Abstract
Objective
To evaluate efficacy and safety of lacosamide (up to 12 mg/kg/day or 400 mg/day) as adjunctive treatment for uncontrolled primary generalised tonic-clonic seizures (PGTCS) in patients (≥4 years) with idiopathic generalised epilepsy (IGE).
Methods
Phase 3, double-blind, randomised, placebo-controlled trial (SP0982; NCT02408523) in patients with IGE and PGTCS taking 1–3 concomitant antiepileptic drugs. Primary outcome was time to second PGTCS during 24-week treatment.
Results
242 patients were randomised and received ≥1 dose of trial medication (lacosamide/placebo: n=121/n=121). Patients (mean age: 27.7 years; 58.7% female) had a history of generalised-onset seizures (tonic-clonic 99.6%; myoclonic 38.8%; absence 37.2%). Median treatment duration with lacosamide/placebo was 143/65 days. Risk of developing a second PGTCS during 24-week treatment was significantly lower with lacosamide than placebo (Kaplan-Meier survival estimates 55.27%/33.37%; HR 0.540, 95% CI 0.377 to 0.774; p<0.001; n=118/n=121). Median time to second PGTCS could not be estimated for lacosamide (>50% of patients did not experience a second PGTCS) and was 77.0 days for placebo. Kaplan-Meier estimated freedom from PGTCS at end of the 24-week treatment period (day 166) for lacosamide/placebo was 31.3%/17.2% (difference 14.1%; p=0.011). More patients on lacosamide than placebo had ≥50% (68.1%/46.3%) or ≥75% (57.1%/36.4%) reduction from baseline in PGTCS frequency/28 days, or observed freedom from PGTCS during treatment (27.5%/13.2%) (n=119/n=121). 96/121 (79.3%) patients on lacosamide had treatment-emergent adverse events (placebo 79/121 (65.3%)), most commonly dizziness (23.1%), somnolence (16.5%), headache (14.0%). No patients died during the trial.
Conclusions
Lacosamide was efficacious and generally safe as adjunctive treatment for uncontrolled PGTCS in patients with IGE.
Video Abstract.
Introduction
Idiopathic generalised epilepsies (IGEs) account for 20%–55% of all epilepsies,1 2 and are characterised by different generalised seizure types (absence, myoclonic and primary generalised tonic-clonic seizures (PGTCS)).3 PGTCS are associated with an increased risk of injury4 and sudden unexpected death in epilepsy.5–7 Treatment of PGTCS in patients with IGE is complex because associated seizure types, such as absence or myoclonic seizures, may be aggravated by certain antiepileptic drugs (AEDs).8–10
Lacosamide is approved as monotherapy and adjunctive therapy for patients (≥4 years of age) with focal (partial-onset) seizures in the European Union,11 USA and other countries.
A phase 2, open-label pilot study (SP0961; NCT01118949) and extension study (SP0962; NCT01118962) demonstrated the safety of lacosamide as adjunctive treatment of uncontrolled PGTCS in patients (16–65 years of age) with IGE.12 The purpose of this phase 3, double-blind, placebo-controlled trial (SP0982; NCT02408523) was to evaluate the efficacy and safety of adjunctive lacosamide as treatment for PGTCS in patients (≥4 years of age) with IGE.
Seizure types associated with IGE can be infrequent and difficult to quantify, leading to long trials with slow enrolment, thereby making it difficult to study the efficacy of AEDs by assessing reductions in seizure frequency from baseline.12 13 A post hoc analysis of a double-blind trial in patients with PGTCS showed superiority of lamotrigine over placebo when analysing time to third seizure.13 The authors concluded that time to ‘nth’ seizure could be a viable design for trials of low-frequency events. Clinical experience with adjunctive lacosamide indicated that an effective dose would be achieved more rapidly than with lamotrigine. Therefore, time to second PGTCS was chosen as the primary efficacy outcome in this trial.14 Using this outcome, the frequency of baseline seizures and duration of the prospective baseline can be reduced allowing for enrolment of patients more representative of the broader PGTCS population. Analysing time to second seizure further reduces the trial duration by reducing the treatment period for non-responders, ultimately reducing exposure to ineffective treatment.14 To our knowledge, this was the first trial assessing efficacy of an AED using this outcome.
Methods
Overall trial design and patients
SP0982 (ClinicalTrials.gov: NCT02408523; VALOR) was a phase 3, double-blind, randomised, placebo-controlled, multicentre trial in patients with IGE taking one to three concomitant AEDs. The trial was performed in North America, Latin America, Europe and the Asia-Pacific region. All patients (or their legal representative) provided written informed consent for participation.
Patients were eligible if they were ≥4 years of age with a confirmed diagnosis of IGE experiencing classifiable PGTCS, had this diagnosis at least 24 weeks before visit 1, and disease onset before 30 years of age. Patients must have had at least three evenly spread PGTCS during the 16-week combined baseline (12-week historical baseline plus 4-week prospective baseline) with at least two PGTCS during the historical baseline and at least one during the first and second 8 weeks of the 16-week combined baseline. Patients must have been maintained on a stable dose of one to two non-benzodiazepine AEDs or one to three AEDs including one benzodiazepine AED for at least 28 days before visit 1 and throughout the prospective baseline and treatment period (benzodiazepines had to be for epilepsy indication).
Eligible patients were randomised 1:1 to receive lacosamide or placebo (twice daily) and stratified by baseline PGTCS frequency (≤2 or >2 per 28 days) and age at informed consent (≥4 to <12 years, ≥12 to <18 years and ≥18 years).
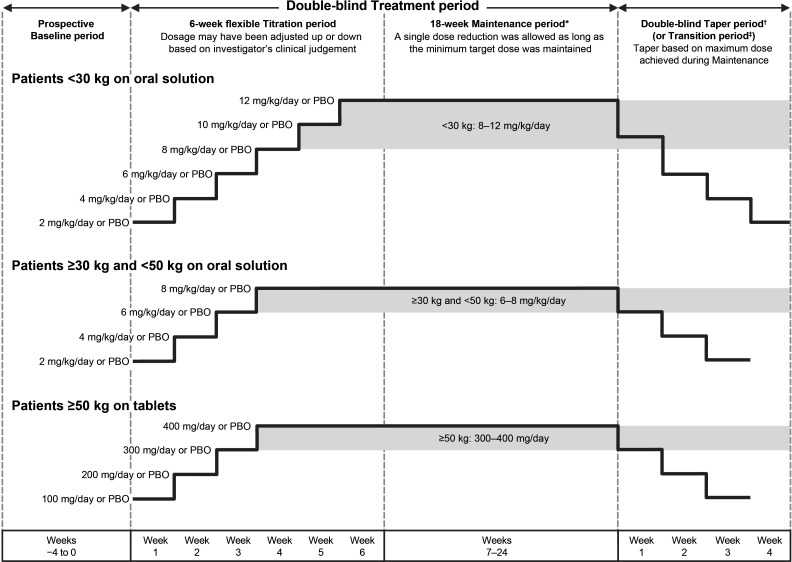
This time-to-event trial enrolled patients in order to observe 125 events (second PGTCS during the 24-week treatment period). The trial was planned to randomise up to 250 patients, and enrolment was discontinued once the 125th event occurred. The trial comprised a 4-week prospective baseline and 6- to 24-week treatment period (6-week titration and up to 18-week maintenance) (figure 1). To ensure a minimum exposure for safety evaluation, patients were required to complete a minimum of 6 weeks of trial treatment.
