeung Andy Wai et al., 2017, Chen and Zeffiro, 2020), combined food visual and taste cues (Huerta et al., 2014, Devoto et al., 2018, Tang et al., 2012), and monetary reward (Oldham et al., 2018) cues. Differences in structural and fMRI meta-analyses findings with regards to the orbitofrontal cortex may be due to the greater susceptibility to signal loss of the orbitofrontal cortex region in fMRI studies and to head motion in fMRI studies of obesity (Siegel et al., 2016, Hodgson et al., 2017). Structural scans assessing gray matter volume are briefer, and cheaper and voxel-based morphometry studies are therefore typically larger than fMRI studies, allowing for better detection of the orbitofrontal cortex in the context of a meta-analysis.
The results of our primary analysis of structural imaging data lend more support for accounts of obesity that emphasize prefrontal (Weygandt et al., 2013, Weygandt et al., 2015, Stojek and MacKillop, 2017) regions rather than striatal differences (Everitt and Robbins, 2016, Berridge and Kringelbach, 2015). Our results also are in line with the conceptualization of obesity is as a chronic medical condition affecting the brain and cognition. This view of obesity has been supported by studies showing relations between greater body mass index and poor verbal memory performance (Masouleh et al., 2016) and poor executive functioning (Walther et al., 2010), which are associated with reduced ventromedial prefrontal cortex (BA10 and BA11) gray matter volume (Masouleh et al., 2016) or reduced orbitofrontal cortex gray matter volume, even when controlling for hypertension (Walther et al., 2010). However, because there is evidence that age contributes to both poor cognitive ability and decreased brain volumes (Minkova et al., 2017) and past studies have shown age to be a contributing factor (Masouleh et al., 2016) or have included only older adults (Walther et al., 2010) we checked for age effects in our primary meta-analysis finding. The association between reduced orbitofrontal cortex gray matter volume and greater body mass index was similar across different age groups, suggesting that our primary meta-analysis finding is independent of age. However, cerebral volume reductions and cognitive problems are not unique to individuals with greater body mass index and may be the result of other medical conditions co-occurring with obesity such as impaired blood pressure, or blood glucose regulation (Schaare et al., 2019, Morville et al., 2018, Markus et al., 2017). Greater body mass index may be viewed as a proxy for individual differences in metabolic functioning, physical inactivity, and overconsumption of poor quality food.
Roll’s hierarchical model of taste most clearly implicates the orbitofrontal cortex (Rolls, 2019). Tier 1 of this model includes the insula and operculum, and is regarded as important in assessing the intensity, temperature, and texture of tastes. Tier 2 features the orbitofrontal cortex (including BA 11, part of the medial orbitofrontal cortex) and the amygdala, and this tier is implicated in monitoring and encoding the reward value of tastes including sensory-specific satiety (Rolls, 2019). Tier 3 includes the medial prefrontal cortex areas (BA10, part of the ventromedial prefrontal cortex, and BA14) and cingulate cortex (BA 24 and BA 32) as well as the striatum/basal ganglia, and lateral hypothalamus/insula regions. Tier 3 is implicated in decision-making and learning regarding food (Rolls, 2019). Our primary finding suggests that the regions associated with greater body mass index overlap with the regions implicated in Tiers 2 and 3 of Rolls’ model (see Supplementary Figure).
Our exploratory analyses of the functional significance of the structural imaging findings based on the Working Memory Task and Reward Task from the Human Connectome Project dataset did not support an association with working memory. By contrast, our results showed the relationship between reduced gray matter volume and greater body mass index was associated with differential reward processing (Wang et al., 2016, Smith et al., 2015), consistent with other functional studies (Verdejo-Roman et al., 2017, Kube et al., 2018, García-García et al., 2019, Bzdok et al., 2013). Animal and human brain lesion studies also support the role of the orbitofrontal cortex in reward processing. Given the absence of gradation in granularity of the orbitofrontal cortex in rodents relative to primates, primate studies offer the closest insights into how this region is implicated in the overconsumption of unhealthy food. Macaques with orbitofrontal cortex BA 11 and BA 13 lesions (Murray et al., 2015, Rudebeck et al., 2017); and humans with orbitofrontal cortex lesions (Reber et al., 2017) are more likely to choose rewards, such as food rewards, which have been previously devalued and differential gray matter volume in these regions predict reward devaluation response (Burke et al., 2014). The orbitofrontal cortex BA 11 and 13 in obesity may be associated with disruption in monitoring sensory-specific satiety, which requires establishing that a food has lost it’s rewarding value after consumption, see monkey studies (Critchley and Rolls, 1996, Pritchard et al., 2007) and human fMRI studies (Rolls et al., 1990, Odoherty et al., 2000, Reber et al., 2017). Macaques with medial frontal pole (BA10) lesions make less adaptive choices, being influenced by irrelevant options (Noonan et al., 2010, Rudebeck and Murray, 2011) and this is also seen in human fMRI and PET studies (Rolls et al., 2010, Rolls et al., 2010, Rogers et al., 1999). In a ‘toxic’ food environment (Brownell & Horgen, 2003), where there are multiple choices of highly rewarding, high-fat, high-sugar foods, overeating may result from or be maintained by maladaptive food choice given prior experience. Macaques exposed to an ‘obesiogenic’ diet, which offered calorically dense food in addition to chow versus those offered chow only (healthy diet) in a randomized controlled trial, gained significantly more weight over a year and exhibited decreased orbitofrontal cortex (BA 11 and 13) to nucleus accumbens functional connectivity as well as greater peripheral inflammation (C-reactive protein), (Godfrey et al., 20180; Godfrey et al., 2020). In this trial, stress appeared to have an interactive effect with the obesiogenic diet unlike the chow-only diet by not resulting in Dopamine 2 receptor binding in this orbitofrontal cortex (BA 11 and 13) region, suggesting that stress and regulation of stress (Morawetz et al., 2020, Chase et al., 2020) may further disrupt orbitofrontal cortex functioning. Human controlled trials of diet interventions examining neural response to palatable food pictures or high-fat high-sweet food have also shown that weight loss is associated with change in orbitofrontal cortex activity (Murdaugh et al., 2012, Chen et al., 2017). Using a region-of-interest analysis, an open trial showed that weight loss was associated with changes in the orbitofrontal cortex (BA 10 and 11) (Neseliler et al., 2019).
