Abstract
Objective
To investigate the immunomodulatory activity of a traditional Sri Lankan concoction of Coriandrum sativum L. and Coscinium fenestratum (Gaertn.) Colebr., which is a Sri Lankan traditional medicine used to relieve inflammation and cold.
Methods
In vivo anti-inflammatory activity was tested using carrageenan-induced rat paw-edema model. Mechanism of anti-inflammatory activity was assessed by investigating the production of nitric oxide (NO), expression of iNOS enzyme, and reactive oxygen species (ROS) by rat peritoneal cells. The membrane stabilizing activity was also tested. The antibody response was determined by assessing the specific haemagglutination antibodies raised against sheep red blood cells.
Results
The three doses of freeze-dried concoction used ((human equivalent dose (HED)—183 mg/kg) 2 × HED and 1/2HED; n = 6 rats/group) showed significant inhibition of paw edema compared to water control at 3rd–5th hours (p < 0.05). Both HED and 1/2HED exhibited marked anti-inflammatory activity (72–83% inhibition at 4th-5th hours; p < 0.05). The HED of the concoction showed significant inhibition of NO (77.5 ± 0.73%, p < 0.001) and ROS production (26.9 ± 2.55%; p < 0.01) by rat peritoneal cells. Inhibition of NO production in the concoction treated rat peritoneal cells was confirmed by the lack of iNOS expression. The concoction also exhibited significant membrane stabilizing activity (IC50 = 0.0006 μg/ml; p = 0.001). HED resulted in a significantly high induction of specific antibody production against SRBC antigens as detected by SRBC haemagglutination assay (mean day 14 titers 253.3 compared to control: 66.7) (p < 0.01).
Conclusions
The traditional Sri Lankan concoction of C. sativum and C. fenestratum demonstrated potent in vivo anti-inflammatory activity, significant reduction of ROS, and NO production by rat peritoneal cells and the lack of iNOS expression confirmed the low NO production. The increased membrane stability also supports the anti-inflammatory activity of the concoction. Further, this concoction induced a significantly high antibody response reflecting its immunostimulatory activity. Together these results scientifically validate the therapeutic use of the concoction of C. sativum and C. fenestratum in Sri Lankan traditional medicinal system for immunomodulatory effects.
1. Introduction
Many medicinal plants are found to have an array of pharmacological properties that could be applied in immunomodulation such as immunostimulants, tonic, neurostimulant, antibacterial, antiviral, antirheumatic, and anticancer [1]. In Sri Lanka, many herbs and medicinal plants are used in Ayurveda and in indigenous medicinal practices for centuries. In traditional medicine, the combination of Coriandrum sativum L. (family: Apiaceae) and Coscinium fenestratum (Gaertn.) Colebr. (family: Menispermaceae) is used as an immunomodulator for various types of ailments including relief of pain, inflammation, cold, and other viral infections for centuries [2]. Immunomodulatory and anti-inflammatory agents are therapeutically important since the pathogenesis of the common cold involves a complex interplay between replicating viruses and the host's inflammatory response [3].
A decoction is made using equal amounts of seeds of C. sativum (coriander; “Kottamalli” or “Kothamburu” in Sinhala and “Kottamalli” in Tamil) and stem of C. fenestratum (calumba wood or tree turmeric, “Veniwalgatta” in Sinhala; “Maramanjal” in Tamil) is a well-known home remedy in Sri Lanka for cold and inflammations, especially during the early stage of infection [2]. These two ingredients are also constituents of the commercially available formulations called “Paspanguwa” along with three other plants parts, Zingiber officinale Roscoe., Oldenlandia corymbosa L., and Solanum surattense Burm.f.) and also in another commercial formulation called Samahan which is a combination of 14 ingredients including these two [4].
The two plant parts, seeds of C. sativum and stem of C. fenestratum, are known to have a range of uses in traditional medicine and in Ayurveda. Coriander is used for treatment for anxiety, flatulence, loss of appetite, and convulsions [5]. Coriander seeds are used as carminative, diuretic, tonic, stimulant, stomachic, cooling agent, aphrodisiac, and analgesic [6]. Coriander has been attributed to have several medicinal uses, having antidiabetic, diuretic, cholesterol lowering, anticancer, anti-inflammatory, antifungal, antihelmintic, antioxidant, and antimicrobial effects [7–11]. Stem of C. fenestratum is thermogenic, ophthalmic, anti-inflammatory, vulnerary, depurative, stomachic, antiseptic, febrifuge, sudorific, and tonic [12, 13]. Stem pieces of C. fenestratum are boiled and one cup is given for a fresh, deep cut, being the most common use against tetanus [2]. The root bark is used for dressing wounds and ulcers. C. fenestratum powder is mixed with ghee and used to apply for quick healing of ulcers. For snake bite poisoning, paste of C. fenestratum and turmeric is applied. C. fenestratum is reported to have anticancer, antimicrobial, antidiabetic, and antioxidant effect and is also used to treat cholera, gastroenteritis, and bleeding piles [14–16]. The seeds of C. sativum and stem of C. fenestratum have previously been shown to have anti-inflammatory activity when tested alone and as ethanolic concoctions of individual ingredients [17, 18]. Some immunostimulatory activity has also been reported with aqueous and ethanolic concoctions of C. sativum when used as a single ingredient [19–22].
The main objective of this study was to scientifically validate the traditional use of this concoction of seeds of C. sativum and stem of C. fenestratum as an immunomodulator. More specifically, we investigated its in vivo anti-inflammatory activity using the carrageenan-induced rat paw-edema model and its effect on some of the immune cellular mechanisms including the production of nitric oxide (NO) and reactive oxygen species (ROS) and the expression of inducible nitric oxide synthase (iNOS) by rat peritoneal cells, membrane stabilizing activity of the concoction, and its immunostimulatory activity in enhancing antibody response.
2. Materials and Methods
2.1. Materials
All chemicals and consumables, unless otherwise stated, were purchase from Sigma Aldrich, USA. Wistar Albino rats and Sheep red blood cells (SRBC) were purchased from Medical Research Institute (MRI), Colombo 08, Sri Lanka. Rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH), rat iNOS, endothelial NOS (eNOS), and neuronal NOS (nNOS) primers and random primers were obtained from Integrated DNA Technologies, USA. RT-PCR and PCR reagents including chloroform, diethyl pyrocarbonate, dNTPs, Go Taq Flexi buffer, isopropyl alcohol, MgCl2, M-MLV reverse transcriptase, RNasin®, RT buffer, and Taq polymerase were purchased from Promega Cooperation. Madison, USA. DNA (100 base pair) ladder was obtained from New England Bio Labs United Kingdom.
The reference drugs, aspirin, indomethacin, and prednisolone and also the syringes, needles, surgical blades, and cannulas (18G) were purchased from State Pharmaceuticals Corporation of Sri Lanka. Tissue culture plates (24 wells and 96 wells round and flat bottom), plates for Enzyme Linked Immunosorbant Assay (ELISA), round bottom tissue culture plates were purchased from Nunc, USA, and nitrocellulose filters (2 μM) and Whatman filter papers (No. 1) were obtained from Whatman Int. Ltd., UK. Reusable rat feeding needle was purchased from Orchid Scientifics, India.
