Abstract
Objectives
To evaluate the long-term efficacy and safety of canakinumab to treat patients with colchicine-resistant familial Mediterranean fever (crFMF) during Epoch 4 (weeks 41 to 113) of the CLUSTER study.
Methods
Patients received open-label canakinumab 150 or 300 mg, every 4 or 8 weeks during a 72-week period. We evaluated disease activity every 8 weeks using the physician global assessment (PGA) of disease activity, counting the number of flares, and measuring concentrations of C reactive protein (CRP) and serum amyloid A (SAA). Safety was studied by determination and classification of observed adverse events (AEs). We analysed safety and efficacy separately in two subgroups of patients receiving a cumulative dose of less than 2700 mg, or equal or more than 2700 mg.
Results
Of the 61 patients that started the CLUSTER study, 60 entered Epoch 4 and 57 completed it. During the 72-week period, 35/60 (58.3%) patients experienced no flares, and 23/60 (38.3%) had one flare, as compared with a median of 17.5 flares per year reported at baseline. PGA scores indicated no disease activity for the majority of patients throughout the study. Median CRP concentrations were always lower than 10 mg/L, while median SAA concentrations remained over the limit of normal (10 mg/L) but under the 30 mg/L threshold. No new or unexpected AEs were reported.
Conclusion
crFMF patients treated with canakinumab during 72 weeks experienced a minimal incidence of flares and good control of clinical disease activity, with no new safety concerns reported.
Keywords: familial mediterranean fever, autoimmune diseases, cytokines, treatment
Key messages.
What is already known about this subject?
Colchicine is the cornerstone of current therapy for familial Mediterranean fever (FMF); however, a subset of patients are resistant or intolerant to it.
Canakinumab, a therapeutic anti-interleukin-1β monoclonal antibody, is effective in controlling and preventing flares in patients with colchicine-resistant FMF (crFMF).
What does this study add?
This study evaluated the long-term efficacy and safety of canakinumab to treat patients with crFMF during a 72-week period, with dose regimens adjusted individually. The results show that patients treated with canakinumab had a good control of disease, with low incidence of flares and median C reactive protein serum values in the normal range throughout the study, whereas median serum amyloid A levels remained over the limit of normal (10 mg/L) but under the 30 mg/L threshold.
No new or unexpected adverse events were reported, and no apparent correlation between their occurrence and the dose regimen was observed.
How might this impact on clinical practice or future developments?
These results underscore the potential of canakinumab as a long-term therapy for patients with crFMF, and show that individual dose adjustment can be important for optimising its therapeutic effect.
Introduction
Familial Mediterranean fever (FMF) is an autoinflammatory hereditary disease characterised by recurrent attacks of fever and serositis (peritonitis, pleuritis and/or acute synovitis), with increased blood concentrations of acute phase reactants, including C reactive protein (CRP) and serum amyloid A protein (SAA).1 2 Renal secondary amyloidosis is the major complication of FMF, leading to end-stage renal disease.2–4
FMF is associated with the presence of pathogenic mutations in the MEFV gene which encodes pyrin, a protein expressed in cells of the innate immune system.5 6 These mutations lead to excessive activation of the pyrin inflammasome with subsequent release of large amounts of interleukin 1 beta (IL-1β).7 Dysregulated IL-1β plays a pivotal role in the pathogenesis of FMF.8
According to the current European League Against Rheumatism (EULAR) recommendations, the aim of FMF treatment is to control acute attacks, minimise chronic subclinical inflammation and its sequelae, mainly secondary amyloidosis, and improve the patient’s quality of life (QoL).3 9 Colchicine is the cornerstone of current therapy for FMF; its regular use prevents attacks, suppresses chronic subclinical inflammation, prevents amyloidosis and improves QoL.3 10 However, a subset of patients fail to respond, or are intolerant to colchicine. Several studies have shown that IL-1β inhibition improves clinical and laboratory features in patients with colchicine-resistant FMF (crFMF).11–15 Results up to week 40 of the phase III CLUSTER trial (NCT02059291) demonstrated that canakinumab, a fully human anti-IL-1β monoclonal antibody, was effective to control inflammation and prevent flares in patients with crFMF.16 Here we report results from Epoch 4, a 72-week period of open-label treatment designed to study the long-term safety and efficacy of canakinumab in patients with crFMF.
Methods
Study design
The CLUSTER study (NCT02059291, https://clinicaltrials.gov/) evaluated the efficacy and safety of canakinumab in patients with three recurrent fever syndromes: crFMF, mevalonate kinase deficiency (also known as the hyperimmunoglobulinaemia D syndrome) and the tumour necrosis factor receptor-associated periodic syndrome. It included three cohorts of patients, one per condition, and each cohort followed the same study design, as previously reported.16 The CLUSTER study was divided in four epochs: a screening period of up to 12 weeks (Epoch 1), a randomised, double-blind, placebo-controlled period of 16 weeks (Epoch 2), a randomised withdrawal, open-label period of 24 weeks (Epoch 3), and an open-label treatment period of 72 weeks (Epoch 4). In this article, we report results of patients with crFMF in Epoch 4 (weeks 41 to 113 of the trial).
At the start of Epoch 3, a proportion of the patients were randomised 1:1 to receive either canakinumab 150 mg or placebo every 8 weeks (q8w), and the rest were treated with open-label canakinumab (150 mg or 300 mg every 4 weeks (q4w)). Patients experiencing a flare (defined as physician global assessment (PGA) score ≥2 and CRP serum levels>30 mg/L) were eligible to start or up-titrate canakinumab up to 300 mg q4w. Thus, at the end of Epoch 3, patients were receiving either placebo q8w, canakinumab 150 mg (q4w or q8w) or canakinumab 300 mg (q4w or q8w).