Figure 1.
Trial design. *Patients were required to achieve and maintain a minimum lacosamide (or matching PBO) dose for at least the final 3 days of week 6 to be eligible for entry into the maintenance period. †The highest possible dose per body weight category is shown for each taper period week. ‡Patients on lacosamide remained on their maintenance dose at entry into the transition period (as indicated by the grey background box), whereas patients in the PBO group initiated lacosamide in a double-blind fashion. On completion of the transition period, eligible patients entered the open-label extension on a weight-based dose (<30 kg: 10 mg/kg/day; ≥30–<50 kg: 8 mg/kg/day; ≥50 kg: 400 mg/day). PBO, placebo.
During titration, doses were uptitrated from a starting dose of 2 mg/kg/day or 100 mg/day in weekly increments to the target maintenance dose range (8–12 mg/kg/day for paediatric patients weighing <30 kg; 6–8 mg/kg/day for paediatric patients weighing ≥30 kg to <50 kg; 300–400 mg/day for adults and paediatric patients weighing ≥50 kg). The treatment period continued until one of the following occurred: completion of ≥6 weeks of the treatment period and occurrence of two or more PGTCS, completion of 24 weeks of the treatment period without occurrence of two PGTCS, or the 125th event occurred in the trial. Eligible patients who chose to enter the open-label extension (EP0012; NCT02408549) completed a 4-week blinded transition, while patients who chose not to continue completed an up to 4-week blinded taper followed by a 30-day safety follow-up.
Outcomes
The primary efficacy outcome was time to second PGTCS during the 24-week (166 days) treatment period. Secondary efficacy outcomes were freedom from PGTCS (estimated using Kaplan-Meier analysis) and time to first PGTCS during the 24-week (166-day) treatment period. Other seizure-related efficacy outcomes were percent change in PGTCS frequency per 28 days from combined baseline, percentage of patients with at least 50%/75% reduction in PGTCS frequency compared with combined baseline, percentage of patients with observed freedom from PGTCS, percentage of patients with observed freedom from all generalised seizures, percent change in days with absence/myoclonic seizures per 28 days relative to prospective baseline, and percentage of patients with at least 50%/75% reduction in absence/myoclonic seizure days compared with prospective baseline.
The key safety outcome was treatment-emergent adverse events (TEAEs) reported spontaneously by the patient and/or caregiver or observed by the investigator.
Statistical analyses
Safety and some efficacy outcomes were assessed in the safety set (SS), which comprised all randomised patients who had been treated with at least one dose of trial medication. Most efficacy outcomes were assessed in the full analysis set (FAS), which included patients who took at least one dose of trial medication and had at least one seizure diary assessment during the treatment period.
For the primary outcome, 125 events (second PGTCS during 24-week treatment period) were necessary to observe a HR of 0.56 with a power of 90% and two-sided test at a significance level of 5%. The observed HR was based on survival rates from a previous trial comparing lamotrigine and placebo.13
Time to second PGTCS was evaluated using a Cox proportional hazards regression model.15 HR, 95% CIs for HR and p value are reported. A Kaplan-Meier plot, estimates for median time to second PGTCS and 95% CIs are provided. The number of events (for titration period, first 12 weeks of treatment period and 24-week treatment period) and the percentage of censored patients (patients who completed Treatment period without having a second PGTCS) are reported. Time to first PGTCS was assessed similarly (without p value).
Prespecified subgroup analyses of efficacy outcomes were performed by age group (paediatric: <18 years; adult: ≥18 years), baseline PGTCS frequency (≤2 and >2 per 28 days) and number of concomitant AEDs (1, 2, ≥3) at trial entry. Additional subgroup analyses were performed by number of lifetime AEDs (1, 2, ≥3) (analysed post hoc) and use of sodium channel blocking AEDs, valproate or levetiracetam at trial entry. Subgroup analyses of safety outcomes were performed by the age group and by number of concomitant AEDs at trial entry.
For additional information on methods, see online supplementary material.
jnnp-2020-323524supp001.pdf (316.2KB, pdf)
Results
Patient disposition and demographics
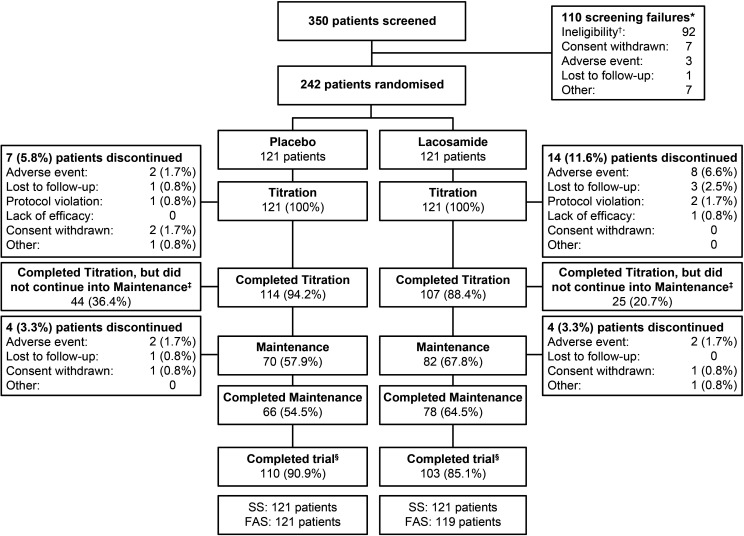
Between April 2015 and May 2019, 350 patients were screened at 115 sites. Overall, 242 patients were randomised; 85.1% (103/121) of patients in the lacosamide group and 90.9% (110/121) in the placebo group met a protocol-defined endpoint (figure 2). A total of 14.9% (18/121) and 9.1% (11/121) of patients discontinued the trial, respectively. The majority of discontinuations occurred during the titration period. Adverse events were the most common reason for trial discontinuation (lacosamide: 8.3%; placebo: 3.3%).
Figure 2.
Patient disposition. *Two of these patients were successfully rescreened and randomised into the trial; †37 patients were baseline failures because of their PGTCS frequency during the combined baseline; ‡41/44 patients on placebo and 22/25 patients on lacosamide did not continue into maintenance because they had a second PGTCS during titration, one patient on lacosamide was labelled as a completer due to a site error, and the five remaining patients did not continue into maintenance because the 125th event had occurred in the trial; §Patients who met a protocol-defined endpoint (completion of ≥6 weeks of the treatment period and occurrence of two or more PGTCS, completion of 24 weeks of the treatment period without occurrence of two PGTCS, or the 125th event occurred in the trial). FAS, full analysis set; PGTCS, primary generalised tonic-clonic seizure; SS, safety set.
All 242 randomised patients received at least one dose of lacosamide or placebo and were included in the SS. Of these, 240 patients (lacosamide: 119; placebo: 121) had at least one seizure diary assessment during the treatment period and were included in the FAS.
Baseline demographics and epilepsy characteristics were similar between the lacosamide and placebo group (table 1). Most patients had a history of tonic-clonic seizures; one patient in the lacosamide group was rediagnosed with focal seizures during the trial.
Table 1.
