Abstract
Nasopharyngeal carcinoma (NPC) is a rare malignancy arising from the nasopharyngeal epithelium and belongs to the group of head and neck cancer types, which are usually associated with viral and/or environmental influences, as well as heredity causes. A recent study reported that the long non-coding RNA (lncRNA) HOXA cluster antisense RNA 2 (HOXA-AS2) may be a prognostic biomarker in NPC; however, the specific mechanisms underlying NCP progression are yet to be determined. The aim of the present study was to investigate the biological role of HOXA-AS2 in NPC. In the present study, the gene expression levels of HOXA-AS2, miR-519, hypoxia-inducible factor (HIF-1α) and programmed death-ligand 1 (PD-L1) were detected using reverse transcription-quantitative PCR (RT-qPCR) analysis and western blotting. Bioinformatics analysis and a dual luciferase reporter assay were performed to predict and confirm the direct interactions between HOXA-AS2 and microRNA (miR)-519, as well as between miR-519 and HIF-1α. A MTT assay was used to detect the cell viability, while cell migratory and invasive abilities were assessed using wound healing and Transwell assays. HOXA-AS2 and HIF-1α were found to be significantly upregulated in NPC tumor tissues, as well as in NPC cell lines. The overexpression of HOXA-AS2 significantly enhanced NPC progression, including the cell proliferative, migratory and invasive abilities. HOXA-AS2 was identified to directly bind to miR-519, whereas a miR-519 inhibitor significantly rescued the HOXA-AS2 knockdown-attenuated progression of NPC. Moreover, miR-519 could bind to HIF-1α and PD-L1. Overexpression of HIF-1α and PD-L1 significantly promoted NPC progression and partially recovered the phenotype of NPC cells attenuated by HOXA-AS2 knockdown. In conclusion, the present study demonstrated that HOXA-AS2/miR-519/HIF-1α and/or HOXA-AS2/miR-519/PD-L1 may be a novel mechanism regulating the progression of NPC, which may facilitate the development of targeted clinical therapy.
Keywords: nasopharyngeal carcinoma, progression, HOXA cluster antisense RNA 2, microRNA-519, hypoxia-inducible factor-1α, programmed death-ligand 1
Introduction
Nasopharyngeal carcinoma (NPC) is a type of head and neck squamous cell carcinoma which had an incidence of 3.26/100,000 in China in 2013 (1). Previous studies have revealed that multiple factors synergistically affect the origin and progression of NPC, including viruses (specifically Epstein-Barr virus), genetic changes and environmental factors (2–4). In addition, an increased understanding of the underlying molecular mechanisms may lead to the development of more effective clinical therapies.
Long non-coding RNAs (lncRNAs) lack a protein-coding capacity, but serve crucial roles in controlling gene expression during cell development and differentiation (5). HOXA cluster antisense RNA 2 (HOXA-AS2) has been implicated in a variety of cancer types, such as gastric cancer, colorectal cancer and pancreatic cancer (6–8). However, to the best of our knowledge, there is currently no research on HOXA-AS2-mediated NPC progression.
MicroRNAs (miRNAs/miRs) are short non-coding RNAs with a length of ~22 nucleotides, which may act as tumor suppressors to target important oncogenes or act as oncogenes to target crucial tumor suppressors (9). Numerous miRNAs, such as miR-101, miR-19, miR-127 and miR-98, have been identified to be involved in the progression of various cancer types, such as hepatocellular carcinoma and non-small cell lung cancer miR-519 has been extensively investigated, particularly its interaction with ELAV like RNA binding protein 1 expression in regulating cancer cell progression (10–12). Moreover, Yu et al (13) reported that miR-519 suppressed NPC cell proliferation by targeting URG4/URGCP. However, studies on miR-519-related mechanisms in cancer, particularly NPC, are currently lacking.
Hypoxia-inducible factor 1 (HIF-1) is a heterodimeric protein that consists of two proteins, HIF-1α and HIF-1β (14). HIF-1α activates the transcription of genes that are involved in crucial aspects of cancer biology, including angiogenesis, cell survival, glucose metabolism and invasion (15). Xie et al (16) found that HIF-1α was associated with poor overall survival, poor progression-free survival, a higher rate of lymph node metastasis and a more advanced tumor stage in NPC. Furthermore, Cha et al (17) observed that miR-519 could suppress HIF-1α expression and tumor angiogenesis, which suggested that may be a potential direct interaction between miR-519 and HIF-1α in NPC cells.
Programmed death-ligand 1 (PD-L1) serves an immune-suppressive role by binding to its receptor PD-1 and blocking T-cell activation (18,19). It was recently revealed that PD-L1 expression is closely associated with NPC prognosis and metastasis (20,21). For example, Zhou et al (22) demonstrated that PD-L1 predicted poor prognosis of NPC. In addition, Fei et al (23) observed that PD-L1 activated epithelial-to-mesenchymal transition in NPC cells via PI3K/AKT signaling pathway.
The aim of the present study was to investigate the biological role of HOXA-AS2 in NPC. To the best of our knowledge, the present study was the first to investigate the role of the HOXA-AS2/miR-519/HIF-1α or PD-L1 axis in regulating NPC progression, using bioinformatics analysis and relevant in vitro experiments. The current findings may provide a basis for the development of HOXA-AS2-targeted clinical therapy.
