Abstract
Introduction
Several reports have shown that Rab14 is dysregulated in human cancers suggesting that it is an oncogenic protein closely related to tumorigenesis. However, whether Rab14 plays a role in the development and progression of human non-small cell lung cancer (NSCLC) remains unclear.
Methods
Rab14 protein levels were examined in 115 cases of NSCLC tissues and 6 cancer cell lines. Rab14 knockdown was performed in H1299 and A549 cell lines. Rab14 plasmid transfection was performed in the LK2 cell line. The biological roles and mechanisms of Rab14 were examined using MTT, colony formation, Matrigel invasion assay, migration assay, cell cycle analysis, Western blotting, and RT-qPCR.
Results
We found that Rab14 was upregulated in 65 of 115 lung cancer tissues. Rab14 high expression was significantly correlated with advanced TNM stage and nodal metastasis. Rab14 protein levels were higher in lung cancer cell lines than in normal bronchial cell line. Functionally, Rab14 overexpression increased growth rate, colony formation, invasion/migration ability and cell cycle progression, while Rab14 siRNA decreased the cell proliferation rate, colony numbers and inhibited invasion/migration ability and cell cycle progression. Rab14 upregulated cyclin D1, cyclin E, connective tissue growth factor (CTGF) and downregulated p27 protein and mRNA levels in both A549 and H1299 cell lines, while Rab14 siRNA produced the opposite effects. Further study showed that Rab14 overexpression increased luciferase reporter activity from transcriptional enhanced associate domain (TEAD) protein. Accordingly, Rab14 increased total Yes-associated protein (YAP) and nuclear YAP protein while decreased phosphorylated (p)-YAP and cytoplasmic YAP protein expression. Cycloheximide treatment showed that Rab14 downregulated the level of YAP degradation. Depletion of YAP using siRNA abolished the influence of Rab14 on cyclin D1, cyclin E, and CTGF. YAP knockdown also partly abolished the effects of Rab14 on cell proliferation and invasion.
Conclusion
In summary, our data showed that Rab14 is overexpressed in human NSCLC. Rab14 facilitated proliferation and invasion, possibly through regulation of YAP signaling.
Keywords: Rab14, non-small cell lung cancer, YAP, Hippo
Introduction
Non-small cell lung cancer (NSCLC) is one of the most common causes of cancer-related deaths.1,2 Although treatment strategies and approaches have greatly improved, NSCLC prognosis remains poor due to high rates of recurrence and tumor metastasis.3–5 It is therefore important to identify effective molecular targets involved in the progression and invasion of NSCLCs.
Rab14 is an important member of RAS oncogene superfamily of proteins, which consists of small G-proteins that participate in many physiological and pathological processes through regulating membrane vesicle transport and signal transduction.6–9 Increasing evidence has shown that Rab14 is involved in carcinogenesis. Rab14 was also found overexpressed and then activated Wnt signaling in human ovarian cancers.10 Rab14 overexpression has also been found in gastric cancer, where it facilitates proliferation and invasion by activating AKT signaling pathway.9 Rab14 also functions as an oncogene in oral squamous cell carcinoma and its silencing suppressed proliferation and enhanced chemo-sensitivity.11 The above reports indicated that Rab14 may act as an oncogene in human carcinomas. To date, the expression profile, clinical significance and biological effects of Rab14 in NSCLC remain unexplored.
In the current study, the expression pattern and clinical significance of Rab14 in were evaluated in NSCLC tissues. In addition, the biological effects of Rab14 on cell proliferation, clonogenic capacity, invasive activity, cell cycle, and its underlying mechanisms were also investigated.
Materials and Methods
Specimens
The study protocol was approved by the institutional review board of China Medical University. The specimens of NSCLC and adjacent normal tissue were obtained from lung cancer patients who were diagnosed in the hospital between January 2010 and October 2015. Written informed consent was provided by the patients. The study was conducted in accordance with the Declaration of Helsinki. The cancer specimens were evaluated according to WHO classification guidelines. Five fresh NSCLC cancer tissues and corresponding normal tissues were stored at −80°C after resection for extraction of RNA.
Immunohistochemistry
Paraffin sections (4μm) were prepared and deparaffinized using xylene and graded alcohol. Citrate buffer was used for antigen retrieval. The ready-to-use goat serum (Maixin, Fuzhou, China) was used for blockage of non-specific antigen. The sections were washed and then incubated with Rab14 primary antibody (1:400, Proteintech, USA) overnight at 4°C. Then immunohistochemical staining was performed using the Elivision Super Kit (Maixin, Fuzhou, China) according to the instructions. Rab14 was localized to cytoplasm and the stained cancer cells were assessed under an Olympus microscope. Staining of Rab14 was scored by evaluating the intensity and percentage of cells showing positive staining. Cytoplasmic staining was considered as positive. The intensity of its cytoplasmic staining was scored as 0 (negative), 1 (weak), 2 (strong). Percentage scores were assigned as 1:1–25%, 2: 26–50% 3: 51–75% and 4: 76–100%. The two scores were multiplied to give a final score of 0 to 8. Rab14 was determined as either low expression: score <4 or high expression: score ≥4.
Cell Culture and Transfection
Lung cancer cell lines H460, H358, LK2, A549, H1299, H292 and the normal human bronchial epithelial (HBE) cell line BEAS-2B were purchased from Shanghai Cell Bank, Chinese Academy of Sciences. Cells were cultured in RPMI-1640 medium with fetal bovine serum (FBS, 10%) in an saturated humidity incubator. LK2 cells were maintained in RPMI −1640 medium until the confluence reached 60%. pCMV6-Rab14 plasmid/empty vector was transfected using Lipofectamine 3000 (Invitrogen, USA). The plasmids were constructed by Origene. siGENOME siRNA for Rab14 (Dharmacon, USA) and the non-targeting siRNA were used for knockdown using Dharmafect1 (Dharmacon, USA) according to the manufacturer′s instructions.