Baseline demographics and epilepsy characteristics (SS)
| Placebo (n=121) | Lacosamide (n=121) | |
| Patient demographics | ||
| Age, mean (SD), years | 27.6 (12.5) | 27.8 (13.1) |
| <18 years, n (%) | 25 (20.7) | 24 (19.8) |
| ≥18 to <65 years, n (%) | 95 (78.5) | 96 (79.3) |
| ≥65 years, n (%) | 1 (0.8) | 1 (0.8) |
| Female, n (%) | 76 (62.8) | 66 (54.5) |
| Epilepsy characteristics | ||
| Time since first diagnosis, mean (SD), years | 15.4 (13.0) | 15.5 (13.1) |
| Median (range), years | 11.3 (0.5 to 60.7) | 11.4 (0.8 to 64.9) |
| Age at diagnosis, mean (SD), years | 12.9 (5.9) | 12.9 (6.8) |
| PGTCS frequency per 28 days during combined baseline, median (range) | 1.24 (0.7 to 19.4) | 1.25 (0.3 to 12.3) |
| Seizure classification history at any time before trial entry*, n (%) | ||
| Any partial-onset seizures (focal seizures) | 0 | 1 (0.8)† |
| Simple partial (focal aware) | 0 | 1 (0.8)† |
| Any generalised seizures | 121 (100) | 121 (100) |
| Absence | 41 (33.9) | 49 (40.5) |
| Atypical absence | 2 (1.7) | 2 (1.7) |
| Myoclonic | 48 (39.7) | 46 (38.0) |
| Clonic | 2 (1.7) | 3 (2.5) |
| Tonic | 1 (0.8) | 2 (1.7) |
| Tonic-clonic | 121 (100) | 120 (99.2)† |
| Atonic | 3 (2.5) | 2 (1.7) |
| Unclassified epileptic seizures | 0 | 2 (1.7) |
| No of prior AEDs and benzodiazepines‡, n (%) | ||
| 0 | 70 (57.9) | 63 (52.1) |
| 1–3 | 37 (30.6) | 47 (38.8) |
| 4–6 | 13 (10.7) | 9 (7.4) |
| ≥7 | 1 (0.8) | 2 (1.7) |
| No of concomitant AEDs and benzodiazepines at trial entry§, n (%) | ||
| 1 | 44 (36.4) | 35 (28.9) |
| 2 | 55 (45.5) | 62 (51.2) |
| ≥3 | 22 (18.2) | 23 (19.0) |
| Concomitant AEDs and benzodiazepines taken during the treatment period by ≥5% of all patients, n (%) | ||
| Valproate | 68 (56.2) | 59 (48.8) |
| Levetiracetam | 48 (39.7) | 56 (46.3) |
| Lamotrigine | 37 (30.6) | 36 (29.8) |
| Topiramate | 15 (12.4) | 16 (13.2) |
| Clonazepam | 16 (13.2) | 12 (9.9) |
| Clobazam | 13 (10.7) | 9 (7.4) |
| Zonisamide | 7 (5.8) | 7 (5.8) |
| Carbamazepine | 5 (4.1) | 9 (7.4) |
| Ongoing comorbid conditions at screening visit, n (%) | ||
| Patients with at least one ongoing medical condition | 75 (62.0) | 69 (57.0) |
| Medical conditions in ≥5% of all patients | ||
| Headache | 9 (7.4) | 13 (10.7) |
| Depression | 8 (6.6) | 12 (9.9) |
| Migraine | 8 (6.6) | 7 (5.8) |
| Obesity | 8 (6.6) | 5 (4.1) |
| Anxiety | 4 (3.3) | 8 (6.6) |
| Back pain | 5 (4.1) | 7 (5.8) |
*Patients could have more than one response in a classification level and/or category; seizure types are listed per the trial protocol (International League Against Epilepsy 1981 classification32) with the newer terminology33 provided in parentheses.
†One patient had a history of partial-onset seizures and was excluded from the per-protocol set.
‡AEDs stopped at least 28 days before visit 1.
§Number of concomitant AEDs and benzodiazepines at trial entry were unknown for one patient in the lacosamide group.
AED, antiepileptic drug; PGTCS, primary generalised tonic-clonic seizure; SS, safety set.
Overall, 28.1% of patients in the lacosamide group and 34.7% in the placebo group were reported to have juvenile myoclonic epilepsy and 10.7% and 12.4% were reported to have juvenile absence epilepsy, respectively (online supplementary table S1).
Efficacy
Primary generalised tonic-clonic seizures
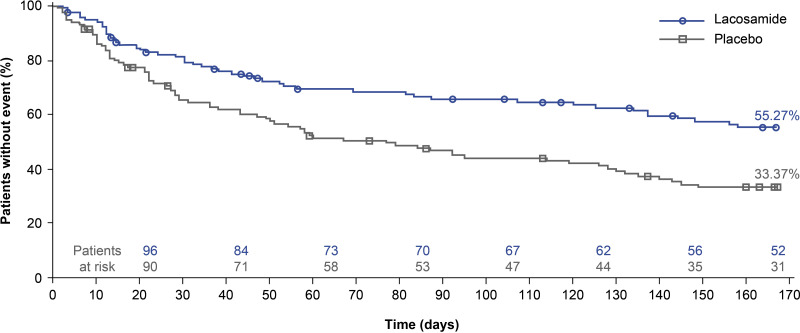
The risk of developing a second PGTCS during the 24-week treatment period was significantly lower in patients randomised to lacosamide than placebo (figure 3, table 2). The Kaplan-Meier survival estimates at the end of the 24-week treatment period were 55.27% with lacosamide and 33.37% with placebo (HR 0.540; 95% CI 0.377 to 0.774; p<0.001). The median time to second PGTCS could not be estimated for lacosamide (because ˃50% of patients did not experience a second PGTCS by day 166) and was 77.0 days (95% CI 49.0 to 128.0) for placebo.
Figure 3.
Kaplan-Meier estimates for time to second PGTCS (125 events) (FAS). One patient in the lacosamide group was randomised after the 125th event and does not appear in this analysis. Symbols represent censored patients (patients who completed the treatment period without having a second PGTCS). FAS, full analysis set; PGTCS, primary generalised tonic-clonic seizure.
Table 2.