Overall, there is evidence that reward evaluation and decision-making systems may be disrupted by the variety and availability of palatable foods in the food environment and this may lead to weight gain (Rolls, 2007aa, Rolls, 2019bb, Rolls, 2019cc). Longitudinal neuroimaging diet trials are needed, however, to assess if food reward devaluation changes are associated with alterations in orbitofrontal cortex functioning and concurrent weight loss.
4.2. Limitations
The quality of a meta-analysis is as only good as the quality of studies that are included. Controlling for total intracranial volume and for sex in voxel-based morphometry studies is important (Nordenskjöld et al., 2013, Ruigrok et al., 2014) and not all studies controlled for these variables. Too few studies examined the association between increased gray matter volume and obesity to permit analysis within the GingerALE framework. Too few studies examined both body mass index and variables that are potential confounding correlates with body mass index to allow the definitive conclusion that the gray matter volume differences found are only due to body mass index. Variables that may be potentially confounded with body mass index include: age, total gray matter volume, biological sex, ancestry or race, income level, education, and country of study (Flegal et al., 1998, Flegal et al., 2002, Mokdad et al., 2003, Hedley et al., 2004, Centers of Disease Control, 2020, Jones-Smith et al., 2011, McLaren, 2007, Kinge et al., 2015), see Supplementary Table 1. Because there are so many potential variables confounded with body mass index, and so few studies examining the gray matter volume relationship with body mass index, we were only able to explore one of these potential confounding factors -- age -- in a preliminary way, see Supplementary Results. More structural imaging studies examining the effect of body mass index on the brain and other associated variables (Tahmasian et al., 2020) are needed, especially heeding to current suggestions in the field to consistently report effect sizes and to use conservative and consistent corrections for multiple comparisons (Eickhoff et al., 2016, Muller et al., 2018). Future meta-analytic work in this area – and neuroimaging more broadly – would benefit from a recent shift toward authors’ sharing their unthresholded statistical maps (Gorgolewski et al., 2015, Smith and Delgado, 2017, Botvinik-Nezer et al., 2020). We cannot draw conclusions about the association between underweight body mass index and gray matter volume as we only examined healthy weight relative to overweight or obese body mass index groups. Theorizing why right orbitofrontal cortex atrophy occurs with higher body mass index is speculative without longitudinal evidence to assess whether these differences are seen prior to weight gain or occur as a result of weight gain. Our exploratory findings examining the functional significance of the orbitofrontal cortex are best seen as hypothesis-generating rather than hypothesis-testing, given that the choice of thresholding was somewhat arbitrary.
4.3. Conclusion
Our findings provide clear evidence for an association between reduced orbitofrontal cortex gray matter and human obesity using a meta-analysis of voxel-based morphometry studies. This extends our view of the part played by the orbitofrontal cortex in reward functioning and highlights the importance of orbitofrontal cortex structure in neural models of obesity. Although requiring replication, our exploratory results suggest that reduced orbitofrontal cortex gray matter with greater body mass index may be important before midlife and may not be only associated with ageing. Our follow up analysis of a young adult sample suggest how the prefrontal regions associated with reduced orbitofrontal cortex gray matter given higher body mass index may be associated with differential reward processing rather than working memory functioning. Future research is needed to elucidate the functional significance of orbitofrontal cortex atrophy in obesity, and to assess changes in gray matter volume at different ages and with weight change longitudinally. Our meta-analysis also suggest that the orbitofrontal cortex (BA 10 and BA 11) may be an important site for neuromodulation techniques to address overeating.
Funding sources
This work was supported by a grant from Temple University (College of Liberal Arts Research Awards grant: ‘Using Human Connectome Project Data to understand normal brain function in healthy adults’) to EYC. Publication of this article was funded in part by the Temple University Libraries Open Access Publishing Fund. This work was supported, in part, by grants from the National Institutes of Health (R21-MH113917 and R03-DA046733 to DVS). Data were provided [in part] by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. Funding sources had no involvement in the design, analysis or interpretation of the data.
CRediT authorship contribution statement
Eunice Y. Chen: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing, Visualization, Project administration. Simon B. Eickhoff: Formal analysis, Methodology, Writing - review & editing. Tania Giovannetti: Writing - review & editing. David V. Smith: Conceptualization, Formal analysis, Resources, Writing - review & editing.