Materials and methods
Clinical specimens and cell lines
Under the approval of the Ethics Committee of the Zhuji People's Hospital of Zhejiang Province, 15 paired tumor tissues and adjacent healthy tissues were collected from 15 patients with NPC (9 males and 6 females) with a mean age of 43 years (age range, 31–59 years) between March 2012 and August 2014 from Weifang Traditional Chinese Hospital (Weifang, China) and written informed consent was obtained from the participants. Tumor tissues were obtained using fiberoptic nasopharyngoscopy at the tumor growth site, and the adjacent side (2-cm away from the tumor) with an observed normal mucosal morphology was used for the adjacent healthy tissues. None of the patients with NPC had received chemotherapy, immunotherapy or radiotherapy prior to surgery.
NPC cancer cell lines (SUNE1 and SUNE2) and a normal nasopharyngeal epithelial cell line (NP69) were purchased from BeNa Culture Collection, as was the 293T cell line, for in vitro experiments. The cell lines were cultured in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Thermo Fisher Scientific, Inc.), and placed at 37°C in a humidified incubator containing 5% CO2.
Stable cell line generation and transfection
The short hairpin (sh)RNA specific to HOXA-AS2 (shHOXA-AS2; 5′-UCAGCUGAUGGCGUAUCCAUGAU-3′) and its negative control (shNC; 5′-GAUUCCCCGGACUUCUCACAG-3′), miR-519-mimics (5′-GUGAUGAAACAACCUGUACUU-3′) and its negative control (NC mimics; 5′-UACCACUGACAAUCGCUACUG-3′), and miR-519-inhibitor (5′-ACCCUUAAUCGACGUCGGGAG-3′) and its negative control (NC inhibitor; 5′-GAGUAGAAGUUGUAAUCUGUC-3′) were synthesized by TsingKe Biotechnology Co., Ltd. The full length of HIF-1α or PD-L1 was subcloned into pcDNA3.1 (Shanghai GenePharma Co., Ltd.) to overexpress HIF-1α or PD-L1, with empty pcDNA3.1 serving as the control. Transfection of the cells with shHOXA-AS2 (10 nM) or shNC (10 nM) and the miR-519-mimics (10 nM) or NC mimics (10 nM) and miR-519-inhibitor (10 nM) or NC inhibitor (10 nM) was conducted with Lipofectamine 2000 transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.). All functional experiments were performed 48 h post-transfection.
Bioinformatic analysis and dual luciferase reporter assays
StarBase version 2.0 (http://starbase.sysu.edu.cn) was used to predict the downstream target of HOXA-AS2 or miR-519. A dual luciferase reporter assay was used to assess the direct binding site between HOXA-AS2 and miR-519, as well as between miR-519 and HIF-1α. Briefly, 293T cells were transfected with the pmirGLO reporter vectors (Promega Corporation) containing wild-type or mutant HOXA-AS2 and 3′-untranslated region (UTR) of HIF-1α, using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.). Subsequently, 293T cells were co-transfected with the pmirGLO reporter vectors and miR-519 mimic or miR-519 inhibitor. After 48 h incubation at 37°C with 5% CO2, luciferase activity was evaluated using the Dual-Luciferase Reporter Analysis system (Promega Corporation). Firefly luciferase activity was normalized to Renilla luciferase (Promega Corporation) gene activity.
RNA isolation, reverse transcription-quantitative PCR (RT-qPCR) and quantification
Total RNA from tissues and cell lines was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. RT was conducted using the Takara PrimeScript kit (Takara Biotechnology Co., Ltd.) at 37°C for 15 min. Subsequently, the RT-qPCR assay was performed using the ViiATM 7 Real-Time PCR system (Thermo Fisher Scientific, Inc.) with SYBR-Green Master Mix to detect gene expression levels. The following amplification conditions were used: Pre-denaturation at 95°C for 15 sec, followed by 40 cycles of denaturation at 94°C for 30 sec, annealing at 60°C for 20 sec and extension at 72°C for 40 sec. Relative gene expression was calculated using the 2−ΔΔCq method (24). GAPDH and U6 were set as an internal control. The primer sequences were as follows: HOXA-AS2 forward, 5′-CCCGTAGGAAGAACCGATGA-3′ and reverse, 5′-TTTAGGCCTTCGCAGACAGC-3′; miR-519 forward, 5′-CATGCTGTGACCCTCCAAAG-3′ and reverse, 5′-GAGAAAACAAACAGAAAGCGCT-3′; PD-L1 forward, 5′-TGCGGACTACAAGCGAATCA-3′ and reverse, 5′-GATCCACGGAAATTCTCTGGTT-3′; HIF-1α forward, 5′-ACTTGGACGCTCTGCCTATG-3′ and reverse, 5′-TTGCGGGGGTTGTAGA-3′; GAPDH forward, 5′-GAAGAGAGAGACCCTCACGCTG-3′ and reverse, 5′-ACTGTGAGGAGGGGAGATTCAGT-3′; and U6 forward, 5′-CTCGCTTCGGCAGCACATATACTA-3′ and reverse, 5′-ACGAATTTGCGTGTCATCCTTGCG-3′.