Analyses of time to second PGTCS during 24-week treatment period (FAS)
| Placebo | Lacosamide* | HR† (95% CI) | |||||
| N | No of events | KM survival estimate, % | N | No of events | KM survival estimate, % | ||
| All patients‡ | 121 | 76§ | 33.37 | 118 | 49¶ | 55.27 | 0.540 (0.377 to 0.774); p<0.001 |
| Age group** | |||||||
| Paediatric (<18 years) | 25 | 14 | 41.54 | 24 | 9 | 61.03 | 0.650 (0.271 to 1.561) |
| Adult (≥18 years) | 96 | 62 | 31.25 | 94 | 40 | 53.60 | 0.527 (0.354 to 0.786) |
| Baseline PGTCS frequency†† | |||||||
| ≤2 per 28 days | 95 | 56 | 37.45 | 93 | 34 | 60.31 | 0.501 (0.327 to 0.767) |
| >2 per 28 days | 26 | 20 | 17.72 | 25 | 15 | 37.59 | 0.653 (0.334 to 1.277) |
| No of concomitant AEDs at trial entry‡ | |||||||
| 1 | 44 | 22 | 44.77 | 34 | 12 | 63.22 | 0.570 (0.279 to 1.165) |
| 2 | 55 | 37 | 30.24 | 61 | 26 | 53.72 | 0.539 (0.323 to 0.900) |
| ≥3 | 22 | 17 | 19.39 | 22 | 11 | 44.43 | 0.440 (0.201 to 0.965) |
| No of lifetime AEDs‡‡‡ | |||||||
| 1 | 28 | 15 | 37.56 | 21 | 9 | 55.56 | 0.578 (0.243 to 1.375) |
| 2 | 54 | 34 | 35.35 | 46 | 14 | 66.33 | 0.374 (0.194 to 0.723) |
| ≥3 | 39 | 27 | 28.28 | 52 | 26 | 45.04 | 0.574 (0.333 to 0.988) |
| SCB use at trial entryत | |||||||
| Yes | 46 | 37 | 17.36 | 46 | 22 | 45.88 | 0.428 (0.248 to 0.739) |
| No | 75 | 39 | 43.39 | 72 | 27 | 60.80 | 0.630 (0.385 to 1.032) |
| Valproate use at trial entry‡ | |||||||
| Yes | 67 | 38 | 40.78 | 59 | 20 | 62.59 | 0.475 (0.276 to 0.819) |
| No | 54 | 38 | 24.10 | 59 | 29 | 48.09 | 0.595 (0.364 to 0.972) |
| Levetiracetam use at trial entry‡ | |||||||
| Yes | 48 | 31 | 31.09 | 53 | 24 | 52.77 | 0.641 (0.373 to 1.101) |
| No | 73 | 45 | 34.97 | 65 | 25 | 57.31 | 0.497 (0.304 to 0.812) |
*One patient in the lacosamide group was randomised after the 125th event and does not appear in this analysis.
†HR <1 indicates time to second PGTCS was improved for lacosamide compared with placebo.
‡Comparison of lacosamide versus placebo was based on a Cox proportional hazards regression model with an effect for treatment, stratifying for the following combinations of patients’ baseline PGTCS frequency and age group: ≤2 PGTCS per 28 days in the combined baseline period and paediatric; ≤2 PGTCS per 28 days in the combined baseline period and adult; >2 PGTCS per 28 days in the combined baseline period.
§Of these events, 45 occurred during the titration period (by day 42) and 61 during the first 12 weeks of the treatment period (by day 84).
¶Of these events, 29 occurred during the titration period (by day 42) and 38 during the first 12 weeks of the treatment period (by day 84).
**Comparison based on a Cox proportional hazards regression model with an effect for treatment, stratifying for baseline PGTCS frequency: ≤2 PGTCS and >2 PGTCS per 28 days in the combined baseline period.
††Comparison based on a Cox proportional hazards regression model with an effect for treatment, stratifying for age group in the group with ≤2 PGTCS per 28 days in the combined baseline and with no stratification in the group with >2 PGTCS per 28 days.
‡‡AEDs stopped before or ongoing at lacosamide initiation.
§§SCB AEDs used in this trial were: carbamazepine, oxcarbazepine, phenytoin and lamotrigine.
AED, antiepileptic drug; FAS, full analysis set; KM, Kaplan-Meier; PGTCS, primary generalised tonic-clonic seizure; SCB, sodium channel blocking AED.
The results of all sensitivity analyses of time to second PGTCS were consistent with the primary analysis (see online supplementary material). Survival estimates were numerically higher with lacosamide than placebo in all subgroup analyses (table 2).
The stratified Kaplan-Meier estimates16 of the proportion of patients free from PGTCS for the 24-week treatment period were higher with lacosamide (31.3%, 95% CI 22.8% to 39.9%) than placebo (17.2%, 95% CI 10.4% to 24.0%) (online supplementary table S2). The difference in stratified PGTCS freedom rate between lacosamide and placebo was 14.1% (95% CI 3.2% to 25.1%; p=0.011).
Kaplan-Meier survival estimates at end of the 24-week treatment period indicated a lower risk of developing a first PGTCS with lacosamide than placebo (30.97% vs 17.27%; HR 0.683, 95% CI 0.507 to 0.921; p=0.012) (online supplementary figure S1). The median time to first PGTCS was 36.0 days (95% CI 25.0 to 78.0) with lacosamide and 20.0 days (95% CI 13.0 to 34.0) with placebo.
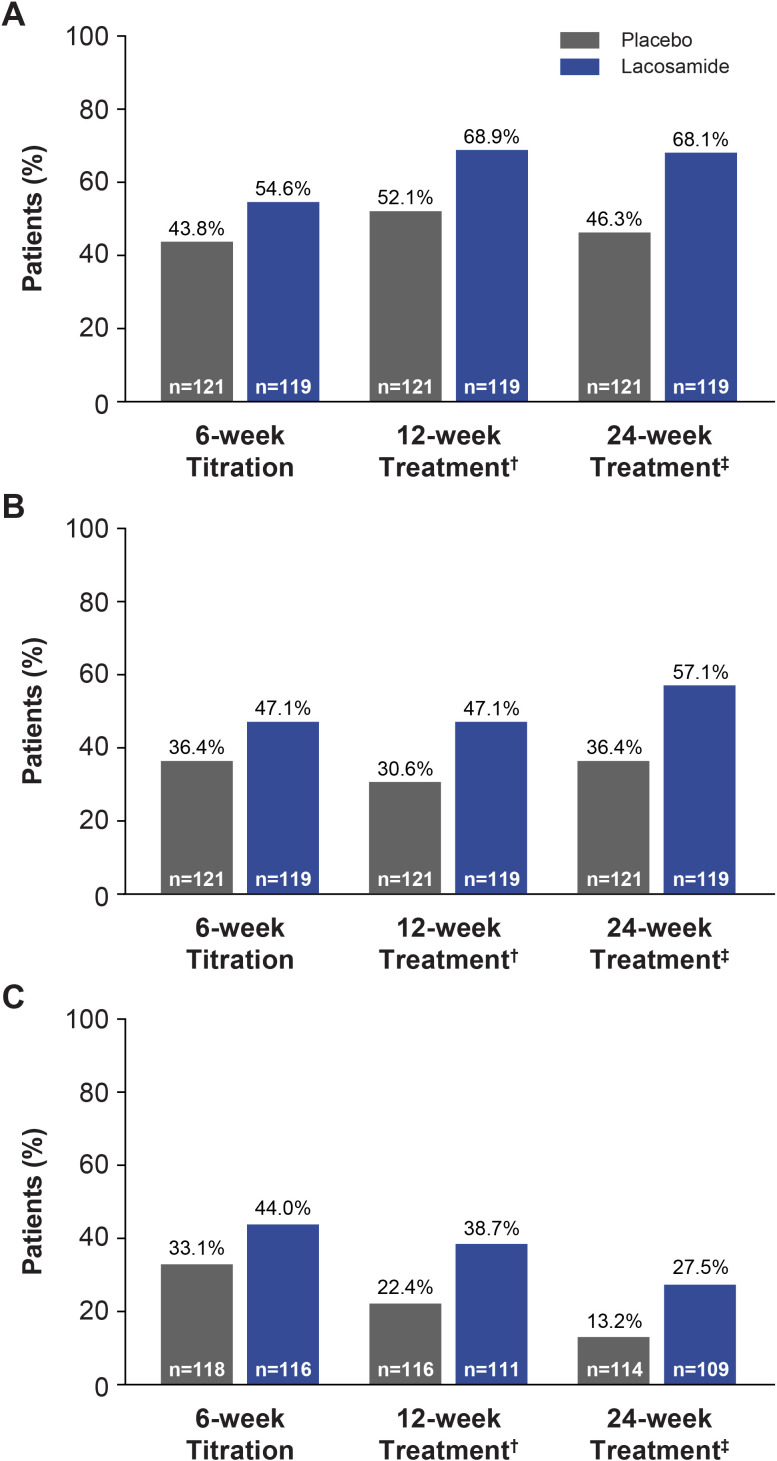
Greater median percent changes in PGTCS frequency per 28 days from combined baseline were observed with lacosamide than placebo during all time periods (titration period: −66.37 vs −42.71; first 12 weeks of treatment period: −71.33 vs −55.69; 24-week treatment period: −77.92 vs −43.24; ranges for all time periods: −100.0 to 943.6 vs −100.0 to 715.4) (online supplementary table S3). The 50% and 75% responder rates for reduction in PGTCS frequency from combined baseline and observed freedom from PGTCS were greater with lacosamide than placebo during all time periods (figure 4).