Patients who completed Epoch 3 on placebo entered Epoch 4 and attended scheduled visits but did not receive canakinumab unless they experienced a flare, in which case they started open-label treatment with canakinumab 150 mg q8w. All other patients entering Epoch 4 continued on the same canakinumab regimen they were receiving at the end of Epoch 3. If patients experienced a flare during Epoch 4, stepwise up-titration (ie, 150 mg q8w to 150 mg q4w to 300 mg q4w) was allowed (maximum 300 mg q4w). Down-titration was not allowed in Epoch 4. During the whole study, doses were adjusted by weight in patients with body weight lower than 40 kg, who received canakinumab at either 2 mg/kg (instead of 150 mg) or 4 mg/kg (instead of 300 mg). Most patients (58/61) were receiving colchicine treatment at study entry, and they were instructed to continue this treatment at a stable dose during the trial. Overall, seven patients did not take colchicine during Epoch 4, reported reasons were lack of efficacy (two patients), lack of tolerability (one patient) and not known (four patients). Sixty-two centres in 16 countries participated in the study. The institutional review board or independent ethics committee at each centre approved the study. Patients or guardians, as appropriate, provided written informed consent.
Objectives
The primary objective of the study was to demonstrate that canakinumab treatment at a dose of 150 mg q4w is superior to placebo in achieving a clinically meaningful reduction of disease activity, defined as resolution of the baseline flare at day 15 and no new disease flare over 16 weeks of treatment (end of Epoch 2). This primary endpoint was met for the three cohorts of patients, and results have been previously reported.16 Secondary objectives for Epoch 4 were to evaluate the long-term safety and tolerability of canakinumab, and exploratory objectives included the evaluation of long-term efficacy by assessing the number of flares per patient, the PGA of disease activity and the analysis of CRP and SAA serum levels over time.
Patients
The detailed inclusion and exclusion criteria for patients in the CLUSTER study have been reported previously.16 Eligible patients with crFMF had a diagnosis of FMF according to Tel Hashomer criteria17 and resistance or intolerance to colchicine. Patients were considered resistant to colchicine if they had historical data documenting ≥1 flare/month despite effective doses of colchicine (from 1.5 mg to 3.0 mg/day or equivalent paediatric age/weight-adjusted regimen). Patients were not involved in the design or conduct of the study, development of outcomes or dissemination of study results.
Assessments
Visits to assess efficacy and safety were scheduled at 8-week intervals. PGA of disease activity was performed by the investigator as previously reported.16 CRP and SAA were measured at the local and central laboratories. A new disease flare was defined as PGA ≥2 and CRP ≥30 mg/L.
Safety assessments consisted of collecting all adverse events (AEs) with their severity, and the regular monitoring of haematology, blood chemistry (including creatinine clearance), vital signs and body weight.
Statistical analysis
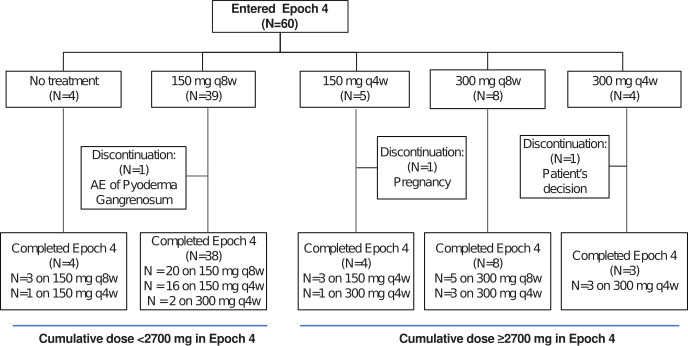
The safety set for Epoch 4 was used for all safety and efficacy analyses, and consisted of all patients with crFMF who received study treatment in Epoch 4 and had ≥1 post-baseline safety assessment. Efficacy and safety data were analysed in two groups of patients classified according to the cumulative dose received over 72 weeks in Epoch 4, <2700 mg, and ≥2700 mg. The cut-off was chosen because 2700 mg was the minimal cumulative dose received by patients starting Epoch 4 with intermediate dose regimens (150 mg q4w or 300 mg q8w). All patients included in the <2700 mg group started Epoch 4 on the lower dose regimen (150 mg q8w) or without treatment (figure 1). Descriptive statistics were used to summarise baseline demographics and disease characteristics, and for presenting efficacy results. The association between baseline characteristics and the requirement for increased doses of canakinumab was assessed using the Kruskal-Wallis and the Mann-Whitney U tests, with patients<40 kg being excluded as they received weight-adjusted doses.
Figure 1.
Patient flow diagram showing treatment regimens at the beginning and the end of Epoch 4. AE, adverse event; N, number of patients; q4w, every 4 weeks; q8w, every 8 weeks.
Results
Patient disposition and baseline characteristics
Of the 61 crFMF patients who were enrolled in the CLUSTER trial,16 60 entered Epoch 4, and 57 completed the study. Figure 1 shows the patient disposition with the treatment regimens received in a flow diagram. Three patients (5%) discontinued the study in Epoch 4, one of them due to his own decision, another due to pregnancy, and a third one due to an AE of pyoderma gangrenosum. Overall, 44 patients received <2700 mg canakinumab and 16 received ≥2700 mg.
Demographics and disease characteristics at baseline (ie, when patients with an active flare were randomised to canakinumab or placebo) are presented in table 1. The patient population had a median age of 18 years, with three (5%) patients<6 years old. Fourteen (23%) patients had body weight lower than 40 kg and therefore received weight-adjusted doses as described in the Methods section. Twenty-seven per cent of patients were previously treated with biologics, mainly anakinra. Patients had frequent flares before entering the trial, with a median of 17.5 flares per year.