Western blotting
Proteins were extracted using RIPA buffer (Beyotime Institute of Biotechnology). Protein concentration was measured with the bicinchoninic acid assay (Beyotime Institute of Biotechnology). Following denaturation, 10 µg protein/lane was separated by 10% SDS-PAGE. Proteins were transferred onto PVDF membranes and blocked in 5% non-fat milk for 2 h at room temperature. The membranes were incubated with primary antibodies against HIF-1α (1:1,000; cat. no. sc-13515; Santa Cruz Biotechnology, Inc.), PD-L1 (1:1,000; cat. no. ab205921; Abcam) and GAPDH (1:1,000; cat. no. sc-47724; Santa Cruz Biotechnology, Inc.) overnight at 4°C. Following primary incubation, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:1,000; goat anti-mouse IgG; cat. no. ab205719 and goat anti-rabbit IgG; cat. no. ab205718; both Abcam) for 2 h at room temperature. Protein bands were visualized using the Pierce ECL Western Blotting kit (Pierce; Thermo Fisher Scientific, Inc.). Protein expression was quantified using Image-Pro® Plus software (version 6.0; Media Cybernetics, Inc.). GAPDH was used as an endogenous control for data normalization.
MTT assay
Transfected cells were seeded into 96-well plates at 3×103 cells/well and cultured at 37°C. Following incubation for 0, 24, 48 and 72 h, 20 µl MTT (Sigma-Aldrich; Merck KGaA) was added into each well and incubated for a further 4 h at 37°C according to the manufacturer's instructions. Subsequently, the medium was removed and 150 µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) was added to dissolve the formazan crystals. The absorbance was measured at 450 nm using a microplate reader (Molecular Devices LLC).
Wound healing assay
Transfected SUNE1 and SUNE2 cells were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) supplemented without serum at a density of 1×104 cells/ml in a humidified atmosphere of 5% CO2 at 37°C, and grown to a fully confluent monolayer. After 6 h, culture medium was replaced with serum-free medium and a sterile tip was used to create a single-line scratch. The plates were then washed twice with PBS to remove detached cells. After 24 h, the medium was replaced with PBS, and the wound gap was observed. Images of the migrating cells were acquired at 0 and 24 h using a light microscope (Nikon Corporation; magnification, ×200) and measured using ImageJ software version 1.8 (National Institutes of Health).
Cell invasion assay
Cell invasion was determined using Transwell chambers (8 µm pore size; EMD Millipore) precoated with 100 µl Matrigel (BD Biosciences) for 1 h at room temperature. SUNE1 and SUNE2 cells (1×104 cells) were added to the top chamber containing 150 µl RPMI-1640 without FBS. An extra 550 µl RPMI-1640 medium with 10% FBS was added to the bottom chamber. After 24 h, 4% paraformaldehyde was added to fix the cells at room temperature for 20 min, followed by staining with 0.1% crystal violet (Sigma-Aldrich; Merck KGaA) for 20 min at room temperature. The invading cells were placed under a light microscope (Nikon Corporation; magnification, ×200) and photographed.
Statistical analysis
The software package SPSS 22.0 (IBM Corp.) was used for subsequent statistical analysis. Each experiment was repeated ≥3 times and the data are presented as the mean ± SD. Comparisons between NPC tissues and adjacent healthy tissues were performed using a paired Student's t-test, while comparisons between the experimental and control groups were performed using an unpaired Student's t-test. Comparisons among multiple groups were performed using one-way ANOVA followed by Tukey's test. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-519 inhibitor rescues HOXA-AS2 knockdown-attenuated progression of NPC
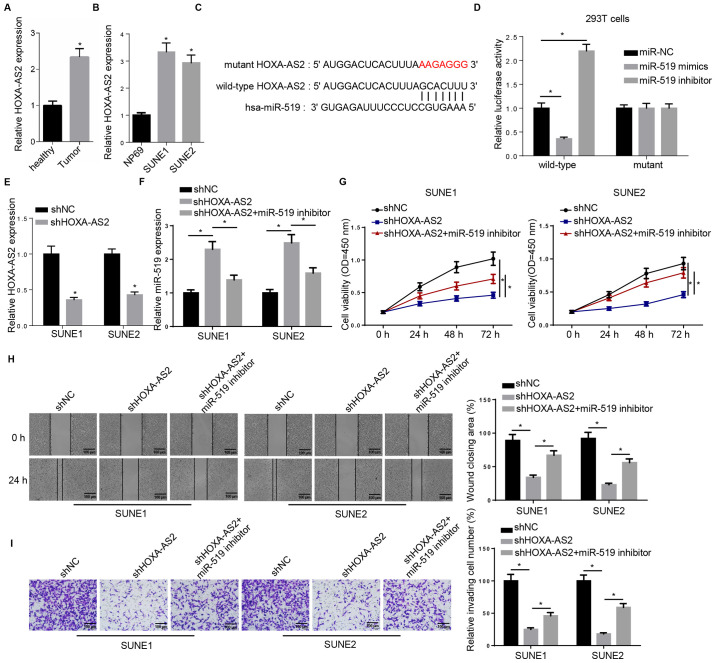
The expression of HOXA-AS2 was first examined in both tissues and cell lines. Consistent with previous studies, the RT-qPCR results demonstrated a significantly higher expression of HOXA-AS2 in NPC tissues compared with the adjacent healthy tissues (Fig. 1A). The same result was also observed in NPC cell lines (SUNE1 and SUNE2) compared with the normal nasopharyngeal epithelial cell line (Fig. 1B). Thus, the aberrant expression pattern between tumor and healthy specimens indicated the important role of HOXA-AS2 in NPC progression.