Figure 4.
(A) 50% responder rates for PGTCS, (B) 75% responder rates for PGTCS and (C) freedom from PGTCS* (FAS). *Percentages are based on the number of patients who had either two PGTCS or completed the time period of interest or completed the trial due to occurrence of the 125th event; †6-week titration period + first 6 weeks of maintenance period; ‡6-week titration period +18-week maintenance period. FAS, full analysis set; PGTCS, primary generalised tonic-clonic seizure.
Absence and myoclonic seizures
Fifty-one patients in the lacosamide group and 42 patients in the placebo group had a history of absence seizures or reported absence seizures during the combined baseline or treatment period. In these patients, the 50% responder rates were 15.7%, 19.6% and 19.6% with lacosamide and 16.7%, 14.3% and 16.7% with placebo during the titration period, first 12 weeks of the treatment period, and 24-week treatment period, respectively. The 75% responder rates were 13.7%, 13.7% and 17.6% with lacosamide and 7.1%, 11.9% and 11.9% with placebo, respectively. Among patients with absence seizure days during the prospective baseline, numerically greater median percent reductions in number of days with absence seizures per 28 days were observed with lacosamide (n=22) than placebo (n=22) (24-week treatment period: −30.1 vs −15.3) (online supplementary table S4).
Among patients who had a history of myoclonic seizures or reported myoclonic seizures during the combined baseline or treatment period, the 50% responder rates were 21.3%, 23.4% and 27.7% with lacosamide (n=47) and 26.5%, 26.5% and 28.6% with placebo (n=49) during the titration period, first 12 weeks of the treatment period and 24-week treatment period, respectively. The 75% responder rates were 12.8%, 14.9% and 14.9% with lacosamide and 16.3%, 18.4% and 18.4% with placebo, respectively. Among patients with myoclonic seizure days during the prospective baseline, numerically lower median percent reductions in number of days with myoclonic seizures per 28 days were observed with lacosamide (n=24) than placebo (n=25) (24-week treatment period: −54.6 vs −65.7) (online supplementary table S4).
All generalised seizures
More patients on lacosamide than placebo achieved observed seizure freedom from all generalised seizures during the titration period (39/116 (33.6%) vs 32/118 (27.1%)), first 12 weeks of the treatment period (31/111 (27.9%) vs 20/116 (17.2%)) and 24-week treatment period (23/109 (21.1%) vs 15/114 (13.2%)) (SS).
Safety
Exposure
Overall, patients were exposed to lacosamide and placebo for a total of 37.2 and 31.1 patient-years, respectively (SS). The median trial medication duration was 143.0 days (range: 1.0 to 176.0) with lacosamide and 65.0 days (7.0 to 176.0) with placebo. The majority of patients on tablets (weighing ≥50 kg) in both the lacosamide (54/75 (72.0%)) and placebo (51/64 (79.7%)) groups took a modal maintenance dose of 400 mg/day.
Seizure worsening
Overall, 10.1% of patients on lacosamide had a≥50% increase in PGTCS frequency from combined baseline to the titration period, first 12 weeks of the treatment period, and 24-week treatment period compared with 14.9%, 15.7% and 16.5%, respectively, on placebo.
A total of 2.0% (1/51) patients on lacosamide and 7.1% (3/42) on placebo had a ≥50% increase in absence seizure days from prospective baseline to the titration period, first 12 weeks of the treatment period and 24-week treatment period. No patients on lacosamide or placebo had a new occurrence of absence seizures.
A total of 8.5% (4/47) patients on lacosamide and 4.1% (2/49) on placebo had a ≥50% increase in myoclonic seizure days from prospective baseline to the titration period, first 12 weeks of the treatment period and 24-week treatment period. One patient (2.1%) on lacosamide and one patient (2.0%) on placebo had a new occurrence of myoclonic seizures. Three (2.5%) patients on lacosamide had a TEAE of myoclonic epilepsy and one (0.8%) patient on placebo had a TEAE of myoclonus; none of these TEAEs were serious.
Treatment-emergent adverse events
Overall, 96 (79.3%) patients on lacosamide and 79 (65.3%) on placebo had TEAEs during the treatment period (table 3). The most frequently reported TEAEs (≥10%) with lacosamide were dizziness, somnolence and headache. The incidences of dizziness and headache were numerically higher with lacosamide than placebo.
Table 3.
Treatment-emergent adverse events (SS)
| Placebo (n=121) | Lacosamide (n=121) | |
| Any TEAEs, n (%) | 79 (65.3) | 96 (79.3) |
| Drug-related TEAEs* | 42 (34.7) | 56 (46.3) |
| Serious TEAEs | 4 (3.3) | 8 (6.6) |
| Severe TEAEs | 3 (2.5) | 6 (5.0) |
| Discontinuations due to TEAEs | 5 (4.1) | 11 (9.1) |
| TEAEs† experienced during the treatment period by ≥5% of patients in either treatment group, n (%) | ||
| Dizziness | 7 (5.8) | 28 (23.1) |
| Somnolence | 17 (14.0) | 20 (16.5) |
| Headache | 12 (9.9) | 17 (14.0) |
| Nausea | 7 (5.8) | 12 (9.9) |
| Vertigo | 2 (1.7) | 8 (6.6) |
| Nasopharyngitis | 4 (3.3) | 8 (6.6) |
| Fatigue | 6 (5.0) | 8 (6.6) |
| Vomiting | 1 (0.8) | 7 (5.8) |
| Upper respiratory tract infection | 6 (5.0) | 3 (2.5) |
| Drug-related TEAEs*† experienced during the treatment period by ≥5% of patients in either treatment group, n (%) | ||
| Dizziness | 4 (3.3) | 21 (17.4) |
| Somnolence | 14 (11.6) | 16 (13.2) |
| Nausea | 3 (2.5) | 9 (7.4) |
| Vertigo | 2 (1.7) | 7 (5.8) |
| Vomiting | 0 | 6 (5.0) |
| Fatigue | 3 (2.5) | 6 (5.0) |
*TEAEs considered drug-related by the investigator (if relationship to trial medication was missing, TEAE was considered drug related).