Acknowledgements
We also like to thank Susan Murray, Melinda Karth, Michelle Karth and Naomi Szanto for assisting with literature searches and screening abstracts and thank Cutey McChen for her support and interest in this, her final paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102420.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alves P.N., Foulon C., Karolis V., Bzdok D., Margulies D.S., Volle E., Thiebaut de Schotten M. An improved neuroanatomical model of the default-mode network reconciles previous neuroimaging and neuropathological findings. Commun. Biol. 2019;2:370. doi: 10.1038/s42003-019-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Berridge Kent C, Kringelbach Morten L. Pleasure systems in the brain. Neuron. 2015;86:646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond David J, Ha Tae Hyon, Lang Donna J, Wayne Su., Torres Ivan J, Honer William G, Lam Raymond W, Yatham Lakshmi N. Body mass index-related regional gray and white matter volume reductions in first-episode mania patients. Biol. Psychiatry. 2014;76:138–145. doi: 10.1016/j.biopsych.2013.08.030. [DOI] [PubMed] [Google Scholar]
- Botvinik-Nezer Rotem, Holzmeister Felix, Camerer Colin F., Dreber Anna, Huber Juergen, Johannesson Magnus, Kirchler Michael, Iwanir Roni, Mumford Jeanette A., Alison Adcock R. Variability in the analysis of a single neuroimaging dataset by many teams. Nature. 2020:1–7. doi: 10.1038/s41586-020-2314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S.J., Benedict C., Burgos J., Kempton M.J., Kullberg J., Nordenskjold R., Kilander L., Nylander R., Larsson E.M., Johansson L., Ahlstrom H., Lind L., Schioth H.B. Late-life obesity is associated with smaller global and regional gray matter volumes: a voxel-based morphometric study. Int. J. Obesity. 2013;37:230–236. doi: 10.1038/ijo.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell Kelly, Horgen Katherine B. McGraw Hill Professional; USA: 2003. Food Fight. [Google Scholar]
- Burke Sara N., Thome Alex, Plange Kojo, Engle James R., Trouard Theodore P., Gothard Katalin M., Barnes Carol A. Orbitofrontal cortex volume in area 11/13 predicts reward devaluation, but not reversal learning performance, in young and aged monkeys. J. Neurosci. 2014;34:9905–9916. doi: 10.1523/JNEUROSCI.3918-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok Danilo, Langner Robert, Schilbach Leonhard, Engemann Denis A, Laird Angela R, Fox Peter T, Eickhoff Simon. Segregation of the human medial prefrontal cortex in social cognition. Front. Hum. Neurosci. 2013;7:232. doi: 10.3389/fnhum.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers of Disease Control About Adult BMI. 2020. http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html Accessed 02/08/2020.
- Centers of Disease Control National Health and Nutrition Examination Survey, 2017–2018. 2020. https://www.cdc.gov/nchs/about/factsheets/factsheet_nhanes.htm Accessed 7/30/2020.
- Chase Henry W., Grace Anthony A., Fox Peter T., Phillips Mary L., Eickhoff Simon B. 'Functional differentiation in the human ventromedial frontal lobe: a data-driven parcellation. Hum. Brain Mapp. 2020;41:3266–3283. doi: 10.1002/hbm.25014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Eunice Y, Olino Thomas M, Conklin Chris J, Mohamed Feroze B, Scott Hoge W., Foster Gary D, Arlt Jean M, Eneva Kalina, Kidd Judy R, Kidd Kenneth R. Genetic and and neural predictors of behavioral weight loss treatment: a preliminary study. Obesity. 2017;25:66–75. doi: 10.1002/oby.21691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Eunice Y, Zeffiro Thomas A. Hunger and BMI modulate neural responses to sweet stimuli: fMRI meta-analysis. Int. J. Obesity. 2020;1–17 doi: 10.1038/s41366-020-0608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole Timothy J, Freeman Jennifer V, Preece Michael A. Body mass index reference curves for the UK, 1990. Arch. Dis. Child. 1995;73:25–29. doi: 10.1136/adc.73.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley Hugo D, Rolls Edmund T. Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. J. Neurophysiol. 1996;75:1673–1686. doi: 10.1152/jn.1996.75.4.1673. [DOI] [PubMed] [Google Scholar]
- de la Vega Alejandro, Chang Luke J, Banich Marie T, Wager Tor D, Yarkoni Tal. Large-scale meta-analysis of human medial frontal cortex reveals tripartite functional organization. J. Neurosci. 2016;36:6553–6562. doi: 10.1523/JNEUROSCI.4402-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto F., Zapparoli L., Bonandrini R., Berlingeri M., Ferrulli A., Luzi L., Banfi G., Paulesu E. Hungry brains: a meta-analytical review of brain activation imaging studies on food perception and appetite in obese individuals. Neurosci. Biobehav. Rev. 2018;94:271–285. doi: 10.1016/j.neubiorev.2018.07.017. [DOI] [PubMed] [Google Scholar]