Figure 1.
miR-519 inhibitor rescues HOXA-AS2 knockdown-attenuated progression of NPC. (A) RT-qPCR analysis of HOXA-AS2 expression in NPC tumor tissues (n=15) and the adjacent healthy tissues (n=15). (B) RT-qPCR analysis of HOXA-AS2 expression in the normal nasopharyngeal epithelial cell line (NP69) and the NPC cancer cell lines (SUNE1 and SUNE2). (C) Bioinformatics analysis identified the binding site between miR-519 and HOXA-AS2. (D) Dual luciferase reporter assay demonstrated the direct interaction between miR-519 and HOXA-AS2. (E) RT-qPCR analysis of the expression of HOXA-AS2 in SUNE1 and SUNE2 cells transfected with shNC and shHOXA-AS2. (F) RT-qPCR analysis of miR-519 expression in cells transfected with shNC, shHOXA-AS2 and shHOXA-AS2 + miR-519 inhibitor. (G) MTT assay results of viability of cells transfected with shNC, shHOXA-AS2 and shHOXA-AS2 + miR-519 inhibitor at 0, 24, 48 and 72 h. (H) Wound healing assay and quantification demonstrated the migratory abilities of cells transfected with shNC, shHOXA-AS2 and shHOXA-AS2 + miR-519 inhibitor at 0 and 24 h. (I) Transwell invasion assay and quantification of the invasive abilities of cells transfected with shNC, shHOXA-AS2 and shHOXA-AS2 + miR-519 inhibitor. Data are presented as the mean ± SD. *P<0.05. miR, microRNA; NPC, nasopharyngeal carcinoma; HOXA-AS2, HOXA cluster antisense RNA 2; sh, short hairpin RNA; NC, negative control; RT-qPCR, reverse transcription-quantitative PCR; OD, optical density.
starBase was used to identify miRNA-lncRNA interactions, and it was found that there was a complementary binding site between HOXA-AS2 and miR-519, which suggested that miR-519 could bind to the 3′-untranslated region (UTR) of HOXA-AS2 (Fig. 1C). The wild-type and mutant type of HOXA-AS2 were constructed and transfected into 293T cells, and then miR-519 mimics or inhibitor were introduced into 293T cells. Subsequently, a dual luciferase reporter assay was performed to assess these findings. It was demonstrated that the miR-519 failed to bind to the 3′-UTR mutant type of HOXA-AS2, which did not affect luciferase activity. However, in wild-type HOXA-AS2-transfected 293T cells, the luciferase activity was significantly decreased by adding exogenous miR-519 mimics to 293T cells, whereas the luciferase activity was significantly increased by the miR-519 inhibitor (Fig. 1D). Collectively, bioinformatics analysis and dual luciferase reporter assay results suggested that HOXA-AS2 could directly interact with miR-519.
The possible impact of the interaction between HOXA-AS2 and miR-519 on NPC progression was further investigated. The RT-qPCR results demonstrated that HOXA-AS2 expression was significantly downregulated in shHOXA-AS2-transfected SUNE1 and SUNE2 cells compared with sh negative control (shNC; Fig. 1E). In addition, the co-transfection with the miR-519 inhibitor abolished HOXA-AS2 knockdown-mediated promoting effects on the expression of miR-519 in SUNE1 and SUNE2 cells (Fig. 1F). The MTT assay identified that the miR-519 inhibitor partly recovered the cell viability attenuated by shHOXA-AS2 (Fig. 1G). Moreover, co-transfection with miR-519 inhibitor significantly enhanced the migratory and invasive abilities of shHOXA-AS2-transfected SUNE1 and SUNE2 cells, as detected using wound healing and Transwell assays (Fig. 1H and I).
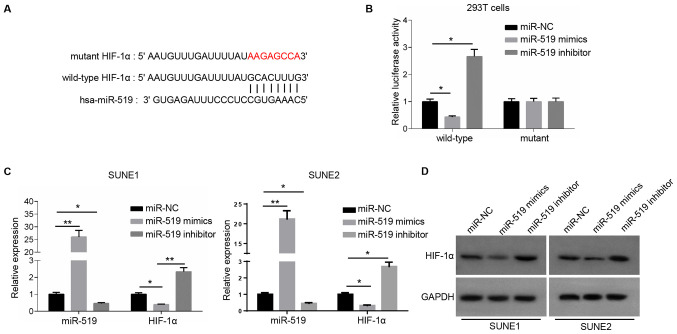
miR-519 directly binds to HIF-1α
It was demonstrated that HOXA-AS2 could directly bind with miR-519 to regulate NPC cancer cell progression. However, further research was required to elucidate the role of their interaction in NPC. Therefore, the potential targets of miR-519 were also detected using starBase and dual luciferase reporter assay to confirm their direct associations. There were a number of potential targets (such as forkhead box Q1, PD-L1 and RCC1 domain-containing protein 1) of miR-519, and it was identified that an important oncogene, HIF-1α, had a complementary binding site with miR-519 (Fig. 2A). miR-519 could not bind to the mutant type of HIF-1α and did not affect the luciferase activity, while the luciferase activity decreased significantly by introducing miR-519 mimics into the wild-type 3′-UTR of HIF-1α-transfected 293T cells compared with miR-NC (Fig. 2B).