Western Blotting and Immunoprecipitation
The lung cancer cells were collected using pre-cooled lysis buffer. The total protein samples were quantified and mixed with appropriate loading buffer. Then equal amount of proteins were subjected to SDS-PAGE for protein separation. The proteins were transferred to a PVDF membrane and then were blocked with 5% BSA buffer against non-specific binding at room temperature for 2 hours. Primary antibodies were against Rab14 (1:1000; Proteintech), cyclin D1, cyclin E, p27, CTGF, YAP, p-YAP, E-cadherin, N-cadherin, Vimentin, Snail (1:1000, Cell Signaling, USA), and GAPDH (1:2000; Cell Signaling Technology, USA). Blots were incubated with primary antibodies overnight at 4°C. The membranes were then washed with TTBS solution and incubated with the secondary HRP conjugated antibodies (1:2000, Santa Cruz, USA) for 2 hours. The Western blot bands were visualized using Pierce HRP substrate, and the pictures were captured using DNR Bio-Imager (Israel).
For immunoprecipitation, Magnetic Beads (Bio-Rad) were incubated with antibodies and unbound antibodies were washed away. Then beads-antibody complex was incubated with target protein. The beads were magnetized using magnetic rack and supernatant was discarded. Then elution buffer was used to collect purified target protein for Western blot analysis.
Quantitative Realtime PCR (SYBR Green Method)
RNAiso reagent (TaKaRa, Dalian, China) was used for the extraction of total RNA. Then RNA was quantified using a Nanodrop2000c photospectrometer (ThermoFisher, USA) and reversed transcribed using a TaKaRa RT kit (Dalian, China) at 85°C for 2 min and 37°C for 30 min. Gene amplification was performed by qPCR using SYBR Green Master mix (TaKaRa, Dalian, China) according to the manufacturer′s instruction. The thermocycling conditions for qPCR were as follows: denaturation at 96°C for 3 minutes, followed by 30 cycles of 96°C for 20 seconds and 62°C for 30 seconds. Relative expression of Rab14 was normalized to the endogenous control β-actin and calculated according to 2−ΔΔCt method. The primer sequences are as follows: Rab14 forward, 5ʹ-CATGGCAACTGCACCATACAAC-3ʹ, Rab14 reverse, 5ʹ-GCAAGATTTTCCTACTCCCATGTC-3ʹ; Cyclin D1, forward5ʹ-TGGAGGTCTGCGAGGAACA-3ʹ, Cyclin D1 reverse5ʹ-TTCATCTTAGAGGCCACGAACAT-3ʹ, Cyclin E forward5ʹ-AGCCAGCCTTGGGACAATAAT-3ʹ, Cyclin E reverse5ʹ-GAGCCTCTGGATGGTGCAAT-3ʹ, p27 forward5ʹ-CTGCAACCGACGATTCTTCTACT-3ʹ, p27 reverse5ʹ-CTTCTGAGGCCAGGCTTCTT-3ʹ, CTGF forward5ʹ-GTTACCAATGACAACGCCTCCT-3ʹ, CTGF reverse5ʹ-TGCACTTTTTGCCCTTCTTAATGT-3ʹ, β-actin forward, 5ʹ-ATAGCACAGCCTGGATAGCAACGTAC 3ʹ, β-actin reverse, 5ʹ-CACCTTCTACAATGAGCTGCGTGTG 3ʹ.
MTT Cell Proliferation Assay
For MTT assay: The transfected cells (3000/per well) were seeded in a 96-well plate and cultured in an incubator overnight. The cells were cultured for an additional 24, 48, 72, 96 and 120 hours respectively, and then MTT solution was added into the wells. The medium was discarded after incubation for 4 hours, and 150μL of DMSO was added into each well to dissolve the formazan. The absorbance values at 490nm were recorded using a microplate reader.
For the clonogenic activity: The transfected cells (1000) were seeded in a 6-cm dish and incubated for about 14 days. The medium was replaced every two days to keep growing. These culture was stained with Giemsa and then placed under a microscope for counting the colony number.
Matrigel Invasion/Transwell Assay
A 24-well transwell chamber was used for invasion assays. Twenty microlitre of Matrigel (BD bioscience) was added in the upper transwell chamber for 4 hours. Cells were washed gently with medium without serum, and then placed in the upper chamber at a density of 1×104/well. RPMI-1640 medium with 10% FBS was added to the lower chamber. The transwell chamber was placed in the incubator for 24 hours. The cells invading to the bottom surface of the upper wells were fixed in paraformaldehyde and then stained with hematoxylin for 20 min at room temperature. Finally, the invasive cells were washed with flowing water and counted under a light microscope.
Wound Healing Assay
Cells were seeded in plates as a monolayer, which was scratched with a pipette tip. Cells were maintained in culture medium without FBS. Images of the cells were taken using a camera at the 0- and 24-h time points. The gap distance of the wound was measured and the relative migration rate was calculated.
Cell Cycle and Apoptosis Analysis
For cell cycle analysis, cultured cells were harvested and fixed using 1% paraformaldehyde, washed with PBS and stained in 5 mg/mL propidium iodide for 30 minutes. Flow cytometry was performed using flow cytometer. To determine the apoptosis percentage, Annexin V/PI staining kit (BD bioscience) was used to stain apoptotic cells. Then cells were analyzed with flow cytometer.
Luciferase Reporter Assay
Lung cancer cells were co-transfected with 0.2 μg of a firefly luciferase reporter gene containing sequence for transcriptional enhanced associate domain (TEAD) and 0.02 μg of Renilla luciferase reporter. Transfections were performed for 18 h using Lipofectamine 3000 according to the manufacturer’s instructions. Renilla luciferase signals were detected using a Berthold Luminometer (Berthold Technologies, Germany) and firefly luciferase activity was detected using the Dual-Luciferase Reporter Assay System (Promega, USA). Relative firefly/Renilla signals were normalized as fold changes.
In vivo Tumor Growth Assay
The Rab14 plasmid was transfected into LK2 cells, which were treated with G418 Neomycin for 2 weeks. All animal experiments and procedures conformed to the institutional animal care guidelines. A xenograft tumor was established by subcutaneous right armpit injections of stable cell lines (8 million cells) to BALB/c athymic nude mice. Tumor growth was measured every 5 days. Animals were sacrificed and tumors were removed after 25 days.