Table 1.
Baseline demographic and disease characteristics (safety set)*
| Characteristics | Patients (n=60) |
| Median age, years (Q1, Q3) | 18.0 (14.0 to 29.5) |
| Female, n (%) | 28 (46.7) |
| Caucasian, n (%) | 49 (81.7) |
| Median duration of disease, years (Q1, Q3) | 13.8 (9.3 to 24.2) |
| Median number of flares per year (Q1, Q3) | 17.5 (12.0 to 27.5) |
| Active disease at maximum colchicine dose, n (%) | 59 (98.3) |
| CRP (mg/L), median (Q1, Q3) | 102 (56.7 to 202.6) |
| SAA (mg/L), median (Q1, Q3) | 618 (265.5 to 1266.0) |
| PGA score (disease activity), n (%) | |
| 0 (None) | 0 |
| 1 (Minimal) | 0 |
| 2 (Mild) | 9 (15.0) |
| 3 (Moderate) | 34 (56.7) |
| 4 (Severe) | 17 (28.3) |
| Arthralgia/arthritis, n (%) | |
| None | 21 (35.0) |
| Minimal | 8 (13.3) |
| Mild | 12 (20.0) |
| Moderate | 16 (26.7) |
| Severe | 3 (5.0) |
| MEFV genotype, n (%) | |
| M694V/M694V | 42 (70.0) |
| M694V/M694I | 3 (5.0) |
| Other genotypes with mutations in exon 10 | 13 (21.7) |
| No mutations in exon 10† | 2 (3.3) |
| Prior use of biologics, n (%) | 16 (26.7) |
| Anakinra | 15 (25.0) |
*Baseline characteristics at study entry (day 0) for crFMF patients of the safety set of Epoch 4 (from week 41 to week 113). As described in the methods, active disease was an eligibility criterion to enter the study.
†Two Japanese patients with no mutations in exon 10 were included in the CLUSTER trial, but not randomised in Epoch 2 and treated with open-label canakinumab. These patients were included in all analyses of the safety population in Epoch 4.
crFMF, colchicine-resistant familial Mediterranean fever; CRP, C-reactive protein; n, number of patients; PGA, physician global assessment; SAA, serum amyloid A.
Control of disease activity
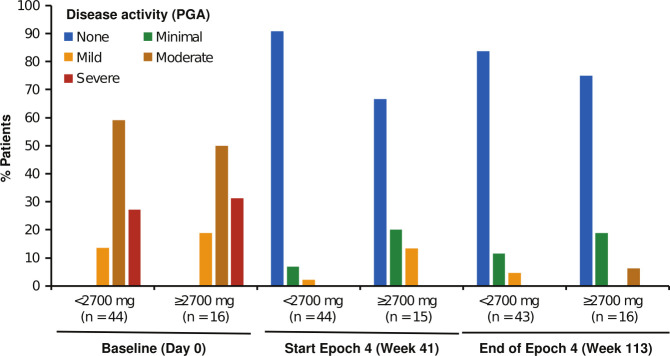
During the 72-week treatment period of Epoch 4, the majority of the patients with crFMF had either no flares (35/60, 58%) or a single flare (23/60, 38%), with two and three flares reported by one patient each, and a median of zero flares per year. The incidence of flares was similar in the two cumulative dose groups, with no flares reported by 26/44 (59%) patients in the <2700 mg group and 9/16 (57%) patients in the ≥2700 mg group. Low PGA scores were maintained throughout Epoch 4 in most patients (figure 2), with more than 90% of patients showing no or minimal disease activity at study end. PGA scores were not apparently higher in the group receiving ≥2700 mg, indicating that good control of disease could be achieved in patients requiring higher doses of canakinumab. Of note, 6/7 patients who did not receive colchicine during Epoch 4 reported no disease flares, and their PGA indicated no (5/7) or minimal (2/7) disease activity at study end.
Figure 2.
Disease activity as measured by PGA over time. Percentages of patients were calculated using as denominator the total number of patients evaluated at each time point (n), for the groups of patients who received cumulative doses <2700 mg (n=44) and ≥2700 mg (n=16) during Epoch 4. PGA, physician’s global assessment.
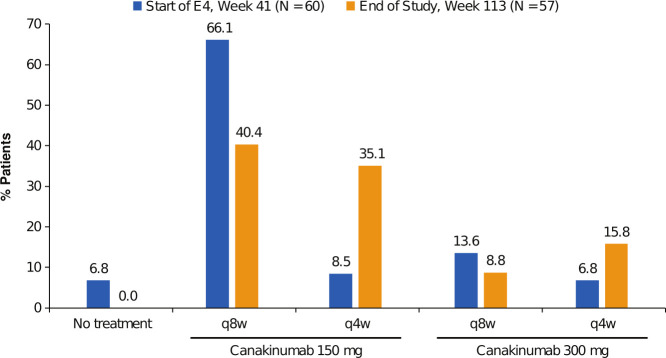
To achieve good control of disease activity, the dosing of canakinumab during Epoch 4 could be increased as described in the Methods section. Figure 3 shows the proportion of patients treated with the different dose regimens at the beginning and at the end of the 72-week period. Most patients entered Epoch 4 on the lower dose regimen of 150 mg q8w (70%), and in many patients (40%), this regimen was sufficient to control disease activity until study end. A similar proportion of patients (44%) received intermediate dose regimens (150 mg q4w or 300 mg q8w) to control disease activity by the end of study, whereas up-titration to the highest dose regimen 300 mg q4w was required in 16% of the patients. All four patients who started the 72-week period with no canakinumab treatment, experienced a flare and started canakinumab during the study. Concerning patients not treated with colchicine, 3/7 remained in the lower 150 mg q8w dose until study end, and one required the maximum 300 mg q4w dose.