- Diehl Maria M, Lempert Karolina M, Parr Ashley C, Ballard Ian, Steele Vaughn R, Smith David V. Toward an integrative perspective on the neural mechanisms underlying persistent maladaptive behaviors. Eur. J. Neurosci. 2018;48:1870. doi: 10.1111/ejn.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Nichols T.E., Laird A.R., Hoffstaedter F., Amunts K., Fox P.T., Bzdok D., Eickhoff C.R. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage. 2016;137:70–85. doi: 10.1016/j.neuroimage.2016.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff Simon B, Laird Angela R, Mickle Fox P., Lancaster Jack L, Fox Peter T. Implementation errors in the GingerALE Software: description and recommendations. Hum. Brain Mapp. 2017;38:7–11. doi: 10.1002/hbm.23342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff Simon B, Laird Angela R, Grefkes Christian, Wang Ling E, Zilles Karl, Fox Peter T. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt Barry J. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories–indications for novel treatments of addiction. Eur. J. Neurosci. 2014;40:2163–2182. doi: 10.1111/ejn.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt Barry J, Robbins Trevor W. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci. Biobehav. Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Everitt Barry J, Robbins Trevor W. Drug addiction: updating actions to habits to compulsions ten years on. Annu. Rev. Psychol. 2016;67:23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- Figley Chase R, Asem Judith SA, Levenbaum Erica L, Courtney Susan M. Effects of body mass index and body fat percent on default mode, executive control, and salience network structure and function. Front. Neurosci. 2016;10:234. doi: 10.3389/fnins.2016.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal K.M., Carroll M.D., Kuczmarski R.J., Johnson C.L. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int. J. Obesity. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- Flegal K.M., Carroll M.D., Ogden C.L., Johnson C.L. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- García-García Isabel, Michaud Andreanne, Dadar Mahsa, Zeighami Yashar, Selin Neseliler D., Collins Louis, Evans Alan C, Dagher Alain. Neuroanatomical differences in obesity: meta-analytic findings and their validation in an independent dataset. Int. J. Obesity. 2019;43:943–951. doi: 10.1038/s41366-018-0164-4. [DOI] [PubMed] [Google Scholar]
- Godfrey J.R., Diaz M.P., Pincus M., Kovacs-Balint Z., Feczko E., Earl E., Miranda-Dominguez O., Fair D., Sanchez M.M., Wilson M.E., Michopoulos V. Diet matters: Glucocorticoid-related neuroadaptations associated with calorie intake in female rhesus monkeys. Psychoneuroendocrinology. 2018;91:169–178. doi: 10.1016/j.psyneuen.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey Jodi R., Pincus Melanie, Kovacs-Balint Zsofia, Feczko Eric, Earl Eric, Miranda-Dominguez Oscar, Fair Damien A., Jones Sara R., Locke Jason, Sanchez Mar M., Wilson Mark E., Michopoulos Vasiliki. Obesogenic diet-associated C-reactive protein predicts reduced central dopamine and corticostriatal functional connectivity in female rhesus monkeys. Brain Behav. Immun. 2020;88:166–173. doi: 10.1016/j.bbi.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski Krzysztof J, Varoquaux Gael, Rivera Gabriel, Schwarz Yannick, Ghosh Satrajit S, Maumet Camille, Sochat Vanessa V, Nichols Thomas E, Poldrack Russell A, Poline Jean-Baptiste. NeuroVault. org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain, Frontiers. Neuroinformatics. 2015;9:8. doi: 10.3389/fninf.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y.K., Sasaki H., Takao H., Yoshikawa T., Hayashi N., Mori H., Kunimatsu A., Aoki S., Ohtomo K. The relationship of waist circumference and body mass index to grey matter volume in community dwelling adults with mild obesity. Obesity Sci. Pract. 2018;4:97–105. doi: 10.1002/osp4.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Qinghua, Chen Chuansheng, Dong Qi, Xue Gui, Chen Chunhui, Zhong-Lin Lu., Bechara Antoine. Gray and white matter structures in the midcingulate cortex region contribute to body mass index in Chinese young adults. Brain Struct. Funct. 2015;220:319–329. doi: 10.1007/s00429-013-0657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley Allison A., Ogden Cynthia L., Johnson Clifford L., Carroll Margaret D., Curtin Lester R., Flegal Katherine M. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA: J. Am. Medical Assoc. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- Herrmann Martin J, Tesar Ann-Katrin, Beier Jennifer, Berg Max, Warrings Bodo. Grey matter alterations in obesity: a meta-analysis of whole-brain studies. Obes. Rev. 2019;20:464–471. doi: 10.1111/obr.12799. [DOI] [PubMed] [Google Scholar]
- Hodgson Karen, Poldrack Russell A, Curran Joanne E, Knowles Emma E, Mathias Samuel, Göring Harald HH, Yao Nailin, Olvera Rene L, Fox Peter T, Almasy Laura. Shared genetic factors influence head motion during MRI and body mass index. Cereb. Cortex. 2017;27:5539–5546. doi: 10.1093/cercor/bhw321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea Robyn A, Szabo-Reed Amanda N, Lepping Rebecca J, Perea Rodrigo, Breslin Florence, Martin Laura E, Brooks William M, Donnelly Joseph E, Savage Cary R. Voxel-based morphometry reveals brain gray matter volume changes in successful dieters. Obesity. 2016;24:1842–1848. doi: 10.1002/oby.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann Annette, Busse Franziska, Mathar David, Mueller Karsten, Lepsien Joeran, Schloegl Haiko, Kabisch Stefan, Kratzsch Jürgen, Neumann Jane, Stumvoll Michael. Obesity-related differences between women and men in brain structure and goal-directed behavior. Front. Hum. Neurosci. 2011;5:58. doi: 10.3389/fnhum.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta Claudia I, Sarkar Pooja R, Duong Timothy Q, Laird Angela R, Fox Peter T. Neural bases of food perception: Coordinate-based meta-analyses of neuroimaging studies in multiple modalities. Obesity. 2014;22:1439–1446. doi: 10.1002/oby.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowitz Deborah, Wittfeld Katharina, Terock Jan, Freyberger Harald Jürgen, Hegenscheid Katrin, Völzke Henry, Habes Mohamad, Hosten Norbert, Friedrich Nele, Nauck Matthias. Association between waist circumference and gray matter volume in 2344 individuals from two adult community-based samples. Neuroimage. 2015;122:149–157. doi: 10.1016/j.neuroimage.2015.07.086. [DOI] [PubMed] [Google Scholar]