2.2. Preparation of the Concoction of C. sativum and C. fenestratum
Seeds of C. sativum and stems of C. fenestratum were purchased from a reputed Ayurvedic store in Colombo, Sri Lanka, and authenticated by Dr. Chandima Wijesiriwardena at the Industrial Technology Institute, Colombo, Sri Lanka. Voucher specimens of C. sativum and C. fenestratum were deposited at the Institute of Biochemistry, Molecular Biology, and Biotechnology (IBMBB), University of Colombo, Sri Lanka.
The concoction was made according to traditional Sri Lankan medicinal practice [2], by boiling 30 g each of C. sativum seeds and C. fenestratum stem in 1920 ml of water in a copper vessel till it reached approximately 240 ml. The concoction was filtered using Whatman No. 1 filter paper and freeze dried (Freezone 4.5-Labconco Corporation, USA). The human equivalent dose (HED) was calculated using the following formula [23].
| (1) |
2.3. Experimental Animals
Wistar strain adult male and female rats weighing 150–250 g were purchased from the Medical Research Institute, Colombo, Sri Lanka. Rats were acclimatized for one week and randomly grouped (n = 6) according to their weights. Rats were housed in the animal house of IBMBB, University of Colombo, under standard conditions (temperature 28–31°C, photoperiod approximately, 12 hours natural day light per day, relative humidity 50–55%). The animals were fed with pellet food purchased from Diamond Stores, Colombo 06, Sri Lanka, and clear drinking water ad libitum.
All experiments were conducted in accordance with the internationally accepted laboratory animal use and care, based on 3 Rs. Ethical clearance was obtained from the Research, Ethics and Higher Degrees committee of the IBMBB, University of Colombo. Animals were subjected to mild ether anesthesia for all procedures.
2.4. Assessment of In Vivo Anti-Inflammatory Activity of the Concoction by Using Carrageenan-Induced Rat Paw-Edema Assay
Three doses of freeze-dried concoction-human equivalent dose (HED-183 mg/kg), high dose (2 × HED-366 mg/kg), and a low dose (1/2HED - 92 mg/kg), were orally administered to three groups of rats (n = 6/group). Indomethacin (5 mg/kg) was used as the reference drug (positive control) and water (2 ml) was administered to the control group. Paw volumes were measured hourly, after the carrageenan (0.1 ml of 1% carrageenan) injection on the left hind paw by using a digital Plethysmometer (Panlab sl., Barcelona, Spain) as described previously [24, 25].
2.5. Assessment for Nitric Oxide Production by Rat Peritoneal Cells
Peritoneal cells were collected as described previously [26]. Three groups of rats were orally treated with HED (the optimum dose selected), prednisolone as reference drug (10 mg/kg), and water as control. One hour after the oral treatment, 1 ml of 0.1% carrageenan (1 mg/ml) was injected to the rat peritoneal cavity. Two hours after this, 40 ml of sterile phosphate buffered saline (PBS) was injected and approximately 35 ml of fluid was drained from peritoneal cavity. The drained peritoneal fluid was centrifuged at 500g for 10 minutes and resuspended in 1 ml of RPMI-1640 medium containing 1% bovine serum albumin (BSA) and total cell and differential cell counts were taken using a Neubauer's haemocytometer (Neubauer, Germany).
Rat peritoneal cells as described above were used to evaluate the inhibitory effect of the HED against the production of nitric oxide. Cell suspension (200 μl of 1 × 106/ml) was plated in 96 well tissue culture (TC) plates where 6 wells were maintained for each rat. The TC plate was incubated for 24 hours at 37°C in a 5% CO2 incubator. After 24 hours, the supernatant was collected, centrifuged at 10,000g for 10 minutes, and stored at −20°C for quantification of nitrite levels. Griess assay was used to quantify nitrite levels in rat peritoneal culture supernatants by mixing 100 μl of culture supernatant with equal volume of Griess solution (equal amounts of 1% Sulphanilamide and 0.1% N-(naphtyl) ethlenediamine hydrochloride) [27]. Optical density at 540 nm was measured 15 minutes after adding the Griess solution using an ELISA microplate reader (ELx 800-Universal Microplate Reader, Biotek Instruments, Canada). A dilution series of NaNO2 standards from 100 to 0.781 μM were used to prepare nitrite standard curve. The amount of nitrites in μM was computed from the standard curve plotted for NaNO2.
2.6. Assessment for the Expression of iNOS by Rat Peritoneal Cells
Total RNA was extracted from rat peritoneal phagocytic cells using the TRIzol reagent (1 ml of TRIzol to 1 × 106 cells) according to the manufacturer's instructions (Invitrogen, USA). Extracted RNA was quantified and then cDNA was synthesized using RNA (2 μg), dNTPs mixture (2 mM), random primers (500 ng), RNAsin (25 units), M-MLV reverse transcriptase enzyme (200 units), and the RT buffer (1X) and PCR was carried out for selected genes iNOS, eNOS, nNOS, and GAPDH independently using the same cDNA. Primers for rat iNOS were selected according to Linenluke et al. and thermal cycle parameters were initial denaturation at 94°C for 5 min followed by 35 cycles of 94°C for 1 min, 62°C for 1 min, 72°C for 1 min, and the final extension of 72°C for 10 min [28]. Primers for other constitutive forms of NOS, eNOs, and nNOS were selected as indicated in Liu et al. (annealing temperature for rat eNOS was 60°C, whereas the annealing temperature for rat nNOS was 62°C) [29]. Rat GAPDH gene which was used as the control or house-keeping gene was also amplified with the primers indicated in Wu et al. [30]. All amplified PCR products were resolved in 2% agarose gel and visualized by the UV transilluminator (Vilber–Laumart gel documentation system).
2.7. Assessment of ROS Production in Rat Peritoneal Cells
Peritoneal cells collected as described in Section 2.5 were used to evaluate the effect of the HED against the production of ROS. Concentration of cell suspensions were adjusted to 4 × 105 cells/ml using complete RPMI containing 10% fetal bovine serum (cRPMI) and 8 × 104 cells in 200 μl of cell suspension were plated in 24-well culture plate and the final volume/well was increased to 400 μl with 200 μl of cRPMI added to each well. For each rat, 3 wells were maintained. Diphenyleneiodonium chloride (DPI) was used as in vitro positive control. For this, 200 μl of cell suspension obtained from a rat treated with water was plated with 200 μl of 10 μM DPI in cRPMI. Plate was incubated for 1 hour at 37°C with 5% CO2 to allow the cell attachment. After one hour, 200 μl of supernatant was removed, 200 μl of 2 mg/ml of nitro blue tetrazolium (NBT) with 12 μg/ml phorbol 12-myristate 13-acetate was added to each well and the plate was incubated for 30 minutes at 37°C with 5% CO2. After half an hour supernatant was removed and plate was washed twice with prewarmed (37°C) PBS. The plate was fixed using 70% methanol and allowed to dry and 120 μl of 2 M KOH and 140 μl of absolute dimethyl sulphoxide were added to each well and the plate was placed on a shaker for 10 minutes. Dissolved formazan (200 μl) was transferred into 96-well ELISA plate and absorbance was read at 620 nm. The concentration of O2− was calculated using standard NBT curve as described previously [31].