Figure 3.
Distribution of canakinumab treatment regimens at the beginning and end of Epoch 4. Patients that entered Epoch 4 continued the same canakinumab regimen that they were receiving when completed Epoch 3, and patients that experienced a flare of disease were eligible for stepwise up-titration (ie, 150 mg q8w to 150 mg q4w to 300 mg q4w) to a maximum regimen of 300 mg q4w. E4, Epoch 4; N, number of patients; q4w, every 4 weeks; q8w, every 8 weeks.
We found no associations between the requirement of high doses of canakinumab and the type of mutation in the MEFV gene, or the duration of disease reported at baseline. A direct association was observed between patient’s body weight and the need for higher doses of canakinumab (p<0.01). Five of 13 patients over 73 kg vs 1/30 patients under 73 kg required the highest 300 mg q4w dose (p=0.01).
Analysis of CRP and SAA blood concentrations
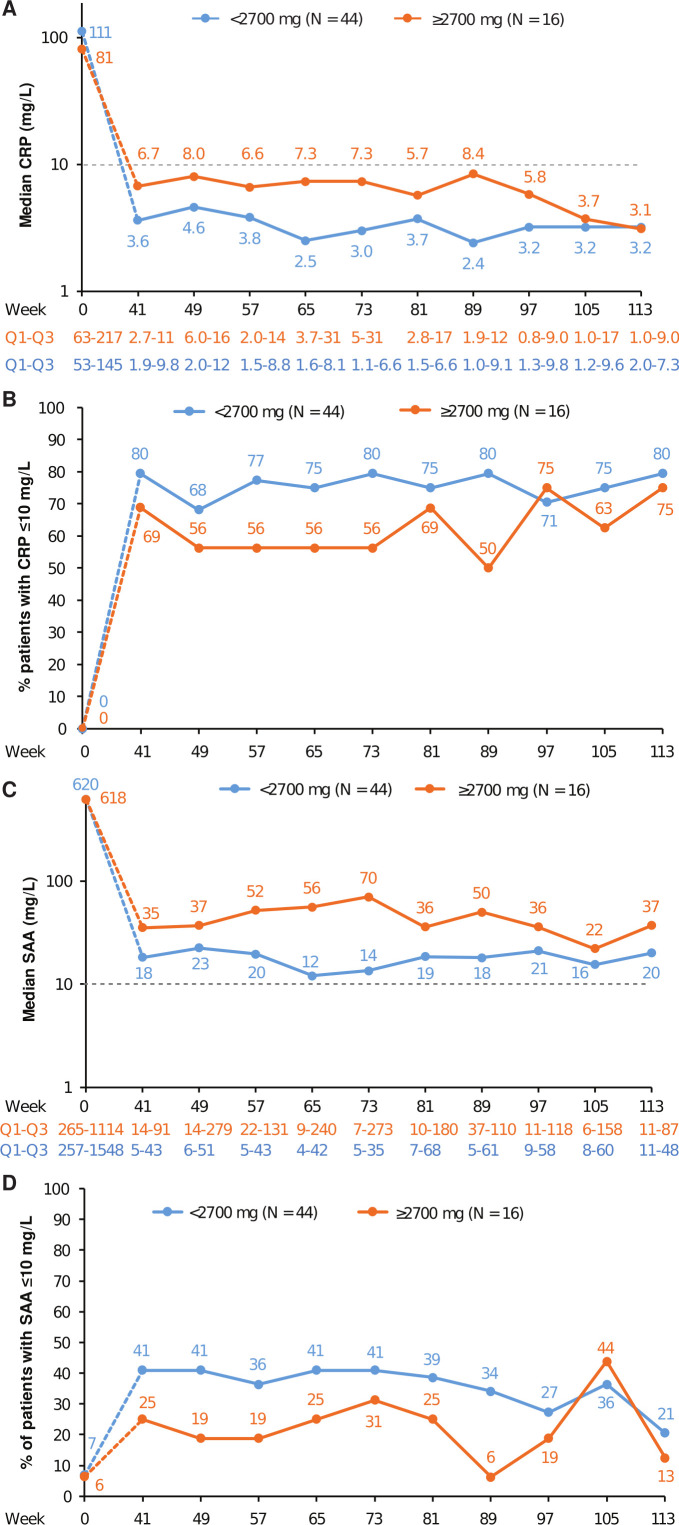
In Epoch 2, a rapid decrease of CRP serum levels from baseline was observed in patients treated with canakinumab.16 During Epoch 4, low levels of CRP were generally maintained for 72 weeks, with median CRP concentrations lower than 10 mg for all measurements between week 41 and week 113 (figure 4A). Of note, median CRP values seemed slightly higher during most of Epoch 4 in the group of patients receiving higher cumulative doses of canakinumab, but values became very similar during the last 16 weeks of the study. As depicted in figure 4B, most patients in both cumulative dose groups presented with CRP concentrations lower than 10 mg/L throughout Epoch 4.
Figure 4.