†MedDRA (V.16.1) preferred term.
SS, safety set; TEAE, treatment-emergent adverse event.
Eight (6.6%) patients on lacosamide had a total of 14 serious TEAEs; one patient each reported abdominal pain, pain in extremity, somnolence, status epilepticus and transaminases increased; one patient had serious TEAEs of dizziness, nausea, somnolence and vomiting; one had serious TEAEs of contusion, grand mal convulsion and headache; and one had serious TEAEs of asthenia and dizziness. Four (3.3%) patients on placebo had a total of four serious TEAEs; one patient each had upper respiratory tract infection, femur fracture, road traffic accident and liver function test abnormal.
Eleven (9.1%) patients on lacosamide and five (4.1%) patients on placebo discontinued due to TEAEs. The only TEAEs leading to discontinuation in more than one patient on lacosamide were dizziness (2 (1.7%)) and suicidal ideation (2 (1.7%)). The only TEAE leading to discontinuation in two or more patients on placebo was rash (2 (1.7%)). No patients died during the trial.
Three (2.5%) patients on lacosamide and two (1.7%) patients on placebo reported rash (considered drug related by the investigator in one patient on lacosamide and two patients on placebo), two (1.7%) patients on lacosamide reported pruritus (considered drug related in one patient), and one (0.8%) patient on placebo reported macular rash (considered drug related). With the exception of one moderate case of rash in a patient on placebo, all of these skin reactions were mild in intensity and had resolved at the end of the trial. One patient on lacosamide and two patients on placebo discontinued due to rash. No patients reported drug reaction with eosinophilia and systemic symptoms, toxic epidermal necrolysis or Stevens-Johnson syndrome.
In the lacosamide group, TEAEs were more common during titration (79/121 (65.3%)) than maintenance (44/82 (53.7%)). In the placebo group, incidence of TEAEs was similar in both periods (67/121 (55.4%) vs 39/70 (55.7%)).
In the placebo group, more patients taking three concomitant AEDs than patients taking one or two concomitant AEDs reported TEAEs during the treatment period. No such trend was seen in the lacosamide group (online supplementary table S5).
Additional safety, quality of life and health outcomes are included in online supplementary material.
Paediatric subgroup
Efficacy
In this trial, 24/121 patients on lacosamide and 25/121 on placebo were <18 years of age. In paediatric patients, the risk of developing a second PGTCS during the 24-week treatment period was lower in patients on lacosamide than placebo (table 2). The Kaplan-Meier estimates of the proportion of patients free from PGTCS for the 24-week treatment period were higher with lacosamide (20.0%, 95% CI 3.6% to 36.4%) than placebo (12.0%, 95% CI 0.0% to 24.7%). The median PGTCS frequency per 28 days at combined baseline was 1.01 (range: 0.7 to 7.5) with lacosamide and 1.00 (0.7 to 19.4) with placebo. Greater median percent changes in PGTCS frequency per 28 days from combined baseline to the 24-week treatment period were observed with lacosamide (−80.64%, range: −100.0% to 281.8%) than placebo (−31.20%, −100.0% to 715.4%). The 50% and 75% responder rates for reduction in PGTCS frequency during the 24-week treatment period were also higher with lacosamide (70.8% and 62.5%, respectively) than placebo (44.0% and 36.0%, respectively).
The median number of days with absence seizures during prospective baseline was 0.0 days (range: 0 to 18) in the lacosamide group (n=15) and 1.0 days (0 to 6) in the placebo group (n=10). The median percent change in days with absence seizures to the 24-week treatment period was −51.8% (range: −78% to −26%) with lacosamide and −44.6% (range: −96% to 137%) with placebo. The 50% and 75% responder rates for reduction in days with absence seizures from prospective baseline to the 24-week treatment period were 6.7% and 6.7% with lacosamide (n=15) and 20.0% and 20.0% with placebo (n=10).
The median number of days with myoclonic seizures during prospective baseline was 3.0 days (range: 0 to 17) in the lacosamide group (n=5) and 1.1 days (0 to 28) in the placebo group (n=7). The median percent change in days with myoclonic seizures from prospective baseline to the 24-week treatment period was 11.5% (range: −96% to 197%) with lacosamide and −34.0% (range: −100% to 0%) with placebo. The 50% and 75% responder rates for reduction in days with myoclonic seizures to the 24-week treatment period were 20.0% and 20.0% with lacosamide (n=5) and 14.3% and 14.3% with placebo (n=7).
Safety
Overall, 22/24 (91.7%) paediatric patients on lacosamide and 15/25 (60.0%) on placebo had TEAEs during the treatment period. The most common TEAEs with lacosamide (≥10%) were dizziness (7 (29.2%) vs 1 (4.0%) with placebo), somnolence (7 (29.2%) vs 1 (4.0%)), headache (3 (12.5%) vs 2 (8.0%)) and nasopharyngitis (3 (12.5%) vs 1 (4.0%)) and nausea (3 (12.5%) vs 0).
Discussion
The results of this trial indicated that adjunctive lacosamide was efficacious in reducing PGTCS frequency in patients (≥4 years of age) with IGE, and was generally well tolerated. Treatment with lacosamide resulted in a significantly lower risk of developing a second PGTCS (HR 0.540; p<0.001) and a significantly higher rate of 166 days freedom from PGTCS (31.3% vs 17.2%; difference 14.1%; p=0.011) than placebo.
Consistent improvements were also observed with lacosamide versus placebo across other efficacy endpoints assessing PGTCS seizure frequency. Median percent reduction in PGTCS frequency (−77.92% vs −56.7% to −77.6%), 50% responder rates (68.1% vs 56.4% to 72.2%) and observed seizure freedom rates (27.5% vs 12.8% to 24.1%) for the entire treatment period were within the upper ranges reported in previous randomised, double-blind, placebo-controlled trials of other AEDs that demonstrated efficacy as adjunctive treatment of PGTCS.17–22 The placebo responses seen in this trial were relatively high (median percent reduction in PGTCS frequency: −43.24%; 50% responder rate: 46.3%; observed seizure freedom rate: 13.2%). However, similarly high placebo responses were also seen in previous trials of adjunctive levetiracetam and lamotrigine treatment in patients with PGTCS.18 20 High placebo responses are not unexpected, given the known variability of seizure frequency in patients with IGE and PGTCS, and the fact that patients in clinical trials are often more adherent to their AED regimen.18 An open-label pilot and extension study assessed the safety of lacosamide for adjunctive treatment of uncontrolled PGTCS in patients with IGE.12 Although these studies were not designed to assess efficacy, reductions in PGTCS, myoclonic and absence seizure frequencies were observed. Several case reports and small-scale studies also suggested that lacosamide may be an effective treatment for patients with IGE.23–28
In the pilot lacosamide study, five (10%) patients had an increase in absence seizures (reported as TEAEs).12 However, because of the uncontrolled nature of the study, it was not clear whether this was due to lacosamide or the natural course of the condition. In a small-case series supported by video-electroencephalogram, the authors concluded that lacosamide can be a reasonable option for patients with IGE and drug-resistant PGTCS, but recommended follow-up in patients with a history of absence seizures who could be at higher risk of seizure worsening.28
In the current trial, 37% of patients had a history of absence seizures and 39% had a history of myoclonic seizures. Median percent reductions in the number of days with absence seizures were numerically higher with lacosamide than placebo and median percent reductions in the number of days with myoclonic seizures were numerically lower with lacosamide than placebo. Responder rates for reduction in days with absence and myoclonic seizures were generally similar with lacosamide and placebo. There was no evidence of an increased risk of seizure worsening for PGTCS and absence seizures with lacosamide and only few patients reported worsening of myoclonic seizures. One patient on lacosamide and three patients on placebo had a ≥50% increase in absence seizure days, while four patients on lacosamide and two patients on placebo had a ≥50% increase in myoclonic seizure days from the combined baseline. If seizure worsening occurred, it was observed during the titration period and no further increase was seen during the first 12 weeks of the treatment period and 24-week treatment period.