2.8. Assessment of Membrane Stabilizing Activity of Concoction
This assay was performed by heat-induced haemolysis of rat erythrocytes as described previously [26]. Ten-fold dilution series of concoction was made using PBS for concentrations from 0.0001 to 1000 μg/ml. Dilutions of Aspirin was also made using PBS for the same concentrations and used as the standard drug. PBS was used as control. Rat erythrocytes washed and resuspended in PBS (20 μl) was added to each tube containing 980 μl of each concentration of test, aspirin and control samples. Samples were first incubated at 37°C for 15 min. Cell suspensions were centrifuged at 1500g for 3 min, the supernatants were removed and the cells were resuspended in 1 ml of PBS. Samples were then incubated at 54°C for 25 min to initiate heat-induced haemolysis and centrifuged at 1500g for 5 min. Supernatants (200 μl) were transferred into an ELISA plate and the optical density (OD) was measured at 540 nm. Percentage inhibition of haemolysis was calculated with respect to the controls and inhibitory concentration (IC50) values were derived. Percent inhibition of haemolysis = [(OD control − OD sample/OD control] × 100.
2.9. Assessment of the Effect of the Concoction on Rat Antibody Production and Detection of Antibodies by Haemagglutination Test
This experiment was designed to investigate the effect of oral treatment of rats with the concoction on their specific antibody production against SRBC antigens. The SRBC immunization was performed according to a modification of the previously described method [32–34]. Two groups of rats (n = 6/group) were orally treated with HED of concoction and water on days 1, 2, 3, 7, 8, and 9. A preparation of 0.5 × 109 cells of freeze-thawed SRBC was injected intraperitoneally on days 1 and 7. Serum collected on days 0, 7, and 14 was tested for anti-SRBC antibodies using SRBC haemagglutination assay [35]. Day 0 (preimmune) sera were used as the negative control, while days 7 and 14 sera were collected to ascertain the levels of antibodies after the exposure to SRBC antigen. Haemagglutination plates were incubated at 37°C for 16 hours.
2.10. Statistical Analysis
Data were analyzed using the statistical package SPSS 17. Data were expressed as mean ± SD/SEM. One-way ANOVA was carried out; p ≤ 0.05 were considered as significant. The Mann–Whitney U test and independent t-test were carried out for small sampled tests. One-way ANOVA followed by post hoc Turkey was carried out to compare the inhibition of in vivo anti-inflammatory activity. Pearson corelation was calculated for dose dependency.
3. Results
3.1. In Vivo Anti-Inflammatory Activity of the Concoction
All three doses of the concoction showed significant anti-inflammatory activity which was comparable to the positive control indomethacin (Figure 1). There was a significant decrease in paw volumes in all three groups, compared to water control at 3rd, 4th, and 5th hours (p < 0.05). The doses, 1/2HED and HED, overlapped at the first and the third hour and the ½HED showed the highest percentage inhibition in paw volumes at the fourth (83.5%) and the fifth (80.1%) hours followed by HED (72%). The percent inhibition of the double dose (2 × HED) increased gradually and reached its maximum at the 5th hour (55.6%); however, its overall anti-inflammatory effect was low compared to the other two doses (1/2HED and HED). This resulted in an inverse dose-dependent activity at 4th (r = −0.99; p=0.03) and 5th (r = −1.00; p=0.001) hours. Since HED showed a significant level of anti-inflammatory activity in the first and second phases of inflammation, it was selected as the optimum dose for further assays. The concoction reported hereafter in the results section is the HED of the concoction.
Figure 1.
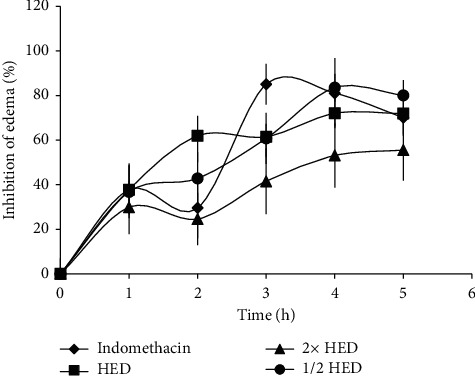
The inhibition of rat paw edema by the concoction of C. sativum and C. fenestratum. Inhibition of paw volume was assessed from rats treated with 1/2HED, HED and 2HED of the concoction, indomethacin (5 mg/kg), and water. HED: Human equivalent dose. Values represent mean ± SEM; n = 6 rats per group.
3.2. Inhibition of Nitric Oxide Production and iNOS Expression by Rat Peritoneal Cells
As shown in Figure 2, nitrite level was significantly inhibited by the concoction and percent inhibition was 77.5 ± 0.73% (p < 0.001). The reference drug and prednisolone had inhibited nitrite levels similarly by 91.5 ± 0.69% (p < 0.001).
Figure 2.
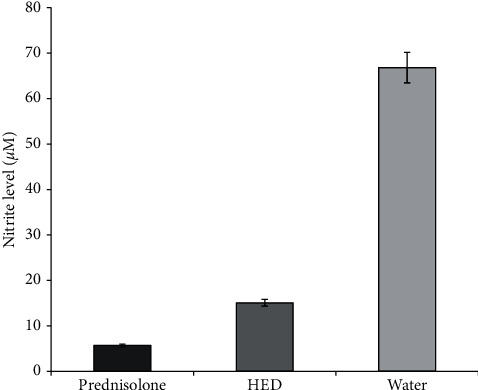
Effect of the human equivalent dose (HED) of the concoction on NO production by rat peritoneal cells. NO production by peritoneal cells obtained from rats treated with HED of the concoction and prednisolone (10 mg/ml) and from control group was assessed. Data represents mean ± SEM.
As shown in Figure 3, amplifications of GAPDH were observed in all three samples (RNA obtained from the rats injected with carrageenan and treated with the reference drug (prednisolone), water, and concoction). Amplification of iNOS was observed only in the control (water treated) and was absent in the concoction treated and the group treated with the reference drug. Amplification of nNOS was clearly observed for concoction treaded group, whereas amplification of both nNOS and eNOS was weak in the group treated with the reference drug.
Figure 3.