CRP and SAA blood levels in crFMF patients over time. (A) Median CRP concentrations. The Y-axis is presented using a logarithmic scale and the dashed line indicates the upper limit of normal value (10 mg/L). The table under the graphic presents the IQR (Q1 to Q3) for each time point. The number of patients with data available for each time point (n) ranged from 41 to 44 (<2700 mg group) and from 15 to 16 (≥2700 mg group). For the overall population, median values remained under the 30 mg/L threshold throughout the study (data not shown). Of note, high baseline values were expected since active disease was an eligibility criterion to enter the study. (B) Proportion of patients with CRP concentrations ≤10 mg/L. Percentages of patients with CRP concentration in the normal range were calculated using as denominator the number of patients for whom measurements were available at each time point (n), which ranged as described for figure 4A. (C) Median SAA levels. SAA median concentrations presented as for figure 4A, N varied from 41 to 44 for the <2700 mg dose group and from 13 to 16 for the ≥2700 mg dose group. (D) Proportion of patients with SAA level ≤10 mg/L. Percentages of patients with SAA under the limit of normal presented as in figure 4B, with N ranging as in figure 4C. crFMF, colchicine-resistant familial Mediterranean fever; CRP, C-reactive protein; SAA, serum amyloid A.
Median SAA levels observed throughout Epoch 4 remained over the 10 mg/L limit of normal, but under the 30 mg/L threshold. Median SAA values were different in the two cumulative dose groups (figure 4C), they ranged from 12 to 23 mg/L in the <2700 mg group and mostly from 36 to 56 mg/L in the ≥2700 mg group. Percentages of patients with values under the 10 mg/L limit seemed to be higher in the <2700 mg group than in the ≥2700 mg group (figure 4D). Considering the nine visits in Epoch 4, three or more SAA measurements with values higher than 50 and 70 mg/L were reported in 25/60 (42%) and 18/60 (30%) patients, respectively. These proportions did not appear to be different in the few patients not treated with colchicine (3/7 and 2/7 patients, respectively). Of note, most patients (51/60) had normal renal function at study baseline, as measured by creatinine clearance (chronic kidney disease stage 1: >90 mL/min/1.73 m2). The mean and median values of creatinine clearance for these patients remained normal at every time point of the study, with no relevant differences between the two cumulative dose groups. In the nine patients with decreased creatinine clearance, no clear trend to improvement or worsening of renal function was observed (data not shown).
Safety
The median duration of exposure to canakinumab in Epoch 4 was 511.5 days, and the total exposure was 79.4 patients-years. The exposure-adjusted rate of AEs was 1.53 per 100 patient-days (table 2), and it appeared to be higher in the ≥2700 mg group (1.92) than in the <2700 mg group (1.38). The majority of AEs (90.6%) were mild to moderate in severity. Some common AEs were related to symptoms of FMF flares, including abdominal pain and fever, and were reported more frequently in the ≥2700 mg group, contributing to the difference in the overall rate between the two groups. The most frequently reported system organ class was infections and infestations, with events observed in 70% of patients, and upper respiratory tract infection was the most frequently reported preferred term in this class (15% of patients). Grade 1 neutropaenia was reported in three (5.2%) patients, grade 2 in four patients (6.9%), and there were no cases of grade 3 or higher. One event (pyoderma gangrenosum) led to discontinuation of treatment, it occurred in one of the two Japanese patients with no mutations in exon 10 involved in the study, and it was considered of moderate severity and not related to canakinumab by the investigator. Twenty-three serious AEs (SAEs) were reported in 13 (21.7%) patients, seven of which were considered probably related to FMF flares. SAEs were reported in 11 (25%) patients in the <2700 mg group (including four patients with events probably related to FMF) and in two (12%, with no events probably related to FMF) in the ≥2700 mg group. There were six cases of serious infections (one each: acute sinusitis, cellulitis, gastroenteritis, infectious colitis, peritonitis and urinary tract infection), five of which were observed in the <2700 group. No opportunistic infections, no complications related to amyloidosis and no deaths were reported during the study.
Table 2.
Exposure-adjusted incidence of AEs per 100 pt-days, and total number of AEs in Epoch 4 (safety set)
| Cumulative dose <2700 mg (n=44) Exp.=20 619 pt-days* Event rate (n)† |
Cumulative dose >2700 mg (n=16) Exp.=8375 pt-days Event rate (n) |
Total (n=60) Exp.=28 994 pt-days Event rate (n) |
|
| Total AEs | 1.38 (297) | 1.92 (161) | 1.53 (458) |
| Total AEs excluding FMF and fever | 1.21 (262) | 1.50 (126) | 1.30 (388) |
| Infections | 0.37 (79) | 0.33 (28) | 0.36 (107) |
| Most common AEs‡ | |||
| FMF§ | 0.12 (26) | 0.36 (30) | 0.19 (56) |
| Abdominal pain | 0.03 (6) | 0.12 (10) | 0.06 (16) |
| Headache | 0.06 (13) | 0.06 (5) | 0.06 (18) |
| Back pain | 0.03 (7) | 0.05 (4) | 0.04 (11) |
| URTI¶ | 0.04 (9) | 0.08 (7) | 0.05 (16) |
| Fever | 0.04 (9) | 0.06 (5) | 0.05 (14) |
| Arthralgia | 0.04 (9) | 0.06 (5) | 0.05 (14) |
| SAEs | 0.09 (20) | 0.04 (3) | 0.08 (23) |
| Serious infections | 0.02 (5) | 0.001 (1) | 0.02 (6) |
| AEs leading to discontinuation | <0.01 (1) | 0.0 (0) | <0.01 (1) |
*Exp. to canakinumab in each group, in pt-days.
†n: total number of events in the 72-week period.
‡AEs (preferred term) occurring in ≥15% of the total population.
§Some cases of FMF flares were reported as AEs using this preferred term.
¶URTI, upper respiratory tract infection.
AE, adverse event; exp., exposure; FMF, familial Mediterranean fever; pt-days, patient days; SAE, serious AEs.