Statistical Analysis
Data were statistically analyzed using SPSS version 19.0 software (IBM, USA). χ2 tests were used to analyze the correlations between Rab14 levels and clinicopathological factors. Student’s t-test was used to evaluate the significant difference between treated groups and the corresponding control groups. p<0.05 was considered to indicate statistically significant.
Results
Rab14 Expression is Elevated in NSCLC
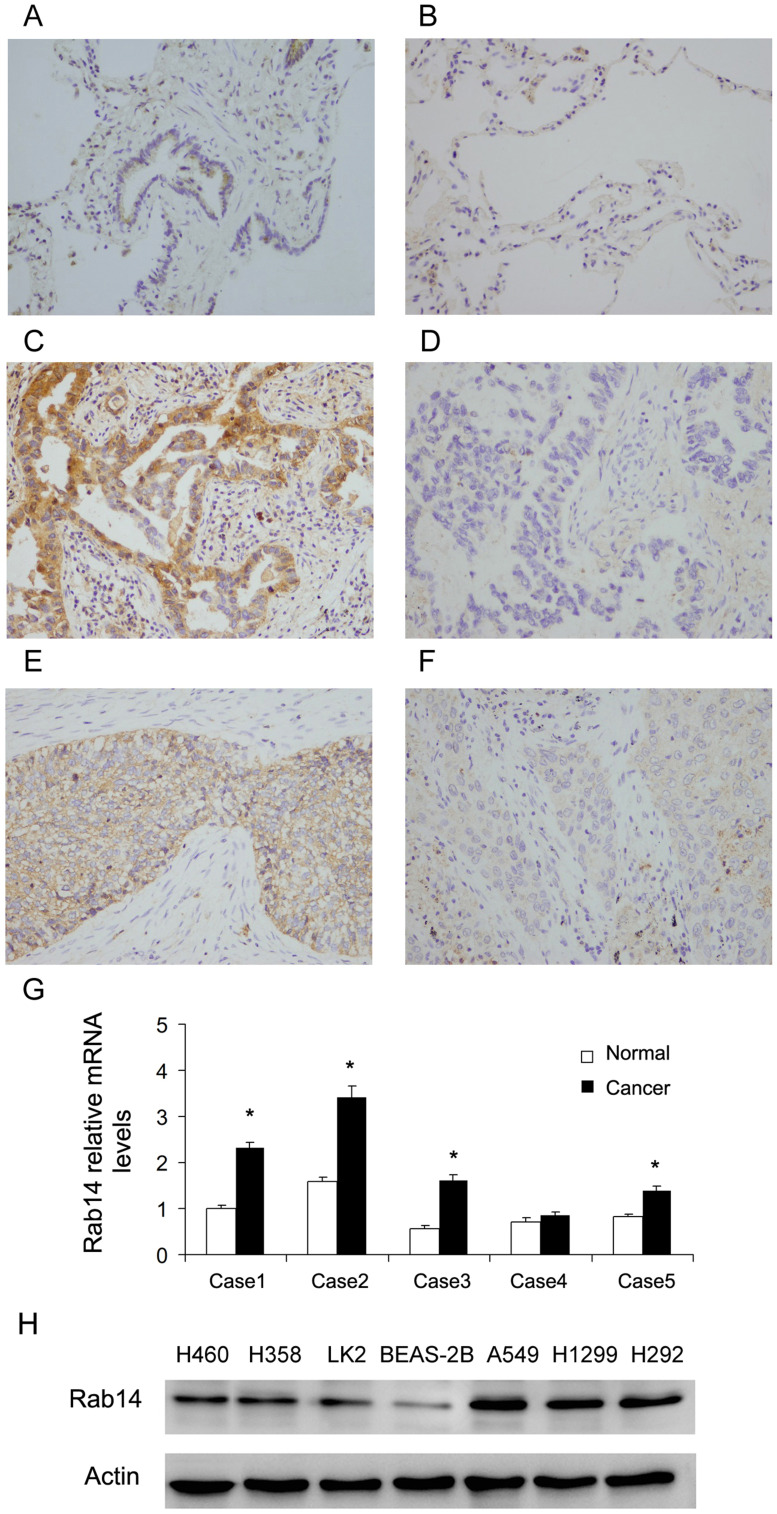
Rab14 protein levels in NSCLC tissues (115 cases) and normal lung tissues (18 cases) were analyzed using immunohistochemistry. The normal bronchial epithelial tissues (Figure 1A) and alveolar tissues (Figure 1B) showed weak/negative staining. High expression of Rab14 was observed in 65 out of 115 (56.5%) the NSCLC tissues (Figure 1C–F). Rab14 high expression was significantly correlated with advanced TNM stage (p=0.0169) and lymph nodal metastasis (p=0.0123, Table 1). We also performed sub-analyses in squamous cell carcinoma and adenocarcinoma. In adenocarcinoma, Rab14 overexpression correlated with differentiation and positive lymph node metastasis (Supplementary Table 1). In squamous cell carcinoma, Rab14 overexpression significantly correlated with TNM stage and T status (Supplementary Table 2). We also examined Rab14 mRNA levels in 5 cases of NSCLC tissues with paired normal lung tissues (Figure 1G). In four-fifths cases, Rab14 mRNA expression was significant higher in cancer tissues compared with adjacent normal lung tissues. Collectively, these data indicated that Rab14 was upregulated and its expression correlated with malignant features in human NSCLCs.
Figure 1.
Rab14 expression is upregulated in NSCLC tissues. (A) Rab14 weak staining in normal bronchial epithelial tissue. (B) Rab14 negative staining in normal alveolar tissue. (C) Rab14 high expression in a case of lung adenocarcinoma. (D) Rab14 negative staining in a case of lung adenocarcinoma. (E) Rab14 high expression in a case of squamous cell carcinoma. (F) Rab14 low expression in a case of squamous cell carcinoma. (Magnification: 400x). (G) RT-qPCR showed that Rab14 mRNA level in 4/5 fresh NSCLC tissues was higher than that in paired adjacent normal tissues. (H) Rab14 protein expression in lung cancer cell lines H460, H358, LK2, A549, H1299, H292 and normal cell line BEAS-2B. *p<0.05.