- Jenkinson Mark, Beckmann Christian F., Behrens Timothy E.J., Woolrich Mark W., Smith Stephen M. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jones-Smith Jessica C, Gordon-Larsen Penny, Siddiqi Arjumand, Popkin Barry M. Cross-national comparisons of time trends in overweight inequality by socioeconomic status among women using repeated cross-sectional surveys from 37 developing countries, 1989–2007. Am. J. Epidemiol. 2011;173:667–675. doi: 10.1093/aje/kwq428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson Henry K, Tuulari Jetro J, Hirvonen Jussi, Lepomäki Virva, Parkkola Riitta, Hiltunen Jaana, Hannukainen Jarna C, Soinio Minna, Pham Tam, Salminen Paulina. Obesity is associated with white matter atrophy: a combined diffusion tensor imaging and voxel-based morphometric study. Obesity. 2013;21:2530–2537. doi: 10.1002/oby.20386. [DOI] [PubMed] [Google Scholar]
- Kennedy James T, Collins Paul F, Luciana Monica. Higher adolescent body mass index is associated with lower regional gray and white matter volumes and lower levels of positive emotionality. Front. Neurosci. 2016;10:413. doi: 10.3389/fnins.2016.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinge Jonas Minet, Strand Bjørn Heine, Vollset Stein Emil, Skirbekk Vegard. Educational inequalities in obesity and gross domestic product: evidence from 70 countries. J Epidemiol Community Health. 2015;69:1141–1146. doi: 10.1136/jech-2014-205353. [DOI] [PubMed] [Google Scholar]
- Kube Jana, Mathar David, Horstmann Annette, Kotz Sonja A., Villringer Arno, Neumann Jane. Altered monetary loss processing and reinforcement-based learning in individuals with obesity. Brain Imaging Behav. 2018;12:1431–1449. doi: 10.1007/s11682-017-9786-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski, R.J., Ogden, C.L., Grummer-Strawn, L.M., Flegal, K.M., Guo, S.S, Wei, R., Mei, Z., Curtin, L.R., Roche, A.F., Johnson, C.L., December 4, 2000 (Revised). Centers of Disease Control Growth Charts: United States advance data from vital and health statistics. In. National Center for Health Statistics, Centers of Disease Control and Prevention.
- Kurth F., Levitt J.G., Phillips O.R., Luders E., Woods R.P., Mazziotta J.C., Toga A.W., Narr K.L. Relationships between gray matter, body mass index, and waist circumference in healthy adults. Hum Brain Mapp. 2013;34:1737–1746. doi: 10.1002/hbm.22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster Jack L, Tordesillas-Gutiérrez Diana, Martinez Michael, Salinas Felipe, Evans Alan, Zilles Karl, Mazziotta John C, Fox Peter T. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavagnino L., Arnone D., Cao B., Soares J.C., Selvaraj S. Inhibitory control in obesity and binge eating disorder: a systematic review and meta-analysis of neurocognitive and neuroimaging studies. Neurosci. Biobehav. Rev. 2016;68:714–726. doi: 10.1016/j.neubiorev.2016.06.041. [DOI] [PubMed] [Google Scholar]
- Markus M.R.P., Ittermann T., Wittfeld K., Schipf S., Siewert-Markus U., Bahls M., Bülow R., Werner N., Janowitz D., Baumeister S.E., Felix S.B., Dörr M., Rathmann W., Völzke H., Grabe H.J. Prediabetes is associated with lower brain gray matter volume in the general population. The Study of Health in Pomerania (SHIP) Nutr. Metab Cardiovasc. Dis. 2017;27:1114–1122. doi: 10.1016/j.numecd.2017.10.007. [DOI] [PubMed] [Google Scholar]
- Masouleh Shahrzad Kharabian, Arélin Katrin, Horstmann Annette, Lampe Leonie, Kipping Judy A, Luck Tobias, Riedel-Heller Steffi G, Schroeter Matthias L, Stumvoll Michael, Villringer Arno. Higher body mass index in older adults is associated with lower gray matter volume: implications for memory performance. Neurobiol. Aging. 2016;40:1–10. doi: 10.1016/j.neurobiolaging.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Mathar David, Horstmann Annette, Pleger Burkhard, Villringer Arno, Neumann Jane. Is it worth the effort? Novel insights into obesity-associated alterations in cost-benefit decision-making. Front. Behav. Neurosci. 2016;9:360. doi: 10.3389/fnbeh.2015.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren Lindsay. Socioeconomic status and obesity. Epidemiol. Rev. 2007;29:29–48. doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- Minkova Lora, Habich Annegret, Peter Jessica, Kaller Christoph P, Eickhoff Simon B, Klöppel Stefan. Gray matter asymmetries in aging and neurodegeneration: A review and meta-analysis. Hum. Brain Mapp. 2017;38:5890–5904. doi: 10.1002/hbm.23772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. The PRISMA Group. The preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad A.H., Ford E.S., Bowman B.A., Dietz W.H., Vinicor F., Bales V.S., Marks J.S. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- Morawetz Carmen, Riedel Michael C., Salo Taylor, Berboth Stella, Eickhoff Simon B., Laird Angela R., Kohn Nils. Multiple large-scale neural networks underlying emotion regulation. Neurosci. Biobehav. Rev. 2020;116:382–395. doi: 10.1016/j.neubiorev.2020.07.001. [DOI] [PubMed] [Google Scholar]