Discussion
Previously reported results up to week 40 of the CLUSTER study demonstrated that canakinumab is effective in controlling flares in patients with crFMF.16 Here, we report results from week 40, showing that continuous treatment with canakinumab allows crFMF patients to maintain long-term control of clinical symptoms. This was most clearly illustrated by the fact that >90% of the patients experienced no flares or one flare throughout the 72-week period, while a median of 17.5 flares per year was reported before baseline. To reach control of disease, the dosage of canakinumab was individually adapted during the study, and results showed that required doses varied between individuals. Elevated body weight was directly associated with the requirement of high doses of canakinumab, suggesting that adjustment of the regimen according to body weight may be important to achieve good control of disease activity. These findings should be taken into account when choosing individual canakinumab dosing in clinical practice. In any case, PGA and other assessments of disease activity showed that patients requiring high doses of canakinumab could reach similar control of symptoms as those needing lower doses. Of note, all four patients who started Epoch 4 with no canakinumab treatment experienced flares, suggesting that continuous treatment is required for sustained control of disease symptoms.
Median serum CRP levels remained low during the whole treatment period, also indicating a positive effect of treatment on the subclinical inflammation observed in FMF patients between flares. Median SAA levels were moderately higher than the limit of normal, particularly in those patients who required higher doses of canakinumab. It can be speculated that the use of even higher doses of the drug may help to achieve normalisation of SAA levels, but this would require further investigations. Of note, most patients recruited in the study (58/60) carry exon 10 high-penetrance mutations, known to be associated with severe disease. We observed no substantial changes in median creatinine clearance values during the trial. Currently, it is not known to what extent canakinumab treatment can prevent amyloidosis and renal complications.
The results of this study support IL-1β inhibition with canakinumab as a valid therapeutic option for patients with FMF with an inadequate response to colchicine. Colchicine compliance should be taken into account when assessing resistance to colchicine, since poor colchicine compliance is frequent and may lead to inadequate responses.3 It is essential to reduce the frequency of FMF attacks in patients who do not respond to colchicine to avoid complications of persistent inflammation, including renal failure due to secondary amyloidosis.9 18 However, since the effect of canakinumab on amyloidosis is unknown, colchicine treatment is to be continued in patients treated with canakinumab. This needs to be clearly communicated to patients, since the absence of attacks may increase the risk of reduced adherence to colchicine treatment. This study included seven patients with crFMF that were not receiving colchicine in Epoch 4. Only one of them reported a flare, and no apparent differences with the rest of the patients were observed for any of the clinical parameters studied, although the small number of patients precludes any firm conclusions.
There were no new or unexpected safety findings in Epoch 4. Overall, no association between the occurrence of serious infections, or other SAEs, and increased cumulative canakinumab doses was apparent.
Limitations of the present study include the open-label nature of canakinumab treatment, the limited number of patients involved and the absence of a control group of patients not treated with canakinumab. In addition, a more standardised definition of inactive disease would be helpful to better define the target of canakinumab treatment in crFMF. In conclusion, the results of this study showed that patients with crFMF treated with canakinumab at doses ranging from 150 mg q8w to 300 mg q4w maintained a good control of clinical disease activity with highly reduced occurrence of flares during a 72-week period, and underscore the potential of canakinumab as a long-term therapy for these patients.
Acknowledgments