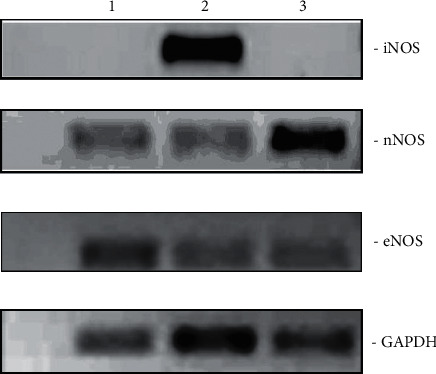
NOS gene expression in peritoneal cells from carrageenan-injected rats orally administered with prednisolone, lane 1; water, lane 2; and the concoction, lane 3. iNOS: inducible NOS; nNOS: neuronal NOS; eNOS: endothelial NOS; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
3.3. Inhibition of In Vivo ROS Production by Rat Peritoneal Cells
As shown in Figure 4, concoction had significantly inhibited the O2− production and percent inhibition was 26.9 ± 2.55% (p=0.002). The two positive controls, in vivo (prednisolone) and in vitro (DPI), had also inhibited the O2− production significantly and percent inhibitions of O2− production were 47.8 ± 1.78% and 48.5 ± 1.97%, respectively (p < 0.001).
Figure 4.
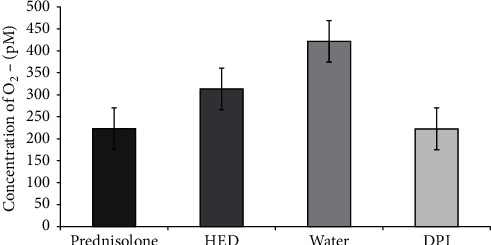
Effect of the human equivalent dose (HED) of the concoction on ROS production by rat peritoneal cells. ROS production by peritoneal phagocytic cells harvested from rats treated with HED of the concoction, prednisolone (10 mg/ml), and water as control. Cells treated with diphenyleneiodonium chloride (DPI) used as in vitro positive control.
3.4. Membrane Stabilizing Activity of the Concoction
As shown in Figure 5, the concoction showed 88.34% of inhibition for heamolysis at 0.1 mg/ml and comparable to the reference drug aspirin (88.36%; p=0.001) and IC50 value of the concoction was 0.0006 μg/ml.
Figure 5.
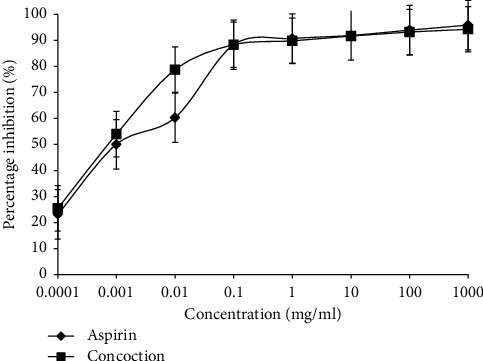
Membrane stabilizing activity of the concoction. Percent inhibition of haemolysis of rat red blood cells treated with the concoction and aspirin (positive control) was assessed. Data represent average from three repeat experiments, n = 6. Data represents mean ± SEM.
3.5. Effect of Oral Administration of the Concoction on Rat Antibody Production
Effect of oral administration of the concoction on rat antibody production was evaluated by assessing the SRBC haemagglutination titers. The sera obtained on day 0 showed no antibodies against SRBC in both groups. On day 7, the SRBC haemagglutination titers in both concoction and control groups of rats were low and comparable (p > 0.05), but both groups showed agglutination (Table 1). Titers of the concoction group on days 7 and 14 increased significantly from 100 (mean) to 253.33 (p=0.03), whereas no significant change was observed in the control group. By day 14, the group of rats treated with the concoction showed a significant increase in their SRBC haemagglutination titers compared to day 14 sera of the control (mean value 253.3 and 66.7, respectively; p=0.004). This showed that after two sets of 3-day oral treatment (and with two sets of antigen exposures), the concoction was able to induce a significant increase in the specific antibody response against SRBC.
Table 1.
Effect of oral administration of the concoction of C. sativum and C. fenestratum on production of haemagglutination antibodies against SRBC.
| Dose | SRBC haemagglutination titer | Significance | |
|---|---|---|---|
| Day 7 | Day 14 | ||
| Mean ± SEM | Mean ± SEM | (p)∗ | |
| HED | 100.0 ± 21.7 | 253.3 ± 43.4 | 0.030 |
| Control | 106.7 ± 26.7 | 66.7 ± 20.0 | 0.223 |
| Significance (p)† | 0.872 | 0.004 | — |
∗Paired t-test. †Independent sample t-test for comparing HED with control. SRBC: sheep red blood cells, SEM: standard error of the mean, and HED: Human equivalent dose.
4. Discussion
This study was designed to determine the immunomodulatory activity of the concoction of C. sativum and C. fenestratum. This was first ascertained by the in vivo anti-inflammatory activity using the carrageenan-induced rat paw edema model and the results showed a significant anti-inflammatory activity of the concoction. Since HED of concoction showed a significant level of anti-inflammatory activity at both first and second phases of inflammation, it was selected as the optimum dose for subsequent experiments to determine possible mechanisms of its anti-inflammatory activity. Further, this study demonstrated significant inhibition of nitric oxide and superoxide anion production by rat peritoneal cells and also in vitro membrane stabilizing capacity reflecting their possible contribution to the anti-inflammatory mechanisms. The specific inhibition of the expression of iNOS by the oral administration of the concoction confirmed the marked decrease of NO production by the rat peritoneal cells. The immunomodulatory activity was also shown by the enhancing effect on specific immune responses as evident by the high antibody titers raised against SRBC antigens following oral administration of concoction. This study reports for the first time the immunomodulatory activity of the concoction of C. sativum and C. fenestratum, including in vivo anti-inflammatory activity with mechanisms, that is, inhibition of ROS and RNS production and also the inhibition of iNOS gene expression by rat peritoneal cells and the immunomodulation to enhance antigen specific antibody response. Further, the findings of this study validate the use of the concoction (or decoction of C. sativum and C. fenestratum) for its traditional claims and use for treatment of cold and as a treatment at early stage of infections.
In the carrageenan-induced paw-edema assay, the two doses (HED and 1/2HED) of the concoction showed significant anti-inflammatory effect during both early and late phases and there was marked inhibition during the late phase. The difference in the phases of the assay may occur due to different chemical and cellular components which come into action in the early, intermediate, and late phases of inflammation [36]. The inhibition in the first phase of inflammation is attributed to activities against serotonin and histamine, whereas inhibition during second phase is mainly due to activities against prostaglandins and suppression of mononuclear leukocyte migration [36]. In contrast to the biphasic pattern, the high inhibition during 2nd hour (intermediate phase) shown by the HED of the concoction indicates that it may have inhibited the kinins which are known to play a role in between the first and second phases [37]. The inverse dose response observed in the present study with the 2 × HED exhibiting a lower anti-inflammatory activity is consistent with the results of a previous study where the potency of a high dose of an alcoholic preparation of coriander (500 mg/ml) was less compared to a low dose (200 mg/ml) [37]. In addition, two other studies have reported anti-inflammatory activity of ethanolic extract of C. sativum [17, 38]. Ammar et al. have attributed the inhibitory effects of coriander fruits (one of the components in the concoction) to inhibition of all mediators released before the second phase as well as prostaglandin, the mediator in the second phase. Phytochemical studies on bioactive extracts of coriander have revealed the presence of unsaturated fatty acids, flavanoids, that is, quarcertin which may together produce an anti-inflammatory effect [37].