Figure 2.
miR-519 directly binds to HIF-1α. (A) Bioinformatics analysis results demonstrating the binding site between miR-519 and HIF-1α. (B) Dual luciferase reporter assay of the luciferase activity of wild-type and mutant 3′-untranslated region of HIF-1α in 293T cells transfected with NC, miR-519 mimics and miR-519 inhibitor. (C) Reverse transcription-quantitative PCR results of miR-519 and HIF-1α expression levels in SUNE1 and SUNE2 cells transfected with NC, miR-519 mimics and miR-519 inhibitor. (D) Western blotting results of the expression of HIF-1α in SUNE1 and SUNE2 cells transfected with NC, miR-519 mimics and miR-519 inhibitor. GAPDH was the loading control. Data are presented as the mean ± SD. *P<0.05 and **P<0.01. miR, microRNA; NC, negative control; HIF-1α, hypoxia-inducible factor-1α.
Subsequently, the expression of miR-519 was significantly increased or decreased in NPC cells transfected with miR-519 mimics or miR-519 inhibitor (Fig. 2C). RT-qPCR analysis and western blotting were used to examine HIF-1α expression. The results indicated that the mRNA and protein expression levels of HIF-1α were significantly lower in miR-519 mimics-transfected NPC cells, and significantly higher in miR-519 inhibitor-transfected NPC cells compared with the miR-NC (Fig. 2C and D). Collectively, these results suggested that miR-519 was able to directly bind to HIF-1α and significantly inhibit its expression.
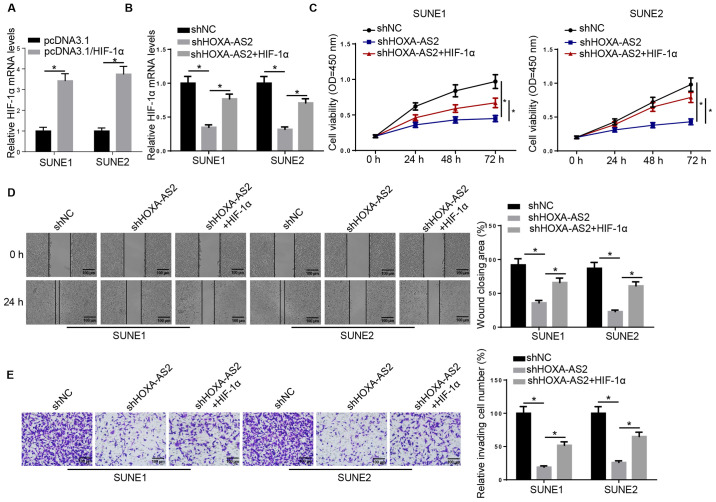
HIF-1α overexpression partially recovers HOXA-AS2-regulated NPC progression
Next, it was examined whether HOXA-AS2 can exert its oncogenic effects on NPC progression via the miR-519/HIF-1α axis. RT-qPCR results demonstrated that the expression of HIF-1α was significantly increased in SUNE1 and SUNE2 cells transfected with HIF-1α overexpression plasmid (Fig. 3A). HIF-1α overexpression plasmid was introduced into shHOXA-AS2-expressing SUNE1 and SUNE2 cells and the results indicated that HIF-1α overexpression abolished the inhibitory effect of HOXA-AS2 knockdown on the expression of HIF-1α (Fig. 3B). The MTT assay demonstrated that overexpression of HIF-1α (shHOXA-AS2 + HIF-1α) was able to partially recover the cell proliferative ability attenuated by shHOXA-AS2 alone (Fig. 3C). It was further indicated that overexpression of HIF-1α in shHOXA-AS2-transfected cells enhanced cell migratory and invasive abilities, which were significantly decreased by shHOXA-AS2 alone (Fig. 3D and E). Therefore, HIF-1α may act as a potential downstream effector for HOXA-AS2/miR-519 in NPC cells.
Figure 3.
HIF-1α overexpression partially recovers HOXA-AS2-regulated nasopharyngeal carcinoma progression. (A) RT-qPCR analysis demonstrated the expression of HIF-1α in SUNE1 and SUNE2 cells transfected with pcDNA3.1 and pcDNA3.1/HIF-1α, or (B) transfected with shNC, shHOXA-AS2 and shHOXA-AS2 + HIF-1α. (C) MTT assay results demonstrated the cell viability of cells transfected with shNC, shHOXA-AS2 and shHOXA-AS2 + HIF-1α at 0, 24, 48 and 72 h. (D) Wound healing assay and quantification of migratory abilities of cells transfected with shNC, shHOXA-AS2 and shHOXA-AS2 + HIF-1α at 0 and 24 h. (E) Transwell invasion assay and quantification of invasive abilities of cells transfected with shNC, shHOXA-AS2 and shHOXA-AS2 + HIF-1α. Data are presented as the mean ± SD. *P<0.05. HOXA-AS2, HOXA cluster antisense RNA 2; sh, short hairpin RNA; NC, negative control; RT-qPCR, reverse transcription-quantitative PCR; OD, optical density; HIF-1α, hypoxia-inducible factor-1α.