The authors thank all additional investigators for their participation in the study: Michel Moutschen, Anne-Sophie Sauvage, Jean-Baptiste Giot, Gilles Darcis, Liege, Belgium; Bernard Lauwerys, Benedicte Brichard, Cecile Boulanger, Gabriel Levy, Bruxelles, Belgium; Liesbet Henckaerts, Daniel Knockaert, Leuven, Belgium; Jeroen Van Der Hilst, Peter Messiaen, Hasselt, Belgium; Susanne Benseler, Paivi Miettunen, Nicole Johnson, Nadia Luca, Heinrike Schmeling, Calgary, Canada; Lori Tucker, Kristin Houghton, Kimberly Morishita, Vancouver, Canada; Pierre Quartier, Ouafa Ben-Brahim, Candice Meyzer, Richard Mouy, Michaela Semeraro, Brigitte Bader-Meunier, Agnes Mogenet, Bernard Lacour, Paris, France; Perrine Dusser, Mariam Piram, Linda Rossi, Madeleine Fénéant-Thibault, Le Kremlin Bicetre, France; Alexandre Belot, Marie Caroline Chastang, Agnes Duquesnes, Caroline Freychet, Audrey Laurent, Marine Desjonqueres, Bron Cedex, France; Tu-Anh Tran, Nimes Cedex, France; Tilmann Kallinich, Kirsten Minden, Mareike Lieber, Anna Raab, Gonza Ngoumou, Anne Sae Lim von Stuckrad, Berlin, Germany; Jasmin Kuemmerle-Deschner, Nikolaus Rieber, Sandra Hansmann, Vanya Icheva, Renate Kaulitz, Martin Ebinger, Joachim Riethmuller, Tom Schleich, Ines Maria Magunia, Nicole Anders, Tübingen, Germany; Barbara Willig, Antonia Kienast, Hamburg, Germany; Eugen Feist, Claudia Kedor, Berlin, Germany; Hendrik Schulze-Koops, Matthias Witt, Jan Leipe, Matthias Grünke, Daniel Teupser, Myriam Liz Grana, München, Germany; Gerd Horneff, Tilman Geikowski, Stefanie Wintrich, Joachim Peitz, Carina Schultz, M. Georg Just, St. Augustin, Germany; Annette Jansson, Veit Grote, Sabine Greil, Fabienne Faber, Julia Birnbaum, Daniel Teupser, Munchen, Germany; Elisabeth Weissbarth-Riedel, Anja Froehlich, Hamburg, Germany; Ulrich Neudorf, Elke Lainka, Frauke Hamsen, Essen, Germany; Tamas Constantin, Diana Garan, Andrea Ponyi, Viktoria Kemeny, Budapest, Hungary; Ilonka Orban, Krisztina Sevcic, Budapest, Hungary; Vincent Tormey, Galway, Ireland; Avi Livneh, Ilan Ben Zvi, Shay Padeh, Kosta Esev, Shiri Spielman, Eitan Giat, Olga Kukuy, Shay Kivity, Chagai Grossman, Merav Lidar, Gil Bornstein, Maya Gerstein, Irit Tirosh, Neta Gotlieb, Ramat Gan, Israel; Yonatan Butbul, Riva Brik, Mona Helou, Karolina Gorodetzky, Haifa, Israel; Philip Hashkes, Ori Toker, Ruby Haviv, Jerusalem, Israel; Itzhak Rosner, Doron Rimar, Lisa Kaly, Michael Rozenbaum, Svetlana Petrovich, Haifa, Israel; Liora Harel, Tal Idlitz Marcus, Mohamad Saied, Rotem Tal, Avraham Zeharia, Petach-Tikva, Israel; Virginia Messia, Manuela Pardeo, Giuseppe Pontrelli, Antonella Insalaco, Alessandra Simonetti, Susanna Livadiotti, Giorgia Grutter, Roma, Italy; Maria Alessio, Francesca Orlando, Federica Fontana, Roberto Della Casa, Napoli, Italy; Laura Obici, Stefano Perlini, Grazia Bossi, Pavia, Italy; Marco Cattalini, Martina Soliani, Giulia Zani, Paola Poli, Erika Barzani, Laura Palumbo, Francesca Ricci, Anna Lucia Foresti, Giuseppe Milesi, Alessandra Manerba, Brescia, Italy; Marco Gattorno, Alberto Martini, Roberta Caorsi, Martina Finetti, Silvia Federici, Marco Gattorno, Maurizio Marasini, Stefania Viola, Alessia Omenetti, Riccardo Papa, Clara Malattia, Francesca Minoia, Genova, Italy; Romina Gallizzi, Mirella Crapanzano, Caterina Pidone, Francesco DeLuca, Messina, Italy; Alberto Tommasini, Emanuela Berton, Giulia Gortani, Andrea Taddio, Serena Pastore, Carlo DePieri, Bianca D'Agata Mottolese, Trieste, Italy; Raffaele Manna, Lucia Cerrito, Elena Verrecchia, Maria Giovinale, Roma, Italy; Alfonso Collana, Roberto Barcellona, Stefania Di Noto, Sciacca, Italy; Ryoki Hara, Masaaki Mori, Kenichi Nishimura, Tomo Nozawa, Masako Kikuchi, Kanagawa, Japan; Takahiro Yasumi, Toshio Heike, Ryuta Nishikomori, Tomoki Kawai, Takayuki Tanaka, Kyoto, Japan; Hidetoshi Takada, Masataka Ishimura, Katsuhide Eguchi, Fukuoka, Japan; Utako Kaneko, Yohei Ikezumi, Takeshi Yamada, Niigata, Japan; Anna Simon, Evertine Abbink, Veroniek Harbers, Karin Mulders-Manders, Simone Hins, Adrianne Hofboer, Inge ter Horst, Nijmegen, Netherlands; Joost Frenkel, Nico Wulffraat, Bas Vastert, Joost Swart, Annet van Royen Kerkhof, Ellen Schatorjé, Utrecht, Netherlands; Marina Stanislav, Evgeny Fedorov, Svetlana Salugina, Yulia Korsakova, Moscow, Russia; Anna Shcherbina, Anna Kozlova, Natalia Kuzmenko, Moscow, Russia; Inmaculada Calvo, Berta Lopez, Isabel Gonzalez, Laura Fernandez, Adriana Rodriguez Vidal, Valencia, Spain; Alina Boteanu, Mari Luz Gamir, Maria Angeles Blazquez, Sara Murias, Rosa Merino, Rosa Alcobendas, Agustin Remesal, Madrid, Spain; Jordi Anton, Estibaliz Iglesias Jimenez, Joan Calzada Hernandez, Rosa Bou Torrent, Segundo Bujan, Fernando Martinez, Barcelona, Spain; Pablo Mesa del Castillo Bermejo, Paula Alcañiz Rodriguez, Murcia, Spain; Michael Hofer, Annette von Scheven-Gête, Beatrice Rolland Gosselin, Andreas Woerner, Raffaella Carlomagno, Aikaterini Theodoropoulou, Lausanne, Switzerland; Ahmet Gul, Murat Erdugan, Bahtiyar Toz, Serdal Ugurlu, Ozgur Kasapcopur, Kenan Barut, Sezgin Sahin, Amra Adrovic, Istanbul, Turkey; Umut Kalyoncu, Omer Karadag, Ezgi Batu, Zehra Avci, Ankara, Turkey; Paul Brogan, Despina Eleftheriou, Charalampia Papadapoulou, Helen Lachmann, Taryn Youngstein, Julian Gillmore, Philip Hawkins, London, UK; Sinisa Savic, Anoop Mistry, Gururaj Arumugakani, Leeds, UK; Andreas Reiff, Bracha Shaham, Diane Brown, Michal Cidon, Shirley Parks, Los Angeles, USA; Meredith Riebschleger, Amr Sawalha, Ann Arbor, USA. We also thank Rajeeb Ghosh of Novartis Healthcare and Marco Migliaccio for medical writing assistance, which was funded by Novartis Pharma AG, Basel, Switzerland.