- Morville Tobias, Madsen Kristoffer, Siebner Hartwig R., Hulme Oliver J. Reward signalling in brainstem nuclei under glycemic flux. bioRxiv. 2018:243006. doi: 10.1371/journal.pone.0243899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller Karsten, Möller Harald E, Horstmann Annette, Busse Franziska, Lepsien Jöran, Blüher Matthias, Stumvoll Michael, Villringer Arno, Pleger Burkhard. Physical exercise in overweight to obese individuals induces metabolic-and neurotrophic-related structural brain plasticity. Front. Hum. Neurosci. 2015;9:372. doi: 10.3389/fnhum.2015.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller V.I., Cieslik E.C., Laird A.R., Fox P.T., Radua J., Mataix-Cols D., Tench C.R., Yarkoni T., Nichols T.E., Turkeltaub P.E., Wager T.D., Eickhoff S.B. Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 2018;84:151–161. doi: 10.1016/j.neubiorev.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh Donna L., Cox James E., Cook III Edwin W., Weller Rosalyn E. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59:2709–2721. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray Elisabeth A., Moylan Emily J., Saleem Kadharbatcha S., Basile Benjamin M., Turchi Janita. Specialized areas for value updating and goal selection in the primate orbitofrontal cortex. eLife. 2015:4. doi: 10.7554/eLife.11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neseliler Selin, Wen Hu., Larcher Kevin, Zacchia Maria, Dadar Mahsa, Scala Stephanie G., Lamarche Marie, Zeighami Yashar, Stotland Stephen C., Larocque Maurice, Marliss Errol B., Dagher Alain. Neurocognitive and hormonal correlates of voluntary weight loss in humans. Cell Metab. 2019;29:39–49.e4. doi: 10.1016/j.cmet.2018.09.024. [DOI] [PubMed] [Google Scholar]
- Noonan M.P., Walton M.E., Behrens T.E., Sallet J., Buckley M.J., Rushworth M.F. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc Natl Acad Sci. 2010;107:20547–20552. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordenskjöld Richard, Malmberg Filip, Larsson Elna-Marie, Simmons Andrew, Brooks Samantha J, Lind Lars, Ahlström Håkan, Johansson Lars, Kullberg Joel. Intracranial volume estimated with commonly used methods could introduce bias in studies including brain volume measurements. Neuroimage. 2013;83:355–360. doi: 10.1016/j.neuroimage.2013.06.068. [DOI] [PubMed] [Google Scholar]
- Nouwen Arie, Chambers Alison, Chechlacz Magdalena, Higgs Suzanne, Blissett Jacqueline, Barrett Timothy G, Allen Harriet A. Microstructural abnormalities in white and gray matter in obese adolescents with and without type 2 diabetes. NeuroImage: Clin. 2017;16:43–51. doi: 10.1016/j.nicl.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odoherty J., Rolls E.T., Francis S., Bowtell R., McGlone F., Kobal G., Renner B., Ahne G. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. NeuroReport. 2000;11:893–897. doi: 10.1097/00001756-200003200-00046. [DOI] [PubMed] [Google Scholar]
- Oldham S., Murawski C., Fornito A., Youssef G., Yücel M., Lorenzetti V. The anticipation and outcome phases of reward and loss processing: a neuroimaging meta-analysis of the monetary incentive delay task. Hum. Brain Mapp. 2018;39:3398–3418. doi: 10.1002/hbm.24184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opel N., Redlich R., Kaehler C., Grotegerd D., Dohm K., Heindel W., Kugel H., Thalamuthu A., Koutsouleris N., Arolt V. Prefrontal gray matter volume mediates genetic risks for obesity. Mol. Psychiatry. 2017;22:703. doi: 10.1038/mp.2017.51. [DOI] [PubMed] [Google Scholar]
- Opel Nils, Redlich Ronny, Grotegerd Dominik, Dohm Katharina, Heindel Walter, Kugel Harald, Arolt Volker, Dannlowski Udo. Obesity and major depression: body-mass index (BMI) is associated with a severe course of disease and specific neurostructural alterations. Psychoneuroendocrinology. 2015;51:219–226. doi: 10.1016/j.psyneuen.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Pannacciulli N., Del Parigi A., Chen K., Le D.S., Reiman E.M., Tataranni P.A. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Pritchard Thomas C, Schwartz Gary J, Scott Thomas R. Taste in the medial orbitofrontal cortex of the macaque. Ann. N. Y. Acad. Sci. 2007;1121:121–135. doi: 10.1196/annals.1401.007. [DOI] [PubMed] [Google Scholar]