It is noteworthy that a comparative study with ethanolic extracts of Curcuma aromatica Salisb. and C. fenestratum have reported that C. fenestratum at a dose of 8 mg/kg exhibited in vivo anti-inflammatory to a lesser extent (34% inhibition at 3 hours). The anti-inflammatory activity was attributed to the presence of tannins and flavonoids [18]. Similarly, another comparison with ethanolic extracts of fruits of Coriandrum sativum leaves of Datura stramonium and Azadirachta indica at 200 mg/kg doses has shown that C. sativum had the less potent anti-inflammatory activity (41% inhibition at 3 hours) compared to A. indica which had the highest activity as reported in this study [17]. The present study used the traditional preparation of the concoction which has shown a much higher anti-inflammatory activity with half HED dose (92 mg/kg) having 84% inhibition. These differences in anti-inflammatory activity may either be attributed to the different types of extraction types having different constituents with their intrinsic potencies. It may be possible that the aqueous extract used in the present study has more potent constituents or that when used as a combination they may show a synergistic effect. This anti-inflammatory effects supports the widespread use of this combination in traditional medicinal practice in Sri Lanka and its use at the early stages of viral infections causing cold related symptoms.
Recent studies have shown that seed oil of Coriandrum sativum contains 53 compounds of which the major compounds are linalool, geranyl acetate, and γ-terpinene, β-pinene, m-cymene, citronellal, citronellol, citral, geraniol, citronellyl acetate, α-cedrene, and α-farnesene and β-sesquiphellandrene [39]. The major alkaloids in Coscinium fenestratum are yellow crystalline berberine, protoberberine, and jatrorrhizine. Many other alkaloids, mainly of the protoberberine type, isolated from stem are magnoflorine, berberrubine, thalifendine, palmitine, and oxyberberine [40–46]. The stem also contains ceryl-alcohol, saponin, hentriacontane, sitosterol, palmitic acid, oleic acid, and sitosterol glucoside [40, 43]. Other compounds reported from the stem are N,N-dimethyllindacarpine, oxypalmitine, (-)-8-oxotetrahydrothalifendine, (-)-8-oxoisocorypalmine and either (-)-8-oxothaicanine or (-)-8-oxo-3-hydroxy-2,4,9,10-tetramethoxyberberine and (-)-8-oxocanadine [45], 12,13-dihydro-8-oxoberberine, 5,6,13,13 a-tetrahydro-9,10, dimethoxydibenzo (a,g) 1,3-benzodioxolo (5,6a) quinalizine-8-one, stigmasterol [44], berlambine, dihydroberlambine, and noroxyhydrastinine [47]. Despite these phytochemical analyses, to date there is no reported study on attributing the anti-inflammatory activity to specific isolated components. Studies on identification of active components of the concoction are in progress. The consistency of the components in the concoction has been confirmed with the identical pattern obtained from thin layer chromatography (TLC) fingerprints (data not shown).
Recruitment of phagocytic cells (neutrophils and macrophages) are a characteristic feature of the late phase of inflammation [36]. In our previous studies on Ixora coccinea, we have shown that inhibition of the cell migration to the peritoneal cavity or to the site of inflammation as a mechanism that makes a significant contribution to the anti-inflammatory effect [26]. In the early stage of action, polymorphonuclear cells predominate whereas mononuclear cells are more potent at the late stage [37]. In the present study, the HED of the concoction which exhibited the optimum anti-inflammatory activity was used to assess its effect on production of ROS (O2−) and RNS (NO) by peritoneal cells. The significant decrease of both ROS and RNS reflects the cellular mechanisms that support the in vivo anti-inflammatory action shown in the paw-edema assay. Our previous studies on methnoloic leaf extract of I. coccinea and aqueous leaf extracts Vitex negundo have also shown similar inhibitory activity of NO and ROS production by rat peritoneal cells [26, 48].
This study showed a considerable reduction in the nitrite levels in rat peritoneal cells obtained after the oral treatment of the concoction. The nitrite levels of the concoction treated rat cells are comparable to that of prednisolone treated group which has shown higher inhibition of NO production. Nitric oxide produced by inducible NOS plays an important role in inflammation and in regulation of the immune system [49]. Oral administration with the concoction showed a specific effect on expression of iNOS, whereas it had no effect on the expression of either nNOS or eNOS genes, the constitutive forms of NOS, which are necessary for the normal cell functions. In contrast, the carrageenan-induced and water treated control group was positive for iNOS gene expression. The reference drug used as the positive control has also shown a significant inhibitory effect on iNOS gene expression as well as the constitutive forms of NOS. The presence of GAPDH gene expression which is a house keeping gene [50] in all three groups was indicative of normal cell functions despite the specific effect on iNOS gene expression. These findings confirm that the significantly reduced nitric oxide production in the concoction treated group was due to the specific inhibition of expression iNOS gene. It is also important to note that the concoction treatment has less or no inhibitory effects on the constitutive forms of NOS unlike the reference drug used as the positive control.
The reduced or excess production of iNOS is known to leads to many immunological disorders. It is also responsible for the deleterious effects in inflammation [51, 52]. Many plant components, such as flavonoids, sesquiterpene, and polyphenols, have been shown to inhibit the iNOS expression [53]. C. sativum and C. fenestratum both contain many flavonoids, alkoloids, and phenolics [14, 54], which could have contributed to the low production of nitric oxide. However, no specific components have been shown to have the iNOS inhibitory activity from either C. sativum or C. fenestratum.
One of the most immediate responses of monocytes to a variety of pathogenic stimuli is the production of the potent oxygen free radical, superoxide anion. The enzyme complex primarily responsible for the production of this highly reactive oxygen species is the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex. Superoxide anion is the first ROS produced and it can combine with nitric oxide to produce peroxinitrite which is highly deleterious to tissues [55]. Quantitative assessment of superoxide anion production by NBT assay showed the capability of concoction to inhibit superoxide anion production in rat peritoneal cells. Thus, the ability of the concoction to inhibit production of both NO and superoxide anion would contribute to preventing the formation of peroxinitrite. Our previous studies using the quantitative NBT assay have shown similar ROS inhibitory activity with methanolic leaf extracts of Ixora coccinea [25].
The concoction showed a significant membrane stabilizing activity, which indicates that it may be another mechanism that would contribute to the anti-inflammatory effects observed. At the onset of an inflammation, the cells undergo activation and release inflammatory mediators. Stabilization of cell and cell organelle membranes would prevent the release of inflammatory mediators. Membrane stabilization has been identified as a mechanism of anti-inflammatory activity of other medicinal plants such as Solanum aethiopicum and Basella alba [56, 57].