Footnotes
Handling editor: Josef S Smolen
Twitter: @drsezaozen, @Kahlenberglab
Contributors: The study was designed by academic authors and Novartis. All authors attest to the completeness and veracity of data and data analyses. All authors had full access to study data, reviewed and revised the manuscript. Authors have approved the final version of the manuscript to be published. All authors were involved in the decision to submit the manuscript for publication.
Funding: This study was funded by Novartis Pharma.
Competing interests: SO has been a consultant for Novartis and Speaker’s Bureau for Sobi; EB-C has been a consultant for Novartis; IF has been an Advisor for Novartis; GA has received research grants from Novartis; HO has no potential conflict of interest; SV has no potential conflict of interest; KM has received research grant from Novartis; JMK has been an Advisor for AstraZeneca, Bristol Myers Squibb, Boehringer Ingleheim and Eli Lilly; JMK has received research grants from Bristol Myers Squibb; ED is an employee of Novartis; FDB has received research grants from Novartis, Sobi, Novimmune, Abbvie, Roche and Sanofi; IK-P has been a consultant for Novartis, LBF, Sobi, CHUGAI, Pfizer and Abbvie; IK-P has received research grant (non-financial) from Sobi.
Patient consent for publication: Not required.
Ethics approval: The study was conducted according to the ethical principles of the Declaration of Helsinki. The study protocol was approved by the Independent Ethics Committee or Institutional Review Board at each site.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. Additional data are available on reasonable request.
References
- 1. Ben-Chetrit E, Levy M. Familial Mediterranean fever. Lancet 1998;351:659–64. 10.1016/S0140-6736(97)09408-7 [DOI] [PubMed] [Google Scholar]
- 2. Padeh S, Berkun Y. Familial Mediterranean fever. Curr Opin Rheumatol 2016;28:523–9. 10.1097/BOR.0000000000000315 [DOI] [PubMed] [Google Scholar]
- 3. Ozen S, Kone-Paut I, Gül A. Colchicine resistance and intolerance in familial Mediterranean fever: definition, causes, and alternative treatments. Semin Arthritis Rheum 2017;47:115–20. 10.1016/j.semarthrit.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 4. Twig G, Livneh A, Vivante A, et al. . Mortality risk factors associated with familial Mediterranean fever among a cohort of 1.25 million adolescents. Ann Rheum Dis 2014;73:704–9. 10.1136/annrheumdis-2012-202932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aksentijevich IC, Deng M.;, Z.; Sood R. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell 1997;90:797–807. 10.1016/s0092-8674(00)80539-5 [DOI] [PubMed] [Google Scholar]
- 6. Touitou I. The spectrum of familial Mediterranean fever (FMF) mutations. Eur J Hum Genet 2001;9:473–83. 10.1038/sj.ejhg.5200658 [DOI] [PubMed] [Google Scholar]
- 7. Park YH, Wood G, Kastner DL, et al. . Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol 2016;17:914–21. 10.1038/ni.3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Migita K, Izumi Y, Fujikawa K, et al. . Dysregulated mature IL-1β production in familial Mediterranean fever. Rheumatology 2015;54:660–5. 10.1093/rheumatology/keu359 [DOI] [PubMed] [Google Scholar]
- 9. Ozen S, Demirkaya E, Erer B, et al. . EULAR recommendations for the management of familial Mediterranean fever. Ann Rheum Dis 2016;75:644–51. 10.1136/annrheumdis-2015-208690 [DOI] [PubMed] [Google Scholar]
- 10. Alghamdi M. Familial Mediterranean fever, review of the literature. Clin Rheumatol 2017;36:1707–13. 10.1007/s10067-017-3715-5 [DOI] [PubMed] [Google Scholar]
- 11. Gül A, Ozdogan H, Erer B, et al. . Efficacy and safety of canakinumab in adolescents and adults with colchicine-resistant familial Mediterranean fever. Arthritis Res Ther 2015;17:243. 10.1186/s13075-015-0765-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brik R, Butbul-Aviel Y, Lubin S, et al. . Canakinumab for the treatment of children with colchicine-resistant familial Mediterranean fever: a 6-month open-label, single-arm pilot study. Arthritis Rheumatol 2014;66:3241–3. 10.1002/art.38777 [DOI] [PubMed] [Google Scholar]
- 13. Alpay N, Sumnu A, Calışkan Y, et al. . Efficacy of anakinra treatment in a patient with colchicine-resistant familial Mediterranean fever. Rheumatol Int 2012;32:3277–9. 10.1007/s00296-010-1474-6 [DOI] [PubMed] [Google Scholar]
- 14. Meinzer U, Quartier P, Alexandra J-F, et al. . Interleukin-1 targeting drugs in familial Mediterranean fever: a case series and a review of the literature. Semin Arthritis Rheum 2011;41:265–71. 10.1016/j.semarthrit.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 15. Ozen S, Bilginer Y, Aktay Ayaz N, et al. . Anti-interleukin 1 treatment for patients with familial Mediterranean fever resistant to colchicine. J Rheumatol 2011;38:516–8. 10.3899/jrheum.100718 [DOI] [PubMed] [Google Scholar]
- 16. De Benedetti F, Gattorno M, Anton J, et al. . Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N Engl J Med 2018;378:1908–19. 10.1056/NEJMoa1706314 [DOI] [PubMed] [Google Scholar]
- 17. Livneh A, Langevitz P, Zemer D, et al. . Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum 1997;40:1879–85. 10.1002/art.1780401023 [DOI] [PubMed] [Google Scholar]
- 18. Bilginer Y, Akpolat T, Ozen S. Renal amyloidosis in children. Pediatr Nephrol 2011;26:1215–27. 10.1007/s00467-011-1797-x [DOI] [PMC free article] [PubMed] [Google Scholar]