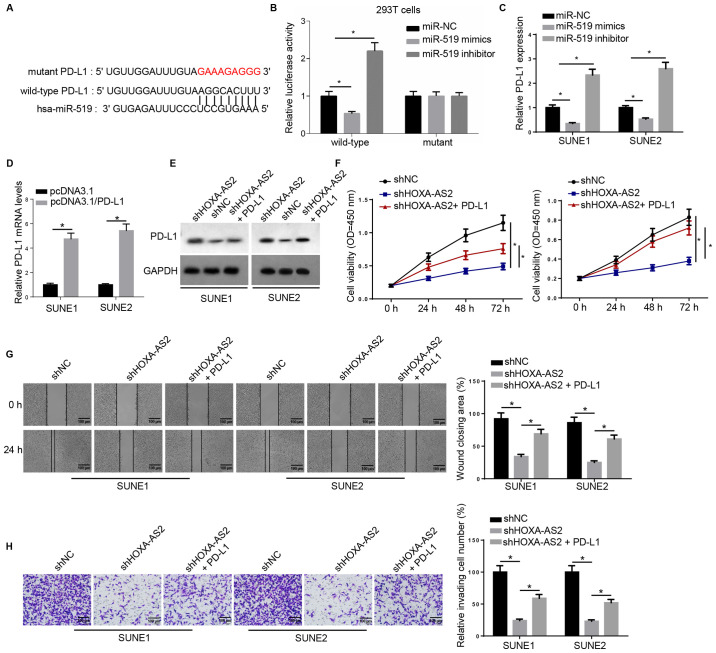
PD-L1 acts as a downstream effector for HOXA-AS2/miR-519 in NPC
miR-519 was also predicted to interact with a key immune checkpoint, PD-L1, using the online program starBase (Fig. 4A). Luciferase activity was significantly downregulated by miR-519 in wild-type 3′-UTR of PD-L1-transfected 293T cells, whereas no significant differences were observed in the relative luciferase activity in mutant PD-L1 (Fig. 4B). Moreover, RT-qPCR was used to examine PD-L1 expression, and found that PD-L1 mRNA expression was negatively regulated by miR-519 (Fig. 4C).
Figure 4.
PD-L1 acts as downstream effector for HOXA-AS2/miR-519 in NPC. (A) Bioinformatics analysis identified the binding site between miR-519 and PD-L1. (B) Dual luciferase reporter assay of the luciferase activity of wild-type and mutant 3′-untranslated region of PD-L1 in 293T cells transfected with NC, miR-519 mimics and miR-519 inhibitor. (C) Reverse transcription-quantitative PCR results of PD-L1 expression in SUNE1 and SUNE2 cells transfected with NC, miR-519 mimics and miR-519 inhibitor, or (D) transfected with pcDNA3.1 and pcDNA3.1/PD-L1. (E) Western blotting results of PD-L1 expression in cells transfected with shNC, shHOXA-AS2 and shHOXA-AS2 + PD-L1. GAPDH was the loading control. (F) MTT assay results of cell viability of cells transfected with shNC, shHOXA-AS2 and shHOXA-AS2 + PD-L1 at 0, 24, 48 and 72 h. (G) Wound healing assay and quantification of migratory abilities of cells transfected with shNC, shHOXA-AS2 and shHOXA-AS2 + PD-L1 at 0 and 24 h. (H) Transwell invasion assay and quantification of invasive abilities of cells transfected with shNC, shHOXA-AS2 and shHOXA-AS2 + PD-L1. Data are presented as the mean ± SD. *P<0.05. HOXA-AS2, HOXA cluster antisense RNA 2; sh, short hairpin RNA; NC, negative control; OD, optical density; PD-L1, programmed death-ligand 1.
RT-qPCR results indicated that the expression of PD-L1 was significantly increased in SUNE1 and SUNE2 cells transfected with PD-L1 overexpression plasmid (Fig. 4D). Western blotting demonstrated that PD-L1 protein expression was markedly decreased in cells with shHOXA-AS2, while this effect was reversed by PD-L1 overexpression (Fig. 4E). Moreover, PD-L1 overexpression significantly promoted the cell proliferative ability that was attenuated by HOXA-AS2 knockdown (Fig. 4F). It was also identified that overexpression of PD-L1 in shHOXA-AS2-transfected NPC cells significantly enhanced cell migration and invasion compared with NPC cells transfected with shHOXA-AS2 alone (Fig. 4G and H). Collectively, these findings suggested that PD-L1 was also a critical downstream biomolecule regulating HOXA-AS2-mediated NPC progression.
Discussion
Continued advances in genomics and transcriptomics have accelerate the discovery and emergence of potential biomarkers of various diseases (25). Furthermore, molecular therapies based on these key genes or pathways are becoming increasingly important in clinical therapy. Compared with other common cancer types, NPC is rare but often difficult to diagnose early (26). While the global incidence of NPC appears to be lower compared with that of other types of cancer, it is more frequently encountered in Asia (27). Considering the limitations of the current research on NPC, it is important to study the molecular mechanisms underlying NPC initiation, progression and metastasis in greater depth.
lncRNAs and miRNAs often exert their oncogenic or tumor-suppressive effects on cancer cells via multiple mechanisms. For example, HOXA-AS2 can promote the progression of breast cancer, osteosarcoma, papillary thyroid cancer and hepatocellular carcinoma by interacting with miR-520c (28–31). In the present study, it was demonstrated that a high expression level of HOXA-AS2 was associated with an increased cell viability, as well as significantly promoted NPC cell migration and invasion; as HOXA-AS2 knockdown reduced these effects.
miRNAs generally function in RNA silencing and post-transcriptional regulation of gene expression, and several miRNAs, such as miR-273, miR-127 and miR-136, are evolutionarily conserved (32). Jalali et al (33) revealed that lncRNAs potentially interact with other classes of non-coding RNAs, including miRNAs, and modulate their regulatory role via interactions. Therefore, in the present study starBase was used to predict the potential targets of HOXA-AS2, and miR-519 was selected. The results identified a direct interaction between HOXA-AS2 and miR-519. Moreover, it was found that overexpression of miR-519 inhibited HOXA-AS2 expression, thus inhibiting NPC progression.