It is noteworthy that our findings on the concoction in the rat experimental system on enhanced antibody production are consistent with some of the previous studies when only the C. sativum extracts were used. Different preparations of aqueous and ethanolic extract of C. sativum had shown immunostimulant activity in mice [19], chickens [20], and fish [21, 22]. It is possible that the immunostimulatory activity observed in the concoction of C. sativum or C. fenestratum is attributable to the effect from C. sativum. Alternately, it may be due to a combined effect of C. sativum or C. fenestratum since some of the immunostimulatory effects observed in the present study was comparatively higher compared to those observed when C. sativum was used by itself. Further studies are in progress to investigate on the exact active component(s) responsible of the increased antibody production and the specific mechanisms of activation of lymphocytes in producing an enhanced antibody response.
Interestingly, the concoction or the combined extract used in the present study exhibited both anti-inflammatory as well as immunostimulatory effects showing a broad spectrum of immunomodulation. This shows the capacity of the concoction to modulate the innate immune response as well as induce an enhanced antibody response against specific antigens SRBC. These immunomodulatory effects may have direct relevance to its traditional use in treatment of inflammation and cold where it could suppress inflammation relieving the cold symptoms, while the specific antibodies raised may prevent having antiviral effects preventing viral replication and further progression of the disease symptoms. According to traditional medical practitioners, the concoction is prescribed when an individual gets first symptoms of an infection meaning during or soon after an antigen exposure. It is also one of the home remedies practiced for years for treatment at early stage in infection [2]. It is noteworthy that rats were orally treated with the decoction just prior to the SRBC antigen exposure and in consecutive 2 days in both immunizations (day 1 and day 7). Thus, these rats were treated in a similar manner to prescribed ailment before checking their antibody levels and a marked elevation in antibody levels was observed within 14 days (for HED of concoction). This indicates the concoction in its traditionally prescribed dose (HED) has the capacity to boost the immune system to protect against an infection or by strengthening the immune system to combat the disease effectively by elevating antibodies against the specific antigen. This also further emphasizes an adjuvant effect by this concoction on antibody production against a specific antigen preparation. In a previous study, leaf extract of Vitex negundo and our previous studies have reported its adjuvant effect (to standard anti-inflammatory drugs) [48, 58]. These results emphasize further studies on identifying the bioactive components and other cellular mechanisms of lymphocyte activation such as the early and increased expression of costimulatory molecules that would enhance the immune response.
5. Conclusions
This study has shown the immunomodulatory activity of a concoction of C. sativum and C. fenestratum by possessing both anti-inflammatory (inhibition of inflammation, nitric oxide, superoxide anion production, and membrane stabilizing activity) and immunostimulatory (enhancement of antibody production) activities. The markedly reduced nitrite levels and superoxide anion and lack of iNOS gene expression in rat peritoneal cells and increased membrane stability may be the key immune cellular mechanisms which support this anti-inflammatory activity of the concoction. Furthermore, the significant elevation of antibody levels clearly supports the immunostimulatory activity which also shows long-term protection against specific antigens. Therefore, this study for the first time scientifically validates the therapeutic claim of Sri Lankan traditional use of the concoction of coriander (C. sativum) and veniwalgata (C. fenestratum) for immunomodulatory effects.
Acknowledgments
The authors are grateful to Dr. Chandima Wijesiriwardena at Industrial Technology Institute, Colombo 07, Sri Lanka, for authenticating the medicinal plant material and Peshala Gunasekera for providing the HPLC analysis of the different preparations of hot water extract. G. Janutharsan is acknowledged for their support in handling animals. The source of support is as follows: MSc programme funds of the Institute of Biochemistry, Molecular Biology, and Biotechnology (IBMBB), University of Colombo.
Data Availability
The data used to support the findings of this study are available from the first author and corresponding author upon request.
Additional Points
(1) The concoction of Coriandrum sativum and Coscinium fenestratum shows a significant dose-dependent reduction of inflammation as shown in the rat paw edema model. (2) Several in vitro mechanisms were shown to contribute to the anti-inflammatory effect: (a) marked inhibition of NO and ROS production by rat peritoneal cells, (b) absence of iNOS expression in rat peritoneal cells confirm the inhibition of NO production, and (c) significant membrane stabilizing activity. (3) The concoction of C. sativum and C. fenestratum showed a significantly higher antibody response against specific antigens.
Conflicts of Interest
Shashika Dinethri Kothalawala is a Ph.D. Trainee, Institute for Veterinary Anatomy-Histology and Embryology, Justus Liebig University, Giessen, Germany. Jayamini C Harasgama is a PhD Trainee, Department of Marine Life Sciences and Fish Vaccine Research Center, Jeju National University, Republic of Korea. Galbada Arachchige Sirimal Premakumara is a Senior Lecturer, Department of Basic Sciences and Social Science, Faculty of Nursing, University of Colombo, Colombo 3. The other authors declare no conflicts of interest.
Authors' Contributions
SDK, DE, OVDSJW, WDR, GASP, and SMH designed the study. SDK, DE, and JCH collected the medicinal plant material, and SDK, DE, JCH, LR, and RN carried out the experimental work. SDK and DE wrote the first draft of the manuscript. SDK, DE, JCH, LR, RN, OVDSJW, and SMH performed the data analysis. SDK, WDR, GASP, and SMH critically revised the manuscript. All authors contributed to the final version of the manuscript. The manuscript has been read and approved by all the authors and each author believes that the manuscript represents honest work.