Table 1.
Distribution of Rab14 Status in NSCLC According to Clinicopathological Characteristics
| Characteristics | Number of Patients | Rab14 Low Expression | Rab14 High Expression | P |
|---|---|---|---|---|
| Age | ||||
| <60 | 67 | 28 | 39 | 0.6663 |
| ≥60 | 48 | 22 | 26 | |
| Gender | ||||
| Male | 75 | 36 | 39 | 0.1804 |
| Female | 40 | 14 | 26 | |
| Differentiation | ||||
| Well | 38 | 19 | 19 | 0.3216 |
| Moderate- Poor | 77 | 31 | 46 | |
| Histology | ||||
| Adenocarcinoma | 62 | 28 | 34 | 0.6937 |
| Squamous cell carcinoma | 53 | 22 | 31 | |
| TNM stage | ||||
| I | 43 | 26 | 17 | 0.0169 |
| II | 40 | 14 | 26 | |
| III | 32 | 10 | 22 | |
| Tumor status | ||||
| T1 | 35 | 12 | 23 | 0.1884 |
| T2–T4 | 80 | 38 | 42 | |
| Nodal metastasis | ||||
| Negative | 56 | 31 | 25 | 0.0123 |
| Positive | 59 | 19 | 40 |
Rab14 Facilitates Growth and Invasion of Lung Cancer Cells
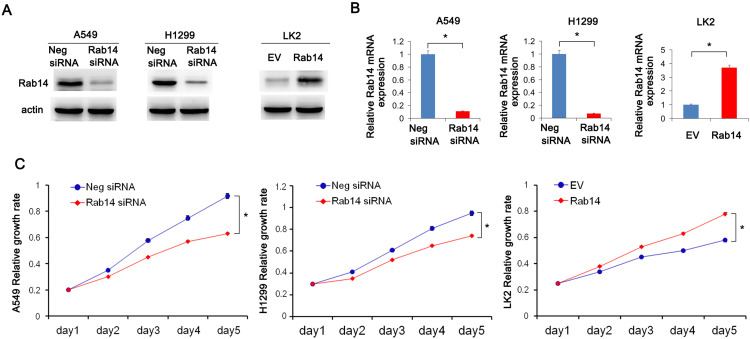
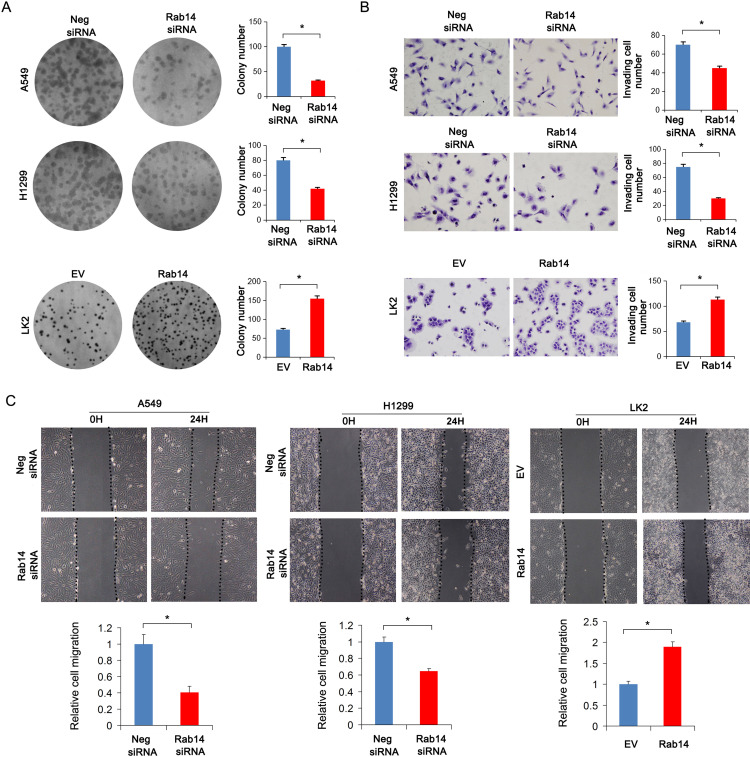
Rab14 protein levels in BEAS-2B and lung cancer cell lines including H460, H358, LK2, A549, H1299 and H292 were detected using Western blot (Figure 1H). The cancer cell lines had relatively high protein expression. Rab14 overexpression was performed in LK2 cell line which had relatively low endogenous Rab14. Rab14 siRNA knockdown was performed in A549 and H1299 cell lines which had relatively high endogenous expression. Western blot and RT-qPCR were used to evaluate the transfection efficiency, which showed that Rab14 plasmid and siRNA significantly changed the endogenous expression of Rab14 as expected (Figure 2A and B). MTT assay showed that Rab14 overexpression increased proliferation rate while Rab14 siRNA decreased it (Figure 2C). Colony formation results showed that the colony number increased after Rab14 overexpression while decreased after Rab14 depletion (Figure 3A). In addition, the results of Matrigel invasion assays demonstrated that the invading cell numbers increased after Rab14 overexpression but decreased after Rab14 knockdown in lung cancer cell lines according to the results of Matrigel invasion assay (Figure 3B). To determine the effect of Rab14 on migration, we performed wound healing assays. Rab14 transfection facilitated cell migration while Rab14 knockdown inhibited cell migration. The relative migration rate is shown in Figure 3C. For consistency and comparison, overexpression experiment was also done in A549 and H1299 cells. As shown in Supplementary Figure 1A and B, Rab14 overexpression increased proliferation rate and invading ability in both A549 and H1299 cells. The effect of Rab14 overexpression/depletion on apoptosis was also examined. As shown in Supplementary Figure 1C, the Rab14 overexpression did not change apoptosis significantly. Rab14 depletion slightly increased apoptosis rate in both cell line. We also investigated the effect of Rab14 on chemo-sensitivity using paclitaxel (2μM). MTT assays showed that Rab14 depletion increased inhibition rate (Supplementary Figure 2A).