Lacosamide was generally safe and well tolerated in patients with IGE and PGTCS. The most common TEAEs with lacosamide were dizziness, somnolence and headache, consistent with those reported previously in patients with focal seizures29–31 and in the open-label pilot study in patients with IGE and uncontrolled PGTCS.12
Findings in the subgroup of paediatric patients were consistent with the overall population. However, patient numbers were low and results should be interpreted with caution as this trial was not powered to evaluate significance in subgroups.
The results of this trial support the use of adjunctive oral lacosamide for treatment of uncontrolled PGTCS in patients ≥4 years of age with IGE.
Acknowledgments
The authors thank the patients and their caregivers and the clinical project team, in addition to the investigators and their teams who contributed to this trial (coinvestigator appendix). The authors acknowledge Michaela Fuchs, PhD, CMPP (Evidence Scientific Solutions, Horsham, UK) for writing assistance, which was funded by UCB Pharma. Publication coordination was provided by Fabien Debailleul, PhD (UCB Pharma, Brussels, Belgium).
Footnotes
Contributors: DGV contributed to the conception and design of the trial, and was the coordinating investigator. DGV, SK, TJO and MW participated in acquisition of data as trial investigators. RR was the trial physician. MB was the clinical programme director. PW was the trial statistician. All authors participated in analysis and interpretation of results and critical revision of the article for intellectual content. All authors approved the final version of the manuscript for publication.
Funding: This trial was sponsored by UCB Pharma.
Competing interests: DGV received speaker honoraria from Eisai, Greenwich Biosciences, Lundbeck, Sunovion and UCB Pharma; his institution received payments for his services as a principal investigator on randomised controlled trials sponsored by Biogen, Eisai, SK Life Science and UCB Pharma; he served as an advisor to Otsuka Pharmaceutical Development and Commercialisation and SK Life Science. SK received speaker honoraria from Desitin, Eisai and UCB Pharma. TJO received research funding from Anavex, Biogen, Eisai, Praxis Precision Medicines, UCB Pharma and Zynerba. MW received speaker honoraria from Eisai, Otsuka Pharmaceutical and UCB Pharma. MB, BS-B, PW and RR are employees of UCB Pharma.
Patient consent for publication: Not required.
Ethics approval: The trial was conducted in accordance with Good Clinical Practice, the Declaration of Helsinki and local laws. The trial protocol, amendments and patient-informed consent were reviewed by a national, regional or independent ethics committee or institutional review board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. Underlying data from this manuscript may be requested by qualified researchers 6 months after product or indication approval in the USA and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymised individual patient-level data and redacted trial documents, which may include: analysis-ready datasets, study protocol, annotated case report form, statistical analysis plan, dataset specifications and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a prespecified time, typically 12 months, on a password-protected portal.
Contributor Information
SP0982 co-investigators:
Sami Aboumatar, Naoki Akamatsu, Soniza Alves-Leon, Alexis Arzimanoglou, Saeed Ata, Michal Bajaček, Michal Bar, Roy Beran, Samuel Berkovic, Luiz Eduardo Betting, Ilan Blatt, Arnaud Biraben, Victor Biton, Enver Bogdanov, Jana Chamilova, Yanhui Chen, Yong Won Cho, I-Ching Chou, Mario Coletti Moja, Liliana Cucos, Anna Członkowska, Veerle De Herdt, Philippe Derambure, Nasrollah Eslami, Jose Carlos Estevez, Viktor Farkas, Bernardo Flasterstein, Cassiano Mateus Forcelini, Michael Frucht, Waldemar Fryze, Feng Gao, Ronit Gilad, Hajo Hamer, Koji Iida, Hitoshi Ikeda, Atsushi Imamura, Yuwu Jiang, Roman Kalinowski, Janusz Kapustecki, Sasho Kastrev, Dong Wook Kim, Heung Dong Kim, Pavel Klein, Pedro Kowacs, Jacek Kowalski, Yuichi Kubota, David Kudrow, Patrick Kwan, Wang-Tso Lee, Benjamin Legros, George Li, Kátia Lin, Lubomír Lipovský, Carmen Gabriela Lupusoru, Edward Maa, Louis Maillard, Dimitar Maslarov, Natalia Maslova, Anatol Mickielewicz, Gennadiy Mishin, Hugo Ceja Moreno, Lola Morgan, Ewa Motta, Najib Murr, Eiji Nakagawa, Victoria Nekrasova, Michael Newmark, Yu-Tze Ng, Hirotomo Ninomiya, Soheyl Noachtar, Liliana Maria Nussbaum, Teiichi Onuma, Ilona Pałka-Kisielowska, André Luis Palmini, Axel Panzer, Jaime Parra, José Pimentel, Irina Poverennova, R Eugene Ramsay, Juan Jesus Rodríguez Uranga, Joanne Rogin, William Rosenfeld, Estevo Santamarina, Mona Sazgar, Andreas Schulze-Bonhage, Pedro Serrano, Baljeet Sethi, Jerry Shih, Hideaki Shiraishi, Nataliya Sivakova, Lech Szczechowski, Mutaz Tabbaa, Shigeki Tanaka, Shigeya Tanaka, Jun Tohyama, Wim Van Paesschen, Blanca Vazquez, László Vécsei, Larisa Volkova, Kimberly Wagner, Xuefeng Wang, Yi Wang, Zan Wang, Robert Wechsler, Aleksandra Wójcik-Pokora, Michael Xu, Elza Márcia Yacubian, Takamichi Yamamoto, William Yorns, Monika Záhumenská, and Jana Zarubova
References
- 1. Marini C, King MA, Archer JS, et al. . Idiopathic generalised epilepsy of adult onset: clinical syndromes and genetics. J Neurol Neurosurg Psychiatry 2003;74:192–6. 10.1136/jnnp.74.2.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gastaut H, Gastaut JL, Gonçalves e Silva GE, et al. . Relative frequency of different types of epilepsy: a study employing the classification of the International League Against Epilepsy. Epilepsia 1975;16:457–61. 10.1111/j.1528-1157.1975.tb06073.x [DOI] [PubMed] [Google Scholar]
- 3. Bosak M, Pawełczak D, Słowik A. Status epilepticus in patients with genetic (idiopathic) generalized epilepsy. Neuropsychiatr Dis Treat 2019;15:1585–92. 10.2147/NDT.S209084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asadi-Pooya AA, Nikseresht A, Yaghoubi E, et al. . Physical injuries in patients with epilepsy and their associated risk factors. Seizure 2012;21:165–8. 10.1016/j.seizure.2011.10.009 [DOI] [PubMed] [Google Scholar]
- 5. Hesdorffer DC, Tomson T, Benn E, et al. . Do antiepileptic drugs or generalized tonic-clonic seizure frequency increase SUDEP risk? A combined analysis. Epilepsia 2012;53:249–52. 10.1111/j.1528-1167.2011.03354.x [DOI] [PubMed] [Google Scholar]
- 6. Harden C, Tomson T, Gloss D, et al. . Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors. Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2017;88:1674–80. 10.1212/WNL.0000000000003685 [DOI] [PubMed] [Google Scholar]
- 7. DeGiorgio CM, Markovic D, Mazumder R, et al. . Ranking the leading risk factors for sudden unexpected death in epilepsy. Front Neurol 2017;8:473. 10.3389/fneur.2017.00473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perucca E, Gram L, Avanzini G, et al. . Antiepileptic drugs as a cause of worsening seizures. Epilepsia 1998;39:5–17. 10.1111/j.1528-1157.1998.tb01268.x [DOI] [PubMed] [Google Scholar]
- 9. Thomas P, Valton L, Genton P. Absence and myoclonic status epilepticus precipitated by antiepileptic drugs in idiopathic generalized epilepsy. Brain 2006;129:1281–92. 10.1093/brain/awl047 [DOI] [PubMed] [Google Scholar]
- 10. Somerville ER. Some treatments cause seizure aggravation in idiopathic epilepsies (especially absence epilepsy). Epilepsia 2009;50 Suppl 8:31–6. 10.1111/j.1528-1167.2009.02233.x [DOI] [PubMed] [Google Scholar]
- 11. Vimpat Vimpat® (lacosamide) Summary of Product Characteristics, 2020. Available: https://www.ema.europa.eu/documents/product-information/vimpat-epar-product-information_en.pdf [Accessed 29 Jul 2020].