- Radua J., Mataix-Cols D., Phillips Mary L, El-Hage W., Kronhaus D.M., Cardoner N., Surguladze S. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry. 2012;27:605–611. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Radua Joaquim, Mataix-Cols David. Meta-analytic methods for neuroimaging data explained. Biol. Mood Anxiety Disord. 2012;2:6. doi: 10.1186/2045-5380-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua Joaquim, Rubia Katya, Canales-Rodríguez Erick Jorge, Pomarol-Clotet Edith, Fusar-Poli Paolo, Mataix-Cols David. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front. Psychiatry. 2014;5:13. doi: 10.3389/fpsyt.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber Justin, Feinstein Justin S., Odoherty John P., Liljeholm Mimi, Adolphs Ralph, Tranel Daniel. Selective impairment of goal-directed decision-making following lesions to the human ventromedial prefrontal cortex. Brain. 2017;140:1743–1756. doi: 10.1093/brain/awx105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel Michael C, Yanes Julio A, Ray Kimberly L, Eickhoff Simon B, Fox Peter T, Sutherland Matthew T, Laird Angela R. Dissociable meta-analytic brain networks contribute to coordinated emotional processing. Hum. Brain Mapp. 2018;39:2514–2531. doi: 10.1002/hbm.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R.D., Owen A.M., Middleton H.C., Williams E.J., Pickard J.D., Sahakian B.J., Robbins T.W. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J. Neurosci. 1999;19:9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T., Grabenhorst F., Deco G. Choice, difficulty, and confidence in the brain. Neuroimage. 2010;53:694–706. doi: 10.1016/j.neuroimage.2010.06.073. [DOI] [PubMed] [Google Scholar]
- Rolls Edmund T. Oxford University Press, Oxford University; 2019. The Orbitofrontal Cortex. [Google Scholar]
- Rolls Edmund T. The orbitofrontal cortex and emotion in health and disease, including depression. Neuropsychologia. 2019;128:14–43. doi: 10.1016/j.neuropsychologia.2017.09.021. [DOI] [PubMed] [Google Scholar]
- Rolls Edmund T, Grabenhorst Fabian, Deco Gustavo. Decision-making, errors, and confidence in the brain. J. Neurophysiol. 2010;104:2359–2374. doi: 10.1152/jn.00571.2010. [DOI] [PubMed] [Google Scholar]
- Rolls Edmund T, Yaxley Simon, Sienkiewicz Zenon J. Gustatory responses of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. J. Neurophysiol. 1990;64:1055–1066. doi: 10.1152/jn.1990.64.4.1055. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. Understanding the mechanisms of food intake and obesity. Obes. Rev. 2007;8:67–72. doi: 10.1111/j.1467-789X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Rudebeck P.H., Murray E.A. Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J. Neurosci. 2011;31:10569–10578. doi: 10.1523/JNEUROSCI.0091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck P.H., Saunders R.C., Lundgren D.A., Murray E.A. Specialized representations of value in the orbital and ventrolateral prefrontal cortex: desirability versus availability of outcomes. Neuron. 2017;95 doi: 10.1016/j.neuron.2017.07.042. 1208-20.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok Amber NV, Salimi-Khorshidi Gholamreza, Lai Meng-Chuan, Baron-Cohen Simon, Lombardo Michael V, Tait Roger J, Suckling John. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaare H.L., Kharabian Masouleh S., Beyer F., Kumral D., Uhlig M., Reinelt J.D., Reiter A.M.F., Lampe L., Babayan A., Erbey M., Roebbig J., Schroeter M.L., Okon-Singer H., Müller K., Mendes N., Margulies D.S., Witte A.V., Gaebler M., Villringer A. Association of peripheral blood pressure with gray matter volume in 19- to 40-year-old adults. Neurology. 2019;92:e758–e773. doi: 10.1212/WNL.0000000000006947. [DOI] [PubMed] [Google Scholar]
- Shott Megan E, Cornier Marc-Andre, Mittal Vijay A, Pryor Tamara L, Orr Joseph M, Brown Mark S, Frank Guido KW. Orbitofrontal cortex volume and brain reward response in obesity. Int. J. Obesity. 2015;39:214–221. doi: 10.1038/ijo.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel Joshua S, Mitra Anish, Laumann Timothy O, Seitzman Benjamin A, Raichle Marcus, Corbetta Maurizio, Snyder Abraham Z. Data quality influences observed links between functional connectivity and behavior. Cereb. Cortex. 2016;27:4492–4502. doi: 10.1093/cercor/bhw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.V., Delgado M.R. Reward processing. In: Toga Arthur W., editor. Brain Mapping. Academic Press; Waltham: 2015. [Google Scholar]
- Smith Dana G, Robbins Trevor W. The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biol. Psychiatry. 2013;73:804–810. doi: 10.1016/j.biopsych.2012.08.026. [DOI] [PubMed] [Google Scholar]
- Smith David V, Delgado Mauricio R. Meta-analysis of psychophysiological interactions: revisiting cluster-level thresholding and sample sizes. Hum. Brain Mapp. 2017;38:588–591. doi: 10.1002/hbm.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J., Cornier M.A., Eichman L.C., Thomas E.A., Bechtell J.L., Tregellas J.R. Brain structure predicts risk for obesity. Appetite. 2012;59:859–865. doi: 10.1016/j.appet.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojek Monika MK, MacKillop James. Relative reinforcing value of food and delayed reward discounting in obesity and disordered eating: a systematic review. Clin. Psychol. Rev. 2017;55:1–11. doi: 10.1016/j.cpr.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Tahmasian Masoud, Samea Fateme, Khazaie Habibolah, Zarei Mojtaba, Masouleh Shahrzad Kharabian, Hoffstaedter Felix, Camilleri Julia, Peter Kochunov B.T., Yeo Thomas, Eickhoff Simon Bodo, Valk Sofie Louise. The interrelation of sleep and mental and physical health is anchored in grey-matter neuroanatomy and under genetic control. Commun. Biol. 2020;3:171. doi: 10.1038/s42003-020-0892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki Y., Kinomura S., Sato K., Inoue K., Goto R., Okada K., Uchida S., Kawashima R., Fukuda H. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring) 2008;16:119–124. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- Tang D.W., Fellows L.K., Small D.M., Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol. Behav. 2012;106:317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Turkeltaub Peter E, Eden Guinevere F, Jones Karen M, Zeffiro Thomas A. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub Peter E, Eickhoff Simon B, Laird Angela R, Fox Mick, Wiener Martin, Fox Peter. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuulari J.J., Karlsson H.K., Antikainen O., Hirvonen J., Pham T., Salminen P., Helmio M., Parkkola R., Nuutila P., Nummenmaa L. Bariatric surgery induces white and grey matter density recovery in the morbidly obese: a voxel-based morphometric study. Hum. Brain Mapp. 2016;37:3745–3756. doi: 10.1002/hbm.23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel Martijn P, Sporns Olaf. Network hubs in the human brain. Trends Cogn. Sci. 2013;17:683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- van der Laan L.N., de Ridder D.T.D., Viergever M.A., Smeets P.A.M. The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage. 2011;55:296–303. doi: 10.1016/j.neuroimage.2010.11.055. [DOI] [PubMed] [Google Scholar]
- Essen Van, David C., Smith Stephen M., Barch Deanna M., Behrens Timothy E.J., Yacoub Essa, Ugurbil Kamil, Minn W.U., Consortium for the H.C.P. The WU-Minn human connectome project: an overview. Neuroimage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen Maria G, Albrecht Jessica, Zelano Christina, Boesveldt Sanne, Breslin Paul, Lundström Johan N. Identification of human gustatory cortex by activation likelihood estimation. Hum. Brain Mapp. 2011;32:2256–2266. doi: 10.1002/hbm.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Roman J., Fornito A., Soriano-Mas C., Vilar-Lopez R., Verdejo-Garcia A. Independent functional connectivity networks underpin food and monetary reward sensitivity in excess weight. Neuroimage. 2017;146:293–300. doi: 10.1016/j.neuroimage.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Veronese Nicola, Facchini Silvia, Stubbs Brendon, Luchini Claudio, Solmi Marco, Manzato Enzo, Sergi Giuseppe, Maggi Stefania, Cosco Theodore, Fontana Luigi. Weight loss is associated with improvements in cognitive function among overweight and obese people: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2017;72:87–94. doi: 10.1016/j.neubiorev.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Walther K., Birdsill A.C., Glisky E.L., Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum. Brain Mapp. 2010;31:1052–1064. doi: 10.1002/hbm.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Kainan S., Smith David V., Delgado Mauricio R. Using fMRI to study reward processing in humans: past, present, and future. J. Neurophysiol. 2016;115:1664–1678. doi: 10.1152/jn.00333.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Shaoming, Tepfer Lindsey J., Taren Adrienne A., Smith David V. Functional parcellation of the default mode network: a large-scale meta-analysis. bioRxiv. 2020:225375. doi: 10.1038/s41598-020-72317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise Christopher M, Piaggi Paolo, Reinhardt Martin, Chen Kewei, Savage Cary R, Krakoff Jonathan, Pleger Burkhard. The obese brain as a heritable phenotype–a combined morphometry and twin study. Int. J. Obesity. 2017;41:458–466. doi: 10.1038/ijo.2016.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weygandt M., Mai K., Dommes E., Leupelt V., Hackmack K., Kahnt T., Rothemund Y., Spranger J., Haynes J.D. The role of neural impulse control mechanisms for dietary success in obesity. Neuroimage. 2013;83:669–678. doi: 10.1016/j.neuroimage.2013.07.028. [DOI] [PubMed] [Google Scholar]
- Weygandt Martin, Mai Knut, Dommes Esther, Ritter Kerstin, Leupelt Verena, Spranger Joachim, Haynes John-Dylan. Impulse control in the dorsolateral prefrontal cortex counteracts post-diet weight regain in obesity. Neuroimage. 2015;109:318–327. doi: 10.1016/j.neuroimage.2014.12.073. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 1995. Physical status: the use and interpretation of anthropometry. Report of a World Health Organization Expert Committee. In: World Health Organization Technical Report Series 854. World Health Organization, Geneva. [PubMed]
- Yang Yingkai, Shields Grant S, Guo Cheng, Liu Yanling. Executive function performance in obesity and overweight individuals: a meta-analysis and review. Neurosci. Biobehav. Rev. 2018;84:225–244. doi: 10.1016/j.neubiorev.2017.11.020. [DOI] [PubMed] [Google Scholar]
- Yao Lizheng, Li Wang, Dai Zhenyu, Dong Congsong. Eating behavior associated with gray matter volume alternations: a voxel based morphometry study. Appetite. 2016;96:572–579. doi: 10.1016/j.appet.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Yarkoni Tal, Poldrack Russell A, Nichols Thomas E, Van Essen David C, Wager Tor D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung Andy Wai Kan, Goto Tazuko K., Keung Leung Wai. Basic taste processing recruits bilateral anteroventral and middle dorsal insulae: an activation likelihood estimation meta-analysis of fMRI studies. Brain Behav. 2017;7:e00655. doi: 10.1002/brb3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.