HIF-1α is a well-known functional gene and it has been extensively investigated. HIF-1α was reported to participate in inflammation (34) and is associated with the response to hypoxia (35). It has been previously shown that HIF-1α is important in tumor progression and cancer therapy (36–38). The current results suggested that miR-519 could directly bind to and significantly inhibit the expression of HIF-1α. In addition, the in vitro experiments demonstrated that overexpression of HIF-1α could recover NPC cancer cell progression attenuated by miR-519.
As well as HIF-1α, the immune checkpoint pathway PD-L1/PD-1 was identified to be involved in HOXA-AS2- and miR-519-mediated NPC progression in the present study. Previous studies indicated that abnormal expression of PD-L1 was associated with NPC prognosis and metastasis (20,21). The current findings were in agreement with the aforementioned previous studies. However, considering the complex mechanism involved in tumorigenesis, other potential downstream effectors should be investigated and in vivo experiments must be performed in future studies.
In conclusion, the results of the present study suggested a novel HOXA-AS2/miR-519/HIF-1α axis that may be underlying NPC progression, and provided additional insights into the development of HOXA-AS2-based clinical therapy. However, some limitations remain to be further addressed: Firstly, the lack of microdissection and immunohistochemistry is a weakness of the study. Secondly, whether other miRNAs or downstream effectors are crucial to HOXA-AS2-regulated phenotypes of NPC must be elucidated in future studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
SW and SY designed the present study. SW and HY performed all the experiments, analyzed the data and prepared the figures. SW and SY drafted the initial manuscript. SY reviewed and revised the manuscript. All authors have read and revised the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Zhuji People's Hospital of Zhejiang Province (approval no. 2012A0122003) and written informed consent was obtained from the participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wei KR, Zheng RS, Zhang SW, Liang ZH, Li ZM, Chen WQ. Nasopharyngeal carcinoma incidence and mortality in China, 2013. Chin J Cancer. 2017;36:90. doi: 10.1186/s40880-017-0257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 3.Jia WH, Qin HD. Non-viral environmental risk factors for nasopharyngeal carcinoma: A systematic review. Semin Cancer Biol. 2012;22:117–126. doi: 10.1016/j.semcancer.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Yu MC. Diet and nasopharyngeal carcinoma. FEMS Microbiol Immunol. 1990;2:235–242. doi: 10.1111/j.1574-6968.1990.tb03524.x. [DOI] [PubMed] [Google Scholar]
- 5.Fatica A, Bozzoni I. Long non-coding RNAs: New players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 6.Xie M, Sun M, Zhu YN, Xia R, Liu YW, Ding J, Ma HW, He XZ, Zhang ZH, Liu ZJ, et al. Long noncoding RNA HOXA-AS2 promotes gastric cancer proliferation by epigenetically silencing P21/PLK3/DDIT3 expression. Oncotarget. 2015;6:33587–33601. doi: 10.18632/oncotarget.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong G, Wu X, Cheng B, Li L, Li X, Li Z, Nong Q, Chen X, Liu Y, Wang S. Knockdown of HOXA-AS2 suppresses proliferation and induces apoptosis in colorectal cancer. Am J Transl Res. 2017;9:4545–4552. [PMC free article] [PubMed] [Google Scholar]
- 8.Lian Y, Li Z, Fan Y, Huang Q, Chen J, Liu W, Xiao C, Xu H. The lncRNA-HOXA-AS2/EZH2/LSD1 oncogene complex promotes cell proliferation in pancreatic cancer. Am J Transl Res. 2017;9:5496–5506. [PMC free article] [PubMed] [Google Scholar]
- 9.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 10.Ristimäki A. Tumor suppressor effect of the microRNA miR-519 is mediated via the mRNA-binding protein HuR. Cell Cycle. 2010;9:1234. doi: 10.4161/cc.9.7.11322. [DOI] [PubMed] [Google Scholar]
- 11.Abdelmohsen K, Kim MM, Srikantan S, Mercken EM, Brennan SE, Wilson GM, de Cabo R, Gorospe M. miR-519 suppresses tumor growth by reducing HuR levels. Cell Cycle. 2010;9:1354–1359. doi: 10.4161/cc.9.7.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelmohsen K, Srikantan S, Kuwano Y, Gorospe M. miR-519 reduces cell proliferation by lowering RNA-binding protein HuR levels. Proc Natl Acad Sci USA. 2008;105:20297–20302. doi: 10.1073/pnas.0809376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu G, Zhang T, Jing Y, Bao Q, Tang Q, Zhang Y. miR-519 suppresses nasopharyngeal carcinoma cell proliferation by targeting oncogene URG4/URGCP. Life Sci. 2017;175:47–51. doi: 10.1016/j.lfs.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- 15.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 16.Xie W, Liu L, He H, Yang K. Prognostic value of hypoxia-inducible factor-1 alpha in nasopharyngeal carcinoma: A meta-analysis. Int J Biol Markers. 2018 Jun 10; doi: 10.1177/1724600818778756. (Epub ahead of print). doi: doi.org/10.1177/1724600818778756. [DOI] [PubMed] [Google Scholar]