Figure 2.
Rab14 promotes cell proliferation. (A) pCMV6-Rab14 transfection upregulated Rab14 protein level significantly in LK2 cells while siRNA transfection downregulated Rab14 protein levels in both A549 and H1299 cells. (B) Rab14 mRNA levels increased in LK2 cells transfected with Rab14 plasmid. In A549 and H1299 cells, Rab14 mRNA levels decreased after siRNA transfection. (C) MTT results showed that proliferation rate increased after pCMV6-Rab14 transfection in LK2 cells, while Rab14 depletion inhibited proliferation rate in A549 and H1299 cells. Experiments were performed in triplicate *p<0.05.
Figure 3.
Rab14 promotes colony formation, invasion and migration. (A) Colony number increased after pCMV6-Rab14 transfection while decreased after Rab14 siRNA transfection. (B) Transwell assay showed that Rab14 overexpression increased the number of invading cells in LK2 while Rab14 depletion decreased invading numbers in A549 and H1299 cells. (C) Rab14 overexpression promoted cell migration while Rab14 depletion suppressed migration. Experiments were performed in triplicate *p<0.05.
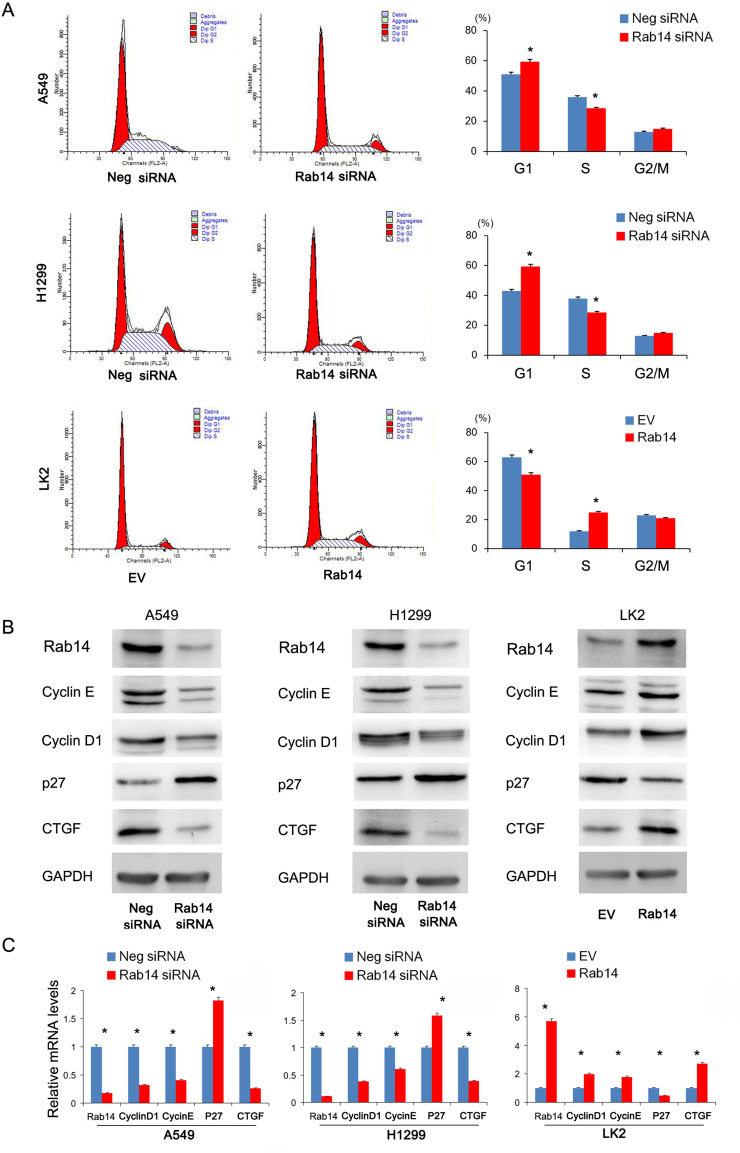
Rab14 Regulates Cell Cycle and Related Protein Levels in Lung Cancer Cells
We then examined whether Rab14 overexpression or depletion affected the cell cycle progression in lung cancer cells. We found that the percentage of S phase increased, while the percentage of G1 phase cells decreased after Rab14 overexpression. Rab14 depletion showed the opposite effects (Figure 4A). To further elucidate the underlying mechanisms of Rab14, we checked for changes in cell cycle related proteins. Using Western blot analysis, we demonstrated that Rab14 overexpression upregulated protein levels of cyclin E, cyclin D1, while downregulating p27 in LK2 cell line (Figure 4B). Rab14 depletion suppressed cyclin E, cyclin D1, while increasing p27 expression in both A549 and H1299 cell lines. In addition, we found that Rab14 could positively regulated CTGF expression. RT-qPCR showed that Rab14 overexpression increased cyclin E, cyclin D1 and CTGF, but decreased p27 mRNA expression (Figure 3C). To find out whether the change of migration and invasion is related to epithelial mesenchymal transformation (EMT), we examined the effect of Rab14 on EMT markers and related transcription factor. As shown in Supplementary Figure 2A, Rab14 depletion did not change the levels of E-cadherin, N-cadherin, Vimentin, and Snail in both A549 and H1299 cell lines.
Figure 4.
Rab14 regulates cell cycle and related protein. (A) Flow cytometry results showed that Rab14 transfection decreased G1 phase percentage and increased S phase percentage in LK2 cells. Rab14 siRNA increased G1 phase percentage and decreased S phase percentage in A549 and H1299 cells. (B) Rab14 overexpression upregulated Rab14, Cyclin E, cyclin D1, CTGF protein levels while downregulating p27 level in LK2 cells. Rab14 depletion downregulated Rab14, cyclin E, cyclin D1, CTGF while upregulating p27 protein levels in A549 and H1299 cells. (C) Rab14, Cyclin D1, cyclin E, CTGF mRNA levels increased while p27 level decreased in LK2 cells transfected with Rab14 plasmid. Rab14 depletion decreased Rab14, cyclin D1, cyclin E, CTGF while increased p27 mRNA levels in both A549 and H1299 cells. Experiments were performed in triplicate *p<0.05.