- 12. Wechsler RT, Yates SL, Messenheimer J, et al. . Lacosamide for uncontrolled primary generalized tonic-clonic seizures: an open-label pilot study with 59-week extension. Epilepsy Res 2017;130:13–20. 10.1016/j.eplepsyres.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 13. French JA, Temkin NR, Hammer AE, et al. . Time to Nth seizure analysis of lamotrigine as adjunctive therapy in subjects with primary generalized tonic-clonic seizures. Epilepsia 2007;48(S6)(abstract 1.211):77–8.17241211 [Google Scholar]
- 14. Warnock R, Yates S, Schmid M, et al. . Rationale and study design for a novel phase 3, randomized, double-blind trial of adjunctive lacosamide in patients with idiopathic generalized (genetic) epilepsy and uncontrolled primary generalized tonic-clonic seizures. Epilepsia 2015;56(S1):215; abstract p0878. [Google Scholar]
- 15. Cox DR. Regression models and life-tables. J R Stat Soc Series B 1972;34:187–202. 10.1111/j.2517-6161.1972.tb00899.x [DOI] [Google Scholar]
- 16. LaVange LM, Durham TA, Koch GG. Randomization-based nonparametric methods for the analysis of multicentre trials. Stat Methods Med Res 2005;14:281–301. 10.1191/0962280205sm397oa [DOI] [PubMed] [Google Scholar]
- 17. Biton V, Montouris GD, Ritter F, et al. . A randomized, placebo-controlled study of topiramate in primary generalized tonic-clonic seizures. Neurology 1999;52:1330–7. 10.1212/WNL.52.7.1330 [DOI] [PubMed] [Google Scholar]
- 18. Biton V, Sackellares JC, Vuong A, et al. . Double-blind, placebo-controlled study of lamotrigine in primary generalized tonic-clonic seizures. Neurology 2005;65:1737–43. 10.1212/01.wnl.0000187118.19221.e4 [DOI] [PubMed] [Google Scholar]
- 19. Trevathan E, Kerls SP, Hammer AE, et al. . Lamotrigine adjunctive therapy among children and adolescents with primary generalized tonic-clonic seizures. Pediatrics 2006;118:e371–8. 10.1542/peds.2006-0148 [DOI] [PubMed] [Google Scholar]
- 20. Berkovic SF, Knowlton RC, Leroy RF, et al. . Placebo-controlled study of levetiracetam in idiopathic generalized epilepsy. Neurology 2007;69:1751–60. 10.1212/01.wnl.0000268699.34614.d3 [DOI] [PubMed] [Google Scholar]
- 21. Biton V, Di Memmo J, Shukla R, et al. . Adjunctive lamotrigine XR for primary generalized tonic-clonic seizures in a randomized, placebo-controlled study. Epilepsy Behav 2010;19:352–8. 10.1016/j.yebeh.2010.07.022 [DOI] [PubMed] [Google Scholar]
- 22. French JA, Krauss GL, Wechsler RT, et al. . Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy: a randomized trial. Neurology 2015;85:950–7. 10.1212/WNL.0000000000001930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Afra P, Strom L, Bainbridge J, et al. . Role of lacosamide as adjunctive treatment of adults with primary generalized epilepsy, 2010. Available: https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/12753 [Accessed 15 Apr 2020].
- 24. Arnold S, Beige A. Adjunctive treatment with lacosamide: an option for generalized epilepsy? 2010. Available: https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/12800 [Accessed 15 Apr 2020].
- 25. Afra P, Adamolekun B. Lacosamide treatment of juvenile myoclonic epilepsy. Seizure 2012;21:202–4. 10.1016/j.seizure.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 26. Zangaladze A, Skidmore C. Lacosamide use in refractory idiopathic primary generalized epilepsy. Epilepsy Behav 2012;23:79–80. 10.1016/j.yebeh.2011.10.023 [DOI] [PubMed] [Google Scholar]
- 27. Miskin C, Khurana DS, Valencia I, et al. . Efficacy and tolerability of lacosamide in the treatment of children with refractory generalized epilepsy. J Child Neurol 2016;31:925–8. 10.1177/0883073816630084 [DOI] [PubMed] [Google Scholar]
- 28. Abarrategui B, García-García ME, Toledano R, et al. . Lacosamide for refractory generalized tonic-clonic seizures of non-focal origin in clinical practice: a clinical and VEEG study. Epilepsy Behav Case Rep 2017;8:63–5. 10.1016/j.ebcr.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ben-Menachem E, Biton V, Jatuzis D, et al. . Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia 2007;48:1308–17. 10.1111/j.1528-1167.2007.01188.x [DOI] [PubMed] [Google Scholar]
- 30. Halász P, Kälviäinen R, Mazurkiewicz-Beldzińska M, et al. . Adjunctive lacosamide for partial-onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia 2009;50:443–53. 10.1111/j.1528-1167.2008.01951.x [DOI] [PubMed] [Google Scholar]
- 31. Chung S, Sperling MR, Biton V, et al. . Lacosamide as adjunctive therapy for partial-onset seizures: a randomized controlled trial. Epilepsia 2010;51:958–67. 10.1111/j.1528-1167.2009.02496.x [DOI] [PubMed] [Google Scholar]
- 32. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 1981;22:489–501. 10.1111/j.1528-1157.1981.tb06159.x [DOI] [PubMed] [Google Scholar]
- 33. Fisher RS, Cross JH, French JA, et al. . Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:522–30. 10.1111/epi.13670 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2020-323524supp001.pdf (316.2KB, pdf)