- 17.Cha ST, Chen PS, Johansson G, Chu CY, Wang MY, Jeng YM, Yu SL, Chen JS, Chang KJ, Jee SH, et al. MicroRNA-519c suppresses hypoxia-inducible factor-1alpha expression and tumor angiogenesis. Cancer Res. 2010;70:2675–2678. doi: 10.1158/0008-5472.CAN-09-2448. [DOI] [PubMed] [Google Scholar]
- 18.Giatromanolaki A, Koukourakis IM, Balaska K, Mitrakas AG, Harris AL, Koukourakis MI. Programmed death-1 receptor (PD-1) and PD-ligand-1 (PD-L1) expression in non-small cell lung cancer and the immune-suppressive effect of anaerobic glycolysis. Med Oncol. 2019;36:76. doi: 10.1007/s12032-019-1299-4. [DOI] [PubMed] [Google Scholar]
- 19.Liang Z, Tian Y, Cai W, Weng Z, Li Y, Zhang H, Bao Y, Li Y. High-affinity human PD-L1 variants attenuate the suppression of T cell activation. Oncotarget. 2017;8:88360–88375. doi: 10.18632/oncotarget.21729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang ZL, Liu S, Wang GN, Zheng SH, Ding SR, Tao YL, Chen C, Liu SR, Yang X, Chang H, et al. The prognostic significance of PD-L1 and PD-1 expression in patients with nasopharyngeal carcinoma: A systematic review and meta-analysis. Cancer Cell Int. 2019;19:141. doi: 10.1186/s12935-019-0863-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao Y, Chan KI, Xiao G, Chen Y, Qiu X, Hao H, Mak SC, Lin T. Expression and clinical significance of PD-L1 and BRAF expression in nasopharyngeal carcinoma. BMC Cancer. 2019;19:1022. doi: 10.1186/s12885-019-6276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Shi D, Miao J, Wu H, Chen J, Zhou X, Hu D, Zhao C, Deng W, Xie C. PD-L1 predicts poor prognosis for nasopharyngeal carcinoma irrespective of PD-1 and EBV-DNA load. Sci Rep. 2017;7:43627. doi: 10.1038/srep43627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fei Z, Deng Z, Zhou L, Li K, Xia X, Xie R. PD-L1 induces epithelial-mesenchymal transition in nasopharyngeal carcinoma cells through activation of the PI3K/AKT pathway. Oncol Res. 2019;27:801–807. doi: 10.3727/096504018X15446984186056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Ilyas M. Next-generation sequencing in diagnostic pathology. Pathobiology. 2017;84:292–305. doi: 10.1159/000480089. [DOI] [PubMed] [Google Scholar]
- 26.Luftig M. Heavy LIFting: Tumor promotion and radioresistance in NPC. J Clin Invest. 2013;123:4999–5001. doi: 10.1172/JCI73416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 28.Fang Y, Wang J, Wu F, Song Y, Zhao S, Zhang Q. Long non-coding RNA HOXA-AS2 promotes proliferation and invasion of breast cancer by acting as a miR-520c-3p sponge. Oncotarget. 2017;8:46090–46103. doi: 10.18632/oncotarget.17552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Zhang R, Cheng G, Xu R, Han X. Long non-coding RNA HOXA-AS2 promotes migration and invasion by acting as a ceRNA of miR-520c-3p in osteosarcoma cells. Cell Cycle. 2018;17:1637–1648. doi: 10.1080/15384101.2018.1489174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia F, Chen Y, Jiang B, Du X, Peng Y, Wang W, Huang W, Feng T, Li X. Long noncoding RNA HOXA-AS2 promotes papillary thyroid cancer progression by regulating miR-520c-3p/S100A4 pathway. Cell Physiol Biochem. 2018;50:1659–1672. doi: 10.1159/000494786. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Xu J, Zhang S, An J, Zhang J, Huang J, Jin Y. HOXA-AS2 promotes proliferation and induces epithelial-mesenchymal transition via the miR-520c-3p/GPC3 axis in hepatocellular carcinoma. Cell Physiol Biochem. 2018;50:2124–2138. doi: 10.1159/000495056. [DOI] [PubMed] [Google Scholar]
- 32.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 33.Jalali S, Bhartiya D, Lalwani MK, Sivasubbu S, Scaria V. Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PLoS One. 2013;8:e53823. doi: 10.1371/journal.pone.0053823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/S0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaGory EL, Giaccia AJ. The ever-expanding role of HIF in tumour and stromal biology. Nat Cell Biol. 2016;18:356–365. doi: 10.1038/ncb3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unwith S, Zhao H, Hennah L, Ma D. The potential role of HIF on tumour progression and dissemination. Int J Cancer. 2015;136:2491–2503. doi: 10.1002/ijc.28889. [DOI] [PubMed] [Google Scholar]
- 38.Yu T, Tang B, Sun X. Development of inhibitors targeting hypoxia-inducible factor 1 and 2 for cancer therapy. Yonsei Med J. 2017;58:489–496. doi: 10.3349/ymj.2017.58.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.