References
- 1.Shah A. S., Wakade A. S., Juvekar A. R. Immunomodulatory activity of methanolic extract of Murraya koenigii (L) Spreng. leaves. Indian Journal of Experimental Biology. 2008;46(7):505–509. [PubMed] [Google Scholar]
- 2.Wimalasekara S. In: Osuthuru Visithuru. Part 3. Gunatilleke S., editor. Colombo, Sri Lanka: Department of Ayurveda; 1994. [Google Scholar]
- 3.Heikkinen T., Järvinen A. The common cold. The Lancet. 2003;361(9351):51–59. doi: 10.1016/s0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goonaratna C., Sooriyarachchi M. The effect of a herbal formulation on the incidence and severity of upper respiratory symptoms in healthy volunteers: an open-label, randomised controlled clinical trial. Ceylon Medical Journal. 2012;57(1):19–32. doi: 10.4038/cmj.v57i1.4197. [DOI] [PubMed] [Google Scholar]
- 5.Emamghoreishi M., Khasaki M., Aazam M. F. Coriandrum sativum: evaluation of its anxiolytic effect in the elevated plus-maze. Journal of Ethnopharmacology. 2005;96(3):365–370. doi: 10.1016/j.jep.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Chaudry N. M. A., Tariq P. Bactericidal activity of black pepper, bay leaf, aniseed and coriander against oral isolates. Pakistan Journal of Pharmaceutical Science. 2006;19(3):214–218. [PubMed] [Google Scholar]
- 7.Reuter J., Huyke C., Casetti F., et al. Anti-inflammatory potential of a lipolotion containing coriander oil in the ultraviolet erythema test. Journal of the German Society Dermatology. 2008;34:847–851. doi: 10.1111/j.0105-1873.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- 8.Hamodi S. J., Essa H., Al-Mashhadani E. H., Al-Jaff F. K., Al-Mashhadani H. E. Effect of coriander seed (Coriandrum sativum L.) as diet ingredient on broilers performance under high ambient temperature. International Journal of Poultry Science. 2010;9:968–971. [Google Scholar]
- 9.Nadeem M., Muhammad Anjum F., Issa Khan M., Tehseen S., El‐Ghorab A., Iqbal Sultan J. Nutritional and medicinal aspects of coriander (Coriandrum sativum L.): a review. British Food Journal. 2013;115(5):743–755. doi: 10.1108/00070701311331526. [DOI] [Google Scholar]
- 10.Jesuthasan A. S., Uluwaduge D. I. Ethnobotanics used in folk medicine of tamil culture in Sri Lanka: a scientific review. Journal of Integrative Medicine. 2017;15(1):19–26. doi: 10.1016/s2095-4964(17)60317-0. [DOI] [PubMed] [Google Scholar]
- 11.Elmas L., Secme M., Mammadov R., Fahrioglu U., Dodurga Y. The determination of the potential anticancer effects of Coriandrum sativum in PC‐3 and LNCaP prostate cancer cell lines. Journal of Cellular Biochemistry. 2019;120(3):3506–3513. doi: 10.1002/jcb.27625. [DOI] [PubMed] [Google Scholar]
- 12.Warrier P. K., Nambiar V. P. K., Ramankutty C. Indian Medicinal Plants. Hyderabad, India: Orient Blackswan; 1994. [Google Scholar]
- 13.Taher M., Amri M. S., Susanti D., Kudos M. B. A., Shafawi A. N., Yazid S. N. Coscinium fenestratum: a review on phytochemicals and pharmacological properties. In: Swamy M. K., Akhtar M. S., editors. Natural Bio-Active Compounds. Berlin, Germany: Springer Nature Singapore Pvt. Ltd.; 2019. pp. 107–125. [Google Scholar]
- 14.Tushar K. V., George S., Remashree A. B., Balachandran I. Coscinium fenestratum (Gaertn) Colebr. a review on this rare critically endangered and highly traded medicinal species. Journal of Plant Sciences. 2008;3:133–145. [Google Scholar]
- 15.Rojsanga P., Sukhthankar M., Krisanapun C., Gritsanapan W., Lawson D. B., Baek S. J. In vitro anti-proliferative activity of alcoholic stem extract of Coscinium fenestratum in human colorectal cancer cells. Experimental and Therapeutic Medicine. 2010;1(1):181–186. doi: 10.3892/etm_00000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rai R. V., Rajesh P. S., Kim H.-M. Medicinal use of Coscinium fenestratum (Gaertn.) Colebr.: an short review. Oriental Pharmacy and Experimental Medicine. 2013;13(1):1–9. doi: 10.1007/s13596-012-0094-y. [DOI] [Google Scholar]
- 17.Sonika G., Manubala R., Deepak J. Comparative studies on anti-inflammatory activity of Coriandrum sativum, Datura stramonium and Azadirachta indica. Asian Journal of Experimental Biological Sciences. 2010;1(1):151–154. [Google Scholar]
- 18.Sudharshan S. J., Prashith K. T. R., Sujatha M. L. Anti-inflammatory activity of Curcuma aromatica Salisb and Coscinium fenestratum Colebr: a comparative study. Journal of Pharmacy Research. 2010;3(1):24–25. [Google Scholar]
- 19.Sharma V., Kansal L., Sharma A., Lodi S., Sharma S. H. Ameliorating effect of Coriandrum sativum extracts on hematological and immunological variables in an animal model of lead intoxication. Journal of Pharmacy and Allied Health Sciences. 2011;1(1):16–29. doi: 10.3923/jpahs.2011.16.29. [DOI] [Google Scholar]
- 20.Hosseinzadeh H., Qotbi A. A. A., Seidavi A., Norris D., Brown D. Effects of different levels of coriander (Coriandrum sativum) seed powder and extract on serum biochemical parameters, microbiota, and immunity in broiler chicks. The Scientific World Journal. 2014;2014:11. doi: 10.1155/2014/628979.628979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Innocent B. X., Fathima M. S. A., Lakshmi D. Studies on the immunostimulant activity of Coriandrum sativum and resistance to Aeromonas hydrophila in Catla catla. Journal of Applied Pharmaceutical Science. 2011;1(7):132–135. [Google Scholar]
- 22.Ahmed S. A. A., Reda R. M., ElHady M. Immunomodulation by Coriandrum sativum seeds (Coriander) and its ameliorative effect on lead‐induced immunotoxicity in Nile tilapia (Oreochromis niloticus L.) Aquaculture Research. 2020;51(3):1077–1088. doi: 10.1111/are.14454. [DOI] [Google Scholar]
- 23.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB Journal. 2007;3:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 24.Winter C. A., Risley E. A., Nuss G. W. Carrageenin-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Experimental Biology and Medicine. 1962;111:3544–3547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 25.Ratnasooriya W. D., Deraniyagala S. A., Galhena G., Liyanage S. S. P., Bathige S. D. N. K., Jayakody J. R. A. C. Anti-inflammatory activity of the aqueous leaf extract of Ixora coccinea. Pharmaceutical Biology. 2005;43(2):147–152. doi: 10.1080/13880200590919483. [DOI] [Google Scholar]
- 26.Handunnetti S. M., Kumara R. R., Deraniyagala S. A., Ratnasooriya W. D. Anti-inflammatory activity of Ixora coccinea methanolic leaf extract. Pharmacognosy Research. 2009;1(2):80–90. [Google Scholar]