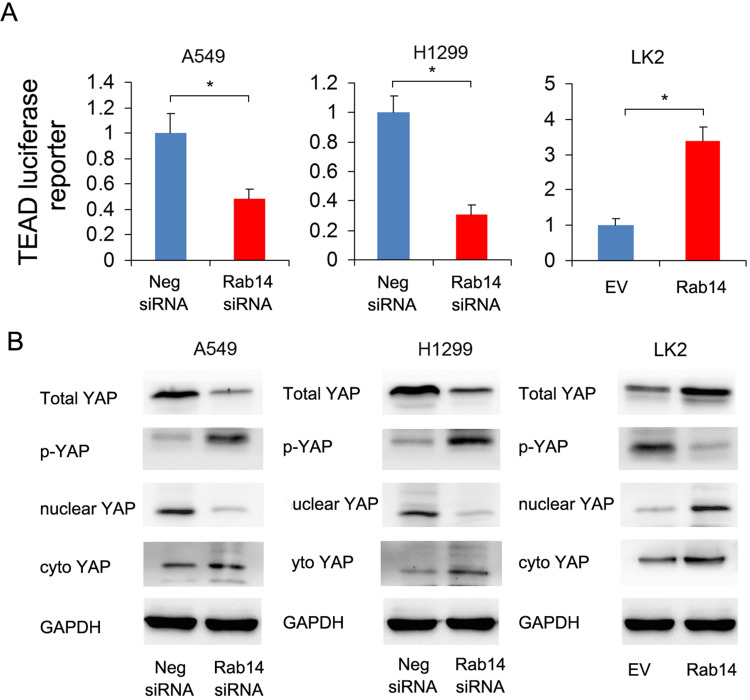
Because CTGF is a downstream target of the Hippo signaling pathway, we hypothesized that Rab14 might regulate Hippo signaling in lung cancer cells. We thus examined Hippo activity by luciferase reporter assay and the changes in YAP protein levels by Western blot. YAP, which interacts with TEAD in the nucleus and activates transcription, is a major downstream effector of Hippo signaling. Luciferase reporter assay showed that Rab14 overexpression enhanced TEAD reporter activity while Rab14 depletion showed the opposite effects (Figure 5A). Rab14 overexpression upregulated total YAP while downregulating inactive p-YAP. Nuclear cytoplasmic fractionation showed that Rab14 overexpression increased nuclear YAP and slightly decreased cytoplasmic YAP (Figure 5B), suggesting its possible role in YAP nuclear/cytoplasmic re-distribution. Thus, it is possible that Rab14 mediated cell proliferation and invasion in lung cancer cells may depend on Hippo signaling pathway.
Figure 5.
Rab14 regulates YAP signaling. (A) Rab14 overexpression enhanced TEAD luciferase reporter activity while Rab14 depletion suppressed TEAD luciferase reporter activity. Experiments were performed in triplicate. (B) Rab14 overexpression increased total YAP, nuclear YAP and decreased p-YAP, cytoplasmic YAP protein levels in LK2 cells. Rab14 depletion downregulated total YAP, nuclear YAP and upregulated p-YAP, cytoplastic YAP levels in A549 and H1299 cells. * p<0.05.
Rab14 Regulates Proliferation and Related Proteins Through Hippo/YAP Signaling
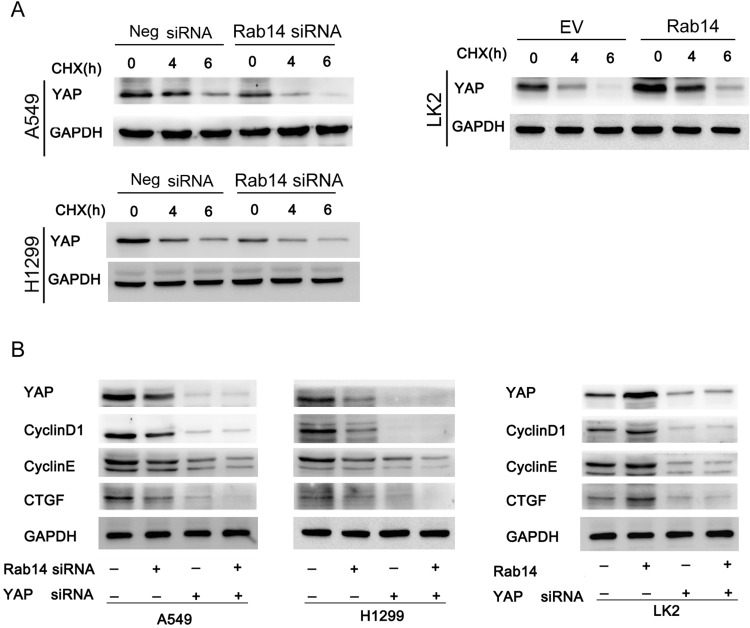
To confirm the involvement of Rab14 in YAP regulation, we further analyzed the change in YAP degradation after treatment with cycloheximide (CHX), a protein synthesis inhibitor. As shown in Figure 6A, Rab14 depletion accelerated YAP degradation while Rab14 overexpression decreased it. We then used YAP siRNA in cancer cells co-transfected with Rab14 plasmid and siRNA. YAP siRNA significantly suppressed the expression of cyclin D1, cyclin E and CTGF (Figure 4D). YAP siRNA significantly ameliorated the effect of Rab14 plasmid/siRNA on cyclin D1, cyclin E and CTGF. We also examined the effect of knockdown YAP on the cell proliferation and migration ability following overexpression/knockdown of Rab14. As shown in Supplementary Figure 3A and B. YAP siRNA reduced cell proliferation and invasion. YAP knockdown could abolish the positive effects of Rab14 on proliferation and invasion. These results indicated that the effect of Rab14 on biological roles of NSCLC was at least partly dependent on YAP. To explore if there is possible interaction between YAP and Rab14, we performed co-immunoprecipitation followed by Western blot. As shown in Supplementary Figure 3C, there is no obvious interaction between them. To examine the effect of Rab14 on tumor growth in vivo, we established Rab14 overexpressing LK2 cells by G418 selection. As shown in Supplementary Figure 3D, the in vivo growth rate and tumor sizes of Rab14 overexpressing cells were much larger than control cells.
Figure 6.
Rab14 regulates cyclin D1, cyclin E and CTGF through YAP. (A) Lung cancer cells were transfected with pCMV6-Rab14/Rab14 siRNA, and then treated with cycloheximide (CHX) for 0, 4, 6 hours respectively. Western blot results showed that Rab14 depletion accelerated YAP degradation in A549 and H1299 cells while Rab14 overexpression decreased YAP degradation in LK2 cells. (B) YAP siRNA was used in cells transfected with Rab14 plasmid and siRNA. YAP siRNA significantly decreased expression of cyclin D1, cyclin E and CTGF. In cells transfected with YAP siRNA, the effect of Rab14 plasmid/siRNA on cyclin D1, cyclin E and CTGF protein was significantly ameliorated.
Discussion
The present study found that Rab14 is upregulated in human non-small cell lung cancer tissues and significantly facilitated cancer cell growth and invasion. Rab14 also increased expression of YAP. As the role of Rab14 in cancer progression has mainly focused on its regulation of intracellular transportation vesicles, its effect on other pathways has not been examined to the best of our knowledge. Our data for the first time indicates the involvement of the Hippo pathway in the biological impact of Rab14 on lung cancer development.
Recent studies have suggested that Rab14 is upregulated in several cancers, including esophageal cancer,12 cervical cancer,13 liver cancer,14 pancreatic cancer,8 gastric cancer.9 To date, its clinical significance in NSCLCs remains unexplored. Our results showed a significant association between Rab14 levels and TNM stage/nodal metastasis, which suggested that Rab14 could be a biomarker indicating malignancy.
We then overexpressed Rab14 in LK2 cell line and knocked down its expression in both H1299 and A549 cells. Our data showed that Rab14 overexpression promoted cell growth activity, colony formation and in vivo tumor growth. Rab14 also increased the invasion and migration of lung cancer cells. Rab14 knockdown also increased chemosensitivity of NSCLC cells. Further analysis showed that Rab14 facilitated cell cycle transition. Mechanistically, Rab14 overexpression enhanced the expression of cyclin E, cyclin D1, CTGF and repressed p27 at both the mRNA and protein levels. Cyclin E and cyclin D1 are important cell cycle related oncoproteins involved in carcinogenesis.15–18 p27 is known as a cyclin-dependent kinase inhibitor, the loss of which indicated poor prognosis of NSCLC patients.19 Our data provided evidence of the growth promoting role of Rab14 in NSCLC cells. CTGF has many biological functions including tumor growth and invasion and serves as a major downstream target of Hippo signaling.20 Our results also showed that Rab14 upregulated CTGF, indicating the potential involvement of Hippo signaling.
YAP, the main effector of Hippo signaling, interacts with TEAD and induces transcriptional activation, which could be detected by TEAD specific luciferase reporter activity.21 Using TEAD luciferase reporter activity, we showed that Rab14 inhibited Hippo signaling by increasing YAP/TEAD activity. Western blot showed that Rab14 upregulated YAP protein expression. YAP has been demonstrated as an oncoprotein in NSCLC and can regulate cyclin D1, cyclin E and CTGF.22 We also found that Rab14 reduced YAP phosphorylation. Phosphorylated YAP binds to 14-3-3 protein and remains in the cytoplasm for degradation23,24 while dephosphorylated YAP translocates into the nucleus and binds to TEAD proteins to activate downstream targets.23,24 Accordingly, cycloheximide treatment showed that Rab14 inhibited YAP degradation. To further validate the essential role of YAP in Rab14 induced downstream effects on cell cycle proteins and CTGF, we used siRNA to knockdown endogenous YAP. Our data showed that YAP silencing significantly reduced effects of Rab14 on cyclin D1, cyclin E, CTGF, as well as cell proliferation and invasion. These findings suggested a link between Rab14, YAP signaling, NSCLC proliferation and invasion.
The exact mechanism of Rab14 on YAP signaling remains unknown. YAP is a transcription factor that translocate into the nucleus and regulate gene expression with TEAD. This translocation event is essential for downstream gene regulation by YAP. Phosphorylated YAP can be sequestered in the cytoplasm and subsequently degraded. Transport of YAP is an active process that requires endocytosis. It has been reported that Rab14 plays an important role in endocytic trafficking.25–27 It is possible that Rab14 facilitates YAP translocation into nucleus, thus decreases its degradation and activates downstream transcriptional activity of YAP/TEAD signaling. Further studies need to be conducted to confirm this possibility.
In conclusion, the current study revealed a novel role for Rab14 in NSCLC by showing its oncogenic characteristics and its regulation of the YAP signaling. Our data indicate a new mechanism for Rab14 and suggest it may be a potential therapeutic target in NSCLC.
Funding Statement
The present study was supported by the National Natural Science Foundation of China (No. 81471898).
Disclosure
We declare that we have no conflicts of interest.