- 27.Maccioni M., Cabezas L. E., Rivero V. E. Effects of prostatein, the major protein produced by the rat ventral prostate, on phagocytic cell functions. American Journal of Reproductive Immunology. 2003;50(6):473–480. doi: 10.1046/j.8755-8920.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- 28.Linenluke B., Stojanovic T., Fiebig T., Fayyazi A., German T., Hacker M. Thalidomide impairment of trinitrobenzene sulphonic acid induced colitis in the rat-role of endothelial cell-leukocyte infiltration. British Journal of Pharmacology. 2001;133(8):1414–1423. doi: 10.1038/sj.bjp.0704193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Wan Q., Guan Q., Gao L., Zhao J. High-fat diet feeding impairs both the expression and activity of AMPKa in rats’ skeletal muscle. Biochemical and Biophysical Research Communications. 2006;339(2):701–707. doi: 10.1016/j.bbrc.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 30.Wu X.-H., Liu C. P., Xu K. F., et al. Reversal of hyperglycemia in diabetic rats by portal vein transplantation of islet-like cells generated from bone marrow mesenchymal stem cells. World Journal of Gastroenterology. 2007;13(24):3342–3347. doi: 10.3748/wjg.v13.i24.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wickremasinghe R., Kumara R. R., De Silva D. E., Ratnasooriya W. D., Handunnetti S. M. Inhibition of phagocytic and intracellular killing activity of human neutrophils by aqueous and methanolic leaf extracts of Ixora coccinea. Journal of Ethnopharmacology. 2014;153(3):900–907. doi: 10.1016/j.jep.2014.03.064. [DOI] [PubMed] [Google Scholar]
- 32.Azadmehr A., Hajiaghaee Z., Afshari A., Amirghofran Z., Refiean-Kopaei M., Darani H. Y. Evaluation of in vivo immune response activity and in vitro anti-cancer effect by Scrophularia megalantha. Journal of Medicinal Plants Research. 2011;5(11):2365–2368. [Google Scholar]
- 33.Gabhe S., Tatke P., Khan T. Evaluation of the immunomodulatory activity of the methanol extract of Ficus benghalensis roots in rats. Indian Journal of Pharmacology. 2006;38(4):271–275. doi: 10.4103/0253-7613.27024. [DOI] [Google Scholar]
- 34.Ranjith M. S., Ranjitsingh A. J. A., Shankar S. G., Vijayalakshmi G. S., Deepa K., Sidhu H. S. Enhanced phagocytosis and antibody production by Tinospora cordifolia: a new dimension in immunomodulation. African Journal of Biotechnology. 2008;7:81–85. [Google Scholar]
- 35.Massey E. D., Mangiafico J. A. Microagglutination test for detecting and measuring serum agglutinins of Francisella tularensis. Applied Microbiology. 1947;27(1):25–27. doi: 10.1128/am.27.1.25-27.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vinegar R., Truax J. F., Selph J. L., Jhonston P. R., Venable A. L., McKenzi K. K. Path way to carrageenan-induced inflammation in the hind limb of rat. Federation Proceedings. 1987;48:118–126. [PubMed] [Google Scholar]
- 37.Ammar N. M., Al Okbi S. Y., Mohamed D. A. Study of the anti-inflammatory activity of some medicinal edible plants growing in Egypt. Journal of Islamic Academy of Science. 1997;10:113–122. [Google Scholar]
- 38.Mohan N. P. V., Suganthi V., Gowri S. Evaluation of anti-inflammatory activity in ethanolic extract of Coriandrum sativum L. using carrageenan induced paw edema in albino rats. Der Pharma Chemica. 2013;5:139–143. [Google Scholar]
- 39.Bhuiyan M. N. I., Begum J., Sultana M. Chemical composition of leaf and seed essential oil of Coriandrum sativum L. from Bangladesh. Bangladesh Journal Pharmacology. 2009;4:150–153. doi: 10.3329/bjp.v4i2.2800. [DOI] [Google Scholar]
- 40.Katti M. C. T., Shintre V. P. Chemical examination of the stems of Coscinium fenestratum, Coleb. Archives of Pharmaceutical Research. 1930;268(5):314–321. doi: 10.1002/ardp.19302680505. [DOI] [Google Scholar]
- 41.Child N. W. R. N. The berberine content of Coscinium fenestratum (Colebr.) Current Science. 1943;12:255–256. [Google Scholar]
- 42.Varier N. S., Pillai P. P. A note on the alkaloids of Coscinium fenestratum (Colebr.) Current Science. 1943;12:228–229. [Google Scholar]
- 43.Siwon J., Verpoorte R., van Essen G., Svendsen A. Studies on Indonesian medicinal plants. III. The alkaloids of Coscinium fenestratum. Planta Medica. 1980;38(1):24–32. doi: 10.1055/s-2008-1074833. [DOI] [PubMed] [Google Scholar]
- 44.Malhotra S., Taneja S. C., Dhar K. L. Minor alkaloid from Coscinium fenestratum. Phytochemistry. 1989;28(7):1998–1999. doi: 10.1016/s0031-9422(00)97910-x. [DOI] [Google Scholar]
- 45.Pinho P. M. M., Pinto M. M. M., Kijjoa A., Pharadai K., Díaz J. G., Herz W. Protoberberine alkaloids from Coscinium fenestratum. Phytochemistry. 1992;31(4):1403–1407. doi: 10.1016/0031-9422(92)80301-t. [DOI] [Google Scholar]
- 46.Agusta A. Coscinium fenestratum (Gaertner) Colebr. In: Lemens R. M. H. J., Bunyapraphatsara N., editors. Plant Resources in South East Asia: Medicinal and Poisonous Plants. Vol. 3. 2003. pp. 139–140. [Google Scholar]
- 47.Datta S. C., Mathur R. K., Baruah J. N. Minor alkaloids of Coscinium fenestratum root. Indian Drugs. 1987;25:p. 350. [Google Scholar]
- 48.Kariyawasam K. W. J. C., Sirisena P. D. N. N., Nanayakkara H. L. C., Ratnasooriya W. D., Handunnetti S. M. Vitex negundo L. leaf extract inhibits IL-6 and TNF-α secretion and phagocytosis in human leukocytes. Journal of Herbal Medicine. 2020;21 doi: 10.1016/j.hermed.2020.100341.100341 [DOI] [Google Scholar]
- 49.Tripathi P., Tripathi P., Kashyap L., Singh V. The role of nitric oxide in inflammatory reactions. FEMS Immunology & Medical Microbiology. 2007;51(3):443–452. doi: 10.1111/j.1574-695x.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- 50.Barber R. D., Harmer D. W., Coleman R. A., Clark B. J. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiological Genomics. 2005;21(3):389–395. doi: 10.1152/physiolgenomics.00025.2005. [DOI] [PubMed] [Google Scholar]
- 51.Moncada S., Higgs E. A. The discovery of nitric oxide and its role in vascular biology. British Journal of Pharmacology. 2006;147(1):S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghasemi A., Hedayati M., Biabani H. Protein precipitation methods evaluated for determination of serum nitric oxide end products by the Griess assay. Research Journal of Medical Sciences. 2007;2(15):29–32. [Google Scholar]
- 53.Sultana S., Ripa F. A., Hamid K. Comparative antioxidant activity study of some commonly used spices in Bangladesh. Pakistan Journal of Biological Sciences. 2010;13(7):340–343. doi: 10.3923/pjbs.2010.340.343. [DOI] [PubMed] [Google Scholar]
- 54.Pacher P., Beckman J. S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological Reviews. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sautabin L. Prostaglandins and nitric oxide as molecular targets of anti-inflammatory therapy. Fitotherapia. 2000;71:48–57. doi: 10.1016/s0367-326x(00)00181-7. [DOI] [PubMed] [Google Scholar]
- 56.Anosike C. A., Obidoa O., Ezeanyika L. U. S. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum) DARU Journal of Pharmaceutical Sciences. 2012;20(1):p. 76. doi: 10.1186/2008-2231-20-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar V., Bhat Z. A., Kumar D., Bohra P., Sheela S. In-vitro anti-inflammatory activity of leaf extracts of Basella alba Linn. Var. Alba. International Journal of Drug Development & Research. 2011;3(2):176–179. [Google Scholar]
- 58.Tandon V. R., Gupta R. K. Vitex negundo Linn (VN) leaf extract as an adjuvant therapy to standard anti-inflammatory drugs. The Indian Journal of Medical Research. 2006;124(4):447–450. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the first author and corresponding author upon request.