References
- 1.Ryan KJ, Nero D, Feinberg BA, et al. Real-world incidence and cost of pneumonitis post-chemoradiotherapy for stage III non-small-cell lung cancer. Future Oncol. 2020;16(1):4303–4313. doi: 10.2217/fon-2019-0524 [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Yan S, Dong L, Li X. A-kinase-interacting protein 1 overexpression correlates with deteriorative tumor features and worse survival profiles, and promotes cell proliferation but represses apoptosis in non-small-cell lung cancer. J Clin Lab Anal. 2020;34(2):e23061. doi: 10.1002/jcla.23061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jankowska-Polanska B, Polanski J, Chabowski M, Rosinczuk J, Mazur G. Influence of coping strategy on perception of anxiety and depression in patients with non-small cell lung cancer. Adv Exp Med Biol. 2019. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, He Z, Yang S, et al. The impact of previous therapy strategy on the efficiency of anlotinib hydrochloride as a third-line treatment on patients with advanced non-small cell lung cancer (NSCLC): a subgroup analysis of ALTER0303 trial. Transl Lung Cancer Res. 2019;8(5):575–583. doi: 10.21037/tlcr.2019.09.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamura M, Matsumoto I, Tanaka Y, et al. Prognostic factor and treatment strategy for clinical N1 non-small cell lung cancer. Gen Thorac Cardiovasc Surg. 2020;68:261–265. doi: 10.1007/s11748-019-01205-4 [DOI] [PubMed] [Google Scholar]
- 6.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81(1):153–208. doi: 10.1152/physrev.2001.81.1.153 [DOI] [PubMed] [Google Scholar]
- 7.Chao H, Deng L, Xu F, et al. RAB14 activates MAPK signaling to promote bladder tumorigenesis. Carcinogenesis. 2019;40:1341–1351. doi: 10.1093/carcin/bgz039 [DOI] [PubMed] [Google Scholar]
- 8.Ge J, Ge C. Rab14 overexpression regulates gemcitabine sensitivity through regulation of Bcl-2 and mitochondrial function in pancreatic cancer. Virchows Arch. 2019;474(1):59–69. doi: 10.1007/s00428-018-2455-5 [DOI] [PubMed] [Google Scholar]
- 9.Guo B, Wang W, Zhao Z, et al. Rab14 act as oncogene and induce proliferation of gastric cancer cells via AKT signaling pathway. PLoS One. 2017;12(1):e0170620. doi: 10.1371/journal.pone.0170620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou R, Jiang L, Yang Z, Wang S, Liu Q. Rab14 is overexpressed in ovarian cancers and promotes ovarian cancer proliferation through Wnt pathway. Tumour Biol. 2016;37(12):16005–16013. doi: 10.1007/s13277-016-5420-4 [DOI] [PubMed] [Google Scholar]
- 11.Lian Q, Ma DM, Chen MG, Chen K, Li XJ. Silencing Rab14 represses the proliferation and migration of oral squamous cell carcinoma, and enhances cisplatin sensitivity. Am J Transl Res. 2017;9:4195–4205. [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H-F, Zhen Q, Fan Y-K. LINC00963 predicts poor prognosis and promotes esophageal cancer cells invasion via targeting miR-214-5p/RAB14 axis. Eur Rev Med Pharmacol Sci. 2020;24(1):164–173. doi: 10.26355/eurrev_202001_19907 [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Liang B, Hou S. TMPO-AS1 promotes cervical cancer progression by upregulating RAB14 via sponging miR-577. J Gene Med. 2019;21(11):e3125. doi: 10.1002/jgm.3125 [DOI] [PubMed] [Google Scholar]
- 14.Chen TW, Yin FF, Yuan YM, et al. CHML promotes liver cancer metastasis by facilitating Rab14 recycle. Nat Commun. 2019;10:2510. doi: 10.1038/s41467-019-10364-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohammedi L, Doula FD, Mesli F, Senhadji R. Cyclin D1 overexpression in algerian breast cancer women: correlation with CCND1 amplification and clinicopathological parameters. Afr Health Sci. 2019;19(2):2140–2146. doi: 10.4314/ahs.v19i2.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi C, Park P, Cho C, Choi C. Cyclin D1 is associated with radiosensitivity of triple-negative breast cancer cells to proton beam irradiation. Int J Mol Sci. 2019;20(19):4943. doi: 10.3390/ijms20194943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Xu D, Li X, et al. Latest overview of the cyclin-dependent kinases 4/6 inhibitors in breast cancer: the past, the present and the future. J Cancer. 2019;10(26):6608–6617. doi: 10.7150/jca.33079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rugo HS. Cyclin-dependent kinase 4/6 inhibition in the treatment of hormone receptor-positive breast cancer. Clin Adv Hematol Oncol. 2019;17(10):555–558. [PubMed] [Google Scholar]
- 19.Sterlacci W, Fiegl M, Hilbe W, et al. Deregulation of p27 and cyclin D1/D3 control over mitosis is associated with unfavorable prognosis in non-small cell lung cancer, as determined in 405 operated patients. J Thorac Oncol. 2010;5(9):1325–1336. doi: 10.1097/JTO.0b013e3181e77efc [DOI] [PubMed] [Google Scholar]
- 20.Di Benedetto A, Mottolese M, Sperati F, et al. The hippo transducers TAZ/YAP and their target CTGF in male breast cancer. Oncotarget. 2016;7(28):43188–43198. doi: 10.18632/oncotarget.9668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dupont S. Luciferase reporter assays to determine YAP/TAZ activity in mammalian cells. Methods Mol Biol. 2019;1893:121–135. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010;101(5):1279–1285. doi: 10.1111/j.1349-7006.2010.01511.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh H, Irvine KD. In vivo regulation of yorkie phosphorylation and localization. Development. 2008;135(6):1081–1088. doi: 10.1242/dev.015255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vassilev A. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15(10):1229–1241. doi: 10.1101/gad.888601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed SE, Hodgson LR, Song S, et al. A role for Rab14 in the endocytic trafficking of GLUT4 in 3T3-L1 adipocytes. J Cell Sci. 2013;126(9):1931–1941. doi: 10.1242/jcs.104307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linford A, Yoshimura S, Nunes Bastos R, et al. Rab14 and its exchange factor FAM116 link endocytic recycling and adherens junction stability in migrating cells. Dev Cell. 2012;22(5):952–966. doi: 10.1016/j.devcel.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su H, Liu B, Frohlich O, Ma H, Sands JM, Chen G. Small GTPase Rab14 down-regulates UT-A1 urea transport activity through enhanced clathrin-dependent endocytosis. FASEB J. 2013;27(10):4100–4107. doi: 10.1096/fj.13-229294 [DOI] [PMC free article] [PubMed] [Google Scholar]